Abstract
Trigeminal autonomic cephalalgias (TACs) are a group of primary headaches including cluster headache (CH), paroxysmal hemicrania (PH) and short-lasting unilateral neuralgiform headache with conjunctival injection and tearing (SUNCT). Another form, hemicrania continua (HC), is also included this group due to its clinical and pathophysiological similarities. CH is the most common of these syndromes, the others being infrequent in the general population. The pathophysiology of the TACs has been partly elucidated by a number of recent neuroimaging studies, which implicate brain regions associated with nociception (pain matrix). In addition, the hypothalamic activation observed in the course of TAC attacks and the observed efficacy of hypothalamic neurostimulation in CH patients suggest that the hypothalamus is another key structure. Hypothalamic activation may indeed be involved in attack initiation, but it may also lead to a condition of central facilitation underlying the recurrence of pain episodes. The TACs share many pathophysiological features, but are characterised by differences in attack duration and frequency, and to some extent treatment response. Although alternative strategies for the TACs, especially CH, are now emerging (such as neurostimulation techniques), this review focuses on the available pharmacological treatments complying with the most recent guidelines. We discuss the clinical efficacy and tolerability of the currently used drugs. Due to the low frequency of most TACs, few randomised controlled trials have been conducted. The therapies of choice in CH continue to be the triptans and oxygen for acute treatment, and verapamil and lithium for prevention, but promising results have recently been obtained with novel modes of administration of the triptans and other agents, and several other treatments are currently under study. Indomethacin is extremely effective in PH and HC, while antiepileptic drugs (especially lamotrigine) appear to be increasingly useful in SUNCT. We highlight the need for appropriate studies investigating treatments for these rare, but lifelong and disabling conditions.
Keywords: Headache, preventive treatments, symptomatic treatments, treatment guidelines, trigeminal autonomic cephalalgias.
INTRODUCTION
The trigeminal autonomic cephalalgias (TACs) are a group of primary headaches characterised by unilateral pain associated with ipsilateral cranial autonomic features, such as conjunctival injection, lacrimation and nasal symptoms [1, 2]. According to the International Classification of Headache Disorders, 3rd edition, beta version (ICHD-IIIbeta) [3], this group includes cluster headache (CH), paroxysmal hemicrania (PH), short-lasting unilateral neuralgiform headache with conjunctival injection and tearing (SUNCT), short-lasting unilateral neuralgiform headache with cranial autonomic features (SUNA). Recently, hemicrania continua (HC), another form of primary headache [4] has been grouped with the TACs due to its clinical and pathophysiological features that are very similar to those of the above-mentioned forms. Neuroimaging, neuroendocrine, neurochemical and neuropharmacological findings have considerably increased understanding of the TACs in recent years. CH is the most frequent TAC; the others are uncommon, even in specialist centres [1]. CH has a mean prevalence of 0.1% in the general population [5] and it shows a clear male predominance [6], with a male/female ratio ranging from 2.5:1 to 7.1:1 [7, 8]. The lifetime incidence of CH, reported in a recent meta-analysis, was 124 per 100,000 and the one-year incidence was 53 per 100,000 [9]. Both PH and SUNCT have a reported prevalence of 0.5 per 1000 [6], although the true rate could actually be higher as these forms are frequently misdiagnosed as trigeminal neuralgia or CH. Paroxysmal hemicrania has been long considered a female problem, although a recent study failed to confirm this [10]. There is no available information about the prevalence of HC and SUNCT/SUNA, but these forms are rare: only several hundred cases have been reported in total. CH may have a genetic basis, but the mode of transmission appears to be variable and the degree of penetrance is unclear [11-13]. No specific genes have yet been clearly associated with this disorder [14]. There is no evidence of a genetic component in PH, SUNCT or HC.
The TACs share several common features, but differ in attack frequency and duration, as well as in response to treatments. All these forms are characterised by predominantly severe, sometimes excruciating, pain, which can lead to high disability and a poor quality of life [15]. In the TACs, as in other, more common, primary headaches (i.e. migraine and tension-type headache), the aim of treatment is twofold: to eliminate acute pain using symptomatic drugs, and to prevent pain (i.e. reduce the frequency and intensity of attacks) using prophylactic drugs. Several neurostimulation techniques have been developed for some of these forms, especially CH [16]. We discuss these briefly, even though they are outside the scope of this paper. In this review, we outline the clinical features and pathophysiology of the TACs. We then look at the pharmacological strategies, both traditional and new, used in these conditions.
CLINICAL FEATURES OF THE TRIGEMINAL AUTONOMIC CEPHALALGIAS
CH is characterised by severe or unbearable unilateral pain, typically in the retro-orbital and frontotemporal areas, associated with ipsilateral symptoms and signs of cranial autonomic dysfunction, i.e. conjunctival injection, tearing, eyelid oedema, miosis, ptosis, nasal congestion, rhinorrhoea and facial sweating. Patients also typically feel restless and agitated during CH attacks. The pain in CH is generally considered more severe than any other form of primary headache pain, as well as one of the most disabling pains a human can experience. The attacks last 15 to 180 minutes, and show a characteristic circadian periodicity. Patients may have up to eight attacks per day. CH is so called because the attacks tend to occur in clusters or bouts of varying duration. In the subtype known as episodic CH (ECH), bouts, or cluster periods, last 7-365 days and are separated by pain-free remission periods of more than one month; in chronic CH (CCH), they recur over a period of more than one year without remission periods or with remission periods lasting less than one month [3]. Even though most CH attacks are spontaneous, some may be triggered by alcohol intake, especially during cluster periods. Attacks can also be triggered by volatile substances, such as solvents and oil-based paints, and by nitroglycerin (NTG), acting as nitric oxide (NO) donors [17,18]. A higher frequency of attacks has been observed during sleep, particularly the first REM sleep [19, 20]. CH is diagnosed clinically on the basis of the current criteria [3], but its features explain why there is often a considerable diagnostic delay; the condition can initially go unrecognised or be misdiagnosed as migraine or sinusitis.
Paroxysmal hemicrania (PH) is characterised by relatively short-lasting attacks (2-30 minutes) of very severe unilateral pain in the retro-orbital or frontotemporal regions. The pain may also radiate to the neck or ipsilateral shoulder, and usually has an abrupt onset and cessation. Most PH attacks are spontaneous, although they can be triggered by rotating the neck or flexing the head to the headache side, or by pressing on the transverse processes of C4-C5, the C2 root, or the great occipital nerve (GON). Mild residual pain may persist between attacks, and interparoxysmal allodynia and hyperalgesia have been observed in patients who had a personal or a family history of migraine [10]. Attacks occur with a frequency of between 5 per day to more than half of the time, but do not show a clear circadian rhythm. The most common autonomic symptoms associated with PH attacks are tearing and nasal congestion, followed by conjunctival injection and rhinorrhoea. The symptoms typically respond to indomethacin [21]. About 20% of patients have episodic PH (EPH), in which periods (lasting at least a week) of recurrent attacks are followed by remission periods (lasting at least a month). Most patients (80%) have chronic PH (CPH); in this form attacks recur fora year without remissions, or with remissions lasting less than a month.
As previously mentioned, the TACs and HC share many common features [4, 22]. Like migraine and PH, HC is predominant in females. HC is characterised by continuous head pain with superimposed exacerbations of the pain. These exacerbations occur with varying frequency, ranging from many times per week to few times per month. The continuous pain, located in the temporal or periorbital area, is mild or moderate in intensity, with no headache-related disability. It is often unilateral, even though cases of side-switching pain [23] and bilateral pain [24] have been reported. Absolute response to indomethacin is a mandatory diagnostic feature, required by the current criteria [3]. During the exacerbation periods, the pain is moderate or severe, lasts hours or days and is associated with migrainous or autonomic symptoms (photophobia and phonophobia, nausea and vomiting, tearing and nasal congestion, rarely auras) [25, 26]. Differential diagnosis between PH and HC can be problematical, as the interparoxysmal pain that occurs in the TACs (mainly PH) can mimic the continuous pain of HC.
Finally, SUNCT is characterised by short lasting (1-600 seconds) attacks of severe lateralised pain that occur with a very high frequency (between 1 per day and more than half of the time). In SUNCT, on the other hand, attacks, or “headache stabs”, can last up to 10 minutes [27] and even up to 20 minutes in some patients [28]; the pain can be experienced anywhere in the head, and the attacks are often triggered by cutaneous stimuli [27]. Tearing and conjunctival injection are generally the only associated autonomic symptoms; in symptomatically more complex forms (SUNA), other parasympathetic signs may occur, such as nasal congestion and rhinorrhea, and only one or neither of conjuntival injection and tearing. Since the cranial autonomic symptoms are known to be due to overexpression of the trigeminal autonomic reflex, it is not uncommon for autonomic symptoms, such as nasal congestion, rhinorrhoea, eyelid oedema and facial flushing to be bilateral during attacks.
In typical cases, the differential diagnosis of CH is with secondary headaches and with other primary headaches, in particular migraine without aura, trigeminal neuralgia, and other short-lasting autonomic headaches. Secondary headaches, e.g. caused by an inflammatory process of the cavernous sinus or of the paranasal sinuses, can mimic the signs and symptoms of CH and sometimes of other TACs. It is more difficult to differentiate between CH and other TACs. A shorter duration and higher frequency of attacks in the absence of a clear periodicity or clusters would seem to point to a diagnosis of PH; however, the possibility of overlap and misdiagnosis between these forms remains high. In such cases, the most useful feature to consider is the response to indomethacin (≥150 mg per os or ≥100 mg i.m.), and its administration (INDOtest) can also be utilised as an ex juvantibus rule [29]. SUNCT also shares clinical characteristics with CH. In this form, however, the pain attacks recur very frequently and tearing and conjunctival injection are generally the only associated autonomic symptoms; furthermore, there is no circadian rhythmicity. On the other hand, however, other parasympathetic signs may be present (i.e. suggesting a diagnosis of SUNA) Fig. (1).
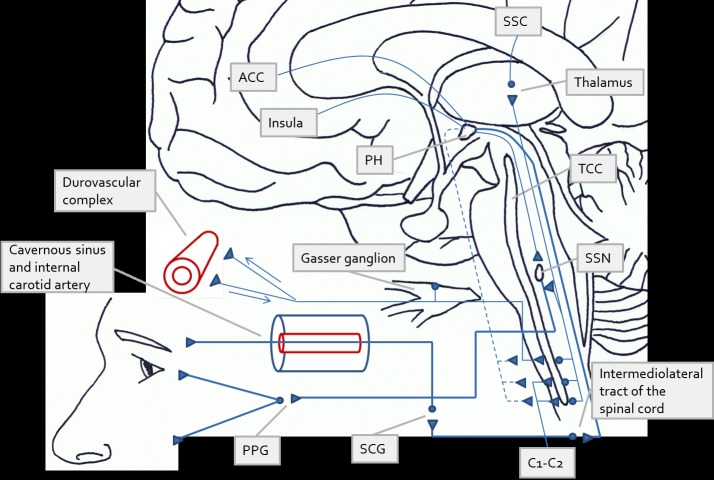
Fig. (1).
Diagram summarising the pathophysiology of cluster headache (CH) and other trigeminal autonomic cephalalgias (TACs) according to the most recent views and insights. The origin of the pain in CH and in the TACs may be peripheral or central. In the first case, the headache attack is suggested to originate from activation of the afferent trigeminal fibres induced by irritation of the structures of the face or of the cranial vault. In the second case (central origin), the attack is thought to be the consequence of direct activation of the posterior hypothalamus (PH), as findings of functional imaging studies have consistently shown. In both circumstances, activation of the superior salivatory nucleus – by the PH, or through the trigeminal-autonomic (or trigeminovascular) reflex (indirect activation)– results in an increased firing of parasympathetic fibres and thus in ipsilateral autonomic signs (conjunctival injection, tearing, nasal congestion and rhinorrhoea). Neurogenic inflammation is also produced by neurotransmitter release at the parasympathetic terminals, and the subsequent irritation of the trigeminal sensory nerves potentiates the vascular response via antidromic CGRP release. Symptoms such as miosis and ptosis (i.e. incomplete Horner’s syndrome) are suggested to result from parasympathetic-induced vasodilation of the internal carotid artery and functional impairment of the oculosympathetic fibres running through the cavernous sinus. Intense pain stimuli are carried through projections first to the trigeminal-cervical complex and then to the thalamus, up to the cortical sensory areas involved in pain processing. The PH is functionally connected to the ipsilateral trigeminal system and has an inhibitory role (dashed lines). Dysfunction of these projections may induce a permissive state not only facilitating attack occurrence, but also influencing the duration of single attacks. Attack duration is the main distinguishing feature of the different TACs. ACC=anterior cingulate cortex, SSC=somatosensory cortex, PH=posterior hypothalamus, TCC=trigeminal-cervical complex, SSN=superior salivatory nucleus, SCG=superior cervical ganglion, PPG=pterygopalatine ganglion.
PATHOPHYSIOLOGY OF THE TRIGEMINAL AUTONOMIC CEPHALALGIAS
The pathophysiological mechanisms underlying the TACs are only partly understood. Several hypotheses have been advanced, including vasomotor changes (vasodilation), inflammation, immune changes, hypothalamic dysfunction and autonomic system imbalance. These processes and mechanisms may well be interrelated, and different central and peripheral neuromodulatory pathways may participate in one or more of them.
It is generally agreed that the pain in CH is due to activation of the trigeminovascular system [30, 31], and that this system may be driven simultaneously in the brainstem and craniofacial sympathetic nerve fibres, thereby giving rise both to pain and to local autonomic phenomena [32]. In more detail, retrograde activation of the trigeminal fibres triggers release of several vasoactive substances. One of these is calcitonin gene-related peptide (CGRP), a neuropeptide belonging to a family of peptides (including calcitonin, adrenomedullin and amylin) that are widely distributed both in the central nervous system (CNS) and in nerve fibres originating from the trigeminal ganglion and innervating blood vessels. Calcitonin gene-related peptide induces intracranial vasodilation and is involved in pain transmission [33, 34]. It can produce sterile neurogenic inflammation with vasodilation, oedema and protein discharge at dural level. Pain signals, evoked by this inflammation, are then directed through the trigeminal ganglion to the trigeminal-cervical complex (TCC) and thence to the thalamus and the cerebral cortex. The fact that CGRP blood levels are reduced after oxygen or sumatriptan administration, and that this reduction is associated with pain remission, constitutes evidence of the crucial role of CGRP in the pathophysiology of CH [35, for review]. Calcitonin gene-related peptide can be considered a marker of activation of the trigeminovascular system. Substance P is another algogenic peptide that has long been considered to play a key role in CH [36], as well as in other primary headaches. The ipsilateral ophthalmic artery has been shown to be dilated during CH attacks [37], although this is a pattern shared bydifferent headache syndromes [38]. In addition, even though vasodilation may activate the trigeminovascular system [39], cerebral blood flow studies do not support a primary role for vasodilation in CH [40, 41]. Capsaicin has been shown to induce pain in healthy humans via vasodilation of cranial vessels, but this finding may reflect activation of the trigeminal-parasympathetic reflex [38].
The cranial autonomic symptoms and signs observed during CH attacks may result from functional activation of the superior salivatory nucleus (SSN) whose parasympathetic outflow, predominantly via the sphenopalatine ganglion, causes parasympathetic symptoms ipsilateral to the pain, such as tearing, conjunctival injection, nasal congestion and rhinorrhoea. These effects are thought to be produced mostly by the release of acetylcholine and vasoactive intestinal peptide (VIP). Thus, the concurrent increase in CGRP and VIP levels observed during CH attacks suggests the presence of a trigeminal-parasympathetic reflex: the trigeminal fibres may thus interact not only with the TCC, but also with the SSN, resulting in parasympathetic activation. On the other hand, the partial Horner’s syndrome observed during some attacks could indicate a peripheral origin. Vasodilation and perivascular oedema of the internal carotid, produced by the neurogenic inflammation, may indeed affect the function of the perivascular sympathetic plexus, leading to ipsilateral miosis and ptosis. However, it remains possible that the autonomic imbalance, associated with a hypothalamic disturbance, may also have a central origin [39, 42]. In any case, it is still not known what initially induces the activation of either the trigeminovascular system or the trigeminal-parasympathetic reflex [36].
Early studies suggested a role for inflammatory mechanisms in CH [43-46]. Steroids usually have positive effects, albeit only in interrupting the active phase of the disease [47]. Recurrent venous vasculitis in the cavernous sinus has also been hypothesised [48, 49], although recent evidence argues against this [50, 51]. In addition, a SPECT/MRI study [52] failed to show plasma protein extravasation into the cavernous sinus of CH patients during an attack.
