Abstract
Background
We hypothesized intrauterine growth restricted offspring (IUGR) demonstrate higher rates of aggression and higher dominance ranks than control (CTR) offspring with normal weight at term; if aggressive behavior is advantageous during resource scarcity, developmental programming may lead to an association between aggression and IUGR.
Methods
We studied 22 group-housed baboons (ages 3-5 years). CTR (male n=8, female n=5) mothers ate ad libitum. IUGR (male n=4, female n=5) mothers were fed 70% feed eaten by CTR mothers during pregnancy and lactation.
Results
IUGR showed higher rates of aggressive displays (p<0.01) and friendly displays (p<0.02). Dominance ranks and physical aggression rates did not differ between groups.
Conclusions
High rates of IUGR aggressive display might reflect developmental programming of behavioral phenotypes enhancing fitness. Friendly displays may reflect reconciliation. Potential mechanisms include neurodevelopment and learning. Exploration of IUGR as a risk factor for behavioral patterns is important for developing diagnostic and therapeutic strategies.
Keywords: Developmental programming, social behavior, Papio, nutrient restriction, maternal nutrition, behavioral phenotype, nonhuman primate, dominance
Introduction
Intrauterine growth restriction (IUGR) is a persistent problem for humans, and is associated with increased fetal morbidity and mortality [86, 104]. Poor maternal nutrition during pregnancy and lactation, which can lead to IUGR, has been linked to increased risk of mood disorders, impaired cognition, and schizophrenia in offspring [43, 100, 110]. Thus, it is important to explore the relationship between intrauterine experience and later behavioral and cognitive health.
Translational studies with baboons and other nonhuman primates can determine the origins and outcomes of IUGR. We have developed a baboon (Papio hamadryas) model of moderate maternal nutrient restriction during pregnancy and lactation that produces IUGR offspring [8, 21, 26, 58-61, 67, 70, 71, 88, 91]. In this model, throughout pregnancy and lactation, the experimental group of mothers is provided with 70% of the global feed eaten by control (CTR) mothers fed ad libitum. Offspring are IUGR at birth, weighing significantly less than CTR [59]. All definitions of IUGR incorporate birthweight [86]; however, growth restriction likely continues after birth into the period of nursing in our experimental model as well as the human situation, since poor maternal diet is known to reduce milk output, with implications for offspring growth [87].
Developmental programming can be defined as “a response to a specific challenge to the mammalian organism during a critical developmental time window that alters the trajectory of development with persistent effects on offspring phenotype and predisposition to future disease” [80:3]. Several studies have shown that poor maternal nutrition leads to developmental programming of physiological coping mechanisms by altering the trajectory of development of metabolic regulatory systems. These outcomes have been considered adaptive in some cases, but can also be maladaptive. When beneficial, they have been considered to produce a phenotype better fitted to an environment of decreased later-life nutrient availability [9, 16, 37, 38, 62, 83, 108]. Behavioral components of developmental programming have been little explored. Aggressive behavior, especially in the context of feeding competition, may be advantageous in environments in which food is scarce. In other words, in times of resource scarcity, it may be adaptive to engage in aggressive behavior that potentially alleviates nutritional stress [31, 105]. Evidence from humans and animals subjected to undernutrition in childhood and adulthood supports the notion that dietary restriction may lead to increased aggression [reviewed by 105]. In baboons, aggressive behavior often occurs in the context of the dominance hierarchy; baboons exhibit linear dominance hierarchies in both wild and captive populations [3, 14, 19, 47]. Female offspring inherit ranks very close to those of their mothers, while male rank is more flexible, depending more on individual characteristics [3, 47]. Since aggressive behavior and dominance rank may be linked, we evaluated both in our IUGR and CTR baboons.
We previously demonstrated that male IUGR offspring demonstrate an altered behavioral phenotype characterized by increased impulsivity at 3 years of age [88]. In this study, we evaluated aggressive, affiliative, and rank-related behaviors in socially-housed control and IUGR offspring. Our first hypothesis was that IUGR baboons demonstrate higher rates of aggressive behavior compared to controls. Our second hypothesis was that IUGR baboons were higher ranked than controls.
Materials and Methods
Humane Care Guidelines
All procedures were approved by the Texas Biomedical Research Institute Institutional Animal Care and Use Committee and conducted in AAALAC approved facilities [92].
Sample
Behavioral observations were conducted on 22 age-matched baboons (ages 3-5 yrs, human equivalent 12-20 yrs) group-housed at the Southwest National Primate Research Center (SNPRC). IUGR (male n = 4, female n = 5) were offspring of mothers consuming 70% of the same feed (Purina Monkey Diet 5038, Purina, St Louis, MO, USA) eaten by CTR mothers in pregnancy and lactation, on a weight-adjusted basis [for feeding and housing protocol, see 58, 92]. CTR (male n = 8, female n = 5) were offspring of mothers fed ad libitum. Offspring were fed diet 5038 ad libitum after weaning at nine months of age. We had unequal numbers of subjects in the groups for several reasons. First, since females were assigned to ad libitum or moderately reduced dietary levels from shortly after the beginning of pregnancy, before sex of fetuses was known, we were unable to control the number of males and females born in each diet treatment group. Second, we used the maximum number of individuals available for each group to improve the ability of each group's mean behavior rates to reflect the population as a whole. Third, several individuals were dropped from the study early on, due to conflicting study protocol and housing constraints.
Behavioral Data Collection
Behavioral data were collected by a single observer (HFH) using The Observer XT (version 10.1) system for a total of 162 hours (mean 7.29 hrs per individual) of observations. Behavioral sampling was conducted by continuous focal animal follows of 20 min duration [4]. A subset of behaviors was chosen from the extensive savannah baboon ethogram of Coelho and Bramblett [23]. We looked at three classes of behavior: aggressive display behavior, initiation of physical aggression, and affiliative display behavior. Both aggressive and non-aggressive behaviors were considered to achieve a more complete understanding of any behavioral patterns present. Other types of behavior examined included behaviors of dominance and submission, activity patterns, and play behavior. For detailed lists and definitions of these behaviors, see [23] and [44]. Aggressive and affiliative behaviors are defined in detail below, since these behaviors are the focus of this study.
Definitions of behavior classes (Table 1):
Table 1. Definitions of behaviors [23, 44].
| Behavior Class | Behavior | Definition |
|---|---|---|
| Aggressive Display Behavior | Aggressive grunt | A series of short, rapid grunts being performed by an animal who is seemingly hostile |
|
|
||
| Canine display | Facial expression in which an actor's mouth is fully open with teeth, and sometimes gums, exposed and directed toward another animal | |
|
|
||
| Eyelid flash | An actor lowers eyelids with exposure of white pigment just above the eyes towards the direction of a recipient | |
|
|
||
| Mantle shake | The rapid shaking of the head and shoulders, oscillating movement of the head and shoulders about the spinal axis | |
|
|
||
| Multi-aggressive display | General category for rapid agonistic performances, consisting of several agonistic behaviors occurring simultaneously | |
|
|
||
| Piloerection | Actor's hair is moved from a flat position to a raised position | |
|
|
||
| Initiation of Physical Aggression | Aggressive chase | Aggressive rapid forward movement by an actor towards a recipient who flees |
|
|
||
| Attack | An actor sets upon another individual aggressively, initiating a fight | |
|
|
||
| Bite | The clamping of the mouth on the body of a recipient followed by a pain or fear response from the recipient who is bitten | |
|
|
||
| Hit | A slap or cuff | |
|
|
||
| Friendly Display Behavior | Chatter (aka friendly grunt) | A series of rapid monosyllabic sounds, usually of low volume |
|
|
||
| Lipsmack | Rapid, repetitive opening and closing of lips; may also include rapid, repetitive opening and closing of lips on a flattened and projecting tongue | |
Aggressive Display Behavior (ADB)
An actor directs a species-typical ritualized threat at a recipient, with no physical contact between actor and recipient. The following species-typical threats were coded as ADB: canine display, eyelid flash, piloerection, mantle shake, aggressive grunt, and multi-aggressive display.
Initiation of Physical Aggression (IPA)
An actor directs physical aggression toward a recipient. The following behaviors were coded as IPA: aggressive chase, attack, bite, and hit.
Friendly Display Behavior (FDB)
An actor directs a species-typical affiliative display toward a recipient. The following behaviors were coded as FDB: chatter and lipsmack.
Determination of Dominance Rank
Dominance rank was assessed using David's Score (DS), which is considered a highly reliable method [27, 35]. Readers are referred to Gammell et al. [35] for a clear description of methods used to calculate DS. Briefly, for each dyadic interaction in which a dominance-related behavior is observed, a “winner” and “loser” are identified. DS is calculated based on the proportions of wins and losses of each individual, weighted by the interactions of other individuals. The DS scores of the individuals are used to place them in rank order, with the highest DS score as the top rank. Rank category (high, medium, low) was assigned by graphing the DS values and determining visual tertiles.
