Abstract
The granules of mast cells contain a myriad of mediators that are stored and protected by the sulfated glycosaminoglycan (GAG) chains that decorate proteoglycans. Whereas heparin is the GAG predominantly associated with mast cells, mast cell proteoglycans are also decorated with heparan sulfate and chondroitin sulfate (CS). This study investigated a unique CS structure produced by mast cells that was detected with the antibody clone 2B6 in the absence of chondroitinase ABC digestion. Mast cells in rodent tissue sections were characterized using toluidine blue, Leder stain and the presence of mast cell tryptase. The novel CS epitope was identified in rodent tissue sections and localized to cells that were morphologically similar to cells chemically identified as mast cells. The rodent mast cell-like line RBL-2H3 was also shown to express the novel CS epitope. This epitope co-localized with multiple CS proteoglycans in both rodent tissue and RBL-2H3 cultured cells. These findings suggest that the novel CS epitope that decorates mast cell proteoglycans may play a role in the way these chains are structured in mast cells.
Keywords: glycosaminoglycan, chondroitin sulfate, proteoglycans, mast cells, endoglycosidase
Introduction
Mast cells are a heterogeneous population derived from hematopoietic progenitor cells. They are widely known for their production and release of histamine in an allergic response, as well as their role in inflammation, and are predominantly found in tissues that interface the host and the external environment, such as the lungs, gastrointestinal tract and skin (Metcalfe et al. 1997). They are often characterized based on their location, as well as the proteases and cytokines they produce and store in their α-granules.
Serglycin is the most abundant proteoglycan produced by mast cells, which has a significant role in the packaging of proteases in the α-granules (Åbrink et al. 2004). A sub-set of mast cells, based on species and tissue of origin (Rönnberg et al. 2012), produce heparin that decorates the proteoglycan (PG) serglycin (Kolset and Gallagher 1990; Metcalfe et al. 1997), whereas mast cell-derived serglycin may be decorated with both oversulfated chondroitin sulfate (CS) and heparin (Stevens et al. 1988; Kjellén et al. 1989). In contrast, mucosal mast cells produce serglycin decorated exclusively with CS, including CS-A, CS-E and CS-B (Stevens et al. 1986), rather than heparin (Enerbäck et al. 1985). In addition to heparin and CS, heparan sulfate (HS) also decorates PGs that are produced by mast cells (Yurt et al. 1977; Stevens et al. 1986; Seldin et al. 1985). It was previously demonstrated by Seldin et al. that glycosaminoglycan (GAG) chains on PGs produced by the rodent mast cell-like RBL cells were 64% CS, with the remaining 36% being either heparin or HS (Seldin et al. 1985). Mast cells also produce the CS PGs versican (Rönnberg et al. 2012) and bikunin (Ide et al. 1999), though the structure of the CS chains attached to these PGs has not been examined. RBL-2H3 cells in culture produce keratan sulfate, CS, HS and heparin from cells in both resting and activated states (Jung et al. 2013). In addition to mast cells, macrophages are also associated with sites of inflammation. Monocytes, the precursors to macrophages, contain CS-A though, with in vitro culture, transition to CS-E (Kolset et al. 1983). Macrophages derived from monocytes have also been shown to increase their amount of CS upon lipopolysaccharide stimulation (Uhlin-Hansen et al. 1989).
The discovery of new CS structures has been enabled by a number of antibodies with epitopes against the varying CS structures. The antibody clone CS-56 detects CS-A and CS-D (Avnur and Geiger 1984; Ito et al. 2005). The antibody clone LY-111 was developed against CS-A (Yada et al. 1992) and recognizes both CS-A and CS-D. The antibody clone MO-225 recognizes CS-D as well as CS-E (Yamagata et al. 1987). Additionally, a number of different antibodies have been developed against native CS/DS epitopes (Sorrell et al. 1990; Sorrell et al. 1993), as well as a suite of antibodies raised against chondroitinase (C’ase) ABC-generated neo-epitopes (Couchman et al. 1984; Caterson et al. 1985), which have become known as anti-CS stub antibodies. The anti-CS stub antibody clones, 1B5, 2B6 and 3B3, detect the terminal unsaturated disaccharide structures following a linkage region that contains an unsulfated, 4-sulfated or 6-sulfated GalNAc, respectively. These stub neo-epitopes are generated by exhaustive digestion with the bacterial endoglycosidase C’ase ABC. It has also been shown that the clone 3B3 detects the non-reducing terminal saturated CS disaccharide consisting of glucuronic acid (GlcA) that is adjacent to the 6-sulfated N-acetylgalactosamine (GalNAc) in the absence of C’ase ABC digestion (Caterson et al. 1990).
The objective of this study was to evaluate the presence of CS epitopes in tissues around material implants. In doing this, we have identified a novel CS epitope detected by the antibody clone 2B6 in the absence of endoglycosidase digestion. Furthermore, we have confirmed the presence of this novel CS epitope in cultured RBL-2H3 cells, and investigated the CSPGs with which this novel CS epitope structure may be associated.
Materials & Methods
Tissue Sections from a Rat Implantation Model
Details of the rodent implantation model used to investigate the host response to implanted materials has previously been described (Farrugia et al. 2014). Briefly, material films (1 × 0.5 cm) were subcutaneously implanted into the lumbar region of female Sprague-Dawley rats. Animals were sacrificed at 7 days after implantation, and the implant was excised and processed for histological evaluation. The in vivo implantation study was performed in an accredited facility by the Association for Assessment and Accreditation of Laboratory Animal Care under Institutional Animal Care and Use Committee approval. Histological results were evaluated for each test condition from multiple animals, and observations were similar between replicates. The results presented are from one material implant that is representative of the study and enables the evaluation of co-localization of markers of interest.
Histological Analysis
Grafts were fixed in 10% neutral-buffered formalin, paraffin embedded and sectioned (4–5 µm) as previously described (Farrugia et al. 2014). Sections were washed twice, 5 min each, with xylene to remove paraffin and the slides were immersed in a series of ethanol solutions for 3 min each [twice in 100% (v/v), once in 95% (v/v), once in 70% (v/v)], followed by several exchanges of water. Histological characterization was undertaken through the use of Leder stain, toluidine blue, and immunolocalization of PGs, glycosaminoglycans and markers of interest. Following rehydration, slides were stained with Leder stain, which detects the presence of chloroacetate esterase (Naphthol AS-D chloroasetate (NADC); 91C kit, Sigma-Aldrich, St Louis, MO) and was carried out as per the manufacturer’s protocol. Briefly, following rehydration, sections were fixed in the citrate-acetone-formaldehyde solution (30 sec), washed in deionized water, and immediately placed into the NADC solution and incubated for 15 min at 37°C. Slides were then rinsed in pre-warmed deionized water for 2 min followed by counterstaining with hematoxylin (Gill #3) for a further 2 min, and then rinsed with deionized water. Following rehydration, sections were stained with toluidine blue solution (0.1% w/v, pH 2.0) for 2 min, washed in RO water three times, dipped into 95% EtOH, followed by 100% EtOH twice, cleared in xylene and mounted. Antigen epitope retrieval was undertaken by immersing the slides after rehydration in 0.01 M sodium citrate (pH 6), followed by heat treatment in a decloaking chamber (Applied Medical, Santa Margarita, CA) at 120°C for 4 min. The slides were then rinsed with deionized water followed by blocking of endogenous peroxidase with 3% (v/v) H2O2 for 5 min. Some slides were also treated with C’ase ABC (0.05 U/mL; Seikagaku Corp. Tokyo, Japan) in 0.1 M Tris-Acetate buffer (pH 8) for 3 hr at 37°C. The slides were washed with 50 mM Tris-HCl, 0.15 M NaCl, 0.05% (w/v) Tween-20, pH 7.6 (TBST), and then blocked with 1% (w/v) bovine serum albumin (BSA) in TBST for 1 hr at room temperature. The slides were incubated with primary antibodies diluted in 1% w/v BSA in TBST at 4°C for 16 hr. Primary antibodies and the concentrations used are detailed in Table 1. Slides were also probed with mouse IgG and IgM whole antibodies (5 µg/ml; Invitrogen, Carlsbad, CA) as isotype controls, as well as blocking solution in place of primary antibodies. Slides were then washed twice with TBST before incubation with the appropriate biotinylated secondary antibodies (1:500; GE Healthcare, Sydney, Australia) for 1 hr at room temperature. Slides were washed twice with TBST, incubated for 30 min with streptavidin-HRP (1:250; GE Healthcare, Sydney, Australia), rinsed four times with TBST, and then processed for color development with NovaRED chromogen stain (Vector Laboratories; Burlingame CA). The slides were then counterstained with hemotoxylin (Gill’s #3, Vector Laboratories) for 6 sec and rinsed with deionized water. Slides were dehydrated through a graded ethanol series and mounted.
