Abstract
Fertility is a complex process and infertility can have many causes. Sperm protein reactive with antisperm antibody (SPRASA)/sperm lysozyme-like protein 1 is a protein discovered as the target of autoantibodies in infertile men and previously thought to be expressed only in sperm. Using a bovine in vitro fertilization model, we have shown that SPRASA antiserum reduced sperm binding to zona-free oocytes and the development of embryos to morulae but did not affect the postfertilization cleavage rate to 2 cells or sperm motility. We demonstrated that SPRASA was expressed in ovarian follicles, corpora lutea, and oocytes by a combination of reverse transcription-polymerase chain reaction and immunohistochemistry. Female mice immunized with SPRASA had profound infertility following timed matings and those mice that did become pregnant had reduced fetal viability. The levels of antibodies reactive with SPRASA in 204 fertile and 202 infertile couples were elevated in 3 infertile but no fertile women. Together, these results indicate that SPRASA has a role in female fertility.
Keywords: SLLP1, SPACA3, SPRASA, contraceptive, oocyte, infertility
Introduction
Infertility affects 1 in 6 couples1 and for many of those couples, despite extensive investigations, the cause of their infertility remains undetermined. Although infertility is a major public health issue, conversely, a large number of fertile couples choose to inhibit their fertility by using contraception. However, existing methods of contraception have significant limitations and it is estimated that approximately half of the pregnancies in the United States are unintended.2 Contraceptive failure is a major problem especially with short-acting contraceptive techniques.3 Gaining a better understanding of the molecules and processes involved in fertility is likely to have the dual benefits of improving diagnostics and therapies for infertile couples, while also aiding in the development of new contraceptive strategies.
The processes involved in successful reproduction are complex involving an array of components and interactions, and the biology of many of these processes is poorly understood. During mammalian fertilization, the sperm must penetrate the cumulus surrounding the oocyte and undergo the acrosome reaction to allow fusion with the oolemma.4 The acrosome reaction exposes the inner acrosomal membrane, externalizing components essential for gamete fusion. Current evidence indicates that both gametes have binding proteins that may interact with each other, although the specific proteins and their roles in sperm–oocyte binding are not well understood.4 Following gamete fusion, mitosis begins and the early embryo is formed. During early development, oocyte-derived genes and proteins are essential for embryo survival, before the switching on of the embryonic genome and transition to genes generated by the embryo itself.5
A wide variety of proteins may participate in sperm–oocyte membrane interactions. Sperm protein reactive with antisperm antibody (SPRASA) also known as sperm lysozyme-like protein 16 is a little studied protein encoded by the SPACA3 gene7 that appears to have testis-specific expression restricted to the acrosome.6,8,9 SPRASA shows similar exon–intron organization and sequence conservation to c-type lysozymes, suggesting that SPRASA belongs to the c-type lysozyme superfamily6,8 but without bacteriolytic activity.6 The SPRASA protein has at least 2 isoforms containing either a predicted transmembrane region or a signal peptide with a cytoplasmic N-terminus.6,9 The function of SPRASA is unknown, but preliminary data suggest that SPRASA may be important in fertilization. We have previously identified SPRASA as the antigenic target of antisperm antibodies from infertile couples.8 Others have shown that an antiserum reactive with SPRASA inhibits the binding of acrosome-reacted human sperm to hamster oocytes,6 and that the treatment of mouse oocytes with either an antiserum reactive with SPRASA or a recombinant SPRASA resulted in inhibition of sperm–oolemma binding.9 Recently, a potential oolemma binding partner to SPRASA, sperm acrosomal SLLP1 binding (SAS1B), has been identified in mice.10 Inhibition of SAS1B in vitro by antibodies or in vivo in knockout mice was shown to reduce fertilization and fertility, respectively.10 It has been proposed that SPRASA may have similar binding specificities to c-type lysozymes and bind hyaluronan of oocytes to facilitate sperm–oocyte fusion.9 Interestingly, yeast-2-hybrid systems have also shown that SPRASA is able to directly interact with zona pellucida 3 (ZP3).11 SPRASA remains localized to the equatorial region of the sperm after its binding to the oolemma, supporting its role in oocyte binding and fusion.9
In this study, we have used a bovine in vitro fertilization (IVF) model to further investigate the role of SPRASA in sperm–oocyte binding, fertilization, and embryonic development and have determined novel expression patterns of SPRASA in oocytes, ovarian follicles, and corpora lutea in 3 model species. We have also examined the effect of inhibiting ovarian SPRASA in vivo by immunizing female mice. Finally, to investigate the possibility that antibodies reactive with SPRASA could be a potential marker of human infertility, we have compared the level of SPRASA-reactive antibodies in infertile and fertile couples.
Materials and Methods
Ethical Approval
All animal work was conducted in accordance with the New Zealand Animal Welfare Act 1999. All animal care and procedures were approved by The University of Auckland Animal Ethics Committee (approval numbers R562 and R911). The investigation of women from infertile and fertile couples was approved by the Northern Regional Ethics Committee (Auckland, New Zealand; approval number AKY/03/12/317).
Generation of SPRASA Antiserum and Control Antiserum
Two antisera reactive with SPRASA were prepared. The immunization protocol and collection of sera followed the method of Harlow and Lane.12 For use in the bovine model, antiserum was prepared by immunizing New Zealand white rabbits (n = 2; AgResearch, Hamilton, New Zealand) with recombinant human SPRASA (exons 2-5; 76% homology to bovine SPRASA; donated by John Steemson, The University of Auckland). Serum was also collected from preimmune rabbits prior to immunization to act as a negative control in subsequent experiments. For use in immunohistochemical investigation of SPRASA expression in the ovaries of cats and dogs, antiserum was prepared by immunizing Wistar rats (n = 2; Vernon Jansen Unit, The University of Auckland) with recombinant cat SPRASA (GenScript, New Jersey).
Preparation of Sperm
Viable bull sperm was prepared from straws of frozen semen (donated by Ambreed NZ Limited, Hamilton, New Zealand) as described.13
Bovine Oocyte Collection and Maturation
Bovine ovaries were obtained from animals killed for food production (Auckland Meat Processors, New Zealand) and cumulus–oocyte complexes were prepared as described.14 Cumulus–oocyte complexes were matured for 22 to 24 hours at 38.5°C in 5% CO2. Cumulus cells were removed by repeated pipetting in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid-buffered synthetic oviductal fluid (HSOF).
Bovine Oocyte–Sperm Binding Assays
In order to determine the effect of SPRASA antiserum on sperm–ZP binding and sperm–oolemma binding, zona-intact and zona-free oocyte binding assays were performed in the presence of SPRASA or control antiserum. To investigate sperm–zona binding, sperm and oocytes were coincubated with antiserum for the duration of the assay. A total of 25 zona-intact oocytes (5 oocytes per experiment, experiments repeated 5 times) and sperm (concentration 1 × 106 sperm/mL) were incubated in IVF media (25 mmol/L NaHCO3, 0.33 mmol/L pyruvate, 1.71 mmol/L CaCl2·H2O, 3.3 mmol/L Na lactate, 107.7 mmol/L NaCl, 7.15 mmol/L KCl, 1.19 mmol/L KH2PO4, 5 µg/mL heparin, 0.2 µmol/L penicilliamine, 0.1 µmol/L hypotaurine, 8 mg/mL bovine serum albumin [BSA], and 0.5% penicillin/streptomycin; p/s) droplets containing antiserum at 1:50 dilution for 2 hours at 38.5°C in 5% CO2.
