Abstract
Performance of immunofluorescence staining on archival formalin-fixed paraffin-embedded human tissues is generally not considered to be feasible, primarily due to problems with tissue quality and autofluorescence. We report the development and application of procedures that allowed for the study of a unique archive of thymus tissues derived from autopsies of individuals exposed to atomic bomb radiation in Hiroshima, Japan in 1945. Multiple independent treatments were used to minimize autofluorescence and maximize fluorescent antibody signals. Treatments with NH3/EtOH and Sudan Black B were particularly useful in decreasing autofluorescent moieties present in the tissue. Deconvolution microscopy was used to further enhance the signal-to-noise ratios. Together, these techniques provide high-quality single- and dual-color fluorescent images with low background and high contrast from paraffin blocks of thymus tissue that were prepared up to 60 years ago. The resulting high-quality images allow the application of a variety of image analyses to thymus tissues that previously were not accessible. Whereas the procedures presented remain to be tested for other tissue types and archival conditions, the approach described may facilitate greater utilization of older paraffin block archives for modern immunofluorescence studies.
Keywords: archival tissue, immunofluorescence, image analysis, thymus, tissue processing
Introduction
Archival tissues are a rich source of potential material for retrospective and comparative studies. However, these tissues, especially those collected decades ago, were often fixed and stored under a variety of what would now be considered sub-optimal conditions, including the use of unbuffered fixation methods and storage under non-climate-controlled conditions. As a result, these tissues are generally considered to be unsuitable for modern immunofluorescence (IF) methods. Given the volume of additional information that can be generated by the application of the many currently available IF-based microscopy and image analysis techniques, the inability to use these archival tissue blocks for this type of staining and analysis represents a lost opportunity for research.
The Radiation Effects Research Foundation (RERF) in Hiroshima, Japan, houses an extensive archive of tissue blocks collected from surgery and autopsy of survivors of the atomic bomb blasts at Hiroshima and Nagasaki. This archive thus represents an excellent example of precious and unique tissue samples that are of great potential interest and scientific value, but were collected and stored decades ago under less than optimal conditions. This study was devised to develop protocols that could generate high-quality single- and dual-color IF images suitable for image analysis from these tissues, some of which were collected and stored as early as 1955.
“Autofluorescence”, due to the intrinsic fluorescence of natural tissue components, is a well-recognized problem that can limit the quality of immunofluorescence studies. Such autofluorescence has primarily been attributed to endogenous flavins, reduced NAD(P)H, lipofuscins, and fibers such as reticulin, collagen, and elastin (Viegas et al. 2007). Any red blood cells (RBCs) present may also contribute significantly to autofluorescence (Baschong et al. 2001). The use of neutral-buffered formalin as a fixative can induce additional autofluorescence, since formaldehyde reacts with adjacent amine groups through Schiff acid-base reactions to generate adducts that are intensely fluorescent. Together, intrinsic and formalin-induced autofluorescence overlaps extensively with the 420 to 650 nm emission wavelengths used by most modern fluorophores (reviewed in Viegas et al. 2007). Numerous attempts have been made to address this problem, primarily to allow direct immunofluorescence studies of formalin-fixed paraffin-embedded murine tissues. Some studies have incorporated broad spectrum, light-absorbing dyes (e.g., Sudan Black B; Baschong et al. 2001; Oliveira et al. 2010), which can prevent excitation of fluorescent molecules in the tissue. Other approaches have included excitation-induced photobleaching (e.g., high intensity exposure to ultraviolet radiation; Viegas et al. 2007), oxidation/reduction (redox) reactions (e.g., exposure to sodium borohydride; Beisker et al. 1987) to destroy autofluorescent molecules, or solubilization and removal of fluorescent molecules (e.g., ammonia-ethanol; Baschong et al. 2001). Overall, the ability of these methodologies to reduce autofluorescence in any given tissue type has depended heavily on tissue vascularity and lipofuschin content (Viegas et al. 2007).
The generally larger connective tissue (e.g., collagen and elastin fiber) content of human tissues compared with mouse tissues further predisposes the human tissues to problematic intrinsic autofluorescence. Older archival tissues pose additional challenges for immunofluorescence studies due to the degradation or loss of antibody epitopes with changes in temperature or to ongoing redox reactions due to endogenous reactive groups or environmental conditions, which add to those initially induced by formalin-induced cross-linking. Numerous methods have been developed to enhance contrast or to enhance tissue reactivity with an antibody via antigen retrieval (reviewed in Shi et al. 2011), and this is mostly targeted toward bright-field applications of immunohistochemistry. However, to our knowledge, none of these previous studies have provided methodologies that could simultaneously decrease autofluorescence and increase antibody signals in decades-old human archival tissues, particularly when additional changes may have been induced in the tissue due to uncontrolled storage conditions or the initial use of non-buffered formalin.
Materials & Methods
Tissue History
Thymus tissues used for this study were derived from autopsies of individuals who were previously exposed to atomic bomb radiation in Hiroshima, Japan, in 1945. Subjects were 15 to 50 years old at the time of their deaths in 1955 to 1972 (Table 1). Tissues were fixed in unbuffered formalin, processed into paraffin blocks, and initially stored in cardboard boxes at ambient temperature (0 to 35°C) at RERF. Supplemental heat and air conditioning were installed in RERF storage rooms during the 1970’s, with 24-hr temperature control beginning in 1999. Thus, blocks were initially stored at uncontrolled ambient temperatures for approximately the first 5 to 20 years, then stored with daytime temperature control only for approximately 15 years, followed by 24-hr temperature control (20°C to 25°C) for an additional ~15 years before they were accessed for this study. Additional tissues used for IF staining controls were pediatric and adult thymus tissues fixed in neutral-buffered formalin, processed into paraffin blocks, and stored under climate-controlled conditions (20°C to 25°C) at the Duke University Medical Center for 20 to 25 years before use in this study.
Table 1.
