Abstract
More than 100 years after it was first invented, the 12-lead electrocardiogram (ECG) continues to occupy an important place in the diagnostic armamentarium of the practicing clinician. With the recognition of relatively rare but important clinical entities such as Wolff–Parkinson–White and the long QT syndrome, this clinical tool was firmly established as a test for assessing risk of sudden cardiac death (SCD). However, over the past two decades the role of the ECG in risk prediction for common forms of SCD, for example in patients with coronary artery disease, has been the focus of considerable investigation. Especially in light of the limitations of current risk stratification approaches, there is a renewed focus on this broadly available and relatively inexpensive test. Various abnormalities of depolarization and repolarization on the ECG have been linked to SCD risk; however, more focused work is needed before they can be deployed in the clinical arena. The present review summarizes the current knowledge on various ECG risk markers for prediction of SCD and discusses some future directions in this field.
Keywords: Sudden cardiac death, Electrocardiogram, Risk
Introduction
Recent analyses of global patterns show that heart disease continues to be the leading killer worldwide1 and sudden cardiac death (SCD) accounts for at least 50% of cardiovascular deaths.2 With continuing low rates of survival following sudden cardiac arrest,3 considerable efforts have been directed towards risk prediction. The left ventricular ejection fraction, which is presently used to identify candidates for the primary prevention implantable cardioverter-defibrillator (ICD), has significant limitations4,5 and there is a great need to identify relevant, practically useful markers which can improve SCD risk stratification. The 12-lead electrocardiogram (ECG) is a widely available, inexpensive, non-invasive tool, familiar to all physicians. As a time-tested modality reflecting the electrical activity of the heart, it seems intuitive that it may contribute significantly to identifying risk of SCD, which is primarily the result of electrical (rhythm) disturbances in the heart. This review addresses the role of the 12-lead ECG in SCD and attempts to lay out its relevance in clinical practice. We focused mainly on the resting ECG; other parameters such as heart rate (HR) variability, turbulence, and T wave alternans are beyond the scope of this review and have been dealt with elsewhere.6
Inherited arrhythmia syndromes
The inherited arrhythmia syndromes are relatively rare in the general population and often have a clear genetic basis in the form of mutations which affect cardiac ion channels11 or result in a cardiomyopathy (Table 1).8 The role of the ECG in these syndromes is quite distinct from the more common forms of SCD. The ECG often provides definitive clues and is the main avenue for establishing the diagnosis.8 For example, right bundle branch block with specific patterns of ST elevation identifies types of Brugada syndrome (Figure 1),8 while right precordial T wave inversion and epsilon wave may indicate arrhythmogenic right ventricular cardiomyopathy (ARVC).9 Furthermore, provocative testing with drugs can unmask typical appearances in the ECG. Sodium channel blocking agents such as procainamide can uncover a Brugada pattern12 and epinephrine challenge has been used to diagnose long QT syndrome (LQTS).13 The ECG can also help in prognostication; different morphologic patterns of QT prolongation, for instance, identify the type of LQTS, and the absolute value of the corrected QT interval (QTc) is linked to the risk of arrhythmia.14 The QTc is also a simple tool to monitor the risk for acquired, especially drug-induced LQTS which may at times unmask an otherwise clinically silent polymorphism in an LQTS gene.15 Thus, the ECG is often a key aid to the clinician in managing these syndromes.
Table 1.
Electrocardiographic abnormalities in the inherited arrhythmia syndromes
| Syndrome | ECG features |
|---|---|
| Long QT syndrome7 | QTc ≥ 500 ms in the absence of other causes LQT2—notched/bifid T wave LQT3—long isoelectric ST segment |
| Brugada syndrome8 | Right bundle branch block and ST elevation in right precordial leads Type I—coved ST elevation ≥2 mma Types II and III—saddleback pattern |
| Catecholaminergic polymorphic VT8 | Classic bidirectional VT during exercise |
| Short QT syndrome8 | QTc ≤ 330 ms or QTc < 360 ms with genetic mutation, aborted SCD or family history |
| Arrhythmogenic right ventricular dysplasia/cardiomyopathy9 | T wave inversion in right precordial leads Epsilon wave |
| Hypertrophic cardiomyopathy10 | ECG-LVH; ST-T wave abnormalities |
| Early repolarization syndrome8 | J-point elevation ≥1 mm in ≥2 contiguous inferior and/or lateral leads ‘Benign’ pattern: ascending ST segment ‘Malignant’ pattern: horizontal/descending ST segment |
VT, ventricular tachycardia; SCD, sudden cardiac death; LVH, left ventricular hypertrophy.
aProvocative testing with sodium channel blockers can unmask type I pattern in those with normal ECG or type II/III pattern at baseline.
Figure 1.
