Significance
The parasitic mite Varroa destructor and the deformed wing virus (DWV) are linked in a mutualistic symbiosis. The mite acts as vector of the viral pathogen, whereas the DWV-induced immunosuppression in honey bees favors mite feeding and reproduction. This functional interaction underpins a rapidly escalating immunosuppression, which can be primed and/or aggravated by a wealth of stress factors that co-trigger colony losses. Our experimental results explain the pivotal role proposed for the Varroa–DWV association in the induction of honey bee colony losses. Here we provide a functional framework for studying the dynamics of this multifactorial syndrome and defining effective strategies to reduce its negative impact on the beekeeping industry.
Keywords: Apis mellifera, Varroa destructor, deformed wing virus, mutualistic symbiosis, honeybee colony losses
Abstract
Honey bee colony losses are triggered by interacting stress factors consistently associated with high loads of parasites and/or pathogens. A wealth of biotic and abiotic stressors are involved in the induction of this complex multifactorial syndrome, with the parasitic mite Varroa destructor and the associated deformed wing virus (DWV) apparently playing key roles. The mechanistic basis underpinning this association and the evolutionary implications remain largely obscure. Here we narrow this research gap by demonstrating that DWV, vectored by the Varroa mite, adversely affects humoral and cellular immune responses by interfering with NF-κB signaling. This immunosuppressive effect of the viral pathogen enhances reproduction of the parasitic mite. Our experimental data uncover an unrecognized mutualistic symbiosis between Varroa and DWV, which perpetuates a loop of reciprocal stimulation with escalating negative effects on honey bee immunity and health. These results largely account for the remarkable importance of this mite–virus interaction in the induction of honey bee colony losses. The discovery of this mutualistic association and the elucidation of the underlying regulatory mechanisms sets the stage for a more insightful analysis of how synergistic stress factors contribute to colony collapse, and for the development of new strategies to alleviate this problem.
The mismatch between the increasing demand for pollination in agriculture and the capacity of honey bees to provide such ecological service severely undermines the sustainability of our food production system (1). Indeed, apiculture is facing a major crisis owing to concurrent factors, such as landscape deterioration, agrochemicals, and parasites (2). Honey bee colony losses have been a major problem since the beginning of modern apiculture (3); however, in 2006, the dramatic dimension of this phenomenon attracted public attention and increasing interest in the scientific community. Several years of intense investigation did not reveal a specific causal agent, but a multifactorial origin was proposed for this syndrome, which is often associated with high levels of parasites in combination with pathogens (2, 4). It was evident that the immunocompetence of honey bees in collapsing colonies was impaired, undermining their capacity to face the biotic stress factors occurring in the hive ecosystem. Indeed, a number of studies have identified important roles of the parasitic mite Varroa destructor (5) and vectored viral pathogens, particularly the deformed wing virus (DWV) (6), in contributing to significant changes in the global viral landscape and a continuing decline in honey bee health (7–9).
We recently contributed to this research issue, proposing a functional model describing how the delicate immune balance underpinning the covert infections of DWV can be destabilized by Varroa feeding, resulting in intense viral proliferation (10). That earlier study provided evidence supporting a major role of DWV in the immune suppression process, characterized by a negative impact on a member of the NF-κB protein family (10). Separate independent work further corroborated this evidence, showing that viral infection in honey bees interfered with the expression of genes that participate in the Toll pathway (11, 12). This finding supports the hypothesis that in honey bees, and more generally in insects, inducible antiviral barriers besides RNAi-mediated mechanisms (11–18) may have an important role. Indeed, in collapsing colonies, these latter barriers under NF-κB or JAK-STAT control appear to be targeted, whereas the RNAi machinery seems to be unaffected (19).
The occurrence, often asymptomatic, of DWV in nearly all honey bee colonies (6), favored by the active vectoring activity of Varroa mite (5), represents a constant threat that can become a severe problem in the presence of additional stress factors, such as, among others, pesticides and poor nutrition, which can promote viral replication (8, 20). Therefore, multiple stress agents exert a synergistic action that compromises the delicate immune balance underpinning the covert DWV infections, and may well account for the multifactorial origin of colony losses.
Collectively, the available experimental evidence indicates that DWV adopts a virulence strategy, still obscure from a molecular standpoint, that targets antiviral barriers under control of the Toll pathway. This virulence strategy can have multifaceted functional implications as a consequence of the central role of NF-κB in immunity and cross-modulated physiological pathways (8), as well as in control of the behavior-specific neurogenomic states in the brain that rely on specific transcriptional modules controlled by this transcription factor (21).
The active role of V. destructor in the dispersal and enhanced replication of the virus, triggered by parasite feeding (7, 10, 12, 22), indicates that the mite–virus association has clear benefits for the latter, whereas an adaptive value for the mite, if any, remains unknown. The feeding behavior of V. destructor is complex, characterized by prolonged use of a feeding hole created by the mother mite on abdominal sternites of the bee pupa, through which both the mother and its offspring repeatedly feed on the bee’s hemolymph (23). Any humoral and cellular immune reaction in the host, such as hemolymph clotting, melanization, or encapsulation, that directly interferes with food uptake and use may in principle result in reduced mite fitness. Therefore, based on current knowledge about DWV-induced immunosuppression, a positive influence of viral infection on mite feeding and reproduction can be hypothesized. However, notwithstanding the key importance of the Varroa–DWV association, the intimate aspects of their interaction have been largely overlooked.
Here we focus on the functional basis of this tight association, examining the impact of DWV infection on multiple immune barriers under the Toll pathway and assessing whether this has any effect on Varroa mite fitness. These are very relevant issues that, if properly addressed, can provide mechanistic insights of key importance to understand the dynamics of mite–virus association and developing new strategies to alleviate its dramatic impact on honey bee colonies.
Results
DWV Infection and Honey Bee Immunosuppression.
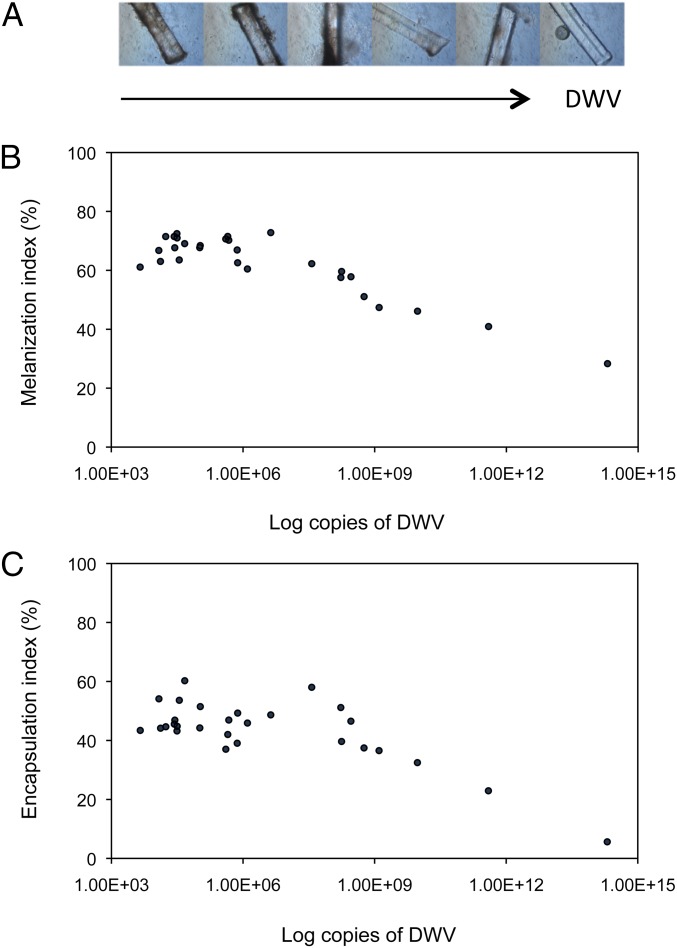
To study the DWV-induced immunosuppression, we first assessed at the phenotypic level how the cellular and humoral components of the immune response vary across different DWV infection levels, irrespective of the presence of Varroa, in the absence of other viral pathogens. We did this by scoring the degree of encapsulation and melanization of a nylon thread at 24 h after its implantation into the body of a fifth instar honey bee larva, whose infection level, scored as the number of DWV genome copies, was determined by quantitative RT-PCR (qRT-PCR). The melanization and encapsulation response in experimental larvae were negatively correlated with DWV titer (melanization: ρ = −0.656, n = 28, P < 0.001; encapsulation: ρ = −0.390, n = 28, P = 0.040) (Fig. 1). It is worth noting that any possible effect of mite parasitism at this stage can be virtually excluded, because mite feeding begins at the prepupal stage, within the sealed brood cell.
