Significance
C/D box small nucleolar RNAs (SNORDs) are abundant, short, nucleoli-residing, noncoding RNAs that guide the methyltransferase fibrillarin to perform 2′-O-methylation of target RNAs. We identified 29 SNORDs present in a fibrillarin-containing fraction as well as a fibrillarin-free fraction enriched in spliceosomes. One of these SNORDs, SNORD27, directs rRNA methylation and regulates alternative pre-mRNA splicing (AS) of E2F7 pre-mRNA, a transcriptional repressor of cell cycle-regulated genes. SNORD27 likely regulates E2F7 pre-mRNA AS by masking splice sites through base pairing. This previously unidentified function of SNORDs increases the number of factors regulating AS, a critical step in the expression of the vast majority of human genes, and highlights a potential coupling between AS, cell cycle, proliferation, and ribosome biogenesis.
Keywords: alternative splicing, gene regulation, snoRNAs, pre-mRNA processing
Abstract
C/D box small nucleolar RNAs (SNORDs) are small noncoding RNAs, and their best-understood function is to target the methyltransferase fibrillarin to rRNA (for example, SNORD27 performs 2′-O-methylation of A27 in 18S rRNA). Unexpectedly, we found a subset of SNORDs, including SNORD27, in soluble nuclear extract made under native conditions, where fibrillarin was not detected, indicating that a fraction of the SNORD27 RNA likely forms a protein complex different from canonical snoRNAs found in the insoluble nuclear fraction. As part of this previously unidentified complex, SNORD27 regulates the alternative splicing of the transcription factor E2F7 pre-mRNA through direct RNA–RNA interaction without methylating the RNA, likely by competing with U1 small nuclear ribonucleoprotein (snRNP). Furthermore, knockdown of SNORD27 activates previously “silent” exons in several other genes through base complementarity across the entire SNORD27 sequence, not just the antisense boxes. Thus, some SNORDs likely function in both rRNA and pre-mRNA processing, which increases the repertoire of splicing regulators and links both processes.
Small nucleolar RNAs (snoRNAs) are 60- to 300-nt-long noncoding RNAs that accumulate in the nucleolus. Based on conserved sequence elements, snoRNAs are classified as C/D box small nucleolar RNAs (SNORDs) or H/ACA box snoRNAs(SNORAs). SNORDs contain sequence elements termed C (RUGAUGA) and D (CUGA) boxes, usually present in duplicates (C′ and D′ boxes), and up to two antisense boxes that hybridize to the target RNA (1). In humans, SNORDs are usually derived from introns. After the splicing reaction, introns are excised as lariats, which are then opened by the debranching enzyme and subsequently degraded. Intronic SNORDs escape this degradation by forming a protein complex that consists of non-histone chromosome protein 2-like 1 (NHP2L1, 15.5K, SNU13), nucleolar protein 5A (NOP56), nucleolar protein 5 (NOP58), and fibrillarin (2–4). The SNORD protein complex forms through the entry of the snoRNA and fibrillarin to a complex containing NHP2L1, NOP58, and at least five assembly factors (5). The SNORD acts as a scaffold for the final protein complex formation and also controls recognition of other RNAs using the antisense boxes. The antisense boxes recognize sequences in rRNA, resulting in the fifth nucleotide upstream of the D or D′ box being 2′-O-methylated by fibrillarin (1). Structural studies indicate that the active form of SNORDs is dimeric (6).
The conserved overall structure of SNORDs allows the identification of their putative target RNA binding sites. However, numerous SNORDs without obvious target RNAs have been identified (7–10) and are termed “orphan snoRNAs.” Genome-wide deep sequencing experiments identified shorter but stable SNORD fragments that were found in all species tested, ranging from mammals to the protozoan Giardia lamblia (11) and Epstein–Barr virus (12). Fragments longer than 27 nt generated by SNORDs will likely not bind argonaute proteins that bind to 21- to 22-nt-long microRNAs. These differences in size indicate that most SNORD fragments are not microRNAs, which have an average length of 21–22 nt (13–16), and suggests that these fragments may have additional functions.
The association of some SNORDs with specific diseases suggests that they may possess functions in addition to directing the 2′-O-methylation of rRNA. For example, the loss of SNORD116 expression is a decisive factor in Prader–Willi syndrome, the most common genetic cause for hyperphagia and obesity (17, 18). SNORD60 is involved in intracellular cholesterol trafficking, which is independent of its suggested function in rRNA methylation (19), and SNORDs U32a, U33, and U35a mediate lipotoxic stress, possibly through their cytosolic function (20). Although SNORDs are considered housekeeping genes, the expression of some SNORDs is altered in cancer. For example, changes in the expression of SNORD27, -30, -25, and -31 mark the progression of smoldering multiple myeloma (21), and SNORD50 deletions are associated with prostate and breast cancer (22–24). Genome-wide comparison of SNORD expression between cancer and normal cells showed the presence of two classes of SNORDs that differ in their terminal stems but are made from the same SNORD hosting intron (25).
Studies in yeast have shown that cellular nutritional status can control the formation of SNORDs. A complex of four proteins [Rvb1, Rvb2, Tah1, and Phi1 (R2TP)] stabilizes NOP58 under conditions of growth. Under starving conditions, the R2TP complex localizes to the cytosol, destabilizing NOP58 formation and inhibiting small nucleolar ribonucleoprotein (snoRNP) formation (26, 27), suggesting that there is a cell program that regulates snoRNP composition.
Taken together, these findings suggest that cells modify SNORDs expression, that SNORDs are structurally more diverse than previously recognized, and that SNORDs have additional cellular functions other than 2′-O-methylation of rRNAs.
A previously described noncanonical function of SNORDs is their influence on alternative exon selection. Alternative pre-mRNA splicing is a mechanism that controls the inclusion of exons and the retention of introns in mature mRNA. More than 94% of human genes are estimated to undergo alternative splicing (28, 29), which greatly increases the complexity of the transcriptome (30). For example, the neuron-specific SNORD115 promotes the inclusion of an alternative exon in the serotonin receptor 2C pre-mRNA (31), and SNORD88C regulates alternative splicing of FGFR3 pre-mRNA (32). Although proof of principle experiments have shown that methylation of the branch point adenosine by engineered snoRNAs can change alternative splicing (33–35), the mechanism by which SNORDs regulate splice site selection in vivo is not clear.
Here, we used a native isolation procedure to characterize SNORDs from the nucleoplasma (36, 37) that were soluble under physiological salt extraction conditions. These SNORDs were found in complexes with splicing factors, where fibrillarin was not detected. We characterized the cancer-relevant SNORD27 in detail and found that it regulates alternative splicing of the E2F7 transcription factor pre-mRNA through direct RNA interaction and suppresses silent exon inclusion in MAP4K3, ZBTB37, FER, and ABCA8 pre-mRNAs. These silent exons are not expressed in the presence of SNORD27 and were previously not described. The data show that SNORDs are structurally diverse and have, in addition to their role in rRNA modification, an unexpected widespread function in pre-mRNA processing.
Results
Subset of SNORDs Is Present in a Nucleoplasmic Fraction Devoid of Fibrillarin.
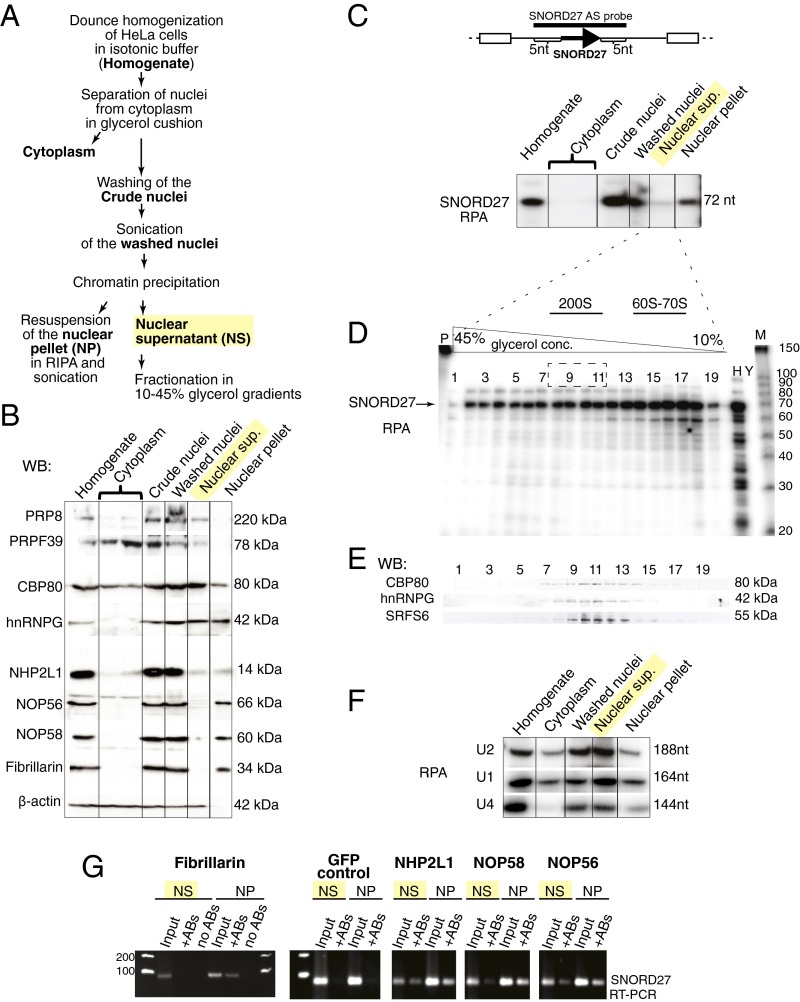
To determine the possible function of SNORDs outside the nucleolus, we prepared a soluble nuclear extract using native salt conditions (Materials and Methods and Fig. 1A) (36, 37). In contrast to the method by Dignam et al. (38) that uses 420 mM KCl and 1 mM DTT for extraction, this method preserves higher-order splicing complexes, as previously determined by EM (36, 37). Western blot analysis showed that native nuclear supernatant contained splicing factors PRP8 and PRPF39 and pre-mRNA–associated proteins CBP80 and heterogeneous ribonucleoprotein G (hnRNPG) as expected (Fig. 1B). SNORDs associate with fibrillarin, NOP56, NOP58, and NHP2L1 to form a canonical snoRNP (3). In this complex, the methyltransferase fibrillarin catalyzes 2′-O-methylation of the targeted ribose residue (6). We noted that the nuclear supernatant contained small amounts of the SNORD-associated proteins NHP2L1, NOP56, and NOP58. Fibrillarin, however, was not detectable (Fig. 1B). All four constitutive components of SNORD-RNPs were present in the resuspended nuclear pellet (Fig. 1B).
Fig. 1.
A portion of SNORD27 is present in a soluble nuclear fraction where fibrillarin is not detected. (A) The schematic representation of HeLa cell fractionation under native conditions. (B) Western blot analysis of the fractionation described in A; 5% of the total volume of each fraction prepared was analyzed by Western blot. The components of spliceosomes (PRP8 and PRPF39), splicing regulators (CBP80/NCBP1 and hnRNPG), and constitutive SNORD binding proteins (NHP2L1/15.5K, NOP56, NOP58, and fibrillarin) were detected by specific antibodies. (C) Distribution of SNORD27 in cellular fractions determined by RPA. The diagram shows the SNORD27 expression cassette and the location of the RPA probe. The arrow indicates SNORD27, and boxes indicate hosting exons. The RPA probe spanned the annotated SNORD27 sequence and 5 nt from the surrounding intron. (D) Separation of the soluble nuclear supernatant fraction in a glycerol gradient. Nuclear supernatant (C) was separated on 10–45% glycerol gradient. The gradient was subdivided into 20 fractions; RNA from 50% of each fraction was isolated and analyzed by RPA with an SNORD27 antisense probe. The arrow shows full-length SNORD27. (E) Individual glycerol gradient fractions were analyzed by Western blotting using antibodies recognizing markers for pre-mRNA processing: CBP80, hnRNPG, and SRSF6. (F) The distribution of U1, U2, and U4 snRNAs in the fractions obtained by HeLa cell fractionation (A) was determined by RPA. (G) Association of SNORD27 with constitutive SNORD binding proteins. Endogenous fibrillarin and Flag-tagged NHP2L1, NOP58, and NOP56 were immunoprecipitated and copurified. SNORD27 was then detected by RT-PCR. Cells transfected with GFP were used as a control. +Abs, immunoprecipitated with the depicted antibodies; H, 10 µg total HeLa RNA; M, molecular weight size marker; NP, nuclear pellet; NS, nuclear supernatant; P, untreated probe; WB, Western blot; Y, 10 µg total yeast RNA.
