Abstract
Objective:
Endometriosis is linked to altered cell proliferation and stem cell markers c-kit/stem cell factor (SCF) in ectopic endometrium. Our aim was to investigate whether c-kit/SCF also plays a role in eutopic endometrium.
Design:
Eutopic endometrium obtained from 35 women with endometriosis and 25 fertile eumenorrheic women was analyzed for in situ expression of SCF/c-kit, Ki67, RAC-alpha serine/threonine-protein kinase (Akt), phosphorylated RAC-alpha serine/threonin-protein kinase (pAkt), Glycogen synthase kinase 3 beta (GSK3β), and phosphorylated glycogen synthase kinase 3 beta (pGSK3β), throughout the menstrual cycle.
Results:
Expression of Ki67 and SCF was higher in endometriosis than in control tissue (P < .05) and greater in secretory rather than proliferative (P < .01) endometrium in endometriosis. Expression of c-kit was also higher in endometriosis although similar in both phases. Expression of Akt and GSK3β was identical in all samples and cycle phases, whereas pAkt and pGSK3β, opposed to control tissue, remained overexpressed in the secretory phase in endometriosis.
Conclusion:
Unceasing cell proliferation in the secretory phase of eutopic endometriosis is linked to deregulation of c-kit/SCF-associated signaling pathways.
Keywords: stem cell factor, c-kit receptor, eutopic endometrium, endometriosis, Akt/GSK3β
Introduction
Endometriosis is a gynecologic pathology characterized by inflammation and invasion of endometrial cells into other tissues. It represents a widespread condition with a relevant impact on women health.1–3 The pathophysiology of the genesis and progression of the disease is unclear and poorly understood. Endometriosis arises from eutopic endometrial cells with increased proliferation and adhesion ability in response to abnormal steroidogenic function, angiogenic factors, cytokines, and others.4 In fact it has been demonstrated that augmented cell viability in eutopic endometrium is a consequence of reduction in cell death by apoptosis and an increase in cell proliferation.5
Epithelial cells in normal endometrium and ectopic endometrial tissue of endometriotic lesions express stem cell markers oct-4 and c-kit.6,7 c-Kit is a proto-oncogene that encodes for a tyrosine kinase receptor,8,9 its ligand is the stem cell factor (SCF).10 Stem cell factor and its receptor have been detected in pregnant endometrium, placental tissues, and in throphoblasts,11,12 suggesting a role in growth, proliferation, and differentiation processes.13–15 Stem cell factor has also been found in the peritoneal fluid of patients with endometriosis.16 c-kit/SCF signal transduction pathway is regulated through Ras/Erk, Src-kinase, or Jak/Stat,17–19 but interestingly in endometrioid and high-grade serous ovarian carcinomas, the phosphatidylinositol 3-kinase (PI3K)/Akt pathway and GSK3β have also been clearly identified.20,21 Endometriosis is considered a precancerous lesion associated with ovarian cancer,22,23 but strangely the expression and regulation of c-kit/SCF in eutopic endometriosis have not been distinctly examined. Therefore, our aim was to evaluate SCF and c-kit expression and their possible association with the Akt/GSK3β pathway in human eutopic endometrium biopsies of women with endometriosis.
Materials and Methods
Patients and Tissue Collection
All tissue samples of this case–control study were obtained with the informed consent of the patients. The research ethics committee of the Hospital de la Mujer, Secretaría de Salud, Mexico, Federal District, Mexico (permit id: HMINV-2012-07) approved the study, which was conducted in accordance with international laws on procedures for human tissue handling of Helsinki declaration (1964). The control group consisted of 25 women aged 19 to 40 years old with no endometriosis manifestations who had an endometrial biopsy due to abnormal uterine bleeding, endometrial dating, evaluation of uterine response to hormone therapy (evidence of ovulation), evaluation of long-term amenorrhea, and exclude pelvic diseases. Nevertheless, endometriosis could not be entirely ruled out because laparoscopic visualization of the pelvis was not undertaken in these women. The histopathology analysis of these biopsies confirmed that 13 were in the proliferative phase and 12 in the secretory phase. When in doubt for possible “red lesions,” the “suspicious” biopsy samples are routinely subjected to a PGP9.5 immunostain to detect nerve fibers; none of the control group biopsies were positive for this marker. Endometrial tissue specimens were obtained from 35 women, 30 of them were nonsmokers, who underwent laparoscopy for stage III and IV endometriosis classified according to the American Society for Reproductive Medicine.24 The most frequent presenting symptoms were menorrhagia, pelvic pain, or both. Eighty-five percent of the patients underwent laparoscopic evaluation of the pelvis; the remaining patients underwent an ultrasound evaluation. Median age of patients was 37 (range 25-52) years old. The diagnosis was histologically confirmed, but we did not find a relation between the Altman Self-Rating Mania Scale score and the patient’s age. The surgical procedures were carried out by an expert gynecological surgeon in the Department of Obstetrics and Gynecology of the Hospital de la Mujer, Mexico City, Mexico, from January 2011 through October 2012. None of the women had received hormonal medication in the 3 months prior to the surgical procedure, and because of the most recent evidence,25 their estrogen and progesterone serum concentrations that are regularly determined by the hospital’s routine laboratory were within normal values. Menstrual cycle dating was determined by the clinical history and by histological description according to Noyes criteria.26 Patients and control healthy women were considered to be Mexican Mestizo, and their body mass index of patients and control women was within the 20 to 31 kg/m2 units range.
