Abstract
The intracellular serine protease inhibitors (serpins) are an important family of proteins that protect cells form proteinase-mediated injury. Understanding the tissue and cellular expression pattern of this protein family can provide important insights into their physiologic roles. For example, high expression in epithelial tissues, such as lung, may suggest a biologic function in cellular defense, secretion, or selective absorption. Although the expression pattern of many of the intracellular serpins has been well described, one member of this class, SERPINB12, has not been carefully examined. We generated a mouse monoclonal antibody directed against human SERPINB12 and delineated its specificity and tissue and cell type distribution pattern through immunoblotting and immunohistochemistry, respectively. This monoclonal antibody was human specific and did not cross-react with other human intracellular serpins or mouse Serpinb12. SERPINB12 was found in nearly all the tissues investigated. In addition, this serpin was found in multiple cell types within individual tissues but primarily the epithelium. These data suggest that SERPINB12, like some other intracellular serpins, may play a vital role in barrier function by providing protection of epithelial cells.
Keywords: serpin, human, immunohistochemistry, epithelium, protease, tissue array
The serine protease inhibitor (SERPIN) protein superfamily regulates diverse biological functions, including matrix remodeling, fibrinolysis, thrombosis, and tumor progression (Silverman et al. 2001; Silverman et al. 2010; Whisstock et al. 2010). Serpins are the largest and most widely distributed types of protease inhibitors that are found in all domains of life (Silverman et al. 2001). A database search of all taxa for protein-coding genomic sequences reveals now almost 7000 members of this highly diverse protein superfamily (http://www.ncbi.nlm.nih.gov/gene).
Within the serpin superfamily resides the clade B or intracellular serpin family (Silverman et al. 2004). The clade B serpins, which reside in the cytosol and nucleus of cells, lack a cleavable N-terminal signal peptide as well as N- and C-terminal extensions but do contain a variable-length loop between helices C and D (Remold-O’Donnell 1993). Of the 13 clade B serpins in humans, most inhibit serine and/or lysosomal cysteine peptidases, with the exception of SERPINB5 (maspin) (Kaiserman and Bird 2010) and SERPINB11 (Askew et al. 2007). Although the precise function of all the human clade B serpins is unknown, evidence from human cell lines and animal models suggests that they appear to protect cells from endogenous peptidase-mediated injury (Bird et al. 1998; Silverman et al. 2004; Yasumatsu et al. 2006; Luke et al. 2007). Interestingly, many of the intracellular serpins, such as SERPINB1 (MNE1) -B2 (PAI-2), -B3 (SCCA1), -B4 (SCCA2), -B6 (PI-6), and -B13 (hurpin), localize to the epithelia, suggesting that they may also play cytoprotective roles against exogenous microbial or viral proteases (Cataltepe, Schick, et al. 2000; Kaiserman and Bird 2005).
In 2001, we identified one of the last human clade B serpins, SERPINB12 (Askew et al. 2001). SERPINB12 is a potent inhibitor of trypsin-like proteases and by RT-PCR appeared to have a wide tissue distribution pattern (Askew et al. 2001). Because most of the other human clade B serpins are more restricted in their tissue expression, we sought to better define expression of SERPINB12 by generating a monoclonal antibody (MAb). While we were preparing to examine the specificity of this IgG1 mouse anti-human SERPINB12 monoclonal antibody in depth, this reagent was used to define the SERPINB12 distribution pattern as part of a tissue-based expression map of the human proteome (Uhlen et al. 2015), as part of the Human Protein Atlas project (www.proteinatlas.org). The goal of this study was to delineate the specificity of our mouse anti-human SERPINB12 monoclonal antibody and confirm and possibly expand this serpin’s tissue and cellular distribution using blocking studies and a broader array of tissue samples. We show that this monoclonal antibody was specific for human SERPINB12 and did not interact with mouse Serpinb12 or other types of human clade B serpins. SERPINB12 was detected in the epithelium of the gastrointestinal, respiratory, and reproductive tracts. In addition, SERPINB12 was highly expressed in the liver and many glandular tissues of the endocrine and reproductive systems. Taken together, we concluded SERPINB12 is broadly expressed and is likely to protect cells from both endogenous and exogenous peptidases.
Materials and Methods
Production of Recombinant Serpin Proteins
Both human SERPINB12 and mouse Serpinb12 cDNAs were amplified using primers (human forward, 5′-TACTCGGATCCATGGACTCTCTTGTTACAGCAAACACC-3′; reverse,5′-TCGTACTCGAGTTAAGGAGAGCAGACCCTGCC-3′; mouse forward, 5′-CGGATCCGTAACCAGTTTTACAATGGAC-3′; reverse, 5′- ATAAGAATGCGGCCGCGCCAGAGTAGATTCAAGGGC-3′)that facilitated an in-frame insertion into the PGEX-6P bacterial expression vector (GE Healthcare; Piscataway, NJ). The amplified human SERPINB12 product was cloned using the restriction endonculeases BamHI and XhoI, and the mouse Serpinb12 product was cloned using BamHI and NotI restriction enzymes (New England BioLabs; Ipswich, MA). DNA sequencing (Genewiz; South Plainfield, NJ) verified both coding sequences were inserted in-frame.
The human GST-SERPINB12 and mouse GST-Serpinb12 recombinant proteins were purified from BL21 Escherichia coli (Amsbio; Lake Forest, CA). Bacterial cells were inoculated in 10 ml 2XY broth (16 g tryptone, 10 g yeast extract, 5 g NaCl in 1 L water) containing 100 µg/µl ampicillin overnight at 37C. Overnight culture was diluted 1 in 50 into fresh 2XY containing 100 µg/µl ampicillin and shaken at 37C until A600 = 0.4 to 0.6. The cells were then removed and shaken at room temperature for another 4 hr without IPTG induction. Cells were collected by centrifugation (4000 × g for 30 min at 4C). Cells were lysed using SoluLyse reagent (Amsbio; Lake Forest, CA) with addition of cOmplete protease-inhibitor cocktail (Roche Life Sciences; Indianapolis, IN). The cellular debris was pelleted by centrifugation (12,000 × g for 10 min at 4C), and the resultant supernatant was added to Glutathione Sepharose 4B (GE Healthcare; Indianapolis, IN). GST-SERPINB12 and GST-Serpinb12 were eluted off Sepharose using GST elution buffer (10 mM reduced glutathione, 50 mM Tris-HCL [pH 8], 100 mM NaCl).