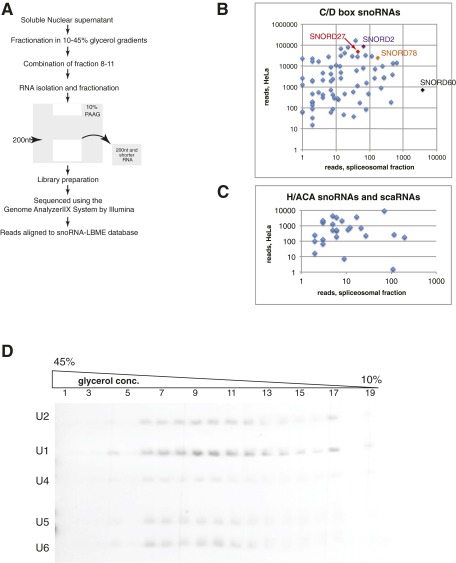
The native nuclear supernatant was further fractionated using 10–45% (vol/vol) glycerol gradients (Fig. 1 C–E), and RNAs smaller than 200 nt from combined fractions 8–11 were analyzed by RNA sequencing (RNA-seq) (Fig. S1). In previous experiments, fractions corresponding to the analogous positions in the gradient were enriched for the five spliceosomal U snRNAs (37, 39–41) (Fig. S1D), various pre-mRNAs (36, 42, 43), and regulatory splicing factors (41, 44, 45) (Fig. 1E) and contained active spliceosomes (37).
Fig. S1.
SNORDs determined by RNA-seq in the nuclear soluble fractions. (A) Experimental outline. The soluble nuclear fraction (nuclear supernatant) was fractionated in a 10–45% glycerol gradient. RNAs smaller than 200 nt from combined fractions 8–11 were extracted from 10% polyacrylamide gel and analyzed by RNA-seq. (B) Enrichment of some SNORDs in the spliceosomal fractions. The number of total reads mapped to SNORDs expressed in HeLa cells is plotted against the number of total reads mapped to SNORDs found in spliceosomal fractions (Table S1). (C) Same as B but for H/ACA snoRNAs and small Cajal body-specific RNAs (scaRNAs). (D) Distribution of spliceosomal U snRNAs in soluble nuclear fraction separated by a glycerol gradient. Nuclear supernatants prepared from HeLa cells were fractionated in a 10–45% glycerol gradient. Fractions enriched for supraspliceosomes (8–11) were combined and refractionated on a second glycerol gradient (37, 38). The distribution of five spliceosomal U snRNAs across the gradient was analyzed by Northern blot of RNA extracted from each fraction of the gradient.
Strikingly, in the fractions where fibrillarin was not detectable, we found a large number of full-length and shortened SNORDs, indicating that a large number of SNORDs exists in forms different from canonical fibrillarin-containing snoRNPs (Table S1).
For additional analysis, only the well characterized SNORDs annotated in the sno-LBME database (46) were considered. Using a threshold of 20 reads, we found 29 SNORDs in the spliceosomal fraction; 93 SNORDs were absent from the spliceosomal fraction but were expressed in HeLa cells as observed from the ENCODE sequencing data (47), and 137 SNORDs were not expressed in HeLa cells and subsequently, were not detected in the HeLa spliceosomal fraction. Thus, ∼24% of the SNORDs expressed in HeLa cells were found in spliceosomal fractions. In contrast, we found only five H/ACA snoRNAs and one small Cajal body-specific RNA (scaRNA) that showed 20 or more reads in the spliceosomal fractions, which represent less than 5% of the total number of known H/ACA snoRNAs and scaRNAs. Therefore, the majority of snoRNA reads were mapped to SNORDs and not mapped to other snoRNAs. Although we found a weak correlation between general SNORDs abundance and presence in the spliceosomal fractions (Spearman’s rank correlation coefficient rs = 0.3; P < 0.0056), a number of snoRNAs was clearly enriched in the spliceosomal fractions (Fig. S1B and Table S1). We detected the same proportion of orphan snoRNAs to total snoRNAs in both spliceosomal fractions and total HeLa RNA. In both cases, orphan snoRNAs comprised ∼11% of the total RNAs (when counting one snoRNA per cluster). Thus, close to one-quarter of expressed SNORDs are found in a fibrillarin-free fraction, without enrichment of orphan SNORDs.
We next determined the presence of SNORD27 in different cellular fractions using an RNase protection assay (RPA), because this SNORD is deregulated in smoldering multiple myeloma (21). However, although SNORD27 was mostly present in the resuspended nuclear pellet, it was also detectable in the native nuclear supernatant (Fig. 1C). Next, nuclear supernatants were further fractionated using 10–45% glycerol gradients. The expected protected fragment of SNORD27 is 72 nt long and present in all fractions (Fig. 1D). In addition, multiple shorter fragments were present in the gradients, with apparent lengths of 22, 30, and 50 nt that reflected some of the fragments observed in the RNA-seq experiments. Some of these RNAs are present in fractions 8–11 that contain most of the in vivo spliceosomes (37, 48) as determined by the presence of five spliceosomal U small nuclear ribonucleoproteins (snRNPs) (Fig. S1D) and regulatory splicing factors (Fig. 1E). As a control, we performed RPA using total HeLa RNA as well as yeast tRNA. The total HeLa RNA was isolated using TRIzol extraction, which solubilizes all RNAs, including those associated with nucleoli. In this preparation, the full-length SNORD27 as well as multiple shorter fragments were detected (Fig. 1D). The presence of the spliceosomal components U1, U2, and U4 snRNAs in the soluble nuclear fractions was confirmed by RPA (Fig. 1F), suggesting that a subset of SNORDs cosediments with spliceosomes.
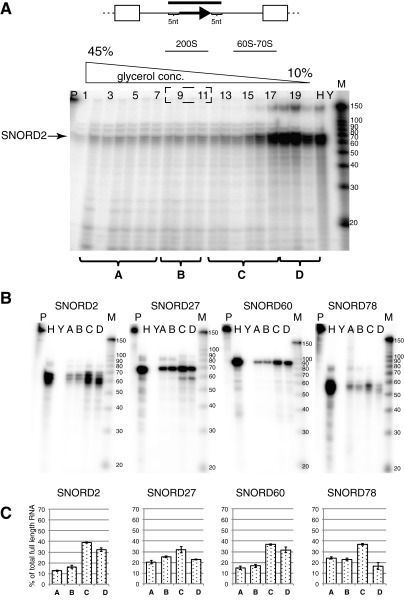
To test whether other SNORDs determined by RNA-seq (Table S1) were present in a fibrillarin-free fraction, we analyzed the sedimentation of SNORD2, SNORD60, and SNORD78 following the previously established characterization of spliceosome-containing fractions (37), (Fig. S1). The gradient was subdivided into four parts: A–D. Fraction A contained proteins that sedimented faster than 200S and was devoid of spliceosomes; fraction B contained supraspliceosomes that sedimented in the 200S region (37, 48, 49), fraction C contained native spliceosomes that sedimented at 60–70S (37, 41, 50), and fraction D contained proteins and small RNAs that appeared as free proteins or assembled into small RNP complexes. SNORD2, SNORD60, and SNORD78 were present in fractions B and C associated with spliceosomes but devoid of fibrillarin (Figs. S2 and S3). This distribution suggests that numerous SNORDs exist in the cell without being associated with fibrillarin, but possibly, they may be associated with spliceosomes.
Fig. S2.
RNase protection analysis of soluble nucleoplasmic SNORDs (2, 27, 60, 74). (A) RPA detecting SNORD2 in the 10–45% glycerol gradient separating the soluble native nuclear extract. A–D correspond to the pooled fractions used in B and Fig. S3. (B) RPA of the soluble nuclear fractions of SNORD2, SNORD27, SNORD60, and SNORD78 in pooled gradient fractions. Fractions from the gradient in Fig. 1D were combined into four samples: A: 1–7; B: 8–11; C: 12–17; and D: 18–20. H, HeLa RNA; M, molecular weight size marker; P, untreated probe; Y, total yeast RNA. (C) Quantification of the RPA from the gradient. Three independent RPA experiments were quantified using the phosphoimager. The percentage of total signal from the majorly protected band is shown. Error bars represent the SD.
Fig. S3.
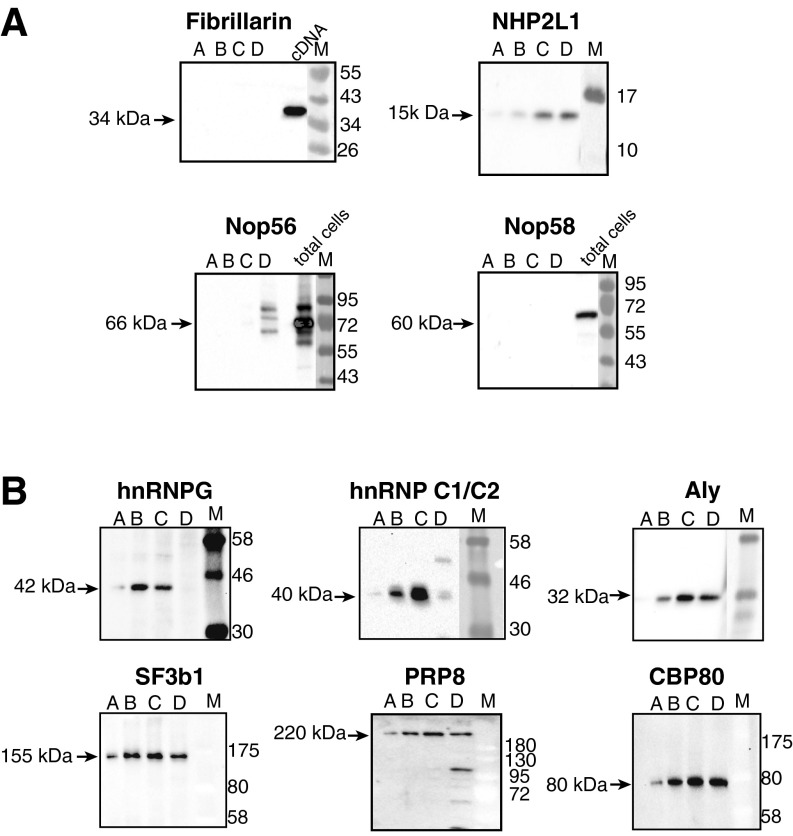
hnRNPs cosediment with nucleoplasmic SNORDs. Aliquots of fractions A–D were analyzed by Western blotting using indicated antisera. (A) Western blot using antisera against fibrillarin, NOP58, NOP56, and NHP2L1. Total lysate of WT cells and cells transfected with cDNA coding for fibrillarin were used as markers of protein mobility. (B) Western blot using antisera against hnRNPs and splicing regulators: heterogeneous ribonucleoprotein G (hnRNPG), heterogeneous ribonucleoprotein C1/C2 (hnRNPC1/C2), ALY/REF, NCBP1, PRP8, and SF3B1. M, molecular weight size marker.
We next tested the association of SNORD27 with SNORD-associated proteins directly. We performed immunoprecipitations using native nuclear supernatant and resuspended nuclear pellet using antibodies against Flag-tagged NHP2L1, NOP58, and NOP56 as well as endogenous fibrillarin. The immunoprecipitates were analyzed by RT-PCR using primers against SNORD27 (Fig. 1G). As shown in Fig. 1G, SNORD27 is readily detectable in all immunoprecipitates made from resuspended nuclear pellet. Confirming our previous results, we could not detect SNORD27 in fibrillarin immunoprecipitates from soluble nuclear extract, which is consistent with the lack of fibrillarin detection in nuclear supernatant based on Western blot results (Fig. 1B). Trace amounts of SNORD27 are detectable with NOP58 and NOP56, and a larger amount immunoprecipitates with NHP2L1.
We next analyzed the location of fibrillarin, NHP2L1, NOP58, and NOP56 in native soluble nuclear extract separated on glycerol gradients. Reflecting the immunoprecipitations, fibrillarin and NOP58 are absent from individual gradient fractions. NHP2L1 and NOP56 are concentrated in the lower-density part at the top of the gradient but absent in higher-density parts (Fig. S3A), suggesting that SNORD27 forms diverse protein–RNA complexes. In contrast, hnRNPG, heterogeneous ribonucleoprotein C1/C2 (hnRNPC1/C2), and ALY/REF as well as splicing factors SF3B1 and PRP8 and the nuclear cap binding protein CBP80 are present in all fractions, with hnRNPG and hnRNPC1/C2 present predominantly in fractions B and C (Fig. S3B).
Taken together, these data suggest that SNORD27 is present in two biochemically separable fractions. In the insoluble nuclear fractions, it is associated with fibrillarin, NOP56, NOP58, and NHP2L1 and likely forms a canonical snoRNP. In the native soluble nuclear fraction, however, SNORD27 is free from fibrillarin, and a part of SNORD27 associates with NOP56 and NHP2L1, suggesting that SNORD27 could have a function outside the canonical snoRNP.
SNORD27 Regulates Alternative Splicing of E2F7 Pre-mRNA.