Immunohistochemistry
Endometrial biopsies were fixed in neutral-buffered 4% ρ-formaldehyde at 4°C overnight and were subsequently paraffin embedded. Before performing immunohistochemistry, sections of the tissues were stained with hematoxylin–eosin to corroborate the clinical diagnosis. Serial sections, 4-μm thick, were used for immunohistochemistry. Paraffin-embedded sections were dewaxed and rehydrated in decreasing concentrations of ethanol. Antigen retrieval was achieved by boiling the samples in 0.01 mol/L citrate of sodium (pH 6) for 20 to 40 minutes. The quenching of endogenous peroxidase was achieved by incubation with 0.3% hydrogen peroxide in methanol for 30 minutes at room temperature. After washing with phosphate-buffered saline (PBS), the slides were incubated with 2% albumin solution for 2 hours at room temperature and extensively washed with PBS. The slides were incubated at 4°C in a humid chamber overnight with primary antibody diluted in Tris-buffered saline containing 1% bovine serum albumin. Mouse monoclonal antihuman SCF (Santa Cruz Biotechnology, Santa Cruz Biotechnology, Inc., Dallas, Texas) diluted 1:200, mouse monoclonal anti-Ki67 (Biocare Medical, Biocare Medical, Concord, California) diluted 1:100, and a rabbit monoclonal anti-c-kit/CD117 (Biocare Medical, Biocare Medical, Concord, California) diluted 1:50 were used. At the end of the incubation period, the samples were extensively washed in PBS and labeled with an avidin–biotin peroxidase detection system for 2 hours (1:200; VECTASTAIN ABC Elite kit; Vector Laboratories, Inc, Burlington, Vermont) at room temperature. After extensive washing, 3,3′-diaminobenzidine (Vector Laboratories, Inc, Burlingame, California) was used as a chromogen. The samples were counterstained with hematoxylin and evaluated with a Nikon Eclipse E600 microscope (Nikon Instruments Inc., Melville, New York). Photographs from 5 different fields per slide were taken randomly. The number and percentage of immunopositive Ki67, SCF, and c-kit positive cells were quantified by image analysis using the NIH Image J software (developed at the U.S. National Institutes of Health, USA superseded by Image J and available on the internet at http://rsb.info.nih.gov/nih-image/) that transforms the image into an 8-bit image; the program then calculates the intensity of the stained area measuring the amount of pixels/square inch and converts the readings into a white to black numerical value, expressed as optical density (OD). The results so expressed were used for analysis purposes, as described previously.27
Immunofluorescence
Tissue sections prepared identically to that described previously, with the exception of the peroxidase blockage, were incubated at 4°C in a humid chamber overnight with primary antibody diluted in PBS. Rabbit polyclonal, anti-Akt1/2/3 (sc-8312, 1:100), anti-p-Akt1/2/3 (sc-7985-R, 1:100), anti-GSK3β (sc-9166, 1:100), and anti-p-GSK3β (sc-11757-R, 1:100), all from Santa Cruz Biotechnology, were used. At the end of the incubation period, the samples were washed thrice in PBS followed by 2 hours incubation with a 1:200 dilution of the specific goat antirabbit immunoglobulin G-rhodamine-conjugated antibody (Merck Millipore). The samples were counterstained with Hoechst 33342 benzimide (Life Technologies, Thermo Fisher Scientific, Waltham, Massachusetts) according to the manufacturer’s instruction. Stained slides were stored at 4°C before being evaluated with a Nikon Eclipse E600 fluorescence microscope. Similar to the evaluation performed for the immunohistochemistry analysis, and also in 5 different fields per slide, but using the RBG stack instead of the 8 bits, the intensity of immunopositive cells was evaluated using the Image J software.
Statistical Analysis
Data were examined to determine their normal distribution using the DÁgostino-Pearson normality test included in the software that we use. Once corroborated, the data were analyzed by an analysis of variance test, followed by a post hoc test using the Bonferroni comparisons. All the statistical analysis was performed with GraphPad Prism 5.0 software (San Diego, California). All values are expressed as the mean ± standard deviation. Differences were considered statistically significant when P < .05.
Results
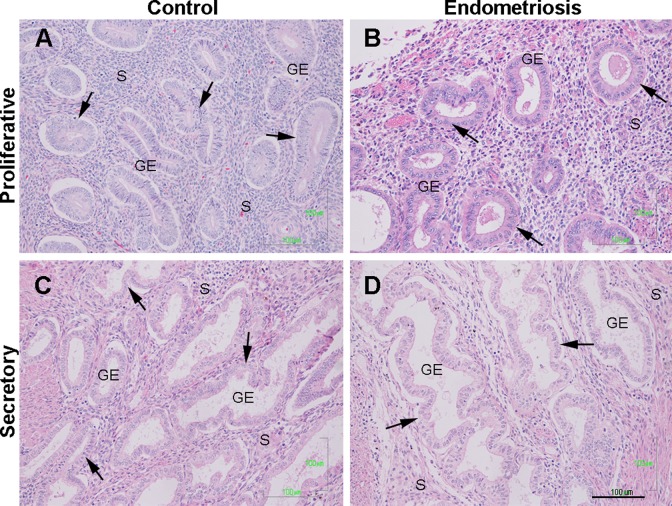
In the proliferative phase of both, case and control samples, columnar surface epithelium, long curving glands, uniform and small stroma cells with round nuclei, and numerous mitotic cells both in the glandular epithelium and stroma were observed. In the secretory phase, the columnar epithelium was cylindrical, the glands exhibited tortuosities and exhibited secretory vacuoles at the apical pole. The stroma showed edematous changes. Because of this, we considered that the eutopic endometrial morphology of control and endometriosis samples were similar in the proliferative as well as in the secretory phases (Figure 1A–D).