Characteristics of Tissues Studied.
| Tissue ID | Age of Block (years) | Successful IF? |
|---|---|---|
| B2 | 60 | Yes |
| B5 | 54 | Yes |
| B7 | 57 | Yes |
| B9 | 54 | Yes |
| B11 | 52 | Yes |
| B13 | 55 | No |
| B14 | 52 | Yes |
| B16 | 44 | No |
| B18 | 53 | Yes |
| B19 | 53 | Yes |
Human Subjects
The use of RERF tissue specimens in this study was approved by the Human Investigation Committee and the Ethics Committee for Genome Research at the RERF. All tissues were de-identified prior to analyses and remained in Japan throughout the entire study. Control tissues from the Duke repository were obtained as anonymous discarded tissues for which consent was not required (Duke IRB exemption #474).
Hematoxylin and Eosin Staining and Bright-field Immunohistochemistry
Four-µm sections of thymus were cut and affixed onto glass slides designed to enhance tissue adhesion (Platinum pro; Matsunami Glass Ind., Kishiwada, Japan). Slides were deparaffinized by immersion in xylene (10 min × 2), and rehydrated using graded ethanols (100% for 5 min × 2; 95% for 5 min × 2; 70% for 5 min × 2) followed by washes with distilled water (DW) (3 min) and PBS (5 min × 2). Hematoxylin and eosin (H&E) staining was performed using standard methods (Kiernan 2008). For bright-field immunohistochemical staining with cytokeratin antibodies, antigen retrieval consisted of immersing slides in L.A.B solution (Polysciences Inc.; Warrington, PA) for 30 min at 95°C, with a 45 min cool-down. Endogenous peroxidases were blocked by incubation in 3% H2O2 in methanol for 15 min followed by washing (5 min × 2) with Tris-buffered saline (TBS) (Sigma-Aldrich; St. Louis, MO). Sections were incubated overnight at 4°C with a mouse anti-human cytokeratin antibody cocktail (clones AE1/AE3, Dako, Glostrup, Denmark; 1:200 in TBS). Unbound antibody was removed by washing with TBS (5 min × 2). Sections were then incubated with anti-mouse antibody conjugated with horseradish peroxidase (EnVision+ System-HRP; Dako) for 1 hr at 37°C, followed by washing with TBS (5 min × 2). For anti-CD1a antibody, antigen retrieval consisted of equilibration with 0.05 M Tris-HCl (pH 7.6; 5 min × 2), enzymatic digestion with 0.0025% Proteinase K (Qiagen; Hilden, Germany) in DW for 10 min, washing with TBS (5 min × 2), and heating in Dako Target Retrieval Solution, pH 9.0 (30 min, 95°C) followed by a 45-min cool-down and DW washes (5 min × 3). Slides were blocked by incubation in Blocking Solution (EnVision+ System-HRP) for 5 min at room temperature, followed by TBS washes (5 min × 2), and then incubated overnight at 4°C with monoclonal rabbit anti-human anti-CD1a antibody (clone EP3622, Epitomics, Burlingame, CA; dilution 1:400 in PBS) followed by TBS washes. Anti-rabbit-HRP (EnVision+ System-HRP) was applied for 1 hr at 37°C followed by TBS washes. For both antibodies, bound antibody was visualized by incubation in liquid DAB+ substrate-chromogen solution (EnVision+ System-HRP) for 15 to 60 min. Slides were rinsed with DW before they were counterstained with Mayer’s Hematoxylin solution (~30 sec), thoroughly rinsed with DW, then dehydrated to xylene and permanently mounted. Sections of human skin were used as controls for bright-field immunohistochemical staining.
Slide Preparation for Immunofluorescence Studies
Four-µm sections of thymus were cut and affixed onto Platinum pro glass slides. For removal of paraffin, the slides were immersed in xylene (5 min × 2), followed by graded ethanols (5 min × 2 in 100%, 5 min × 2 in 95%, then 5 min × 2 in 70% ethanol). Staining results were compared for deparaffinized sections that were rehydrated by sequential immersion in either DW, PBS, PBS with 5% sucrose, or PBS with 0.05% Tween 20 (5 min × 2, each), or incubated in PBS with 5% sucrose overnight at room temperature.
Antigen Retrieval to Enhance Immunofluorescence Signal Intensity
A variety of antigen retrieval treatments to enhance the intensity of the primary antibody binding were applied prior to the autofluorescence-reducing treatment, as summarized in Table 2. If the treatment involved heat, slides were allowed to gradually cool in the same buffer for 45 min after heating.
Table 2.
Effects of Treatments on Autofluorescence and Antibody Signals.