The presence of SNORD27 in nuclear fractions devoid of fibrillarin suggests that it has a function apart from rRNA 2′-O-methylation. We previously found that the orphan SNORD115 changes alternative splicing of the serotonin receptor 2C and several other pre-mRNAs, possibly through base pairing of SNORD115 to a splicing regulatory region (31, 51). We, therefore, looked for complementarities between SNORD27 and pre-mRNAs across the genome.
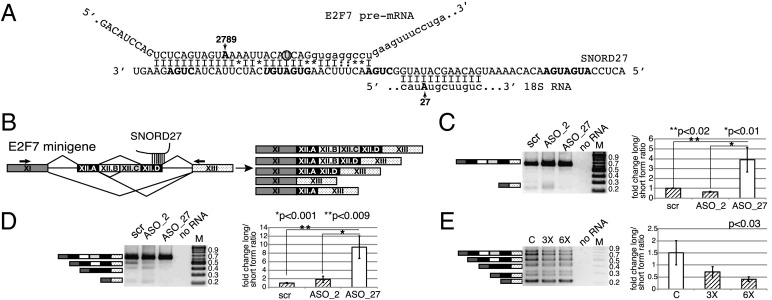
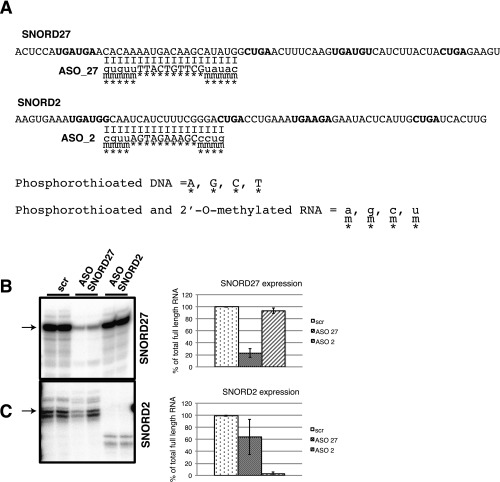
Because very little is currently known about the binding between SNORDs and their noncanonical targets, we performed a genome-wide search for potential targets using complementarities to the entire sequence of SNORD27 and not just the antisense box involved in recognizing rRNA. We searched for targets of any length with at least 22 matching nucleotides and up to two mismatches and allowed any number of noncanonical (U:G) pairings (Table S2), concentrating on complementarities in the vicinity of alternative exons. One of the best complementarities was found between SNORD27 and an alternatively spliced exon of the E2F7 gene. The complementarity consisted of 29 nucleotides with two G:U pairs, four mismatched bases, and no gaps. Importantly, the sequence complementarity extended to five nucleotides of the alternative 5′ splice site (Fig. 2A). The alternative exon is longer (425 nt) than the average human cassette exon (147 nt) (52) and contains several internal splice sites (Fig. 2B and Table S3A). To test the functionality of the sequence complementarity to SNORD27, we knocked down SNORD27 using modified chimeric antisense oligonucleotides. This approach was previously developed to knock down noncoding RNAs (ncRNAs), including intronic SNORDs, in HeLa cells (53). The oligonucleotides contained 10 phosphothioate DNA nucleotides surrounded by 5 2′-O-methyl–modified phosphorothioate RNA nucleotides at both the 5′ and 3′ ends. The oligonucleotides were designed to bind the antisense box of SNORD27 and SNORD2 located between C and D′ boxes (Fig. S4). The oligonucleotides reduced the amount of SNORD27 by 80% (Fig. S4B). Oligonucleotides knocking down SNORD2 by 95% were used as a negative control (Fig. S4C). The knockdown of SNORD27 reduced alternative exon skipping fourfold (Fig. 2C). To allow for mechanistic studies, a minigene containing the alternative exon and its two flanking constitutive exons was constructed (Fig. 2B). Transfecting this construct into HeLa cells showed a similar pattern to the endogenous splicing event. However, use of an internal splice site in the exon was more prevalent in the minigene. Similar to the endogenous gene, an SNORD27 knockdown reduced alternative exon skipping (Fig. 2D).
Fig. 2.
SNORD27 influences splicing of the E2F7 pre-mRNA. (A) Sequence alignment between SNORD27, E2F7, and 18S rRNA. SNORD27 is shown in the 3′→5′ direction; all other RNAs are shown in the 5′→3′ direction. The C and D boxes are indicated in bold. The nonconsensus U in the C′ box is indicated in bold italic. The adenosine residue A27 of the 18S rRNA that undergoes 2′-O-methylation is indicated in bold. The putative 2′-O-methylation site on E2F7’s A2789 (RefSeq NM_2033942) is indicated. Capital letters indicate the E2F7 exon, and lowercase letters indicate the intron. The SNP rs310831 [CAT > CAA (H > Q)] in E2F7 is indicated by a circle. (B) Gene structure and splicing pattern of the E2F7 gene region regulated by SNORD27. The location of the SNORD27 binding site is schematically indicated. Angled lines indicate splicing patterns. The splice products are shown in Right. (C) Effect of SNORD27 knockdown on endogenous E2F7 splicing. HeLa cells were transfected with SNORD27 (ASO_27) or SNORD2 (ASO_2) antisense or random base oligonucleotides. The location of the antisense oligonucleotides and the knockdown efficiency of the snoRNAs are shown in Fig. S4. The RNA was analyzed by RT-PCR using primers indicated in B. Graphs show the quantification of the splicing products. The ratio of the exon inclusion to exon skipping of untreated cells was set to one. The fold change is shown. Error bars are SDs of at least four independent experiments. The P value was determined using a two-tailed t test. (D) Analysis of SNORD27 knockdown on E2F7 minigene splicing. The gene region shown in B was cloned under a CMV promoter. HeLa cells were cotransfected with E2F7 minigene and SNORD27 (ASO_27) or SNORD2 (ASO_2) antisense or random base oligonucleotides. The RNA was analyzed similar to in C. (E) Effect of SNORD27 overexpression on E2F7 splicing. An increasing amount of SNORD27 expression construct was cotransfected with the E2F7 minigene in porcine cells (Porcine Aortic Endothelial). C indicates no SNORD27 construct transfected, and 3× and 6× indicate threefold or sixfold molar ratio of SNORD27 construct to E2F7 minigene, respectively. The RNA was analyzed similar to in C. The P value was determined using one-way ANOVA test. M, molecular weight size marker; scr, random base oligonucleotide.
Fig. S4.
Knockdown of SNORD2 and SNORD27. (A) Sequence complementarity between the knockdown oligonucleotide and targets. Stars under the nucleotides indicate the phosphorothioate modification; m indicates the 2′-O-methylation. Knockdown of (B) SNORD27 and (C) SNORD2 detected by RPA. The results of RPA are shown in Left, and their quantification is in Right. scr, Random base oligonucleotide.
SNORD27 depletion thus leads to an almost constitutive use of the alternative exon harboring the predicted binding site. Cotransfection of E2F7 and SNORD27 expression constructs did not increase E2F7 alternative exon skipping in HeLa cells, likely because of the high level of endogenous expression of SNORD27. However, the E2F7 sequence complementarity of SNORD27 is only poorly conserved between pig and human (Table S3B); therefore, we cotransfected porcine (Porcine Aortic Endothelial) cells with human E2F7 and human SNORD27 expression constructs to avoid interference with endogenous SNORD27. We observed an SNORD27 concentration-dependent increase in the E2F7 exon skipping. At the highest ratio, the alternative exon was predominantly skipped, further confirming that SNORD27 blocks use of the alternative splice site (Fig. 2E).
In summary, these data show that SNORD27 blocks use of an alternative exon in E2F7 pre-mRNA.
Sequence Complementarity Between SNORD27 and E2F7 Is Necessary for Splicing Regulation.
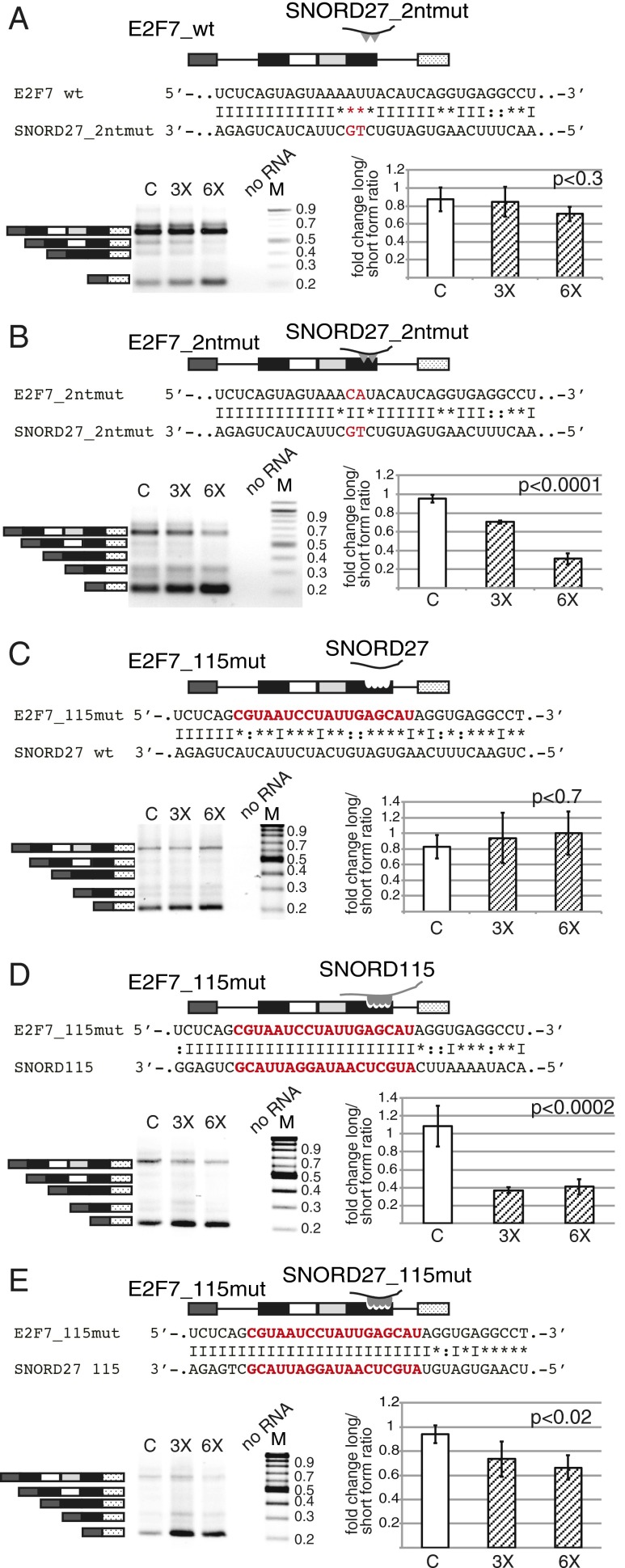
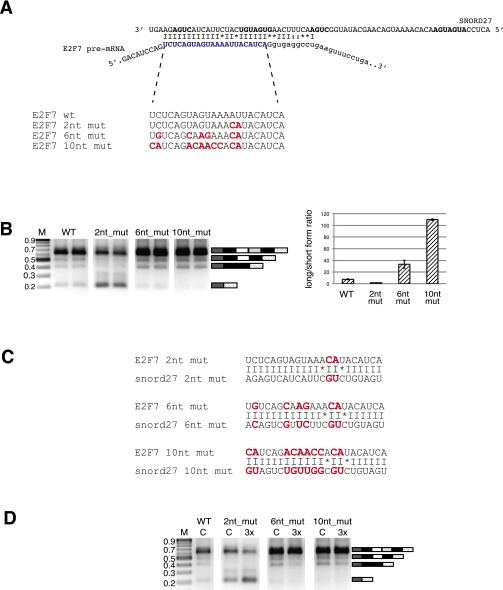
To determine whether a direct SNORD27–E2F7 RNA interaction is necessary for regulation, we used a compensatory mutation approach. We mutated two nucleotides in the region of sequence complementarity between SNORD27 and E2F7 (Fig. 3A). Transfecting the WT SNORD27 had no statistically significant effect on the splicing pattern of the E2F7 minigene carrying these two nucleotide mutations (Fig. 3A). In contrast, using an SNORD27 compensatory mutation strongly induced exon skipping (Fig. 3B). Additional mutations led to almost full alternative exon inclusion (Fig. S5A), and cotransfection of SNORD27 carrying the compensatory mutations did not change the splicing pattern (Fig. S5C). These data show that SNORD27–target RNA binding is necessary for regulation, indicating a direct binding in vivo.
Fig. 3.