To investigate sperm–oolemma binding, the ZP was dissolved by incubating oocytes in 0.5% (w/v) pronase (Sigma, Australia) for 2 to 5 minutes, followed by extensive washes in HSOF. Oocytes were transferred to IVF medium and allowed to recover for 30 minutes. Sperm were capacitated for 4 hours in IVF medium and induced to undergo the acrosome reaction with 5 µmol/L progesterone (Sigma) in IVF medium for 20 minutes as published.15 A total of 25 zona-free oocytes (5 oocytes per experiment, experiments repeated 5 times) and acrosome-reacted sperm (1 × 106 sperm/mL) were incubated in IVF media droplets containing antiserum at 1:50 dilution for 2 hours in a humidified incubator at 38.5°C in 5% CO2.
Fixation and Staining of Oocytes From Oocyte–Sperm Binding Assays
For both zona-intact and zona-free binding assays, sperm–oocyte complexes were washed twice in HSOF to remove loosely bound sperm. Sperm–oocyte complexes were fixed in 4% paraformaldehyde (PFA; Sigma) in phosphate-buffered saline (PBS; 120 mmol/L, NaCl, 2.7 mmol/L KCl, 1.5 mmol/L Na2HPO4, and 8 mmol/L KH2PO4, pH 7.4) and stained with 5 µg/mL Hoechst 33342 (Sigma) for 10 minutes. Sperm–oocyte complexes were washed in PBS and transferred onto poly-l-lysine (Sigma) coated microscope slides (Biolab, Auckland, New Zealand). Coverslips were mounted using 100% glycerol (BDH, Auckland). Images were captured on the Coolpix 990 digital camera (Nikon, Japan), and the number of fluorescent sperm nuclei attached per oocyte (see Supplementary Figure 1; count including both bound sperm and sperm that had fused with the plasma membrane) was counted.
Assessment of Sperm Motility
Viable sperm (concentration 1 × 106 sperm/mL) was incubated with SPRASA or control antiserum (1:50 dilution) for 3 hours at 38.5°C in 5% CO2. To assess motility, 10 µL of sperm suspension was placed onto prewarmed slides after 0, 1/2, 1, 2, and 3 hours. The number of motile sperm was counted in 5 low power microscopic fields to classify 200 sperm. The count was repeated on a second 10 µL of sperm suspension, and the percentages in each independent count were compared. Counts were accepted as accurate if they fell within the 95% confidence intervals.16 Final percentages presented are the average of 2 independent counts.
Bovine IVF Model
A bovine IVF model was used to determine the effect of SPRASA antiserum on fertilization, cleavage, and embryo development. Oocytes and sperm (concentration 1 × 106 sperm/mL) were incubated in a droplet of IVF medium for 18 to 22 hours at 38.5°C in 5% CO2. For each treatment group (see below), either SPRASA or control antisera were included at a 1:50 dilution.
The following 3 treatment groups were investigated: (1) sperm incubated with antiserum for 30 minutes, washed, then incubated with oocytes (n = 100); (2) oocytes (n = 100) incubated with antiserum for 30 minutes, washed, and incubated with sperm; and (3) sperm and oocytes (n = 100) coincubated with antiserum for IVF culture.
Synthetic oviductal fluid (SOF; 25 mmol/L NaHCO3, 0.33 mmol/L pyruvate, 1.71 mmol/L CaCl2·H2O, 1.5 mmol/L glucose, 3.3 mmol/L Na lactate, 0.49 mmol/L MgCl2.6H2O, 107.7 mmol/L NaCl, 7.15 mmol/L KCl, 1.19 mmol/L KH2PO4, 1 mmol/L l-glutamine, 1% minimum essential medium nonessential amino acids, 1% basal medium eagle essential amino acids, and 8 mg/mL BSA, 0.5% p/s) droplets were equilibrated for 2 hours at 38.5°C in 5% CO2, 8% O2, and 87% N2 (BOC, Auckland, New Zealand). Presumptive embryos were selected, washed twice in HSOF, and transferred into SOF droplets. Embryos were allowed to develop for 8 days, and the same embryos were followed from fertilization until the end of the experiment. Fertilization rate (number of fertilized embryos divided by total number of oocytes) was assessed by the extrusion of the second polar body 18 to 22 hours after IVF. Cleavage rate (number of 2 cell embryos divided by total number of oocytes) was determined by the number of 2 cell embryos on day 1 of IVF. Embryo development rate was assessed for morula rate (number of morula divided by total number of oocytes) and blastocyst rate (number of blastocysts divided by total number of oocytes) development on days 5 and 8 of IVF, respectively.
Expression of SPACA3/SPRASA in Oocytes and Ovaries
To determine whether oocytes express SPRASA, zona-intact and zona-free bovine oocytes were fixed in 4% PFA in PBS for an hour and washed in 3% fetal calf serum (Invitrogen, Auckland, New Zealand) and 0.1% Tween-20 (Thermo Fisher, Auckland, New Zealand) in PBS (blocking solution). Oocytes were incubated in blocking solution overnight at 4°C, 50 mmol/L glycine (Sigma) in blocking solution for 15 minutes, with antiserum at 1:100 dilution in blocking solution for an hour, with biotinylated antirabbit immunoglobulin G (IgG; Jackson Immuno Research, Australia) at 1:1000 dilution in blocking solution for 30 minutes, and with streptavidin-conjugated 5-(4,6-dichlorotriazinyl) aminofluorescein (Jackson Immuno Research) at 1:500 dilution in blocking solution for 30 minutes. All incubations were carried out at room temperature unless noted, and oocytes were washed in blocking solution between incubations. Oocytes were mounted on microscope slides with Citifluor (Emgrid, Australia). Staining was visualized on a Zeiss LSM 510 Microscope (Carl Zeiss Microscopy, New Zealand).
To investigate SPRASA expression in ovaries, the ovaries of 11 cats and 8 dogs were collected after routine surgical sterilization. To determine whether messenger RNA (mRNA) encoding SPRASA was expressed, total RNA was extracted from the ovaries of 7 cats and 5 dogs and converted to complementary DNA. Polymerase chain reaction (PCR) amplification was carried out using SPACA3, glyceraldehyde 3-phosphate dehydrogenase, or β-actin-specific PCR primers. Details of PCR conditions and primers are given in Supplementary Table 1. To determine the protein-level expression of SPRASA, immunohistochemical investigation was carried out on the ovaries of 6 cats and 5 dogs. The ovaries were fixed in 4% PFA (Sigma) in PBS overnight at 4°C, incubated in 70% ethanol (BDH Laboratory Supplies, Global Science, New Zealand) for 1 hour, incubated in 80% ethanol for 1 hour, incubated in 2 changes of 95% ethanol for 1 hour each, incubated in 3 changes of 100% ethanol for 1 hour each, and incubated in 2 changes of xylene (Sigma Aldrich, New Zealand) for 1 hour each. The ovaries were embedded in paraffin wax (Sigma Aldrich), 6 μmol/L sections were cut and transferred onto poly-l-lysine (Sigma)-coated microscope slides. Sections were dewaxed by immersion in 2 changes of xylene for 5 minutes each and rehydrated by immersion in 2 changes of 95% and 80% ethanol for 5 minutes each followed by immersion in water for 10 minutes. Rehydrated sections were then stained with SPRASA antiserum or nonimmune mouse serum and counterstained with hematoxylin.
Expression of SPACA3/SPRASA in Sperm and Sperm Precursor Cells
To investigate SPRASA expression in sperm and sperm precursor cells, the testis of 5 cats and 10 dogs were collected after routine surgical sterilization. The expression of SPACA3 mRNA and SPRASA protein was determined for cat and dog ovaries.
