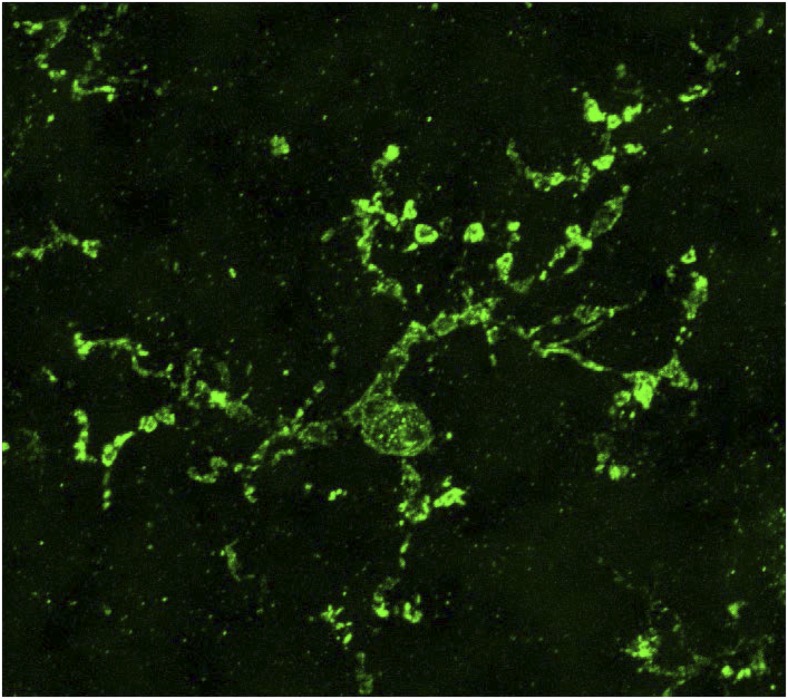
Microglia, central nervous system (CNS) resident mononuclear phagocytes, are broadly distributed throughout the brain and spinal cord. They originate from yolk sac-derived progenitors and colonize the CNS rudiment, where they subsequently expand and differentiate (1, 2). Microglia constitute ∼10% of the cells in the CNS and play critical roles in immune surveillance and clearance of invading pathogens (3, 4). In addition, microglia influence synapse formation (5) and participate in neuronal network refinement through phagocytosis of less active presynaptic boutons (6, 7). During homeostasis, microglia clear the debris that results from neuronal cell loss and glial turnover. Microglia are activated in response to brain injury or disease and may offer therapeutic opportunities for a broad spectrum of CNS disorders, ranging from multiple sclerosis to Alzheimer’s disease and autism (8–10). However, rigorous investigations into the role of microglia, and attempts to manipulate them for therapeutic purposes, have been limited by a lack of biomarkers to distinguish microglia from other closely related myeloid subsets. An ideal microglial marker would be selectively and stably expressed by all microglia, conserved across species, and localized to the cell surface to facilitate antibody-based cell sorting. The search may finally be over. In PNAS, Bennett et al. (11) identify transmembrane protein 119 (Tmem119) as a microglia-specific biomarker specific for mouse and human (Fig. 1). The authors developed anti-Tmem119 mAbs suitable for cell sorting and immunohistological staining. Equipped with anti-Tmem119, they isolated (i) developing, (ii) resting, and (iii) activated microglia and performed deep RNA-sequencing. Data mining of microglia transcriptomes provides rich insights into gene expression, identifies many gene products associated with neurological disorders, and defines transcriptional changes as microglia mature or become “classically” activated. Importantly, Tmem119 immunoreactivity (IR) was carefully examined under various injury paradigms that trigger CNS infiltration by bone marrow (BM)-derived immune cells and found to be a stable marker selective for microglia.
Fig. 1.
High-magnification anti-Tmem119 immunofluorescence labeling of adult human neocortex depicting morphological features of an individual microglial cell, including many long and highly ramified processes. (Magnification: 3,000×.) Photomicrograph (maximum-intensity projection confocal image) adapted from ref. 11.
