Significance
Genetic mutations that impair basal ganglia (BG) function affect sequential movements, but the link between genotype, BG circuit dysfunction, and altered behavior remains unclear. Here, we found that viral expression of a genetic mutation that causes Huntington’s disease (HD) in a vocalization-related region of the songbird BG leads to the selective loss of striatal medium spiny neurons, abnormal temporal patterns of cortico-BG activity during vocalization, and highly unstable vocal sequences. Moreover, silencing activity in the cortical component of this circuit stabilized vocalizations, supporting the idea that the genetic mutation that causes HD affects complex motor sequences by altering temporal patterns of cortico-BG activity.
Keywords: Huntington’s disease, basal ganglia, songbird, motor sequence, vocalization
Abstract
The basal ganglia (BG) promote complex sequential movements by helping to select elementary motor gestures appropriate to a given behavioral context. Indeed, Huntington’s disease (HD), which causes striatal atrophy in the BG, is characterized by hyperkinesia and chorea. How striatal cell loss alters activity in the BG and downstream motor cortical regions to cause these disorganized movements remains unknown. Here, we show that expressing the genetic mutation that causes HD in a song-related region of the songbird BG destabilizes syllable sequences and increases overall vocal activity, but leave the structure of individual syllables intact. These behavioral changes are paralleled by the selective loss of striatal neurons and reduction of inhibitory synapses on pallidal neurons that serve as the BG output. Chronic recordings in singing birds revealed disrupted temporal patterns of activity in pallidal neurons and downstream cortical neurons. Moreover, reversible inactivation of the cortical neurons rescued the disorganized vocal sequences in transfected birds. These findings shed light on a key role of temporal patterns of cortico-BG activity in the regulation of complex motor sequences and show how a genetic mutation alters cortico-BG networks to cause disorganized movements.
The execution of complex behaviors, such as speech or musicianship, depends on the ability to organize elementary motor “gestures” into precisely timed sequences of movements. In the vertebrate brain, the basal ganglia (BG) play a key role in regulating complex motor sequences, as revealed by the behavioral disruptions characteristic of neurological diseases that affect BG function (1–3). A particularly striking example is Huntington’s disease (HD), which is characterized anatomically by extensive cell death in the striatum, the primary input layer of the BG network (4), and behaviorally by hyperkinesia and chorea, which can interfere with the ability to produce coordinated, sequential movements (5, 6). Despite numerous animal models of HD, the detailed neural mechanisms by which striatal dysfunction leads to disorganized movements have remained elusive. One challenge is that HD also affects brain regions other than the BG, making it difficult to identify causal links between BG circuit dysfunction and behavioral symptoms. Another challenge, especially in rodent models of HD, is the limited understanding of whether and how BG circuitry contributes to specific behaviors.
The zebra finch is a small Australian songbird that presents several advantages for addressing these challenges. Adult male zebra finches sing a learned song comprising a reproducible sequence of stereotyped syllables (i.e., a motif), and they sing both spontaneously and abundantly, allowing for extensive characterization of syllables and syllable sequences. Moreover, an anatomically differentiated network of interconnected brain nuclei important to singing distinguishes the zebra finch brain (7, 8). An anterior forebrain pathway within this song system shares many similarities to mammalian cortico-BG networks (Fig. 1A), including a BG nucleus (area X) that contains both medium spiny neurons (MSNs) and pallidal neurons, which closely resemble their mammalian counterparts in their intrinsic, synaptic, and circuit properties (9–13). Pallidal neurons in area X communicate via the thalamus to a cortical premotor nucleus [lateral magnocellular nucleus of the anterior nidopallium (LMAN)] that plays a critical role in modulating song variability (14–18). The dedicated nature of this pathway for singing provides a rare opportunity to explore a detailed mechanism by which deficits in the BG act across broader cortico-BG networks to cause specific behavioral symptoms. Furthermore, the spatially distributed organization of the component song nuclei is well suited to the use of viral methods for selectively expressing genetic constructs within a single node in this network (19–22).
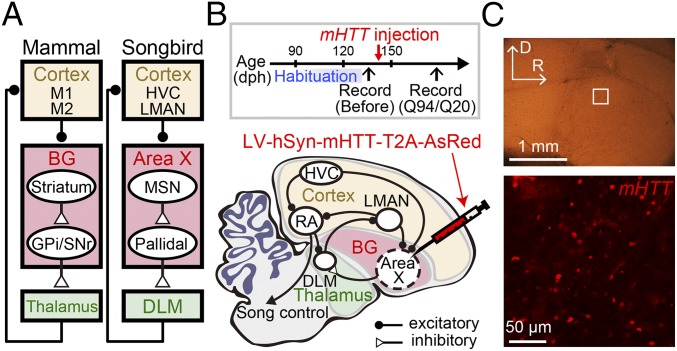
Fig. 1.
Expression of mHTT in the songbird BG. (A) Simplified schematic showing some of the common features of mammalian and songbird cortico-BG networks. (B) Time line of experiments (Top) and a schematic showing a part of the neural circuitry involved in singing behaviors in the songbird (Bottom; sagittal view with rostral to the right and dorsal upward). LV containing a mutant form of mHTT and a fluorescent reporter, AsRed, with insertion of a self-cleaving T2A sequence was injected into the songbird BG (area X) of adult male zebra finches. The songs before injection (habituation) were highly stable (Fig. S1). Behavioral or neural changes were assessed at >30 d after injection. dph, days posthatch. (C) Square region in area X in a sagittal brain slice (Top; D, dorsal; R, rostral) was imaged 34 d after injection with a confocal microscope (Bottom) to confirm expression of mHTT-Q94, as revealed by coexpressed AsRed (see also Fig. S1).
Intriguingly, a transgenic zebra finch model of HD was recently generated that shares several similarities with human HD (23), lending support to the idea that songbirds can afford useful models in which to identify how genetic mutations that cause HD alter BG circuitry to affect behavior. Indeed, these transgenic HD songbirds showed deficits in song learning; produced songs with abnormal syllable and sequence variability as adults; and displayed other symptoms, including body rigidity, tremor, and cervical dystonia. However, as with transgenic rodent models of HD and patients with HD, the brain-wide expression of the genetic mutation that causes HD in these transgenic songbirds makes it challenging to understand the link between genotype, circuit pathology, and behavioral phenotype. Here, we overcame this challenge by virally expressing a mutant gene fragment that causes HD [exon 1 of the huntingtin gene (mHTT)] in area X of adult male zebra finches. This focal genetic perturbation caused selective deficits in vocalization: The transfected birds vocalized more abundantly and sang aberrant songs in which syllable sequences, but not individual syllables, were selectively destabilized. These vocal changes were accompanied by the selective loss of MSNs in area X, a marked reduction of inhibitory synapses on pallidal neurons, and disrupted temporal patterns of singing-related activity in pallidal and LMAN neurons. Moreover, reversibly inactivating LMAN was sufficient to restore normal vocal behavior. These findings indicate that focal expression of mHTT in the BG disrupts motor sequences by altering temporal patterns of cortico-BG activity, which may afford a novel target for ameliorating disorganized movements in BG diseases.
Results
Expression of mHTT in the Songbird BG.
Human HD is caused by expanded (>34) Gln (CAG) repeats in exon 1 of the huntingtin gene (mHTT). We transfected area X neurons in adult zebra finches (139 ± 19 d posthatch, mean ± SD; n = 9 birds) with lentivirus (LV) containing a mutant form of mHTT with either 94 or 20 CAG repeats (mHTT-Q94 or HTT-Q20) and a cleavable fluorescent reporter, AsRed, under the control of the human synapsin 1 promoter (Fig. 1B). Between 20 and 30 d following LV injection into area X, a time course similar to other studies that have used LV to express transgenes in songbirds (19–22), a subset of neurons expressed the AsRed reporter (Fig. 1C). These experiments indicate that LV-mediated gene transfer can be used to express mHTT in the songbird BG region area X.
Selective Disruption of Syllable Sequence Organization in mHTT Birds.
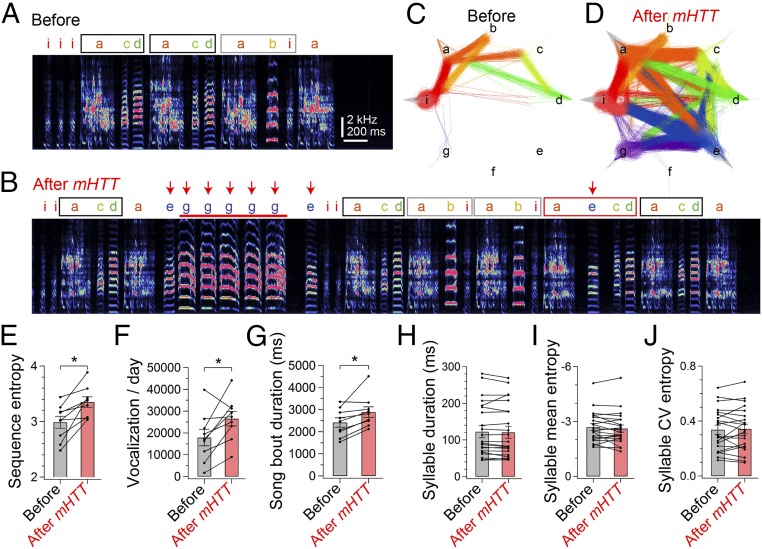
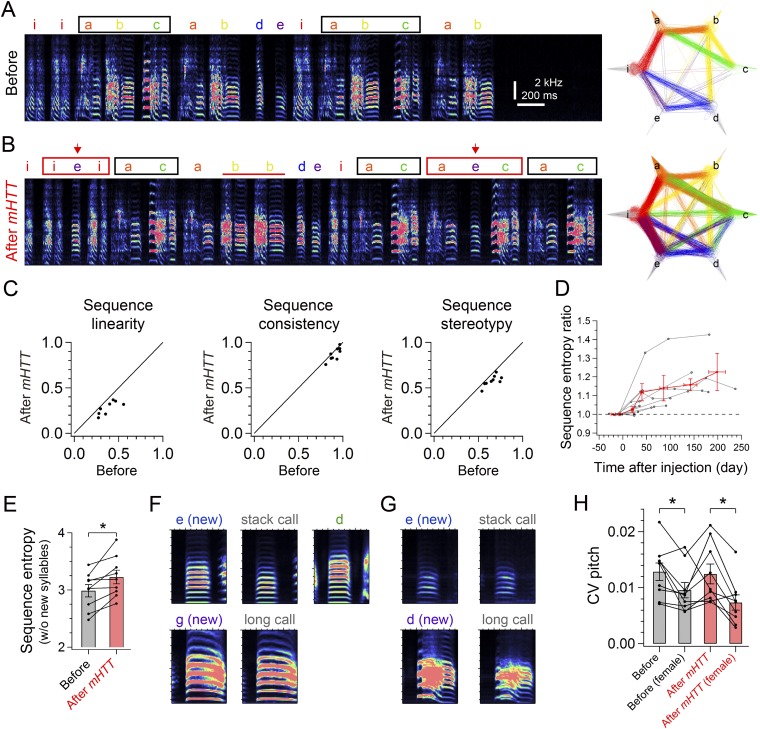
We used LV-mediated gene transfer to address how expression of mHTT affects previously learned songs in adult zebra finches. Adult male zebra finches typically sing stable stereotyped motifs, with one or more motifs sung in quick succession to form a song bout (Fig. 2 A and C). Before viral injections, we confirmed that all of the birds used in our study sang a stable sequence of stereotyped syllables (Fig. S1; all songs were recorded in social isolation). Thirty to sixty days following bilateral injection of mHTT-Q94 in area X, the singing behavior of all birds underwent marked changes (Fig. 2 and Fig. S2; all songs were recorded in social isolation). The most notable change was that syllable sequences became highly unstable from one motif to the next, an effect that could be attributed to an increase in previously rare syllable transitions as well as the emergence of novel syllable transitions, some of which involved newly incorporated vocalizations (Fig. 2 A–D). In some instances, this sequence instability made comparison of motifs with those motifs produced before viral injection difficult (Fig. S2 A and B). We quantified these sequence-level effects using a variety of metrics, including sequence entropy, linearity, consistency, and stereotypy, all of which revealed significant changes (Fig. 2E and Fig. S2C). Long-term recordings revealed that changes in sequence stability could persist for many months after viral injection (Fig. S2D). These sequence-level effects remained even if song bouts that included newly incorporated syllables were excluded from the analysis (Fig. S2E), indicating that the original syllable sequences also became unstable. Moreover, calculating sequence entropy separately for each syllable revealed that transitions from most (∼72%) syllables became more variable, suggestive of a global sequencing defect. We also noted that some newly integrated syllables closely resembled calls (i.e., vocalizations produced outside song bouts) that the bird had produced before viral injections, whereas others had spectral features distinct from the bird’s calls (Fig. S2 F and G). Therefore, expression of mHTT-Q94 in area X can lead to the inappropriate incorporation of calls and novel vocalizations into the song motif, and this newly integrated vocal material can also contribute to sequence instability.