Table 1.
Details of the Primary Antibodies Utilized for Immunohistological Staining.
| Primary Antibody Epitope | Species | Isotype | Clone / Catalogue No. | Supplier | Dilution | Endoglyco-sidase Digestion* |
|---|---|---|---|---|---|---|
| Mast Cell Tryptase | Mouse | IgG | AA1 | Abcam | 2 µg/ml | – |
| Chondroitin-4 sulfate stub | Mouse | IgG | 2B6 | 1:500 | Yes | |
| Chondroitin-6 sulfate stub | Mouse | IgM | 3B3 | 1:500 | Yes | |
| Serglycin | Rabbit | Polyclonal | sc-292311 | Santa Cruz Biotechnology, Dallas, TX | 1:100 | – |
| Perlecan | Rabbit | Polyclonal | CCN-1 | ** | 1:1000 | – |
| Versican | Mouse | IgG | 5D5 | *** | 1:250 | Yes |
| Bikunin | Rabbit | Polyclonal | Ab43073 | Abcam, Cambridge, MA | 1:400 | – |
| Aggrecan | Mouse | IgG1 | 969D4D11 | Invitrogen, Carlsbad, CA | 0.2 µg/ml | Yes |
| CD86 | Rabbit | IgG | EP1158Y | Abcam | 1:200 | – |
| CD206 | Rabbit | Polyclonal | ab64693 | Abcam | 1:500 | – |
Digestion with chondroitinase ABC, 0.05 U/ml, pH 8.0, 0.1 M Tris-Acetate buffer.
Raised in house against immunopurified human coronary artery endothelial cell-derived perlecan (Lord et al. 2014; Whitelock et al. 1999).
A gift from Dr Firoz Rahemtulla, University of Alabama, Birmingham, AL, USA
Culture and Activation of Mast Cell Line RBL-2H3
Rat basophilic leukemia cells (RBL-2H3) were maintained in RPMI-1640 medium containing 10% (v/v) fetal bovine serum (FBS), 100 U/ml penicillin and 100 µg/ml of streptomycin and maintained in a humidified incubator (5% CO2 / 95% air atmosphere at 37°C). Media was replenished every 3 to 4 days. Siriganian (SG) activation buffer (119 mM NaCl, 5 mM KCl, 25 mM PIPES, 5.6 mM Dextrose, 0.4 mM MgCl2, pH 7.25) was prepared and filter sterilized, and combined with 0.1% (v/w) BSA and 1 mM CaCl2 before use. Mast cells were chemically activated by adding a protein kinase C activator, 50 nM phorbol 12-myristate 13-acetate (PMA), 500 nM of a calcium ionophore, A23187 (Sigma-Aldrich), and 0.1% (v/w) BSA in SG buffer, and equilibrated to 37°C. Before activation, cells were washed twice with SG buffer (containing BSA and CaCl2). Cells were activated for 2 hr.
Flow Cytometry
RBL-2H3 cells suspended in sterile SG buffer at a concentration of 3 × 106 cells/ml were analyzed in either their resting or chemically activated state (PMA/A23187). Cells were incubated in each condition for 2 hr at 37°C, and then washed in DPBS and centrifuged at 1200 rpm for 5 min. Cells were then fixed and half were permeabilized using 300 mM sucrose, 50 mM NaCl, 3 mM MgCl2, 2 mM HEPES, 0.5% (w/v) Triton X-100 for 5 min on ice and blocked using 1% BSA/PBS for 20 min at room temperature. Five hundred μl of cell suspension (5 × 105 cells) was incubated with primary antibodies for 16 hr at 4°C. Primary antibodies included the 4-sulfated CS stub antibody described above (clone 2B6) as well as the mouse monoclonal anti-CS type A and C antibody (1:500, clone CS-56; Sigma-Aldrich), which detects the disaccharide A-D sequence [GlcUA-GalNAc(4S)-GlcUA(2S)-GalNAc(6S)], and isotype controls, whole mouse IgG and IgM (1:500; Invitrogen). Cells were then incubated with Alex Fluor 488-conjugated secondary antibodies (1:500, goat anti-mouse IgG/IgM; Invitrogen) for 30 min at room temperature and analyzed using a flow cytometer (BD Biosciences; Franklin Lakes, NJ) with 104 events collected per sample. Data were analyzed using the commercial software, FCS 4 Express.
Immunocytochemistry
RBL-2H3 cells, at a density of 1 × 104 cells/well, were seeded into glass chamberwell slides (Thermo Scientific; Rochester, NY) for 2 hr in either SG buffer or SG buffer with activators (PMA/A23187) in a humidified incubator (5% CO2 /95% air atmosphere at 37°C). Cells were washed with PBS and fixed using 4% paraformaldehyde for 15 min at 37°C and permeabilized as described above. After washing with 50 mM Tris-HCl, 0.15 M NaCl, pH 7.6 (TBS), non-specific binding sites were blocked with 5% goat serum in TBS containing 0.1% (w/v) Tween-20 (TBST) for 1 hr at room temperature followed by incubation with the primary antibodies at room temperature for 2 hr. Primary antibodies used included mouse monoclonal anti-4 sulfated and anti-6 sulfated CS stub antibodies (1:500, clones 2B6 and 3B3, respectively), with cells pre-treated with and without C’ase ABC digestion (0.05 U/mL, 0.1 M Tris Acetate, pH 8). Slides were rinsed twice with TBST and incubated with Alexa Fluor 488 anti-mouse antibodies (1:500, Life Technologies) for 1 hr at RT before rinsing twice with TBST. Nuclei were stained with DAPI (2.5 µg/ml) for 20 min in the dark before two final washes with TBS. Slides were mounted with Prolong gold (Invitrogen) and then visualized using a confocal microscope (FluoView FV1200; Olympus Optical Co. Ltd, Tokyo, Japan).
Co-localization experiments were carried out as described above with the following modifications. Following blocking of non-specific binding with 5% goat serum in TBST, slides were probed with antibody clone 2B6 (1:500) and antibodies against CSPGs serglycin (1:500; SC-292311, Santa Cruz Biotechnology, Dallas, TX) or perlecan [1:1000, CCN-1, raised in house against immunopurified human coronary artery endothelial cell derived perlecan (Lord et al. 2014, Whitelock et al. 1999)] at room temperature for 2 hr. Slides were rinsed twice with TBST and incubated with Alexa Fluor 488 anti-mouse IgG and Alexa Fluor 594 anti-rabbit antibodies (1:500, Life Technologies) for 1 hr at room temperature before rinsing twice with TBST. Nuclei were stained with DAPI (2.5 µg/ml). Slides were then mounted and visualized using a confocal microscope. Co-localization was quantified using the Manders co-localization coefficient that provides a measure of co-occurrence of two channels independent of signal proportionality and is reported as the fraction of pixels with positive values in both channels.