Mutational analysis of SNORD27–E2F7 interaction. The gels show RT-PCR analysis of HeLa cells cotransfected with SNORD27 WT or mutant construct and E2F7 WT or mutant minigene. The diagrams schematically show constructs that were used for cotransfection. The sequence alignment shows complementarity between cotransfected pairs, where the mutated sequences are shown in red. C indicates no SNORD27 construct transfected, and 3× and 6× indicate threefold or sixfold molar ratio of SNORD27 construct to E2F7 minigene, respectively. The ratio of the exon inclusion to exon skipping of untreated cells was set to one. The fold change is shown. Error bars are SDs of at least four independent experiments. The P values were determined using one-way ANOVA test. (A) WT E2F7 minigene was cotransfected with SNORD27_2nt mutant (binding site was changed by mutating two nucleotides in the complementary region that generated a 4-nt mismatch). (B) The E2F7 minigene was mutated (E2F7_2ntmut) to compensate for the changes in the SNORD27_2nt mutant. (C) The pre-mRNA serotonin receptor 2C sequences were introduced into the E2F7 minigene (E2F7_115mut) and cotransfected with WT SNORD27. (D) The E2F7_115mut minigene was cotransfected with the SNORD115 construct. (E) The E2F7_115mut minigene was cotransfected with an SNORD27 construct containing the SNORD115 (MBII-52) binding site (SNORD27_115). M, molecular weight size marker.
Fig. S5.
Mutation in the E2F7 region showing sequence complementarity toward SNORD27. (A) Alignment of E2F7 pre-mRNA and SNORD27. The three mutated E2F7 sequences are shown below. (B) Effect of the mutations on E2F7 splicing patterns. HeLa cells were transfected with E2F7 mutated minigenes, and total RNA was analyzed by RT-PCR, similar to that shown in Fig. 2. The two samples represent replicates of the same condition. The quantification of splice isoforms ratio is shown in Right. (C) Alignment of mutated E2F7 pre-mRNA and SNORD27. The E2F7 minigene was mutated to compensate for the changes in the SNORD27 mutants. The sequence alignment shows complementarity between cotransfected pairs, where the mutated sequences are shown in red. (D) Effect of E2F7 compensatory mutations. HeLa cells were cotransfected with E2F7 mutated minigenes, with 3× molar excess of SNORD27 minigenes having compensatory mutations; RNA was analyzed by RT-PCR similar to B. M, molecular weight size marker.
We next tested whether the E2F7 alternative exon could be regulated by a heterologous SNORD. We tested SNORD115, which has been shown to regulate alternative splicing of the serotonin receptor 2C pre-mRNA. We introduced pre-mRNA serotonin receptor 2C sequences into the E2F7 minigene and the SNORD115 antisense sequence into SNORD27. Introducing the serotonin receptor sequences into the E2F7 exon resulted in its skipping, and SNORD27 overexpression resulted in a slight activation of the exon (Fig. 3C). However, introducing the 18-nt sequence complementarity between serotonin receptor 2C pre-mRNA and SNORD115 into the E2F7 alternative exon activated the skipping of this exon (Fig. 3D), similar to cotransfecting the mutated E2F7 exon with SNORD27 containing SNORD115 sequences (Fig. 3E). These responses to compensatory mutations suggest that RNA binding sites can be swapped between pre-mRNA and their regulating SNORDs. However, the exact outcome of the regulation is influenced by the sequence context.
SNORD27 Associates with E2F7 mRNA in the Nucleus.
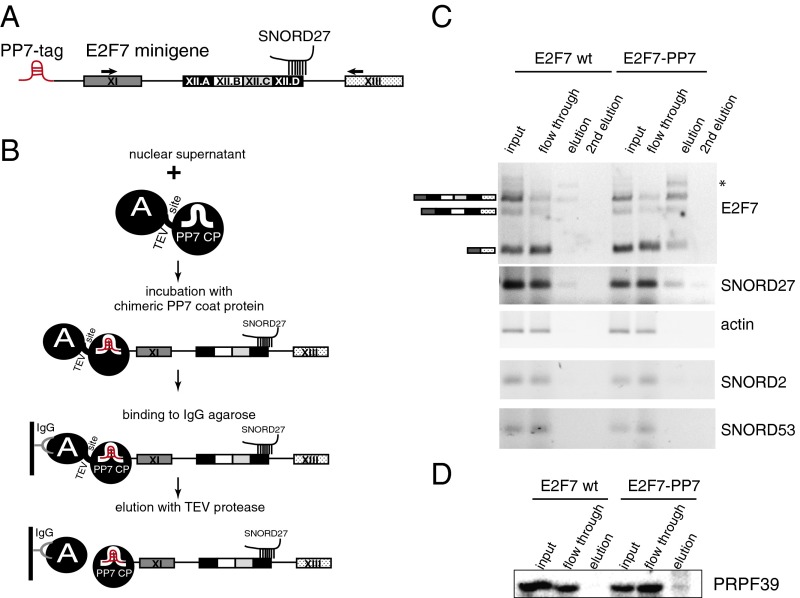
The compensatory mutations strongly suggest a direct interaction between SNORD27 and E2F7 pre-mRNA that could regulate splicing. We further tested an association between SNORD27, E2F7, and splicing factors using biochemical pulldown experiments. To allow for RNA pull-down, we introduced the binding site for Pseudomonas aeruginosa phage 7 (PP7) coat protein at 5′ end of E2F7 minigene (PP7-tag) (40, 54) (Fig. 4A). This system was previously used for affinity purification of native RNP complexes (54). The PP7-tagged construct was transfected into HeLa cells, and a soluble native nuclear fraction was prepared (Fig. 1A). The supernatant was incubated with a PP7 binding protein fused to two Z domains of protein A by a Tobacco etch virus protease (TEV) cleavage site (54), and RNA and associated factors were captured using IgG-agarose beads. E2F7 RNA and associated factors were released using the TEV protease under native conditions (Fig. 4B).
Fig. 4.
SNORD27 binds to E2F7 pre-mRNA in vivo. (A) Schematic representation of the E2F7-PP7 minigene with a PP7 RNA tag at the 5′ end. The location of the SNORD27 binding site and amplification primers are indicated. (B) Purification scheme. Soluble nuclear fractions were obtained from HeLa cells transfected with PP7-tagged E2F7 minigene and incubated with PP7 binding protein fused to protein A. Complexes were isolated using IgG-coated beads and eluted using TEV-protease cleavage. The diagram does not depict the spliceosomal complex within which the eluted transcript is associated. (C) RT-PCR of RNAs eluted after PP7 tag purification. E2F7 and SNORD27 were amplified by RT-PCR, and SNORD53 and β-actin were used as negative controls. *Location of an unspecific extra band. (D) Western blot showing the elution profile of the spliceosome-associated protein PRPF39. Lanes correspond to those in C.
After eluting bound RNA with TEV protease, we could readily detect E2F7 mRNA as well as SNORD27 in the eluates, whereas an E2F7 minigene without PP7 tag produced no signal in the eluates (Fig. 4C). An extra faint band, detected above the RT-PCR product of unspliced E2F7 RNA, likely represents a precursor that has high unspecific binding to the beads, because it was detected in eluates of both untagged control and PP7-tagged E2F7.
Next, we analyzed the same eluates using Western blot with an antibody against the U1 component PRPF39, which again gave a specific signal in the PP7-E2F7 minigene eluates (Fig. 4D). These findings indicate that SNORD27 binds to E2F7 RNA during the splicing reaction.
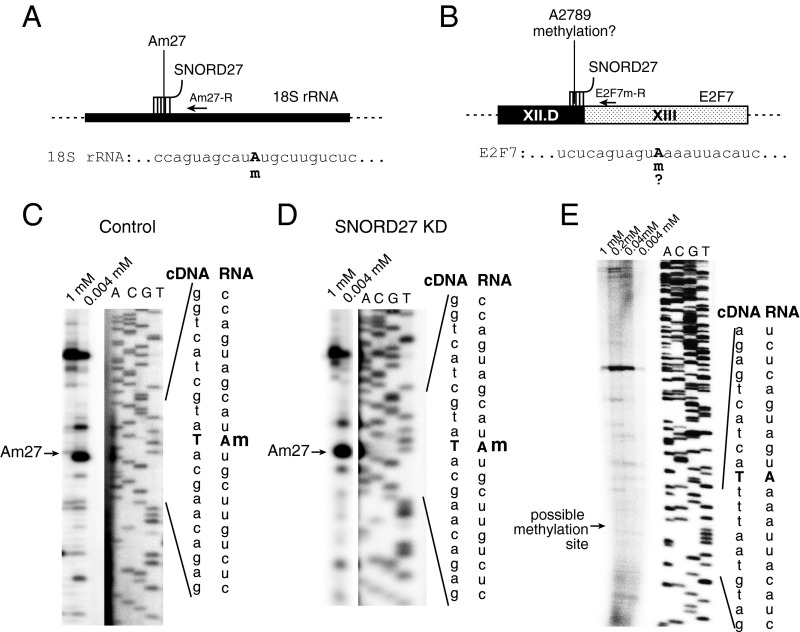
E2F7 mRNA Is Not 2′-O-Methylated in the SNORD27 Binding Site.
Although we could not detect fibrillarin in the soluble nuclear supernatants, the direct interaction between SNORD27 and E2F7 could indicate E2F7 2′-O-methylation by using catalytic amounts of fibrillarin, which could impact splicing. We tested this possibility using primer extension analysis under limited nucleotide concentration. In this assay, RNA is reverse transcribed with a decreasing concentration of deoxynucleotides, resulting in a pause at the methylation site under limiting conditions (Fig. 5 A and B). We used this assay to determine the methylation status at A27 of 18S rRNA and observed a clear pause at the predicted site at 4 µM dNTP concentration (Fig. 5C). In contrast, there is no stop at the E2F7 sequence located 5 nt upstream of the D box (Fig. 5E). SNORD27 knockdown did not change the methylation pattern of 18S RNA, indicating that the splicing changes that we observed are not caused by a deregulation of rRNA (Fig. 5D). Although in some instances, this method fails to detect a 2′-O-methylation (55), the absence of a reverse transcription stop together with fibrillarin not being detected in nuclear supernatant strongly suggest that E2F7 pre-mRNA is not methylated.
Fig. 5.
SNORD27 does not cause 2′-O-methylation of E2F7-RNA. The complementarity between SNORD27 and its targets is indicated in Fig. 2A. (A) Location of the reverse primer Am27-R and SNORD27 binding site in the 18S rRNA. The nucleotide A27 predicted to undergo 2′-O-methylation is indicated with an m. (B) Location of the reverse primer E2F7m-R and a predicted 2′-O-methylation site in the E2F7 RNA. The nucleotide A2789 (RefSeq NM_2033942) predicted to undergo 2′-O-methylation is indicated with an m. (C) Primer extension using 18S rRNA with excess or limited amount of dNTPs using primer Am27-R. (D) Primer extension using 18S rRNA derived from cells treated with SNORD27 knockdown oligonucleotide. (E) Primer extension using E2F7 RNA with excess or limited amount of dNTPs using primer E2F7m-R. The gels show the cDNA sequence corresponding to the RNA sequence shown on the right. KD, knockdown.
SNORD27 Suppresses Silent Exon Inclusion in Multiple Pre-mRNAs.
We next tested more computationally predicted interactions between SNORD27 and pre-mRNAs (Table S2) by comparing splicing patterns between naive HeLa cells and cells undergoing SNORD27 knockdown.
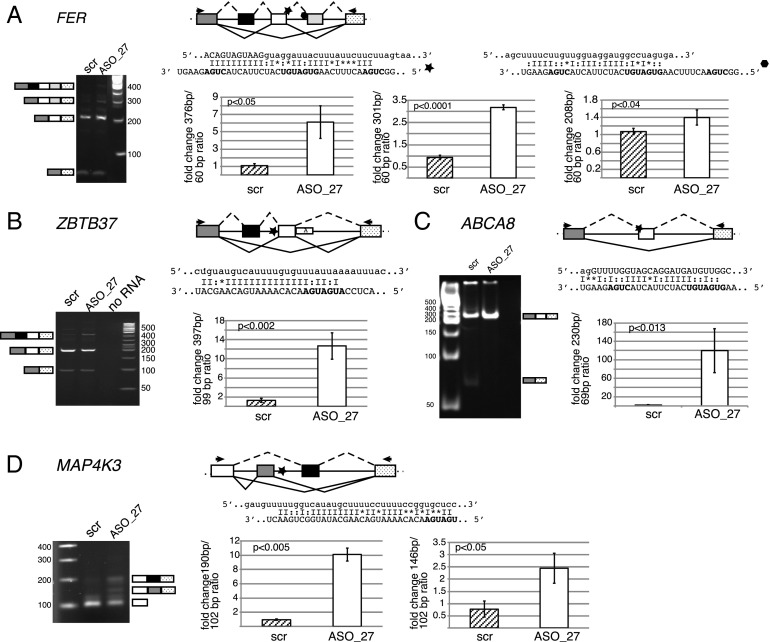
From 30 SNORD27 predicted binding sites near alternative exons, eight exons showed alternative splicing in HeLa cells. In these events, we observed changes in the splicing patterns for FER, ZBTB37, MAP4K3, and ABCA8 (Fig. 6) after SNORD27 knockdown. SNORD27 knockdown resulted in several unexpected bands. Sequencing revealed the existence of nonannotated exons (Fig. 6 and Table S3C), for which no EST evidence was available. Thus, one of the functions of SNORD27 seems to be to repress the inclusion of putative exons. These putative silent exons have canonical 5′ and 3′ splice sites but were not previously detected in mRNAs.
