Significance
Sialic acids are terminal glycan structures present on cellular glycoproteins and often overexpressed on certain pathogens and tumors. Sialic acids interact with sialic acid-binding Ig-type lectin (siglec) receptors, suggesting a potential regulatory role in homeostasis or pathology-mediated immune modulation. Here, we show that modification of antigens with sialic acids alters their immunogenicity. Sialylated antigens impose a regulatory program on dendritic cells (DCs) via Siglec-E. DCs loaded with sialylated antigens induce de novo regulatory T (Treg) cells and inhibit the generation of new effector T cells as well as the function of existing ones. This dual tolerogenic DC function is maintained under inflammatory conditions and, therefore, sialylation of antigens could provide a novel way to induce antigen-specific immune tolerance to treat patients who suffer from autoimmunity and allergies.
Keywords: sialic acids, regulatory T cells, dendritic cells, tolerance, glycans
Abstract
Sialic acids are negatively charged nine-carbon carboxylated monosaccharides that often cap glycans on glycosylated proteins and lipids. Because of their strategic location at the cell surface, sialic acids contribute to interactions that are critical for immune homeostasis via interactions with sialic acid-binding Ig-type lectins (siglecs). In particular, these interactions may be of importance in cases where sialic acids may be overexpressed, such as on certain pathogens and tumors. We now demonstrate that modification of antigens with sialic acids (Sia-antigens) regulates the generation of antigen-specific regulatory T (Treg) cells via dendritic cells (DCs). Additionally, DCs that take up Sia-antigen prevent formation of effector CD4+ and CD8+ T cells. Importantly, the regulatory properties endowed on DCs upon Sia-antigen uptake are antigen-specific: only T cells responsive to the sialylated antigen become tolerized. In vivo, injection of Sia-antigen–loaded DCs increased de novo Treg-cell numbers and dampened effector T-cell expansion and IFN-γ production. The dual tolerogenic features that Sia-antigen imposed on DCs are Siglec-E–mediated and maintained under inflammatory conditions. Moreover, loading DCs with Sia-antigens not only inhibited the function of in vitro–established Th1 and Th17 effector T cells but also significantly dampened ex vivo myelin-reactive T cells, present in the circulation of mice with experimental autoimmune encephalomyelitis. These data indicate that sialic acid-modified antigens instruct DCs in an antigen-specific tolerogenic programming, enhancing Treg cells and reducing the generation and propagation of inflammatory T cells. Our data suggest that sialylation of antigens provides an attractive way to induce antigen-specific immune tolerance.
Antigen-presenting cells (APCs), and predominantly dendritic cells (DCs), are crucial to maintain immune homeostasis, because these cells determine whether or not an immune reaction is mounted against pathogens or self or innocuous foreign antigens. In healthy individuals, encounter with innocuous (self-)antigens will lead to induction of regulatory T (Treg) cells, which in turn dampen excessive immune responses, a process that fails in autoimmune patients (1).
Ways to promote this immune-inhibitory signature of DCs may therefore provide new therapeutic strategies to fight autoimmune disorders. Although different methods are described to push DCs into an immune-inhibitory mode, none of these methods is antigen-specific and may therefore exert off-target effects. Additional evidence suggests that merely generating a large pool of Treg cells is not sufficient to control aberrant immunity: the inflammatory milieu in the affected areas promotes a strong APC-mediated activation of antigen-specific autoreactive T cells, which makes these autoreactive T cells refractory to suppression by Treg cells (2–5). These findings suggest that strategies that aim at controlling autoimmunity should not only involve inducing Treg-cell populations but also dampening autoreactive T cells, thereby creating a milieu that allows Treg cells to exert their suppressive function. Certain pathogenic infections and tumors are marked by the presence of tolerogenic DCs and T cells (6–9). An often overlooked feature shared by these pathological conditions is the presence of aberrant glycosylation patterns (7, 10–12). In particular, increased levels of sialic acids have been demonstrated in these situations. On tumors, enhanced sialylation driven by enhanced expression and activity of β-galactoside α2,6-sialyltransferase 1 (ST6Gal-1) and/or α-N-acetylgalactosaminide α2,6-sialyltransferase 1 (ST6GalNAc-I) often correlates with tumor invasion and poor prognosis (13–15). Additionally, the hypersialylated pathogens Campylobacter jejuni and Neisseria meningitides were shown to negatively affect human APC function and consequently subvert immune responses (7, 16–19).
Sialic acids are the outermost monosaccharides on glycan chains of glycoproteins and glycolipids, attached to the underlying glycans with α2,3, α2,6, or α2,8 linkage (14) and as such form the recognition elements for sialic acid-binding Ig-like lectins (siglecs) (14, 20). Siglecs are predominantly expressed by innate immune cells, such as DCs, macrophages, and B cells (20). On these cells, siglecs function as endocytic receptors as well as can regulate activation status and cytokine secretion. Most siglecs are characterized by the presence of one or more immunoreceptor tyrosine-based inhibitory motifs (ITIMs) in their intracellular domain (21) and, thus, siglec triggering often counteracts activatory signals elicited by receptors containing immunoreceptor tyrosine-based activatory motifs (ITAMs) (20). Although engagement of the hCD33rSiglecs on innate cells by sialylated antigens has been shown to negatively modulate the proinflammatory functions of APCs, effects on T-cell responses have not yet been investigated in detail.
Because the immune-inhibitory effects induced by sialylated pathogens and tumors may be attributed to different configurations of sialic acid-containing glycoproteins or glycolipids, we set out to characterize the effects of sialic acids on DCs and T-cell responses using a well-characterized neoglycoconjugate approach based on the model antigens ovalbumin (OVA) or the encephalitogenic peptide derived from myelin oligodendrocyte glycoprotein (MOG35–55) that we modified with either α2,3- or α2,6-linked sialyl-lactose (hereafter named Sia-antigens). Our data reveal that internalization of Sia-antigen by DCs endows them with the ability to promote the differentiation of naive CD4+ T cells into Treg cells at the expense of functional CD4+ and CD8+ effector T cells, both in vitro and in vivo. We provide evidence that this feature is antigen-specific and even effective under inflammatory conditions. Moreover, our findings demonstrate that Sia-antigen–loaded DCs also dampen the function of established effector T cells, suggesting that sialylation of antigens provides a means to dampen excessive T-cell pathologies.
Results
Sia-Antigen–Pulsed DCs Promote de Novo Induction of Foxp3+CD4+ Treg Cells.
