Abstract
We previously showed that expression of ghrelin messenger RNA is significantly increased in the ovaries of cycling pigs but not in prepubertal animals and that ghrelin stimulates estradiol (E2) secretion by ovarian follicles in prepubertal animals. The present study investigated in vitro the role of ghrelin in regulating the ovarian steroidogenesis during estrus cycle in mature pigs. Small (SFs), medium (MFs), and large (LFs) ovarian follicles were collected on days 4 to 6, 10 to 12, and 16 to 18 of the estrous cycle from cycling pigs and exposed to 20, 100, and 500 pg/mL ghrelin for 24 hours. In additional experiments, MFs were exposed to ghrelin plus 100 ng/mL follicle-stimulating hormone (FSH) or luteinizing hormone (LH). Levels of progesterone (P4), testosterone (T), and E2 in culture medium were determined by enzyme-linked immunosorbent assay, and the expression of the steroid pathway enzymes 3β hydroxysteroid dehydrogenase (HSD), 17β-HSD, and cytochrome P450 aromatase (CYP19) was evaluated by Western blotting. Ghrelin had no effect on steroid secretion when present at 20 pg/mL, its concentration in follicular fluid, whereas at 100 pg/mL and 500 pg/mL, its concentration in serum, ghrelin significantly decreased secretion of P4, T, and E2. Moreover, all concentrations of ghrelin decreased steroid secretion in FSH- and LH-stimulated follicles. Western blot analysis showed that ghrelin inhibited expression of 3β-HSD, 17β-HSD, and CYP19 proteins. These results suggest that ghrelin, by direct inhibition of 3β-HSD, 17β-HSD, and CYP19 protein expression, inhibits LH- and FSH-stimulated steroid secretion by ovarian follicles, thus negatively affecting ovarian steroidogenesis in mature pigs.
Keywords: ghrelin, steroid secretion, FSH, LH, porcine ovarian follicles, estrous cycle
Introduction
Ghrelin and its fully functional receptor, GHS-R type 1a (GHSR-1a), are present in many reproductive organs, including the ovaries,1–4 testes,5 placenta,6 and endometrium,7 with ghrelin having direct effects on reproductive functions.8–10 In hypothalamus, ghrelin has been shown to stimulate food intake by neuropeptide Y and agouti-related protein.9 In pituitary, ghrelin directly or indirectly changed gonadotropin secretion.8 Inhibitory activity of ghrelin in the regulation of gonadotropin secretion has been shown in ovarectomized rats,11 sheep,12 monkeys,13 and humans.14,15 Ghrelin and GHSR-1a have been detected in the ovaries of humans and animals. Expression of GHSR-1a was found to parallel follicular development, with stronger immunostaining in the granulosa and thecal layers of healthy antral follicles.4 In human and porcine luteal cells, expression of GHSR-1a has also been observed with ghrelin inhibiting progesterone (P4) production.16,17 In pig ovaries, the level of expression of ghrelin messenger RNA (mRNA) was found to depend on the stage of the estrous cycle, with lowest expression in proestrous and maximum expression in estrous and diestrous.18 We previously showed that ghrelin and GHSR-1a are present in the ovarian follicles of both prepubertal and estrous cycle pigs.19 Moreover, during 24 hours of in vitro ovarian follicles culture, ghrelin was secreted to medium by follicles collected from the ovaries of prepubertal animals20 and by increasing of aromatase activity, stimulated estradiol secretion.21 Additionally, ghrelin was shown to mediate the proliferation and apoptosis of porcine ovary cells by activating the extracellular signal-regulated kinase 1/2 and phosphoinositide-3 kinase pathways.22
Based on previously published data showing that ghrelin gene expression levels were significantly correlated with the size of ovarian follicles from estrous cycle animals, but not in prepubertal pigs,19 in the present study, we tested the hypothesis that ghrelin may be important factor in regulation of steroidogenesis in mature pig. We therefore evaluated the direct in vitro effects of ghrelin on basal and gonadotropin-stimulated steroid secretion and on the expression of 3β hydroxysteroid dehydrogenase (HSD), 17β-HSD, and cytochrome P450 aromatase (CYP19) proteins and enzymes involved in steroidogenesis.