Immunization and Timed Mating of Female Mice
Mice were housed under a light/dark cycle (12 hour/12 hour), in a temperature controlled room (22°C) with standard food (Teklad Global 18% Protein Rodent Diet, 18% protein, and 5% fat; Harlan, Harlan County, Kentucky) and water ad libitum Four-week-old female CD1 mice (Vernon Jansen Unit, The University of Auckland) were immunized subcutaneously with recombinant human SPRASA protein (exons 2-5; donated by John Steemson, The University of Auckland; n = 10) or recombinant keyhole limpet hemocyanin (KLH) protein (Sigma; n = 11). Animals were randomly allocated to experimental or control groups. The immunization protocol followed that described by Harlow and Lane.12 Briefly, each animal received 3 immunizations at 2 weekly intervals. Each immunization contained 25 µg of protein emulsified in adjuvant (total protein per animal = 75 µg). Freund complete adjuvant (Invitrogen) was used in the first immunization and subsequent immunizations used Freund incomplete adjuvant (Invitrogen).
Following immunization, SPRASA-immunized (n = 10) or control KLH-immunized (n = 8) female mice were monitored for entry into pro-oestrous using an EC40 Estrus Cycle Monitor (Fine Science Tools Inc, Canada) following the manufacturer’s instructions. When in pro-oestrous, the females were housed overnight with a single male. The following day, females were checked for coital plugs, the females were removed from the male and their weight was monitored daily until day 12 postcoitus to determine whether pregnancy had occurred. If pregnancy was detected, females were euthanized and pregnancy was confirmed by dissection. Pregnant uteri were weighed, and the number of fetuses were counted and weighed. If pregnancy was not detected, the female was monitored for entry into pro-oestrous and housed with a male as described previously. Mating was repeated up to 5 times if the females did not become pregnant. At the conclusion of the experiment, all animals were euthanased, and their ovaries excised for histologic examination of ovarian follicles.
Quantification of Ovarian Follicles in Immunized Mice
Murine ovaries were prepared for histology, and the number of follicles determined following the method of Myers et al17 with modifications. Briefly, ovaries were excised, and the right ovary was fixed in PFA and embedded in paraffin wax as described for cat and dog ovaries. Every fifth section was stained with hematoxylin and eosin following the method of Fischer.18 The number of primordial, primary, early antral, antral, and preovulatory follicles (classified as described by Myers et al17) with visible nuclei was counted in each section, and total counts were multiplied by the number of sections from each ovary.
Detection of SPRASA-Reactive Antibodies in the Blood of Fertile and Infertile Couples
Blood samples obtained by venipuncture were collected following written informed consent from 102 infertile couples (recruited through 2 fertility services; Fertility Associates, Auckland, New Zealand, and Fertility Plus, Auckland, New Zealand) and 104 fertile couples (recruited from the general Auckland population through media advertisement). Couples were defined as infertile if they were unable to conceive after 1 year of regular intercourse without the use of contraception. The clinically diagnosed disorders affecting the infertile cohort recruited for this study are summarized in Supplementary Table 2. Couples were defined as fertile if they had produced live offspring within the preceding 10 years.
Blood samples were allowed to clot overnight at 4°C and centrifugation at 2400g for 10 minutes in a MicroCL 21 Microcentrifuge (Thermo Fisher). Serum was aspirated, aliquoted, and stored at −80°C until required. For the enzyme-linked immunosorbent assay protocol, microplate wells were incubated overnight at 4°C with 1 µg/mL of recombinant SPRASA protein in carbonate buffer, with blocking solution, with sera diluted 1:100 in blocking solution, with biotinylated antihuman IgG (Jackson Immuno Research) diluted 1:2000 in blocking solution, and with streptavidin-biotinylated horseradish peroxidase (sHRP) diluted 1:5000 in blocking solution. All incubations were for 1 hour at room temperature, and microplates were washed 3 times with PBS-Tween between each incubation. 3,3′,5,5′-Tetramethylbenzidine substrate was added for 30 minutes at room temperature, and the color reaction was stopped by the addition of H2SO4. The absorbance was read at 450 nm on a Benchmark Microplate Reader (Bio-Rad, California).
Serum samples were run in duplicate. To allow for normalization, 7 randomly selected serum controls were run on each assay. The optical density for each sample was divided by the median of the 7 controls to give the normalized value. To identify those women with elevated levels of SPRASA-reactive antibodies, the Tukey method19,20 for identifying outliers was applied to the levels of antibodies in the women’s serum.
Statistical Analysis
Student t test was used to determine the significance of difference in sperm binding to the oocytes, sperm motility, fertilization rate, embryo development, ovarian follicle populations, fertility outcomes, serum leptin levels in immunized mice, and levels of SPRASA-reactive antibodies in the serum of women. Kruskal-Wallis test was used to compare between IVF treatment groups. Statistical analysis of body weight, fat pad weight, and organ weight was performed with the Mann-Whitney U test. A 2-tailed chi-square test was used to compare the total number of pregnant mice in the SPRASA-immunized and control KLH-immunized groups. The levels of SPRASA-reactive antibodies in women and men from infertile and fertile couples are expressed as a box and whisker plot using the Tukey calculation for population outliers.19,20 All analyses were carried out in the computer program GraphPad Prism version 6 (GraphPad Software Inc, California). P < .05 was considered statistically significant. Results are reported as mean ± standard deviation (SD).
Results
The Effect of SPRASA Antiserum on Sperm–Oocyte Binding and Sperm Motility
We have confirmed using immunohistochemistry that SPRASA is expressed in the acrosome of sperm6–9 and in a crescent pattern in sperm precursor cells, likely to be cap-phase spermatids (Supplementary Figure 2). Further, SPACA3 was shown to be expressed in the testes of both juvenile and adult animals by reverse transcription PCR (RT-PCR; Supplementary Figure 2). To determine whether SPRASA has a role in sperm–ZP binding, we examined sperm and oocytes incubated with an SPRASA or control antiserum. There were no significant differences in the number of sperm bound to the ZP between the SPRASA and control antiserum groups (Figure 1A).
Figure 1.
