Abstract
Objectives
This systematic review used meta-analytic procedures to synthesize changes in patient-centered outcomes following medication adherence interventions.
Methods
Strategies to locate studies included online searches of 13 databases and 19 research registries, hand searches of 57 journals, and author and ancestry searches of all eligible studies. Search terms included patient compliance, medication adherence, and related terms. Searches were conducted for all studies published since 1960.
Eligible published or unpublished primary studies tested medication adherence interventions and reported medication knowledge, quality of life, physical function, and symptom outcomes. Primary study attributes and outcome data were reliably coded. Overall standardized mean differences (SMDs) were analyzed using random-effects models. Dichotomous and continuous moderator analyses and funnel plots were used to explore risks of bias.
Results
Thorough searching located eligible 141 reports. The reports included 176 eligible comparisons between treatment and control subjects across 23,318 subjects. Synthesis across all comparisons yielded statistically significant SMDs for medication knowledge (d = 0.449), quality of life (d = 0.127), physical function (d = 0.142), and symptoms (d = 0.182). The overall SMDs for studies focusing on subsamples of patients with specific illnesses were more modest but also statistically significant. Of specific symptoms analyzed (depression, anxiety, pain, energy/vitality, cardiovascular, and respiratory), only anxiety failed to show a significant improvement following medication adherence interventions. Most SMDs were significantly heterogeneous, and risk of bias analyses suggested links between study quality and SMDs.
Conclusions
Modest but significant improvements in patient-centered outcomes followed medication adherence interventions.
Keywords: patient-centered outcomes, medication adherence, meta-analysis, patient knowledge, quality of life, physical function, depression, anxiety, symptoms
Introduction
Inadequate medication adherence limits effective treatment for many diseases. Nonadherence is a pervasive underrecognized cause of poor health outcomes [1, 2]. Taking into account unfilled prescriptions, missed doses, and inadequate persistence, patient medication adherence averages only 50% [2-5]. The health and economic consequences of poor adherence have led to many intervention trials designed to improve medication-taking behavior. In these trials, the primary outcome is medication-taking behavior, but an increasing number of studies are also assessing the impact of interventions on outcomes that are important to patients such as improved quality of life, alleviation of symptoms, increased physical function, and knowledge about their medications [6, 7]. Assessment of patient-centered outcomes in medication adherence studies is related to the overall increased emphasis in health care research on addressing health care from the patient's perspective.
Many extant reviews of medication adherence interventions typically examine the effects of interventions on medication adherence behavior [8-11] or on clinical outcomes [12-17]. However, no previous systematic comprehensive reviews have synthesized patient-centered outcomes following medication adherence interventions. Therefore, rather than investigate adherence behaviors or clinical outcomes, this systematic review and meta-analysis was designed to synthesize findings from primary medication adherence intervention studies reporting results for patient quality of life, physical symptoms and mood, physical function, and knowledge. The project addressed the following three research questions: 1) What are the overall standardized mean difference (SMD) outcomes for quality of life, knowledge, physical function, and symptoms following medication adherence interventions in a diverse sample of acutely and chronically ill patients? 2) What are the SMDs for these outcomes when interventions are delivered to patients having specific types of illnesses such as diabetes or cardiovascular disease? 3) What is the risk of bias in extant studies?
Methods
Standard systematic review and meta-analytic methods were used to conduct the investigation, and PRISMA guidelines were followed in reporting the results [18, 19]. This study was one component of a larger parent comprehensive review of medication adherence intervention research.
Eligibility Criteria
Primary studies were included that tested an intervention designed to increase medication adherence and also reported an outcome measure of knowledge, quality of life, physical function, or symptoms. These outcomes were selected because they are patient-centered outcomes most commonly reported in adherence research. Studies with diverse interventions were included regardless of the extent to which the interventions themselves were patient-centered or focused on patient empowerment or engagement. While this project included studies designed to increase medication adherence, this study did not synthesize medication adherence behavior outcomes, nor did it investigate the relationship between patient-centered outcomes and adherence behavior.