Fig. 1.
Immunocompetence of honey bee larvae as affected by DWV infection. (A) Nylon threads at 24 h after implantation into the body of fifth instar honey bee larvae with increasing DWV infection levels. (B) Level of melanization of a nylon thread implant in honey bee larvae with different levels of viral infection, measured as number of DWV genome copies (ρ = −0.656, n = 28, P < 0.001). (C) Level of encapsulation of a nylon thread implant in honey bee larvae with different levels of viral infection, measured as number of DWV genome copies (ρ = −0.390, n = 28, P = 0.040).
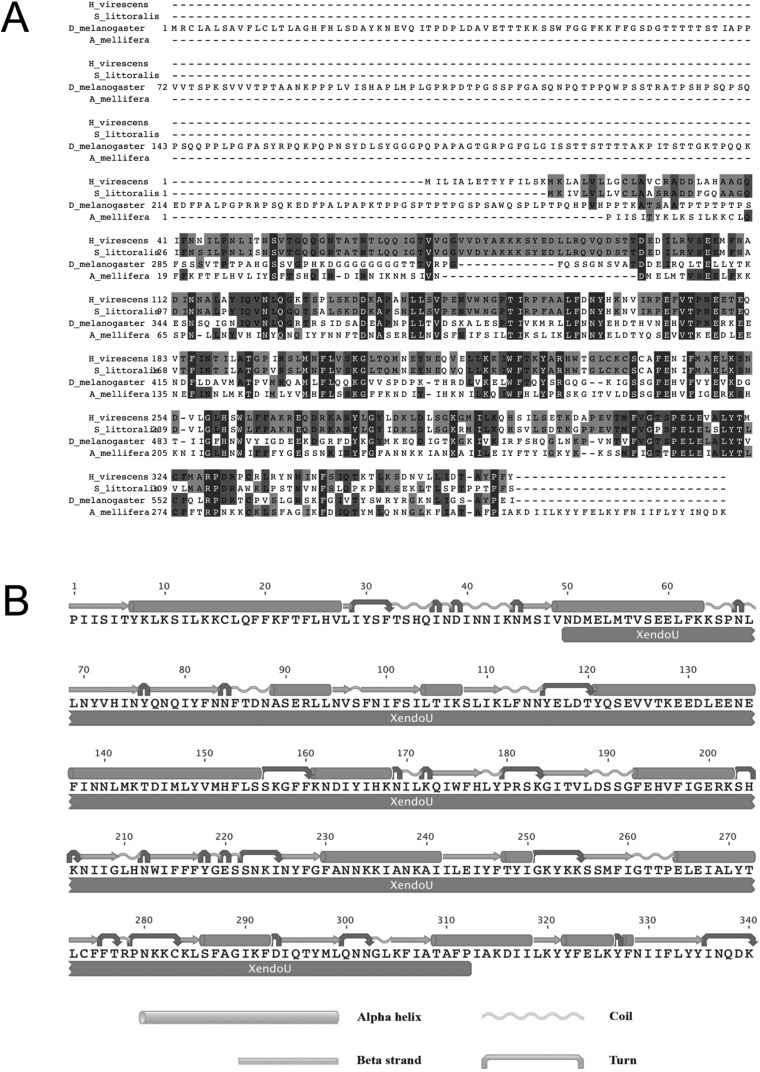
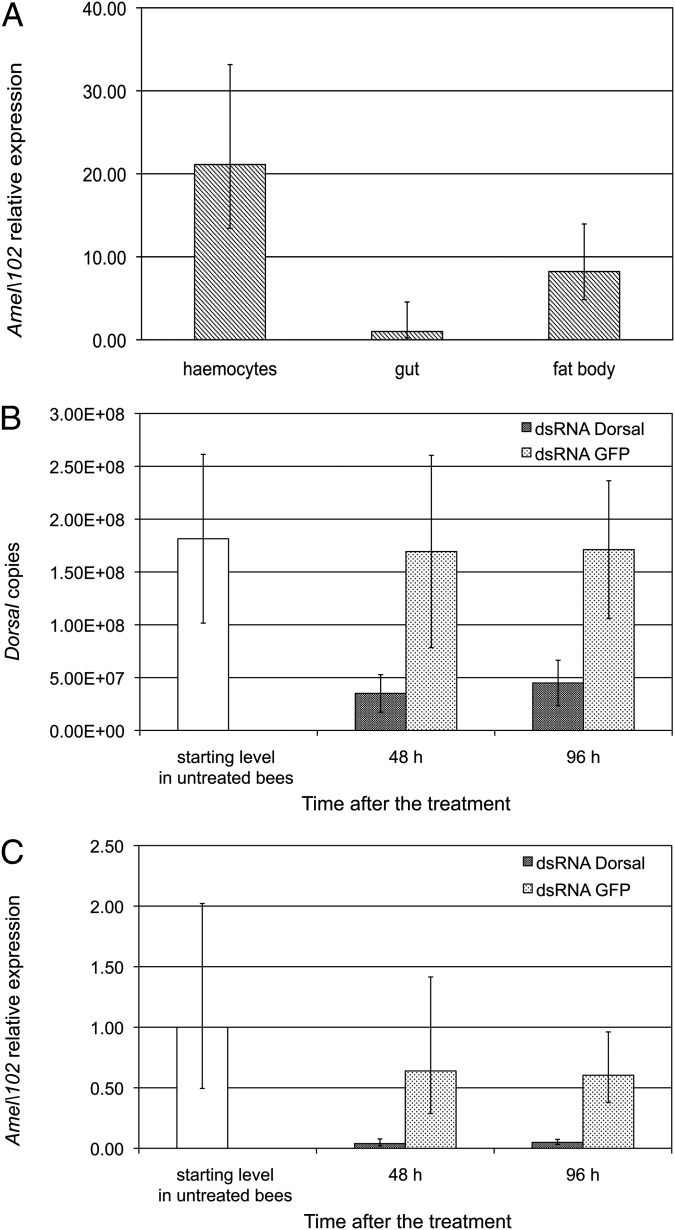
Melanization and encapsulation in insects are mediated by a number of genes that control the formation of a cellular capsule around foreign intruders and the deposition of melanin and other toxic molecules on their surface (24). Moreover, although the processes of clotting and of nodule and capsule formation differ in their final appearance, they share some molecular steps, and both involve melanin biosynthesis (24–26). Among the immune genes involved in these reactions is a sequence of key importance codes for a protein generating amyloid fibers that mediate encapsulation and strictly localized melanization of nonself material in Lepidoptera (27, 28). Here we cloned and characterized the cDNA of the homolog of this gene in Apis mellifera (GenBank accession no. KU513387), hereinafter denoted as Amel\102. This full-length cDNA encodes a predicted protein of 270 aa that shows 36% sequence identity with P102Hv (27) (Fig. S1). The expression profile of Amel\102 in different tissues of honey bee larvae, assessed by qRT-PCR analysis, matched that observed in lepidopteran larvae, showing high levels of expression in the haemocytes and lower levels in the gut and fat body (Fig. 2A; adjusted H = 11.52, df = 2, P = 0.003).