Human recombinant, GST-SERPINB3 (GST-SCCA1), GST-SERPINB4 (GST-SCCA2), and GST-SERPINB13 were generated as described previously (Schick et al. 1997; Schick et al. 1998; Jayakumar et al. 2003). 6× His-SERPINB2 was purchased from ProteinTech (Chicago, IL).
Generation of MAbs Specific for SERPINB12
Monoclonal antibodies were produced in mice by Cell Essentials (Boston, MA) using full-length recombinant 6× His-SERPINB12 antigen. Hybridomas were screened using recombinant GST-SERPINB12 protein. Monoclonal antibodies were collected from hybridoma culture supernatants.
Isotyping of MAbs
Purified antibodies were screened for isotype using an ELISA-based mouse immunoglobulin isotyping kit (Life Technologies; Grand Island, NY) as per the manufacturer’s instructions.
Specificity of SERPINB12 MAbs by Immunoblotting
The recombinant proteins 6× His-SERPINB2, GST-SERPINB3, GST-SERPINB4, GST-SERPIN B12, GST-Serpinb12, and GST-SERPINB13 were separated by SDS-PAGE and immunoblotted using the purified mouse monoclonal SERPINB12 antibody (H3-1B MAb) at 1:5000 dilution, a monoclonal GST-antibody (Thermo Scientific; Waltham, MA) at 1:1000 dilution, or a monoclonal His antibody (GE Healthcare Lifesciences; Pittsburgh, PA) at 1:1000 dilution. All antibodies were diluted with Tris-buffered saline (TBS; 137 mM NaCl, 27 mM KCl, 10 mM Tris-HCl [pH 7.4]) + 0.1% Tween 20 (Sigma-Aldrich; St. Louis, MO) + 1% Block (Bio-Rad; Hercules, CA). All subsequent wash steps were performed as directed by manufacturer instructions. After washing, the primary antibody binding was detected with peroxidase-conjugated bovine anti-mouse (Santa Cruz Biotechnology, Dallas, TX). Signal was detected using Super Signal West Pico Chemiluminescent Substrate (Thermo Scientific) and autoradiography.
Specificity of SERPINB12 MAb by Immunohistochemistry
To determine the specificity of the H3-1B MAb in immunohistochemical staining, tissue arrays were treated as below using the following primary antibody conditions: H3-1B MAb (1 in 100), mouse IgG1 (Dako, Carpinteria, CA) (1 in 100 dilution), or a blocked control, which consisted of preincubation of the H3-1B MAb with recombinant GST-SERPINB12 in a 1:1 molar ratio for 2 hr at 25C prior to diluting 1 in 100 in phosphate-buffered saline (PBS; 137 mM NaCl, 27 mM KCl, 10 mM phosphate buffer [pH 7.4]) + 0.5% BSA. Subsequent primary antibody detection steps were completed as below.
Immunohistochemistry
Commercial multiorgan tissue arrays were purchased from Pantomics (Richmond, CA). The arrays were heated to 60C for 30 min and then deparaffinized using two 5-min xylene washes. Arrays were rehydrated by soaking in 100% ethanol twice for 2 min, 95% ethanol for 5 min, 70% ethanol for 5 min, and finally water for 5 min. Antigen retrieval was performed by boiling arrays in 10 mM citric acid for 10 min. Arrays were blocked in 1% BSA (Sigma-Aldrich) and 5% donkey serum (Santa Cruz Biotechnology) in PBS for 60 min. Primary H3-1B MAb was diluted 1:100 in PBS containing 0.5% BSA and incubated overnight at 4C. Negative control arrays (Secondary alone) were incubated with 0.5% BSA in PBS at 4C. Secondary antibody donkey anti-mouse IgG-HRP (Santa Cruz Biotechnologies) diluted 1:200 in PBS containing 0.5% BSA was incubated with arrays for 60 min. Arrays were then stained with a DAB kit (Vector Laboratories, Burlington, CA) and hematoxylin (Thermo Scientific) as per the manufacturer’s instructions. Tissue arrays were then mounted in water-soluble Gelvatol mounting media (0.2 M Tris base [Thermo Fisher Scientific, Waltham, MA], pH 8.5; polyvinyl alcohol [Sigma-Aldrich]; and glycerol [Sigma-Aldrich] added to appropriate consistency) and imaged using an Olympus BH2 microscope (Olympus, Pennsylvania, PA) mounted with a Jenoptik ProgRes C5 camera (Jenoptik AG, Jena, Germany). Images were acquired using ProgRes CapturePro 2.7 software (Jenoptik AG). Images were enhanced (color balance and contrast) for clarity and cropped using Adobe Photoshop (v13.0; Adobe Systems, San Jose, CA).
Results
Characterization of Anti-SERPINB12 MAb
The H3-1B antibody was determined to be an IgG1 kappa light chain mouse monoclonal antibody. Because human SERPINB12 is most similar to the mouse orthologue, Serpinb12, and the SERPINB4, -B3, and -B13 clade B serpins (Suppl. Table S1), to determine the specificity of the mouse IgG1 H3-1B MAb, we probed parallel immunoblots containing the recombinant serpins 6×His-SERPINB2 (lane 1), GST-SERPINB3 (lane 2), -B4 (lane 3), -B12 (lane 4), -B13 (lane 5), and mouse GST-Serpinb12 (lane 6). The H3-1B MAb detected a band of approximately 72 kDa only in the lane containing GST-SERPINB12 (Fig. 1A). Monoclonal antibodies against GST (Fig. 1B) and 6×His (Fig. 1C) epitope tags served as positive controls for the recombinant GST and His fusion proteins, respectively. A companion Coomassie-stained SDS-PAGE gel confirmed that equivalent amounts of protein were loaded onto the gel (Fig. 1C). These data suggested that the H3-1B MAb only detected SERPINB12 and did not cross-react with other types of clade B serpins and is species specific.