Studies of subjects with psychiatric conditions such as major clinical depression, bipolar disorder, or schizophrenia were excluded, as were studies of subjects who were being treated for substance abuse. Studies examining adherence to medications related to sexual function or reproduction were also excluded.
Only studies that compared intervention subjects to control subjects were included in the analysis sample; single-group pre-post studies were excluded. Studies were included whether or not they reported patients’ levels of adherence at baseline or whether they reported adherence levels post intervention. Small-sample studies, which often have inadequate statistical power, were nonetheless included because meta-analyses do not rely on p values to determine SMDs [20]. Both unpublished and published studies were included [21]. Inclusion of only published research in the meta-analysis may result in overestimation of the true magnitude of the summary SMD because studies with statistically significant findings are more likely to be published than those with nonsignificant results [20, 22, 23]. Overall SMDs for outcomes were calculated with unpublished studies included in the sample as well as excluded from it.
Search Strategies and Information Sources
In searching for eligible studies, multiple search strategies were employed to avoid bias that can result from narrow searches [24]. Searches were conducted by an expert health sciences librarian in the following electronic databases: PUBMED, PsychINFO, MEDLINE, EBSCSO, Cochrane Central Trials Register, CINAHL, Cochrane Database of Systematic Reviews, EBM Reviews, PDQT, ERIC, IndMed, International Pharmaceutical Abstracts, and Communication and Mass Media. The primary MeSH terms used in constructing search strategies were patient compliance and medication adherence. Patient compliance was used to locate studies published before 2009, and medication adherence was used to locate studies published after 2008, when it was introduced as a MeSH term. Other MeSH terms used in search strategies were: drugs, dosage forms, generic, prescription drugs, and pharmaceutical preparations. Text words used in searches were: adherent, adherence, compliant, compliance, noncompliant, noncompliance, nonadherent, nonadherence, improve, promote, enhance, encourage, foster, advocate, influence, incentive, ensure, remind, optimize, increase, address, decrease, impact, prevent, prescription(s), prescribed, drug(s), medication(s), pill(s), tablet(s), and regimen(s). Search terms for knowledge, quality of life, function, and symptoms were not used because these terms are inconsistently applied in computerized indexing systems.
Additional resources were also searched for potentially eligible studies. Abstracts from 48 conferences were examined, and hand searches were conducted in 57 journals. Nineteen research registers, such as the Research Portfolio Online Reporting Tool and clinicaltrials.gov, were also searched. Authors of studies identified through research register searches were contacted for additional studies, as were individuals who had authored more than one eligible primary study in the parent meta-analysis project sample. Ancestry searches were conducted on all eligible primary studies and review articles.
Study Selection