Fig. 2.
Selective disruption of syllable sequences in mHTT-Q94 birds. Spectrogram of a representative song bout from a male adult zebra finch at 8 d before (A) and 47 d after (B) bilateral injection of mHTT in area X (see also Fig. S2 A and B). Characters denote different syllables. Stereotyped song motifs are demarcated with black and gray squares. Integration of new vocalizations (red arrows) accompanied a new syllable sequence (red square) and increased occurrence of syllable repetition (red horizontal bar). A syllable transition diagram shows all syllable trajectories on the day in A (C) and B (D). Each song bout starts with a gray inward line, and lines were then drawn between consecutive syllables to show a syllable trajectory of the song bout, which ends with an outward line. Colors denote preceding syllables. Repetitions of syllables are shown as curved lines with brighter colors. After injection of mHTT, sequence entropy (E), total vocalizations on the day (F), and mean song bout duration (G) were increased, indicating disrupted syllable sequencing [sequence entropy: t(8) = 4.059, P = 0.010; vocalization: t(8) = 2.785, P = 0.024; bout duration: t(8) = 3.46, P = 0.011]. *P < 0.050. Spectral features such as duration (H), mean entropy (I), and CV of entropy (J) were not significantly changed by injection of mHTT [duration: t(20) = 0.833, P = 0.415; mean entropy: t(20) = 1.122, P = 0.275; CV entropy: t(20) = 0.418, P = 0.681] (see also Figs. S2 and S3).
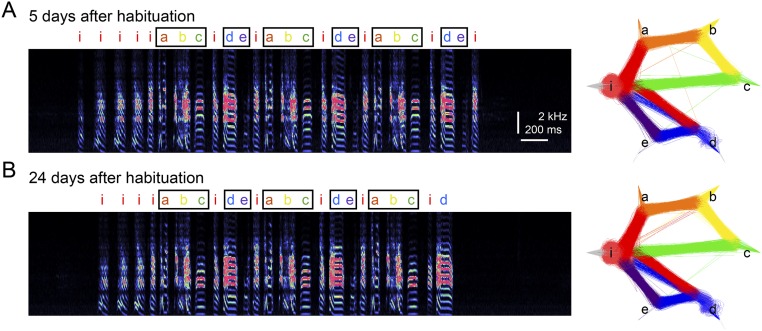
Fig. S1.
Stable syllable sequences during the habituation period. (A) Spectrogram of a representative song bout (Left) and a syllable transition diagram (Right) from a bird at 5 d after the start of habituation to the recording chamber. Song motifs were shown with black squares. (B) Same as A, but at 24 d after habituation.
Fig. S2.
Behavioral data from mHTT birds. Spectrogram of a representative song bout (Left) and a syllable transition diagram (Right) from a bird recorded 8 d before (A) and 137 d after (B) bilateral injection of mHTT in area X. Note that the dominant song motif (black square) was reduced from three to two syllables after injection, but syllable transitions became variable with irregular syllable repetitions (red horizontal bar) and syllable insertions (red arrow), forming new syllable transitions (red square). (C) Decrease in linearity [71.9 ± 4.2%; t(8) = 6.656, P < 0.001], consistency [95.7 ± 1.5%; t(8) = 2.941, P = 0.022], and stereotypy [88.4 ± 2.3%; t(8) = 5.152, P = 0.002] after injection of mHTT. (D) Long-term change of sequence entropy in mHTT birds (gray) and its average in each time window (red), shown normalized to the value at 1–15 d before injection. (E) Sequence entropy calculated with song bouts that did not include newly incorporated syllables [t(8) = 3.558, P = 0.007]. (F) Mean spectrograms of 30 renditions of syllables and calls of the mHTT bird shown in Fig. 2 A–D. A newly incorporated syllable (Top Left, e) had different spectral features from a stack call (Top Middle) or an original syllable (Top Right, d). (Bottom) Another newly integrated syllable (g) was highly similar to a long call. Each panel shows a 200-ms spectrogram (from 50 ms before to 150 ms after the vocal onset) with the frequency range of 0.5–10 kHz. (G) Same as in F, but with data from the other mHTT bird shown in Fig. 6. A newly integrated syllable (Top Left, e) was highly similar to a stack call (Top Right). Another newly incorporated syllable (Bottom Left, d) had slightly different spectral features from a long call (Bottom Right). (H) CV pitch of syllables before and after mHTT injection in the presence or absence of a female [effect of injection: F(1,24) = 0.72, P = 0.402; effect of a female: F(1,24) = 7.54, P = 0.010; interaction: F(1,24) = 0.35, P = 0.561]. Error bars indicate SEM. *P < 0.050.
In addition to increased instability of syllable sequences, we noted increases in the daily amount of vocalization and in the duration of individual song bouts (Fig. 2 F and G), the latter of which could be attributed to an increase in the number of syllables produced during each bout [mean syllables per bout; before: 15.2 ± 1.9, after: 18.1 ± 2.7; t(8) = 2.70, P = 0.030]. There was also an increased occurrence of song bouts containing repeated syllables [repetitions occurred in 8.4 ± 4.1% of bouts before injection and in 34.1 ± 8.8% of bouts after injection; t(7) = 4.205, P = 0.003] and a trend toward increased numbers of times that the affected syllable was repeated [before: 2.13 ± 0.10 syllables; after: 2.48 ± 0.22 syllables; t(6) = 2.045, P = 0.087]. In contrast, we did not see changes in repetition of introductory notes, which are unlearned vocalizations produced before a motif [before: 2.10 ± 0.20 introductory notes, after: 2.11 ± 0.23 introductory notes; t(8) = 1.816, P = 0.923]. Transfecting area X neurons with HTT-Q20 did not produce sequence- or bout-level changes in song behavior (Fig. S3), indicating that these vocal changes were caused by expanded CAG repeats in mHTT-Q94 rather than by nonspecific effects of viral injection or damage resulting from stereotaxic surgery.
Fig. S3.
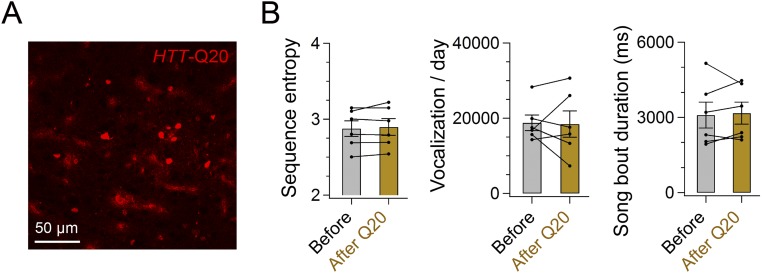
Behavioral data from HTT-Q20 birds. (A) Expression of HTT-Q20 as revealed by coexpressed AsRed. The cell density was 673.8 ± 189.6 cells per square millimeter (n = 4 hemispheres from two birds). (B) Sequence entropy [t(5) = 1.020, P = 0.354], vocalizations per day [t(5) = 0.120, P = 0.909], and song bout duration [t(5) = 0.359, P = 0.734] were unchanged >30 d after injection of HTT-Q20. Error bars indicate SEM.
In contrast to these sequence- and bout-level changes, syllable features in mHTT-Q94 birds, such as duration, Wiener entropy, and variability [coefficient of variance (CV) entropy], remained unaffected (Fig. 2 H–J). Moreover, in the presence of a female, mHTT-Q94 birds reduced the variability in the pitch of individual syllables (Fig. S2H) and increased the number of introductory notes [no female: 4.89 ± 1.20, female: 8.31 ± 1.48; t(7) = 6.719, P = 0.001], two context-dependent features of vocal performance important to effective courtship in normal males (24, 25). We also noted that mHTT-Q94 birds exhibited reduced sequence entropy in the presence of a female [no female: 3.34 ± 0.11, female: 3.13 ± 0.15; t(7) = 2.710, P = 0.030]. No other gross behavioral changes, such as tremor or cervical dystonia, were detected in mHTT-Q94 birds. Taken together, these results indicate that expressing mHTT-Q94 in area X selectively impairs the bird’s ability to organize syllable sequences.
Preferential Loss of Striatal Neurons and Reduction of Inhibitory Synapses on Pallidal Neurons in mHTT Birds.