Fig. S1.
(A) Alignment of H. virescens, S. littoralis, D. melanogaster, and A. mellifera 102 protein sequences. Black and gray shadings indicate identity and high conservation of amino acids, respectively. (B) Predicted secondary structure and conserved domains of Amel\102 protein.
Fig. 2.
Amel\102 transcriptional profile and regulation. (A) Amel\102 transcription profile in honey bee larval tissues (adjusted H = 11.52, df = 2, P = 0.003). (B) Transcription level of dorsal-1A in honey bees maintained for different time intervals on a sucrose/protein solution containing a dsRNA targeting this gene (dsRNA dorsal) or dsRNA GFP as a control. (C) Transcription level of Amel\102 as affected by silencing of dorsal-1A. The error bars indicate the SD of the mean. The significant drop in dorsal-1A transcription (H = 11.57, df = 1, P < 0.001) was associated with a significant transcriptional down-regulation of Amel\102 (H = 14.29, df = 1, P < 0.001).
To assess whether Amel\102 is an inducible immune gene under NF-κB control, we silenced this transcription factor by RNAi and assessed the expression level of Amel\102 by qRT-PCR. The significant down-regulation of dorsal-1A in honey bees exposed to dsRNA targeting this gene (Fig. 2B; H = 11.57, df = 1, P < 0.001) was associated with a drastic drop in the transcription level of Amel\102 (Fig. 2C; H = 14.29, df = 1, P < 0.001). This clearly indicates that Amel\102 is under NF-κB control.
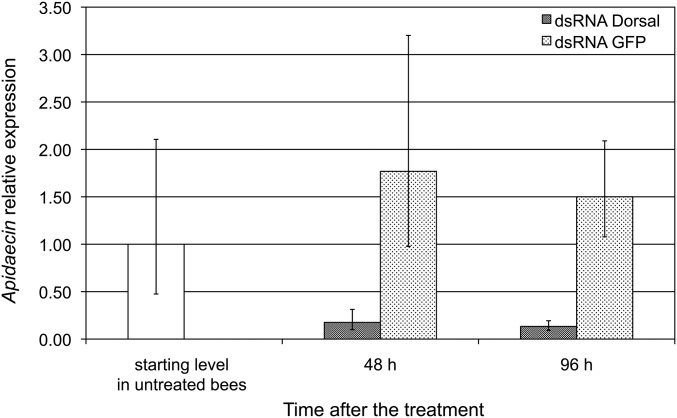
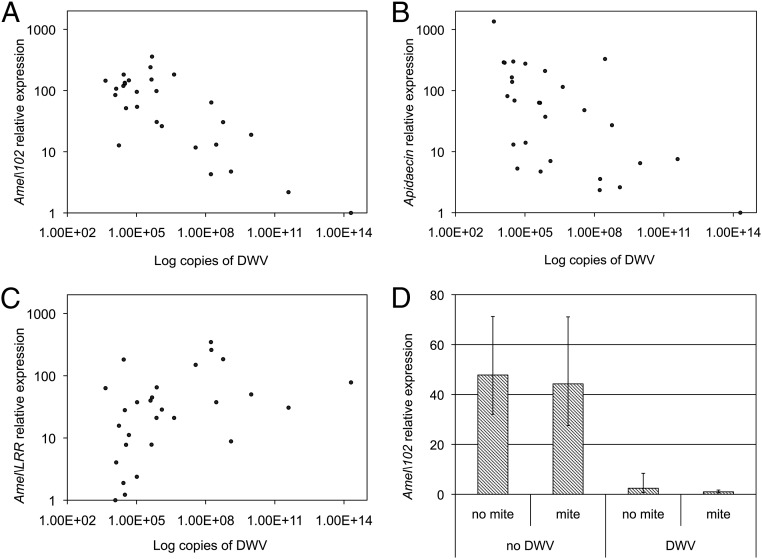
To demonstrate the possible occurrence of an upstream viral action on the Toll pathway with effects on various immune responses under NF-κB control, we scored the impact of different levels of DWV infection on the transcription of Amel\102 and apidaecin, a gene encoding an antimicrobial peptide under NF-κB control (29), as also supported by its strong down-regulation in response to dorsal-1A silencing performed in our experiment (Fig. S2; H = 14.29, df = 1, P < 0.001). We found that the transcription level of Amel\102 was negatively correlated with the level of DWV infection (Fig. 3A; ρ = −0.575, n = 28, P = 0.001). This result is consistent with the expected central role of the Amel\102 gene in melanization and encapsulation, as well as the negative effect of DWV on such processes. Moreover, a negative correlation was also found between the transcription level of apidaecin and the level of DWV infection (Fig. 3B; ρ = −0.636, n = 28, P < 0.001). Collectively, these results corroborate the importance of viral infection on honey bee immunosuppression, and clearly indicate that the adverse effect of DWV on the bees’ immune response is caused by an upstream alteration of the Toll pathway.
Fig. S2.
Transcription level of apidaecin as affected by silencing of dorsal-1A. Error bars indicate the SD of the mean (H = 14.29, df = 1, P < 0.001).
Fig. 3.
Gene expression in honey bee larvae as affected by DWV infection. (A–C) Effect of DWV infection level on transcription of (A) Amel\102 (ρ = −0.575, n = 28, P = 0.001), (B) apidaecin (ρ = −0.636, n = 28, P < 0.001), and (C) Amel\LRR (ρ = 0.511, n = 28, P = 0.005). (D) Amel\102 transcription as affected by DWV infection (F = 66.37, df = 1, P < 0.001) and feeding activity by the Varroa mite (F = 2.74, df = 1, P = not significant; interaction: F = 1.69, df = 1, P = not significant). Error bars indicate SD.
Because NF-κB activation in honey bees is negatively modulated by a leucine reach repeat protein (Amel\LRR) (30), we investigated whether the transcription rate of its encoding gene can be influenced by the level of viral infection. Indeed this was the case, as demonstrated by a positive correlation between the number of DWV genome copies and the transcription rate of Amel\LRR (Fig. 3C; ρ = 0.511, n = 28, P = 0.005). This result indicates that DWV infection could have a negative effect on NF-κB activation by enhancing the transcription of its negative modulator Amel\LRR. The possibility that DWV adopts a virulence strategy somewhat similar to that recently described for Salmonella, which targets an NLR protein that negatively regulates NF-κB signaling (31), is an intriguing hypothesis meriting further research efforts.