Nitric oxide (NO) has been shown to be also involved in the pathophysiology of CH [53], acting as a potent vasodilator, but also playing a role in central and peripheral modulation of nociception [54], especially in both initiation and maintenance of hyperalgesia [55-57]. These processes are probably associated with activation of the calcium-dependent NO synthase (NOS) isoforms [58]. Nitric oxide appears to have a modulatory role in the spinal trigeminal nucleus, as NOS inhibition is associated with reduced activity of neurons with meningeal input in this nucleus [59]. Interestingly, CGRP and NOS co-localise in many trigeminal ganglion neurons [60]. It has been suggested that NO induces release of CGRP [61], although other evidence fails to support this suggestion [62]. Systemic NTG activates neuronal groups in selected brain areas critical in nociception, and particularly in the transmission of cephalic pain, such as the nucleus trigeminalis caudalis, and it induces specific changes in the content of brain neurotransmitters involved in pain processing [63]. Administration of NTG triggers spontaneous-like attacks in CH during the active phase but not during remission, thus representing an experimental model of induced headache [53, 64]. Nitric oxide may also act as an inhibitor of cytochrome oxidase, increasing the cellular oxygen demand [65]. Neuronal NOS (nNOS) is an isoform expressed in most regions of the CNS; interestingly, the hypothalamus contains a large number of nNOS-containing neurons [66]. In view of the periodicity of CH attacks and the finding of several hormonal changes in this condition, the activity of the hypothalamic suprachiasmatic nucleus has been suggested to be deranged in CH patients [67, 68]. The hypothalamus may show abnormal production of NO. A basal hyperfunction of the L-arginine-NO pathway was suggested to occur in both phases of CH [69], but a later study failed to confirm this [70]. A recent study [71] showed higher cerebrospinal fluid (CSF) levels of stable products of NO oxidation (nitrite and nitrate) in CH patients in the active period than inpatients in remission and control subjects. The CH patients also had significantly enhanced nitrite and nitrate CSF levels in remission compared with the controls. These apparent discrepancies regarding the role of NO may be explained by methodological differences (studies on plasma rather than CSF, and in spontaneous rather than NTG-induced attacks). On the other hand, the level of NO production has been shown to correlate with disease activity in inflammatory disorders [72], and increased nitrinergic activity may be an expression of enhanced inflammatory activity in CH. In CH, there could be a certain threshold before the trigeminovascular system is activated, which would explain why attacks occur during the active period and not in remission; CH patients may therefore be sensitised to CH attacks by a mechanism related to high NO levels [73]. High NO levels may also contribute to the generation and maintenance of central hyperalgesia [55-57], and activation of the trigeminovascular system induced by the release of algogenic neuropeptides (substance P, CGRP) may induce neurogenic inflammation, sensitising vessels and meninges and triggering vasodilation. Interestingly, dexamethasone treatment inhibits nNOS activity in the mouse [74]; the effectiveness of steroids in humans with CH may therefore be due toreduced production of NO, leading to decreased inflammation and activation of the trigeminal system.
The hypothesis that CH has a primary central origin was supported by early observations that lithium is an effective prophylactic drug for both ECH and CCH attacks [75,76]. For several reasons, the hypothalamus is indeed at the centre of scientific interest in CH and other TACs (Table 1). Cluster headache is a biorhythmic disorder, since attacks often occur with a strict circadian periodicity and the clusters often occur during spring and autumn, suggesting disruption of the organism’s internal temporal homeostasis. Substantial early neuroendocrine evidence supported a role for the hypothalamus in CH [67]. The locus coeruleus and dorsal raphe nucleus of the brainstem send noradrenergic and serotoninergic fibres to the hypothalamus [77]. Dysfunction of these nuclei could alter the monoaminergic regulation of the hypothalamus and underlie the development of CH [78, 79]. A direct connection also exists between the posterior hypothalamus and the TCC [77]: injection of orexins A and B, and of the gamma aminobutyric (GABA)-A receptor antagonist bicuculline into the posterior hypothalamus is followed by activation of the TCC [80,81]. In addition, the hypothalamus has an important role in pain perception. Stimulation of the anterior hypothalamus suppresses responses to painful stimuli of wide dynamic range neurons in the dorsal horn [82]. Similarly, the pain threshold is increased following injection of opioids into the posterior, pre-optic and arcuate nuclei of the hypothalamus [83]. Recently, an asymmetric facilitation of trigeminal nociceptive processing predominantly at brainstem level was detected in patients with CH, especially in the active phase [84]. Central facilitation of nociception therefore appears to be an important part of the pathophysiology of CH. In the 1970s, successful treatment of intractable facial pain with posteromedial hypothalamotomy indicated that the posterior hypothalamus is involved in pain control in humans [85]. Electrode stimulation of the posterior hypothalamus was later proposed as a treatment for chronic CH in drug-resistant patients [86]. This stereotactic technique has proved to be effective in controlling headache attacks in most patients, providing further convincing evidence that the hypothalamus plays a major role in CH mechanisms [87]. In this regard, pituitary diseases have been recently reported to present as a TAC in several patients [2], but it is unclear whether this may be linked to involvement of the hypothalamus and/or to the neuroendocrine derangement reported in these forms [67].
Table 1.
Features suggesting a hypothalamic involvement in CH.
| Circadian periodicity | Frequent occurrence of pain episodes at fixed times during the day and night, with high intraindividual reproducibility. |
| Cluster periodicity | Occurrence of clusters in autumn or spring in most patients |
| Autonomic features ipsilateral to pain | Cranial ipsilateral trigeminal autonomic symptoms associated with pain during the attacks (conjunctival injection, lacrimation, nasal congestion, rhinorrhea, ptosis and facial sweating) |
| Sleep pattern | Occurrence of pain attacks during sleep, particularly in the REM phase, with wake-ups |
| Neuroimaging findings | Increased gray matter in the inferior posterior hypothalamus on VBM; activation of the ipsilateral posterior hypothalamus on fMRI and H215O PET; hypermetabolism of the hypothalamus on FDG-PET; altered functional connectivity using a hypothalamic seed ROI on resting-state fMRI; decreased hypothalamic N-acetylaspartate:creatine and choline:creatine levels on MRS |
| Neuroendocrine correlates | Several hormone changes in CH patients, including testosterone, prolactin, melatonin, luteinizing hormone, follicle-stimulating hormone, growth hormone, orexins, and hypothalamo-pituitary-adrenal (HPA) axis function |
| Response to treatments | Effectiveness of drugs influencing hormone and neurotransmitter pathways in the hypothalamus. Effectiveness of deep brain stimulation (DBS) of posterior hypothalamus in patients unresponsive to medications. |
Most of the recent data on hypothalamic involvement in CH and TACs come from neuroimaging studies. Following the initial PET observation of inferior hypothalamic grey matter activation ipsilateral to NTG-induced pain in CH patients [68], functional neuroimaging techniques have, in recent years, allowed significant advances [reviewed in 88]. One major finding in the TACs is the presence of posterior hypothalamic activation during attacks. Most PET and functional MRI (fMRI) studies show hypothalamic hyper-activity (ipsilateral to the headache side in CH, contralateral in PH, and bilateral in SUNCT) during attacks. This activation is absent during pain-free periods in episodic CH, and is not specific to the TACs, having also been described in other pain conditions, such as migraine [89]. It is also unclear whether it reflects true activation of the hypothalamic region or, rather, involvement of the ventral tegmental area or other structures close to the hypothalamus [90, 88]. Nevertheless, hypothalamic activation may mirror a general antinociceptive response in healthy humans, and this response may be particularly altered in the TACs. In addition, the hypothalamic hyperactivation appears to persist during occipital nerve stimulation in chronic CH patients, regardless of the effectiveness of the procedure [91]. This may facilitate, centrally, activation of both the trigeminal system and the parasympathetic reflex,inducing pain and autonomic symptoms, as previously proposed [92].It is still not clear whether the hypothalamus is the real generator of CH mechanisms, or instead plays a secondary role (as a brain region participating in the pain network). However, some of the inconsistent findings in this regard might be explained by the use of different methods and timing of investigation (i.e. active vs remission phases, during attacks vs pain-free condition) [93]. The main features pointing to hypothalamic involvement in CH are reported in Table 1. Another major finding of neuroimaging studies in theTACs is the involvement of brain regions participating in human nociceptive processing, such as the anterior cingulate cortex, prefrontal cortex, thalamus, periaqueductal grey, basal ganglia, insula and cerebellum. These areas (collectively known as the pain matrix) have consistently shown increases in blood flow during attacks [38, 94, 95] and metabolic normalisation after occipital nerve stimulation in CH [96] and after indomethacin administration in PH [97]. These data suggest that metabolic changes are associated with disturbed nociception in acute and chronic pain conditions like the TACs. Several neuroimaging findings also implicate the central descending opiatergic pain control system in CH. Hypometabolism can be detected in the perigenual anterior cingulate cortex in episodic CH patients [98] and this pattern is reversed by clinically effective occipital nerve stimulation [96]. Furthermore, the opioid receptor availability in the rostral anterior cingulate cortex and the hypothalamus, as detected with PET, decreases with duration of CH [99]. Non-invasive neuroimaging techniques are expected to go on elucidating the mechanisms underlying the TACs in the near future.
In an animal model using brainstem stimulation to activate the trigeminal autonomic reflex arc and recording of neurons of the trigeminocervical complex, it has been recently found that brainstem activation may be the driver of both sensory and autonomic symptoms in the TACs, and that this may partly involve the parasympathetic outflow to the cranial vasculature. This pathway may play a critical role in the TACs, and represent a target for symptomatic treatments [100]. Finally, with particular regard to SUNCT and SUNA, a unifying pathophysiological model (2) suggests the occurrence of different degrees of interaction between peripheral and central mechanisms, as in the case of trigeminal neuralgia. Focal demyelination of the trigeminal sensory root and posterior hypothalamic dysfunction may contribute to the development of disease, the latter mechanism being more pronounced in patients with more cranial autonomic symptoms.
In summary, the TACs may share similar patho-physiological mechanisms, also because some overlapexists between the various forms in the response to treatments. For instance, complete response to indomethacin is a diagnostic criterion for PH, but clinical experience often shows that this drug can also improve CH [101].
THE MANAGEMENT OF CLUSTER HEADACHE
Effective treatment of CH demands the active participation of the patient who should be reassured about the benign nature of the condition and informed about the factors that may precipitate attacks during an active phase, in particular alcoholic beverages, which should therefore be avoided. Patients should also be informed that drugs for preventing or interrupting attacks are available, but also that, as yet, there exist no treatments able to prevent active periods or modify the natural history of the disease.
The therapeutic management of CH is usually divided into acute, transitional and prophylactic treatments.
Treatment of the Acute Phase
Since CH pain is immediately severe and develops rapidly, it requires a fast-acting drug. Initially, oral medications (especially NSAIDs) are usually taken by patients, but they are not able to provide rapid and effective pain control as the therapeutic agent needs to be promptly bioavailable; therefore it should be given parenterally. The objectives of a correct and effective symptomatic treatment are (1) to treat the attack when it starts; (2) to obtain pain relief as soon as possible (possibly within 15 min of administration of the drug); (3) to avoid or minimise adverse events [8]. Several drugs have been proposed for the acute treatment of CH, but very few have proved to be really effective.
Sumatriptan
Sumatriptanis the first of a relatively new class of drugs (the triptans) that act on the serotonin (5-hydroxytriptamine, 5-HT) receptors 5-HT1B and 5-HT1D [102]. As 5HT1B receptor agonists, the triptans have a vasoconstrictor effect on small and medium-sized arteries, such as those supplying the cerebral cortex, but also other regions including the coronary artery bed. In addition, as 5HT1D receptor agonists, the triptans inhibit the neuronal release of vasoactive peptides, such as CGRP, substance P and neurokinin A [103]. Findings in animal models of migraine indicate that the action of sumatriptan in aborting pain takes place primarily at a proximal site, i.e.the trigeminal afferent; furthermore, early administration of sumatriptan can inhibit neurotransmission to second order neurons in the TCC, thus preventing sensitisation of central neurons [104]. Some of these mechanisms are certainly relevant to the effect of sumatriptan on pain in CH. Subcutaneous sumatriptan has a Tmax of 12 min. It shows low plasma protein binding (between 14 and 21%)and has a half-life of approximately two hours. Sumatriptan is metabolised in the liver and gastointestinal tract by monoamine oxidase type A. Its major metabolite is the inactive indole acetic acid derivative, which accounts for approximately 40% of the total dose. Sumatriptan metabolites are excreted by both the kidney and the liver. Phar-macokinetic differences between the oral, nasal spray and rectal formulations (in particular, different distribution phases during elimination of the drug) mean that the above pharmacokinetic data for subcutaneous sumatriptan cannot be generalised to other routes of administration. Sumatriptan is considered the drug of first choice in the symptomatic treatment of CH on the basis of randomised, placebo-controlled trials (RCT) at the dose of 6 mg or 12 mg [105, 106]. The higher dose was no more effective than the lower dose in controlling the attacks and, moreover, was associated with more adverse effects [106]. Studies investigating the long-term efficacy and safety of subcutaneous sumatriptan have given positive results, with headache relief obtained in 96% of attacks [107], no reduction of efficacy with continued use, and no increase in adverse effects with higher frequencies of use [107, 108].
Patients with episodic and chronic CH and attacks lasting at least 45 minutes were treated with 20 mg intranasal sumatriptan in an RCT [109], with a significantly higher headache response for the drug than for placebo (responder rates: 57% vs 26%). Another open-label study reported lower efficacy of intranasal versus subcutaneous sumatriptan [110]. In addition to its open-label design, a major limitation of this study was that outcomes were evaluated at a relatively early time point (15 minutes after treatment).
A new needle-free method of delivering subcutaneous sumatriptan has recently become available in the U.S.A. The method, which eliminates both the needle and the associated disposal issues, improves drug delivery and showed acceptable patient tolerability in clinical trials [111]. A multicentre study, conducted to determine its ease of use and patient preferences, gave good results in migraine patients and possibly CH patients [112]. Although this method delivers relatively lower levels of the drug, it allows rapid attainment of Cmax, which is a significant advantage over the other routes (oral, subcutaneous, and intranasal).
In summary, subcutaneous sumatriptan is effective in the acute treatment of CH giving either partial relief of pain or complete headache remission within 15 minutes of injection. The most common adverse events, mild to moderate in 90% of cases [113], are local reactions at the injection site, dizziness, paraesthesia, cold or warm sensations and irritation of the nostril in the case of the intranasal formulation [113]. Sumatriptan is contraindicated in patients with coronary artery disease or cerebrovascular disease, thus a clinical evaluation of the risk of vascular diseases is mandatory in all patients before prescribing the drug. Serious cardiovascular events are mostly seen in patients with pre-existing major risk factors or established cardiac or cerebrovascular disease. A recent systematic review of observational studies did not report strong cardiovascular safety issues for triptans [114]. Chest-related symptoms commonly occurring in patients receiving sumatriptan could be due to multiple other causes or to mechanisms unrelated to 5HT1B activity in the coronary arteries. Furthermore, the early warnings about the potential for the development of serotonin syndrome when selective serotonin reuptake inhibitors (SSRIs)/selective norepinephrine reuptake inhibitors (SNRIs) are co-prescribed with triptans [115], have been not later supported by clinical evidence [116]. This has probably represented a case of overstated drug risk unduly influencing the utilization of a beneficial treatment.
Zolmitriptan
Oral zolmitriptan, evaluated as an acute treatment for CH in a RCT [117], was found to be superior to placebo in the episodic form (ECH), but not in the chronic form (CCH). The most commonly reported adverse effects were paraesthesia, heaviness, asthenia, nausea, dizziness and chest tightness. The efficacy of zolmitriptan nasal spray for the acute treatment of CH was also observed in two controlled studies [118,119]. The drug was well tolerated. The most commonly reported adverse effects were an unpleasant taste (22%), nasal cavity discomfort (12%) and somnolence (8%). A meta-analysis of two studies and a Cochrane analysis of six randomised studies, controlled versus placebo, demonstrated that sumatriptan and zolmitriptan are superior to placebo and show efficacy comparable to that of sumatriptan nasal spray [120, 113]. Like sumatriptan, zolmitriptan is contraindicated in patients with known vascular disease risks or established vascular disease and in these cases other acute treatments should be preferred.
Oxygen
Oxygen by inhalation is recognised as one of the two most effective abortive treatments for CH after injectable sumatriptan. Oxygen therapy for acute CH was first proposed in the 1950s [121], and became one of the acute treatments of choice in the early 1980s [122]. The efficacy of 100% oxygen inhalation at a maximum of 7 litres per minute (L/min) for 15 minutes observed in early studies was later confirmed in a controlled crossover study versus room air [123]. A large placebo-controlled crossover trial of 109 patients [124] compared the efficacy of 100% oxygen given via a non-rebreathing face mask at 12 L/min for 15 minutes with that of air inhalation in the treatment of four separate CH attacks. The two treatments differed significantly: pain freedom or substantial pain relief were obtained by 78% of the oxygen-treated versus 20% of the air-treated patients. If the patient fails to respond, the usual recommended flow may be increased to 14-15 L/min [125]. Hyperbaric oxygen has also been studied as an acute treatment for CH. In a placebo-controlled study [126], it not only interrupted attacks, but even ended the cluster period in three outof six patients. A gender difference in response to oxygen has been reported in clinical practice: 59% in female CH patients and up to 87% in male CH patients [127]. Moreover, data from the United States Cluster Headache Survey [128] revealed that a positive smoking history does not significantly alter the efficacy of inhaled oxygen and that most CH patients obtain complete head pain relief within 20 minutes of starting oxygen inhalation.
It is still not known exactly how inhaled oxygen interrupts a CH attack. Early studies suggested arterial vasoconstriction as the underlying mechanism of action, since the CH patients benefiting the most were those showing the greatest reduction in cerebral blood flow after oxygen inhalation [129]. Hyperoxia was later shown to inhibit plasma protein extravasation elicited by electrical stimulation of the rat trigeminal ganglion [130]. Another experimental study suggested that oxygen might act by reducing firing of the cranial autonomic pathway, in particular of the SSN [131], in other words by reducing the parasympathetic outflow; this would explain why inhaled oxygen is effective in migraine with severe autonomic features. On the other hand, the poor efficacy of oxygen in other TACs does not support this hypothesis. It is thus likely that different mechanisms are involved in the therapeutic action of oxygen, i.e. reduction of the parasympathetic outflow and control of the neurogenic inflammation caused by activation of the trigeminovascular reflex.