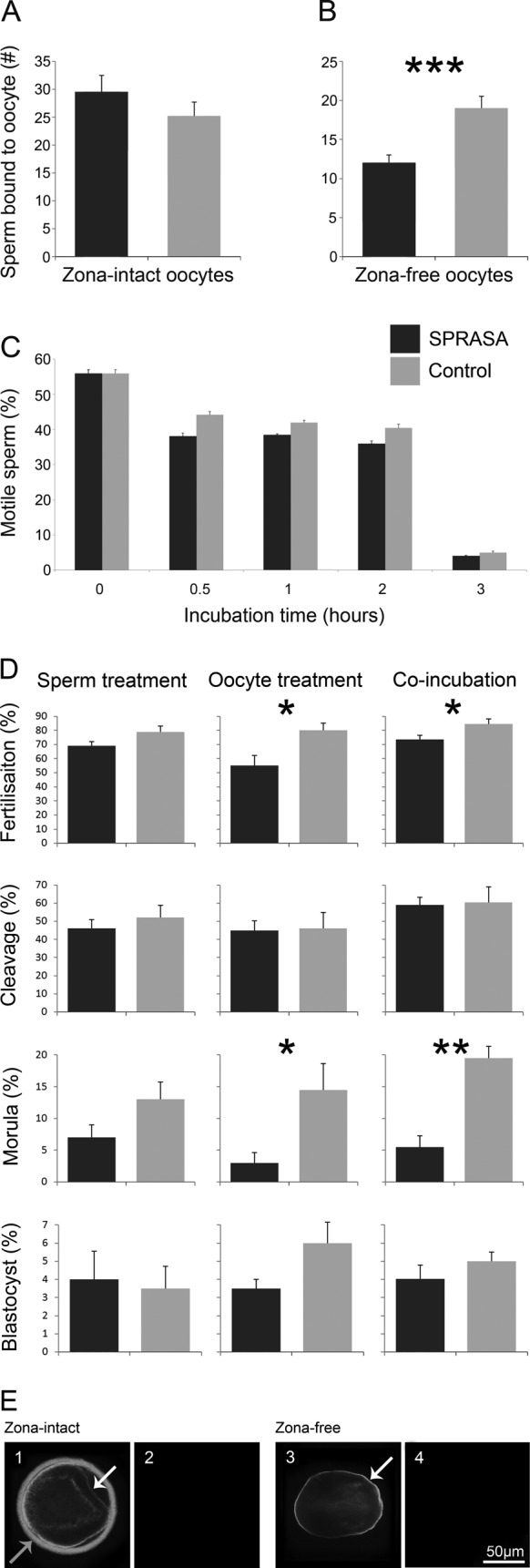
The SPRASA antiserum significantly reduced the numbers of sperm binding to the oolemma, the fertilization rate, and the development to morula stage; SPRASA was localized to the zona pellucida and oolemma of bovine oocytes. Bovine oocytes and sperm were used to access the effect of SPRASA antiserum on sperm binding to oocytes, sperm motility, fertilization, and embryonic development; as well as to localize SPRASA expression. For binding assays, sperm and oocytes were coincubated with an SPRASA or control antiserum for 2 hours. Sperm–oocyte complexes were stained and the number of sperm nuclei attached per oocyte was counted. For assessment of sperm motility, viable sperm was incubated with SPRASA or control antiserum for 3 hours. The number of motile sperm were counted after 0, 1/2, 1, 2, and 3 hours. For IVF, oocytes and sperm were incubated for 18 to 22 hours. Three treatment groups were investigated: (1; sperm treatment) sperm incubated with antiserum for 30 minutes, washed, then incubated with oocytes; (2; oocyte treatment) oocytes incubated with antiserum for 30 minutes, washed, and incubated with sperm; and (3; coincubation) sperm and oocytes coincubated with antiserum for IVF culture. Presumptive embryos were selected and allowed to develop for 8 days. To determine whether oocytes express SPRASA, oocytes were fixed and incubated with antiserum, then detected visualized using fluorescent microscopy. A, The SPRASA antiserum did not affect the number of sperm bound to zona-intact oocytes but (B) significantly reduced the numbers of acrosome-reacted sperm binding to the oolemma of zona-free oocytes. C, The percentage of motile sperm was not affected by incubation with SPRASA antiserum. D, The fertilization rate was significantly reduced when oocytes, or sperm and oocytes, were incubated with SPRASA antiserum prior to fertilization with untreated bovine sperm. Development to morula stage was significantly inhibited by SPRASA antiserum when the antiserum was incubated with either oocytes alone, or with oocytes and sperm, but not when sperm was treated with the antiserum. Data are presented as percentage ± standard deviation (SD); *P = .05; **P = .01; ***P = .001. E, Immunofluorescent confocal photomicrographs demonstrating the localization of SPRASA on oocytes. Zona-intact or zona-free oocytes were incubated with (E1 and E3) SPRASA antiserum or (E2 and E4) control antiserum. SPRASA was localized to the zona pellucida (grey arrow) and oolemma (white arrow) of zona-intact oocytes and to the oolemma of zona-free oocytes. Scale bar = 50 µm and applies to all images. SPRASA indicates sperm protein reactive with antisperm antibody. IVF indicates in vitro fertilization; SPRASA, sperm protein reactive with antisperm antibody.
To determine whether SPRASA has a role in sperm–oolemma binding, progesterone-induced acrosome-reacted sperm and zona-free oocytes were coincubated with SPRASA or control antiserum. There were significantly fewer sperm binding to the oolemma when sperm and oocytes were incubated with SPRASA compared to control antiserum (P = .001; Figure 1B).
To examine the effect of SPRASA antiserum on sperm motility, motile sperm were counted after incubation with SPRASA or control antiserum. No significant differences were seen in the percentage of motile sperm, when sperm were incubated with SPRASA or control antiserum for between 0 and 3 hours (Figure 1C).
The Effect of SPRASA Antiserum on Fertilization, Cleavage, and Embryo Development
To examine whether SPRASA is involved in fertilization, cleavage, or embryonic development, gametes were treated with SPRASA or control antiserum, and IVF culture was carried out. Antiserum was used to treat (1) sperm and (2) oocytes separately and (3) both sperm and oocytes together. There were no significant differences when these treatment groups were compared within SPRASA or control antiserum treatments (data not shown).
There was a significant reduction in the rate of fertilization when oocytes (SPRASA: 54.13 ± 25.38; control: 79.76 ± 19.33; P = .04) or sperm and oocytes (SPRASA: 72.85 ± 11.83; control: 85.77 ± 11.78; P = .05) but not sperm alone (SPRASA: 68.63 ± 12.47; control: 78.41 ± 10.01; P = .11) were treated with SPRASA-reactive antiserum compared to control antiserum. There was no significant difference in the cleavage rates between SPRASA antiserum and control antiserum treatment in any group.
Treating oocytes (SPRASA: 3.56 ± 5.09; control: 14.38 ± 16.25; P = .03) or sperm and oocytes (SPRASA: 5.64 ± 6.16; control: 19.43 ± 16.86; P = .01) but not sperm alone (SPRASA: 6.82 ± 8.79; control: 12.50 ± 12.97; P = .20) with SPRASA antiserum significantly inhibited development to the morula stage, compared to control antiserum. There was no further inhibition of development to the blastocyst stage between SPRASA antiserum and control antiserum treatment (Figure 1D).
Localization of SPRASA on Oocytes
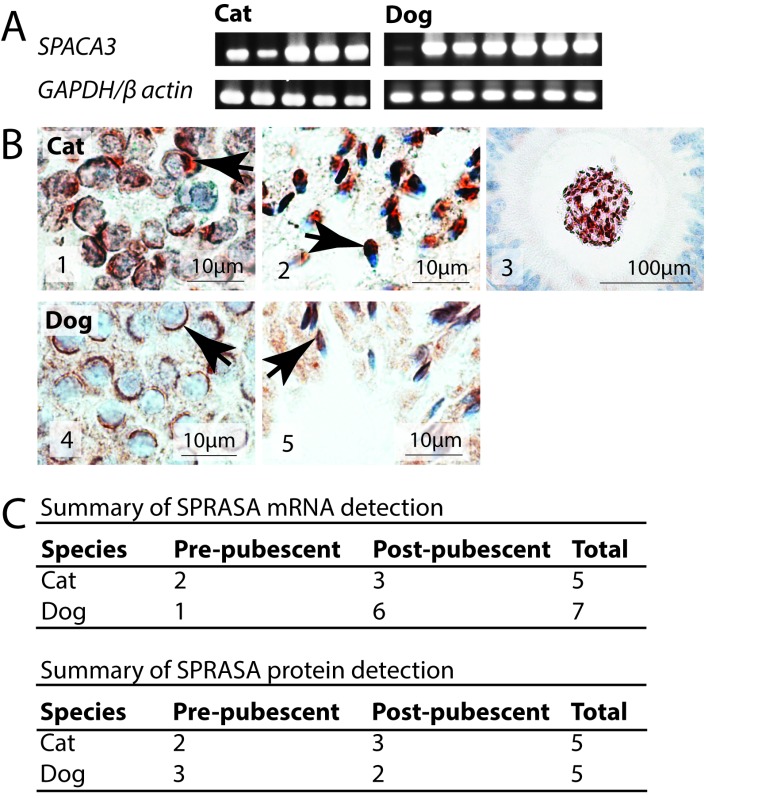
That incubating oocytes with SPRASA antiserum significantly inhibited embryo development in the bovine IVF model (Figure 1D), suggesting that oocytes may express SPRASA. Immunofluorescent staining showed that SPRASA was localized to the ZP and the oolemma of bovine oocytes (Figure 1E). In order to confirm that SPRASA was expressed by oocytes and to confirm that this expression was not limited to bovine oocytes, we examined the expression of SPACA3/SPRASA in the ovaries of cats and dogs by RT-PCR and immunohistochemistry. SPACA3/SPRASA mRNA and protein were present in the ovaries of both cats and dogs in both juvenile and sexually mature animals. In the ovary, immunohistochemistry showed that SPRASA was present in ovarian follicles at all stages of development and was localized to the ooplasm and granulosa cells with weak staining in theca cells. Corpora leutea also stained strongly for SPRASA (Figure 2).