To assess whether the observed immunosuppression was due exclusively to the action of DWV infection, we performed an additional experiment in vitro. Honey bee larvae were exposed to a controlled Varroa infestation, and the expression level of Amel\102, as affected by mite feeding, was assessed in the presence or absence of DWV infection. The lowest transcription rates of Amel\102, which appears to be under NF-κB control, were observed in DWV-infected larvae, irrespective of the presence or absence of Varroa (comparison of DWV infection levels: F = 66.37, df = 1, P < 0.001; comparison of mite infestation levels: F = 2.74, df = 1, P = not significant; interaction: F = 1.69, df = 1, P = not significant) (Fig. 3D). Therefore, this gene appears to be a good molecular marker of honey bee immunocompetence as affected by DWV infection. The matching pattern of variation shown by Amel\102 transcription and the immune parameters scored above (Fig. 1) indicates that this gene, as in lepidopterans, plays an important role in the modulation of both humoral and cellular immune responses and is targeted by DWV, which adversely affects NF-κB signaling through a molecular mechanism that remains to be studied.
Effect of Honey Bee Immunosuppression on Varroa Fitness.
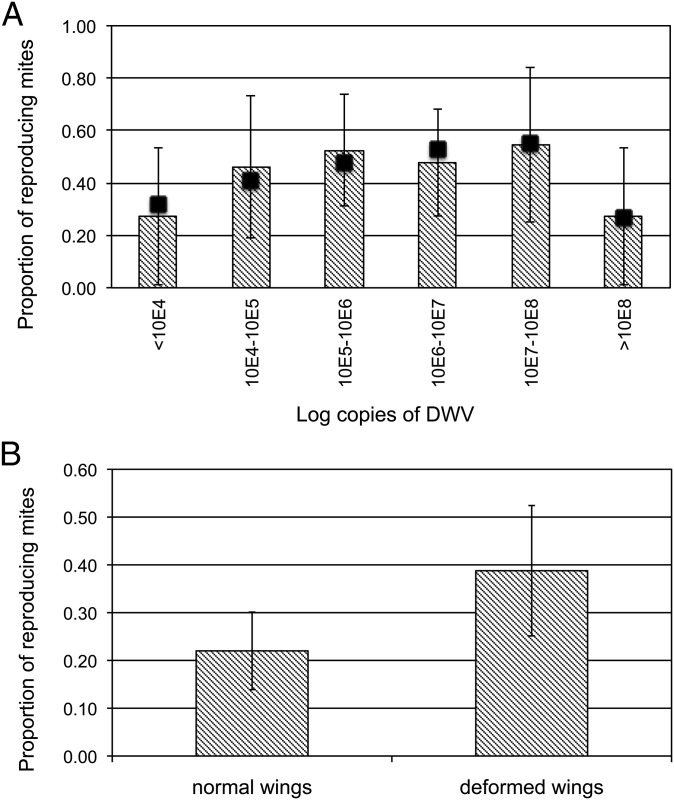
To assess whether the immunosuppressant action of DWV can facilitate mite feeding and, consequently, increase mite fitness, we assessed mite reproduction in honey bees showing variable levels of DWV infection. To do so, we artificially infested fifth instar honey bee larvae with one mite each and kept them under laboratory conditions for 12 d (Fig. S3). After pupation, we noted the possible presence of offspring generated by each mother mite during the honey bee’s metamorphosis, and assessed the viral infection level of the honey bee, as the number of DWV genome copies, by qRT-PCR. We replicated this experimental setup twice, resulting in 90 bee samples. The proportion of reproducing mites (i.e., fertility) increased with DWV infection level up to a threshold of 108 genome copies per honey bee, after which very high viral loads seemed to exert a negative impact on mite reproduction (Fig. 4A; equation of the curve describing the observed trend: y = −0.0122x2 + 0.1979x − 0.2437, R2 = 0.868, df = 3, P = 0.048). Overall, mite fertility was similar to that reported previously under the same experimental conditions (32), and clearly varied according to the level of DWV infection.
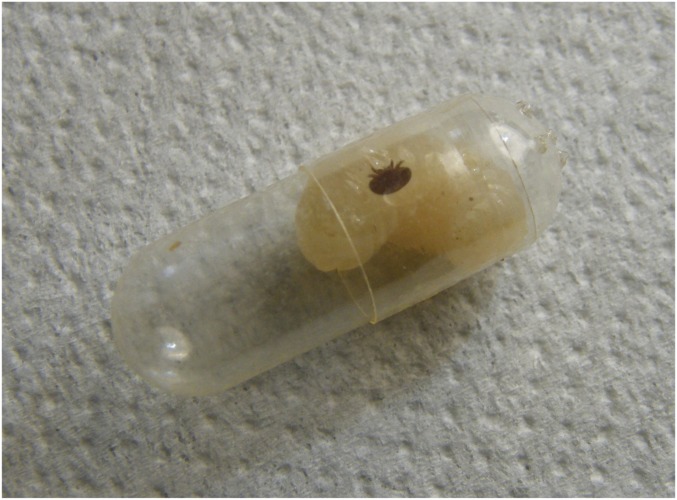
Fig. S3.
V. destructor adult on a honey bee larva kept in the gelatin capsule used for in vitro experiments on mite reproduction.
Fig. 4.
Viral infection and mite reproduction. (A) Proportion of reproducing Varroa mites (i.e., fertility) on honey bees with different levels of DWV infection. The equation of the curve describing the observed trend and the correlation coefficient are y = −0.0122x2 + 0.1979x – 0.2437 and R2 = 0.868 (df = 3, P = 0.048). The black squares represent the values expected under this model. (B) Mite fertility on honey bees with deformed or normal wings at eclosion (χ2 = 4.64, df = 1, P = 0.031). Error bars indicate the 95% confidence limits of the proportions.
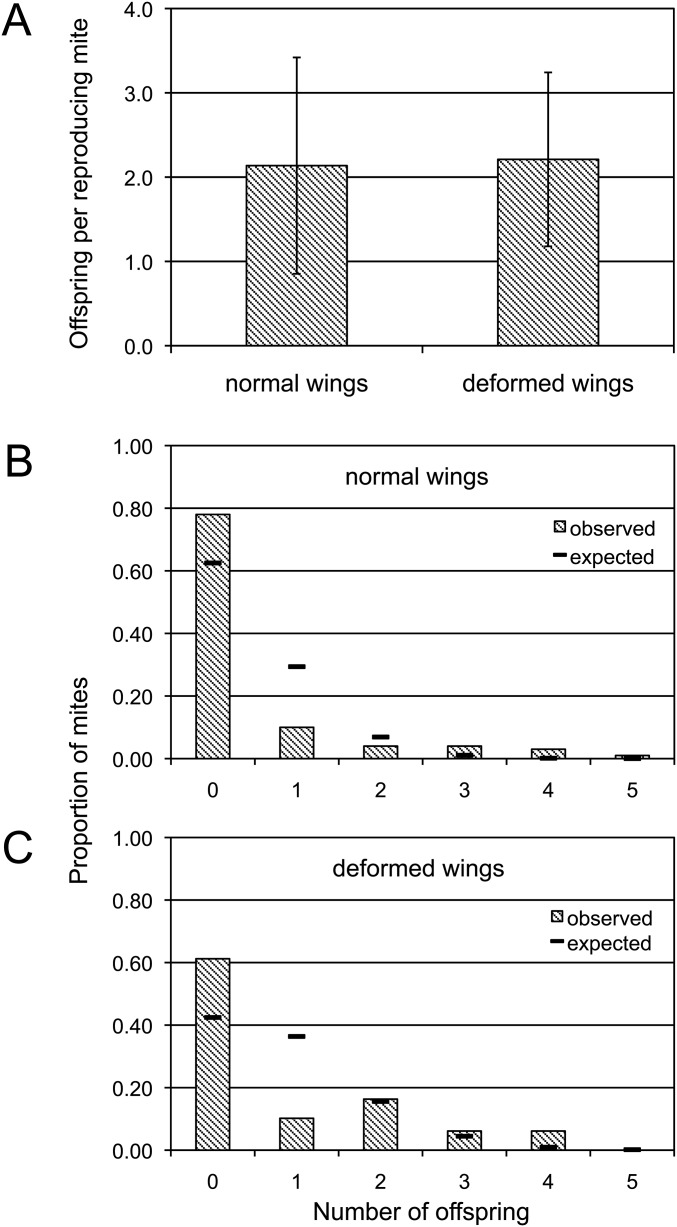
The occurrence of crippled wings at eclosion is a characteristic symptom of an overt virus infection in honey bees, induced by a high DWV load (6). In fact, we found a significant correlation between viral load and wing deformity in 46 samples from the previous experiment, in which the condition of wings was evident at the time of sampling (χ2 = 5.894, df = 1, P = 0.015). Therefore, to check the observed positive relationship between viral infection and mite reproduction, we performed an additional in vitro experiment in which we assessed the reproduction of mites feeding on immature honey bees with either deformed or normal wings at the adult stage (Fig. S4). We studied a total of 149 honey bees, 49 of which had deformed wings at eclosion. The fertility rate approached 40% in mites infesting larvae giving rise to deformed wing bees, but was limited to only 22% when the host larvae developed into normal wing bees (Fig. 4B; χ2 = 4.64, df = 1, P = 0.031). Conversely, the number of offspring generated by each reproducing mite (i.e., fecundity) did not seem to be affected (Fig. S5).