Materials and Methods
Reagents
M199 medium and phosphate-buffered saline were purchased from CytoGen, Poland. Antibiotic–antimycotic solution (100×), Tris, Na-deoxycholate, Nonidet NP-40, sodium dodecyl sulfate (SDS), protease inhibitor (EDTA-free), dithiothreitol, Tween 20, bromophenol blue, anti-β-actin antibody (A5316), follicle-stimulating hormone (FSH) from porcine pituitary (F2293), and luteinizing hormone (LH) from sheep pituitary (L5269) were obtained from Sigma Chemical Co (St Louis, Missouri). Human ghrelin (031-30; Phoenix Europe GmbH, Germany) was utilized in this experiment because porcine ghrelin was not readily available at the onset of this experiment. Human ghrelin differs from porcine ghrelin by 3 amino acids.23 Anti-3β-HSD (sc-30820), anti-17β-HSD (sc-26963), and anti-CYP19 (sc-14244) antibodies, horseradish peroxidase-conjugated antibody (sc-2020), and Western blotting luminol reagent (sc-2048) were obtained from Santa Cruz Biotechnology (Germany).
Collection and Treatment of Ovarian Follicles
Crossbred gilts (Large White × Polish Landrace), approximately 7 to 8 months of age and weighing 130 to 140 kg, were used in the experiment. Ovaries were collected in a bottle filled with sterilized ice-cold saline with antibiotic–antimycotic solution and were transported to the laboratory. Approximately 1 hour elapsed from slaughter to collection in the laboratory. Small (SFs; 2-4 mm), medium (MFs; 4-7 mm), and large (MFs; 8-12 mm) follicles were obtained from the ovaries of cycling animals on days 4 to 6, 10 to 12, and 16 to 18 of the estrous cycle, respectively.24 The approximate stage of the estrous cycle of the ovaries was determined using criteria described by Akins and Morrisette25 and based on our experience and knowledge.
To evaluate the effect of ghrelin on basal steroid secretion, SF (n = 16), MF (n = 16), and LF (n = 16) were selected from 6 pigs. Ghrelin action on steroid secretion was independent of follicles size, so we decided the next experiments: steroidogenic enzymes (3β-HSD, 17β-HSD, and CYP19) protein expression and effect of ghrelin on gonadotropin-stimulated steroidogenesis perform only on MF. Additionally, Li et al26 suggested that serum ghrelin is not affected by exogenous gonadotropins. To analyze the effect of ghrelin on gonadotropin-stimulated steroid secretion and steroidogenic enzymes protein expression, MF (n = 40) from 5 pigs were collected. Each experiment was repeated 3 times.
After isolation, the follicles were slit using small scissors to facilitate penetration of the compounds into the tissue and to remove oocytes and follicular fluids. Small pieces (2 mm) of whole follicular walls, including theca and granulosa cells, were separately placed in 24-well plates in M199 medium without phenol red, supplemented with antibiotic–antimycotic solution and incubated with ghrelin (20, 100 and 500 pg/mL) alone or in combination with FSH (100 ng/mL) and/or LH (100 ng/mL) for 24 hours at 37°C. The concentrations of ghrelin used in this study were based on plasma ghrelin concentrations in normal women (250-500 pg/mL) and concentration in serum levels in prepubertal pigs (106 pg/mL)27 and in follicular fluid collected from porcine ovarian follicles (20 pg/mL).20 Unfortunately, thus far, there has been a lack of available data on plasma ghrelin concentrations in adult pigs. The medium was separated from the follicles and stored at −20°C for steroid hormone (progesterone [P4], testosterone [T], and estradiol [E2]) determination. The follicles were frozen at −70°C for 3β-HSD, 17β-HSD, and CYP19 protein expression analysis.
Steroid Analysis
The concentrations of P4, T, and E2 in the media were determined by enzyme immunoassay using commercial enzyme-linked immunosorbent assay kits (DRG Diagnostic, Germany), with limits of assay sensitivity of 0.045 ng/mL, 0.083 ng/mL, and 9.714 pg/mL, respectively, and ranges of 0 to 40 ng/mL, 0 to 16 ng/mL, and 0 to 2000 pg/mL, respectively. The inter- and intraexperiment coefficients of variation were 4.34% and 6.99%, respectively, for P4; 6.71% and 3.28%, respectively, for T; and 6.72% and 2.71%, respectively, for E2. All samples were assayed in duplicate in the same assay.
Western Blot
Immunoblotting of follicular proteins was performed as described previously.19 Briefly, equal aliquots of protein (30 μg) were separated by 12% SDS polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. The membranes were blocked by incubation with 5% nonfat dry milk in Tris-buffered saline containing 0.1% Tween-20 (TBS-T buffer) and incubated overnight at 4°C with antibodies to 3β-HSD (a goat polyclonal IgG; recommended for detection of 3β-HSD of mouse, rat, human, equine, canine, bovine, and porcine), 17β-HSD (a goat polyclonal IgG; recommended for detection of 17β-HSD of mouse, rat, and human), and CYP19 (a goat polyclonal IgG; recommended for detection of CYP19 of mouse, rat, human, equine, canine, bovine, porcine, and avian), each diluted 1:200. After washing in TBS-T, the membranes were incubated with secondary antibody. An anti-β-actin antibody diluted 1:3000 was used as a loading control. Immune complexes were detected by chemiluminescence and visualized using the ChemiDoc-It Imaging System (UVP, LLC, Upland). The bands were quantified densitometrically and analyzed using Image Lab 2.0 software (BioRad, Poland).