Results
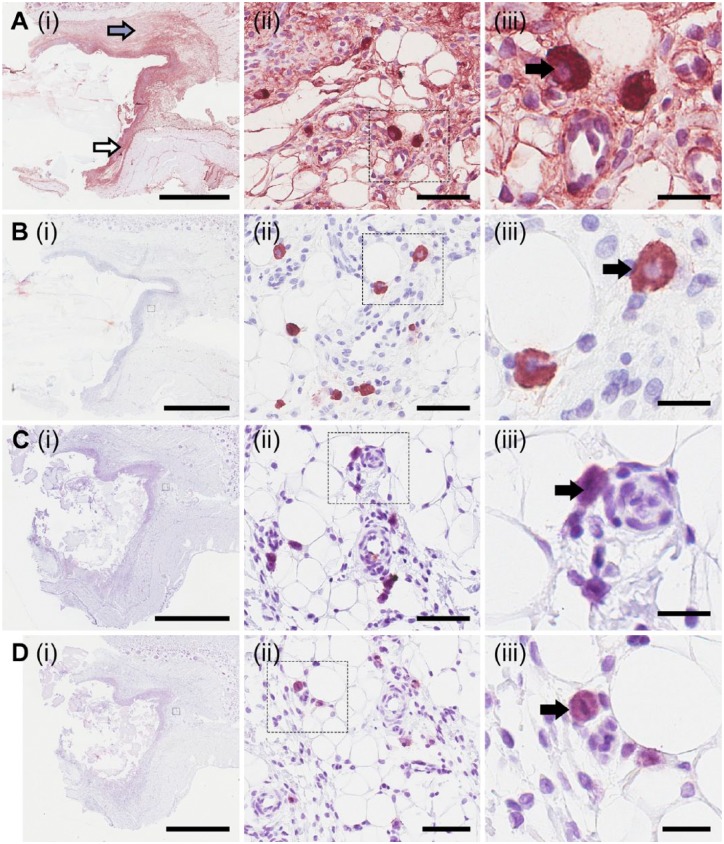
Sections probed with antibodies that detect the epitopes 2B6 and 3B3 showed positive staining after bacterial endoglycosidase digestion with C’ase ABC (Fig. 1). Positive staining of 2B6 after C’ase ABC digestion, denoted as 2B6+ herein, was predominantly localized within the fibrous capsule surrounding the material implant (Fig. 1A(i), indicated by the white arrow), which formed in response to the implant. 2B6+ staining was also found at lower intensity in the tissue adjacent to the fibrous capsule (Fig. 1A(i), indicated by the grey arrow). Observation of these sections at higher magnifications revealed a population of intensely stained cells as compared with the surrounding tissue (Fig. 1A(ii) and 1A(iii), indicated by the black arrow). These cells had a distinct morphology and were approximately 10–20 µm in diameter. Surprisingly, these cells were also detected with the antibody clone 2B6 without prior endoglycosidase digestion, denoted as 2B6- herein (Fig. 1B(ii) and 1B(iii)); this suggests that the epitope detected by the 2B6 antibody may be a native CS epitope in addition to the 4-sulfated CS neo-epitope stub generated by C’ase ABC digestion. The fibrous capsule and surrounding tissue did not react with 2B6- (Fig. 1B(i)), with the exception of the 2B6- cells.
Figure 1.
Characterization and localization of chondroitin sulfate structures. Using immunohistological methods, sections were probed for the presence of epitopes, detectable using antibody clones 2B6 (A and B) and 3B3 (C and D). Sections were probed with the antibody clones with (A and C) and without (B and D) prior endoglycosidase digestion with chondroitinase ABC. Small boxes marked in the images in the first column are sequentially enlarged in the middle and right columns. Arrows indicate 2B6+ staining at the fibrous capsule (white arrow) and positive staining at a lower intensity in tissue adjacent to the fibrous capsule (grey arrow). Black arrows indicate positive staining of cells when probed with 2B6 with (A(iii)) and without C’ase ABC digestion (B(iii)), or 3B3 with (C(iii)) and without C’ase ABC digestion (D(iii)). Scale (i) 3 mm; (ii) 60 µm; (iii) 20 µm.
Cells in proximity to blood vessels were stained positively for the antibody clone 3B3 with (3B3+, Fig. 1C(iii)) and without (3B3-, Fig. 1D(iii)) C’ase ABC digestion. These cells exhibited a similar morphology to the cells that contain the 2B6- epitope (Fig. 1A(iii) and 1B(iii)). Antibody clone 3B3 was not reactive with the fibrous capsule or the surrounding tissue either with (Fig. 1C) and without (Fig. 1D) treatment with C’ase ABC. These results suggest, in the absence of endoglycosidase digestion, the presence of either the 6-sulfated CS neo-epitope stub generated by C’ase ABC digestion or the presence of the non-reducing terminal end-saturated CS disaccharide consisting of glucuronic acid (GlcA), which is adjacent to the 6-sulfated N-acetylgalactosamine (GalNAc), or a combination of both of these epitopes.
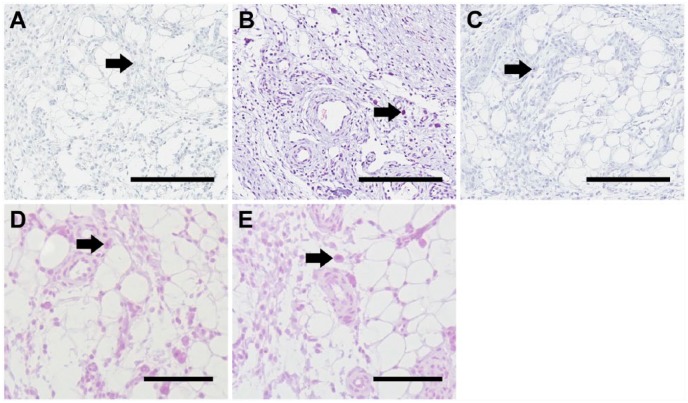
To control for staining with the antibody clones 2B6 and 3B3, tissue sections were probed with biotinylated secondary antibodies, mouse IgG (Fig. 2A) and mouse IgM (Fig. 2B), where slides were incubated prior to the biotinylated secondary antibodies with blocking solution. The tissue sections revealed no significant staining when probed with either the biotinylated anti-mouse IgG or IgM secondary antibody. Cells previously shown to express the 2B6- or 3B3- epitopes are indicated by black arrows. These data demonstrate that non-specific binding of the biotinylated secondary antibodies did not occur. Tissue sections probed with whole mouse IgG (Fig. 2D) and IgM (Fig. 2E) antibodies, as isotype control, demonstrated that non-specific immunoglobulin binding did not occur.
Figure 2.
Immunohistochemical controls included probing the tissue sections without primary antibodies with biotinylated mouse IgG (A), mouse IgM (B) and rabbit (C) secondary antibodies. Isotype controls were carried out with sections probed with mouse IgG (D) and IgM (E). Cells previously shown to express the 2B6- or 3B3- epitopes are indicated by black arrows. Scale, 200 µm.
The type of inflammatory cell involved in these tissue regions was of interest. To investigate the types of inflammatory cells present, tissue sections were probed for the presence of macrophages using the cell-surface markers CD86 and CD206 for the detection of type 1 (Fig. 3A) and type 2 macrophages (Fig. 3B), respectively (Wolf et al. 2014). The fibrous capsule was positively stained for the presence of type 1 macrophages, where their presence was also observed in the surrounding tissue. The cells that expressed the 2B6- epitope were positive for CD86 on the cell surface (Fig. 3A(iii), indicated by the black arrow). The localization of CD206 was shown to be within the fibrous capsule as well as within the adjacent tissue (Fig. 3B(i)). Similar to CD86, cells that expressed the 2B6- epitope were shown to have slight staining for CD206 (Fig. 3B(iii), indicated by the black arrow). Controls with biotinylated anti-rabbit secondary antibody only (Fig. 2C) did not show positive staining, indicating that non-specific binding of biotinylated anti-rabbit antibody did not occur.