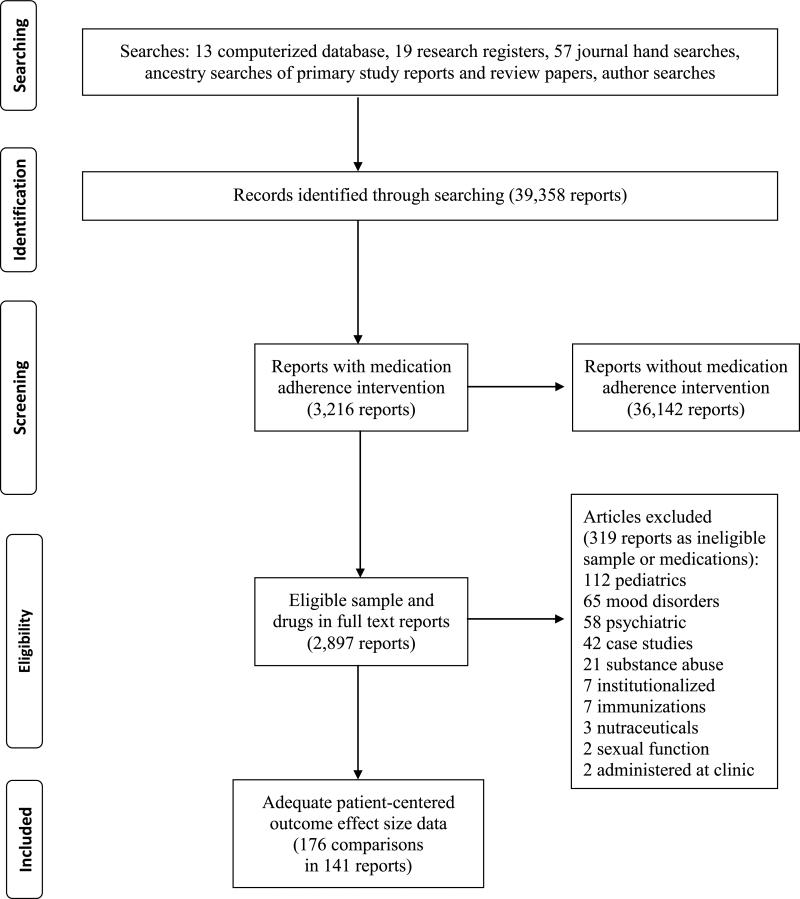
Figure 1 depicts the flow of potentially eligible primary studies through the project [19]. Citations were imported into bibliographic software and tracked using study-specific customized fields. In evaluating citations, the following eligibility criteria were applied: presence of an intervention to increase medication adherence; treatment vs. control comparison; and outcome data for knowledge, quality of life, function, or symptoms. When necessary, corresponding authors were contacted to obtain missing information to permit SMD calculation [18].
Figure 1.
Identification and flow of potentially eligible studies through the systematic review
Data Collection
The coding frame to extract primary study characteristics and outcomes was developed from the research team's prior projects, existing meta-analyses on related topics, and experts’ opinions. The coding frame was pilot tested with 20 studies. Report features were coded (year of distribution, publication status), as were patient attributes (gender, age, ethnicity, health status) and intervention features. Methodological attributes of studies were coded, including assignment of patients to groups, allocation concealment, type of control group (attention control vs. true control), masking of data collectors, sample size, attrition rates, and intention-to-treat analyses [19]. In order to calculate the SMD for each study, outcome data such as sample sizes, means, and measures of variability were coded.
Data coding was conducted by research staff members who received extensive training and ongoing supervision during the data collection period. All variables in studies were independently coded by two people; discrepancies between coders were discussed and corrected to achieve 100% agreement [25]. Coded information to be used for SMD calculations was additionally examined for accuracy by a doctorally trained staff member. To ensure sample independence, studies with any authors in common were examined to identify potentially overlapping samples. This was accomplished by comparing study attributes such as the period of time over which the investigation was conducted, the location of participant recruitment, the research protocol, and intervention content. In cases where sample independence could not be verified across studies, authors were contacted for clarification. If multiple reports on a particular study were available, all were used during coding to obtain as complete a data set as possible. In cases where multiple related papers presented data from which a SMD could be calculated for any given comparison, data were used from the paper reporting the largest sample size and the most distal data collection point.
Statistical Analysis
SMDs (d) were calculated for each comparison. The SMD is the difference between the treatment group vs. control group post-intervention means divided by the pooled standard deviation [18, 26, 27]. A positive value for d indicates a more favorable result for the treatment group compared to the control group. In determining the overall SMD for each of the outcomes, individual SMDs were weighted by the inverse of variance to give larger sample studies more influence [26]. Corresponding 95% confidence intervals were constructed for each overall SMD.
