Abstract
Some 200 years ago, workers developed gingivitis, periodontal disease, alveolar crest bone sequestra, and draining fistulae after exposure to phosphorous fumes and phosphorous paste in the manufacture of the friction match. Many also suffered loss of teeth and pathologic fracture of the mandible. Known as “phossy jaw,” the constellation rather abruptly vanished following the International Berne Convention of 1906. Today, “bis-phossy jaw” (bisphosphonate-induced osteonecrosis of the jaw) has surfaced with pathologic fractures and other features common to its predecessor, “phossy jaw.” This modern equivalent is reported with ever-increasing frequency and is presented here in the format of a brief historical review and a case report that includes segmental en bloc extirpation of necrotic mandible and pain-free salvage. Computerized imagery and three-dimensional printing technology were successfully chosen to create and apply a custom titanium bone plate, without free-tissue transfer.
Keywords: phossy jaw, bis-phossy jaw, bisphosphonate-induced necrosis of the jaw, John Walker, friction match, patient-specific implant (PSP)
Man had long sought creation and easy passage of flame to wood. But, until the ingenuity of an upper northeastern Englishman, John Walker, it had not occurred: Walker created the friction match. “Ignitable sticks” were novel, without a doubt, even in the midst of other striking innovations in the 19th century. Factories quickly appeared because of popular demand, but in ca. 1860, after phosphorous was added to friction-match paste, osteonecrosis of the jaws (“phossy jaw”) developed in a significant percentage of workers, usually within 3 to 5 years of exposure.
A modern equivalent, “bis-phossy jaw,” now increasingly presents for operative repair, as an aging population evolves, as the number of patients diagnosed with disease-induced bone fragility increases, and as exposure to bisphosphonates is prolonged. One such case is presented, in which segmental resection of necrotic and fractured mandible, brief use of an external fixator, and reconstruction were achieved. Three-dimensional printing technology and a custom titanium bone plate permitted the repair without free-tissue transfer.
“Phossy Jaw”: John Walker (1781–1859) and the Friction Match
John Walker was born in 1781 in the town of Stockton-on-Tees, the third of three sons (and two daughters) of Mary Peacock and John Walker, Sr., a proprietor of grocers and wine merchants in the area. He and his siblings attended the local grammar schools of Stockton, on the northern bank of the river Tees (Figs. 1 and 2). Stockton-on-Tees dated back to the 14th century and had been occupied, at one time, by Scots.1 2 3 4 5 6 7 8 9 10
Fig. 1.

High Street, Stockton-on-Tees. (Image courtesy: Pictures Archive, Stockton Central Library.)
Fig. 2.

Corner of Dovecoton and High Streets, Stockton-on-Tees. (Image courtesy: Pictures Archive, Stockton Central Library.)
Little is otherwise known of John Walker's childhood, but at the age of 15 years, he was articled to Watson Alcock, principal surgeon, and twice mayor of Stockton-on-Tees. The surgical apprenticeship provoked an interest in pharmacy and chemistry, leading Walker to study at Durham University (Fig. 3a, b), some 25 miles to the north.
Fig. 3.

(a) Historic University of Durham. (Image courtesy: Palace Green Library, Durham University, and BBC News.) (b) Cosins's Library, 1842. (Image courtesy: University of Durham.)
John Walker (Fig. 4) ended a protracted period of academic and itinerant study at the age of 31 years, and in 1812, he returned to his hometown, soon to open his own shop as a “chemist and druggist” at 59 High Street (Fig. 5). Twelve years later (1824), positioned at the hearth of his fireplace, Walker experimented with various chemical compounds that he thought might break into flames when dried and scraped on a rough surface, yet do so without explosion.
Fig. 4.

John Walker (1781–1859) (Image courtesy: en.wikipedia.org.)
Fig. 5.

Walker shop on High Street. (Image courtesy: heritage.stockton.gov.uk.)
The chemical paste most safely combustible was sulfur based, and Walker was able with little refinement to begin selling “ignitable wooden sticks” from display cases in his shop on High Street. The matches were offered in small tins containing 50 matches (Fig. 6a).
Fig. 6.