Figure 3.
Localization of macrophages. Using immunohistological methods, sections were probed to investigate the presence of macrophages, type 1 (A) and type 2 (B). Small boxes marked in the images in the first column are sequentially enlarged in the middle and right columns. Black arrow indicates cells that express the 2B6- epitope that had slight staining for CD86 (A(iii)) and CD206 (B(iii)). Scale (i) 3 mm; (ii) 60 µm; (iii) 20 µm.
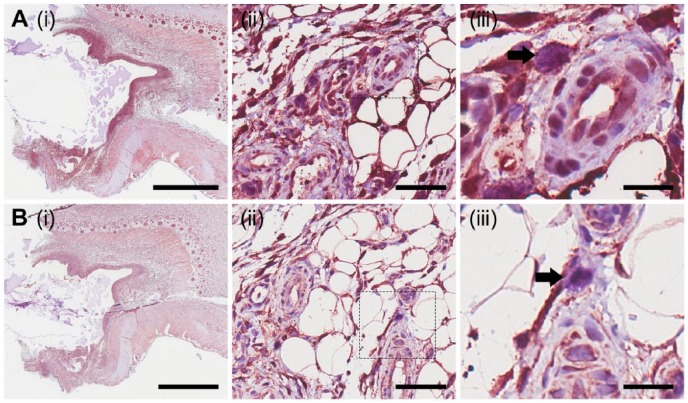
In addition to the infiltration of macrophages, mast cells also infiltrate to the implantation site of a foreign material (Farrugia et al. 2014). Sections were stained with toluidine blue (Fig. 4A), and Leder stain (Fig. 4B) to determine the presence of mast cells, and probed for the presence of mast cell tryptase (Fig. 4C), a serine proteinase that is stored in mast cell α-granules and released upon activation. Cells within the tissue sections showed punctate staining with toluidine blue (Fig. 4A(ii) and 4A(iii)). Higher magnification (Fig. 4A(iii)) depicts violet staining of these cells, as indicated by the black arrow. Leder-stained cells were found within the fibrous capsule that had formed around the implanted material, as well as in close proximity to blood vessels (Fig. 4B(ii)). Cells previously shown to be stained with toluidine blue were found to be magenta following staining with Leder stain (Fig. 4B(iii)), confirming that these cells were mast cells. Intracellular staining of mast cell tryptase was observed in cells within the fibrous capsule as well as in the adjacent tissue (Fig. 4C). Similar to toluidine blue- and Leder-stained sections, a population of cells in proximity to blood vessels was positive for mast cell tryptase (Fig. 4C(ii) and 4C(iii)). These results demonstrate that the population of cells with positive staining for toluidine blue, Leder stain and mast cell tryptase are mast cells. The cells chemically characterized as mast cells were observed to be of similar morphology and size (10–20 µm) to cells that expressed the 2B6- and 3B3- epitopes. This suggests that the cells found to express the epitope detected by 2B6- may be mast cells.
Figure 4.
Characterization and localization of mast cells. Mast cells were chemically characterized via staining with toluidine blue (A), Leder stain (B) and immunohistological methods to probe for the presence of mast cell tryptase (C). Small boxes marked in the images in the first column are sequentially enlarged in the middle and right columns. Black arrows point to punctate violet staining for mast cells (A(iii)), Leder stain positive cells (B(iii)), and mast cell tryptase positive cells (C(iii)). Scale (i) 3 mm; (ii) 60 µm; (iii) 20 µm.
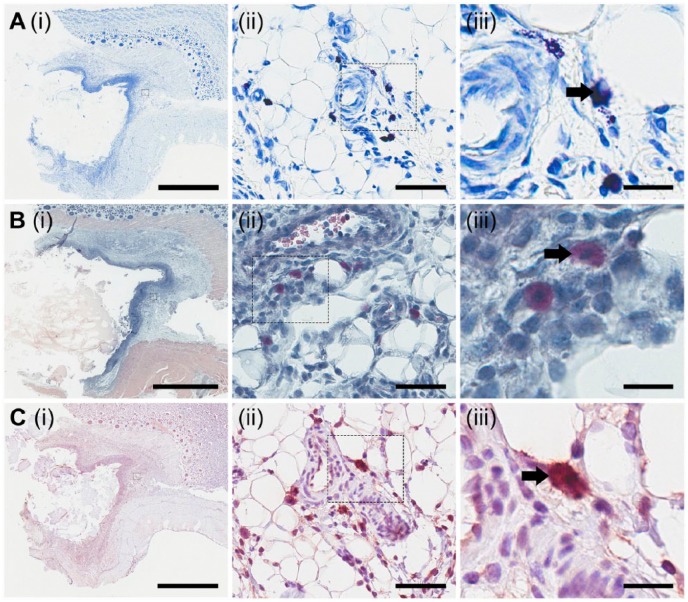
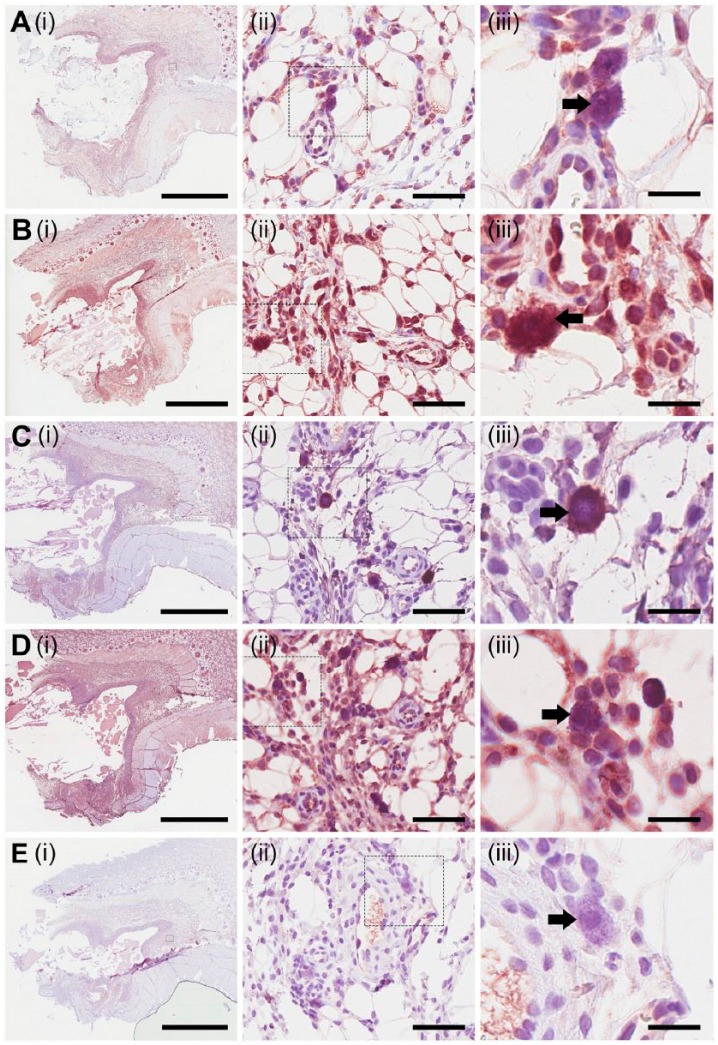
Tissue sections were probed to detect the presence of the CSPGs serglycin (Fig. 5A), perlecan (Fig. 5B), versican (Fig. 5C), bikunin (Fig. 5D), and aggrecan (Fig. 5E). Serglycin was localized within the fibrous capsule that surrounded the implanted materials, as well as in tissue adjacent to the fibrous capsule, although with decreased expression (Fig. 5A(i)). Serglycin was shown to be present in 2B6- cells (Fig. 5A(iii), indicated by the black arrow). Perlecan was detected in the fibrous capsule (Fig. 5B(i)). Cells that contained the 2B6 epitope were also positive for perlecan, with intense cellular staining observed (Fig. 5B(iii), indicated by the black arrow). Versican was found to be present in relatively low levels in the tissue adjacent to the fibrous capsule surrounding the implanted material (Fig. 5C(i)). On further investigation, versican was present with intracellular staining in 2B6- cells (Fig. 5C(iii), indicated by the black arrow). Bikunin was expressed in the fibrous capsule as well as in the adjacent tissue (Fig. 5D(i)). Similar to serglycin, perlecan and versican, bikunin was shown to be present in 2B6- cells (Fig. 5D(iii), indicated by the black arrow). Aggrecan is a CSPG primarily associated with cartilage. Sections probed for the presence of aggrecan showed minimal staining within tissue adjacent to the fibrous capsule (Fig. 5E(i)), and previously shown to express the 2B6- epitope did not stain positively for aggrecan (Fig. 5E(iii), indicated by the black arrow). These results demonstrate that a number of different CSPGs were present in the fibrous capsule in response to the implanted materials with a similar localization to cells that express the 2B6- epitope. This suggests that the 2B6- epitope may be associated with a number of different CSPGs.