Because clinical and methodological heterogeneity is common in behavior change research, heterogeneity was expected in the sample and was addressed in five ways. First, random-effects models were used for the analyses because they take into account variance beyond that explained by within-study sampling error. The random-effects model acknowledges that variation in SMDs also occurs due to between-study differences such as intervention content, participant characteristics, and methodological features [28]. Second, both location and variability parameters are reported. Third, the conventional heterogeneity statistic Q was computed to test for the presence of heterogeneity among the studies included in the meta-analysis sample. To quantify the extent of heterogeneity across studies, the heterogeneity index I2 also was calculated [20]. Fourth, a limited sensitivity analysis was conducted to determine whether common risks of bias that were detected in the studies were influencing the magnitude of the summary SMD values. Finally, results of the meta-analysis were interpreted in the context of discovered heterogeneity. All calculations and analyses were performed using the software Comprehensive Meta-Analysis.
Risk of Bias
Because methodological quality can vary across studies and therefore potentially influence results, the risk of bias was minimized to the extent possible, and appropriate analyses to detect bias were conducted on the final sample of studies. To avoid bias commonly associated with single-group pre-post studies, only controlled trials were included in the meta-analysis. To minimize bias toward inclusion of studies with larger SMDs, which are often the easiest to locate when conducting searches, comprehensive search strategies were used to produce the broadest possible sample [24]. For this reason, unpublished as well as published studies were included in the sample because the most consistent difference between them is the statistical significance of published findings [21]. To statistically manage for variations in sample size, study SMDs were weighted so that the more precise estimates (due to larger sample size) exerted proportionally more influence on results [28].
Risk of bias due to study quality was determined by coding for the presence or absence of common indicators of methodological strength, including random assignment of participants to groups, allocation concealment, use of attention controls, data collector masking, sufficient sample size, intention-to-treat analyses, and low attrition rates [19, 29]. Exploratory moderator analysis was conducted to identify whether these methodological differences were potential sources of variation in the individual SMDs calculated for each study and could account for some of the observed heterogeneity across studies. Dichotomous moderators were examined using a meta-analytic analog of ANOVA to test the between-group heterogeneity statistic Qbetween [20]. Continuous moderators were examined by testing the unstandardized regression slope using a meta-analytic analogue of regression analysis [20].
To detect possible publication bias in the sample of studies, funnel plots were constructed in which each observed study SMD was plotted against its standard error [30]. In the absence of publication bias, the observed study SMDs will be distributed symmetrically around the overall average SMD, with larger studies having less sampling error clustered at the funnel's narrow end and smaller studies with more variation at the wider end. Asymmetry in the distribution suggests the presence of bias. Begg's test was used examine whether asymmetry in the plot was due to chance alone [20].
Results
Comprehensive searching yielded 141 reports of eligible studies. Two reports were published in the 1970s, 10 during the 1980s, and 105 were published after 2000. The sample also included 10 dissertations, two presentations, and two unpublished studies.
Some reports included multiple comparisons. For example, two treatment groups may have been compared to control groups. The total meta-analysis sample therefore consisted of k = 176 comparisons. The total number of participants across all included studies was 23,818 and consisted of 12,715 treatment and 10,603 control subjects. Most investigations were conducted in North America (k = 86) or Europe (k = 48); fewer were conducted in Asia (k = 17), Australia (k = 13), Africa (k = 10), or South America (k = 2). The majority of investigations (k = 127) reported funding for projects.
Descriptive statistics for included studies are provided in Table 1. Participant sample sizes ranged from 10 to 1,340, with a median of 87 subjects. The median of the mean age of subjects across studies was 57 years. Women were well represented, with studies including a median of 56% female participants. Ethnicity of participants was poorly reported, with only 60 studies providing this information; among studies reporting race/ethnicity, samples included a median of 55% non-Caucasian participants. The number of medications taken by subjects was likewise poorly reported, with only 32 studies providing data. A number of studies focused entirely on subjects possessing a specific health problem, with the most common being cardiovascular disease (k = 44), pulmonary problems (k = 40), HIV/AIDS (k = 18), and diabetes (k = 17).
Table 1.