(a) 1827 Walker match tin. (Image courtesy: en.wikipedia.org.) (b) 1832 London box of “friction lights,” giving credit to Walker. (Image courtesy: Pictures Archive, Stockton Central Library, Stockton-on-Tees; photograph by Cliff Thornton.)
John Walker laid no formal claims to novelty and, thus unarmed with patent protection, copycats quickly arose in Glasgow and London. Competitors beyond the Stockton borough initially acknowledged the Walker intellectual “ownership” on their cardboard-box packaging (Fig. 6b).
At the time of John Walker's mid-19th-century death (1859) and burial in the Parish Yard of the Church of St. Mary the Virgin, in nearby Norton, yellow (the so-called “white”) phosphorus was added to the sulfur-based matchstick coating to trigger more volatile flame. Encouraged by ease of use and increased demand, the new matches became highly sought commodities, and manufacturing changed from small entrepreneurial shops to large factories in London, Glasgow, Amsterdam, Paris, Vienna, and Berlin.
The phosphorus-based vats released toxic fumes into the factory air (particularly in the dipping centers and drying rooms), and “dippers and handlers” exposed to the fumes and the fat-soluble matchstick paste within 3 to 5 years suffered gingivitis, sequestration of alveolar crest bone, and osteonecrosis of mandibular and maxillary bone stock (“phossy jaws”). Oral and orocutaneous fistulae were common in the afflicted (Fig. 7).5 In the more unduly exposed, the tracheopulmonary tree became a second end point of toxicity, and cough, sputum production, and hemoptysis were common (“phossy lung”). Systemic toxicity developed in the more chronically afflicted, provoking seizures (“phossy brain”) and causing leukopenia and anemia (“phossy marrow”). (Fig. 8) Factory owners, seeking to control the environmental risks, required serial oral examination of those at greatest risk (Fig. 9a, b).6 The incidence of “phossy jaw” and other toxic responses abated rather rapidly after yellow (“white”) phosphorous in matchstick paste was proscribed by the International Berne (Switzerland) Convention of 1906.1
Fig. 7.

Factory worker with “phossy jaw,” with visible oro-cutaneous fistulae. (Image courtesy: Andrea Brady and Krupskaya Press.)
Fig. 8.
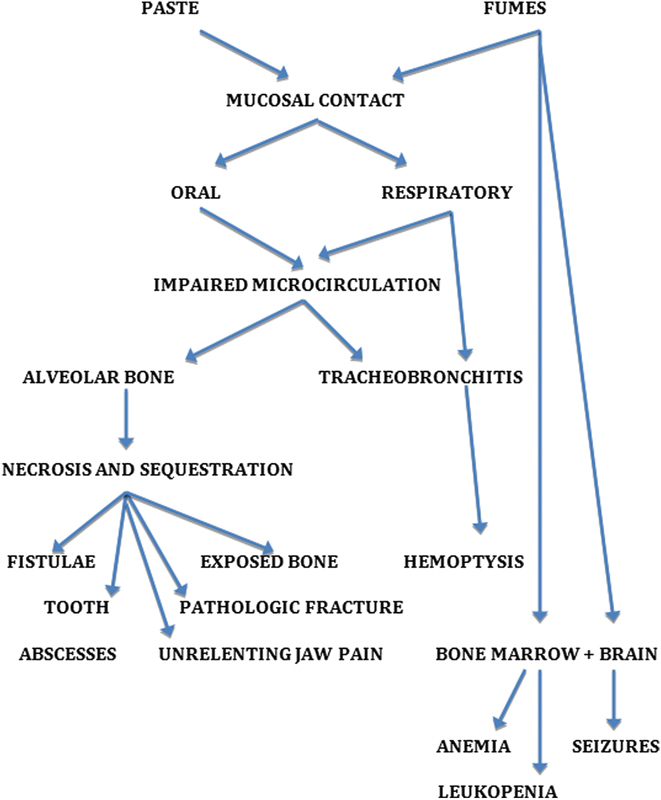
End points of yellow (“white”) phosphorous toxicity, leading to “phossy jaw,” “phossy lung,” “phossy marrow,” and “phossy brain.”
Fig. 9.