Fig. S4.
A honey bee with deformed wings obtained from a larva artificially infested with a Varroa mite, which reproduced within the rearing cell. The mother mite and an immature offspring are seen on the abdomen.
Fig. S5.
Fecundity of mites infesting honey bees showing normal and deformed wings at eclosion (A) and the distribution of mites according to the number of offspring (B and C). (B) Mites kept on bees with normal wings on eclosion. (C) Mites on bees with deformed wings. Error bars indicate the SD. The hyphens in B and C indicate the proportion expected according to the Poisson distribution. The significant deviation observed in both normal-winged bees and deformed-winged bees (χ2 = 18.02, df = 1, P < 0.001 and χ2 = 30.32, df = 2, P < 0.001, respectively) suggests that the first and subsequent ovipositions are not independent events, and that reproduction needs to be triggered once for continuing as long as conditions are suitable.
Collectively, our data demonstrate that mite fertility is enhanced by high DWV titers, and support the hypothesis that viral infection promotes Varroa fitness.
Discussion
The results of this study show that honey bees with increasing DWV loads have reduced immunocompetence at both the humoral and cellular levels. The negative impact of the combined action of Varroa and DWV on honey bee immunity has been proposed based on the results of several studies showing variable levels of transcriptional down-regulation of immune genes in most cases. The very close relationship between the mite and the virus, along with the complexity of the results from transcriptomic studies, have generated a somewhat contrasting picture, however. The initially proposed primary role for the parasitic mite in the induction of this immune syndrome (33) has been challenged by several studies that apparently do not support this hypothesis (12, 22, 34–36), even though the possibility that Varroa feeding and its saliva may modulate immune effector molecules cannot be ruled out, as suggested by the effect of this secretion on insect hemocytes in vitro (37). Moreover, a direct immunosuppressive activity of DWV targeting the antiviral barriers under NF-κB control has been proposed in the framework of a bistable dynamic model that assumes a transition from immunostimulation to immunosuppression as the DWV titer increases and then exceeds a critical threshold (Ct) (10). This working hypothesis is in tune with more recent data indicating that in honey bees from colonies affected by colony collapse disorder, which regularly show high levels of viral infection, the immune response mediated by the RNAi machinery is apparently unaffected (19). Furthermore, immune genes in the Toll pathway are down-regulated in honey bees exposed to Varroa and bearing high DWV loads (12) or challenged with nonspecific dsRNAs (11).
Our present data, obtained with a more focused functional approach, demonstrate the immunosuppressive role of DWV and show that its virulence strategy for overcoming the specific antiviral barriers under the Toll pathway by disrupting NF-κB signaling inevitably has a multifaceted influence on different arms of the immune response. This has a direct impact on the fitness of the Varroa mite, possibly facilitating the mite’s ectoparasitic trophic activity. Thus, the viral pathogen has a positive influence on mite feeding and consequently on its reproduction, as measured in this study. Given the strong impact of mite feeding on viral replication (10, 12), a loop of reciprocal stimulation of the two symbionts is evident, which largely accounts for their central role in honey bee colony losses (7, 10).
The basal level of infertility in Varroa mite populations can be quite high and may vary for reasons not completely understood, often related to undefined host factors (5). We suggest that the presence and level of infection by DWV may partly account for this variation, which could be due to different levels of host immunosuppression. Even though this positive effect of DWV infection on mite reproduction can be partly induced by enhanced feeding efficiency, we cannot rule out the possibility of a greater nutritional suitability of infected hosts, which is an issue meriting further research efforts aimed at fully characterizing the host regulation strategy adopted by the Varroa mite.
The concept of host regulation is adopted to describe a wide range of host physiological and behavioral changes induced by parasites of arthropods, which have been especially well investigated in insect parasitoids (38). These carnivorous insects, particularly parasitic wasps of the order Hymenoptera, are able to colonize and exploit living insect hosts using a wealth of virulence factors (38). The ovarian secretions injected by adult females during oviposition may contain symbiont viruses (39), which in some parasitic wasps of caterpillars are members of the unique family Polydnaviridae (39–41). The ancestor of these viral symbionts in the genus Bracovirus was a viral pathogen of the host, in the Nudivirus group, now stably integrated into the wasp genome and used as a tool to deliver virulence factors (41, 42). The Varroa–DWV association could be considered a similar symbiotic relationship, but at an early stage and with a less intimate level of integration, where the vector role of Varroa is paid back by a DWV-induced fitness enhancement mediated by host immunosuppression. This seems to be part of a unique evolutionary pattern promoting the “alliance” of parasitic arthropods with viral pathogens of the host to overcome its immune barriers (39).
The results of our present study shed new light on the Varroa–DWV association, supporting a previously unrecognized mutualistic symbiosis. This information accounts for the central importance of the mite–virus complex in the induction of honey bee colony losses, and sets the stage for studies aiming to develop new management strategies for one of the most dangerous parasite–pathogen associations for the beekeeping industry. Indeed, any environmental stress that interferes with honey bee immunocompetence and promotes DWV replication in individuals bearing covert infections (8, 10, 30) also has an indirect effect on Varroa, which is favored by the escalating viral-induced immunosuppression. This may account in part for the recently observed positive correlation between Varroa populations and honey bee exposure to neonicotinoids (43, 44), which are known to promote viral replication in DWV-infected honey bees (30); however, additional field evidence is needed to support this hypothesis. A more thorough understanding of the molecular mechanisms underlying this self-boosted process that enhances the fitness of both symbionts, as well as its modulation by interacting stress factors, will be crucial to understand the complex dynamics of honey bee colony losses and developing novel strategies to alleviate its significant negative impact on agriculture and environment.
Materials and Methods
Biological Material.
The honey bees and mites used in this study were from A. mellifera ligustica colonies maintained in the experimental apiary of the Università degli Studi di Napoli “Federico II” and from A. mellifera ligustica × A. mellifera carnica colonies maintained in the experimental apiary of the Università degli Studi di Udine.