Figure 1.
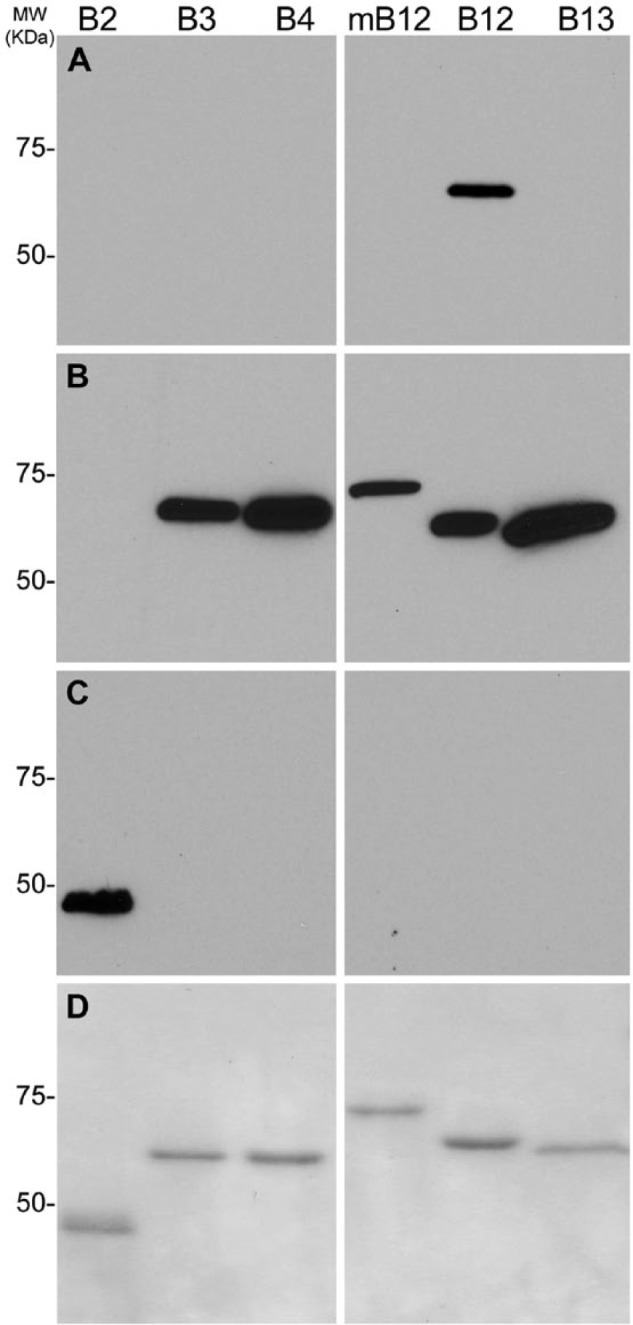
Specificity of H3-1B monoclonal antibody against SERPINB12. (A) Immunoblot of H3-1B against 6×His-SERPINB2 (B2), GST-tagged recombinant proteins: GST-SERPINB3 (B3), -B4 (B4), -B13 (B13), -B12 (B12), and GST-Serpinb12 (mB12). Note that only SERPINB12 is detected. (B) Parallel immunoblot of monoclonal anti-GST antibody (1:1000 dilution) with the same recombinant serpins (B2, B3, B4, B12, B13, and mB12) showing detection of all GST-tagged proteins (B3, B4, B12, B13, and mB12). (C) Parallel immunoblot of monoclonal anti-His antibody (1 in 100 dilution) with the same recombinant serpins (B2, B3, B4, B12, B13, and mB12) showing detection of only 6×His SERPINB2. (D) Parallel Coomassie SDS-PAGE to show approximately equal amounts of proteins loaded. MW markers for all immunoblots and SDS-PAGE are in KDa.
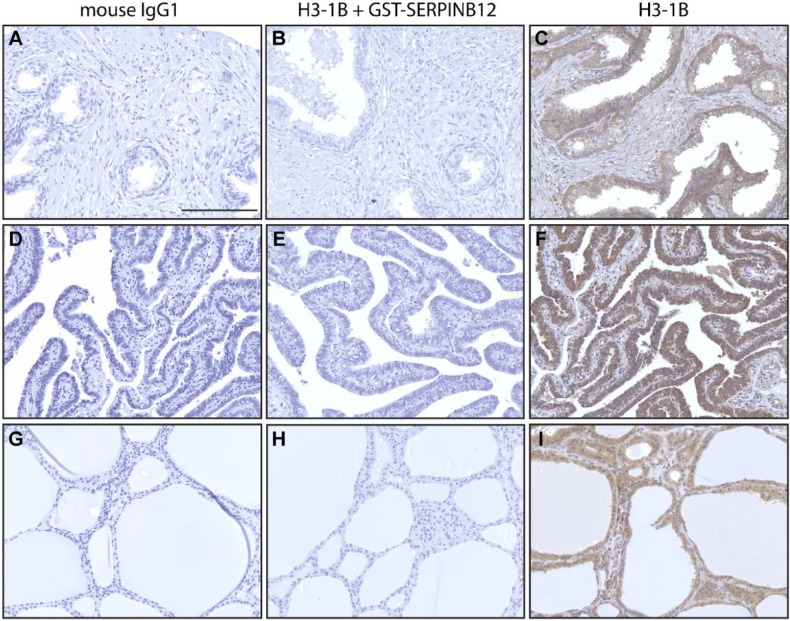
To determine specificity of the antibody by immunohistochemical methods, tissue arrays were incubated with mouse IgG1 (Fig. 2A,D,G), H3-1B MAb (Fig. 2C,F,I), and H3-1B MAb preincubated with recombinant GST-SERPINB12 in a 1:1 molar ratio for 2 hr at 25C (Fig. 2B,E,H). Staining is only seen in the H3-1B MAb-alone treated tissue array. In separate sets of experiments using different types of tissues, the specificity of the primary antibody MAb was controlled for by substitution with either mouse IgG1 or an unrelated monoclonal antibody (CD163) (data not shown). Similarly, the blocking experiments with GST-SERPINB12 were controlled for by substitution with BSA. None of the other primary antibodies gave a signal, and BSA did not block the activity of H3-1B (data not shown). Taken together, these data confirmed that that the H3-1B MAb was highly specific for human SERPINB12 in tissue samples.