| Effect on Signal |
||||
|---|---|---|---|---|
| Tissue Treatments | Time | Temp | CK14 | CD1a |
| To rehydrate tissue | ||||
| DW | 5 min × 2 | room temp | + | + |
| PBS | 5 min × 2 | room temp | + | + |
| PBS with 5% sucrose | 5 min × 2 | room temp | + | + |
| PBS with 0.05% Tween20 | 5 min × 2 | room temp | + | + |
| PBS with 5% sucrose overnight at RT | o/n | room temp | – | – |
| To enhance signal intensity | ||||
| 10% citraconic acid (pH 6.0) | 45 min | 95°C | + | – |
| 10 mM Tris (pH 10.0) | 45 min | 95°C | + | ± |
| 1 mM EDTA (pH 8.0) | 45 min | 95°C | + | ± |
| 10 mM citrate buffer (pH 6.0) | 45 min | 95°C | – | – |
| TE buffer (pH 7.0) | 45 min | 95°C | + | ± |
| TE buffer (pH 8.0) | 45 min | 95°C | + | ± |
| TE buffer (pH 9.0) | 45 min | 95°C | + | + |
| TE buffer (pH 10.0) | 45 min | 95°C | + | + |
| Autoclave for 20 min at 121°C, TE buffer (pH 9.0) | 20 min | 121°C | + | – |
| 5% DTT with 5% 2-ME | 5 min | 95°C | + | ± |
| 0.05% Triton X-100 in TE buffer (pH 9.0) | 45 min | 95°C | + | + |
| 0.4% pepsin in 0.01 N HCl | 5 min | 37°C | – | – |
| trypsin (0.05%) in 0.5mM EDTA (pH8.0) | 5 min | 37°C | – | – |
| trypsin (0.05%) in 0.5 mM EDTA (pH 8.0) | 20 min | 37°C | – | – |
| proteinase K (0.005%) in PBS | 10 min | room temp | – | – |
| To reduce autofluorescence | ||||
| 0.25% NH3 in 70% ethanol (EtOH) | 1 hr | room temp | ++ | + |
| 3% H2O2 in methanol before 1st ab | 15 min | room temp | + | ++ |
| 3% H2O2 in methanol before 2nd ab | 15 min | room temp | – | ++ |
| Sodium borohydride (NaBH4, 1 mg/ml) | 10 min × 3 | on ice | + | + |
| 2.0 mM glycine | 1 hr | room temp | ± | ++ |
| 0.3% Sudan black B in 70% EtOH | 1 hr | room temp | + | + |
| Antibody staining protocols | ||||
| Anti-CK14 (1:25) | o/n | 4°C | + | |
| Anti-CD1a (1:25) | o/n | 4°C | + | |
| Mixture of anti-CK14 and anti-CD1a | o/n | 4°C | – | + |
| Anti-CK14 2 hr 4°C first, then anti-CD1a o/n 4°C | 2 hr, then o/n | 4°C | – | + |
| Anti-CK14 4 hr 4°C first, then anti-CD1a o/n 4°C | 4 hr, then o/n | 4°C | – | + |
| Anti-CK14 4 hr 37°C first, then anti-CD1a o/n 4°C | 4 hr, then o/n | 37°C, 4°C | – | + |
| Anti-CK14 6 hr 4°C first, then anti-CD1a o/n 4°C | 6 hr, then o/n | 4°C | + | + |
| Anti-CK14 o/n 4°C first, then anti-CD1a o/n 4°C | o/n × 2 | 4°C | + | + |
Key: (+) indicates beneficial effect, (±) indicates minimal to no effect, and (–) indicates detrimental effect; o/n indicates overnight; temp, temperature; 2-ME, 2-mercaptoethanol. TE buffer = 10 mM Tris + 1 mM EDTA. The treatments used in the final optimized protocol are highlighted in bold.
Treatments to Reduce Autofluorescence
To reduce autofluorescence, tissue sections were treated with one or more of the following after antigen retrieval procedures but before incubation with antibodies: 1) 0.25% NH3 in 70% ethanol (EtOH) for 1 hr at room temperature, followed by washing with 50% EtOH for 10 min, then with washing buffer (0.05% Tween 20 in PBS) for 5 min × 5; 2) 3% H2O2 in methanol for 15 min before the 1st antibody, followed by washing with washing buffer (5 min × 5); 3) 3% H2O2 in methanol for 15 min before the 2nd antibody, followed by washing with washing buffer (5 min × 5); 4) Sodium borohydride (NaBH4, 1 mg/ml) on ice for 10 min × 3, followed by washing with washing buffer (5 min × 5); or 5) 2.0 mM glycine, at room temperature for 60 min followed by washing with DW for 5 min, then washing buffer (10 min × 2). In some cases, sections were instead or additionally stained with 0.3% Sudan Black B in 70% EtOH for 1 hr after antibody incubation and prior to cover slip mounting. Excess Sudan black was wiped using a swab, followed by a jet wash with washing buffer.
Antibody Staining Protocols
Slides were blocked by incubation in 10% goat serum (Invitrogen; Carlsbad, CA) for 1 hr at room temperature, followed by washing with washing buffer (5 min × 3). For this study, staining was optimized using different combinations of incubation times and temperatures, as described in Table 2. In the optimized protocol, sections were incubated with anti-CK14 (clone LL002, IgG3, Thermo Scientific, Fremont, CA; 1:25 dilution) for 6 hr at 4°C and/or with anti-CD1a (clone O10, IgG1, Thermo Scientific; 1:25 dilution) overnight at 4°C. Primary antibodies were removed and sections were washed with washing buffer (5 min × 4), before application of secondary antibodies (for anti-CK14: Alexa Fluor 488-conjugated goat anti-mouse IgG3, (AF488); for anti-CD1a: Alexa Fluor 594-conjugated goat anti-mouse IgG1 (AF594); Invitrogen) both at 1:100 dilution. After incubation for 1 hr at room temperature, sections were washed with washing buffer (5 min × 3), then mounted using anti-fade mounting solution with DAPI (ProLong® Gold, P36935, Invitrogen) and a cover-slip.
Imaging and Microscopy
Stained samples were observed under a fluorescence microscope (BZ-9000; Keyence, Osaka, Japan) using “green” and “red” filter sets with the following specifications: Green filter: Emission 470/40 (maximum/bandpass in nm; effective range, 450–490 nm), Excitation 525/50 (500–550 nm) and Red filter: Emission 560/40 (540–580 nm), Excitation 630/75 (592.5–667.5 nm). The dichroic mirror for green was 400 nm and for red was 585 nm. As stated by the supplier, the green filter set is recommended for the detection of AF488 (absorption maximum 496 nm/emission maximum 519 nm) and the red filter set is recommended for the detection of AF594 (absorption maximum 590 nm/emission maximum 617 nm).
Images were analyzed using BZ-II image analyzer software (Keyence). Briefly, fluorescence images in both green and red channels were acquired at 20× magnification via Z-sectioning (Z stack acquisition; 1 µm × 4 steps). Each green and red image was stitched and automatically focused by the BZ-II software using the Full Focus function; this functions samples the locations with the best focus at each coordinate amongst the grouped images captured with a combination of the Z-stack and Merge functions, and then merges them. A series of these operations created wide-view magnified green and red images. The Haze Reduction and Black Balance functions provided by the software were used to remove blurring from images and enhance fluorescence signals by providing a uniformly black background.