Statistical Analysis
Each treatment was conducted in 4 wells, and each experiment was repeated 3 independent times. Statistical analysis was performed using Statistical 6.0. Data were analyzed by 1-way analysis (for ghrelin effect on basal steroidogenesis) or 2-way analysis (for ghrelin effect on gonadotropins-stimulated steroidogenesis) of variance followed by the Turkey honestly significant difference multiple range test. All data are expressed as the mean ± standard deviation. *P < .05, **P < .01, and ***P < .001 were considered statistically significant. Different letters indicate significant differences between groups (P < .001).
Results
Effect of Ghrelin on Steroid Hormone Secretion by Ovarian Follicles
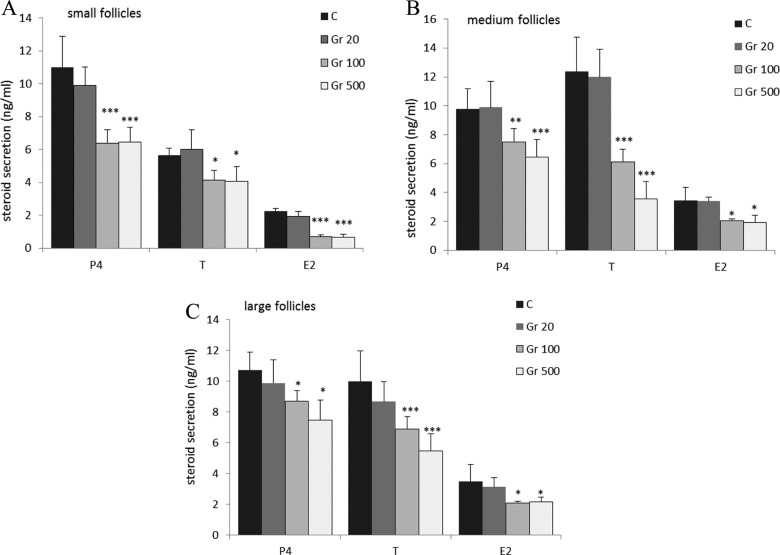
To determine whether ghrelin directly affects steroid secretion, small-, medium-, and large-sized porcine ovarian follicles were incubated for 24 hours with 20, 100, and 500 pg/mL ghrelin. At 20 pg/mL, noted in follicular fluid, ghrelin had no effect on secretion of P4, T, or E2 from follicles of any size. At both physiological concentrations, however, ghrelin significantly inhibited the secretion of P4, T, and E2 from all 3 follicle sizes: SF (P4: 6.395 ± 0.8 and 6.447 ± 0.9 vs 10.984 ± 1.9 ng/mL; T: 4.145 ± 0.6 and 4.070 ± 0.9 vs 5.664 ± 0.4 ng/mL; E2: 0.708 ± 0.09 and 0.646 ± 0.2 vs 2.233 ± 0.2 ng/mL after 100 and 500 pg/mL of ghrelin, respectively), MF (P4: 7.512 ± 0.9 and 6.463 ± 1.2 vs 9.793 ± 1.4 ng/mL; T: 6.1 ± 0.9 and 3.554 ± 1.1 vs 12.36 ± 2.4 ng/mL; E2: 2.055 ± 0.12 and 1.915 ± 0.5 vs 3.461 ± 0.9 ng/mL, respectively), and LF (P4: 8.695 ± 0.7 and 7.49 ± 1.3 vs 10.715± 1.2 ng/mL; T: 6.885 ± 0.8 and 5.48 ± 1.1 vs 9.995 ± 2 ng/mL; E2: 2.09 ± 0.1 and 2.166 ± 0.3 vs 3.5 ± 1.1 ng/mL, respectively; P < .001; Figure 1).
Figure 1.
Effect of ghrelin on the secretion of progesterone (P4), testosterone (T), and estradiol (E2) from (A) small- (2-4 mm), (B) medium- (4-7 mm), and (C) large- (8-12 mm) sized follicles. Follicles were incubated with 20, 100, or 500 pg/mL ghrelin, and the secretion of the 3 steroid hormones was assayed by enzyme-linked immunosorbent assay (ELISA). (***P < .001; **P < .01; *P < .05 compared to untreated control follicles).