Figure 2.
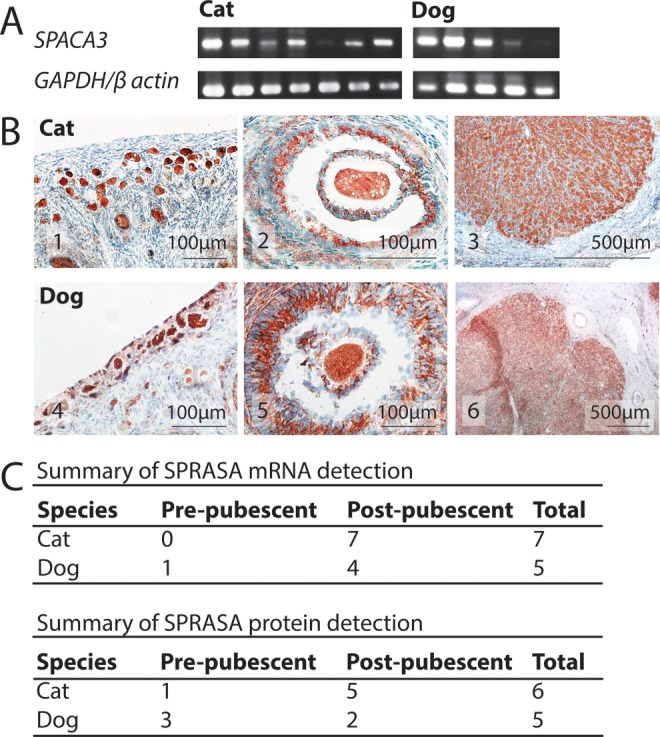
SPRASA/SPACA3 is present in ovarian follicles in cats and dogs regardless of sexual maturity. A, Agarose gel images of SPACA3, GAPDH, and β-actin polymerase chain reaction (PCR) amplicons from individual cat and dog ovaries. The GAPDH and β-actin are included as positive controls. B, Photomicrographs of (B1-B3) cat ovary, (B4-B6) dog ovary showing the localization of SPRASA. B1 and B4, SPRASA expression in primordial follicles, (B2 and B5) SPRASA expression associated with an antral follicle, and (B3 and B6) SPRASA expression in a corpus luteum. SPRASA = red; nuclei = blue. C, The ovaries of 11 cats and 8 dogs were investigated for the expression of SPACA3/SPRASA messenger RNA (mRNA) and protein. SPRASA was present in the ovaries of all animals regardless of the sex, species, and in both juvenile and sexually mature animals. GAPDH indicates glyceraldehyde 3-phosphate dehydrogenase; SPRASA, sperm protein reactive with antisperm antibody. (The color version of this article is available at http://rs.sagepub.com.)
Immunization of Female Mice with SPRASA Reduces Their Fertility
To assess the effect of neutralizing SPRASA in vivo, 10 female mice were immunized with recombinant SPRASA and 8 control female mice were immunized with the irrelevant antigen, KLH. The immunized mice were timed mated up to 5 times. There was a significant reduction in the total number of pregnant mice in the SPRASA-immunized mice (P = .001). All the control KLH-immunized mice (100%) became pregnant after 2 matings. In contrast, only 3 (30%) SPRASA-immunized mice become pregnant after 2 matings, with the remaining 7 (70%) SPRASA-immunized mice failing to become pregnant after at least 4 matings (Table 1).
Table 1.
Immunization With SPRASA Leads to a Reduction in Fertility in Female Mice.a,b
| Immunization | Number of Mice Pregnant After Each Mating | ||||||
|---|---|---|---|---|---|---|---|
| First | Second | Third | Forth | Fifth | Summary | ||
| SPRASA | 2/10 | 1/8 | 0/8 | 0/8 | 0/5 | 3/10 (30%) | P = .001 |
| KLH | 4/8 | 4/4 | – | – | – | 8/8 (100%) | |
Abbreviations: KLH, keyhole limpet hemocyanin; SPRASA, sperm protein reactive with antisperm antibody.
aAnimals were euthanized to confirm pregnancy. Pregnancy is defined as confirmed fetuses at day 12 postcoitus.
bThe P value refers to a chi-square test comparing the total number of pregnant mice over all matings in the SPRASA-immunized and control KLH-immunized groups.
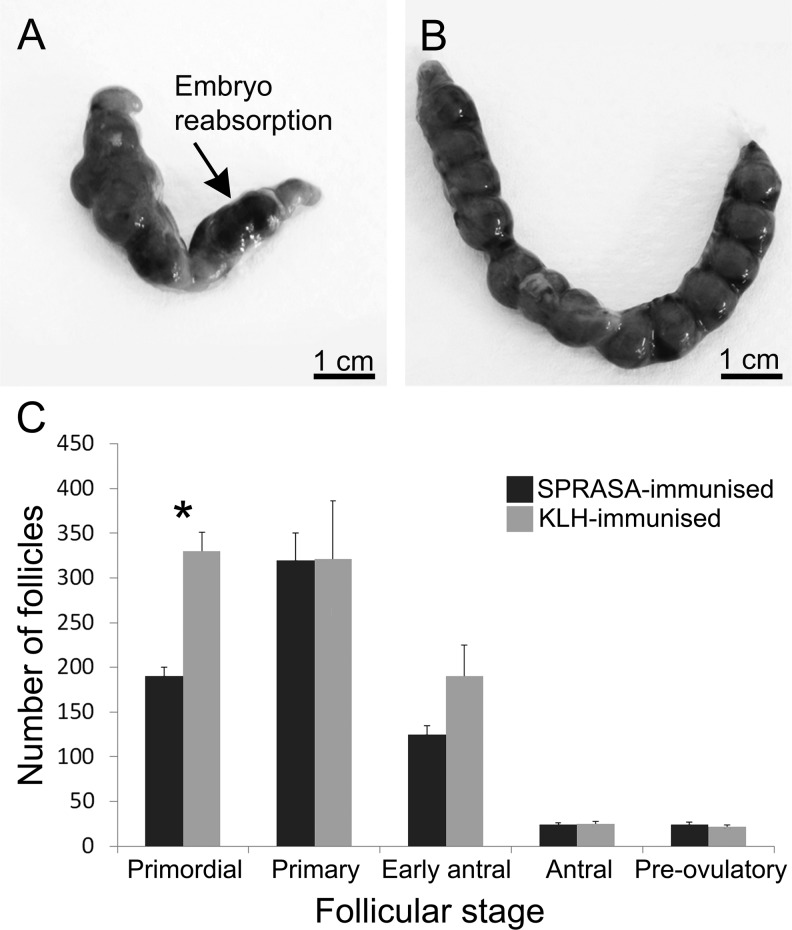
At euthanasia (day 12 postcoitus), gross examination of the uteri of those SPRASA-immunized mice that were pregnant revealed that several embryos were obviously nonviable and were reabsorbing. In contrast, there were no resorbing embryos in the control KLH-immunized mice (Figure 3A and B). No differences were observed between SPRASA-immunized and control KLH-immunized mice in embryo number or embryo weight (data not shown).