The development of microglia-specific tools has been hampered by the close resemblance between microglia and their hematopoietic relatives, particularly monocyte-derived macrophages. Consequently, there is a high degree of overlap in expression of cell surface molecules. For example, CD11b, CD115, Iba-1, and F4/80 are common to monocytes/macrophages and microglia alike. The fractalkine receptor (CX3CR1) is strongly expressed by microglia but also by subsets of circulating monocytes that repopulate macrophage populations in noninflamed peripheral organs during homeostasis (12). In fact, CX3CR1 expression defines the two major subsets of circulating monocytes in both humans and mice. Some investigators have found myeloid cells (presumably microglia) in the juxtavascular regions of the CNS parenchyma that express CD11c, a marker traditionally associated with dendritic cells (13). CD11c+ microglia would be difficult to distinguish from monocyte-derived dendritic cells that accumulate in the CNS during multiple sclerosis (14, 15). To further complicate matters, microglia are “plastic” cells: they assume different states of activation with distinct gene-expression patterns, cell surface phenotypes, and functions (4). “Resting” microglia are stationary cells, constantly sampling the local microenvironment with their long and highly ramified extensions. They are unable to phagocytose debris and lack many surface receptors required for antigen presentation. In contrast, activated microglia are highly motile phagocytic cells that engage in antigen presentation and proinflammatory pathways (3). The expression of several markers that have been used to identify microglia changes with activation, obscuring their specificity in the setting of nervous system injury or disease. CD45 is expressed at low or intermediate levels on resting microglia, but is up-regulated with activation or aging. This makes activated microglia difficult to distinguish from BM-derived monocytes in the inflamed CNS, both of which are CD45high. Expression levels of MHC class II and CD11b on microglia fluctuate in a similar manner (16).
In their quest to find microglia-specific markers, Bennett et al. (11) compared microglia-enriched gene-expression profiles with existing datasets of highly pure CNS cells and freshly isolated immune cells. Follow-up studies, including in situ hybridization and quantitative PCR, revealed highly enriched expression of Tmem119 in parenchymal microglia but not in hematopoietic macrophage populations in the choroid plexus or meninges. In addition, Tmem119 expression was absent in BM, spleen, liver, and blood. Tmem119 is a type 1A membrane protein. Anti-Tmem119 labeling of brain tissue shows colocalization with CX3CR1. Microglia are conventionally identified as CX3CR1+CD11b+ CD45lo-int cells by flow-cytometric analysis of CNS mononuclear cells (17). However, all of those markers are also expressed by at least some subsets of hematopoietic myeloid cells. Is Tmem119 subject to the same limitations? Bennett et al. (11) conducted Tmem119 immunolabeling in three experimental paradigms known to trigger neuroinflammation, including (i) sciatic nerve injury, (ii) intraperitoneal lipopolysaccharide injection to activate parenchymal microglia, and (iii) optic nerve crush injury. The authors convincingly demonstrate that Tmem119 IR is restricted to microglia and not associated with BM-derived monocytes/ macrophages under any of those conditions.
BM-derived phagocytes do not contribute to the pool of established microglia in the unperturbed CNS (18). However, peripheral myeloid cell engraftment of the CNS does occur following insults that compromise the blood–brain barrier and alter the parenchymal milieu. Although this is most evident in the context of neuroinflammatory diseases, such as multiple sclerosis and infectious encephalomyelitis, monocytes can infiltrate the CNS in response to brain and spinal cord trauma, infectious encephalomyelitis, cerebrovascular ischemia, CNS malignancy, and neurodegenerative diseases, such as Alzheimer’s and amytrophic lateral sclerosis (18–23). Unlike other tissue-resident macrophages, microglial precursors do not transition through a monocytic stage (24). Mature microglia also differ from most macrophages (including perivascular macrophages in the CNS) in that they survive high-dose ionizing irradiation and constitute a self-renewing population that is independent of adult hematopoiesis in the steady state (25, 26). These observations suggest that the CNS microenvironment provides signals that induce and sustain properties that are unique to microglia. To directly test whether the CNS microenvironment induces Tmem119 expression in BM-derived cells, chimeric mice were constructed by reconstituting lethally irradiated wild-type hosts with GFP+ BM donor cells (11). At 3 and 6 mo following irradiation and BM transplantation, a significant fraction of Iba1+ cells in the CNS were derived from GFP+ donor cells, including numerous cells along blood vessels, meninges, and in the parenchyma. Strikingly, none of these GFP+ cells stained positive for Tmem119, even several months following engraftment in the CNS (11). Together, these studies show that Tmem119 is superior to currently used methods for microglia identification.