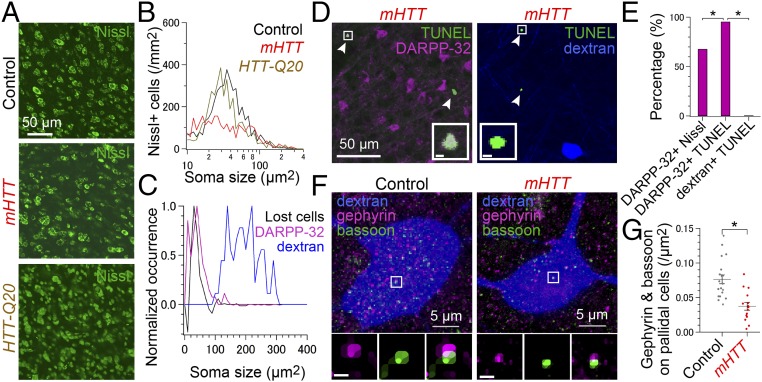
A major feature of HD is striatal atrophy resulting from the death of striatal MSNs (4), raising the possibility that the behavioral changes we detected in mHTT-Q94 birds resulted from the loss of striatal neurons. However, because smaller (i.e., soma area of <100 μm2) striatal MSNs and larger pallidal neurons are intermingled in area X (10), injecting LV-mHTT-Q94 could potentially exert nonspecific cytotoxic effects on both striatal and pallidal neurons. Consistent with the idea that mHTT-Q94 expression in area X selectively kills MSNs, Nissl staining of fixed tissue sections revealed that the density of smaller neurons (i.e., soma area of <100 μm2) in area X was reduced in mHTT-Q94 birds [Fig. 3 A and B; ∼42% loss; control: 4,147 ± 269 cells per square millimeter, mHTT-Q94: 2,389 ± 343 cells per square milliliter; t(6) = 3.932, P = 0.008], but not in HTT-Q20 birds [HTT-Q20: 3,595 ± 331 cells per square millimeter; t(6) = 1.288, P = 0.245]. Notably, the size distribution of cells that were lost following LV-mHTT-Q94 injection in area X was similar to the size distribution of MSN cell bodies, as measured by 32 kDa dopamine- and cAMP-regulated phosphoprotein (DARPP-32) immunofluorescence (Fig. 3C), and did not overlap with the size distribution of pallidal cell bodies, which were labeled by injecting retrograde tracer into their axonal terminal field in the song motor thalamus [i.e., the medial nucleus of the dorsolateral thalamus (DLM)]. These findings support the idea that expressing mHTT-Q94 in area X results in the loss of striatal MSNs, but spares the pallidal cell population.
Fig. 3.
Cellular and synaptic deficits in area X of mHTT birds. Nissl-stained area X neurons (A) and their soma size histograms averaged across control birds (black, n = 4), mHTT birds (red, n = 4), and HTT-Q20 birds (orange, n = 2) (B). (C) Peak-normalized soma size histogram of the difference between black and red traces in B (black, indicating lost cells), DARPP-32–positive MSNs (magenta, n = 2 birds) and dextran-labeled pallidal neurons (blue, n = 2 birds). (D) TUNEL-positive dying neurons (green, arrowhead) in mHTT birds were DARPP-32–positive MSNs (Left, pseudocolored magenta) but not dextran-labeled pallidal neurons (Right, pseudocolored blue). (Insets) Squared region. (Scale bars, 2 μm.) (E) Fraction of DARPP-32–positive MSNs was greater in TUNEL-positive neurons than in Nissl-positive neurons [χ2(3) = 25.7, P < 0.001], suggesting the preferential degeneration of MSNs. The fraction of DARPP-32–positive MSNs was greater than dextran-labeled pallidal neurons in TUNEL-positive neurons [χ2(3) = 101.0, P < 0.001] in area X of mHTT birds. (F) Maximum-projected images showing inhibitory synapses detected as colocalization of gephyrin (pseudocolored magenta) and bassoon puncta (green) on a dextran-labeled pallidal neuron (pseudocolored blue) in a control bird (Left) and mHTT bird (Right). (Insets) Median-filtered images of gephyrin (Left), bassoon (Center), and merged (Right) in the squared region. (Scale bars, 0.5 μm.) (G) Reduced inhibitory synapses on pallidal neurons in mHTT-Q94 birds [control: n = 19 cells from two birds, mHTT-Q94: n = 15 cells from two birds; t(32) = 4.517, P < 0.001]. *P < 0.050 (see also Fig. S4).
To explore cell death triggered in area X by expressing mHTT-Q94, we labeled area X cells using the TUNEL system, which detects cell death by labeling cleaved DNA (26). We detected more TUNEL-positive cells in area X of birds injected with mHTT-Q94 [control: 1.2 ± 1.2 cells per square millimeter, mHTT-Q94: 36.3 ± 11.8 cells per square millimeter; t(9) = 2.688, P = 0.025]. Moreover, almost all (105 of 110 cells, n = 3 birds) of the TUNEL-positive cells in mHTT-Q94 birds were also positive for the MSN marker DARPP-32, whereas none (0 of 21 cells, n = 2 birds) of the TUNEL-positive cells were pallidal neurons, as identified via retrograde label from DLM (Fig. 3 D and E). In area X of birds injected with LV-mHTT-Q94, DARPP-32–positive cells constituted a higher fraction in the TUNEL-positive cell populations (105 of 110 cells) than in the Nissl-stained neuron populations (53 of 78 neurons, n = 2 birds) (Fig. 3E). This result indicates that expressing mHTT-Q94 in area X preferentially induces cell death in striatal MSNs, because nonspecific cell death would result in a similar fraction of DARPP-32–positive cells in the TUNEL-positive and Nissl-stained neuron populations.
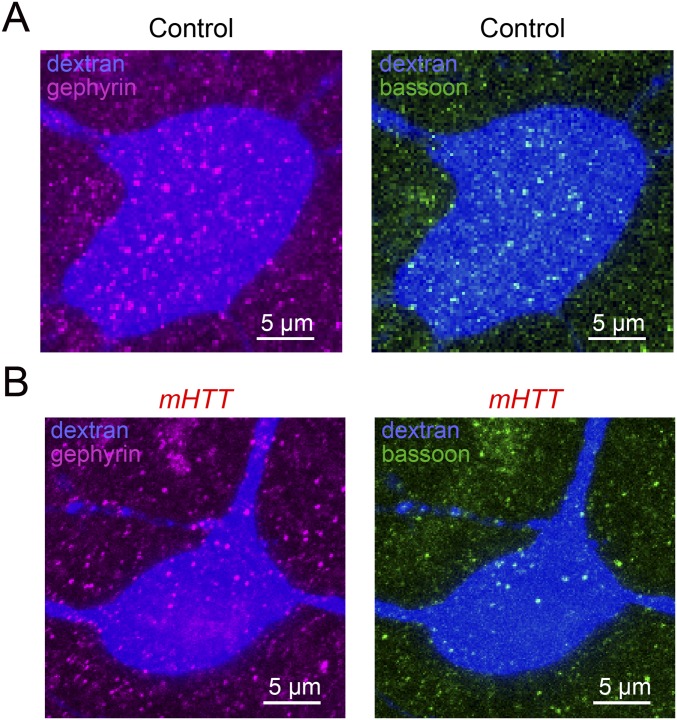
Similar to mammalian striatopallidal organization, MSNs make inhibitory synapses onto pallidal neurons in area X (12). The loss of MSNs following mHTT-Q94 expression in area X raises the possibility that inhibitory synapses on pallidal neurons are also reduced in number. Consistent with this idea, the cell bodies and proximal dendrites of DLM-projecting pallidal neurons in mHTT-Q94 birds had half the number of inhibitory synapses, as measured by the immunocytochemical colocalization of inhibitory postsynaptic markers (gephyrin) and nonspecific presynaptic markers (bassoon) (Fig. 3 F and G and Fig. S4). Therefore, expressing mHTT-Q94 in area X reduces perisomatic inhibitory synapses on DLM-projecting pallidal neurons in parallel with reducing the numbers of MSNs, which are a major source of these inhibitory synapses. Because DLM-projecting pallidal neurons serve as the sole output of area X, MSN cell death followed by inhibitory synaptic reorganization on pallidal neurons could affect singing by altering the output of the BG network.
Fig. S4.
Additional histological data. Maximum-projected images of anti-gephyrin (Left) and anti-bassoon (Right) signals on a dextran-labeled pallidal neuron (blue) in a control bird (A) and mHTT bird (B) shown in Fig. 3F.
Temporally Disrupted Cortico-BG Network Activity in mHTT Birds.
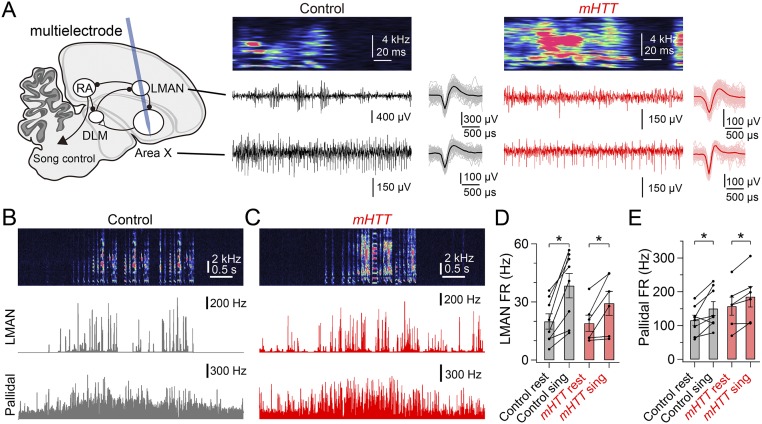
Pallidal neurons in area X influence song indirectly through LMAN, a premotor cortical structure that serves as an output of the cortico-BG network (17, 27). To examine how expressing mHTT-Q94 in area X affects cortico-BG network activity, we used a multielectrode array and spike-sorting methods to record pallidal and LMAN neuron activity simultaneously in freely behaving adult male zebra finches (Fig. S5A; all recordings were made from adult male zebra finches in social isolation). Both pallidal and LMAN neurons in mHTT-Q94 birds exhibited increased firing rates with singing, as was also observed in normal birds (Fig. S5 B–E).
Fig. S5.
Recordings of LMAN and area X pallidal neuron activity in singing birds. (A) Schematic showing a typical path of the multielectrode array (blue) implanted in LMAN and area X (Left) with representative recordings in a control bird (Center) and mHTT bird (Right), each of which shows a syllable spectrogram (Top), LMAN activity (Middle), and area X pallidal activity (Bottom). (Insets) Averaged waveforms of 100 randomly chosen sorted spikes. In a control bird (B) and mHTT bird (C), representative recordings of a song bout (Top) and instantaneous firing rate of LMAN (Middle) and pallidal activity (Bottom) are shown. (D) Mean LMAN firing rate (FR) during resting (rest) and singing (sing) states in control and mHTT-Q94 birds. In both conditions, the LMAN FR was greater during singing than resting [control: t(7) = 5.010, P = 0.002, n = 8 cells from three birds; mHTT-Q94: t(5) = 2.681, P = 0.031, n = 6 cells from three birds). (E) Mean pallidal firing rate during resting (rest) and singing (sing) states in control and mHTT birds. In both conditions, the pallidal FR was greater during singing than resting [control: t(7) = 2.840, P = 0.025, n = 8 cells from four birds; mHTT-Q94: t(5) = 4.092, P = 0.009, n = 6 cells from three birds]. Error bars indicate SEM. *P < 0.050.