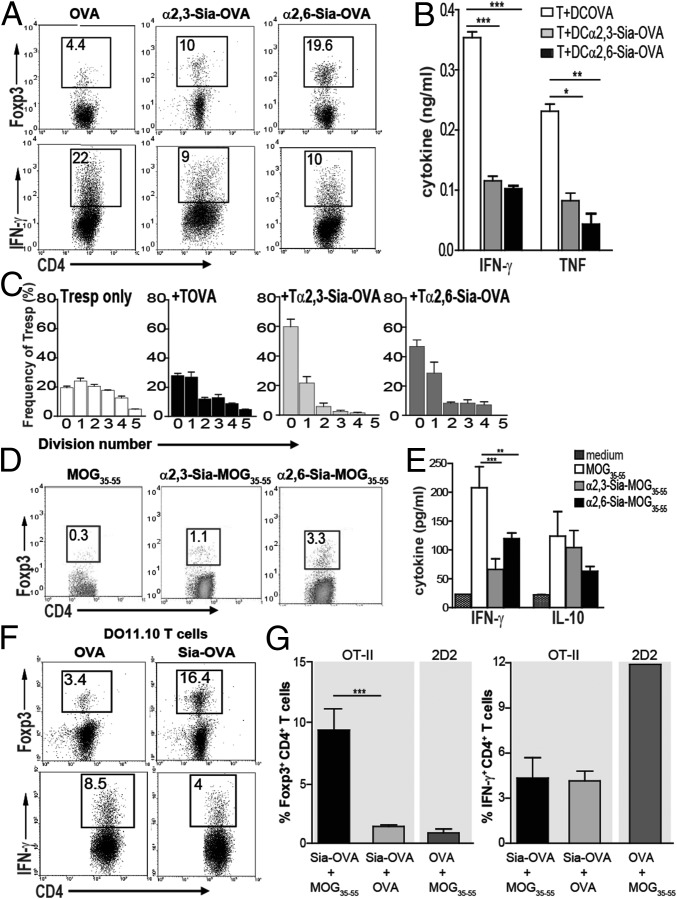
Because hypersialylated tumors and pathogens have been linked with tolerogenic DCs and T cells, we hypothesized that sialic acids present on glycosylated antigens may serve as an inhibitory signal and down-modulate inflammatory T-cell responses. To examine whether T-cell polarization is influenced by DCs exposed to sialylated antigens, we generated neoglycoconjugates by maleimide-thiol coupling of α2,3- or α2,6-linked sialyl-lactose to either OVA (yielding α2,3- or α2,6-Sia-OVA, respectively, Fig. S1). Subsequently, splenic CD11c+ DCs were pulsed with α2,3- or α2,6-Sia-OVA and cocultured with naive CD4+CD62LhiCD25− OT-II T cells. In these DC–T-cell cocultures, the Sia-OVA–pulsed DCs induced a two- to fivefold increase in Foxp3+CD4+ T-cell numbers (Fig. 1A) and reduced the percentage of IFN-γ–producing effector T cells compared with OVA-pulsed DCs that did not differentiate naive T cells into Foxp3+ T cells but into IFN-γ–producing effector T cells instead (Fig. 1A). These data were further strengthened by reduced TNF and IFN-γ levels in supernatants from Sia-OVA–pulsed DC cocultures (Fig. 1B). Comparable results were obtained using bone marrow-derived DCs (BMDCs) (Fig. S2A). Functional analysis of the induced Foxp3+CD4+ T cells revealed the presence of suppressive properties (Fig. 1C). Addition of T cells primed with Sia-OVA–loaded DCs markedly inhibited the proliferation of carboxyfluorescein succinimidyl ester (CFSE)-labeled responder T (Tresp) cells: only a few cells underwent one to two divisions, and the majority did not divide (Fig. 1C). In contrast, CD4+ T cells primed with OVA-pulsed DCs (Tova cells) did not inhibit the proliferation of Tresp cells. Under these conditions, and similarly to control cultures to which no T cells were added, Tresp cells were able to undergo up to five cell divisions.
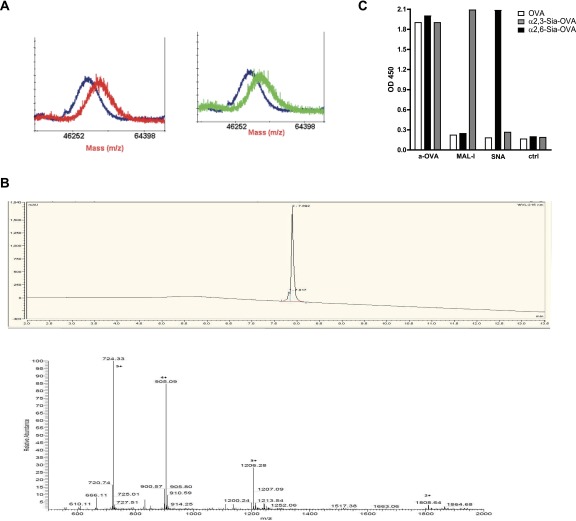
Fig. S1.
Detection of α2,3- and α2,6-linked sialic acids on sialylated antigens. (A) The presence of α2,3- or α2,6-linked sialic acids on OVA was analyzed by MALDI-TOF comparing the mass of the nonmodified OVA (blue line) to the α2,3- (red line) or α2,6-coupled OVA (green line). We confirmed an average substitution of 5.5 sialic acids per OVA. (B) We confirmed the purity (Upper) and mass (Lower) of Sia-MOG by HPLC (Upper, purity > 90%) and mass spectrometry (Lower, visible m/z: 1808 [M+2H]2+; 1206 [M+3H]3+; 905 [M+4H]4+; 724 [M+5H]5+). (C) The antigens were coated, and the presence of α2,3- or α2,6-linked sialic acids was detected by ELISA using MAL-I and SNA lectins, respectively. Additionally, OVA was detected using an anti-OVA antibody. Data are means ± SEM of duplicate measurements and representative of five independent experiments.
Fig. 1.
Sia-OVA promotes DC-mediated Treg-cell differentiation and prevents effector T-cell generation. (A) Foxp3 and IFN-γ expressed by OT-II T cells cocultured with OVA-, α2,3-Sia-OVA–, or α2,6-Sia-OVA–loaded splenic DCs. The percentage of positive cells is indicated (n = 5). (B) IFN-γ and TNF in supernatants of DC-differentiated OT-II T cells (mean ± SEM; n = 5). (C) Suppressive activity of T cells primed with OVA-, α2,3-Sia-OVA–, or α2,6-Sia-OVA–loaded DCs in a coculture with CFSE-labeled CD4+ Tresp cells (mean frequency Tresp cells/division ± SEM; n = 2). (D) Foxp3 expression in 2D2 T cells differentiated by MOG35–55–, α2,3-Sia-MOG35–55–, or α2,6-Sia-MOG35–55–loaded DCs (n = 3). (E) TNF and IL-10 in supernatants of DC-differentiated 2D2 T cells (mean ± SEM; n = 3). (F) Foxp3 and IFN-γ expression by DO11.10 CD4+ T cells upon coculture with Sia-OVA– or OVA-loaded DCs. The percentage of positive cells is indicated (mean ± SEM; n = 2). (G) Naive CD4+ 2D2 and OT-II T cells were cocultured with DCs loaded with different antigens. The percentage of Foxp3+ and IFN-γ+ T cells is depicted (mean ± SEM; n = 2). ***P < 0.001; **P < 0.01; *P < 0.05.
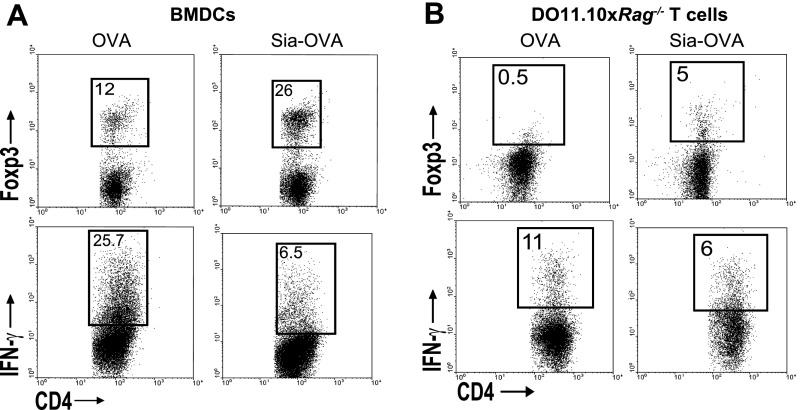
Fig. S2.
Sia-OVA–loaded BMDCs promote de novo Foxp3+ T-cell generation and prevents effector T-cell generation. (A and B) Flow cytometric detection of Foxp3 and IFN-γ in OT-II (A) or DO11.10×Rag−/− CD4+ (B) T cells cocultured with OVA-, α2,3-Sia-OVA, or α2,6-Sia-OVA–loaded BMDCs. Percentages of positive cells are indicated (n = 5 and n = 2).
Similarly, sialylation of MOG35–55, a well-known target of autoreactive T cells in experimental autoimmune encephalomyelitis (EAE), a murine model of multiple sclerosis (22), transformed this peptide into a tolerogenic antigen. DCs pulsed with α2,3- or α2,6-Sia-MOG35–55 induced naive MOG-responsive CD4+ 2D2 T cells (23) to express Foxp3 and prevented differentiation into IFN-γ–producing effector T cells (Fig. 1 D and E). Notably, CD4+ T cells primed by Sia-MOG35–55 do not produce more IL-10 than MOG35–55–loaded DCs (Fig. 1E). Because both α2,3- and α2,6-linked Sia-antigens evoked equivalent tolerogenic responses, only the data for α2,6-linked Sia-antigens, designated Sia-OVA or Sia-MOG35–55, are shown for the remainder of the study.
