Abstract
Increasing epidemiologic evidence suggests that metformin, a well-established AMPK activator and the most favorable first-line anti-diabetic drug, reduces stroke incidence and severity. However, the mechanism for this remains unclear. Moreover, previous experimental studies have reported controversial results about the effects of metformin on stroke outcomes during the acute phase. However, recent studies have consistently suggested that AMPK-mediated microglia/macrophage polarization and angioneurogenesis may play essential roles in metformin-promoted, long-term functional recovery following stroke. The present review summarizes the neuropharmacological actions of metformin in experimental stroke with an emphasis on the recent findings that the cell-specific effects and duration of AMPK activation are critical to the effects of metformin on stroke outcomes.
Keywords: AMPK activation, anti-diabetic drug, metformin, neuropharmacological actions.
INTRODUCTION
Stroke is the third leading cause of death and the leading cause of disabilities (e.g. Paralysis, ataxia, paresthesia and aphasia) in adults. Globally, stroke results in approximately 4.4 million deaths per year [1]. Moreover, the stroke incidence is expected to continually rise as life expectancy increases. Although medical care has markedly improved during the last few decades [2], it only decreases the number of deaths from stroke while leading to increased numbers of stroke survivors experiencing life-long disability [3, 4]. In spite of its huge socioeconomic burden, stroke still lacks effective treatments. Therefore, novel drug therapies are urgently needed for stroke.
Metformin, a drug that has been used in clinics for over 50 years, is the most favorable first-line drug for treating type 2 diabetes [5-7]. Globally, more than 100 million diabetes patients are prescribed this drug annually. Recently, metformin was reported to exert off-label beneficial effects, including anti-cancer effects [8], therapeutic effects in neurological disorders [9, 10] and favorable effects on the cardiovascular and cerebrovascular system [11]. Epidemiologic studies have shown that metformin reduces the stroke incidence and severity [12, 13]. Although diabetes and hyperglycemia are well-known risk factors for stroke, the beneficial effects of metformin on stroke outcomes are independent of its glucose-reducing effects [12]. These results indicate that metformin could be developed into a disease-modifying drug to treat stroke. This review summarizes the recent experimental research on metformin’s effects on stroke outcomes with an emphasis on recent findings that the cell-specific effects and duration of AMPK (5’-monophosphate-activated protein kinase) activation are critical to metformin’s effects on stroke outcomes.
MOLECULAR MECHANISMS OF METFORMIN ACTIONS
Metformin was discovered before the era of mechanism-based drug discovery. Intensive efforts have been made to uncover the mechanisms for the pharmacological actions of metformin (Fig. 1). The therapeutic actions of metformin in reducing hepatic glucose production are thought to be mediated by AMPK activation [7, 14]. AMPK, a master cellular sensor and regulator of energy homeostasis, consists of an α catalytic subunit and two regulatory subunits (β and γ) [15]. AMPK is activated when the AMP/ATP levels are high and when AMP binds to the γ subunit of the AMPK complex. AMP binding induces an allosteric change of the α subunit, which in turn exposes the activation site on the α subunit to be targeted by the upstream kinases [16, 17]. However, metformin does not directly activate AMPK nor does it act by targeting the upstream kinases [18]. Instead, metformin acts through respiratory chain inhibition to increase AMP:ATP ratios, leading to AMPK activation. Live kinase B (LKB) is the upstream kinase responsible for metformin activation of AMPK [19]. Multiple downstream mechanisms of AMPK activation may mediate the effects of metformin on stroke outcomes. For instance, AMPK activation has been reported to protect against cerebral ischemia by activating the nuclear factor erythroid 2-related factor (Nrf2) antioxidant pathway or inhibiting the NF- κB cascade to suppress post-ischemic neuroinflammation [20, 21].
Fig. (1).