Figure 1.
Histological architecture of control and eutopic endometriosis endometrium. Representative image of the histological organization of glandular epithelium (GE) and stroma (S) of normal (A and C) and eutopic endometrium with endometriosis (B and D) in the menstrual cycle phases. Arrows shown typical GE during the proliferative (A and B) and the secretory (C and D) phases, in control and endometriosis endometrium, respectively. Scale bar = 100 μm. Biopsy samples were hematoxylin–eosin stained. (The color version of this article is available at http://rs.sagepub.com.)
Expression of Ki67, SCF, and c-Kit Receptor
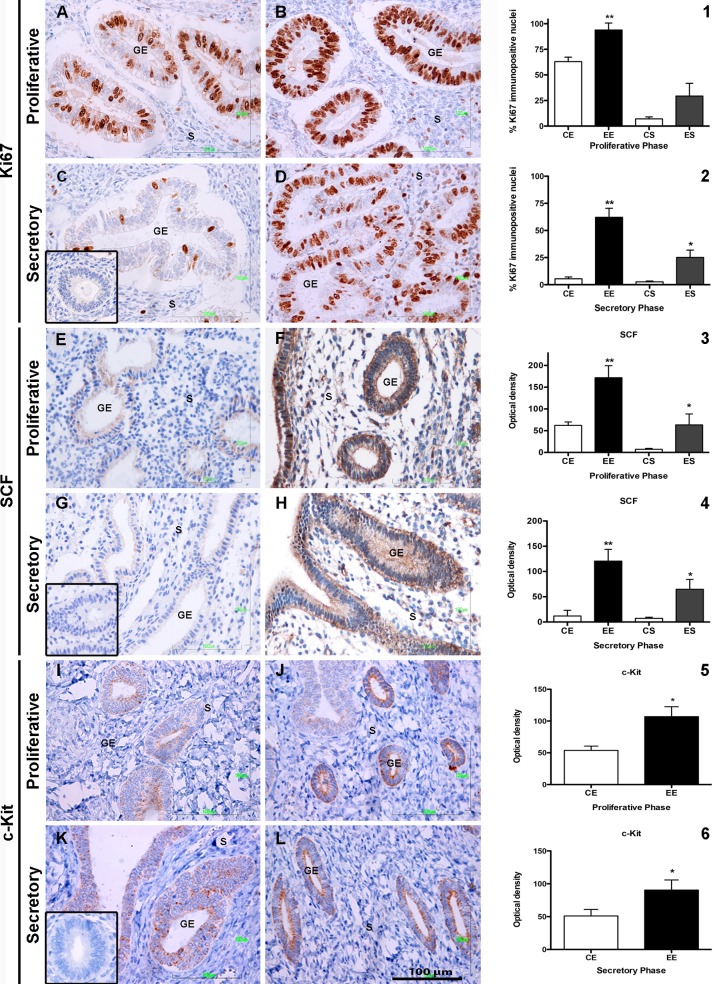
A significant increase in the number of Ki67 immunopositive nuclei was observed in the glandular epithelium during the proliferative phase of women with endometriosis. No histological differences were observed in stromal cells of endometriosis and control samples in the same phase (Figure 2A and B). The mean percentage of Ki67 immunopositive cells in the endometriosis samples was 93.84% ± 19% versus 62% ± 94% in the control group (P < .01; Figure 2). Unexpectedly, a highly significant increase in the mean percentage of Ki67 immunopositive cells 62.14% ± 8.27% versus 5.4% ± 1.64% (P < .01) was observed in the glandular epithelium of endometriosis samples during the secretory phase (Figure 2D) in comparison with control samples (Figure 2C); the difference was also observed in the stroma and it was also significant (25.14% ± 6.76% vs 2.58% ± 0.8%, P < .05; Figure 2).
Figure 2.
Expression of Ki67, SCF, and c-kit in control and eutopic endometriosis endometrium. Representative image of Ki67 expression in control and eutopic endometriosis is shown in the proliferative phase (A and B, respectively) and in the secretory phase (C and D). The percentage of Ki67 immunopositive nuclei in EE and ES compared to CE and CS is shown in both the proliferative (1) and the secretory phases (2). A representative image of stem cell factor (SCF) expression under identical parameters as those of Ki67 is shown in (E) to (H), the percentage of SCF immunopositive cells in EE and ES compared to CE and CS is shown in both the proliferative (3) and the secretory phases (4). The expression of c-kit is shown in (I) to (L). The percentage of c-kit immunopositive cells in CE and EE is shown in both the proliferative (5) and the secretory phases (6). An isotype match IgG stain was used as negative control for each of the antibodies used, and the result is shown in the inset in the low left side of the (C), (G), and (K) images. The S and GE within each figure mark the stroma and the glandular epithelium. Magnification ×400, scale bar = 100 μm. The histograms in the right side of the images represent the mean ± standard deviation of the values obtained for the endometrial biopsy samples of all the endometriosis (n = 35) and the control (n = 25) groups for each immunostain in the proliferative or the secretory phases. *P < .05 and **P < .01. CE indicates control epithelium; EE, endometriosis epithelium; CS, control stroma; ES, endometriosis stroma; IgG, immunoglobulin G.