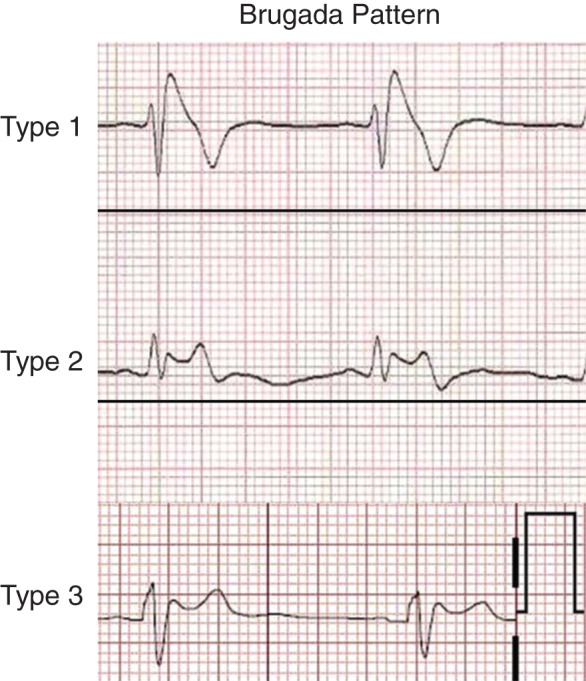
Electrocardiogram patterns in Brugada syndrome. Reproduced with permission from Ref. 71
Common forms of sudden cardiac death
Most SCD (≈80%) in the general population is associated with coronary artery disease (CAD),16 while a smaller proportion is due to other cardiomyopathies. In patients with CAD, the major mechanisms of SCD include acute ischaemia secondary to plaque rupture and also chronic scar-related re-entrant ventricular arrhythmias. This in turn may be influenced by genetic susceptibility and autonomic tone.17 Assessment of SCD risk is complex since this condition often represents the end result of multiple processes acting in concert.18 Various components of the surface ECG may potentially reflect different aspects of the SCD risk cascade (Table 2). Therefore, a sizeable body of research has focused on identification of potential markers from the ECG which may help to identify the individual at highest risk.
Table 2.
Electrocardiographic markers linked to SCD risk in the general population and potential pathophysiologic mechanisms
| ECG marker | Possible mechanism(s) |
|---|---|
| Resting heart rate | ?Increased sympathetic tone |
| Abnormalities of depolarization | |
| Pathologic Q waves/QRS score | Myocardial scar, LV dysfunction, re-entrant arrhythmia |
| QRS duration | Prolonged depolarization–?facilitation of re-entry |
| QRS fragmentation | Fibrosis, inhomogeneous myocardial conduction, re-entry |
| Increased myocardial voltage (ECG-LVH) | ?Increased LV mass ?Other abnormal electrical remodelling |
| Abnormalities of repolarization | |
| QTc/JTc | Prolonged repolarization |
| QT/JT dispersion | Heterogeneity of repolarization, ?Phase 2 re-entry |
| T-peak to T-end interval | Transmural dispersion of repolarization |
| Early repolarization pattern | ?Phase 2 re-entry |
| QRS–T angle | Abnormal depolarization, heterogeneity of repolarization |
SCD, sudden cardiac death; ECG, electrocardiogram; LV, left ventricular; LVH, left ventricular hypertrophy; QTc/JTc, corrected QT/JT interval.
Resting heart rate
A simple marker, a higher resting HR has been linked to SCD risk in several studies. In the Paris Prospective Study, subjects with an HR >75 b.p.m. had an almost four-fold risk of SCD compared with those with an HR <65.19 Higher HR predicts appropriate shocks, ventricular fibrillation (VF), and sustained ventricular tachycardia (VT) in patients with ICDs.20 Heart rate is closely tied to autonomic tone which influences the risk of arrhythmia.21 However, there has been some concern that HR may be a non-specific marker22; furthermore, it is influenced by several factors. Drugs are an especially important confounder and it is encouraging that data from the Oregon Sudden Unexpected Death Study (Oregon-SUDS) showed that the HR–SCD association persisted even after accounting for common HR modulating drugs.23 Similar in-depth investigations probing both specificity and mechanisms of the HR-SCD association are needed.
Abnormalities of depolarization
Various abnormalities related to the QRS complex, which reflects ventricular depolarization, can help to evaluate SCD risk in an individual. Q waves, representing loss of myocardium and possibly LV dysfunction and scar, have been associated with an increased risk.24 In an attempt to extend this concept and make it more quantitative, various QRS scores have been calculated and shown to correlate with ventricular arrhythmic events.25,26 However, both the aforementioned markers may simply convey the SCD risk conferred by CAD and potentially lack the ability to discriminate risk of SCD vs. heart failure death.27 Markers which can help to achieve finer resolution in risk assessment beyond CAD in terms of a direct mechanistic link to arrhythmia are clearly desirable.
