Abstract
Background
Daikenchuto (DKT), a traditional Japanese herbal medicine, is widely used for treatment of gastrointestinal disorders. We evaluated the efficacy and safety of DKT for abdominal bloating in patients with chronic constipation.
Objective
To evaluate the efficacy and safety of DKT for the treatment of abdominal bloating.
Methods
After discontinuing as-needed use of laxatives, 10 patients received oral DKT for 14 days (15 g/d). To evaluate small intestinal bacteria overgrowth (SIBO), a glucose breath test was performed before and after treatment with DKT. Before beginning the treatment, 4 patients (40%) had a diagnosis of SIBO based on a positive glucose breath test result. In both the SIBO and non-SIBO groups, bowel movement frequency and stool form remained unchanged after DKT treatment.
Results
For all patients, median total Gastrointestinal Symptoms Rating Scale score and the median Gastrointestinal Symptoms Rating Scale indigestion and constipation subscales were significantly decreased, whereas the median visual analog score for decreased abdominal bloating was significantly increased. Improvements of those symptoms were the same in both the SIBO and non-SIBO groups, indicating that DKT does not have effects on small intestine bacteria. No serious side effects were reported.
Conclusions
DKT treatment improved quality of life for patients with chronic constipation regardless of the presence of SIBO and showed no effects on small intestine bacteria. UMIN Clinical Trial Registry identifier: UMIN000008070.
Key words: abdominal bloating, chronic constipation, daikenchuto, small intestinal bacterial overgrowth
Introduction
The main treatment modalities for patients with chronic constipation include laxatives, antiflatulence drugs, enterokinesis-regulating agents, cholinolytic drugs, and high-molecular-weight polymer administrations, as well as enemas and other methods. However, extended use of those treatments may cause abdominal pain and/or diarrhea, which can lower patient satisfaction. In patients with chronic constipation, abdominal bloating may have a serious negative influence on quality of life (QOL) to a greater degree than abnormal bowel habits and there is no standard regimen available for this distressing condition. Daikenchuto (DKT) (TJ-100), a traditional Japanese herbal medicine, has been widely used in Japan for treatment of gastrointestinal disorders such as postoperative ileus and irritable bowel syndrome. Previous studies have reported that DKT increased the level of calcitonin gene-related peptide and adrenomedullin, leading to increased intestinal blood flow in healthy subjects.1 And recent study2 revealed that DKT serves as a novel therapeutic agent for postoperative ileus characterized by its anti-inflammatory potency. DKT’s anti-inflammatory action is partly mediated by activation of alpha7 nicotinic acetylcholine receptors, which in turn ameliorated gastrointestinal motility disorder in postoperative ileus in mouse.2
DKT is composed of 3 crude agents in fixed proportions: Zingiberis rhizoma (processed ginger), Ginseng radix (Panax ginseng), and Zanthoxyli fructus (Japanese pepper). It is available in the form of extracted granules that are initially prepared by decocting the 3 crude agents in a 10-fold volume of purified water and keeping the mixture at 95°C for 1 hour, after which it is filtered and spray-dried to yield an extract powder. Subsequently, maltose syrup powder is added at a ratio of 1:8, then mixed and granulated to produce the final product. The current indication of DKT is for relief of abdominal coldness and pain accompanied by flatulence.
The purpose of our study was to evaluate the efficacy and safety of DKT for treatment of abdominal bloating in patients with chronic constipation.
Patients and Methods
Study design and subjects
This prospective single-center randomized open trial was conducted to evaluate the efficacy and safety of DKT for abdominal bloating accompanied by chronic constipation. The study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of Izumo-City General Medical Center, and registered in the UMIN Clinical Trial Registry (UMIN000008070). Study participants were enrolled from April 2011 to March 2013. Ten patients (3 men and 7 women; mean age 58.4 [19.5] years) with abdominal bloating received oral administrations (15 g/d 3 times/d before meals) of DKT (Tsumura & Co, Tokyo, Japan) for 14 consecutive days, during which any as-needed use of laxatives was discontinued (Table). Participating patients fulfilled the following criteria: abdominal bloating; able to cease use of laxatives, including on-demand use, during the study period; and liver function at less than the upper limit of normal set by our institution, determined within 6 months. Patients with organic colonic lesions or a history of total gastrectomy or colectomy were excluded from the study. Functional constipation patients were excluded, as were patients with organic causes of constipation such as diabetes mellitus, Parkinson disease, or a neurologic aliment. Patients who had taken kampo medicines, antibiotics, Lactobacillus, antacids, prokinetics, calcium polycarbophil, anticholinergics, laxatives, antidepressants, or antianxiety drugs within 2 weeks were also excluded from our study.
Table 1.