The expression of SCF in the glandular epithelium and stroma cells of endometriosis samples was also enhanced in the proliferative phase in comparison with control biopsies (Figure 2E and F). The differences were highly significant (171.9 ± 27.5 OD vs 62.19 ± 7.91 OD, P < .01) for glandular epithelium; differences were also found in relation to stroma SCF expression (63.31 ± 15.09 OD vs 7.25 ± 2.09 OD; P < .05; Figure 2). It was interesting to observe that in the secretory phase of endometriosis biopsies, highly significant differences were also observed; the noticeable increase in the expression of SCF both in the glandular epithelium (120.6 ± 23.17 OD vs 11.97 ± 9.87 OD, P < .01) and in the stroma cells (64.82 ± 19.43 OD vs 7.25 ± 2.09 OD, P < .05; Figure 2G and H) in comparison to control biopsies.
The expression of c-kit receptor was observed only in the glandular epithelium of endometriosis biopsies both in the proliferative and the secretory phases (Figure 2I–L). The differences were statistically significant (93.38 ± 16.82 vs 53.76 ± 6.74 OD for the proliferative phase and 90.47 ± 15.36 vs 51.69 ± 10.90 OD; P < .05) in comparison with the control biopsies. An interesting observation was the occasional presence of c-kit positive cells in the stroma of control biopsies.
Expression of p-Akt and p-GSK3β
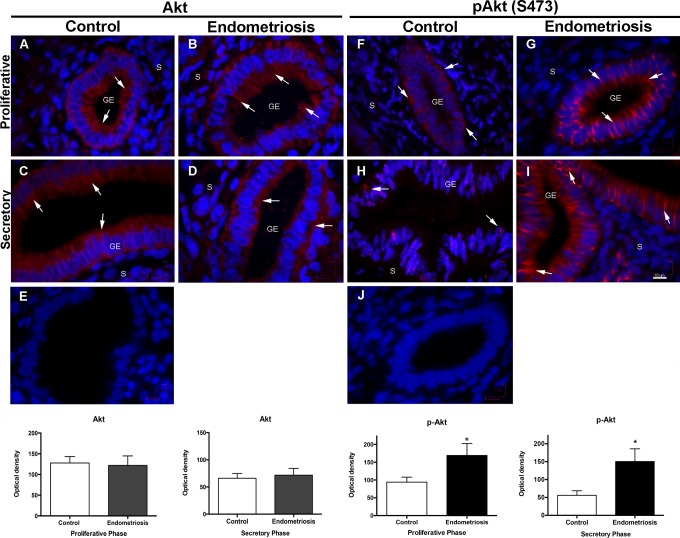
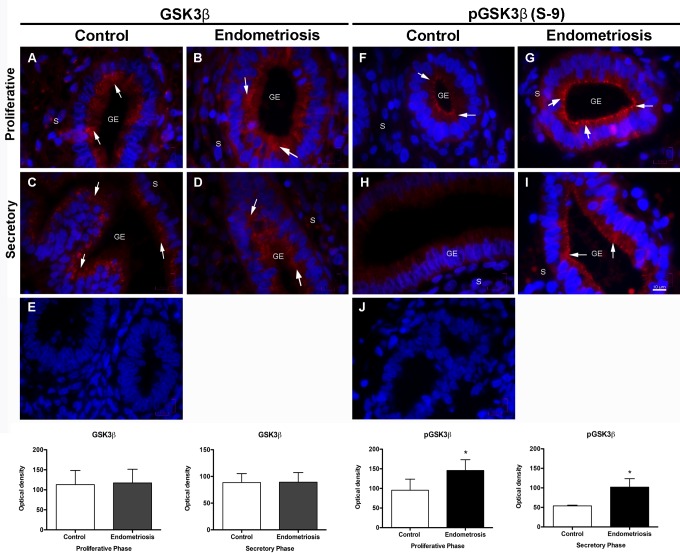
No significant differences in the expression of Akt and GSK3β were observed in the proliferative or the secretory phases of control and endometriosis biopsies. Nevertheless, there was a significantly increased expression of the phosphorylated Akt (S473) in the proliferative phase of endometriosis biopsies in comparison with the controls (P < .05); interestingly, this overexpression remained increased in the secretory phase of endometriosis biopsies, whereas, as expected, the control biopsies showed a nearly 50% reduction in pAkt expression; the differences were highly significant (Figure 3; P < .01). An identical behavior with the same statistical differences was observed when pGSK3β (S9) expression was evaluated (Figure 4).
Figure 3.