A longer QRS duration (QRSD) has been shown to be associated with the risk of SCD irrespective of the presence of bundle branch block28 and even when controlled for the presence of CAD.29 A prospective population-based study in Finland showed a 27% increase in SCD risk for each 10 ms increase in the QRSD.30 In addition, dynamic prolongation of QRSD is known to occur in acute myocardial ischaemia31; whether this is mechanistically linked to the occurrence of VF in myocardial infarction needs further exploration. While prolonged QRSD has been well-studied as a risk factor for SCD, there are limited data on the relevance of specific QRS morphologies. A large population-based study reported that intraventricular conduction delay was associated with arrhythmic death, with a weaker association for left bundle branch block, while right bundle branch block was not predictive.32
Fragmentation of the QRS complex (fQRS), defined as the presence of various RSR′ patterns, has recently generated considerable interest as a potential risk marker. This ECG finding is thought to result from disrupted electrical conduction in areas of diseased or fibrosed myocardium33; thus, a pathophysiological correlation to re-entrant arrhythmias seems logical. Das et al.34 were the first to demonstrate that fQRS was better than Q waves in detecting myocardial scar. Subsequent studies have shown fQRS to be related to SCD as well as ICD shocks.35–37 These studies reported that fQRS in certain myocardial anatomic territories predicts risk, the mechanisms of which remain unclear. However, increasing evidence of a prognostic role for fQRS beyond the patient with CAD, such as in individuals with ARVC38 and cardiac amyloidosis,39 suggests that it has the potential to emerge as a useful risk marker for SCD risk.40
Another often overlooked risk factor in the context of SCD is the presence of left ventricular hypertrophy (LVH) on the ECG. While ECG-LVH has been shown to be associated with SCD risk,41 conventional thinking has been that it is simply an imperfect indicator of increased LV mass; thus being considered secondary in importance to the echocardiogram.42 However, a recent analysis among SCD cases showed very low overlap between ECG and echo-LVH, with more than half of those who had increased QRS voltage not manifesting echocardiographic LVH. Moreover, ECG-LVH was independently associated with SCD risk even after adjustment for echo-LVH.43 A subsequent study demonstrated similar findings using ECG vs. cardiac magnetic resonance diagnosed LVH with respect to the risk of developing new atrial fibrillation.44 These reports suggest that increased myocardial voltage may be reflecting adverse ‘electrical’ remodelling, conveying a risk that could be distinct from that conferred by anatomic LV hypertrophy.
Abnormalities of repolarization
Prolongation of the QT interval has long been associated with an increased risk of ventricular arrhythmias; although, this has been mostly in the context of the LQTSs or QT prolonging drugs. However, findings accumulated over the last decade suggest that this may also be an important risk factor for SCD in the community. Indeed, evidence from the Oregon-SUDS suggested that while diabetes and QT-affecting drugs contributed to SCD risk, idiopathic QT prolongation unrelated to either of these factors was the strongest predictor of SCD in CAD, conferring up to a five-fold increased risk.45 Other studies have reported the utility of the QT interval for risk prediction in CAD patients. Kinoshita et al.46 found a QTc of ≥450 ms in men and ≥470 ms in women to be an independent predictor of SCD in subjects undergoing bypass surgery. A recent meta-analysis incorporating 23 studies confirmed an increased risk of SCD when comparing subjects in the highest vs. lowest categories of QTc; thus relative variations even within the traditionally defined normal limits for the QTc may be relevant in terms of risk in the population.47 Since assessing the QT interval in patients with prolonged QRSD is generally not recommended, the JT interval may be a more useful measure in such patients48; this measurement is also more suited to understanding the independent contributions of abnormal depolarization and repolarization to SCD risk.29
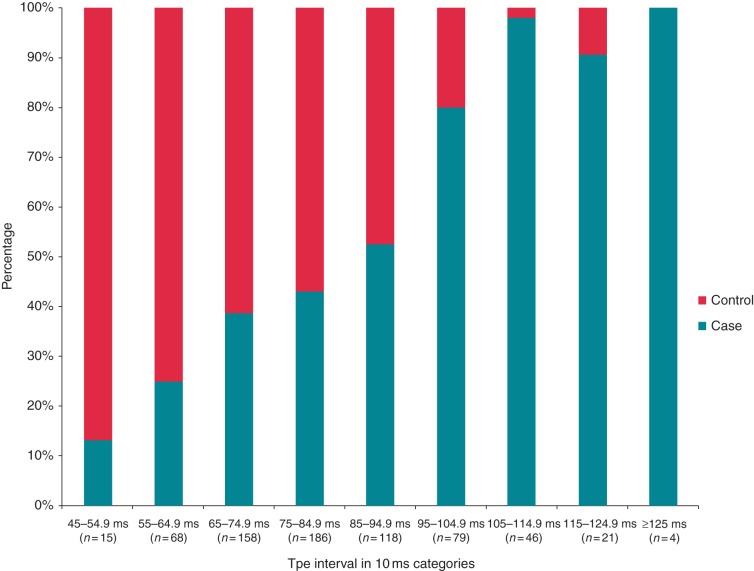
Another novel ECG marker that has emerged recently is the T-peak to T-end interval (Tpe). This has been thought to be a measurement of the transmural dispersion of repolarization, though this explanation has been disputed.49 A prolonged Tpe may reflect a period where the epicardium has fully repolarized, but the subendocardium is still recovering. This could create an electrical substrate for after depolarization driven re-entrant ventricular arrhythmias.50,51 A recent community-based study showed the Tpe to be significantly prolonged in cases of SCD compared with controls with CAD (Figure 2). Importantly, Tpe was more strongly associated with SCD in patients with prolonged (compared with normal) QRSD, where measurement of QTc is challenging. Additionally, it remained associated with SCD among subjects with normal QTc.52 Taken together; Tpe may be a useful complementary addition to the QTc in assessing abnormal repolarization. Studies have also shown the Tpe or the Tpe/QT ratio to be a predictor of arrhythmic events in inherited conditions such as Brugada syndrome53 and hypertrophic cardiomyopathy.54
Figure 2.