Results
Comparison of Staining of Archival Tissues Stored under Different Conditions using Standard Immunohistochemical Staining Protocols
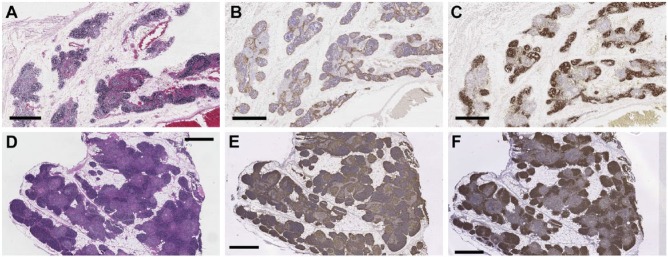
Thymus tissues obtained from the RERF tissue archives were fixed in unbuffered formalin and initially stored under uncontrolled conditions, whereas tissues from the Duke tissue repository were fixed in neutral-buffered formalin and stored under climate-controlled conditions. Despite their differences in fixation, longer length of time since embedding, and history of uncontrolled storage conditions, most tissues from the RERF archive generated high-quality H&E and immunohistochemical staining (Fig. 1A–1C) that was of a similar quality as that achieved with the Duke tissues (Fig. 1D–1F).
Figure 1.
H&E and bright-field immunohistochemical staining of archival thymus tissues. (A–C) RERF thymus tissues were fixed in unbuffered formalin, processed into paraffin blocks, and stored for ~50 years under initially uncontrolled conditions before they were stained with (A) H&E, or with (B) anti-CK14, or (C) anti-CD1a antibodies in immunoperoxidase assays, as described in the Materials & Methods. The results closely modeled those obtained (D–F) using thymus tissues that were fixed with neutral-buffered formalin and stored under climate-controlled conditions for 20–25 years before they were stained with (D) H&E, (E) anti-CK14, or anti-CD1a (F) antibodies using identical methods. Scale, 1 mm.
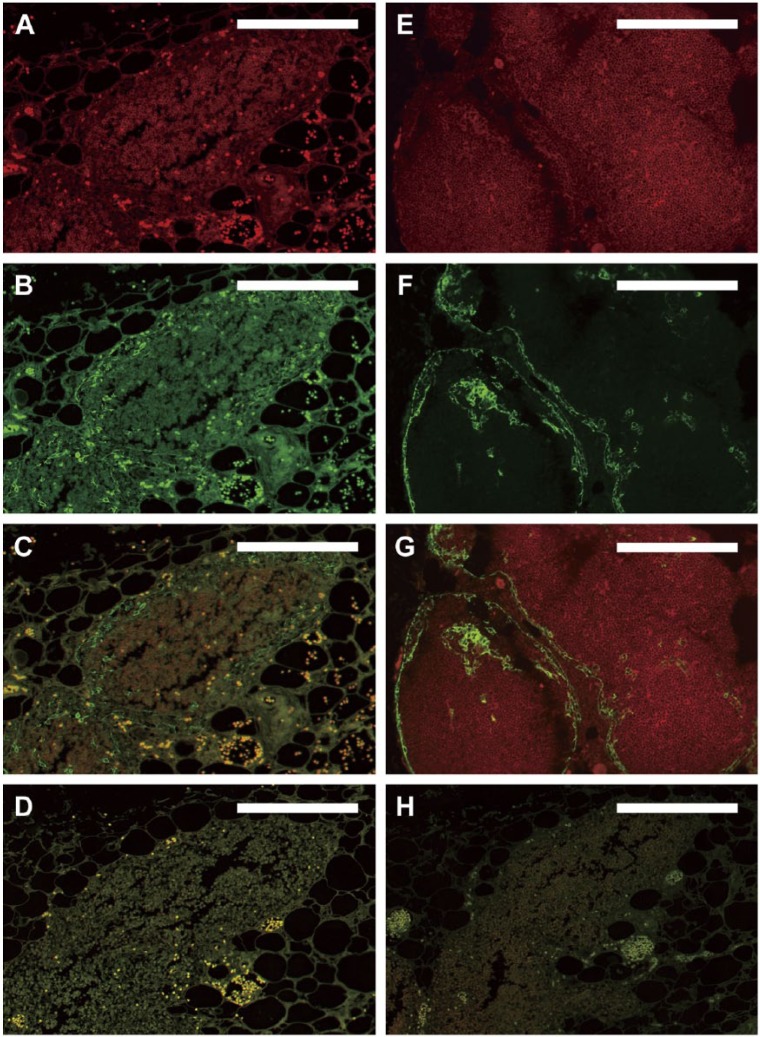
Before applying immunofluorescence staining to the older archival tissues, a range of combinations of primary and secondary antibodies were evaluated to optimize fluorescence signals on more modern tissues (Fig. 2E–G). Based on this work, the specific primary and secondary antibodies chosen for the IF studies were different from those used for the bright-field staining (see Materials & Methods). Since the primary goal was co-localization of cortical thymocytes (CD1a positive) and thymic epithelium (cytokeratin positive) to assess changes in thymic architecture via image analysis, this report focuses on dual-color IF studies. However, the methods developed are equally applicable for studies that require only single color IF.
Figure 2.
Immunofluorescence using standard protocols. Dual-color immunofluorescence images were derived from older RERF archival thymus tissues fixed in unbuffered formalin and stored under initially uncontrolled conditions (A–D, H). (A–C) Fluorescence following reaction with anti-CD1a antibody (red, A), anti-CK14 antibody (green, B), and the merged images (C) on a single slide, in the absence of autofluorescence-reducing techniques. A separate section of the same tissue that was deparaffinized but not reacted with antibodies is shown in (D) to document autofluorescence in the absence of background antibody staining. (A–D) High autofluorescence and poor signal-to-noise ratio initially observed with the older archival tissues that prevented definitive identification of cortical thymocytes in (A) and thymic epithelium in (B). For comparison, thymus tissues fixed with neutral-buffered formalin and stored under climate-controlled conditions for 25 years before staining (E–G) show optimal dual-color immunofluorescence images under the same staining conditions used for panels (A–C). Strong specific staining of cortical thymocytes (red, E) and thymic epithelial cells (green, F; merged images in G) is observed in these tissues without the need for additional autofluorescence-reducing techniques. The staining pattern shown in (E–G) thus served as a gold standard for determining optimal IF staining of the older archival tissues from RERF throughout the remainder of the study. (H) Same tissue as in (A–D) with omission of primary antibodies but with application of NH3/EtOH, H2O2, and Sudan Black B treatments, as described in the text. Scale, 0.2 mm.