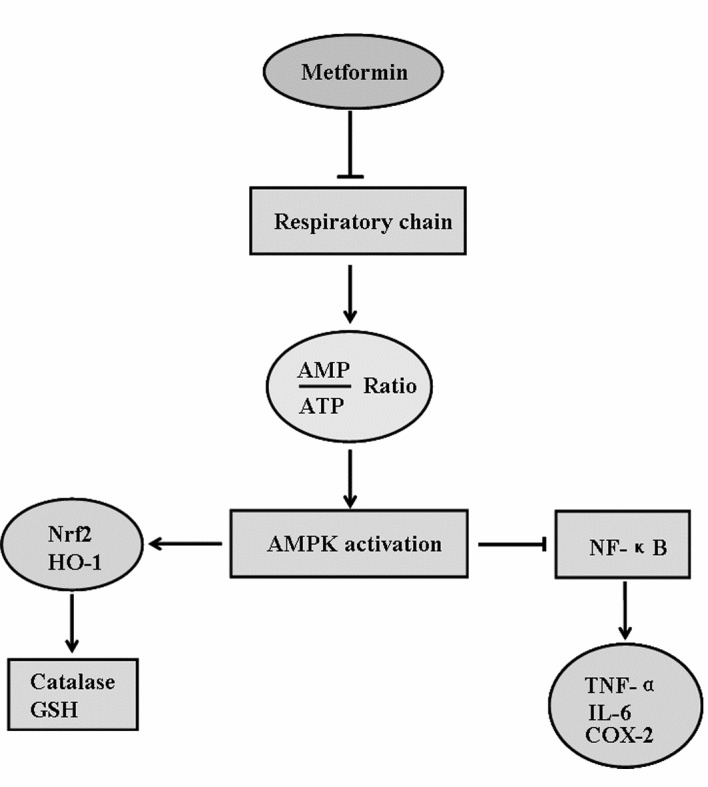
Molecular mechanisms underlying the effects of metformin on stroke outcomes.
In addition to AMPK activation, metformin may act via other mechanism to impact stroke outcomes. For instance, it is likely that metformin confers neuroprotection against cerebral ischemia by regulating the incretin axis since metformin has been reported to acutely increase the plasma level of glucagon-like peptide-1, which is known to exert pleiotropic neurological actions [22]. However, currently there is no evidence suggesting that the incretin axis mediates meformin effects on stroke outcomes. Thus, further investigation is needed. Because AMPK is the most well-established target kinase through which metformin exerts its pharmacological actions and because numerous studies have suggested that AMPK is a crucial mediator in stroke pathogenesis [23], the review mainly focuses on how metformin acts through AMPK activation to affect stroke outcomes.
Several subunits of AMPK have been found in the brain that are involved in stroke pathogenesis. For instance, the α2 subunit of AMPK is highly expressed in neurons and astrocytes [24]. AMPK α2 gene deletion has been shown to decrease infarct damage, whereas AMPK α1 gene deletion does not affect infarction following experimental stroke [25]. Several AMPK β subunits are expressed in the astrocytes, including β2 and β1, although astrocytic expression of the latter is lower than β2 in astrocytes [23]. The γ subunits of AMPK are primarily expressed in neurons, with the exception of γ2, which is mainly located in glia [23]. Indeed, a clinical study has related the polymorphism of AMPK γ2 gene to cognitive impairment and diabetes in old age [26], suggestive of a potential role of AMPK γ2 in stroke outcomes.
AMPK activation is remarkably increased in the brain following cerebral ischemia [27]. However, it is highly debated whether post-ischemic AMPK activation plays a beneficial or detrimental role after cerebral ischemia [23]. The different cellular combination of AMPK subunits may account for the diverse effects of AMPK activation following stroke [25, 28]. On the other hand, the diverse effects of AMPK may also critically depend on the cellular types and duration of AMPK activation. For instance, as mentioned previously, although systematic gene deletion of AMPK α2 confers neuroprotection following experimental stroke, cell-type specific deletion of AMPK α2 in astrocytes is detrimental after cerebral ischemia, leading to increased infarction and poorer behavioral outcomes [23]. Consistent with the diverse roles of AMPK activation in cerebral ischemia, the current literature also reveals that metformin, as an AMPK activator, has diverse effects on stroke outcomes.
Recent studies suggest that the cell type specificity and the duration of AMPK activation may account for the differential effects of metformin. Metformin may also exert differential effects in the brain vs. periphery or during the acute phase vs. recovery phase following stroke.