Oxygen can be used in patients with high vascular risk in whom acute treatment with the triptans is contraindicated. Caution should, however, be exercised in patients with chronic obstructive pulmonary disease, because of the risk of respiratory depression.
Ergotamine and Dihydroergotamine
Ergot derivatives were among the first drugs made available for the treatment of CH, with beneficial effects reported in 70% patients in a controlled study [122]. Dihydroergotamine (DHE) is available in various formulations: intravenous, intramuscular, subcutaneous and intranasal. Although the efficacy of injectable DHE has never been tested in controlled studies, clinical observations suggest that DHE could be effective in acute CH treatment and give better responses when administered intravenously as opposed to intramuscularly or subcutaneously. That said, a controlled study [132] evaluating the efficacy of intranasal DHE 1 mg for acute CH treatment in 25 patients reported a moderately positive response: pain intensity was decreased but attack duration was not.
The effect of the ergots (like that of the triptans) is based primarily on their interaction with the 5-HT receptors. At least seven classes and 14 subtypes of 5-HT receptors are presently known, each of which exerts different biological effects. In general, in the CNS, the 5-HT1 receptors are inhibitory whereas the 5-HT2-7 receptors are excitatory [133]. E and DHE interact with adrenergic and dopaminergic receptors, as well as with 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1F, 5-HT2A, 5-HT2C, 5-HT3, and 5-HT4 [133, 134]. In migraine, the clinical effects of these drugs reflect agonism primarily at the 5-HT1B/D receptors, and to a lesser extent at 5-HT1F receptors. The action at 5-HT1B receptors results in constriction of extracerebral blood vessels in the meninges, which are innervated by algogenic nerve fibres, whereas the action at5-HT1D receptors appears to produce presynaptic inhibition of trigeminal peptide release, affecting TCC nociceptive transduction and inhibiting nausea and vomiting via interaction in the brainstem (nucleus tractus solitarius) [135]. The final phenomena (vasoconstriction, reduced neurogenic inflammation, reduced central nociceptive signal transmission, reduced autonomic associated symptoms) explain the effects in migraine, but some of these mechanisms may well underlie the effects of ergots in CH.
The use of ergots, particularly E, is limited by potential serious adverse effects related to their α-adrenergic-induced vasoconstrictive action. These adverse effects are more severe than those of triptans, since the 5-HT1B receptors are preferentially expressed in intracranial extracerebral arteries compared with the periphery, where 5-HT2A receptors predominate. Ergots should never be used in patients with coronary, cerebral or peripheral vascular disease [133].
Local Anaesthetics
Early uncontrolled studies evaluating the therapeutic efficacy of topical lidocaine suggested that it could have a role in the acute treatment of CH. Use of a 4% lidocaine solution, applied locally to the sphenopalatine fossa in patients with NTG-induced CH attacks [136], or self-applied intranasally in the nostril ipsilateral to the pain [137], proved to be effective in variable percentages of patients. Better results were found in a placebo-controlled study, in which 10% lidocaine was applied bilaterally to the sphenopalatine fossa under anterior rhinoscopy in CH patients with NTG-induced attacks [138]. In patients with ECH or CCH, the application of a solution of cocaine 10% in both nostrils was shown to interrupt CH attacks both in an open study [139] and subsequently in a controlled study versus placebo [138]. No significant adverse events were recorded with the exception of a mild state of arousal in a patient who had abused the drug. Cocaine exerts sympathomimetic effects by modulating reuptake of noradrenaline in nerve endings, whereas lidocaine appears to exert its effects via conduction-blocking properties. Furthermore, these findings, suggesting that the sphenopalatine ganglion is involved in pain mechanisms, indicate that these anaesthetic agents might have a role in the symptomatic treatment of CH. In the case of cocaine, the risk of addiction, especially in a disabling condition like CH, should be obviously borne in mind, and its administration should be restricted to selected cases.
Somatostatin and its Analogues
Two RCTs have been conducted on the effect of somatostatin or one of its analogues, octreotide, in the treatment of acute CH attacks. In the first study intravenous somatostatin (25 μg in 50 ml saline) was more effective than placebo in inducing significant pain reduction in 20 minutes [140]. In the second, 100 µg octreotide (a somatostatin analogue with a longer half-life) was administered subcutaneously and produced a significant response in 30 minutes [141]. The mechanism of action of these peptides is unknown, but somatostatin has been shown to inhibit the release of numerous vasoactive peptides, including CGRP [142]. In addition, neurons containing somatostatin are found in the regions of the CNS involved in nociception, including the TCC, the periaqueductal grey, and the hypothalamus, which are also involved in CH pathophysiology [141]. Since they do not have vasoconstrictive effects, somatostatin and octreotide can also be used for the acute treatment of CH in patients with high vascular risk as a valid (albeit not equally effective) alternative to subcutaneous sumatriptan. The most common side effects of these agents are hyperglycaemia, nausea, abdominal pain, diarrhea and meteorism.
In conclusion, for the acute treatment of CH attacks, the first-line interventions supported by the highest level of evidence (A) are subcutaneous sumatriptan 6 mg, intranasal sumatriptan 20 mg, intranasal zolmitriptan 5 or 10 mg, and 100% oxygen, while subcutaneous octreotide and intranasal lidocaine 4-10% are supported by a lower level of evidence (B) [8, 143]. These treatments and levels of evidence are summarised in Table 2. However, the choice of treatment must also be made taking into account the variability in individual response. In this regard, in a prospective study in CH patients, older age emerged as a predictor for decreased response to the triptans, whereas nausea, vomiting and restlessness predicted a poor response to oxygen [144]. Other important variables are the presence of clinical comorbidities andthe patient’s preferred route of self-administration of a given treatment.
Table 2.
Levels of recommendation for symptomatic (a) and preventive (b) treatment of cluster headache (CH) [8,145].
| Drug | Dosage | Level of Recommendation | Comments |
|---|---|---|---|
| (a) Symptomatic treatments Sumatriptan Sumatriptan Zolmitriptan Oxygen inhalation Octreotide Lidocaine |
6 mg s.c 20 mg nasal spray 5–10 mg nasal spray 7-10 l/min for 15 min 100 µg s.c. 1 ml (4-10%) nasal spray |
A A A A B B |
Slower onset of action than sumatriptan s.c. Comparable in efficacy to sumatriptan nasal spray Flow rates up to 15 l/min have been effective Can be used in patients with cardiovascular diseases |
| Drug | Dosage (per day) | Level of Recommendation | Comments |
| (b) Preventive treatments for cluster headache | |||
| Verapamil Lithium carbonate Valproic acid Topiramate Baclofen Melatonin |
200-900 mg per os 600-900 mg per os 500-2000 mg per os 50-200 mg per os 15-30 mg per os 10 mg per os |
A B C B C C |
Less effective than lithium in chronic CH Elective efficacy in chronic CH |
Level A rating requires at least 1 convincing class I study or at least 2 consistent, convincing class II studies.
Level B rating requires at least 1 convincing class II study or overwhelming class III evidence.
Level C rating requires at least 2 convincing class III studies.
Preventive Treatment
Preventive treatment is a fundamental part of the management of active CH. Different drugs and approaches for acute CH treatment, like the triptans and oxygen, have been found to be safe and well tolerated even when used frequently or in prolonged treatments. Thus, in ECH, a symptomatic treatment alone may be suitable for active phases of short duration (mini-clusters). However, there is no evidence that symptomatic agents can influence the natural onset and evolution of typical cluster periods. For this reason, prophylactic treatments are necessary, administered with the aim of achieving: 1) rapid disappearance of attacks and resolution of active periods; 2) reduced frequency, intensity and duration of attacks [4, 8]. On the other hand, while the real effectiveness of a given treatment can be ascertained in chronic CH, it is more difficult to evaluate in the episodic form, since active periods can always subside spontaneously.
CH prophylaxis should be governed by a few general rules [8, 145]: 1) preventive treatment should start early in the active phase, and continue for at least two weeks after the disappearance of attacks; 2) the treatment should be reduced gradually and ultimately suspended, and if the attacks reappear, dosages must be increased back to therapeutic levels; 3) treatment should be re-started at the onset of a subsequent active period; 4) in the choice of the treatment, several factors should be taken into account, such as the patient’s age and lifestyle (e.g. alcohol intake should be avoided during a cluster period), the expected duration of the cluster period, the type of CH (episodic or chronic),the response to previous treatments, any reported side effects, any contraindications to recommended drugs, any comorbid diseases; 7) polytherapy, tested in only a few trials, is indicated only in patients resistant to monotherapy or patients who do not tolerate the recommended drugs at the optimal dosages.
The prophylaxis of CH may be divided into a transitional and a long-term prophylaxis.
Transitional Prophylaxis
The preventive treatments often have a delayed onset of action; furthermore, in order to avoid adverse effects they (may) need to be titrated gradually until the effective dose is reached. For these reasons, a patient may lack prophylactic coverage for days or weeks. The aim of transitional prophylaxis is to interrupt pain attacks rapidly and to maintain pain relief until the prophylactic drug has become effective.
Corticosteroids
Oral prednisone was evaluated in an uncontrolled study as a transitional treatment in patients with ECH and CCH, at doses ranging from 10 mg/day to 80 mg/day. A loading dose of prednisone was given for 3-10 days and then tapered over 10-30 days [146]. A significant reduction (72% of patients) or complete remission (58%) of attacks within 3-10 days was observed. These results suggested that doses of 40 mg or higher were needed to control the attacks. High doses of intravenous corticosteroids (methylprednisolone 30 mg/kg over 3 hours) interrupted the attacks in most patients for at least two days, after which they returned [147]. Some patients instead showed a complete cluster remission. Methylprednisolone i.v. (250 mg in 100 ml saline) followed by prednisone per os (10 mg/day) was found to induce a further benefit in patients already treated with optimal doses of verapamil [148]. The precise mechanisms underlying the steroid effect in CH are unknown. However, as previously mentioned, inflammatory and/or altered immune mechanisms have long been hypothesised in CH [43-46,48,49]. In addition, enhanced inflammatory activity in the trigeminovascular system and increased NO production may occur in CH [73], and steroids have been also found to reduce NO production via inhibition of nNOS activity in animal models [74]. Greater occipital nerve block using corticosteroids (triamcinolone, betamethasone) combined with local anesthetics (lidocaine), or corticosteroid alone (cortivazol, methylprednisolone), has been shown to be effective in CH patients, although the precise mechanisms of corticosteroid effects are largely unknown. Long-acting preparations and relatively high doses would appear to be more appropriate according to controlled trials [149].
Dihydroergotamine and Ergotamine Tartrate
In addition to its use as a symptomatic treatment, the use of DHE as a transitional therapy has also been investigated. In open-label studies, repetitive i.v. DHE (0.5 mg 3 times per day) and i.v. DHE (0.5 + 1 mg) and DHE nasal spray (1 mg) or s.c. DHE (0.5-1 mg) were found to be effective in most of ECH and CCH patients [150, 151]. Adverse effects were mild and only a few patients had to discontinue the drug.In other clinical studies, ergotamine tartrate was evaluated as a transitional therapy. It was administered at a total daily dose of 3-4 mg for 2-3 weeks and proved moderately effective [152, 153].
Long-term Prophylaxis
Long-term pharmacological prophylaxis of CH includes several treatments capable of modifying the natural behaviour of CH. These drugs, whose action targets the cluster periods, reduce the frequency and the severity of CH attacks; they are thought to interfere with the mechanisms underlying the disease. Many patients (those with a particularly high annual rate of attacks) often find their quality of life significantly improved by long-term prophylaxis. In addition to the need (already mentioned) for a concurrent transitional therapy, it is sometimes necessary to combine different drugs in order to obtain good control of both the attacks and the clusters.
Verapamil
Verapamil is the most widely used drug in maintenance prophylaxis of CH patients [8]. This calcium antagonist interferes with slow calcium channels (voltage-gated channels). Administered for two weeks at a dose of 360 mg per day it was shown, in a placebo-controlled study, to be aneffective and safe treatment for reducing headache frequency in ECH patients [154]. Some patients even became completely pain free, while half of them experienced a substantial benefit as early as the first week of treatment. In addition, verapamil was shown to be effective in a considerable number of CCH patients in two open studies [155,156] and, compared with lithium carbonate, to be faster acting and associated with fewer side effects (response rate:50% vs 37%) [157]. The dosages used in these studies (up to 960-1200 mg) were higher than those used for the episodic form.
The extensive use of verapamil in maintenance prophylaxis is also due its wide therapeutic window and high safety profile. In addition, verapamil used in combination with other drugs rarely results in notable adverse interactions. The most frequent adverse effects – hypotension, constipation, peripheral oedema and bradycardia – are all due to its anti-arrhythmic, vasodilating and negative inotropic effects. For this reason, patients with low blood pressure, a low heart rate or a branch block should be carefully evaluated before beginning treatment with verapamil. In such cases it is advisable to obtain a baseline ECG before initiating verapamil therapy and to repeat it regularly both during the drug titration up to the effective dose and during the home treatment.
With regard to its mechanism of action, some observations indicate that verapamil has minimal effects on vascular structures. In CH, it induces changes in cerebral blood flow that are smaller than those induced by other calcium antagonists. This suggests that the effectiveness of verapamil in CH is not due to effects on the vascular bed, but rather to other effects [158]. In this respect, verapamil modulates the activity of central neurons via interactions with muscarinic, serotoninergic and dopaminergic receptors [159, 160], and inhibits presynaptic adrenergic receptors, thereby increasing noradrenaline release. Of note, this latter effect is particularly important at the hypothalamic level. Furthermore, verapamil has been found to inhibit dopamine release via antagonism at the D2 receptors [161]. Another important effect involves the opioid system, which participates in the modulation of pain pathways, via changes in the analgesic effect of morphine and restoration of the pain control system [162]. Probably due to this effect, verapamil appears to be faster acting than lithium both in CH prophylaxis and in the treatment of depression.
Lithium Carbonate
The use of lithium in CH was first prompted by the early observation of its effectiveness in another classical cyclic condition, i.e. bipolar disorder (BD) [75]. Some similarities between CH and periodic affective illness (relating, for example, to the light/dark cycle, sleep-wake cycles and effects of common treatments) have been reported [163]. The effects of lithium in BD are supported by evidence accumulated over more than 60 years; consequently, lithium is the first-line treatment in the majority of BD treatment guidelines and remains the ‘gold standard’ for both research studies and clinical practice [164]. In CH patients, lithium appears to be particularly useful in the chronic form, as an early study showed a significant improvement in nearly all the patients treated [165]. Usually, this improvement is seen within a week of starting the treatment and continues throughout administration of the drug, although mild headache attacks can occur during treatment. The efficacy of lithium carbonate was confirmed in other studies in a high proportion of ECH and CCH patients [166]. In a further study, lithium carbonate (300 mg, 3 times daily) led to reductions in both headache index and analgesic consumption, similar to those obtained with verapamil; however, lithium carbonate needs a longer time to reach its complete pharmacological effect and hasa narrow therapeutic window [158]. Treatment with lithium is currently rated as level of evidence B in the EFNS guidelines [8].
The mechanism of action of lithium in CH (as in BD) remains largely unknown. The observation that lithium does not prevent alcohol-induced CH attacks suggested that this drug acts on the central mechanisms responsible for spontaneous attacks, but has minimal or no effects on peripheral disease mechanisms [167]. Robust evidence implicates a number of neurotransmitters in this action on the central mechanisms. Lithium has been shown to influence the excitatory neurotransmitter glutamate, whose concentrations appear to be increased in mania and decreased in depression [164]. In animal models, lithium administration increases the availability of glutamate in the post-synaptic neuron, an effect mediated by N-methyl D-aspartate receptor stimulation [168] and by inhibition of its uptake via glutamate transporters [169]. Interestingly, chronic lithium administration eventually restores glutamate uptake to ‘normal’ levels, a change that is again in keeping with its longer-term mood-stabilising properties [169]. Dopamine (DA) is another neurotransmitter found to be increased in patients with mania and therefore a potential target for the action of lithium [170], via modulation of the synthesis and/or release of DA in the presynaptic terminal [171, 172] and via inhibition of G-protein functioning and adenlyl cyclase and cyclic adenosine monophosphate pathways [173]. Lithium also increases the concentrations of GABA, thereby counteracting mania [170]. However, while these findings may, in part, explain the antidepressant and mood-stabilising properties of lithium, the effects of this drug in CH remain unclear. Lithium has recently been shown to exert a neuroprotective effecton glycogen synthase kinase-3, a system directly involved in modulating synaptic plasticity and cell structure and resilience; lithium may prevent cell death caused by excessive excitatory neurotransmission, such as during a manic episode [170, 174]. Another likely theory regarding the action of lithium is the ‘inositol depletion hypothesis’ [174]. Inositol is responsible for maintaining phospholipid concentrations of cell membranes and the efficiency of cellular signalling. In both in vivo and in vitro studies, lithium has been shown to interfere with this system, acting in regions of the brain where inositol is in excess [175]. Again, these effects of lithium appear to be relevant to the mechanisms of depression, but it is not known to what extent they may impinge on CH pathophysiology. With regard to the involvement of the hypothalamus in CH, it is interesting that lithium has been found to accumulate in this brain region, particularly in the area concerned with the regulation of body temperature [176]. There is also evidence that lithium modulates serotonin pathways in several brain regions, facilitating tryptophan uptake [177] and inhibiting the spontaneous release of serotonin in many areas, including the hypothalamus [178]. In addition, lithium interferes with the encephalin system, and with the structure of sleep and sleep/wake rhythms [179].