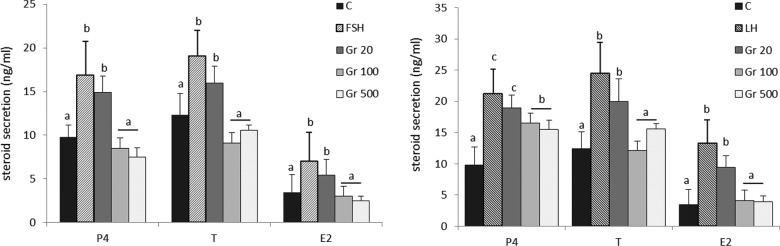
We also tested the effects of ghrelin on FSH- and LH-stimulated hormone secretion. Both FSH and LH significantly increased the secretion of P4, T, and E2 by medium-sized follicles (Figure 2). Ghrelin in doses of 100 and 500 pg/mL significantly decreased FSH- and LH-stimulated steroid secretion (P < .001) whereas at a dose of 20 pg/mL had no effect.
Figure 2.
Effect of ghrelin on the secretion of progesterone (P4), testosterone (T), and estradiol (E2) from medium-sized ovarian follicles stimulated with (A) follicle-stimulating hormone (FSH) or (B) luteinizing hormone (LH). Follicles were incubated with FSH (100 ng/mL) or LH (100 ng/mL) plus 20, 100, or 500 pg/mL ghrelin, and the secretion of the 3 steroid hormones was assayed by enzyme-linked immunosorbent assay (ELISA). Different letters indicate significant differences among each steroids analysis (P < .001).
Effect of Ghrelin on Expression of 3β-HSD, 17β-HSD, and CYP19 Proteins
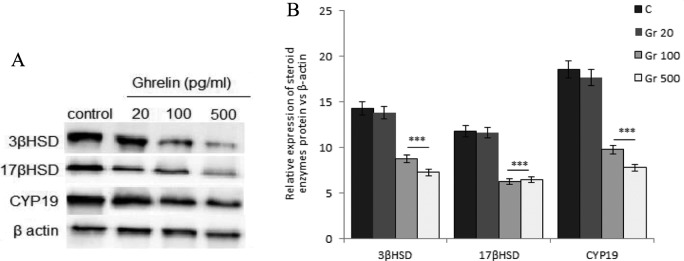
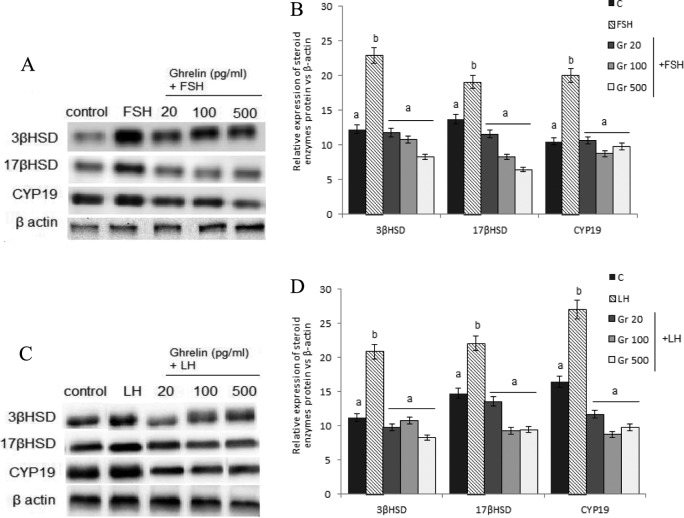
Western blotting assays showed that in medium-sized follicles, 100 and 500 pg/mL ghrelin decreased the expression of the 3 proteins, 3β-HSD, 17β-HSD, and CYP19, whereas 20 pg/mL ghrelin had no effect (Figure 3). Moreover, both FSH and LH significantly increased the expression of these 3 proteins, whereas all 3 concentrations of ghrelin significantly reduced their gonadotropin-stimulated expression (Figure 4).
Figure 3.
Effect of ghrelin on the expression of 3β hydroxysteroid dehydrogenase (HSD; 42 kDa), 17β-HSD (34.5 kDa), and cytochrome P450 aromatase (CYP19; 50 kDa) in medium-sized ovarian follicles. Follicles were incubated with 20, 100, or 500 pg/mL ghrelin, and the expression of the 3 proteins was assayed by Western blotting. A, Each panel shows a representative Western blot and a (B) densitometric calculation of protein expression. (***P < .001 compared with untreated control follicles).
Figure 4.