THE PREVENTATIVE EFFECTS OF METFORMIN ON STROKE OUTCOMES
Metformin has been epidemiologically shown to reduce the incidence of stroke, and the effects are independent of the glucose-lowering effect [12]. However, experimental stroke yields inconsistent results regarding whether pre-treatment of metformin is a protective effect in stroke models. It has been reported that acute metformin treatment for 3 days before experimental stroke remarkably exacerbates infarct damage at 24 hours after middle cerebral artery occlusion (MCAO) [28]. In contrast, chronic treatment with metformin for 3 weeks before experimental stroke decreases acute infarction at 24 hours after MCAO [28]. Consistent with the notion that AMPK activation is deleterious to acute stroke outcomes, the authors showed that acute treatment of metformin enhanced cerebral AMPK activation following MCAO and AMPK gene deletion abolished the detrimental effects conferred by acute treatment of metformin; while chronic metformin treatment reduced AMPK activation after stroke [28]. In part, AMPK activation may exacerbate lactate accumulation and metformin may increase infarct damage in this way. Metformin-induced changes in AMPK activation following cerebral ischemia seem to be mediated by neuronal nitric oxidase (nNOS) because deletion of nNOS abolishes both deleterious effects conferred by acute treatment of metformin and neuroprotective effects by chronic treatment of metformin. Taken together, this study seems to suggest that the effects of metformin pretreatment depend on the duration of metformin treatment.
Consistently, it has been reported that chronic (2 weeks) pretreatment of metformin via the gavage route reduces neuronal ischemic damage in the hippocampus in a global cerebral ischemia model [20, 29]. However, in this global cerebral ischemia model, chronic pretreatment of metformin did not decrease AMPK activation in the ischemic brain, which is in sharp contrast to the aforementioned study [28]. Instead, in this study, chronic metformin pretreatment increases cerebral AMPK activation following ischemia, suggesting that metformin may confer neuroprotection by activating AMPK [29]. Further investigation is needed to investigate whether the discrepancy between the two studies may be attributed to the type of ischemic insults (focal vs. global ischemia), cerebral regions (cortex vs. hippocampus) and the duration of ischemia.
Although acute pretreatment of metformin is reported to exacerbate acute infarction following transient focal cerebral ischemia, a recent publication showed that acute pretreatment of metformin 24 hours prior to permanent MCAO induced preconditioning, conferring robust neuroprotection at 24 hours after cerebral ischemia [30]. In this study, the preconditioning effects of metformin are mediated by AMPK activation-dependent autophagy. Once again, the study suggests that metformin pretreatment exerts preventative effects on stroke by activating AMPK. These results are in contrast with the aforementioned publication [28] as well as the finding that metformin pretreatment acts through AMPK activation to abolish neuroprotection conferred by hypoxia preconditioning following MCAO [7]. The factors that account for the discrepancy between these studies probably include the different doses of metformin (10 mg/kg vs. 100 mg/kg) and different stroke models employed (permanent vs. transient MCAO models). The high doses of metformin used in the previous study [28] may induce over-activation of AMPK, leading to prolonged astrocytic glycolysis and a subsequent progressive acidosis [28].
Diabetes mellitus is a well-established risk factor for stroke. It has been reported that more than 30% stroke patients have diabetes mellitus, and patients with diabetes mellitus are at a high risk for hemorrhagic transformation [31]. Metformin is a favorable first-line anti-diabetic drug. To recapitulate the clinical condition, it is essential to use combination models of stroke and diabetes to investigate whether metformin exerts therapeutic effects in stroke patients with diabetes mellitus. The Goto-Kzizaki rat is a well-established rodent model for diabetes mellitus. Goto-Kzizaki rats display enhanced levels of vascular remodeling markers and dysfunctional cerebral neovascularization, which leads to hemorrhagic transformation and greater neurological deficits following cerebral ischemic although Goto-Kzizaki rats sustain smaller infarction sizes [32]. Pretreatment with 4 weeks of metformin can significantly decrease vascular remodeling and the severity of hemorrhagic transformation in Goto-Kzizaki rats. However, the vasoprotective effects of metformin following cerebral ischemia in Goto-Kzizaki rats with diabetes mellitus may be related to metformin’s effect on glycemic control. Nevertheless, these studies suggest that metformin is potentially a preventative therapy against stroke in patients with diabetes.