Data for determining rank order were collected at the same time as data used to calculate behavioral frequencies, as described above. Each time a dominance-related behavior was observed, the identity and roles of the interaction partners were recorded. “Win” behaviors were displace, mount, receive present, aggressive chase, hit/slap, bite, and attack. “Loss” behaviors were to be displaced, receive mount, present, flee from aggressor, and be attacked. Mounting and presenting were not counted as dominance-related behaviors among the males because they often occurred during play and were not always in the same direction as agonistic encounters [73].
For the males, there were many individuals not included in this study living in the social groups under observation. All of these males were considered in rank calculations since DS is dependent on interactions of all individuals in a social group. Non-study males were not observed systematically; we merely recorded their roles in interactions with focal animals. Interaction matrices produced for calculating DS in this study are available in [44].
Body Weight
Body weights (kg) from eight points in the baboons' lives were analyzed: birthweight, weight at 3 months of age, weight at 6 months of age, weight at 9 months of age, weight at 1 year of age, weight at 2 years of age, weight at 3 years of age, and weight at 4 years of age. Three male CTR baboons were omitted from body weight analyses because body weight data from the appropriate time points were not available.
Statistical Analyses
For every baboon, The Observer XT calculated rates at which different behaviors were performed. Rate was defined as the average number of times a behavior was performed during a 20-minute observation period. Rates of behaviors from the same class were summed to calculate the behavior class rate.
Statistical analyses were performed in SPSS Version 21.0. We used 2-way ANOVA to test for differences in rates of behavior classes between groups, using sex and offspring growth treatment (IUGR, CTR) as independent variables and behavior class rates as dependent variables.
Differences between IUGR and CTR baboons in dominance rank were calculated using two-tailed Student's t-tests. Sexes were analyzed separately for rank since they lived in separate enclosures and because wild baboon males and females exhibit different rank structures [3, 47, 95].
We tested for differences in body weight (kg) between IUGR and CTR offspring with two-tailed Student's t-tests. Male and female body weights were considered separately because baboons are sexually dimorphic in body mass [47]; preliminary analyses showed that males in our sample weighed significantly more than females by one year of age.
Linear regression was used to examine the relationship between the continuous variables weight and DS, with weight at various ages as independent variables and DS as the dependent variable.
Data are provided as mean (x̄) ± standard deviation. Significance was set at p < 0.05.
Student's t-test showed there was no difference in age between IUGR (x̄ = 3.766 ± 0.5130) and CTR (x̄ = 3.588 ± 0.6889), so age is not considered in the following analysis.
Results
Aggressive Display Behavior (ADB)
There was a main effect of IUGR on ADB (F(1,18) = 9.599, MSE = 0.657, p < 0.01, PES = 0.348, OP = 0.834) (Fig. 1). There was also a main effect of sex (F(1,18) = 5.814, p < 0.03, PES = 0.244, OP = 0.626). The interaction of sex and IUGR was significant as well (F(1,18) = 5.613, p < 0.03, PES = 0.238, OP = 0.611). Male CTR (x̄ = 1.125 ± 0.5451) and female CTR (x̄ = 1.110 ± 0.4891) performed ADB at similar rates, but rates were higher in female IUGR (x̄ = 1.370 ± 0.9985) and nearly three times higher in male IUGR (x̄ = 3.075 ± 1.2639). Thus, both male and female IUGR individuals show higher frequency of ADB when compared to CTR, but the difference is more pronounced in the males. Because frequency of ADB was so high in IUGR males, overall rates of aggressive display are higher in males than they are in females.
Figure 1.
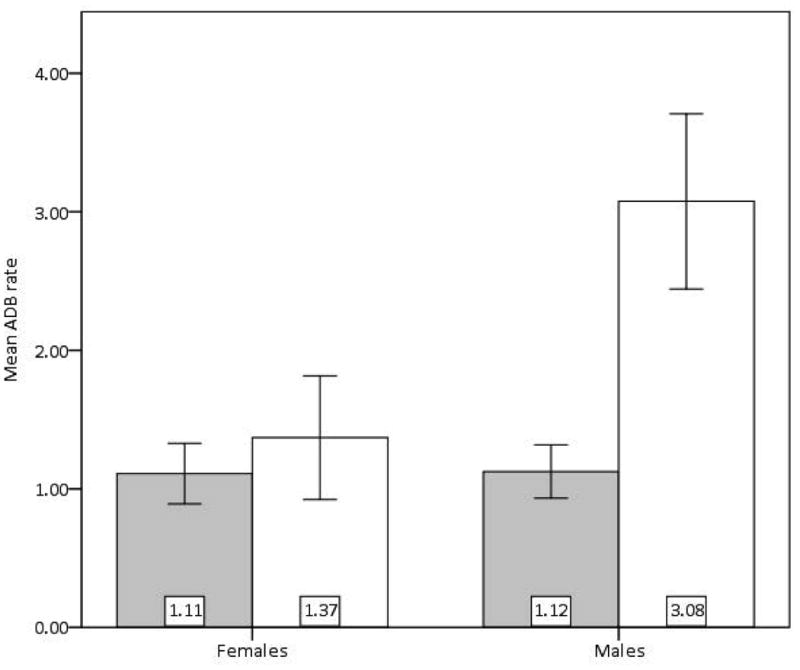
IUGR (open bars; female n = 5, male n = 4) demonstrate higher mean rate per 20-min observation of Aggressive Display Behavior (ADB) than do CTR (shaded bars; female n = 5, male n = 8). Error Bars: +/- 1 SEM, F(1,18) = 9.599, p < 0.01.
Initiation of Physical Aggression (IPA)
There was no main effect of IUGR (p = 0.32) or sex (p = 0.64) on IPA, nor was the interaction of sex and IUGR significant (p = 0.94). Male CTR (x̄ = 0.775 ± 0.5898) and female CTR (x̄ = 0.670 ± 0.7589) performed IPA at rates similar to male IUGR (x̄ = 0.525 ± 0.6185) and female IUGR (x̄ = 0.380 ± 0.3818).
Friendly Display Behavior (FDB)
There was a main effect of IUGR on FDB (F(1,18) = 6.941, MSE = 0.434, p < 0.02, PES = 0.278, OP = 0.703) (Fig. 2). The interaction of sex and IUGR was not significant (p = 0.18), and there was no main effect of sex (p = 0.29). IUGR males (x̄ = 1.700 ± 1.0304) and IUGR females (x̄ = 0.980 ± 0.7396) performed FDB at higher rates than did CTR males (x̄ = 0.531 ± 0.3515) or CTR females (x̄ = 0.620 ± 0.6291).
Figure 2.
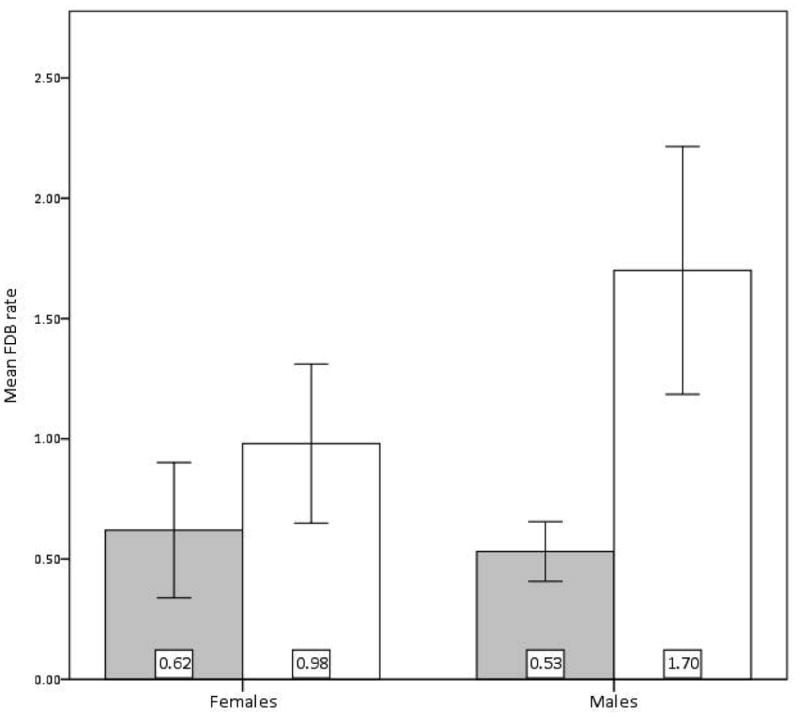
IUGR (open bars; female n = 5, male n = 4) demonstrate higher mean rate per 20-min observation of all Friendly Display Behavior (FDB) than do CTR (shaded bars; female n = 5, male n = 8). Error Bars: +/- 1 SEM, F(1,18) = 6.941, p < 0.02.
Offspring Dominance Rank
Females
All 10 females lived in the same cage. Therefore, data were placed in a single matrix of interactions and ranked 1-10 based on DS score (Table 2). One IUGR female was in the high rank category, at rank #2. Three IUGR females were in the medium rank category, and no IUGR females were in the low rank category. Two CTR females were in the high rank category (ranks #1 & #3). One CTR female was in the medium rank category and two CTR females were in the low rank category. The results of t-tests indicated that neither DS (CTR x̄ = 1.86 ± 32.78, IUGR x̄ = -2.46 ± 24.53, t(8) = 0.236, p = 0.819) nor rank category (CTR x̄ = 2.0, IUGR x̄ = 2.0, t(8) = 0.00, p = 1.00) differed between IUGR and CTR.