Fig. 6.
SNORD27 regulates multiple exons. Validation of bioinformatically predicted SNORD27 binding sites (Table S2). HeLa cells were transfected with SNORD27 antisense (ASO_27) or random base oligonucleotide (scr), and RNA was analyzed by RT-PCR. The fragments of each investigated gene are shown schematically. Rectangles and lines represent exons and introns, respectively. Black boxes represent silent exons, white and light gray boxes represent alternative exons, and dark gray and dotted boxes constitutive exons. The stars and hexagons show the locations of the SNORD27 binding sites. Solid lines indicate the splicing patterns observed in HeLa cells, and dashed lines indicate the splicing patterns in HeLa cells with SNORD27 knockdown. Sequence alignments show complementarities between SNORD27 and regulated pre-mRNAs. SNORD27 is shown in the 3′→5′ direction; all other RNAs are in the 5′→3′ direction. The C and D boxes are indicated in bold. Canonical base pairing is indicated with a line, G:U base pairing is indicated with a dot, and mismatches are indicated with stars. The graphs in Right show the quantification of the splicing products. The diagram above each graph shows which ratio was determined. The ratio of exon inclusion to exon skipping of untreated cells was set to one. The fold change is shown. Error bars are SDs of three independent experiments. The P values were determined using two-tailed t tests. Effect of SNORD27 knockdown on alternative splicing of the following gene transcripts: (A) FER, (B) ZBTB37, (C) ABCA8, and (D) MAP4K3.
Discussion
SNORDs are highly abundant and among the best-studied ncRNAs. In the classic model, the snoRNA forms a complex with NOP58, NOP56, NHP2L1, and the 2′-O-methyltransferase fibrillarin. The RNA guides this enzymatic activity predominantly to rRNAs using one or two accessible snoRNA parts, the antisense boxes. However, about one-half of 267 human snoRNAs show no sequence complementarity toward other ncRNAs and thus, are orphan, suggesting additional functions (25, 56). Genome-wide analysis of SNORD–rRNA interaction revealed additional base-pairing regions and suggested an asymmetric binding of proteins to human SNORDs (57). These results indicate that there are differences between SNORDs from different organisms and between individual SNORDs. In addition, orphan SNORDs have shown functions in alternative splicing, cholesterol traffic, microRNAs production, and lipid toxicity (reviewed in ref. 58), but their mechanism of action remained unclear.
To obtain mechanistic insight into these noncanonical functions of SNORDs, we isolated nucleoplasmic SNORDs under native conditions and fractionated them using glycerol gradients. RNA-seq of fractions that contained the pre-mRNA splicing machinery but were devoid of fibrillarin showed that a subset of SNORDs is present in these fractions, including SNORDs known to target rRNA. Because the canonical SNORD protein fibrillarin was not detectable, these SNORDs do not form canonical RNPs and do not function in RNA methylation. Not all SNORDs expressed in HeLa cells are present in these native fractions, and some SNORDs seems to be enriched (Fig. S1B and Table S1), which argues against a dissociation of snoRNPs during the isolation procedure that would affect all SNORDs. SNORDs were more abundant in this preparation than H/ACA box snoRNAs. This enrichment may reflect the biogenesis of SNORDs, in that they are released by exonucleases acting on introns released by the spliceosome. In contrast, the generation of H/ACA box snoRNAs is less dependent on splicing (59). Thus, it is possible that, because of their biogenesis, some SNORDs can be retained by the spliceosome, where they could play a role in splice site selection. It is also possible that retention occurs through the NHP2L1 protein, which is a component of both the SNORDs and the U4/U6 complex—a hypothesis that needs to be investigated in the future.
We focused on SNORD27 as an example, because it is deregulated during the progression of smoldering multiple myeloma (21). SNORD27 is not an orphan SNORD, because it exhibits a perfect sequence complementarity to 18S RNA and is predicted to methylate A27 (60), which is methylated in human rRNA. Computational searches revealed a 29-nt-long stretch of complementarity between SNORD27 and the pre-mRNA of the E2F7 transcription factor. This complementarity contains two G:U base pairing and four mismatched bases. Importantly, the C′ and D′ boxes of SNORD27 are part of the sequence complementarity. Their inclusion in the binding site further suggests the noncanonical nature of this SNORD27 complex; as in a canonical snoRNP, these sequences bind to their respective C and D boxes and interact with proteins (6). Furthermore, the involvement of C and D boxes in pre-mRNA binding might explain why we could not detect 2′-O-methylation of E2F7 pre-mRNA.
On the pre-mRNA level, the sequence complementarity encompasses seven of nine bases at the 5′ splice site that bind to U1 snRNA during the splicing reaction. Overexpression of SNORD27 leads to skipping of the exon and knockdown of SNORD27 results in increased exon inclusion, suggesting that SNORD27 inhibits alternative exon use. The analysis of compensatory mutations and RNA pulldown experiments suggests that SNORD27 binds directly to E2F7 pre-mRNAs. The location of the binding site near the 5′ splice site suggests that SNORD27 acts through competition with U1 snRNP. The correct regulation of the E2F7 alternative exon is likely important for the cell, because use of the exon changes the reading frame and alters the C terminus of the protein, which is necessary for the transcriptional activation of its target genes. E2F7 has antiproliferative properties (61, 62), and it is possible that SNORD27 changes observed in cancer influence E2F7-dependent cell cycle regulation.
SNORD27 binding sites in 30 human genes were predicted computationally (Table S2). From this list, SNORD27 knockdown identified four genes with alternative splicing that is influenced by SNORD27 in HeLa cells. In one of the identified genes, FER, the sequence complementarity between SNORD27 and the pre-mRNA encompasses the 5′ splice, and therefore, the SNORD27 could act through competition with U1 snRNP. The binding site in the ABCA8 pre-mRNA covers a 3′ splice site, possibly affecting U2AF binding. The other sequence complementarities are in intronic regions, and the mechanism of action is unclear. Detailed mechanism of this regulation remains to be determined, but these results indicate that the mechanistic mode of action may differ depending on the binding site of the snoRNA on the pre-mRNA.
The exons regulated by SNORD27 in the E2F7, FER, ZBTB37, MAP4K3, and ABCA8 genes are all suboptimal. For example, the E2F7 exon is 425 nt in length, which is abnormally long for a cassette exon. The SNORD27-dependent exons in the other genes are flanked by weak splice sites, and there is no EST evidence for their existence in cells. Using RT-PCR, these exons could not be detected without SNORD27 knockdown. A biological function of SNORD27 could thus be the repression of pre-mRNA sequences that have the potential to be recognized as weak alternative exons. Because some of these weak exons are generated through short (Alu) or long (LINE) interspersed elements (63–65), SNORDs might have a more general role in suppressing newly formed exons.
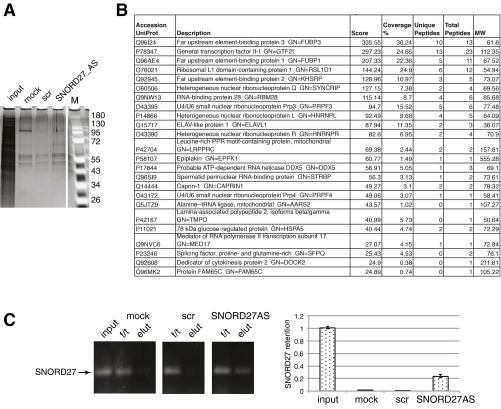
Mechanistically, this regulation of alternative splicing is likely achieved by the formation of a protein–SNORD27 complex, likely including heterogeneous ribonucleoprotein (hnRNP) proteins, that binds to pre-mRNAs. SNORD27 is equally distributed throughout a 10–45% glycerol gradient (Fig. 1D), suggesting that it forms protein complexes of differing composition. In contrast, SNORD2 and SNORD60 peak at low glycerol concentrations, suggesting a unique SNORD–protein complex (Fig. S3). To gain insight into proteins associated with SNORD27, we pulled down SNORD27 from soluble nuclear fractions using RNA capture oligos and identified associated proteins using MS (Fig. S6). These proteins were predominantly RNA binding proteins, including hnRNPs, which might associate with SNORD27. However, the precise nature of these hnRNP–SNORD27 complexes remains to be determined.
Fig. S6.
Affinity isolation of proteins bound to SNORD27 in nuclear supernatants. (A) SNORD27 present in soluble nuclear extract was purified using RNA capture oligonucleotides with the sequence shown in Fig. S4A, except that oligonucleotide was 2′-O-methyl-phosphothioated and 3′-TEG-biotinylated. RNA–protein complexes were isolated using streptavidin. The gel shows staining of the eluted proteins. (B) Proteins that were only detected in pulldowns with SNORD27 antisense oligonucleotide but not detected in negative controls (mock or random base oligonucleotide). Percentage coverage was calculated by dividing the number of amino acids in all found peptides by the total number of amino acids in the entire protein sequence. The scores are calculated based on the log10 of the probability that the observed match between the experimental data and the database sequence is a random event (82). (C) SNORD27 pulldown efficiency. The input, flow-through fractions (f/t), and eluates from the mock, random base, and SNORD27 antisense oligo pulldowns were analyzed using (Left) end point RT-PCR and (Right) real-time PCR with SNORD27-specific primers. M, molecular weight size marker; mock, no RNA capture oligonucleotide present; MW, molecular weight; scr, random base oligonucleotide; SNORD27AS, SNORD27 antisense oligonucleotide.
The sequence complementarities between SNORD27 and all of its targets contained the C and D boxes of SNORD27 (Fig. 7A). The presence of C and D boxes in the binding sites indicates that the whole SNORD sequence has to be analyzed to identify target genes. As with other SNORDs, the RPA of SNORD27 showed the occurrence of smaller fragments (Fig. 1D). It is currently unclear whether the shorter fragments or the full-length snoRNA is active in splice site regulation. Because the full-length snoRNA is more abundant for SNORD27, we assume that the full-length form is the active one.
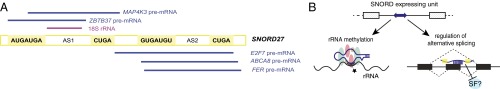
Fig. 7.
A dual role for SNORD27 in rRNA and pre-mRNA processing. (A) Regions of complementarity between SNORD27 and its pre-mRNA and rRNA targets. The C (RUGAUGA), D (CUGA), and antisense boxes (AS1 and AS2) are indicated. Lines indicate the sequence complementarity toward regulated pre-mRNAs and 18S rRNA. The detailed RNA sequences are shown in Figs. 2A and 6. (B) Working model for SNORD27 action on pre-mRNA regulation. (B, Lower Left) SNORD27 can associate with NOP56, NOP58, NHP2L1, and fibrillarin to form a canonical snoRNP that performs 2′-O-methylation on rRNA (B, Lower Right). A portion of the snoRNA associates with RNA binding proteins and regulates pre-mRNA splicing, where it represses exons, possibly by competing with activating splicing factors, such as U1 snRNA, or other splicing factors (SFs).
An evolutionary analysis (Table S3D) shows that the first antisense box binding to 18S rRNA is highly conserved between SNORD27 orthologs. However, sequences corresponding to the E2F7 interaction site are highly divergent during evolution without compensatory mutations in the E2F7 genes (Table S3E). Thus, searches for noncanonical SNORD targets should not take evolutionary conservation into account and should include the whole sequence, not just the antisense boxes. Furthermore, SNORD27 fragments have also been observed in mouse retinas, where 19-, 26-, and 28-nt-long fragments were identified by deep sequencing (66), suggesting a conservation of noncanonical properties. It is possible that SNORD regulation of alternative splicing is species-specific, possibly reflecting the low conservation of alternative frameshifting exons between species (67, 68). In this scenario, newly evolved alternative exon use could be regulated by interaction with SNORDs.
Several SNORDs and H/ACA box snoRNAs have been shown to be precursors for microRNAs (69, 70). Consistent with our observations, Brameier et al. (70) found that SNORD27 [designated as U27 sno-miRNA (for snoRNA derived microRNA)] generates short stable fragments of ∼20 nt in length. However, the physiological function of these fragments remains unclear, because U27 sno-miRNA did not cause an effect in a Renilla/Luciferase reporter system in HeLa cells.
Our finding that numerous SNORDs predicted to modify rRNA, among them SNORD27, SNORD2, SNORD60, and SNORD78, can be detected in fractions where fibrillarin was not detected suggests that canonical SNORDs have biological roles in addition to their involvement in 2′-O-methylation. Importantly, assays that solely rely on detecting the association with fibrillarin (71) will miss these previously unidentified, noncanonical functions, because they do not take localization in a fibrillarin-free fraction into account. It is possible that the unexpected role of SNORD60 in intracellular cholesterol trafficking is caused through a fibrillarin-free RNP acting on pre-mRNAs and not through rRNA methylation (19).