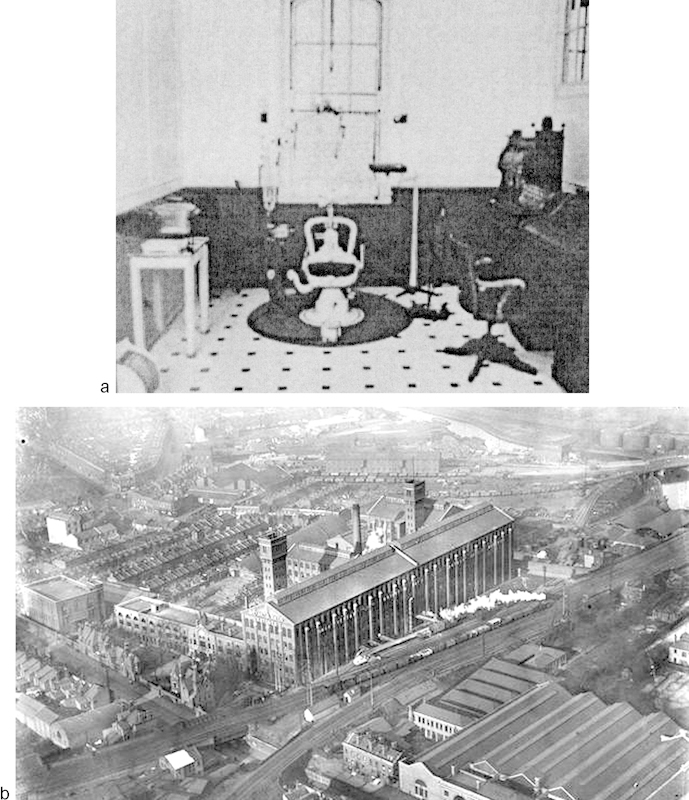
(a) Dental suite in the Bryant & May [Match] Factory, (Bow) London (Image courtesy: Andrea Brady and Krupskaya Press.) (b) The Bryant and May Factory, (Bow) London. (Image courtesy: the Ripper posting June 19, 2014, titled “The Match Girls” http://the-east-end.co.uk/tag/east-end/. Accessed February 1, 2015.)
“Bis-Phossy Jaw” (Bisphosphonate-Induced Osteonecrosis): A Modern Equivalent
Like the friction match, bisphosphonates were developed in the innovative 19th century. The chemicals, with two phosphonate (PO3) groups, were first employed to soften water used, at the time, to hydrate orange groves. Their chemical attributes were investigated in the 1960s, but it was 30 years before their mechanism of effect on bone was delineated by Merck & Company investigators.11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38
Merck launched alendronate (Fosamax; Merck, Kenilworth, NJ) in the 1990s to treat conditions that feature bone fragility12; other pharmaceutical companies followed suit. Osteoporosis, bone metastasis (breast and prostate), multiple myeloma, and Paget primary disease of bone (osteitis deformans) are but a few of the human disorders profoundly improved when bisphosphonates are used, either as a preventive or as treatment.28
The mechanism of action of bisphosphonates remains uncertain. Bench research to date, however, suggests bisphosphonates throttle the osteoclast (warranting dysfunction without triggering cell death) and (in ways not yet certain) disallow inherent angiogenic repair (Fig. 10). Migliorati et al and Lam et al, it follows, speculate side effects of bisphosphonates that lead to osteonecrosis are the result of a combination of (1) inhibition of bone remodeling and (2) decreased endosteal blood flow.19 28
Fig. 10.