Figure 5.
Localization of CS proteoglycans. Using immunohistological methods, sections were probed for the presence of serglycin (A), perlecan (B), versican (C), bikunin (D) and aggrecan (E). Small boxes marked in the images in the first column are sequentially enlarged in the middle and right columns. Black arrows in each of the images for column on the right depict the presence (A–D) or absence (E) of staining in cells previously shown to express the 2B6- epitope. Scale (i) 3 mm; (ii) 60 µm; (iii) 20 µm.
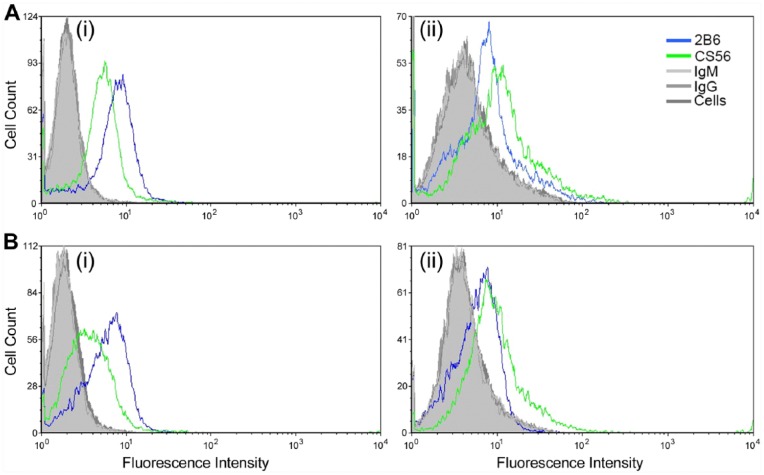
The novel CS epitope recognized by the antibody clone 2B6 in rodent tissues was explored further in the rat-derived mast cell-like line RBL-2H3. We investigated the expression of CS epitopes by RBL-2H3 cells detected with the antibody clones 2B6 and CS-56 via flow cytometry (Fig. 6). Expression of the 2B6- epitope showed a similar fluorescence intensity when the cells were in either their resting or activate states, irrespective of permeabilization prior to antibody probing. In contrast, the fluorescence intensity of the CS-56 antibody reactivity increased upon permeabilization of the cell membrane when cells were in either their resting or activated state. These results demonstrate that the 2B6- and CS-56 epitopes are present in RBL-2H3 cells in different cellular locations. Cells were also incubated with whole mouse IgG and IgM, and blocking solution in place of a primary antibody, followed by incubation with Alex Fluor 488-conjugated secondary antibodies as controls for non-specific immunoglobulin and secondary antibody binding. Background fluorescence levels were detected for each of these controls and compared to cells probed with either antibody clones 2B6 or CS-56. These data demonstrated no immunoglobulin binding, either IgG or IgM, or non-specific binding of the fluorophore-conjugated secondary antibody. These data confirmed the specificity of both antibody clones 2B6 and CS-56 in the RBL-2H3 cells.
Figure 6.
Flow cytometry probing for the presence of the CS epitopes detected by the antibody clones CS56 and 2B6 in the absence of chondroitinase ABC digestion in RBL-2H3 cells in their resting state (A) and following chemical activation (B). Cells were either not permeabilized [A(i) and (B(i)] or permeabilized [A(ii) and (B(ii)] before incubation with primary antibodies.
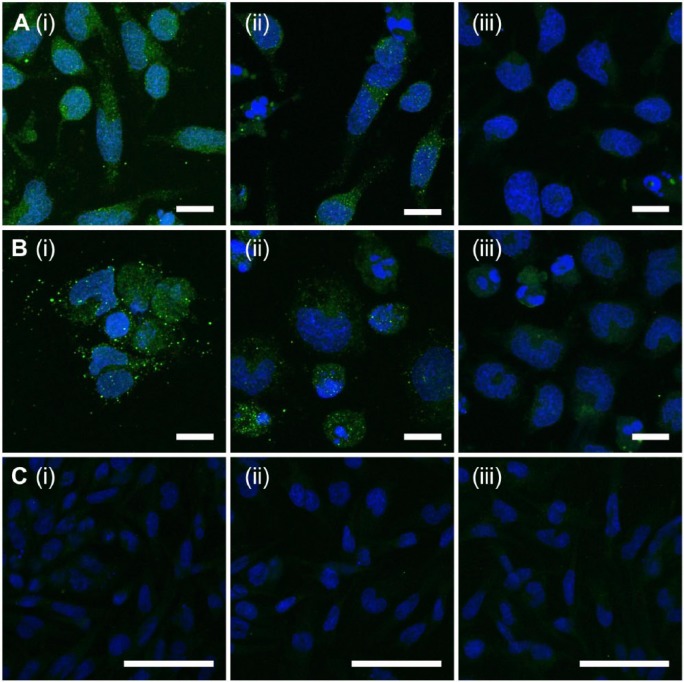
Immunocytochemistry was carried out on RBL-2H3 cells in their resting state and following chemical activation to further investigate the presence and localization of the epitope detected by the antibody clone 2B6. Epitopes detected by the antibody clone 2B6 with (Fig. 7A(i)) and without (Fig. 7A(ii)] endoglycosidase digestion were shown to be present when cells were in their resting state. No significant staining was detected for cells probed with the fluorophore conjugated secondary antibody only (Fig. 7A(iii)). Following chemical activation (Fig. 7B), the presence of the 2B6 epitope was found to be more punctate, particularly when cells were endoglycosidase digested before antibody probing (Fig. 7B(i)). Positive staining for the 2B6- epitope was also observed when cells were in their activated state (Fig. 7B(ii)). Cells probed with the fluorophore-conjugated secondary antibody only showed minimal background fluorescence, with an absence of the punctate staining (Fig. 7B(iii)). RBL-2H3 cells contained neither the 3B3+ (Fig. 7C(i)) nor 3B3- (Fig. 7C(ii)) epitopes. Additionally, cells probed with the fluorophore-conjugated secondary antibody only were also void of staining (Fig. 7C(iii)). As observed within the rodent tissue and supporting the flow cytometry data, the 2B6- epitope was present in rat-derived mast cells and was shown to be not dependent on their activation state. Furthermore, the punctate staining suggested a granular localization of the both the 2B6+ and 2B6- epitopes. These data suggest that RBL-23 cells produce a native CS epitope detected by the antibody clone 2B6 in the absence of C’ase ABC, in addition to the 4-sulfated CS stub generated by endoglycosidase digestion.