Figure 2.
Specificity of the H3-1B MAb by immunohistochemistry. Immunohistochemical peroxidase staining of tissue array sections of prostate (A–C), fallopian tubes (D–F), and thyroid (G–I) with nonspecific mouse IgG1 (A, D, G), H3-1B blocked with recombinant GSTSERPINB12 (B, E, H), and H3-1B (C, F, I). HRP secondary antibody was detected using the DAB peroxidase detection kit. Positive staining is only seen in the H3-1B tissues. Scale bar = 100 µm. Images were captured on an Olympus BH2 microscope mounted with a Jenoptik ProgRes C5 camera. Images were acquired using ProgRes CapturePro 2.7 software.
Immunohistochemical Analysis of Human Tissues Using a SERPINB12-Specific MAb
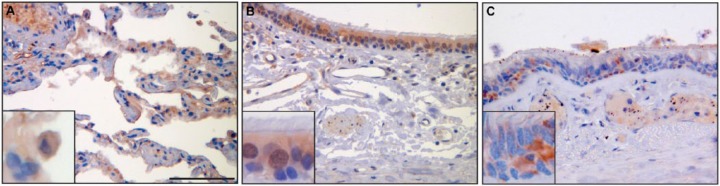
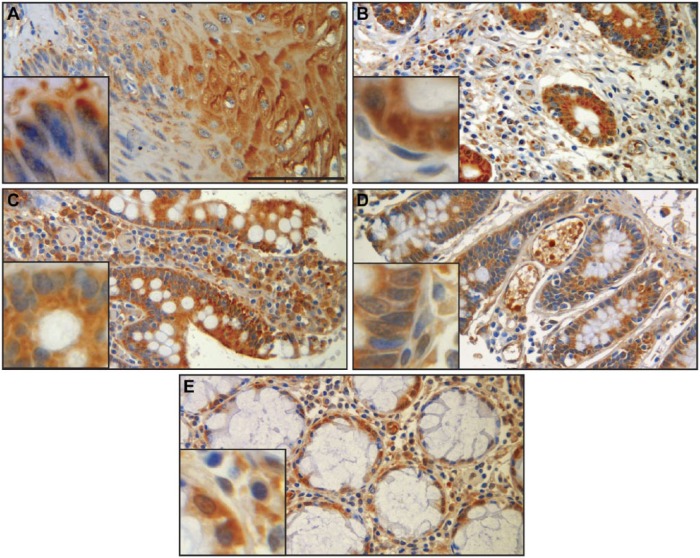
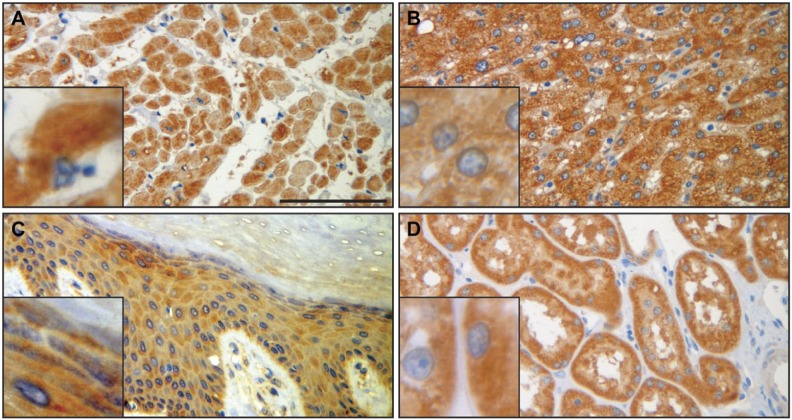
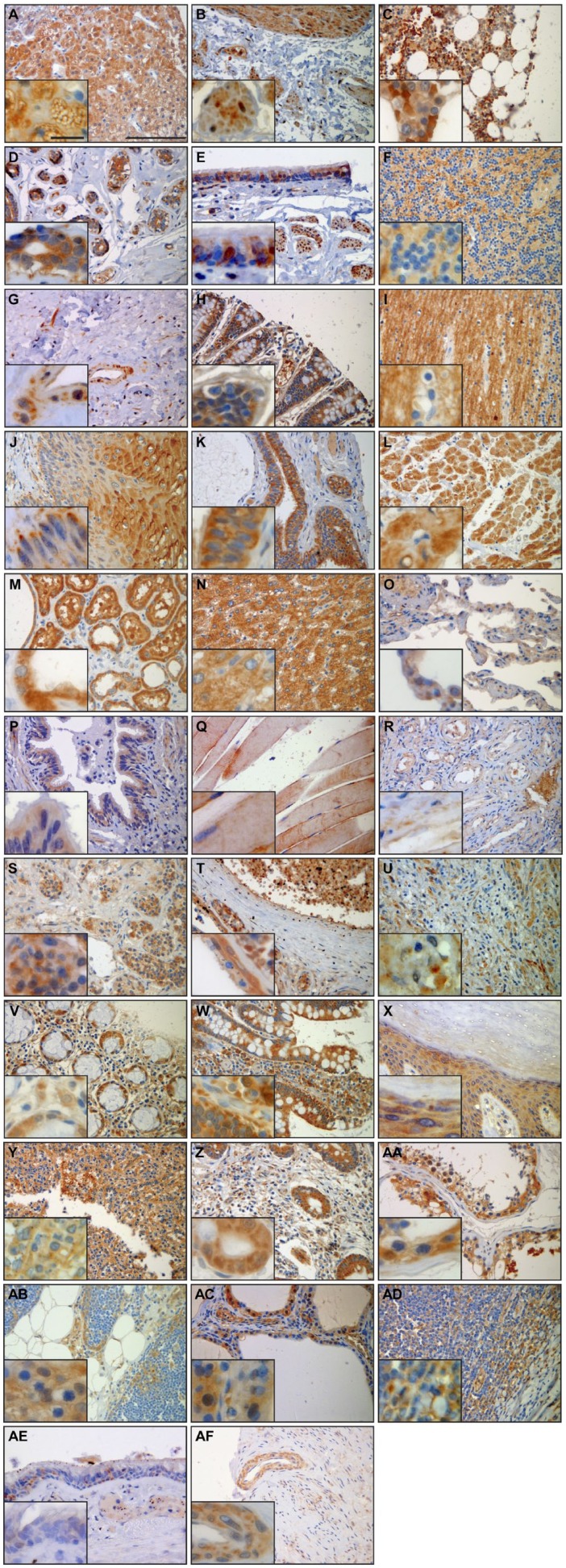
SERPINB12 is expressed in several different cell types of virtually all tissues examined. Of particular interest due to its potential role as a cytoprotective protease inhibitor are the environmentally exposed epithelia of the digestive tract, respiratory tract, and skin. SERPINB12 was found in the squamous epithelium of the esophagus (Fig. 3A), the foveolar epithelium of the stomach (Fig. 3B), and the epithelium of the small intestine (Fig. 3C), colon (Fig. 3D), and rectum (Fig. 3E). In the respiratory tract, SERPINB12 is expressed in the alveolar macrophages (Fig. 4A), the bronchus, conducting airways, and alveolar epithelium (pneumocytes; Fig. 4B), as well as the epithelium of the trachea (Fig. 4C). SERPINB12 is also expressed in the epithelial layer of the skin (Fig. 5C). In addition to these epithelial tissues, SERPINB12 was found in multiple tissue and cell types with varying levels of expression (Table 1) with representative sections illustrated in Fig. 3 (gastrointestinal tissues), Fig. 4 (pulmonary tissues), and Fig. 5 (other tissues with readily detectable expression). The tissue arrays were graded for staining intensity using a qualitative scale; 0 = no staining, 1 = low intensity (light staining), 2 = mild intensity, and 3 = strong expression (dark staining). Representative images, as well as controls, for the full tissue survey are shown in Fig. 6.
Figure 3.
Respiratory tract. SERPINB12 staining in the lung and tracheobronchial tree showed that the distal alveolated lung parenchyma had little staining of alveolar lining cells (A) but did highlight alveolar macrophages (inset) and some endothelium of interstitial blood vessels. The bronchial (B) and tracheal (C) epithelium in general demonstrated weak intensity and variable distribution of cytoplasmic staining. Upon closer inspection, some bronchial epithelial cells appeared to demonstrate nuclear staining; however, this may be related to the overlapping nature of the pseudostratified epithelium (inset). Scale bar = 100 µm. Images were captured on an Olympus BH2 microscope mounted with a Jenoptik ProgRes C5 camera. Images were acquired using ProgRes CapturePro 2.7 software.