As exemplified by SNORD27, an SNORD can function in both pre-mRNA and rRNA metabolism (Fig. 7B). Findings in yeast suggest that cells can regulate the assembly of snoRNAs into SNORD-RNPs through the R2TP complex (26, 27). It is thus possible that a cell can regulate the fraction of SNORDs acting in pre-mRNA or rRNA processing. For example, cells might use more SNORD27 for rRNA modification when there is demand for protein biosynthesis. This model suggests that SNORDs could participate in coordinating mRNA processing with ribosomal biogenesis.
More than 2,500 transcription factors have been identified in humans (72). In contrast, less than 50 sequence-specific splicing regulators have been described (73). It is possible that ncRNAs provide additional sequence-specific splicing regulation, and we suggest that this is a novel role for some SNORDs.
Materials and Methods
Preparation of Soluble Nuclear Supernatant Under Native Conditions.
Nuclear supernatant was prepared as previously described with minor modifications (36, 37). HeLa cells, grown in five 15-cm Petri dishes, were washed two times in VB buffer [0.125 M KCl, 30 mM Tris⋅HCl, pH 7.5, 5 mM Mg(oAc)2, 0.15 mM spermine, 0.05 mM spermidine, 2 mM vanadyl ribonucleosides], resuspended in isotonic SB buffer [10 mM KCl, 30 mM Tris⋅HCl, pH 7.5, 5 mM Mg(OAc)2, 0.15 mM spermine, 0.05 mM spermidine, 2 mM vanadyl ribonucleosides], and opened with 20 strokes in Dounce homogenizer (pestle B). Cell nuclei were layered on the glycerol cushion [25% (vol/vol) glycerol, 10 mM KCl, 30 mM Tris⋅HCl, pH 7.5, 5 mM Mg(oAc)2, 0.15 mM spermine, 0.05 mM spermidine, 2 mM vanadyl ribonucleosides]. After centrifugation for 4 min at 4,000 × g, the aqueous layer was designated as “cytoplasm,” and the pellet was designated as “crude nuclei.” Nuclei pellet was washed two times, resuspended in ST2M buffer (0.1 M NaCl, 10 mM Tris⋅HCl, pH 8.0, 2 mM MgCl2, 0.15 mM spermine, 0.05 mM spermidine, 2 mM vanadyl ribonucleosides), and sonicated using a Bioraptor Plus (Diagenode) instrument (two cycles of 30-s pulse and 30-s break; setting: high). After chromatin precipitation in the presence of 2 mg/mL yeast tRNA (Life Technology), sample was cleared by centrifugation (3 min at 13,500 × g), and resulting supernatant was designated as “nuclear supernatant.” The pellet was resuspended in 1× RIPA buffer (0.15 M NaCl, 50 mM Tris⋅HCl, pH 8.0, 1% Nonidet P-40, 0.5% sodium deoxycholate, protease inhibitor mixture), sonicated using a Bioraptor instrument (three cycles of 30-s pulse and 30-s break; setting: high), and cleared. The supernatant was designated as “resuspended nuclear pellet.”
RNA Isolation and RNA-Seq.
Nuclear supernatant (described above) was fractionated using 10–45% (vol/vol) glycerol gradients (37). Centrifugations were carried out at 4 °C in an SW41 rotor run at 41,000 rpm (287,472 × g) for 90 min. Fractions cosedimenting with splicing factors (8–11; sedimenting at 200S) were pooled, and RNA was isolated and analyzed. To extract RNA associated with the spliceosomes, fractions of the glycerol gradients (520 µL) were mixed with 150 µL extraction buffer (50 mM Tris⋅HCl, pH 7.5, 300 mM NaCl) and 50 µL 10% (wt/vol) SDS, and the RNA was recovered by extraction with phenol and precipitation in ethanol (37). The integrity of the RNA was evaluated by an Agilent 2100 BioAnalyzer. For small RNA library construction, ∼10 µg RNA was used followed by Illumina Directional mRNA-Seq Library Prep. The prerelease protocol was used with the following changes: (i) the poly-A selection and fragmentation of mRNA steps were omitted; and (ii) to enrich for small RNAs, ethanol precipitation was used instead of column fractionation of the T4 polynucleotide kinase-treated RNA. Adaptors were then ligated to the 5′ and 3′ ends of the RNA, and cDNA was prepared from the ligated RNA and amplified to prepare the sequencing library. The amplified sequences were purified by PAGE, and sequences representing RNA smaller than 200 nt were extracted from the gel. The library was sequenced using the Genome AnalyzerIIX System by Illumina.
SI Materials and Methods describes cell lines, analysis of sequencing data, PP7-tagged RNA affinity purification, immunoprecipitation and RNA isolation, methylation analysis, protein analysis by Western blot, purification of the recombinant proteins, RNase protection analysis, list of primers, RT-PCR, purification of RNA and bound proteins using biotinylated antisense oligonucleotides, and MS and protein identifications.
SI Materials and Methods
Cell Lines.
HeLa S3, Porcine Aortic Endothelial, NIH 3T3, and CHO-K1 cells were grown in high-glucose DMEM containing 10% (vol/vol) heat-inactivated FCS at 37 °C in 5% CO2. Transfection experiments were performed using Lipofectamine 2000 (Life Technology) as described in the manufacturer’s protocol.
Analysis of Sequencing Data.
The sequencing gave ∼29 million reads passed filters. These reads were quality trimmed at the 3′ end, and adapter sequences were removed, keeping only reads with lengths of at least 12 nt after trimming. Finally, reads were filtered by fastq_quality_filter of the FASTX package to keep only reads with quality at least 25 at 95% or more of the read. This cleaning process kept ∼19 million reads, which were subsequently mapped to the human genome by TopHat (v2.0.11) (74, 75), giving 14,155,782 mapped reads. These reads were used for the analysis-adapting miRExpress program (v2.1.4) (75) for snoRNAs.
Reads were mapped against the human snoRNAs sequences from snoRNA database (snoRNABase) (46), allowing up to 7% mismatches. In total, 21,428, 8,496, or 1,735 reads (for 12 and above, 20 and above, or 60 and above analyses, respectively) were mapped to snoRNA sequences. No position and length coverage restrictions were applied for counting the total amount of reads that map to a certain snoRNA. Reads were counted as full length when they covered at least 90% of the snoRNA sequence; otherwise, they were counted as short.
To determine RNA expression in HeLa, we downloaded HeLa-S3 cell alignment data for whole-cell small RNA-seq data from the ENCODE (76) website. Then, for each of the replicates , we used the intersectBed tool from the BEDTools suite (77) to count the number of reads falling within the boundaries of the snoRNAs. The libraries used were replicate 1 (hgdownload.cse.ucsc.edu/goldenPath/hg19/encodeDCC/wgEncodeCshlShortRnaSeq/wgEncodeCshlShortRnaSeqHelas3CellShorttotalTapAlnRep1.bam) and replicate 2 (hgdownload.cse.ucsc.edu/goldenPath/hg19/encodeDCC/wgEncodeCshlShortRnaSeq/wgEncodeCshlShortRnaSeqHelas3CellShorttotalTapAlnRep2.bam).
PP7-Tagged RNA Affinity Purification.
A PP7 tag was introduced into 5′ UTR of the E2F7 minigene (E2F7-PP7). A PP7 pulldown was performed as previously described (40). All steps were conducted at 4 °C, with mild rotation. Nuclear supernatant corresponding to two 15-cm Petri dishes was supplemented with 10% (vol/vol) glycerol, 0.1% Nonidet P-40, and complete protease inhibitor; 1 μg TEV and chimeric Pseudomonas aeruginosa phage 7 coat protein was added to the nuclear supernatant followed by incubation for 2 h. Affinity purification was performed on prewashed rabbit IgG agarose beads (Sigma Aldrich) for an additional 2 h. Before overnight incubation with TEV protease [0.2 µg/µL in 10% (vol/vol) glycerol, 10 mM Tris, pH 8, 2 mM MgCl2, 100 mM NaCl, 1 mM DTT], agarose was washed three times with binding buffer and once with TEV buffer. RNA was isolated using TRIzol LS.
Immunoprecipitation and RNA Isolation.
Immunoprecipitations were performed in ST2M+Nonidet P-40 buffer (0.1 M NaCl, 10 mM Tris⋅HCl, pH 8.0, 2 mM MgCl2, 1% Nonidet P-40, complete protease inhibitor mixture; Life Technology) from “nuclear supernatant” and/or “nuclear pellet” corresponding to two 15-cm Petri dishes of the HeLa cells. The nuclear supernatant fraction was supplemented with Nonidet P-40 and protease inhibitor, and the nuclear pellet buffer was replaced with ST2M+Nonidet P-40 buffer using Amicon Pro Centrifugal Filters 3 kDa (Millipore). All steps were conducted at 4 °C, with mild agitation.
Fibrillarin complexes were immunoprecipitated by overnight incubation with 3 µg anti-Fibrillarin antiserum (ab5821; Abcam) and 50 µL Dynabeads Protein A followed by five washing steps with ST2M-Nonidet P-40. RNA was eluted from the beads in 50 µL by incubation with Protease K mixture (0.4 µg/µL Protease K in 30 mM Tris⋅HCl, pH 8.0, 10 mM EDTA, 1% SDS) for 1 h at 50 °C. Resulting supernatant was treated with phenol:chloroform:isoamil alcohol (25:24:1; pH 8.0) and precipitated by 150 µL absolute ethanol in the presence of 300 mM NaCl and 10 µg glycoblue (Ambion).
Flag-tagged versions of NOP56, NOP58, and NHP2L1 were overexpressed in HeLa cells for 24 h before immunoprecipitation (48 µg plasmid and 60 µL Lipofectamin 2000 in 5 mL OptiMEM per dish; media was changed after 6 h of transfection). Nuclear supernatant and nuclear pellet were prepared as described above. RNA–protein complexes were immunoprecipitated using anti-FLAG M2 Magnetic Beads in ST2M+Nonidet P-40 for 1 h. RNA–protein complexes were eluted by incubation of the beads with 100 µL 3× Flag peptide in ST2M+Nonidet P-40 buffer (100 µg/mL). RNA was purified using TRIzol LS (Life Technology).
Methylation Analysis.
The 2′-O-methylation status of the RNA was analyzed using in vitro reverse transcription with 5′-end radiolabeled primer (55, 78). When low concentration of dNTPs is used, reverse transcriptase tends to pause and terminate cDNA synthesis near 2′-O-methylated nucleotides. The products of the reaction resolved on the sequencing gel show strong stop signal 1 nt downstream from the actual position of the methylation. Oligonucleotide labeling, primer extension reaction, and sequencing reactions were performed using previously published protocols (78). We used 5 and 100 µg total HeLa RNA to determine methylation status of the 18S rRNA and E2F7 mRNA, respectively. All samples were treated with DNase-free RNase A before gel electrophoresis.
Protein Analysis by Western Blot.
Same volumes of separate and combined gradient fractions were resolved in 10% (wt/vol) polyacrylamide gel (PAAG) and transferred to nylon membrane. Specific proteins were detected by ECL detection after standard immunoblotting with the following antibodies: rabbit polyclonal anti-Fibrillarin (ab5821; Abcam), rabbit polyclonal anti-NOP56 (A302-721A; Bethyl Laboratories), rabbit polyclonal anti-NOP58 (ab98160; Abcam), rabbit polyclonal anti-NHP2L1 (ab95958; Abcam), rabbit polyclonal anti-NCBP1 (CBP80; ab42389; Abcam), mouse monoclonal anti-ALY/REF (SC-32311; Santa Cruz), rabbit polyclonal anti-hnRNPG (44), mouse monoclonal anti-hnRNPC1/C2 (sc-32308; Santa Cruz), mouse monoclonal anti-hnRNPU (SC-32315; Santa Cruz), rabbit polyclonal anti-SF3b1 (79), rabbit polyclonal anti-PRPF39 (ab97654; Abcam), and rabbit monoclonal PRP8 (H-300; Santa Cruz). Primary antibodies were detected by donkey anti-rabbit and anti-mouse antibodies conjugated with HRP.
Purification of the Recombinant Proteins.
TEV and chimeric Pseudomonas aeruginosa phage 7 coat proteins were purified as previously described (54, 80).
RNase Protection Analysis.