In clinical practice, serum lithium levels must be carefully monitored both during the drug titration phase and during the home treatment. Since lithium has a low safety profile, electrolyte levels and kidney and thyroid function should be checked periodically. Similarly, extreme vigilance is mandatory during the co-administration of lithium with other drugs, such as diuretics and non-steroidal anti-inflammatory drugs. The most frequent adverse events of lithium include tremor, gastrointestinal disturbances, dizziness and polyuria.
Other Treatments
Many other drugs have been evaluated for use in long-term prophylaxis of CH. Since evidence of their efficacy in this setting is not completely satisfactory, they should only be considered for CH in patients in whom the first-line therapies, verapamil and lithium, are not well tolerated or even contraindicated.
Antiepileptic Drugs
AEDs have been shown to be effective drugs for the prevention of migraine. Although the available data are limited, more recent evidence suggests that they could also play a role in the prevention of CH. This is important, given the limited availability of some CH medications and the risk of long-term toxicity associated with others. Gradual titration is recommended to achieve optimal tolerability of AEDs, although some evidence shows that faster titration can be well tolerated in patients with CH.
Topiramate has been shown, in two open studies, to have some therapeutic efficacy at different dosages (50 and 125 mg/day and 25-200 mg/day, respectively), leading to remission within about three weeks and reduced duration of the cluster period [180, 181]. However, on the basis of current evidence (level B), topiramate can only be considered as a second-line therapy [8, 182]. Major adverse effects are weight loss and the risk of recurrent stones in patients with a positive history of nephrolithiasis or cholelithiasis; other common complaints include cognitive dysfunction, paresthesia, alteration in taste, fatigue and dizziness.
Valproic acid, in an open study, was found to be effective at doses of 500 to 2000 mg daily [183]. A later RCT did not confirm these results [184], although this may have been due to an unusually high response rate in the placebo group (62%). The current guidelines rate valproic acid as a third-line therapy in CH (level of evidence: C) [8]. During treatment with valproic acid it is very important to monitor not only blood levels of the drug, but also liver function given the potential risk of hepatic failure. Common adverse effects include weight gain, fatigue, tremor, hair loss and nausea. Monitoring with complete blood counts is also useful during valproic acid therapy.
Gabapentin was tested at doses of 800-3600 mg/day in three different open trials, following a report of its successful administrationin a single CH case [185]. The drug interrupted the cluster period in at least 50% of patients, and significantly reduced the frequency of the attacks and intensity of the pain in many others [186-188]. The more common adverse effects of gabapentin include somnolence and fatigue, dizziness, weight gain, peripheral oedema and ataxia; however, the drug is usually well tolerated.
Serotonin Antagonists
Methysergide (8-16 mg/day) was consistently found to be effective in a high proportion of CH patients in early open trial studies [189, 190]. However, its prolonged use can produce pulmonary and retroperitoneal fibrosis [191]. Moreover, its negative interactions with the triptans (the main symptomatic drugs in CH) make it difficult to manage in clinical practice. Side effects are frequent (up to 45%of patients) and include nausea, dizziness, abdominal pain, restlessness, somnolence and cramps.
In a controlled study, another serotonin antagonist, pizotifen, administered at a dose of 1-4 mg/day, was shown to significantly reduce attack frequency in 36% of patients and to interrupt the cluster period in 21% [192].
Histamine sulphate (i.v.), used in intractable CH patients, reduced the frequency of attacks by up to 100% in a third of the cases and by up to 50% in another third; it proved in effective in the remaining third [193].
Melatonin, investigated in a RCT at a daily dose of 10 mg vs placebo for two weeks in 20 ECH patients, induced a significant and relatively rapid reduction of the headache frequency [194]. However, these results were not confirmed in a later study investigating the use of melatonin as an adjunctive treatment in ECH [195].
Clonidine, given as a 5-7.5 mg transdermal patch, was studied in two open studies in ECH and CCH patients and found to impact positively on attack frequency, attack duration and pain intensity [196]. However, a later study in ECH patients did not confirm these results [197]. Tiredness and decreased blood pressure levels were the most frequent adverse events noted in these studies.
Baclofen (10 mg 3 times daily, orally), in an open study, induced remission in most CH patients without significant side effects [198].
Capsaicin is a derivative of homovanillic acid found in hot peppers. Capsaicin is a known neuropeptide depletor that has been shown to cause the release of substance P and other neuropeptides from primary sensory neurons. It eventually causes desensitisation by depleting the nerve terminals of substance P and CGRP [199]. Repeated intranasal capsaicin application was initially found to be effective on the frequency of ECH and CCH attacks when administered bilaterally at a dose of 300 μg per nostril [200]. Capsaicin was subsequently shown to be effective when administered in the nostril ipsilateral to the pain but not in the contralateral nostril [201]. CCH patients were headache free for a maximum of 40 days, but then attacks invariably recurred.
Botulinum toxin type A, injected at a dose of 50 UI ipsilateral to the pain as add-on therapy in a limited number of ECH and CCH patients, showed inconsistent results in an open study [202]. At variance with migraine, further data are thus required to support the efficacy of this therapeutic approach in CH.
Triptans
Interest in the use of the triptans as a preventive treatment for CH is increasing, and the topic was recently addressed in a dedicated review [203]. Observations of the triptans playing an extremely helpful role in the acute treatment of CH prompted the suggestion that they might also have a role in the long-term prophylaxis of CH. Surprisingly, in a controlled study, sumatriptan, the most effective acute CH drug, provided no benefit in CCH patients when administered orally at a dose of 100 mg [204]. In open studies, noratriptan and eletriptan were instead shown to be helpful and well tolerated as additional therapies in both long-term and transitional prophylaxis [205,206]. In addition, frovatriptan, the triptan with the longest half-life (26 hours), was shown to be effective and safe at a dose of 5 mg/day in CH patients transitioning in to longer-term preventive therapy [207]. However, a recent RCT failed to replicate these results in short-term prophylaxis in ECH [208]. There is no evidence in the literature supporting the use of zolmitriptan, rizatriptan or almotriptan as prophylactic agents for CH. It has also been pointed out that it is particularly difficult to conduct clinical trials with valid designs when investigating drugs (triptans or others) in the prophylaxis of CH according to the present guidelines [208]. In conclusion, in the absence of controlled studies, the triptans may be used in the preventive management of CH as a second-line, short-term, bridging monotherapy or as an add-on treatment only in complicated cases [203].
Civamide, a cis-isomer of capsaicin, is a transient receptor potential vanilloid receptor modulator, which selectively depresses activity in type-C nociceptive fibres and causes release and subsequent depletion of neuropeptides via a mechanism of desensitisation to further release), including substance P and CGRP [209]. Intranasal civamide, compared with placebo [210], resulted in a >50% decrease in the frequency of CH attacks. Moreover, most of the reported adverse effects, such as nasal burning, lacrimation, pharyngitis and rhinorrhoea, were mostly linked to the local application of the drug. This promising treatment is under active investigation.
Kudzu. Kudzu is a vine indigenous to Asian countries, traditionally used in Chinese medicine with different indications. It contains high levels of phytoestrogens, mostly isoflavones. Kudzu has been reported to reduce intensity, frequency and duration of CH attacks [211]. The underlying mechanisms of action are still unknown, but kudzu has been shown to modulate oestrogen receptors centrally [212]. Kudzu also appears to reduce alcohol intake [213], which is a known trigger of CH attacks.
The main preventive agents used in CH with their levels of evidence are summarised in Table 2. These drugs have widely different molecular targets, and this reflects the multifactorial nature of CH.
Neurostimulation Techniques
In recent years, neurostimulation techniques have emerged as promising treatments for intractable CCH and look set to play an increasingly important role in the clinical management of CH. Several strategies are being investigated, including deep brain stimulation (DBS) of the hypothalamus, occipital nerve stimulation (ONS) and sphenopalatine ganglion (SPG) stimulation [214]. DBS has been investigated in open [86, 214] and sham-controlled [215] studies and it showed beneficial effects, but also significant surgical risks. ONS induced an at least 50% reduction in attack frequency in 67% of CCH patients [216]. However, all the ONS studies were small, uncontrolled studies; in addition, a high frequency of adverse effects was reported [217, 218]. More recently, acute stimulation of the SPG was shown to be effective in several patients [219]; in another study, on-demand SPG stimulation produced either acute pain relief or significant effects on attack prevention in CCH sufferers, and showed an acceptable safety profile compared with other surgical procedures [220]. However, to date there are no certain predictors of the effect of neurostimulation techniques, and this issue requires further investigation.
TREATMENT OF THE OTHER TACs
In the other TACs, i.e. PH, HC and SUNCT, the extreme brevity of the attacks renders any acute attack treatment almost vain; furthermore, in clinical trials, any effects attributed to a given drug may actually be spontaneous effects. Therefore, the aim of treatment in these cases is to break the recurring pattern of attacks. Because of the low prevalence of these forms and the limited number of patients tested, it is only recently that attempts have been made to define levels of recommendation for the drugs used in the preventive treatment of these TACs [145].
Paroxysmal Hemicrania and Hemicrania Continua
Few studies have addressed the treatment of PH and HC, and those that have done generally had open and non-controlled designs. No reliable information is therefore available about the required doses, treatment duration, andpatient follow-up. By definition, PH is responsive to indomethacin and this peculiar feature is a mandatory diagnostic criterion [3]. Accordingly, the diagnosis should be reconsidered in patients not responding to indomethacin at effective dosages (200-225 mg) [8, 221, 222]. An excellent and prompt response to indomethacin is also a main feature of HC. Functional imaging studies have provided some clues as to the mechanism underlying this response, revealing (in both syndromes) activation not only in the posterior hypothalamus, but also in the ventral midbrain [95]. The ventral midbrain may therefore represent a potential target of indomethacin. The suggested initial dose of indomethacin in PH and HC is 25 mg three times per day for three days, but this dosage can be increased with an additional dose of 25 mg every three days. Most patients respond completely within 24-48 hours to a dose of 150 mg a day. Lack of response to therapeutic doses of indomethacin should rule out the diagnosis, or suggest a symptomatic form of PH and HC, i.e. due to underlying causes [221]. Since the most common side effects of indomethacin are peptic ulcers and other gastrointestinal disorders, patients usually require co-administration of proton pump inhibitors or H2 receptor antagonists. In patients with episodic PH or with remitting forms of HC, treatment with indomethacin at effective doses should be prolonged beyond the typical attack period and then gradually tapered. CPH and non-remitting HC often need a long-lasting treatment, although prolonged remissions after discontinuing the drug have been reported.
Cyclooxygenase-2 selective inhibitors (rofecoxib, celecoxib) have repeatedly been reported to be effective in PH [223-227]. However, the increased risk of myocardial infarctions and strokes associated with their prolonged use urges caution. A combination of piroxicam and β-cyclodestrine alleviated the clinical symptoms in both CPH and HC patients [228]. Similarly, melatonin, possibly affecting central nociceptive transmission via potentiation of endogenous opioid pathways, was reported to reduce the intensity of pain in HC patients [229]. Verapamil was observed in one study to be an effective alternative to indomethacin [230] and good results were also obtained with topiramate in CPH [231]. Finally, in one study, blockade of the GON with local injection of steroids and lidocaine provided prolonged benefit in PH patients [232].
SUNCT
As in the other TACs, observational studies in SUNCT are rare, and the current evidence is mainly based on anecdotal observations and case reports. However, in single cases and small groups of patients some effects have been observed using verapamil [233], and i.v. or oral steroids [234, 235]. Intravenous lidocaine was found to provide notable relief of pain and autonomic symptoms [236]. Most data concern preventive treatments with AEDs. Carbamazepine, at doses of 200-2000 mg/day [237-243] and topiramate at doses of 50-200 mg/day [244-246] reportedly improve the clinical symptoms to various extents. Gabapentin, administered either alone at doses of 800-2700 mg/day [247-249] orat a dose of 400 mg in combination with oxcarbazepine 600 mg/day [250], appears to be useful as a long-term treatment, giving a 60% response rate in SUNA (versus 45% in SUNCT). These findings suggest that it shows better and less selective effectiveness in the forms with more autonomic symptoms. However, lamotrigine, due to its efficacy coupled with its notable safety and tolerability, has been the focus of most clinical reports [235]. Used at doses of 100-400 mg/day this drug has consistently proved effective in relieving pain in SUNCT [251-257], also as a long-term treatment [258]. On the basis of the above evidence, therapeutic guidelines for SUNCT and SUNA have been proposed [259]. Lamotrigine must be titrated up to the effective dose very slowly to avoid severe adverse effects, mostly involving the skin (such as Stevens-Johnson syndrome). The levels of evidence for treatments used in PH and SUNCT, according to the recently published Italian guidelines [145]. The reported beneficial effect of antiepileptic drugs in SUNCT and SUNA may reflect similarities in the pathophysiological mechanisms between these disorders and trigeminal neuralgia.