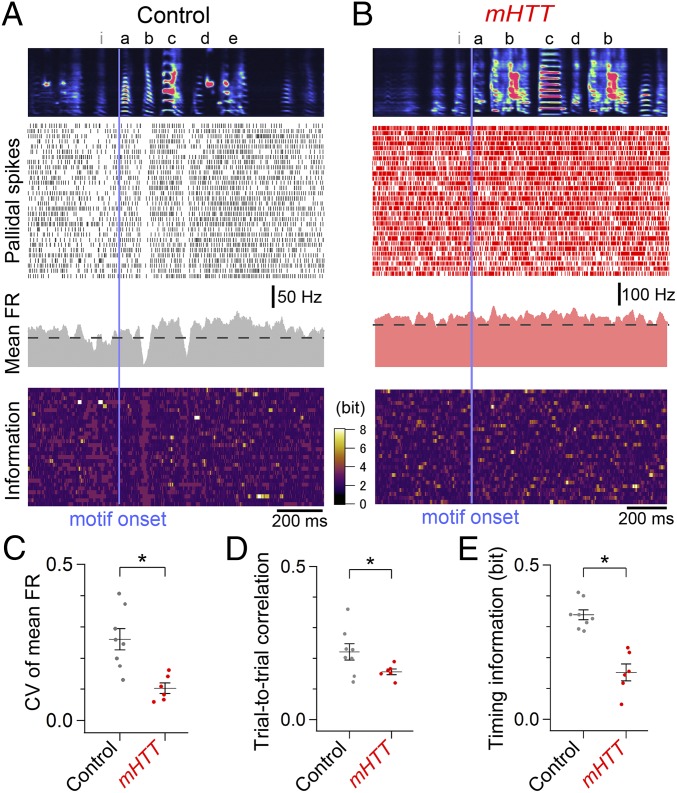
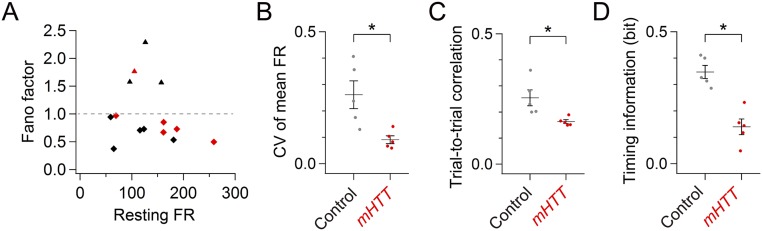
However, abnormal activity patterns in both cell types became evident when they were aligned to a specific vocal sequence (the dominant motif: the most frequently produced motif) (Materials and Methods and Figs. 4 and 5). Whereas pallidal neurons in normal birds exhibited substantial modulation of firing rates at specific times during the motif (Fig. 4A), this temporal modulation was greatly reduced in mHTT-Q94 birds (Fig. 4 B and C). We also noted a reduction of the within-cell, trial-to-trial correlation of pallidal activity in mHTT-Q94 birds (Fig. 4D), suggesting that expressing mHTT-Q94 in area X disrupts the temporal precision of pallidal activity during singing. To estimate how changes in the temporal patterning of pallidal activity relate to syllable sequences, we calculated the mutual information between pallidal activity and consecutive time sequences in the motif. Indeed, this “timing information” was significantly decreased in mHTT-Q94 birds (Fig. 4E), indicating that pallidal activity conveys less information of moment-by-moment sequences in the song motif. Because pallidal neurons in area X can be divided into two distinct anatomical classes, only one of which projects to the thalamic nucleus DLM (28), a remaining issue is whether the firing properties of DLM-projecting pallidal cells are affected. We applied a Fano factor analysis, which has previously been used to distinguish the two pallidal cell types (29), to establish that the singing-related activity of putative DLM-projecting pallidal neurons was temporally disrupted in mHTT-Q94 birds (Fig. S6). These results show that expressing mHTT-Q94 in area X disrupts the temporal precision of pallidothalamic activity, which may degrade timing information about specific vocal sequences.
Fig. 4.
Reduction of precise temporal modulation of pallidal activity in mHTT birds. Pallidal activity was aligned to the onset of the dominant song motif (vertical bar) in a control bird (A) and mHTT bird (B), showing averaged spectrogram of the first 30 renditions of songs that include the dominant song motif (Top), a spike raster of the corresponding pallidal neuron activity (Upper Middle), the cell’s mean firing rate (FR) (Lower Middle), and information on the cell’s activity calculated as -log2p(a), where p(a) is the probability of observing the spike number a in a 20-ms bin during the motif (Bottom). Compared with control birds, pallidal activity in mHTT birds exhibited a reduction in the CV of the mean FR (C), trial-to-trial correlation (D), and timing information, calculated as mutual information between pallidal activity and motif timing (E) [CV of mean FR: t(12) = 3.877, P = 0.002; correlation: t(12) = 2.525, P = 0.049; timing information: t(12) = 5.646, P < 0.001]. *P < 0.050 (see also Figs. S5 and S6).
Fig. 5.
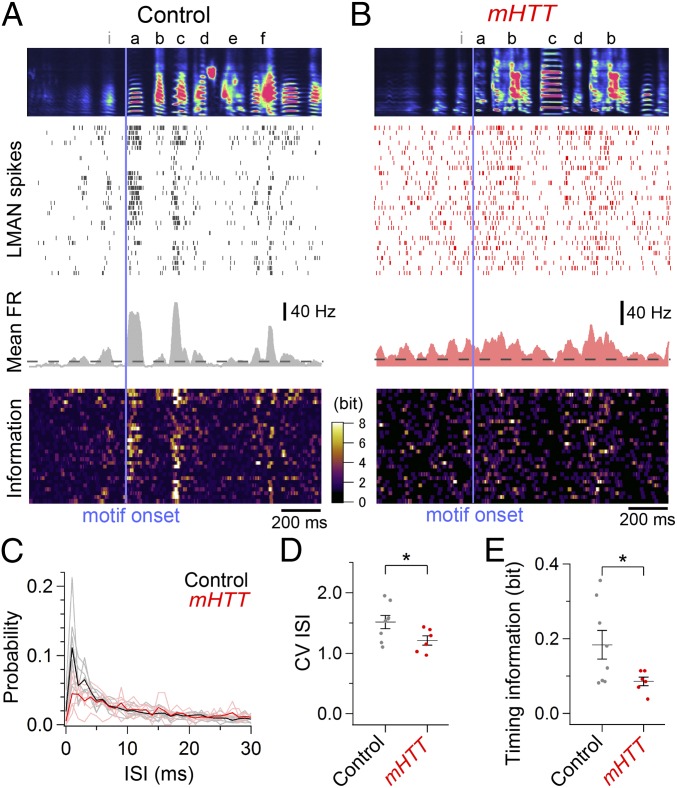
Abnormally regular LMAN activity with less timing information in mHTT birds. LMAN activity was aligned to the onset of the dominant song motif (vertical bar) in a control bird (A) and mHTT bird (B), showing averaged spectrogram of the first 30 renditions of songs that includes the dominant song motif (Top), corresponding spike rasters of LMAN activity (Upper Middle), mean FR across renditions (Lower Middle), and information on the cell’s activity as in Fig. 4 A and B (Bottom). (C) Mean ISI histogram of LMAN activity in control (black, n = 8 cells from three birds) and mHTT (red, n = 6 cells from three birds) birds. Bright lines indicate individual data. Compared with control birds, LMAN activity in mHTT birds exhibited a reduction in the CV of the ISI (D) and timing information (E) [CV ISI: t(12) = 2.286, P = 0.041; timing information: t(12) = 2.304, P = 0.040]. *P < 0.050 (see also Figs. S5 and S7).
Fig. S6.
Analysis of putative DLM-projecting pallidal neuron activity. (A) Scatter plot of pallidal neuron activity showing the mean FR during the nonsinging state (resting FR) and mean Fano factor during singing. Putative DLM-projecting pallidal activity (diamonds) and non–DLM-projecting pallidal activity (triangles) from control (black) and mHTT birds (red) could be segregated with a mean Fano factor of 1.0 (horizontal dashed line) (29). The Fano factor was calculated for the first 500 ms of the dominant motif as variance divided by mean (σ2/μ) of a 20-ms binned spike count (a sliding step size of 1 ms) across 30 iterations. Quantification of precise temporal modulation of putative DLM-projecting pallidal neuron activity in control and mHTT birds (same dataset as in Fig. 4) shows a reduction in the CV of the mean FR (B), trial-to-trial correlation (C), and timing information (D) in mHTT birds [CV of mean FR: t(8) = 2.649, P = 0.029; correlation: t(8) = 2.498, P = 0.037; timing information: t(8) = 5.294, P < 0.001]. Error bars indicate SEM. *P < 0.050.
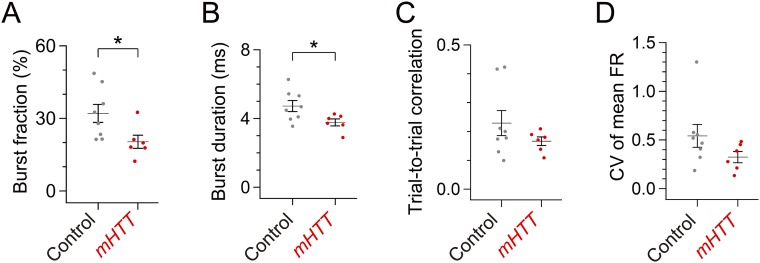
We also detected changes in the temporal patterns of singing-related LMAN activity following expression of mHTT in area X. We found that singing-related burst firing of LMAN neurons, which is known to be regulated by BG circuitry (22, 30), was less frequent in mHTT-Q94 birds and that bursts were of shorter duration when they did occur (Fig. 5 A–C and Fig. S7 A and B). Because the mean firing rate in LMAN was not altered in mHTT-Q94 birds [Fig. S5D; t(12) = 1.027, P = 0.325], LMAN firing was more evenly distributed throughout different syllables in the motif, as reflected in the reduced CV in interspike intervals (ISIs; Fig. 5D). Moreover, we also noted reduced timing information in LMAN neurons of mHTT-Q94 birds (Fig. 5E), indicating that the abnormally regular LMAN activity has less selectivity to specific syllables, as we observed with pallidal neuron activity in mHTT-Q94 birds. However, unlike for pallidal neurons, the within-cell, trial-to-trial correlation of LMAN activity was preserved in mHTT-Q94 birds (Fig. S7C), and LMAN firing still exhibited substantial modulation during singing (Fig. S7D). Therefore, LMAN neurons in mHTT-Q94 birds transmit abnormal timing information to downstream song motor structures on a background of normal trial-to-trial firing rate correlations and substantial firing rate modulations.