RNase protection analysis was performed with uniformly 32P-labeled probes using the RPA III Kit (Ambion). The following sequences were cloned in reverse orientation in pCRII-TOPO or pcDNA3.1-TOPO, digested with SpeI or XhoI endonucleases, and used to generate RNase protection probes by in vitro transcription with T7 RNA polymerase. Uppercase and lowercase letters correspond to the mature SNORDs and intronic sequences, respectively. Probes for protection and minigenes were deposited into Addgene:
>SNORD2: ccttaAAGTGAAATGATGGCAATCATCTTTCGGGACTGACCTGAAATGAAGAGAATACTCATTGCTGATCACTTGattat;
>SNORD27: taaccACTCCATGATGAACACAAAATGACAAGCATATGGCTGAACTTTCAAGTGATGTCATCTTACTACTGAGAAGTgagag;
>SNORD60: AGTCTGTGATGAATTGCTTTGACTTCTGACACCTCGTATGAAAACTGCACGTGCAGTCTGATTATTTAGCAAGACTGAGGCTT; and
>SNORD78: ggggtttGTGTAATGATGTTGATCAAATGTCTGACCTGAAATGAGCATGTAGACAAAGGTAACACTGAAGAAccctgtg.
List of Primers.
pre-mRNA alternative splicing assay: E2F7-F2 (GGAAAGGCAACAGCAAACTC), E2F7-R2 (CGGGTGTCTTGAAAAACGTC); ZBTB375f (GCCAACAAGCAGTGAAGTTG), ZBTB375r1 (CCACCGCTCAGATACTTCCT); FERf (CGCATTCTAGACTCCCGAAG), FERr (CCTCCACACTGCACAAGTGA); MAP4K3f (CCCGACAGTGATGGTTTTT), MAP4K3r (ACCACCTTGGTGTCCTTGTC ); and Abca8F (CTTGCTTCCATTTTCCCAGA), Abca8R (AGGCCCAAGTTTGTTGACAC). The 18S A27 methylation assay: Am27-R (TCTGATAAATGCACGCATCC), and the E2F7 methylation assay: E2F7m-R (CAAAGCGGCAGGTTAGTCAG).
Reverse Transcription Polymerase Chain Reaction (RT-PCR).
Total cells RNA was isolated using the GenElute Mammalian Total RNA Miniprep Kit (Sigma Aldrich). Reverse transcription was performed with the SuperScript III Reverse Transcriptase Kit (Life Technology) using 1 µg total RNA and 10 pmol reverse sequence-specific primers. The reaction was incubated at 50 °C for 50 min; 1/20th of the reverse transcription reaction (RT) was used for end point PCR with Platinum Taq Polymerase (Life Technology), 0.2 mM dNTPs, and 0.2 µM primers in standard Platinum Taq buffer or quantitative PCR with PerfeCTa SYBR Green SuperMix Low ROX (Quanta). The amplification for end point PCR was performed in an Eppendorf PCR System, and for quantitative PCR, it was performed in a Stratagene Mx3005P instrument with the following conditions: initial denaturation for 1 min at 94 °C, optimized number of cycles for 30 s at 94 °C and 30 s at 55 °C, and extension of 1 min at 72 °C. PCR products were resolved in 2% (wt/vol) agarose gels and stained by ethidium bromide. The quantification of the splicing products was done using the program ImageJ. The ratio of the exon inclusion to exon skipping of untreated cells was set to one. Error bars are SDs of at least four independent experiments. P values were calculated using one-way ANOVA test or two-tailed t test.
Purification of RNA and Bound Proteins Using Biotinylated Antisense Oligonucleotides.
All steps were conducted at 4 °C, with mild agitation. Pulldown was performed with RNA oligonucleotide [2′-O-methylated phosphorothioate with tetra-ethyleneglycol biotin (TEG-Biotin) on 3′-end], having 20 nucleotides complementary to the first antisense box of SNORD27 RNA. M-280 Streptavidin Dynabeads (Life Technology) were washed three times with 10 vol ST2M+0.1NP40 buffer [0.1 M NaCl, 10 mM Tris⋅HCl, pH 8.0, 2 mM MgCl2, 0.1% Nonidet P-40, complete protease inhibitor mixture (Life Technology), 1 µg/µL RNase Inhibitor (New England Biolabs)] and preblocked by incubation with 500 ng/µL yeast tRNA and 1 mg/mL BSA in ST2M+0.1NP40 buffer for 1 h. Each sample was incubated with an aliquot of beads corresponding to 30 µL initial suspension. Nuclear supernatant corresponding to five 15-cm Petri dishes of the HeLa cells in ST2M+0.1NP40 buffer was depleted from unspecific streptavidin binding material by incubation with prewashed Dynabeads for 1 h. Depleted nuclear supernatant was incubated with 1 µg SNORD27 antisense oligonucleotide (2 ng/µl final) or random base oligonucleotide for 5 h followed by additional incubation for 1 h with preblocked Dynabeads and five washings with 1 mL ST2M+0.1NP40 buffer. Two-thirds of the beads were treated with RNase A/T1 (1:10 dilution; Ambion) for 1 h at 37 °C followed by the addition of 1× Laemmli SDS/PAGE buffer and incubation for 10 min at 95 °C. One-half of the material was loaded on 10% PAAG. Gel was stained overnight by SyproRuby (Life Technology) according to the manufacturer’s protocol. RNA was eluted from one-third of the total amount of beads as described for immunoprecipitation.
MS and Protein Identifications.
Liquid chromatography–MS/MS analysis was performed using an LTQ-Orbitrap Mass Spectrometer (Thermo Fisher Scientific) coupled with an Eksigent Nanoflex cHiPLC System (Eksigent) through a nanoelectrospray ionization source (82). The peptide samples were separated with a reversed-phase cHiPLC column (75 μm × 150 mm) at a flow rate of 300 nL/min. Mobile phase A was water with 0.1% (vol/vol) formic acid, whereas B was acetonitrile with 0.1% (vol/vol) formic acid. A 50-min gradient condition was applied: initial 3% mobile phase B was increased linearly to 50% in 24 min and further, to 85% and 95% for 5 min each before it was decreased to 3% and reequilibrated. The mass analysis method consisted of one segment with eight scan events. The first scan event was an Orbitrap MS Scan (100−1,600 m/z), with 60,000 resolution for parent ions followed by data-dependent MS/MS for fragmentation of the seven most intense ions with the collision-induced dissociation method. The liquid chromatography–MS/MS data were submitted to a local mascot server for MS/MS protein identification by Proteome Discoverer (version 1.3; Thermo Fisher Scientific) against the Homo sapiens (human) taxonomy subset of the Swissprot database. Parameters used in the MASCOT MS/MS ion search were trypsin digest with maximum of two miscleavages, cysteine carbamidomethylation, methionine oxidation, a maximum of 10-ppm MS error tolerance, and a maximum of 0.8-Da MS/MS error tolerance. A decoy database was built and searched. Filter settings that determine false discovery rates are used to distribute the confidence indicators for the peptide matches. Peptide matches that pass the filter associated with the strict false discovery rate (with a target setting of 0.01) are assigned as high confidence. For the MS/MS ion search, proteins with two or more high-confidence peptides were considered unambiguous identifications without manual inspection. Proteins identified with one high-confidence peptide were manually inspected and confirmed.
Supplementary Material
Acknowledgments
The authors thank N. J. McGlincy, C. Waechter, K. Collins, and G. Grohs for discussions. We thank the University of Kentucky Proteomics Core, which was supported by National Institute of General Medical Sciences Centers of Biomedical Research Excellence Grant P20GM103486-09 and the Office of the Vice President for Research of the University of Kentucky as well as High-End Instrumentation Grant S10RR029127. This work was supported by Postdoctoral Fellowship 13POST16820024 from the American Heart Association (to M.F.); MINECO (Ministerio de Economía y Competitividad) Spanish Government Grant BIO2014-52566-R (to A.P. and E.E.); Consolider RNAREG Grant CSD2009-00080 (to A.P. and E.E.); Sandra Ibarra Foundation for Cancer Grant FSI2013 (to A.P. and E.E.); AGAUR (Agència de Gestió d’Ajuts Universitaris i de Recerca) Grant 2014-SGR1121 (to A.P. and E.E.); USA-Israel Binational Science Foundation, Transformative Grant 2010508 (to R.S. and S.S.); NIH Grants 01GM079549 (to R.S.) and 01GM083187 (to S.S.); and the Foundation for Prader-Willi Research (S.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1519292113/-/DCSupplemental.
References
- 1.Filipowicz W, Pelczar P, Pogacic V, Dragon F. Structure and biogenesis of small nucleolar RNAs acting as guides for ribosomal RNA modification. Acta Biochim Pol. 1999;46(2):377–389. [PubMed] [Google Scholar]
- 2.Filipowicz W, Pogacić V. Biogenesis of small nucleolar ribonucleoproteins. Curr Opin Cell Biol. 2002;14(3):319–327. doi: 10.1016/s0955-0674(02)00334-4. [DOI] [PubMed] [Google Scholar]
- 3.Watkins NJ, Bohnsack MT. The box C/D and H/ACA snoRNPs: Key players in the modification, processing and the dynamic folding of ribosomal RNA. Wiley Interdiscip Rev RNA. 2012;3(3):397–414. doi: 10.1002/wrna.117. [DOI] [PubMed] [Google Scholar]
- 4.Hirose T, Shu MD, Steitz JA. Splicing-dependent and -independent modes of assembly for intron-encoded box C/D snoRNPs in mammalian cells. Mol Cell. 2003;12(1):113–123. doi: 10.1016/s1097-2765(03)00267-3. [DOI] [PubMed] [Google Scholar]
- 5.Bizarro J, et al. Proteomic and 3D structure analyses highlight the C/D box snoRNP assembly mechanism and its control. J Cell Biol. 2014;207(4):463–480. doi: 10.1083/jcb.201404160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lapinaite A, et al. The structure of the box C/D enzyme reveals regulation of RNA methylation. Nature. 2013;502(7472):519–523. doi: 10.1038/nature12581. [DOI] [PubMed] [Google Scholar]
- 7.Hüttenhofer A, et al. RNomics: An experimental approach that identifies 201 candidates for novel, small, non-messenger RNAs in mouse. EMBO J. 2001;20(11):2943–2953. doi: 10.1093/emboj/20.11.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jády BE, Kiss T. Characterisation of the U83 and U84 small nucleolar RNAs: Two novel 2′-O-ribose methylation guide RNAs that lack complementarities to ribosomal RNAs. Nucleic Acids Res. 2000;28(6):1348–1354. doi: 10.1093/nar/28.6.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavaillé J, et al. Identification of brain-specific and imprinted small nucleolar RNA genes exhibiting an unusual genomic organization. Proc Natl Acad Sci USA. 2000;97(26):14311–14316. doi: 10.1073/pnas.250426397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vitali P, et al. Identification of 13 novel human modification guide RNAs. Nucleic Acids Res. 2003;31(22):6543–6551. doi: 10.1093/nar/gkg849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saraiya AA, Wang CC. snoRNA, a novel precursor of microRNA in Giardia lamblia. PLoS Pathog. 2008;4(11):e1000224. doi: 10.1371/journal.ppat.1000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutzinger R, et al. Expression and processing of a small nucleolar RNA from the Epstein-Barr virus genome. PLoS Pathog. 2009;5(8):e1000547. doi: 10.1371/journal.ppat.1000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taft RJ, et al. Small RNAs derived from snoRNAs. RNA. 2009;15(7):1233–1240. doi: 10.1261/rna.1528909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawaji H, et al. Hidden layers of human small RNAs. BMC Genomics. 2008;9:157. doi: 10.1186/1471-2164-9-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott MS, Avolio F, Ono M, Lamond AI, Barton GJ. Human miRNA precursors with box H/ACA snoRNA features. PLOS Comput Biol. 2009;5(9):e1000507. doi: 10.1371/journal.pcbi.1000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ono M, et al. Identification of human miRNA precursors that resemble box C/D snoRNAs. Nucleic Acids Res. 2011;39(9):3879–3891. doi: 10.1093/nar/gkq1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding F, et al. SnoRNA Snord116 (Pwcr1/MBII-85) deletion causes growth deficiency and hyperphagia in mice. PLoS One. 2008;3(3):e1709. doi: 10.1371/journal.pone.0001709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duker AL, et al. Paternally inherited microdeletion at 15q11.2 confirms a significant role for the SNORD116 C/D box snoRNA cluster in Prader-Willi syndrome. Eur J Hum Genet. 2010;18(11):1196–1201. doi: 10.1038/ejhg.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brandis KA, et al. Box C/D small nucleolar RNA (snoRNA) U60 regulates intracellular cholesterol trafficking. J Biol Chem. 2013;288(50):35703–35713. doi: 10.1074/jbc.M113.488577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michel CI, et al. Small nucleolar RNAs U32a, U33, and U35a are critical mediators of metabolic stress. Cell Metab. 2011;14(1):33–44. doi: 10.1016/j.cmet.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.López-Corral L, et al. Genomic analysis of high-risk smoldering multiple myeloma. Haematologica. 2012;97(9):1439–1443. doi: 10.3324/haematol.2011.060780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong XY, et al. Implication of snoRNA U50 in human breast cancer. J Genet Genomics. 2009;36(8):447–454. doi: 10.1016/S1673-8527(08)60134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong XY, et al. SnoRNA U50 is a candidate tumor-suppressor gene at 6q14.3 with a mutation associated with clinically significant prostate cancer. Hum Mol Genet. 2008;17(7):1031–1042. doi: 10.1093/hmg/ddm375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siprashvili Z, et al. The noncoding RNAs SNORD50A and SNORD50B bind K-Ras and are recurrently deleted in human cancer. Nat Genet. 2016;48(1):53–58. doi: 10.1038/ng.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deschamps-Francoeur G, et al. Identification of discrete classes of small nucleolar RNA featuring different ends and RNA binding protein dependency. Nucleic Acids Res. 2014;42(15):10073–10085. doi: 10.1093/nar/gku664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kakihara Y, Makhnevych T, Zhao L, Tang W, Houry WA. Nutritional status modulates box C/D snoRNP biogenesis by regulated subcellular relocalization of the R2TP complex. Genome Biol. 2014;15(7):404. doi: 10.1186/s13059-014-0404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prieto MB, Georg RC, Gonzales-Zubiate FA, Luz JS, Oliveira CC. Nop17 is a key R2TP factor for the assembly and maturation of box C/D snoRNP complex. BMC Mol Biol. 2015;16:7. doi: 10.1186/s12867-015-0037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan Q, et al. Alternative splicing of conserved exons is frequently species-specific in human and mouse. Trends Genet. 2005;21(2):73–77. doi: 10.1016/j.tig.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Wang ET, et al. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456(7221):470–476. doi: 10.1038/nature07509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelemen O, et al. Function of alternative splicing. Gene. 2013;514(1):1–30. doi: 10.1016/j.gene.2012.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kishore S, Stamm S. The snoRNA HBII-52 regulates alternative splicing of the serotonin receptor 2C. Science. 2006;311(5758):230–232. doi: 10.1126/science.1118265. [DOI] [PubMed] [Google Scholar]