Characteristics of Primary Studies Included in the Meta-analysis Sample
| Characteristic | k | Min | Q 1 | Mdn | Q 3 | Max |
|---|---|---|---|---|---|---|
| Mean subject age (years) | 171 | 25.8 | 44.7 | 57 | 66 | 84.5 |
| Total post-test sample size per study | 176 | 10 | 49 | 87 | 155 | 1,340 |
| Percentage attrition | 147 | 0 | 0 | 10 | 22 | 81 |
| Percentage female | 172 | 0 | 41 | 56 | 63 | 100 |
| Percentage non-Caucasian adults | 60 | 0 | 26 | 55 | 75 | 100 |
| Number of prescribed medications | 32 | 2 | 3.3 | 5.25 | 6.3 | 10.4 |
Note. Includes all studies that contributed at least one effect size to the primary analyses.
k = number of comparisons providing data on characteristic; Min=minimum, Q1=first quartile, Q3=third quartile, Mdn=median, Max=maximum.
Thirty studies reported a theoretical or conceptual framework for interventions. The most common theories were motivational interviewing in seven studies and social cognitive theory in six studies. With regard to intervention content, the most common interventions were providing education about medication (k = 137) or disease (k = 83) and giving written materials to patients (k = 79). Other frequently reported interventions included helping patients solve adherence problems (k = 45) or overcome barriers to adherence (k = 20) and providing social support (k = 37). Some interventions involved having patients self-monitor disease signs/symptoms (k = 39) or medication-taking behavior (k = 14). In some studies, the interventionist provided feedback to patients about their adherence behavior (k = 7) and about disease signs (k = 15). Other patient-level interventions reported by at least 10 studies included provision of medication calendars (k = 12), rewards for adherence (k = 11), medication side-effect management (k = 12), teaching medication administration skills (k = 12), and improving patient-provider communication (k = 12). A number of interventions focused on improving integration of health care services (k = 29). Most interventions were face-to-face (k = 143) and were delivered primarily by pharmacists (k = 79) or physicians (k = 29).
Intervention dose was incompletely reported across studies. Among studies reporting an intervention dose, the mean number of sessions was 4.9 (k = 128), and mean session length was 54 minutes (k = 39). Time to follow-up was likewise incompletely reported. For example, only 46 of 86 studies reporting a quality of life outcome indicated when this variable was assessed after the intervention was completed. The median number of days to follow-up was 92 for quality of life and symptoms of depression or anxiety, 28 for all other non-mood related symptoms, and 14 for both knowledge and physical function.
Knowledge, Quality of Life, and Physical Function Outcomes
The results of random-effects analysis across studies of knowledge, quality of life, and physical function outcomes following medication adherence interventions are reported in Table 2. All three of these outcomes improved in patients receiving medication adherence interventions compared to patients in control groups. The greatest impact was on knowledge, with an overall SMD of d = 0.449. More modest SMDs were found for quality of life (d = 0.127) and physical function (d = 0.142). Results for all three outcomes reached statistical significance.
Table 2.