Fig. S7.
Additional analysis of LMAN activity. (A) Burst firing of LMAN neurons was less frequent in mHTT birds [fraction of ISIs <5 ms: t(12) = 2.527, P = 0.027]. Burst firing tended to be less frequent even if we restricted our analysis to the number of bursts per second [control: 7.50 ± 1.48 bursts per second, mHTT-Q94: 3.77 ± 0.20 bursts per second; t(12) = 2.126, P = 0.055]. (B) Mean duration of LMAN bursts was shorter in mHTT birds [t(12) = 2.505, P = 0.028]. (C) Trial-to-trial correlation of LMAN activity was not different between control and mHTT birds [t(12) = 1.331, P = 0.208]. (D) CV of mean FR of LMAN activity was not different between control and mHTT birds [t(12) = 1.650, P = 0.127]. Error bars indicate SEM. *P < 0.050.
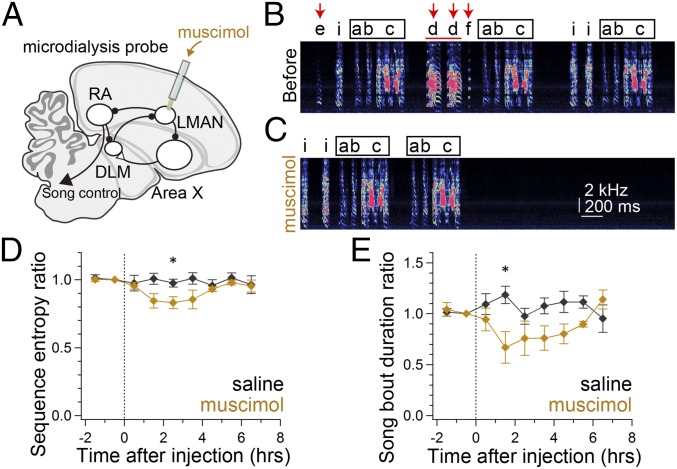
Inactivating LMAN Activity Reversibly Rescues Disrupted Song Behavior in mHTT Birds.
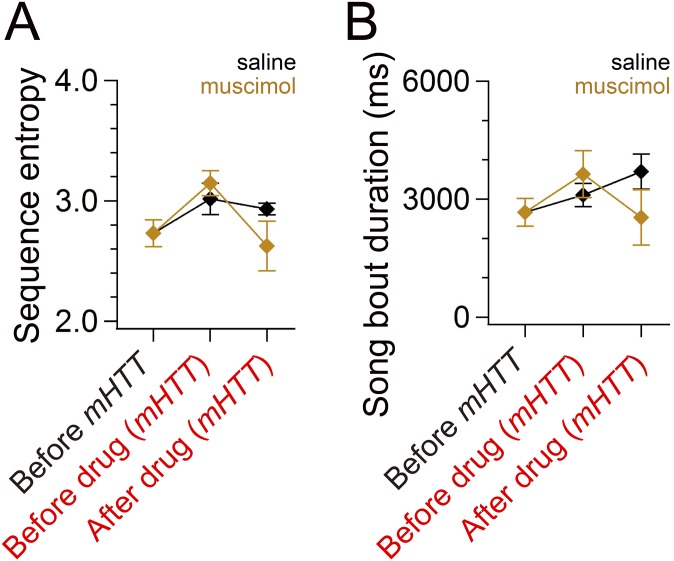
To test directly the idea that the temporally disrupted LMAN activity is responsible for disorganized song in mHTT-Q94 birds, we reversibly inactivated LMAN by muscimol application through bilaterally implanted microdialysis probes (Fig. 6A). Notably, LMAN inactivation reduced sequence entropy to nearly normal levels (Fig. 6 B–D and Fig. S8A) and also reduced overall song bout duration in mHTT-Q94 birds (Fig. 6E and Fig. S8B). The shorter song bout duration was attributed to reduced syllable numbers in each song bout [before muscimol: 20.0 ± 2.7 syllables per bout, after muscimol: 16.7 ± 2.6 syllables per bout; t(3) = 3.590, P = 0.037]. We also noted that there was a tendency toward less frequent syllable repetitions, but this difference was not significant [before muscimol: 35.1 ± 15.8%, after muscimol: 7.3 ± 2.7%; t(3) = 1.961, P = 0.145]. Inactivating LMAN also reduced the variability in the fundamental frequency of individual syllables [CV of pitch; pre: 0.013 ± 0.003, post: 0.006 ± 0.001; t(5) = 2.571, P = 0.050], consistent with the known function of LMAN in modulating syllable variability (15, 31, 32). Taken together, these findings indicate that temporally disrupted LMAN activity is critical for driving disorganized song behavior in mHTT-Q94 birds.
Fig. 6.
Inactivating LMAN rescues aberrant song behavior in mHTT birds. (A) Schematic showing infusion of 5 mM muscimol with a microdialysis probe implanted into LMAN to inactivate LMAN activity. A representative song bout sung 1 h before (B) and 2 h after (C) infusion of muscimol in a mHTT bird is shown. Characters denote song syllables, and the square indicates the dominant motif. Increased sequence variability with irregular syllable insertion (red arrows in B) and repetition (red horizontal bar in B) decreased after muscimol infusion (C). Sequence entropy (D) and song bout duration (E) are shown as a function of time after infusion of saline (black) or muscimol (orange) in mHTT birds (n = 4 birds). Asterisks show significance (*P < 0.050), with a paired t test, after the significant main effect of condition was confirmed with two-way ANOVA [sequence entropy [F(1,58) = 10.41, P = 0.002; bout duration: F(1,58) = 11.05, P = 0.002] (see also Fig. S8).
Fig. S8.
Recovery of songs of mHTT birds to normal levels by inactivating LMAN activity. Inactivation of LMAN activity recovered sequence entropy [A; before mHTT and 2 h after infusion of muscimol: t(3) = 1.035, P = 0.377] and song bout duration [B; before mHTT and 1 h after infusion of muscimol; t(3) = 0.284, P = 0.795] nearly to the original levels (same dataset as in Fig. 6). Error bars indicate SEM.
Discussion
Here, we show that expressing mHTT in area X, a region of the songbird BG specialized for singing behavior, destabilizes syllable sequences and increases overall vocal activity, but leaves the structure of individual syllables intact. A cellular correlate of these behavioral changes includes the selective loss of striatal MSNs in area X paralleled by a marked reduction in inhibitory synapses on pallidal neurons that serve as the BG output. These cellular and synaptic changes were sufficient to alter the activity across a larger expanse of the BG-cortical network, because pallidal neurons and downstream LMAN neurons in mHTT birds exhibited abnormal patterns of singing-related activity. Moreover, reversibly inactivating LMAN rescued aberrant vocal behavior in mHTT birds, indicating that changes to the timing of activity in the cortico-BG network drive changes in vocal sequencing. The present findings that aberrant temporal patterns of cortico-BG activity disrupt complex motor sequences may inform strategies for ameliorating disorganized movements in BG diseases.
Parallels to HD and Selective Impairment of Sequential Movements in mHTT Birds.
BG circuits are implicated in the organization of motor sequences, in part, by selecting for elementary gestures appropriate to a given behavioral context (i.e., action selection) (1–3). Here, we found that focally expressing mHTT in area X of the zebra finch affects syllable sequence organization by destabilizing syllable sequences and decreasing song termination, and also by increasing the tendency to repeat syllables. Sequence destabilization could be attributed to an increase in previously rare syllable transitions as well as the expression of novel syllable transitions, some of which involved the incorporation of new syllables into a motif. These behavioral changes are reminiscent of disorganized movements in humans with HD, including increased spontaneous movements, disorganized sequences of elementary gestures, and insertion of inappropriate gestures into sequential movements (6, 33).
These results also extend the recent finding that a transgenic zebra finch model of HD exhibits various HD-like symptoms (23) by demonstrating that focal expression of mHTT in area X selectively affects motor sequences related to singing behavior, thus providing a more specific link between the genetic insult, circuit defects, and aberrant behavior. Here, we observed only syllable sequence destabilization following expression of mHTT in area X, whereas the transgenic HD songbird displays sequence and syllable instability, as well as body rigidity, tremor, and cervical dystonia. Presumably, the additional behavioral symptoms that manifest in the transgenic finch result from the expression of mHTT in other song and motor regions of the brain outside of area X, many of which also express the mHTT gene at high levels (23). Another distinction is that the viral approach allowed for temporally restricted expression of mHTT in adult finches after they had already successfully learned and stabilized their songs, thus avoiding the potential confound in the transgenic finch that some of its song abnormalities are secondary consequences of impaired learning. Consequently, a useful aspect of the viral approach is that it establishes the sufficiency of spatially and developmentally restricted expression of mHTT in area X of the adult finch in driving sequence-specific changes to song.
Previous studies indicate that the ability to organize multiple movements temporally can be disrupted in humans with HD, leaving elementary movements relatively unaffected (5, 6, 34). Similarly, birds expressing mHTT in area X exhibited sequence-level changes to song, but the structure and variability of individual syllables remained unaffected. The lack of changes to previously learned syllables and the continued ability of mHTT birds to modulate variability as a function of social context further support a model in which the variability of individual syllables and of the sequences in which they occur are at least partly under independent control (16, 35). A possible clinical parallel is that humans with HD often exhibit grammatical errors and can struggle to produce complex sentences, even though simple phonemic generation can be preserved (33). In summary, these various observations strengthen the notion that the BG play a critical role in organizing and terminating motor sequences and show that focally expressing mHTT in the BG results in elevated levels of aberrant sequential behavior, reminiscent of the hyperkinetic, disorganized movements characteristic of HD.
Circuit Basis for Impaired Motor Sequences in mHTT Birds.
The song-related effects of expressing mHTT in area X exert selective behavioral and anatomical effects that contrast with the behavioral and anatomical effects elicited by area X lesions, thus helping to illuminate how different components of the BG circuitry regulate sequential movements. First, sequence variability, vocal activity, and song bout duration increase in mHTT finches but are often unaffected following area X lesions in adult birds (36, 37; but see 38, 39). Moreover, variability and duration of individual syllables are unaffected in mHTT birds but are altered following area X lesions (30, 38). The song behavior of mHTT birds could not simply be attributed to nonspecific cytotoxic effects of viral injection, infection, or overexpression of mHTT, because expressing exon 1 with 20 CAG repeats [i.e., a quantity fivefold greater than the avian repeat number (23)] was without any discernible behavioral effect. More fundamentally, chemical or electrolytic lesions placed in area X destroy both MSNs and pallidal neurons, whereas expressing mHTT in area X selectively kills MSNs without reducing the numbers of pallidal neurons. Interestingly, chemical inactivation of the globus pallidus internus does not disrupt learned motor sequences in monkeys, suggesting that pallidal neuron activity may be unnecessary for the maintenance of previously learned behaviors (40). In mHTT birds, the intact population of pallidal neurons may exacerbate aberrant vocal behavior by transmitting temporally disrupted patterns of activity to downstream thalamic and cortical premotor neurons.