Table 2. Dominance rank and David's Score (DS) by social group.
| Social group | Rank | DS | Rank category | Trmt |
|---|---|---|---|---|
| Females | 1 | 44.35 | High | CTR |
|
|
||||
| 2 | 34.35 | High | IUGR | |
|
|
||||
| 3 | 22.55 | High | CTR | |
|
|
||||
| 4 | 8 | Med | IUGR | |
|
|
||||
| 5 | 0.63 | Med | CTR | |
|
|
||||
| 6 | -11.81 | Med | IUGR | |
|
|
||||
| 7 | -13.14 | Med | IUGR | |
|
|
||||
| 8 | -20.66 | Low | CTR | |
|
|
||||
| 9 | -29.69 | Low | IUGR | |
|
|
||||
| 10 | -37.57 | Low | CTR | |
|
|
||||
| Male group 1 | 1 | 33.86 | High | CTR |
|
|
||||
| 2 | 24.34 | High | CTR | |
|
|
||||
| 3 | 8.4 | High | IUGR | |
|
|
||||
| 14 | -6.4 | Low | CTR | |
|
|
||||
| Male group 2 | 1 | 30.75 | High | IUGR |
|
|
||||
| 2 | 26 | High | CTR | |
|
|
||||
| 4 | 9 | High | CTR | |
|
|
||||
| 14 | -17.75 | Low | CTR | |
|
|
||||
| Male group 3 | 1 | 37.8 | High | IUGR |
|
|
||||
| 2 | 25.8 | High | IUGR | |
|
|
||||
| 3 | 13.3 | High | CTR | |
|
|
||||
| 9 | -13.05 | Low | CTR | |
Males
Since the males lived in three different social groups, a separate matrix of interactions was constructed for each group. Ranks based on DS score were assigned within groups (Table 2).
In the first male group, the IUGR individual was rank #3, in the high rank category. Two CTR individuals outranked this IUGR individual. The third CTR individual was rank #14, in the low rank category.
In the second male group, the IUGR individual was the top ranked male. Two CTR individuals were ranked just below this male in the high rank category. The third CTR individual was rank #14, in the low rank category.
In the third male group, the two IUGR individuals were ranked #1 and #2. One CTR was also in the high rank category, with rank #3. The other CTR individual was rank #9, in the low rank category.
In conclusion, all IUGR males were in the high rank category. CTR males were in both high and low rank categories. All medium-ranked individuals were cagemates not part of the overall study (see Methods).
Rank category was not normally distributed and could not be adjusted with transformation, probably as a result of the presence of non-study males living with the study participants. DS score was normally distributed, but the difference between CTR (x̄ = 8.66 ± 19.27) and IUGR (x̄ = 25.69 ± 12.53) was not significant (t(10) = -1.587, p = 0.144).
Other Behavioral Differences
There was no main effect of IUGR on rates of play behaviors, rank-related behaviors, or any other types of behavior other than ADB and FDB. Mean durations of time spent in different activity states did not differ between groups either [44]. In other words, there are no indications in these data that IUGR engaged in overall higher rates of activity than did CTR.
Main effects of sex, but not IUGR, were found in numerous individual behaviors. Males engaged in higher rates (p < 0.05) of the following behaviors: grapple aggression, play chase, solitary locomotor play, manual manipulate self, manual manipulate peer, oral inspect peer, and nail bite. Females engaged in higher rates of the following behaviors: flee from aggressor, present, receive present, be displaced, eat, groom.
Body Weight (kg)
Body weights at various ages are presented in Table 3 (males) and Table 4 (females).
Table 3. Male body weight (kg) in IUGR versus CTR from birth to four years of age.
| Age | Trmt | Mean | Std Dev | Sig | % different |
|---|---|---|---|---|---|
| Birth | CTR | 0.92 | 0.11 | 0.23 | 12% |
|
|
|||||
| IUGR | 0.82 | 0.11 | |||
|
|
|||||
| 3 mos | CTR | 1.59 | 0.25 | 0.10 | 19% |
|
|
|||||
| IUGR | 1.31 | 0.16 | |||
|
|
|||||
| 6 mos | CTR | 2.32 | 0.17 | 0.01 | 27% |
|
|
|||||
| IUGR | 1.77 | 0.25 | |||
|
|
|||||
| 9 mos | CTR | 3.06 | 0.15 | 0.05 | 16% |
|
|
|||||
| IUGR | 2.61 | 0.40 | |||
|
|
|||||
| 1 yr | CTR | 4.27 | 0.40 | 0.06 | 19% |
|
|
|||||
| IUGR | 3.55 | 0.58 | |||
|
|
|||||
| 2 yrs | CTR | 7.36 | 0.93 | 0.17 | 12% |
|
|
|||||
| IUGR | 6.55 | 0.55 | |||
|
|
|||||
| 3 yrs | CTR | 10.31 | 1.39 | 0.33 | 8% |
|
|
|||||
| IUGR | 9.56 | 0.26 | |||
|
|
|||||
| 4 yrs | CTR | 15.92 | 2.71 | 0.24 | 13% |
|
|
|||||
| IUGR | 13.99 | 1.48 | |||
Table 4. Female body weight (kg) in IUGR versus CTR from birth to four years of age.
| Age | Trmt | Mean | Std Dev | Sig | % different |
|---|---|---|---|---|---|
| Birth | CTR | 0.87 | 0.12 | 0.30 | 12% |
|
|
|||||
| IUGR | 0.78 | 0.15 | |||
|
|
|||||
| 3 mos | CTR | 1.57 | 0.20 | 0.11 | 23% |
|
|
|||||
| IUGR | 1.25 | 0.34 | |||
|
|
|||||
| 6 mos | CTR | 2.24 | 0.28 | 0.31 | 14% |
|
|
|||||
| IUGR | 1.94 | 0.55 | |||
|
|
|||||
| 9 mos | CTR | 2.79 | 0.42 | 0.11 | 22% |
|
|
|||||
| IUGR | 2.34 | 0.38 | |||
|
|
|||||
| 1 yr | CTR | 3.62 | 0.36 | 0.35 | 6% |
|
|
|||||
| IUGR | 3.41 | 0.28 | |||
|
|
|||||
| 2 yrs | CTR | 6.06 | 0.78 | 0.61 | 4% |
|
|
|||||
| IUGR | 6.28 | 0.56 | |||
|
|
|||||
| 3 yrs | CTR | 8.82 | 0.87 | 0.48 | 5% |
|
|
|||||
| IUGR | 9.29 | 0.98 | |||
|
|
|||||
| 4 yrs | CTR | 12.45 | 1.69 | 0.51 | 5% |
|
|
|||||
| IUGR | 13.13 | 1.40 | |||
For birthweight, there was no main effect of IUGR for males (p = 0.23) or females (p = 0.30). By three months of age, the magnitude of difference between IUGR and CTR in body weight increased, but there was still no main effect of IUGR in males (p = 0.10) or females (p = 0.11). At six months of age, there was a main effect of IUGR in males (p = 0.01), but not in females (p = 0.31). At nine months of age, there again was a main effect of IUGR in males (p = 0.05), but not in females (p = 0.11).
At one year of age, there was a near-significant main effect of IUGR among the males (p = 0.06), but not among the females (p = 0.35). There was no main effect of IUGR in either sex at ages two years (males p = 0.17; females p = 0.61), three years (males p = 0.33; females p = 0.48), or four years (males p = 0.24; females p = 0.51).
Although few significant differences were found between IUGR and CTR in body weight, note that mean body weight of CTR males was higher than that of IUGR males at every age, while in females, IUGR mean body weight was higher than CTR at years 2, 3, and 4.
Linear regression determined that body weight was not predictive of DS at any age, in either sex, with one exception: in females, birthweight was correlated with DS (F(1,8) = 7.166, R2 = 0.472, p = 0.03).
Discussion
Offspring Growth
In contemporary North America and Europe, 4-8% of infants are classified as low birthweight or small for gestational age [54, 86]. Many etiological factors contribute to low birthweight. Maternal factors associated with increased risk of producing offspring IUGR include nutritional deficiency, hypertensive disease, renal disease, pregnancy at high altitude, and use of tobacco, alcohol, and other drugs; fetal factors associated with increased IUGR risk include multiple gestations, placental abnormalities, infections, and chromosomal abnormalities [86]. Most of these correlations also involve poor fetal nutrition. One extensively studied example of low birthweight leading to adverse life-course health outcomes is the Dutch Hunger Winter (November 1944 to May 1945), when birthweight declined by about 10% [96, 98]; individuals born during this time are at increased risk of chronic diseases, such as type 2 diabetes [38, 83], obesity [84, 85], and cardiovascular disease [76, 89]. This reduction in birth weight is similar to the IUGR observed in offspring in the present study, who weighed 11-12% less at birth than controls [59]. The growth restriction continued after birth into the period of nursing, presumably because mothers on the nutrient reduced diet produced less milk [87]. For both males and females, the magnitude of difference in body weight increased over the first nine postnatal months, with IUGR weighing less than CTR, although the difference reached statistical significance only in the males (Tables 3 & 4). After nine months of age, the IUGR offspring began to catch up in size. This is the age at which young baboons are removed from their natal groups with their mothers to new age- and sex-matched social groups. Among the males, CTR weighed more on average than IUGR at every age. Among the females, CTR weighed more on average than IUGR only until two years of age; at ages 2, 3, and 4 years, IUGR females weighed more on average than CTR females, although the difference was small and not significant.