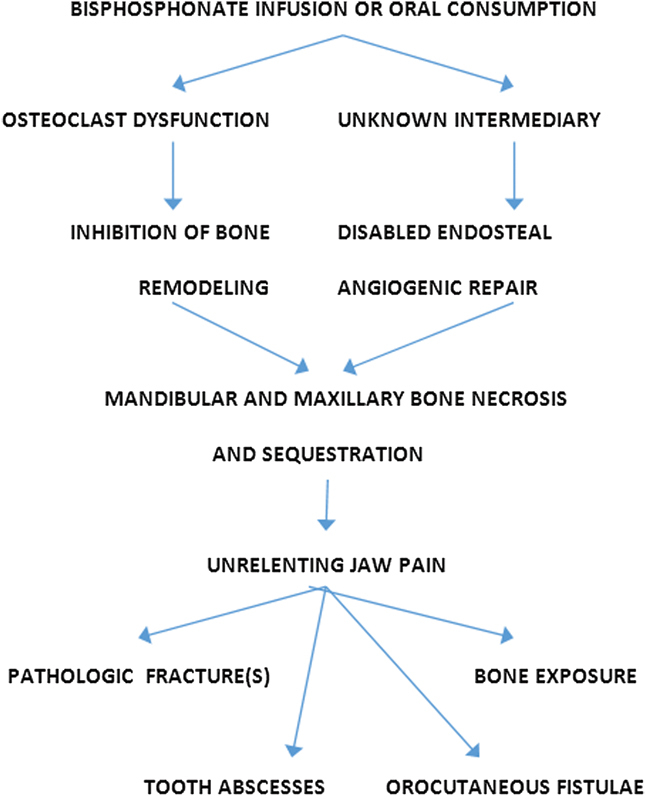
Pathophysiology and end points of bisphosphonate-induced toxicity, leading to “bis-phossy jaw.”
The clinical effects of bisphosphonates, like the experience in factory workers exposed to white phosphorous, persist for prolonged periods of time (decades), even in cases in which treatment has been discontinued.25 26 The longevity of mal effects has led to comparative studies of the binding of various bisphosphonates to bone. Potency, duration of oral and intravenous administration (particularly 3–5 years), local oral anatomy and disease, and age are just some of the variables that appear to affect clinical outcomes, whether after infusion or oral consumption.
Case Report
A 68-year-old nondiabetic female with osteoporosis (on 10 mg alendronate/Fosamax daily for 2 years) and a history of breast cancer presented to her local dentist, complaining of unrelenting pain in the left lower jaw. Believing the pain was odontogenic in nature, mandibular teeth numbers 20, 21, 22, and 28 were extracted under local anesthesia, rendering the mandible edentulous.
Pain in the body of the mandible persisted despite the extractions, and socket number 20 failed to heal after oral rinses, serial curettage, and multiple courses of clindamycin. In short order, the patient was referred to the Otolaryngology-Head & Neck Service at Regional Medical Center Bayonet Point. Upon presentation, a 1.0 × 2.5 cm area of exposed bone was noted in the region of the body of the left mandible, accompanied by intense pain, fever, granulation tissue, pustular drainage, and malodor. Cultures revealed normal mouth flora.
Sequestrectomy and histologic study of specimens failed to reveal breast cancer metastatic to the body of the mandible. Computed tomography (CT) confirmed extensive bone necrosis and a suspected pathologic fracture of the left body of the mandible. Tracheostomy, resection of necrotic left mandibular body to the point of active bleeding, copious irrigation, and placement of an external fixator (Joe Hall Morris appliance39) followed, as did a percutaneous endoscopic gastrostomy (PEG) for nutrition. Oral consumption of alendronate was stopped.
Some 90 days later, the compromised oral soft tissue appeared to have sufficiently healed. The prior surgical site was approached from below, by way of a large cervical apron flap, sweeping downward from each mastoid tip to the sternoclavicular notch. A left DePuy Synthes (Depuy Synthes, West Chester, PA)–Materialise (Materialise, Glen Burie, MD) patient-specific titanium implant (based on mirrored images of the right mandible) was secured to bone stock, distal, and mesial to the resection. Temporary fixation of a patient-specific mandibular positioning guide, custom screw-hole guides (for precise drill-hole location), and trocar guide cylinders (with irrigation ports) facilitated the accuracy of the reconstruction. Four screws secured the implant to the left ramus and five to the right symphysis and parasymphysis; bicortical screw length varied, as predetermined by computer analysis (Figs. 11a, b and 12a, b). The past 18 months have been free of sequelae. The remaining bone stock fails to reveal areas of necrosis, and avoidance of alendronate continues. The PEG tube has been removed. Fabrication of a complete lower denture would be considered in ensuing months.
Fig. 11.