THE THERAPEUTIC EFFECTS OF POST-STROKE TREATMENT OF METFORMIN
In addition to the preventative effects of metformin, another equally or more important issue is whether metformin, administered following the onset of stroke, exerts therapeutic effects. Several studies have investigated its issue. In contrast to the complicated effects of metformin pretreatment, currently available experimental stroke studies consistently suggest that post-stroke treatment of metformin is beneficial in not only reducing acute infarction but in promoting long-term functional recovery following stroke. These facts indicate that metformin could be translated into a stroke therapeutics. It is reported that metformin, systematically administered immediately after experimental stroke via an intra-peritoneal injection, significantly decreases acute infarction in mice, and reducing stroke-induced enhancement of blood glucose levels is likely the underlying mechanism [33]. However, when administered locally into the brain via an intraventricular route, post-stroke metformin treatment exacerbates acute infarction by activating cerebral AMPK [33]. The peripheral vs. cerebral effects of metformin seem to account for the differential effects of metformin administered via different routes. Additionally, this study still suggests a detrimental role of cerebral AMPK activation following stroke.
However, in contrast to the complicated effects of metformin on acute infarct damage, AMPK activation induced by metformin following stroke exerts beneficial effects on long-term post-stroke recovery. Several recent publications, including one from our group, have shown that metformin, when administered within clinically relevant therapeutic windows, acts through cerebral AMPK activation and accelerates functional recovery following stroke [34-36]. Louise D. McCullough, whose group first showed that cerebral activation of AMPK exacerbates stroke outcomes, reported that the post-stroke treatment of metformin is beneficial to stroke recovery in 2010. In 2014, her group further showed that chronic treatment with metformin, starting at 3 days after experimental stroke, remarkably improves post-stroke angiogenesis and recovery following stroke. Mechanistically, metformin-enhanced angiogenesis and functional recovery is dependent on AMPK because the beneficial effects of metformin following stroke are lost in AMPK-α2 gene-deletion mice [35]. Simultaneously, a publication from our group also showed that post-stroke functional recovery and brain tissue repair, such as angiogenesis and neurogenesis, are significantly enhanced by chronic treatment with metformin starting at 1 day after experimental stroke. By using in vivo and in vitro cellular models, we further showed that metformin acts through cerebral AMPK activation to induce alternative (M2) action of microglia/infiltrated macrophages in the brain following stroke, which significantly contributes to promoting the effects of metformin on post-stroke brain repair, including angiogenesis. This study provides evidence that cell-specific effects, such as neuronal vs. glial effects, are critical to metformin’s effects on stroke outcomes [34]. Our published results were further confirmed in a recent publication showing that metformin administered after reperfusion improves post-stroke neurogenesis and angiogenesis in the brain via an AMPK activation-dependent mechanism [36]. Moreover, in a rat stroke model with diabetes mellitus, metformin, administered through drinking water at 1-2 days after stroke, displays therapeutic effects on sensorimotor recovery, cognitive deficits and anxiety-like symptoms [37]. In this diabetic model, the beneficial effects of metformin on long-term stroke recovery seem to be mediated by the euglycemic effects of metformin. Taken together, these studies consistently suggest that metformin, when administered within the clinically relevant therapeutic window, exerts therapeutic effects on long-term functional recovery. This is critical to the translation of the beneficial effects of metformin into clinical therapies given the fact that numerous stroke therapies have failed in clinical trials because of narrow therapeutic windows.
CONCLUSION
Metformin, a well-established AMPK activator and most favorable first-line anti-diabetic drug, has been shown epidemiologically to exert beneficial effects on stroke patients. However, the currently available experimental studies reveal that metformin displays complicated effects on stroke outcomes, as summarized in Table 1. First, it is likely that the metformin’s effects on stroke outcomes are determined by the timing and duration of metformin administration because most currently available studies from experimental stroke show that post-stroke chronic treatment of metformin displays beneficial effects on long-term stroke recovery, while acute administration of metformin, especially before stroke, exerts dose-dependently effects on acute infarction. Second, metformin’s effects following stroke are cell-specific or tissue-specific. Most experimental studies suggest that neuronal AMPK activation induced by metformin during the acute phase is detrimental, while glial AMPK activation plays a beneficial role. Moreover, experimental evidence also suggests that cerebral AMPK activation by metformin is deleterious to stroke outcomes, while peripheral AMPK activation by metformin alleviates stoke-enhanced serum glucose levels and therefore plays a beneficial role. Despite the complicated effects of metformin on acute stroke outcomes, the currently available experimental evidence consistently suggests that post-stroke chronic treatment promotes long-term functional recovery and brain repair within clinically relevant therapeutic windows. Therefore, future study is needed to validate the translational potential of metformin in patients with or without diabetes. Furthermore, further investigation is required to determine how metformin acts through tissue- or cell-specific effects to affect stroke outcomes. Dissecting the intertwined mechanisms underlying metformin’s effects may lead to novel stroke therapies.