Figure 4.
Gastrointestinal tract. Immunohistochemistry using H3-1B shows staining in all epithelial compartments within the major segments of the gastrointestinal tract. The squamous epithelium of the esophagus (A) and glandular epithelium of the stomach (B), small intestine (C), colon (D), and rectum (E) exhibit diffuse, variably intense cytoplasmic staining (insets). Some stromal elements also exhibit staining, including lamina propria stromal cells and macrophages. Scale bar = 100 µm. Images were captured on an Olympus BH2 microscope mounted with a Jenoptik ProgRes C5 camera. Images were acquired using ProgRes CapturePro 2.7 software.
Figure 5.
SERPINB12 immunohistochemistry of heart (A), liver (B), skin epidermis (C), and kidney (D) shows diffuse, variably intense granular cytoplasmic staining in the cells of all four tissue types. Insets highlight the cytoplasmic staining. Cardiomyocytes (A) and hepatocytes (B) demonstrated the most intense staining. Scale bar = 100 µm. Images were captured on an Olympus BH2 microscope mounted with a Jenoptik ProgRes C5 camera. Images were acquired using ProgRes CapturePro 2.7 software.
Table 1.
SERPINB12 Tissue Distribution Summary.
| Tissue | Scoringa | Cell Type Specificity | HPAb Detection |
|---|---|---|---|
| Adrenal gland | 2.0 | Adrenal cortical cells | High |
| Bladder | 1.0 | Detrusor muscle | Medium |
| Bone marrow | 0.5 | Hematopoietic cells | Low |
| Breast | 1.0 | Large duct epithelium; terminal duct lobular unit | Medium |
| Bronchus | 1.0 | Bronchiolar epithelium; smooth muscle | Medium |
| Cerebellum | 1.3 | Granular cells; Purkinje cells; neurons/axons | High |
| Cerebral cortex | 0.5 | Axons; neuropil | Low |
| Esophagus | 1.7 | Squamous epithelium; smooth muscle | Medium |
| Fallopian tube | 1.5 | Epithelium; vascular tissue; smooth muscle | Medium |
| Heart | 2.0 | Myocytes | Medium |
| Intestine, colon | 1.1 | Epithelium | Medium |
| Intestine, rectum | 1.0 | Epithelium | Medium |
| Intestine, small intestine | 2.0 | Epithelium | Medium |
| Kidney | 2.5 | Tubular epithelium | Medium |
| Liver | 2.0 | Hepatocytes | Medium |
| Lung | 1.0 | Epithelium; alveolar macrophages | Low |
| Muscle, skeletal | 2.0 | Myocytes | Low |
| Ovary | 1.0 | Fibrovascular tissue | ND |
| Pancreas | 2.0 | Acinar cells; duct cells | Medium |
| Pituitary gland | 1.3 | Pituicytes | NA |
| Placenta | 1.0 | Cytotrophoblasts | Medium |
| Prostate | 1.0 | Fibromuscular stroma | Low |
| Skin | 1.0 | Squamous epithelium; eccrine duct | Medium |
| Spleen | 0.0 | ND | |
| Stomach | 1.5 | Superficial foveolar epithelium | High |
| Testis | 1.0 | Sertoli cells | Low |
| Thymus | 1.7 | Macrophages; epithelium | NA |
| Thyroid | 1.0 | Follicular epithelium | Medium |
| Tonsil | 1.2 | Squamous epithelium; macrophages | Low |
| Trachea | 1.0 | Epithelium | NA |
| Uterus | 1.2 | Endometrium; smooth muscle | Medium |
NA, not available; ND, not detected.