Expression of Akt and pAkt (S473) in control and eutopic endometriosis endometrium. Representative images of Akt and pAkt (S473) expression in the proliferative phase of control endometrium (A and F) and eutopic endometriosis (B and G) endometrium. C and H, Akt and pAkt (S473) expression during the secretory phase in the control endometrium and (D and I) in eutopic endometrium with endometriosis. E and J, An isotype match immunoglobulin G stain used as negative control for each of the antibodies. Arrows show the Akt and pAkt (S473) localization mainly in the glandular epithelia. Magnification ×1000, scale bar = 10 μm. The histograms represent the mean ± standard deviation of all the values obtained for the endometrial biopsy samples of the endometriosis (n = 35) and the control (n = 25) groups for each immunostain in the proliferative or the secretory phases. *P < .05. GE indicates glandular epithelium; S, stroma.
Figure 4.
Expression of GSK3β and pGSK3β (S9) in control and eutopic endometriosis endometrium. Representative images of GSK3β and pGSK3β (S9) expression in the proliferative phase of control endometrium (A and F), and eutopic endometriosis (B and G) endometrium. C and H, GSK3β and pGSK3β (S9) expression during the secretory phase in the control endometrium and (D and I) in eutopic endometrium with endometriosis. Arrows show GSK3β and pGSK3β (S9) localization. E and J, An isotype match immunoglobulin G stain used as negative control for each of the antibodies. Magnification ×1000, scale bar = 10 μm. The histograms represent the mean ± standard deviation of all the values obtained for the endometrial biopsy samples of the endometriosis (n = 35) and the control (n = 25) groups for each immunostain in the proliferative or the secretory phases. *P < .05. GE indicates glandular epithelium; S, stroma.
Discussion
Endometriosis is a chronic inflammatory disease characterized by increased cellular proliferation, adhesion, and invasion of tissue similar to uterine endometrium in the eutopic endometrium28 and places other than those physiologically appropriate.
Our results did not show evidence of histological differences between endometriosis eutopic endometrium and control biopsies. This suggests that in patients with endometriosis, the relative concentration of steroid hormones is not altered, but it does not rule out the firm concept that it is an estrogen-dependent pathology with progesterone resistance due to low progesterone-receptor levels.29–31 In fact it appears that the regulation of the different subtypes of estrogen receptor and progesterone receptor isoforms mediates distinct responses to steroid hormones and differs between normal and eutopic endometria,32 although the most recent evidence shows that the elevated endometrial concentrations of estrone and estradiol in endometriotic tissue are determined by local metabolism rather than circulating levels.25
The proliferation of epithelial and stromal cells in endometriosis biopsies was increased in the proliferative phase, as determined by the cell cycle marker Ki67, but it was unexpectedly higher in the secretory phase of endometriosis biopsies. Our results are in accordance with those of Mourtzikou et al,33 for control biopsies, but not as far as endometriosis is concerned. Expression of Ki67 is dependent on estrogens and is also linked to SCF/c-kit in other tissues and some cancers.34,35 Increased cell proliferation has been related to a reduction in cell death and an increase in cell proliferation due to abundance of c-myc,5 although a differential regulation of micro-RNAs in the secretory and proliferative phase of the cycle, which is under hormonal control,36 has also been considered.
It has been suggested that different stem cell populations contribute to endometriosis pathogenesis.37 Stem cell factor and its receptor c-kit regulate the differentiation, proliferation, and survival of stem/pluripotent cells in normal endometrium and play an important role in follicular development.38 The expression of SCF increases in the proliferative phase and diminishes in the secretory phase11 of normal endometrial phases. An interesting although relatively uncommon result was that some of the biopsies of the women with and without endometriosis showed occasional c-kit+ cells in the stroma, which seem to belong to some kind of undifferentiated mesenchymal stromal cells that require c-kit for maintenance.39 This cells are possibly those considered as human endometrial side population cells that correspond to somatic stem cells and express c-kit40; they can be found in stromal or epithelial endometrial compartments, but these clonogenic epithelial cells are weakly supported by SCF, especially those found in the stroma.41 We could not establish a relation between the number of these putative stem cells and the degree of endometriosis; but nevertheless, it is possible that these cells that regulate the effect of growth factors on proliferation and differentiation of endometrial cells39 have a central role in endometriosis. Schwab et al42 have shown that clonogenicity does not vary from the proliferative to the secretory stage of the menstrual cycle or between active cycling and inactive endometrium for both epithelial and stromal cells. Thus, suggesting a complex regulation of these putative stem cells in the endometrium42 possible dependent on local production of hormones and growth factors.