CONCLUSIONS
Although alternative approaches (such as neuro-stimulation techniques) are emerging for the TACs, especially for CH, most of the currently available therapeutic strategies in these syndromes are pharmacological. The clinical efficacy and tolerability of the most widely used drugs are supported by a limited number of RCTs, open studies in small case series, and single-case reports. Albeit with these limitations, the elective approaches in CH continue to be the triptans and oxygen for acute treatment, steroids for transitional prophylaxis, and verapamil and lithium for prevention. Promising results have recently been obtained with novel modes of administration of the triptans (needle-free methods) and with other agents, and some possible future treatments (e.g. civamide) are currently under study. Indomethacin is extremely effective in PH and HC, while AEDs (especially lamotrigine) appear to be increasingly useful in SUNCT. Neuroimaging studies are expected to go on providing insights into TAC patho-physiology, ultimately allowing the discovery ofnovel therapeutic approaches. Any treatment should be investigated according to dedicated guidelines for clinical trials, and longitudinal assessment of the natural course of the disorder should be performed. Future RCTs dealing with the TACs should evaluate the efficacy of CGRP inhibitors or CGRP receptor antagonists, a relatively new class of migraine abortive drugs. With regard to CH forms that do not respond to treatments, definition of refractoriness and inclusion of refractory chronic CH in the current headache classification may result in a better management of this disease [260]. Finally, further investigation of sleep and circadian physiology inpatients suffering with TACs may provide additional pathophysiological information, useful for developing new treatments for these rare, but lifelong and disabling conditions.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Matharu M.S., Goadsby P.J. Trigeminal Autonomic Cephalalgias: Diagnosis and Management. In: Silbertstein S.D., Lipton R.B., Dodick D.W., editors. Wolff’s Headache and Other Head Pain. 8th ed. New York: Oxford University Press; 2008. pp. 379–430. [Google Scholar]
- 2.Goadsby P.J., Cittadini E., Cohen A.S. Trigeminal autonomic cephalalgias: paroxysmal hemicrania, SUNCT/SUNA, and hemicrania continua. Semin. Neurol. 2010;30(2):186–191. doi: 10.1055/s-0030-1249227. [DOI] [PubMed] [Google Scholar]
- 3.Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders. 3rd. Cephalalgia; 2013. [Google Scholar]
- 4.Goadsby P.J., Cittadini E., Burns B., Cohen A.S. Trigeminal autonomic cephalalgias: diagnostic and therapeutical developments. Curr. Opin. Neurol. 2008;21(3):323–330. doi: 10.1097/WCO.0b013e3282fa6d76. [DOI] [PubMed] [Google Scholar]
- 5.Russell M.B. Epidemiology and genetics of cluster headache. Lancet Neurol. 2004;3(5):279–283. doi: 10.1016/S1474-4422(04)00735-5. [DOI] [PubMed] [Google Scholar]
- 6.Sjaastad O., Bakketeig L.S. Cluster headache prevalence. Vågå study of headache epidemiology. Cephalalgia. 2003;23(7):528–533. doi: 10.1046/j.1468-2982.2003.00585.x. [DOI] [PubMed] [Google Scholar]
- 7.Bahra A., May A., Goadsby P.J. Cluster headache: a prospective clinical study with diagnostic implications. Neurology. 2002;58(3):354–361. doi: 10.1212/WNL.58.3.354. [DOI] [PubMed] [Google Scholar]
- 8.May A., Leone M., Afra J., Linde M., Sándor P.S., Evers S., Goadsby P.J., EFNS Task Force EFNS guidelines on the treatment of cluster headache and other trigeminal-autonomic cephalalgias. Eur. J. Neurol. 2006;13(10):1066–1077. doi: 10.1111/j.1468-1331.2006.01566.x. [DOI] [PubMed] [Google Scholar]
- 9.Fischera M., Marziniak M., Gralow I., Evers S. The incidence and prevalence of cluster headache: a meta-analysis of population-based studies. Cephalalgia. 2008;28:614–18. doi: 10.1111/j.1468-2982.2008.01592.x. [DOI] [PubMed] [Google Scholar]
- 10.Cittadini E., Matharu M.S., Goadsby P.J. Paroxysmal hemicrania: a prospective clinical study of 31 cases. Brain. 2008;131(Pt 4):1142–1155. doi: 10.1093/brain/awn010. [DOI] [PubMed] [Google Scholar]
- 11.Schürks M. Genetics of cluster headache. Curr. Pain Headache Rep. 2010;14(2):132–139. doi: 10.1007/s11916-010-0096-8. [DOI] [PubMed] [Google Scholar]
- 12.Leone M., Russell M.B., Rigamonti A., Attanasio A., Grazzi L., D’Amico D., Usai S., Bussone G. Increased familial risk of cluster headache. Neurology. 2001;56(9):1233–1236. doi: 10.1212/WNL.56.9.1233. [DOI] [PubMed] [Google Scholar]
- 13.Russell M.B., Andersson P.G., Thomsen L.L., Iselius L. Cluster headache is an autosomal dominantly inherited disorder in some families: a complex segregation analysis. J. Med. Genet. 1995;32(12):954–956. doi: 10.1136/jmg.32.12.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baumber L., Sjöstrand C., Leone M., Harty H., Bussone G., Hillert J., Trembath R.C., Russell M.B. A genome-wide scan and HCRTR2 candidate gene analysis in a European cluster headache cohort. Neurology. 2006;66(12):1888–1893. doi: 10.1212/01.wnl.0000219765.95038.d7. [DOI] [PubMed] [Google Scholar]
- 15.Rozen T.D., Fishman R.S. Cluster headache in the United States of America: demographics, clinical characteristics, triggers, suicidality, and personal burden. Headache. 2012;52(1):99–113. doi: 10.1111/j.1526-4610.2011.02028.x. [DOI] [PubMed] [Google Scholar]
- 16.Magis D., Jensen R., Schoenen J. Neurostimulation therapies for primary headache disorders: present and future. Curr. Opin. Neurol. 2012;25(3):269–276. doi: 10.1097/WCO.0b013e3283532023. [DOI] [PubMed] [Google Scholar]
- 17.Olesen J. The role of nitric oxide (NO) in migraine, tension-type headache and cluster headache. Pharmacol. Ther. 2008;120(2):157–171. doi: 10.1016/j.pharmthera.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Sances G., Tassorelli C., Pucci E., Ghiotto N., Sandrini G., Nappi G. Reliability of the nitroglycerin provocative test in the diagnosis of neurovascular headaches. Cephalalgia. 2004;24(2):110–119. doi: 10.1111/j.1468-2982.2004.00639.x. [DOI] [PubMed] [Google Scholar]
- 19.Russell D. Cluster headache: severity and temporal profiles of attacks and patient activity prior to and during attacks. Cephalalgia. 1981;1(4):209–216. doi: 10.1046/j.1468-2982.1981.0104209.x. [DOI] [PubMed] [Google Scholar]
- 20.Dexter J.D., Weitzman E.D. The relationship of nocturnal headaches to sleep stage patterns. Neurology. 1970;20(5):513–518. doi: 10.1212/WNL.20.5.513. [DOI] [PubMed] [Google Scholar]
- 21.Antonaci F., Sjaastad O. Chronic paroxysmal hemicrania (CPH): a review of the clinical manifestations. Headache. 1989;29(10):648–656. doi: 10.1111/j.1526-4610.1989.hed2910648.x. [DOI] [PubMed] [Google Scholar]
- 22.Pareja J.A., Vincent M., Antonaci F., Sjaastad O. Hemicrania continua: diagnostic criteria and nosologic status. Cephalalgia. 2001;21(9):874–7. doi: 10.1046/j.1468-2982.2001.00276.x. [DOI] [PubMed] [Google Scholar]
- 23.Newman L.C., Lipton R.B., Russell M., Solomon S. Hemicrania continua: attacks may alternate sides. Headache. 1992;32(5):237–238. doi: 10.1111/j.1526-4610.1992.hed3205237.x. [DOI] [PubMed] [Google Scholar]
- 24.Pasquier F., Leys D., Petit H. “Hemicrania continua”: the first bilateral case? Cephalalgia. 1987;7(3):169–170. doi: 10.1046/j.1468-2982.1987.0703169.x. [DOI] [PubMed] [Google Scholar]
- 25.Newman L.C., Lipton R.B., Solomon S. Hemicrania continua: ten new cases and a review of the literature. Neurology. 1994;44(11):2111–2114. doi: 10.1212/WNL.44.11.2111. [DOI] [PubMed] [Google Scholar]
- 26.Peres M.F., Siow H.C., Rozen T.D. Hemicrania continua with aura. Cephalalgia. 2002;22(3):246–248. doi: 10.1046/j.1468-2982.2002.00325.x. [DOI] [PubMed] [Google Scholar]
- 27.Cohen A.S., Matharu M.S., Goadsby P.J. Short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing (SUNCT) or cranial autonomic features (SUNA)--a prospective clinical study of SUNCT and SUNA. Brain. 2006;129(Pt 10):2746–2760. doi: 10.1093/brain/awl202. [DOI] [PubMed] [Google Scholar]
- 28.Matharu M.S., Cohen A.S., Boes C.J., Goadsby P.J. Short-lasting unilateral neuralgiform headache with conjunctival injection and tearing syndrome: a review. Curr. Pain Headache Rep. 2003;7(4):308–318. doi: 10.1007/s11916-003-0052-y. [DOI] [PubMed] [Google Scholar]
- 29.Antonaci F., Costa A., Ghirmai S., Sances G., Sjaastad O., Nappi G. Parenteral indomethacin (the INDOTEST) in cluster headache. Cephalalgia. 2003;23(3):193–196. doi: 10.1046/j.1468-2982.2003.00495.x. [DOI] [PubMed] [Google Scholar]
- 30.Dodick D.W., Rozen T.D., Goadsby P.J., Silberstein S.D. Cluster headache. Cephalalgia. 2000;20(9):787–803. doi: 10.1046/j.1468-2982.2000.00118.x. [DOI] [PubMed] [Google Scholar]
- 31.Goadsby P.J., Bahra A., May A. Mechanisms of cluster headache. Cephalalgia. 1999;19(Suppl. 23):19–21. doi: 10.1177/0333102499019s2305. [DOI] [PubMed] [Google Scholar]
- 32.Goadsby P.J., Lipton R.B. A review of paroxysmal hemicranias, SUNCT syndrome and other short-lasting headaches with autonomic feature, including new cases. Brain. 1997;120(Pt 1):193–209. doi: 10.1093/brain/120.1.193. [DOI] [PubMed] [Google Scholar]
- 33.Poyner D.R. Calcitonin gene-related peptide: multiple actions, multiple receptors. Pharmacol. Ther. 1992;56(1):23–51. doi: 10.1016/0163-7258(92)90036-Y. [DOI] [PubMed] [Google Scholar]
- 34.Wimalawansa S.J. Calcitonin gene-related peptide and its receptors: molecular genetics, physiology, pathophysiology, and therapeutic potentials. Endocr. Rev. 1996;17(5):533–585. doi: 10.1210/edrv-17-5-533. [DOI] [PubMed] [Google Scholar]
- 35.Tfelt-Hansen P., Le H. Calcitonin gene-related peptide in blood: is it increased in the external jugular vein during migraine and cluster headache? A review. J. Headache Pain. 2009;10(3):137–143. doi: 10.1007/s10194-009-0112-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.May A. Cluster headache: pathogenesis, diagnosis, and management. Lancet. 2005;366(9488):843–855. doi: 10.1016/S0140-6736(05)67217-0. [DOI] [PubMed] [Google Scholar]
- 37.Waldenlind E., Ekbom K., Torhall J. MR-angiography during spontaneous attacks of cluster headache: a case report. Headache. 1993;33(6):291–5. doi: 10.1111/j.1526-4610.1993.hed3306291.x. [DOI] [PubMed] [Google Scholar]
- 38.May A., Bahra A., Büchel C., Frackowiak R.S., Goadsby P.J. PET and MRA findings in cluster headache and MRA in experimental pain. Neurology. 2000;55(9):1328–1335. doi: 10.1212/WNL.55.9.1328. [DOI] [PubMed] [Google Scholar]
- 39.Goadsby P.J., Edvinsson L. Human in vivo evidence for trigeminovascular activation in cluster headache. Neuropeptide changes and effects of acute attacks therapies. Brain. 1994;117(Pt 3):427–434. doi: 10.1093/brain/117.3.427. [DOI] [PubMed] [Google Scholar]
- 40.Krabbe A.A., Henriksen L., Olesen J. Tomographic determination of cerebral blood flow during attacks of cluster headache. Cephalalgia. 1984;4(1):17–23. doi: 10.1046/j.1468-2982.1984.0401017.x. [DOI] [PubMed] [Google Scholar]
- 41.Nelson R., du Boulay G.H., Marshall J., Russell R.W., Symon L., Zilkha E. Cerebral blood flow studies in patients with cluster headache. 1980. [DOI] [PubMed]
- 42.Meyer E.L., Waldenlind E., Marcus C. Diminished nocturnal lipolysis in cluster headache: a sign of central sympathetic dysregulation? Neurology. 2003;61(9):1250–1254. doi: 10.1212/01.WNL.0000091860.92717.36. [DOI] [PubMed] [Google Scholar]
- 43.Gawel M.J., Krajewski A., Luo Y.M., Ichise M. The cluster diathesis. Headache. 1990;30(10):652–655. doi: 10.1111/j.1526-4610.1990.hed3010652.x. [DOI] [PubMed] [Google Scholar]
- 44.Martelletti P., Stirparo G., De Stefano L., Di Sabato F., Giacovazzo M., Rinaldi-Garaci C. Defective expression of IL-2 receptors on peripheral blood lymphocytes from patients with cluster headache. Headache. 1990;30(4):228–231. doi: 10.1111/j.1526-4610.1990.hed3004228.x. [DOI] [PubMed] [Google Scholar]
- 45.Martelletti P., Granata M., Giacovazzo M. Serum interleukin-1 beta is increased in cluster headache. Cephalalgia. 1993;13(5):343–345. doi: 10.1046/j.1468-2982.1993.1305343.x. [DOI] [PubMed] [Google Scholar]
- 46.Empl M., Förderreuther S., Schwarz M., Müller N., Straube A. Soluble interleukin-2 receptors increase during the active periods in cluster headache. Headache. 2003;43(1):63–68. doi: 10.1046/j.1526-4610.2003.03011.x. [DOI] [PubMed] [Google Scholar]
- 47.Antonaci F., Costa A., Candeloro E., Sjaastad O., Nappi G. Single high-dose steroid treatment in episodic cluster headache. Cephalalgia. 2005;25(4):290–295. doi: 10.1111/j.1468-2982.2004.00855.x. [DOI] [PubMed] [Google Scholar]
- 48.Hannerz J. Orbital phlebography and signs of inflammation in episodic and chronic cluster headache. Headache. 1991;31(8):540–542. doi: 10.1111/j.1526-4610.1991.hed3108540.x. [DOI] [PubMed] [Google Scholar]
- 49.Hardebo J. How cluster headache is explained as intracavernous inflammatory process lesioning sympathetic fibers. 1994. [DOI] [PubMed]
- 50.Sianard-Gainko J., Milet J., Ghuysen V., Schoenen J. Increased parasellar activity on gallium SPECT is not specific for active cluster headache. Cephalalgia. 1994;14(2):132–133. doi: 10.1111/j.1468-2982.1994.1402132.x. [DOI] [PubMed] [Google Scholar]
- 51.Remahl I.N., Waldenlind E., Bratt J., Ekbom K. Cluster headache is not associated with signs of a systemic inflammation. Headache. 2000;40(4):276–282. doi: 10.1046/j.1526-4610.2000.00041.x. [DOI] [PubMed] [Google Scholar]
- 52.Schuh-Hofer S., Siekmann W., Offenhauser N., Reuter U., Arnold G. Effect of hyperoxia on neurogenic plasma protein extravasation in the rat dura mater. Headache. 2006;46(10):1545–1551. doi: 10.1111/j.1526-4610.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- 53.Olesen J. The role of nitric oxide (NO) in migraine, tension-type headache and cluster headache. Pharmacol. Ther. 2008;120(2):157–171. doi: 10.1016/j.pharmthera.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 54.Luo Z.D., Cizkova D. The role of nitric oxide in nociception. Curr. Rev. Pain. 2000;4(6):459–466. doi: 10.1007/s11916-000-0070-y. [DOI] [PubMed] [Google Scholar]
- 55.Meller S.T., Gebhart G.F. Nitric oxide (NO) and nociceptive processing in the spinal cord. Pain. 1993;52(2):127–136. doi: 10.1016/0304-3959(93)90124-8. [DOI] [PubMed] [Google Scholar]
- 56.Ferreira J., Santos A.R., Calixto J.B. The role of systemic, spinal and supraspinal L-arginine-nitric oxide-cGMP pathway in thermal hyperalgesia caused by intrathecal injection of glutamate in mice. Neuropharmacology. 1999;38(6):835–842. doi: 10.1016/S0028-3908(99)00006-4. [DOI] [PubMed] [Google Scholar]
- 57.Tassorelli C., Greco R., Wang D., Sandrini M., Sandrini G., Nappi G. Nitroglycerin induces hyperalgesia in rats--a time-course study. Eur. J. Pharmacol. 2003;464(2-3):159–162. doi: 10.1016/S0014-2999(03)01421-3. [DOI] [PubMed] [Google Scholar]
- 58.Strosznajder J., Chalimoniuk M., Samochocki M. Activation of serotonergic 5-HT1A receptor reduces Ca(2+)- and glutamatergic receptor-evoked arachidonic acid and No/cGMP release in adult hippocampus. Neurochem. Int. 1996;28(4):439–444. doi: 10.1016/0197-0186(95)00103-4. [DOI] [PubMed] [Google Scholar]
- 59.De Col R., Koulchitsky S.V., Messlinger K.B. Nitric oxide synthase inhibition lowers activity of neurons with meningeal input in the rat spinal trigeminal nucleus. Neuroreport. 2003;14(2):229–232. doi: 10.1097/00001756-200302100-00014. [DOI] [PubMed] [Google Scholar]