Tissue arrays scored as no staining (0), mild (1), moderate (2), or strong (3). Scores given are average of multiple sections and tissue arrays (n≥2).
From the Human Protein Atlas (HPA; www.proteinatlas.org).
Figure 6.
Summary of immunohistochemistry with anti-SERPINB12 monoclonal antibody (H3-1B); ×20 magnification with ×40 magnification insets. (A) Adrenal gland, (B) bladder, (C) bone marrow, (D) breast, (E) bronchus, (F) cerebellum, (G) cervix, (H) colon, (I) cortex, (J) esophagus, (K) fallopian tube, (L) heart, (M) kidney, (N) liver, (O) lung, (P) trachea/bronchus, (Q) muscle-skeletal, (R) ovary, (S) pituitary, (T) placenta, (U) prostate, (V) rectum, (W) small intestine, (X) skin, (Y) spleen, (Z) stomach, (AA) testis, (AB) thymus, (AC) thyroid, (AD) tonsils, (AE) trachea, and (AF) uterus. ×20 scale bars 100 µm and all ×40 scale bars 30 µm. Images were captured on an Olympus BH2 microscope mounted with a Jenoptik ProgRes C5 camera. Images were acquired using ProgRes CapturePro 2.7 software.
Expression of SERPINB12 by System
Musculoskeletal System
SERPINB12 was moderately expressed in myocytes of skeletal muscle and weakly detected in the hematopoietic cells of bone marrow.
Cardiovascular System
In the heart, SERPINB12 was moderately expressed and localized to the cardiomyocytes (Fig. 5A).
Digestive System
SERPINB12 was expressed in all tissues of the digestive system as discussed above. In addition to the epithelia, SEPRINB12 was mildly expressed in the smooth muscle of the esophagus (Fig. 4A) and hepatocytes of the liver (Fig. 5B).
Skin
SERPINB12 was expressed at low levels in both the squamous epithelium and eccrine duct epithelium (Fig. 5C).
Endocrine System
SERPINB12 was mildly expressed in acinar cells and duct cells of pancreas. In addition, low-level expression was seen in the pituicytes of the pituitary gland and the follicular epithelium of the thyroid. Interestingly, moderate diffuse staining was seen in cortical cells within the adrenal gland.
Female Reproductive System
Low-level SERPINB12 expression levels were detected in the endometrium and smooth muscle of the uterus, the fibrovascular tissue of the ovary, the epithelium, vascular tissue, smooth muscle of the fallopian tube, and large duct epithelium and terminal duct lobular unit of the breast.
Male Reproductive System
SERPINB12 was detected at low levels in fibromuscular stroma of the prostate, as well as the Leydig and Sertoli cells of the testis.
Urinary Tract
High expression levels of SERPINB12 were detected in tubular epithelium of the kidney (Fig. 5D). In addition, the detrusor muscle of the bladder had low levels of SERPINB12 expression.
Neurological System
Low-level expression of SERPINB12 was detected in granular cells, Purkinje cells, and neurons/axons of the cerebellum and axons and neuropil of the cerebral cortex.
Lung/Respiratory System
In addition to the moderate expression detected in epithelium throughout respiratory tract discussed above, SERPINB12 was also found in both smooth muscle and alveolar macrophages.
Other Systems
Mild expression of SERPINB12 was detected in macrophages and epithelium of the thymus. Low-level expression of this serpin was also seen in the cytotrophoblasts of the placenta, as well as the squamous epithelium and macrophages of the tonsils. No expression of SERPINB12 was detected in the spleen.
Discussion
SERPINB12 is a human clade B/intracellular inhibitor of trypsin-like proteinases and shares a ~40% to 50% amino acid identity with the other 12 family members (Askew et al. 2001). SERPINB12 also shares ~70% primary amino acid identity with its mouse orthologue (Askew et al. 2004). The MAbs raised against SERPINB12 were isotyped and screened for broad specificity via immunoblotting. Of the MAbs raised, the IgG1 H3-1B MAb was the most specific under limited conditions. Further examination of the specificity of MAb H3-1B by immunoblotting and immunohistochemistry showed that this MAb was specific for human SERPINB12. Although not all clade B serpins were examined for cross-reactivity, the orthologous mouse Serpinb12, the three human clade B serpins with the highest amino acid identity, and the outlier SERPINB2 were not detected by immunoblotting of recombinant proteins. Moreover, preincubation of the H3-1B MAb with recombinant human GST-SERPINB12 abolished all staining by immunohistochemistry. These data suggest that the H3-1B MAb is specific for human SERPINB12 and does not cross-react with its mouse orthologue or any of the human clade B serpins tested.