Application of the initially “optimized” primary and secondary antibody combinations to the older RERF archival tissues using standard IF protocols yielded extremely poor images. Both CD1a and CK14 fluorescence signals were low, with very high generalized background due to autofluorescence (Fig. 2A–2D). This resulted in low signal to noise ratios (compare with Fig. 2E–2G) that made definitive cell identification and application of image analysis impossible. This difference in quality of IF staining in the older RERF archival tissues as compared with the Duke tissues was likely due to a combination of fixation of the RERF tissues in unbuffered formalin, their prolonged storage, and/or their initially uncontrolled storage conditions.
Testing Methods to Reduce Autofluorescence
The primary concern for successful IF using the older archival tissues was reducing autofluorescence. Therefore, a variety of methods were identified and tested to address this concern (Table 2). Several different tissue rehydration methods were tested. Brief immersion of the deparaffinized sections in DW, PBS, PBS with 5% sucrose, (5 min × 2, each), or overnight incubation in PBS with 5% sucrose failed to reduce autofluorescence and similarly had no effect on IF signal intensity. However, rehydration of deparaffinized sections by brief immersion in PBS with 0.05% Tween-20 (5 min × 2, each) was found to reduce autofluorescence slightly, in part by removing small particles that adhered to the sticky slide glass that was required to maintain adhesion of the tissue to the slide (Table 2).
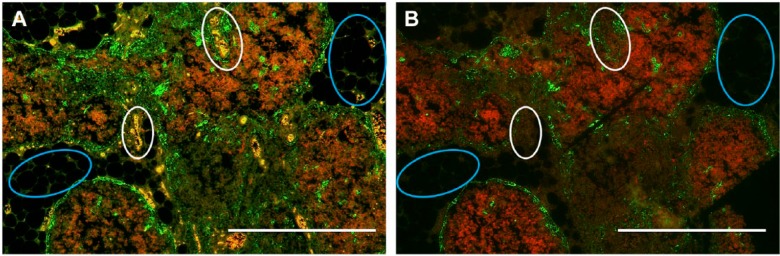
The autofluorescence observed was occasionally derived from red blood cells (RBCs) and elastic fibers present in the sections (Fig. 3A). The use of broad spectrum-absorbing black dyes, such as Sudan Black B, has previously been suggested as a method to quench autofluorescence derived from natural tissue components (Baschong et al. 2001; Sutterlin et al. 2004; Oliveira et al. 2010). In this study, the application of Sudan Black B staining after antibody incubation and prior to cover slip mounting was found to markedly reduce autofluorescence generated by these natural sources (Fig. 3B). Although a 0.1% solution of Sudan Black B was previously reported to successfully reduce autofluorescence in other tissues (Baschong et al. 2001; Viegas et al. 2007), we found that use of 0.3% Sudan Black B was required to achieve a consistent and reproducible decrease in the autofluorescence of our tissue samples.
Figure 3.
Sudan black staining quenches natural autofluorescence. (A) White circles indicate foci of red blood cells (RBCs) and blue circles indicate elastin fibers that generate strong autofluorescence and obscure the signal resulting from bound anti-CK14 antibody in the tissue shown in Figure 2. A serial section with Sudan black staining applied after antibody staining but before coverslipping (B) shows that this treatment decreases autofluorescence of these natural components to almost non-detectable levels. Scale, 0.5 mm.
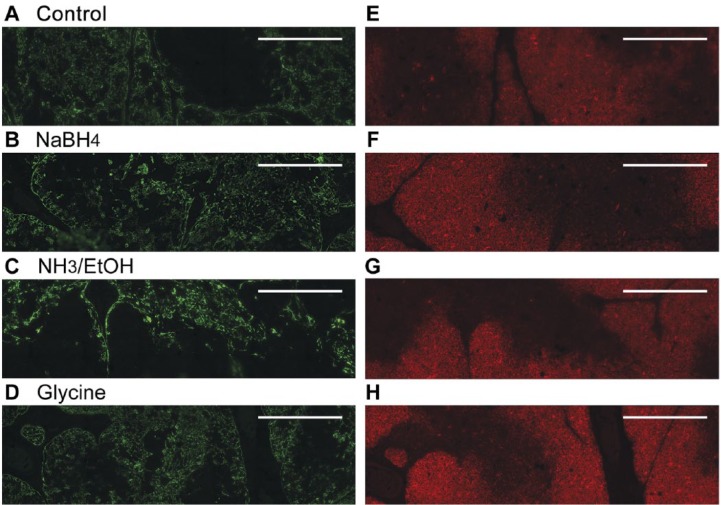
However, considerable autofluorescence remained, hypothesized to be due to chemical changes in the tissue resulting from their initial fixation using unbuffered formalin. To reverse such changes, tissue sections were treated with a mild reducing or oxidizing agent (sodium borohydride, NH3 in ethanol, H2O2, or glycine) after antigen retrieval (see below), but prior to incubation with primary antibody (Fig. 4). Treatment with glycine provided the best results for the CD1a signal (Fig. 4H), but was less effective in reducing autofluorescence as seen with the green filter set (Fig. 4D). Overall, treatment with NH3/EtOH provided the best results for dual staining with both anti-CD1a and anti-CK14 antibodies (Fig. 4C, 4G). Figure 2H shows the marked reduction in autofluorescence that is achievable through combination of NH3/EtOH, H2O2, and Sudan Black B treatments in the absence of primary antibodies.
Figure 4.
Treatment with a mild reducing agent reduces autofluorescence without markedly decreasing antibody signals. Representative dual immunofluorescence images obtained with anti-CK14 antibody (green, A–D) or anti-CD1a antibody (red, E–H) are shown without (A, E) and with additional steps to reduce autofluorescence: NaBH4 (B, F), NH3/ethanol (C, G), or glycine (D, H). See Materials & Methods for details of treatments. The NH3/EtOH treatment decreased autofluorescence so that internal cortical areas now appear appropriately negative with anti-CK14 antibody (green, panel C) and medullary areas now appear appropriately negative with anti-CD1a antibody (red, panel G). Scale, 0.5 mm.