Random-effects Medication Knowledge, Quality of Life, and Function Effect Size Estimates and Statisticsa
| k | Effect Size | p (ES) | 95% Confidence Interval | Standard Error | I2 | Q | p (Q) | |
|---|---|---|---|---|---|---|---|---|
| Medication knowledge, all samples | 60 | 0.449 | <.001 | 0.334, 0.564 | 0.059 | 76.510 | 251.175 | <.001 |
| Medication knowledge, cardiovascular samples | 14 | 0.611 | <.001 | 0.115, 1.106 | 0.253 | 94.386 | 231.568 | <.001 |
| Medication knowledge, pulmonary samples | 6 | 0.547 | .009 | 0.139, 0.954 | 0.208 | 78.557 | 23.318 | <.001 |
| Medication knowledge, diabetes samples | 3 | 0.303 | .484 | −0.544, 1.149 | 0.432 | 94.839 | 38.751 | <.001 |
| Medication knowledge, HIV samples | 3 | 0.455 | .014 | 0.092, 0.819 | 0.185 | 0 | 1.930 | .381 |
| Medication knowledge, non HIV infection samples | 8 | 0.699 | .045 | 0.015, 1.383 | 0.349 | 87.161 | 54.523 | <.001 |
| Quality of life, all samples | 86 | 0.127 | <.001 | 0.068, 0.186 | 0.030 | 51.071 | 173.723 | <.001 |
| Quality of life, cardiovascular samples | 23 | 0.183 | .001 | 0.071, 0.295 | 0.057 | 41.524 | 37.622 | .020 |
| Quality of life, pulmonary samples | 33 | 0.148 | .002 | 0.052, 0.244 | 0.049 | 35.372 | 49.514 | .025 |
| Quality of life, diabetes samples | 7 | 0.303 | .111 | −0.069, 0.675 | 0.190 | 90.927 | 66.132 | <.001 |
| Quality of life, HIV samples | 8 | 0.112 | .426 | −0.163, 0.386 | 0.140 | 73.995 | 28.887 | <.001 |
| Physical function, all samples | 29 | 0.142 | .005 | 0.044, 0.241 | 0.050 | 58.666 | 67.740 | <.001 |
| Physical function, cardiovascular samples | 13 | 0.190 | .034 | 0.014, 0.365 | 0.089 | 67.288 | 36.684 | <.001 |
k denotes number of comparisons, effect size (ES) is the standardized mean difference (d), I2 is the percentage of total variation among studies’ observed effect sizes due to heterogeneity, Q is a conventional homogeneity statistic.
Statistics reported when at least three effect sizes were available.
When overall SMDs were determined for studies targeting patients with specific diseases, values for knowledge were still greater than those for quality of life and physical function, regardless of disease type. Knowledge values for specific patient groups ranged from 0.303 to 0.699, with only the SMD for diabetes studies failing to achieve statistical significance; however, this SMD was estimated across only three primary studies. Quality of life SMDs for specific disease groups ranged from 0.112 to 0.303 and were statistically significant for comparisons in which the analysis sample included more than 10 primary studies.
Physical function was reported less often than knowledge and quality of life, and the only specific patient group for which it was reported was in individuals with cardiovascular disease. For cardiovascular disease-focused studies, the overall SMD was 0.190, which was statistically significant (p = .034, k = 13.). Those SMDs calculated from only a few primary studies (e.g., knowledge in HIV samples, quality of life in diabetes samples) should be interpreted with caution.
With a few exceptions, substantial heterogeneity was found across studies as evidenced by statistically significant Q tests (Table 2). The magnitude of I2 in these cases indicates that a large proportion of the variability in SMDs across studies is due to between-study differences.
Symptom Outcomes
Overall SMDs for those patient symptoms that could be coded from three or more studies are shown in Table 3. Evaluable symptoms were those associated with depression and anxiety as well as patients’ levels of energy and pain. Overall SMD for the impact of interventions on respiratory symptoms was determined from studies focused on patients with pulmonary diseases; likewise, cardiac symptom results were determined from studies focused on patients with cardiovascular disease.
Table 3.