Effect of ghrelin on the expression of 3β hydroxysteroid dehydrogenase (HSD; 42 kDa), 17β-HSD (34.5 kDa), and cytochrome P450 aromatase (CYP19; 50 kDa) in medium-sized ovarian follicles stimulated with (A and B) follicle stimulating hormone (FSH) or (C and D) luteinizing hormone (LH). Follicles were incubated with 20, 100, or 500 pg/mL ghrelin in the presence of FSH (100 ng/mL) or LH (100 ng/mL), and the expression of 3 proteins was assayed by Western blotting. A and C, Each panel shows a representative Western blot and a (B and D) densitometric calculation of protein expression. Different letters indicate significant differences among each steroid enzymes’ protein expression (P < .001).
Discussion
This study showed that ghrelin significantly decreased steroid secretion by ovarian follicles, independent of follicle size, in cycling pigs and that it directly inhibited follicular expression of 3 proteins, 3β-HSD, 17β-HSD, and CYP19, involved in steroid synthesis. We previously showed that, in prepubertal pigs, ghrelin stimulated estradiol secretion by increasing CYP19 protein expression.21 Thus, taken together, these findings indicate that ghrelin plays different roles in ovarian steroidogenesis in cycling and prepubertal pigs. This difference is likely associated with differences in steroid hormonal milieu during puberty and the estrus cycle and not with differences in ghrelin concentrations in ovarian follicles. We suggest that difference in ghrelin action on porcine ovarian steroidogenesis is dependent on animal reproductive status and ghrelin/GHSR-1a receptor regulation by growth hormone,28 insulin growth factor (IGF),29 glucocorticoids,30 thyroid hormone,31 and sex steroids.32 Interventions associated with estrogen level variations (eg, ovariectomy and estradiol treatment) influence changes in ghrelin levels in the pubertal stage in females.
In the present study, it is observed that inhibitory effect of ghrelin on basal and gonadotropin-stimulated steroidogenesis is in good agreement with results showing that injection of ghrelin significantly reduced the serum concentration of E2 through an estrous cycle in rats and decreased estrogen receptor β (ERβ) mRNA expression in the ovary.33 In addition, ghrelin was found to inhibit secretion of E2 in cultured human luteinizing granulosa cells collected from women with infertility due to uni- or bilateral tubal disorder.34 Moreover, ghrelin was shown to inhibit secretion of P4 in porcine luteal cells by inhibiting 3β-HSD activity and protein expression17 and to inhibit basal and human chorionic gonadotropin -stimulated P4 secretion by human luteal cells.16 Also, in cultured chicken ovarian cells35 and fragments of ovaries isolated from adult transgenic rabbits,36 ghrelin reduced ovarian hormone secretion. Pretreatment of rabbits with ghrelin stimulated, suppressed, or even reversed subsequent LH and IGF-I effects on hormone secretion by cultured granulosa cells.37
The present study indicated very clearly that ghrelin may be one of the hormones responsible for regulation of ovarian steroidogenesis in mature pig. Similar to other appetite hormones or adipokines, ghrelin has autocrine or paracrine effects on porcine ovarian steroid synthesis. Obestatin, a newly discovered metabolic hormone produced in the stomach, directly controls porcine ovarian cell functions: it can stimulate proliferation (accumulation of proliferating cell nuclear antigen, cyclin B1, and mitogen-activated protein kinase), apoptosis (expression of p53, caspase 3, and Bax), and the secretion of P4.38 Opposite effect of adiponectin on porcine ovarian steroid synthesis was observed in prepubertal39 and cycling animals.40 In granulosa cells collected from prepubertal pigs, adiponectin modulates steroid synthetic protein gene expression, increasing steroidogenic acute regulatory protein transcript abundance and reducing CYPP450 aromatase.39 A previously published study by Maleszka et al40 demonstrated that in cultured porcine granulosa cells, adiponectin has no effect on basal and FSH-stimulated P4 secretion but increased basal not FSH-stimulated E2 secretion. Additionally, in porcine theca interna cells, adiponectin reduced basal secretion of T and has no effect on secretion of P4, androstenedione, and E2.40 Next, resistin also directly regulated porcine ovarian function, but the effect on steroid secretion is similar in prepubertal and mature animals. In ovary collected from both prepubertal and mature pigs, resistin increased basal41,42 but decreased gonadotropin-stimulated androgen secretion (unpublished results). Stimulatory action of leptin-induced FSH and LH on E2 production during the early and mid-follicular phase and on P4 production just before ovulation, respectively, has been observed in adult pigs during the estrous cycle,43 which suggested that leptin positively affects the estrus cycle in pigs. The presented date clearly showed that ghrelin at 100 and 500 pg/mL doses, by direct inhibition of 3β-HSD, 17β-HSD, and CYP19 protein expression, decreased gonadotropin-stimulated steroid secretion by ovarian follicles, thus negatively affecting the ovarian physiology in mature pigs. Interestingly, ghrelin at 20 pg/mL had no effect on basal steroidogenesis, while in gonadotropin-stimulated cultures, lack of steroids secretion but inhibitory effect on protein expression of steroidogenic enzymes was observed. This is probably connected with the fact that steroidogenic response of follicular cells to gonadotropins may be modulated by other endocrine and paracrine factors, for example, ovarian steroids, inhibin, activin, and various peptide factors.44 Additionally, it is probably due to short time period of incubation and the lowest doses of ghrelin. A certain period of time is necessary from the stimulation of the expression of enzymes’ protein to visible effect on steroids secretion.