Methods to Enhance Antibody Signal Intensity
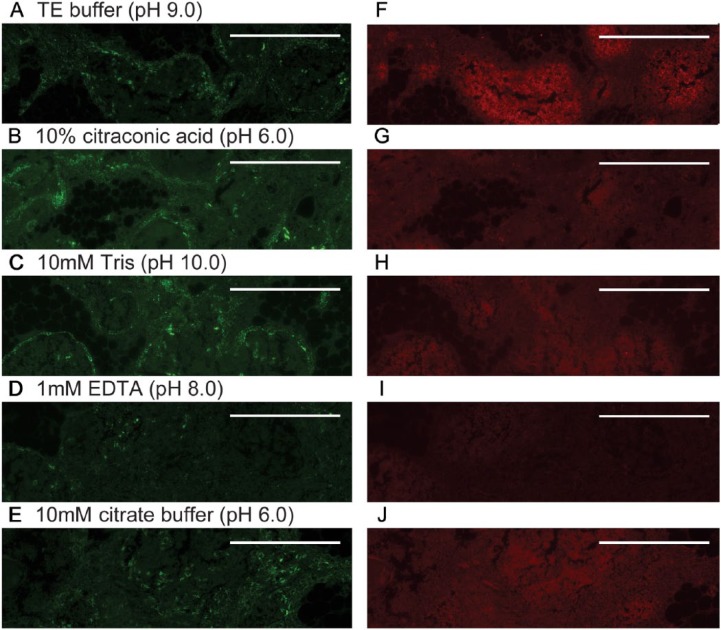
The methods described above reduced the autofluorescence that previously limited the use of IF, but obtaining high-quality IF images from these archival tissues also required enhancing the intensity of fluorescence signals. A number of antigen retrieval methods were tested in this study to enhance intensity of antibody signals (see Materials & Methods). Examples of the effects of several of these methods on immunofluorescence intensity are demonstrated in Figs. 5 and 6. For the anti-CK14 and anti-CD1a antibodies used, antigen retrieval with TE buffer (pH 9.0) at 95°C for 45 min, with gradual cooling for 45 min gave the best results (Fig. 5A, 5F) and was used for the remainder of the study.
Figure 5.
Methods to enhance antibody reactivity/signals. Representative dual immunofluorescence images obtained with anti-CK14 (green, A–E) or anti-CD1a antibody (red, F–J) are shown after heating at 95°C for 45 min in various buffers in an attempt to enhance antibody reactivity/signals. Buffers used were: TE buffer, pH 9.0 (A, F); 10% citraconic acid, pH 6.0 (B, G); 10 mM Tris, pH 10.0 (C, H); 1 mM EDTA, pH 8.0 (D, I); and 10 mM citrate buffer, pH 6.0 (E, J). All tissues shown were stained with 0.3% Sudan Black B to reduce autofluorescence, but did not receive NH3/EtOH or 3% H2O2 treatments. Scale, 0.5 mm.
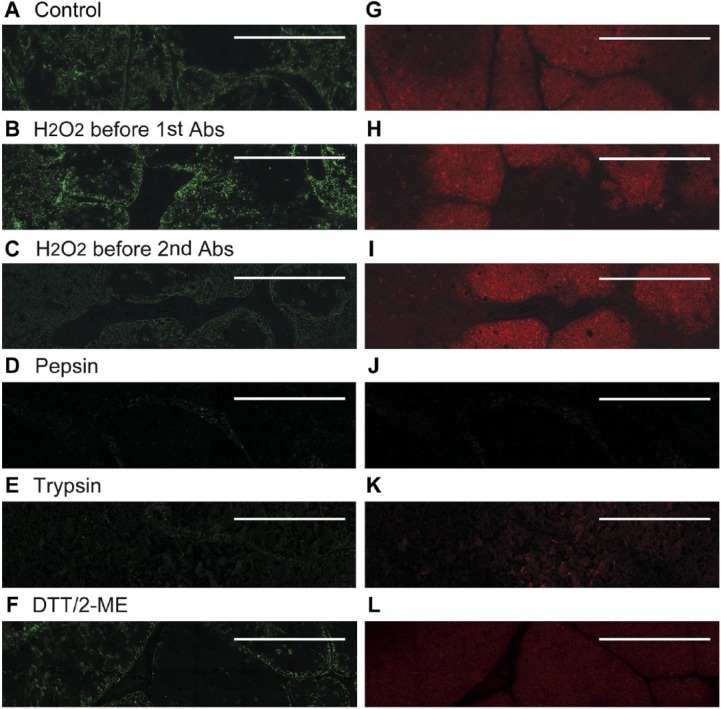
Figure 6.
Methods to enhance antibody reactivity/signals. Representative immunofluorescence images obtained with anti-CK14 antibody (A–F) or anti-CD1a antibody (G–L) are shown without (A, G) and with additional steps to enhance antibody reactivity/signals: H2O2 in methanol prior to primary antibody (B, H): H2O2 in methanol prior to secondary antibody (C, I); pepsin (D, J); trypsin (E, K); or DTT/2-ME (F, L). See Materials & Methods for details of treatments. Scale, 0.5 mm.