Characteristics of enrolled patients.
| Characteristic | Result |
|---|---|
| Number of patients | 10 |
| Age, y | |
| Mean (SD) | 58.4 (20.6) |
| Range | 34-85 |
| Gender | |
| Men | 3 |
| Women | 7 |
| Body mass index | |
| Mean (SD) | 23.6 (3.8) |
| Range | 18.7−31.2 |
Evaluation of abdominal symptoms
QOL was evaluated at the baseline, then on days 7 and 14 using the Japanese version of the Gastrointestinal Symptoms Rating Scale (GSRS) and abdominal bloating and overall treatment effect were assessed using a visual analog scale (VAS) score (0–100; 0 = none). GSRS and VAS were used in evaluating feelings of fullness.[3], [4] The frequency of bowel movements, stool consistency and form (Bristol Stool Scale),5 and abdominal symptoms were evaluated using a face scale with a daily diary.
Glucose breath test (GBT)
To evaluate small intestinal bacteria overgrowth (SIBO), a GBT was performed before and after administration of DKT using a Gastrolyzer (Bedfont Scientific Ltd, Kent, England). Diagnosis of SIBO was defined by a change in hydrogen concentration of >10 ppm in expired air after being given 100 g glucose.
Statistical analysis
Data for comparisons between groups were analyzed using χ2, ANOVA, and Wilcoxon’s signed rank tests. Categorical data were compared using Wilcoxon’s rank sum test; Student t test; or, where unequal variances occurred, Welch’s test. P values < 0.05 were considered significant. All statistical analyses were performed using SPSS (version 12.0 for PC, SPSS Japan Inc. Tokyo, Japan).
Results
Four patients (40%) were positive for SIBO based on GBT results. In an analysis of all patients, no changes were observed in frequency of bowel movements or stool form after DKT treatment. In contrast, there was a significant decrease in median total GSRS score (Figure 1), and median GSRS indigestion and constipation subscales (ANOVA P < 0.001), whereas acid reflex, diarrhea, and abdominal pain subscales did not show significant changes (Figure 2). For abdominal bloating, the median VAS score was notably reduced from 76 to 30 (P = 0.005), whereas the score for overall treatment effect increased from 13 to 51 (P = 0.005). Furthermore, the severity of abdominal pain and bloating assessed by a face scale significantly decreased (P = 0.039 and P = 0.008, respectively) (Figure 3). Improvements in these symptoms were observed in both the SIBO and non-SIBO groups. In addition, QOL scores improved (decreased median total GSRS score, median GSRS constipation subscales, and median VAS score for abdominal bloating and increased median VAS score for overall treatment effect) in both groups. In the SIBO group, GBT results showed similar patterns before and after administration of DKT. DKT did not affect bowel movement frequency or stool form in all patients (Figure 4), nor after dividing patients into SIBO and non-SIBO groups. Furthermore the GBT results showed similar patterns before and after administration of DKT in the SIBO group and non-SIBO groups (Figure 5). No adverse effects were observed in any of the patients during the 14-day treatment period.
Figure 1.
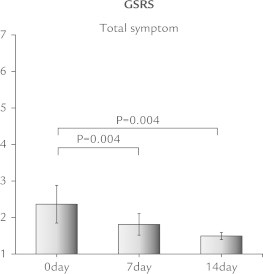
There was a significant decrease in median total Gastrointestinal Symptoms Rating Scale (GSRS) score.
Figure 2.
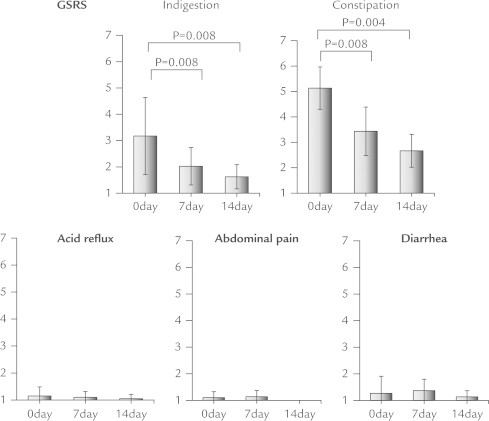
There were significant decreases in indigestion and constipation subscales of the Gastrointestinal Symptoms Rating Scale (GSRS), whereas acid reflex, diarrhea, and abdominal pain subscales did not show significant changes.
Figure 3.
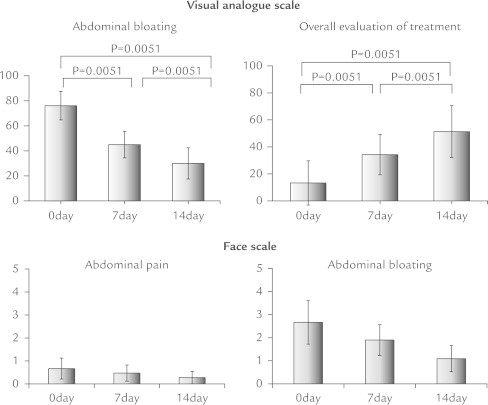
For abdominal bloating the median visual analog score was notably reduced, whereas the score for overall treatment effect increased. The severity of abdominal pain and bloating assessed by a face scale significantly decreased.