Distribution of Tpe among cases of SCD vs. control subjects. Reproduced with permission from Ref. 52. Tpe, T-peak to T-end interval; SCD, sudden cardiac death.
The early repolarization (ER) pattern or J point elevation, previously considered to be a benign finding, was reported to have a link with idiopathic VF by Haissaguerre et al.55 Subsequently, its broader association with more common forms of SCD was demonstrated in subjects with acute coronary syndromes (ACSs).56 It has been recognized that there are benign and malignant variations of ER based on the presence of ascending vs. horizontal or descending ST segments, respectively57; however, the mechanisms underlying their contribution to arrhythmia risk need to be better elucidated to understand the true potential of this finding in risk prediction.58
An interesting index involving parameters of both depolarization and repolarization is the QRS–T angle which can be thought of as the difference between the net directions of depolarization and repolarization in the heart. While the spatial QRS–T angle is a vectorcardiographic measure that needs additional computer-based analysis, the frontal QRS–T angle is more readily calculated as the difference between the QRS and T wave axis in the frontal plane. In large population-based studies, an increased QRS–T angle has been associated with an increased SCD risk59,60; however, whether it provides an incremental value beyond a simply abnormal T-wave axis is unclear.60
Using multiple markers: the concept of cumulative risk
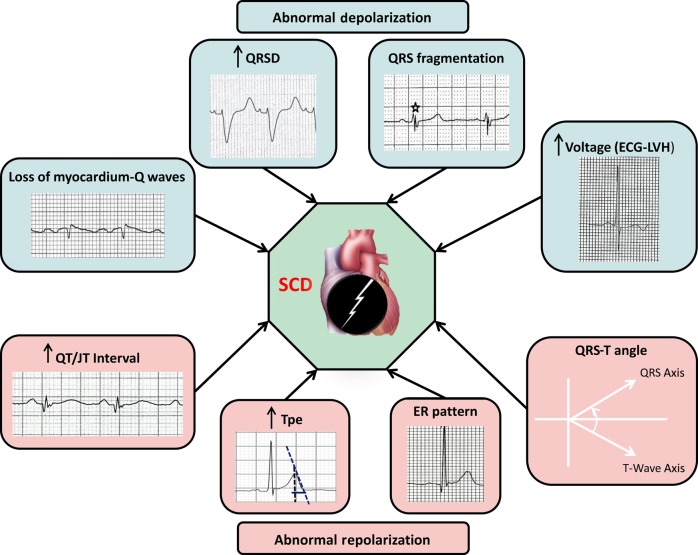
While several of the markers discussed so far are promising from a mechanistic/pathophysiologic viewpoint, it is unlikely that any single one of them would have adequate discriminative power to be of value to the clinician as a risk predictor.61 As SCD represents the convergence of multiple risk-inducing processes, it stands to reason that using multiple risk markers, reflecting different facets of the heart's electrical activity would convey more information than a single marker (Figure 3). Validation of this approach in large population-based studies would be needed to finally develop an ‘electrical’ risk score derived from the ECG. This could potentially be deployed even at the level of the primary care physician to trigger appropriate referral of an at-risk individual. Early proof-of-concept for a cumulative risk approach using a combination of variables has been seen in analyses from the defibrillator trials5,62 and hopefully this strategy can be extended to incorporate data from various simple diagnostic tools. The ECG likely represents only one arm of a multi-pronged approach to SCD risk prediction and future research needs to focus on combining information from the ECG with risk markers derived from other areas including clinical markers, genetics, biomarkers, and imaging.17 Especially in the context of more common forms of SCD such as among patients with significant CAD, parameters of diagnostic utility such as sensitivity, specificity, and predictive values for individual ECG markers are difficult to estimate and are therefore not reported in the existing literature.
Figure 3.
Using cumulative effect of multiple ECG risk markers for SCD risk assessment. SCD, sudden cardiac death.
Electrocardiogram in the field and ‘Dynamic Monitoring’
So far, the ECG as a risk tool has been only considered in the context of long-term risk prediction and prevention. One form of SCD not well addressed by this approach is the occurrence of VF during acute MI which often results in death prior to reaching the hospital. However, increasing performance of pre-hospital 12-lead ECGs in the field by emergency medical services programmes, especially for ACSs,63 has the potential to introduce a new dimension of ‘rapid’ analysis in the field with a view to possibly identifying the ACS individual at risk of impending VF. This can help to trigger more aggressive triage and intervention which may be life-saving. One study suggested that the total magnitude of ST elevation in all leads can predict VF in patients with ST elevation MI.64 Moreover, continuous monitoring in the field has the potential to help identify dynamic changes immediately preceding onset of VF which may give valuable insights into pathophysiology. Newer sources of data such as from long-term event monitors and implantable loop recorders may similarly yield dynamic information that was previously unobtainable. Dynamic and static monitoring are complementary approaches which can aid SCD risk assessment. The genesis of arrhythmia is said to involve interplay between a susceptible substrate and a trigger which actually results in an acute event. It can be postulated that while parameters from an ECG at one point in time can inform long-term risk of susceptibility to arrhythmia (‘static’ risk), dynamic monitoring in the acute setting, including ECG in the field at the time of an ACS and various forms of ambulatory ECG monitoring can help understand changes preceding arrhythmia65 which could potentially be surrogate markers for triggers of arrhythmia. Furthermore, variability in parameters may at times be more informative than a single value. For example, Holter monitoring in heart failure patients has shown that dynamic changes in HR and repolarization measures correlate with prognosis.66 Dedicated research in this area is needed to realize its full potential.