Although pallidal neurons are preserved in mHTT birds, inhibitory cells and synapses in area X undergo extensive reconfiguration. An analysis of cell death- and cell type-specific markers indicates that expressing mHTT in area X selectively triggers cell death in MSNs, similar to the selective loss of striatal MSNs in HD (4, 41). One consequence of MSN cell death is the marked reduction of inhibitory puncta on DLM-projecting pallidal neuron cell bodies and proximal dendrites, which presumably reflects the loss of inhibitory input from MSNs, and perhaps from other inhibitory neurons in area X. Although syllable repetitions in zebra finches with area X lesions have been speculated to arise from the replacement of MSNs through ongoing neurogenesis (38), the persistent reduction in inhibitory synapses on the DLM-projecting pallidal neurons we observed suggests that the primary factor driving abnormal vocal behavior is the massive loss of MSNs, which is a hallmark of HD. Ultimately, because pallidal neurons fire action potentials spontaneously and at high rates, and because MSN neurons generate precisely timed action potential bursts during singing (13), this loss of MSN input is likely to diminish or otherwise alter precisely timed singing-related activity in pallidal neurons.
Indeed, extracellular recordings made from pallidal neurons in mHTT finches revealed reduced temporal modulation during singing, consistent with the idea that diminished inhibition results in less precisely controlled pallidal neuron activity. More specifically, although pallidal neuron firing rates increased during song, the normal patterns of sharp and transient decreases in firing rate were absent, which could be accounted for by diminished inhibitory input from MSN cells. Notably, transient decreases in pallidal neuron firing rate are sufficient to generate action potential bursts in their postsynaptic targets in the song motor thalamus (i.e., DLM) (42), which, in turn, excite the LMAN neurons that are an important source of vocal variability (14, 16, 43). Therefore, expressing mHTT in area X has the potential to alter activity not only in the BG but also in the cortical neurons that are indirect targets of pallidal neurons and that more directly affect vocalization. In support of this view, LMAN neurons in mHTT birds exhibited reduced bursting activity and less variable spike timing, and ultimately conveyed less timing information during singing. Furthermore, inactivating LMAN stabilized syllable sequences, but sharply reduced song bout duration and overall vocal activity, suggesting that abnormal patterns of LMAN activity drive abnormal vocal behavior in mHTT birds. These findings provide insights into how a spatially restricted genetic insult can ramify through the local BG circuitry and across a broader cortico-BG network to affect a complex sequential behavior and raise the possibility that precisely manipulating this network activity may help to restore normal motor function in HD.
Possible Mechanisms by Which Cortico-BG Networks Influence Sequential Movements.
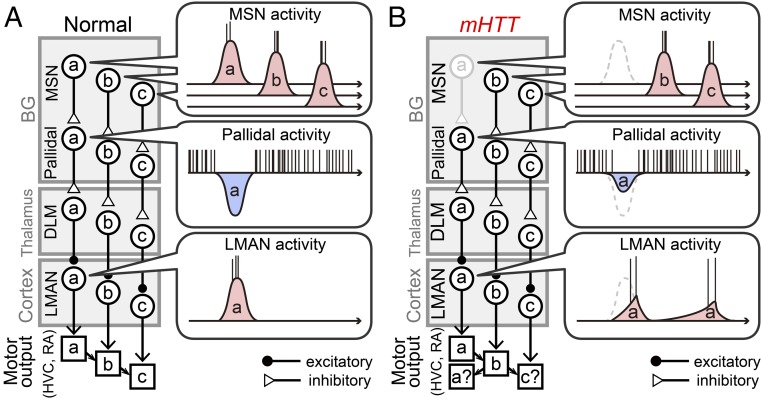
A remaining question is how abnormal LMAN activity patterns affect sequential vocal behavior. Individual LMAN axons bifurcate as they exit the caudal margin of the nucleus, with separate branches terminating in the song motor cortical region [the robust nucleus of arcopallium (RA)] and in area X (18, 44, 45). The LMAN-RA pathway is known to be an important source of syllable variability (15) and could also potentially affect sequence variability through recurrent pathways involving the thalamus and the song premotor nucleus HVC (46), which lies upstream of RA and is thought to regulate song’s temporal features, including intersyllable transitions (47–51). However, syllable variability was unaffected in mHTT birds, raising the question of how the propagation of altered LMAN activity through RA could affect sequence variability without also increasing the variability of individual syllables. One possibility is that distinct features of LMAN activity differently affect syllable sequences and variability through the LMAN-RA pathway: Disrupted temporal patterns could destabilize syllable sequences without affecting syllable variability, whereas unaltered trial-to-trial correlations and persistent firing rate modulation may support normal levels of syllable variability regardless of reduced bursting activity. However, another intriguing possibility is that abnormal activity patterns in LMAN disrupt syllable sequences by interfering with HVC activity. Indeed, a recent study revealed an indirect pathway from LMAN to HVC that includes area X, the ventral pallidum, and a midbrain cell group (A11) (16). In this view, temporally abnormal activity patterns in LMAN can promote the production of certain syllables at wrong times in the sequence by acutely altering premotor commands at the level of HVC (Fig. 7). Furthermore, altered LMAN activity patterns may also indirectly affect vocal sequences by inducing plastic changes in HVC. Indeed, LMAN lesions can prevent synaptic changes in HVC normally triggered by deafening (52), suggesting that LMAN activity can modulate synaptic organization in HVC through the BG and midbrain pathway. In mammals, striatal plasticity is known to induce dynamic changes in cortical signaling (53) and is required for the emergence of timing signals in the BG and midbrain during motor sequence learning (54). Thus, the mHTT-dependent loss of MSNs could engage both acute and long-term mechanisms that disrupt the precisely timed signals that normally flow through cortico-BG networks, resulting in disorganized motor sequences.
Fig. 7.
Model of how MSN loss can acutely disrupt motor sequences. A simplified schematic depicts a cortico-BG module that conveys information about a motor sequence in a normal bird (A) and mHTT bird (B). In a normal bird, each MSN fires a short burst of action potentials that is hypothesized to encode information about a specific vocal motor gesture (a, b, or c) that is conveyed to pallidal, DLM, and LMAN neurons. In the mHTT bird, loss of the MSN that encodes a (gray) reduces the inhibitory modulation of related pallidal neuron activity, which subsequently disrupts the temporal pattern of the associated LMAN neuron’s activity. The mistimed signals of the LMAN neuron may disturb the motor signals in the downstream song premotor region HVC, or possibly through RA (Discussion), promoting an insertion of motor output a at a wrong time when these pathways are about to induce motor output of c, disrupting the normal pattern of syllable sequences. Note that this model does not necessarily disrupt the spectral features of syllable a by LMAN activity and can be compatible with the unaltered trial-to-trial correlation of LMAN activity in mHTT birds (Fig. 5), which may account for the retained trial-to-trial variability of syllable a regardless of the reduced burst firing.
Materials and Methods
Detailed procedures are provided in SI Materials and Methods. All experimental procedures were performed in accordance with the NIH guidelines and approved by the Duke University Medical Center Animal Care and Use Committee.
Song Analysis.
For song analysis, days before and >30 d after injection were randomly chosen [mHTT-Q94: 7.2 ± 3.9 d before and 61.7 ± 45.8 d after injection; HTT-Q20: 5.8 ± 2.9 d before and 70.8 ± 23.6 d after injection (mean ± SD); n = 9], and all of the recorded files on the day were processed with a custom MATLAB program (MathWorks) to extract and classify song syllables as described previously (52). To compare syllable features before and after mHTT injection, shared syllables (excluding introductory notes) from 100 randomly chosen renditions were analyzed. To extract song bouts, we first determined the most frequently observed syllable sequence on that day as the dominant motif. Then, song bouts were defined as successive vocalizations with intersyllable intervals of <400 ms and that included at least three syllables from the dominant motif, allowing us to exclude call bouts. To compare sequence features before and after mHTT injection, 100 randomly chosen song bouts were analyzed. Sequence entropy S was calculated as follows based on the empirically estimated transition probability matrix:
where pi is the probability of observing each syllable transition type. Linearity, consistency, and stereotypy were calculated with syllables, including introductory notes in individual song bouts, and averaged across the 100 randomly chosen bouts.
Data Analysis.
To examine spike activity aligned to the dominant motif, the first 30 recordings after implantation containing the dominant motif were identified, and analyses were confined to the first 500 ms after the motif onset. Instantaneous firing rate was calculated as the reciprocal of the ISI and smoothed with a band-pass filter (25–75 Hz). Mean firing rate was the average of binned firing rates in a 20-ms sliding bin with a sliding step of 1 ms. The trial-to-trial correlation was the average of the peak between a ±10-ms time shift in cross-correlation values calculated between all of the possible combinations of the 30 iterations of smoothed instantaneous firing rate after mean subtraction. Timing information I(T;A) was calculated as mutual information between timing T in the motif and spike activity A based on binned firing rates:
where p(t) is the probability of observing a specific timing t in the motif, p(a) is the probability of observing the spike number a in the motif, and p(t|a) is the probability of observing the activity in a specific timing t in the motif given the spike number of a. Because p(t) is a constant of 1/500, the first term on the right side is 8.966. Qualitatively, low timing information means syllable timings cannot be decoded from the observed spike activity.
Statistics.
Error bars and values in the text indicate mean ± SEM unless otherwise noted. Paired or unpaired t tests were used to assess the effects of mHTT. To compare the proportion of TUNEL-positive MSNs, a χ2 test was used. Repeated two-way ANOVA was performed in MATLAB to examine the effects of mHTT on social modulation of CV pitch and the effects of LMAN inactivation.
SI Materials and Methods
Animals.
Adult male zebra finches (143 ± 39 d posthatch, mean ± SD; n = 38) were obtained from the Duke University Medical Center breeding facility. All experimental procedures were in accordance with the NIH guidelines and approved by the Duke University Medical Center Animal Care and Use Committee. Adult male zebra finches were isolated in a sound-attenuating chamber under a 14/10-h light/dark cycle, where their songs were automatically recorded with Sound Analysis Pro (Ofer Tchernichovski, Hunter College, New York) (55). All songs were recorded in social isolation except in Fig. S2H.