Sia-OVA had comparable effects on naive T-cell polarization using BMDCs isolated from BALB/c mice as C57BL/6-derived DCs: 16% of DO11.10 T cells cocultured with Sia-OVA-BMDCs were committed to the Foxp3+ lineage, whereas almost fivefold fewer T cells became Foxp3+ when cocultured with OVA-loaded BMDCs (Fig. 1F). DO11.10 T cells primed with OVA-loaded BMDCs became committed to the IFN-γ–producing Th1 lineage (8.5%; Fig. 1F). These data indicate that sialylated antigens induced the maturation of Foxp3+ T cells irrespective of a Th1- or Th2-prone microenvironment. Using CD4+ T cells from DO11.10×Rag−/− mice, we established that the Sia-OVA-DC–induced Foxp3+CD4+ T cells were de novo-generated (Fig. S2B). We next assessed whether the immunosuppressive properties gained by DCs upon internalization of sialylated antigens are antigen-specific or whether these DCs become broad immunosuppressive and also silence unrelated T-cell responses. Hereto, DCs loaded with Sia-OVA and nonmodified MOG35–55 were subsequently cocultured with purified CD4+ 2D2 T cells. As a control, DCs were loaded with OVA and MOG or cocultured with naive CD4+ OT-II T cells. Clearly, 2D2 T cells are not polarized into Foxp3+ T cells by DCs pulsed with MOG and the nonrelated Sia-OVA (Fig. 1G). The 2D2 T cells become IFN-γ–producing effector T cells, similarly as when encountering DCs pulsed with MOG35–55 only (Fig. 1E). Only OT-II T cells become tolerized when encountering Sia-OVA–pulsed DCs, even when these DCs internalized additional nonmodified antigens. Together, these data reveal that uptake of sialic acid-modified antigens by DCs results in the generation of tolerogenic DCs that promote de novo Treg-cell induction and prevent effector T-cell generation in an antigen-specific fashion.
Induction of Immune Tolerance Is Specific for Sialic Acid Carbohydrate Structures.
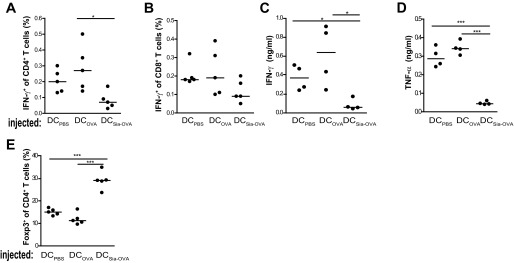
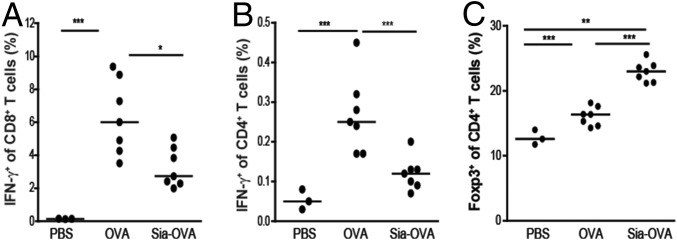
Given these specific tolerogenic effects of Sia-antigen–loaded DCs on T cells in vitro, we next examined whether administration into C57BL/6 mice also resulted in immune tolerance in vivo. Indeed, adoptive transfer of Sia-OVA–pulsed DCs into C57BL/6 mice before immunization significantly reduced IFN-γ–producing CD4+ and CD8+ T-cell numbers, which was mirrored by low amounts of TNF and IFN-γ in the culture supernatants of ex vivo-restimulated splenocytes (Fig. S3 A–D). These effects were not induced by injecting OVA-pulsed DCs. Importantly, the reduction in effector T-cell numbers in Sia-OVA-DC–injected mice was accompanied by significantly higher frequencies of Foxp3+CD4+ T cells (Fig. S3E). Thus, DCs that have internalized sialylated antigens also mediate T-cell tolerance in vivo. Because these data confirmed that in vitro-generated Sia-OVA–loaded DCs maintain the capacity to induce suppressive Treg cells in vivo, we continued to investigate whether administration of Sia-OVA could modulate endogenous DCs. For this purpose, C57BL/6 mice were injected with Sia-OVA 1 wk before OVA immunization. Pretreatment of the mice with Sia-OVA dampened subsequent induction of effector T-cell formation, as indicated by the lower proportions of IFN-γ–producing CD8+ and CD4+ T cells detected ex vivo (Fig. 2 A and B). This inhibitory effect was not observed in mice that received OVA or PBS. Notably, inhibition of effector T-cell expansion in mice injected with Sia-OVA was accompanied by a higher frequency of Foxp3+CD4+ T cells (Fig. 2C). Thus, DCs also become tolerogenic in vivo upon uptake of Sia-OVA, resulting in Treg-cell expansion and effector T-cell dampening.
Fig. S3.
Injection of Sia-OVA–loaded DCs prevents the effector immune response in vivo. For adoptive transfer of antigen-loaded DCs, the DCs were pulsed overnight with 200 µg/mL Sia-OVA or OVA, and 3 × 105 DCs were injected i.v. into each recipient mouse. On day 7, mice were immunized s.c. with 100 µg of OVA/50 µg of CpG. Frequencies of IFN-γ–expressing CD4+ T cells (A) and CD8+ T cells (B) in spleen were determined. Dots represent individual mice (n = 5/group); bars indicate the median. (C and D) IFN-γ and TNF secreted by PMA/ionomycin-restimulated splenocytes (mean ± SEM; n = 5/group). (E) Frequency of Foxp3+ among CD4+ splenocytes. Dots represent individual mice (n = 5/ group); bars indicate the median. *P < 0.05; ***P < 0.001.
Fig. 2.
Sia-OVA induces tolerance and prevents the effector immune response in vivo. C57BL/6 mice received 50 µg of Sia-OVA or OVA 1 wk before sensitization with OVA/poly(I:C)/anti-CD40. Frequencies of IFN-γ+CD8+ T cells (A), IFN-γ+CD4+ T cells (B), and Foxp3+CD4+T cells (C) among total splenocytes were determined by flow cytometry. Dots represent individual mice (n = 7/group; bars indicate the median). *P < 0.05; ***P < 0.001; ns, not significant.
DCs Become Tolerogenic upon Internalization of Sialylated Antigens.