- 60.Hou M., Kanje M., Longmore J., Tajti J., Uddman R., Edvinsson L. 5-HT(1B) and 5-HT(1D) receptors in the human trigeminal ganglion: co-localization with calcitonin gene-related peptide, substance P and nitric oxide synthase. Brain Res. 2001;909(1-2):112–120. doi: 10.1016/S0006-8993(01)02645-2. [DOI] [PubMed] [Google Scholar]
- 61.Strecker T., Dux M., Messlinger K. Nitric oxide releases calcitonin-gene-related peptide from rat dura mater encephali promoting increases in meningeal blood flow. J. Vasc. Res. 2002;39(6):489–496. doi: 10.1159/000067206. [DOI] [PubMed] [Google Scholar]
- 62.Schwenger N., Dux M., de Col R., Carr R., Messlinger K. Interaction of calcitonin gene-related peptide, nitric oxide and histamine release in neurogenic blood flow and afferent activation in the rat cranial dura mater. Cephalalgia. 2007;27(6):481–491. doi: 10.1111/j.1468-2982.2007.01321.x. [DOI] [PubMed] [Google Scholar]
- 63.Tassorelli C., Blandini F., Costa A., Preza E., Nappi G. Nitroglycerin-induced activation of monoaminergic transmission in the rat. Cephalalgia. 2002;22(3):226–232. doi: 10.1046/j.1468-2982.2002.00355.x. [DOI] [PubMed] [Google Scholar]
- 64.Sances G., Tassorelli C., Pucci E., Ghiotto N., Sandrini G., Nappi G. Reliability of the nitroglycerin provocative test in the diagnosis of neurovascular headaches. Cephalalgia. 2004;24(2):110–119. doi: 10.1111/j.1468-2982.2004.00639.x. [DOI] [PubMed] [Google Scholar]
- 65.Cooper C.E., Davies N.A., Psychoulis M., Canevari L., Bates T.E., Dobbie M.S., Casley C.S., Sharpe M.A. Nitric oxide and peroxynitrite cause irreversible increases in the K(m) for oxygen of mitochondrial cytochrome oxidase: in vitro and in vivo studies. Biochim. Biophys. Acta. 2003;1607(1):27–34. doi: 10.1016/j.bbabio.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 66.McCann S.M., Kimura M., Karanth S., Yu W.H., Rettori V. Nitric oxide controls the hypothalamic-pituitary response to cytokines. Neuroimmunomodulation. 1997;4(2):98–106. doi: 10.1016/S0140-6736(98)02470-2. [DOI] [PubMed] [Google Scholar]
- 67.Nappi G., Micieli G., Sandrini G., Martignoni E., Lottici P., Bono G. Headache temporal patterns: towards a chronobiological model. Cephalalgia. 1983;3(1) Suppl. 1:21–30. doi: 10.1177/03331024830030s102. [DOI] [PubMed] [Google Scholar]
- 68.May A., Bahra A., Büchel C., Frackowiak R.S., Goadsby P.J. Hypothalamic activation in cluster headache attacks. Lancet. 1998;352(9124):275–278. doi: 10.1016/S0140-6736(98)02470-2. [DOI] [PubMed] [Google Scholar]
- 69.D’Amico D., Leone M., Ferraris A., Catania A., Carlin A., Grazzi L., Bussone G. Role of nitric oxide in cluster headache. Ital. J. Neurol. Sci. 1999;20(2) Suppl.:S25–S27. doi: 10.1007/PL00014993. [DOI] [PubMed] [Google Scholar]
- 70.Costa A., Ravaglia S., Sances G., Antonaci F., Pucci E., Nappi G. Nitric oxide pathway and response to nitroglycerin in cluster headache patients: plasma nitrite and citrulline levels. 2003. [DOI] [PubMed]
- 71.Steinberg A., Wiklund N.P., Brundin L., Remahl A.I. Levels of nitric oxide metabolites in cerebrospinal fluid in cluster headache. Cephalalgia. 2010;30(6):696–702. doi: 10.1177/0333102409351799. [DOI] [PubMed] [Google Scholar]
- 72.Rejdak K., Eikelenboom M.J., Petzold A., Thompson E.J., Stelmasiak Z., Lazeron R.H.C., Barkhof F, Polman C.H., Uitdehaag B.M.J., Giovannoni G. CSF nitric oxide metabolites are associated with activity and progression of multiple sclerosis. 2004. [DOI] [PubMed]
- 73.Steinberg A., Nilsson Remahl A.I. Role of nitric oxide in cluster headache. Curr. Pain Headache Rep. 2012;16(2):185–190. doi: 10.1007/s11916-012-0250-6. [DOI] [PubMed] [Google Scholar]
- 74.Personett D., Fass U., Panickar K., McKinney M. Retinoic acid-mediated enhancement of the cholinergic/neuronal nitric oxide synthase phenotype of the medial septal SN56 clone: establishment of a nitric oxide-sensitive proapoptotic state. J. Neurochem. 2000;74(6):2412–2424. doi: 10.1046/j.1471-4159.2000.0742412.x. [DOI] [PubMed] [Google Scholar]
- 75.Ekbom K. Lithium in the treatment of chronic cluster headache. Headache. 1977;17(1):39–40. doi: 10.1111/j.1526-4610.1977.hed1701039.x. [DOI] [PubMed] [Google Scholar]
- 76.Kudrow L. Lithium prophylaxis for chronic cluster headache. Headache. 1977;17(1):15–18. doi: 10.1111/j.1526-4610.1977.hed1701015.x. [DOI] [PubMed] [Google Scholar]
- 77.Malick A., Strassman R.M., Burstein R. Trigeminohypothalamic and reticulohypothalamic tract neurons in the upper cervical spinal cord and caudal medulla of the rat. J. Neurophysiol. 2000;84(4):2078–2112. doi: 10.1152/jn.2000.84.4.2078. [DOI] [PubMed] [Google Scholar]
- 78.Hodge C.J., Jr, Apkarian A.V., Stevens R., Vogelsang G., Wisnicki H.J. Locus coeruleus modulation of dorsal horn unit responses to cutaneous stimulation. Brain Res. 1981;204(2):415–420. doi: 10.1016/0006-8993(81)90600-4. [DOI] [PubMed] [Google Scholar]
- 79.Basbaum A.I., Moss M.S., Glazer E.J. Opiate and stimulation produced analgesia: the contribution of monoamines. Adv. Pain Res. Ther. 1983;5:323–339. [Google Scholar]
- 80.de Lecea L., Kilduff T.S., Peyron C., Gao X., Foye P.E., Danielson P.E., Fukuhara C., Battenberg E.L., Gautvik V.T., Bartlett F.S., II, Frankel W.N., van den Pol A.N., Bloom F.E., Gautvik K.M., Sutcliffe J.G. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. USA. 1998;95(1):322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Holland P., Goadsby P.J. The hypothalamic orexinergic system: pain and primary headaches. Headache. 2007;47(6):951–962. doi: 10.1111/j.1526-4610.2007.00842.x. [DOI] [PubMed] [Google Scholar]
- 82.Millan M.J. The induction of pain: an integrative review. Prog. Neurobiol. 1999;57(1):1–164. doi: 10.1016/S0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- 83.Yaksh T.L. Central pharmacology of nociceptive processing. In: Wall P.D., Melzack R., editors. Textbook of pain. Edinburgh: Churchill Livingstone; 1999. pp. 253–308. [Google Scholar]
- 84.Holle D., Zillessen S., Gaul C., Naegel S., Kaube H., Diener H.C., Katsarava Z., Obermann M. Habituation of the nociceptive blink reflex in episodic and chronic cluster headache. Cephalalgia. 2012;32(13):998–1004. doi: 10.1177/0333102412453955. [DOI] [PubMed] [Google Scholar]
- 85.Sano K., Sekino H., Hashimoto I., Amano K., Sugiyama H. Posteromedial Hypothalamotomy in the treatment of tractable pain. Confin. Neurol. 1975;37(1-3):285–290. doi: 10.1159/000102762. [DOI] [PubMed] [Google Scholar]
- 86.Leone M., Franzini A., Bussone G. Stereotactic stimulation of posterior hypothalamic gray matter in a patient with intractable cluster headache. N. Engl. J. Med. 2001;345(19):1428–1429. doi: 10.1056/NEJM200111083451915. [DOI] [PubMed] [Google Scholar]
- 87.Leone M., Franzini A., Cecchini A.P., Broggi G., Bussone G. Hypothalamic deep brain stimulation in the treatment of chronic cluster headache. Ther. Adv. Neurol. Disorder. 2010;3(3):187–195. doi: 10.1177/1756285610370722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iacovelli E., Coppola G., Tinelli E., Pierelli F., Bianco F. Neuroimaging in cluster headache and other trigeminal autonomic cephalalgias. J. Headache Pain. 2012;13(1):11–20. doi: 10.1007/s10194-011-0403-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Denuelle M., Fabre N., Payoux P., Chollet F., Geraud G. Hypothalamic activation in spontaneous migraine attacks. Headache. 2007;47(10):1418–1426. doi: 10.1111/j.1526-4610.2007.00776.x. [DOI] [PubMed] [Google Scholar]
- 90.Sánchez del Rio M., Alvarez Linera J. Functional neuroimaging of headaches. Lancet Neurol. 2004;3(11):645–651. doi: 10.1016/S1474-4422(04)00904-4. [DOI] [PubMed] [Google Scholar]
- 91.Magis D., Gerardy P.Y., Remacle J.M., Schoenen J. Sustained effectiveness of occipital nerve stimulation in drug-resistant chronic cluster headache. Headache. 2011;51(8):1191–1201. doi: 10.1111/j.1526-4610.2011.01973.x. [DOI] [PubMed] [Google Scholar]
- 92.Leone M., Bussone G. Pathophysiology of trigeminal autonomic cephalalgias. Lancet Neurol. 2009;8(8):755–764. doi: 10.1016/S1474-4422(09)70133-4. [DOI] [PubMed] [Google Scholar]
- 93.Holle D., Obermann M. Cluster headache and the hypothalamus: causal relationship or epiphenomenon? Expert Rev. Neurother. 2011;11(9):1255–1263. doi: 10.1586/ern.11.115. [DOI] [PubMed] [Google Scholar]
- 94.Morelli N., Pesaresi I., Cafforio G., Maluccio M.R., Gori S., Di Salle F., Murri L. Functional magnetic resonance imaging in episodic cluster headache. J. Headache Pain. 2009;10(1):11–14. doi: 10.1007/s10194-008-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Matharu M.S., Cohen A.S., McGonigle D.J., Ward N., Frackowiak R.S., Goadsby P.J. Posterior hypothalamic and brainstem activation in hemicrania continua. Headache. 2004;44(8):747–761. doi: 10.1111/j.1526-4610.2004.04141.x. [DOI] [PubMed] [Google Scholar]
- 96.Magis D., Bruno M.A., Fumal A., Gérardy P.Y., Hustinx R., Laureys S., Schoenen J. Central modulation in cluster headache patients treated with occipital nerve stimulation: an FDG-PET study. BMC Neurol. 2011;11:25. doi: 10.1186/1471-2377-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Matharu M.S., Cohen A.S., Frackowiak R.S., Goadsby P.J. Posterior hypothalamic activation in paroxysmal hemicrania. Ann. Neurol. 2006;59(3):535–545. doi: 10.1002/ana.20763. [DOI] [PubMed] [Google Scholar]
- 98.Sprenger T., Ruether K.V., Boecker H., Valet M., Berthele A., Pfaffenrath V., Wöller A., Tölle T.R. Altered metabolism in frontal brain circuits in cluster headache. Cephalalgia. 2007;27(9):1033–1042. doi: 10.1111/j.1468-2982.2007.01386.x. [DOI] [PubMed] [Google Scholar]
- 99.Sprenger T., Willoch F., Miederer M., Schindler F., Valet M., Berthele A., Spilker M.E., Förderreuther S., Straube A., Stangier I., Wester H.J., Tölle T.R. Opioidergic changes in the pineal gland and hypothalamus in cluster headache: a ligand PET study. Neurology. 2006;66(7):1108–1110. doi: 10.1212/01.wnl.0000204225.15947.f8. [DOI] [PubMed] [Google Scholar]
- 100.Akerman S., Holland P.R., Summ O., Lasalandra M.P., Goadsby P.J. A translational in vivo model of trigeminal autonomic cephalalgias: therapeutic characterization. Brain. 2012;135(Pt 12):3664–3675. doi: 10.1093/brain/aws249. [DOI] [PubMed] [Google Scholar]
- 101.Prakash S., Dholakia S.Y., Shah K.A. A patient with chronic cluster headache responsive to high-dose indomethacin: is there an overlap with chronic paroxysmal hemicrania? Cephalalgia. 2008;28(7):778–781. doi: 10.1111/j.1468-2982.2008.01581.x. [DOI] [PubMed] [Google Scholar]
- 102.Buzzi M.G., Moskowitz M.A. Evidence for 5-HT1B/1D receptors mediating the antimigraine effect of sumatriptan and dihydroergotamine. Cephalalgia. 1991;11(4):165–168. doi: 10.1046/j.1468-2982.1991.1104165.x. [DOI] [PubMed] [Google Scholar]
- 103.Moskowitz M.A. Neurogenic inflammation in the pathophysiology and treatment of migraine. Neurology. 1993;43(6) Suppl. 3:S16–S20. [PubMed] [Google Scholar]
- 104.Burstein R., Levy D., Jakubowski M. Effects of sensitization of trigeminovascular neurons to triptan therapy during migraine. Rev. Neurol. (Paris) 2005;161(6-7):658–660. doi: 10.1016/S0035-3787(05)85109-4. [DOI] [PubMed] [Google Scholar]
- 105.The Sumatriptan Cluster Headache Study Group Treatment of acute cluster headache with sumatriptan. N. Engl. J. Med. 1991;325(5):322–326. doi: 10.1056/NEJM199108013250505. [DOI] [PubMed] [Google Scholar]
- 106.Ekbom K., Monstad I., Prusinski A., Cole J.A., Pilgrim A.J., Noronha D., The Sumatriptan Cluster Headache Study Group Subcutaneous sumatriptan in the acute treatment of cluster headache: a dose comparison study. Acta Neurol. Scand. 1993;88(1):63–69. doi: 10.1111/j.1600-0404.1993.tb04189.x. [DOI] [PubMed] [Google Scholar]
- 107.Ekbom K., Krabbe A., Micieli G., Prusinski A., Cole J.A., Pilgrim A.J., Noronha D., Micelli G. Cluster headache attacks treated for up to three months with subcutaneous sumatriptan (6 mg). Sumatriptan Cluster Headache Long-term Study Group. Cephalalgia. 1995;15:230–236. doi: 10.1046/j.1468-2982.1995.015003230.x. [DOI] [PubMed] [Google Scholar]
- 108.Göbel H., Lindner V., Heinze A., Ribbat M., Deuschl G. Acute therapy for cluster headache with sumatriptan: findings of a one-year long-term study. Neurology. 1998;51(3):908–911. doi: 10.1212/WNL.51.3.908. [DOI] [PubMed] [Google Scholar]
- 109.van Vliet J.A., Bahra A., Martin V., Ramadan N., Aurora S.K., Mathew N.T., Ferrari M.D., Goadsby P.J. Intranasal sumatriptan in cluster headache: randomized placebo-controlled double-blind study. Neurology. 2003;60(4):630–633. doi: 10.1212/01.WNL.0000046589.45855.30. [DOI] [PubMed] [Google Scholar]
- 110.Hardebo J.E., Dahlöf C. Sumatriptan nasal spray (20 mg/dose) in the acute treatment of cluster headache. Cephalalgia. 1998;18(7):487–489. doi: 10.1046/j.1468-2982.1998.1807487.x. [DOI] [PubMed] [Google Scholar]
- 111.Freitag F.G. Sumatriptan needle-free subcutaneous (Sumavel(®) DosePro™) approved for the acute treatment of migraine, with or without aura, and cluster headaches. Expert Rev. Neurother. 2011;11(4):481–490. doi: 10.1586/ern.11.41. [DOI] [PubMed] [Google Scholar]
- 112.Brandes J.L., Cady R.K., Freitag F.G., Smith T.R., Chandler P., Fox A.W., Linn L., Farr S.J. Needle-free subcutaneous sumatriptan (Sumavel™ DosePro™): bioequivalence and ease of use. Headache. 2009;49:1435–1444. doi: 10.1111/j.1526-4610.2009.01530.x. [DOI] [PubMed] [Google Scholar]
- 113.Law S., Derry S., Moore R.A. Triptans for acute cluster headache. Cochrane Database Syst. Rev. 2013;7(7):CD008042. doi: 10.1002/14651858.cd008042.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Roberto G., Raschi E., Piccinni C., Conti V., Vignatelli L., D'Alessandro R., De Ponti F., Poluzzi E. Adverse cardiovascular events associated with triptans and ergotamines for treatment of migraine: systematic review of observational studies. Cephalalgia. 2014:0333102414550416. doi: 10.1177/0333102414550416. [DOI] [PubMed] [Google Scholar]
- 115.Evans R.W. The FDA alert on serotonin syndrome with combined use of SSRIs or SNRIs and Triptans: an analysis of the 29 case reports. MedGenMed. 2007;9(3):48. [PMC free article] [PubMed] [Google Scholar]
- 116.Gillman P.K. Triptans, serotonin agonists, and serotonin syndrome (serotonin toxicity): a review. Headache. 2010;50(2):264–272. doi: 10.1111/j.1526-4610.2009.01575.x. [DOI] [PubMed] [Google Scholar]
- 117.Bahra A., Gawel M.J., Hardebo J.E., Millson D., Breen S.A., Goadsby P.J. Oral zolmitriptan is effective in the acute treatment of cluster headache. Neurology. 2000;54(9):1832–1839. doi: 10.1212/WNL.54.9.1832. [DOI] [PubMed] [Google Scholar]
- 118.Cittadini E., May A., Straube A., Evers S., Bussone G., Goadsby P.J. Effectiveness of intranasal zolmitriptan in acute cluster headache: a randomized, placebo-controlled, double-blind crossover study. Arch. Neurol. 2006;63(11):1537–1542. doi: 10.1001/archneur.63.11.nct60002. [DOI] [PubMed] [Google Scholar]
- 119.Rapoport A.M., Matthew N.T., Silberstein S.D., Dodick D., Tepper S.J., Sheftell F.D., Bigal M.E. Zolmitriptan nasal spray in the acute treatment of cluster headache: a double-blind study. 2007. [DOI] [PubMed]
- 120.Hedlund C., Rapoport A.M., Dodick D.W., Goadsby P.J. Zolmitriptan nasal spray in the acute treatment of cluster headache: a meta-analysis of two studies. Headache. 2009;49(9):1315–1323. doi: 10.1111/j.1526-4610.2009.01518.x. [DOI] [PubMed] [Google Scholar]
- 121.Horton B.T. Histaminic cephalgia. J. Lancet. 1952;72(2):92–98. [PubMed] [Google Scholar]