Table 1.
Pharmacological effects of metformin on stroke outcomesa.
| Duration | Stroke Model | Route | Histological Outcomes | Functional Recovery | Proposed Mechanisms | Refs | |
|---|---|---|---|---|---|---|---|
| Pre-treatment with metformin |
24 h | pMCAO | ip | Decreasing acute infarction | Reduced neurological deficits at 24 h or 96 h | Inducing autophagy | [30] |
| 3 d | tMCAO | ip | Increasing acute infarction | - | Increasing lactate level in the brain | [28] | |
| 2 weeks | Global ischemia | po | Decreasing apoptosis | - | AMPK activation | [29] | |
| Post-treatment with metformin | 3 d | tMCAO | ip | Decreasing acute infarction | - | Peripheral AMPK activation | [33] |
| 1 d | tMCAO | icv | Increasing acute infarction | - | Cerebral AMPK activation | [33] | |
| 14 d | tMCAO | ip | Decreasing brain atrophy; enhancing neurogenesis and angiogenesis | - | AMPK and eNOS activation | [36] | |
| 3 weeks | tMCAO | ip | Enhancing angiogenesis | Improved | AMPK activation | [35] | |
| 30 d | tMCAO | ip | No effect on acute infarction, but enhancing long-term neurogenesis and angiogenesis | Improved | Microglial AMPK activation | [34] |
ip,intraperitoneally; icv, intracerebroventricularly; po, orally; pMCAO, permanent middle cerebral ischemia; tMCAO, transient middle cerebral ischemia.
SOURCES OF FUNDING
The project is supported by the grants from National Science Foundation of China (81130023, 81373382, 81371278, 81171246), National Basic Research Plan (2011CB504403), the Priority Academic Program Development of Jiangsu Higher Education Institutions of China (PAPD) and funding from BM2013003.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
References
- 1.Halpin H.A., Morales-Suárez-Varela M.M., Martin-Moreno J.M. Chronic disease prevention and the new public health. Public Health Rev. 2010;32:125–154. [Google Scholar]
- 2.Strong K., Mathers C., Bonita R. Preventing stroke: saving lives around the world. Lancet Neurol. 2007;6(2):182–187. doi: 10.1016/S1474-4422(07)70031-5. [DOI] [PubMed] [Google Scholar]
- 3.Towfighi A., Saver J.L. Stroke declines from third to fourth leading cause of death in the United States: historical perspective and challenges ahead. Stroke. 2011;42(8):2351–2355. doi: 10.1161/STROKEAHA.111.621904. [DOI] [PubMed] [Google Scholar]
- 4.Burke J.F., Lisabeth L.D., Brown D.L., Reeves M.J., Morgenstern L.B. Determining stroke’s rank as a cause of death using multicause mortality data. Stroke. 2012;43(8):2207–2211. doi: 10.1161/STROKEAHA.112.656967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nathan D.M., Buse J.B., Davidson M.B., Ferrannini E., Holman R.R., Sherwin R., Zinman B., American Diabetes Association. European Association for the Study of Diabetes Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2009;52(1):17–30. doi: 10.1007/s00125-008-1157-y. [DOI] [PubMed] [Google Scholar]
- 6.Inzucchi S.E., Bergenstal R.M., Buse J.B., Diamant M., Ferrannini E., Nauck M., Peters A.L., Tsapas A., Wender R., Matthews D.R. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2012;55(6):1577–1596. doi: 10.1007/s00125-012-2534-0. [DOI] [PubMed] [Google Scholar]
- 7.Rena G., Pearson E.R., Sakamoto K. Molecular mechanism of action of metformin: old or new insights? Diabetologia. 2013;56(9):1898–1906. doi: 10.1007/s00125-013-2991-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowker S.L., Yasui Y., Veugelers P., Johnson J.A. Glucose-lowering agents and cancer mortality rates in type 2 diabetes: assessing effects of time-varying exposure. Diabetologia. 2010;53(8):1631–1637. doi: 10.1007/s00125-010-1750-8. [DOI] [PubMed] [Google Scholar]
- 9.Patrone C., Eriksson O., Lindholm D. Diabetes drugs and neurological disorders: new views and therapeutic possibilities. Lancet Diabetes Endocrinol. 2014;2(3):256–262. doi: 10.1016/S2213-8587(13)70125-6. [DOI] [PubMed] [Google Scholar]