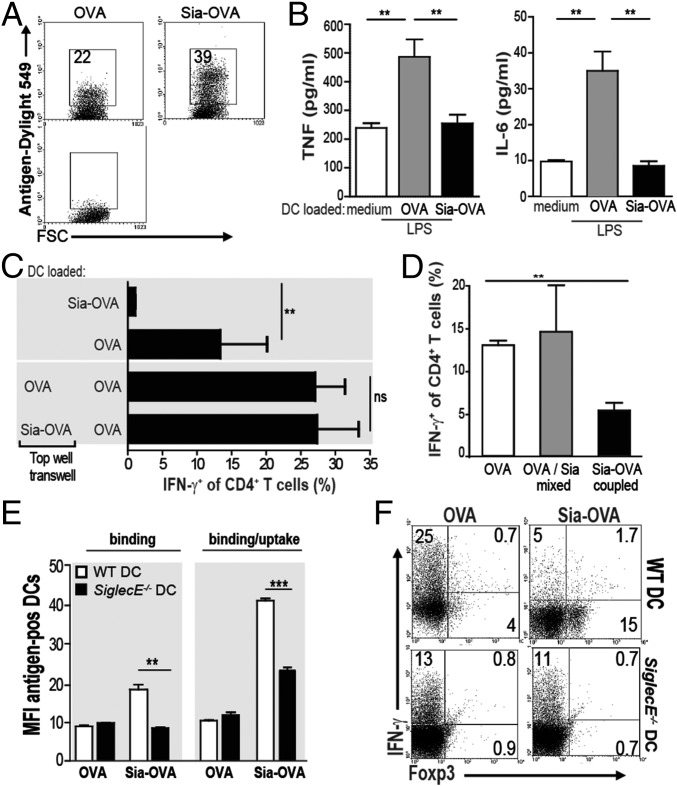
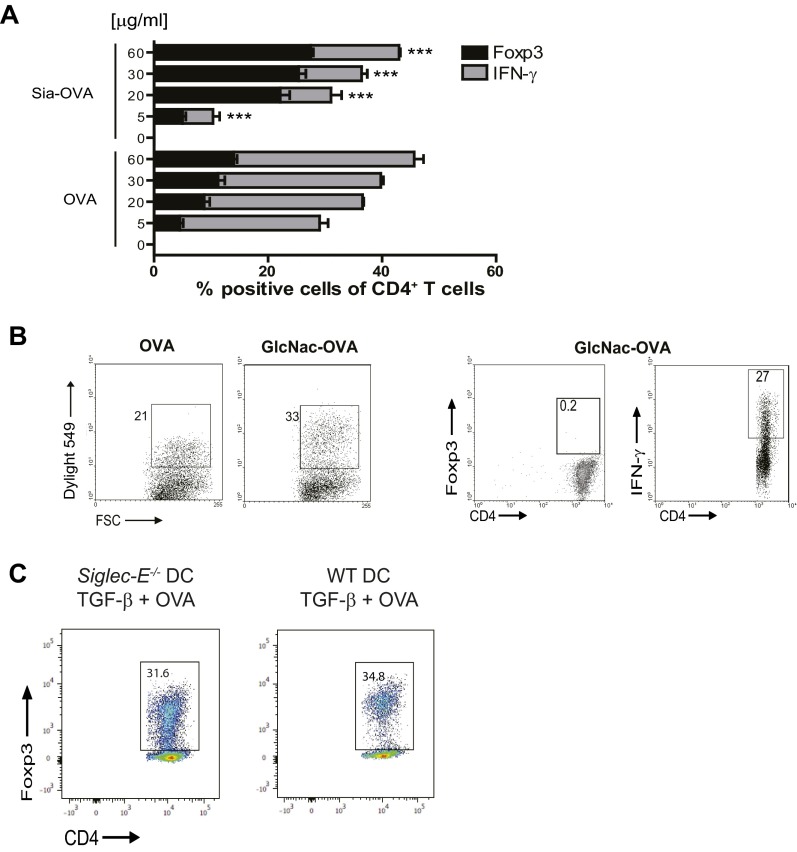
Induction of CD4+ Treg cells is known to predominantly occur after exposure of naive CD4+ T cells to low concentrations of antigens (24). However, sialylation of OVA did not hamper OVA uptake by DCs (Fig. 3A). In fact, higher amounts of Sia-OVA than OVA were taken up by DCs. Although DCs loaded with very low amounts of Sia-OVA (i.e., ≤5 µg/mL) did not promote Foxp3+CD4+ T-cell induction to a greater extent than OVA-loaded DCs, a clear inhibition of effector T-cell generation was observed with these low concentrations of antigen (Fig. S4A). The effects observed on T-cell responses were highly specific for sialic acids. Whereas GlcNAc modification improved internalization of OVA (Fig. S4B and ref. 25) similar to Sia-OVA, DCs loaded with mannose receptor targeting GlcNAc-OVA promoted Th1 cell skewing and not Treg-cell induction (Fig. S4B). Together, these data reveal that the induction of dual tolerogenic features on DCs is specific for sialylated antigens. Because DCs are key orchestrators of T-cell responses, we further examined the effects of Sia-antigen internalization on the instructive signals DCs provide to T cells. We observed that these DCs secreted lower amounts of proinflammatory IL-6 and TNF than DCs loaded with OVA (Fig. 3B). To assess whether the tolerogenic effects of Sia-OVA–loaded DCs are mediated via soluble factors, OVA-loaded DCs and naive OT-II T cells were separated from Sia-OVA–loaded DCs using Transwell chambers. In this case, OVA-loaded DCs did not acquire tolerogenic and suppressive effects during T-cell differentiation (Fig. 3C). These data suggest that Sia-antigen–loaded DCs mediate tolerance in a cell–cell contact-dependent fashion. Notably, DCs only gained tolerogenic properties when sialic acids were covalently coupled to OVA and not when the sialic acids and OVA were provided separately to DCs (Fig. 3D). Thus, although our data demonstrate that antigens modified with sialic acids are efficiently taken up by DCs, it remained unclear how these Sia-antigens relay tolerogenic signals into DCs. We hypothesized that siglecs may play a role in the binding and internalization of Sia-OVA, as well as potentially triggering a signaling cascade that induces modulation of the DC function (21).
Fig. 3.
Sia-OVA-DC–mediated Treg-cell generation depends on antigen dose and soluble factors. (A) Binding/uptake of Dylight549-labeled Sia-OVA or OVA by DCs analyzed using flow cytometry (n = 3). (B) Secretion of TNF and IL-6 by DCs incubated with OVA/LPS or Sia-OVA/LPS (mean ± SEM; n = 5). (C) Frequency of IFN-γ+ OT-II cells after differentiation by DCOVA (lower wells) in the presence or absence or Sia-OVA–loaded DCs in the top well of Transwells (mean ± SEM of triplicates; n = 4). (D) IFN-γ+ OT-II T-cell frequencies induced by BMDCs loaded with OVA, Sia-OVA, or a mixture of OVA and sialic acids (mean ± SEM; n = 3). (E) Mean fluorescence intensity (MFI) of bound and/or internalized Dylight549-labeled Sia-OVA or OVA by WT and Siglec-E−/− DCs. (Means ± SEM; n = 4). (F) Foxp3 and IFN-γ expression in OT-II T cells differentiated by antigen-loaded WT or Siglec-E−/− BMDCs (n = 2). ***P < 0.001; **P < 0.01.
Fig. S4.
Treg-cell generation depends on the dose of sialylated antigens and is intrinsic to sialic acids. (A) Percentage of Foxp3+ and IFN-γ+ in OT-II T cells that were differentiated by DCs pulsed with the indicated concentrations of Sia-OVA or OVA (mean ± SEM; n = 3). (B) Binding/uptake of Dylight549-labeled OVA or GlcNAc-OVA by DCs (n = 4). GlcNAc-OVA–pulsed DCs were cocultured with naive OT-II T cells, and frequencies of Foxp3+ and IFN-γ+ CD4+ T cells were determined (n = 3). (C) Siglec-E−/− or WT BMDCs were treated with 10 ng/mL TGF-β for 24 h and subsequently loaded with OVA (30 µg/mL) prior to coculture with naive OT-II T cells. Five days later, the frequency of Foxp3+ cells was determined using flow cytometry. Numbers in plots indicate the percentage of Foxp3+CD4+ T cells. ***P < 0.001.
Because the CD33-related Siglec-E is expressed on immature murine DCs and is known to preferentially bind α2,3- and α2,6-linked sialic acids (21), we investigated whether Siglec-E could be the receptor on immature murine DCs that binds Sia-OVA. Comparison of the binding and uptake of Sia-OVA by Siglec-E−/− DCs and wild-type (WT) DCs revealed that both processes were substantially reduced in Siglec-E−/− DCs (Fig. 3E). Furthermore, the absence of Siglec-E on DCs profoundly arrested Treg-cell induction in response to Sia-OVA (Fig. 3F): 15% of CD4+ T cells differentiated into Foxp3+ T cells when cocultured with Sia-OVA–loaded WT DCs compared with only 0.5% in cultures containing Sia-OVA–loaded Siglec-E−/− DCs. Moreover, Siglec-E−/− DCs did not prevent the generation of IFN-γ–producing effector CD4+ T cells. In fact, Siglec-E−/− DCs loaded with either native OVA or Sia-OVA equally skewed naive CD4+ T cells toward IFN-γ–producing effector cells (i.e., 13% vs. 11%, respectively; Fig. 3F). To rule out that an intrinsic defect of Siglec-E−/− DCs to promote Treg-cell induction underlies the failure of Siglec-E−/− DCs to induce Treg cells in response to Sia-antigen, we treated immature Siglec-E−/− DCs with TGF-β or IL-10 for 24 h while loading with OVA before coculture with naive CD4+CD62LHigh OT-II cells (26). Analysis of Foxp3 expression showed that cocultures with Siglec-E−/− DCs contained comparable frequencies of Foxp3+ CD4+ T cells as those with WT DCs (Fig. S4C), indicating that Siglec-E−/− DCs are able to induce Treg cells similar to WT DCs.