Figure 3.
Effect of immunization with SPRASA on embryos and ovarian follicle numbers. A and B, Representative photographs of uteri from (A) SPRASA-immunized and (B) control KLH-immunized mice day 12 postcoitus. Embryo reabsorptions seen in SPRASA-immunized mice are indicated by an arrow. Scale bar = 1 cm. C, Ovaries were harvested from SPRASA-immunized (dark gray; n = 3) and control KLH-immunized (light gray; n = 3) mice at dioestrous, prepared for histology and sectioned to completion. Follicles at each stage were quantified in every fifth section, and follicle counts were expressed as the mean number of follicles per ovary ± standard deviation (SD), *P ≤ .05. KLH indicates keyhole limpet hemocyanin; SPRASA, sperm protein reactive with antisperm antibody.
Given that we have shown SPRASA to be expressed in ovarian follicles, we examined the ovaries of the immunized mice to investigate whether immunization of the animals had reduced their fertility by destroying the follicular pool. The ovaries of all mice appeared grossly normal. There was no significant difference in the numbers of early antral, antral, or preovulatory follicles between the SPRASA-immunized and control KLH-immunized mice (Figure 3C). However, the number of primordial follicles was significantly reduced (P = .003) in SPRASA-immunized mice (193.33 ± 11.67; n = 3) compared to control KLH-immunized (330.00 ± 28.87; n = 3) mice. Copora lutea were also present in the SPRASA and control immunized mice but were not quantified.
High Levels of SPRASA-Reactive Antibodies Were Present in the Sera of Some Infertile Women
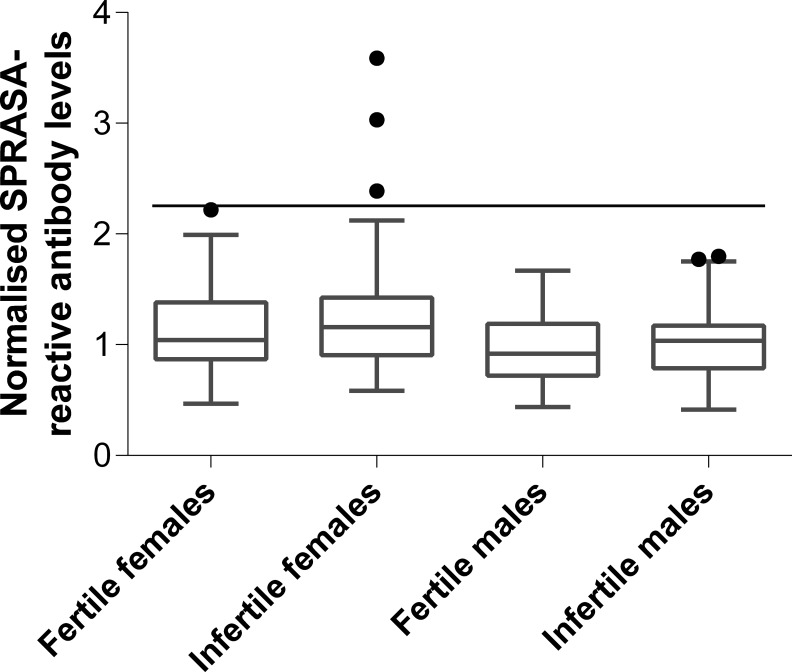
In order to determine whether SPRASA-reactive antibodies were associated with infertility, sera from infertile (n = 102) or fertile (n = 104) couples were screened for the presence of SPRASA-reactive IgG antibodies. The levels of SPRASA-reactive antibodies were not significantly different between fertile (mean 1.13 ± 0.37) and infertile women (1.25 ± 0.48; P = .05) or fertile (0.95 ± 0.29) and infertile men (1.03 ± 0.28; P = .06). However, 3 infertile women, but no fertile women nor any men, had elevated levels of SPRASA-reactive antibodies (Figure 4). Of these 3 infertile women, 1 had endometriosis while the other 2 women were apparently normal, but their male partners had abnormal semen or sperm.
Figure 4.
Three infertile women were found to have elevated levels of antibodies reactive with SPRASA. Serum collected from women and men from infertile (n = 102) and fertile (n = 104) couples was tested by enzyme-linked immunosorbent assay for immunoreactivity against SPRASA. Serum levels of antibodies were considered to be elevated if they were above the threshold for outliers based on the levels of antibodies in the infertile women, as determined by the Tukey method (black line). SPRASA indicates sperm protein reactive with antisperm antibody.
Discussion
Function of SPRASA and Sperm
SPRASA was discovered as the target of antisperm antibodies from some infertile men.8 Antisperm antibodies have been documented to disrupt a variety of steps during fertilization.21,22 In this study, we demonstrated that SPRASA antiserum did not affect the motility of sperm. This was not unexpected as we and others have shown that SPRASA is located on the inner acrosomal membrane of sperm6–9 and thus is not exposed to antibodies in the external environment while the acrosome remains intact. This confirms that antibodies reactive with SPRASA are unlikely to disrupt sperm transport and that any effect they have on fertility would be more likely to occur at a later stage. In vitro fertilization assays are commonly employed to assess the function of sperm proteins with the use of experimentally raised antibodies against specific proteins. In this study, sperm–ZP binding was not affected when bovine gametes were incubated with SPRASA antiserum, suggesting that SPRASA is not involved in the primary recognition and binding of the ZP that occurs between acrosome intact sperm and the oocyte. This finding is consistent with the functions of other intra-acrosomal proteins,22–26 as SPRASA epitopes localized to the inner acrosomal membrane would not be exposed prior to the acrosome reaction.
In contrast, following the acrosome reaction, intra-acrosomal proteins become surface exposed and can be involved in secondary or tight binding that occurs between acrosome-reacted sperm and the ZP, as well as subsequent binding and fusion with the oolemma.27 This study confirms that SPRASA is involved in sperm–oolemma binding, as SPRASA antiserum significantly reduced the number of sperm bound to the oolemma. This is again consistent with the findings of several reports examining intra-acrosomal proteins24,26,28–30 and agrees with previous investigations of SPRASA localization and function by others.6,9
SPRASA Is Expressed by Ovaries/Oocytes/Ovarian Follicles
Prior to this study, SPRASA was believed to be expressed only in the testes/sperm. We have demonstrated that SPACA3/SPRASA is also expressed by bovine, cat and dog ovaries/oocytes/ovarian follicles at the oolemma and zona pellucida. SPRASA is also expressed weakly in theca cells and strongly in the luteinized cells of corpora lutea. Furthermore, we have found mRNA expression of SPRASA in murine ovaries.31 Other proteins originally thought to be sperm specific such as spermadhesin and PH-20 have been shown to be expressed in both male and female reproductive tracts and to have potential roles in reproduction in both sexes.32,33 The function of SPRASA on the oocyte is unclear, but it seems likely that this role is additional to sperm–oocyte interactions. Our demonstration that antibodies reactive with SPRASA inhibit later embryonic development may point to another, as yet unknown, function. Furthermore, our finding that SPRASA is expressed by oocytes, as well as sperm, suggests that antisperm/antiovarian antibodies specific for SPRASA may be a contributing factor for infertility in women. This is further supported by our finding of elevated levels of SPRASA-reactive antibodies in the sera of 3 infertile women. That SPRASA-reactive antibodies were not statistically associated with infertility in general is not surprising as there are multiple known and unknown causes of infertility.