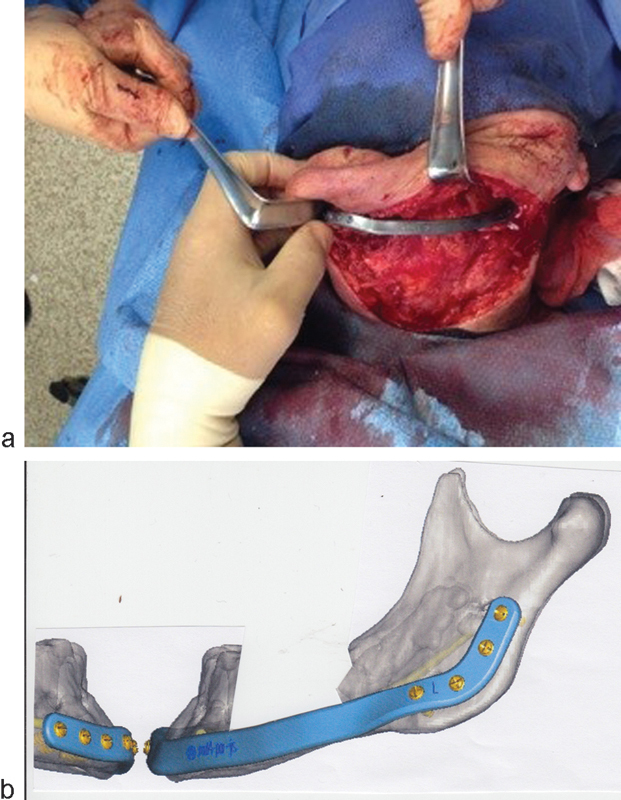
(a and b) Extirpation and stabilization with an external fixator, awaiting soft-tissue healing.
Fig. 12.
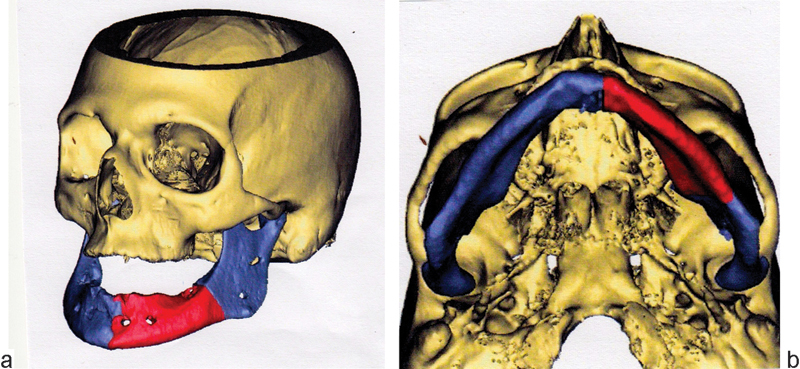
(a and b) Computer model of resection, positioning guide, and end result after placement of patient-specific implant.
Discussion
Hellstein and Marek in 2005 rhetorically ask if bisphosphonate-induced osteonecrosis is “the phossy jaw of the 21st century.” We believe it is and find the long-forgotten parallels uncanny. Further, we are disinclined to use the words “bisphosphonate-associated” and more inclined to acknowledge a stronger linkage by instead using “bisphosphonate-induced.” We concur with Jiminez-Soriano and Bagan, Hellstein and Marek, and Abu-id et al: “bis-phossy” is a catchy, insightfully descriptive term that implies the consequential relationship.22 23 33
The mandible, mandibular vestibule, and anterior floor of the mouth enjoy a rich blood supply by way of terminal branches of the external carotid artery. Of particular note, distribution is by endosteal and by outer and inner periosteal systems, the latter distinguished by a three-tiered vasculature, above and below the mylohyoideus musculature40 (Fig. 13a). The terminal arborization is not a new revelation: it was noted by Cryer in 191641 and dramatized by the studies of Knapp et al and Dempster and Enlow when they injected the inferior alveolar arteries with India ink, after extraction of organic material with ethylendiamine42 43 44 45 (Fig. 13b).
Fig. 13.