Conclusions and future directions
The 12-lead ECG continues to be a useful tool for identification of individuals at increased risk of SCD. At present, the utility of this modality is undisputed for making a diagnosis and assessing risk in patients with primary and inherited arrhythmia syndromes, e.g. Wolf–Parkinson–White and the long QT/Brugada syndromes; and even the acquired forms of drug-induced LQTS. Several ECG markers have also been associated with an increased risk of more common forms of SCD, for example that associated with CAD or diabetes, but these are not yet ready for prime time. As clinicians we would like to put this widely available and inexpensive tool to use in decision-making for who should receive a primary prevention defibrillator. It is likely that these ECG variables will not be ‘stand alone’ predictors of increased SCD risk but will rather be utilized to predict risk in a cumulative manner, even in combination with other risk stratification tools. Given the pitfalls of sole reliance on EF and burgeoning healthcare costs associated with ICDs,67 there is an urgent need to evolve alternate approaches which may involve data from real-world populations/registries to evaluate the additional value of various risk predictors beyond EF. Newer statistical approaches using reclassification and discrimination improvements are likely to be useful in assessing the added benefit conferred by a new marker to the existing risk model.68 The above limitations notwithstanding, the simplicity and ready availability of the ECG is likely to ensure an expanded role for this clinical tool in SCD risk prediction. Additionally, rapid advances in mobile and wireless technology,69 as well as development of remote monitoring systems for implantable devices70 promise a new era of ECG-based surveillance which could take SCD risk prediction to the next level.
Funding
This work was funded in part by National Heart, Lung, and Blood Institute grants R01HL105170 and R01HL122492 to S.S.C. S.S.C. holds the Pauline and Harold Price Chair in Cardiac Electrophysiology at the Heart Institute, Cedars-Sinai Medical Center, Los Angeles, CA.
Conflict of interest: none declared.
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zipes DP, Wellens HJ. Sudden cardiac death. Circulation 1998;98:2334–51. [DOI] [PubMed] [Google Scholar]
- 3.Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA 2008;300:1423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gehi A, Haas D, Fuster V. Primary prophylaxis with the implantable cardioverter-defibrillator: the need for improved risk stratification. JAMA 2005;294:958–60. [DOI] [PubMed] [Google Scholar]
- 5.Buxton AE, Lee KL, Hafley GE, Pires LA, Fisher JD, Gold MR, et al. Limitations of ejection fraction for prediction of sudden death risk in patients with coronary artery disease: lessons from the MUSTT study. J Am Coll Cardiol 2007;50:1150–7. [DOI] [PubMed] [Google Scholar]
- 6.Goldberger JJ, Cain ME, Hohnloser SH, Kadish AH, Knight BP, Lauer MS, et al. American Heart Association/American College of Cardiology Foundation/Heart Rhythm Society Scientific Statement on Noninvasive Risk Stratification Techniques for Identifying Patients at Risk for Sudden Cardiac Death. A scientific statement from the American Heart Association Council on Clinical Cardiology Committee on Electrocardiography and Arrhythmias and Council on Epidemiology and Prevention. J Am Coll Cardiol 2008;52:1179–99. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Timothy KW, Vincent GM, Lehmann MH, Fox J, Giuli LC, et al. Spectrum of ST-T-wave patterns and repolarization parameters in congenital long-QT syndrome: ECG findings identify genotypes. Circulation 2000;102:2849–55. [DOI] [PubMed] [Google Scholar]
- 8.Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, et al. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Europace 2013;15:1389–406. [DOI] [PubMed] [Google Scholar]
- 9.Quarta G, Ward D, Tome Esteban MT, Pantazis A, Elliott PM, Volpe M, et al. Dynamic electrocardiographic changes in patients with arrhythmogenic right ventricular cardiomyopathy. Heart 2010;96:516–22. [DOI] [PubMed] [Google Scholar]
- 10.Perez-Riera AR, de Lucca AA, Barbosa-Barros R, Yanowitz FG, de Cano SF, Cano MN, et al. Value of electro-vectorcardiogram in hypertrophic cardiomyopathy. Ann Noninvasive Electrocardiol 2013;18:311–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schimpf R, Veltmann C, Wolpert C, Borggrefe M. Arrhythmogenic hereditary syndromes: Brugada syndrome, long QT syndrome, short QT syndrome and CPVT. Minerva Cardioangiol 2010;58:623–36. [PubMed] [Google Scholar]
- 12.Shimizu W, Antzelevitch C, Suyama K, Kurita T, Taguchi A, Aihara N, et al. Effect of sodium channel blockers on ST segment, QRS duration, and corrected QT interval in patients with Brugada syndrome. J Cardiovasc Electrophysiol 2000;11:1320–9. [DOI] [PubMed] [Google Scholar]