Song Analysis.
To compare syllable features before and after mHTT injection, shared syllables (excluding introductory notes) from 100 randomly chosen renditions were analyzed to calculate mean duration, mean entropy (Wiener entropy), and mean CV entropy (entropy variance) based on a power spectrum generated from an 11.6-ms sliding window with a step size of 0.5 ms. The CV entropy was calculated as the CV of Wiener entropy within each syllable. The CV of pitch was calculated in Sound Analysis Pro as the CV of fundamental frequency across >10 randomly chosen iterations of a part of those syllables with clear harmonic structure. Directed songs were recorded by presenting a female zebra finch to a previously isolated male for <3 min for each trial. All of the analyzed songs are undirected songs unless otherwise noted. To compare sequence features before and after mHTT injection, 100 randomly chosen song bouts were analyzed to report sequence entropy, mean song bout duration, syllable repetition, linearity, consistency, and stereotypy. Linearity was calculated as internal linearity [(no. of syllable types − 1)/no. of transition types] (56) with syllables, including introductory notes, but repetition of introductory notes (“ii”) was excluded so that the syllable sequence “iiiabcd” gives the linearity score of 1. The consistency score was calculated [no. of typical transitions/no. of transitions], where typical transitions were determined for each preceding syllable as the most frequently observed syllable transitions in the song. The stereotypy score was the average of linearity and consistency scores. Linearity, consistency, and stereotypy were calculated in each song bout and averaged across 100 randomly chosen bouts. Repetition of a syllable, excluding introductory notes, was detected in 100 randomly chosen bouts to calculate the mean number of times that the affected syllable was repeated in each repetition.
Viral Injection.
Adult male zebra finches were kept in a recording chamber for >7 d before the viral injection to allow sufficient habituation. Injection procedures are similar to the injection procedures described previously (22). Briefly, birds were anesthetized with isoflurane inhalation [2% (vol/vol) O2] and placed in a custom stereotaxic apparatus with a heat blanket. Area X was first mapped based on stereotaxic coordinates (5.3 mm rostral, 1.7 mm lateral, and 3.0 mm ventral; head angle of 40°; stereotaxic coordinates in this study were measured from the posterior edge of the bifurcation of the midsagittal sinus) and characteristic high-frequency units in multiunit recordings (Carbostar-1; Kation Scientific). To facilitate extensive viral transfection in area X, viral aliquots of 161 nL were delivered to six different sites in each area X (966 nL per hemisphere in total) using a Nanoject-II (Drummond Scientific). LV was obtained from the Duke Viral Vector Core at a concentration of ∼106 focus-forming units per milliliter. Behavioral and physiological changes were assessed >30 d after the bilateral injection.
Histology.
Birds were deeply anesthetized with i.m. injection of 20 μL Euthasol (Virbac) and transcardially perfused with PBS, followed by 4% (wt/vol) paraformaldehyde (PFA) in PBS. The removed brain was postfixed with 4% (wt/vol) PFA in PBS for >2 h and cryoprotected with 30% (wt/vol) sucrose and 4% (wt/vol) PFA in PBS overnight. Frozen sagittal sections (thickness of 40–50 μm) were prepared with a sledge microtome (Reichert) and collected in PBS. For immunohistochemistry, sections were washed twice in PBS, blocked with 1% (wt/vol) BSA and 2% (wt/vol) normal goat serum in PBS with 0.3% Triton X-100 (PBST) for 1 h, and incubated with primary Abs in PBST at 4 °C overnight. Then, sections were washed three times in PBS and incubated with secondary Abs in PBS at room temperature for 1 h, followed by three washes in PBS. The following combinations of Abs were used: rabbit anti–DARPP-32 Abs (1:500, ab40801; Abcam) and donkey anti-rabbit IgG Abs with DyLight 405 or Alexa 488 (1:500; Jackson ImmunoResearch), rabbit anti-gephyrin Abs (1:750, ab32206; Abcam) and donkey anti-rabbit IgG Abs with DyLight 405 (1:500; Jackson ImmunoResearch), and mouse anti-bassoon Abs (1:500, ab82958; Abcam) and goat anti-mouse IgG Abs with Alexa 488 (1:500; Jackson ImmunoResearch). Sections were imaged with a confocal microscope (710 LSM; Zeiss) after coverslipping with Fluoromount-G (SouthernBiotech). To label DLM-projecting pallidal neurons, dextran Alexa Fluor 647 (D-22914; Molecular Probes) was injected into DLM 4–7 d before the perfusion. The target site in DLM was based on stereotaxic coordinates (1.8 mm rostral, 1.1 mm lateral, and 4.1 mm ventral; head angle of 30°) and high-frequency units concomitant with low-frequency large units in multiunit recordings. Labeling with the TUNEL system (Promega) or Nissl stain (1:200 NeuroTrace, N-21480; Molecular Probes) was performed after immunohistochemistry according to the protocol provided by the supplier. For quantification of labeled neurons other than Nissl stain, we confined the analysis to near the surface of sections because some agents, such as anti–DARPP-32 Abs, did not penetrate the section sufficiently.
Image Analysis.
To quantify the number and soma size of neurons, confocal images near the center of area X (0.202 mm2) were binarized with thresholding, noise was removed with a despeckle filter, and puncta with an area of >10 μm2 were counted with ImageJ (v1.48; NIH) after correcting misaligned contours. To quantify the synaptic colocalization, images were acquired with 0.45-μm steps, and all optical sections containing dextran signal on the cell body of a pallidal neuron were maximum-projected and used for analysis. First, an outline of the cell body and dendrites was traced in the maximum-projected image within a circle roughly twice the diameter of the cell body centered on the soma. Then, colocalization of gephyrin, bassoon, and dextran signal was automatically detected within the traced region using the Puncta Analyzer plug-in for ImageJ v1.29 after background subtraction with a rolling ball radius of 50 and thresholding. Parameters for synaptic analysis were determined in a section from a normal bird and kept constant throughout the analysis.
Extracellular Recording in Singing Birds.
A 16-channel silicone probe (A1x16-5mm-100-177-CM16LP; NeuroNexus) was implanted to LMAN and/or area X in a hemisphere based on stereotaxic coordinates (5.3 mm rostral and 1.7 mm lateral; head angle of 40°) and characteristic LMAN and pallidal units. Habituation of the bird to a dummy weight (1.5–2 g) for >7 d before the implantation shortened the recovery period. Data were collected with a universal serial bus (USB) interface board (RHD2000; Intan Technologies) after band-pass filtering (0.2–10 kHz) and sampling at 20 kHz with a small amplifier board (RHD2132 16-Channel; Intan Technologies) on the bird’s head. Spikes were sorted offline with a custom MATLAB program based on OSort v3.0 (Ueli Rutishauser, Cedars-Sinai Medical Center, Los Angeles) (57). All of the sorted units had a signal-to-noise ratio of >3 (mean peak/noise SD). Unit activity in area X was classified as a pallidal unit if the resting mean firing rate was >50 Hz (13). After the recording, the brain was cut and Nissl-stained to confirm the recording sites.
LMAN Inactivation.
Microdialysis probes were bilaterally implanted into LMAN based on stereotaxic coordinates (4.9 mm rostral, 1.85 mm lateral, and 2.4 mm ventral; head angle of 40°) and by the presence of bursting units in multiunit recordings (Carbostar-1). After a recovery period of 2–3 d, probes were loaded with saline or 5 mM muscimol in saline, followed by a wash with saline after >6 h. Infusion of muscimol into LMAN for >2 h eliminated the multiunit activity in LMAN without reducing the multiunit activity in the medial magnocellular nucleus of the anterior nidopallium (MMAN) (112.0 ± 0.9% of the activity before infusion; n = 2 anesthetized birds), which is distant by >1 mm from LMAN and may also be involved in regulating vocal sequences (56).
Acknowledgments
We thank James McNamara, Kevin Franks, Nicole Calakos, Cagla Eroglu, Jeff Beck, and David Schneider for reading and commenting on the manuscript; Marguerita Klein in the Duke Viral Vector Core for preparing viruses; and Chia-Hui Chen for helping with viral injection. This study was supported by research grants from the Hereditary Disease Foundation (to R.M.), by NIH Grant DC02524 (to R.M.), by a fellowship from the Ruth K. Broad Foundation (to M.T.), and by a fellowship from the Astellas Foundation for Research on Metabolic Disorders (to M.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1523754113/-/DCSupplemental.