Our results showed that SCF expression was significantly increased in the glandular epithelium and stromal cells, whereas its c-kit receptor was only increased in the glandular epithelium, both in the proliferative and the secretory phases of women with endometriosis. Temporal changes in SCF and c-kit receptor expression during the normal menstrual cycle11 and in benign endometrial tissues43 have been reported, suggesting their possible involvement in endometrial function. Nevertheless, c-kit expression is controversial since its expression in the endometrium of women with endometriosis does not seem to differ from women without endometriosis.44
Our results showed an important increase in pAkt and pGSK3β expression in the glandular epithelium during the proliferative and secretory phases of endometriosis. The SCF/c-kit interaction is involved in many cellular functions such as survival, proliferation, and apoptosis through the PI3K/Akt pathway45 and GSK3β, a key regulatory kinase that participates in numerous signaling pathways. This signaling pathway participates in epithelial proliferation in uterine endometrium.46,47 Akt increases endometrial cell survival, and β-estradiol-induced Akt phosphorylation of stromal cells may be involved in altered apoptosis/proliferation regulation in endometriosis.48 This suggests that SCF/c-kit could promote epithelial cell proliferation and survival through Akt/GSK3β signaling, not only in the proliferative but also in the secretory phase of endometriosis. GSK3β is constitutively active, and its activity is negatively regulated primarily through Ser9 phosphorylation49,50 followed by Tyr216 phosphorylation both of which are mediated by Akt.51 We showed that Akt and GSK3β remained phosphorylated during the secretory phase in endometriosis biopsies. Phosphorylation of Akt upregulates the expression of cell survival genes and downregulates apoptosis.52 Our results confirm the observations made by Silveira et al53 but might be considered puzzling since progesterone inhibits the AKT/GSK3β pathway related to epithelial proliferation54 but GSK3β maintains progesterone receptor A stability.55 Our results reinforce the importance of the PI3K/Akt pathway in endometriosis but contrary to the results of Cinar et al,48 we showed that the sustained pAkt expression in both phases of the cycle, especially in the secretory phase, is nonestradiol dependent, thus advocating Akt as a major participant in the pathogenesis of endometriosis. The overall results of our work indicate that there is an increased expression of SCF/C-kit and an impaired signaling by the perpetuation of Akt and GSK3β phosphorylation in eutopic endometriosis that might maintain epithelial cell proliferation in the secretory phase of the menstrual cycle. We acknowledge that our results are solely based on immunohistochemistry stain data and although functional experiments, such as the therapeutic inhibition of Akt,56 would be welcomed, so far they are not being considered in endometriosis.
Acknowledgments
The authors thank Raquel Guerrero Alquicira for excellent technical assistance.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Yanira Franco Murillo is a recipient of a doctoral fellowship from CONACyT and the Posgrado en Ciencias Biológicas, Facultad de Ciencias, UNAM, Mexico, Federal District, Mexico.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Macer ML, Taylor HS. Endometriosis and infertility: a review of the pathogenesis and treatment of endometriosis-associated infertility. Obstet Gynecol Clin North Am. 2012;39(4):535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Acien P, Velasco I. Endometriosis: a disease that remains enigmatic. ISRN Obstet Gynecol. 2013;2013:242149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu DT, Hitchcock A. Endometriosis: its association with retrograde menstruation, dysmenorrhoea and tubal pathology. Br J Obstet Gynaecol. 1986;93(8):859–862. [DOI] [PubMed] [Google Scholar]
- 4. Liu H, Lang JH. Is abnormal eutopic endometrium the cause of endometriosis? The role of eutopic endometrium in pathogenesis of endometriosis. Med Sci Monit. 2011;17(4):RA92–RA99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johnson MC, Torres M, Alves A, et al. Augmented cell survival in eutopic endometrium from women with endometriosis: expression of c-myc, TGF-beta1 and bax genes. Reprod Biol Endocrinol. 2005:3:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pacchiarotti A, Caserta D, Sbracia M, Moscarini M. Expression of oct-4 and c-kit antigens in endometriosis. Fertil Steril. 2011;95(3):1171–1173. [DOI] [PubMed] [Google Scholar]
- 7. Cho NH, Park YK, Kim YT, Yang H, Kim SK. Lifetime expression of stem cell markers in the uterine endometrium. Fertil Steril. 2004;81(2):403–407. [DOI] [PubMed] [Google Scholar]
- 8. Roskoski R., Jr Signaling by Kit protein-tyrosine kinase—the stem cell factor receptor. Biochem Biophys Res Commun. 2005;337(1):1–13. [DOI] [PubMed] [Google Scholar]