- 13.Ackerman MJ, Khositseth A, Tester DJ, Hejlik JB, Shen WK, Porter CB. Epinephrine-induced QT interval prolongation: a gene-specific paradoxical response in congenital long QT syndrome. Mayo Clin Proc 2002;77:413–21. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz PJ. Practical issues in the management of the long QT syndrome: focus on diagnosis and therapy. Swiss Med Wkly 2013;143:w13843. [DOI] [PubMed] [Google Scholar]
- 15.Yang P, Kanki H, Drolet B, Yang T, Wei J, Viswanathan PC, et al. Allelic variants in long-QT disease genes in patients with drug-associated torsades de pointes. Circulation 2002;105:1943–8. [DOI] [PubMed] [Google Scholar]
- 16.Adabag AS, Peterson G, Apple FS, Titus J, King R, Luepker RV. Etiology of sudden death in the community: results of anatomical, metabolic, and genetic evaluation. Am Heart J 2010;159:33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chugh SS. Early identification of risk factors for sudden cardiac death. Nat Rev Cardiol 2010;7:318–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chugh SS, Reinier K, Teodorescu C, Evanado A, Kehr E, Al Samara M, et al. Epidemiology of sudden cardiac death: clinical and research implications. Prog Cardiovasc Dis 2008;51:213–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jouven X, Zureik M, Desnos M, Guerot C, Ducimetiere P. Resting heart rate as a predictive risk factor for sudden death in middle-aged men. Cardiovasc Res 2001;50:373–8. [DOI] [PubMed] [Google Scholar]
- 20.Cale R, Mendes M, Brito J, Sousa P, Carmo P, Almeida S, et al. Resting heart rate is a powerful predictor of arrhythmic events in patients with dilated cardiomyopathy and implantable cardioverter-defibrillator. Rev Port Cardiol 2011;30:199–212. [PubMed] [Google Scholar]
- 21.Hohnloser SH, Klingenheben T, van de Loo A, Hablawetz E, Just H, Schwartz PJ. Reflex versus tonic vagal activity as a prognostic parameter in patients with sustained ventricular tachycardia or ventricular fibrillation. Circulation 1994;89:1068–73. [DOI] [PubMed] [Google Scholar]
- 22.Jouven X, Escolano S, Celermajer D, Empana JP, Bingham A, Hermine O, et al. Heart rate and risk of cancer death in healthy men. PLoS ONE 2011;6:e21310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teodorescu C, Reinier K, Uy-Evanado A, Gunson K, Jui J, Chugh SS. Resting heart rate and risk of sudden cardiac death in the general population: influence of left ventricular systolic dysfunction and heart rate-modulating drugs. Heart Rhythm 2013;10:1153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith WC, Kenicer MB, Tunstall-Pedoe H, Clark EC, Crombie IK. Prevalence of coronary heart disease in Scotland: Scottish Heart Health Study. Br Heart J 1990;64:295–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strauss DG, Selvester RH, Lima JA, Arheden H, Miller JM, Gerstenblith G, et al. ECG quantification of myocardial scar in cardiomyopathy patients with or without conduction defects: correlation with cardiac magnetic resonance and arrhythmogenesis. Circ Arrhythm Electrophysiol 2008;1:327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strauss DG, Poole JE, Wagner GS, Selvester RH, Miller JM, Anderson J, et al. An ECG index of myocardial scar enhances prediction of defibrillator shocks: an analysis of the Sudden Cardiac Death in Heart Failure Trial. Heart Rhythm 2011;8:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loring Z, Zareba W, McNitt S, Strauss DG, Wagner GS, Daubert JP. ECG quantification of myocardial scar and risk stratification in MADIT-II. Ann Noninvasive Electrocardiol 2013;18:427–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morin DP, Oikarinen L, Viitasalo M, Toivonen L, Nieminen MS, Kjeldsen SE, et al. QRS duration predicts sudden cardiac death in hypertensive patients undergoing intensive medical therapy: the LIFE study. Eur Heart J 2009;30:2908–14. [DOI] [PubMed] [Google Scholar]
- 29.Teodorescu C, Reinier K, Uy-Evanado A, Navarro J, Mariani R, Gunson K, et al. Prolonged QRS duration on the resting ECG is associated with sudden death risk in coronary disease, independent of prolonged ventricular repolarization. Heart Rhythm 2011;8:1562–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurl S, Makikallio TH, Rautaharju P, Kiviniemi V, Laukkanen JA. Duration of QRS complex in resting electrocardiogram is a predictor of sudden cardiac death in men. Circulation 2012;125:2588–94. [DOI] [PubMed] [Google Scholar]
- 31.Wiegerinck RF, Galvez-Monton C, Jorge E, Martinez R, Ricart E, Cinca J. Changes in QRS duration and R-wave amplitude in electrocardiogram leads with ST segment elevation differentiate epicardial and transmural myocardial injury. Heart Rhythm 2010;7:1667–73. [DOI] [PubMed] [Google Scholar]