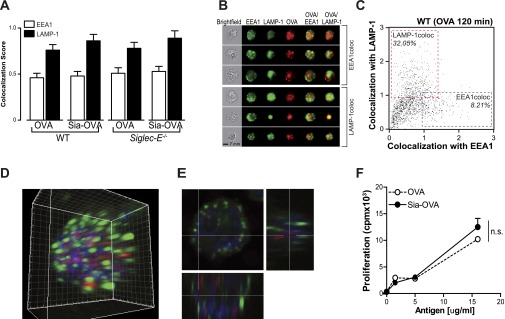
We next investigated the possibility that native and sialylated antigens might follow a different intracellular route, consequently affecting presentation in MHC molecules and T-cell activation. We therefore used imaging flow cytometry, a method that allows high-throughput image analysis of cell in flow with near-confocal resolution, to analyze the intracellular routing of fluorescent labeled Sia-OVA and compare it to the routing of native OVA. Costaining with markers for early endosomal (EEA-1) (open bars) and lysosomal (LAMP-1) (solid bars) compartments illustrated the presence of both Sia-OVA and OVA within late endosomes/lysosomes 2 h after pulse as indicated by the higher colocalization score with LAMP-1 than with EEA-1 (Fig. S5 A and B). Moreover, the percentage of cells with a high OVA–LAMP-1 colocalization was roughly four times higher than of cells with high OVA–EEA-1 colocalization (Fig. S5C). Colocalization of Sia-OVA (red) with EEA-1 (green) and LAMP-1 (blue) was additionally analyzed using a confocal laser-scanning microscopy (CLSM). The 3D rendering of a 20-µm Z-stack clearly showed the lack of association of OVA with early endosomes but the enclosure of OVA within LAMP-1 positive vesicles (Fig. S5 D and E). Thus, these data show that sialylation does not affect the intracellular routing of antigens. Moreover, the fact that both Sia-OVA and native OVA end up in the compartment that generates MHC-II–binding peptides is in line with our finding that CD4+ T-cell proliferation induced by Sia-OVA–loaded DCs is comparable to that of OVA-loaded DCs (Fig. S5F). Together, these data imply that Sia-antigens predominantly impose tolerogenic function on DCs via its interaction with Siglec-E on the DC surface.
Fig. S5.
Sialylation of antigens does not affect the intracellular routing, processing, and presentation of antigens in MHC-II. (A) WT or Siglec-E−/− BMDCs were incubated with atto633-labeled Sia-OVA or native OVA, and colocalization with EEA-1 (open bars) and LAMP-1 (solid bars) was assessed after 2 h using imaging flow cytometry. (B) Representative images of cells with high colocalization of OVA with EEA-1 or LAMP-1. (C) Representative bivariate plot of colocalization scores of OVA with EEA-1 or LAMP-1 in WT BMDCs. (D and E) WT or Siglec-E−/− BMDCs were incubated with atto633-labeled Sia-OVA for 2 h, and colocalization with EEA-1 (green) and LAMP-1 (blue) was analyzed using CLSM. The 3D rendering of a 20-μm Z-stack is shown. (E) Cross-section focusing in an OVA-rich area. (F) Proliferation of OT-II cells after 3 d of coculture with Sia-OVA or native OVA-loaded DCs (mean ± SEM; n = 3); ns, not significant (Student’s t test).
Sia-Antigen also Relays Tolerogenic Properties to DCs Under Inflammatory Conditions.
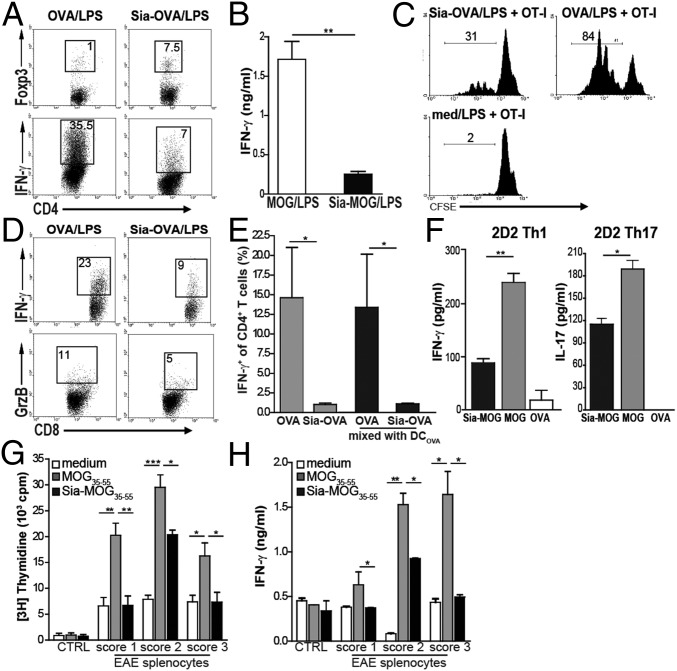
In the experiments described above, DCs were modulated by sialylated antigens under homeostatic conditions. To determine whether Sia-OVA also imposes tolerogenic function on DCs under inflammatory conditions, as occurs during infections or in the early stages of neoplasia, we assessed naive CD4+ T-cell differentiation by DCs pulsed with Sia-OVA or Sia-MOG35–55 in the presence of LPS. Whereas naive CD4+ T cells markedly differentiated into effector T cells when primed with OVA/LPS or MOG35–55/LPS–pulsed DCs, the ability to induce Treg cells and control effector T-cell generation was maintained by Sia-antigen/LPS–loaded DCs (Fig. 4 A and B). Also, the ability of CD8+ T cells to expand and become lytic effectors, as shown by IFN-γ and granzyme B production, was significantly reduced when DCs had internalized sialylated antigens (Fig. 4 C and D), indicating a strong inhibitory effect of sialylated antigens on the capacity of DCs to initiate cytotoxic T lymphocyte (CTL) responses. Sia-OVA–loaded DCs also dampened effector T-cell formation when cultured at a 1:1 ratio with OVA-loaded DCs (Fig. 4E). These findings suggest that sialylation of antigens could also be exploited to dampen existing effector T cells. We therefore generated Th1 and Th17 cells from naive 2D2 CD4+ T cells in vitro and observed that secretion of effector cytokines by Th1 and Th17 cells was significantly reduced when encountering Sia-MOG35–55–loaded DCs but not when reactivated by MOG-loaded DCs (Fig. 4F). To examine whether Sia-antigens could inhibit MOG-specific effector T cells induced upon the in vivo induction of EAE, we restimulated splenocytes from mice with different EAE clinical scores, representing different stages of effector T-cell number and activity, with Sia-MOG– or MOG35–55–loaded DCs. MOG35–55–loaded DCs induced ex vivo proliferation and IFN-γ production by splenocytes collected from mice that were at the onset of disease (score 1) or at a more severe phase of the disease (scores 2 or 3). Notably, these processes were significantly suppressed when using Sia-MOG35–55–loaded DCs (Fig. 4 G and H). These data indicate that sialic acid-modified antigens instruct DCs in an antigen-specific tolerogenic program, reducing inflammatory CD8+ and CD4+ T-cell responses.
Fig. 4.
Sia-antigen also modulates DCs in an inflammatory environment. DCs were loaded with antigens in the presence of LPS and cocultured with OT-II or OT-I T cells. (A) Flow cytometric analysis of Foxp3 and IFN-γ expression; the percentage of positive cells is indicated. (B) IFN-γ produced by 2D2 T cells cocultured with DCs pulsed with MOG35–55/LPS, α2,3-Sia-MOG35–55/LPS, or α2,6-Sia-MOG35–55/LPS (mean ± SEM; n = 3). CFSE-labeled OT-I T cells cocultured with Sia-OVA/LPS or OVA/LPS–loaded DCs. (C and D) Percentages of divided cells (C) and of IFN-γ+ and GrB+ OT-I cells (D) are shown (n = 3). (E) Proportion of IFN-γ+CD4+ T cells in cultures with DCs loaded with Sia-OVA or OVA mixed 1:1 with DCOVA. Control cultures contain only DCSia-OVA or DCOVA. (F) IFN-γ or IL-17 production by in vitro-generated Th1 and Th17 cells after reactivation by antigen-loaded DCs. (G and H) Ex vivo restimulation of spleens from mice induced for EAE with Sia-MOG35–55–loaded (black bars) or MOG35–55–loaded (gray bars) DCs. Medium-treated DCs (white bars) and spleens of naive mice were assessed as controls. Four days later, MOG-specific T-cell proliferation (G) and IFN-γ in culture supernatants (H) was determined (means ± SEM; n = 3 mice/condition). ***P < 0.001; **P < 0.01; *P < 0.05.