In summary, we found that ghrelin directly inhibits the expression of the steroidogenic enzymes 3β-HSD, 17β-HSD, and CYP19, resulting in a downregulation of steroid hormone secretion from the ovarian follicles of cycling pigs. Inhibition of LH- and FSH-stimulated steroid secretion by ghrelin in ovarian follicles may negatively affect the regular estrous cycle in these animals.
Acknowledgments
The authors wish to thank Monika Dubiel for her technical support in performing ELISAs.
Footnotes
Authors’ Note: This study was presented in part at the 3rd Winter Workshop of the Society for Biology of Reproduction, January 1 and 2, 2013, Zakopane, Poland, and 3rd World Congress of Reproductive Biology, September 02-04, 2014, Edinburgh, Scotland.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article: This study was supported partly by the DS/MND/WBiNoZ/IZ/8/2012, and partly by K/ZDS/004194 Jagiellonian University in Krakow, Poland.
Reference
- 1. Caminos JE, Tena-Sempere M, Gaytán F, et al. Expression of ghrelin in the cyclic and pregnant rat ovary. Endocrinology. 2003;144(4):1594–1602. [DOI] [PubMed] [Google Scholar]
- 2. Du C, Xilingaowa, Cao G, Wang C, et al. Expression of the orexigenic peptide ghrelin in the sheep ovary. Dom Anim Endocrinol. 2009;36(2):89–98. [DOI] [PubMed] [Google Scholar]
- 3. Du C, Li H, Cao G, Xilingaowa, Wang C, Li C. Expression of the orexigenic peptide ghrelin and the type 1a growth hormone secretagogue receptor in sheep oocytes and pre-implantation embryos produced in vitro. Reprod Dom Anim. 2010;45(1):92–98. [DOI] [PubMed] [Google Scholar]
- 4. Gaytan F, Barreiro M, Chopin L, et al. Immunolocalization of ghrelin and its functional receptor, the type 1a growth hormone secretagogue receptor, in the cyclic human ovary. J Clin Endocrinol Metab. 2003;88(2):879–887. [DOI] [PubMed] [Google Scholar]
- 5. Ishikawa T, Fujioka H, Ishimura T, Takenaka A, Fujisawa M. Ghrelin expression in human testis and serum testosterone level. J Androl. 2007;28(2):320–324. [DOI] [PubMed] [Google Scholar]
- 6. Gualillo O, Caminos J, Blanco M, et al. Ghrelin, a novel placental-derived hormone. Endocrinology. 2001;142(2):788–794. [DOI] [PubMed] [Google Scholar]
- 7. Tawadros N, Salamonsen LA, Dimitriadis E, Chen C. Facilitation of decidualization by locally produced ghrelin in the human endometrium. Mol Hum Reprod. 2007;13(7):483–489. [DOI] [PubMed] [Google Scholar]
- 8. Rak-Mardyla A. Ghrelin role in hypothalamus-pituitary-ovarian axis. J Physiol Pharmacol. 2013;64(6):695–704. [PubMed] [Google Scholar]
- 9. Lu S, Guan JL, Wang QP, et al. Immunocytochemical observation of ghrelin-containing neurons in the rat arcuate nucleus. Neurosci Lett. 2002;321(3):157–160. [DOI] [PubMed] [Google Scholar]
- 10. Szczepankiewicz D, Skrzypski M, Pruszynska-Oszmalek E, et al. Importance of ghrelin in hypothalamus-pituitary axis on growth hormone release during normal pregnancy in the rat. J Physiol Pharmacol. 2010;61(4):443–449. [PubMed] [Google Scholar]
- 11. Furuta M, Funabashi T, Kimura F. Intracerebroventricular administration of ghrelin rapidly suppresses pulsatile luteinizing hormone secretion in ovariectomized rats. Biochem Biophys Res Commun. 2001;288(4):780–785. [DOI] [PubMed] [Google Scholar]
- 12. Iqbal J, Kurose Y, Canny B, Clarke IJ. Effects of central infusion of ghrelin on food intake and plasma levels of growth hormone, luteinizing hormone, prolactin, and cortisol secretion in sheep. Endocrinology. 2006;147(1):510–519. [DOI] [PubMed] [Google Scholar]