- 32.Aro AL, Anttonen O, Tikkanen JT, Junttila MJ, Kerola T, Rissanen HA, et al. Intraventricular conduction delay in a standard 12-lead electrocardiogram as a predictor of mortality in the general population. Circ Arrhythm Electrophysiol 2011;4:704–10. [DOI] [PubMed] [Google Scholar]
- 33.Das MK, Saha C, El Masry H, Peng J, Dandamudi G, Mahenthiran J, et al. Fragmented QRS on a 12-lead ECG: a predictor of mortality and cardiac events in patients with coronary artery disease. Heart Rhythm 2007;4:1385–92. [DOI] [PubMed] [Google Scholar]
- 34.Das MK, Khan B, Jacob S, Kumar A, Mahenthiran J. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation 2006;113:2495–501. [DOI] [PubMed] [Google Scholar]
- 35.Brenyo A, Pietrasik G, Barsheshet A, Huang DT, Polonsky B, McNitt S, et al. QRS fragmentation and the risk of sudden cardiac death in MADIT II. J Cardiovasc Electrophysiol 2012;23:1343–8. [DOI] [PubMed] [Google Scholar]
- 36.Terho HK, Tikkanen JT, Junttila JM, Anttonen O, Kentta TV, Aro AL, et al. Prevalence and prognostic significance of fragmented QRS complex in middle-aged subjects with and without clinical or electrocardiographic evidence of cardiac disease. Am J Cardiol 2014;114:141–7. [DOI] [PubMed] [Google Scholar]
- 37.Pei J, Li N, Gao Y, Wang Z, Li X, Zhang Y, et al. The J wave and fragmented QRS complexes in inferior leads associated with sudden cardiac death in patients with chronic heart failure. Europace 2012;14:1180–7. [DOI] [PubMed] [Google Scholar]
- 38.Canpolat U, Kabakci G, Aytemir K, Dural M, Sahiner L, Yorgun H, et al. Fragmented QRS complex predicts the arrhythmic events in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. J Cardiovasc Electrophysiol 2013;24:1260–6. [DOI] [PubMed] [Google Scholar]
- 39.Perlini S, Salinaro F, Cappelli F, Perfetto F, Bergesio F, Alogna A, et al. Prognostic value of fragmented QRS in cardiac AL amyloidosis. Int J Cardiol 2013;167:2156–61. [DOI] [PubMed] [Google Scholar]
- 40.Rosengarten JA, Scott PA, Morgan JM. Fragmented QRS for the prediction of sudden cardiac death: a meta-analysis. Europace 2015;17:969–77. [DOI] [PubMed] [Google Scholar]
- 41.Wachtell K, Okin PM, Olsen MH, Dahlof B, Devereux RB, Ibsen H, et al. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive therapy and reduction in sudden cardiac death: the LIFE Study. Circulation 2007;116:700–5. [DOI] [PubMed] [Google Scholar]
- 42.Devereux RB, Casale PN, Wallerson DC, Kligfield P, Hammond IW, Liebson PR, et al. Cost-effectiveness of echocardiography and electrocardiography for detection of left ventricular hypertrophy in patients with systemic hypertension. Hypertension 1987;9(2 Pt 2):II69–76. [DOI] [PubMed] [Google Scholar]
- 43.Narayanan K, Reinier K, Teodorescu C, Uy-Evanado A, Chugh H, Gunson K, et al. Electrocardiographic versus echocardiographic left ventricular hypertrophy and sudden cardiac arrest in the community. Heart Rhythm 2014;11:1040–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chrispin J, Jain A, Soliman EZ, Guallar E, Alonso A, Heckbert SR, et al. Association of electrocardiographic and imaging surrogates of left ventricular hypertrophy with incident atrial fibrillation: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2014;63:2007–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chugh SS, Reinier K, Singh T, Uy-Evanado A, Socoteanu C, Peters D, et al. Determinants of prolonged QT interval and their contribution to sudden death risk in coronary artery disease: the Oregon Sudden Unexpected Death Study. Circulation 2009;119:663–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kinoshita T, Asai T, Suzuki T, Matsubayashi K, Horie M. Time course and prognostic implications of QT interval in patients with coronary artery disease undergoing coronary bypass surgery. J Cardiovasc Electrophysiol 2012;23:645–9. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Post WS, Blasco-Colmenares E, Dalal D, Tomaselli GF, Guallar E. Electrocardiographic QT interval and mortality: a meta-analysis. Epidemiology 2011;22:660–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crow RS, Hannan PJ, Folsom AR. Prognostic significance of corrected QT and corrected JT interval for incident coronary heart disease in a general population sample stratified by presence or absence of wide QRS complex: the ARIC Study with 13 years of follow-up. Circulation 2003;108:1985–9. [DOI] [PubMed] [Google Scholar]
- 49.Opthof T, Coronel R, Wilms-Schopman FJ, Plotnikov AN, Shlapakova IN, Danilo P, Jr, et al. Dispersion of repolarization in canine ventricle and the electrocardiographic T wave: Tp-e interval does not reflect transmural dispersion. Heart Rhythm 2007;4:341–8. [DOI] [PubMed] [Google Scholar]