The H3-1B MAb was licensed to a commercial vendor (Santa Cruz Biotechnology) and used by the Human Protein Atlas project (HPA; www.proteinatlas.org). Using different tissue samples and immunohistochemical techniques, we demonstrated that there was excellent concordance in the tissue distribution profiles between our studies and those described by the HPA (Uhlen et al. 2015). However, we detected SERPINB12 in the ovaries, where it was not detected in the HPA study in this organ. In addition, we detected SERPINB12 expression in the thymus and trachea, which was not studied in the HPA project. Additionally, the overall expression levels were similar between the two studies, with some notable exceptions: the adrenal gland, cerebellum, and stomach were scored as high-level expression by the HPA, whereas in our study, these organs were given a low-medium expression level score. In the current study, the organ with highest expression score was the kidney, with expression restricted to the tubular epithelium. Moreover, subtle differences exist in the cell-type specificity in some of the organs. For example, in the bladder, we detected SERPINB12 only in the detrusor muscle cells, whereas the HPA states expression in urothelial cells. Many of the small discrepancies can be attributed to the different sections used for the two separate studies, and thus, these data together can provide a much more complete expression profile of this almost ubiquitously expressed intracellular serpin. In addition, there is a good correlation with the RT-PCR data previously published by our laboratory, with almost all tissues having detectable mRNA levels except thymus, colon, and skeletal muscle (Askew et al. 2001). All these tissues were positive in our immunohistochemical survey with the H3-1B MAb, as well as in the HPA database, at low to medium levels. Also of note, the spleen was positive for mRNA levels but was negative by immunohistochemistry in both studies. There is growing evidence that there is often poor correlation of protein and mRNA levels due to mRNA decay, translation, and protein degradation (Lundberg et al. 2010; Vogel et al. 2010; Schwanhausser et al. 2011; Schwanhausser et al. 2013).
SERPINB12 was primarily expressed in the epithelia of most organs, including the respiratory tract, the digestive system, and skin. This is similar to the tissue and cell-type expression profile of some of the other human intracellular serpin members (e.g., SERPINB3, -B4, -B6, and -B13) detected within the epithelia exposed to the environment (Silverman et al. 2004). However, subtle differences exist. For example, SERPINB3 and -B4 were coexpressed in the suprabasal layers of stratified squamous epithelium (Cataltepe, Gornstein, et al. 2000), whereas SERPINB12 was expressed in both the suprabasal and basal layers. Unlike the other intracellular serpins, SERPINB12 was also expressed in the liver parenchyma, multiple endocrine tissues, and a restricted set of central nervous system tissues and kidney/urinary collecting system. Of note, the only tissue where SERPINB12 was not detected in this survey was the spleen.
SERPINB12 was detected in multiple sites within the lung. In addition to the epithelial cells, this serpin was also expressed in alveolar macrophages. These macrophages harbor and secrete a multitude of elastinolytic proteases to digest the extracellular matrix during infection. These proteases include the trypsin-like serine proteases (Russell et al. 2002) such as human airway trypsin-like protease (Orikawa et al. 2012), which may be potential targets of SERPINB12 based on its in vitro protease inhibitory profile (Askew et al. 2001). This localization of SERPINB12 may suggest that this serpin could play a cytoprotective role of macrophages from its own endogenous proteases along with other protease inhibitors. In addition, unlike previously described clade B serpins (Cataltepe, Gornstein, et al. 2000), SERPINB12 was detected in airway epithelium, including pneumocytes. These epithelial cells have multiple roles, including surfactant production. This suggests that SERPINB12 may have roles in multiple biological processes such as protein processing.
SERPINB12 is an inhibitor of trypsin and plasmin in vitro (Askew et al. 2001), which are produced and secreted from the pancreas and liver, respectively. These organs also express high levels of SERPINB12, suggesting that this serpin may also play a cytoprotective role in pancreatic cells and hepatocytes from endogenous proteolytic digestion via these two highly active and pervasive enzymes.
The abundant tissue distribution of SERPINB12 suggests that this intracellular serpin has an extremely important physiological role. Its expression in both the epithelia and the macrophages suggests a role in host defense, either by inhibition of exogenous viral and bacterial proteases or a cytoprotective role of vital cells from endogenous proteases needed to combat infection. In addition, the expression of SERPINB12 in the same tissues as its in vitro protease targets suggests a cytoprotective role of the secretory cells producing these enzymes.
In summary, we have described the intensity and extent of SERPINB12 expression using immunohistochemistry in a wide array of human organs and tissues. The expression of SERPINB12 in virtually all tissues suggests that this intracellular serpin may have diverse and wide-ranging biological roles and may be an important therapeutic target for multiple disease processes.
Footnotes
Author Contributions: JZN, MG, and LEJ executed the immunoblotting and immunohistochemistry. The pathology examination, cell type identification, and scoring were performed by JAO. GAS and CJL planned the experiments and analyzed the data. The manuscript was written by JZN, GAS, and CJL.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The IgG1 mouse anti-human SERPINB12 monoclonal antibody H3-1B is sold under license by Santa Cruz Biotechnology, Dallas, Texas (catalogue number: sc-32234) to GAS.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH grants: T32 AR052282 and DK081422
References
- Askew DJ, Askew YS, Kato Y, Luke CJ, Pak SC, Bromme D, Silverman GA. (2004). The amplified mouse squamous cell carcinoma antigen gene locus contains a serpin (serpinb3b) that inhibits both papain-like cysteine and trypsin-like serine proteinases. Genomics 84:166-175. [DOI] [PubMed] [Google Scholar]