- 13. Vulliémoz NR, Xiao E, Xia-Zhang L, Germond M, Rivier J, Ferin M. Decrease in luteinizing hormone pulse frequency during a five-hour peripheral ghrelin infusion in the ovariectomized rhesus monkey. J Clin Endocrinol Metab. 2004;89(11):5718–5723. [DOI] [PubMed] [Google Scholar]
- 14. Kluge M, Schusser P, Uhr M, Yassouridis A, Steiger A. Ghrelin suppress secretion of luteinining hormone in humans. J Clin Endocrinol Metab. 2007;92(8):3202–3205. [DOI] [PubMed] [Google Scholar]
- 15. Lanfranco F, Bonelli L, Baldi M, Me E, Broglio F, Ghigo E. Acylated ghrelin inhibits spontaneous luteinizing hormone pulsatility and responsiveness to naloxone but not that to gonadotropin-releasing hormone in young men: evidence for a central inhibitory action of ghrelin on the gonadal axis. J Clin Endocrinol Metab. 2008;93(9):3633–3639. [DOI] [PubMed] [Google Scholar]
- 16. Tropea A, Tiberi F, Minici F, et al. Ghrelin affects the release of luteolytic and luteotropic factors in human luteal cells. J Clin Endocrinol Metab. 2007;92(8):3239–3245. [DOI] [PubMed] [Google Scholar]
- 17. Rak-Mardyła A, Gregoraszczuk EL, Karpeta A, Duda M. Expression of ghrelin and the ghrelin receptor in different stages of porcine corpus luteum development and the inhibitory effects of ghrelin on progesterone secretion, 3β-hydroxysteroid dehydrogenase (3β-HSD) activity and protein expression. Theriogenology. 2012;77(8):1505–1512. [DOI] [PubMed] [Google Scholar]
- 18. Zhang W, Lei Z, Su J, Chen S. Expression of ghrelin in the porcine hypothalamo-pituitary-ovary axis during the estrous cycle. Anim Reprod Sci. 2008;109(1-4):356–367. [DOI] [PubMed] [Google Scholar]
- 19. Rak-Mardyła A, Gregoraszczuk E. Expression of ghrelin and its receptor during different physiological stages in pig ovary. J Physiol Pharmacol. 2012;63(2):95–99. [PubMed] [Google Scholar]
- 20. Rak A, Gregoraszczuk EL. Ghrelin levels in prepubertal pig ovarian follicles. Act Vet Hung. 2009;57(1):109–113. [DOI] [PubMed] [Google Scholar]
- 21. Rak A, Gregoraszczuk EL. Modulatory effect of ghrelin in prepubertal porcine ovarian follicles. J Physiol Pharmacol. 2008;59(4):781–793. [PubMed] [Google Scholar]
- 22. Rak-Mardyła A, Gregoraszczuk E. ERK 1/2 and PI-3 kinase pathways as a potential mechanism of ghrelin action on cell proliferation and apoptosis in the porcine ovarian follicular cells. J Physiol Pharmacol. 2010;61(4):451–458. [PubMed] [Google Scholar]
- 23. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–660. [DOI] [PubMed] [Google Scholar]
- 24. Gregoraszczuk E, Bylica A, Gertler A. Response of porcine theca and granulosa cells to GH during short-term in vitro culture. Anim Reprod Sci. 2000;58(1–2):113–125. [DOI] [PubMed] [Google Scholar]
- 25. Akins EL, Morrisette MC. Gross ovarian changes during estrus cycle of swine. Amer J Vet Res. 1968;29(10):1952–1955. [PubMed] [Google Scholar]
- 26. Li L, Ferin M, Sauer MV, Lobo RA. Serum and follicular fluid ghrelin levels negatively reflect human oocyte quality and in vitro embryo development. Fertil Steril. 2011;96(4):1116–1120. [DOI] [PubMed] [Google Scholar]
- 27. Govoni N, De Iasio R, Cocco C, et al. Gastric immunolocalization and plasma profiles of acyl-ghrelin in fasted and fasted-refed prepuberal gilts. J Endocrinol. 2005;186(3):505–513. [DOI] [PubMed] [Google Scholar]
- 28. Nass R, Gilrain J, Anderson S, et al. High plasma growth hormone (GH) levels inhibit expression of GH secretagogue receptor messenger ribonucleic acid levels in the rat pituitary. Endocrinology. 2000;14:2084–2089. [DOI] [PubMed] [Google Scholar]