Discussion
Here, we show, for the first time to our knowledge, that DCs become tolerogenic upon uptake of soluble sialylated antigens. Moreover, we provide evidence for induction of antigen-specific immune tolerance under inflammatory conditions using sialylated antigens. We observed that under inflammatory conditions, effector T-cell functions were also dampened by Sia-antigen–loaded DCs. Importantly, under these circumstances Sia-antigen–loaded DCs retained the capacity to polarize naive CD4+ T cells toward Treg cells. To the best of our knowledge, this tolerizing effect under inflammatory conditions has not been demonstrated for other tolerizing compounds. The possibility of inducing tolerance in vivo in an antigen-specific manner via this new strategy of sialylation of antigens may open up new avenues for treating patients who suffer from unwanted immune reactions such as autoimmunity and allergies.
Tolerogenic DCs, either occurring in vivo or generated in vitro, are characterized by an antiinflammatory phenotype attributable to low expression of costimulatory molecules and the ability to promote Treg-cell induction. Although uptake of Sia-antigen by DCs in vitro did not result in expression of surface markers associated with “classic” tolerogenic DCs, DCs are clearly tolerogenic in function upon internalization of sialylated antigens. We previously observed a similar absence of clear effects on the phenotype of human monocyte-derived DCs after incubation with the highly sialylated pathogen Neisseria gonorrhea, although T-cell polarizing capacity was affected (16, 27). Despite producing lower amounts of the proinflammatory cytokines IL-6 and TNF, we did not observe significant differences in the quantity of IL-10 or TGF-β produced by Sia-OVA–loaded DCs compared with OVA-loaded DCs. Therefore, it is possible that Sia-OVA–pulsed DCs induce Treg-cell induction and prevent effector T-cell generation via cell surface receptor–ligand interactions, rather than secretion of antiinflammatory cytokines. This possibility is further substantiated by our findings that Sia-OVA–loaded DCs did not confer tolerogenic capacity onto CD4+ T cells when physically separated or when the supernatant of Sia-OVA–loaded DCs was used to treat OVA-loaded DCs.
Our findings indicate that the differentiation of naive CD4+CD25− T cells into Treg cells and reduced generation of effector IFN-γ+ T cells by Sia-OVA–pulsed DCs were not dependent on the mouse strain, because DCs and T cells from both C57BL/6 and BALB/c mice underwent equivalent responses. Additionally, we even observed de novo Treg-cell generation using naive CD4+ T cells from DO11.10×Rag−/− mice, which lack natural Treg (nTreg) cells. However, we anticipate that DCs tolerized by the uptake of Sia-antigen in vivo may also propagate nTreg cells, because the detection of Foxp3+CD4+ T cells does not discriminate between induced Treg (iTreg) cells and nTreg cells. Nevertheless, the net result of Sia-antigen internalization by DCs is the formation of increased numbers of T cells with suppressive capacity that can inhibit detrimental effector T-cell responses. In this process, Siglec-E plays an important role. In fact, our data indicate that DCs are endowed with tolerogenic characteristics via Siglec-E signaling upon internalization of sialylated antigens. This finding is underscored by our observations that intracellular trafficking of antigens is not altered by sialylation of antigens and that the presence of Siglec-E is required on DCs to induce Treg cells in response to Sia-antigens.
Besides affecting CD4+ T-cell responses, DCs loaded with antigens modified with sialic acids also strongly restrained CD8+ T-cell responses. Both the expansion of CD8+ T cells as well as the acquisition of cytotoxic effector functions was significantly dampened, even under inflammatory conditions. Similarly, reduced CTL generation was observed in vivo when pretreating mice with Sia-antigen or Sia-antigen–loaded DCs. How CD8+ T-cell responses are regulated in vivo by Sia-antigen pretreatment is unclear. The CD8+ T-cell responses can either be controlled by Sia-antigen–induced Treg cells or via direct effects of the Sia-antigen–tolerized DCs, as shown in vitro. Because autoreactive CTLs have been shown to contribute to the pathogenesis in type 1 diabetes and multiple sclerosis (28–31), treatment with sialylated antigens could thus also be effective to dampen these pathologies.
CTLs also play crucial functions in the immune response against viral infections. However, certain viruses such as herpes simplex virus (HSV)-1 and -2 adopt latency as a strategy to evade immune defense and persist in the host (32). Infections caused by these viruses are characterized by low antigen expression and a low level of CD8+ T-cell activation (33). Because high levels of sialylated antigens have been found on the envelope glycoproteins of HSV-1/-2, it can be hypothesized that sialic acids on HSV contribute to HSV immune escape by tolerizing DCs. Although the focus of the present study was to examine the effect of sialylated antigens on DCs and T-cell responses, B-cell responses may also be modulated. Liposomes coated with high-affinity sialic acid derivatives that target CD22 have been shown to induce B-cell tolerance (34).
Many siglecs act as negative regulators of immune responses via the expression of ITIM motifs in their cytoplasmic tails. Therefore, it is likely that the observed tolerogenic effect of Sia-antigen results from interaction of Sia-antigen with siglec receptors on DCs. Our studies showed a significant reduction in the binding and uptake of Sia-OVA by Siglec-E−/− DCs and, concomitant, drastic inhibition of Treg-cell generation. This finding suggests a relevant role for the Siglec-E receptor in the interaction of Sia-antigens with DCs. Moreover, in view of these findings, Sia-antigen–mediated Treg-cell induction and T-effector inhibition may occur via two different modes, with Siglec-E mainly implicated in Treg-cell polarization. Loading WT DCs with low concentrations of Sia-OVA antigen did not lead to enhanced generation of Treg cells but still dampened effector T-cell generation. It can be speculated that low antigen concentrations do not evoke clustering of Siglec-E, which is necessary to relay intracellular ITIM signaling (21).
In healthy individuals, the most important role of Treg cells is to maintain immune tolerance to self- and innocuous exogenous antigens as well as the intestinal microflora to prevent the development of autoimmune and allergic diseases (35). Defects in Treg-cell number and/or function have been shown to contribute to autoimmune diseases (4). Thus, therapies directed at resolving Treg-cell defects have the potential to prevent and also cure such diseases.
Modification of specific antigens using sialic acids has a number of advantages over current therapeutic strategies aimed at establishing large cohorts of Treg cells: this method provides DCs with a dual-tolerogenic function, because in addition to induction of Treg cells, these DCs simultaneously inhibit the generation of IFN-γ–producing T cells. Thus, using sialylated antigens as a therapy may directly dampen excessive inflammation, a process that occurs during autoimmunity. Furthermore, in contrast to current adjuvant approaches such as administration of retinoic acid or TGF-β, nonspecific suppression is minimized, because the specific antigen is directly modified with sialic acids.
Moreover, we provide evidence for induction of antigen-specific immunotolerance under inflammatory conditions using sialylated antigens. Importantly, under these circumstances Sia-antigen–loaded DCs retained the capacity to polarize naive CD4+ T cells toward Treg cells. To the best of our knowledge, this sustained antigen-specific tolerizing effect has not been demonstrated for other tolerizing compounds.
In conclusion, our data demonstrate that sialylation alters the immunogenicity of an antigen and provides a novel way to induce tolerogenic DCs for the treatment of autoimmune diseases and allergies.
Experimental Procedures
Mice.
C57BL/6 mice were used at 8–12 wk of age. OT-II, DO11.10, DO11.10×Rag2−/−, and 2D2 TCR transgenic mice were bred in the animal facilities of VU Medical Center (VUmc), Erasmus University Medical Center (Erasmus MC), and the TWINCORE Institute under specific pathogen-free conditions. All experiments were approved by the Animal Experiments Committee of the Erasmus MC and VUmc and performed in accordance with national and international guidelines and regulations.
Dendritic Cells.
Spleens from C57BL/6 mice were enriched for CD11c+ cells by magnetic separation according to the manufacturer’s protocol (Miltenyi Biotec). BMDCs were cultured from bone marrow of WT or Siglec-E−/− mice as described by Lutz et al. (36) with minor modifications (25).
Antibodies.