- 9. Sharkey AM, Jokhi PP, King A, Loke YW, Brown KD, Smith SK. Expression of c-kit and kit ligand at the human maternofetal interface. Cytokine. 1994;6(2):195–205. [DOI] [PubMed] [Google Scholar]
- 10. Yarden Y, Kuang WJ, Yang-Feng T, et al. Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987;6(11):3341–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kauma S, Huff T, Krystal G, Ryan J, Takacs P, Turner T. The expression of stem cell factor and its receptor, c-kit in human endometrium and placental tissues during pregnancy. J Clin Endocrinol Metab. 1996;81(3):1261–1266. [DOI] [PubMed] [Google Scholar]
- 12. Saito S, Enomoto M, Sakakura S, Ishii Y, Sudo T, Ichijo M. Localization of stem cell factor (SCF) and c-kit mRNA in human placental tissue and biological effects of SCF on DNA synthesis in primary cultured cytotrophoblasts. Biochem Biophys Res Commun. 1994;205(3):1762–1769. [DOI] [PubMed] [Google Scholar]
- 13. Li J, Goodyer CG, Fellows F, Wang R. Stem cell factor/c-kit interactions regulate human islet-epithelial cluster proliferation and differentiation. Int J Biochem Cell Biol. 2006;38(5–6):961–972. [DOI] [PubMed] [Google Scholar]
- 14. Lennartsson J, Ronnstrand L. The stem cell factor receptor/c-kit as a drug target in cancer. Curr Cancer Drug Targets. 2006;6(1):65–75. [DOI] [PubMed] [Google Scholar]
- 15. Ulivi P, Zoli W, Medri L, et al. c-kit and SCF expression in normal and tumor breast tissue. Breast Cancer Res Treat. 2004;83(1):33–42. [DOI] [PubMed] [Google Scholar]
- 16. Osuga Y, Koga K, Tsutsumi O, et al. Stem cell factor (SCF) concentrations in peritoneal fluid of women with or without endometriosis. Am J Reprod Immunol. 2000;44(4):231–235. [DOI] [PubMed] [Google Scholar]
- 17. Liang J, Wu YL, Chen BJ, Zhang W, Tanaka Y, Sugiyama H. The c-kit receptor-mediated signal transduction and tumor-related diseases. Int J Biol Sci. 2013;9(5):435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yasuda A, Sawai H, Takahashi H, et al. Stem cell factor/c-kit receptor signaling enhances the proliferation and invasion of colorectal cancer cells through the PI3K/Akt pathway. Dig Dis Sci. 2007;52(9):2292–2300. [DOI] [PubMed] [Google Scholar]
- 19. Silva CM. Role of STATs as downstream signal transducers in Src family kinase-mediated tumorigenesis. Oncogene. 2004;23(48):8017–8023. [DOI] [PubMed] [Google Scholar]
- 20. Wu R, Hu TC, Rehemtulla A, Fearon ER, Cho KR. Preclinical testing of PI3K/AKT/mTOR signaling inhibitors in a mouse model of ovarian endometroid adenocarcinoma. Clin Cancer Res. 2011;17(23):7359–7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Espinosa I, Catasus L, Canet B, DÁngelo E, Muñoz J, Prat J. Gene expression analysis identifies two groups of ovarian high-grade serous carcinomas with different prognosis. Mod Pathol 2011;24(6):846–854. [DOI] [PubMed] [Google Scholar]
- 22. Lai CR, Hsu CY, Chen YJ, Yen MS, Chao KC, Li AF. Ovarian cancers arising from endometriosis: a microenvironmental biomarker study including ER, HNF1ss, p53, PTEN, BAF250a, and COX-2. J Chin Med Assoc. 2013;76(11):629–634. [DOI] [PubMed] [Google Scholar]
- 23. Siufi Neto J, Kho RM, Dos Santos Siufi DF, Baracat EC, Anderson KS, Abrao MS. Cellular, histologic, and molecular changes associated with endometriosis and ovarian cancer. J Minim Invasive Gynecol. 2014;21(1):55–63. [DOI] [PubMed] [Google Scholar]
- 24. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67(5):817–821. [DOI] [PubMed] [Google Scholar]
- 25. Huhtinen K, Desai R, Stahle M, et al. Endometrial and endometriotic concentrations of estrone and estradiol are determined by local metabolism rather than circulating levels. J Clin Endocrinol Metab. 2012;97(11):4228–4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122(2):262–263. [DOI] [PubMed] [Google Scholar]
- 27. Mendoza-Rodriguez CA, Merchant-Larios H, Segura-Valdez ML, et al. c-fos and estrogen receptor gene expression pattern in the rat uterine epithelium during the estrous cycle. Mol Reprod Dev. 2003;64(4):379–388. [DOI] [PubMed] [Google Scholar]
- 28. Delbandi AA, Mahmoudi M, Shervin A, et al. Eutopic and ectopic stromal cells from patients with endometriosis exhibit differential invasive, adhesive, and proliferative behavior. Fertil Steril. 2013;100(3):761–769. [DOI] [PubMed] [Google Scholar]
- 29. Al-Sabbagh M, Lam EW, Brosens JJ. Mechanisms of endometrial progesterone resistance. Mol Cell Endocrinol. 2012;358(2):208–215. [DOI] [PubMed] [Google Scholar]
- 30. Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab. 2000;85(8):2897–2902. [DOI] [PubMed] [Google Scholar]
- 31. Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev. 2013;34(1):130–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shao R, Cao S, Wang X, Feng Y, Billig H. The elusive and controversial roles of estrogen and progesterone receptors in human endometriosis. Am J Transl Res. 2014;6(2):104–113. [PMC free article] [PubMed] [Google Scholar]
- 33. Mourtzikou A, Kosmas K, Marouga A, Stamouli M, Pouliakis A, Karakitsos P. The use of an immunocytochemical double-labeling staining can display the distribution of Bcl-2/Ki-67 cells in endometrial adenocarcinomas as well as in normal endometrium. Clin Lab. 2012;58(1–2):133–144. [PubMed] [Google Scholar]