In brief, to obtain both the fifth instar honey bee larvae and the mites needed for artificial infestation and in vitro rearing experiments, all of the sealed cells of several brood combs were marked on the evening before the experiment. The next morning, the brood combs were transferred to the laboratory, and all of the unmarked cells that had been sealed during the preceding 12–15 h were unsealed with forceps. The frames were incubated at 35 °C and 75% relative humidity, to allow the honey bee larvae to emerge from cells together with possible infesting mites. When needed, honey bee larvae were isolated in gelatin capsules (6.5 mm diameter) together with one mite each, and then kept in an incubator at 34–35 °C and 75–80% relative humidity.
Encapsulation and Melanization Assay.
Fifth instar honey bee larvae (n = 40) were obtained from freshly capped cells. A piece of nylon thread (0.08 mm) was inserted into the hemocelic cavity under a stereomicroscope at 20× magnification. After 24 h, the implants were removed and photographed under a light microscope (400×). Image analysis was performed using GIMP version 2.8 (GNU Image Manipulation Program; www.gimp.org). The degree of encapsulation was scored as the percentage of nonwhite pixels (i.e., covered by hemocytes). The melanization index (a percentage) was calculated as (1 − x/255)100, where x represents the mean degree of gray intensity (a numerical reading ranging from 0 for black to 255 for white) of the pixels in the area covered by hemocytes. The rest of the body was immediately stored at −80 °C for the subsequent molecular analysis. All samples were analyzed by qRT-PCR to assess for the possible presence of black queen cell virus and sacbrood virus, which were previously found in the same area, although at a comparatively much lower prevalence than DWV (10). A total of 28 larvae were considered for the analysis, after discarding nine larvae showing prolonged bleeding after the implantation and three larvae showing the presence of viral pathogens other than DWV.
Cloning of the Amel\102 Gene.
To clone the A. mellifera homolog of the 102Hv gene, we first blasted the protein sequence of 102Hv (GenBank accession no. CBY85302.1) against the “nonredundant protein sequences” (nr) database of the honey bee genome. A sequence (XP_003251941.1) showing 36% identity with 102Hv was identified, and the corresponding cDNA was obtained by qRT-PCR and elongated by 5′ and 3′ RACE, using specific primer pairs (Table S1). This cDNA is contained in a contig (11266) of the whole genome shotgun sequence (GenBank accession no. AADG06011266.1).
Table S1.
Primer pairs used in this study
| Analysis | Name | Sequence, bp | Fragment, bp |
| RACE 5′ | Amel\102 GSP | ATGGTTTCATTTATATCCTCGA (22) | 473 |
| Amel\102 NESTED GSP | GATATAATGTTATATGTCATGC (22) | ||
| RACE 3′ | Amel\102 GSP | CCTCGAAGTAAAGGAATAACTG (22) | 444 |
| Amel\102 NESTED GSP | GAACATGTATTTATAGGAGAAAG (23) | ||
| qRT-PCR | DWV, forward | GCGCTTAGTGGAGGAAATGAA (21) | 69 |
| DWV, reverse | GCACCTACGCGATGTAAATCTG (22) | ||
| qRT-PCR | Amel\102, forward | CAACTCCAGAATTGGAAATAGCA (23) | 160 |
| Amel\102, reverse | TTTGCAATAGGAAAAGCAGTTG (22) | ||
| qRT-PCR | Actin, forward | GATTTGTATGCCAACACTGTCCTT (24) | 69 |
| Actin, reverse | TTGCATTCTATCTGCGATTCCA (22) | ||
| qRT-PCR | Dorsal, forward | TCGGATGGTGCTACGAGCGA | 153 |
| Dorsal, reverse | AGCATGCTTCTCAGCTTCTGCCT | ||
| qRT-PCR | Apidaecin, forward | TTTTGCCTTAGCAATTCTTGTTG | 81 |
| Apidaecin, reverse | GAAGGTCGAGTAGGCGGATCT | ||
| qRT-PCR | Amel\LRR, forward | CTTGGTGAAGGCCTTGATG | 87 |
| Amel\LRR, reverse | ATGCAAAGAGCTATCATCA |
Expression Analysis of the Amel\102 Gene.
We measured the expression profile of Amel\102 in various tissues of honey bee larvae by qRT-PCR. For this, we used a micropipette to collect the hemolymph exuding from a puncture made with a sterile needle in the dorsal vessel in the fourth abdominal segment of fifth instar honey bee larvae (n = 15), which was transferred in 40 µL of PBS (137 mM NaCl, 2.7 mM KCl, and 10 mM phosphate buffer, pH 7.4). After bleeding, experimental larvae were dissected under an optical stereomicroscope (20×) to separate the gut and the fat body. Hemolymph samples were centrifuged at 600 × g for 6 min, to isolate the hemocytes. All tissues to be analyzed were immediately transferred in a cold extraction buffer for subsequent total RNA extraction.
To assess whether Amel\102, apidaecin, and Amel\LRR expression is influenced by DWV infection, we performed qRT-PCR to determine transcription levels in honey bee larvae with different levels of viral genome copies in the samples used for the encapsulation and melanization experiments. To examine whether immunosuppression was due exclusively to the action of DWV infection, we performed an additional experiment in which honey bee larvae were exposed to a controlled Varroa infestation. At the end of the experiment, we measured the expression level of Amel\102, as affected by mite feeding, in the presence and absence of DWV infection. To do so, we collected fifth instar honey bee larvae from freshly capped cells and introduced them singly into gelatin capsules (6.5 mm diameter), along with a single Varroa mite obtained from a highly infested colony. An equal number of uninfested fifth instar honey bee larvae served as controls. Rearing capsules were then transferred into an incubator and kept at 35 °C and 80% relative humidity. After 24 h, honey bee larvae were flash-frozen at −80 °C for the molecular analysis. Ten individuals for each experimental combination of Varroa infestation and DWV infection were selected at random, and the transcription level of Amel\102 was measured.
Virus Infection and Mite Reproduction.
We artificially infested fifth instar honey bee larvae with one mite each and kept them in gelatin capsules at 35 °C and 75% relative humidity (32). After 12 d, at the end of the pupal stage, cells were opened and inspected under a stereomicroscope to assess the possible occurrence of honey bee wing deformity and mite reproduction. The honey bees were then stored at −80 °C for subsequent qRT-PCR to assess the viral genome copy number.
This experiment was replicated twice. A total of 90 honey bees in which the viral infection level as well as mite reproduction could be assessed were obtained and used in the analysis. Five honey bees that appeared to be uninfected were not used in this analysis.
Because the relationship between wing deformity at the adult stage and viral load is well known from the literature (6) and confirmed by the present study, we conducted another experiment in which artificially infested honey bee larvae were checked after reaching the adult stage (when wing deformity can be unequivocally assessed). The status of their wings correlated with the reproduction of the mites feeding on them. We replicated the experiment six times, obtaining 149 adult honey bees, of which 100 had normal wings and 49 had crippled wings.
qRT-PCR.
qRT-PCR was performed following standard methods, as described in detail in SI Materials and Methods.
RNAi.
Double-stranded honey bee dorsal-1A (A. mellifera Dorsal variant A, mRNA, GI:58585243, 2389 bp) was prepared using the MEGAscript RNAi Kit (Ambion), following the manufacturer’s standard protocol. The target sequence was PCR-amplified with specific primers, carrying a 5′ tail of the T7 promoter at both ends and used as template for T7-dependent in vitro transcription. The following primers were used: forward, 5′-TAATACGACTCACTATAGGGAGACAATCCAGCACTTATTC-3′; reverse, 5′-TAATACGACTCACTATAGGGAGCCTGAATAGTGTTATTAGC-3′. The reaction product was subjected to DNase digestion and purified, and the final preparation was dissolved in nuclease-free water.