Figure 4.
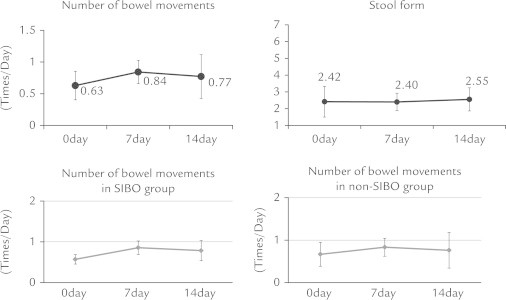
Daikenchuto did not affect bowel movement frequency or stool form in all patients, nor after dividing into the small intestinal bacteria overgrowth (SIBO) and non-SIBO groups.
Figure 5.
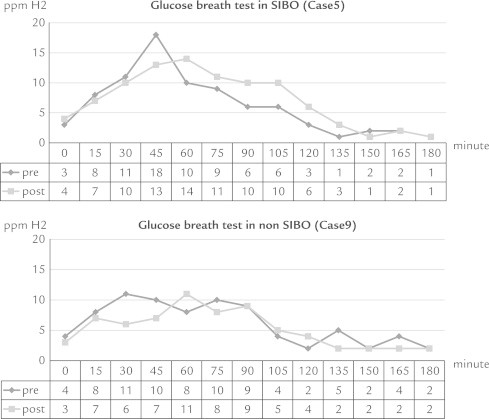
The glucose breath test results (in parts per million of hydrogen [ppm H2]) showed similar patterns before and after administration of daikenchuto in the small intestinal bacteria overgrowth (SIBO) group and non-SIBO groups.
Discussion
The purpose of our study was to evaluate the efficacy of DKT for abdominal symptoms in patients with chronic constipation. Functional constipation has a complex pathophysiology. Prior clinical studies found that DKT alleviated constipation in patients with Parkinson disease6 and was also effective for postoperative ileus.7 In other studies,[8], [9] DKT stimulated gastrointestinal motility in healthy subjects and decreased rectal compliance and sensation in patients with constipation.10
Although good findings have been reported, the mechanisms that generate clinical benefits related to DKT remain unidentified. A previous report noted the effects of DKT for reduction of bloating and abdominal pain in patients with chronic constipation who required the stimulant sennoside.4 Sennoside is frequently given for chronic constipation. However, protracted use of laxatives, including sennoside, increases the frequency of bowel movements and may impair patient QOL.
We enrolled patients with chronic constipation who were willing to discontinue the use of laxatives, including on-demand use, and evaluated the efficacy of DKT therapy without concomitant drugs. We found a noteworthy decrease in median total GSRS score and median GSRS indigestion and constipation subscales, along with improvements in bloating and abdominal pain symptoms. On the other hand, no significant changes were observed for bowel movement frequency or stool form. Furthermore, bloating was not improved with defecation. Bloating in individuals with chronic constipation can have various causes other than stool stagnation and may be affected by DKT.
Possible pathogenic factors related to abdominal bloating include overproduction of abdominal gas, hypersensitivity to bowel distention, and excessive distention of the colon due to constipation. According to a past report,11 DKT may directly stimulate colonic motility and reduce colonic gas volume in patients after stroke, whereas another suggested that a 5-day administration of DKT stimulates the small intestine and enhances ascending colon transit in healthy adults.12 Such effects have also been reported in dogs and guinea pigs.13
SIBO is a pathologic condition in which abdominal gas is produced in the upper small intestine, possibly causing unpleasant abdominal symptoms. As with abdominal bloating, effective treatments for SIBO have yet to be established. A recent study14 found that gut microbiome diversity was lacking in a rat model of inflammatory bowel disease, whereas DKT prevented bacterial translocation by suppression of cytokines and apoptosis in a fast-stress applied rat model.
In our study there was no increase in bowel movement frequency despite improvements in bloating and abdominal pain in both the SIBO and non-SIBO groups. Furthermore, the GBT results showed similar patterns before and after administration of DKT in the SIBO group and non-SIBO groups. These results suggest that a 2-week administration of DKT does not affect bacteria in the small intestine.
Possible factors that may have led to our findings are the small sample size and lack of comparison with a placebo. In addition, the data were analyzed based on patient symptom scores. Additional studies with longer follow-up periods and a placebo are required to evaluate the prolonged influence of DKT on small intestine bacteria.
Conclusions
DKT administration resulted in improved QOL in patients with and without SIBO who had chronic constipation. Although the present 2-week administration protocol seemed to have no effects on small intestine bacteria, further investigations are necessary to clarify the feasibility of DKT as treatment for abdominal bloating in patients with chronic constipation.