- 10.Howland R.H. A “glucose eater” drug as a therapeutic agent in psychiatry. J. Psychosoc. Nurs. Ment. Health Serv. 2013;51(9):13–16. doi: 10.3928/02793695-20130805-01. [DOI] [PubMed] [Google Scholar]
- 11.Roussel R., Travert F., Pasquet B., Wilson P.W., Smith S.C., Jr, Goto S., Ravaud P., Marre M., Porath A., Bhatt D.L., Steg P.G. Reduction of Atherothrombosis for Continued Health (REACH) Registry Investigators. Metformin use and mortality among patients with diabetes and atherothrombosis. Arch. Intern. Med. 2010;170(21):1892–1899. doi: 10.1001/archinternmed.2010.409. [DOI] [PubMed] [Google Scholar]
- 12.UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352(9131):854–865. doi: 10.1016/S0140-6736(98)07037-8. [DOI] [PubMed] [Google Scholar]
- 13.Cheng Y.Y., Leu H.B., Chen T.J., Chen C.L., Kuo C.H., Lee S.D., Kao C.L. Metformin-inclusive therapy reduces the risk of stroke in patients with diabetes: a 4-year follow-up study. J. Stroke Cerebrovasc. Dis. 2014;23(2):e99–e105. doi: 10.1016/j.jstrokecerebrovasdis.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J., Wu M., Ventre J., Doebber T., Fujii N., Musi N., Hirshman M.F., Goodyear L.J., Moller D.E. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 2001;108(8):1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardie D.G., Ross F.A., Hawley S.A. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012;13(4):251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheung P.C., Salt I.P., Davies S.P., Hardie D.G., Carling D. Characterization of AMP-activated protein kinase gamma-subunit isoforms and their role in AMP binding. Biochem. J. 2000;346(Pt 3):659–669. doi: 10.1042/bj3460659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oakhill J.S., Chen Z.P., Scott J.W., Steel R., Castelli L.A., Ling N., Macaulay S.L., Kemp B.E. beta-Subunit myristoylation is the gatekeeper for initiating metabolic stress sensing by AMP-activated protein kinase (AMPK). Proc. Natl. Acad. Sci. U.S.A. 2010;107(45):19237–19241. doi: 10.1073/pnas.1009705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawley S.A., Ross F.A., Chevtzoff C., Green K.A., Evans A., Fogarty S., Towler M.C., Brown L.J., Ogunbayo O.A., Evans A.M., Hardie D.G. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010;11(6):554–565. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaw R.J., Lamia K.A., Vasquez D., Koo S.H., Bardeesy N., Depinho R.A., Montminy M., Cantley L.C. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310(5754):1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashabi G., Khalaj L., Khodagholi F., Goudarzvand M., Sarkaki A. Pre-treatment with metformin activates Nrf2 antioxidant pathways and inhibits inflammatory responses through induction of AMPK after transient global cerebral ischemia. Metab. Brain Dis. 2014 doi: 10.1007/s11011-014-9632-2. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y., Tang G., Li Y., Wang Y., Chen X., Gu X., Zhang Z., Wang Y., Yang G.Y. Metformin attenuates blood-brain barrier disruption in mice following middle cerebral artery occlusion. J. Neuroinflammation. 2014;11:177. doi: 10.1186/s12974-014-0177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maida A., Lamont B.J., Cao X., Drucker D.J. Metformin regulates the incretin receptor axis via a pathway dependent on peroxisome proliferator-activated receptor-α in mice. Diabetologia. 2011;54(2):339–349. doi: 10.1007/s00125-010-1937-z. [DOI] [PubMed] [Google Scholar]
- 23.Manwani B., McCullough L.D. Function of the master energy regulator adenosine monophosphate-activated protein kinase in stroke. J. Neurosci. Res. 2013;91(8):1018–1029. doi: 10.1002/jnr.23207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turnley A.M., Stapleton D., Mann R.J., Witters L.A., Kemp B.E., Bartlett P.F. Cellular distribution and developmental expression of AMP-activated protein kinase isoforms in mouse central nervous system. J. Neurochem. 1999;72(4):1707–1716. doi: 10.1046/j.1471-4159.1999.721707.x. [DOI] [PubMed] [Google Scholar]