Figure 7.
Immunolocalization of epitopes detected using the antibody clones 2B6 and 3B3 produced by mast cells (RBL-2H3). Cells were probed for the presence of 2B6 with (A(i) and B(i)) and without (A(ii) and B(ii)) endoglycosidase digestion with chondroitinase ABC. Localization of 2B6 was investigated when cells were in their resting (A) or chemically activated (B) states. Cells were probed for the presence of 3B3 with (C(i)) and without (C(ii)) chondroitinase ABC digestion. Localization was investigated when cells were in their resting state. Negative controls were not incubated in the presence of the primary antibody before incubation with Alexa Fluor 488-conjugated anti-mouse secondary antibody (A(iii), B(iii) and C(iii)). Scale (A, B) 10 µm; (C) 50 µm.
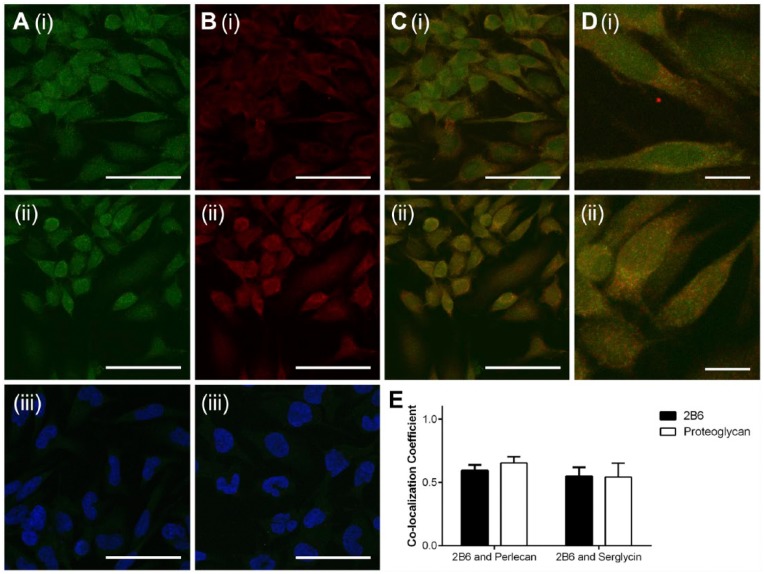
To further investigate the CSPGs that may be associated with the novel CS epitope, RBL-2H3 cells were stained for the presence of 2B6- and either serglycin or perlecan (Fig. 8). Positive staining for the 2B6- epitope was observed (Fig. 8A(i) and 8A(ii)). These cells also showed reactivity for both perlecan (Fig. 8B(i)) and serglycin (Fig. 8B(ii)). Cells probed with anti-mouse Alexa Fluor 488 (Fig. 8A(iii)) or anti-rabbit Alexa Fluor 594 (Fig. 8B(iii)), where the blocking solution was used in place of a primary antibody, showed minimal staining, which indicated that non-specific binding of the secondary antibodies did not occur. Co-localization of the 2B6- epitope with perlecan (Fig. 8C(i) and 8D(i)) or serglycin (Fig. 8C(ii) and 8D(ii)) was observed, as indicated by yellow staining. Both perlecan and serglycin were detected in regions of positive staining that co-localized with the 2B6- epitope, as well as regions where the 2B6- epitope did not co-localize with either CSPG (Fig. 8D(i) and 8D(ii)). Quantification of co-localization was carried out using the Manders co-localization coefficient (Fig. 8E). An average coefficient of 0.59 was shown for co-localization of the 2B6- epitope with perlecan, and 0.65 for perlecan with 2B6-. The Manders co-localization coefficient of 0.55 was shown for 2B6- with serglycin, and 0.54 for serglycin with 2B6-. These results demonstrate that the 2B6- epitope did not solely co-localize with either of the CSPGs and that neither perlecan nor serglycin solely co-localized with the 2B6- epitope. These results demonstrate the 2B6- does not associate with a specific CSPG and suggests these CSPGs may not be decorated exclusively with a CS that contains the 2B6- epitope.
Figure 8.
Co-localization of CS proteoglycans with 2B6 in the absence of chondroitinase ABC digestion. Cells were probed for the presence of 2B6 (A(i) and (ii)) and either perlecan (B(i)) or serglycin (B(ii)). Cells were then probed with anti-mouse Alexa Fluor 488 or anti-rabbit Alexa Fluor 594 secondary antibodies to detect the presence of 2B6 or the CSPGs, respectively. Merged channels demonstrate co-localization of 2B6 and perlecan (C(i) and D(i)), as well as 2B6 and serglycin (C(ii) and (D(ii)). Co-localization of 2B6 and serglycin/perlecan was quantified using the Manders co-localization coefficient (E). Scale (A, B, C) 50 µm; (D) 10 µm.
Discussion
The current study investigated the presence of a novel CS epitope detected using the antibody clone 2B6 in the absence of C’ase ABC digestion in mammalian tissue and derived cells. The mammalian tissue was obtained from a rodent implantation model, where the inflammatory response to implanted materials, including Surgicel and chitosan, was evaluated (Farrugia et al. 2014). In this study mast cells infiltrated into the wound site in response to all of the implanted materials and the expression of the 2B6- epitope was not dependent on the material implanted. The implantation of a foreign material will invoke an inflammatory response involving a range of cells including neutrophils, monocytes, macrophages and mast cells. Macrophages, and their precursor monocytes, are inflammatory cell types that are also known to contain serglycin decorated with CS-E (Uhlin-Hansen et al. 1989). Unlike in mast cells, where serglycin is stored in its granules and released upon activation, serglycin is continuously secreted from macrophages and is involved in the regulation of tumor necrosis factor-α (Zernichow et al. 2006). Serglycin has been shown to be produced by various inflammatory cells where it is involved with the storage and secretion of various proteases and cytokines (Grujic et al. 2005; Niemann et al. 2007).
The role that mast cells play in the immune system is largely due to the constituents of their secretory granules, which include various enzymes, cytokines, and proteases (Wernersson and Pejler 2014). Heparanase is contained in the secretory granules with roles in the cleavage of heparin and HS, wherein the ability of heparanase to intracellularly cleave heparin in mast cells (Wang et al. 2011) has been demonstrated and shown to influence the storage and release of secretory granular components. Cleavage of macromolecular heparin by heparanase is crucial for accelerating the release of heparin and chymase from the secretory granules to the extracellular matrices (Higashi et al. 2014). Heparanase, however, does not cleave CS chains, and therefore would not be responsible for the results presented with regard to the CS structure detected by 2B6.
The 2B6 antibody detects the unsaturated terminal disaccharide stub structure containing 4-sulfated GalNAc, which is revealed after bacterial C’ase ABC digestion (Couchman et al. 1984; Caterson et al. 1985). Evidence of the 2B6- epitope has previously been reported in human osteoarthritic cartilage (Asari et al. 1996), with the epitope localized within the chondrocytes; however, the possible function of this epitope was not further explored. The data presented in this study demonstrates that cells in rodent tissue that are morphologically similar to and chemically characterized as mast cells produce a CS structure detected by the 2B6 antibody without endoglycosidase digestion. The bacterial endoglycosidase, chondroitinase (C’ase) ABC, belongs to the family of lysases where endolytic cleavage of CS chains occurs at β(1 → 4) galactosaminic bonds between GalNAc and GlcA. This results in an unsaturated terminal disaccharide structure after the linkage region. A suite of antibodies—2B6, 3B3 and 1B5 (Couchman et al. 1984, Caterson et al. 1985)—enables the detection of the terminal unsaturated disaccharide structures that remain following C’ase ABC digestion, and determination of whether these structures are sulfated, 4S or 6S, or unsulfated. The results presented here demonstrate that mammalian cells produce an epitope recognized by the antibody clone 2B6 in the absence of endoglycosidase digestion. This suggests that the 2B6- epitope may be generated in situ or that the structure that was detected may occur naturally. To the best of our knowledge, there is no known mammalian CS digesting enzyme that reveals the epitope of antibody clone 2B6.