To better compare genetic profiles of microglia in the developing, mature, and inflamed brain, Bennet et al. (11) FAC-sorted CNS mononuclear cells using anti-Tmem119. Isolated cells were subjected to deep sequencing and comparative analysis of transcriptomes. The transcriptome of murine microglia isolated on
In PNAS, Bennett et al. identify transmembrane protein 119 (Tmem119) as a microglia-specific biomarker specific for mouse and human.
postnatal day 14 resembled that of analogous cells isolated from the adult CNS, suggesting that microglia are fully mature by that age. Remarkably, a number of human disease-related genes were strongly enriched in microglia, providing a platform to investigate the potential role of microglia in disparate brain conditions, ranging from developmental and metabolic disorders to epilepsy, neurodegenerative diseases, and psychiatric disorders. Microglia become rapidly activated following stimulation via pattern-recognition receptors. “Classic” activation by peripheral injection of lipopolysaccharide into adult mice caused rapid induction of proinflammatory genes. However, Tmem119 IR expression remained stable, underscoring its suitability as a marker of microglia irrespective of activation state or alterations in the CNS microenvironment.
The literature on microglial phenotype and function has grown dramatically over the past 10 y (10, 27). Future research will be greatly aided by the discovery of Tmem119 as a stable and specific microglial marker. Hence, the development of cell type-specific gene knock-out, inducible gene knock-out, and transgenic mouse models using the Tmem119 promoter will allow researchers to assess the contribution of specific gene products to the function of microglia during homeostasis and in disease pathogenesis. The location of Tmem119 on the cell surface will facilitate microglial cell isolation from the healthy and diseased CNS tissue (human or experimental animal models), for in-depth studies of the transcriptome (coding and noncoding RNAs), proteome, epigenetic, and posttranslational modifications and functional properties. The data generated are expected to accelerate the identification of novel disease mechanisms and molecular targets for therapeutic interventions following CNS injury or disease.
A number of interesting questions arise from the Bennett et al. (11) study. First, what is the function of Tmem119 in microglia? Is Tmem119 a surface receptor? If so, what are its binding partners and downstream signaling mechanisms? Which factors induce Tmem119 expression during microglia development? Are those factors released by, or expressed on the surface of macroglia or neurons? Are they specific to the developing CNS? Similar to macrophages, microglia undoubtedly can assume different functional phenotypes, including proinflammatory and alternatively activated subsets (4). For example, postinflammation, microglia participate in neural tissue repair and demonstrate immunoregulatory properties. Is Tmem119 IR altered during the polarization of microglia toward different functional lineages? All of these questions may soon be addressed by studies using Tmem119-specific antibodies as well as Tmem119−/− mutant mice. In sum, Tmem119 provides the research community with much needed molecular handles to identify and manipulate microglia with great specificity in CNS health and disease.
Acknowledgments
The authors’ research is supported by the Charles A. Dana Foundation (B.M.S. and R.J.G.); the Dr. Miriam and Sheldon G. Adelson Medical Foundation on Neural Repair and Rehabilitation (R.J.G.); Veteran’s Administration Merit Review Awards 1I01RX000416 and 1I01BX001387 (to R.J.G. and B.M.S.); and National Institutes of Health Grants R01NS081281 (to R.J.G.) and R01NS057670 (to B.M.S.).
Footnotes
The authors declare no conflict of interest.
See companion article on page E1738.