References
- 1.Mink JW. The basal ganglia: Focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50(4):381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- 2.Graybiel AM. The basal ganglia and chunking of action repertoires. Neurobiol Learn Mem. 1998;70(1-2):119–136. doi: 10.1006/nlme.1998.3843. [DOI] [PubMed] [Google Scholar]
- 3.Costa RM. Plastic corticostriatal circuits for action learning: What’s dopamine got to do with it? Ann N Y Acad Sci. 2007;1104:172–191. doi: 10.1196/annals.1390.015. [DOI] [PubMed] [Google Scholar]
- 4.Gil JM, Rego AC. Mechanisms of neurodegeneration in Huntington’s disease. Eur J Neurosci. 2008;27(11):2803–2820. doi: 10.1111/j.1460-9568.2008.06310.x. [DOI] [PubMed] [Google Scholar]
- 5.Agostino R, Berardelli A, Formica A, Accornero N, Manfredi M. Sequential arm movements in patients with Parkinson’s disease, Huntington’s disease and dystonia. Brain. 1992;115(Pt 5):1481–1495. doi: 10.1093/brain/115.5.1481. [DOI] [PubMed] [Google Scholar]
- 6.Thompson PD, et al. The coexistence of bradykinesia and chorea in Huntington’s disease and its implications for theories of basal ganglia control of movement. Brain. 1988;111(Pt 2):223–244. doi: 10.1093/brain/111.2.223. [DOI] [PubMed] [Google Scholar]
- 7.Mooney R. Neural mechanisms for learned birdsong. Learn Mem. 2009;16(11):655–669. doi: 10.1101/lm.1065209. [DOI] [PubMed] [Google Scholar]
- 8.Nottebohm F, Arnold AP. Sexual dimorphism in vocal control areas of the songbird brain. Science. 1976;194(4261):211–213. doi: 10.1126/science.959852. [DOI] [PubMed] [Google Scholar]
- 9.Doupe AJ, Perkel DJ, Reiner A, Stern EA. Birdbrains could teach basal ganglia research a new song. Trends Neurosci. 2005;28(7):353–363. doi: 10.1016/j.tins.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Reiner A, Laverghetta AV, Meade CA, Cuthbertson SL, Bottjer SW. An immunohistochemical and pathway tracing study of the striatopallidal organization of area X in the male zebra finch. J Comp Neurol. 2004;469(2):239–261. doi: 10.1002/cne.11012. [DOI] [PubMed] [Google Scholar]
- 11.Farries MA, Perkel DJ. A telencephalic nucleus essential for song learning contains neurons with physiological characteristics of both striatum and globus pallidus. J Neurosci. 2002;22(9):3776–3787. doi: 10.1523/JNEUROSCI.22-09-03776.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farries MA, Ding L, Perkel DJ. Evidence for “direct” and “indirect” pathways through the song system basal ganglia. J Comp Neurol. 2005;484(1):93–104. doi: 10.1002/cne.20464. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg JH, Fee MS. Singing-related neural activity distinguishes four classes of putative striatal neurons in the songbird basal ganglia. J Neurophysiol. 2010;103(4):2002–2014. doi: 10.1152/jn.01038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kao MH, Doupe AJ, Brainard MS. Contributions of an avian basal ganglia-forebrain circuit to real-time modulation of song. Nature. 2005;433(7026):638–643. doi: 10.1038/nature03127. [DOI] [PubMed] [Google Scholar]
- 15.Ölveczky BP, Andalman AS, Fee MS. Vocal experimentation in the juvenile songbird requires a basal ganglia circuit. PLoS Biol. 2005;3(5):e153. doi: 10.1371/journal.pbio.0030153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamaguchi K, Mooney R. Recurrent interactions between the input and output of a songbird cortico-basal ganglia pathway are implicated in vocal sequence variability. J Neurosci. 2012;32(34):11671–11687. doi: 10.1523/JNEUROSCI.1666-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bottjer SW, Halsema KA, Brown SA, Miesner EA. Axonal connections of a forebrain nucleus involved with vocal learning in zebra finches. J Comp Neurol. 1989;279(2):312–326. doi: 10.1002/cne.902790211. [DOI] [PubMed] [Google Scholar]
- 18.Luo M, Ding L, Perkel DJ. An avian basal ganglia pathway essential for vocal learning forms a closed topographic loop. J Neurosci. 2001;21(17):6836–6845. doi: 10.1523/JNEUROSCI.21-17-06836.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts TF, Tschida KA, Klein ME, Mooney R. Rapid spine stabilization and synaptic enhancement at the onset of behavioural learning. Nature. 2010;463(7283):948–952. doi: 10.1038/nature08759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts TF, Gobes SM, Murugan M, Ölveczky BP, Mooney R. Motor circuits are required to encode a sensory model for imitative learning. Nat Neurosci. 2012;15(10):1454–1459. doi: 10.1038/nn.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haesler S, et al. Incomplete and inaccurate vocal imitation after knockdown of FoxP2 in songbird basal ganglia nucleus Area X. PLoS Biol. 2007;5(12):e321. doi: 10.1371/journal.pbio.0050321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murugan M, Harward S, Scharff C, Mooney R. Diminished FoxP2 levels affect dopaminergic modulation of corticostriatal signaling important to song variability. Neuron. 2013;80(6):1464–1476. doi: 10.1016/j.neuron.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu WC, et al. Human mutant huntingtin disrupts vocal learning in transgenic songbirds. Nat Neurosci. 2015;18(11):1617–1622. doi: 10.1038/nn.4133. [DOI] [PubMed] [Google Scholar]
- 24.Woolley SC, Doupe AJ. Social context-induced song variation affects female behavior and gene expression. PLoS Biol. 2008;6(3):e62. doi: 10.1371/journal.pbio.0060062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riebel K. Song and female mate choice in zebra finches: A review. Adv Stud Behav. 2009;40:197–238. [Google Scholar]
- 26.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hessler NA, Doupe AJ. Singing-related neural activity in a dorsal forebrain-basal ganglia circuit of adult zebra finches. J Neurosci. 1999;19(23):10461–10481. doi: 10.1523/JNEUROSCI.19-23-10461.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldberg JH, Adler A, Bergman H, Fee MS. Singing-related neural activity distinguishes two putative pallidal cell types in the songbird basal ganglia: Comparison to the primate internal and external pallidal segments. J Neurosci. 2010;30(20):7088–7098. doi: 10.1523/JNEUROSCI.0168-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woolley SC, Rajan R, Joshua M, Doupe AJ. Emergence of context-dependent variability across a basal ganglia network. Neuron. 2014;82(1):208–223. doi: 10.1016/j.neuron.2014.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kojima S, Kao MH, Doupe AJ. Task-related “cortical” bursting depends critically on basal ganglia input and is linked to vocal plasticity. Proc Natl Acad Sci USA. 2013;110(12):4756–4761. doi: 10.1073/pnas.1216308110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stepanek L, Doupe AJ. Activity in a cortical-basal ganglia circuit for song is required for social context-dependent vocal variability. J Neurophysiol. 2010;104(5):2474–2486. doi: 10.1152/jn.00977.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kao MH, Wright BD, Doupe AJ. Neurons in a forebrain nucleus required for vocal plasticity rapidly switch between precise firing and variable bursting depending on social context. J Neurosci. 2008;28(49):13232–13247. doi: 10.1523/JNEUROSCI.2250-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chenery HJ, Copland DA, Murdoch BE. Complex language functions and subcortical mechanisms: Evidence from Huntington’s disease and patients with non-thalamic subcortical lesions. Int J Lang Commun Disord. 2002;37(4):459–474. doi: 10.1080/1368282021000007730. [DOI] [PubMed] [Google Scholar]
- 34.Serrien DJ, Burgunder JM, Wiesendanger M. Grip force scaling and sequencing of events during a manipulative task in Huntington’s disease. Neuropsychologia. 2001;39(7):734–741. doi: 10.1016/s0028-3932(00)00153-6. [DOI] [PubMed] [Google Scholar]
- 35.Ali F, et al. The basal ganglia is necessary for learning spectral, but not temporal, features of birdsong. Neuron. 2013;80(2):494–506. doi: 10.1016/j.neuron.2013.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scharff C, Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: Implications for vocal learning. J Neurosci. 1991;11(9):2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sohrabji F, Nordeen EJ, Nordeen KW. Selective impairment of song learning following lesions of a forebrain nucleus in the juvenile zebra finch. Behav Neural Biol. 1990;53(1):51–63. doi: 10.1016/0163-1047(90)90797-a. [DOI] [PubMed] [Google Scholar]
- 38.Kubikova L, et al. Basal ganglia function, stuttering, sequencing, and repair in adult songbirds. Sci Rep. 2014;4:6590. doi: 10.1038/srep06590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kobayasi KI, Okanoya K. Context-dependent song amplitude control in Bengalese finches. Neuroreport. 2003;14(3):521–524. doi: 10.1097/00001756-200303030-00045. [DOI] [PubMed] [Google Scholar]
- 40.Desmurget M, Turner RS. Motor sequences and the basal ganglia: Kinematics, not habits. J Neurosci. 2010;30(22):7685–7690. doi: 10.1523/JNEUROSCI.0163-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reiner A, et al. Differential loss of striatal projection neurons in Huntington disease. Proc Natl Acad Sci USA. 1988;85(15):5733–5737. doi: 10.1073/pnas.85.15.5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Person AL, Perkel DJ. Pallidal neuron activity increases during sensory relay through thalamus in a songbird circuit essential for learning. J Neurosci. 2007;27(32):8687–8698. doi: 10.1523/JNEUROSCI.2045-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boettiger CA, Doupe AJ. Intrinsic and thalamic excitatory inputs onto songbird LMAN neurons differ in their pharmacological and temporal properties. J Neurophysiol. 1998;79(5):2615–2628. doi: 10.1152/jn.1998.79.5.2615. [DOI] [PubMed] [Google Scholar]
- 44.Nixdorf-Bergweiler BE, Lips MB, Heinemann U. Electrophysiological and morphological evidence for a new projection of LMAN-neurones towards area X. Neuroreport. 1995;6(13):1729–1732. doi: 10.1097/00001756-199509000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Vates GE, Nottebohm F. Feedback circuitry within a song-learning pathway. Proc Natl Acad Sci USA. 1995;92(11):5139–5143. doi: 10.1073/pnas.92.11.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ashmore RC, Renk JA, Schmidt MF. Bottom-up activation of the vocal motor forebrain by the respiratory brainstem. J Neurosci. 2008;28(10):2613–2623. doi: 10.1523/JNEUROSCI.4547-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Long MA, Jin DZ, Fee MS. Support for a synaptic chain model of neuronal sequence generation. Nature. 2010;468(7322):394–399. doi: 10.1038/nature09514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hahnloser RH, Kozhevnikov AA, Fee MS. An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature. 2002;419(6902):65–70. doi: 10.1038/nature00974. [DOI] [PubMed] [Google Scholar]
- 49.Vu ET, Mazurek ME, Kuo YC. Identification of a forebrain motor programming network for the learned song of zebra finches. J Neurosci. 1994;14(11 Pt 2):6924–6934. doi: 10.1523/JNEUROSCI.14-11-06924.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bouchard KE, Brainard MS. Neural encoding and integration of learned probabilistic sequences in avian sensory-motor circuitry. J Neurosci. 2013;33(45):17710–17723. doi: 10.1523/JNEUROSCI.2181-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amador A, Perl YS, Mindlin GB, Margoliash D. Elemental gesture dynamics are encoded by song premotor cortical neurons. Nature. 2013;495(7439):59–64. doi: 10.1038/nature11967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamaguchi K, Tschida KA, Yoon I, Donald BR, Mooney R. Auditory synapses to song premotor neurons are gated off during vocalization in zebra finches. eLife. 2014;3:e01833. doi: 10.7554/eLife.01833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koralek AC, Jin X, Long JD, 2nd, Costa RM, Carmena JM. Corticostriatal plasticity is necessary for learning intentional neuroprosthetic skills. Nature. 2012;483(7389):331–335. doi: 10.1038/nature10845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jin X, Costa RM. Start/stop signals emerge in nigrostriatal circuits during sequence learning. Nature. 2010;466(7305):457–462. doi: 10.1038/nature09263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tchernichovski O, Nottebohm F, Ho CE, Pesaran B, Mitra PP. A procedure for an automated measurement of song similarity. Anim Behav. 2000;59(6):1167–1176. doi: 10.1006/anbe.1999.1416. [DOI] [PubMed] [Google Scholar]
- 56.Foster EF, Bottjer SW. Lesions of a telencephalic nucleus in male zebra finches: Influences on vocal behavior in juveniles and adults. J Neurobiol. 2001;46(2):142–165. doi: 10.1002/1097-4695(20010205)46:2<142::aid-neu60>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 57.Rutishauser U, Schuman EM, Mamelak AN. Online detection and sorting of extracellularly recorded action potentials in human medial temporal lobe recordings, in vivo. J Neurosci Methods. 2006;154(1-2):204–224. doi: 10.1016/j.jneumeth.2005.12.033. [DOI] [PubMed] [Google Scholar]