Hyaluronidase-4, a member of the hyaluronidase family, has shown to behave as a chondroitin hydrolyze in C. elegans (Kaneiwa et al. 2008). Most hyaluronidases degrade both hyaluronidase and CS, including hyaluronidase-1 and testicular hyaluronidase, particularly CS-A (Honda et al. 2012), where HYAL-4 cleaves galactosaminidic bonds of CS, specifically GalNAc(4S)-GlcUA and GalNAc(6S)-GlcUA, with an optimal pH of 4.5 – 5 (Kaneiwa et al. 2010). Thus, it is hypothesized that the 2B6- epitope detected may be the result of hyaluronidase cleavage of CS. We investigated the CSPG with which the novel CS epitope may associate, and show that serglycin, perlecan, versican and bikunin localize in cells of similar morphology as those that contain the novel CS epitope. Furthermore, co-localization of 2B6- epitope with both perlecan and serglycin in RBL-2H3 cells indicated that the 2B6- epitope was present on both of these CSPGs. This demonstrates that the unique CS structure is not specific to a certain CSPG, and supports the hypothesis that the novel epitope is generated by a mammalian chondroitinase or synthesized by the cells.
Serglycin is the prominent CSPG produced by mast cells; although, the staining results presented to demonstrate the presence of serglycin within the rodent tissue were relatively faint. It is noted that the primary antibody used to probe for the presence of serglycin was raised against an amino acid sequence of full length human serglycin (aa 1–158) and not a rodent sequence, which could explain this result. Murine mast cell tryptase 5 binds to heparin-containing PGs (Matsumoto et al. 1995), demonstrating the importance of GAGs in the protection and packing of contents of mast cell granules. Furthermore, serglycin is important for the assembly of secretory granules, the transport of secretory compounds into the granules, as well as the maturation of granules (Braga et al. 2007). The roles of GAGs in mast cell granules include the storage (Åbrink et al. 2004) and retention (Henningsson et al. 2006) of proteases through complex formation (Serafin et al. 1986). Heparin is important in the storage of mast cell proteases (Humphries et al. 1999); N-deacetylase/N-sulphotransferase-2 knockout mice are unable to express heparin and show defects in their granules, including reduced storage of proteases and histamine (Forsberg et al. 1999). Proteases and cytokines are packaged in an inactive state in mast cell granules at pH 5.5 through strong ionic interactions with GAGs (De Young et al. 1987). Heparanase is involved in the cleavage of heparin/HS from the PGs in mast cell granules and the release of bound proteases and cytokines into the surrounding tissue. It is hypothesized that this process also occurs for CS in the mast cell granules and that cleavage occurs by a member of the hyaluronidase family, thus enabling the release of CS-bound proteases and cytokines. Further investigation is underway to test this hypothesis.
Acknowledgments
Authors acknowledge technical support from staff within the Biomedical Imaging Facility (BMIF), and the Histological Microscopy Unit (HMU) at the University of New South Wales.
Footnotes
Author Contributions: BF, JW, BC and ML contributed to the experimental design, data interpretation, and preparation of the manuscript. RO’G contributed to data interpretation and preparation of the manuscript. BF carried out the experiments. All authors have read and approved the final manuscript.
Competing Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by funding from the Australian Research Council under the Linkage Project (LP0776293) scheme.
References
- Åbrink M, Grujic M, Pejler G. (2004). Serglycin is essential for maturation of mast cell secretory granule. J Biol Chem 279:40897-40905. [DOI] [PubMed] [Google Scholar]
- Asari A, Akizuki S, Itoh T, Kominami E, Uchiyama Y. (1996). Human osteoarthritic cartilage exhibits the 2B6 epitope without pretreatment with chondroitinase ABC. Osteoarthritis Cartilage 4:149-152. [DOI] [PubMed] [Google Scholar]
- Avnur Z, Geiger B. (1984). Immunocytochemical localization of native chondroitin-sulfate in tissues and cultured cells using specific monoclonal antibody. Cell 38:811-822. [DOI] [PubMed] [Google Scholar]
- Braga T, Grujic M, Lukinius A, Hellman L, Åbrink M, Pejler G. (2007). Serglycin proteoglycan is required for secretory granule integrity in mucosal mast cells. Biochem J 403:49-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterson B, Christner JE, Baker JR, Couchman JR. (1985). Production and characterization of monoclonal antibodies directed against connective tissue proteoglycans. Fed Proc 44:386-393. [PubMed] [Google Scholar]
- Caterson B, Mahmoodian F, Sorrell JM, Hardingham TE, Bayliss MT, Carney SL, Ratcliffe A, Muir H. (1990). Modulation of native chondroitin sulphate structure in tissue development and in disease. J Cell Sci 97(Pt 3):411-417. [DOI] [PubMed] [Google Scholar]
- Couchman JR, Caterson B, Christner JE, Baker JR. (1984). Mapping by monoclonal antibody detection of glycosaminoglycans in connective tissues. Nature 307:650-652. [DOI] [PubMed] [Google Scholar]
- De Young MB, Nemeth EF, Scarpa A. (1987). Measurement of the internal pH of mast cell granules using microvolumetric fluorescence and isotopic techniques. Arch Biochem Biophys 254:222-233. [DOI] [PubMed] [Google Scholar]
- Enerbäck L, Kolset SO, Kusche M, Hjerpe A, Lindahl U. (1985). Glycosaminoglycans in rat mucosal mast cells. Biochem J 227:661-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrugia BL, Whitelock JM, Jung M, Mcgrath B, O’grady RL, Mccarthy SJ, Lord MS. (2014). The localisation of inflammatory cells and expression of associated proteoglycans in response to implanted chitosan. Biomaterials 35:1462-1477. [DOI] [PubMed] [Google Scholar]
- Forsberg E, Pejler G, Ringvall M, Lunderius C, Tomasini-Johansson B, Kusche-Gullberg M, Eriksson I, Ledin J, Hellman L, Kjellen L. (1999). Abnormal mast cells in mice deficient in a heparin-synthesizing enzyme. Nature 400:773-776. [DOI] [PubMed] [Google Scholar]
- Grujic M, Braga T, Lukinius A, Eloranta M-L, Knight SD, Pejler G, Åbrink M. (2005). Serglycin-deficient cytotoxic t lymphocytes display defective secretory granule maturation and granzyme B storage. J Biol Chem 280:33411-33418. [DOI] [PubMed] [Google Scholar]
- Henningsson F, Hergeth S, Cortelius R, Åbrink M, Pejler G. (2006). A role for serglycin proteoglycan in granular retention and processing of mast cell secretory granule components. FEBS J 273:4901-4912. [DOI] [PubMed] [Google Scholar]
- Higashi N, Waki M, Sue M, Kogane Y, Shida H, Tsunekawa N, Hasan A, Sato T, Kitahara A, Kasaoka T, Hayakawa Y, Nakajima M, Irimura T. (2014). Heparanase-mediated cleavage of macromolecular heparin accelerates release of granular components of mast cells from extracellular matrices. Biochem J 458:291-299. [DOI] [PubMed] [Google Scholar]
- Honda T, Kaneiwa T, Mizumoto S, Sugahara K, Yamada S. (2012). hyaluronidases have strong hydrolytic activity toward chondroitin 4-sulfate comparable to that for hyaluronan. Biomolecules 2:549-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries DE, Wong GW, Friend DS, Gurish MF, Qiu W-T, Huang C, Sharpe AH, Stevens RL. (1999). Heparin is essential for the storage of specific granule proteases in mast cells. Nature 400:769-772. [DOI] [PubMed] [Google Scholar]