References
- 1.Gomez Perdiguero E, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518(7540):547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alliot F, Godin I, Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res. 1999;117(2):145–152. doi: 10.1016/s0165-3806(99)00113-3. [DOI] [PubMed] [Google Scholar]
- 3.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 4.Ransohoff RM, Perry VH. Microglial physiology: Unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 5.Parkhurst CN, et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155(7):1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stevens B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131(6):1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 7.Paolicelli RC, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333(6048):1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 8.Goldmann T, Prinz M. Role of microglia in CNS autoimmunity. Clin Dev Immunol. 2013;2013:208093. doi: 10.1155/2013/208093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koyama R, Ikegaya Y. Microglia in the pathogenesis of autism spectrum disorders. Neurosci Res. 2015;100:1–5. doi: 10.1016/j.neures.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Olmos-Alonso A, et al. 2016. Pharmacological targeting of CSF1R inhibits microglial proliferation and prevents the progression of Alzheimer’s-like pathology. Brain 139(Pt 3):891–907.
- 11.Bennett ML, et al. New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci USA. 2016;113:E1738–E1746. doi: 10.1073/pnas.1525528113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19(1):71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 13.Prodinger C, et al. CD11c-expressing cells reside in the juxtavascular parenchyma and extend processes into the glia limitans of the mouse nervous system. Acta Neuropathol. 2011;121(4):445–458. doi: 10.1007/s00401-010-0774-y. [DOI] [PubMed] [Google Scholar]
- 14.Deshpande P, King IL, Segal BM. Cutting edge: CNS CD11c+ cells from mice with encephalomyelitis polarize Th17 cells and support CD25+CD4+ T cell-mediated immunosuppression, suggesting dual roles in the disease process. J Immunol. 2007;178(11):6695–6699. doi: 10.4049/jimmunol.178.11.6695. [DOI] [PubMed] [Google Scholar]
- 15.King IL, Dickendesher TL, Segal BM. Circulating Ly-6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. Blood. 2009;113(14):3190–3197. doi: 10.1182/blood-2008-07-168575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perry VH, Matyszak MK, Fearn S. Altered antigen expression of microglia in the aged rodent CNS. Glia. 1993;7(1):60–67. doi: 10.1002/glia.440070111. [DOI] [PubMed] [Google Scholar]
- 17.Ford AL, Goodsall AL, Hickey WF, Sedgwick JD. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. J Immunol. 1995;154(9):4309–4321. [PubMed] [Google Scholar]
- 18.Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10(12):1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 19.Thériault P, ElAli A, Rivest S. The dynamics of monocytes and microglia in Alzheimer’s disease. Alzheimers Res Ther. 2015;7(1):41. doi: 10.1186/s13195-015-0125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malm T, Koistinaho M, Muona A, Magga J, Koistinaho J. The role and therapeutic potential of monocytic cells in Alzheimer’s disease. Glia. 2010;58(8):889–900. doi: 10.1002/glia.20973. [DOI] [PubMed] [Google Scholar]
- 21.Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011;14(9):1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- 22.Solomon JN, et al. Origin and distribution of bone marrow-derived cells in the central nervous system in a mouse model of amyotrophic lateral sclerosis. Glia. 2006;53(7):744–753. doi: 10.1002/glia.20331. [DOI] [PubMed] [Google Scholar]
- 23.Chiba T, Umegaki K. Pivotal roles of monocytes/macrophages in stroke. Mediators Inflamm. 2013;2013:759103. doi: 10.1155/2013/759103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoeffel G, et al. (2015) C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity 42(4):665–678. [DOI] [PMC free article] [PubMed]
- 25.Ginhoux F, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lassmann H, Schmied M, Vass K, Hickey WF. Bone marrow derived elements and resident microglia in brain inflammation. Glia. 1993;7(1):19–24. doi: 10.1002/glia.440070106. [DOI] [PubMed] [Google Scholar]
- 27.Aguzzi A, Barres BA, Bennett ML. Microglia: Scapegoat, saboteur, or something else? Science. 2013;339(6116):156–161. doi: 10.1126/science.1227901. [DOI] [PMC free article] [PubMed] [Google Scholar]