Use of Deconvolution Microscopy Methods to Improve Image Quality
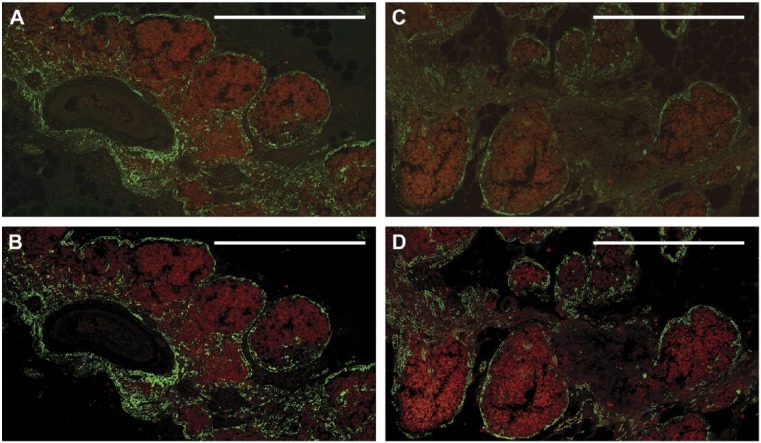
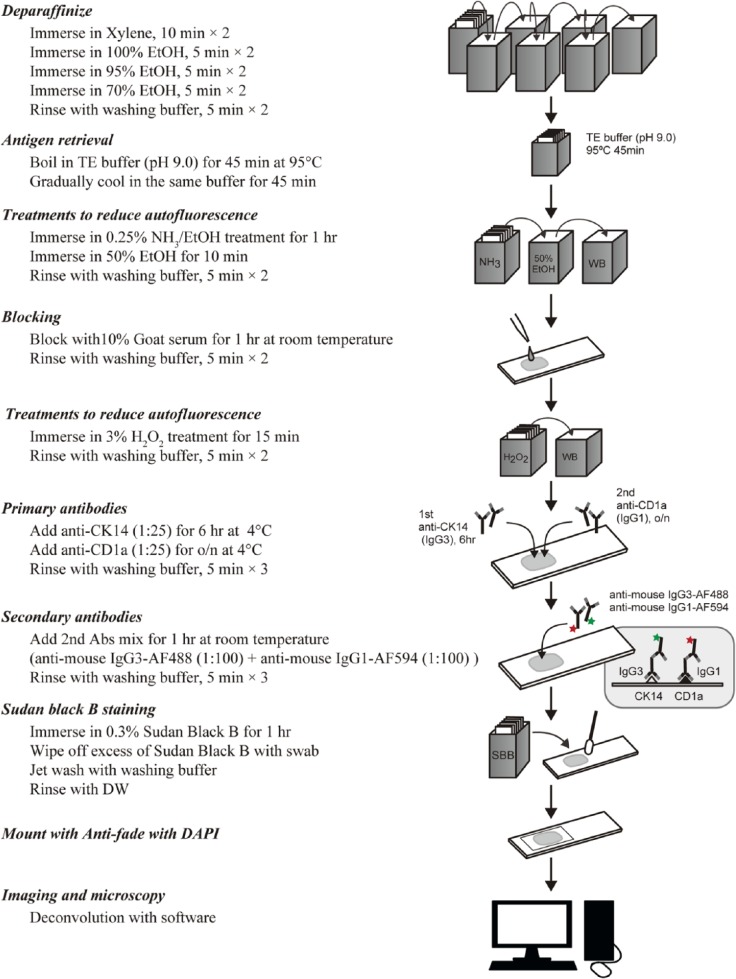
Significantly improved images were obtained after employing the methods to reduce autofluorescence and to enhance signal via enhancing primary antibody binding and reducing non-specific binding, as described above. However, image analysis typically requires very high-quality images, with extremely low background and high contrast. To further improve the image quality, we employed deconvolution microscopy techniques. As shown in Fig. 7, the application of deconvolution microscopy sharpened the images, resulted in further increases in the signal-to-noise ratio, and generated high-quality, dual-color fluorescence images that were suitable for advanced image analysis techniques. Based on all the techniques described, the optimized staining protocol that was developed to both minimize autofluorescence and maximize fluorescent antibody signals is summarized in Fig. 8. Using this protocol, high-quality, dual fluorescence images could be obtained for 8 of 10 tissue blocks studied, which had been stored for 44 to 60 years (Table 1). For the two remaining tissues, high-quality images were obtained for only one of the two antibodies used (for anti-CD1a in case B13 and anti-CK14 in case B16). However, both of these tissues demonstrated appropriate staining for both anti-CK14 and anti-CD1a antibodies using single-color bright-field immunohistochemical staining and different primary antibody clones, as described in the Materials & Methods.
Figure 7.
Application of deconvolution microscopy further reduces background and improves the signal-to-noise ratio in dual-color immunofluorescence. The two different thymus tissues shown were each fixed in unbuffered formalin and stored as described in the Materials & Methods for 52 years before staining with anti-CK14 (green) and anti-CD1a (red) antibodies. Treatments to reduce autofluorescence and enhance signal intensity incubation were NH3/EtOH, 3% H2O2, and 0.3% Sudan Black B, as described in the Materials & Methods. Prior to deconvolution, fluorescence signals are blurred and unclear (A, C). The addition of deconvolution microscopy (B, D) further improves the images, resulting in a sharper signal that enhances image quality. Signal intensities and clarity are now similar to those seen with the more recent tissues (compare with Fig. 2G), and allow for the differential localization of cell types. Scale, 0.5 mm.
Figure 8.
Optimized protocol to minimize autofluorescence and maximize antibody signals. Shown is a summary of the protocol used to generate high-quality, dual fluorescence images from archival thymus tissues. Abbreviations: o/n, overnight; Abs, antibodies; DW, distilled water.
Discussion
We have developed a protocol for generating high-quality, high-contrast, dual-color immunofluorescence images from sub-optimally stored formalin-fixed, paraffin-embedded archival thymus tissues. The broad range of strategies tested to address problematic autofluorescence and low signal intensity in paraffin blocks from the RERF archive of tissues from atomic bomb survivors were initially chosen empirically, based on the past experience of the authors, since mechanisms that contribute to autofluorescence and poor antibody reactivity with older archival tissues are still not clearly understood. Some of these strategies were initially developed for another purpose, but were tested because they were known to be compatible with subsequent immunostaining of the tissue and had at least some theoretical explanation for potential efficacy. The strategies reported here represent a significant advance in methodology that may potentially be of value to increase the utilization of previously inaccessible archival tissue sources for modern IF studies. The innovation of this technique is the inclusion of multiple independent steps designed to quench autofluorescence and enhance signals, including using NH3/EtOH to achieve a decrease in fluorescent moieties followed by fluorescence quenching with Sudan Black. Fluorescence images were then obtained using deconvolution microscopy to further enhance the signal-to-noise ratio. The resulting images are of high quality and appropriate for use in a variety of image analysis techniques, thus allowing the application of advanced image analysis techniques to archival tissues that were previously thought to be too degraded for study. Although this study was limited to thymus tissues, the techniques developed are likely to also be applicable to other types of older archival tissues that similarly exhibit poor reactivity with antibodies or problematic autofluorescence due to fixation or storage or due to the presence of blood or elastin fibers. However, additional studies will be required to fully assess the utility of the protocol presented here over a broad range of tissue types and fixation/storage conditions.