- 25.Li J., Zeng Z., Viollet B., Ronnett G.V., McCullough L.D. Neuroprotective effects of adenosine monophosphate-activated protein kinase inhibition and gene deletion in stroke. Stroke. 2007;38(11):2992–2999. doi: 10.1161/STROKEAHA.107.490904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim E., Lee S.H., Lee K.S., Cheong H.K., Namkoong K., Hong C.H., Oh B.H. AMPK γ2 subunit gene PRKAG2 polymorphism associated with cognitive impairment as well as diabetes in old age. Psychoneuroendocrinology. 2012;37(3):358–365. doi: 10.1016/j.psyneuen.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 27.McCullough L.D., Zeng Z., Li H., Landree L.E., McFadden J., Ronnett G.V. Pharmacological inhibition of AMP-activated protein kinase provides neuroprotection in stroke. Stroke. 2005;38(11):2992–2999. doi: 10.1074/jbc.M409985200. [DOI] [PubMed] [Google Scholar]
- 28.Li J., Benashski S.E., Venna V.R., McCullough L.D. Effects of metformin in experimental stroke. Stroke. 2010;41(11):2645–2652. doi: 10.1161/STROKEAHA.110.589697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashabi G., Khodagholi F., Khalaj L., Goudarzvand M., Nasiri M. Activation of AMP-activated protein kinase by metformin protects against global cerebral ischemia in male rats: interference of AMPK/PGC-1α pathway. Metab. Brain Dis. 2014;29(1):47–58. doi: 10.1007/s11011-013-9475-2. [DOI] [PubMed] [Google Scholar]
- 30.Jiang T., Yu J.T., Zhu X.C., Wang H.F., Tan M.S., Cao L., Zhang Q.Q., Gao L., Shi J.Q., Zhang Y.D., Tan L. Acute metformin preconditioning confers neuroprotection against focal cerebral ischaemia by pre-activation of AMPK-dependent autophagy. Br. J. Pharmacol. 2014;171(13):3146–3157. doi: 10.1111/bph.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Idris I., Thomson G.A., Sharma J.C. Diabetes mellitus and stroke. Int. J. Clin Pract. 2006. [DOI] [PubMed]
- 32.Elgebaly M.M., Prakash R., Li W., Ogbi S., Johnson M.H., Mezzetti E.M., Fagan S.C., Ergul A. Vascular protection in diabetic stroke: role of matrix metalloprotease-dependent vascular remodeling. J. Cereb. Blood Flow Metab. 2010;30(12):1928–1938. doi: 10.1038/jcbfm.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harada S., Fujita-Hamabe W., Tokuyama S. The importance of regulation of blood glucose levels through activation of peripheral 5' -AMP-activated protein kinase on ischemic neuronal damage. Brain Res. 2010;1351:254–263. doi: 10.1016/j.brainres.2010.06.052. [DOI] [PubMed] [Google Scholar]
- 34.Jin Q., Cheng J., Liu Y., Wu J., Wang X., Wei S., Zhou X., Qin Z., Jia J., Zhen X. Improvement of functional recovery by chronic metformin treatment is associated with enhanced alternative activation of microglia/macrophages and increased angiogenesis and neurogenesis following experimental stroke. Brain Behav. Immun. 2014;40:131–142. doi: 10.1016/j.bbi.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Venna V.R., Li J., Hammond M.D., Mancini N.S., McCullough L.D. Chronic metformin treatment improves post-stroke angiogenesis and recovery after experimental stroke. Eur. J. Neurosci. 2014;39(12):2129–2138. doi: 10.1111/ejn.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Y., Tang G., Zhang Z., Wang Y., Yang G.Y. Metformin promotes focal angiogenesis and neurogenesis in mice following middle cerebral artery occlusion. Neurosci. Lett. 2014;579:46–51. doi: 10.1016/j.neulet.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 37.Prakash R., Li W., Qu Z., Johnson M.A., Fagan S.C., Ergul A. Vascularization pattern after ischemic stroke is different in control versus diabetic rats: relevance to stroke recovery. Stroke. 2013;44(10):2875–2882. doi: 10.1161/STROKEAHA.113.001660. [DOI] [PMC free article] [PubMed] [Google Scholar]