- 122.Kudrow L. Response of cluster headache attacks to oxygen inhalation. 1981. [DOI] [PubMed]
- 123.Fogan L. Treatment of cluster headache. A double-blind comparison of oxygen v air inhalation. Arch. Neurol. 1985;42(4):362–363. doi: 10.1001/archneur.1985.04060040072015. [DOI] [PubMed] [Google Scholar]
- 124.Cohen A.S., Burns B., Goadsby P.J. High-flow oxygen for treatment of cluster headache: a randomized trial. JAMA. 2009;302(22):2451–2457. doi: 10.1001/jama.2009.1855. [DOI] [PubMed] [Google Scholar]
- 125.Rozen T.D. High oxygen flow rates for cluster headache. 2004. [DOI] [PubMed]
- 126.Di Sabato F., Fusco B.M., Pelaia P., Giacovazzo M. Hyperbaric oxygen therapy in cluster headache. Pain. 1993;52(2):243–245. doi: 10.1016/0304-3959(93)90137-E. [DOI] [PubMed] [Google Scholar]
- 127.Rozen T.D., Niknam R., Shechter A.L., Silberstein S.D. Gender differences in clinical characteristics and treatment response in cluster headache patients. Cephalalgia. 1999;19:323. [Google Scholar]
- 128.Rozen T.D., Fishman R.S. Inhaled oxygen and cluster headache sufferers in the United States: use, efficacy and economics: results from the United States Cluster Headache Survey. Headache. 2011;51(2):191–200. doi: 10.1111/j.1526-4610.2010.01806.x. [DOI] [PubMed] [Google Scholar]
- 129.Hardebo J.E., Ryding E. Cerebral blood flow response to oxygen in cluster headache. In: Olesen J., editor. Migraine and other mechanisms. New York: Raven; 1991. pp. 311–314. [Google Scholar]
- 130.Schuh-Hofer S., Siekmann W., Offenhauser N., Reuter U., Arnold G. Effect of hyperoxia on neurogenic plasma protein extravasation in the rat dura mater. Headache. 2006;46(10):1545–1551. doi: 10.1111/j.1526-4610.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- 131.Akerman S., Holland P.R., Lasalandra M.P., Goadsby P.J. Oxygen inhibits neuronal activation in the trigeminocervical complex after stimulation of trigeminal autonomic reflex, but not during direct dural activation of trigeminal afferents. Headache. 2009;49(8):1131–1143. doi: 10.1111/j.1526-4610.2009.01501.x. [DOI] [PubMed] [Google Scholar]
- 132.Andersson P.G., Jespersen L.T. Dihydroergotamine nasal spray in the treatment of attacks of cluster headache. A double-blind trial versus placebo. Cephalalgia. 1986;6(1):51–54. doi: 10.1046/j.1468-2982.1986.0601051.x. [DOI] [PubMed] [Google Scholar]
- 133.Bigal M.E., Tepper S.J. Ergotamine and dihydroergotamine: a review. Curr. Pain Headache Rep. 2003;7(1):55–62. doi: 10.1007/s11916-003-0011-7. [DOI] [PubMed] [Google Scholar]
- 134.Silberstein S.D., McCrory D.C. Ergotamine and dihydroergotamine: history, pharmacology, and efficacy. Headache. 2003;43(2):144–166. doi: 10.1046/j.1526-4610.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 135.Baron E.P., Tepper S.J. Revisiting the role of ergots in the treatment of migraine and headache. Headache. 2010;50(8):1353–1361. doi: 10.1111/j.1526-4610.2010.01662.x. [DOI] [PubMed] [Google Scholar]
- 136.Kittrelle J.P., Grouse D.S., Seybold M.E. Cluster headache. Local anesthetic abortive agents. Arch. Neurol. 1985;42(5):496–498. doi: 10.1001/archneur.1985.04060050098017. [DOI] [PubMed] [Google Scholar]
- 137.Robbins L. Intranasal lidocaine for cluster headache. 1995. [DOI] [PubMed]
- 138.Costa A., Pucci E., Antonaci F., Sances G., Granella F., Broich G., Nappi G. The effect of intranasal cocaine and lidocaine on nytroglicerin-induced attacks in cluster headache. Cephalagia. 2000;20:85–91. doi: 10.1046/j.1468-2982.2000.00026.x. [DOI] [PubMed] [Google Scholar]
- 139.Barré F. Cocaine as an abortive agent in cluster headache. Headache. 1982;22(2):69–73. doi: 10.1111/j.1526-4610.1982.hed2202069.x. [DOI] [PubMed] [Google Scholar]
- 140.Sicuteri F., Geppetti P., Marabini S., Lembeck F. Pain relief by somatostatin in attacks of cluster headache. Pain. 1984;18(4):359–365. doi: 10.1016/0304-3959(84)90048-4. [DOI] [PubMed] [Google Scholar]
- 141.Matharu M.S., Levy M.J., Meeran K., Goadsby P.J. Subcutaneous octreotide in cluster headache: randomized placebo-controlled double-blind crossover study. Ann. Neurol. 2004;56(4):488–494. doi: 10.1002/ana.20210. [DOI] [PubMed] [Google Scholar]
- 142.Helyes Z., Pintér E., Németh J., Kéri G., Thán M., Oroszi G., Horváth A., Szolcsányi J. Anti-inflammatory effect of synthetic somatostatin analogues in the rat. Br. J. Pharmacol. 2001;134(7):1571–1579. doi: 10.1038/sj.bjp.0704396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Brainin M., Barnes M., Baron J.C., Gilhus N.E., Hughes R., Selmaj K., Waldemar G., Guideline Standards Subcommittee of the EFNS Scientific Committee Guidance for the preparation of neurological management guidelines by EFNS scientific task forces--revised recommendations 2004. Eur. J. Neurol. 2004;11(9):577–581. doi: 10.1111/j.1468-1331.2004.00867.x. [DOI] [PubMed] [Google Scholar]
- 144.Schürks M., Rosskopf D., de Jesus J., Jonjic M., Diener H.C., Kurth T. Predictors of acute treatment response among patients with cluster headache. Headache. 2007;47(7):1079–1084. doi: 10.1111/j.1526-4610.2007.00862.x. [DOI] [PubMed] [Google Scholar]
- 145.Sarchielli P., Granella F., Prudenzano M.P., Pini L.A., Guidetti V., Bono G., Pinessi L., Alessandri M., Antonaci F., Fanciullacci M., Ferrari A., Guazzelli M., Nappi G., Sances G., Sandrini G., Savi L., Tassorelli C., Zanchin G. Italian guidelines for primary headaches: 2012 revised version. J. Headache Pain. 2012;13(2) Suppl. 2:S31–S70. doi: 10.1007/s10194-012-0437-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Couch J.R., Jr, Ziegler D.K. Prednisone therapy for cluster headache. Headache. 1978;18(4):219–221. doi: 10.1111/j.1526-4610.1978.hed1804219.x. [DOI] [PubMed] [Google Scholar]
- 147.Antonaci F., Costa A., Candeloro E., Sjaastad O., Nappi G. Single high-dose steroid treatment in episodic cluster headache. Cephalalgia. 2005;25(4):290–295. doi: 10.1111/j.1468-2982.2004.00855.x. [DOI] [PubMed] [Google Scholar]
- 148.Mir P., Alberca R., Navarro A., Montes E., Martínez E., Franco E., Cayuela A., Lozano P. Prophylactic treatment of episodic cluster headache with intravenous bolus of methylprednisolone. Neurol. Sci. 2003;24(5):318–321. doi: 10.1007/s10072-003-0182-3. [DOI] [PubMed] [Google Scholar]
- 149.Becker W.J. Cluster headache: conventional pharmacological management. Headache. 2013;53(7):1191–1196. doi: 10.1111/head.12145. [DOI] [PubMed] [Google Scholar]
- 150.Mather P.J., Silberstein S.D., Schulman E.A., Hopkins M.M. The treatment of cluster headache with repetitive intravenous dihydroergotamine. Headache. 1991;31(8):525–532. doi: 10.1111/j.1526-4610.1991.hed3108525.x. [DOI] [PubMed] [Google Scholar]
- 151.Magnoux E., Zlotnik G. Outpatient intravenous dihydroergotamine for refractory cluster headache. Headache. 2004;44(3):249–255. doi: 10.1111/j.1526-4610.2004.04055.x. [DOI] [PubMed] [Google Scholar]
- 152.Halker R., Vargas B., Dodick D.W. Cluster headache: diagnosis and treatment. Semin. Neurol. 2010;30(2):175–185. doi: 10.1055/s-0030-1249226. [DOI] [PubMed] [Google Scholar]
- 153.Ekbom K., Hardebo J.E. Cluster headache: aetiology, diagnosis and management. Drugs. 2002;62(1):61–69. doi: 10.2165/00003495-200262010-00003. [DOI] [PubMed] [Google Scholar]
- 154.Leone M., D’Amico D., Frediani F., Moschiano F., Grazzi L., Attanasio A., Bussone G. Verapamil in the prophylaxis of episodic cluster headache: a double-blind study versus placebo. 2000. [DOI] [PubMed]
- 155.Gabai I.J., Spierings E.L. Prophylactic treatment of cluster headache with verapamil. Headache. 1989;29(3):167–168. doi: 10.1111/j.1526-4610.1989.hed2903167.x. [DOI] [PubMed] [Google Scholar]
- 156.Blau J.N., Engel H.O. Individualizing treatment with verapamil for cluster headache patients. Headache. 2004;44(10):1013–1018. doi: 10.1111/j.1526-4610.2004.04196.x. [DOI] [PubMed] [Google Scholar]
- 157.Bussone G., Leone M., Peccarisi C., Micieli G., Granella F., Magri M., Manzoni G.C., Nappi G. Double blind comparison of lithium and verapamil in cluster headache prophylaxis. 1990. [DOI] [PubMed]
- 158.Jónsdóttir M., Meyer J.S., Rogers R.L. Efficacy, side effects and tolerance compared during headache treatment with three different calcium blockers. Headache. 1987;27(7):364–369. doi: 10.1111/j.1526-4610.1987.hed2707364.x. [DOI] [PubMed] [Google Scholar]
- 159.El-Fakahany E., Richelson E. Effect of some calcium antagonists on muscarinic receptor-mediated cyclic GMP formation. J. Neurochem. 1983;40(3):705–710. doi: 10.1111/j.1471-4159.1983.tb08036.x. [DOI] [PubMed] [Google Scholar]
- 160.Taylor J.E., Defeudis F.V. Inhibition of [3H]spiperone binding to 5-HT2 receptors of rat cerebral cortex by the calcium antagonists verapamil and D600. Eur. J. Pharmacol. 1984;106(1):215–216. doi: 10.1016/0014-2999(84)90703-9. [DOI] [PubMed] [Google Scholar]
- 161.Maisel A.S., Motulsky H.J., Insel P.A. Hypotension after quinidine plus verapamil. Possible additive competition at alpha-adrenergic receptors. N. Engl. J. Med. 1985;312(3):167–170. doi: 10.1056/NEJM198501173120308. [DOI] [PubMed] [Google Scholar]
- 162.Kavaliers M. Calcium channel blockers inhibit the antagonistic effects of Phe-Met-Arg-Phe-amide (FMRFamide) on morphine- and stress-induced analgesia in mice. Brain Res. 1987;415(2):380–384. doi: 10.1016/0006-8993(87)90225-3. [DOI] [PubMed] [Google Scholar]
- 163.Costa A., Leston J.A., Cavallini A., Nappi G. Cluster headache and periodic affective illness: common chronobiological features. Funct. Neurol. 1998;13(3):263–272. [PubMed] [Google Scholar]
- 164.Malhi G.S., Tanious M. Optimal frequency of lithium administration in the treatment of bipolar disorder: clinical and dosing considerations. CNS Drugs. 2011;25(4):289–298. doi: 10.2165/11586970-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 165.Ekbom K. Lithium for cluster headache: review of the literature and preliminary results of long-term treatment. 1981. [DOI] [PubMed]
- 166.Steiner T.J., Hering R., Couturier E.G., Davies P.T., Whitmarsh T.E. Double-blind placebo-controlled trial of lithium in episodic cluster headache. Cephalalgia. 1997;17(6):673–675. doi: 10.1046/j.1468-2982.1997.1706673.x. [DOI] [PubMed] [Google Scholar]
- 167.Kudrow L. Cluster headache: mechanisms and management. Oxford University Press; 1980. [Google Scholar]
- 168.Hokin L.E., Dixon J.F., Los G.V. A novel action of lithium: stimulation of glutamate release and inositol 1,4,5 trisphosphate accumulation via activation of the N-methyl D-aspartate receptor in monkey and mouse cerebral cortex slices. Adv. Enzyme Regul. 1996;36:229–244. doi: 10.1016/0065-2571(95)00021-6. [DOI] [PubMed] [Google Scholar]
- 169.Dixon J.F., Hokin L.E. Lithium acutely inhibits and chronically up-regulates and stabilizes glutamate uptake by presynaptic nerve endings in mouse cerebral cortex. Proc. Natl. Acad. Sci. USA. 1998;95(14):8363–8368. doi: 10.1073/pnas.95.14.8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Post R.M., Speer A.M., Hough C.J., Xing G. Neurobiology of bipolar illness: implications for future study and therapeutics. Ann. Clin. Psychiatry. 2003;15(2):85–94. doi: 10.3109/10401230309085674. [DOI] [PubMed] [Google Scholar]
- 171.Ferrie L., Young A.H., McQuade R. Effect of chronic lithium and withdrawal from chronic lithium on presynaptic dopamine function in the rat. J. Psychopharmacol. (Oxford) 2005;19(3):229–234. doi: 10.1177/0269881105051525. [DOI] [PubMed] [Google Scholar]
- 172.Ferrie L., Young A.H., McQuade R. Effect of lithium and lithium withdrawal on potassium-evoked dopamine release and tyrosine hydroxylase expression in the rat. Int. J. Neuropsychopharmacol. 2006;9(6):729–735. doi: 10.1017/S1461145705006243. [DOI] [PubMed] [Google Scholar]
- 173.Manji H.K., Lenox R.H. Signaling: cellular insights into the pathophysiology of bipolar disorder. Biol. Psychiatry. 2000;48(6):518–530. doi: 10.1016/S0006-3223(00)00929-X. [DOI] [PubMed] [Google Scholar]
- 174.Ikonomov O.C., Manji H.K. Molecular mechanisms underlying mood stabilization in manic-depressive illness: the phenotype challenge. Am. J. Psychiatry. 1999;156(10):1506–1514. doi: 10.1176/ajp.156.10.1506. [DOI] [PubMed] [Google Scholar]
- 175.Silverstone P.H., McGrath B.M., Kim H. Bipolar disorder and myo-inositol: a review of the magnetic resonance spectroscopy findings. Bipolar Dis. 2005;7:1–10. doi: 10.1111/j.1399-5618.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- 176.Klemfuss H. Rhythms and the pharmacology of lithium. Pharmacol. Ther. 1992;56(1):53–78. doi: 10.1016/0163-7258(92)90037-Z. [DOI] [PubMed] [Google Scholar]
- 177.Swann A.C., Heninger G.R., Marini J.L., Sheard M.H., Maas J.W. Lithium effects on high-affinity tryptophan uptake: evidence against a stabilization mechanism. Brain Res. 1980;194(1):287–292. doi: 10.1016/0006-8993(80)91346-3. [DOI] [PubMed] [Google Scholar]
- 178.Friedman E., Wang H.Y. Effect of chronic lithium treatment on 5-hydroxytryptamine autoreceptors and release of 5-[3H]hydroxytryptamine from rat brain cortical, hippocampal, and hypothalamic slices. J. Neurochem. 1988;50(1):195–201. doi: 10.1111/j.1471-4159.1988.tb13249.x. [DOI] [PubMed] [Google Scholar]
- 179.Kripke D.F., Judd L.L., Hubbard B., Janowsky D.S., Huey L.Y. The effect of lithium carbonate on the circadian rhythm of sleep in normal human subjects. Biol. Psychiatry. 1979;14(3):545–548. [PubMed] [Google Scholar]
- 180.Wheeler S.D., Carrazana E.J. Topiramate-treated cluster headache. Neurology. 1999;53(1):234–236. doi: 10.1212/WNL.53.1.234. [DOI] [PubMed] [Google Scholar]
- 181.Leone M., Dodick D., Rigamonti A., D’Amico D., Grazzi L., Mea E., Bussone G. Topiramate in cluster headache prophylaxis: an open trial. Cephalalgia. 2003;23(10):1001–1002. doi: 10.1046/j.1468-2982.2003.00665.x. [DOI] [PubMed] [Google Scholar]
- 182.Láinez M.J., Pascual J., Pascual A.M., Santonja J.M., Ponz A., Salvador A. Topiramate in the prophylactic treatment of cluster headache. Headache. 2003;43(7):784–789. doi: 10.1046/j.1526-4610.2003.03137.x. [DOI] [PubMed] [Google Scholar]
- 183.Hering R., Kuritzky A. Sodium valproate in the treatment of cluster headache: an open clinical trial. Cephalalgia. 1989;9(3):195–198. doi: 10.1046/j.1468-2982.1989.903195.x. [DOI] [PubMed] [Google Scholar]
- 184.El Amrani M., Massiou H., Bousser M.G. A negative trial of sodium valproate in cluster headache: methodological issues. Cephalalgia. 2002;22(3):205–208. doi: 10.1046/j.1468-2982.2002.00349.x. [DOI] [PubMed] [Google Scholar]
- 185.Ahmed F. Chronic cluster headache responding to gabapentin: a case report. 2000. [DOI] [PubMed]
- 186.Leandri M., Luzzani M., Cruccu G., Gottlieb A. Drug-resistant cluster headache responding to gabapentin: a pilot study. Cephalalgia. 2001;21(7):744–746. doi: 10.1046/j.1468-2982.2001.00260.x. [DOI] [PubMed] [Google Scholar]
- 187.Schuh-Hofer S., Israel H., Neeb L., Reuter U., Arnold G. The use of gabapentin in chronic cluster headache patients refractory to first-line therapy. Eur. J. Neurol. 2007;14(6):694–696. doi: 10.1111/j.1468-1331.2007.01738.x. [DOI] [PubMed] [Google Scholar]
- 188.Vuković V., Lovrencić-Huzjan A., Budisić M., Demarin V. Gabapentin in the prophylaxis of cluster headache: an observational open label study. Acta Clin. Croat. 2009;48(3):311–314. [PubMed] [Google Scholar]
- 189.Curran D.A., Hinterberger H., Lance J.W. Methysergide. Res. Clin. Stud. Headache. 1967;1:74–122. [Google Scholar]
- 190.Lovshin L.L. Treatment on histaminic cephalalgia with Methysergide (UML-491) (based on 159 cases). Dis. Nerv. Syst. 1963;24:120–124. [Google Scholar]