Autofluorescence generated by formalin-fixed, paraffin-embedded human tissues has traditionally been thought to be too intense to allow such tissues to be studied by immunofluorescence. This report describes the results from applying multiple treatments that both decrease autofluorescence and enhance the generation of specific antibody signals. The final optimized staining protocol is presented in Fig. 8. A key point in applying this protocol to other sources of archival tissues and tissue types is that each antibody or combination of antibodies must be tested to determine an optimal combination of treatments. As described above, the anti-CK14 and anti-CD1a antibodies used for this study each required slightly different methods for optimal results in single-color staining, but a single treatment could be identified that allowed dual-color IF.
The RERF archival tissues used in this study were fixed in unbuffered formalin, which may be in part responsible for the enhanced autofluorescence that was observed. Unbuffered formalin was commonly used in the past, and acid/base chemical changes that contribute to autofluorescence are likely a common condition in tissue archives that are older than 20–25 years. Part of the success of the NH3/EtOH treatment in reducing autofluorescence may be related to an ability to compensate for the lack of buffering at initial fixation, by post-fixation chemical modification and/or reduction of fixation-induced fluorescent moieties. The broad absorbance throughout the visible spectrum that results in the black color of Sudan Black B dye allows it to absorb incident light and thus minimize fluorescence excitation at a variety of wavelengths. We found that application of 0.3% Sudan Black B was able to reduce the relatively weak residual autofluorescence signals that remained following NH3/EtOH treatment without a substantial reduction in the desired and strong specific fluorescence signals. Thus, the combination of these two techniques markedly improved the quality of the resulting images.
We wish to draw specific attention to two places where our optimized staining protocol differs from usual practices. We used 0.3% Sudan Black B as a treatment to reduce autofluorescence, rather than the 0.1% that was previously reported (Viegas et al. 2007) to be ineffective in reducing autofluorescence from murine tissues such as liver and kidney that are prone to high autofluorescence. Second, all slides in this study were washed after the (serum) blocking incubation, which is not necessarily done for all studies. Since all slides in our study were washed in this fashion, further studies by other investigators with less restricted access to problematic archival tissues will be required to determine whether this washing step removed substances that either inhibited antibody signals or contributed to autofluorescence, increased background staining due to non-specific antibody binding, or had no substantial effect on the final image quality.
In summary, we have developed a methodology for generating high-quality, high-contrast, dual-color immunofluorescence images from sub-optimally stored formalin-fixed, paraffin-embedded archival thymus tissues via a combination of multiple methods for reducing tissue autofluorescence and enhancing antibody signals. The approaches detailed in this study can provide a guide for the production of successful immunofluorescence images from a wide range of archival formalin-fixed, paraffin-embedded tissues. Given the wide range of image analysis techniques now available, this protocol should make archival tissues that are currently considered unusable widely accessible for these types of studies.
Acknowledgments
The authors thank Mika Kai, Mariko Kuroda, Mika Yamaoka, and Mayumi Maki (RERF) for expert technical assistance with antibody staining. We also thank Kengo Yoshida, Ph.D. (RERF) for case ascertainment and Gregory Sempowski, Ph.D. (Duke University Medical Center) and Seishi Kyoizumi, Ph.D., Yoichiro Kusunoki Ph.D., and Kei Nakachi, Ph.D. (RERF) for reviewing the data, editing the manuscript, and for helpful discussions.
Footnotes
Author Contributions: JK, NM, and LH designed the studies; JK and RI performed the immunohistochemistry and imaging and supervised the technical staff involved in the project; JK, NM, and LH drafted the manuscript. All authors have read and approved the final manuscript.
Competing Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Radiation Effects Research Foundation (RERF), Hiroshima and Nagasaki, Japan, is a public interest foundation funded by the Japanese Ministry of Health, Labour and Welfare (MHLW) and the U.S. Department of Energy (DOE). This publication was supported by RERF Research Protocol 2-15. The work reported here was supported by a United States National Institute of Allergy and Infectious Disease/National Institutes of Health contract (No. HHSN272200900059C) to the RERF. The views of the authors do not necessarily reflect those of the two governments.
References
- Baschong W, Suetterlin R, Laeng RH. (2001). Control of autofluorescence of archival formaldehyde-fixed, paraffin-embedded tissue in confocal laser scanning microscopy (CLSM). J Histochem Cytochem 49:1565-1572. [DOI] [PubMed] [Google Scholar]
- Beisker W, Dolbeare F, Gray JW. (1987). An improved immunocytochemicalprocedure for high-sensitivity detection of incorporated bromodeoxyuridine. Cytometry 8:235-239. [DOI] [PubMed] [Google Scholar]
- Kiernan JA. (2008). Histological and Histochemical Methods: Theory and Practice. 4th edition, Bloxham: Scion. [Google Scholar]
- Oliveira VC, Carrara RC, Simoes DL, Saggioro FP, Carlotti CG, Covas DT, Neder L. (2010). Sudan Black B treatment reduced autofluorescence and improves resolution of in situ hybridization specific fluorescent signals of brain sections. Histol Histopathol 25:1017-1024. [DOI] [PubMed] [Google Scholar]
- Shi S-R, Shi Y, Taylor CR. (2011). Antigen Retrieval Immunohistochemistry: Review and future prospects in research and diagnosis over two decades. J Histochem Cytochem 59:13-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetterlin R, Baschong W, Laeng RH. (2004). Immunofluorescence and confocal laser scanning microscopy of chronic myeloproliferative disorders on archival formaldehyde-fixed bone marrow. J Histochem Cytochem 52:347-335. [DOI] [PubMed] [Google Scholar]
- Viegas MS, Martins TC, Seco F, do Carmo A. (2007). An improved and cost-effective methodology for the reduction of autofluorescence in direct immunofluorescence studies on formalin-fixed paraffin-embedded tissues. Eur J Histochem 51:59-56. [PubMed] [Google Scholar]