Conflicts of interest
The authors have indicated that they have no conflicts of interests regarding the content of this article.
Acknowledgments
The authors thank the staff and patients who participated in this study and Mark Benton, who provided language help. This study was supported by Second Department of Internal Medicine, Shimane University School of Medicine, which provided contributions to the consumables used. No funding source had involvement in the design, analysis, or writing of the manuscript. M. Yuki designed the research study, carried out the research, and drafted the manuscript. Y. Kinoshita designed the research study and wrote the manuscript. Y. Kamazawa performed the statistical analysis. M. Kusunoki, Y. Takahashi, and S. Nakashima collected the samples. I. Ikuma decided to submit the manuscript for publication. G. Uno performed the statistical analysis. T. Shizuku designed the research study and wrote the article. Y. Kobayashi conceived the study, provided coordination, and helped to draft the manuscript. All authors read and approved the final manuscript.
References
- 1.Sato Y., Katagiri F., Inoue S. Dai-kenchu-to raises levels of calcitonin gene-related peptide and substance P in human plasma. Bio Pharm Bull. 2004;27:1875–1877. doi: 10.1248/bpb.27.1875. [DOI] [PubMed] [Google Scholar]
- 2.Endo M., Hori M., Ozaki H. Daikenchuto, a traditional Japanese herbal medicine, ameliorates postoperative ileus by anti-inflammatory action through nicotinic acetylcholine receptors. J Gastroenterol. 2014;49:1026–1039. doi: 10.1007/s00535-013-0854-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hongo M., Fukuhara S., Green J. QOL (quality of life) in gastroenterological series; evaluation of QOL by Gastrointestinal Symptom Rating Scale (GSRS) [in Japanese] Diagnosis and Therapy. 1999;87:731–736. [Google Scholar]
- 4.Horiuchi A., Nakayama Y., Tanaka N. Effect of traditional Japanese medicine, Daikenchuto (TJ-100) in patients with chronic constipation. Gastroenterology Res. 2010;3:151–155. doi: 10.4021/gr219w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis S.J., Heaton K.W. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 6.Sakakibara R., Odaka T., Lui Z. Dietary herb extract dai-kenchu-to ameliorates constipation in parkinsonian patients (Parkinson’s disease and multiple system atrophy) Mov Disord. 2005;20:261–262. doi: 10.1002/mds.20352. [DOI] [PubMed] [Google Scholar]
- 7.Itoh T., Yamakawa J., Mai M. The effect of the herbal medicine dai-kenchu-to on post-operative ileus. J Int Med Res. 2002;30:428–432. doi: 10.1177/147323000203000410. [DOI] [PubMed] [Google Scholar]
- 8.Kawahara H., Yanaga K. The herbal medicine Dai-Kenchu-To directly stimulates colonic motility. Surg Today. 2009;39:175–177. doi: 10.1007/s00595-008-3810-y. [DOI] [PubMed] [Google Scholar]
- 9.Kawasaki N., Nakada K., Suzuki Y. Effect of Dai-kenchu-to on gastrointestinal motility and gastric emptying. Int J Surg. 2009;7:218–222. doi: 10.1016/j.ijsu.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Iturrino J., Camilleri M., Wong B.S. Randomised clinical trial: the effects of daikenchuto, TU-100, on gastrointestinal and colonic transit, anorectal and bowel function in female patients with functional constipation. Aliment Pharmacol Ther. 2013;37:776–785. doi: 10.1111/apt.12264. [DOI] [PubMed] [Google Scholar]
- 11.Numata T., Takayama S., Tobita M. Traditional Japanese medicine daikenchuto improves functional constipation in poststroke patients. Evid Based Complement Alternat Med. 2014;2014:231258. doi: 10.1155/2014/231258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manabe N., Camilleri M., Rao A. Effect of daikenchuto (TU-100) on gastrointestinal and colonic transit in humans. Am J Physiol Gastrointest Liver Physiol. 2010;298:G970–G975. doi: 10.1152/ajpgi.00043.2010. [DOI] [PubMed] [Google Scholar]
- 13.Satoh K., Hashimoto K., Hayakawa T. Mechanism of atropine-resistant contraction induced by Dai-kenchu-to in guinea pig ileum. Jpn J Pharmacol. 2001;86:32–37. doi: 10.1254/jjp.86.32. [DOI] [PubMed] [Google Scholar]
- 14.Yoshikawa K., Shimada M., Kuwahara T. Effect of Kampo medicine “Dai-kenchu-to” on microbiome in the intestine of the rats with fast stress. J Med Invest. 2013;60:221–227. doi: 10.2152/jmi.60.221. [DOI] [PubMed] [Google Scholar]