Hypothesis 1: Aggression
Our first hypothesis was that IUGR baboons engaged in more aggressive behavior than did CTR baboons. We found that IUGR baboons engaged in higher rates of ADB, when compared to CTR. The effect was more pronounced in males than females. IUGR males displayed aggressively three times as often as did CTR males. The partial eta squared (PES) value, which is a measure of effect size, indicated that 35% of the variance could be explained by IUGR alone. This incidence can be classified as a medium sized effect [24]. A medium effect size can be interpreted as a strong signal of the potentially profound effect of the intrauterine and lactational environment on individual behavioral development, particularly given the relatively moderate level of reduced nutrition experienced by the IUGR baboons during a limited, but important, developmental period of their lives.
IPA was similar between groups. The finding of an effect on ADB, but not IPA, is consistent with data from wild baboons indicating most aggressive interactions consist of ritualized threats (e.g., canine displays; Fig. 3) rather than contact aggression [17, 66, 97, 99]. Canine displays were the most frequently observed type of threat display. A canine display is a facial expression in which the baboon's mouth is fully open with teeth, and sometimes gums, exposed and directed toward another animal. It may be distinct from yawning because baboons do not always show their teeth or direct the yawn at another individual [HFH personal observation, 7, 57]. Two studies in nonhuman primates report higher rates of yawning, without distinguishing it from canine displaying, under conditions of crowding [11, 48], suggesting high rates of canine displaying may be related to increased levels of tension.
Figure 3.

Two male baboons display their not-yet-fully-developed canines. Canine displaying is a common type of baboon aggressive display. Photos by HF Huber.
IUGR offspring also engaged in higher rates of the behavior class FDB, which we speculate may be indicative of concomitantly higher rates of reconciliation. Primates, including baboons, are well known for engaging in peaceful post-conflict reunions, which are known as behaviors of reconciliation [94, 109]. However, we cannot analyze reconciliation patterns with the current data because we did not keep track of post-conflict proximity of individuals involved in agonistic encounters, nor did we record the identity of individuals to whom potentially reconciliatory gestures were directed. Wild olive baboons are equally likely to reconcile after low intensity aggression (e.g., threat displays) as they are to reconcile after high intensity aggression (e.g., chasing and biting) [18]. Lipsmack and chatter are both affiliative social actions. Baboons usually perform lipsmack as a friendly greeting, while chatter often occurs after the smoothing of a conflict [20, 25]. It is possible that IUGR individuals make up for bouts of aggressiveness with these affiliative gestures. They exhibit higher rates of threat displays in particular, and chatter and lipsmack represent an opposite type of display, with friendly instead of threatening intent [10, 18, 20, 28]. However, the effect size was small for the difference between IUGR and CTR in friendly displaying.
Hypothesis 2: Dominance Rank
Our second hypothesis was that IUGR baboons would achieve higher ranks than CTR baboons. We predicted this for both males and females, even though female rank is generally considered to be inherited and stable [3, 47, 95]. Since these baboons have not lived with their mothers for several years, instead living in age- and sex-matched groups, it is possible that female rank is flexible rather than stable. There are few data indicating whether captive female baboons who do not live within matrilines inherit and maintain their mothers' ranks [but see 81].
Our data provided no evidence to support the hypothesis that IUGR baboons were higher ranked than CTR. For females and males, rank category (high, medium, low) and DS were similar between IUGR and CTR. However, it is worth noting that not a single IUGR individual, male or female, was low ranking. Every single IUGR male was high ranking. All IUGR females were either high or medium ranking. CTR males were found in both the high and low rank categories; all medium-ranked males were non-study cage mates. CTR females were found in all three rank categories. When looked at this way, it appears that IUGR may attain high ranks, even though the correlation was not significant, potentially due to the small sample size.
Since no clear differences between IUGR and CTR were found in rank order, it is not surprising that IUGR and CTR baboons do not exhibit differences in rates of rank-related behaviors. There was no main effect of IUGR on any rank-related behavior.
Body weight had little effect on the rank order of the baboons. In the males, body weight did not affect DS at any age. Other studies examining the relationship between body weight and rank in male baboons report mixed findings, with one report finding no effect of weight on rank [53], and another finding the opposite [30]. In females, however, birthweight was predictive of DS. Since female baboons inherit their mothers' ranks [3, 47, 95], it is feasible that high rank mothers gave birth to heavier offspring [6], regardless of diet, and their daughters inherited their high rank. As the difference in weight between IUGR and CTR females increased in the months following birth, the effects of IUGR may have outweighed the effects of mother's rank, leading to there no longer being a correlation between rank and body weight after birth. This is speculative because we do not have data about mothers' ranks. None of the baboons had reached full adult size, which also may help explain why body weight was not an important predictor of rank.
Other Behavioral Findings
IUGR and CTR rates of play behaviors, rank-related behaviors, and activity patterns were similar. The differences found between males and females, irrespective of growth treatment, can mostly be explained based on species-typical sex-based differences in baboon behavior or the peculiarities of the housing situation [see 44 for further discussion]. The exception is the behavior nail bite. It is possible that nail biting is displacement behavior [103]. Males did this more often than did females. If nail biting is displacement behavior, rather than normal grooming behavior, it may be related to the high rate of threat displays in the males. Nail biting could be an anxiety response related to high levels of aggression, since displacement behavior is thought to increase in situations of uncertainty and/or anxiety, in both humans and nonhuman primates [103].
Growth, Aggression, and Rank in the Theoretical Contexts of Primate Behavior and Developmental Programming
Wild baboons are food limited and thought to experience high levels of feeding competition [6, 14]. In a species and habitat in which resources are contestable and monopolizable, competition and conflict are frequent and inevitable [3]. Because aggression is frequent in both sexes [40, 66], we predicted increased aggressive behavior in IUGR animals of both sexes. We found higher rates of aggression in both sexes, but the difference was more pronounced in the males.
Hypothetically, male baboons might benefit more than females by having an aggressive behavioral phenotype. Males usually leave their natal group around eight years of age and must establish themselves in new groups [2, 74, 78]. Female baboons usually remain with their natal group and live within matrilines; female rank is inherited from mothers and stable throughout life, while male rank is flexible [3, 47, 95]. Low ranking females have reduced access to resources when compared to high ranking females; high ranking mothers have higher reproductive success and their offspring grow more quickly [3, 5, 6, 14, 15, 19, 75].
Mothers of the IUGR cohort, who were under 30% dietary reduction during pregnancy and lactation, may be similar to low ranking females in the wild. Barton and Whiten [15] report for a wild population of olive baboons that low ranking females ate 30% less than high ranking females did, although the nutrient composition of the diet was the same. This is similar to the difference between the diets of the mothers of the IUGR and CTR baboons in our study. When a wild low ranking pregnant baboon gives birth to a daughter, no matter what her daughter's behavior, including levels of aggression, the daughter is destined to be low rank; this effect has been demonstrated in long-term studies of wild populations [reviewed by 47]. However, if the mother has a son, he is born low ranking, but has the opportunity to change his rank beginning in young adulthood. An aggressive behavioral phenotype may benefit him by improving his likelihood of establishing himself in a new group and moving up in the ranks, increasing his access to food and other resources. Even though male ranks were not different between IUGR and CTR, the limited analyses indicate a possible trend toward higher rank in IUGR individuals. This combined with the strong evidence for increased aggressive behavior supports the presence of an aggressive behavioral phenotype in IUGR baboons, particularly in males.
In regards to growth, male IUGR were at their lightest weight relative to CTR at six months of age, when CTR males were 27% heavier than IUGR. However, by four years of age, the magnitude of difference between IUGR and CTR males had decreased to 13%. In the females, the largest difference in weight occurred at three months of age, when CTR were 23% heavier than IUGR. By one year of age, the difference between IUGR and CTR females was just 6%. It seems that both male and female IUGR baboons experienced substantial “catch-up growth” [68], although female IUGR seem to have caught up more than did male IUGR. The catch-up of the IUGR baboons accelerated after weaning. Long-term data from wild yellow baboons of Amboseli show animals that had a slow start did not catch up in size later in life [3]. Alberts and Altmann [3] suggest wild baboons probably cannot obtain enough food for compensatory growth. The captive baboons in this study had high nutritional access after weaning, so may have had greater ability to catch up in growth than do wild baboons. This greater ability to catch up may derive from multi-generational nutritional effects. Wells [108] argues maternal capital is transmitted across generations, not just from one mother to her offspring. “Capital” encompasses all resources parents have at their disposal for spending on growth and development of offspring. A lineage that starts with high maternal capital can continue to have higher maternal capital than a lineage that starts with low capital over many generations [108]. Because female rank is inherited and stable, females in low ranking lineages may be from long lines of females with reduced nutritional access [3, 6, 19]. These females from low ranking lineages may be an example of transmission of low maternal capital across generations. The animals in this study were exposed to a single generation of nutrient restriction, which occurred only in mothers during pregnancy and lactation; these mothers were not from mothers who also had reduced nutrient access, so they are not from a low capital lineage. This may be one reason the IUGR offspring caught up in growth, when low ranking wild baboons do not.