- Ide H, Itoh H, Yoshida E, Kobayashi T, Tomita M, Maruyama H, Osada Y, Nakahata T, Nawa Y. (1999). Immunohistochemical demonstration of inter-α-trypsin inhibitor light chain (bikunin) in human mast cells. Cell Tissue Res 297:149-154. [DOI] [PubMed] [Google Scholar]
- Ito Y, Hikino M, Yajima Y, Mikami T, Sirko S, Von Holst A, Faissner A, Fukui S, Sugahara K. (2005). Structural characterization of the epitopes of the monoclonal antibodies 473HD, CS-56, and MO-225 specific for chondroitin sulfate D-type using the oligosaccharide library. Glycobiology 15:593-603. [DOI] [PubMed] [Google Scholar]
- Jung M, Lord MS, Cheng B, Lyons JG, Alkhouri H, Hughes JM, Mccarthy SJ, Iozzo RV, Whitelock JM. (2013). Mast cells produce novel shorter forms of perlecan that contain functional endorepellin: a role in angiogenesis and wound healing. J Biol Chem 288:3289-3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneiwa T, Mizumoto S, Sugahara K, Yamada S. (2010). Identification of human hyaluronidase-4 as a novel chondroitin sulfate hydrolase that preferentially cleaves the galactosaminidic linkage in the trisulfated tetrasaccharide sequence. Glycobiology 20:300-309. [DOI] [PubMed] [Google Scholar]
- Kaneiwa T, Yamada S, Mizumoto S, Montaño AM, Mitani S, Sugahara K. (2008). Identification of a novel chondroitin hydrolase in caenorhabditis elegans. J Biol Chem 283:14971-14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjellén L, Pettersson I, Lillhager P, Steen ML, Pettersson U, Lehtonen P, Karlsson T, Ruoslahti E, Hellman L. (1989). Primary structure of a mouse mastocytoma proteoglycan core protein. Biochem J 263:105-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolset SO, Gallagher JT. (1990). Proteoglycans in haemopoietic cells. Biochim Biophys Acta 1032:191-211. [DOI] [PubMed] [Google Scholar]
- Kolset SO, Kjellén L, Seljelid R, Lindahl U. (1983). Changes in glycosaminoglycan biosynthesis during differentiation in vitro of human monocytes. Biochem J 210:661-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord MS, Chuang CY, Melrose J, Davies MJ, Iozzo RV, Whitelock JM. (2014). The role of vascular-derived perlecan in modulating cell adhesion, proliferation and growth factor signaling. Matrix Biol 35:112-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto R, Šali A, Ghildyal N, Karplus M, Stevens RL. (1995). packaging of proteases and proteoglycans in the granules of mast cells and other hematopoietic cells: A cluster of histidines on mouse mast cell protease 7 regulates its binding to heparin serglycin proteoglycans. J Biol Chem 270:19524-19531. [DOI] [PubMed] [Google Scholar]
- Metcalfe DD, Baram D, Mekori YA. (1997). Mast cells. Physiol Rev 77:1033-1079. [DOI] [PubMed] [Google Scholar]
- Niemann CU, Åbrink M, Pejler G, Fischer RL, Christensen EI, Knight SD, Borregaard N. (2007). Neutrophil elastase depends on serglycin proteoglycan for localization in granules. Blood 109:4478-4486. [DOI] [PubMed] [Google Scholar]
- Rönnberg E, Melo FR, Pejler G. (2012). Mast Cell Proteoglycans. J Histochem Cytochem 60:950-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seldin DC, Austen KF, Stevens RL. (1985). Purification and characterization of protease-resistant secretory granule proteoglycans containing chondroitin sulfate di-B and heparin-like glycosaminoglycans from rat basophilic leukemia cells. J Biol Chem 260:11131-11139. [PubMed] [Google Scholar]
- Serafin WE, Katz HR, Austen KF, Stevens RL. (1986). Complexes of heparin proteoglycans, chondroitin sulfate E proteoglycans, and [3H]diisopropyl fluorophosphate-binding proteins are exocytosed from activated mouse bone marrow-derived mast cells. J Biol Chem 261:15017-15021. [PubMed] [Google Scholar]
- Sorrell JM, Carrino DA, Caplan AI. (1993). structural domains in chondroitin sulfate identified by anti-chondroitin sulfate monoclonal antibodies. Immunosequencing of chondroitin sulfates. Matrix 13:351-361. [DOI] [PubMed] [Google Scholar]
- Sorrell JM, Mahmoodian F, Schafer IA, Davis B, Caterson B. (1990). Identification of monoclonal antibodies that recognize novel epitopes in native chondroitin/dermatan sulfate glycosaminoglycan chains: their use in mapping functionally distinct domains of human skin. J Histochem Cytochem 38:393-402. [DOI] [PubMed] [Google Scholar]
- Stevens RL, Fox CC, Lichtenstein LM, Austen KF. (1988). Identification of chondroitin sulfate E proteoglycans and heparin proteoglycans in the secretory granules of human lung mast cells. Proc Natl Acad Sci U S A 85:2284-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens RL, Lee TD, Seldin DC, Austen KF, Befus AD, Bienenstock J. (1986). Intestinal mucosal mast cells from rats infected with Nippostrongylus brasiliensis contain protease-resistant chondroitin sulfate di-B proteoglycans. J Immunol 137:291-295. [PubMed] [Google Scholar]
- Uhlin-Hansen L, Eskeland T, Kolset SO. (1989). Modulation of the expression of chondroitin sulfate proteoglycan in stimulated human monocytes. J Biol Chem 264:14916-14922. [PubMed] [Google Scholar]
- Wang B, Jia J, Zhang X, Zcharia E, Vlodavsky I, Pejler G, Li J-P. (2011). Heparanase affects secretory granule homeostasis of murine mast cells through degrading heparin. J Allergy Clin Immunol 128:1310-1317.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernersson S, Pejler G. (2014). Mast cell secretory granules: armed for battle. Nat Rev Immunol 14:478-494. [DOI] [PubMed] [Google Scholar]
- Whitelock JM, Graham LD, Melrose J, Murdoch AD, Iozzo RV, Underwood PA. (1999). Human perlecan immunopurified from different endothelial cell sources has different adhesive properties for vascular cells. Matrix Biol 18:163-178. [DOI] [PubMed] [Google Scholar]
- Wolf MT, Dearth CL, Ranallo CA, Lopresti ST, Carey LE, Daly KA, Brown BN, Badylak SF. (2014). Macrophage polarization in response to ECM coated polypropylene mesh. Biomaterials 35:6838-6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yada T, Arai M, Suzuki S, Kimata K. (1992). Occurrence of collagen and proteoglycan forms of type IX collagen in chick embryo cartilage. Production and characterization of a collagen form-specific antibody. J Biol Chem 267:9391-9397. [PubMed] [Google Scholar]
- Yamagata M, Kimata K, Oike Y, Tani K, Maeda N, Yoshida K, Shimomura Y, Yoneda M, Suzuki S. (1987). A monoclonal antibody that specifically recognizes a glucuronic acid 2-sulfate-containing determinant in intact chondroitin sulfate chain. J Biol Chem 262:4146-4152. [PubMed] [Google Scholar]
- Yurt RW, Leid RW, Spragg J, Austen KF. (1977). Immunologic Release of Heparin from Purified Rat Peritoneal Mast Cells. J Immunol 118:1201-1207. [PubMed] [Google Scholar]
- Zernichow L, Åbrink M, Hallgren J, Grujic M, Pejler G, Kolset SO. (2006). serglycin is the major secreted proteoglycan in macrophages and has a role in the regulation of macrophage tumor necrosis factor-α secretion in response to lipopolysaccharide. J Biol Chem 281:26792-26801. [DOI] [PubMed] [Google Scholar]