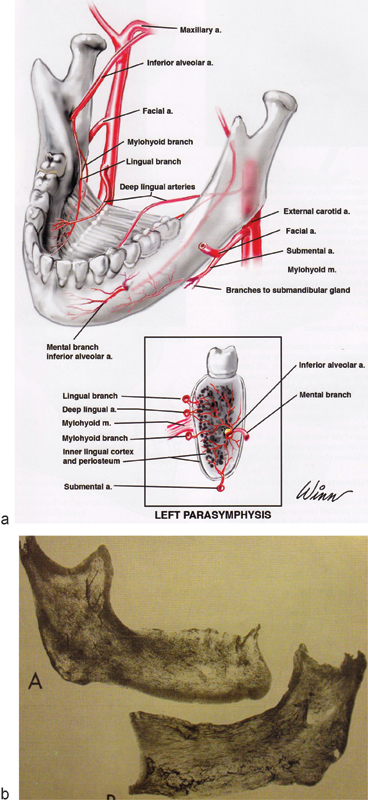
(a) Illustration of endosteal and periosteal blood supply of the mandible. (Image courtesy: William Winn, Richard A. Pollock, and Thieme Medical Publishing.) (b) India ink studies reveal extensive terminal arborization of endosteal vessels of the mandible, after injecting the inferior alveolar arteries and after preinjecting the vessels with ethylenediamine. (Image courtesy: Anatomical Record.)
The biomechanism by which mandibular bone and its microvasculature is singled out as an end point of toxicity requires much further study, although the suspected sequence and pathogenesis of bis-phossy jaw can be depicted, as it is in Fig. 10. Future clinical and bench studies might include angiography of the endosteal vasculature during and after bisphosphonate administration; serial biennial cone-beam CT monitoring of the mandible in patients consuming bisphosphonate; and the viability of bone grafts despite patients having discontinued bisphosphonates.
Clinical research would do well to explain the proclivity of the jaws and the adjacent soft tissue to osteonecrosis compared with skull and long bones.20 22 28 30
Savvy dental practitioners continue to be aware of unrelenting pain as a marker of compromised bone and cognizant of tardy healing of gingiva in patients consuming bisphosphonates. They would astutely offer preventive care early and weigh extraction and surgical intervention according to perceived risk.6 13 16 21 31 35 36 37
Temporary use of an external fixator in this case begged time for healing of the soft-tissue envelope. Deferment of definitive repair also allowed design of a patient-specific device and outcomes model. The timing and successful mechanics of surgical intervention in this case may or may not be directly applicable, however, to other patients.
We are particularly aware of the unrelenting, decades-long proclivity of bisphosphonates to bind to bone, as are the patient and her referring doctors. As such the residual bone and soft tissue of the ipsilateral and the contralateral mandible and perhaps even the anterior floor of the mouth may become compromised. Should evidence of necrosis recur, secondary, even tertiary, procedures may unfortuitously follow.
References
- 1.John Walker Available at: http://archives.rootsweb.ancestry.com/th/read/GENBRIT/2000-12/0977572827. Available at: http://www.historyofmatches.com/matches-inventors/john-walker/. Accessed August 1, 2014
- 2.Stockton-on-Tees Available at: https://www.thisisstockton.gov.uk/historystocktonpicturesarchive. (photos 19th century, courtesy thisisstockton.org). Accessed August 1, 2014
- 3.Phossy jaw Available at: http://en.wikipedia.org/wiki/Phossy_jaw. Accessed August 1, 2014
- 4.Stephen L, Lee S. London: Smith, Elder & Co.; 1885. Dictionary of National Biography. [Google Scholar]
- 5.Brady A Wildfire: A Verse Essay San Francisco: Krupskaya Press; 2010. Available at: http://www.academia.edu/1904531/Andrea_Bradys_Wildfire_Generation_in_Destruction. Accessed November 26, 2014
- 6.Legge T M. Report of cases of phosphorus necrosis. J Industrial Hygiene. 1920;2:50–52. [Google Scholar]
- 7.Ward E F. Phosphorus necrosis in the manufacture of fireworks. J Ind Hyg Toxicol. 1928;10:314–330. [Google Scholar]
- 8.Heimann H. Chronic phosphorus poisoning. J Ind Hyg Toxicol. 1946;28:142–150. [PubMed] [Google Scholar]
- 9.Hughes J PW, Baron R, Buckland D H. et al. Phosphorus necrosis of the jaw: a present-day study. Br J Ind Med. 1962;19:83–99. doi: 10.1136/oem.19.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miles A E. Phosphorus necrosis of the jaw: ‘phossy jaw’. Br Dent J. 1972;133(5):203–206. doi: 10.1038/sj.bdj.4802906. [DOI] [PubMed] [Google Scholar]