- 29. Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Oikawa S. Insulin-like growth factor-I down-regulates ghrelin receptor (growth hormone secretagogue receptor) expression in the rat pituitary. Regul Pept. 2005;127(1–3):203–206. [DOI] [PubMed] [Google Scholar]
- 30. Thomas G, Bennett P, Carmignac D, Robinson I. Glucocorticoid regulation of growth hormone (GH) secretagogue induced growth responses and GH secretagogue receptor expression in the rat. Growth Horm IGF Res. 2000;10(1):45–52. [DOI] [PubMed] [Google Scholar]
- 31. Kamegai J, Tamura H, Ishii S, Sugihara H, Wakabayashi I. Thyroid hormones regulate pituitary growth hormone secretagogue receptor gene expression. J Neuroendocrinol. 2001;13(3):275–278. [DOI] [PubMed] [Google Scholar]
- 32. Kamegai J, Wakabayashi I, Kineman R, Frohman L. Growth hormone-releasing hormone receptor (GHRH-R) and growth hormonesecretagogue receptor (GHS-R) mRNA levels during postnatal development in male and female rats. J Neuroendocrinol. 1999;11(4):299–306. [DOI] [PubMed] [Google Scholar]
- 33. Fang F, Wang L, Zhang Y, Li Y, Su S, Zhang X. Role of ghrelin on estrogen and progesterone secretion in the adult rat ovary during estrous cycle. Syst Biol Reprod Med. 2012;58(2):116–119. [DOI] [PubMed] [Google Scholar]
- 34. Viani I, Vottero A, Tassi F, et al. Ghrelin inhibits steroid biosynthesis by cultured granulosa-lutein cells. J Clin Endocrinol Metab. 2008;93(4):1476–1481. [DOI] [PubMed] [Google Scholar]
- 35. Sirotkin AV, Grossmann R. Effects of ghrelin and its analogues on chicken ovarian granulosa cells. Domest Anim Endocrinol. 2008;34(2):125–134. [DOI] [PubMed] [Google Scholar]
- 36. Sirotkin AV, Chrenek P, Darlak K, Valenzuela F, Kuklova Z. Some endocrine traits of transgenic rabbits. II. Changes in hormone secretion and response of isolated ovarian tissue to FSH and ghrelin. Physiol Res. 2008;57(5):745–751. [DOI] [PubMed] [Google Scholar]
- 37. Sirotkin AV, Rafay J, Kotwica J, Darlak K, Valenzuela F. Role of ghrelin in regulating rabbit ovarian function and the response to LH and IGF-I. Domest Anim Endocrinol. 2009;36(3):162–172. [DOI] [PubMed] [Google Scholar]
- 38. Mészárosová M, Sirotkin AV, Grossmann R, Darlak K, Valenzuela F. The effect of obestatin on porcine ovarian granulosa cells. Anim Reprod Sci. 2008;108(1–2):196–207. [DOI] [PubMed] [Google Scholar]
- 39. Ledoux S, Campos DB, Lopes FL, Dobias-Goff M, Palin MF, Murphy BD. Adiponectin induces periovulatory changes in ovarian follicular cells. Endocrinology. 2006;147(11):5178–5186. [DOI] [PubMed] [Google Scholar]
- 40. Maleszka A, Smolinska N, Nitkiewicz A, et al. Adiponectin Expression in the Porcine Ovary during the Oestrous Cycle and Its Effect on Ovarian Steroidogenesis. Int J Endocrinol. 2014;2014:957076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rak-Mardyła A, Duda M, Gregoraszczuk EL. A role for resistin in the ovary during the estrous cycle. Horm Metab Res. 2014;46(7):1–6. [DOI] [PubMed] [Google Scholar]
- 42. Rak-Mardyła A, Durak M, Gregoraszczuk EL. Effects of resistin on porcine ovarian follicle steroidogenesis in prepubertal animals: an in vitro study. Reprod Biol Endocrinol. 2013;11:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gregoraszczuk EŁ, Wójtowicz AK, Ptak A, Nowak K. In vitro effect of leptin on steroids’ secretion by FSH- and LH-treated porcine small, medium and large preovulatory follicles. Reprod Biol. 2003;3(3):227–239. [PubMed] [Google Scholar]
- 44. Hanukoglu I. Steroidogenic enzymes: structure, function, and role in regulation of steroid hormone biosynthesis. J Steroid Biochem Mol Biol. 1992;43(8):779–804. [DOI] [PubMed] [Google Scholar]