- 32.Scott MS, et al. Human box C/D snoRNA processing conservation across multiple cell types. Nucleic Acids Res. 2012;40(8):3676–3688. doi: 10.1093/nar/gkr1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao X, Yu YT. Targeted pre-mRNA modification for gene silencing and regulation. Nat Methods. 2008;5(1):95–100. doi: 10.1038/nmeth1142. [DOI] [PubMed] [Google Scholar]
- 34.Ge J, Liu H, Yu YT. Regulation of pre-mRNA splicing in Xenopus oocytes by targeted 2′-O-methylation. RNA. 2010;16(5):1078–1085. doi: 10.1261/rna.2060210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stepanov GA, et al. Artificial box C/D RNAs affect pre-mRNA maturation in human cells. BioMed Res Int. 2013;2013:656158. doi: 10.1155/2013/656158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spann P, Feinerman M, Sperling J, Sperling R. Isolation and visualization of large compact ribonucleoprotein particles of specific nuclear RNAs. Proc Natl Acad Sci USA. 1989;86(2):466–470. doi: 10.1073/pnas.86.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Azubel M, Habib N, Sperling R, Sperling J. Native spliceosomes assemble with pre-mRNA to form supraspliceosomes. J Mol Biol. 2006;356(4):955–966. doi: 10.1016/j.jmb.2005.11.078. [DOI] [PubMed] [Google Scholar]
- 38.Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sperling R, Spann P, Offen D, Sperling J. U1, U2, and U6 small nuclear ribonucleoproteins (snRNPs) are associated with large nuclear RNP particles containing transcripts of an amplified gene in vivo. Proc Natl Acad Sci USA. 1986;83(18):6721–6725. doi: 10.1073/pnas.83.18.6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kotzer-Nevo H, de Lima Alves F, Rappsilber J, Sperling J, Sperling R. Supraspliceosomes at defined functional states portray the pre-assembled nature of the pre-mRNA processing machine in the cell nucleus. Int J Mol Sci. 2014;15(7):11637–11664. doi: 10.3390/ijms150711637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yitzhaki S, Miriami E, Sperling R, Sperling J. Phosphorylated Ser/Arg-rich proteins: Limiting factors in the assembly of 200S large nuclear ribonucleoprotein particles. Proc Natl Acad Sci USA. 1996;93(17):8830–8835. doi: 10.1073/pnas.93.17.8830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sperling R, et al. Abundant nuclear ribonucleoprotein form of CAD RNA. Mol Cell Biol. 1985;5(3):569–575. doi: 10.1128/mcb.5.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sebbag-Sznajder N, et al. Regulation of alternative splicing within the supraspliceosome. J Struct Biol. 2012;177(1):152–159. doi: 10.1016/j.jsb.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heinrich B, et al. Heterogeneous nuclear ribonucleoprotein G regulates splice site selection by binding to CC(A/C)-rich regions in pre-mRNA. J Biol Chem. 2009;284(21):14303–14315. doi: 10.1074/jbc.M901026200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang YH, et al. ZRANB2 localizes to supraspliceosomes and influences the alternative splicing of multiple genes in the transcriptome. Mol Biol Rep. 2013;40(9):5381–5395. doi: 10.1007/s11033-013-2637-9. [DOI] [PubMed] [Google Scholar]
- 46.Lestrade L, Weber MJ. snoRNA-LBME-db, a comprehensive database of human H/ACA and C/D box snoRNAs. Nucleic Acids Res. 2006;34(Database issue):D158–D162. doi: 10.1093/nar/gkj002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Consortium EP. ENCODE Project Consortium An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489(7414):57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sperling J, Azubel M, Sperling R. Structure and function of the Pre-mRNA splicing machine. Structure. 2008;16(11):1605–1615. doi: 10.1016/j.str.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 49.Shefer K, Sperling J, Sperling R. The supraspliceosome—a multi-task machine for regulated pre-mRNA processing in the cell nucleus. Comput Struct Biotechnol J. 2014;11(19):113–122. doi: 10.1016/j.csbj.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raitskin O, Cho DS, Sperling J, Nishikura K, Sperling R. RNA editing activity is associated with splicing factors in lnRNP particles: The nuclear pre-mRNA processing machinery. Proc Natl Acad Sci USA. 2001;98(12):6571–6576. doi: 10.1073/pnas.111153798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kishore S, et al. The snoRNA MBII-52 (SNORD 115) is processed into smaller RNAs and regulates alternative splicing. Hum Mol Genet. 2010;19(7):1153–1164. doi: 10.1093/hmg/ddp585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bolisetty MT, Beemon KL. Splicing of internal large exons is defined by novel cis-acting sequence elements. Nucleic Acids Res. 2012;40(18):9244–9254. doi: 10.1093/nar/gks652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ideue T, Hino K, Kitao S, Yokoi T, Hirose T. Efficient oligonucleotide-mediated degradation of nuclear noncoding RNAs in mammalian cultured cells. RNA. 2009;15(8):1578–1587. doi: 10.1261/rna.1657609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hogg JR, Collins K. RNA-based affinity purification reveals 7SK RNPs with distinct composition and regulation. RNA. 2007;13(6):868–880. doi: 10.1261/rna.565207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maden BE. Mapping 2′-O-methyl groups in ribosomal RNA. Methods. 2001;25(3):374–382. doi: 10.1006/meth.2001.1250. [DOI] [PubMed] [Google Scholar]
- 56.Dupuis-Sandoval F, Poirier M, Scott MS. The emerging landscape of small nucleolar RNAs in cell biology. Wiley Interdiscip Rev RNA. 2015;6(4):381–397. doi: 10.1002/wrna.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Nues RW, et al. Box C/D snoRNP catalysed methylation is aided by additional pre-rRNA base-pairing. EMBO J. 2011;30(12):2420–2430. doi: 10.1038/emboj.2011.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Falaleeva M, Stamm S. In: Fragments of Small Nucleolar RNAs as a New Source for Non-Coding RNAs. Regulatory RNAs, Basics, Methods and Applications. Mallick B, Ghosh Z, editors. Springer; Berlin: 2012. pp. 49–71. [Google Scholar]
- 59.Richard P, Kiss AM, Darzacq X, Kiss T. Cotranscriptional recognition of human intronic box H/ACA snoRNAs occurs in a splicing-independent manner. Mol Cell Biol. 2006;26(7):2540–2549. doi: 10.1128/MCB.26.7.2540-2549.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tycowski KT, Shu MD, Steitz JA. A mammalian gene with introns instead of exons generating stable RNA products. Nature. 1996;379(6564):464–466. doi: 10.1038/379464a0. [DOI] [PubMed] [Google Scholar]
- 61.Carvajal LA, Hamard PJ, Tonnessen C, Manfredi JJ. E2F7, a novel target, is up-regulated by p53 and mediates DNA damage-dependent transcriptional repression. Genes Dev. 2012;26(14):1533–1545. doi: 10.1101/gad.184911.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aksoy O, et al. The atypical E2F family member E2F7 couples the p53 and RB pathways during cellular senescence. Genes Dev. 2012;26(14):1546–1557. doi: 10.1101/gad.196238.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lev-Maor G, Sorek R, Shomron N, Ast G. The birth of an alternatively spliced exon: 3′ splice-site selection in Alu exons. Science. 2003;300(5623):1288–1291. doi: 10.1126/science.1082588. [DOI] [PubMed] [Google Scholar]
- 64.Mersch B, Sela N, Ast G, Suhai S, Hotz-Wagenblatt A. SERpredict: Detection of tissue- or tumor-specific isoforms generated through exonization of transposable elements. BMC Genet. 2007;8:78. doi: 10.1186/1471-2156-8-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sela N, Mersch B, Hotz-Wagenblatt A, Ast G. Characteristics of transposable element exonization within human and mouse. PLoS One. 2010;5(6):e10907. doi: 10.1371/journal.pone.0010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Soundara Pandi SP, Chen M, Guduric-Fuchs J, Xu H, Simpson DA. Extremely complex populations of small RNAs in the mouse retina and RPE/choroid. Invest Ophthalmol Vis Sci. 2013;54(13):8140–8151. doi: 10.1167/iovs.13-12631. [DOI] [PubMed] [Google Scholar]
- 67.Merkin J, Russell C, Chen P, Burge CB. Evolutionary dynamics of gene and isoform regulation in Mammalian tissues. Science. 2012;338(6114):1593–1599. doi: 10.1126/science.1228186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang C, Krainer AR, Zhang MQ. Evolutionary impact of limited splicing fidelity in mammalian genes. Trends Genet. 2007;23(10):484–488. doi: 10.1016/j.tig.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 69.Ender C, et al. A human snoRNA with microRNA-like functions. Mol Cell. 2008;32(4):519–528. doi: 10.1016/j.molcel.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 70.Brameier M, Herwig A, Reinhardt R, Walter L, Gruber J. Human box C/D snoRNAs with miRNA like functions: Expanding the range of regulatory RNAs. Nucleic Acids Res. 2011;39(2):675–686. doi: 10.1093/nar/gkq776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bortolin-Cavaillé ML, Cavaillé J. The SNORD115 (H/MBII-52) and SNORD116 (H/MBII-85) gene clusters at the imprinted Prader-Willi locus generate canonical box C/D snoRNAs. Nucleic Acids Res. 2012;40(14):6800–6807. doi: 10.1093/nar/gks321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Babu MM, Luscombe NM, Aravind L, Gerstein M, Teichmann SA. Structure and evolution of transcriptional regulatory networks. Curr Opin Struct Biol. 2004;14(3):283–291. doi: 10.1016/j.sbi.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 73.Chen M, Manley JL. Mechanisms of alternative splicing regulation: Insights from molecular and genomics approaches. Nat Rev Mol Cell Biol. 2009;10(11):741–754. doi: 10.1038/nrm2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim D, et al. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14(4):R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang WC, et al. miRExpress: Analyzing high-throughput sequencing data for profiling microRNA expression. BMC Bioinformatics. 2009;10:328. doi: 10.1186/1471-2105-10-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rosenbloom KR, et al. ENCODE data in the UCSC Genome Browser: Year 5 update. Nucleic Acids Res. 2013;41(Database issue):D56–D63. doi: 10.1093/nar/gks1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Quinlan AR, Hall IM. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26(6):841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kiss T, Jády BE. Functional characterization of 2′-O-methylation and pseudouridylation guide RNAs. Methods Mol Biol. 2004;265:393–408. doi: 10.1385/1-59259-775-0:393. [DOI] [PubMed] [Google Scholar]
- 79.Convertini P, et al. Sudemycin E influences alternative splicing and changes chromatin modifications. Nucleic Acids Res. 2014;42(8):4947–4961. doi: 10.1093/nar/gku151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kapust RB, et al. Tobacco etch virus protease: Mechanism of autolysis and rational design of stable mutants with wild-type catalytic proficiency. Protein Eng. 2001;14(12):993–1000. doi: 10.1093/protein/14.12.993. [DOI] [PubMed] [Google Scholar]
- 81.Trapnell C, Pachter L, Salzberg SL. TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25(9):1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pappin DJ, Hojrup P, Bleasby AJ. Rapid identification of proteins by peptide-mass fingerprinting. Curr Biol. 1993;3(6):327–332. doi: 10.1016/0960-9822(93)90195-t. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.