- 191.Graham J.R., Suby H.I., LeCompte P.R., Sadowsky N.L. Fibrotic disorders associated with methysergide therapy for headache. N. Engl. J. Med. 1966;274(7):359–368. doi: 10.1056/NEJM196602172740701. [DOI] [PubMed] [Google Scholar]
- 192.Ekbom K. Prophylactic treatment of cluster headache with a new serotonin antagonist, BC 105. Acta Neurol. Scand. 1969;45(5):601–610. doi: 10.1111/j.1600-0404.1969.tb01270.x. [DOI] [PubMed] [Google Scholar]
- 193.Diamond S., Freitag F.G., Prager J., Gandhi S. Treatment of intractable cluster. Headache. 1986;26(1):42–46. doi: 10.1111/j.1526-4610.1986.hed2601042.x. [DOI] [PubMed] [Google Scholar]
- 194.Leone M., D’Amico D., Moschiano F., Fraschini F., Bussone G. Melatonin versus placebo in the prophylaxis of cluster headache: a double-blind pilot study with parallel groups. Cephalalgia. 1996;16(7):494–496. doi: 10.1046/j.1468-2982.1996.1607494.x. [x]. [DOI] [PubMed] [Google Scholar]
- 195.Pringsheim T., Magnoux E., Dobson C.F., Hamel E., Aubé M. Melatonin as adjunctive therapy in the prophylaxis of cluster headache: a pilot study. Headache. 2002;42(8):787–792. doi: 10.1046/j.1526-4610.2002.02181.x. [DOI] [PubMed] [Google Scholar]
- 196.D’Andrea G., Perini F., Granella F., Cananzi A., Sergi A. Efficacy of transdermal clonidine in short-term treatment of cluster headache: a pilot study. Cephalalgia. 1995;15(5):430–433. doi: 10.1046/j.1468-29821995.1505430.x. [DOI] [PubMed] [Google Scholar]
- 197.Leone M., Attanasio A., Grazzi L., Libro G., D’Amico D., Moschiano F., Bussone G. Transdermal clonidine in the prophylaxis of episodic cluster headache: an open study. Headache. 1997;37(9):559–560. doi: 10.1046/j.1526-4610.1997.3709559.x. [DOI] [PubMed] [Google Scholar]
- 198.Hering-Hanit R., Gadoth N. Baclofen in cluster headache. Headache. 2000;40(1):48–51. doi: 10.1046/j.1526-4610.2000.00009.x. [DOI] [PubMed] [Google Scholar]
- 199.Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol. Rev. 1991;43(2):143–201. [PubMed] [Google Scholar]
- 200.Sicuteri F., Fusco B.M., Marabini S., Campagnolo V., Maggi C.A., Geppetti P., Fanciullacci M. Beneficial effect of capsaicin application to the nasal mucosa in cluster headache. Clin. J. Pain. 1989;5(1):49–53. doi: 10.1097/00002508-198903000-00010. [DOI] [PubMed] [Google Scholar]
- 201.Fusco B.M., Marabini S., Maggi C.A., Fiore G., Geppetti P. Preventative effect of repeated nasal applications of capsaicin in cluster headache. Pain. 1994;59(3):321–325. doi: 10.1016/0304-3959(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 202.Sostak P., Krause P., Förderreuther S., Reinisch V., Straube A. Botulinum toxin type-A therapy in cluster headache: an open study. J. Headache Pain. 2007;8(4):236–241. doi: 10.1007/s10194-007-0400-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Pageler L., Limmroth V. Oral triptans in the preventive management of cluster headache. Curr. Pain Headache Rep. 2012;16(2):180–184. doi: 10.1007/s11916-012-0251-5. [DOI] [PubMed] [Google Scholar]
- 204.Monstad I., Krabbe A., Micieli G., Prusinski A., Cole J., Pilgrim A., Shevlin P. Preemptive oral treatment with sumatriptan during a cluster period. Headache. 1995;35(10):607–613. doi: 10.1111/j.1526-4610.1995.hed3510607.x. [DOI] [PubMed] [Google Scholar]
- 205.Mulder L.J., Spierings E.L. Naratriptan in the preventive treatment of cluster headache. Cephalalgia. 2002;22(10):815–817. doi: 10.1046/j.1468-2982.2002.00424.x. [DOI] [PubMed] [Google Scholar]
- 206.Zebenholzer K., Wöber C., Vigl M., Wessely P. Eletriptan for the short-term prophylaxis of cluster headache. Headache. 2004;44(4):361–364. doi: 10.1111/j.1526-4610.2004.04079.x. [DOI] [PubMed] [Google Scholar]
- 207.Siow H.C., Pozo-Rosich P., Silberstein S.D. Frovatriptan for the treatment of cluster headaches. Cephalalgia. 2004;24(12):1045–1048. doi: 10.1111/j.1468-2982.2004.00734.x. [DOI] [PubMed] [Google Scholar]
- 208.Pageler L., Katsarava Z., Lampl C., Straube A., Evers S., Diener H.C., Limmroth V. Frovatriptan for prophylactic treatment of cluster headache: lessons for future trial design. 2011. [DOI] [PubMed]
- 209.Rapoport A.M., Bigal M.E., Tepper S.J., Sheftell F.D. Intranasal medications for the treatment of migraine and cluster headache. CNS Drugs. 2004;18(10):671–685. doi: 10.2165/00023210-200418100-00004. [DOI] [PubMed] [Google Scholar]
- 210.Saper J.R., Klapper J., Mathew N.T., Rapoport A., Phillips S.B., Bernstein J.E. Intranasal civamide for the treatment of episodic cluster headaches. Arch. Neurol. 2002;59(6):990–994. doi: 10.1001/archneur.59.6.990. [DOI] [PubMed] [Google Scholar]
- 211.Sewell R.A. Response of Cluster Headache to Kudzu. 2009. [DOI] [PubMed]
- 212.Fitzpatrick L.A. Selective estrogen receptor modulators and phytoestrogens: new therapies for the postmenopausal women. Mayo Clin. Proc. 1999;74(6):601–607. doi: 10.4065/74.6.601. [DOI] [PubMed] [Google Scholar]
- 213.Lukas S.E., Penetar D., Berko J., Vicens L., Palmer C., Mallya G., Macklin E.A., Lee D.Y. An extract of the Chinese herbal root kudzu reduces alcohol drinking by heavy drinkers in a naturalistic setting. Alcohol. Clin. Exp. Res. 2005;29(5):756–762. doi: 10.1097/01.ALC.0000163499.64347.92. [DOI] [PubMed] [Google Scholar]
- 214.Pedersen J.L., Barloese M., Jensen R.H. Neurostimulation in cluster headache: a review of current progress. Cephalalgia. 2013;33(14):1179–1193. doi: 10.1177/0333102413489040. [DOI] [PubMed] [Google Scholar]
- 215.Fontaine D., Lazorthes Y., Mertens P., Blond S., Géraud G., Fabre N., Navez M., Lucas C., Dubois F., Gonfrier S., Paquis P., Lantéri-Minet M. Safety and efficacy of deep brain stimulation in refractory cluster headache: a randomized placebo-controlled double-blind trial followed by a 1-year open extension. J. Headache Pain. 2010;11(1):23–31. doi: 10.1007/s10194-009-0169-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Magis D., Schoenen J. Advances and challenges in neurostimulation for headaches. Lancet Neurol. 2012;11(8):708–719. doi: 10.1016/S1474-4422(12)70139-4. [DOI] [PubMed] [Google Scholar]
- 217.Burns B., Watkins L., Goadsby P.J. Treatment of intractable chronic cluster headache by occipital nerve stimulation in 14 patients. Neurology. 2009;72(4):341–345. doi: 10.1212/01.wnl.0000341279.17344.c9. [DOI] [PubMed] [Google Scholar]
- 218.Fontaine D., Christophe Sol J., Raoul S., Fabre N., Geraud G., Magne C., Sakarovitch C., Lanteri-Minet M. Treatment of refractory chronic cluster headache by chronic occipital nerve stimulation. Cephalalgia. 2011;31(10):1101–1105. doi: 10.1177/0333102411412086. [DOI] [PubMed] [Google Scholar]
- 219.Ansarinia M., Rezai A., Tepper S.J., Steiner C.P., Stump J., Stanton-Hicks M., Machado A., Narouze S. Electrical stimulation of sphenopalatine ganglion for acute treatment of cluster headaches. Headache. 2010;50(7):1164–1174. doi: 10.1111/j.1526-4610.2010.01661.x. [DOI] [PubMed] [Google Scholar]
- 220.Schoenen J., Jensen R.H., Lantéri-Minet M., Láinez M.J., Gaul C., Goodman A.M., Caparso A., May A. Stimulation of the sphenopalatine ganglion (SPG) for cluster headache treatment. Pathway CH-1: a randomized, sham-controlled study. Cephalalgia. 2013;33(10):816–830. doi: 10.1177/0333102412473667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sjaastad O., Vincent M. Indomethacin responsive headache syndromes: chronic paroxysmal hemicrania and Hemicrania continua. How they were discovered and what we have learned since. Funct. Neurol. 2010;25(1):49–55. [PubMed] [Google Scholar]
- 222.Pareja J.A., Caminero A.B., Franco E., Casado J.L., Pascual J., Sánchez del Río M. Dose, efficacy and tolerability of long-term indomethacin treatment of chronic paroxysmal hemicrania and hemicrania continua. Cephalalgia. 2001;21(9):906–910. doi: 10.1046/j.1468-2982.2001.00287.x. [DOI] [PubMed] [Google Scholar]
- 223.Porta-Etessam J., Cuadrado M., Rodríguez-Gómez O., García-Ptacek S., Valencia C. Are Cox-2 drugs the second line option in indomethacin responsive headaches? J. Headache Pain. 2010;11(5):405–407. doi: 10.1007/s10194-010-0225-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lisotto C., Maggioni F., Mainardi F., Zanchin G. Rofecoxib for the treatment of chronic paroxysmal hemicrania. Cephalalgia. 2003;23(4):318–320. doi: 10.1046/j.1468-2982.2003.00500.x. [DOI] [PubMed] [Google Scholar]
- 225.Siow H.C. Seasonal episodic paroxysmal hemicrania responding to cyclooxygenase-2 inhibitors. Cephalalgia. 2004;24(5):414–415. doi: 10.1111/j.1468-2982.2003.00695.x. [DOI] [PubMed] [Google Scholar]
- 226.Mathew N.T., Kailasam J., Fischer A. Responsiveness to celecoxib in chronic paroxysmal hemicrania. Neurology. 2000;55(2):316. doi: 10.1212/WNL.55.2.316. [DOI] [PubMed] [Google Scholar]
- 227.Evers S., Husstedt I.W. Alternatives in drug treatment of chronic paroxysmal hemicrania. Headache. 1996;36(7):429–432. doi: 10.1046/j.1526-4610.1996.3607429.x. [DOI] [PubMed] [Google Scholar]
- 228.Sjaastad O., Antonaci F. A piroxicam derivative partly effective in chronic paroxysmal hemicrania and hemicrania continua. Headache. 1995;35(9):549–550. doi: 10.1111/j.1526-4610.1995.hed3509549.x. [DOI] [PubMed] [Google Scholar]
- 229.Rozen T.D. Melatonin responsive hemicrania continua. 2006. [DOI] [PubMed]
- 230.Evers S., Husstedt I.W. Alternatives in drug treatment of chronic paroxysmal hemicrania. Headache. 1996;36(7):429–432. doi: 10.1046/j.1526-4610.1996.3607429.x. [DOI] [PubMed] [Google Scholar]
- 231.Cohen A.S., Goadsby P.J. Paroxysmal hemicrania responding to topiramate. J. Neurol. Neurosurg. Psychiatry. 2007;78(1):96–97. doi: 10.1136/jnnp.2006.096651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Afridi S.K., Shields K.G., Bhola R., Goadsby P.J. Greater occipital nerve injection in primary headache syndromes--prolonged effects from a single injection. Pain. 2006;122(1-2):126–129. doi: 10.1016/j.pain.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 233.Narbone M.C., Gangemi S., Abbate M. A case of SUNCT syndrome responsive to verapamil. Cephalalgia. 2005;25(6):476–478. doi: 10.1111/j.1468-2982.2004.00876.x. [DOI] [PubMed] [Google Scholar]
- 234.Trauninger A., Alkonyi B., Kovács N., Komoly S., Pfund Z. Methylprednisolone therapy for short-term prevention of SUNCT syndrome. Cephalalgia. 2010;30(6):735–739. doi: 10.1111/j.1468-2982.2009.01971.x. [DOI] [PubMed] [Google Scholar]
- 235.Bussone G., Rapoport A. Acute and preventive treatment of cluster headache and other trigeminal autonomic cephalgias. Handb. Clin. Neurol. 2010;97:431–442. doi: 10.1016/S0072-9752(10)97036-X. [DOI] [PubMed] [Google Scholar]
- 236.Arroyo A.M., Durán X.R., Beldarrain M.G., Pinedo A., García-Moncó J.C. Response to intravenous lidocaine in a patient with SUNCT syndrome. Cephalalgia. 2010;30(1):110–112. doi: 10.1111/j.1468-2982.2009.01871.x. [DOI] [PubMed] [Google Scholar]
- 237.Calvo J.F., Bruera O.C., de Lourdes Figuerola M., Gestro D., Tinetti N., Leston J.A. SUNCT syndrome: clinical and 12-year follow-up case report. Cephalalgia. 2004;24(10):900–902. doi: 10.1111/j.1468-2982.2004.00755.x. [DOI] [PubMed] [Google Scholar]
- 238.Putzki N., Nirkko A., Diener H.C. Trigeminal autonomic cephalalgias: a case of post-traumatic SUNCT syndrome? Cephalalgia. 2005;25(5):395–397. doi: 10.1111/j.1468-2982.2004.00860.x. [DOI] [PubMed] [Google Scholar]
- 239.Pareja J.A., Sjaastad O. SUNCT syndrome in the female. Headache. 1994;34(4):217–220. doi: 10.1111/j.1526-4610.1994.hed3404217.x. [DOI] [PubMed] [Google Scholar]
- 240.Raimondi E., Gardella L. SUNCT syndrome. Two cases in Argentina. Headache. 1998;38(5):369–371. doi: 10.1046/j.1526-4610.1998.3805369.x. [DOI] [PubMed] [Google Scholar]
- 241.Becser N., Berky M. SUNCT syndrome: a Hungarian case. Headache. 1995;35(3):158–160. doi: 10.1111/j.1526-4610.1995.hed3503158.x. [DOI] [PubMed] [Google Scholar]
- 242.Selekler H.M., Efendi H., Alemdar M. Short-lasting unilateral neuralgiform headache with severe lacrimation and mild conjunctival injection. Cephalalgia. 2005;25(4):317–320. doi: 10.1111/j.1468-2982.2004.00854.x. [DOI] [PubMed] [Google Scholar]
- 243.Schwaag S., Frese A., Husstedt I.W., Evers S. SUNCT syndrome: the first German case series. Cephalalgia. 2003;23(5):398–400. doi: 10.1046/j.1468-2982.2003.00551.x. [DOI] [PubMed] [Google Scholar]
- 244.Rossi P., Cesarino F., Faroni J., Malpezzi M.G., Sandrini G., Nappi G. SUNCT syndrome successfully treated with. 2003. [DOI] [PubMed]
- 245.Matharu M.S., Boes C.J., Goadsby P.J. SUNCT syndrome: prolonged attacks, refractoriness and response to topiramate. 2002. [DOI] [PubMed]
- 246.Kuhn J., Vosskaemper M., Bewermeyer H. SUNCT syndrome: a possible bilateral case responding to topiramate. 2005. [DOI] [PubMed]
- 247.Porta-Etessam J., Benito-Leon J., Martinez-Salio A., Berbel A. Gabapentin in the treatment of SUNCT syndrome. 2002. [DOI] [PubMed]
- 248.Graff-Radford S.B. SUNCT syndrome responsive to gabapentin (Neurontin). Cephalalgia. 2000;20(5):515–517. doi: 10.1046/j.1468-2982.2000.00065.x. [DOI] [PubMed] [Google Scholar]
- 249.Hunt C.H., Dodick D.W., Bosch E.P. SUNCT responsive to gabapentin. 2002. [DOI] [PubMed]
- 250.Marziniak M., Breyer R., Evers S. SUNCT syndrome successfully treated with the combination of oxcarbazepine and gabapentin. Pain Med. 2009;10(8):1497–1500. doi: 10.1111/j.1526-4637.2009.00729.x. [DOI] [PubMed] [Google Scholar]
- 251.Gutierrez-Garcia J.M. SUNCT syndrome responsive to lamotrigine. Headache. 2002;42(8):823–825. doi: 10.1046/j.1526-4610.2002.02187.x. [DOI] [PubMed] [Google Scholar]
- 252.Leone M., Rigamonti A., Usai S., Damico D., Grazzi L., Bussone G. Two new SUNCT cases responsive to lamotrigine. Cephalalgia. 2000;20(9):845–847. doi: 10.1046/j.1468-2982.2000.00128.x. [DOI] [PubMed] [Google Scholar]
- 253.D’Andrea G., Granella F., Ghiotto N., Nappi G. Lamotrigine in the treatment of SUNCT syndrome. Neurology. 2001;57(9):1723–1725. doi: 10.1212/WNL.57.9.1723. [DOI] [PubMed] [Google Scholar]
- 254.Chakravarty A., Mukherjee A. SUNCT syndrome responsive to lamotrigine: documentation of the first Indian case. 2003. [DOI] [PubMed]
- 255.D’Andrea G., Granella F., Cadaldini M. Possible usefulness of lamotrigine in the treatment of SUNCT syndrome. Neurology. 1999;53(7):1609. doi: 10.1212/WNL.53.7.1609. [DOI] [PubMed] [Google Scholar]
- 256.Vikelis M., Xifaras M., Mitsikostas D.D. SUNCT syndrome in the elderly. Cephalalgia. 2005;25(11):1091–1092. doi: 10.1111/j.1468-2982.2005.00971.x. [DOI] [PubMed] [Google Scholar]
- 257.Malik K., Rizvi S., Vaillancourt P.D. The SUNCT syndrome: successfully treated with lamotrigine. Pain Med. 2002;3(2):167–168. doi: 10.1046/j.1526-4637.2002.02025.x. [DOI] [PubMed] [Google Scholar]
- 258.Piovesan E.J., Siow C., Kowacs P.A., Werneck L.C. Influence of lamotrigine over the SUNCT syndrome: one patient follow-up for two years. Arq. Neuropsiquiatr. 2003;61(3A):691–694. doi: 10.1590/S0004-282X2003000400032. [DOI] [PubMed] [Google Scholar]
- 259.Cohen A.S., Matharu M.S., Goadsby P.J. Suggested guidelines for treating SUNCT and SUNA. Cephalagia. 2005;25:1200. [Google Scholar]
- 260.Mitsikostas D.D., Edvinsson L., Jensen R.H., Katsarava Z., Lampl C., Negro A., Osipova V., Paemeleire K., Siva A., Valade D., Martelletti P. Refractory chronic cluster headache: a consensus statement on clinical definition from the European Headache Federation. J. Headache Pain. 2014;15:79. doi: 10.1186/1129-2377-15-79. [DOI] [PMC free article] [PubMed] [Google Scholar]