Availability of capital is not the only means by which developmental programming is transmitted across generations. Epigenomic programming through DNA methylation and chromatin modification are also involved. Increased aggressive behavior is strongly associated with altered functioning of the serotonin (5-HT) system in a variety of taxa [reviewed by 93, 102]. Serotonergic functioning has been shown to be influenced by DNA methylation. For example, Wang et al. [106] found that adult human males with a history of childhood aggression had lower in vivo 5-HT synthesis in the orbitofrontal cortex, and key genes in the 5-HT pathway showed differential DNA methylation. Alasaari et al. [1] examined nurses in low and high stress work environments for differences in serotonin transporter gene (SLC6A4) promoter methylation, finding lower promoter methylation levels in nurses in the high stress environment. Aspects of maternal care are also important. Rat pups of mothers that performed pup licking and grooming (LG) and arched back nursing (ABN), which are species-typical behaviors, at either high or low rates show different stress responses; offspring of low-LG-ABN mothers grow up to be more fearful, with associated stable alterations of DNA methylation and chromatin structure [101, 107].
The content of mothers' milk also plays a role in behavioral development. Variation in the energy available in mothers' milk has been associated with different behavioral phenotypes [41, 42]. Hinde et al. [42] found that in young rhesus macaques (Macaca mulatta), greater exposure to cortisol in milk was associated with behavioral temperaments characterized by greater nervousness and less confidence. They speculate that young and poor condition mothers may be programming their offspring through cortisol signaling to prioritize growth over energetically expensive behaviors like play and exploration, to accommodate for the mothers' inability to produce as much milk and nutrient density, given their own competing need for energetic resources [42].
Findings from other studies of our baboon model of IUGR demonstrate that early nutritional experiences lead to differential epigenetic modification and neurodevelopment. Ye et al. [111] demonstrated disrupted serotonergic development in IUGR offspring, marked by reduced mRNA expression, reduced protein expression, and reduced serotonergic neuron numbers in the median and dorsal raphe. Antonow-Schlorke et al. [8] found delayed frontal cortex development in fetal brains of IUGR baboons, including impaired cell proliferation, increased proliferative cell death, impaired neuronal maturation, and suppressed myelinogenesis. These dramatic changes to neurodevelopment are likely to have long-term effects on brain function [8, 65].
The expected effects on mental function can be seen in reports of IUGR and CTR performance in cognitive assessments. Rodriguez et al. [88] found in IUGR baboons reduced motivation, increased impulsivity, and less accuracy in choosing correct stimuli. Keenan et al. [51] report that IUGR baboons show low arousal, poor attention and persistence, and difficulty modulating activity levels, a set of behaviors paralleled in human children with oppositional defiant disorder (ODD) and attention deficit hyperactivity disorder (ADHD), the most common psychopathologies in human children [56]. These behavioral and cognitive changes in IUGR compared to CTR baboons may be explained at least partly by epigenetic influences on neurodevelopment and serotonergic functioning [8, 111].
Physiological mechanisms implicated in behavioral changes in IUGR versus CTR baboons likely are intertwined with learning-based mechanisms. Baboons are behaviorally flexible with a high capacity for learning different social styles [13, 77, 90, 72]. Their behavioral development is influenced by their rearing experiences [12, 22]. It is possible that IUGR individuals learn increased aggression from their nutrient-reduced mothers. Numerous studies have reported increased aggressive behavior in individuals with low intake of certain nutrients [39, 45, 49, 50, 55, 82]. Even increasing dietary fiber has been shown to produce alterations to maternal behavior [29]. In the study described herein, mothers continued under nutrient restriction during lactation, when infants would have witnessed aggressive behaviors of their mothers. This learning could take place during the weaning process, which is thought to act as a blueprint for later social interactions [28].
The interweaving of physiology and experiential learning in behavioral development is supported by the work of Kinnally et al. [52], who recorded the amount of aggression infant rhesus macaques received from their mothers, and then tested for differences between infants who received different levels of aggression. They found infants who received more aggression from their mothers exhibited lower post-stressor 5-HT expression. Thus, the IUGR baboons may have picked up their pattern of increased aggression from their mothers during weaning.
In sum, neurodevelopment, serotonergic function, and growth may be programmed in response to maternal behavioral and physiological capital, producing variation in offspring behavioral phenotypes. Whether elevated rates of aggression in IUGR baboons reflects an aggressive behavioral phenotype that enhances survival and reproductive success, we do not yet know. IUGR individuals may experience improved access to resources by being predisposed to engage in aggressive competition. Alternatively, increased aggression could be a maladaptive developmental outcome associated with poor nutrition. For example, individuals in utero during the Dutch Hunger Winter are at increased risk of antisocial personality disorder, which is unlikely to be an adaptive condition [69]. Behavioral phenotypes can potentially be neutral or negative, resulting from conserved growth at the expense of compromised behavioral development. To test directly whether the observed behavioral pattern is adaptive, long-term data regarding survival rates, reproductive success, nutritional access, and rates of aggression and affiliation in a large sample of primates are necessary. Although this study was inspired by the notion that increased aggressive behavior has the potential to be adaptive under circumstances of resource scarcity, we do not have the data at this time to determine whether the observed behavioral pattern is adaptive, maladaptive, or neutral. The mere presence of a trait does not necessarily imply an adaptive purpose [36].
Conclusions
The IUGR baboons in this study, compared to CTR, engaged in more aggressive and affiliative behaviors. There are numerous reports of altered behavior and cognition of humans who experienced fetal growth restriction or poor nutrition early in life [e.g., 32-34, 46, 63, 64, 79]. This suggests that observations reported here in baboons are relevant to human behaviors subsequent to IUGR. Humans are even more behaviorally variable and flexible than are baboons, and hence it is possible that behavioral predispositions will be more difficult to reveal given the extensive range of environmental influences on human behavior. This high degree of flexibility and learning in human behavior is likely amenable to early life interventions to influence the direction of behavioral development. Thus, observations of behavioral patterns in nonhuman primates can be useful in defining the need for, and type of, interventional strategies in children.
Unraveling the dynamic relationship between maternal nutrition and offspring behavioral development is essential for understanding the spectrum of ways early experiences affect lifetime behavior. With more specific understanding of the effects of low nutritional intake on fetal development, mothers will better be able to assess the benefits of different nutritional strategies during pregnancy. Mapping the details of how IUGR can be a risk factor for behavioral patterns will lead to interventional strategies, which may come in the form of nutritional recommendations for pregnant mothers or behavioral therapies for at-risk children that lead to healthier individuals not just during growth and development, but throughout life.
Acknowledgments
We thank Karen Moore and Susan Jenkins for their help in manuscript preparation and data management.
Funding: This work was supported by funding from NIH/NICHD 2P01HD021350 (Mechanisms of Placental, Fetal Brain, and Renal Outcomes of IUGR), 5R24 RR021367 (Nutrient Restriction and Developmental Programming), and R21HD057480 (Effects of Prenatal Nutrition on Developmental Outcomes in the Juvenile Baboon).
Footnotes
Institution at Which Work Was Performed: This work was performed at the Southwest National Primate Research Center, San Antonio, TX, USA.