- Askew DJ, Cataltepe S, Kumar V, Edwards C, Pace SM, Howarth RN, Pak SC, Askew YS, Bromme D, Luke CJ, Whisstock JC, Silverman GA. (2007). SERPINB11 is a new noninhibitory intracellular serpin: common single nucleotide polymorphisms in the scaffold impair conformational change. J Biol Chem 282:24948-24960. [DOI] [PubMed] [Google Scholar]
- Askew YS, Pak SC, Luke CJ, Askew DJ, Cataltepe S, Mills DR, Kato H, Lehoczky J, Dewar K, Birren B, Silverman GA. (2001). SERPINB12 is a novel member of the human ov-serpin family that is widely expressed and inhibits trypsin-like serine proteinases. J Biol Chem 276:49320-49330. [DOI] [PubMed] [Google Scholar]
- Bird CH, Sutton VR, Sun J, Hirst CE, Novak A, Kumar S, Trapani JA, Bird PI. (1998). Selective regulation of apoptosis: the cytotoxic lymphocyte serpin proteinase inhibitor 9 protects against granzyme B–mediated apoptosis without perturbing the Fas cell death pathway. Mol Cell Biol 18:6387-6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataltepe S, Gornstein ER, Schick C, Kamachi Y, Chatson K, Fries J, Silverman GA, Upton MP. (2000). Co-expression of the squamous cell carcinoma antigens 1 and 2 in normal adult human tissues and squamous cell carcinomas. J Histochem Cytochem 48:113-122. [DOI] [PubMed] [Google Scholar]
- Cataltepe S, Schick C, Luke CJ, Pak SCO, Goldfarb D, Chen P, Tanasiyevic MJ, Posner MR, Silverman GA. (2000). Development of specific monoclonal antibodies and a sensitive discriminatory immunoassay for the circulating tumor markers SCCA1 and SCCA2. Clin Chim Acta 295:107-127. [DOI] [PubMed] [Google Scholar]
- Jayakumar A, Kang Y, Frederick MJ, Pak SC, Henderson Y, Holton PR, Mitsudo K, Silverman GA, El-Naggar AK, Bromme D, et al. (2003). Inhibition of the cysteine proteinases cathepsins K and L by the serpin headpin (SERPINB13): a kinetic analysis. Arch Biochem Biophys 409:367-374. [DOI] [PubMed] [Google Scholar]
- Kaiserman D, Bird PI. (2005). Analysis of vertebrate genomes suggests a new model for clade B serpin evolution. BMC Genomics 6:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiserman D, Bird PI. (2010). Control of granzymes by serpins. Cell Death Differ 17:586-595. [DOI] [PubMed] [Google Scholar]
- Luke CJ, Pak SC, Askew YS, Naviglia TL, Askew DJ, Nobar SM, Vetica AC, Long OS, Watkins SC, Stolz DB, et al. (2007). An intracellular serpin regulates necrosis by inhibiting the induction and sequelae of lysosomal injury. Cell 130:1108-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg E, Fagerberg L, Klevebring D, Matic I, Geiger T, Cox J, Algenas C, Lundeberg J, Mann M, Uhlen M. (2010). Defining the transcriptome and proteome in three functionally different human cell lines. Mol Syst Biol 6:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orikawa H, Kawaguchi M, Baba T, Yorita K, Sakoda S, Kataoka H. (2012). Activation of macrophage-stimulating protein by human airway trypsin-like protease. FEBS Lett 586:217-221. [DOI] [PubMed] [Google Scholar]
- Remold-O’Donnell E. (1993). The ovalbumin family of serpin proteins. FEBS Lett. 315:105-108. [DOI] [PubMed] [Google Scholar]
- Russell RE, Thorley A, Culpitt SV, Dodd S, Donnelly LE, Demattos C, Fitzgerald M, Barnes PJ. (2002). Alveolar macrophage-mediated elastolysis: roles of matrix metalloproteinases, cysteine, and serine proteases. Am J Physiol Lung Cell Mol Physiol 283:L867-L873. [DOI] [PubMed] [Google Scholar]
- Schick C, Bromme D, Bartuski AJ, Uemura Y, Schechter NM, Silverman GA. (1998). The reactive site loop of the serpin SCCA1 is essential for cysteine proteinase inhibition. Proc Natl Acad Sci USA 95:13465-13470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schick C, Kamachi Y, Bartuski AJ, Cataltepe S, Schechter NM, Pemberton PA, Silverman GA. (1997). Squamous cell carcinoma antigen 2: a novel serpin that inhibits the chymotrypsin-like proteinases cathepsin G and mast cell chymase. J Biol Chem 272:1849-1855. [DOI] [PubMed] [Google Scholar]
- Schwanhausser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, Chen W, Selbach M. (2011). Global quantification of mammalian gene expression control. Nature 473:337-342. [DOI] [PubMed] [Google Scholar]
- Schwanhausser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, Chen W, Selbach M. (2013). Corrigendum: global quantification of mammalian gene expression control. Nature 495:126-127. [DOI] [PubMed] [Google Scholar]
- Silverman GA, Bird PI, Carrell RW, Church FC, Coughlin PB, Gettins PG, Irving JA, Lomas DA, Luke CJ, Moyer RW, et al. (2001). The serpins are an expanding superfamily of structurally similar but functionally diverse proteins: evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J Biol Chem 276:33293-33296. [DOI] [PubMed] [Google Scholar]
- Silverman GA, Whisstock JC, Askew DJ, Pak SC, Luke CJ, Cataltepe S, Irving JA, Bird PI. (2004). Human clade B serpins (ov-serpins) belong to a cohort of evolutionarily dispersed intracellular proteinase inhibitor clades that protect cells from promiscuous proteolysis. Cell Mol Life Sci 61:301-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman GA, Whisstock JC, Bottomley SP, Huntington JA, Kaiserman D, Luke CJ, Pak SC, Reichhart JM, Bird PI. (2010). Serpins flex their muscle: I. Putting the clamps on proteolysis in diverse biological systems. J Biol Chem 285:24299-24305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, Sjostedt E, Asplund A, et al. (2015). Proteomics: tissue-based map of the human proteome. Science 347:1260419. [DOI] [PubMed] [Google Scholar]
- Vogel C, Abreu Rde S, Ko D, Le SY, Shapiro BA, Burns SC, Sandhu D, Boutz DR, Marcotte EM, Penalva LO. (2010). Sequence signatures and mRNA concentration can explain two-thirds of protein abundance variation in a human cell line. Mol Syst Biol 6:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whisstock JC, Silverman GA, Bird PI, Bottomley SP, Kaiserman D, Luke CJ, Pak SC, Reichhart JM, Huntington JA. (2010). Serpins flex their muscle: II. Structural insights into target peptidase recognition, polymerization, and transport functions. J Biol Chem 285:24307-24312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumatsu R, Altiok O, Benarafa C, Yasumatsu C, Bingol-Karakoc G, Remold-O’Donnell E, Cataltepe S. (2006). SERPINB1 upregulation is associated with in vivo complex formation with neutrophil elastase and cathepsin G in a baboon model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 291:L619-L627. [DOI] [PubMed] [Google Scholar]