- 34. Li W, Jia M, Qin X, Hu J, Zhang X, Zhou G. Harmful effect of ERbeta on BCRP-mediated drug resistance an cell proliferation in ERa/PR-negative breast cancer. FEBS J. 2013;280(23):6128–6140. [DOI] [PubMed] [Google Scholar]
- 35. Krasagakis K, Kruger-Krasagakis S, Eberle J, Tsatsakis A, Tosca AD, Stathopoulos EN. Co-expression of KIT receptor and its ligand stem cell factor in Merkel cell carcinoma. Dermatology. 2009;218(1):37–43. [DOI] [PubMed] [Google Scholar]
- 36. Belleudi F, Cardinali G, Kovacs D, Picardo M, Torrisi MR. KGF promotes paracrine activation of the SCF/c-KIT Axis from human keratinocytes to melanoma cells. Transl Oncol. 2010;3(2):80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Forte A, Cipollaro M, Galderisi U. Genetic, epigenetic and stem cell alterations in endometriosis: new insights and potential therapeutic perspectives. Clin Sci. 2014;126(2):123–138. [DOI] [PubMed] [Google Scholar]
- 38. Oliveira FR, Dela Cruz C, Del Puerto HL, Vilamil QT, Reis FM, Camargos AF. Stem cells: are they the answer to the puzzling etiology of endometriosis? Histol Histopathol. 2012;27(1):23–29. [DOI] [PubMed] [Google Scholar]
- 39. Suphanantachat S, Iwata T, Ishihara J, Yamato M, Okano T, Izumi Y. A role for c-kit in the maintenance of undifferentiated human mesenchymal stromal cells. Biomaterials. 2014;35(11):3618–3626. [DOI] [PubMed] [Google Scholar]
- 40. Cervelló I, Gil-Sanchis C, Mas A, et al. Human endometrial side population cells exhibit genotypic, phenotypic and functional features of somatic stem cells. PLoS One. 2010;5(6):e10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chan RW, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004;70(6):1738–1750. [DOI] [PubMed] [Google Scholar]
- 42. Schwab KE, Chan RW, Gargett CE. Putative stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil Steril. 2005;84(suppl 2):1124–1130. [DOI] [PubMed] [Google Scholar]
- 43. Mitsunari M, Harada T, Tanikawa M, Iwabe T, Taniguchi F, Terakawa N. The potential role of stem cell factor and its receptor c-kit in the mouse blastocyst implantation. Mol Hum Reprod. 1999;5(9):874–879. [DOI] [PubMed] [Google Scholar]
- 44. Elmore LW, Domson K, Moore JR, Kornstein M, Burks RT. Expression of c-kit (CD117) in benign and malignant human endometrial epithelium. Arch Pathol Lab Med. 2001;125(1):146–151. [DOI] [PubMed] [Google Scholar]
- 45. Uzan C, Cortez A, Dufournet C, Fauvet R, Siffroi JP, Darai E. Endometrium from women with and without endometriosis, and peritoneal, ovarian and bowel endometriosis, show different c-kit protein expression. J Reprod Immunol. 2005;65(1):55–63. [DOI] [PubMed] [Google Scholar]
- 46. Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2011;11(3):297–305. [DOI] [PubMed] [Google Scholar]
- 47. Toyofuku A, Hara T, Taguchi T, Katsura Y, Ohama K, Kudo Y. Cyclic and characteristic expression of phosphorylated Akt in human endometrium and decidual cells in vivo and in vitro. Hum Reprod. 2006;21(5):1122–1128. [DOI] [PubMed] [Google Scholar]
- 48. Cinar O, Seval Y, Uz YH, et al. Differential regulation of Akt phosphorylation in endometriosis. Reprod Biomed Online. 2009;19(6):864–871. [DOI] [PubMed] [Google Scholar]
- 49. Chen B, Pan H, Zhu L, Deng Y, Pollard JW. Progesterone inhibits the estrogen-induced phosphoinositide 3-kinase → AKT → GSK-3beta → cyclin D1 → pRB pathway to block uterine epithelial cell proliferation. Mol Endocrinol. 2005;19(8):1978–1990. [DOI] [PubMed] [Google Scholar]
- 50. Forde JE, Dale TC. Glycogen synthase kinase 3: a key regulator of cellular fate. Cell Mol Life Sci. 2007;64(15):1930–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol. 2001;65(4):391–426. [DOI] [PubMed] [Google Scholar]
- 52. Kockeritz L, Doble B, Patel S, Woodgett JR. Glycogen synthase kinase-3—an overview of an over-achieving protein kinase. Curr Drug Targets. 2006;7(11):1377–1388. [DOI] [PubMed] [Google Scholar]
- 53. Silveira CG, Abrao MS, Dias JA, Jr, et al. Common chromosomal imbalances and stemness-related protein expression markers in endometriotic lesions from different anatomical sites: the potential role of stem cells. Hum Reprod. 2012;27(11):3187–3197. [DOI] [PubMed] [Google Scholar]
- 54. Matsuzaki S, Canis M, Darcha C, Pouly JL, Mage G. HOXA-10 expression in the mid-secretory endometrium of infertile patients with either endometriosis, uterine fibromas or unexplained infertility. Hum Reprod. 2009;24(12):3180–3187. [DOI] [PubMed] [Google Scholar]
- 55. Wang S, Li Y, Hsu PH, Lee SY, Kim Y, Lee EY. Progesterone receptor A stability is mediated by glycogen synthase kinase-3b in the Brca-1 deficient mammary gland. J Biol Chem. 2013;288(36):26265–26274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Folkes AJ, Ahmadi K, Alderton WK, et al. The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3,2-d]pyrimide (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J Med Chem. 2008;51(18):5522–5532. [DOI] [PubMed] [Google Scholar]