References
- 1.Alasaari JS, Lagus M, Ollila HM, Toivola A, Kivimäki M, Vahtera J, Kronholm E, Härmä M, Puttonen S, Paunio T. Environmental stress affects DNA methylation of a CpG rich promoter region of serotonin transporter gene in a nurse cohort. PLoS ONE. 2012;7(9):e45813. doi: 10.1371/journal.pone.0045813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alberts SC, Altmann J. Balancing costs and opportunities: dispersal in male baboons. Am Nat. 1995;145(2):279–306. [Google Scholar]
- 3.Alberts SC, Altmann J. The Amboseli Baboon Research Project: 40 years of continuity and change. In: Kappeler PM, Watts DP, editors. Long-Term Field Studies of Primates. Berlin: Springer Berlin Heidelberg; 2012. pp. 261–287. [Google Scholar]
- 4.Altmann J. Observational study of behavior: sampling methods. Behaviour. 1974;49(3/4):227–267. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- 5.Altmann J, Alberts SC. Intraspecific variability in fertility and offspring survival in a nonhuman primate: behavioral control of ecological and social sources. In: Wachter KW, Bulatao RA, editors. Offspring: Human Fertility Behavior in Biodemographic Perspective. Washington, D.C: National Academies Press; 2003. [PubMed] [Google Scholar]
- 6.Altmann J, Alberts SC. Growth rates in a wild primate population: ecological influences and maternal effects. Behav Ecol Sociobiol. 2005;57(5):490–501. [Google Scholar]
- 7.Altmann SA. The structure of primate social communication. In: Altmann SA, editor. Social Communication among Primates. Chicago, IL: University of Chicago Press; 1967. pp. 325–362. [Google Scholar]
- 8.Antonow-Schlorke I, Schwab M, Cox LA, Li C, Stuchlik K, Witte OW, Nathanielsz PW, McDonald TJ. Vulnerability of the fetal primate brain to moderate reduction in maternal global nutrient availability. PNAS. 2011;108(7):3011–3016. doi: 10.1073/pnas.1009838108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armitage JA, Khan IY, Taylor PD, Nathanielsz PW, Poston L. Developmental programming of the metabolic syndrome by maternal nutritional imbalance: How strong is the evidence from experimental models in mammals? J Physiol. 2004;561(2):355–377. doi: 10.1113/jphysiol.2004.072009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aureli F, Cords M, van Schaik CP. Conflict resolution following aggression in gregarious animals: A predictive framework. Anim Behav. 2002;64(3):325–343. [Google Scholar]
- 11.Aureli F, de Waal FBM. Inhibition of social behavior in chimpanzees under high-density conditions. Am J Primatol. 1997;41(3):213–228. doi: 10.1002/(SICI)1098-2345(1997)41:3<213::AID-AJP4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 12.Bardi M, Bode AE, Ramirez SM, Brent LY. Maternal care and development of stress responses in baboons. Am J Primatol. 2005;66(3):263–278. doi: 10.1002/ajp.20143. [DOI] [PubMed] [Google Scholar]
- 13.Barrett L, Gaynor D, Henzi SP. A dynamic interaction between aggression and grooming reciprocity among female chacma baboons. Anim Behav. 2002;63(6):1047–1053. [Google Scholar]
- 14.Barton RA. Sociospatial mechanisms of feeding competition in female olive baboons, Papio anubis. Anim Behav. 1993;46(4):791–802. [Google Scholar]
- 15.Barton RA, Whiten A. Feeding competition among female olive baboons, Papio anubis. Anim Behav. 1993;46(4):777–789. [Google Scholar]
- 16.Bateson P, Barker D, Clutton-Brock T, Deb D, D'Udine B, Foley RA, Gluckman P, Godfrey K, Kirkwood T, Lahr MM, McNamara J, Metcalfe NB, Monaghan P, Spencer HG, Sultan SE. Developmental plasticity and human health. Nature. 2004;430(6998):419–421. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- 17.Bernstein IS. Social Mechanisms in the Control of Primate Aggression. In: Campbell CJ, Fuentes A, MacKinnon KC, Panger M, Bearder SK, editors. Primates in Perspective. New York: Oxford University Press; 2007. pp. 562–571. [Google Scholar]
- 18.Castles DL, Whiten A. Post-conflict behaviour of wild olive baboons. I. Reconciliation, redirection and consolation. Ethology. 1998;104(2):126–147. [Google Scholar]
- 19.Cheney DL, Seyfarth RM, Fischer J, Beehner J, Bergman T, Johnson SE, Kitchen DM, Palombit RA, Rendall D, Silk JB. Factors affecting reproduction and mortality among baboons in the Okavango Delta. Botswana Int J Primatol. 2004;25(2):401–428. [Google Scholar]
- 20.Cheney DL, Seyfarth RM, Silk JB. The role of grunts in reconciling opponents and facilitating interactions among adult female baboons. Anim Behav. 1995;50(1):249–257. [Google Scholar]
- 21.Choi J, Li C, McDonald TJ, Comuzzie A, Mattern V, Nathanielsz PW. Emergence of insulin resistance in juvenile baboon offspring of mothers exposed to moderate maternal nutrient reduction. Am J Physiol Regul Integr Comp Physiol. 2011;301(3):R757–R762. doi: 10.1152/ajpregu.00051.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coelho AM, Bramblett CA. Effects of rearing on aggression and subordination in Papio monkeys. Am J Primatol. 1981;1(4):401–412. doi: 10.1002/ajp.1350010405. [DOI] [PubMed] [Google Scholar]
- 23.Coelho AM, Bramblett CA. Behaviour of the genus Papio: Ethogram, taxonomy, methods, and comparative measures. In: Seth PK, Seth S, editors. Perspectives in Primate Biology. New York: Today and Tomorrow's Printers and Publishers; 1989. [Google Scholar]
- 24.Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York, NY: Psychology Press; 1988. [Google Scholar]
- 25.Colmenares F. Greeting behaviour in male baboons, I: Communication, reciprocity and symmetry. Behaviour. 1990;113(1/2):81–116. [Google Scholar]
- 26.Cox LA, Nijland MJ, Gilbert JS, Schlabritz-Loutsevitch NE, Hubbard GB, McDonald TJ, Shade RE, Nathanielsz PW. Effect of 30 per cent maternal nutrient restriction from 0.16 to 0.5 gestation on fetal baboon kidney gene expression. J Physiol. 2006;572(1):67–85. doi: 10.1113/jphysiol.2006.106872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Vries H. On using the DomWorld model to evaluate dominance ranking methods. Behaviour. 2009;146(6):843–869. [Google Scholar]
- 28.de Waal FBM. The integration of dominance and social bonding in primates. Q Rev Biol. 1986;61(4):459–479. doi: 10.1086/415144. [DOI] [PubMed] [Google Scholar]
- 29.Fairbanks LA, Blau K, Jorgensen MJ. High-fiber diet promotes weight loss and affects maternal behavior in vervet monkeys. Am J Primatol. 2010;72(3):234–241. doi: 10.1002/ajp.20772. [DOI] [PubMed] [Google Scholar]
- 30.Fairbanks LA, Jorgensen MJ, Huff A, Blau K, Hung Y-Y, Mann JJ. Adolescent impulsivity predicts adult dominance attainment in male vervet monkeys. Am J Primatol. 2004;64(1):1–17. doi: 10.1002/ajp.20057. [DOI] [PubMed] [Google Scholar]
- 31.Fessler DMT. Pseudoparadoxical impulsivity in restrictive anorexia nervosa: A consequence of the logic of scarcity. Int J Eat Disord. 2002;31(4):376–388. doi: 10.1002/eat.10035. [DOI] [PubMed] [Google Scholar]
- 32.Freeman HE, Klein RE, Townsend JW, Lechtig A. Nutrition and cognitive development among rural Guatemalan children. Am J Public Health. 1980;70(12):1277–1285. doi: 10.2105/ajph.70.12.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galler JR, Barrett LR. Children and famine: long-term impact on development. Ambulatory Child Health. 2001;7(2):85–95. [Google Scholar]
- 34.Galler JR, Ramsey F. A follow-up study of the influence of early malnutrition on development: Behavior at home and at school. J Am Acad Child Adolesc Psychiatry. 1989;28(2):254–261. doi: 10.1097/00004583-198903000-00018. [DOI] [PubMed] [Google Scholar]
- 35.Gammell MP, de Vries H, Jennings DJ, Carlin CM, Hayden TJ. David's score: A more appropriate dominance ranking method than Clutton-Brock et al.'s index. Anim Behav. 2003;66(3):601–605. [Google Scholar]
- 36.Gould SJ, Lewontin RC. The spandrels of San Marco and the panglossian paradigm: a critique of the adaptationist programme. Proc R Soc Lond B. 1979;205(1161):581–598. doi: 10.1098/rspb.1979.0086. [DOI] [PubMed] [Google Scholar]
- 37.Hales C, Barker D. Type 2 (non-insulin-dependent) diabetes mellitus: The thrifty phenotype hypothesis. Diabetologia. 1992;35(7):595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- 38.Hales C, Barker D. The thrifty phenotype hypothesis: Type 2 diabetes. Br Med Bull. 2001;60(1):5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 39.Hamazaki T, Hamazaki K. Fish oils and aggression or hostility. Prog Lipid Res. 2008;47(4):221–232. doi: 10.1016/j.plipres.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Hausfater G. Dominance and reproduction in Baboons (Papio cynocephalus) Contrib Primatol. 1975;7:1–150. [PubMed] [Google Scholar]