SPRASA Has Potential Roles in Fertilization and Embryonic Development
SPRASA appears to be a protein with multiple functions in fertility. In vitro, inhibiting SPRASA significantly reduced the fertilization rate, whereas subsequent development to the 2 cell embryo was not affected, suggesting that inhibiting SPRASA may retard sperm penetration into the oocyte and/or subsequent early phases of fertilization. That blocking SPRASA did not completely inhibit fertilization suggests that there is likely to be redundancy in the receptors for sperm/oolemma binding, and that SPRASA is only one of the molecules involved in this event. It is also possible that the antiserum we employed in this study was not entirely effective at blocking the function of SPRASA. The mechanisms of action of antibodies reactive with sperm antigens are not well understood, but it has been suggested that some antibodies may inhibit sperm–oocyte interactions and other fertility events at multiple points during fertilization.34
In addition to inhibiting fertilization, SPRASA antiserum disrupted early embryonic development to the morula stage, but this effect was only apparent when oocytes or oocytes and sperm but not sperm alone were treated with SPRASA antiserum. Oocyte proteins are critical for early embryonic development.5 We have shown that SPRASA is expressed by the oocyte; the inhibition of morula stage development by SPRASA antiserum indicates that oocyte-expressed SPRASA may function in early embryonic development. However, activation of the embryonic genome and degradation of oocyte-derived proteins are well advanced by the morula stage.5,35 Thus, the effect of SPRASA antiserum on morula development suggests SPRASA may also be expressed by the embryo, and this requires further investigation.
As our in vitro experiments suggest that antibodies reactive with SPRASA reduce fertility in vitro, we conducted further experiments to confirm that inhibiting SPRASA would also reduce fertility in vivo. When SPRASA-immunized female mice were mated to normal males, there was a 70% reduction in fertility. In many cases, this was profound infertility, with 7 of the 10 SPRASA-immunized females failing to become pregnant after 4 to 5 timed matings. This infertility was not due to a failure to copulate since all females were confirmed to be in pro-oestrous, and coital plugs were observed postmating, indicating that immunization with SPRASA did not affect mating behavior.
Since immunization with ovarian antigens, such as zona pellucida proteins, can lead to the destruction of ovarian follicles,36–45 we compared the ovaries of SPRASA-immunized and control females. We found no significant differences in the numbers of ovarian follicles at all stages of development, except for primordial follicles. We also observed (but did not quantify) corpora lutea in the ovaries of the SPRASA-immunized mice, suggesting normal ovarian function was maintained. Although the reduction in the number of primordial follicles may possibly have led to a long-term effect on the fertility of the animals, this is unlikely to have accounted for the profound infertility exhibited in the SPRASA-immunized mice, since the numbers of primary, early antral, antral, and preovulatory follicles were unaffected by immunization. In keeping with our in vitro observations, these results suggest that the infertility in response to immunization with SPRASA was due to defects in fertilization and/or subsequent embryonic/fetal development, rather than due to defects in the production of oocytes. Although some of the SPRASA-immunized mice did become pregnant, many of the fetuses in the pregnant SPRASA-immunized mice were nonviable, confirming that SPRASA has a function in the embryo/fetus in addition to its known role in fertilization.9 Further work is needed to determine the nature of the function of SPRASA postfertilization.
Summary
Prior to this study, SPRASA was believed to be expressed only by sperm and to function only in sperm/oocyte binding.9 We have demonstrated that SPRASA is also expressed by ovarian follicles and corpora lutea, and that this protein has potential functions in both fertilization and subsequent embryonic/fetal development in several species. We have extended our previous finding that elevated SPRASA-reactive antibodies are associated with infertility in men to demonstrate that autoantibodies reactive with SPRASA are also present in some infertile women but not in fertile women. Further work will be needed to determine the mechanism by which SPRASA antibodies inhibit fertility and the role that SPRASA plays in normal embryonic/fetal development.
Supplementary Material
Supplementary Material
Acknowledgments
We would like to thank Auckland Meat Packers, Ambred (Hamilton), the staff of the Vernon Jansen Unit of the Faculty of Medical and Health Sciences, The University of Auckland, Kim Telford and the staff of Unitec Veterinary Hospital, April Jones, The Ardmore Vet, and Satya Amirapu, Histology Laboratory, School of Medical Sciences, The University of Auckland.
Footnotes
Authors’ Note: This work was performed at the Department of Obstetrics and Gynaecology, School of Medicine, Faculty of Medical and Health Sciences, The University of Auckland, Private Bag 92019, Auckland, New Zealand.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by grants from the Marsden Fund of the Royal Society of New Zealand, Michelson Prize & Grants, a program of the Found Animals Foundation, and the Staff Research Fund of The University of Auckland.
Supplemental Material: The online supplements are available at http://rs0.sagepub.com/supplemental.
References
- 1. Thoma ME, McLain AC, Louis JF, et al. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013;99(5):1324–1331 e1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception. 2011;84 (5):478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long-acting reversible contraception. N Engl J Med. 2012;366 (21):1998–2007. [DOI] [PubMed] [Google Scholar]
- 4. Florman HM, Ducibella T. Knobil and Neill's Physiology of Reproduction. Amsterdam, the Netherlands; Boston, MA: Elsevier, c2006; 2006. [Google Scholar]
- 5. Li L, Zheng P, Dean J. Maternal control of early mouse development. Development. 2010;137 (6):859–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mandal A, Klotz KL, Shetty J, et al. SLLP1, a unique, intra-acrosomal, non-bacteriolytic, c lysozyme-like protein of human spermatozoa. Biol Reprod. 2003;68 (5):1525–1537. [DOI] [PubMed] [Google Scholar]
- 7. Wang QT, Piotrowska K, Ciemerych MA, et al. A genome-wide study of gene activity reveals developmental signaling pathways in the preimplantation mouse embryo. Developmental Cell. 2004;6 (1):133–144. [DOI] [PubMed] [Google Scholar]
- 8. Chiu WW, Erikson EK, Sole CA, Shelling AN, Chamley LW. SPRASA, a novel sperm protein involved in immune-mediated infertility. Hum Reprod. 2004;19 (2):243–249. [DOI] [PubMed] [Google Scholar]
- 9. Herrero MB, Mandal A, Digilio LC, Coonrod SA, Maier B, Herr JC. Mouse SLLP1, a sperm lysozyme-like protein involved in sperm–egg binding and fertilization. Dev Biol. 2005;284 (1):126–142. [DOI] [PubMed] [Google Scholar]