Individual frames were removed from the colony and stored in an incubator overnight at 34 °C and 90% relative humidity. Emerging bees were maintained as groups of 30 individuals in sterile boxes and fed daily with 2 mL of a 50% sucrose/protein solution, containing 50 µg of dsRNA of dorsal-1A. Controls were fed with a similar solution containing a dsRNA of mGFP6, obtained as described above. Samples were collected from five bees at the beginning of the experiment, to assess the starting level of scored parameters, and again after 48 h and 96 h of exposure to the dsRNA feeding solution. Samples were stored at −80 °C until use for RNA extraction.
The transcription levels of dorsal-1A and apidaecin and the number of DWV genome copies were determined by SYBR Green qRT-PCR, as described above.
Bioinformatic and Statistical Analyses.
Alignment of Heliothis virescens, Spodoptera littoralis, Drosophila melanogaster, and A. mellifera protein sequences was performed using the ClustalW algorithm. Secondary structure prediction of A. mellifera P102 was carried out with InterProScan tool and the EMBOSS: garnier algorithm. Analyses were performed using Geneious version 6.1.6 (Biomatters; available from www.geneious.com).
All of the correlation analyses were carried out using the Spearman rank-sum method. The expression profile of Amel\102 in different honey bee tissues was analyzed using Kruskal–Wallis one-way ANOVA.
In the RNAi experiments, gene expression and viral replication in bees fed with dsRNA of dorsal-1A, or dsRNA of GFP as controls, were compared using the Scheirer–Ray–Hare extension of the Kruskal–Wallis test. Five bees per each treatment were used in the analysis. Amel\102 transcription as affected by DWV infection and feeding activity by Varroa was analyzed using the Sheirer–Ray–Hare extension of the Kruskal–Wallis test.
The polynomial curve fitting the distribution of Varroa mite fertility rates associated with different levels of honey bee viral infection, expressed as the logarithm of the average infection level of the bees in each class, was calculated using the least squares method. The relationship between viral load and wing deformity was assessed by logistic regression. The fertility of Varroa mites on honey bees with normal and crippled wings was analyzed using the χ2 test. The 95% confidence limits of the fertility reported in Fig. 4 were calculated using the following equation: 1.96 * √P * (1 − P)/n.
SI Materials and Methods
Total RNA for qRT-PCR was isolated from individual honey bees using TRIzol reagent (Invitrogen), according to the manufacturer’s instructions. The concentration and purity of total RNA were determined by spectrophotometry (Nanodrop ND100; Thermo Fisher Scientific). Differential relative expression of Amel\102 was tested by means of SYBR Green qRT-PCR, using the primer pairs reported in Table S1. Relative gene expression data were analyzed using the 2ΔΔCt method. To verify that the amplification efficiencies of the target and reference genes (β-actin) were approximately equal, the amplification of five 10-fold dilutions of the total RNA sample (from 1,000 to 0.1 ng per reaction) in triplicate were analyzed. The efficiency plot for log input total RNA vs. ΔCt had a slope lower than ±0.1 (slope = −0.066, R2 = 0.0961).
The quantification of DWV genome copies in individual honey bees was performed by SYBR Green qRT-PCR. Titers of DWV were determined by relating the Ct values of unknown samples to an established standard curve. The standard curve was established by plotting the logarithm of seven 10-fold dilutions of a starting solution containing 21.9 ng of plasmid DNA pCR II-TOPO (TOPO-TA cloning) with a DWV insert (from 21.9 ng to 21.9 fg), against the corresponding Ct value as the average of three repetitions. The PCR efficiency (E = 107.5%) was calculated based on the slope and coefficient of correlation (R2) of the standard curve, according to the following formula: E = 10(−1/slope) − 1 (slope = −3.155, y-intercept = 41.84, R2 = 0.999). Similarly, for quantification of dorsal-1A transcript numbers, the standard curve was established by plotting the logarithm of nine 10-fold dilutions of a starting solution containing 127.4 ng of plasmid DNA (TOPO-TA cloning) with a dorsal-1A insert (from 127.4 ng to 1.3 fg) against the corresponding Ct value as the average of three repetitions. The PCR efficiency (E = 93.2%) was calculated as described above for DWV (slope = 23.495, y-intercept = 46.19, R2 = 0.996). Amplifications were performed using the StepOne Real-Time PCR System (Life Technologies) with the following thermal cycling profiles: one cycle at 48 °C for 15 min for reverse transcription, one cycle at 95 °C for 10 min, 40 cycles at 95 °C for 15 s and 60 °C for 1 min, and one cycle at 68 °C for 7 min, using the Power SYBR Green RNA-to-Ct 1-Step Kit (Thermo Fisher Scientific). All primer pairs were designed using PrimerExpress 3.0 software (Life Technologies) following the standard procedure. Negative (H2O) and positive controls (previously identified positive samples) were included in each qRT-PCR run.
Acknowledgments
We thank Spencer Behmer (Texas A&M University), Michael Strand (University of Georgia), and Silvia Gigliotti (Institute of Biosciences and Bioresources - Consiglio Nazionale delle Ricerche, IBBR-CNR) for their critical read of the manuscript, and Ilaria Di Lelio and Adriana Marinelli (University of Napoli “Federico II”) for their help and suggestions during the development of this study. The research leading to these results was funded by the European Union Seventh Framework Programme (FP7/2007-2013), under Grant 613960 (SMARTBEES).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1523515113/-/DCSupplemental.