The phycoerythrin (PE)-labeled antibodies were anti-CD8b (H35-17.2) and anti-CD4 (GK1.5); the APC-labeled antibodies were anti-CD62L (MEL-14), anti-Foxp3 (FJK-169), and anti-IFN-γ (XMG1.2). Anti-CD62L and –IFN-γ antibodies were purchased from BD Pharmingen (BD Biosciences); others were obtained from eBiosciences.
Modification of Antigens with Sialylated Glycans.
To obtain Sia-OVA and Sia-MOG, maleimide-activated 6′-sialyl-N-acetyllactosamine (SLN306; Neu5Acα2,6Galβ1,4Glc; DEXTRA Labs) and 3′-sialyl-N-acetyllactosamine (SLN302; Neu5Acα2,3Galβ1,4Glc) were conjugated to thio-activated OVA (Calbiochem) and to MOG35–55 peptide, which is described in detail in SI Materials and Methods.
Th-Cell Differentiation and Testing of Suppressive Capacity.
Naive CD4+CD62LhiCD25− T cells were purified from spleen and LN cell suspensions using the Dynal mouse CD4+CD62L+ T-cell isolation kit II mouse (Miltenyi Biotec) or by sorting on a MoFlo (DakoCytomation). Naive CD4+CD62L+CD25− T cells (5 × 104) were added to wells containing DCs (1 × 104) that were pulsed with the indicated concentrations of Sia-antigen or native antigen 3 h prior. After 2 d, 10 U/mL recombinant mouse IL-2 (Invitrogen) was added, and T-cell polarization was evaluated on day 6 by intracellular staining for Foxp3 and IFN-γ following 5 h of restimulation with phorbol 12-myristate 13-acetate (PMA) (30 μg/mL)/ionomycin (500 ng/mL; Sigma) in the presence of Brefeldin A (5 µg/mL; Sigma). To determine whether Sia-OVA–loaded DCs induce Treg-cell differentiation via a soluble factor, the cells were cultured in 0.4-μm pore size Transwell plates (Millipore). Suppressive capacity of Sia-OVA-DC–primed T cells was determined using 4-d cocultures with 1 × 104 OVA-loaded DCs and CFSE-labeled responder OT-II T cells.
Cytokine Analysis.
Cytokines were determined by cytometric bead arrays (CBA) using the CBA Th1/2/17 kit and mouse inflammation kit (BD Biosciences) or by ELISA using specific antibody pairs (eBiosciences) following the manufacturers’ instructions.
In Vivo Treatment.
For modulation of endogenous DCs, C57BL/6 mice were injected i.v. with 50 µg of Sia-OVA or OVA and primed 1 wk later by s.c. injection of 200 µg of OVA/25 µg of polyinosinic–polycytidylic acid [poly(I:C)]/25 µg of anti-CD40. Seven days after priming, the mice were killed, and spleens were analyzed for the frequency of Treg cells and effector T cells after restimulation with either 2 µg/mL SIINFEKL or 200 µg/mL EKLTEWTSSNMEER OVA peptides.
Confocal Microscopy and Imaging Flow Cytometry.
Intracellular routing of Sia-OVA or native OVA in DCs was analyzed confocal laser-scanning microscopy and ImageStream X (Amnis) imaging flow cytometer as described in SI Materials and Methods.
Statistical Analysis.
Prism 5.0 software (GraphPad) was used for statistical analysis. The Student’s t test and one-way ANOVA with Bonferroni correction were used to determine statistical significance. Statistical significance was defined as P < 0.05.
SI Materials and Methods
Modification of Antigens with Sialylated Glycans.
To obtain Sia-OVA and Sia-MOG, maleimide-activated 6′-sialyl-N-acetyllactosamine (SLN306; Neu5Acα2,6Galβ1,4Glc; DEXTRA Labs) and 3′-sialyl-N-acetyllactosamine (SLN302; Neu5Acα2,3Galβ1,4Glc) were conjugated to thio-activated OVA (Calbiochem) and to MOG35–55 peptide with an added cysteine to the N terminus, through a thiol-ene reaction. MOG35–55 peptide was produced by solid-phase peptide synthesis using fluorenylmethyloxycarbonyl chemistry with a Symphony peptide synthesizer (Protein Technologies).
The glycans were activated with the bifunctional cross linker 4-N-maleimidophenyl butyric acid hydrazide (MPBH) (Pierce) and OVA was activated with the linker N-succinimidyl S-acetylthioacetate (SATA) (Pierce). The hydrazide moiety of MPBH was covalently linked to the reducing end of the carbohydrate via reductive amination at a 3:1 molar ratio, OVA was reacted with SATA via the amino groups on its surface at a 6:1 molar ratio, and the final reaction of OVA-SATA with the derivatized carbohydrate was performed at a molar ratio of 1: 10. The final reaction of MOG35–55 with the derivatized carbohydrates was performed at a molar ratio of 1:1.5.
Briefly, SATA, dissolved in DMSO, was added to a filtered solution of OVA in phosphate buffer (pH 8.2; filtered to 200 nm). After vigorous stirring, the resulting OVA-SATAAc solution was purified using a PD10 column (GE Healthcare) and diluted with 10% vol/vol of 0.5 M NH2OH⋅HCl solution (pH 7.2). After another 40 min of vigorous stirring and a second purification using a disposable size-exclusion cartridge (PD10 column; GE Healthcare Life Sciences), OVA-SATASH was ready for coupling with the MPBH-glycans. A mixture of MPBH (3 eq), 3′-sialyl-N-acetyllactosamine (or 6′-sialyl-N-acetyllactosamine) (1 eq), and picoline borane complex (10 eq; Sigma-Aldrich) dissolved in DMSO/AcOH (8:2) was incubated for 2 h at 65 °C, cooled to room temperature (RT), 1.4 mL of ice-cold isopropanol (anhydrous; Sigma-Aldrich) was added, and then incubated at –20 °C for 1 h. Subsequently, the precipitated MPBH–carbohydrates were pelleted, washed twice with cold isopropanol, and dissolved in 50 µL of PBS. The derivatization was confirmed by electrospray ionization mass spectrometry. The obtained MPBH–3′sialyl-N-acetyllactosamine and MPBH–6′-sialyl-N-acetyllactosamine were used immediately for coupling to OVA-SATASH and to MOG35–55 peptide. Conjugation of OVA-SATASH and MOG35–55 to the activated glycans was performed o/n at 4 °C, and the neoglycoconjugates were purified by size exclusion chromatography. The concentrations of OVA and MOG were determined using the bicinchoninic acid assay (Pierce).
The presence of α2,6- or α2,3-linked sialic acids on the antigens was confirmed by matrix-assisted laser desorption/ionization coupled to time-of-flight (MALDI-TOF), HPLC, and ELISA (Fig. S1). In brief, 10 µg/mL Sia-antigen was coated directly onto ELISA plates (Nunc Maxisorb; Nunc), followed by incubation with the biotinylated plant lectins from Maackia amurensis Lectin-I (MAL-I) and Sambucus nigra (SNA) (Vector Labs) and peroxidase-labeled streptavidin (Sigma-Aldrich).
Confocal Microscopy and Imaging Flow Cytometry.
BMDCs were incubated with 30 μg/mL atto633-labeled Sia-OVA or native OVA for 2 h at 37 °C, fixed and permeabilized for 20 min on ice, and stained with primary and secondary antibodies. Colocalization was analyzed using a Leica AOBS SP2 CLSM system containing a DM-IRE2 microscope with glycerol objective lens (PL APO 63×/NA1.30); images were acquired using Leica confocal software (version 2.61). For imaging flow cytometry, ∼1 × 106 BMDCs were incubated with Sia-OVA or OVA and after 2 h cells were washed twice, fixed in ice-cold 4% (wt/vol) PFA/PBS for 20 min, and permeabilized in 0.1% saponin (Sigma) in PBS for 30 min at RT. Stainings were performed at RT in PBS/0.1% saponin/2% BSA. After staining, cells were washed twice, resuspended in PBS/1% BSA/0.02% NaN3 and kept at 4 °C until analysis. Cells were acquired on the ImageStream X (Amnis) imaging flow cytometer. Analysis was performed using the IDEAS version 6.1 software (Amnis). Cells were first gated based on the Gradient RMS (bright field) feature and then based on area vs. aspect ratio intensity (both on bright field). The first gating identified the cells that appeared in focus, whereas the second excluded doublets and cells other than BMDCs. Three-color colocalization was calculated using the bright detail colocalization 3 feature.