- 11.Toxicological Profile for White Phosphorus Sciences International, Inc. for the United States Department of Health and Human Services, Agency for Toxic Substances and Disease Registry (ATSDR). 1997 [PubMed]
- 12.Fleisch H. Development of bisphosphonates. Breast Cancer Res. 2002;4(1):30–34. doi: 10.1186/bcr414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bone H G, Hosking D, Devogelaer J P. et al. Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350(12):1189–1199. doi: 10.1056/NEJMoa030897. [DOI] [PubMed] [Google Scholar]
- 14.Estilo C L Van Poznak C H Williams T et al. Osteonecrosis of the maxilla and mandible in patients treated with bisphosphonates: a retrospective study J Clin Oncol 200422(14S):8088 [Google Scholar]
- 15.Ruggiero S L, Mehrotra B, Rosenberg T J, Engroff S L. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg. 2004;62(5):527–534. doi: 10.1016/j.joms.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Marx R E, Sawatari Y, Fortin M, Broumand V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg. 2005;63(11):1567–1575. doi: 10.1016/j.joms.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Delmas P D. The use of bisphosphonates in the treatment of osteoporosis. Curr Opin Rheumatol. 2005;17(4):462–466. doi: 10.1097/01.bor.0000163448.51661.87. [DOI] [PubMed] [Google Scholar]
- 18.Migliorati C A, Schubert M M, Peterson D E, Seneda L M. Bisphosphonate-associated osteonecrosis of mandibular and maxillary bone: an emerging oral complication of supportive cancer therapy. Cancer. 2005;104(1):83–93. doi: 10.1002/cncr.21130. [DOI] [PubMed] [Google Scholar]
- 19.Migliorati C A, Casiglia J, Epstein J, Jacobsen P L, Siegel M A, Woo S B. Managing the care of patients with bisphosphonate-associated osteonecrosis: an American Academy of Oral Medicine position paper. J Am Dent Assoc. 2005;136(12):1658–1668. doi: 10.14219/jada.archive.2005.0108. [DOI] [PubMed] [Google Scholar]
- 20.Melo M D, Obeid G. Osteonecrosis of the jaws in patients with a history of receiving bisphosphonate therapy: strategies for prevention and early recognition. J Am Dent Assoc. 2005;136(12):1675–1681. doi: 10.14219/jada.archive.2005.0110. [DOI] [PubMed] [Google Scholar]
- 21.Durie B G Katz M Crowley J Osteonecrosis of the jaw and bisphosphonates N Engl J Med 2005353199–102., discussion 99–102 [DOI] [PubMed] [Google Scholar]
- 22.Jimenez-Soriano Y, Bagan J V. Bisphosphonates, as a new cause of drug-induced jaw osteonecrosis: an update. Med Oral Patol Oral Cir Bucal. 2005;10 02:E88–E91. [PubMed] [Google Scholar]
- 23.Hellstein J W, Marek C L. Bisphosphonate osteochemonecrosis (bis-phossy jaw): is this phossy jaw of the 21st century? J Oral Maxillofac Surg. 2005;63(5):682–689. doi: 10.1016/j.joms.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Woo S B, Hellstein J W, Kalmar J R. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144(10):753–761. doi: 10.7326/0003-4819-144-10-200605160-00009. [DOI] [PubMed] [Google Scholar]
- 25.Ruggiero S L, Fantasia J, Carlson E. Bisphosphonate-related osteonecrosis of the jaw: background and guidelines for diagnosis, staging and management. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(4):433–441. doi: 10.1016/j.tripleo.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Ruggiero S, Gralow J, Marx R E. et al. Practical guidelines for the prevention, diagnosis, and treatment of osteonecrosis of the jaw in patients with cancer. J Oncol Pract. 2006;2(1):7–14. doi: 10.1200/jop.2006.2.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kademani D, Koka S, Lacy M Q, Rajkumar S V. Primary surgical therapy for osteonecrosis of the jaw secondary to bisphosphonate therapy. Mayo Clin Proc. 2006;81(8):1100–1103. doi: 10.4065/81.8.1100. [DOI] [PubMed] [Google Scholar]
- 28.Lam D K, Sándor G KB, Holmes H I, Evans A W, Clokie C ML. A review of bisphosphonate-associated osteonecrosis of the jaws and its management. J Can Dent Assoc. 2007;73(5):417–422. [PubMed] [Google Scholar]
- 29.Mavrokokki T, Cheng A, Stein B, Goss A. Nature and frequency of bisphosphonate-associated osteonecrosis of the jaws in Australia. J Oral Maxillofac Surg. 2007;65(3):415–423. doi: 10.1016/j.joms.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 30.Marx R E. Uncovering the cause of “phossy jaw” Circa 1858 to 1906: oral and maxillofacial surgery closed case files-case closed. J Oral Maxillofac Surg. 2008;66(11):2356–2363. doi: 10.1016/j.joms.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Khosla S, Burr D, Cauley J. et al. Editorial: bisphosphonate-associated osteonecrosis of the jaw (ONJ): report of a task force of the American Society for Bone and Mineral Research. JBMR. 2007;22:1479–1488. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 32.Ault A. Jaw necrosis affects 1 in 1700 on oral bisphosphonates. Internal Medicine News. 2008;41:23. [Google Scholar]
- 33.Abu-Id M H, Warnke P H, Gottschalk J. et al. “Bis-phossy jaws” - high and low risk factors for bisphosphonate-induced osteonecrosis of the jaw. J Craniomaxillofac Surg. 2008;36(2):95–103. doi: 10.1016/j.jcms.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 34.Hoff A O, Toth B B, Altundag K. et al. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res. 2008;23(6):826–836. doi: 10.1359/JBMR.080205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cartsos V M, Zhu S, Zavras A I. Bisphosphonate use and the risk of adverse jaw outcomes: a medical claims study of 714,217 people. J Am Dent Assoc. 2008;139(1):23–30. doi: 10.14219/jada.archive.2008.0016. [DOI] [PubMed] [Google Scholar]
- 36.Carlson E R Basile J D The role of surgical resection in the management of bisphosphonate-related osteonecrosis of the jaws J Oral Maxillofac Surg 200967(5, Suppl):85–95. [DOI] [PubMed] [Google Scholar]
- 37.AAOMS (American Association of Oral and Maxillofacial Surgeons) Position Paper on Bisphosphonate-Related Osteonecrosis Update 2009
- 38.Watters A L, Hansen H J, Williams T. et al. Intravenous bisphosphonate-related osteonecrosis of the jaw: long-term follow-up of 109 patients. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115(2):192–200. doi: 10.1016/j.oooo.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 39.Morris J H. Biphase connector, external skeletal splint for reduction and fixation of mandibular fractures. Oral Surg Oral Med Oral Pathol. 1949;2(11):1382–1398, illust. doi: 10.1016/0030-4220(49)90087-0. [DOI] [PubMed] [Google Scholar]
- 40.Pollock R A, Schubert W. New York: Thieme Medical Publishers; 2012. The mandible (Part 1, Surgical Anatomy and General Considerations, Chapter 5) [Google Scholar]
- 41.Cryer M H. Philadelphia, PA: Lea & Febiger; 1916. The Internal Anatomy of the Face. [Google Scholar]
- 42.Knapp D E, Avery J K, Costich E R. A technique for the study of the internal structure of calcified tissues. J Dent Res. 1958;37(5):880–885. doi: 10.1177/00220345580370051701. [DOI] [PubMed] [Google Scholar]
- 43.Dempster W T, Enlow D H. Osteone organization and the demonstration of vascular canals in the compacta of the human mandible. Anat Rec. 1959;133:268. doi: 10.1002/ar.1091350305. [DOI] [PubMed] [Google Scholar]
- 44.Dempster W T, Enlow D H. Patterns of vascular channels in the cortex of the human mandible. Anat Rec. 1959;135:189–205. doi: 10.1002/ar.1091350305. [DOI] [PubMed] [Google Scholar]
- 45.Cohen L. Methods of investigating the vascular architecture of the mandible. J Dent Res. 1959;38:920–931. doi: 10.1177/00220345590380052201. [DOI] [PubMed] [Google Scholar]