- 50.Gupta P, Patel C, Patel H, Narayanaswamy S, Malhotra B, Green JT, et al. T(p-e)/QT ratio as an index of arrhythmogenesis. J Electrocardiol 2008;41:567–74. [DOI] [PubMed] [Google Scholar]
- 51.Liu T, Brown BS, Wu Y, Antzelevitch C, Kowey PR, Yan GX. Blinded validation of the isolated arterially perfused rabbit ventricular wedge in preclinical assessment of drug-induced proarrhythmias. Heart Rhythm 2006;3:948–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Panikkath R, Reinier K, Uy-Evanado A, Teodorescu C, Hattenhauer J, Mariani R, et al. Prolonged Tpeak-to-tend interval on the resting ECG is associated with increased risk of sudden cardiac death. Circ Arrhythm Electrophysiol 2011;4:441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Letsas KP, Weber R, Astheimer K, Kalusche D, Arentz T. Tpeak-Tend interval and Tpeak-Tend/QT ratio as markers of ventricular tachycardia inducibility in subjects with Brugada ECG phenotype. Europace 2010;12:271–4. [DOI] [PubMed] [Google Scholar]
- 54.Shimizu M, Ino H, Okeie K, Yamaguchi M, Nagata M, Hayashi K, et al. T-peak to T-end interval may be a better predictor of high-risk patients with hypertrophic cardiomyopathy associated with a cardiac troponin I mutation than QT dispersion. Clin Cardiol 2002;25:335–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haissaguerre M, Derval N, Sacher F, Jesel L, Deisenhofer I, de Roy L, et al. Sudden cardiac arrest associated with early repolarization. N Engl J Med 2008;358:2016–23. [DOI] [PubMed] [Google Scholar]
- 56.Tikkanen JT, Wichmann V, Junttila MJ, Rainio M, Hookana E, Lappi OP, et al. Association of early repolarization and sudden cardiac death during an acute coronary event. Circ Arrhythm Electrophysiol 2012;5:714–8. [DOI] [PubMed] [Google Scholar]
- 57.Tikkanen JT, Junttila MJ, Anttonen O, Aro AL, Luttinen S, Kerola T, et al. Early repolarization: electrocardiographic phenotypes associated with favorable long-term outcome. Circulation 2011;123:2666–73. [DOI] [PubMed] [Google Scholar]
- 58.Barra S, Providência R, Paiva L, Nascimento J. Early repolarization patterns and the role of additional proarrhythmic triggers. Europace 2013;15:482–5. [DOI] [PubMed] [Google Scholar]
- 59.Kardys I, Kors JA, van der Meer IM, Hofman A, van der Kuip DA, Witteman JC. Spatial QRS-T angle predicts cardiac death in a general population. Eur Heart J 2003;24:1357–64. [DOI] [PubMed] [Google Scholar]
- 60.Aro AL, Huikuri HV, Tikkanen JT, Junttila MJ, Rissanen HA, Reunanen A, et al. QRS-T angle as a predictor of sudden cardiac death in a middle-aged general population. Europace 2012;14:872–6. [DOI] [PubMed] [Google Scholar]
- 61.Pepe MS, Janes H, Longton G, Leisenring W, Newcomb P. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol 2004;159:882–90. [DOI] [PubMed] [Google Scholar]
- 62.Goldenberg I, Vyas AK, Hall WJ, Moss AJ, Wang H, He H, et al. Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol 2008;51:288–96. [DOI] [PubMed] [Google Scholar]
- 63.Quinn T, Johnsen S, Gale CP, Snooks H, McLean S, Woollard M, et al. Effects of prehospital 12-lead ECG on processes of care and mortality in acute coronary syndrome: a linked cohort study from the Myocardial Ischaemia National Audit Project. Heart 2014;100:944–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Demidova MM, Carlson J, Erlinge D, Platonov PG. Predictors of ventricular fibrillation at reperfusion in patients with acute ST-elevation myocardial infarction treated by primary percutaneous coronary intervention. Am J Cardiol 2015;115:417–22. [DOI] [PubMed] [Google Scholar]
- 65.Shenthar J, Deora S, Rai M, Nanjappa Manjunath C. Prolonged Tpeak-end and Tpeak-end/QT ratio as predictors of malignant ventricular arrhythmias in the acute phase of ST-segment elevation myocardial infarction: A prospective case-control study. Heart Rhythm 2015;12:484–9. [DOI] [PubMed] [Google Scholar]
- 66.Cygankiewicz I, Zareba W, de Luna AB. Prognostic value of Holter monitoring in congestive heart failure. Cardiol J 2008;15:313–23. [PubMed] [Google Scholar]
- 67.Jauhar S, Slotwiner DJ. The economics of ICDs. N Engl J Med 2004;351:2542–4. [DOI] [PubMed] [Google Scholar]
- 68.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157–72. [DOI] [PubMed] [Google Scholar]
- 69.Rosenberg M, Silvestri S, Duran A, Porter J, McAleer J, Papa L. Feasibility and accuracy of using mobile phone images of electrocardiograms to initiate the cardiac catheterization process. J Telemed Telecare 2015;21:100–3. [DOI] [PubMed] [Google Scholar]
- 70.Furukawa T, Maggi R, Bertolone C, Ammirati F, Santini M, Ricci R, et al. Effectiveness of remote monitoring in the management of syncope and palpitations. Europace 2011;13:431–7. [DOI] [PubMed] [Google Scholar]
- 71.Veerakul G, Nademanee K. Brugada syndrome: two decades of progress. Circ J 2012;76:2713–22. [DOI] [PubMed] [Google Scholar]