- 10. Sachdev M, Mandal A, Mulders S, et al. Oocyte specific oolemmal SAS1B involved in sperm binding through intra-acrosomal SLLP1 during fertilization. Dev Biol. 2012;363 (1):40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Naz RK, Dhandapani L. Identification of human sperm proteins that interact with human zona pellucida3 (ZP3) using yeast two-hybrid system. J Reprod Immunol. 2010;84 (1):24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harlow E, Lane D. Antibodies a Laboratory Manual. NY: Cold Spring Harbor; 1988. [Google Scholar]
- 13. Schurmann A, Wells DN, Oback B. Early zygotes are suitable recipients for bovine somatic nuclear transfer and result in cloned offspring. Reproduction. 2006;132 (6):839–848. [DOI] [PubMed] [Google Scholar]
- 14. Grant VJ, Irwin RJ, Standley NT, Shelling AN, Chamley LW. Sex of bovine embryos may be related to mothers’ preovulatory follicular testosterone. Biol Reprod. 2008;78 (5):812–815. [DOI] [PubMed] [Google Scholar]
- 15. Lee CY, Khorasani AM, Dorjee S. Assessment of progesterone-induced acrosome reaction by biotinylated monoclonal antibody probes. Am J Reprod Immunol. 1998;39 (3):164–171. [DOI] [PubMed] [Google Scholar]
- 16. World Health Organization DoRHaR. WHO Laboratory Manual for the Examination of Semen and Sperm–Cervical Mucus Interaction. 4th ed Cambridge: Cambridge University Press; 1999. [Google Scholar]
- 17. Myers M, Britt KL, Wreford NG, Ebling FJ, Kerr JB. Methods for quantifying follicular numbers within the mouse ovary. Reproduction. 2004;127 (5):569–580. [DOI] [PubMed] [Google Scholar]
- 18. Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harbor Protocols. 2008;3 (5):1–2. [DOI] [PubMed] [Google Scholar]
- 19. Tukey JW. Exploratory Data Analysis. Boston, MA: Addison-Wesley; 1977. [Google Scholar]
- 20. Rousseeuw PJ, Ruts I, Tukey JW. The Bagplot: A Bivariate Boxplot. Am Stat. 1999;53(4):382–387. JSTOR 2686061. [Google Scholar]
- 21. Chamley LW, Clarke GN. Antisperm antibodies and conception. Semin Immunopathol. 2007;29 (2):169–184. [DOI] [PubMed] [Google Scholar]
- 22. Inoue N, Ikawa M, Isotani A, Okabe M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature. 2005;434 (7030):234–238. [DOI] [PubMed] [Google Scholar]
- 23. Saxena DK, Tanii I, Oh-oka T, Yoshinaga K, Toshimori K. Behaviour and role of an intra-acrosomal antigenic molecule, acrin 3, during mouse fertilisation in vitro. Zygote. 2000;8 (4):329–338. [DOI] [PubMed] [Google Scholar]
- 24. Coonrod SA, Herr JC, Westhusin ME. Inhibition of bovine fertilization in vitro by antibodies to SP-10. J Reprod Fertil. 1996;107 (2):287–297. [DOI] [PubMed] [Google Scholar]
- 25. Okabe M, Adachi T, Takada K, et al. Capacitation-related changes in antigen distribution on mouse sperm heads and its relation to fertilization rate in vitro. J Reprod Immunol. 1987;11 (2):91–100. [DOI] [PubMed] [Google Scholar]
- 26. Primakoff P, Hyatt H, Myles DG. A role for the migrating sperm surface antigen PH-20 in guinea pig sperm binding to the egg zona pellucida. J Cell Biol. 1985;101 (6):2239–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Buffone MG, Foster JA, Gerton GL. The role of the acrosomal matrix in fertilization. Int J Dev Biol. 2008;52 (5-6):511–522. [DOI] [PubMed] [Google Scholar]
- 28. Howes E, Pascall JC, Engel W, Jones R. Interactions between mouse ZP2 glycoprotein and proacrosin; a mechanism for secondary binding of sperm to the zona pellucida during fertilization. J Cell Sci. 2001;114 (pt 22):4127–4136. [DOI] [PubMed] [Google Scholar]
- 29. Saxena DK, Tanii I, Yoshinaga K, Toshimori K. Role of intra-acrosomal antigenic molecules acrin 1 (MN7) and acrin 2 (MC41) in penetration of the zona pellucida in fertilization in mice. J Reprod Fertil. 1999;117 (1):17–25. [DOI] [PubMed] [Google Scholar]
- 30. Auer J, Camoin L, Courtot AM, Hotellier F, De Almeida M. Evidence that P36, a human sperm acrosomal antigen involved in the fertilization process is triosephosphate isomerase. Mol Reprod Dev. 2004;68 (4):515–523. [DOI] [PubMed] [Google Scholar]
- 31. Prendergast DW, Woad KJ, Chamley LW, Shelling AN. Spatial and temporal expression of the sperm protein SPRASA in mice. Biol Reprod. 2008;78(301):74a. [Google Scholar]
- 32. Ekhlasi-Hundrieser M, Sinowatz F, Greiser De Wilke I, Waberski D, Topfer-Petersen E. Expression of spermadhesin genes in porcine male and female reproductive tracts. Mol Reprod Dev. 2002;61 (1):32–41. [DOI] [PubMed] [Google Scholar]
- 33. Zhang H, Martin-DeLeon PA. Mouse Spam1 (PH-20) is a multifunctional protein: evidence for its expression in the female reproductive tract. Biol Reprod. 2003;69 (2):446–454. [DOI] [PubMed] [Google Scholar]
- 34. Hao Z, Wolkowicz MJ, Shetty J, et al. SAMP32, a testis-specific, isoantigenic sperm acrosomal membrane-associated protein. Biol Reprod. 2002;66 (3):735–744. [DOI] [PubMed] [Google Scholar]
- 35. Hamatani T, Carter MG, Sharov AA, Ko MS. Dynamics of global gene expression changes during mouse preimplantation development. Dev Cell. 2004;6 (1):117–131. [DOI] [PubMed] [Google Scholar]
- 36. Wood DM, Dunbar BS. Direct detection of two cross-reactive antigens between porcine and rabbit zonae pellucidae by radioimmunoassay and immunoelectrophoresis. J Exp Zool. 1981;217 (3):423–433. [DOI] [PubMed] [Google Scholar]
- 37. Skinner SM, Mills T, Kirchick HJ, Dunbar BS. Immunization with zona pellucida proteins results in abnormal ovarian follicular differentiation and inhibition of gonadotropin-induced steroid secretion. Endocrinology. 1984;115 (6):2418–2432. [DOI] [PubMed] [Google Scholar]
- 38. Mahi Brown CA, Yanagimachi R, Hoffman JC, Huang TT., Jr Fertility control in the bitch by active immunization with porcine zonae pellucidae: use of different adjuvants and patterns of estradiol and progesterone levels in estrous cycles. Biol Reprod. 1985;32 (4):761–772. [DOI] [PubMed] [Google Scholar]
- 39. Mahi-Brown CA, Yanagimachi R, Nelson ML, Yanagimachi H, Palumbo N. Ovarian histopathology of bitches immunized with porcine zonae pellucidae. Am J Reprod Immunol Microbiol. 1988;18 (3):94–103. [DOI] [PubMed] [Google Scholar]
- 40. Sacco AG, Yurewicz EC, Subramanian MG. Effect of varying dosages and adjuvants on antibody response in squirrel monkeys (Saimiri sciureus) immunized with the porcine zona pellucida Mr = 55,000 glycoprotein (ZP3). Am J Reprod Immunol. 1989;21 (1):1–8. [DOI] [PubMed] [Google Scholar]
- 41. Sehgal S, Gupta SK, Bhatnagar P. Long-term effects of immunization with porcine zona pellucida on rabbit ovaries. Pathology. 1989;21 (2):105–110. [DOI] [PubMed] [Google Scholar]
- 42. Upadhyay SN, Thillaikoothan P, Bamezai A, Jayaraman S, Talwar GP. Role of adjuvants in inhibitory influence of immunization with porcine zona pellucida antigen (ZP-3) on ovarian folliculogenesis in bonnet monkeys: a morphological study. Biol Reprod. 1989;41 (4):665–673. [DOI] [PubMed] [Google Scholar]
- 43. Hasegawa A, Koyama K, Inoue M, Takemura T, Isojima S. Antifertility effect of active immunization with ZP4 glycoprotein family of porcine zona pellucida in hamsters. J Reprod Immunol. 1992;22 (2):197–210. [DOI] [PubMed] [Google Scholar]
- 44. Fayrer Hosken RA, Dookwah HD, Brandon CI. Immunocontrol in dogs. Animal Reprod Sci. 2000;60-61:365–373. [DOI] [PubMed] [Google Scholar]
- 45. Wood DM, Liu C, Dunbar BS. Effect of alloimmunization and heteroimmunization with zonae pellucidae on fertility in rabbits. Biol Reprod. 1981;25 (2):439–450. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials