Abstract
Despite significant research efforts aimed at understanding the neurobiological underpinnings of mood (depression, bipolar disorder) and psychotic disorders, the diagnosis and evaluation of treatment of these disorders are still based solely on relatively subjective assessment of symptoms as well as psychometric evaluations. Therefore, biological markers aimed at improving the current classification of psychotic and mood-related disorders, and that will enable patients to be stratified on a biological basis into more homogeneous clinically distinct subgroups, are urgently needed. The attainment of this goal can be facilitated by identifying biomarkers that accurately reflect pathophysiologic processes in these disorders. This review postulates that the field of psychotic and mood disorder research has advanced sufficiently to develop biochemical hypotheses of the etiopathology of the particular illness and to target the same for more effective disease modifying therapy. This implies that a “one-size fits all” paradigm in the treatment of psychotic and mood disorders is not a viable approach, but that a customized regime based on individual biological abnormalities would pave the way forward to more effective treatment. In reviewing the clinical and preclinical literature, this paper discusses the most highly regarded pathophysiologic processes in mood and psychotic disorders, thereby providing a scaffold for the selection of suitable biomarkers for future studies in this field, to develope biomarker panels, as well as to improve diagnosis and to customize treatment regimens for better therapeutic outcomes.
Keywords: Antidepressant, biomarker panel, GABA-glutamate, genomics-proteomics, immune-inflammation-redox, kynureninecytokine, neurotransmitters, nitric oxide, schizophrenia.
INTRODUCTION
Major depression (MD), bipolar disorder (BPD) and psychotic disorders (e.g. schizophrenia) are often misdiagnosed, leading to inadequate treatment and devastating consequences [1, 2]. MD is among the most debilitating diseases worldwide, with a life-time prevalence of up to 20% [3], and even though major advances have been made in developing new drugs, less than 50% of patients achieve remission after antidepressant treatment [4]. Bipolar disorder affects approximately 1.2% of the population worldwide [5], and differs from MD with a unique hallmark of mania (elevated mood or euphoria, hyper-activity with a lack of need for sleep, and an increased optimism) which frequently leads to a deficit in the patient’s judgment [6]. On the other hand, schizophrenia is a debilitating neuropsychiatric disorder, typically emerging during adolescence or early adulthood and continuing to plague patients suffering from the disease to varying degrees throughout their lifetime [7]. Approximately 1% of the general population worldwide is affected by the disorder and the life expectancy of patients with schizophrenia has been demonstrated to be nearly 20% shorter than that of the general population [8].
Despite an abundance of research, the pathogenesis and aetiology of mood and psychotic disorders remain unclear, challenging the diagnosis and treatment of these disorders [9], mainly for the following reasons:
- Diagnoses of typical psychiatric disorders are primarily based on operationalized behavioural diagnostic systems either as self-reported symptoms by patients or observations by clinicians, being confirmed against diagnostic criteria set out in the Diagnostic and Statistical Manual of Mental Disorders 4th/ 5th ed. (DSMIV/V) and International Statistical Classification of Diseases, 10th Revision [10].
- Laboratory diagnostic and screening tools, such as a non-invasive blood-based test, remain elusive [11], while mood and psychotic symptoms may overlap with other neurological and psychiatric problems [9].
Clinically useful biomarkers in these disorders could therefore significantly improve diagnosis and treatment and has been one of the holy grails of MD, BPD and schizophrenia research [12]. However, the likelihood of any single biomarker achieving a high enough degree of sensitivity and specificity for mood and psychotic disorders is relatively low. Biomarker panels may represent an attainable alternate to a single-biomarker approach [13]. Common features of this method include correlates attributed to the individual which may determine the presence or absence of a state of sickness or that may even predict response to treatment [13]. Biomarkers may also indicate the presence of a pathophysiological process that can be addressed with a preventive treatment [14], as well as highlight “state” and/or “trait” markers. Therefore, the identification of biomarkers prior to onset of depressive and bipolar symptoms or psychosis has enormous potential importance for the design of future preventive strategies.
The Biomarkers Definitions Working Group of the National Institutes of Health Group [15] (2001) defined a biomarker as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention” [15]. Biomarkers should also provide high levels of sensitivity and specificity (>80%) in the detection and classification of MD, BPD and psychotic disorders [16], in order to be clinically useful.
There are many potential biomarkers for mood and psychotic disorders and previous studies have tested specific biomarkers based on the hypotheses of monoamine dysfunction, altered immune-inflammatory processes, neuroendocrine dysfunction and disturbances in neuroplasticity [17]. However, considering the most recent neuroanatomical basis for these illnesses, as well as relevant hypotheses of their aetiology, this review will provide an overview of potential biomarkers that could contribute to an initial multi-analyte biomarker panel of mood and psychotic disorders. Such biomarkers include molecules and/or processes directly or indirectly connected with growth factors, neurotransmitters, oxidative stress, inflammation, neuro-imaging, genetic, proteomic and neuronal resilience markers. In order to do this, we have collated neuroimaging and neurobiological findings from clinical studies as well as data from validated translational animal models, to assist in developing a putative, uniform biomarker panel for MD, BPD and schizophrenia.
There can be no doubt as to the value of translational animal models in drug discovery and in identifying novel neurobiological targets. Considering mood and psychotic disorders, these models include for example social isolation rearing (SIR) and the glutamate N-methyl D-aspartate (NMDA) antagonist models of schizophrenia [18, 19], and the Flinders Sensitive Line (FSL) or chronic mild stress (CMS) rat models of MD [20, 21]. Developing an appropriate animal model to mimic BPD has proven to be an arduous task, it being difficult to establish a model that combines symptoms of MD, mania and euthymia in an alternating manner as is observed in BPD. Instead, animal models have been developed to express features central to either MD or mania using pharmacologic (amphetamines and ouabain), environmental (e.g. behavioral despair; sleep deprivation), or genetic (e.g. FSL rat) models. Therefore in parallel with our analysis of the clinical scenario, we will also closely scrutinize appropriate animal models for correlation with clinical findings. This review will cover MD, BPD and schizophrenia with respect to the noted biomarkers and across clinical and pre-clinical correlates.
THE NEUROANATOMY AND NEUROCIRCUITRY OF PSYCHIATRIC ILLNESS
Clinical Correlates
Neuroimaging methods, such as structural magnetic resonance imaging (MRI), functional magnetic resonance imaging (fMRI), diffusion tensor imaging (DTI) and positron emission tomography (PET), provide important evidence for underlying biological factors of MD, BPD and psychotic disorders [22]. Generally, areas of the limbic system, the hippocampus and frontal cortical areas are under scrutiny with regard to structural and functional neuroimaging research in mood disorders [23]. Importantly, there is increasing awareness of the interplay between specific neurocircuitry of the brain and behavioural pathology.
Depression
Neuroimaging studies have been central in identifying the key structures involved in the pathophysiology of MD, showing decreases in hippocampal volume of up to 15% in depressed patients [24], as well as reductions in grey-matter volume and glial density in the prefrontal cortex and the hippocampus [25]. Other studies in MD indicated large volume reductions in the anterior cingulate cortex (ACC) and orbitofrontal cortex (OFC) accompanied by lesser reductions in the prefrontal cortex, along with moderate reductions in the hippocampus, the putamen, and the caudate nucleus [26]. Lorenzetti et al. [27] reported volume reductions of the hippocampus, basal ganglia, the OFC and subgenual prefrontal cortex in patients suffering from MD, while more persistent forms of MD (which may include recurrent episodes or relapses and extended illness duration) are accompanied by an increased effect on regional brain volumes [28]. While reductions in hippocampal volume in MD may have a genetic component [29], it is also a function of illness duration [30] as well as poor compliance [28]. Moreover, PET studies have revealed consistently increased regional blood flow and glucose metabolism in the amygdala, orbital cortex, and medial thalamus but decreased blood flow in the dorsomedial/dorsal-anterolateral prefrontal cortex and anterior cingulate cortex in un-medicated MD patients [31]. N-acetyl-aspartate (NAA), an indicator of neuronal viability, was also reduced in frontal cortex and in subcortical regions of MD patients [32, 33].
Regarding antidepressant therapy, Frodl et al. [34] demonstrated increases in hippocampal volume in patients who were subjected to continual treatment with antidepressants for three years, while Mayberg et al. reported that patients who responded to antidepressant treatment presented with increased anterior cingulate metabolism at baseline when compared to non-responders and to healthy controls [35]. Importantly, Macqueen et al. noted a greater degree of hippocampal shrinkage in patients with a prior history of switching antidepressants, indicating the possible deleterious effect of non-compliance on hippocampal integrity [28]. Furthermore, it has been proposed by structural neuroimaging studies that the volume of regional structures, for example the ACC and hippocampus, may provide an estimate of response to treatment [36]. Clinical response has also been demonstrated
to be predicted by activity in the rostral ACC region as measured by electroencephalography (EEG) studies which identified activity localised to the rostral ACC region as being predictive of a clinical response to antidepressant medication [37]. Finally, favourable treatment outcomes have repeatedly been demonstrated to be associated with integrity of perigenual anterior cingulate volume [38]. Moreover, direct electrical stimulation of the striatum has been found to elicit a positive response in patients suffering from resistant MD and is bolstered by reports proposing the striatum as an important relay system between limbic and cortical structures [39]. ACC activity has also been positively related to a variety of treatment responses, including antidepressant pharmacotherapy and sub-chronic and experimental treatment strategies, including sleep deprivation [40], which suggests that ACC response is generalised across different treatment types.
Bipolar Disorder
In patients with BPD, neuroimaging studies have found enlargement of the amygdala [41] and reductions in the dorsal and ventral prefrontal cortices [42] while PET studies have found decreases in cortical metabolism and increased normalized subcortical metabolism in depressed patients with BPD [43]. Two meta-analyses of neuroimaging studies concluded that patients with BPD suffered from hypo-activation and gray matter reductions in cortical-cognitive brain structures and increased activation of the para-hippocampal gyrus and amygdala [44].
Schizophrenia
In schizophrenia and other psychotic disorders, regions such as the ACC and dorsolateral prefrontal cortex (DLPC) have been emphasised [45]. Using cognitive paradigms, fMRI studies have demonstrated alterations in cerebellar activity in patients with schizophrenia, anxiety disorders and dementia (see review [46]). However, Farrow et al. [47] found that lateral and medial frontal regions and bilateral posterior temporal lobe regions feature structural losses in schizophrenia, whereas alterations in patients suffering from BPD were limited to bilateral inferior temporal gyri while deficits observed subsequently were limited to the ACC. Temporal lobe regions present with decreased activation in patients suffering from schizophrenia [48]. Additionally, EEG studies have demonstrated a reduction in the P300 wave amplitude, elicited in the process of decision making, in BPD and schizophrenia patients compared to control subjects [49]. Studies utilizing structural MRI have consistently observed temporal lobe abnormalities in schizophrenia, although results in BPD are less dependable [50]. Previous fMRI studies have also consistently reported anomalies in the prefrontal cortex in patients suffering either from a first episode or established schizophrenia [51, 52]. However, some of the evidence points to dorsolateral hyper-frontality, and especially for tasks which demand working memory, as well as increased activity in parietal regions [53]. Considering the progression from the prodromal phase to established chronic illness, patients with first episode and established schizophrenia show a gradual deterioration in frontal and striatal activation [54]. The most consistent findings in schizophrenia relating to cognition are detriments in executive tasks requiring prefrontal cortical function, eg. a self-ordered working memory task [55] or anti-saccade eye movements [56], olfactory identification [57], and tasks that rely on rapid processing of information (eg. story recall) [58]. A recent 1H-MRS study in schizophrenia patients measuring NAA and N-acetylaspartylglutamate (NAAG) found a significant increase in NAAG/NAA ratio in the ACC but no difference in the left frontal lobe, although an inverse correlation between frontal lobe NAAG and negative symptoms was observed [59].
Pre-Clinical Correlates
Depression
Reductions in hippocampal volume have been observed in FSL rats, a genetic model of MD, when compared to Flinders Resistant Line (FRL) controls and is associated with a decrease in the number of neurons and synapses in the hippocampus – these alterations are reversed after chronic imipramine therapy [60].
Bipolar Disorder
In the ouabain-induced rat model of bipolar mania, PET imaging suggests reduced cerebral glucose metabolism, and is prevented by pre-treatment with lithium which concurs with similar decreases in cerebral metabolism noted in BPD patients [61]. Furthermore, lithium prevented stress-induced alterations in the amygdala by preventing increases in dendritic branching of pyramidal neurons in this structure [62]. Unfortunately, a paucity of MRI studies remains a shortcoming in animal models of BPD.
Schizophrenia
Previous studies indicated that the SIR model, a neurodevelopmental animal model of schizophrenia, presents with significantly reduced PFC volume, reduced accumbal dendritic length and spine density, cytoskeletal alterations and loss of parvalbumin (PV)-containing interneurons [18, 63, 64]. Among the most robust pathologies observed in schizophrenia is a decrease in gamma-aminobutyric acid (GABA) signaling (discussed in section 3.1.4), deficits of which are limited to the class of GABAergic interneurons containing the calcium binding protein PV [65]. These neurons synapse on the cell body or axon initial segment of glutamatergic neurons and thus are positioned to potently regulate pyramidal cell output. Furthermore a decrease in PV interneuron functionality may lead to reduced inhibitory control over pyramidal cell activity and also reduce coordination in activity of large brain networks [66].
SIR rats without an enriched environment also present with a decrease in dendritic spine density in the dorsolateral striatum when compared to rats from an enriched environment [67, 68]. Moreover, a NMDA receptor antagonist model of schizophrenia, viz. the phencyclidine (PCP) model, presents with decreased synaptic spine density on frontal cortical neurones [64]. Interestingly, rats treated chronically with MK-801 (another NMDA receptor antagonist model) also show a reduction in the amount of PV-containing neurones in the dentate gyrus and CA1 region of the hippocampus, although this is not accompanied by alterations in the PFC [69]. Furthermore, chronic intermittent exposure to PCP decreases NAA and NAAG levels in the temporal cortex, while it raises hippocampal NAAG levels [70]. Similarly, SIR reduces NAA in the temporal cortex without changes observed in the hippocampus, striatum or frontal cortex [71]. These changes may indicate neuronal dysfunction that mirrors alterations observed in schizophrenia, as discussed in the clinical section [59].
In order to determine which regions exhibit the most disease-relevant information as well as the most potential for predictive capacity, these neuroanatomical correlates need to be linked to biological and genetic markers to more accurately predict both the pathology underlying the disease and the clinical outcomes. Predicting clinical response will assist in early identification and to further stratify patients who may benefit from more intensive, alternative, or combined therapies.
BIOLOGICAL, GENETIC AND PROTEIN BIOMARKERS
Neuroendocrine and Circadian Rhythms
Various hormones, especially the HPA-axis, thyroid hormones, insulin, as well as altered circadian rhythm, have a pronounced influence on neurodevelopment and the neurobiology of MD, BPD and schizophrenia [72]. They also influence other hormones such as sex steroids, orexin, arginine vasopressin etc. that are also implicated in these disorders but for reasons of space cannot be covered here.
Circadian Rhythms
Clinical Correlates
Altered circadian rhythms occupy a critical role in how the brain copes with stressful experiences and ultimately in regulating behavioural responses [73, 74]. The influence of circadian rhythm on mood and behaviour has received much attention in recent years and implicates not only hormonal dysregulation in these disorders, but also includes disturbances in neurochemical, redox and inflammatory cascades in its sphere of influence [75, 76]. Indeed, these processes will be discussed in subsequent sections of this review. Output from the suprachiasmatic nucleus (SCN) of the hypothalamus, the master biological clock, is under regulation by serotonergic (5-HT2c) and melatonergic (MT1/2) receptors, the expression of which are regulated by various clock genes. Indeed, melatonin-mediated regulation of hippocampal plasticity as well as clock gene expression in hippocampal neurons suggests a hitherto poorly recognized aspect in our understanding and treatment of these disorders [78]. Altered SCN output to other hypothalamic centres, but also monoaminergic cell bodies in the brain stem, will lead to wide-spread disturbances in neuroendocrine as well as monoaminergic function [78]. It is therefore not surprising that a significant amount of preclinical and clinical data has described the association between altered circadian rhythms with genetic, environmental and developmental abnormalities precedent to the development of MD, BPD (mania symptoms) and schizophrenia (see recent reviews by Wulff et al. [75] and Karatsoreos [76]). It is of relevance that agomelatine, a recently available antidepressant that acts via re-entrainment of circadian rhythms, may also have a therapeutic role in disorders other than MD, in particular BPD and schizophrenia [77].
Pre-Clinical Correlates
Biomarkers of circadian rhythms in animals remain a shortcoming, with such studies relying heavily on endocrine markers (as will be clear in the following section). Nevertheless, alterations in this system have on numerous occasions been shown to not only be of importance in humans suffering from MD, BPD and schizophrenia, but also in translational animal models for these disorders. Most animal models selectively bred to display characteristics of MD feature disturbed diurnal rhythms, eg. the FSL rat [79], Wistar Kyoto rat [80] and mice bred for spontaneous helplessness [81]. Also, depriving animals of REM sleep has been suggested to model mania [82]. In animal models of schizophrenia, blind-drunk (Bdr) mice demonstrate fragmented rest and activity rhythms under a light/dark cycle – which is reminiscent of altered sleeping patterns in schizophrenic patients [83].
Cortisol
Clinical Correlates
A dysfunctional hypothalamic-pituitary-adrenal (HPA) axis has been implicated in MD, BPD and also schizophrenia [84-86], affecting adrenocorticotrophic hormone (ACTH) release and cortisol secretion from the adrenal cortex [87]. Elevated salivary levels of cortisol after waking may represent a biomarker for depression in adolescence [88]. Importantly, an abnormal cortisol response, such as a flatter diurnal cortisol pattern, implies an abnormal stress reactivity that correlates with a greater severity of depression [73, 74], suggesting that altered circadian rhythms occupies a critical role in how the brain copes with stressful experiences and ultimately in regulating mood. Although the dexamethasone suppression test has attracted interest as a promising diagnostic test for MD, there has not been a consistent approach to evaluate its clinical usefulness [89].
Steen et al. [90] found no significant difference in cortisol release during a mental challenge in schizophrenia and BPD patients, although blunted cortisol release was observed in male patients compared to controls in both disorders [90]. A significant increase in systemic cortisol metabolism in both schizophrenia and BPD patients has been described, with results in patients with schizophrenia vs. controls being most consistent [87]. Interestingly, studies in children at risk for developing psychosis lend further support to the suggestion that illness onset is predated by a degree of HPA axis abnormalities, rather than being a subsequent epiphenomenon [91]. A blunted cortisol awakening response may embody an early marker of susceptibility to develop psychosis which may even be genetically mediated, whilst increases in diurnal cortisol levels may develop only proximate to disease onset [91]. These studies reaffirm the status of the HPA-axis, particularly cortisol levels and metabolism, as a putative biomarker in MD, BPD and schizophrenia, and warrants further study.
Pre-Clinical Correlates
FSL rats have been found to be hypocortisolemic, while Wistar Kyoto rats present with increased levels of corticotropin releasing hormone (CRH) and ACTH. Brain levels of dehydroepiandrosterone (DHEA), an adrenal androgen known to have antidepressant-like effects, has been demonstrated to be decreased in both FSL and Wistar Kyoto rats vs. healthy controls, Sprague Dawley and Wistar rats respectively [92]. Mice showing high reactivity to stress also present with symptoms resembling that in depressed patients and were demonstrated to have a flattened diurnal rhythm of glucocorticoid secretion [93]. Similarly, mice exposed to 6 weeks CMS presented with high plasma corticosterone levels and decreased hippocampal expression of glucocorticoid receptors [94]. Sleep deprivation, which has been used to induce an animal model of mania, leads to a marked increase in CRH [95]. Cortisol levels have also been found to be increased both in the frontal cortex and periphery of rats exposed to prenatal stress, which has relevance in that prenatal stress may predict the development of these disorders. In addition to increased corticosterone release, Ward et al. [96] found rats exposed to prenatal stress to also have adrenal hypertrophy with increased expression of CRF-1 receptors in the amygdala [97]. Furthermore, olanzapine treatment was able to reverse the increased cortisol observed in the prefrontal cortex following prenatal stress [98].
Thyroid Hormones
Clinical Correlates
The relation between thyroid dysfunction and mental disorders has long been recognized, ranging from depression [99], anxiety [100] and schizophrenia [101]. A recent study explored thyroid-stimulating hormone (TSH) in patients with acute schizophrenia, unipolar depression, bipolar depression and bipolar mania and, apart from measuring TSH disturbances in all the disorders, observed a definite higher prevalence of thyroid dysfunction in patients with both unipolar and bipolar mood disorders vs. controls [102]. Another study observed significant thyroid dysfunction (hypothyroidism and hyperthyroidism) in schizophrenia as well as BPD patients [88]. Interestingly, autoimmune thyroid disease was more common in schizophrenia [103], emphasising an immune-inflammatory basis for the illness. Santos et al. [104] reviewed research on thyroid function in schizophrenia, relating interrelations between the pituitary-thyroid axis and major neuro-signaling systems involved in schizophrenia (including serotonin (5-HT), dopamine (DA), glutamate and GABA networks), as well as myelination and inflammatory processes. These processes are all convergent on the pathology of this disorder, as will be discussed. The authors conclude that thyroid hormone deregulation is a common feature in schizophrenia. Together, these studies emphasize the relevance of thyroid hormonal status as possible biomarkers in MD, BPD and schizophrenia, although further work in this regard is required to establish its putative role as a biomarker.
Pre-Clinical Correlates
Wistar Kyoto rats, an animal model of depression, have increased TSH levels that, together with ACTH, remains elevated after the diurnal peak [80]. To the best of our knowledge, the current body of literature on preclinical BPD and schizophrenia research does not contain significant data on thyroid hormones in animal models of mood and psychotic disorders.
Neurochemical Markers
The majority of drugs used clinically to treat MD, BPD and psychotic disorders such as schizophrenia target monoamine (DA, 5-HT and noradrenaline (NA)) receptors, reuptake transporters and monoamine metabolism [105, 106]. DA-ergic, 5-HT-ergic and/or NA-ergic neurotransmission affects behaviour by regulating motivation, reward seeking, aggression, and activity level – all symptoms that play an important role in the pathophysiology of these disorders [107, 108]. However, the cause of mood disorders is far from being a simple dysregulation of central monoamines. For example, monoamine oxidase inhibitors and monoamine reuptake inhibitors produce immediate increases in monoamine transmission [109], whereas their mood-enhancing properties are only fully realised following 4-6 weeks of sustained treatment. In fact, some patients do not show adequate improvement even after many months of treatment [110]. This indicates that enhanced serotonergic or noradrenergic neurotransmission per se is not immediately responsible for the clinical actions of these drugs [111]. Indeed, neurotrophins, neurogenesis and the concepts of neuroplasticity has now taken centre stage in our understanding of mood and psychiatric disorders and the mechanism of action of antidepressants [109, 112, 112a]. Thus, and apart from some data based on NA (see later), selecting an antidepressant based on its monoamine selectivity remains to be substantiated. The same can be said for antipsychotic drugs. Furthermore, a realization that neuroendocrine and metabolic dysfunction also contribute to the eventual development of these disorders, has provided a new framework for understanding their neurobiology and treatment. Nevertheless, their contribution towards the understanding and treatment of these disorders warrants closer scrutiny with respect to viable clinical biomarkers.
Dopamine
Clinical Correlates
Depression
Depressive symptoms (e.g. avolition, guilt, suicidality and social withdrawal) are ascribed to frontal cortical hypo-dopaminergia [109]. Striatal DA levels in MD are also reduced [113], being linked to symptoms such as anhedonia, reduced motivation and decreased energy levels. Patients presenting with MD episodes have been demonstrated to have significantly decreased dopamine transporter (DAT) binding potential, with binding potential correlating to receptor density and affinity [114]. Anhedonic MD patients exhibit significantly decreased levels of DAT in basal ganglia which are in accordance with the hypothesis linking impaired DA transmission to an impaired reward system [115]. It has also been suggested that decreased striatal D2 receptor density may underlie depressive symptoms, while increased striatal D2 receptor density/affinity has been observed in patients after successful SSRI treatment [116] which coincides with evidence of 5-HT modulating DA pathways [117].
Bipolar Disorder
Pharmacological evidence supports evidence that excessive DA neurotransmission mediates manic symptoms in BPD patients [118], while DA receptor D2 antagonists are robust anti-manic agents [119].
Schizophrenia
The DA hypothesis of schizophrenia proposes that a hyper-dopaminergic state in the striatum mediates positive symptom expression, while a hypo-dopaminergic state in the frontal cortex mediates cognitive and negative symptoms [120]. In line with this hypothesis, post-mortem studies in schizophrenia patients have described frontal cortical hypo-dopaminergia [121] and elevated DA levels in the striatum [120]. However, a previous study reviewing clinical evidence for DA involvement in schizophrenia came to the conclusion that multiple ‘‘hits’’ (i.e. adverse environment, infection, chronic substance abuse etc.) interact to result in DA dysregulation, thereby producing the final common pathway to psychosis in schizophrenia [122]. In fact, MD [123, 124] and BPD [125, 126] are also correlated to early life trauma. It is pathways related to the latter that are deemed critical prodromal events in early life adversity, such as neurotoxicity, oxidative stress and inflammation that may hold the clue to identifying more appropriate biomarkers for these illnesses. Investigators have noted a strong correlation between D2 receptor binding and response to an antipsychotic, with a minimum 70% receptor occupancy necessary for antipsychotic action [127]. In fact, the success of treatment with antipsychotic agents depend on dopamine D2 receptor blockade, while Howes and Kapur [122] recently suggested various genetic and environmental factors to be implicated in compromising the brain and ultimately leading to dysregulation of DA.
Preclinical Correlates
Depression
CMS decreases in vivo DA release [129] and leads to a decrease in D2 and D3 receptors in the limbic forebrain which is reversed by chronic treatment with imipramine [130]. A decrease in DA release in the nucleus accumbens has been observed as well as increased DA levels in limbic regions in FSL rats (together with elevated 5-HT and NA; see below) – possibly due to an increased synthesis and decreased release of DA [131].
Bipolar Disorder
Models of mania which incorporate dopaminergic agents, eg. amphetamine, have been demonstrated to be superior to other similar models [132]. Alpha-methyl-para-tyrosine (AMPT) mediated catecholamine depletion mitigates some mania-related characteristics in DAT knockdown (KD) mice [133], while treatment with valproate reverses locomotor hyperactivity in these animals [134]. Also, treatment with lithium and valproate reverses increased extracellular DA and oxidative damage in a dextro-amphetamine-induced rat model of mania [135]. Furthermore, hyperactive rats exposed to CMS display significantly reduced HVA (homovanillic acid – DA metabolite) compared to DA in the nucleus accumbens, indicating decreased DA release in this brain region [136].
Schizophrenia
Previous evidence on the SIR model has indicated elevated striatal and decreased frontal cortical DA, dihidroxylphenylacetic acid (Dopac) and homovanillic acid (HVA) levels [137]; increased or decreased frontal cortical DA and unchanged striatal DA [138]; as well as decreased frontal cortical and elevated nucleus accumbens DA, Dopac and HVA [139, 140]. Another study in the SIR model observed reduced PFC D1 receptor density [141]. However, changes in mesolimbic dopamine D2 receptor expression are inconsistent, describing down-regulation in striatum [142], but no change in mesolimbic [143], hippocampal, PFC or amygdala areas [144]. SIR also induces a hyper-responsiveness in DA release in the PFC in response to systemic administration of the atypical antipsychotics clozapine and olanzapine, but not haloperidol [145]. Moreover, microdialysis data show that both basal and stress-induced PFC DA levels are reduced in rats chronically treated with PCP [146, 147].
Serotonin (5-HT)
Clinical Correlates
Depression
Although 5HT2c antagonists are ineffective alone in the treatment of MD, they do show benefit when combined with other mood-regulating mechanisms, such as 5-HT reuptake inhibition (SRIs) or melatonin agonism (e.g., agomelatine) [78]. Since 5-HT2C receptor activation inhibits NA and DA release [78], the suppression of these monoamines by elevated 5-HT contradicts traditional views that antidepressant response typically involves an increase in brain 5-HT, as well as NA and DA. Indeed elevated 5-HT-mediated suppression of DA and NA release will be counter-productive [148], such as causing emotional detachment and failure to address the anhedonic symptoms of MD [78]. Clearly there are valid reasons to doubt whether an elevation in brain 5-HT is in any way essential for antidepressant response. In fact, a sustained increase in 5-HT does not appear to be a requirement for anxiolytic/antidepressant effects of an SRI [149]. Further on this point, 5-HT agonists are ineffective as antidepressants while the 5-HT reuptake enhancer, tianeptine, is an effective antidepressant despite having the exact opposite effect on synaptic levels of 5-HT than SRIs [150]. This evidence contradicts the simplistic view that brain 5-HT needs to be elevated to improve mood, and has been instrumental in fueling the search for new generation antidepressants.
Post-mortem studies have indicated that suicidal patients with MD present with low cerebrospinal fluid (CSF) levels of 5-hydroxyindole-acetic acid (5-HIAA), the metabolite of 5-HT [151, 152], along with increased 5-HT2A receptor binding sites in platelets [153] and prefrontal cortical sites [154] as well as increased limbic and decreased frontal cortical 5-HT1A receptors (reviewed in [155]). MD patients also present with reduced 5-HT2A receptor density in the frontal cortex [155a]. Interestingly, limbic density and activity of monoamine oxidase (MAO) is elevated in MD [156, 157] which in turn will influence a number of components of monoamine signalling.
Bipolar Disorder
The role of 5-HT in the pathogenesis of BPD is less studied, although a post-mortem study has indicated that subjects with DSM-III-R diagnoses of BPD who died while depressed had significantly reduced levels of 5-HIAA in frontal (−54%) and parietal cortex (−64%) [158]. A deficit in 5-HT uptake sites has also been observed in the brains of depressed BPD patients after death [159]. Furthermore, 5-HIAA levels were found to be decreased in the CSF of depressed BPD patients [160] and elevated in manic BPD patients [161].
Schizophrenia
Post-mortem studies in patients with schizophrenia [162, 163] as well as psychotic patients [164] have observed reduced frontal cortex 5-HT2A and increased 5-HT1A receptor density. Another study also indicated increased striatal but diminished frontal cortical 5-HT uptake sites in schizophrenia patients [165]. In line with these findings, CSF, genetic and neuroimaging studies have demonstrated an increase in central 5-HT-ergic neurotransmission in schizophrenia [166, 167] and typified by the serotonergic psychedelics such as lysergide. A previous review suggested that the positive symptoms observed in schizophrenia (delusions, hallucinations etc.) could be associated with an excess of 5-HT in the striatum [168]. Despite the above evidence for 5-HT involvement in schizophrenia, clinical studies have found selective 5HT2A/2C antagonists to be ineffective as antipsychotics (reviewed in [169]) and that alterations of brainstem 5-HT transporters are generally not associated with schizophrenia [170].
Preclinical Correlates
Depression
Excessive activation of the 5-HT2C receptor is anxiogenic [171] while 5-HT2C receptor antagonists are rapid acting with sustained anxiolytic actions [172]. 5HT and 5HIAA levels were noted to be higher in limbic structures in the brains of FSL rats compared to normal Sprague Dawley rats [173], while 5-HT2/3 receptor density is compromised in the nucleus accumbens leading to a lack of DA-5-HT interaction [174]. Furthermore, CMS leads to an increase in 5HT2a receptors in the cortex which is reversed by imipramine treatment [20]. However, contrary to human subjects, FSL rats present with decreased 5-HT synthesis [175] and SERT-expression [176].
Bipolar Disorder
5-HT-related data are limited in animal models of BPD and mania. However, a mutPOLG transgenic (Tg) mouse model of BPD has been demonstrated to have enhanced 5-HT turnover, accompanied by reduced 5-HT levels, in the amygdala and hippocampus when compared to non-Tg animals [177].
Schizophrenia
Studies on the SIR model has observed decreased cortical (or striatal) 5-HT/5-HIAA [138, 178], decreased frontal cortical and elevated nucleus accumbens and striatal 5-HT and 5-HIAA levels [140, 179]. Deficits in prefrontal 5-HT following SIR is also linked to the behavioural impairments associated with schizophrenia [180]. Evidence of altered 5-HT levels in the NMDA receptor antagonist model is limited with only one study indicating that 5-HT3 receptor antagonists can attenuate the behavioural hyperactivity caused by PCP [181].
Noradrenaline (NA)
Clinical Correlates
Depression
NA is of major importance in MD (reviewed in [182]). Previous studies have observed reduced levels of NA transporters in the locus coeruleus [183], altered density and sensitivity of frontal cortical α2A-adrenoceptors [184, 185], and a reduction of NA levels in non-compliant MD patients [186]. Further, a positive relationship between urine NA levels and MD has been confirmed [187]. Symptoms of anxiety were also associated with increased NA excretion in the urine [187]. Moreover, studies have demonstrated that low urinary excretion of the NA metabolite, 3-methoxy-4-hydroxyphenylglycol (MHPG), predict a positive response to NA-selective drugs such as imipramine, nortriptyline, desipramine, or maprotiline [188, 189]. These studies illustrate the significance of urinary noradrenergic measurements as a biomarker in guiding treatment selection and predicting efficacy. Expression of adrenoreceptor density has also been investigated in individuals suffering from MD. However, even though dysregulation in alpha and beta-adrenoreceptor systems have been noted, it remains unclear whether alterations in the expression of these receptors are causative in the pathology of MD. Considering the heterogeneity of the disorder, the value of adrenoreceptor dysregulation as a biomarker is unclear [190].
Bipolar Disorder
NA studies in BPD are limited although an increased turnover of NA has been shown to be central to the pathology of the disorder [158]. Furthermore, post-mortem studies in schizophrenia associated with the positive symptoms of the illness describe elevated brain NA levels as mentioned above [191]. NA has also been shown to be one of the primary neurotransmitters targeted during carbamazepine therapy in BPD patients [192].
Schizophrenia
An earlier review found consistent evidence that the positive and negative symptoms observed in schizophrenia are associated with over-activity and under-activity of central NA, respectively [191]. Moreover, increased NA reactivity and/or tone have been linked to anxiety observed in schizophrenia [193].
Preclinical Correlates
Depression
Data relating to NA as a biomarker in preclinical models of MD are limited, although increased catecholamines, including NA, has been reported in limbic regions in FSL rats [194].
Bipolar Disorder
Interestingly, very little data is currently available in animal models of BPD to support the role of NA as a biomarker, although a preclinical study has suggested a noradrenergic role for lamotrigine, producing an anti-immobility effect in the mouse forced swimming test (FST) while investigating the depressive facet of the disorder [195].
Schizophrenia
Similarly, evidence in support of NA in a schizophrenia animal model is extremely limited. However, a recent study on the SIR model in our laboratory has demonstrated elevated frontal cortical NA as well as striatal NA and MHPG, with decreased frontal cortical MHPG levels, in SIR rats [179]. Earlier SIR studies found an increase in NA turnover in the hippocampus, cerebellum and cortex of Wistar rats [196].
Glutamate and Gamma-Aminobutyric Acid (GABA)
Glutamate and aspartate, and GABA and glycine, are the preeminent excitatory and inhibitory amino acids respectively, in the brain. Their diffuse presence in interneurons (GABA) or as relay neurons and interneurons (glutamate) allows them to play a profound role in regulating the function of most neurotransmitter systems in the brain. As a result of their ubiquitous presence they are implicated in the neurobiology of probably all central nervous system disorders, in particular MD, BPD and schizophrenia. GABA-glutamate interactions have importance in kindling, a mechanism suggested to underlie the development of rapid cycling of mood or psychotic episodes, and how stressful life events adversely impact long-term outcome. GABA pathways exert a permissive role on the kindling action of glutamate, with excessive glutamatergic activity associated with synaptic remodeling and neurodegeneration.
Clinical Correlates
Depression
Abnormalities resulting in an increase in glutamate transmission have been reported in patients with MD [197]. Elevated levels of glutamate act on extrasynaptic NMDA receptors leading to an influx of Ca2+ into the neurons, which results in the toxic accumulation of reactive oxygen species (ROS) [198], with increased nitric oxide (NO) production playing a key role in MD pathology and treatment response [199, 200]. We have earlier proposed that the NO pathway may play an important role in relapse and treatment resistance [201] as well as influencing the effect of non-compliance on treatment outcome [202]. In MD, glutamatergic hyper-function seems to be closely related to the lack of 5-HT-ergic and noradrenergic neurotransmission noted to underlie the core symptoms of MD. Indeed, studies examining peripheral blood of MD patients have demonstrated the glutamatergic system to be overly activated [203, 204]. Elevated glutamate levels have also been found in the occipital cortex of un-medicated subjects with MD [205]. Accordingly, reduced glycine binding (where it acts to abrogate NMDA receptor activity) has been described in the frontal and temporal cortex of suicide victims and MD patients [206, 207], leading to hyperglutamatergia. Glutamate, in combination with quinolinic acid (QA), a glutamate agonist derived from the kynureine pathway (see later), may contribute to excitotoxicity in the central nervous system [208]. While several factors may influence the levels of kynurenine and its metabolites (eg. inflammation), a decrease in tryptophan (TRP) may generally be observed in patients suffering from MD resulting in reduced 5-HT levels. In general, depression is associated with lowered TRP, increased indoleamine 2,3 dioxygenase (IDO) activity as well as reduced levels of kynurenic acid [209, 210]. Furthermore, microglial levels of QA have been demonstrated to be upregulated in MD [211].
Bipolar Disorder
A recent review of magnetic resonance spectroscopy (MRS) studies in patients with BPD observed the cingulate and prefrontal cortices to contain higher glutamate levels, and possibly associated with illness state [212], while a decrease in NMDA receptor binding has also been noted in the CA3 region of the hippocampus [213]. In a post-mortem morphological study, an increase in QA positive microglia has been observed in the subgenual anterior cingulate cortex of BPD patients, commensurate with increased glutamatergic activity [211]. Drug therapy with the pyrimidine compound, cytidine, reduces glutamine/glutamate levels in BPD and possibly related to symptom severity, suggesting that the presence of glutamatergic dysfunction is an important factor in the underlying pathology of BPD [214]. Furthermore, the presence of genetic mutations affecting the glutamate pathway has also been suggested to be implicated in BPD [215].
Schizophrenia
Release of DA from cortical and limbic striatal structures are controlled by glutamate-GABA-glutamate feedback loops situated on pyramidal cells of the frontal cortex, the disturbances of which underlie the behavioral manifestations of schizophrenia [216, 217]. GABA’ergic interneurons in the brain stem monoaminergic nuclei, viz. raphe nuclei, locus coeruleus, ventral tegmentum, also modulate ascending serotonergic, noradrenergic and dopaminergic pathways, resulting in tonic inhibition of NA and DA release in the PFC, resulting in the emotional, mood and cognitive deficits associated with MD and schizophrenia [78].
The “glutamate” hypothesis of schizophrenia emerged from the observation that NMDA receptor inhibition induces schizophrenia-like behaviors in humans. Cortical hypoglutamatergia compromises DA release in the ventral tegmentum leading to meso-limbic hyperdopaminergic (positive symptoms) and meso-cortical hypodopaminergia (negative symptoms) [217]. Mitochondrial dysfunction, pro-inflammatory cytokines and increased IDO-mediated conversion of tryptophan to QA (supported by clinical evidence for elevated QA [218]), the latter an NMDA receptor agonist, may be directly or indirectly implicated in eliciting glutamate hyperactivity thereby increasing NMDA receptor activation, altered redox balance and oxidative stress [217]. Schizophrenia has also been likened to the kindling phenomenon, a process of increased excitatory glutamatergic activity coupled with a relative loss of inhibitory GABA’ergic tone [217].
In post-mortem schizophrenia studies, deficits of glutamate systems have been described in the temporal cortex, medial temporal lobe and striatal regions [219, 220], together with losses of glutamate uptake sites [221] and increases in NMDA receptors in the same brain regions [207]. Previous studies also emphasize the impact of NO metabolism via glutamate and GABA on NMDA receptor mediated neurotransmission in schizophrenia [222, 223]. NO is an important second messenger for the glutamate NMDA receptor pathway, and its overproduction is implicated in schizophrenia. Excessive NO release include impairment of NMDA-receptor mediated neurotransmission, disturbed DA metabolism, excessive ROS generation and mitochondrial dysfunction with cell-death (reviewed in [222, 223]). However, altered NO metabolism is not unique to, or indicative of, schizophrenia as disturbances in this signalling cascade has been noted in MD and BPD [222], as noted earlier. A recent clinical study also indicated elevated GABA and glutamate levels in the medial prefrontal cortex of un-medicated patients, with no alterations in medicated schizophrenia patients, suggesting possible normalization of GABA and glutamate with antipsychotic treatment [224].
Preclinical Correlates
Depression
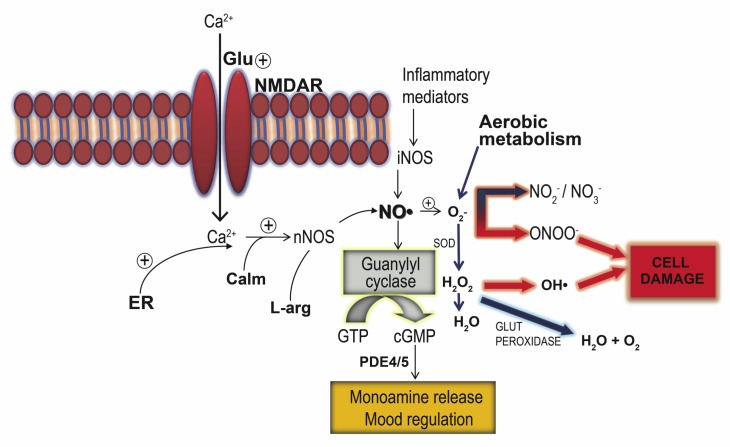
Under conditions of chronic stress, elevated glucocorticoid levels enhance glutamatergic transmission by increasing the expression of the glutamate ionotropic NMDA receptors, as well as increasing the synthesis and extracellular concentrations of glutamate [225]. Abnormalities resulting in an increase in glutamate–NO transmission have been reported in FSL rats [226]. The possible mechanisms whereby NO can contribute to mood disorders is obscure, although persistent research has highlighted various possibilities including the actions of the NO/cyclic guanosine 3'5'-monophosphate (cGMP) pathway. Modulators of the NO-pathway have also gained relevance in MD research due to NO-inhibitors demonstrating antidepressant effects in models predictive of antidepressant activity [227, 228]. By activating soluble guanylate cyclase (sGC) which converts guanosine 5'-triphosphate (GTP) to the intracellular messenger cGMP [229, 230], NO is enabled to mediate many cellular processes, particularly the regulation of ion channels, activation and inhibition of cyclic nucleotide hydrolysis by phosphodiesterase, activation of G-kinase and modulation of neurotransmitter release [229, 231]. Interestingly, neuronal nitric oxide synthase (nNOS) inhibitors (eg. methylene blue) [232] as well as PDE5 inhibitors (e.g. sildenafil) [233] have antidepressant and anxiolytic effects in animal models [234, 235] (Fig. 1), as do clinically relevant antidepressants [228]. These effects however are due to interference with constitutive nNOS-mediated effects and not inducible NOS (iNOS), which rather plays an important role in chronic stress associated with inflammation [231] (Fig. 1). Stressed vs. naive FSL/FRL rats show elevated hippocampal glutamate-NO signalling [224], indicating that a chronic environmental stressor is required in order to demonstrate altered glutamate-NO signalling in FSL rats [236]. This is congruent with the fact that MD involves a prior and/or ongoing chronic stressor [237, 238]. Considering these findings, it is not surprising that NMDA antagonists such as dizocilpine (MK-801) [239], ketamine [240], memantine [241] and others [242] exert antidepressant effects, while disinhibition of glutamate-NO signalling follows antidepressant discontinuation after chronic treatment [243, 244]. A possible explanation could be that NMDA receptor antagonists increase 5-HT levels in the brain [245], while also having a modulatory effect on pathways involved in neuroplasticity and cellular resilience [202].
Fig. (1).
Glutamate-mediated effects on the cGMP-NO system leading to monoamine release that in turn can be targeted by pharmacological means, eg. PDE5 and NOS inhibitors, as well as known antidepressants. However this pathway can also lead to oxidative stress if excessive glutamate-mediated NO synthesis combines with O2- from aerobic metabolism. Also depicted is the effect of inflammatory mediators that promote iNOS-mediated NO synthesis thereby promoting the formation of cell-damaging reactive oxygen and nitrogen species. These pro-oxidative mechanisms can be abrogated by endogenous antioxidant systems such as superoxide dismutase (SOD) and glutathione that act as a sink to quench excessive NO and/or O2-.
Abbreviations: calmodulin (Calm); cyclic guanosine monophosphate (cGMP); endoplasmic reticulum (ER); glutamate (Glu); glutathione peroxidase (glut peroxidase); guanosine triphosphate (GTP); inducible nitric oxide synthase (iNOS); l-arginine (L-arg); neuronal nitric oxide synthase (nNOS); nitric oxide (NO); NMDA receptor (NMDAR); phosphodiesterase (PDE); superoxide dismutase (SOD); superoxide (O2-); hydrogen peroxide (H2O2).
Preliminary evidence also supports the use of NMDA antagonists such as ketamine in treatment-resistant MD [246]. How this happens still needs illumination, although animal studies have begun to delve into the possible mechanisms involved [247]. The latter work has indicated a mutual cooperation with glutamate AMPA receptors [247], resulting in activation of mammalian target of rapamycin (mTOR) [248] and inhibition of glycogen synthase kinase-3β (GSK-3β) [249] signalling (Fig. 2). The mTOR pathway plays a pivotal role in protein synthesis by stimulating mRNA translation via interaction with its downstream targets [248], and leads to prolonged elevation of synapse-associated proteins in the prefrontal cortex [248]. Diminished activity of the mTOR pathway could underlie synaptic deficits in the PFC as previously reported in MD [250]. Furthermore, this evidence is supported by the behavioural responses to ketamine being blocked in mice which express constitutively active GSK-3β [251]. Considering the contributory role of oxidative stress in MD, inactivation of GSK-3β is linked to the regulation of redox homeostasis via stress responsive genes that protect cells against inflammation and oxidative stress [252, 253] (Fig. 2).
Fig. (2).
An overview of key neuroprotective and neurotoxic molecules involved in drug (antidepressant, lithium etc.)-induced neuroplasticity. The monoaminergic system mainly exerts its effect on BDNF expression via the cAMP cascade, while BDNF in turn exerts its effect on monoaminergic neurons through the TrkB-receptor via the MAPK/ERK cascade and the phospholipase C (PLC) signalling system. Adapted from [342]. Abbreviations: beta adrenoceptor (βAR); alpha-2 adrenoceptor (α2AR); BDNF receptor (TrkB), see text for further details/ abbreviations not noted here.
Bipolar Disorder
The standard treatment for BPD, lithium salts, target the glutamate-NO system [254, 255]. Unfortuantely, current literature lacks sufficient data to elaborate on the role of glutamate/GABA as a biomarker in preclinical models of mania and BPD.
Schizophrenia
Decreased glutamate release has been observed in the frontal cortices of Homer1 mutant mice, a putative animal model of schizophrenia [256], while chronic phencyclidine (PCP) administration in rats is associated with a decreased expression of glutamate receptors in the prefrontal cortex [257] and a reduced number of cortical and hippocampal PV-immunoreactive neurones [64]. Confirming this, partial deletion of the NMDA receptor in mice is associated with behavioural alterations akin to that observed in PCP treated mice [258], while increased NMDA receptor binding has been described in the frontal cortex of SIR animals [141].
Neuronal Growth Factors
Growth factors are intricately involved in the survival, growth and differentiation of specific groups of neurons. Their relevance is gaining in importance in the light of increasing evidence that mood and psychotic disorders are associated with structural brain changes and that alterations in growth factors may precipitate or exacerbate depressive, BPD and psychotic episodes [13, 259, 260].
Brain-Derived Neurotrophic Factor (BDNF)
Clinical Correlates
Depression
Extensive studies have established that altered BDNF plays a pivotal role in MD. BDNF and the transcription factor, cyclic adenosine monophosphate (cAMP) response element binding protein (CREB), are intimately linked biochemically (see Fig. 2), playing a critical role in cellular resilience and neuroplasticity. Antidepressant treatment up-regulates CREB in the cortex and hippocampus of humans [261]. Both serum BDNF levels and CREB phosphorylation and protein levels are reduced in depressed individuals [262]. Moreover, an inverse relationship exists between serum levels of BDNF and the severity of MD [263], while antidepressant treatment is able to reverse the deficit in BDNF observed in MD [264, 265] and to increase phosphorylation and binding of CREB [266, 267]. BDNF is expressed throughout the body [268], but the exact origins of circulating BDNF remain elusive. BDNF has been shown to originate from several sources including brain neurons, vascular endothelial cells and platelets. It has also been shown to cross the blood-brain barrier [269] so that plasma BDNF levels may reflect central BDNF levels [270]. BDNF regulates synaptic plasticity in neuronal networks and appears to be a particularly relevant factor for mood disorders with associated cognitive dysfunction [271 -273].
CREB is responsible for regulating BDNF expression [274]. Activation of CREB is associated with the regulation of synaptic plasticity as well as transcription of specific target genes involved in the production of proteins, BDNF being one example [275, 276]. Post-mortem studies have reported decreased hippocampal BDNF in MD patients who committed suicide, but elevated levels in patients who were being treated with antidepressant agents at the time of death [268, 277]. Considering the growing evidence for an interaction between MD and metabolic and redox-related conditions [278-280], our group recently showed that altered serum BDNF may be linked to metabolic and redox factors, with BDNF levels indicating either a counter-regulatory action on the effects of glutathione oxidation or that BDNF may mediate the redox effects itself, leading to the development of a mood disorder [281].
Various factors associated with an increased risk of developing MD, e.g. smoking [282, 283] and type II diabetes mellitus [284], have been linked to BDNF deficits. Thus decreased levels of BDNF have been found in smoking individuals when compared to non-smokers [285, 286], while smoking cessation leads to improved BDNF levels [285, 286]. Likewise, serum levels of BDNF have been shown to be significantly lower in subjects with Type II diabetes when compared to healthy controls [287, 288], while cerebral output of BDNF is inhibited in the presence of high blood glucose levels [287]. These findings reiterate the causal link between metabolic diseases, altered BDNF and the development of MD noted earlier. On the other hand, for example, physical exercise has been shown to increase BDNF [289, 290], to be neuroprotective, to improve mood and to have antidepressant effects [291, 292].
Bipolar Disorder
Decreased peripheral BDNF levels have been observed in BPD patients, possibly associated with the pathophysiology and severity of manic symptoms [293]. Exercise has also been shown to decrease depressive symptoms in BPD patients and to even increase the frequency of mania [294]. The latter would indicate that elevated levels of BDNF may not always be beneficial, as has been proposed in a study in patients with MD [281]. The authors suggest that, by adversely affecting resilience, BDNF facilitates activity-dependent plasticity that may translate to a variable effect on mood and other plasticity-dependent functions. In fact, BDNF has been noted to induce paradoxical depressogenic effects [281]. As a mediator of synaptic plasticity, maladaptive secretion of BDNF (eg. a response to environmental adversity) may set in motion counterregulatory responses that are counterproductive.
Schizophrenia
Decreased peripheral BDNF levels have been observed in schizophrenia [295]. Importantly, a recent study indicated that clinically stable schizophrenia patients present with significantly increased serum levels of BDNF after exposure to cognitive training targeted at improving neuroplasticity [296]. Furthermore, post-mortem studies reported a decreased concentration of BDNF-positive neurons [297] and BDNF concentrations in brain tissue of schizophrenic patients, which include the cortical areas and the hippocampi [298].
Preclinical Correlates
Depression
Antidepressant treatment up-regulates CREB in the cortex and hippocampus of rats [299], while an overexpression of CREB in the dentate gyrus results in antidepressant effects in the FST and a learned helplessness paradigm – both animal models of MD [300]. In the latter, decreased hippocampal BDNF levels were described. Although contrary to that in human subjects, some studies have also noted increased serum levels of BDNF [301, 302]. In FSL rats, serum and whole blood BDNF levels have been found to be significantly increased compared to control but significantly decreased in the hippocampus, with no differences noted in the frontal cortex and CSF [303], suggesting that BDNF is differentially regulated in hippocampus, serum, and whole blood in these animals. The latter is not unlike similar paradoxical findings in humans, where BDNF has been suggested to play a counter-regulatory role [281]. Preclinical studies have indicated that BDNF administration produces antidepressant-like behaviour [304], while antidepressants and electroconvulsive therapy increase BDNF levels [305]. After animals were subjected to repeated stress, they constantly presented with decreased BDNF levels as measured in the hippocampus and serum, while corticosterone levels returned to normal levels, suggesting that changes in brain plasticity occur following a second stressful event [306]. The presence of decreased serum BDNF levels accompanied by normal serum cortisol levels may therefore represent a relevant biomarker for identifying individuals who are more likely to develop depressive symptoms in the subset of a population which may be predisposed to developing affective disorders. These alterations may even be expanded to other disorders provoked by stressful life events, for instance schizophrenia [307].
With smoking having been found to affect BDNF levels in humans, decreased levels of BDNF have also been found in rats repeatedly exposed to nicotine [308]. Similarly, physical exercise also increases BDNF in animals [289, 290].
Bipolar Disorder
BDNF levels are decreased in both the amygdala and hippocampus of rats in the ouabain model of mania, and reversed by lithium [309]. Moreover, in an amphetamine-induced model of mania, BDNF was also decreased in the hippocampus and increased by valproate and lithium [119].
Schizophrenia
Neonatal PCP administration produced a sustained elevation of BDNF in the hippocampus and the entorhinal cortex of 8-week-old rats [310]. However, studies in the SIR model observed significantly reduced medial PFC BDNF levels [311] as well as decreased hippocampal BDNF [312].
Insulin-Like Growth Factor
Insulin-like growth factor-1 (IGF-1) is involved in regulating peripheral cell growth and metabolism [313]; and plays a crucial role in the growth and differentiation of nerves and also in the synthesis and release of neurotransmitters [314].
Clinical Correlates
Depression
Unfortunately, there have not been sufficient clinical studies to determine whether peripheral IGF-1 is altered in MD patients or following antidepressant administration.
Bipolar Disorder
In BPD patients, a previous study observed altered IGF signalling in post-mortem brain tissue [315].
Schizophrenia
Antipsychotic-free schizophrenia patients have been found to present with a decrease in plasma IGF levels [316].
Preclinical Correlates
Depression
Unfortunately, we are not aware of any extensive IGF-related data in established animal models of MD. Nevertheless, preclinical studies have indicated that peripheral IGF-1 administration reduces immobility in the FST [317], increases central BDNF mRNA [318] and produces antidepressant-like behavioural responses in mice exposed to CMS [317]. Moreover, after chronically treating rats with antidepressants, elevated IGF-1 expression was observed in the brains of these animals [319]. Finally, IGF-1 has been found to regulate adult hippocampal neurogenesis in rats [320].
Bipolar Disorder, Schizophrenia
To the best of our knowledge there is no pre-clinical data in established animal models of IGF-1 as a preclinical biomarker in BPD or schizophrenia.
Vascular Endothelial Growth Factor
Vascular endothelial growth factor (VEGF) acts as a neurotrophic factor, is a cytokine implicated in angiogenesis [321] and has been related to the vascular niche hypothesis of adult neurogenesis [322]. This hypothesis attributes increases in the proliferation of neurons in the adult hippocampus to VEGF-induced angiogenesis. VEGF is purported to play a role in several features associated with neuronal growth, including neuronal regeneration and differentiation as well as axonal outgrowth [323].
Clinical Correlates
Depression
MD patients present with higher plasma VEGF levels which can be reversed with antidepressant treatment [324], while earlier studies have confirmed said increase in MD [325]. Furthermore, remitted MD patients have significantly elevated VEGF levels, while MD patients with a family history of psychiatric disorders also have higher baseline levels of VEGF, compared to MD patients without a family history and healthy controls [326]. This may be indicative of a role for VEGF in the pathology of MD, possibly hinting of a neuroprotective role to counter reduced neurogenesis in MD.
Bipolar Disorder
BPD patients present with higher plasma VEGF levels during acute episodes vs. healthy controls [327], emphasizing that a depressive and manic episode in mood disorders may be associated with the neuroprotective role of VEGF. Interestingly, a recent study indicated that VEGF mRNA levels were significantly decreased in BPD patients treated with lithium vs. healthy controls [328], suggesting that VEGF may be a useful marker in BPD and as an indicator of lithium response.
Schizophrenia
VEGF data in schizophrenia patients are limited, although previous studies have not observed any differences in serum VEGF in schizophrenia vs. healthy individuals [285]. However, significantly reduced levels of VEGF mRNA have been observed in the DLPFC of patients with schizophrenia [329].
Preclinical Correlates
Depression
The relationship between central and peripheral levels of VEGF still needs clarification. In a genetic rat model of MD, Elfving and colleagues found decreased levels of VEGF in the brain but no variations in serum VEGF levels [301].
Bipolar Disorder
To the best of our knowledge, there is no data available on VEGF as a biomarker in preclinical models of BPD.
Schizophrenia
Similarly, no pre-clinical data is currently available on VEGF in the SIR or NMDA antagonist models of schizophrenia. However, a pre-clinical study did observe that VEGF levels are increased in rat hippocampi following 14 days haloperidol or olanzapine treatment [303]. Interestingly, in the case of haloperidol treatment this increase was lost 45 days later, while olanzapine treatment bolstered the initial increase in VEGF [330], reaffirming that first and second generation antipsychotics are not therapeutically equivalent. This underlines VEGF as a possible marker in schizophrenia treatment but not diagnosis per se.
Neuronal Resilience Markers
Several neurochemical markers have been associated with neuroprotective effects and positive antidepressant treatment response. With the increased evidence for a neurodegenerative profile for MD, BPD and schizophrenia and the progressive nature of these illnesses, identifying neuroresilience markers is gaining in relevance. In this regard, resilience markers linked to the BDNF pathway are especially attractive.
Stress and environmental adversity is a common thread throughout all three illnesses under review [252, 331, 332]. Stress-induced increases in glucocorticoid levels have been shown to decrease the synthesis of neurotrophic factors, particularly BDNF, which is an effective neuroprotective factor and protagonist of neurogenesis [333]. These neurotrophic effects are mainly mediated through inhibition of cell death pathways and activation of mitogen-activated protein kinases (originally extracellular signal-regulated kinases or MAPK/ERK) signalling pathways and phosphotidylinositol-3 kinase (PI-3K)/Akt (protein kinase B) pathways (see Fig. 2) [334]. As noted earlier, BDNF expression is decreased during MD, BPD and schizophrenia, a response that is reversed by effective pharmacological treatment [295, 335-337]. Furthermore, increased structural atrophy observed in treatment resistant MD has been correlated with greater decreases in BDNF levels [338] in patients failing to respond to SRI treatment compared to treatment responsive patients.
The cAMP cascade is activated following increased serotonergic and adrenergic receptor activity which results in downstream activation of CREB [339]. The ensuing elevation in cAMP ultimately leads to increased BDNF expression which subsequently activates the MAPK/ERK pathway, a major pathway involved in cell growth and proliferation [340, 341] (see Fig. 2). Monoaminergic neurons experience an increase in growth following MAPK/ERK pathway activation thereby accounting for how BDNF modulates the monoaminergic system [342].
Activation of the PI-3K cascade by BDNF leads to phosphorylation of Akt, a molecule at the crossroad of cell survival and cell death [343] (see Fig. 2). Activation of Akt following phosphorylation leads to enhanced activity of mTOR which is responsible for regulating the expression of several genes involved in cell growth, particularly a group of synapse-associated genes that have been directly linked to neuroplastic events [248]. Conversely, inactivation of Akt by dephosphorylation leads to a decrease in phosphorylation and subsequent activation of Bcl-xL/Bcl-2-associated death promoter (Bad), a pro-apoptotic molecule [343]. It is therefore evident that in combination, the MAPK/ERK and PI-3K pathways are largely accountable for the neuroplastic events occurring during antidepressant response and, furthermore, directly links the actions of BDNF to these processes.
Clinical Correlates
Depression
Decreased cAMP levels and lower MAPK/ERK pathway activity has been associated with MD, which has been shown to be reversed by increasing BDNF levels [344].
Bipolar Disorder
GSK-3b has been demonstrated to be an important role-player in BPD with lithium, an inhibitor of GSK-3b, having served as a mainstay in the treatment of BPD. It also regulates various proteins and is involved in neuroplasticity and neurotransmission [345]. Therefore, agents involved in the modulation of GSK-3b and its downstream pathways may serve as valuable biomarkers in the diagnosis and treatment of BPD – e.g., several molecules involved in both cell survival and apoptosis, such as CREB [346] and p53 [347], respectively. GSK-3b also plays an important role in the regulation of the Wnt [347] and PI-3K [348] signalling pathways linked to cellular resilience [348, 349] (Fig. 2). It has also been suggested that progranulin (PGRN) may serve as a neurotrophic factor- modulating neurite outgrowth as well as neuronal differentiation and survival [350]. Furthermore, plasma levels of PGRN are decreased in BPD patients [351, 352] and GSK-3β has been implicated in mediating PGRN activity [351]. GSK-3β protein expression is decreased in the platelets of BPD patients [349]. Even though previous studies could not find alterations in brain expression of GSK-3β, decreased protein expression in platelets can be reversed by mood stabilizers – but not antidepressants – thereby emphasizing a valuable role for GSK-3β as a peripheral biomarker and even a state – rather than trait – marker of BPD [353].
Schizophrenia
A recent post-mortem study in patients with schizophrenia found increased levels of various proteins involved in the MAPK- and cAMP-associated pathways, as expressed in frontal cortical structures [354]. In line with these observations are studies indicating alterations in several proteins in the MAPK-associated pathway: extracellular signal-regulated kinase (ERK)-2, immediate early genes c-fos and c-Jun levels were elevated in the thalamus on both protein and transcription level, whereas c-Jun protein and Elk-1, CREB, and ATF-2 protein levels were elevated in the cerebellar vermis [355, 356]. Moreover, other proteins involved in the MAPK pathway, including MEK1, MEK2, RSK1, B-Raf, and CREB were found to be reduced in the frontal cortex of schizophrenia patients [357]. With regards to the cAMP pathways, decreased DA- and cAMP-regulated phosphoprotein Mr 32 kDa (DARPP-32) was observed in the frontal cortex and thalamus of schizophrenia patients [358, 359]. Furthermore, a recent review highlights numerous evidence and theories in support of a novel mTOR based hypothesis of the neuropathology of schizophrenia [360]. Control of protein synthesis is the primary role of this signalling cascade while it is also regulated by known extracellular and environmental factors implicated in the pathology of schizophrenia [360].
Preclinical Correlates
Depression
Blocking MAPK signalling leads to depressive-like behaviour in the FST in rats and inhibits the antidepressant effects of ketamine [340]. These findings provide some insight on how glutamate-NMDA signalling interacts with monoaminergic-cAMP pathways to mitigate a faster onset of action or to treat refractory MD. Furthermore, SIR in rats, a putative neurodevelopmental animal model of MD and schizophrenia [18], leads to an enhanced expression of mitogen-activated kinase phosphatase and apoptosis-related genes in the prefrontal cortices of Sprague-Dawley rats [361].
Bipolar Disorder
Transgenic mice that overexpress GSK-3 present with decreased habituation and an increase in activity that has been related to hyperactivity in mania [362]. β-catenin, a downstream molecule of GSK-3 (Fig. 2), was found to be decreased in the hippocampi of black Swiss mice, a putative model of mania [363], while the behaviour of transgenic mice overexpressing β-catenin was found to have a behavioural phenotype similar to that of lithium-treated animals [364]. Of significance is that lithium stabilizes β-catenin by inhibiting GSK-3β thus reducing neuronal vulnerability to apoptosis [365].
Schizophrenia
Gururajan and Van den Buuse [360] have explained the involvement of mTOR in schizophrenia by referring to numerous animal models of schizophrenia. However, direct measurement of neuronal resilience markers in the SIR model and the NMDA antagonist model is limited, with only one study indicating that MK-801 administration elevates phosphorylation of MAPK in the frontal cortex of rats [19].
Oxidative Stress Markers
Normal oxidative metabolism in cells results in the production of various ROS. Oxidative stress occurs when cellular antioxidant defence mechanisms, such as SOD, catalase, glutathione peroxidase, fail to counterbalance and control endogenous production of ROS such as O2- and H2O2. This leads to a free radical attack on proteins, DNA and lipids [366, 367]. SOD is the primary defense against oxidative stress by converting O2- to H2O2 [368]. Hydrogen peroxide in turn is converted to water and glutathione (GSSG) by catalase and glutathione peroxidase [369], with GSSG rapidly being converted to reduced glutathione (GSH) by glutathione reductase [370]. The brain has relatively low levels of antioxidant defences, as well as a high lipid content that is highly susceptible to attack by ROS [371]. Thus, a reduction in GSH, and an increase in GSSG, is regarded as being indicative of increased oxidative stress.
Many of the changes in oxidative status may be directly related to increased inflammatory response due to the presence of other systemic illnesses, such as endocrine and metabolic disorders and cardiovascular disorders [281]. Furthermore, changes in certain neurotransmitter systems in the brain, especially glutamate and GABA, increase the risk of oxidative stress in the brain and subsequent neuronal oxidation and cell death [279]. Moreover, oxidative stress in its own right may mediate altered monoaminergic activity [372] that underlies the pathology of many neuropsychiatric illnesses associated with oxidative stress [373]. One of the more prominent redox active molecules released by changes in glutamate activity in the brain is NO, and which is well described as being a contributing factor towards the development of MD [374], schizophrenia [223] and possibly BPD [375]. In this regard, both constitutive NOS-, such as nNOS, and iNOS-mediated NO synthesis needs to be considered, with nNOS being more involved in neurotransmission and iNOS in inflammation. Fig. 1 provides an outline of how glutamate, NO and redox systems interact to produce oxidative stress.
Clinical Correlates
Depression
In recent years MD has been associated with several changes in redox status, presenting as either an increase in oxidative stress and/or diminished oxidative defence systems [279]. Elevated plasma malondialdehyde (an indication of lipid peroxidation) levels and susceptibility of red blood cells to oxidation, as well as an increase in serum SOD activity, has been observed in MD patients [376, 377]. However, Srivastava et al. [378] found no alterations in the activities of SOD and glutathione peroxidation in polymorphonuclear leukocytes from patients with MD. In their clinical study, Berk and colleagues noted only limited support for the role of antioxidant and glutathione precursor, N-acetyl cysteine (NAC), as an adjunctive therapy for MD, although further such clinical studies are required [379]. A high incidence of co-morbid metabolic syndrome and MD have been observed [380] with inflammation a major mediator in the development of both MD and metabolic syndrome [381]. In support of this, substantial evidence exists linking insulin- and NO-mediated pathways in the brain. In fact, insulin upregulates expression of nNOS [382] while a role for increased NO and insulin/peroxisome proliferator-activated receptor (PPAR) signalling has been noted following stress, thus presenting as a susceptibility factor in the subsequent development of MD [383].
Bipolar Disorder
BPD patients present with changes in antioxidant enzymes, for example Andreazza et al. [384] reported manic and depressive phases to be associated with increased SOD activity, but unaltered activity in euthymia. This is corroborated in part by Machado-Vieira [260] who found untreated manic bipolar patients to present with increased activity of SOD. Furthermore, patients who were euthymic presented with decreased catalase activity [384], while activity was increased in manic patients who did not receive treatment [260]. Increases in lipid peroxidation due to oxidative stress unrelated to the phase of illness have also been reported [260, 384]. In addition, BPD patients were found to express increased lipid peroxidation in the cingulate cortex [385], while clinical studies have indicated that the antioxidant, NAC, is effective as adjunctive treatment in BPD [386 -388].
Schizophrenia
Evidence has accumulated in recent years that antioxidant systems are impaired in schizophrenia [389]. Gawryluk et al. [390] reported reduced levels of GSH in post-mortem prefrontal cortices of patients with schizophrenia. Do and colleagues [391] found a 52% decrease in GSH levels in the prefrontal cortex of patients with schizophrenia. Interestingly, a significant deficit in total antioxidant status was inversely associated with some domains of cognitive deficits in schizophrenia patients, such as attention and immediate memory [392]. Moreover, plasma SOD activity was negatively correlated with positive symptoms in first-episode schizophrenia patients [393]. Lower levels of total antioxidant status, catalase and glutathione peroxidation has been described in first episode schizophrenia patients, with GSH levels positively associated with executive function [394]. Furthermore, clinical studies have described the clinical utility of NAC as an adjunctive treatment in schizophrenia [387, 395], as well as the combination of ω-3 fatty acids with vitamins E and C [396]. However, we have been unable to demonstrate efficacy for ω-3 polyunsaturated fatty acids (PUFA) plus alpha-lipoic acid in preventing relapse in patients who had responded well to antipsychotic treatment after a single episode of psychosis [397]. Further studies in this regard are nevertheless warranted.
Preclinical Correlates
Depression
With regard to significant animal studies, SOD and catalase activities have been found to be decreased in rats exposed to a chronic stress model of MD, and could be reversed by tianeptine [398]. Similarly, animal studies have found NAC to be as effective an antidepressant as imipramine [399]. Of particular interest is that exposure to ozone worsens anxiety, cognitive and depressive-like behaviour in the FSL rat model of MD, suggesting that genetically susceptible individuals exposed to high levels of oxidative stress are at higher risk of developing mood and/or anxiety disorders [400]. Moreover, exacerbating levels of oxidative stress (eg. with ozone) can attenuate antidepressant action [400]. In fact, stress-related activation of the NMDA-NOS cascade has been proposed to be a vulnerability factor in stress-sensitive FSL rats [236].
Considering the connection between inflammation, NO and insulin/PPAR signalling in MD, it is not surprising that PPARγ has been associated with suppression in immune response through its ability to inhibit the expression of inflammatory cytokines [401] and to have actions on pathways involved in apoptosis, cellular proliferation and cellular resilience [402]. Moreover, it has been demonstrated that metabolites of 5-HT act as PPARγ-agonists in the periphery [403], which further indicates the possibility of an underlying link between biochemical pathways of mood disorders and metabolic syndrome. The recently discovered prostaglandin and endogenous PPARγ ligand, 15d-PGJ2, presents with anti-inflammatory properties [404], increases the neuronal metabolism of glucose, prevents stress-induced suppression of glutamate uptake [405] and has been suggested to be a possible marker for psychiatric diseases [406]. Indeed, animal studies have also decribed the antidepressant activity of PPARγ agonists [407].
Bipolar Disorder
SOD was found to be increased and catalase (CAT) decreased in an ouabain-induced rat model of BPD [408], while lithium and valproate protect against amphetamine-induced oxidative stress in the same model, thus further supporting a role for oxidative stress in BPD [119]. When considering the proposed role of oxidative stress and inflammatory process in BPD it is important to note that some mood stabilizers, eg. lithium, valproate (VPA), carbamazepine, lamotrigine, suppress (brain) pro-inflammatory mediators such as cyclooxygenase-2 (COX-2) and prostaglandins [409 -411], indicating possible anti-inflammatory properties. Moreover, NAC reverses and protects against oxidative protein damage induced by d-amphetamine in a rat model of mania [412].
Schizophrenia
Animal studies, too, have confirmed that schizophrenia involves redox imbalance and oxidative stress. Using the ketamine challenge model, researchers have noted a decreased expression of PV-interneurons (relating to GABA; see section 2 and section 3.1.4) in the hippocampus [413] – a recent review by Bitanihirwe and Woo, 2011 [366] explain that GABAergic pathways innervate primary neurons and could increase intracellular calcium levels and subsequently trigger oxidative damage. Elevations in nicotinamide adenosine dinucleotide phosphate (NADPH) oxidase 2 (Nox2) are observed in the prefrontal cortex of rats exposed to the SIR model of schizophrenia [414]. Nox2 is a major source of ROS and controls glutamate release in the prefrontal cortex ([415]; reviewed in [416]). We have also observed increased SOD activity, a decrease in the ratio of oxidized vs. reduced glutathione and an increase in lipid peroxidation in both the striatum and frontal cortex of SIR rats [373]. Importantly, all the latter alterations could be reversed with clozapine treatment [373]. The latter not only emphasizes the validity of these findings but also highlights the ability of contemporary treatments like clozapine to address disturbances in redox balance. Preclinical studies in the SIR model of schizophrenia also confirmed NAC’s utility as adjunctive treatment to an anti-psychotic [137].
Inflammatory Markers
Increasing evidence indicates that inflammation may have a critical role in the pathophysiology of MD, BPD and schizophrenia [386, 417]. Inflammation is also a closely associated phenomenon with oxidative stress, discussed above. Cytokines play a crucial role as signalling molecules in the immune system and have the ability to cross the blood–brain barrier (BBB), granting it both central and peripheral activity [418]. Cytokines have been demonstrated to exert activity in almost every area relevant to the pathophysiology of MD, BPD and psychotic disorders including neurotransmitter metabolism, neuroendocrine function, and neural plasticity [386, 419-421]. The pro-inflammatory cytokines, such as interleukin (IL)-1, tumor necrosis factor (TNF)-α, and IL-6, can inhibit neurogenesis in vivo [422, 423], induce apoptosis [424, 425] and negatively affect synaptogenesis, synaptic plasticity and connectivity, and also the structure of synaptic membranes [426, 427]. On the other hand, anti-inflammatory cytokines such as IL-10 and IL-4 dampen the immune and inflammatory response [421] so that an inflammatory state is generally determined by an imbalance between pro- and anti-inflammatory mediators. Fig. 3 provides an outline of how inflammatory mediators and oxidative stress are related to regulate immune response and redox status.
Fig. (3).
A simplified diagram of the kynurenine pathway, indicating the principle enzymes, IDO and tryptophan-2,3-dioxygenase (TDO), and the subsequent formation of kynurenine and its metabolites from TRP. The diagram indicates the inter-relationship of kynurenine metabolites, particularly QA, kynurenic acid and 3-hydroxyanthranilic acid. This diagram also depicts the activation and inhibition of IDO via pro- and anti-inflammatory cytokines respectively, as well as the influence of oxidative stress processes that will eventually determine cellular resilience or susceptibility to neurotoxic insults. Increased activity of the tryptophan-kynurenine synthesis (by TDO/IDO) will also diminish the availability of tryptophan for serotonin synthesis via tryptophan-5-hydroxylase, with resulting effects on mood and behavior.
Abbreviations: glutathione (GSH); interferon (IFN)-γ; interleukin (IL)-2 and -4; nitric oxide synthase (NOS); reactive oxygen species (ROS); serotonin (5-HT); tumour necrosis factor (TNF)-α. Adapted from [452, 453].
Clinical Correlates
Depression
A strong relationship has been demonstrated between MD and the presence of inflammation and its associated inflammatory mediators [428, 429]. These mediators include the pro-inflammatory cytokines, IL-1, -2, -6 and -8, interferon (IFN)-γ and TNF-α [430] that, when administered to a healthy individual, may induce sickness behaviour [431, 432]. Sickness behaviour describes a state in which many of the symptoms coincide with that of MD [420]. Furthermore, it has been proposed that constant elevation of cytokine levels may lead to neurotransmitter changes which are interpreted by the brain as stressors that further allow these molecules to contribute to the development of MD [429]. Not only do pro-inflammatory cytokines contribute towards altered neurotransmitter metabolism, neuroendocrine function, synaptic plasticity and behaviour characteristic of MD [419, 433-435], but also stimulate hypothalamic-pituitary-adrenal (HPA)-axis hormones as well as CRH in both the hypothalamus and the amygdala. The latter play an important role in fear responses and anxiety-related behaviour [419].
Cytokine-induced MD is associated with alterations in 5-HT metabolism through the activation of IDO [436], as well as alterations in CRH function [430, 436]. Importantly, MD induced by IFN-γ also involves the activation of iNOS [437], the latter known to play an important role in stress-related inflammation [438]. Peripheral levels of IL-1, IL-6 and TNF-α is increased in patients with MD [299, 439], and these effects are normalized following antidepressant treatment [440, 441]. Increased levels of IL-1 and IL-6 have also been measured in the CSF of patients suffering from MD [442]. Furthermore, an elevation in IL-6 levels has been proposed as an early marker for cognitive symptoms and has been found to correspond to the severity of MD as well as increased activity of the HPA axis [430, 443, 444]. Interestingly, IL-6 levels have been demonstrated to be increased in patients with treatment-resistant depression compared to treatment responders [445], which indicates that altered IL-6 levels in the blood of depressed individuals may serve as a marker of possible response to antidepressant treatment [208]. In line with these findings, a recent clinical study found that administration of the non-specific COX inhibitor acetylsalicylic acid leads to an improved onset of action during fluoxetine treatment and also increased the response rate to the drug when compared to patients receiving only fluoxetine [446].
Bipolar Disorder
BPD may be associated with moderately increased plasma levels of pro-inflammatory cytokines, such as IL-6 and TNF-α, along with increased IL-1, and IL-1 receptor antagonist protein, while elevated mRNA levels have been observed in post-mortem frontal cortex of BPD patients [447 -449].
Schizophrenia
Schizophrenia patients and their first-degree relatives have been found to present with significantly elevated pro- vs. anti-inflammatory cytokines [450], as well as a significant elevation in pro-inflammatory cytokines in first episode psychosis patients, with a positive correlation between IL-6 and duration of illness [418]. IL-6 has been found to be significantly increased in early and late stage schizophrenia, with IL-10 reported to decrease in the late stages [451].
The presence of elevated levels of IL-1 in the CSF has been suggested as a marker of acute psychotic relapse, while immune-inflammatory dysfunctions may be implicated in the underlying processes mediating relapse, considering that the relationship between cytokine abnormalities and acute exacerbations of schizophrenia appear not to be related to treatment with antipsychotics. On this point, certain cytokines have been suggested to represent state markers for acute exacerbations of psychosis (see [217] for review). However, a number of studies have described inconsistent effects on plasma/serum cytokines, namely IL-4 [452], [453], IL-6 (reviewed in [454]), IFN-γ [452, 455]; reviewed in [456], and TNF-α (reviewed in [456]). Furthermore, a positive correlation between the severity of cognitive deficits and levels of IL-1β, IL-6 and TNF-α have been described in schizophrenia [252].
Preclinical Correlates
Depression
Acetylsalicylic acid has proved to be an effective augmentation agent to fluoxetine in rats resistant to standard fluoxetine treatment using the CMS animal model of MD [457]. Furthermore, it has been demonstrated in gene-environment animal models of MD that peripheral levels of inflammatory markers and regulators of metabolic pathways, including glucocorticoids, MAPK’s and cytokines are altered in these animals when compared to healthy controls [458].
Bipolar Disorder
Using the ouabain model of BPD, investigators have noted an increased activation of signalling pathways linked to inflammation in the brain [459].
Schizophrenia
Elevated pro-inflammatory cytokines (TNF-α, IFN-γ) and decreased anti-inflammatory (IL-4) and dual-action (IL-6) cytokines has been described in the SIR model, and which are for the most part reversed with clozapine treatment [137]. More interesting is that NAC is able to augment select behavioural, neurochemical and anti-inflammatory responses to clozapine in SIR animals [137]. Other pre-clinical studies have also indicated that IL-6 and TNF-α have direct inhibitory effects on adult hippocampal neurogenesis [422, 460], which may attenuate antidepressant and antipsychotic efficacy by decreasing hippocampal neurogenesis or interfering with the neurogenic properties of these drugs.
Kynurenine Pathway Markers
An important link with the inflammatory cascade is the kynurenine pathway whereby TRP is catabolized to kynurenine via hepatic TDO and via IDO in the central nervous system, lungs and placenta [461] (Fig. 3). Kynurenine is then metabolised to either kynurenic acid (KYNA) or 3-hydroxykynurine (3-OHK) and then to anthranilic acid, 3-hydroxyanthranilic acid (3-OHAA) and QA [462]. Conversion along this highly regulated pathway accounts for the metabolism of approximately 80% of non-protein bound TRP, an essential amino acid necessary for the synthesis of 5-HT (Fig. 3; [463]). QA, a NMDA receptor agonist and excitotoxin, along with
3OHK, a mediator of neuronal apoptosis, and 3-OHAA, a free radical, are all capable of inducing neurodegenerative changes in the brain [462, 464, 465]. KYNA, on the other hand, acts as an antagonist at the facilitatory glycine site on the NMDA receptor ion channel, thus having potential neuroprotective properties [466]. The activation of IDO by pro-inflammatory cytokines (e.g. INF-γ and TNF-α) in the CNS also leads to increased TRP degradation into kynurenine and QA, thereby reducing the bioavailability of TRP for 5-HT synthesis (Fig. 3) (reviewed in [467]). Hence, increased pro-inflammatory and decreased anti-inflammatory actions in the CNS can contribute to central 5-HT deficiency, which plays an important role in the pathogenesis of MD and BPD but also the negative symptoms of schizophrenia [386, 468, 469].
Clinical Correlates
Depression
Previous studies have observed decreased TRP and 5-HT along with increased kynurenine in the peripheral blood of depressed patients receiving the pro-inflammatory cytokine, IFN-γ [470, 471]. Significant decreases in the concentration of KYNA have also been observed in the plasma of depressed individuals [465]. Alterations in the symptoms of depressed patients were significantly positively correlated with kynurenine and negatively correlated with levels of 5-HT [471]. A previous study also indicated the correlation of increased IL-6 production in vitro with decreased TRP levels in depressed patients, emphasizing the influence of IL-6 on 5-HT metabolism via TRP in these patients [444]. A post-mortem study also indicated elevated QA levels in the brain of patients with MD [472]. This supports the hypothesis that the development of depressive symptoms may be mediated by increased TRP metabolism via IDO along the KYN pathway [436, 473].
Bipolar Disorder
Levels of TRP and kynurenine-dependent TRP index have been demonstrated to be diminished in bipolar mania [467]. Furthermore, increased kynurenine was found post-mortem in the ACC in patients with BPD, which corresponded with increased density and intensity of TDO positive glial cells [474].
Schizophrenia
In patients with schizophrenia clinical post-mortem studies found elevated levels of TRP, 3-OHAA, kynurenine and QA in various brain regions [475, 476]. Moreover, individuals suffering from schizophrenia present with increased levels of TRP in the CSF and plasma whether they received treatment or not [477, 478]. Although increases in KYNA levels have been observed in the post-mortem brain tissue of schizophrenia patients who received treatment [218], Myint and colleagues [479] have described a significant reduction in plasma levels of KYNA accompanied by a decrease in the neuroprotective ratio (a measure of the relationship between KYNA and kynurenine levels) in treated and non-treated patients suffering from schizophrenia.
Preclinical Correlates
Depression
In a stress-induced rat model of MD the kynurenine pathway was observed to be activated, leading to increased expression and activity of hepatic TDO as well as its expression in the cortex [480]. This increased TDO activity was associated with elevated circulating kynurenine concentration and a reduction in circulating TRP concentration [480]. Furthermore, induction of depressive and anxiety-like symptoms via the administration of a viral mimetic led to reductions in BDNF signalling and activation of the kynurenine pathway [481]. Recently, the potential for using the kynurenine 3-monooxygenase inhibitor, Ro 61-8048 (to elevate kynurenine levels), as an antidepressant has been suggested ([482], while the TDO inhibitor, allopurinol, has been noted for its antidepressant-like effects in rats [480].
Bipolar Disorder
Current animal models of BPD have not produced data related to the role of the kynurenine pathway, and data is this regard is eagerly awaited.
Schizophrenia
Recent pre-clinical studies in the SIR model of schizophrenia have described elevated plasma TRP, kynurenine, anthranilic acid, 3-OHAA and QA with reduced KYNA and neuroprotective ratio, with all alterations reversed with the antipsychotic, clozapine [137, 483].
GENETIC MARKERS
Full remission of a psychiatric illness is often impeded by variability in an individual’s response to psychoactive drugs, so that being able to predict responses to psychotropic drugs holds great promise in improving treatment outcome. Genotyping, where proteins involved in neurotransmission and cellular signalling specific for illness pathology and drug response are identified and targeted, will allow the selection of an appropriate psychopharmacologic agent to best suit an individual’s pathologic and metabolic characteristics.
Clinical Correlates
Depression
The development of MD has been demonstrated to be critically influenced by genetic factors which provide the opportunity to investigate mechanisms underlying the disorder [13]. Polymorphisms on genes encoding the 5-HT transporter, 5-HT2A-receptor, BDNF, and tryptophan hydroxylase have been identified as candidate genes implicated in the pathology of MD [484], while several studies have suggested the Met/Met genotype (of the gene coding for COMT) to be predictive of antidepressant response rates [485, 486]. The Val/Met polymorphism in MD has not been consistent and has not been linked to any single effect [487]. Nevertheless, although contradictory findings have been presented, research into polymorphisms on the COMT variant may still hold promise [488].
Bipolar Disorder
Efforts to identify possible genetic markers and isolation of genes implicated in the pathogenesis of BPD have been challenging and at times contradictory [489]. Nevertheless, a recent study by Mahon et al. [490] suggested that abnormalities in lower white matter in the temporal lobe might be a marker for genetic risk of BPD. It has been suggested that the presence of an unknown gene on chromosome 12q22-q23.2 may predispose especially men to develop MD and perhaps even BPD [491]. Various studies have explored a possible link between variations in tryptophan hydroxylase II (TPH2) [492, 493] and the development of BPD, although its link with schizophrenia has been dismissed [494]. As in MD, the Val/Met polymorphism in BPD has not been consistent and has not been linked to any single effect [487].
Schizophrenia
Although the heritability for schizophrenia is estimated to be as high as 70%, the illness clearly does not have a pattern of inheritance in any population or even in single families that is consistent with the effect of a single gene [14]. Moreover, according to Riley and Kendler [495], genetics is not a determinant of schizophrenia but rather a means by which the disease is mediated. It is therefore necessary for several genes to interact and be influenced by environmental factors to lead to a patient presenting with the array of symptoms associated with schizophrenia. Like in MD and BPD, the Val/Met polymorphism is involved in predisposition to the development of schizophrenia [487] but further work is needed. The introduction of the SchizophreniaGene (SzGene) database by Allen et al. has made a substantial contribution to schizophrenia genomics [496]. Using the SzGene database, Sun et al. [497] described a collection of highly ranked genes that may be utilized as a working blueprint in the future. These genes, amongst others, include:
Disrupted in Schizophrenia 1(DISC1). A gene that encodes a protein that has been implicated in neurite outgrowth and cortical development by interacting with other proteins.
Dystrobrevin Binding Protein 1 (DTNBP1). A gene that encodes a protein purported to influence organelle biogenesis associated with melanosomes, platelet dense granules, and lysosomes.
Catechol-O-Methyl Transferase (COMT). A catabolic enzyme involved in the degeneration of various molecules that are biologically active, including DA.
D-Amino acid Oxidase (DAO). This is a NMDA-receptor-mediated signalling gene.
Regulator of G Protein Signalling 4 (RGS4). This is a regulatory molecule that acts as a GTPase activating protein.
Neuregulin 1 (NRG1). This is a protein essential for normal development and function of the nervous system (reviewed in [498]).
Metabotropic Glutamate Receptor 3 (GRM3) gene. This gene, coding for the GRM3 glutamate receptor, is linked to inhibition of the cAMP cascade and has been associated with susceptibility to develop mood disorders.
Another possibly important genetic marker, and that links with oxidative stress, is NOS as discussed earlier. Weber et al. [499] indicated that a genetic variance in nitric oxide synthase NOS1 results in a reduction in the expression of the gene in the prefrontal brain region which adds to schizophrenia burden, and that NOS1 interacts with NOS1 adapter protein (NOS1AP) in doing so. The interaction observed in NOS1, NOS1AP and the PDZ binding domain may therefore establish a novel drug target for treating schizophrenia [499].
Preclinical Correlates
Depression
mRNA expression of neuropeptide Y have been demonstrated to be decreased in various brain regions of FSL rats [500, 501] while basal mRNA levels of several genes involved in the synthesis of neurotransmitters are similarly altered. These include tyrosine hydroxylase (TH), DA β-hydroxylase, phenylethanolamine N-methyltransferase (PNMT) and GTP cyclohydrolase I – all of which were elevated in FSL rats [502]. Also, protein expression of BDNF, CREB and Bcl-2 were reduced in rats after exposure to CMS [503].
Bipolar Disorder
The lack of viable genetic animal models of BPD has left a void in the search for genetic markers at pre-clinical level. However, black Swiss mice (a genetic model of mania) present with increased mRNA expression of β-catenin in the hippocampus, as opposed to other brain regions [363], while increased CSF levels of S100B (a neuronal trophic factor released by astrocytes) has been observed in the ouabain-induced model of mania [504]. Furthermore, transgenic mice expressing increased S100B have been demonstrated to be hyperactive [505].
Schizophrenia
The SIR model has shown increased expression of metabotropic glutamate receptor (mGluR)6 and ionotropic AMPA3 receptor subunit genes in the PFC [361], as well as reduced mGluR1 and mGluR5 expression [506]. These findings are consistent with the proposal that dysregulation of glutamatergic activity may contribute to the behavioural/cognitive deficits associated with SIR [64]. Furthermore, the PCP model produces up regulation of genes coding for frontal cortical NMDA receptors and produces differential expression of frontal cortical genes coding for BDNF [252]. Another clear indication of the involvement of genetic markers in schizophrenia is the numerous genetic animal models with validity for schizophrenia, such as: the DISC1 knockout (KO) model, the DA transporter KO model, the Homer1a KO model, insulin receptor KO model and the mGluR 1 and 5 KO models (reviewed in [507]).
PROTEOMIC MARKERS
The proteome is the entire collection of “proteins encoded by the genome of an organism at a specific point in time, incorporating the set of isoforms, posttranslational modifications, covalent structures and complex protein-protein interactions present therein” [508]. Proteomics provides an insight into the character and interactions of proteins and thus of signalling pathways – understanding a proteome allows for development of effective predictive biomarkers [509].
Clinical Correlates
Depression
Ditzen et al. [510] observed several aspects in the CSF which were significantly altered in depressed patients when compared to controls, including 11 proteins and 144 peptide features. A recent large proteomic study in MD and schizophrenia patients observed that insulin was the marker with the highest statistical significance in MD patients compared to controls [2]. Their findings are consistent with the observation that MD is frequently linked with insulin resistance [511]. The increased comorbidity between type 2 diabetes and MD [512] and the strong link between MD and metabolic syndrome [513] further supports this hypothesis. Moreover, increased levels of chromogranin A, a secretory protein and precursor for functional peptides, are linked to dysregulated insulin levels in patients with schizophrenia [514].
Bipolar Disorder
Novikova et al. [515] identified several protein biomarkers which may possibly be involved in the pathology of BPD, e.g. MB-18.5, CBF2, DECR2, BYSL, ANKARD12, ALDOC and DKK2 (part of the Wnt signalling cascade) [516]. The Wnt cascade is a group of signal transduction pathways comprising proteins that pass signals from outside to inside of a cell through cell surface receptors. These proteins appear to be compromised in patients with BPD [517]. Importantly, these proteins interact with GSK3-B, BDNF, oxidative stress mediators and cytokines relevant to BPD [349].
Schizophrenia
Cyclophilin A is a protein biomarker that plays an important role in cerebral cortical plasticity [518]. Differing levels of this protein has been noted postmortem in patients with schizophrenia and BPD, suggesting contradictory influences on plasticity specifically in the DLPFC in these diseases [515]. We have earlier noted the importance of neurotrophins like BDNF and VEGF in the neurobiology of psychiatric disorders. Proteomic studies [2] have also observed significant differences in various growth factors and neurotrophins in patients with schizophrenia, influencing somatic and dendritic growth in the hippocampus and prefrontal cortex, such as BDNF, VEGF or stem cell factor. To a lesser extent, this may include members of the chemokine/cytokine family. In line with these findings, previous studies have indeed observed decreased VEGF in serum of patients with schizophrenia [259, 519], although this has not always been reproducible [520]. The current body of evidence relating to BDNF in schizophrenia provides little congruence, with increased, decreased, or no change in serum or plasma BDNF levels noted [259, 521]. Another study in schizophrenia patients identified distinctive profiles of peptides and proteins in the CSF [522] that are potentially specific for schizophrenia, including a VEGF-derived peptide sequence consisting of 40 amino acids, a transthyretin protein cluster (a serum and CSF carrier of the thyroid hormone thyroxin), and another smaller protein cluster also associated with transthyretin, with 95% specificity and 80 to 88% sensitivity [522]. Recent studies also observed that serum concentrations of insulin and chromogranin A were increased in schizophrenia patients [514, 523].
Preclinical Correlates
Depression
Yang et al. [524] recently published proteomic data from the CMS rat model of MD, noting decreases in glyoxalase-1 and dihydropyrimidinase-related protein-2 (DRP-2) in the prefrontal cortex, which translates to alterations in energy and glutathione pathways. Carboni and colleagues [458] detected significantly increased levels of leptin, IL-1α and BDNF in FSL compared to FRL rats.
Bipolar Disorder
To the best of our knowledge there are no current proteomic data on animal models of BPD – this may be due to the lack of comprehensive animal models presenting with cyclic behaviour ranging from depressive to manic.
Schizophrenia
Proteomic studies in preclinical models of schizophrenia are limited. One study, however, describes the prohibitin protein, a potential marker of synaptic pathology, to be up-regulated in chronic schizophrenia patients as well as in the ketamine animal model of schizophrenia [525]. Another proteomics study describes the neuroprotective action of abrogated COX-II expression in insulin receptor KO mice as a validated animal model of schizophrenia [526]. Using the PCP animal model to access frontal cortical levels of chromogranin A, B and secretoneurin (large protein molecules of the chromogranin family), acute PCP treatment caused a decrease in secretoneurin, while chronic PCP treatment elevated this protein [527].
Micro-RNAs
Clinical Correlates
MicroRNAs (miRNAs), a large family of small non-coding RNAs, are potent regulators of gene expression with proposed roles in brain development and function [528]. These miRNAs may play a substantial role in the pathophysiology of mood and psychotic disorders and may even have an influence on the effect of drugs used to treat these disorders [529].
Depression
Bocchio-Chiavetto et al. [529] found 28 miRNAs to be up-regulated and 2 miRNAs to be down-regulated following antidepressant treatment – they further demonstrated that these differentiations could be associated with alteration of neuronal pathways and may be involved in the underlying mechanisms of MD.
Schizophrenia and BPD
Perkins et al. [530] observed that from 264 human miRNAs, 16 were differentially expressed, 15 were expressed at lower levels and 1 at a higher level in post mortem prefrontal cortex tissue of schizophrenia patients vs. controls. Similarly, post-mortem studies in schizophrenia or BPD found underexpression of several miRNAs of the adult prefrontal cortex [528], while in a study evaluating 667 miRNAs in postmortem prefrontal cortex tissue of schizophrenia and BPD patients, 22 miRNAs were found differentially expressed between cases and controls, 7 deregulated in schizophrenia and 15 in BPD [531]. Furthermore, these 22 miRNAs were found to target brain specific genes involved in neurodevelopment, behaviour as well as the development of schizophrenia and BPD [531]. It has also been observed that a considerable modification to post-transcriptational regulation may be defined by an overall increase in miRNA expression in two regions of the cerebral cortex in post-mortem brain tissue from patients suffering from schizophrenia [532].
These studies highlight miRNAs as possible biomarkers for mood and psychotic disorders, although it is clear that further research is needed to evaluate the relationship between miRNA alterations and disease development and progression. The fact that altered expression of miRNAs is reflected also in the blood of patients suffering from mood disorders potentially give rise to the idea that peripheral miRNAs may be screened as an aid in the diagnosis and treatment of these disorders. However, as with most peripheral biomarkers, the correlation between central and peripheral expression of miRNAs remains a topic of debate [533, 534].
Pre-Clinical Correlates
By referring to selected animal models of depression, recent reviews have evaluated the molecular biology of miRNAs in relation to the pathophysiology of clinical depression as well as the utility of targeting miRNAs for antidepressant treatment [533, 535]. They confirm the dysregulation of a large number of miRNAs in these depression models. Moreover, miRNAs to some extent may be associated with treatment and onset of BPD and schizophrenia. The reader is advised to consult these papers for further detail.
DEVELOPING A BIOMARKER PANEL
To date the diagnosis and treatment of patients suffering from mood and psychotic disorders have almost exclusively been based on behavioural symptomatology observed in these individuals, with laboratory testing and confirmation of diagnosis being absent due to the diverse aetiologies and underlying neurochemical abnormalities associated with these disorders. However, in recent years several biological markers have been linked to neuronal changes associated with the pathological processes and/or treatment response in these disorders. Being able to identify discrepancies in these markers in humans and using them in the diagnosis and treatment of mood and psychotic disorders will surely improve treatment efficacy and potentially even allow for provisional measures to be taken to counter neuronal deficits and prevent the onset and/or progression of symptoms and structural brain changes associated with these disorders. Quantifying the underlying abnormalities in these disorders may also be helpful in understanding the pathogenesis of these disorders and mechanisms causing the delayed response to drugs observed in their treatment. But then again, as mentioned in the introduction the likelihood of identifying any single biomarker with sensitivity and specificity for MD, BPD and schizophrenia is relatively low so that a feasible alternative to the single-biomarker approach could be the development of biomarker panels.
From the data discussed in this review, we have attempted to distinguish between biomarkers that provide mostly substantial evidence in support of them being a strong or moderate biomarker of either MD, BPD of schizophrenia, as opposed to markers where there is less of an evidence base to link it to the neuroanatomy or pathophysiology of these disorders. Furthermore, we distinguish between markers observed in clinical vs preclinical studies in order to highlight affirmation as well as the possibility/importance of further investigation. Table 1 describes a putative biomarker panel based on the clinical and preclinical data described in this article, where strong, moderate and weak candidate biomarkers are color coded for easy identification and interpetation with the text.
Table 1.
A putative biomarker panel, based on clinical and pre-clinical data for depression, bipolar disorder and schizophrenia.
Altered endocrine responses, indicated in the 2nd row of Table 1, are typical of a number of these illnesses, and can in many instances be related to altered circadian rhythm. Moreover, such changes, for example cortisol, can be instrumental in driving many of the behavioural and pathological changes evident in these disorders, eg. hyper-glutamatergia, structural brain changes, etc. Furthermore, the recent introduction of the 5-HT2c antagonist/M1/2 agonist, agomelatine, as the first antidepressant acting to re-establish altered circadian rhythms provides robust validation of the importance of these processes in the development and treatment of depression [78]. Moreover, its actions on frontal cortical DA function hints of possible value in the treatment of schizophrenia.
Regarding the neuroanatomy and neurocircuitry of MD, BPD and schizophrenia, it is evident (and quite expected) that there are distinct alterations in the volume of certain brain structures in mood and psychotic disorders, highlighting the importance of altered neuroplasticity and structural brain changes in these illnesses, indicated in the 1st row of Table 1. Decreased volumes have been recorded for various limbic structures in both MD and schizophrenia, specifically the prefrontal cortex. Although several observations have been made in BPD, current data is limited or of little clinical value and further research into the neuroanatomy of this disorder is necessary. Overall, there remains speculation as to whether the above-described neuroplastic changes are cause or effect.
Neuroimaging studies, indicated in the 1st row of Table 1, have also given insight into altered neurocircuitry, blood flow and metabolic rate in affected brain structures in these disorders and largely parallel the neurocircuitry described above. Of importance is the decreased activity in cerebellar, prefrontal, frontal and cortical structures in clinical studies of schizophrenia as well as decreases in cerebral glucose metabolism in both preclinical and clinical studies of BPD and alterations in NAA in MD and schizophrenia.
Current pharmacotherapy of MD, BPD and schizophrenia in many ways provides robust construct and predictive validity for the importance of monoamines as biomarkers for these illnesses. At the neurochemical level, indicated in the 3rd row of Table 1, patients with MD present with a decrease in DA, 5-HIAA, NA and MHPG; BPD patients present with an increase in DA, associated with manic symptoms, and a decrease in 5-HIAA; schizophrenia patients present with an increase and decrease in DA in the striatum and prefrontal cortex respectively, along with increased 5-HT transmission and increased NA levels. Decreased levels of DA have been correlated with anhedonic behaviour in MD in both clinical and preclinical studies. Increases in DA as well as NA have, however, been linked to manic symptoms of BPD and models of mania as well as clinical and preclinical studies of schizophrenia. Glutamate on the other hand is increased peripherally and in the cortex of MD and BPD patients, is reversed with ketamine treatment, while in un-medicated schizophrenia patients the literature indicates an increase of glutamate and GABA in post-mortem prefrontal cortex and striatum tissue. However, as noted earlier, schizophrenia is proposed to involve cortical hypoglutamatergia that in turn drives meso-limbic hyperdopaminergia and meso-cortical hypodopaminergia. Decreases in the level of 5HIAA, a 5-HT metabolite, have also been noted in both MD and BPD patients suffering from depressive symptoms, while 5HT transmission is increased in patients suffering from schizophrenia.
With regards to the growth factors, indicated in the 4th row of Table 1, the current literature supports a general decrease in BDNF in MD, BPD and schizophrenia; none to limited data of IGF-1 in MD, no changes of IGF-1 in BPD and decreased IGF-1 in schizophrenia; VEGF was increased in MD and BPD, but mRNA of VEGF was decreased in schizophrenia. While VEGF studies in humans report conflicting results and with preclinical data still lacking, it may still hold promise as a biomarker of psychiatric/mood disorders – especially considering the divergent results seen in MD vs schizophrenia patients. Recently published meta-analyses on BDNF are emphatic that altered serum BDNF is a state marker in MD, BPD and schizophrenia [536], although peripheral BDNF levels are not a sufficient measure of disease severity in MD [337] neither does it adequately discriminate between MD, BPD and schizophrenia. Animal studies tend to echo these sentiments, although serum BDNF levels may act as marker of predisposition to develop symptoms in rats used to model these disorders [306, 307]. Interesting enough, it does differentiate between mood states in BPD [336] and between acute and remitted states in MD [536]. In schizophrenia, a significant positive correlation between BDNF levels and positive and negative syndrome scale (PANSS) positive subscore has been described, as well as higher BDNF levels in the paranoid subtype of schizophrenia [537]. Low BDNF levels at the onset of psychosis may therefore contribute to the pathogenesis of schizophrenia and could perhaps be a candidate biological marker for positive symptoms. It may also play an important role as a marker of disease progression in BPD due to an observed association between peripheral BDNF levels and age and duration of illness [336, 538].
Markers of neuronal resilience, indicated in the 5th row of Table 1, have aroused considerable interest and play an important role in programmed cell death and plasticity. GSK-3 has surfaced as a possible marker in BPD via its regulation of several biochemical pathways, including the Wnt pathway. It has also been connected to the effects of lithium treatment in BPD. Decreased cAMP and activity of the MAPK/ERK pathway has been demonstrated in both humans and animal models of MD and may serve as a valuable biomarker in the disorder. However, data in BPD and schizophrenia is less clear. Increases in MAPK have been noted in animal models of schizophrenia but need further investigation in a clinical setting as well.
Numerous findings in MD, BPD and schizophrenia patients indicate the presence of a prooxidative state, indicated in the 6th row of Table 1. However, elevations in SOD and lipid peroxidation are relatively non-specific, although BPD patients present with an increase and decrease in catalase in manic and euthymic symptoms respectively, making catalase a possible specific marker in the latter disorder. Furthermore, schizophrenia and BPD patients present with decreased levels of GSH [539]. Importantly, studies in translational animal models have provided evidence that the associated oxidative stress occurring with MD-related behaviors can be reversed by antidepressant treatment [400] and that the antioxidant and glutathione precursor, NAC, is antidepressant in rats [399]. Moreover, exacerbated levels of oxidative stress can attenuate antidepressant action [400]. However, preliminary clinical studies have only been able to provide limited support for the use of NAC as an adjunctive therapy for MD [379]. Similar studies in humans and animals have described reversal of redox changes by lithium and/or antipsychotics in BPD [119, 135] and schizophrenia [137, 373, 387, 395], respectively. This review is adamant that both oxidant and antioxidant systems and redox balance play a pathophysiological role in MD, BPD and psychotic disorders such as schizophrenia. This realisation has opened the door to the possible clinical utility of antioxidant drugs (e.g. NAC) in the treatment of these illnesses alone and as an adjunctive treatment [12, 118, 540].
As has been noted, inflammation and oxidative stress are closely linked. Significant increases in the levels of pro-inflammatory cytokines have been reported in humans and animal models of MD, BPD and schizophrenia, indicated in the 7th row of Table 1. Along with the above-mentioned oxidative stress observations is the evidence that MD, BPD and schizophrenia patients present with an elevation in pro-inflammatory cytokines (IL-1, 6, IFN-γ and TNF-α), that is reversed with antidepressant treatment in MD. A recent animal study in the SIR model of schizophrenia indicates that clozapine and NAC can also reverse this pro-inflammatory state [137]. This review suggests that the presence of a pro-inflammatory state is a non-specific pathological marker for MD, BPD and schizophrenia yet underscores the pathological role of inflammation in these disorders, especially give their reversal by typical drug treatment. Furthermore, anti-inflammatory cytokines have been found to be decreased in both humans and animal modeIs of schizophrenia. However it remains unclear whether activation of inflammatory pathways in the CNS during MD, BPD and psychotic disorders is rooted in the periphery (e.g., as a function of overt or nascent medical illness or psychological stress) and/or whether stress or other yet to be identified processes (e.g., vascular insults in late life MD/psychosis) induce inflammatory responses directly within the brain. Strong evidence supports a prenatal inflammatory event as a prodromal event to the development of schizophrenia [541]. Prenatal immune challenge with either a systemic endotoxin or viral mimic vs. an inducer of local inflammation suggests that neurodevelopment of the fetus may rather be affected by circulating cytokines and/or fever as opposed to direct effects evoked on the fetus by the agent responsible for maternal infection [542]. What is evident from work in animals is that these pathological processes seem to have their origin in a disturbance in the mitochondria [179], and explains why redox dysfunction is such a central feature of these illnesses [373]. Cytokine activity may elicit several effects on the brain, affecting the synthesis, release and reuptake of several neurotransmitters, including monamines, which have the ability to influence mood [417].
An interesting observation is that MD and BPD patients present with a decrease in TRP along with an increase in kynurenine and QA levels, while schizophrenia patients (corroborated by animal studies using the SIR model [137]), indicates an increase in TRP, 3OHAA, kynurenine and QA along with a decrease in KYNA [475, 476, 479]. These findings are indicated in the 8th row of Table 1. The decrease in TRP could therefore possibly be specific markers for MD and BPD, with the increase in TRP along with the decrease in KYNA specific to schizophrenia. The relative induction of KYNA versus QA may determine the effects of cytokines on the CNS and remains an important area for future investigation, including the therapeutic targeting of IDO and kynurenine enzymatic pathways in MD, BPD and schizophrenia [465, 467, 475].
Even though these disorders are known to be hereditary to some extent, the exact genetic basis still needs further illucidation, indicated in the 9th row of Table 1. Val/Met polymorphisms have been studied in MD, BPD and schizophrenia and even though various reports have been made, data remains inconclusive. Polymorphisms in 5-HT transporters and receptors, as well as BDNF and tryptophan hydroxylase hold promise in MD and warrants further research to pinpointing exact genetic markers involved in the development of MD [484]. DISC1 is a well researched candidate gene for schizophrenia and affective disorders with a range of functions relating to neurodevelopment, although studies into its role in these disorders remains promising albeit conflicting [543]. Gene variations in the SzGene database [496] also hold promise in schizophrenia research and also needs further research to clarify predisposition in developing schizophrenia. Tracking genetic variants in patient blood may therefore serve to compliment biomarker panels by providing more information relating genotypes to MD, BPD and psychotic disorders and their respective treatment responses.
With regards to proteomic markers, indicated in the 10th row of Table 1, it is clear that utilizing modern proteomic techniques, especially mass spectrometric approaches, may support attempts to understand the biochemical processes that accompany psychiatric disorders and may in turn lead to the development of diagnostics and better therapeutics. In MD, abnormalities in insulin secretion has been observed [511] and the disorder is also accompanied by decreased levels of glyoxalase-1 and dihydropyrimidinase-related protein-2 and increased leptin, IL-1 and BDNF protein levels in animal models [458, 524]. This supports the hypothesis for a shared etiopathology with an inflammatory underbuild in patients with co-morbid MD and metabolic syndrome and/or type II diabetes mellitus, which highlights the proposed utility of the PPARγ-pathway in the treatment of MD [544]. Increased insulin levels have also been reported in patients suffering from schizophrenia and may be accompanied by increases in cyclophillin A [518], suggesting increased support for the role of inflammation in the disorder with cyclophillin A already being linked to a variety of disorders with an inflammatory component – amongs others, type II diabetes [545]. Morever, these findings may relate to the confounding observation of weight gain and metabolic syndrome in this disorder, and that may be worsened by certain antipsychotic drugs [546]. Various proteins involved in the WNT cascade may possibly serve as proteomic biomarkers of BPD which may lead to these markers aiding in the diagnosis and treatment of the disorder and therefore warrants further investigation. Thus, screening peripheral compartments, such as serum and CSF, in patients and controls for altered expression of proteins and metabolites known to be involved in the pathophysiology of the disease or associated with comorbid states could serve in developing a “fingerprint” for identifying persons at risk of developing MD, BPD or schizophrenia. However, it is critical that we bring together knowledge on the biology of these illnesses, co-morbid states, illness severity and treatment resistance to enable proteomic markers to realize this potential.
Finally, studies suggest that micro-mRNAs, indicated in the 11th row of Table 1, may play a valuable role as a biomarker in the diagnosis and treatment of mood and psychotic disorders, however further reseach is warranted and the relation between central and peripheral expression still needs elucidation.
This review has focussed primarily on suitable disease-specific biomarkers with especially predictive validity. However, we have on occasion made reference in the aforegoing sections to physiological markers. It is maybe encumbent to mention that these markers are gaining in interest, with some recently been found to have value in predicting treatment response. Thus for example, we earlier described that clinical response to antidepressants can be predicted by assessing activity in the rostral ACC region via electroencephalography (EEG) [37]. Similarly, we noted that by studying P304 wave amplitude, EEG can be used to assess decision making in BPD and schizophrenia patients [49]. In a study based on the “disconnection hypothesis” of schizophrenia [547], and with accumulating evidence of abnormal functional connectivity in schizophrenia, Takahashi and colleagues argued that neurophysiologic signals may provide a retrospective window with which to view disordered neural dynamics in schizophrenia [548]. Using a novel entropy-based approach for measuring dynamical complexity in physiological systems, they observed abnormal dynamical EEG signal complexity in anterior brain areas in schizophrenia that normalized selectively in fronto-central areas following antipsychotic treatment. This approach has also been proven successful in MD [488]. Another promising neurophysiological marker is electroretinography (ERG), a specialized measure of retinal function, which has been studied in schizophrenia and BPD [549]. ERG abnormalities may reflect altered phospholipid metabolism and/or impaired dopaminergic transmission. With all patients receiving stable psychotropic medications at least for 2 weeks before the first assessment, the authors found that retinal dysfunctions are specific for schizophrenia, as compared with BPD, and are confined to the acute stage of the illness. Another potential physiological marker is event-related-potentials (ERPs) (voltage fluctuations in an EEG depicting neural activity), which are specific for cognitive dysfunction in schizophrenia [550] and also studied in MD [551]. Although physiology markers have not been as extensively studied as biological markers, there is a literature describing their use in animals, such as employing EEG and related markers in translational animal models of MD [552] and schizophrenia [553]. For example, blind-drunk (Bdr) mice demonstrate fragmented rest and activity rhythms under a light/dark cycle, reminiscent of altered sleeping patterns in schizophrenic patients [83], while depriving animals of REM sleep, which can be studied as an EEG-related marker, has been suggested to model mania [82].
DISCUSSION AND CONCLUSION
The search for blood biomarkers can essentially be divided into screening putative markers inferred by our current knowledge of the given illness, e.g. BDNF, CREB etc, or through exploring candidate pathways through the use of “omic” procedures, such as proteomic or transciptomic profiling that offer an unbiased view of these pathways. Either approach has its own set of advantages and disadvantages, yet studies deploying either still lack the critical requirement of reproducibility and selectivity [13]. Another important challenge in identifying possible biomarkers is the predictive efficacy of a specific biomarker in the treatment of MD, BPD and schizophrenia. Very few biomarkers of these illnesses have shown utility in regards to predictive efficacy following drug treatment. Having this in hand offers the possibility of introducing tailored pharmacotherapy. Furthermore, demonstrating a dose-dependent response of these markers under the conditions of treatment may aid in more accurately establishing an appropriate dose selection during clinical trials, thereby optimizing drug discovery and development. Knowing this brings the focus of future research to a more optimized translational approach [554]. Ideally, questions from a clinical situation should be translated into a valid animal model, where such animal data can be integrated with patients’ data in order to identify predictive biomarkers. Thereafter extensive validation should be performed on these biomarkers before diagnostic kits with predictive value can be developed and marketed [554]. In the end, the final requirement is that the aforegoing process should allow clinicians to make evidence-based decisions that will reinforce the decision to treat, with what agent/s, and with a higher likelihood of success than that provided by current approaches. However, there are a number of obstacles to overcome before realising a biomarker panel that is sensitive and specific enough to be implemented as a reliable tool for diagnosis and treatment, which include:
difficulties in translating findings observed in animal models to clinical studies and correlating markers measured in animal subjects with those measured in human patients [13, 555];
employing clinical studies in a larger population in order to validate specific findings [13, 555, 556];
attributing measured biomarkers to one specific pathway [2] as measured in a specific disorder, thus ensuring biomarker specificity [13] and the possible presence of underlying comorbid disorders [13, 547];
the complexity of underlying pathophysiologic and ethiologic origins of these disorders combined with demographics [556], and interpatient variables, eg. smoking [2];
correlating data from observations made in different locations and utilizing different sampling techniques [2];
accounting for disease state [13, 556] and previous/current drug therapy [2, 557];
correlating levels of markers measured in different tissue samples, eg. plasma vs CSF [2, 13];
the influence of the time at which biomarkers are measured, eg. the influence of circadian rhythm and disease state/progression [2, 558];
and the inability to measure biological markers in brain tissue in live patients due to the invasive nature thereof [559].
Nevertheless recent studies using “omics” approaches have demonstrated that careful selection of approporiate biomarker panels can provide good separation between diseased and healthy states, as well as predict response to treatment. Two recent studies by Pajer [555] and Redei [556] set out to investigate the validity of potential biomarkers of MD and found that several of these markers may have possible use in discriminating between depressed and non-depressed individuals and may even predict response to therapy. Furthermore Redei et al. found several blood markers identified in animal models of depression to correlate with levels measured in depressed human individuals. Importantly, these studies affirm the approach taken in this review that clinical data and that obtained from validated translational animal models are supportive and should be used together when developing a biomarker panel [555]. Indeed, the latter studies corroborate that genes expressing transcipts belonging to processes related to transcription, neurodevelopment, neurodegeneration and redox are causally related to at least MD [555], which concurs with molecular mechanisms linked to these processes and highlighted in this review. Although metabolomics per se has not been covered in this review as it addresses much the same processes as do genomic and proteomic methods, studying the metabolome has great potential to map potential biomarkers in neuropsychiatric disorders. Indeed, as has been noted, mood and psychotic disorders are linked to a range of disturbances in metabolic pathways, eg. neurotransmitter systems, TRP-kynurenine metabolism, oxidative stress, etc, so that generating a metabolic signature for a specific disorder will aid in metabolic phenotyping and contribute to discovering disease-specific biomarkers as well as predicting treatment response [560].
Based on this review, the ever-increasing availability of new pre-clinical and clinical studies is beginning to forge a way through the neuropathologic compexity of illnesses like MD, schizophrenia and BPD, so much so that we are in a position to portray how altered neuroendocrine, anatomical, neurochemical and other pathologies can be linked to a specific disorder. The role of the endocrine system has long been linked to mood and psychotic disorders with MD patients presenting with increased saliva cortisol as well as HPA-axis activation. Similarly, patients suffering from BPD and schizophrenia have been found to have increased systemic cortisol metabolism. Dysfunction of the hypothalamic-thyroid axis has been demonstrated in all three disorders.
In MD, decreased hippocampal volume as well as reductions in the size of the prefrontal cortex and basal ganglia is accompanied by reduced levels of monoamine neurotransmitters (NA, 5-HT and DA), decreased levels of the 5-HT metabolite, 5-HIAA, and an increase in the levels of glutamate. However, DA has been noted to be increased in BPD and increased in the striatum and decreased in the frontal cortex of both schizophrenic patients and most animal model studies of the disorder. In contrast with MD and BPD, increased 5-HT transmission and NA levels characterize neurotransmission in schizophrenia and is accompanied by decreased NAAG levels in the temporal cortex while increased in the hippocampus as well as reduced activity in various brain regions, including the cerebellar and temporal lobes, prefrontal cortex, cortex and striatum.
Although a variety of observations have been made regarding BDNF, VEGF and IGF, data currently available report conflicting findings. However, hypotheses and data surrounding these markers make a strong case for their involvement in these disorders – whether as a cause or result of underlying pathology. Continued investigation will, more than likely, eventually lead to pinpointing the exact roles of these markers in the pathophysiology and/or progression of mood and psychotic disorders and establish them as valid biomarkers in the diagnosis and/or treatment of MD, BPD and schizophrenia.
Inflammation has emerged as a central role player in the pathophysiology of all three disorders discussed in this review with levels of pro-inflammatory cytokines being observed to be markedly increased in MD, BPD and schizophrenia and a decrease in anti-inflammatory cytokines also contributing to the inflammatory component of schizophrenia. Inflammation is thus not illness specific but a residual marker of ongoing pathology. Increased levels of kynurenine add to the immune response in these disorders with TRP levels being decreased in MD and BPD but increased in schizophrenia. Closely associated with inflammation, nitrosative and oxidative stress deepens the extent of neuronal stress in these disorders – increased lipid peroxidation accompanied by raised levels of SOD are a feature in all three disorders, accompanied by decreased GSH in schizophrenia. The individual inflammatory components that characterize said inflammation in these disorders may in the end prove to be more illness-specific markers. Thus for example, we noted earlier that a decrease in TRP may be specific markers for MD and BPD, while the increase in TRP and decrease in KYNA is more specific to schizophrenia. Similarly, certain components of the inflammatory response such as NO may be pro- or anti-inflammatory depending on the cellular mileux and/or pathways activated (eg see nNOS vs iNOS-mediated pathways in Fig. 1).
The current body of literature features data on a wide variety of possible biomarkers linked to mood and psychotic disorders. To improve diagnostic techniques and treatment strategies, it is of great importance that possible trait and state markers of these disorders are scrutinized to a point where they can be incorporated into an appropriate panel of biomarkers (as presented in Table 1) which may serve as adjunct to current diagnostic criteria. Furthermore, such a panel may assist in treatment strategies being tailored to the unique context in which mood and psychotic disorders present in each individual. In this manner we may move forward from the current “one-size-fits-all” approach to treating an individual to one that addresses the biological processes underlying the disorder and specific for that particular patient.
ACKNOWLEDGEMENTS
Declared none.
AUTHOR DISCLOSURES
Sources of Funding
The authors declare that this work has been funded by the South African Medical Research Council (BHH). The funder has no other role in this study.
AUTHOR CONTRIBUTIONS
BH Harvey devised the concept and lay-out of the manuscript, as well as finalized the pre-submission version of the manuscript. S Brand prepared the first draft as well as the final version of the manuscript; M Möller contributed to the initial and final versions of the manuscript.
CONFLICTS OF INTEREST
The authors declare that over the past three years Brian Harvey has participated in speakers/advisory boards and received honoraria from Servier, and has received research funding from Lundbeck and Servier. The authors declare that, except for income from the primary employer, research funding to BHH from the South African Medical Research Council as well as the honoraria described above, no other financial support or compensation has been received from any individual or corporate entity over the past three years for research or professional services, and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
REFERENCES
- 1.Smith M.J., Barch D.M., Csernansky J.G. Bridging the gap between schizophrenia and psychotic mood disorders: Relating neurocognitive deficits to psychopathology. Schizophr. Res. 2009;107(1):69–75. doi: 10.1016/j.schres.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Domenici E., Willé D.R., Tozzi F., Prokopenko I., Miller S., McKeown A., Brittain C., Rujescu D., Giegling I., Turck C.W., Holsboer F., Bullmore E.T., Middleton L., Merlo-Pich E., Alexander R.C., Muglia P. Plasma protein biomarkers for depression and schizophrenia by multi analyte profiling of case-control collections. PLoS One. 2010;5(2):e9166. doi: 10.1371/journal.pone.0009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams D.R., González H.M., Neighbors H., Nesse R., Abelson J.M., Sweetman J., Jackson J.S. Prevalence and distribution of major depressive disorder in African Americans, Caribbean blacks, and non-Hispanic whites: results from the National Survey of American Life. Arch. Gen. Psychiatry. 2007;64(3):305–315. doi: 10.1001/archpsyc.64.3.305. [DOI] [PubMed] [Google Scholar]
- 4.Warden D., Rush A.J., Trivedi M.H., Fava M., Wisniewski S.R. The STAR*D project results: A comprehensive review of findings. Curr. Psychiatry Rep. 2007;9:449–459. doi: 10.1007/s11920-007-0061-3. [DOI] [PubMed] [Google Scholar]
- 5.Merikangas K.R., Pato M. Recent developments in the epidemiology of bipolar disorder in adults and children: Magnitude, correlates, and future directions. Clin. Psychol. Sci. Pract. 2009;16:121–133. doi: 10.1111/j.1468-2850.2009.01152.x. [DOI] [Google Scholar]
- 6.Belmaker R.H. Bipolar disorder. N. Engl. J. Med. 2004;351(5):476–486. doi: 10.1056/NEJMra035354. [DOI] [PubMed] [Google Scholar]
- 7.Tien A.Y., Eaton W.W. Psychopathologic precursors and sociodemographic risk factors for the schizophrenia syndrome. Arch. Gen. Psychiatry. 1992;49(1):37–46. doi: 10.1001/archpsyc.1992.01820010037005. [DOI] [PubMed] [Google Scholar]
- 8.Jablensky A., Sartorius N., Ernberg G., Anker M., Korten A., Cooper J.E., Day R., Bertelsen A. Schizophrenia: manifestations, incidence and course in different cultures. A World Health Organization ten-country study. Psychol. Med. Monogr. Suppl. 1992;20:1–97. doi: 10.1017/S0264180100000904. [DOI] [PubMed] [Google Scholar]
- 9.Lakhan S.E., Kramer A. Schizophrenia genomics and proteomics: are we any closer to biomarker discovery? Behav. Brain Funct. 2009;5:2. doi: 10.1186/1744-9081-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frances A.J., Widiger T. Psychiatric diagnosis: lessons from the DSM-IV past and cautions for the DSM-5 future. Annu. Rev. Clin. Psychol. 2012;8:109–130. doi: 10.1146/annurev-clinpsy-032511-143102. [DOI] [PubMed] [Google Scholar]
- 11.Connor T.J., Leonard B.E. Handbook of Experimental Pharmacology. Antidepressants. Past, Present and Future; New York: Springer; 2004. [Google Scholar]
- 12.Dean B. Dissecting the Syndrome of Schizophrenia: Progress toward Clinically Useful Biomarkers. Schizophr. Res. Treat.2011. 2011. p. 614730.. [DOI] [PMC free article] [PubMed]
- 13.Schmidt H.D., Shelton R.C., Duman R.S. Functional biomarkers of depression: Diagnosis, treatment, and pathophysiology.3 . 2011. [DOI] [PMC free article] [PubMed]
- 14.Freedman R., Ross R., Leonard S., Myles-Worsley M., Adams C.E., Waldo M., Tregellas J., Martin L., Olincy A., Tanabe J., Kisley M.A., Hunter S., Stevens K.E. Early biomarkers of psychosis. Dialogues Clin. Neurosci. 2005;7(1):17–29. doi: 10.31887/DCNS.2005.7.1/frreedman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atkinson A.J., Colburn W.A., DeGruttola V.G., DeMets D.L., Downing G.J., Hoth D.F., Oates J.A., Peck C.C., Schooley R.T., Spilker B.A., Woodcock J., Zeger S.L., Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001;69(3):89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 16.Ritsner M.S., Gottesman I.I. 2009. [Google Scholar]
- 17.Domenici E., Muglia P. The search for peripheral disease markers in psychiatry by genomic and proteomic approaches. 2007. [DOI] [PubMed]
- 18.Fone K.C.F., Porkess M.V. Behavioural and neurochemical effects of post-weaning social isolation in rodents-Relevance to developmental neuropsychiatric disorders. 2008. [DOI] [PubMed]
- 19.Ishii D., Matsuzawa D., Kanahara N., Matsuda S., Sutoh C., Ohtsuka H., Nakazawa K., Kohno M., Hashimoto K., Iyo M., Shimizu E. Effects of aripiprazole on MK-801-induced prepulse inhibition deficits and mitogen-activated protein kinase signal transduction pathway. Neurosci. Lett. 2010;471(1):53–57. doi: 10.1016/j.neulet.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Papp M., Klimek V., Willner P. Effects of imipramine on serotonergic and beta-adrenergic receptor binding in a realistic animal model of depression. Psychopharmacology (Berl.) 1994;114(2):309–314. doi: 10.1007/BF02244853. [DOI] [PubMed] [Google Scholar]
- 21.Overstreet D.H., Wegener G. The flinders sensitive line rat model of depression--25 years and still producing. Pharmacol. Rev. 2013;65(1):143–155. doi: 10.1124/pr.111.005397. [DOI] [PubMed] [Google Scholar]
- 22.Schneider B., Prvulovic D., Oertel-Knöchel V., Knöchel C., Reinke B., Grexa M., Weber B., Hampel H. Biomarkers for major depression and its delineation from neurodegenerative disorders. Prog. Neurobiol. 2011;95(4):703–717. doi: 10.1016/j.pneurobio.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Savitz J., Drevets W.C. Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neurosci. Biobehav. Rev. 2009;33(5):699–771. doi: 10.1016/j.neubiorev.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell S., Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J. Psychiatry Neurosci. 2004;29(6):417–426. [PMC free article] [PubMed] [Google Scholar]
- 25.Sheline Y.I. Neuroimaging studies of mood disorder effects on the brain. 2003. [DOI] [PubMed]
- 26.Koolschijn P.C., van Haren N.E., Lensvelt-Mulders G.J., Hulshoff Pol H.E., Kahn R.S. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum. Brain Mapp. 2009;30(11):3719–3735. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lorenzetti V., Allen N.B., Fornito A., Yücel M. Structural brain abnormalities in major depressive disorder: a selective review of recent MRI studies. J. Affect. Disord. 2009;117(1-2):1–17. doi: 10.1016/j.jad.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 28.MacQueen G.M., Campbell S., McEwen B.S., Macdonald K., Amano S., Joffe R.T., Nahmias C., Young L.T. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc. Natl. Acad. Sci. USA. 2003;100(3):1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baaré W.F.C., Vinberg M., Knudsen G.M., Paulson O.B., Langkilde A.R., Jernigan T.L., Kessing L. V. Hippocampal volume changes in healthy subjects at risk of unipolar depression. 2010. [DOI] [PubMed]
- 30.Sheline Y.I., Sanghavi M., Mintun M.A., Gado M.H. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J. Neurosci. 1999;19(12):5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neumeister A., Hu X.Z., Luckenbaugh D.A., Schwarz M., Nugent A.C., Bonne O., Herscovitch P., Goldman D., Drevets W.C., Charney D.S. Differential effects of 5-HTTLPR genotypes on the behavioral and neural responses to tryptophan depletion in patients with major depression and controls. Arch. Gen. Psychiatry. 2006;63(9):978–986. doi: 10.1001/archpsyc.63.9.978. [DOI] [PubMed] [Google Scholar]
- 32.Brambilla P., Stanley J.A., Nicoletti M.A., Sassi R.B., Mallinger A.G., Frank E., Kupfer D., Keshavan M. S., Soares J. C. 2005.
- 33.Gruber S., Frey R., Mlynárik V., Stadlbauer A., Heiden A., Kasper S., Kemp G.J., Moser E. Quantification of metabolic differences in the frontal brain of depressive patients and controls obtained by 1H-MRS at 3 Tesla. Invest. Radiol. 2003;38(7):403–408. doi: 10.1097/01.rli.0000073446.43445.20. [DOI] [PubMed] [Google Scholar]
- 34.Frodl T., Jäger M., Smajstrlova I., Born C., Bottlender R., Palladino T., Reiser M., Möller H.J., Meisenzahl E.M. Effect of hippocampal and amygdala volumes on clinical outcomes in major depression: a 3-year prospective magnetic resonance imaging study. J. Psychiatry Neurosci. 2008;33(5):423–430. [PMC free article] [PubMed] [Google Scholar]
- 35.Mayberg H.S., Brannan S.K., Mahurin R.K., Jerabek P.A., Brickman J.S., Tekell J.L., Silva J.A., McGinnis S., Glass T.G., Martin C.C., Fox P.T. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8(4):1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- 36.Vakili K., Pillay S.S., Lafer B., Fava M., Renshaw P.F., Bonello-Cintron C.M., Yurgelun-Todd D.A. Hippocampal volume in primary unipolar major depression: a magnetic resonance imaging study. Biol. Psychiatry. 2000;47(12):1087–1090. doi: 10.1016/S0006-3223(99)00296-6. [DOI] [PubMed] [Google Scholar]
- 37.Korb A.S., Hunter A.M., Cook I.A., Leuchter A.F. Rostral anterior cingulate cortex theta current density and response to antidepressants and placebo in major depression. 2009. [DOI] [PMC free article] [PubMed]
- 38.Gong Q., Wu Q., Scarpazza C., Lui S., Jia Z., Marquand A., Huang X., McGuire P., Mechelli A. Prognostic prediction of therapeutic response in depression using high-field MR imaging. 2011. [DOI] [PubMed]
- 39.Malone D.A., Jr, Dougherty D.D., Rezai A.R., Carpenter L.L., Friehs G.M., Eskandar E.N., Rauch S.L., Rasmussen S.A., Machado A.G., Kubu C.S., Tyrka A.R., Price L.H., Stypulkowski P.H., Giftakis J.E., Rise M.T., Malloy P.F., Salloway S.P., Greenberg B.D. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol. Psychiatry. 2009;65(4):267–275. doi: 10.1016/j.biopsych.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pizzagalli D.A. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36(1):183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Altshuler L.L., Bartzokis G., Grieder T., Curran J., Jimenez T., Leight K., Wilkins J., Gerner R., Mintz J. An MRI study of temporal lobe structures in men with bipolar disorder or schizophrenia. Biol. Psychiatry. 2000;48(2):147–162. doi: 10.1016/S0006-3223(00)00836-2. [DOI] [PubMed] [Google Scholar]
- 42.López-Larson M.P., DelBello M.P., Zimmerman M.E., Schwiers M.L., Strakowski S.M. Regional prefrontal gray and white matter abnormalities in bipolar disorder. Biol. Psychiatry. 2002;52(2):93–100. doi: 10.1016/S0006-3223(02)01350-1. [DOI] [PubMed] [Google Scholar]
- 43.Ketter T.A., Kimbrell T.A., George M.S., Dunn R.T., Speer A.M., Benson B.E., Willis M.W., Danielson A., Frye M.A., Herscovitch P., Post R.M. Effects of mood and subtype on cerebral glucose metabolism in treatment-resistant bipolar disorder. Biol. Psychiatry. 2001;49(2):97–109. doi: 10.1016/S0006-3223(00)00975-6. [DOI] [PubMed] [Google Scholar]
- 44.Houenou J., Frommberger J., Carde S., Glasbrenner M., Diener C., Leboyer M., Wessa M. 2011. [DOI] [PubMed]
- 45.Banati R., Hickie I.B. Therapeutic signposts: using biomarkers to guide better treatment of schizophrenia and other psychotic disorders. Med. J. Aust. 2009;190(4) Suppl.:S26–S32. doi: 10.5694/j.1326-5377.2009.tb02371.x. [DOI] [PubMed] [Google Scholar]
- 46.Baldaçara L., Borgio J.G., Lacerda A.L., Jackowski A.P. Cerebellum and psychiatric disorders. Rev. Bras. Psiquiatr. 2008;30(3):281–289. doi: 10.1590/S1516-44462008000300016. [DOI] [PubMed] [Google Scholar]
- 47.Farrow T.F.D., Whitford T.J., Williams L.M., Gomes L., Harris A.W.F. Diagnosis-related regional gray matter loss over two years in first episode schizophrenia and bipolar disorder. 2005. [DOI] [PubMed]
- 48.Kiehl K.A., Stevens M.C., Celone K., Kurtz M., Krystal J.H. Abnormal hemodynamics in schizophrenia during an auditory oddball task. Biol. Psychiatry. 2005;57(9):1029–1040. doi: 10.1016/j.biopsych.2005.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Donnell B.F., Vohs J.L., Hetrick W.P., Carroll C.A., Shekhar A. Auditory event-related potential abnormalities in bipolar disorder and schizophrenia. Int. J. Psychophysiol. 2004;53(1):45–55. doi: 10.1016/j.ijpsycho.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 50.Strakowski S.M., DelBello M.P., Sax K.W., Zimmerman M.E., Shear P.K., Hawkins J.M., Larson E.R. Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Arch. Gen. Psychiatry. 1999;56(3):254–260. doi: 10.1001/archpsyc.56.3.254. [DOI] [PubMed] [Google Scholar]
- 51.Davidson L.L., Heinrichs R.W. Quantification of frontal and temporal lobe brain-imaging findings in schizophrenia: a meta-analysis. Psychiatry Res. 2003;122(2):69–87. doi: 10.1016/S0925-4927(02)00118-X. [DOI] [PubMed] [Google Scholar]
- 52.Fusar-Poli P., Broome M.R., Matthiasson P., Williams S.C., Brammer M., McGuire P.K. Effects of acute antipsychotic treatment on brain activation in first episode psychosis: an fMRI study. Eur. Neuropsychopharmacol. 2007;17(6-7):492–500. doi: 10.1016/j.euroneuro.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 53.Whalley H.C., Harris J.C., Lawrie S.M. The neurobiological underpinnings of risk and conversion in relatives of patients with schizophrenia. Int. Rev. Psychiatry. 2007;19(4):383–397. doi: 10.1080/09540260701496869. [DOI] [PubMed] [Google Scholar]
- 54.Morey R.A., Inan S., Mitchell T.V., Perkins D.O., Lieberman J.A., Belger A. Imaging frontostriatal function in ultra-high-risk, early, and chronic schizophrenia during executive processing. Arch. Gen. Psychiatry. 2005;62(3):254–262. doi: 10.1001/archpsyc.62.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pukrop R., Ruhrmann S., Schultze-Lutter F., Bechdolf A., Brockhaus-Dumke A., Klosterkötter J. Neurocognitive indicators for a conversion to psychosis: comparison of patients in a potentially initial prodromal state who did or did not convert to a psychosis. Schizophr. Res. 2007;92(1-3):116–125. doi: 10.1016/j.schres.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 56.Nieman D., Becker H., van de Fliert R., Plat N., Bour L., Koelman H., Klaassen M., Dingemans P., Niessen M., Linszen D. Antisaccade task performance in patients at ultra high risk for developing psychosis. Schizophr. Res. 2007;95(1-3):54–60. doi: 10.1016/j.schres.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 57.Brewer W.J., Wood S.J., McGorry P.D., Francey S.M., Phillips L.J., Yung A.R., Anderson V., Copolov D.L., Singh B., Velakoulis D., Pantelis C. Impairment of olfactory identification ability in individuals at ultra-high risk for psychosis who later develop schizophrenia. Am. J. Psychiatry. 2003;160(10):1790–1794. doi: 10.1176/appi.ajp.160.10.1790. [DOI] [PubMed] [Google Scholar]
- 58.Lencz T., Smith C.W., McLaughlin D., Auther A., Nakayama E., Hovey L., Cornblatt B. A. Generalized and Specific Neurocognitive Deficits in Prodromal Schizophrenia. 2006. [DOI] [PubMed]
- 59.Jessen F., Fingerhut N., Sprinkart A.M., Kühn K.U., Petrovsky N., Maier W., Schild H.H., Block W., Wagner M., Träber F. N-acetylaspartylglutamate (NAAG) and N-acetylaspartate (NAA) in patients with schizophrenia. Schizophr. Bull. 2013;39(1):197–205. doi: 10.1093/schbul/sbr127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen F., Madsen T.M., Wegener G., Nyengaard J.R. Imipramine treatment increases the number of hippocampal synapses and neurons in a genetic animal model of depression. Hippocampus. 2010;20(12):1376–1384. doi: 10.1002/hipo.20718. [DOI] [PubMed] [Google Scholar]
- 61.Hougland M.T., Gao Y., Herman L., Ng C.K., Lei Z. ; Hougland M.T., Gao Y., Herman L., Ng C.K., Lei Z., El-Mallakh R.S. Positron emission tomography with fluorodeoxyglucose-F18 in an animal model of mania. Psychiatry Res. 2008;164(2):166–171. doi: 10.1016/j.pscychresns.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 62.Johnson S.A., Wang J.F., Sun X., McEwen B.S., Chattarji S., Young L.T. Lithium treatment prevents stress-induced dendritic remodeling in the rodent amygdala. Neuroscience. 2009;163(1):34–39. doi: 10.1016/j.neuroscience.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 63.Alquicer G., Morales-Medina J.C., Quirion R., Flores G. Postweaning social isolation enhances morphological changes in the neonatal ventral hippocampal lesion rat model of psychosis. J. Chem. Neuroanat. 2008;35(2):179–187. doi: 10.1016/j.jchemneu.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 64.Jones C.A., Watson D.J., Fone K.C. Animal models of schizophrenia. Br. J. Pharmacol. 2011;164(4):1162–1194. doi: 10.1111/j.1476-5381.2011.01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lewis D.A., Hashimoto T., Volk D.W. Cortical inhibitory neurons and schizophrenia. Nat. Rev. Neurosci. 2005;6(4):312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 66.Lodge D.J., Behrens M.M., Grace A.A. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J. Neurosci. 2009;29(8):2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Comery T.A., Shah R., Greenough W.T. 1995. [DOI] [PubMed]
- 68.Comery T.A., Stamoudis C.X., Irwin S.A., Greenough W.T. Increased density of multiple-head dendritic spines on medium-sized spiny neurons of the striatum in rats reared in a complex environment. Neurobiol. Learn. Mem. 1996;66(2):93–96. doi: 10.1006/nlme.1996.0049. [DOI] [PubMed] [Google Scholar]
- 69.Braun I., Genius J., Grunze H., Bender A., Möller H.J., Rujescu D. Alterations of hippocampal and prefrontal GABAergic interneurons in an animal model of psychosis induced by NMDA receptor antagonism. Schizophr. Res. 2007;97(1-3):254–263. doi: 10.1016/j.schres.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 70.Reynolds L.M., Cochran S.M., Morris B.J., Pratt J.A., Reynolds G.P. Chronic phencyclidine administration induces schizophrenia-like changes in N-acetylaspartate and N-acetylaspartylglutamate in rat brain. Schizophr. Res. 2005;73(2-3):147–152. doi: 10.1016/j.schres.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 71.Harte M.K., Powell S.B., Reynolds L.M., Swerdlow N.R., Geyer M.A., Reynolds G.P. Reduced N-acetylaspartate in the temporal cortex of rats reared in isolation. Biol. Psychiatry. 2004;56(4):296–299. doi: 10.1016/j.biopsych.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 72.Geffken G.R., Ward H.E., Staab J.P., Carmichael S.L., Evans D.L. Psychiatric morbidity in endocrine disorders. Psychiatr. Clin. North Am. 1998;21(2):473–489. doi: 10.1016/S0193-953X(05)70017-4. [DOI] [PubMed] [Google Scholar]
- 73.Souêtre E., Salvati E., Belugou J.L., Pringuey D., Candito M., Krebs B., Ardisson J.L., Darcourt G. Circadian rhythms in depression and recovery: evidence for blunted amplitude as the main chronobiological abnormality. Psychiatry Res. 1989;28(3):263–278. doi: 10.1016/0165-1781(89)90207-2. [DOI] [PubMed] [Google Scholar]
- 74.Doane L.D., Mineka S., Zinbarg R.E., Craske M., Griffith J.W., Adam E.K. Are flatter diurnal cortisol rhythms associated with major depression and anxiety disorders in late adolescence? The role of life stress and daily negative emotion. Dev. Psychopathol. 2013;25(3):629–642. doi: 10.1017/S0954579413000060. [DOI] [PubMed] [Google Scholar]
- 75.Wulff K., Gatti S., Wettstein J.G., Foster R.G. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat. Rev. Neurosci. 2010;11(8):589–599. doi: 10.1038/nrn2868. [DOI] [PubMed] [Google Scholar]
- 76.Karatsoreos I.N. Links between circadian rhythms and psychiatric disease. 2014. [DOI] [PMC free article] [PubMed]
- 77.De Berardis D., Fornaro M., Serroni N., Campanella D., Rapini G., Olivieri L., Srinivasan V., Iasevoli F., Tomasetti C., De Bartolomeis A., Valchera A., Perna G., Mazza M., Di Nicola M., Martinotti G., Di Giannantonio M. Agomelatine beyond borders: current evidences of its efficacy in disorders other than major depression. Int. J. Mol. Sci. 2015;16(1):1111–1130. doi: 10.3390/ijms16011111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harvey B.H., Slabbert F.N. New insights on the antidepressant discontinuation syndrome. Hum. Psychopharmacol. 2014;29(6):503–516. doi: 10.1002/hup.2429. [DOI] [PubMed] [Google Scholar]
- 79.Overstreet D.H. The Flinders sensitive line rats: a genetic animal model of depression. Neurosci. Biobehav. Rev. 1993;17(1):51–68. doi: 10.1016/S0149-7634(05)80230-1. [DOI] [PubMed] [Google Scholar]
- 80.Solberg L.C., Olson S.L., Turek F.W., Redei E. Altered hormone levels and circadian rhythm of activity in the WKY rat, a putative animal model of depression. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281(3):R786–R794. doi: 10.1152/ajpregu.2001.281.3.R786. [DOI] [PubMed] [Google Scholar]
- 81.El Yacoubi M., Bouali S., Popa D., Naudon L., Leroux-Nicollet I., Hamon M., Costentin J., Adrien J., Vaugeois J.M. Behavioral, neurochemical, and electrophysiological characterization of a genetic mouse model of depression. Proc. Natl. Acad. Sci. USA. 2003;100(10):6227–6232. doi: 10.1073/pnas.1034823100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gessa G.L., Pani L., Fadda P., Fratta W. Sleep deprivation in the rat: an animal model of mania. Eur. Neuropsychopharmacol. 1995;5(Suppl.):89–93. doi: 10.1016/0924-977X(95)00023-I. [DOI] [PubMed] [Google Scholar]
- 83.Oliver P.L., Sobczyk M.V., Maywood E.S., Edwards B., Lee S., Livieratos A., Oster H., Butler R., Godinho S.I., Wulff K., Peirson S.N., Fisher S.P., Chesham J.E., Smith J.W., Hastings M.H., Davies K.E., Foster R.G. Disrupted circadian rhythms in a mouse model of schizophrenia. Curr. Biol. 2012;22(4):314–319. doi: 10.1016/j.cub.2011.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Corcoran C., Walker E., Huot R., Mittal V., Tessner K., Kestler L., Malaspina D. The stress cascade and schizophrenia: etiology and onset. Schizophr. Bull. 2003;29(4):671–692. doi: 10.1093/oxfordjournals.schbul.a007038. [DOI] [PubMed] [Google Scholar]
- 85.Daban C., Vieta E., Mackin P., Young A.H. Hypothalamic-pituitary-adrenal axis and bipolar disorder. Psychiatr. Clin. North Am. 2005;28(2):469–480. doi: 10.1016/j.psc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 86.Pariante C.M., Lightman S.L. 2008.
- 87.Steen N.E., Methlie P., Lorentzen S., Dieset I., Aas M., Nerhus M., Haram M., Agartz I., Melle I., Berg J.P., Andreassen O.A. Altered systemic cortisol metabolism in bipolar disorder and schizophrenia spectrum disorders. J. Psychiatr. Res. 2014;52:57–62. doi: 10.1016/j.jpsychires.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 88.Goodyer I.M., Croudace T., Dudbridge F., Ban M., Herbert J. Polymorphisms in BDNF (Val66Met) and 5-HTTLPR, morning cortisol and subsequent depression in at-risk adolescents. Br. J. Psychiatry. 2010;197(5):365–371. doi: 10.1192/bjp.bp.110.077750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mokhtari M., Arfken C., Boutros N. 2013. [DOI] [PubMed]
- 90.Steen N.E., Lorentzen S., Barrett E.A., Lagerberg T.V., Hope S., Larsson S., Berg A.O., Agartz I., Melle I., Berg J.P., Andreassen O.A. Sex-specific cortisol levels in bipolar disorder and schizophrenia during mental challenge--relationship to clinical characteristics and medication. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35(4):1100–1107. doi: 10.1016/j.pnpbp.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 91.Cullen A.E., Zunszain P.A., Dickson H., Roberts R.E., Fisher H.L., Pariante C.M., Laurens K.R. Cortisol awakening response and diurnal cortisol among children at elevated risk for schizophrenia: relationship to psychosocial stress and cognition. Psychoneuroendocrinology. 2014;46:1–13. doi: 10.1016/j.psyneuen.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Malkesman O., Weller A. 2009.
- 93.Touma C., Bunck M., Glasl L., Nussbaumer M., Palme R., Stein H., Wolferstätter M., Zeh R., Zimbelmann M., Holsboer F., Landgraf R. Mice selected for high versus low stress reactivity: a new animal model for affective disorders. Psychoneuroendocrinology. 2008;33(6):839–862. doi: 10.1016/j.psyneuen.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 94.Li M., Fu Q., Li Y., Li S., Xue J., Ma S. Emodin opposes chronic unpredictable mild stress induced depressive-like behavior in mice by upregulating the levels of hippocampal glucocorticoid receptor and brain-derived neurotrophic factor. Fitoterapia. 2014;98:1–10. doi: 10.1016/j.fitote.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 95.Fadda P., Fratta W. Stress-induced sleep deprivation modifies corticotropin releasing factor (CRF) levels and CRF binding in rat brain and pituitary. Pharmacol. Res. 1997;35(5):443–446. doi: 10.1006/phrs.1997.0155. [DOI] [PubMed] [Google Scholar]
- 96.Ward H.E., Johnson E.A., Salm A.K., Birkle D.L. Effects of prenatal stress on defensive withdrawal behavior and corticotropin releasing factor systems in rat brain. Physiol. Behav. 2000;70(3-4):359–366. doi: 10.1016/S0031-9384(00)00270-5. [DOI] [PubMed] [Google Scholar]
- 97.Cratty M.S., Ward H.E., Johnson E.A., Azzaro A.J., Birkle D.L. Prenatal stress increases corticotropin-releasing factor (CRF) content and release in rat amygdala minces. Brain Res. 1995;675(1-2):297–302. doi: 10.1016/0006-8993(95)00087-7. [DOI] [PubMed] [Google Scholar]
- 98.Issa G., Wilson C., Terry A.V., Jr, Pillai A. An inverse relationship between cortisol and BDNF levels in schizophrenia: data from human postmortem and animal studies. Neurobiol. Dis. 2010;39(3):327–333. doi: 10.1016/j.nbd.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 99.Trzepacz P.T., McCue M., Klein I., Levey G.S., Greenhouse J. A psychiatric and neuropsychological study of patients with untreated Graves’ disease. Gen. Hosp. Psychiatry. 1988;10(1):49–55. doi: 10.1016/0163-8343(88)90084-9. [DOI] [PubMed] [Google Scholar]
- 100.Kathol R.G., Delahunt J.W. The relationship of anxiety and depression to symptoms of hyperthyroidism using operational criteria. Gen. Hosp. Psychiatry. 1986;8(1):23–28. doi: 10.1016/0163-8343(86)90060-5. [DOI] [PubMed] [Google Scholar]
- 101.Snabboon T., Khemkha A., Chaiyaumporn C., Lalitanantpong D., Sridama V. Psychosis as the first presentation of hyperthyroidism. Intern. Emerg. Med. 2009;4(4):359–360. doi: 10.1007/s11739-009-0259-y. [DOI] [PubMed] [Google Scholar]
- 102.Wysokiński A., Kłoszewska I. Level of thyroid-stimulating hormone (TSH) in patients with acute schizophrenia, unipolar depression or bipolar disorder. Neurochem. Res. 2014;39(7):1245–1253. doi: 10.1007/s11064-014-1305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Radhakrishnan R., Calvin S., Singh J.K., Thomas B., Srinivasan K. Thyroid dysfunction in major psychiatric disorders in a hospital based sample. Indian J. Med. Res. 2013;138(6):888–893. [PMC free article] [PubMed] [Google Scholar]
- 104.Santos N.C., Costa P., Ruano D., MacEdo A., Soares M.J., Valente J., Pereira A. T., Azevedo M. H., Palha J. A. Revisiting thyroid hormones in schizophrenia. 2012. [DOI] [PMC free article] [PubMed]
- 105.McIntyre A., Gendron A., McIntyre A. Quetiapine adjunct to selective serotonin reuptake inhibitors or venlafaxine in patients with major depression, comorbid anxiety, and residual depressive symptoms: a randomized, placebo-controlled pilot study. Depress. Anxiety. 2007;24(7):487–494. doi: 10.1002/da.20275. [DOI] [PubMed] [Google Scholar]
- 106.Lieberman J.A. Understanding the mechanism of action of atypical antipsychotic drugs. A review of compounds in use and development. Br. J. Psychiatry Suppl. 1993;•••(22):7–18. [PubMed] [Google Scholar]
- 107.Toups M., Trivedi M.H., Trivedi M.D. Biomarkers and the future of treatment for depression. Cerebrum. 2012;2012:6. [PMC free article] [PubMed] [Google Scholar]
- 108.Rapaport M.H., Bresee C. Serial mitogen-stimulated cytokine production from continuously ill patients with schizophrenia. Neuropsychopharmacology. 2010;35(2):428–434. doi: 10.1038/npp.2009.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Krishnan V., Nestler E.J. The molecular neurobiology of depression. Nature. 2008;455(7215):894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Machado-Vieira R., Salvadore G., Diazgranados N., Zarate C.A., Jr Ketamine and the next generation of antidepressants with a rapid onset of action. Pharmacol. Ther. 2009;123(2):143–150. doi: 10.1016/j.pharmthera.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nestler E.J., Barrot M., DiLeone R.J., Eisch A.J., Gold S.J., Monteggia L.M. Neurobiology of depression. Neuron. 2002;34(1):13–25. doi: 10.1016/S0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 112.Della Pasqua O., Santen G.W., Danhof M., Payne J.L., Denicoff K., Gray N.A., Zarate C.A. The missing link between clinical endpoints and drug targets in depression. Trends Pharmacol. Sci. 2010;31(4):144–152. doi: 10.1016/j.tips.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 113.Nestler E.J., Carlezon W.A., Jr The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry. 2006;59(12):1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 114.Meyer J.H., Krüger S., Wilson A.A., Christensen B.K., Goulding V.S., Schaffer A., Minifie C., Houle S., Hussey D., Kennedy S.H. Lower dopamine transporter binding potential in striatum during depression. Neuroreport. 2001;12(18):4121–4125. doi: 10.1097/00001756-200112210-00052. [DOI] [PubMed] [Google Scholar]
- 115.Savitz J., Lucki I., Drevets W.C. 5-HT(1A) receptor function in major depressive disorder. Prog. Neurobiol. 2009;88(1):17–31. doi: 10.1016/j.pneurobio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Klimke A., Larisch R., Janz A., Vosberg H., Müller-Gärtner H.W., Gaebel W. Dopamine D2 receptor binding before and after treatment of major depression measured by [123I]IBZM SPECT. Psychiatry Res. 1999;90(2):91–101. doi: 10.1016/S0925-4927(99)00009-8. [DOI] [PubMed] [Google Scholar]
- 117.Alex K.D., Pehek E.A. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol. Ther. 2007;113(2):296–320. doi: 10.1016/j.pharmthera.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Berk M., Dodd S. Kauer-Sant'Anna, M.; Malhi, G.S.; Bourin, M.; Kapczinski, F.; Norman, T. Dopamine dysregulation syndrome: Implications for a dopamine hypothesis of bipolar disorder. Acta Psychiatr. Scand. 2007;116:41–49. doi: 10.1111/j.1600-0447.2007.01058.x. [DOI] [PubMed] [Google Scholar]
- 119.Frey B.N., Valvassori S.S., Réus G.Z., Martins M.R., Petronilho F.C., Bardini K., Dal-Pizzol F., Kapczinski F., Quevedo J. Effects of lithium and valproate on amphetamine-induced oxidative stress generation in an animal model of mania. J. Psychiatry Neurosci. 2006;31(5):326–332. [PMC free article] [PubMed] [Google Scholar]
- 120.Guillin O., Abi-Dargham A., Laruelle M. Neurobiology of dopamine in schizophrenia. Int. Rev. Neurobiol. 2007;78:1–39. doi: 10.1016/S0074-7742(06)78001-1. [DOI] [PubMed] [Google Scholar]
- 121.Rollema H., Lu Y., Schmidt A.W., Sprouse J.S., Zorn S.H. 2000.
- 122.Howes O.D., Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr. Bull. 2009;35(3):549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kuhlman K.R., Maercker A., Bachem R., Simmen K., Burri A. Developmental and contextual factors in the role of severe childhood trauma in geriatric depression: the sample case of former indentured child laborers. Child Abuse Negl. 2013;37(11):969–978. doi: 10.1016/j.chiabu.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 124.Simeon D., Yehuda R., Cunill R., Knutelska M., Putnam F.W., Smith L.M. Factors associated with resilience in healthy adults. Psychoneuroendocrinology. 2007;32(8-10):1149–1152. doi: 10.1016/j.psyneuen.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 125.Aas M., Aminoff S.R., Vik Lagerberg T., Etain B., Agartz I., Andreassen O.A., Melle I. Affective lability in patients with bipolar disorders is associated with high levels of childhood trauma. Psychiatry Res. 2014;218(1-2):252–255. doi: 10.1016/j.psychres.2014.03.046. [DOI] [PubMed] [Google Scholar]
- 126.Erten E., Funda Uney A., Saatçioğlu Ö., Özdemir A., Fıstıkçı N., Çakmak D. Effects of childhood trauma and clinical features on determining quality of life in patients with bipolar I disorder. J. Affect. Disord. 2014;162:107–113. doi: 10.1016/j.jad.2014.03.046. [DOI] [PubMed] [Google Scholar]
- 127.Harvey B.H., Stein D.J., Emsley R.A. The new-generation antipsychotics - Integrating the neuropathology and pharmacology of schizophrenia. S. Afr. Med. J. 1999;89:661–672. [Google Scholar]
- 128.Kapur S., Mamo D. Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27(7):1081–1090. doi: 10.1016/j.pnpbp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 129.Willner P. 1997.
- 130.Papp M., Klimek V., Willner P. Parallel changes in dopamine D2 receptor binding in limbic forebrain associated with chronic mild stress-induced anhedonia and its reversal by imipramine. Psychopharmacology (Berl.) 1994;115(4):441–446. doi: 10.1007/BF02245566. [DOI] [PubMed] [Google Scholar]
- 131.Yadid G., Nakash R., Deri I., Tamar G., Kinor N., Gispan I., Zangen A. Elucidation of the neurobiology of depression: insights from a novel genetic animal model. Prog. Neurobiol. 2000;62(4):353–378. doi: 10.1016/S0301-0082(00)00018-6. [DOI] [PubMed] [Google Scholar]
- 132.Berk M., Dodd S. Efficacy of atypical antipsychotics in bipolar disorder. Drugs. 2005;65(2):257–269. doi: 10.2165/00003495-200565020-00006. [DOI] [PubMed] [Google Scholar]
- 133.van Enkhuizen J., Geyer M.A., Halberstadt A.L., Zhuang X., Young J.W. Dopamine depletion attenuates some behavioral abnormalities in a hyperdopaminergic mouse model of bipolar disorder. J. Affect. Disord. 2014;155:247–254. doi: 10.1016/j.jad.2013.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ralph-Williams R.J., Paulus M.P., Zhuang X., Hen R., Geyer M.A. Valproate attenuates hyperactive and perseverative behaviors in mutant mice with a dysregulated dopamine system. Biol. Psychiatry. 2003;53(4):352–359. doi: 10.1016/S0006-3223(02)01489-0. [DOI] [PubMed] [Google Scholar]
- 135.da-Rosa D.D., Valvassori S.S., Steckert A.V., Ornell F., Ferreira C.L., Lopes-Borges J., Varela R.B., Dal-Pizzol F., Andersen M.L., Quevedo J. Effects of lithium and valproate on oxidative stress and behavioral changes induced by administration of m-AMPH. Psychiatry Res. 2012;198(3):521–526. doi: 10.1016/j.psychres.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 136.Murray R., Boss-Williams K.A., Weiss J.M. Effects of chronic mild stress on rats selectively bred for behavior related to bipolar disorder and depression. Physiol. Behav. 2013;119:115–129. doi: 10.1016/j.physbeh.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 137.Möller M., Du Preez J.L., Viljoen F.P., Berk M., Emsley R., Harvey B.H. Social isolation rearing induces mitochondrial, immunological, neurochemical and behavioural deficits in rats, and is reversed by clozapine or N-acetyl cysteine. 2013. [DOI] [PubMed]
- 138.Trabace L., Zotti M., Colaianna M., Morgese M.G., Schiavone S., Tucci P., Harvey B.H., Wegener G., Cuomo V. Neurochemical differences in two rat strains exposed to social isolation rearing. Acta Neuropsychiatr. 2012;24(5):286–295. doi: 10.1111/j.1601-5215.2011.00627.x. [DOI] [PubMed] [Google Scholar]
- 139.Powell S.B., Geyer M.A., Preece M.A., Pitcher L.K., Reynolds G.P., Swerdlow N.R. Dopamine depletion of the nucleus accumbens reverses isolation-induced deficits in prepulse inhibition in rats. Neuroscience. 2003;119(1):233–240. doi: 10.1016/S0306-4522(03)00122-2. [DOI] [PubMed] [Google Scholar]
- 140.Brenes J.C., Fornaguera J. The effect of chronic fluoxetine on social isolation-induced changes on sucrose consumption, immobility behavior, and on serotonin and dopamine function in hippocampus and ventral striatum. Behav. Brain Res. 2009;198(1):199–205. doi: 10.1016/j.bbr.2008.10.036. [DOI] [PubMed] [Google Scholar]
- 141.Toua C., Brand L., Möller M., Emsley R.A., Harvey B.H. The effects of sub-chronic clozapine and haloperidol administration on isolation rearing induced changes in frontal cortical N-methyl-D-aspartate and D1 receptor binding in rats. Neuroscience. 2010;165(2):492–499. doi: 10.1016/j.neuroscience.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 142.Hall F.S., Wilkinson L.S., Humby T., Inglis W., Kendall D.A., Marsden C.A., Robbins T.W. Isolation rearing in rats: pre- and postsynaptic changes in striatal dopaminergic systems. Pharmacol. Biochem. Behav. 1998;59(4):859–872. doi: 10.1016/S0091-3057(97)00510-8. [DOI] [PubMed] [Google Scholar]
- 143.Del Arco A., Zhu S., Terasmaa A., Mohammed A.H., Fuxe K. Hyperactivity to novelty induced by social isolation is not correlated with changes in D2 receptor function and binding in striatum. Psychopharmacology (Berl.) 2004;171(2):148–155. doi: 10.1007/s00213-003-1578-8. [DOI] [PubMed] [Google Scholar]
- 144.Malone D.T., Kearn C.S., Chongue L., Mackie K., Taylor D.A. Effect of social isolation on CB1 and D2 receptor and fatty acid amide hydrolase expression in rats. Neuroscience. 2008;152(1):265–272. doi: 10.1016/j.neuroscience.2007.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Heidbreder C.A., Foxton R., Cilia J., Hughes Z.A., Shah A.J., Atkins A., Hunter A.J., Hagan J.J., Jones D.N. Increased responsiveness of dopamine to atypical, but not typical antipsychotics in the medial prefrontal cortex of rats reared in isolation. Psychopharmacology (Berl.) 2001;156(2-3):338–351. doi: 10.1007/s002130100760. [DOI] [PubMed] [Google Scholar]
- 146.Jentsch J.D., Tran A., Le D., Youngren K.D., Roth R.H. Subchronic phencyclidine administration reduces mesoprefrontal dopamine utilization and impairs prefrontal cortical-dependent cognition in the rat. Neuropsychopharmacology. 1997;17(2):92–99. doi: 10.1016/S0893-133X(97)00034-1. [DOI] [PubMed] [Google Scholar]
- 147.Jentsch J.D., Taylor J.R., Roth R.H. Subchronic phencyclidine administration increases mesolimbic dopaminergic system responsivity and augments stress- and psychostimulant-induced hyperlocomotion. Neuropsychopharmacology. 1998;19(2):105–113. doi: 10.1016/S0893-133X(98)00004-9. [DOI] [PubMed] [Google Scholar]
- 148.de Bodinat C., Guardiola-Lemaitre B., Mocaër E., Renard P., Muñoz C., Millan M.J. Agomelatine, the first melatonergic antidepressant: discovery, characterization and development. Nat. Rev. Drug Discov. 2010;9(8):628–642. doi: 10.1038/nrd3274. [DOI] [PubMed] [Google Scholar]
- 149.Popa D., Cerdan J., Repérant C., Guiard B.P., Guilloux J.P., David D.J., Gardier A. M. 2010. [DOI] [PubMed]
- 150.Brink C.B., Harvey B.H., Brand L. Tianeptine: a novel atypical antidepressant that may provide new insights into the biomolecular basis of depression. Recent Patents CNS Drug Discov. 2006;1(1):29–41. doi: 10.2174/157488906775245327. [DOI] [PubMed] [Google Scholar]
- 151.Lidberg L., Belfrage H., Bertilsson L., Evenden M.M., Åsberg M. Suicide attempts and impulse control disorder are related to low cerebrospinal fluid 5-HIAA in mentally disordered violent offenders. Acta Psychiatr. Scand. 2000;101(5):395–402. doi: 10.1034/j.1600-0447.2000.101005395.x. [DOI] [PubMed] [Google Scholar]
- 152.Mann J.J., Malone K.M. Cerebrospinal fluid amines and higher-lethality suicide attempts in depressed inpatients. 1997. [DOI] [PubMed]
- 153.Pandey G.N., Pandey S.C., Janicak P.G., Marks R.C., Davis J.M. Platelet serotonin-2 receptor binding sites in depression and suicide. Biol. Psychiatry. 1990;28(3):215–222. doi: 10.1016/0006-3223(90)90576-N. [DOI] [PubMed] [Google Scholar]
- 154.Hrdina P.D., Demeter E., Vu T.B., Sótónyi P., Palkovits M. 5- HT uptake sites and 5-HT2 receptors in brain of antidepressant-free suicide victims/depressives: increase in 5-HT2 sites in cortex and amygdala. Brain Res. 1993;614:37–44. doi: 10.1016/0006-8993(93)91015-K. [DOI] [PubMed] [Google Scholar]
- 155.Hurlemann R., Matusch A., Kuhn K.U., Berning J., Elmenhorst D., Winz O., Kolsch H., Zilles K., Wagner M., Maier W., Bauer A. 5-HT2A receptor density is decreased in the at-risk mental state. Psychopharmacology (Berl.) 2008;195(4):579–590. doi: 10.1007/s00213-007-0921-x. [DOI] [PubMed] [Google Scholar]
- 156.Meyer J.H., Ginovart N., Boovariwala A., Sagrati S., Hussey D., Garcia A., Young T., Praschak-Rieder N., Wilson A.A., Houle S. Elevated monoamine oxidase A levels in the brain: An explanation for the monoamine imbalance of major depression. Arch. Gen. Psychiatry, . 2006;63:1209–1216. doi: 10.1001/archpsyc.63.11.1209. [DOI] [PubMed] [Google Scholar]
- 157.Meyer J.H., Wilson A.A., Sagrati S., Miler L., Rusjan P., Bloomfield P.M., Clark M., Sacher J., Voineskos A.N., Houle S. Brain monoamine oxidase A binding in major depressive disorder: relationship to selective serotonin reuptake inhibitor treatment, recovery, and recurrence. Arch. Gen. Psychiatry. 2009;66(12):1304–1312. doi: 10.1001/archgenpsychiatry.2009.156. [DOI] [PubMed] [Google Scholar]
- 158.Young L.T., Warsh J.J., Kish S.J., Shannak K., Hornykeiwicz O. Reduced brain 5-HT and elevated NE turnover and metabolites in bipolar affective disorder. Biol. Psychiatry. 1994;35(2):121–127. doi: 10.1016/0006-3223(94)91201-7. [DOI] [PubMed] [Google Scholar]
- 159.Leake A., Fairbairn A.F., McKeith I.G., Ferrier I.N. Studies on the serotonin uptake binding site in major depressive disorder and control post-mortem brain: neurochemical and clinical correlates. Psychiatry Res. 1991;39(2):155–165. doi: 10.1016/0165-1781(91)90084-3. [DOI] [PubMed] [Google Scholar]
- 160.Asberg M., Bertilsson L., Mårtensson B., Scalia-Tomba G.P., Thorén P., Träskman-Bendz L. CSF monoamine metabolites in melancholia. Acta Psychiatr. Scand. 1984;69(3):201–219. doi: 10.1111/j.1600-0447.1984.tb02488.x. [DOI] [PubMed] [Google Scholar]
- 161.Swann A.C., Secunda S., Davis J.M., Robins E., Hanin I., Koslow S.H., Maas J.W. CSF monoamine metabolites in mania. Am. J. Psychiatry. 1983;140(4):396–400. doi: 10.1176/ajp.140.4.396. [DOI] [PubMed] [Google Scholar]
- 162.Burnet P.W., Eastwood S.L., Harrison P.J. 5-HT1A and 5-HT2A receptor mRNAs and binding site densities are differentially altered in schizophrenia. Neuropsychopharmacology. 1996;15(5):442–455. doi: 10.1016/S0893-133X(96)00053-X. [DOI] [PubMed] [Google Scholar]
- 163.Burnet P.W.J., Eastwood S.L., Harrison P.J. [3H]WAY-100635 for 5-HT(1A) receptor autoradiography in human brain: A comparison with [3H]8-OH-DPAT and demonstration of increased binding in the frontal cortex in schizophrenia. Neurochem. Int., 1997;30:565–574. doi: 10.1016/S0197-0186(96)00124-6. [DOI] [PubMed] [Google Scholar]
- 164.Rasmussen H., Erritzoe D., Andersen R., Ebdrup B.H., Aggernaes B., Oranje B., Kalbitzer J., Madsen J., Pinborg L.H., Baaré W., Svarer C., Lublin H., Knudsen G.M., Glenthoj B. Decreased frontal serotonin2A receptor binding in antipsychotic-naive patients with first-episode schizophrenia. Arch. Gen. Psychiatry. 2010;67(1):9–16. doi: 10.1001/archgenpsychiatry.2009.176. [DOI] [PubMed] [Google Scholar]
- 165.Joyce J.N., Shane A., Lexow N., Winokur A., Casanova M.F., Kleinman J.E. Serotonin uptake sites and serotonin receptors are altered in the limbic system of schizophrenics. Neuropsychopharmacology. 1993;8(4):315–336. doi: 10.1038/npp.1993.32. [DOI] [PubMed] [Google Scholar]
- 166.Ngan E.T.C., Yatham L.N., Ruth T.J., Liddle P.F. Decreased serotonin 2A receptor densities in neuroleptic-naive patients with schizophrenia: A pet study using [18F] setoperone. Am. J. Psychiatry. 2000;157:1016–1018. doi: 10.1176/appi.ajp.157.6.1016. [DOI] [PubMed] [Google Scholar]
- 167.Eastwood S.L., Burnet P.W., Gittins R., Baker K., Harrison P.J. Expression of serotonin 5-HT(2A) receptors in the human cerebellum and alterations in schizophrenia. Synapse. 2001;42(2):104–114. doi: 10.1002/syn.1106. [DOI] [PubMed] [Google Scholar]
- 168.Aghajanian G.K., Marek G.J. Serotonin model of schizophrenia: emerging role of glutamate mechanisms. Brain Res. Brain Res. Rev. 2000;31(2-3):302–312. doi: 10.1016/S0165-0173(99)00046-6. [DOI] [PubMed] [Google Scholar]
- 169.Roth B.L., Sheffler D.J., Kroeze W.K. Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nat. Rev. Drug Discov. 2004;3(4):353–359. doi: 10.1038/nrd1346. [DOI] [PubMed] [Google Scholar]
- 170.Laruelle M., Abi-Dargham A., van Dyck C., Gil R., D’Souza D.C., Krystal J., Seibyl J., Baldwin R., Innis R. Dopamine and serotonin transporters in patients with schizophrenia: an imaging study with [(123)I]beta-CIT. Biol. Psychiatry. 2000;47(5):371–379. doi: 10.1016/S0006-3223(99)00257-7. [DOI] [PubMed] [Google Scholar]
- 171.Heisler L.K., Zhou L., Bajwa P., Hsu J., Tecott L.H. Serotonin 5-HT2C receptors regulate anxiety-like behavior. Genes Brain Behav. . 2007;6:491–496. doi: 10.1111/j.1601-183X.2007.00316.x. [DOI] [PubMed] [Google Scholar]
- 172.Dekeyne A., Mannoury la Cour C., Gobert A., Brocco M., Lejeune F., Serres F., Sharp T., Daszuta A., Soumier A., Papp M., Rivet J.M., Flik G., Cremers T.I., Muller O., Lavielle G., Millan M.J. S32006, a novel 5-HT2C receptor antagonist displaying broad-based antidepressant and anxiolytic properties in rodent models. Psychopharmacology (Berl.) 2008;199(4):549–568. doi: 10.1007/s00213-008-1177-9. [DOI] [PubMed] [Google Scholar]
- 173.Zangen A., Overstreet D.H., Yadid G. High serotonin and 5-hydroxyindoleacetic acid levels in limbic brain regions in a rat model of depression: normalization by chronic antidepressant treatment. J. Neurochem. 1997;69(6):2477–2483. doi: 10.1046/j.1471-4159.1997.69062477.x. [DOI] [PubMed] [Google Scholar]
- 174.Murray K.C., Nakae A., Stephens M.J., Rank M., D’Amico J., Harvey P.J., Li X., Harris R.L., Ballou E.W., Anelli R., Heckman C.J., Mashimo T., Vavrek R., Sanelli L., Gorassini M.A., Bennett D.J., Fouad K. Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT2C receptors. Nat. Med. 2010;16(6):694–700. doi: 10.1038/nm.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hasegawa S., Nishi K., Watanabe A., Overstreet D.H., Diksic M. Brain 5-HT synthesis in the Flinders Sensitive Line rat model of depression: an autoradiographic study. Neurochem. Int. 2006;48(5):358–366. doi: 10.1016/j.neuint.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 176.Owens W.A., Aguilar D., Overstreet D.H., Daws L.C. SERTainly slower: Reduced SERT expression and function in the Flinders Sensitive Line (FSL) rat model of depression. Presented at meeting of Society for Neuroscience, DC November, Washington, 2011. ; 2011. [Google Scholar]
- 177. Consideration of the relationship between depression and dementia. Psychogeriatrics, . 2006;6:128–133. doi: 10.1111/j.1479-8301.2006.00151.x. [DOI] [Google Scholar]
- 178.Jaffe E.H., De Frias V., Ibarra C. Changes in basal and stimulated release of endogenous serotonin from different nuclei of rats subjected to two models of depression. Neurosci. Lett. 1993;162(1-2):157–160. doi: 10.1016/0304-3940(93)90584-8. [DOI] [PubMed] [Google Scholar]
- 179.Möller M., Du Preez J.L., Viljoen F.P., Berk M., Harvey B.H. N-Acetyl cysteine reverses social isolation rearing induced changes in cortico-striatal monoamines in rats. Metab. Brain Dis. 2013;28(4):687–696. doi: 10.1007/s11011-013-9433-z. [DOI] [PubMed] [Google Scholar]
- 180.Meltzer H.Y., Li Z., Kaneda Y., Ichikawa J. Serotonin receptors: their key role in drugs to treat schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27(7):1159–1172. doi: 10.1016/j.pnpbp.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 181.Gleason S.D., Shannon H.E. Blockade of phencyclidine-induced hyperlocomotion by olanzapine, clozapine and serotonin receptor subtype selective antagonists in mice. Psychopharmacology (Berl.) 1997;129(1):79–84. doi: 10.1007/s002130050165. [DOI] [PubMed] [Google Scholar]
- 182.Moret C., Briley M. The importance of norepinephrine in depression. Neuropsychiatr. Dis. Treat. 2011;7(Suppl. 1):9–13. doi: 10.1016/S0006-3223(02)01728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Klimek V., Stockmeier C., Overholser J., Meltzer H.Y., Kalka S., Dilley G., Ordway G.A. Reduced levels of norepinephrine transporters in the locus coeruleus in major depression. J. Neurosci. 1997;17(21):8451–8458. doi: 10.1523/JNEUROSCI.17-21-08451.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ordway G.A., Schenk J., Stockmeier C.A., May W., Klimek V. Elevated agonist binding to alpha2-adrenoceptors in the locus coeruleus in major depression. Biol. Psychiatry. 2003;53(4):315–323. doi: 10.1016/S0006-3223(02)01728-6. [DOI] [PubMed] [Google Scholar]
- 185.Valdizán E.M., Díez-Alarcia R., González-Maeso J., Pilar-Cuéllar F., García-Sevilla J.A., Meana J.J., Pazos A. α₂-Adrenoceptor functionality in postmortem frontal cortex of depressed suicide victims. Biol. Psychiatry. 2010;68(9):869–872. doi: 10.1016/j.biopsych.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ruhé H.G., Mason N.S., Schene A.H. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol. Psychiatry. 2007;12(4):331–359. doi: 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- 187.Hughes J.W., Watkins L., Blumenthal J.A., Kuhn C., Sherwood A. Depression and anxiety symptoms are related to increased 24-hour urinary norepinephrine excretion among healthy middle-aged women. J. Psychosom. Res. 2004;57(4):353–358. doi: 10.1016/S0022-3999(04)00064-9. [DOI] [PubMed] [Google Scholar]
- 188. Enhanced norepinephrine output during long-term desipramine treatment: A possible role for the extraneuronal monoamine transporter (SLC22A3). J. Psychiatr. Res . 2008;42:605–611. doi: 10.1016/j.jpsychires.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Schildkraut J.J., Schatzberg A.F., Samson J.A., Rosenbaum A., Bowden C.L. Norepinephrine output and metabolism in depressed patients during antidepressant treatments. 1992. [DOI] [PubMed]
- 190.Cottingham C., Wang Q. α2 adrenergic receptor dysregulation in depressive disorders: Implications for the neurobiology of depression and antidepressant therapy. Neurosci. Biobehav. Rev. 2012;36:2214–2225. doi: 10.1016/j.neubiorev.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yamamoto K., Hornykiewicz O. Proposal for a noradrenaline hypothesis of schizophrenia. 2004. [DOI] [PubMed]
- 192.Dilsaver S.C., Peck J.A., Traumata D., Swan A.C. Treatment with carbamazepine may enhance alpha 2-noradrenergic autoreceptor sensitivity. Biol. Psychiatry. 1993;34(8):551–557. doi: 10.1016/0006-3223(93)90198-M. [DOI] [PubMed] [Google Scholar]
- 193.Lysaker P.H., Salyers M.P. Anxiety symptoms in schizophrenia spectrum disorders: associations with social function, positive and negative symptoms, hope and trauma history. Acta Psychiatr. Scand. 2007;116(4):290–298. doi: 10.1111/j.1600-0447.2007.01067.x. [DOI] [PubMed] [Google Scholar]
- 194.Zangen A., Overstreet D.H., Yadid G. Increased catecholamine levels in specific brain regions of a rat model of depression: Normalization by chronic antidepressant treatment. Brain Res., . 1999;824:243–250. doi: 10.1016/S0006-8993(99)01214-7. [DOI] [PubMed] [Google Scholar]
- 195.Bourin M., Prica C. The role of mood stabilisers in the treatment of the depressive facet of bipolar disorders. 2007. [DOI] [PubMed]
- 196.Miachon S., Rochet T., Mathian B., Barbagli B., Claustrat B. Long-term isolation of Wistar rats alters brain monoamine turnover, blood corticosterone, and ACTH. Brain Res. Bull. 1993;32(6):611–614. doi: 10.1016/0361-9230(93)90162-5. [DOI] [PubMed] [Google Scholar]
- 197.Zarate C.A., Jr, Du J., Quiroz J., Gray N.A., Denicoff K.D., Singh J., Charney D.S., Manji H.K. Regulation of cellular plasticity cascades in the pathophysiology and treatment of mood disorders: role of the glutamatergic system. Ann. N. Y. Acad. Sci. 2003;1003:273–291. doi: 10.1196/annals.1300.017. [DOI] [PubMed] [Google Scholar]
- 198.Coyle J.T., Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262(5134):689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 199.Suzuki E., Yagi G., Nakaki T., Kanba S., Asai M. Elevated plasma nitrate levels in depressive states. J. Affect. Disord. 2001;63(1-3):221–224. doi: 10.1016/S0165-0327(00)00164-6. [DOI] [PubMed] [Google Scholar]
- 200.Dhir A., Kulkarni S.K. Nitric oxide and major depression. Nitric Oxide. 2011;24(3):125–131. doi: 10.1016/j.niox.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 201.Harvey B.H. Affective disorders and nitric oxide: A role in pathways to relapse and refractoriness? 1996.
- 202.Harvey B.H., McEwen B.S., Stein D.J. Neurobiology of antidepressant withdrawal: implications for the longitudinal outcome of depression. Biol. Psychiatry. 2003;54(10):1105–1117. doi: 10.1016/S0006-3223(03)00528-6. [DOI] [PubMed] [Google Scholar]
- 203.Altamura C.A., Mauri M.C., Ferrara A., Moro A.R., D’Andrea G., Zamberlan F. Plasma and platelet excitatory amino acids in psychiatric disorders. Am. J. Psychiatry. 1993;150(11):1731–1733. doi: 10.1176/ajp.150.11.1731. [DOI] [PubMed] [Google Scholar]
- 204.Mauri M.C., Ferrara A., Boscati L., Bravin S., Zamberlan F., Alecci M., Invernizzi G. Plasma and platelet amino acid concentrations in patients affected by major depression and under fluvoxamine treatment. Neuropsychobiology. 1998;37(3):124–129. doi: 10.1159/000026491. [DOI] [PubMed] [Google Scholar]
- 205.Sanacora G., Gueorguieva R., Epperson C.N., Wu Y.T., Appel M., Rothman D.L., Krystal J.H., Mason G.F. Subtype-specific alterations of γ-aminobutyric acid and glutamate in patients with major depression. Arch. Gen. Psychiatry. 2004;61(7):705–713. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- 206.Nowak G., Ordway G.A., Paul I.A. Alterations in the N-methyl-D-aspartate (NMDA) receptor complex in the frontal cortex of suicide victims. Brain Res. 1995;675(1-2):157–164. doi: 10.1016/0006-8993(95)00057-W. [DOI] [PubMed] [Google Scholar]
- 207.Nudmamud-Thanoi S., Reynolds G.P. The NR1 subunit of the glutamate/NMDA receptor in the superior temporal cortex in schizophrenia and affective disorders. Neurosci. Lett. 2004;372(1-2):173–177. doi: 10.1016/j.neulet.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 208.Müller N., Schwarz M.J. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol. Psychiatry. 2007;12(11):988–1000. doi: 10.1038/sj.mp.4002006. [DOI] [PubMed] [Google Scholar]
- 209.Maes M., Galecki P., Verkerk R., Rief W. Somatization, but not depression, is characterized by disorders in the tryptophan catabolite (TRYCAT) pathway, indicating increased indoleamine 2,3-dioxygenase and lowered kynurenine aminotransferase activity. Neuroendocrinol. Lett. 2011;32(3):264–273. [PubMed] [Google Scholar]
- 210.Gabbay V., Klein R.G., Katz Y., Mendoza S., Guttman L.E., Alonso C.M., Babb J.S., Hirsch G.S., Liebes L. The possible role of the kynurenine pathway in adolescent depression with melancholic features. J. Child Psychol. Psychiatry. 2010;51(8):935–943. doi: 10.1111/j.1469-7610.2010.02245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Steiner J., Walter M., Gos T., Guillemin G.J., Bernstein H.G., Sarnyai Z., Mawrin C., Brisch R., Bielau H., Meyer zu Schwabedissen L., Bogerts B., Myint A.M. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? J. Neuroinflammation. 2011;8:94. doi: 10.1186/1742-2094-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Yksel C., Öngür D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. 2010. [DOI] [PMC free article] [PubMed]
- 213.Scarr E., Pavey G., Sundram S., MacKinnon A., Dean B. Decreased hippocampal NMDA, but not kainate or AMPA receptors in bipolar disorder. Bipolar Disord. 2003;5(4):257–264. doi: 10.1034/j.1399-5618.2003.00024.x. [DOI] [PubMed] [Google Scholar]
- 214.Yoon S.J., Lyoo I.K., Haws C., Kim T.S., Cohen B.M., Renshaw P.F. Decreased glutamate/glutamine levels may mediate cytidine’s efficacy in treating bipolar depression: a longitudinal proton magnetic resonance spectroscopy study. Neuropsychopharmacology. 2009;34(7):1810–1818. doi: 10.1038/npp.2009.2. [DOI] [PubMed] [Google Scholar]
- 215.Cherlyn S.Y., Woon P.S., Liu J.J., Ong W.Y., Tsai G.C., Sim K. Genetic association studies of glutamate, GABA and related genes in schizophrenia and bipolar disorder: A decade of advance. Neurosci. Biobehav. Rev. . 2010;34:958–977. doi: 10.1016/j.neubiorev.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 216.Schwartz T.L., Sachdeva S., Stahl S.M. Glutamate neurocircuitry: theoretical underpinnings in schizophrenia. Front. Pharmacol. 2012;3:195. doi: 10.3389/fphar.2012.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Emsley R., Chiliza B., Asmal L., Harvey B.H. The nature of relapse in schizophrenia. BMC Psychiatry. 2013;13:50. doi: 10.1186/1471-244X-13-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Schwarcz R., Rassoulpour A., Wu H.Q., Medoff D., Tamminga C.A., Roberts R.C. Increased cortical kynurenate content in schizophrenia. Biol. Psychiatry. 2001;50(7):521–530. doi: 10.1016/S0006-3223(01)01078-2. [DOI] [PubMed] [Google Scholar]
- 219.Bauer D., Gupta D., Harotunian V., Meador-Woodruff J.H., McCullumsmith R.E. Abnormal expression of glutamate transporter and transporter interacting molecules in prefrontal cortex in elderly patients with schizophrenia. Schizophr. Res. 2008;104(1-3):108–120. doi: 10.1016/j.schres.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Goff D.C., Coyle J.T. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. 2001. [DOI] [PubMed]
- 221.Aparicio-Legarza M.I., Cutts A.J., Davis B., Reynolds G.P. Deficits of [3H]D-aspartate binding to glutamate uptake sites in striatal and accumbens tissue in patients with schizophrenia. Neurosci. Lett. 1997;232(1):13–16. doi: 10.1016/S0304-3940(97)00563-6. [DOI] [PubMed] [Google Scholar]
- 222.Bernstein H.G., Bogerts B., Keilhoff G. The many faces of nitric oxide in schizophrenia. A review. Schizophr. Res. 2005;78(1):69–86. doi: 10.1016/j.schres.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 223.Bernstein H.G., Keilhoff G., Steiner J., Dobrowolny H., Bogerts B. Nitric oxide and schizophrenia: present knowledge and emerging concepts of therapy. CNS Neurol. Disord. Drug Targets. 2011;10(7):792–807. doi: 10.2174/187152711798072392. [DOI] [PubMed] [Google Scholar]
- 224.Kegeles L.S., Mao X., Stanford A.D., Girgis R., Ojeil N., Xu X., Gil R., Slifstein M., Abi-Dargham A., Lisanby S. H., Shungu D. C. Elevated prefrontal cortex γ-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. . Arch. General Psychiatry, . 2012;69:449–459. doi: 10.1001/archgenpsychiatry.2011.1519. [DOI] [PubMed] [Google Scholar]
- 225.Lu J., Goula D., Sousa N., Almeida O.F.X. Ionotropic and metabotropic glutamate receptor mediation of glucocorticoid-induced apoptosis in hippocampal cells and the neuroprotective role of synaptic N-methyl-D-aspartate receptors. 2003. [DOI] [PubMed]
- 226.Wegener G., Harvey B.H., Bonefeld B., Müller H.K., Volke V., Overstreet D.H., Elfving B. Increased stress-evoked nitric oxide signalling in the Flinders sensitive line (FSL) rat: a genetic animal model of depression. Int. J. Neuropsychopharmacol. 2010;13(4):461–473. doi: 10.1017/S1461145709990241. [DOI] [PubMed] [Google Scholar]
- 227.Heiberg I.L., Wegener G., Rosenberg R. Reduction of cGMP and nitric oxide has antidepressant-like effects in the forced swimming test in rats. Behav. Brain Res. 2002;134(1-2):479–484. doi: 10.1016/S0166-4328(02)00084-0. [DOI] [PubMed] [Google Scholar]
- 228.Wegener G., Volke V., Harvey B.H., Rosenberg R. Local but not systemic administration of serotonergic antidepressants decreases hippocampal nitric oxide synthase activity. 2003. [DOI] [PubMed]
- 229.Denninger J.W., Marletta M.A. Guanylate cyclase and the ⋅NO/cGMP signaling pathway. Biochimica et Biophysica Acta (BBA) -. Bioenergetics. 1999;1411:334–350. doi: 10.1016/S0005-2728(99)00024-9. [DOI] [PubMed] [Google Scholar]
- 230.Esplugues J.V. NO as a signalling molecule in the nervous system. Br. J. Pharmacol. 2002;135(5):1079–1095. doi: 10.1038/sj.bjp.0704569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Oosthuizen F., Wegener G., Harvey B.H. Nitric oxide as inflammatory mediator in post-traumatic stress disorder (PTSD): evidence from an animal model. Neuropsychiatr. Dis. Treat. 2005;1(2):109–123. doi: 10.2147/nedt.1.2.109.61049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Harvey B.H., Duvenhage I., Viljoen F., Scheepers N., Malan S.F., Wegener G., Brink C.B., Petzer J.P. Role of monoamine oxidase, nitric oxide synthase and regional brain monoamines in the antidepressant-like effects of methylene blue and selected structural analogues. Biochem. Pharmacol. 2010;80(10):1580–1591. doi: 10.1016/j.bcp.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 233.Beavo J.A., Hardman J.G., Sutherland E.W. Hydrolysis of cyclic guanosine and adenosine 3′,5′-monophosphates by rat and bovine tissues. J. Biol. Chem. 1970;245(21):5649–5655. [PubMed] [Google Scholar]
- 234.Liebenberg N., Harvey B.H., Brand L., Brink C.B. Antidepressant-like properties of phosphodiesterase type 5 inhibitors and cholinergic dependency in a genetic rat model of depression. Behav. Pharmacol. 2010;21(5-6):540–547. doi: 10.1097/FBP.0b013e32833befe5. [DOI] [PubMed] [Google Scholar]
- 235.Liebenberg N., Harvey B.H., Brand L., Wegener G., Brink C.B. Chronic treatment with the phosphodiesterase type 5 inhibitors sildenafil and tadalafil display anxiolytic effects in Flinders Sensitive Line rats. Metab. Brain Dis. 2012;27(3):337–340. doi: 10.1007/s11011-012-9284-z. [DOI] [PubMed] [Google Scholar]
- 236.Wegener G., Harvey B.H., Bonefeld B., Müller H.K., Volke V., Overstreet D.H., Elfving B. Increased stress-evoked nitric oxide signalling in the Flinders sensitive line (FSL) rat: a genetic animal model of depression. Int. J. Neuropsychopharmacol. 2010;13(4):461–473. doi: 10.1017/S1461145709990241. [DOI] [PubMed] [Google Scholar]
- 237.Kessler R.C., Zhao S., Blazer D.G., Swartz M. Prevalence, correlates, and course of minor depression and major depression in the National Comorbidity Survey. J. Affect. Disord. 1997;45(1-2):19–30. doi: 10.1016/S0165-0327(97)00056-6. [DOI] [PubMed] [Google Scholar]
- 238.Kendler K.S., Karkowski L.M., Prescott C.A. Stressful life events and major depression: risk period, long-term contextual threat, and diagnostic specificity. J. Nerv. Ment. Dis. 1998;186(11):661–669. doi: 10.1097/00005053-199811000-00001. [DOI] [PubMed] [Google Scholar]
- 239.Maj J., Rogóz Z., Skuza G., Sowińska H. Effects of MK-801 and antidepressant drugs in the forced swimming test in rats. Eur. Neuropsychopharmacol. 1992;2(1):37–41. doi: 10.1016/0924-977X(92)90034-6. [DOI] [PubMed] [Google Scholar]
- 240.Ostroff R., Gonzales M., Sanacora G. Antidepressant effect of ketamine during ECT. Am. J. Psychiatry. 2005;162(7):1385–1386. doi: 10.1176/appi.ajp.162.7.1385. [3]. [DOI] [PubMed] [Google Scholar]
- 241.Ossowska G., Klenk-Majewska B., Szymczyk G. The effect of NMDA antagonists on footshock-induced fighting behavior in chronically stressed rats. J. Physiol. Pharmacol. 1997;48(1):127–135. doi: 10.1176/appi.ajp.162.7.1385. [DOI] [PubMed] [Google Scholar]
- 242.Kugaya A., Sanacora G. Beyond monoamines: glutamatergic function in mood disorders. CNS Spectr. 2005;10(10):808–819. doi: 10.1017/s1092852900010403. [DOI] [PubMed] [Google Scholar]
- 243.Harvey B.H., Jonker L.P., Brand L., Heenop M., Stein D.J. NMDA receptor involvement in imipramine withdrawal-associated effects on swim stress, GABA levels and NMDA receptor binding in rat hippocampus. Life Sci. 2002;71(1):43–54. doi: 10.1016/S0024-3205(02)01561-8. [DOI] [PubMed] [Google Scholar]
- 244.Harvey B.H., Retief R., Korff A., Wegener G. Increased hippocampal nitric oxide synthase activity and stress responsiveness after imipramine discontinuation: role of 5HT 2A/C-receptors. Metab. Brain Dis. 2006;21(2-3):211–220. doi: 10.1007/s11011-006-9018-1. [DOI] [PubMed] [Google Scholar]
- 245.Yan Q.S., Reith M.E., Jobe P.C., Dailey J.W. Dizocilpine (MK-801) increases not only dopamine but also serotonin and norepinephrine transmissions in the nucleus accumbens as measured by microdialysis in freely moving rats. Brain Res. 1997;765(1):149–158. doi: 10.1016/S0006-8993(97)00568-4. [DOI] [PubMed] [Google Scholar]
- 246.aan het Rot M., Collins K.A., Murrough J.W., Perez A.M., Reich D.L., Charney D.S., Mathew S.J. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol. Psychiatry. 2010;67(2):139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 247.Koike H., Iijima M., Chaki S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav. Brain Res. 2011;224(1):107–111. doi: 10.1016/j.bbr.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 248.Li N., Lee B., Liu R.J., Banasr M., Dwyer J.M., Iwata M., Li X.Y., Aghajanian G., Duman R.S. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Liu R.J., Fuchikami M., Dwyer J.M., Lepack A.E., Duman R.S., Aghajanian G.K. GSK-3 inhibition potentiates the synaptogenic and antidepressant-like effects of subthreshold doses of ketamine. Neuropsychopharmacology. 2013;38(11):2268–2277. doi: 10.1038/npp.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Jernigan C.S., Goswami D.B., Austin M.C., Iyo A.H., Chandran A., Stockmeier C.A., Karolewicz B. The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35(7):1774–1779. doi: 10.1016/j.pnpbp.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Beurel E., Song L., Jope R.S. Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol. Psychiatry. 2011;16(11):1068–1070. doi: 10.1038/mp.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Liu L., Jia F., Yuan G., Chen Z., Yao J., Li H., Fang C. Tyrosine hydroxylase, interleukin-1beta and tumor necrosis factor-alpha are overexpressed in peripheral blood mononuclear cells from schizophrenia patients as determined by semi-quantitative analysis. Psychiatry Res. 2010;176(1):1–7. doi: 10.1016/j.psychres.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 253.Surh Y.J., Kundu J.K., Li M.H., Na H.K., Cha Y.N. Role of Nrf2-mediated heme oxygenase-1 upregulation in adaptive survival response to nitrosative stress. Arch. Pharm. Res. 2009;32(8):1163–1176. doi: 10.1007/s12272-009-1807-8. [DOI] [PubMed] [Google Scholar]
- 254.Harvey B.H., Carstens M.E., Taljaard J.J. Evidence that lithium induces a glutamatergic: nitric oxide-mediated response in rat brain. Neurochem. Res. 1994;19(4):469–474. doi: 10.1007/BF00967326. [DOI] [PubMed] [Google Scholar]
- 255.Ghasemi M., Dehpour A.R. The NMDA receptor/nitric oxide pathway: a target for the therapeutic and toxic effects of lithium. Trends Pharmacol. Sci. 2011;32(7):420–434. doi: 10.1016/j.tips.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 256.Szumlinski K.K., Lominac K.D., Kleschen M.J., Oleson E.B., Dehoff M.H., Schwarz M.K., Seeburg P.H., Worley P.F., Kalivas P.W. Behavioral and neurochemical phenotyping of Homer1 mutant mice: possible relevance to schizophrenia. Genes Brain Behav. 2005;4(5):273–288. doi: 10.1111/j.1601-183X.2005.00120.x. [DOI] [PubMed] [Google Scholar]
- 257.Barbon A., Fumagalli F., La Via L., Caracciolo L., Racagni G., Riva M.A., Barlati S. Chronic phencyclidine administration reduces the expression and editing of specific glutamate receptors in rat prefrontal cortex. Exp. Neurol. 2007;208(1):54–62. doi: 10.1016/j.expneurol.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 258.Mohn A.R., Gainetdinov R.R., Caron M.G., Koller B.H. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98(4):427–436. doi: 10.1016/S0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- 259.Ikeda Y., Yahata N., Ito I., Nagano M., Toyota T., Yoshikawa T., Okubo Y., Suzuki H. Low serum levels of brain-derived neurotrophic factor and epidermal growth factor in patients with chronic schizophrenia. Schizophr. Res. 2008;101(1-3):58–66. doi: 10.1016/j.schres.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 260.Machado-Vieira R., Andreazza A.C., Viale C.I., Zanatto V., Cereser V., Jr, Vargas R.d.S., Kapczinski F., Portela L. V., Souza D. O., Salvador M., Gentil V. Oxidative stress parameters in unmedicated and treated bipolar subjects during initial manic episode: A possible role for lithium antioxidant effects. 2007. [DOI] [PubMed]
- 261.Nibuya M., Nestler E.J., Duman R.S. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J. Neurosci. 1996;16(7):2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Aydemir C., Yalcin E.S., Aksaray S., Kisa C., Yildirim S.G., Uzbay T., Goka E. Brain-derived neurotrophic factor (BDNF) changes in the serum of depressed women. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2006;30(7):1256–1260. doi: 10.1016/j.pnpbp.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 263.Yulug B., Ozan E., Aydin N., Kirpinar I. Brain-derived neurotrophic factor polymorphism: more than a prognostic factor during depression? J. Neuropsychiatry Clin. Neurosci. 2009;21(4):471–472. doi: 10.1176/jnp.2009.21.4.471. [DOI] [PubMed] [Google Scholar]
- 264.Aydemir O., Deveci A., Taneli F. The effect of chronic antidepressant treatment on serum brain-derived neurotrophic factor levels in depressed patients: a preliminary study. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29(2):261–265. doi: 10.1016/j.pnpbp.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 265.Huang T.L., Lee C.T., Liu Y.L. Serum brain-derived neurotrophic factor levels in patients with major depression: effects of antidepressants. J. Psychiatr. Res. 2008;42(7):521–525. doi: 10.1016/j.jpsychires.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 266.Frechilla D., Otano A., Del Río J. Effect of chronic antidepressant treatment on transcription factor binding activity in rat hippocampus and frontal cortex. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1998;22(5):787–802. doi: 10.1016/S0278-5846(98)00040-2. [DOI] [PubMed] [Google Scholar]
- 267.Laifenfeld D., Karry R., Grauer E., Klein E., Ben-Shachar D. Antidepressants and prolonged stress in rats modulate CAM-L1, laminin, and pCREB, implicated in neuronal plasticity. 2005. [DOI] [PubMed]
- 268.Karege F., Vaudan G., Schwald M., Perroud N., La Harpe R. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. 2005. [DOI] [PubMed]
- 269.Pan W., Banks W.A., Fasold M.B., Bluth J., Kastin A.J. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37(12):1553–1561. doi: 10.1016/S0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 270.Karege F., Perret G., Bondolfi G., Schwald M., Bertschy G., Aubry J.M. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109(2):143–148. doi: 10.1016/S0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- 271.Pittenger C., Duman R.S. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33(1):88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 272.Schinder A.F., Poo M. The neurotrophin hypothesis for synaptic plasticity. Trends Neurosci. 2000;23(12):639–645. doi: 10.1016/S0166-2236(00)01672-6. [DOI] [PubMed] [Google Scholar]
- 273.Yamada K., Nabeshima T. Brain-derived neurotrophic factor/ TrkB signaling in memory process. . J. Pharmacol. Sci. 2003; 91:267–270. doi: 10.1254/jphs.91.267es.. [DOI] [PubMed] [Google Scholar]
- 274.Montminy M.R., Gonzalez G.A., Yamamoto K.K. Regulation of cAMP-inducible genes by CREB. Trends Neurosci. 1990;13(5):184–188. doi: 10.1016/0166-2236(90)90045-C. [DOI] [PubMed] [Google Scholar]
- 275.Tao X., Finkbeiner S., Arnold D.B., Shaywitz A.J., Greenberg M.E. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20(4):709–726. doi: 10.1016/S0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 276.Grewal S.S., York R.D., Stork P.J. Extracellular-signal-regulated kinase signalling in neurons. Curr. Opin. Neurobiol. 1999;9(5):544–553. doi: 10.1016/S0959-4388(99)00010-0. [DOI] [PubMed] [Google Scholar]
- 277.Dwivedi Y., Rizavi H.S., Conley R.R., Roberts R.C., Tamminga C.A., Pandey G.N. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch. Gen. Psychiatry. 2003;60(8):804–815. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- 278.Hayley S., Poulter M.O., Merali Z., Anisman H. The pathogenesis of clinical depression: stressor- and cytokine-induced alterations of neuroplasticity. Neuroscience. 2005;135(3):659–678. doi: 10.1016/j.neuroscience.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 279.Harvey B.H. Is major depressive disorder a metabolic encephalopathy? Hum. Psychopharmacol. 2008;23(5):371–384. doi: 10.1002/hup.946. [DOI] [PubMed] [Google Scholar]
- 280.Shelton R.C., Miller A.H. Eating ourselves to death (and despair): The contribution of adiposity and inflammation to depression. . Prog. Neurobiol. 2010;91:275–299. doi: 10.1016/j.pneurobio.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Harvey B.H., Hamer M., Louw R., van der Westhuizen F.H., Malan L. Metabolic and glutathione redox markers associated with brain-derived neurotrophic factor in depressed african men and women: evidence for counterregulation? Neuropsychobiology. 2013;67(1):33–40. doi: 10.1159/000343501. [DOI] [PubMed] [Google Scholar]
- 282.Flensborg-Madsen T., von Scholten M.B., Flachs E.M., Mortensen E.L., Prescott E., Tolstrup J.S. Tobacco smoking as a risk factor for depression. A 26-year population-based follow-up study. J. Psychiatr. Res. 2011;45(2):143–149. doi: 10.1016/j.jpsychires.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 283.Goodwin R.D., Prescott M., Tamburrino M., Calabrese J.R., Liberzon I., Galea S. Smoking is a predictor of depression onset among National Guard soldiers. Psychiatry Res. 2013;206(2-3):321–323. doi: 10.1016/j.psychres.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Golden S.H., Lazo M., Carnethon M., Bertoni A.G., Schreiner P.J., Diez Roux A.V., Lee H.B., Lyketsos C. Examining a bidirectional association between depressive symptoms and diabetes. JAMA. 2008;299(23):2751–2759. doi: 10.1001/jama.299.23.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Kim T.S., Kim D.J., Lee H., Kim Y.K. Increased plasma brain-derived neurotrophic factor levels in chronic smokers following unaided smoking cessation. Neurosci. Lett. 2007;423(1):53–57. doi: 10.1016/j.neulet.2007.05.064. [DOI] [PubMed] [Google Scholar]
- 286.Bhang S.Y., Choi S.W., Ahn J.H. Changes in plasma brain-derived neurotrophic factor levels in smokers after smoking cessation. Neurosci. Lett. 2010;468(1):7–11. doi: 10.1016/j.neulet.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 287.Krabbe K.S., Nielsen A.R., Krogh-Madsen R., Plomgaard P., Rasmussen P., Erikstrup C., Fischer C.P., Lindegaard B., Petersen A.M., Taudorf S., Secher N.H., Pilegaard H., Bruunsgaard H., Pedersen B.K. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia. 2007;50(2):431–438. doi: 10.1007/s00125-006-0537-4. [DOI] [PubMed] [Google Scholar]
- 288.Fujinami A., Ohta K., Obayashi H., Fukui M., Hasegawa G., Nakamura N., Kozai H., Imai S., Ohta M. Serum brain-derived neurotrophic factor in patients with type 2 diabetes mellitus: Relationship to glucose metabolism and biomarkers of insulin resistance. Clin. Biochem. 2008;41(10-11):812–817. doi: 10.1016/j.clinbiochem.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 289.Griffin E.W., Mullally S., Foley C., Warmington S.A., O’Mara S.M., Kelly A.M. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol. Behav. 2011;104(5):934–941. doi: 10.1016/j.physbeh.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 290.Cotman C.W., Berchtold N.C. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25(6):295–301. doi: 10.1016/S0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 291.Byrne A., Byrne D.G. The effect of exercise on depression, anxiety and other mood states: a review. J. Psychosom. Res. 1993;37(6):565–574. doi: 10.1016/0022-3999(93)90050-P. [DOI] [PubMed] [Google Scholar]
- 292.Popper C.W. Mood disorders in youth: exercise, light therapy, and pharmacologic complementary and integrative approaches. Child Adolesc. Psychiatr. Clin. N. Am. 2013;22(3):403–441, v. doi: 10.1016/j.chc.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 293.Machado-Vieira R., Dietrich M.O., Leke R., Cereser V.H., Zanatto V., Kapczinski F., Souza D. O., Portela L. V., Gentil V. Decreased Plasma Brain Derived Neurotrophic Factor Levels in Unmedicated Bipolar Patients During Manic Episode. 2007. [DOI] [PubMed]
- 294.Sylvia L.G., Friedman E.S., Kocsis J.H., Bernstein E.E., Brody B.D., Kinrys G., Kemp D.E., Shelton R.C., McElroy S.L., Bobo W.V., Kamali M., McInnis M.G., Tohen M., Bowden C.L., Ketter T.A., Deckersbach T., Calabrese J.R., Thase M.E., Reilly-Harrington N.A., Singh V., Rabideau D.J., Nierenberg A.A. Association of exercise with quality of life and mood symptoms in a comparative effectiveness study of bipolar disorder. J. Affect. Disord. 2013;151(2):722–727. doi: 10.1016/j.jad.2013.07.031. [DOI] [PubMed] [Google Scholar]
- 295.Green M.J., Matheson S.L., Shepherd A., Weickert C.S., Carr V.J. Brain-derived neurotrophic factor levels in schizophrenia: a systematic review with meta-analysis. Mol. Psychiatry. 2011;16(9):960–972. doi: 10.1038/mp.2010.88. [DOI] [PubMed] [Google Scholar]
- 296.Vinogradov S., Fisher M., Holland C., Shelly W., Wolkowitz O., Mellon S.H. Is Serum Brain-Derived Neurotrophic Factor a Biomarker for Cognitive Enhancement in Schizophrenia? 2009. [DOI] [PMC free article] [PubMed]
- 297.Iritani S., Niizato K., Nawa H., Ikeda K., Emson P.C. Immunohistochemical study of brain-derived neurotrophic factor and its receptor, TrkB, in the hippocampal formation of schizophrenic brains. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27(5):801–807. doi: 10.1016/S0278-5846(03)00112-X. [DOI] [PubMed] [Google Scholar]
- 298.Durany N., Michel T., Zöchling R., Boissl K.W., Cruz-Sánchez F.F., Riederer P., Thome J. Brain-derived neurotrophic factor and neurotrophin 3 in schizophrenic psychoses. Schizophr. Res. 2001;52(1-2):79–86. doi: 10.1016/S0920-9964(00)00084-0. [DOI] [PubMed] [Google Scholar]
- 299.Dowlati Y., Herrmann N., Swardfager W., Liu H., Sham L., Reim E.K., Lanctôt K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 300.Chen A.C., Shirayama Y., Shin K.H., Neve R.L., Duman R.S. Expression of the cAMP response element binding protein (CREB) in hippocampus produces an antidepressant effect. Biol. Psychiatry, . 2001;49:753–762. doi: 10.1016/S0006-3223(00)01114-8. [DOI] [PubMed] [Google Scholar]
- 301.Elfving B., Plougmann P.H., Wegener G. Differential brain, but not serum VEGF levels in a genetic rat model of depression. Neurosci. Lett. 2010;474(1):13–16. doi: 10.1016/j.neulet.2010.02.063. [DOI] [PubMed] [Google Scholar]
- 302.Roceri M., Hendriks W., Racagni G., Ellenbroek B.A., Riva M.A. Early maternal deprivation reduces the expression of BDNF and NMDA receptor subunits in rat hippocampus. Mol. Psychiatry. 2002;7(6):609–616. doi: 10.1038/sj.mp.4001036. [DOI] [PubMed] [Google Scholar]
- 303.Elfving B., Plougmann P.H., Müller H.K., Mathé A.A., Rosenberg R., Wegener G. Inverse correlation of brain and blood BDNF levels in a genetic rat model of depression. Int. J. Neuropsychopharmacol. 2010;13(5):563–572. doi: 10.1017/S1461145709990721. [DOI] [PubMed] [Google Scholar]
- 304.Schmidt H.D., Duman R.S. Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology. 2010;35(12):2378–2391. doi: 10.1038/npp.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Nibuya M., Morinobu S., Duman R.S. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J. Neurosci. 1995;15(11):7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Blugeot A., Rivat C., Bouvier E., Molet J., Mouchard A., Zeau B., Bernard C., Benoliel J.J., Becker C. Vulnerability to depression: from brain neuroplasticity to identification of biomarkers. J. Neurosci. 2011;31(36):12889–12899. doi: 10.1523/JNEUROSCI.1309-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Cannon T.D., Van Erp T.G.M., Bearden C.E., Loewy R., Thompson P., Toga A.W., Huttunen M. O., Keshavan M. S., Seidman L. J., Tsuang M. T. 2003. [DOI] [PubMed]
- 308.Yeom M., Shim I., Lee H.J., Hahm D.H. Proteomic analysis of nicotine-associated protein expression in the striatum of repeated nicotine-treated rats. Biochem. Biophys. Res. Commun. 2005;326(2):321–328. doi: 10.1016/j.bbrc.2004.11.034. [DOI] [PubMed] [Google Scholar]
- 309.Jornada L.K., Moretti M., Valvassori S.S., Ferreira C.L., Padilha P.T., Arent C.O., Fries G.R., Kapczinski F., Quevedo J. Effects of mood stabilizers on hippocampus and amygdala BDNF levels in an animal model of mania induced by ouabain. J. Psychiatr. Res. 2010;44(8):506–510. doi: 10.1016/j.jpsychires.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 310.Jornada L.K., Moretti M., Valvassori S.S., Ferreira C.L., Padilha P.T., Arent C.O., Fries G. R., Kapczinski F., Quevedo J. 2006. [DOI] [PubMed]
- 311.Wall V.L., Fischer E.K., Bland S.T. Isolation rearing attenuates social interaction-induced expression of immediate early gene protein products in the medial prefrontal cortex of male and female rats. Physiol. Behav. 2012;107(3):440–450. doi: 10.1016/j.physbeh.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Scaccianoce S., Del Bianco P., Paolone G., Caprioli D., Modafferi A.M., Nencini P., Badiani A. Social isolation selectively reduces hippocampal brain-derived neurotrophic factor without altering plasma corticosterone. Behav. Brain Res. 2006;168(2):323–325. doi: 10.1016/j.bbr.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 313.Stewart C.E., Rotwein P. Growth, differentiation, and survival: multiple physiological functions for insulin-like growth factors. Physiol. Rev. 1996;76(4):1005–1026. doi: 10.1152/physrev.1996.76.4.1005. [DOI] [PubMed] [Google Scholar]
- 314.Anlar B., Sullivan K.A., Feldman E.L. Insulin-like growth factor-I and central nervous system development. Horm. Metab. Res. 1999;31(2-3):120–125. doi: 10.1055/s-2007-978708. [DOI] [PubMed] [Google Scholar]
- 315.Bezchlibnyk Y.B., Xu L., Wang J.F., Young L.T. Decreased expression of insulin-like growth factor binding protein 2 in the prefrontal cortex of subjects with bipolar disorder and its regulation by lithium treatment. Brain Res. 2007;1147:213–217. doi: 10.1016/j.brainres.2007.01.147. [DOI] [PubMed] [Google Scholar]
- 316.Venkatasubramanian G., Chittiprol S., Neelakantachar N., Naveen M.N., Thirthall J., Gangadhar B.N., Shetty K.T. Insulin and insulin-like growth factor-1 abnormalities in antipsychotic-naive schizophrenia. Am. J. Psychiatry. 2007;164(10):1557–1560. doi: 10.1176/appi.ajp.2007.07020233. [DOI] [PubMed] [Google Scholar]
- 317.Duman C.H., Schlesinger L., Terwilliger R., Russell D.S., Newton S.S., Duman R.S. Peripheral insulin-like growth factor-I produces antidepressant-like behavior and contributes to the effect of exercise. Behav. Brain Res. 2009;198(2):366–371. doi: 10.1016/j.bbr.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Carro E., Nuñez A., Busiguina S., Torres-Aleman I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J. Neurosci. 2000;20(8):2926–2933. doi: 10.1523/JNEUROSCI.20-08-02926.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Khawaja X., Xu J., Liang J.J., Barrett J.E. Proteomic analysis of protein changes developing in rat hippocampus after chronic antidepressant treatment: Implications for depressive disorders and future therapies. J. Neurosci. Res. 2004;75(4):451–460. doi: 10.1002/jnr.10869. [DOI] [PubMed] [Google Scholar]
- 320.Anderson M.F., Åberg M.A., Nilsson M., Eriksson P.S. Insulin-like growth factor-I and neurogenesis in the adult mammalian brain. Brain Res. Dev. Brain Res. 2002;134(1-2):115–122. doi: 10.1016/S0165-3806(02)00277-8. [DOI] [PubMed] [Google Scholar]
- 321.Leung D.W., Cachianes G., Kuang W.J., Goeddel D.V., Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246(4935):1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 322.Palmer T.D., Willhoite A.R., Gage F.H. Vascular niche for adult hippocampal neurogenesis. J. Comp. Neurol. 2000;425(4):479–494. doi: 10.1002/1096-9861(20001002)425:4<479::AID-CNE2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 323.Sun Y., Jin K., Xie L., Childs J., Mao X.O., Logvinova A., Greenberg D.A. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J. Clin. Invest. 2003;111(12):1843–1851. doi: 10.1172/JCI200317977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Iga J., Ueno S., Yamauchi K., Numata S., Tayoshi-Shibuya S., Kinouchi S., Nakataki M., Song H., Hokoishi K., Tanabe H., Sano A., Ohmori T. Gene expression and association analysis of vascular endothelial growth factor in major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2007;31(3):658–663. doi: 10.1016/j.pnpbp.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 325.Kahl K.G., Bens S., Ziegler K., Rudolf S., Kordon A., Dibbelt L., Schweiger U. Angiogenic factors in patients with current major depressive disorder comorbid with borderline personality disorder. Psychoneuroendocrinology. 2009;34(3):353–357. doi: 10.1016/j.psyneuen.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 326.Takebayashi M., Hashimoto R., Hisaoka K., Tsuchioka M., Kunugi H. Plasma levels of vascular endothelial growth factor and fibroblast growth factor 2 in patients with major depressive disorders. J. Neural Transm. 2010;117(9):1119–1122. doi: 10.1007/s00702-010-0452-1. [DOI] [PubMed] [Google Scholar]
- 327.Lee B.H., Kim Y.K. Increased plasma VEGF levels in major depressive or manic episodes in patients with mood disorders. J. Affect. Disord. 2012;136(1-2):181–184. doi: 10.1016/j.jad.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 328.Kikuchi K., Iga J., Tayoshi S., Nakataki M., Watanabe S., Numata S., Ohmori T. Lithium decreases VEGF mRNA expression in leukocytes of healthy subjects and patients with bipolar disorder. Hum. Psychopharmacol. 2011;26(4-5):358–363. doi: 10.1002/hup.1215. [DOI] [PubMed] [Google Scholar]
- 329.Fulzele S., Pillai A. Decreased VEGF mRNA expression in the dorsolateral prefrontal cortex of schizophrenia subjects. 2009. [DOI] [PubMed]
- 330.Pillai A., Mahadik S.P. Differential effects of haloperidol and olanzapine on levels of vascular endothelial growth factor and angiogenesis in rat hippocampus. Schizophr. Res. 2006;87(1-3):48–59. doi: 10.1016/j.schres.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 331.Heim C., Binder E.B. 2012.
- 332.Miller S., Hallmayer J., Wang P.W., Hill S.J., Johnson S.L., Ketter T.A. Brain-derived neurotrophic factor val66met genotype and early life stress effects upon bipolar course. 2013. [DOI] [PMC free article] [PubMed]
- 333.Nestler E.J., Barrot M., DiLeone R.J., Eisch A.J., Gold S.J., Monteggia L.M. Neurobiology of depression. Neuron. 2002;34(1):13–25. doi: 10.1016/S0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 334.Bonni A., Brunet A., West A.E., Datta S.R., Takasu M.A., Greenberg M.E. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286(5443):1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- 335.Duman R.S., Monteggia L.M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry. 2006;59(12):1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 336.Fernandes B.S., Gama C.S., Ceresér K.M., Yatham L.N., Fries G.R., Colpo G., de Lucena D., Kunz M., Gomes F.A., Kapczinski F. Brain-derived neurotrophic factor as a state-marker of mood episodes in bipolar disorders: a systematic review and meta-regression analysis. J. Psychiatr. Res. 2011;45(8):995–1004. doi: 10.1016/j.jpsychires.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 337.Molendijk M.L., Spinhoven P., Polak M., Bus B.A., Penninx B.W., Elzinga B.M. Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N=9484). Mol. Psychiatry. 2014;19(7):791–800. doi: 10.1038/mp.2013.105. [DOI] [PubMed] [Google Scholar]
- 338.Wolkowitz O.M., Wolf J., Shelly W., Rosser R., Burke H.M., Lerner G.K., Reus V.I., Nelson J.C., Epel E.S., Mellon S.H. Serum BDNF levels before treatment predict SSRI response in depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35(7):1623–1630. doi: 10.1016/j.pnpbp.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Duman R.S. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular Med. 2004;5(1):11–25. doi: 10.1385/NMM:5:1:011. [DOI] [PubMed] [Google Scholar]
- 340.Réus G.Z., Vieira F.G., Abelaira H.M., Michels M., Tomaz D.B., dos Santos M.A.B., Carlessi A. S., Neotti M. V., Matias B. I., Luz J. R., Dal-Pizzol F., Quevedo J. MAPK signaling correlates with the antidepressant effects of ketamine. 2014. [DOI] [PubMed]
- 341.Huang E.J., Reichardt L.F. Trk receptors: roles in neuronal signal transduction. Annu. Rev. Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- 342.Duman R.S., Voleti B. Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid-acting agents. Trends Neurosci. 2012;35(1):47–56. doi: 10.1016/j.tins.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Kamada H., Nito C., Endo H., Chan P.H. Bad as a converging signaling molecule between survival PI3-K/Akt and death JNK in neurons after transient focal cerebral ischemia in rats. J. Cereb. Blood Flow Metab. 2007;27(3):521–533. doi: 10.1038/sj.jcbfm.9600367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Dwivedi Y., Rizavi H.S., Roberts R.C., Conley R.C., Tamminga C.A., Pandey G.N. Reduced activation and expression of ERK1/2 MAP kinase in the post-mortem brain of depressed suicide subjects. J. Neurochem. 2001;77(3):916–928. doi: 10.1046/j.1471-4159.2001.00300.x. [DOI] [PubMed] [Google Scholar]
- 345.Rowe M.K., Wiest C., Chuang D.M. GSK-3 is a viable potential target for therapeutic intervention in bipolar disorder. Neurosci. Biobehav. Rev. 2007;31(6):920–931. doi: 10.1016/j.neubiorev.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Bullock B.P., Habener J.F. Phosphorylation of the cAMP response element binding protein CREB by cAMP-dependent protein kinase A and glycogen synthase kinase-3 alters DNA-binding affinity, conformation, and increases net charge. Biochemistry. 1998;37(11):3795–3809. doi: 10.1021/bi970982t. [DOI] [PubMed] [Google Scholar]
- 347.Watcharasit P., Bijur G.N., Zmijewski J.W., Song L., Zmijewska A., Chen X., Johnson G.V., Jope R.S. Direct, activating interaction between glycogen synthase kinase-3beta and p53 after DNA damage. Proc. Natl. Acad. Sci. USA. 2002;99(12):7951–7955. doi: 10.1073/pnas.122062299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Hanada M., Feng J., Hemmings B.A. 2004. [DOI] [PubMed]
- 349.Hu L.W., Kawamoto E.M., Brietzke E., Scavone C., Lafer B. The role of Wnt signaling and its interaction with diverse mechanisms of cellular apoptosis in the pathophysiology of bipolar disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35(1):11–17. doi: 10.1016/j.pnpbp.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 350.Gao X., Joselin A.P., Wang L., Kar A., Ray P., Bateman A., Goate A.M., Wu J.Y. Progranulin promotes neurite outgrowth and neuronal differentiation by regulating GSK-3β. Protein Cell. 2010;1(6):552–562. doi: 10.1007/s13238-010-0067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Galimberti D., Dell’Osso B., Fenoglio C., Villa C., Cortini F., Serpente M., Kittel-Schneider S., Weigl J., Neuner M., Volkert J., Leonhard C., Olmes D.G., Kopf J., Cantoni C., Ridolfi E., Palazzo C., Ghezzi L., Bresolin N., Altamura A.C., Scarpini E., Reif A. Progranulin gene variability and plasma levels in bipolar disorder and schizophrenia. PLoS One. 2012;7(4):e32164. doi: 10.1371/journal.pone.0032164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Kittel-Schneider S., Weigl J., Volkert J., Geßner A., Schmidt B., Hempel S., Kiel T., Olmes D.G., Bartl J., Weber H., Kopf J., Reif A. Further evidence for plasma progranulin as a biomarker in bipolar disorder. J. Affect. Disord. 2014;157:87–91. doi: 10.1016/j.jad.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 353.Pandey G.N., Ren X., Rizavi H.S., Dwivedi Y. Glycogen synthase kinase-3β in the platelets of patients with mood disorders: effect of treatment. J. Psychiatr. Res. 2010;44(3):143–148. doi: 10.1016/j.jpsychires.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 354.Funk A.J., McCullumsmith R.E., Haroutunian V., Meador-Woodruff J.H. Abnormal activity of the MAPK- and cAMP-associated signaling pathways in frontal cortical areas in postmortem brain in schizophrenia. Neuropsychopharmacology. 2012;37(4):896–905. doi: 10.1038/npp.2011.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Kyosseva S.V. Differential expression of mitogen-activated protein kinases and immediate early genes fos and jun in thalamus in schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2004;28(6):997–1006. doi: 10.1016/j.pnpbp.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 356.Todorova V.K., Elbein A.D., Kyosseva S.V. Increased expression of c-Jun transcription factor in cerebellar vermis of patients with schizophrenia. Neuropsychopharmacology. 2003;28(8):1506–1514. doi: 10.1038/sj.npp.1300211. [DOI] [PubMed] [Google Scholar]
- 357.Yuan P., Zhou R., Wang Y., Li X., Li J., Chen G., Guitart X., Manji H.K. Altered levels of extracellular signal-regulated kinase signaling proteins in postmortem frontal cortex of individuals with mood disorders and schizophrenia. J. Affect. Disord. 2010;124(1-2):164–169. doi: 10.1016/j.jad.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Albert K.A., Hemmings H.C., Jr, Adamo A.I., Potkin S.G., Akbarian S., Sandman C.A., Cotman C.W., Bunney W.E., Jr, Greengard P. Evidence for decreased DARPP-32 in the prefrontal cortex of patients with schizophrenia. Arch. Gen. Psychiatry. 2002;59(8):705–712. doi: 10.1001/archpsyc.59.8.705. [DOI] [PubMed] [Google Scholar]
- 359.Feldcamp L.A., Souza R.P., Romano-Silva M., Kennedy J.L., Wong A.H. Reduced prefrontal cortex DARPP-32 mRNA in completed suicide victims with schizophrenia. Schizophr. Res. 2008;103(1-3):192–200. doi: 10.1016/j.schres.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 360.Gururajan A., van den Buuse M. Is the mTOR-signalling cascade disrupted in Schizophrenia? J. Neurochem. 2014;129(3):377–387. doi: 10.1111/jnc.12622. [DOI] [PubMed] [Google Scholar]
- 361.Levine J.B., Youngs R.M., MacDonald M.L., Chu M., Leeder A.D., Berthiaume F., Konradi C. Isolation rearing and hyperlocomotion are associated with reduced immediate early gene expression levels in the medial prefrontal cortex. Neuroscience. 2007;145(1):42–55. doi: 10.1016/j.neuroscience.2006.11.063. [DOI] [PubMed] [Google Scholar]
- 362.Prickaerts J., Moechars D., Cryns K., Lenaerts I., van Craenendonck H., Goris I., Daneels G., Bouwknecht J.A., Steckler T. Transgenic mice overexpressing glycogen synthase kinase 3beta: a putative model of hyperactivity and mania. J. Neurosci. 2006;26(35):9022–9029. doi: 10.1523/JNEUROSCI.5216-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Hannah-Poquette C., Anderson G.W., Flaisher-Grinberg S., Wang J., Meinerding T.M., Einat H. 2011. [DOI] [PubMed]
- 364.Gould T.D., Einat H., O’Donnell K.C., Picchini A.M., Schloesser R.J., Manji H.K. Beta-catenin overexpression in the mouse brain phenocopies lithium-sensitive behaviors. Neuropsychopharmacology. 2007;32(10):2173–2183. doi: 10.1038/sj.npp.1301338. [DOI] [PubMed] [Google Scholar]
- 365.Harvey B.H., Meyer C.L., Gallichio V.S., Manji H.K. Lithium salts in AIDS and AIDS-related dementia. Psychopharmacol. Bull. 2002;36(1):5–26. [PubMed] [Google Scholar]
- 366.Bitanihirwe B.K., Woo T.U. Oxidative stress in schizophrenia: an integrated approach. Neurosci. Biobehav. Rev. 2011;35(3):878–893. doi: 10.1016/j.neubiorev.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Young J., McKinney S.B., Ross B.M., Wahle K.W., Boyle S.P. Biomarkers of oxidative stress in schizophrenic and control subjects. Prostaglandins Leukot. Essent. Fatty Acids. 2007;76(2):73–85. doi: 10.1016/j.plefa.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 368.Bains J.S., Shaw C.A. Neurodegenerative disorders in humans: the role of glutathione in oxidative stress-mediated neuronal death. Brain Res. Brain Res. Rev. 1997;25(3):335–358. doi: 10.1016/S0165-0173(97)00045-3. [DOI] [PubMed] [Google Scholar]
- 369.Griffith O.W. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic. Biol. Med. 1999;27(9-10):922–935. doi: 10.1016/S0891-5849(99)00176-8. [DOI] [PubMed] [Google Scholar]
- 370.Bouligand J., Deroussent A., Paci A., Morizet J., Vassal G. Liquid chromatography-tandem mass spectrometry assay of reduced and oxidized glutathione and main precursors in mice liver. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2006;832(1):67–74. doi: 10.1016/j.jchromb.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 371.Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet. 1994;344(8924):721–724. doi: 10.1016/S0140-6736(94)92211-X. [DOI] [PubMed] [Google Scholar]
- 372.Garcia-Cazorla A., Duarte S., Serrano M., Nascimento A., Ormazabal A., Carrilho I., Briones P., Montoya J., Garesse R., Sala-Castellvi P., Pineda M., Artuch R. Mitochondrial diseases mimicking neurotransmitter defects. Mitochondrion. 2008;8(3):273–278. doi: 10.1016/j.mito.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 373.Möller M., Du Preez J.L., Emsley R., Harvey B.H. Isolation rearing-induced deficits in sensorimotor gating and social interaction in rats are related to cortico-striatal oxidative stress, and reversed by sub-chronic clozapine administration. Eur. Neuropsychopharmacol. 2011;21(6):471–483. doi: 10.1016/j.euroneuro.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 374.Dhir A., Kulkarni S.K. Nitric oxide and major depression. Nitric Oxide. 2011;24(3):125–131. doi: 10.1016/j.niox.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 375.Selek S., Savas H.A., Gergerlioglu H.S., Bulbul F., Uz E., Yumru M. The course of nitric oxide and superoxide dismutase during treatment of bipolar depressive episode. J. Affect. Disord. 2008;107(1-3):89–94. doi: 10.1016/j.jad.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 376.Sarandol A., Sarandol E., Eker S.S., Erdinc S., Vatansever E., Kirli S. Major depressive disorder is accompanied with oxidative stress: short-term antidepressant treatment does not alter oxidative-antioxidative systems. Hum. Psychopharmacol. 2007;22(2):67–73. doi: 10.1002/hup.829. [DOI] [PubMed] [Google Scholar]
- 377.Khanzode S.D., Dakhale G.N., Khanzode S.S., Saoji A., Palasodkar R. Oxidative damage and major depression: the potential antioxidant action of selective serotonin re-uptake inhibitors. Redox Rep. 2003;8(6):365–370. doi: 10.1179/135100003225003393. [DOI] [PubMed] [Google Scholar]
- 378.Srivastava N., Barthwal M.K., Dalal P.K., Agarwal A.K., Nag D., Seth P.K., Srimal R.C., Dikshit M. A study on nitric oxide, beta-adrenergic receptors and antioxidant status in the polymorphonuclear leukocytes from the patients of depression. J. Affect. Disord. 2002;72(1):45–52. doi: 10.1016/S0165-0327(01)00421-9. [DOI] [PubMed] [Google Scholar]
- 379.Berk M., Dean O.M., Cotton S.M., Jeavons S., Tanious M., Kohlmann K., Hewitt K., Moss K., Allwang C., Schapkaitz I., Robbins J., Cobb H., Ng F., Dodd S., Bush A.I., Malhi G.S. The efficacy of adjunctive N-acetylcysteine in major depressive disorder: a double-blind, randomized, placebo-controlled trial. J. Clin. Psychiatry. 2014;75(6):628–636. doi: 10.4088/JCP.13m08454. [DOI] [PubMed] [Google Scholar]
- 380.Knol M.J., Twisk J.W., Beekman A.T., Heine R.J., Snoek F.J., Pouwer F. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia. 2006;49(5):837–845. doi: 10.1007/s00125-006-0159-x. [DOI] [PubMed] [Google Scholar]
- 381.Capuron L., Su S., Miller A.H., Bremner J.D., Goldberg J., Vogt G.J., Maisano C., Jones L., Murrah N.V., Vaccarino V. Depressive symptoms and metabolic syndrome: is inflammation the underlying link? Biol. Psychiatry. 2008;64(10):896–900. doi: 10.1016/j.biopsych.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Yuan Z.R., Liu B., Zhang Y., Yuan L., Muteliefu G., Lu J. Upregulated expression of neuronal nitric oxide synthase by insulin in both neurons and astrocytes. Brain Res. 2004;1008(1):1–10. doi: 10.1016/j.brainres.2004.01.076. [DOI] [PubMed] [Google Scholar]
- 383.García-Bueno B., Pérez-Nievas B.G., Leza J.C. Is there a role for the nuclear receptor PPARγ in neuropsychiatric diseases? Int. J. Neuropsychopharmacol. 2010;13(10):1411–1429. doi: 10.1017/S1461145710000970. [DOI] [PubMed] [Google Scholar]
- 384.Andreazza A.C., Cassini C., Rosa A.R., Leite M.C., de Almeida L.M., Nardin P., Cunha A.B., Ceresér K.M., Santin A., Gottfried C., Salvador M., Kapczinski F., Gonçalves C.A. Serum S100B and antioxidant enzymes in bipolar patients. J. Psychiatr. Res. 2007;41(6):523–529. doi: 10.1016/j.jpsychires.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 385.Wang J.F., Shao L., Sun X., Young L.T. Increased oxidative stress in the anterior cingulate cortex of subjects with bipolar disorder and schizophrenia. Bipolar Disord. 2009;11(5):523–529. doi: 10.1111/j.1399-5618.2009.00717.x. [DOI] [PubMed] [Google Scholar]
- 386.Berk M., Kapczinski F., Andreazza A.C., Dean O.M., Giorlando F., Maes M., Yücel M., Gama C.S., Dodd S., Dean B., Magalhães P.V., Amminger P., McGorry P., Malhi G.S. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci. Biobehav. Rev. 2011;35(3):804–817. doi: 10.1016/j.neubiorev.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 387.Berk M., Copolov D.L., Dean O., Lu K., Jeavons S., Schapkaitz I., Anderson-Hunt M., Bush A.I. N-acetyl cysteine for depressive symptoms in bipolar disorder--a double-blind randomized placebo-controlled trial. Biol. Psychiatry. 2008;64(6):468–475. doi: 10.1016/j.biopsych.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 388.Magalhães P.V., Dean O.M., Bush A.I., Copolov D.L., Malhi G.S., Kohlmann K., Jeavons S., Schapkaitz I., Anderson-Hunt M., Berk M. N-acetylcysteine for major depressive episodes in bipolar disorder. Rev. Bras. Psiquiatr. 2011;33(4):374–378. doi: 10.1590/S1516-44462011000400011. [DOI] [PubMed] [Google Scholar]
- 389.Mahadik S.P., Mukherjee S. Free radical pathology and antioxidant defense in schizophrenia: a review. Schizophr. Res. 1996;19(1):1–17. doi: 10.1016/0920-9964(95)00049-6. [DOI] [PubMed] [Google Scholar]
- 390.Gawryluk J.W., Wang J.F., Andreazza A.C., Shao L., Young L.T. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int. J. Neuropsychopharmacol. 2011;14(1):123–130. doi: 10.1017/S1461145710000805. [DOI] [PubMed] [Google Scholar]
- 391.Do K.Q., Trabesinger A.H., Kirsten-Krüger M., Lauer C.J., Dydak U., Hell D., Holsboer F., Boesiger P., Cuénod M. Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur. J. Neurosci. 2000;12(10):3721–3728. doi: 10.1046/j.1460-9568.2000.00229.x. [DOI] [PubMed] [Google Scholar]
- 392.Zhang X.Y., Chen C., Xiu M.H., Tang W., Zhang F., Liu L., Chen Y., Liu J., Yao J.K., Kosten T.A., Kosten T.R. Plasma total antioxidant status and cognitive impairments in schizophrenia. Schizophr. Res. 2012;139(1-3):66–72. doi: 10.1016/j.schres.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 393.Wu Z., Zhang X.Y., Wang H., Tang W., Xia Y., Zhang F., Liu J., Fu Y., Hu J., Chen Y., Liu L., Chen C., Xiu M.H., Kosten T.R., He J. Elevated plasma superoxide dismutase in first-episode and drug naive patients with schizophrenia: inverse association with positive symptoms. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012;36(1):34–38. doi: 10.1016/j.pnpbp.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 394.Martínez-Cengotitabengoa M., Mac-Dowell K.S., Leza J.C., Micó J.A., Fernandez M., Echevarría E., Sanjuan J., Elorza J., González-Pinto A. Cognitive impairment is related to oxidative stress and chemokine levels in first psychotic episodes. Schizophr. Res. 2012;137(1-3):66–72. doi: 10.1016/j.schres.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 395.Lavoie S., Murray M.M., Deppen P., Knyazeva M.G., Berk M., Boulat O., Bovet P., Bush A.I., Conus P., Copolov D., Fornari E., Meuli R., Solida A., Vianin P., Cuénod M., Buclin T., Do K.Q. Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology. 2008;33(9):2187–2199. doi: 10.1038/sj.npp.1301624. [DOI] [PubMed] [Google Scholar]
- 396.Mahadik S.P., Evans D.R. Is schizophrenia a metabolic brain disorder? Membrane phospholipid dysregulation and its therapeutic implications. Psychiatr. Clin. North Am. 2003;26(1):85–102. doi: 10.1016/S0193-953X(02)00033-3. [DOI] [PubMed] [Google Scholar]
- 397.Emsley R., Chiliza B., Asmal L., du Plessis S., Phahladira L., van Niekerk E., van Rensburg S.J., Harvey B.H. A randomized, controlled trial of omega-3 fatty acids plus an antioxidant for relapse prevention after antipsychotic discontinuation in first-episode schizophrenia. Schizophr. Res. 2014;158(1-3):230–235. doi: 10.1016/j.schres.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 398.Della F.P., Abelaira H.M., Réus G.Z., Ribeiro K.F., Antunes A.R., Scaini G., Jeremias I.C., dos Santos L.M., Jeremias G.C., Streck E.L., Quevedo J. Tianeptine treatment induces antidepressive-like effects and alters BDNF and energy metabolism in the brain of rats. Behav. Brain Res. 2012;233(2):526–535. doi: 10.1016/j.bbr.2012.05.039. [DOI] [PubMed] [Google Scholar]
- 399.Ferreira F.R., Biojone C., Joca S.R., Guimarães F.S. Antidepressant-like effects of N-acetyl-L-cysteine in rats. Behav. Pharmacol. 2008;19(7):747–750. doi: 10.1097/FBP.0b013e3283123c98. [DOI] [PubMed] [Google Scholar]
- 400.Mokoena M.L., Harvey B.H., Viljoen F., Ellis S.M., Brink C.B. Ozone exposure of Flinders Sensitive Line rats is a rodent translational model of neurobiological oxidative stress with relevance for depression and antidepressant response. Psychopharmacology (Berl.) 2015;232(16):2921–2938. doi: 10.1007/s00213-015-3928-8. [DOI] [PubMed] [Google Scholar]
- 401.Ricote M., Li A.C., Willson T.M., Kelly C.J., Glass C.K. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature. 1998;391(6662):79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 402.Sertznig P., Seifert M., Tilgen W., Reichrath J. Present concepts and future outlook: function of peroxisome proliferator-activated receptors (PPARs) for pathogenesis, progression, and therapy of cancer. J. Cell. Physiol. 2007;212(1):1–12. doi: 10.1002/jcp.20998. [DOI] [PubMed] [Google Scholar]
- 403.Waku T., Shiraki T., Oyama T., Maebara K., Nakamori R., Morikawa K. The nuclear receptor PPARγ individually responds to serotonin- and fatty acid-metabolites. EMBO J. 2010;29(19):3395–3407. doi: 10.1038/emboj.2010.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Scher J.U., Pillinger M.H. 15d-PGJ2: the anti-inflammatory prostaglandin? Clin. Immunol. 2005;114(2):100–109. doi: 10.1016/j.clim.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 405.García-Bueno B., Caso J.R., Pérez-Nievas B.G., Lorenzo P., Leza J.C. Effects of peroxisome proliferator-activated receptor gamma agonists on brain glucose and glutamate transporters after stress in rats. Neuropsychopharmacology. 2007;32(6):1251–1260. doi: 10.1038/sj.npp.1301252. [DOI] [PubMed] [Google Scholar]
- 406.García-Bueno B., Madrigal J.L., Lizasoain I., Moro M.A., Lorenzo P., Leza J.C. The anti-inflammatory prostaglandin 15d-PGJ2 decreases oxidative/nitrosative mediators in brain after acute stress in rats. Psychopharmacology (Berl.) 2005;180(3):513–522. doi: 10.1007/s00213-005-2195-5. [DOI] [PubMed] [Google Scholar]
- 407.Eissa Ahmed A.A., Al-Rasheed N.M., Al-Rasheed N.M. Antidepressant-like effects of rosiglitazone, a PPARγ agonist, in the rat forced swim and mouse tail suspension tests. Behav. Pharmacol. 2009;20(7):635–642. doi: 10.1097/FBP.0b013e328331b9bf. [DOI] [PubMed] [Google Scholar]
- 408.Jornada L.K., Valvassori S.S., Steckert A.V., Moretti M., Mina F., Ferreira C.L., Arent C.O., Dal-Pizzol F., Quevedo J. Lithium and valproate modulate antioxidant enzymes and prevent ouabain-induced oxidative damage in an animal model of mania. J. Psychiatr. Res. 2011;45(2):162–168. doi: 10.1016/j.jpsychires.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 409.Bazinet R.P., Rao J.S., Chang L., Rapoport S.I., Lee H.J. Chronic carbamazepine decreases the incorporation rate and turnover of arachidonic acid but not docosahexaenoic acid in brain phospholipids of the unanesthetized rat: relevance to bipolar disorder. Biol. Psychiatry. 2006;59(5):401–407. doi: 10.1016/j.biopsych.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 410.Lee H.J., Ertley R.N., Rapoport S.I., Bazinet R.P., Rao J.S. Chronic administration of lamotrigine downregulates COX-2 mRNA and protein in rat frontal cortex. Neurochem. Res. 2008;33(5):861–866. doi: 10.1007/s11064-007-9526-3. [DOI] [PubMed] [Google Scholar]
- 411.Goldstein B.I., Kemp D.E., Soczynska J.K., McIntyre R.S. Inflammation and the phenomenology, pathophysiology, comorbidity, and treatment of bipolar disorder: a systematic review of the literature. J. Clin. Psychiatry. 2009;70(8):1078–1090. doi: 10.4088/JCP.08r04505. [DOI] [PubMed] [Google Scholar]
- 412.Valvassori S.S., Petronilho F.C., Réus G.Z., Steckert A.V., Oliveira V.B., Boeck C.R., Kapczinski F., Dal-Pizzol F., Quevedo J. Effect of N-acetylcysteine and/or deferoxamine on oxidative stress and hyperactivity in an animal model of mania. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32(4):1064–1068. doi: 10.1016/j.pnpbp.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 413.Harte M.K., Powell S.B., Swerdlow N.R., Geyer M.A., Reynolds G.P. Deficits in parvalbumin and calbindin immunoreactive cells in the hippocampus of isolation reared rats. J. Neural Transm. 2007;114(7):893–898. doi: 10.1007/s00702-007-0627-6. [DOI] [PubMed] [Google Scholar]
- 414.Schiavone S., Sorce S., Dubois-Dauphin M., Jaquet V., Colaianna M., Zotti M., Cuomo V., Trabace L., Krause K.H. Involvement of NOX2 in the development of behavioral and pathologic alterations in isolated rats. Biol. Psychiatry. 2009;66(4):384–392. doi: 10.1016/j.biopsych.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 415.Sorce S., Schiavone S., Tucci P., Colaianna M., Jaquet V., Cuomo V., Dubois-Dauphin M., Trabace L., Krause K.H. The NADPH oxidase NOX2 controls glutamate release: a novel mechanism involved in psychosis-like ketamine responses. J. Neurosci. 2010;30(34):11317–11325. doi: 10.1523/JNEUROSCI.1491-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Schiavone S., Jaquet V., Sorce S., Dubois-Dauphin M., Hultqvist M., Bäckdahl L., Holmdahl R., Colaianna M., Cuomo V., Trabace L., Krause K.H. NADPH oxidase elevations in pyramidal neurons drive psychosocial stress-induced neuropathology. Transl. Psychiatry. 2012;2:e111. doi: 10.1038/tp.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Miller A.H., Maletic V., Raison C.L. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry. 2009;65(9):732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Miller B.J., Buckley P., Seabolt W., Mellor A., Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol. Psychiatry. 2011;70(7):663–671. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Raison C.L., Capuron L., Miller A.H. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Dantzer R., O’Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Potvin S., Stip E., Sepehry A.A., Gendron A., Bah R., Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol. Psychiatry. 2008;63(8):801–808. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 422.Iosif R.E., Ekdahl C.T., Ahlenius H., Pronk C.J., Bonde S., Kokaia Z., Jacobsen S.E., Lindvall O. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J. Neurosci. 2006;26(38):9703–9712. doi: 10.1523/JNEUROSCI.2723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Kaneko N., Kudo K., Mabuchi T., Takemoto K., Fujimaki K., Wati H., Iguchi H., Tezuka H., Kanba S. Suppression of cell proliferation by interferon-alpha through interleukin-1 production in adult rat dentate gyrus. Neuropsychopharmacology. 2006;31(12):2619–2626. doi: 10.1038/sj.npp.1301137. [DOI] [PubMed] [Google Scholar]
- 424.Buntinx M., Moreels M., Vandenabeele F., Lambrichts I., Raus J., Steels P., Stinissen P., Ameloot M. Cytokine-induced cell death in human oligodendroglial cell lines: I. Synergistic effects of IFN-γ and TNF-α on apoptosis. J. Neurosci. Res. 2004;76(6):834–845. doi: 10.1002/jnr.20118. [DOI] [PubMed] [Google Scholar]
- 425.Medina S., Martínez M., Hernanz A. Antioxidants inhibit the human cortical neuron apoptosis induced by hydrogen peroxide, tumor necrosis factor alpha, dopamine and beta-amyloid peptide 1-42. Free Radic. Res. 2002;36(11):1179–1184. doi: 10.1080/107157602100006445. [DOI] [PubMed] [Google Scholar]
- 426.Stellwagen D., Malenka R.C. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440(7087):1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 427.Sunico C.R., Portillo F., González-Forero D., Moreno-López B. Nitric-oxide-directed synaptic remodeling in the adult mammal CNS. J. Neurosci. 2005;25(6):1448–1458. doi: 10.1523/JNEUROSCI.4600-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Capuron L., Su S., Miller A.H., Bremner J.D., Goldberg J., Vogt G.J., Maisano C., Jones L., Murrah N.V., Vaccarino V. Depressive symptoms and metabolic syndrome: is inflammation the underlying link? Biol. Psychiatry. 2008;64(10):896–900. doi: 10.1016/j.biopsych.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Anisman H., Merali Z. Cytokines, stress and depressive illness: brain-immune interactions. Ann. Med. 2003;35(1):2–11. doi: 10.1080/07853890310004075. [DOI] [PubMed] [Google Scholar]
- 430.Schiepers O.J., Wichers M.C., Maes M. Cytokines and major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29(2):201–217. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 431.Capuron L., Miller A.H. Cytokines and psychopathology: lessons from interferon-alpha. Biol. Psychiatry. 2004;56(11):819–824. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 432.Yirmiya R. Behavioral and psychological effects of immune activation: Implications for 'depression due to a general medical condition'. Curr. Opin. Psychiatry. 1997;10:470–476. doi: 10.1097/00001504-199711000-00011. [DOI] [Google Scholar]
- 433.Danner M., Kasl S.V., Abramson J.L., Vaccarino V. Association between depression and elevated C-reactive protein. Psychosom. Med. 2003;65(3):347–356. doi: 10.1097/01.PSY.0000041542.29808.01. [DOI] [PubMed] [Google Scholar]
- 434.Miller A.H., Pariante C.M., Pearce B.D. Effects of cytokines on glucocorticoid receptor expression and function. Glucocorticoid resistance and relevance to depression. Adv. Exp. Med. Biol. 1999;461:107–116. doi: 10.1007/978-0-585-37970-8_7. [DOI] [PubMed] [Google Scholar]
- 435.Musselman D.L., Lawson D.H., Gumnick J.F., Manatunga A.K., Penna S., Goodkin R.S., Greiner K., Nemeroff C.B., Miller A.H. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N. Engl. J. Med. 2001;344(13):961–966. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- 436.Capuron L., Neurauter G., Musselman D.L., Lawson D.H., Nemeroff C.B., Fuchs D., Miller A.H. Interferon-alpha-induced changes in tryptophan metabolism. relationship to depression and paroxetine treatment. Biol. Psychiatry. 2003;54(9):906–914. doi: 10.1016/S0006-3223(03)00173-2. [DOI] [PubMed] [Google Scholar]
- 437.Hashioka S., Klegeris A., Monji A., Kato T., Sawada M., McGeer P.L., Kanba S. 2007. [DOI] [PubMed]
- 438.Harvey B.H., Oosthuizen F., Brand L., Wegener G., Stein D.J. Stress-restress evokes sustained iNOS activity and altered GABA levels and NMDA receptors in rat hippocampus. Psychopharmacology (Berl.) 2004;175(4):494–502. doi: 10.1007/s00213-004-1836-4. [DOI] [PubMed] [Google Scholar]
- 439.Kahl K.G., Bens S., Ziegler K., Rudolf S., Dibbelt L., Kordon A., Schweiger U. Cortisol, the cortisol-dehydroepiandrosterone ratio, and pro-inflammatory cytokines in patients with current major depressive disorder comorbid with borderline personality disorder. Biol. Psychiatry. 2006;59(7):667–671. doi: 10.1016/j.biopsych.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 440.Himmerich H., Milenovic S., Fulda S., Plümäkers B., Sheldrick A.J., Michel T.M., Kircher T., Rink L. Regulatory T cells increased while IL-1ß decreased during antidepressant therapy. 2010. [DOI] [PubMed]
- 441.Song C., Halbreich U., Han C., Leonard B.E., Luo H. Imbalance between pro- and anti-inflammatory cytokines, and between Th1 and Th2 cytokines in depressed patients: the effect of electroacupuncture or fluoxetine treatment. Pharmacopsychiatry. 2009;42(5):182–188. doi: 10.1055/s-0029-1202263. [DOI] [PubMed] [Google Scholar]
- 442.Levine J., Barak Y., Chengappa K.N., Rapoport A., Rebey M., Barak V. Cerebrospinal cytokine levels in patients with acute depression. Neuropsychobiology. 1999;40(4):171–176. doi: 10.1159/000026615. [DOI] [PubMed] [Google Scholar]
- 443.Gimeno D., Marmot M.G., Singh-Manoux A. Inflammatory markers and cognitive function in middle-aged adults: the Whitehall II study. Psychoneuroendocrinology. 2008;33(10):1322–1334. doi: 10.1016/j.psyneuen.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Maes M., Scharpé S., Meltzer H.Y., Bosmans E., Suy E., Calabrese J., Cosyns P. Relationships between interleukin-6 activity, acute phase proteins, and function of the hypothalamic-pituitary-adrenal axis in severe depression. Psychiatry Res. 1993;49(1):11–27. doi: 10.1016/0165-1781(93)90027-E. [DOI] [PubMed] [Google Scholar]
- 445.Maes M., Bosmans E., De Jongh R., Kenis G., Vandoolaeghe E., Neels H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9(11):853–858. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- 446.Mendlewicz J., Kriwin P., Oswald P., Souery D., Alboni S., Brunello N. Shortened onset of action of antidepressants in major depression using acetylsalicylic acid augmentation: a pilot open-label study. Int. Clin. Psychopharmacol. 2006;21(4):227–231. doi: 10.1097/00004850-200607000-00005. [DOI] [PubMed] [Google Scholar]
- 447.Ortiz-Domínguez A., Hernández M.E., Berlanga C., Gutiérrez-Mora D., Moreno J., Heinze G., Pavón L. Immune variations in bipolar disorder: phasic differences. Bipolar Disord. 2007;9(6):596–602. doi: 10.1111/j.1399-5618.2007.00493.x. [DOI] [PubMed] [Google Scholar]
- 448.Drexhage R.C., Knijff E.M., Padmos R.C., Heul-Nieuwenhuijzen Lv., Beumer W., Versnel M.A., Drexhage H.A. The mononuclear phagocyte system and its cytokine inflammatory networks in schizophrenia and bipolar disorder. Expert Rev. Neurother. 2010;10(1):59–76. doi: 10.1586/ern.09.144. [DOI] [PubMed] [Google Scholar]
- 449.Rao J.S., Harry G.J., Rapoport S.I., Kim H.W. Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol. Psychiatry. 2010;15(4):384–392. doi: 10.1038/mp.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Martínez-Gras I., García-Sánchez F., Guaza C., Rodríguez-Jiménez R., Andrés-Esteban E., Palomo T., Rubio G., Borrell J. Altered immune function in unaffected first-degree biological relatives of schizophrenia patients. Psychiatry Res. 2012;200(2-3):1022–1025. doi: 10.1016/j.psychres.2012.05.036. [DOI] [PubMed] [Google Scholar]
- 451.Pedrini M., Massuda R., Fries G.R., de Bittencourt Pasquali M.A., Schnorr C.E., Moreira J.C. F., Teixeira A. L., Lobato M. I. R., Walz J. C., Belmonte-de-Abreu P. S. 2012. [DOI] [PubMed]
- 452.Kim Y.K., Myint A.M., Lee B.H., Han C.S., Lee H.J., Kim D.J., Leonard B. E. 2004.
- 453.Reindl W., Weiss S., Lehr H.A., Förster I. Essential crosstalk between myeloid and lymphoid cells for development of chronic colitis in myeloid-specific signal transducer and activator of transcription 3-deficient mice. Immunology. 2007;120(1):19–27. doi: 10.1111/j.1365-2567.2006.02473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Strober W., Fuss I.J., Blumberg R.S. The immunology of mucosal models of inflammation. 2002. [DOI] [PubMed]
- 455.Arolt V., Rothermundt M., Wandinger K.P., Kirchner H. Decreased in vitro production of interferon-gamma and interleukin-2 in whole blood of patients with schizophrenia during treatment. Mol. Psychiatry. 2000;5(2):150–158. doi: 10.1038/sj.mp.4000650. [DOI] [PubMed] [Google Scholar]
- 456.Takedatsu H., Michelsen K.S., Wei B., Landers C.J., Thomas L.S., Dhall D., Braun J., Targan S.R. TL1A (TNFSF15) regulates the development of chronic colitis by modulating both T-helper 1 and T-helper 17 activation. Gastroenterology. 2008;135(2):552–567. doi: 10.1053/j.gastro.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Wang Y., Yang F., Liu Y.F., Gao F., Jiang W. Acetylsalicylic acid as an augmentation agent in fluoxetine treatment resistant depressive rats. Neurosci. Lett. 2011;499(2):74–79. doi: 10.1016/j.neulet.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 458.Carboni L., Becchi S., Piubelli C., Mallei A., Giambelli R., Razzoli M., Mathé A.A., Popoli M., Domenici E. Early-life stress and antidepressants modulate peripheral biomarkers in a gene-environment rat model of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34(6):1037–1048. doi: 10.1016/j.pnpbp.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 459.Kawamoto E.M., Lima L.S., Munhoz C.D., Yshii L.M., Kinoshita P.F., Amara F.G., Pestana R.R., Orellana A.M., Cipolla-Neto J., Britto L.R., Avellar M.C., Rossoni L.V., Scavone C. Influence of N-methyl-D-aspartate receptors on ouabain activation of nuclear factor-κB in the rat hippocampus. J. Neurosci. Res. 2012;90(1):213–228. doi: 10.1002/jnr.22745. [DOI] [PubMed] [Google Scholar]
- 460.Monje M.L., Toda H., Palmer T.D. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302(5651):1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 461.Stone T.W., Darlington L.G. Endogenous kynurenines as targets for drug discovery and development. Nat. Rev. Drug Discov. 2002;1(8):609–620. doi: 10.1038/nrd870. [DOI] [PubMed] [Google Scholar]
- 462.Stone T.W. Kynurenines in the CNS: from endogenous obscurity to therapeutic importance. Prog. Neurobiol. 2001;64(2):185–218. doi: 10.1016/S0301-0082(00)00032-0. [DOI] [PubMed] [Google Scholar]
- 463.Allegri G., Costa C.V., Bertazzo A., Biasiolo M., Ragazzi E. Enzyme activities of tryptophan metabolism along the kynurenine pathway in various species of animals. Farmaco. 2003;58(9):829–836. doi: 10.1016/S0014-827X(03)00140-X. [DOI] [PubMed] [Google Scholar]
- 464.Schwarcz R. The kynurenine pathway of tryptophan degradation as a drug target. Curr. Opin. Pharmacol. 2004;4(1):12–17. doi: 10.1016/j.coph.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 465.Myint A.M., Kim Y.K., Verkerk R., Scharpé S., Steinbusch H., Leonard B. Kynurenine pathway in major depression: evidence of impaired neuroprotection. J. Affect. Disord. 2007;98(1-2):143–151. doi: 10.1016/j.jad.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 466.Guillemin G.J., Cullen K.M., Lim C.K., Smythe G.A., Garner B., Kapoor V., Takikawa O., Brew B.J. Characterization of the kynurenine pathway in human neurons. J. Neurosci. 2007;27(47):12884–12892. doi: 10.1523/JNEUROSCI.4101-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Myint A.M., Kim Y.K., Verkerk R., Park S.H., Scharpé S., Steinbusch H.W., Leonard B.E. Tryptophan breakdown pathway in bipolar mania. J. Affect. Disord. 2007;102(1-3):65–72. doi: 10.1016/j.jad.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 468.Abi-Dargham A., Laruelle M., Aghajanian G.K., Charney D., Krystal J. The role of serotonin in the pathophysiology and treatment of schizophrenia. J. Neuropsychiatry Clin. Neurosci. 1997;9(1):1–17. doi: 10.1176/jnp.9.1.1. [DOI] [PubMed] [Google Scholar]
- 469.Silver H. Selective serotonin re-uptake inhibitor augmentation in the treatment of negative symptoms of schizophrenia. Expert Opin. Pharmacother. 2004;5(10):2053–2058. doi: 10.1517/14656566.5.10.2053. [DOI] [PubMed] [Google Scholar]
- 470.Capuron L., Raison C.L., Musselman D.L., Lawson D.H., Nemeroff C.B., Miller A.H. Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. Am. J. Psychiatry. 2003;160(7):1342–1345. doi: 10.1176/appi.ajp.160.7.1342. [DOI] [PubMed] [Google Scholar]
- 471.Bonaccorso S., Marino V., Puzella A., Pasquini M., Biondi M., Artini M., Almerighi C., Verkerk R., Meltzer H., Maes M. Increased depressive ratings in patients with hepatitis C receiving interferon-alpha-based immunotherapy are related to interferon-alpha-induced changes in the serotonergic system. J. Clin. Psychopharmacol. 2002;22(1):86–90. doi: 10.1097/00004714-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 472.Leonard B.E., Myint A. Inflammation and depression: is there a causal connection with dementia? Neurotox. Res. 2006;10(2):149–160. doi: 10.1007/BF03033243. [DOI] [PubMed] [Google Scholar]
- 473.Quak J., Doornbos B., Roest A.M., Duivis H.E., Vogelzangs N., Nolen W.A., Penninx B.W., Kema I.P., de Jonge P. Does tryptophan degradation along the kynurenine pathway mediate the association between pro-inflammatory immune activity and depressive symptoms? Psychoneuroendocrinology. 2014;45:202–210. doi: 10.1016/j.psyneuen.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 474.Miller C.L., Llenos I.C., Dulay J.R., Weis S. Upregulation of the initiating step of the kynurenine pathway in postmortem anterior cingulate cortex from individuals with schizophrenia and bipolar disorder. Brain Res. 2006;1073-1074:25–37. doi: 10.1016/j.brainres.2005.12.056. [DOI] [PubMed] [Google Scholar]
- 475.Miller C.L., Llenos I.C., Cwik M., Walkup J., Weis S. Alterations in kynurenine precursor and product levels in schizophrenia and bipolar disorder. Neurochem. Int. 2008;52(6):1297–1303. doi: 10.1016/j.neuint.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 476.Torrey E.F., Yolken R.H., Zito M., Heyes M. Increased CSF and brain quinolinic acid in schizophrenia and bipolar disorder. Schizophr. Res. 1998;29:91–92. doi: 10.1016/S0920-9964(97)88530-1. [DOI] [Google Scholar]
- 477.Issa F., Gerhardt G.A., Bartko J.J., Suddath R.L., Lynch M., Gamache P.H., Freedman R., Wyatt R.J., Kirch D.G. A multidimensional approach to analysis of cerebrospinal fluid biogenic amines in schizophrenia: I. Comparisons with healthy control subjects and neuroleptic-treated/unmedicated pairs analyses. Psychiatry Res. 1994;52(3):237–249. doi: 10.1016/0165-1781(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 478.Ravikumar A., Deepadevi K.V., Arun P., Manojkumar V., Kurup P.A. Tryptophan and tyrosine catabolic pattern in neuropsychiatric disorders. Neurol. India. 2000;48(3):231–238. [PubMed] [Google Scholar]
- 479.Myint A.M., Schwarz M.J., Verkerk R., Mueller H.H., Zach J., Scharpé S., Steinbusch H.W., Leonard B.E., Kim Y.K. Reversal of imbalance between kynurenic acid and 3-hydroxykynurenine by antipsychotics in medication-naïve and medication-free schizophrenic patients. Brain Behav. Immun. 2011;25(8):1576–1581. doi: 10.1016/j.bbi.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 480.Gibney S.M., Fagan E.M., Waldron A.M., O’Byrne J., Connor T.J., Harkin A. Inhibition of stress-induced hepatic tryptophan 2,3-dioxygenase exhibits antidepressant activity in an animal model of depressive behaviour. Int. J. Neuropsychopharmacol. 2014;17(6):917–928. doi: 10.1017/S1461145713001673. [DOI] [PubMed] [Google Scholar]
- 481.Gibney S.M., McGuinness B., Prendergast C., Harkin A., Connor T.J. Poly I:C-induced activation of the immune response is accompanied by depression and anxiety-like behaviours, kynurenine pathway activation and reduced BDNF expression. Brain Behav. Immun. 2013;28:170–181. doi: 10.1016/j.bbi.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 482.Yang J., Li W., Zhou Z., Yang C. Is Ro 61-8048 a potential fast-acting antidepressant? J. Neurol. Sci. 2012;315(1-2):180. doi: 10.1016/j.jns.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 483.Möller M., Du Preez J.L., Emsley R., Harvey B.H. Social isolation rearing in rats alters plasma tryptophan metabolism and is reversed by sub-chronic clozapine treatment. 2012. [DOI] [PubMed]
- 484.Lohoff F.W. Overview of the genetics of major depressive disorder. Curr. Psychiatry Rep. 2010;12(6):539–546. doi: 10.1007/s11920-010-0150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Benedetti F., Colombo C., Pirovano A., Marino E., Smeraldi E. The catechol-O-methyltransferase Val(108/158)Met polymorphism affects antidepressant response to paroxetine in a naturalistic setting. Psychopharmacology (Berl.) 2009;203(1):155–160. doi: 10.1007/s00213-008-1381-7. [DOI] [PubMed] [Google Scholar]
- 486.Benedetti F., Barbini B., Bernasconi A., Fulgosi M.C., Dallaspezia S., Gavinelli C., Locatelli C., Lorenzi C., Pirovano A., Radaelli D., Smeraldi E., Colombo C. Acute antidepressant response to sleep deprivation combined with light therapy is influenced by the catechol-O-methyltransferase Val(108/158)Met polymorphism. J. Affect. Disord. 2010;121(1-2):68–72. doi: 10.1016/j.jad.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 487.Craddock N., Owen M.J., O’Donovan M.C. The catechol-O-methyl transferase (COMT) gene as a candidate for psychiatric phenotypes: evidence and lessons. Mol. Psychiatry. 2006;11(5):446–458. doi: 10.1038/sj.mp.4001808. [DOI] [PubMed] [Google Scholar]
- 488.Spronk D., Arns M., Barnett K.J., Cooper N.J., Gordon E. An investigation of EEG, genetic and cognitive markers of treatment response to antidepressant medication in patients with major depressive disorder: a pilot study. J. Affect. Disord. 2011;128(1-2):41–48. doi: 10.1016/j.jad.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 489.DePaulo J.R., Phillips A.E., Potash J.A., McInnis M.G., McMahon F.J. The current status and prospects for genetic studies of bipolar disorder. Clin. Neurosci. Res. 2001;1:153–157. doi: 10.1016/S1566-2772(00)00019-0. [DOI] [Google Scholar]
- 490.Mahon K., Burdick K.E., Ikuta T., Braga R.J., Gruner P., Malhotra A.K., Szeszko P.R. Abnormal temporal lobe white matter as a biomarker for genetic risk of bipolar disorder. Biol. Psychiatry. 2013;73(2):177–182. doi: 10.1016/j.biopsych.2012.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Abkevich V., Camp N.J., Hensel C.H., Neff C.D., Russell D.L., Hughes D.C., Plenk A.M., Lowry M.R., Richards R.L., Carter C., Frech G.C., Stone S., Rowe K., Chau C.A., Cortado K., Hunt A., Luce K., O’Neil G., Poarch J., Potter J., Poulsen G.H., Saxton H., Bernat-Sestak M., Thompson V., Gutin A., Skolnick M.H., Shattuck D., Cannon-Albright L. Predisposition locus for major depression at chromosome 12q22-12q23.2. Am. J. Hum. Genet. 2003;73(6):1271–1281. doi: 10.1086/379978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Harvey M., Gagné B., Labbé M., Barden N. Polymorphisms in the neuronal isoform of tryptophan hydroxylase 2 are associated with bipolar disorder in French Canadian pedigrees. 2007. [DOI] [PubMed]
- 493.Grigoroiu-Serbanescu M., Diaconu C.C., Herms S., Bleotu C., Vollmer J., Mühleisen T.W., Prelipceanu D., Priebe L., Mihailescu R., Georgescu M.J., Sima D., Grimberg M., Nöthen M. M., Cichon S. Investigation of the tryptophan hydroxylase 2 gene in bipolar I disorder in the Romanian population. 2008. [DOI] [PubMed]
- 494.Tee S.F., Chow T.J., Tang P.Y., Loh H.C. Linkage of schizophrenia with TPH2 and 5-HTR2A gene polymorphisms in the Malay population. Genet. Mol. Res. 2010;9(3):1274–1278. doi: 10.4238/vol9-3gmr789. [DOI] [PubMed] [Google Scholar]
- 495.Riley B., Kendler K.S. Molecular genetic studies of schizophrenia. Eur. J. Hum. Genet. 2006;14(6):669–680. doi: 10.1038/sj.ejhg.5201571. [DOI] [PubMed] [Google Scholar]
- 496.Allen N.C., Bagade S., McQueen M.B., Ioannidis J.P., Kavvoura F.K., Khoury M.J., Tanzi R.E., Bertram L. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat. Genet. 2008;40(7):827–834. doi: 10.1038/ng.171. [DOI] [PubMed] [Google Scholar]
- 497.Sun J., Kuo P.H., Riley B.P., Kendler K.S., Zhao Z. Candidate genes for schizophrenia: a survey of association studies and gene ranking. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2008;147B(7):1173–1181. doi: 10.1002/ajmg.b.30743. [DOI] [PubMed] [Google Scholar]
- 498.Harrison P.J., Law A.J. 2006.
- 499.Weber H., Klamer D., Freudenberg F., Kittel-Schneider S., Rivero O., Scholz C.J., Volkert J., Kopf J., Heupel J., Herterich S., Adolfsson R., Alttoa A., Post A., Grußendorf H., Kramer A., Gessner A., Schmidt B., Hempel S., Jacob C.P., Sanjuán J., Moltó M.D., Lesch K.P., Freitag C.M., Kent L., Reif A. The genetic contribution of the NO system at the glutamatergic post-synapse to schizophrenia: further evidence and meta-analysis. Eur. Neuropsychopharmacol. 2014;24(1):65–85. doi: 10.1016/j.euroneuro.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 500.Caberlotto L., Fuxe K., Overstreet D.H., Gerrard P., Hurd Y.L. Alterations in neuropeptide Y and Y1 receptor mRNA expression in brains from an animal model of depression: region specific adaptation after fluoxetine treatment. Brain Res. Mol. Brain Res. 1998;59(1):58–65. doi: 10.1016/S0169-328X(98)00137-5. [DOI] [PubMed] [Google Scholar]
- 501.Melas P.A., Mannervik M., Mathé A.A., Lavebratt C., Neuropeptide Y. Neuropeptide Y: identification of a novel rat mRNA splice-variant that is downregulated in the hippocampus and the prefrontal cortex of a depression-like model. Peptides. 2012;35(1):49–55. doi: 10.1016/j.peptides.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 502.Serova L., Sabban E.L., Zangen A., Overstreet D.H., Yadid G. Altered gene expression for catecholamine biosynthetic enzymes and stress response in rat genetic model of depression. 1998. [DOI] [PubMed]
- 503.Xiao L., Shu C., Tang J., Wang H., Liu Z., Wang G. Effects of different CMS on behaviors, BDNF/CREB/Bcl-2 expression in rat hippocampus. Biomed. Aging Pathol. 2011;1:138–146. doi: 10.1016/j.biomag.2010.10.006. [DOI] [Google Scholar]
- 504.Machado-Vieira R., Schmidt A.P., Ávila T.T., Kapczinski F., Soares J.C., Souza D.O., Portela L.V. Increased cerebrospinal fluid levels of S100B protein in rat model of mania induced by ouabain. Life Sci. 2004;76(7):805–811. doi: 10.1016/j.lfs.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 505.Gerlai R., Roder J. Abnormal exploratory behavior in transgenic mice carrying multiple copies of the human gene for S100 beta. J. Psychiatry Neurosci. 1995;20(2):105–112. [PMC free article] [PubMed] [Google Scholar]
- 506.Melendez R.I., Gregory M.L., Bardo M.T., Kalivas P.W. Impoverished rearing environment alters metabotropic glutamate receptor expression and function in the prefrontal cortex. Neuropsychopharmacology. 2004;29(11):1980–1987. doi: 10.1038/sj.npp.1300507. [DOI] [PubMed] [Google Scholar]
- 507.Taylor A., Taylor S., Markham J., Koenig J. 2009. [Google Scholar]
- 508.Taurines R., Dudley E., Grassl J., Warnke A., Gerlach M., Coogan A.N., Thome J. Proteomic research in psychiatry. J. Psychopharmacol. (Oxford) 2011;25(2):151–196. doi: 10.1177/0269881109106931. [DOI] [PubMed] [Google Scholar]
- 509.Lee J.M., Han J.J., Altwerger G., Kohn E.C. Proteomics and biomarkers in clinical trials for drug development. J. Proteomics. 2011;74(12):2632–2641. doi: 10.1016/j.jprot.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Ditzen C., Tang N., Jastorff A.M., Teplytska L., Yassouridis A., Maccarrone G., Uhr M., Bronisch T., Miller C.A., Holsboer F., Turck C.W. Cerebrospinal fluid biomarkers for major depression confirm relevance of associated pathophysiology. Neuropsychopharmacology. 2012;37(4):1013–1025. doi: 10.1038/npp.2011.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Tashiro A., Hongo M., Ota R., Utsumi A., Imai T. Hyper-insulin response in a patient with depression. Changes in insulin resistance during recovery from depression. Diabetes Care. 1997;20(12):1924–1925. doi: 10.2337/diacare.20.12.1924. [DOI] [PubMed] [Google Scholar]
- 512.Katon W.J. The comorbidity of diabetes mellitus and depression. Am. J. Med. 2008;121(11) Suppl. 2:S8–S15. doi: 10.1016/j.amjmed.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Dunbar J.A., Reddy P., Davis-Lameloise N., Philpot B., Laatikainen T., Kilkkinen A., Bunker S.J., Best J.D., Vartiainen E., Kai Lo S., Janus E.D. Depression: an important comorbidity with metabolic syndrome in a general population. Diabetes Care. 2008;31(12):2368–2373. doi: 10.2337/dc08-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Guest P.C., Schwarz E., Krishnamurthy D., Harris L.W., Leweke F.M., Rothermundt M., van Beveren N.J., Spain M., Barnes A., Steiner J., Rahmoune H., Bahn S. Altered levels of circulating insulin and other neuroendocrine hormones associated with the onset of schizophrenia. Psychoneuroendocrinology. 2011;36(7):1092–1096. doi: 10.1016/j.psyneuen.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 515.Novikova S.I., He F., Cutrufello N.J., Lidow M.S. Identification of protein biomarkers for schizophrenia and bipolar disorder in the postmortem prefrontal cortex using SELDI-TOF-MS ProteinChip profiling combined with MALDI-TOF-PSD-MS analysis. Neurobiol. Dis. 2006;23(1):61–76. doi: 10.1016/j.nbd.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 516.Krupnik V.E., Sharp J.D., Jiang C., Robison K., Chickering T.W., Amaravadi L., Brown D.E., Guyot D., Mays G., Leiby K., Chang B., Duong T., Goodearl A.D., Gearing D.P., Sokol S.Y., McCarthy S.A. Functional and structural diversity of the human Dickkopf gene family. Gene. 1999;238(2):301–313. doi: 10.1016/S0378-1119(99)00365-0. [DOI] [PubMed] [Google Scholar]
- 517.Gould T.D., Manji H.K. The Wnt signaling pathway in bipolar disorder. Neuroscientist. 2002;8(5):497–511. doi: 10.1177/107385802237176. [DOI] [PubMed] [Google Scholar]
- 518.Arckens L., Van der Gucht E., Van den Bergh G., Massie A., Leysen I., Vandenbussche E., Eysel U.T., Huybrechts R., Vandesande F. Differential display implicates cyclophilin A in adult cortical plasticity. Eur. J. Neurosci. 2003;18(1):61–75. doi: 10.1046/j.1460-9568.2003.02726.x. [DOI] [PubMed] [Google Scholar]
- 519.Futamura T., Toyooka K., Iritani S., Niizato K., Nakamura R., Tsuchiya K., Someya T., Kakita A., Takahashi H., Nawa H. Abnormal expression of epidermal growth factor and its receptor in the forebrain and serum of schizophrenic patients. Mol. Psychiatry. 2002;7(7):673–682. doi: 10.1038/sj.mp.4001081. [DOI] [PubMed] [Google Scholar]
- 520.Hashimoto T., Bergen S.E., Nguyen Q.L., Xu B., Monteggia L.M., Pierri J.N., Sun Z., Sampson A.R., Lewis D.A. Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. J. Neurosci. 2005;25(2):372–383. doi: 10.1523/JNEUROSCI.4035-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Gama C.S., Andreazza A.C., Kunz M., Berk M., Belmonte-de-Abreu P.S., Kapczinski F. Serum levels of brain-derived neurotrophic factor in patients with schizophrenia and bipolar disorder. Neurosci. Lett. 2007;420(1):45–48. doi: 10.1016/j.neulet.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 522.Huang J.T., Leweke F.M., Tsang T.M., Koethe D., Kranaster L., Gerth C.W., Gross S., Schreiber D., Ruhrmann S., Schultze-Lutter F., Klosterkötter J., Holmes E., Bahn S. CSF metabolic and proteomic profiles in patients prodromal for psychosis. PLoS One. 2007;2(8):e756. doi: 10.1371/journal.pone.0000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Guest P.C., Wang L., Harris L.W., Burling K., Levin Y., Ernst A., Wayland M.T., Umrania Y., Herberth M., Koethe D., van Beveren J.M., Rothermundt M., McAllister G., Leweke F.M., Steiner J., Bahn S. Increased levels of circulating insulin-related peptides in first-onset, antipsychotic naïve schizophrenia patients. Mol. Psychiatry. 2010;15(2):118–119. doi: 10.1038/mp.2009.81. [DOI] [PubMed] [Google Scholar]
- 524.Yang Y., Yang D., Tang G., Zhou C., Cheng K., Zhou J., Wu B., Peng Y., Liu C., Zhan Y., Chen J., Chen G., Xie P. Proteomics reveals energy and glutathione metabolic dysregulation in the prefrontal cortex of a rat model of depression. 2013. [DOI] [PubMed]
- 525.Smalla K.H., Mikhaylova M., Sahin J., Bernstein H.G., Bogerts B., Schmitt A., van der Schors R., Smit A.B., Li K.W., Gundelfinger E.D., Kreutz M.R. A comparison of the synaptic proteome in human chronic schizophrenia and rat ketamine psychosis suggest that prohibitin is involved in the synaptic pathology of schizophrenia. Mol. Psychiatry. 2008;13(9):878–896. doi: 10.1038/mp.2008.60. [DOI] [PubMed] [Google Scholar]
- 526.Zhao Y., Patzer A., Herdegen T., Gohlke P., Culman J. Activation of cerebral peroxisome proliferator-activated receptors gamma promotes neuroprotection by attenuation of neuronal cyclooxygenase-2 overexpression after focal cerebral ischemia in rats. FASEB J. 2006;20(8):1162–1175. doi: 10.1096/fj.05-5007com. [DOI] [PubMed] [Google Scholar]
- 527.Marksteiner J., Weiss U., Weis C., Laslop A., Fischer-Colbrie R., Humpel C., Feldon J., Fleischhacker W.W. Differential regulation of chromogranin A, chromogranin B and secretogranin II in rat brain by phencyclidine treatment. Neuroscience. 2001;104(2):325–333. doi: 10.1016/S0306-4522(01)00081-1. [DOI] [PubMed] [Google Scholar]
- 528.Moreau M.P., Bruse S.E., David-Rus R., Buyske S., Brzustowicz L.M. Altered microRNA expression profiles in postmortem brain samples from individuals with schizophrenia and bipolar disorder. Biol. Psychiatry. 2011;69(2):188–193. doi: 10.1016/j.biopsych.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Bocchio-Chiavetto L., Maffioletti E., Bettinsoli P., Giovannini C., Bignotti S., Tardito D., Corrada D., Milanesi L., Gennarelli M. Blood microRNA changes in depressed patients during antidepressant treatment. Eur. Neuropsychopharmacol. 2013;23(7):602–611. doi: 10.1016/j.euroneuro.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 530.Perkins D.O., Jeffries C.D., Jarskog L.F., Thomson J.M., Woods K., Newman M.A., Parker J.S., Jin J., Hammond S.M. microRNA expression in the prefrontal cortex of individuals with schizophrenia and schizoaffective disorder. Genome Biol. 2007;8(2):R27. doi: 10.1186/gb-2007-8-2-r27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Kim A.H., Reimers M., Maher B., Williamson V., McMichael O., McClay J.L., van den Oord E.J., Riley B.P., Kendler K.S., Vladimirov V.I. MicroRNA expression profiling in the prefrontal cortex of individuals affected with schizophrenia and bipolar disorders. Schizophr. Res. 2010;124(1-3):183–191. doi: 10.1016/j.schres.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Beveridge N.J., Gardiner E., Carroll A.P., Tooney P.A., Cairns M.J. Schizophrenia is associated with an increase in cortical microRNA biogenesis. Mol. Psychiatry. 2010;15(12):1176–1189. doi: 10.1038/mp.2009.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Dwivedi Y. Evidence demonstrating role of microRNAs in the etiopathology of major depression. J. Chem. Neuroanat. 2011;42(2):142–156. doi: 10.1016/j.jchemneu.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Varol N., Konac E., Gurocak O.S., Sozen S. The realm of microRNAs in cancers. Mol. Biol. Rep. 2011;38(2):1079–1089. doi: 10.1007/s11033-010-0205-0. [DOI] [PubMed] [Google Scholar]
- 535.Hansen K.F., Obrietan K. MicroRNA as therapeutic targets for treatment of depression. Neuropsychiatr. Dis. Treat. 2013;9:1011–1021. doi: 10.2147/NDT.S34811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Fernandes B.S., Berk M., Turck C.W., Steiner J., Gonçalves C.A. Decreased peripheral brain-derived neurotrophic factor levels are a biomarker of disease activity in major psychiatric disorders: a comparative meta-analysis. Mol. Psychiatry. 2014;19(7):750–751. doi: 10.1038/mp.2013.172. [DOI] [PubMed] [Google Scholar]
- 537.Chen C., Wang J., Wang B., Yang S.C., Zhang C.X., Zheng Y.L., Li Y.L., Wang N., Yang K.B., Xiu M.H., Kosten T.R., Zhang X.Y. Decreased levels of serum brain-derived neurotrophic factor in drug-naïve first-episode schizophrenia: relationship to clinical phenotypes. Psychopharmacology (Berl.) 2009;207(3):375–380. doi: 10.1007/s00213-009-1665-6. [DOI] [PubMed] [Google Scholar]
- 538.Yatham L.N., Kapczinski F., Andreazza A.C., Trevor Young L., Lam R.W., Kauer-Sant’anna M. Accelerated age-related decrease in brain-derived neurotrophic factor levels in bipolar disorder. Int. J. Neuropsychopharmacol. 2009;12(1):137–139. doi: 10.1017/S1461145708009449. [DOI] [PubMed] [Google Scholar]
- 539.Raffa M., Barhoumi S., Atig F., Fendri C., Kerkeni A., Mechri A. Reduced antioxidant defense systems in schizophrenia and bipolar I disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012;39(2):371–375. doi: 10.1016/j.pnpbp.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 540.Berk M., Copolov D., Dean O., Lu K., Jeavons S., Schapkaitz I., Anderson-Hunt M., Judd F., Katz F., Katz P., Ording-Jespersen S., Little J., Conus P., Cuenod M., Do K. Q., Bush A. I. 2008.
- 541.Meyer U. Anti-inflammatory signaling in schizophrenia. Brain Behav. Immun. 2011;25(8):1507–1518. doi: 10.1016/j.bbi.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 542.Fortier M.E., Luheshi G.N., Boksa P. Effects of prenatal infection on prepulse inhibition in the rat depend on the nature of the infectious agent and the stage of pregnancy. Behav. Brain Res. 2007;181(2):270–277. doi: 10.1016/j.bbr.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 543.Duff B.J., Macritchie K.A., Moorhead T.W., Lawrie S.M., Blackwood D.H. Human brain imaging studies of DISC1 in schizophrenia, bipolar disorder and depression: a systematic review. Schizophr. Res. 2013;147(1):1–13. doi: 10.1016/j.schres.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 544.García-Bueno B., Pérez-Nievas B.G., Leza J.C. Is there a role for the nuclear receptor PPARγ in neuropsychiatric diseases? Int. J. Neuropsychopharmacol. 2010;13(10):1411–1429. doi: 10.1017/S1461145710000970. [DOI] [PubMed] [Google Scholar]
- 545.Nigro P., Pompilio G., Capogrossi M.C., Cyclophilin A. Cyclophilin A: a key player for human disease. Cell Death Dis. 2013;4:e888. doi: 10.1038/cddis.2013.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Panariello F., Javaid N., Teo C., Monda M., Viggiano A., de Luca V. The role of orexin system in antipsychotics induced weight gain. Curr. Psychiatry Rev. 2011;7:12–18. doi: 10.2174/157340011795945793. [DOI] [Google Scholar]
- 547.Friston K.J. The disconnection hypothesis. Schizophr. Res. 1998;30(2):115–125. doi: 10.1016/S0920-9964(97)00140-0. [DOI] [PubMed] [Google Scholar]
- 548.Takahashi T., Cho R.Y., Mizuno T., Kikuchi M., Murata T. Antipsychotics reverse abnormal EEG complexity in drug-naïve schizophrenia: A multiscale entropy analysis. 2010. [DOI] [PMC free article] [PubMed]
- 549.Balogh Z., Benedek G., Kéri S. Retinal dysfunctions in schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32(1):297–300. doi: 10.1016/j.pnpbp.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 550.Luck S.J., Mathalon D.H., O’Donnell B.F., Hämäläinen M.S., Spencer K.M., Javitt D.C., Uhlhaas P.J. A roadmap for the development and validation of event-related potential biomarkers in schizophrenia research. Biol. Psychiatry. 2011;70(1):28–34. doi: 10.1016/j.biopsych.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Greimel E., Trinkl M., Bartling J., Bakos S., Grossheinrich N., Schulte-Körne G. 2015. [DOI] [PubMed]
- 552.Steiger A., Kimura M. Wake and sleep EEG provide biomarkers in depression. J. Psychiatr. Res. 2010;44(4):242–252. doi: 10.1016/j.jpsychires.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 553.Yamamoto J. Cortical and hippocampal EEG power spectra in animal models of schizophrenia produced with methamphetamine, cocaine, and phencyclidine. Psychopharmacology (Berl.) 1997;131(4):379–387. doi: 10.1007/s002130050306. [DOI] [PubMed] [Google Scholar]
- 554.Labermaier C., Masana M., Müller M.B. Biomarkers predicting antidepressant treatment response: how can we advance the field? Dis. Markers. 2013;35(1):23–31. doi: 10.1155/2013/984845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Pajer K., Andrus B.M., Gardner W., Lourie A., Strange B., Campo J., Bridge J., Blizinsky K., Dennis K., Vedell P., Churchill G.A., Redei E.E. Discovery of blood transcriptomic markers for depression in animal models and pilot validation in subjects with early-onset major depression. Transl. Psychiatry. 2012;2(4):e101. doi: 10.1038/tp.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Redei E.E., Andrus B.M., Kwasny M.J., Seok J., Cai X., Ho J., Mohr D.C. Blood transcriptomic biomarkers in adult primary care patients with major depressive disorder undergoing cognitive behavioral therapy. Transl. Psychiatry. 2014;4(4):e442. doi: 10.1038/tp.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Savitz J.B., Drevets W.C. Imaging phenotypes of major depressive disorder: genetic correlates. Neuroscience. 2009;164(1):300–330. doi: 10.1016/j.neuroscience.2009.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Leuchter A.F., Cook I.A., Hamilton S.P., Narr K.L., Toga A., Hunter A.M., Faull K., Whitelegge J., Andrews A.M., Loo J., Way B., Nelson S.F., Horvath S., Lebowitz B.D. Biomarkers to predict antidepressant response. Curr. Psychiatry Rep. 2010;12(6):553–562. doi: 10.1007/s11920-010-0160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Hampel H., Frank R., Broich K., Teipel S.J., Katz R.G., Hardy J., Herholz K., Bokde A.L., Jessen F., Hoessler Y.C., Sanhai W.R., Zetterberg H., Woodcock J., Blennow K. Biomarkers for Alzheimer’s disease: academic, industry and regulatory perspectives. Nat. Rev. Drug Discov. 2010;9(7):560–574. doi: 10.1038/nrd3115. [DOI] [PubMed] [Google Scholar]
- 560.Quinones M.P., Kaddurah-Daouk R. Metabolomics tools for identifying biomarkers for neuropsychiatric diseases. 2009. [DOI] [PubMed]