- 41.Hinde K, Capitanio JP. Lactational programming? mother's milk energy predicts infant behavior and temperament in rhesus macaques (Macaca mulatta) Am J Primatol. 2010;72(6):522–529. doi: 10.1002/ajp.20806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hinde K, Skibiel AL, Foster AB, Rosso LD, Mendoza SP, Capitanio JP. Cortisol in mother's milk across lactation reflects maternal life history and predicts infant temperament. Behav Ecol. 2014:aru186. doi: 10.1093/beheco/aru186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoek HW, Brown AS, Susser E. The Dutch Famine and schizophrenia spectrum disorders. Soc Psychiatry Psychiatr Epidemiol. 1998;33(8):373–379. doi: 10.1007/s001270050068. [DOI] [PubMed] [Google Scholar]
- 44.Huber HF. Aggressive Behavioral Phenotype in Intrauterine Growth Restricted (IUGR) Baboons Exposed to Moderate Nutrient Restriction Early in Development [dissertation] Carbondale (IL): Southern Illinois University; 2014. p. 190. [Google Scholar]
- 45.Imai K, Yoshimura S, Hashimoto K, Boorman GA. Effects of dietary restriction on age-associated pathological changes in fischer 344 rats. In: Fishbein L, editor. Biological Effects of Dietary Restriction. Berlin: Springer Berlin Heidelberg; 1991. pp. 87–98. [Google Scholar]
- 46.Jacka FN, Ystrom E, Brantsaeter AL, Karevold E, Roth C, Haugen M, Meltzer HM, Schjolberg S, Berk M. Maternal and early postnatal nutrition and mental health of offspring by age 5 years: A prospective cohort study. J Am Acad Child Adolesc Psychiatry. 2013;52(10):1038–1047. doi: 10.1016/j.jaac.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 47.Jolly CJ. Baboons, mandrills, and mangabeys: Afro-Papionin socioecology in a phylogenetic perspective. In: Campbell CJ, Fuentes A, MacKinnon KC, Panger M, Bearder SK, editors. Primates in Perspective. New York: Oxford University Press; 2007. pp. 240–251. [Google Scholar]
- 48.Judge PG, Griffaton NS, Coelho AM. Conflict management by hamadryas baboons (Papio hamadryas hamadryas) during crowding: A tension-reduction strategy. Am J Primatol. 2006;68(10):993–1006. doi: 10.1002/ajp.20290. [DOI] [PubMed] [Google Scholar]
- 49.Kaplan JR, Fontenot MB, Manuck SB, Muldoon MF. Influence of dietary lipids on agonistic and affiliative behavior in Macaca fascicularis. Am J Primatol. 1996;38(4):333–347. doi: 10.1002/(SICI)1098-2345(1996)38:4<333::AID-AJP4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 50.Kaplan J, Manuck S, Fontenot M, Muldoon M, Shively C, Mann J. The cholesterol-serotonin hypothesis: interrelationships among dietary lipids, central serotonergic activity, and social behavior in monkeys. In: Hillbrand M, Spitz R, editors. Lipids, Health, and Behavior. Washington, D.C: American Psychology Association; 1997. pp. 139–165. [Google Scholar]
- 51.Keenan K, Bartlett TQ, Nijland M, Rodriguez JS, Nathanielsz PW, Zürcher NR. Poor nutrition during pregnancy and lactation negatively affects neurodevelopment of the offspring: Evidence from a translational primate model. Am J Clin Nutr. 2013;98(2):396–402. doi: 10.3945/ajcn.112.040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kinnally EL, Tarara ER, Mason WA, Mendoza SP, Abel K, Lyons LA, Capitanio JP. Serotonin transporter expression is predicted by early life stress and is associated with disinhibited behavior in infant rhesus macaques. Genes Brain Behav. 2010;9(1):45–52. doi: 10.1111/j.1601-183X.2009.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kitchen DM, Seyfarth RM, Fischer J, Cheney DL. Loud calls as indicators of dominance in male baboons (Papio cynocephalus ursinus) Behav Ecol Sociobiol. 2003;53(6):374–384. [Google Scholar]
- 54.Kramer MS. Determinants of low birth weight: methodological assessment and meta-analysis. B World Health Organ. 1987;65(5):663. [PMC free article] [PubMed] [Google Scholar]
- 55.Lane MA, Baer DJ, Rumpler WV, Weindruch R, Ingram DK, Tilmont EM, Cutler RG, Roth GS. Calorie restriction lowers body temperature in rhesus monkeys, consistent with a postulated anti-aging mechanism in rodents. Proc Natl Acad Sci USA. 1996;93(9):4159–4164. doi: 10.1073/pnas.93.9.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lavigne JV, LeBailly SA, Hopkins J, Gouze KR, Binns HJ. The prevalence of ADHD, ODD, depression, and anxiety in a community sample of 4-year-olds. J Clin Child Adolesc Psychol. 2009;38(3):315–328. doi: 10.1080/15374410902851382. [DOI] [PubMed] [Google Scholar]
- 57.Leone A, Ferrari PF, Palagi E. Different yawns, different functions? Testing social hypotheses on spontaneous yawning in Theropithecus gelada. Sci Rep. 2014;4:4010. doi: 10.1038/srep04010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li C, Levitz M, Hubbard GB, Jenkins SL, Han V, Ferry RJ, Jr, McDonald TJ, Nathanielsz PW, Schlabritz-Loutsevitch NE. The IGF axis in baboon pregnancy: Placental and systemic responses to feeding 70% global ad libitum diet. Placenta. 2007;28(11–12):1200–1210. doi: 10.1016/j.placenta.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li C, McDonald TJ, Wu G, Nijland MJ, Nathanielsz PWA. Intrauterine growth restriction alters term fetal baboon hypothalamic appetitive peptide balance. J Endocrinol. 2013;217(3):275–282. doi: 10.1530/JOE-13-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li C, Ramahi E, Nijland MJ, Choi J, Myers DA, Nathanielsz PW, McDonald TJ. Up-regulation of the fetal baboon hypothalamo-pituitary-adrenal axis in intrauterine growth restriction: Coincidence with hypothalamic glucocorticoid receptor insensitivity and leptin receptor down-regulation. Endocrinology. 2013;154(7):2365–2373. doi: 10.1210/en.2012-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li C, Schlabritz-Loutsevitch NE, Hubbard GB, Han V, Nygard K, Cox LA, McDonald TJ, Nathanielsz PW. Effects of maternal global nutrient restriction on fetal baboon hepatic insulin-like growth factor system genes and gene products. Endocrinology. 2009;150(10):4634–4642. doi: 10.1210/en.2008-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lieberman LS. Dietary, evolutionary, and modernizing influences on the prevalence of type 2 diabetes. Annu Rev Nutr. 2003;23(1):345–377. doi: 10.1146/annurev.nutr.23.011702.073212. [DOI] [PubMed] [Google Scholar]
- 63.Liu J. Malnutrition at age 3 years and externalizing behavior problems at ages 8, 11, and 17 years. Am J Psychiatry. 2004;161(11):2005–2013. doi: 10.1176/appi.ajp.161.11.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Low JA, Handley-Derry MH, Burke SO, Peters RD, Pater EA, Killen HL, Derrick EJ. Association of intrauterine fetal growth retardation and learning deficits at age 9 to 11 years. Am J Obstet Gynecol. 1992;167(6):1499–1505. doi: 10.1016/0002-9378(92)91727-r. [DOI] [PubMed] [Google Scholar]
- 65.Machado CJ. Maternal influences on social and neural development in macaque monkeys. In: Clancy KBH, Hinde K, Rutherford JN, editors. Building Babies. New York: Springer; 2013. pp. 259–279. [Google Scholar]
- 66.Markham AC, Alberts SC, Altmann J. Intergroup conflict: ecological predictors of winning and consequences of defeat in a wild primate population. Animal Behaviour. 2012;84(2):399–403. doi: 10.1016/j.anbehav.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McDonald TJ, Wu G, Nijland MJ, Jenkins SL, Nathanielsz PW, Jansson T. Effect of 30 % nutrient restriction in the first half of gestation on maternal and fetal baboon serum amino acid concentrations. Br J Nutr. 2013;109(08):1382–1388. doi: 10.1017/S0007114512003261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Metcalfe NB, Monaghan P. Compensation for a bad start: grow now, pay later? Trends Ecol Evol. 2001;16(5):254–260. doi: 10.1016/s0169-5347(01)02124-3. [DOI] [PubMed] [Google Scholar]
- 69.Neugebauer R, Hoek H, Susser E. Prenatal exposure to wartime famine and development of antisocial personality disorder in early adulthood. JAMA. 1999;282(5):455–462. doi: 10.1001/jama.282.5.455. [DOI] [PubMed] [Google Scholar]
- 70.Nijland MJ, Mitsuya K, Li C, Ford S, McDonald TJ, Nathanielsz PW, Cox LA. Epigenetic modification of fetal baboon hepatic phosphoenolpyruvate carboxykinase following exposure to moderately reduced nutrient availability. J Physiol. 2010;588(8):1349–1359. doi: 10.1113/jphysiol.2009.184168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nijland MJ, Schlabritz-Loutsevitch NE, Hubbard GB, Nathanielsz PW, Cox LA. Non-human primate fetal kidney transcriptome analysis indicates mammalian target of rapamycin (mTOR) is a central nutrient-responsive pathway. J Physiol. 2007;579(3):643–656. doi: 10.1113/jphysiol.2006.122101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ossi-Lupo K. Skill learning for survival in nonhuman primates. In: Lancy DF, Bock J, Gaskins S, editors. The Anthropology of Learning in Childhood. Lanham, MD: Rowman Altamira; 2010. pp. 309–340. [Google Scholar]