References
- 1.Breeze TD, et al. Agricultural policies exacerbate honeybee pollination service supply-demand mismatches across Europe. PLoS One. 2014;9(1):e82996. doi: 10.1371/journal.pone.0082996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ratnieks FLW, Carreck NL. Ecology: Clarity on honey bee collapse? Science. 2010;327(5962):152–153. doi: 10.1126/science.1185563. [DOI] [PubMed] [Google Scholar]
- 3.Underwood R, vanEngelsdorp D. Colony collapse disorder: Have we seen this before? Bee Cult. 2007;35:13–18. [Google Scholar]
- 4.Carreck N, Neumann P. Honey bee colony losses. J Apic Res. 2010;49(1):1–6. [Google Scholar]
- 5.Rosenkranz P, Aumeier P, Ziegelmann B. Biology and control of Varroa destructor. J Invertebr Pathol. 2010;103(Suppl 1):S96–S119. doi: 10.1016/j.jip.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 6.de Miranda JR, Genersch E. Deformed wing virus. J Invertebr Pathol. 2010;103(Suppl 1):S48–S61. doi: 10.1016/j.jip.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Martin SJ, et al. Global honey bee viral landscape altered by a parasitic mite. Science. 2012;336(6086):1304–1306. doi: 10.1126/science.1220941. [DOI] [PubMed] [Google Scholar]
- 8.Nazzi F, Pennacchio F. Disentangling multiple interactions in the hive ecosystem. Trends Parasitol. 2014;30(12):556–561. doi: 10.1016/j.pt.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Kielmanowicz MG, et al. Prospective large-scale field study generates predictive model identifying major contributors to colony losses. PLoS Pathog. 2015;11(4):e1004816. doi: 10.1371/journal.ppat.1004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nazzi F, et al. Synergistic parasite–pathogen interactions mediated by host immunity can drive the collapse of honeybee colonies. PLoS Pathog. 2012;8(6):e1002735. doi: 10.1371/journal.ppat.1002735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flenniken ML, Andino R. Non-specific dsRNA-mediated antiviral response in the honey bee. PLoS One. 2013;8(10):e77263. doi: 10.1371/journal.pone.0077263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryabov EV, et al. A virulent strain of deformed wing virus (DWV) of honeybees (Apis mellifera) prevails after Varroa destructor-mediated, or in vitro, transmission. PLoS Pathog. 2014;10(6):e1004230. doi: 10.1371/journal.ppat.1004230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brutscher LM, Daughenbaugh KF, Flenniken ML. Antiviral defense mechanisms in honey bees. Curr Opin Insect Sci. 2015;10:71–82. doi: 10.1016/j.cois.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zambon RA, Nandakumar M, Vakharia VN, Wu LP. The Toll pathway is important for an antiviral response in Drosophila. Proc Natl Acad Sci USA. 2005;102(20):7257–7262. doi: 10.1073/pnas.0409181102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Souza-Neto JA, Sim S, Dimopoulos G. An evolutionary conserved function of the JAK-STAT pathway in anti-dengue defense. Proc Natl Acad Sci USA. 2009;106(42):17841–17846. doi: 10.1073/pnas.0905006106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabin LR, Hanna SL, Cherry S. Innate antiviral immunity in Drosophila. Curr Opin Immunol. 2010;22(1):4–9. doi: 10.1016/j.coi.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kingsolver MB, Huang Z, Hardy RW. Insect antiviral innate immunity: Pathways, effectors, and connections. J Mol Biol. 2013;425(24):4921–4936. doi: 10.1016/j.jmb.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamiable O, Imler JL. Induced antiviral innate immunity in Drosophila. Curr Opin Microbiol. 2014;20:62–68. doi: 10.1016/j.mib.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chejanovsky N, et al. Characterization of viral siRNA populations in honey bee colony collapse disorder. Virology. 2014;454-455:176–183. doi: 10.1016/j.virol.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Goulson D, Nicholls E, Botías C, Rotheray EL. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science. 2015;347(6229):1255957. doi: 10.1126/science.1255957. [DOI] [PubMed] [Google Scholar]
- 21.Chandrasekaran S, et al. Behavior-specific changes in transcriptional modules lead to distinct and predictable neurogenomic states. Proc Natl Acad Sci USA. 2011;108(44):18020–18025. doi: 10.1073/pnas.1114093108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuster RD, Boncristiani HF, Rueppell O. Immunogene and viral transcript dynamics during parasitic Varroa destructor mite infection of developing honey bee (Apis mellifera) pupae. J Exp Biol. 2014;217(Pt 10):1710–1718. doi: 10.1242/jeb.097766. [DOI] [PubMed] [Google Scholar]
- 23.Donzé G, Guerin PM. Behavioral attributes and parental care of Varroa mites parasitizing honeybee brood. Behav Ecol Sociobiol. 1994;34(5):305–319. [Google Scholar]
- 24.Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 25.Theopold U, Schmidt O, Söderhäll K, Dushay MS. Coagulation in arthropods: Defence, wound closure and healing. Trends Immunol. 2004;25(6):289–294. doi: 10.1016/j.it.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Dushay MS. Insect hemolymph clotting. Cell Mol Life Sci. 2009;66(16):2643–2650. doi: 10.1007/s00018-009-0036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falabella P, et al. Functional amyloids in insect immune response. Insect Biochem Mol Biol. 2012;42(3):203–211. doi: 10.1016/j.ibmb.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Di Lelio I, et al. Functional analysis of an immune gene of Spodoptera littoralis by RNAi. J Insect Physiol. 2014;64:90–97. doi: 10.1016/j.jinsphys.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Evans JD, et al. Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Mol Biol. 2006;15(5):645–656. doi: 10.1111/j.1365-2583.2006.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Prisco G, et al. Neonicotinoid clothianidin adversely affects insect immunity and promotes replication of a viral pathogen in honey bees. Proc Natl Acad Sci USA. 2013;110(46):18466–18471. doi: 10.1073/pnas.1314923110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaki MH, Man SM, Vogel P, Lamkanfi M, Kanneganti TD. Salmonella exploits NLRP12-dependent innate immune signaling to suppress host defenses during infection. Proc Natl Acad Sci USA. 2014;111(1):385–390. doi: 10.1073/pnas.1317643111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nazzi F, Milani N. A technique for reproduction of Varroa jacobsoni Oud. under laboratory conditions. Apidologie. 1994;25:579–584. [Google Scholar]
- 33.Yang X, Cox-Foster DL. Impact of an ectoparasite on the immunity and pathology of an invertebrate: Evidence for host immunosuppression and viral amplification. Proc Natl Acad Sci USA. 2005;102(21):7470–7475. doi: 10.1073/pnas.0501860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gregory PG, Evans JD, Rinderer T, de Guzman L. Conditional immune-gene suppression of honeybees parasitized by Varroa mites. J Insect Sci. 2005;5:7. doi: 10.1093/jis/5.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Navajas M, et al. Differential gene expression of the honey bee Apis mellifera associated with Varroa destructor infection. BMC Genomics. 2008;9:301. doi: 10.1186/1471-2164-9-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Liu X, Zhang W, Han R. Differential gene expression of the honey bees Apis mellifera and A. cerana induced by Varroa destructor infection. J Insect Physiol. 2010;56(9):1207–1218. doi: 10.1016/j.jinsphys.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 37.Richards EH, Jones B, Bowman A. Salivary secretions from the honeybee mite, Varroa destructor: Effects on insect haemocytes and preliminary biochemical characterization. Parasitology. 2011;138(5):602–608. doi: 10.1017/S0031182011000072. [DOI] [PubMed] [Google Scholar]
- 38.Pennacchio F, Strand MR. Evolution of developmental strategies in parasitic hymenoptera. Annu Rev Entomol. 2006;51:233–258. doi: 10.1146/annurev.ento.51.110104.151029. [DOI] [PubMed] [Google Scholar]
- 39.White JA, Giorgini M, Strand MR, Pennacchio F. Arthropod endosymbiosis and evolution. In: Minelli A, Boxshall G, Fusco G, editors. Arthropod Biology and Evolution. Springer-Verlag; Berlin, Germany: 2014. pp. 441–477. [Google Scholar]
- 40.Strand MR, Burke GR. Polydnavirus–wasp associations: Evolution, genome organization, and function. Curr Opin Virol. 2013;3(5):587–594. doi: 10.1016/j.coviro.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 41.Herniou EA, et al. When parasitic wasps hijacked viruses: Genomic and functional evolution of polydnaviruses. Philos Trans R Soc Lond B Biol Sci. 2013;368(1626):20130051. doi: 10.1098/rstb.2013.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bézier A, et al. Polydnaviruses of braconid wasps derive from an ancestral nudivirus. Science. 2009;323(5916):926–930. doi: 10.1126/science.1166788. [DOI] [PubMed] [Google Scholar]
- 43.Alburaki M, et al. Neonicotinoid-coated Zea mays seeds indirectly affect honeybee performance and pathogen susceptibility in field trials. PLoS One. 2015;10(5):e0125790. doi: 10.1371/journal.pone.0125790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dively GP, Embrey MS, Kamel A, Hawthorne DJ, Pettis JS. Assessment of chronic sublethal effects of imidacloprid on honey bee colony health. PLoS One. 2015;10(3):e0118748. doi: 10.1371/journal.pone.0118748. [DOI] [PMC free article] [PubMed] [Google Scholar]