Acknowledgments
We thank our biotechnicians for excellent caretaking of the animals. This work was supported by the Seventh Framework Program of Marie Curie Actions (7th Framework Programme for Research and Technological Development-Carmusys), Koningin Wilhelmina Fonds (Dutch Cancer Foundation) Grant VU2009-2598, Senternovem Grant SII071030, and European Research Council Grants ERCAdvanced339977 and ESTAR14104 “SIAGEN-MS.”
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1507706113/-/DCSupplemental.
References
- 1.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155(3):1151–1164. [PubMed] [Google Scholar]
- 2.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199(7):971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Long SA, Buckner JH. CD4+FOXP3+ T regulatory cells in human autoimmunity: More than a numbers game. J Immunol. 2011;187(5):2061–2066. doi: 10.4049/jimmunol.1003224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol. 2010;10(12):849–859. doi: 10.1038/nri2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beavis PA, et al. Resistance to regulatory T cell-mediated suppression in rheumatoid arthritis can be bypassed by ectopic foxp3 expression in pathogenic synovial T cells. Proc Natl Acad Sci USA. 2011;108(40):16717–16722. doi: 10.1073/pnas.1112722108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belkaid Y. Regulatory T cells and infection: A dangerous necessity. Nat Rev Immunol. 2007;7(11):875–888. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]
- 7.Severi E, Hood DW, Thomas GH. Sialic acid utilization by bacterial pathogens. Microbiology. 2007;153(Pt 9):2817–2822. doi: 10.1099/mic.0.2007/009480-0. [DOI] [PubMed] [Google Scholar]
- 8.Ménétrier-Caux C, et al. Targeting regulatory T cells. Target Oncol. 2012;7(1):15–28. doi: 10.1007/s11523-012-0208-y. [DOI] [PubMed] [Google Scholar]
- 9.Mayer CT, Berod L, Sparwasser T. Layers of dendritic cell-mediated T cell tolerance, their regulation and the prevention of autoimmunity. Front Immunol. 2012;3:183. doi: 10.3389/fimmu.2012.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aarnoudse CA, Garcia Vallejo JJ, Saeland E, van Kooyk Y. Recognition of tumor glycans by antigen-presenting cells. Curr Opin Immunol. 2006;18(1):105–111. doi: 10.1016/j.coi.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Saeland E, et al. Differential glycosylation of MUC1 and CEACAM5 between normal mucosa and tumour tissue of colon cancer patients. Int J Cancer. 2012;131(1):117–128. doi: 10.1002/ijc.26354. [DOI] [PubMed] [Google Scholar]
- 12.Varki A, Gagneux P. Multifarious roles of sialic acids in immunity. Ann N Y Acad Sci. 2012;1253:16–36. doi: 10.1111/j.1749-6632.2012.06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bogenrieder T, Herlyn M. Axis of evil: Molecular mechanisms of cancer metastasis. Oncogene. 2003;22(42):6524–6536. doi: 10.1038/sj.onc.1206757. [DOI] [PubMed] [Google Scholar]
- 14.Varki A, Kannagi R, Toole BP. Glycosylation changes in cancer. In: Varki A, et al., editors. Essentials of Glycobiology. 2nd Ed. Cold Spring Harbor Laboratory Press; Spring Harbor, NY: 2009. Chapter 44. [PubMed] [Google Scholar]
- 15.Swindall AF, et al. ST6Gal-I protein expression is upregulated in human epithelial tumors and correlates with stem cell markers in normal tissues and colon cancer cell lines. Cancer Res. 2013;73(7):2368–2378. doi: 10.1158/0008-5472.CAN-12-3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bax M, et al. Campylobacter jejuni lipooligosaccharides modulate dendritic cell-mediated T cell polarization in a sialic acid linkage-dependent manner. Infect Immun. 2011;79(7):2681–2689. doi: 10.1128/IAI.00009-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guerry P, et al. Phase variation of Campylobacter jejuni 81-176 lipooligosaccharide affects ganglioside mimicry and invasiveness in vitro. Infect Immun. 2002;70(2):787–793. doi: 10.1128/iai.70.2.787-793.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harvey HA, Swords WE, Apicella MA. The mimicry of human glycolipids and glycosphingolipids by the lipooligosaccharides of pathogenic neisseria and haemophilus. J Autoimmun. 2001;16(3):257–262. doi: 10.1006/jaut.2000.0477. [DOI] [PubMed] [Google Scholar]
- 19.van Vliet SJ, García-Vallejo JJ, van Kooyk Y. Dendritic cells and C-type lectin receptors: Coupling innate to adaptive immune responses. Immunol Cell Biol. 2008;86(7):580–587. doi: 10.1038/icb.2008.55. [DOI] [PubMed] [Google Scholar]
- 20.Crocker PR, McMillan SJ, Richards HE. CD33-related siglecs as potential modulators of inflammatory responses. Ann N Y Acad Sci. 2012;1253:102–111. doi: 10.1111/j.1749-6632.2011.06449.x. [DOI] [PubMed] [Google Scholar]
- 21.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7(4):255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 22.Nylander A, Hafler DA. Multiple sclerosis. J Clin Invest. 2012;122(4):1180–1188. doi: 10.1172/JCI58649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bettelli E, et al. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J Exp Med. 2003;197(9):1073–1081. doi: 10.1084/jem.20021603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gottschalk RA, Corse E, Allison JP. TCR ligand density and affinity determine peripheral induction of Foxp3 in vivo. J Exp Med. 2010;207(8):1701–1711. doi: 10.1084/jem.20091999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh SK, et al. Targeting glycan modified OVA to murine DC-SIGN transgenic dendritic cells enhances MHC class I and II presentation. Mol Immunol. 2009;47(2-3):164–174. doi: 10.1016/j.molimm.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 26.Mahnke K, Schmitt E, Bonifaz L, Enk AH, Jonuleit H. Immature, but not inactive: The tolerogenic function of immature dendritic cells. Immunol Cell Biol. 2002;80(5):477–483. doi: 10.1046/j.1440-1711.2002.01115.x. [DOI] [PubMed] [Google Scholar]
- 27.van Vliet SJ, et al. Variation of Neisseria gonorrhoeae lipooligosaccharide directs dendritic cell-induced T helper responses. PLoS Pathog. 2009;5(10):e1000625. doi: 10.1371/journal.ppat.1000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Unger WW, et al. Islet-specific CTL cloned from a type 1 diabetes patient cause beta-cell destruction after engraftment into HLA-A2 transgenic NOD/scid/IL2RG null mice. PLoS One. 2012;7(11):e49213. doi: 10.1371/journal.pone.0049213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friese MA, Fugger L. Autoreactive CD8+ T cells in multiple sclerosis: A new target for therapy? Brain. 2005;128(Pt 8):1747–1763. doi: 10.1093/brain/awh578. [DOI] [PubMed] [Google Scholar]
- 30.Huseby ES, et al. A pathogenic role for myelin-specific CD8(+) T cells in a model for multiple sclerosis. J Exp Med. 2001;194(5):669–676. doi: 10.1084/jem.194.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun D, et al. Myelin antigen-specific CD8+ T cells are encephalitogenic and produce severe disease in C57BL/6 mice. J Immunol. 2001;166(12):7579–7587. doi: 10.4049/jimmunol.166.12.7579. [DOI] [PubMed] [Google Scholar]
- 32.Koelle DM. Immunobiology and host response. In: Arvin A, et al., editors. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge Univ Press; Cambridge, UK: 2007. Chapter 34. [PubMed] [Google Scholar]
- 33.Teuton JR, Brandt CR. Sialic acid on herpes simplex virus type 1 envelope glycoproteins is required for efficient infection of cells. J Virol. 2007;81(8):3731–3739. doi: 10.1128/JVI.02250-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macauley MS, et al. Antigenic liposomes displaying CD22 ligands induce antigen-specific B cell apoptosis. J Clin Invest. 2013;123(7):3074–3083. doi: 10.1172/JCI69187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10(7):490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 36.Lutz MB, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223(1):77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]