- 73.Owens NW. The development of sociosexual behaviour in free-living baboons, Papio anubis. Behaviour. 1976;57(3/4):241–259. doi: 10.1016/0003-3472(75)90103-7. [DOI] [PubMed] [Google Scholar]
- 74.Packer C. Inter-troop transfer and inbreeding avoidance in Papio anubis. Anim Behav. 1979;27(1):1–36. doi: 10.1016/0003-3472(79)90127-1. [DOI] [PubMed] [Google Scholar]
- 75.Packer C, Collins D, Sindimwo A, Goodall J. Reproductive constraints on aggressive competition in female baboons. Obstet Gynecol Surv. 1995;50(6):449–452. doi: 10.1038/373060a0. [DOI] [PubMed] [Google Scholar]
- 76.Painter RC, Rooij SR, de, Bossuyt PM, Simmers TA, Osmond C, Barker DJ, Bleker OP, Roseboom TJ. Early onset of coronary artery disease after prenatal exposure to the Dutch famine. Am J Clin Nutr. 2006;84(2):322–327. doi: 10.1093/ajcn/84.1.322. [DOI] [PubMed] [Google Scholar]
- 77.Paterson JD. Ecologically differentiated patterns of aggressive and sexual behavior in two troops of Ugandan baboons, Papio anubis. Am J Phys Anthropol. 1973;38(2):641–647. doi: 10.1002/ajpa.1330380281. [DOI] [PubMed] [Google Scholar]
- 78.Phillips-Conroy JE, Jolly CJ. Male dispersal and philopatry in the Awash baboon hybrid zone. Primate Report. 2004;68:27–52. [Google Scholar]
- 79.Pollitt E, Saco-Pollitt C, Leibel RL, Viteri FE. Iron deficiency and behavioral development in infants and preschool children. Am J Clin Nutr. 1986;43(4):555–565. doi: 10.1093/ajcn/43.4.555. [DOI] [PubMed] [Google Scholar]
- 80.Rabadán-Diehl C, Nathanielsz P. From mice to men: Research models of developmental programming. J Dev Orig Health Dis. 2013;4(01):3–9. doi: 10.1017/S2040174412000487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ramirez SM, Brent L, Sosa E, Comuzzie A, Rogers J. Determinants of dominance rank in a large sample of captive female baboons. Am J Primatol. 66(S1):107. abstract #93. [Google Scholar]
- 82.Ramsey J, Colman R, Binkley N, Christensen J, Gresl T, Kemnitz J, Weindruch R. Dietary restriction and aging in rhesus monkeys: the University of Wisconsin study. Exp Gerontol. 2000;35(9–10):1131–1149. doi: 10.1016/s0531-5565(00)00166-2. [DOI] [PubMed] [Google Scholar]
- 83.Ravelli A, van der Meulen J, Michels R, Osmond C, Barker D, Hales C, Bleker O. Glucose tolerance in adults after prenatal exposure to famine. The Lancet. 1998;351(9097):173–177. doi: 10.1016/s0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- 84.Ravelli AC, van der Meulen J, Osmond C, Barker DJ, Bleker OP. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr. 1999;70(5):811–816. doi: 10.1093/ajcn/70.5.811. [DOI] [PubMed] [Google Scholar]
- 85.Ravelli G-P, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976;295(7):349–353. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- 86.Resnik R, Creasy RK. Intrauterine growth restriction. In: Creasy RK, Resnik R, Iams JD, Lockwood CJ, Moore TR, editors. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 6th. Philadelphia, PA: Saunders Elsevier; 2009. pp. 635–650. [Google Scholar]
- 87.Roberts SB, Cole TJ, Coward WA. Lactational performance in relation to energy intake in the baboon. Am J Clin Nutr. 1985;41(6):1270–1276. doi: 10.1093/ajcn/41.6.1270. [DOI] [PubMed] [Google Scholar]
- 88.Rodriguez JS, Bartlett TQ, Keenan KE, Nathanielsz PW, Nijland MJ. Sex-dependent cognitive performance in baboon offspring following maternal caloric restriction in pregnancy and lactation. Reprod Sci. 2012;19(5):493–504. doi: 10.1177/1933719111424439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roseboom TJ, van der Meulen JHP, Osmond C, Barker DJP, Ravelli ACJ, Schroeder-Tanka JM, van Montfrans GA, Michels RPJ, Bleker OP. Coronary heart disease after prenatal exposure to the Dutch famine, 1944–45. Heart. 2000;84(6):595–598. doi: 10.1136/heart.84.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sapolsky RM. Social cultures among nonhuman primates. Curr Anthropol. 2006;47(4):641–656. [Google Scholar]
- 91.Schlabritz-Loutsevitch NE, Dudley CJ, Gomez JJ, Heath Nevill C, Smith BK, Jenkins SL, McDonald TJ, Bartlett TQ, Nathanielsz PW, Nijland MJ. Metabolic adjustments to moderate maternal nutrient restriction. Br J Nutr. 2007;98(02):276–284. doi: 10.1017/S0007114507700727. [DOI] [PubMed] [Google Scholar]
- 92.Schlabritz-Loutsevitch NE, Howell K, Rice K, Glover EJ, Nevill CH, Jenkins SL, Cummins LB, Frost PA, McDonald TJ, Nathanielsz PW. Development of a system for individual feeding of baboons maintained in an outdoor group social environment. J Med Primatol. 2004;33(3):117–126. doi: 10.1111/j.1600-0684.2004.00067.x. [DOI] [PubMed] [Google Scholar]
- 93.Siegel A, Victoroff J. Understanding human aggression: new insights from neuroscience. Int J Law Psychiatry. 2009;32(4):209–215. doi: 10.1016/j.ijlp.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 94.Silk JB. The form and function of reconciliation in primates. Annu Rev Anthropol. 2002;31:21–44. [Google Scholar]
- 95.Silk JB, Altmann J, Alberts SC. Social relationships among adult female baboons (Papio cynocephalus) I. Variation in the strength of social bonds. Behav Ecol Sociobiol. 2006;61(2):183–195. [Google Scholar]
- 96.Smith CA. The effect of wartime starvation in Holland upon pregnancy and its product. Am J Obstet Gynecol. 1947;53(4):599–608. doi: 10.1016/0002-9378(47)90277-9. [DOI] [PubMed] [Google Scholar]
- 97.Smuts BB, Watanabe JM. Social relationships and ritualized greetings in adult male baboons (Papio cynocephalus anubis) International Journal of Primatology. 1990;11(2):147–172. [Google Scholar]
- 98.Stein Z, Susser M. The Dutch Famine, 1944–1945 and the reproductive process. I. Effects on six indices at birth. Pediatr Res. 1975;9(2):70–76. doi: 10.1203/00006450-197502000-00003. [DOI] [PubMed] [Google Scholar]
- 99.Strum SC. Almost Human: A Journey Into the World of Baboons. Chicago: University of Chicago Press; 2001. [1987] [Google Scholar]
- 100.Susser E, Hoek HW, Brown A. Neurodevelopmental disorders after prenatal famine: The story of the Dutch Famine Study. Am J Epidemiol. 1998;147(3):213–216. doi: 10.1093/oxfordjournals.aje.a009439. [DOI] [PubMed] [Google Scholar]
- 101.Szyf M, McGowan P, Meaney MJ. The social environment and the epigenome. Environ Mol Mutagen. 2008;49(1):46–60. doi: 10.1002/em.20357. [DOI] [PubMed] [Google Scholar]
- 102.Takahashi A, Quadros IM, Almeida RMM, Miczek KA. Brain serotonin receptors and transporters: initiation vs. termination of escalated aggression. Psychopharmacology. 2011;213(2-3):183–212. doi: 10.1007/s00213-010-2000-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Troisi A. Displacement activities as a behavioral measure of stress in nonhuman primates and human subjects. Stress. 2002;5(1):47–54. doi: 10.1080/102538902900012378. [DOI] [PubMed] [Google Scholar]
- 104.Vandenbosche RC, Kirchner JT. Intrauterine growth retardation. Am Fam Physician. 1998;58(6):1384–1390. 1393–1394. [PubMed] [Google Scholar]
- 105.Vitousek KM, Manke FP, Gray JA, Vitousek MN. Caloric restriction for longevity: II—The systematic neglect of behavioural and psychological outcomes in animal research. Eur Eat Disord Rev. 2004;12(6):338–360. [Google Scholar]
- 106.Wang D, Szyf M, Benkelfat C, Provençal N, Turecki G, Caramaschi D, Côté SM, Vitaro F, Tremblay RE, Booij L. Peripheral SLC6A4 DNA methylation is associated with in vivo measures of human brain serotonin synthesis and childhood physical aggression. PLoS ONE. 2012;7(6):e39501. doi: 10.1371/journal.pone.0039501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Weaver ICG, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7(8):847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 108.Wells JCK. Maternal capital and the metabolic ghetto: An evolutionary perspective on the transgenerational basis of health inequalities. Am J Hum Biol. 2010;22(1):1–17. doi: 10.1002/ajhb.20994. [DOI] [PubMed] [Google Scholar]
- 109.Wittig RM, Crockford C, Wikberg E, Seyfarth RM, Cheney DL. Kin-mediated reconciliation substitutes for direct reconciliation in female baboons. Proc R Soc Lond B Biol Sci. 2007;274(1613):1109–1115. doi: 10.1098/rspb.2006.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xu M-Q, Sun W-S, Liu B-X, Feng G-Y, Yu L, Yang L, He G, Sham P, Susser E, Clair DS, He L. Prenatal malnutrition and adult schizophrenia: Further evidence from the 1959-1961 Chinese Famine. Schizophr Bull. 2009;35(3):568–576. doi: 10.1093/schbul/sbn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ye W, Xie L, Li C, Nathanielsz PW, Thompson BJ. Impaired development of fetal serotonergic neurons in intrauterine growth restricted baboons. J Med Primatol. 2014;43(4):284–287. doi: 10.1111/jmp.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]