Random-effects Symptom Effect Size Estimates and Statisticsa
| k | Effect Size | p (ES) | 95% Confidence Interval | Standard Error | I2 | Q | p (Q) | |
|---|---|---|---|---|---|---|---|---|
| All symptoms, all samples | 62 | 0.182 | <.001 | 0.105, 0.259 | 0.039 | 64.224 | 170.506 | <.001 |
| Depressive symptoms, all samples | 31 | 0.222 | <.001 | 0.127, 0.318 | 0.049 | 29.721 | 42.687 | .062 |
| Depressive symptoms, cardiovascular samples | 5 | 0.329 | .003 | 0.113, 0.545 | 0.110 | 0 | 3.107 | .540 |
| Depressive symptoms, diabetes samples | 8 | 0.300 | <.001 | 0.187, 0.414 | 0.058 | 0 | 3.179 | .868 |
| Depressive symptoms, HIV samples | 10 | 0.182 | .160 | −0.072, 0.436 | 0.129 | 60.149 | 22.584 | .007 |
| Anxiety symptoms, all samples | 10 | 0.026 | .765 | −0.146, 0.198 | 0.088 | 24.338 | 11.895 | .219 |
| Anxiety symptoms, HIV samples | 5 | −0.101 | .319 | −0.299, 0.098 | 0.101 | 0 | 3.869 | .424 |
| Vitality/energy symptoms, all samples | 25 | 0.172 | .011 | 0.040, 0.304 | 0.067 | 78.698 | 112.663 | <.001 |
| Vitality/energy symptoms, cardiovascular | 12 | 0.215 | .038 | 0.012, 0.419 | 0.104 | 78.305 | 50.704 | <.001 |
| Pain, all sample | 26 | 0.119 | .008 | 0.030, 0.207 | 0.045 | 45.379 | 45.770 | .007 |
| Pain, cardiovascular samples | 10 | 0.180 | .043 | 0.006, 0.354 | 0.089 | 51.656 | 18.617 | .029 |
| Respiratory symptoms, pulmonary samples | 19 | 0.283 | <.001 | 0.130, 0.437 | 0.078 | 58.217 | 43.079 | .001 |
| Cardiac symptoms, cardiovascular samples | 5 | 0.450 | .125 | −0.125, 1.025 | 0.293 | 71.655 | 14.112 | .007 |
k denotes number of comparisons, effect size (ES) is the standardized mean difference (d), I2 is the percentage of total variation among studies’ observed effect sizes due to heterogeneity, Q is a conventional homogeneity statistic.
Statistics reported when at least five effect sizes were available.
When analyzed across all studies, depressive symptoms improved following medication adherence interventions with an overall SMD of 0.222. Depressive symptom results were statistically significant for studies of patients with cardiovascular disease (d = 0.329, p <.003) and diabetes (d = 0.300, p < .001), but they were not significant for patients with HIV (d = 0.182, p = .160). Anxiety symptoms were not significantly improved across any patient samples.
Energy/vitality significantly improved after medication adherence interventions across all patient samples (d = 0.172, p = .011) and among patients with cardiovascular disease (d = 0.215, p =.038). Positive SMDs for pain were also found across all studies reporting pain data (d = 0.119, p = .008) and across studies focusing specifically on cardiovascular patients (d = 0.180, p =.043).
Respiratory symptoms were significantly improved following medication adherence interventions in studies of patients with pulmonary disease (d = 0.283, p <.001). While cardiac symptoms improved in patients with cardiovascular disease, the improvement was not statistically significant (d = 0.450, p = .125); however, only five studies were available for this analysis.
Substantial between-studies heterogeneity was found for most symptoms as indicated by Q tests and I2 values (Table 3). The exceptions were for depressive symptoms among cardiovascular and diabetes patients and for anxiety symptoms across all patient samples and HIV patients. Again, findings from analyses with small k should be interpreted with caution.
Risk of Bias
To explore whether study quality was associated with SMDs, moderator analyses of common indicators of methodological quality were conducted. More than two-thirds of studies in the sample used random assignment of subjects (k = 128). Moderator analyses comparing studies with and without random assignment revealed no statistically significant differences in SMDs for knowledge, quality of life, and function. Some risk of bias related to nonrandom subject assignment was detected for symptom outcomes. Studies with random assignment reported larger SMDs (d = 0.243) than studies without random assignment (d = 0.033).
Allocation concealment was reported in 47 studies. Results were not significantly different between studies with and without concealment for all four outcomes (p =.373 to .990). Moderator analysis of studies with attention controls vs. true controls was not conducted because only four studies in the sample employed attention controls. Data collector masking was reported by 53 comparisons; SMDs between studies with and without masking were not significantly different for any of the outcomes (p = .386 to .714). Sixty-four comparisons used intention-to-treat analyses. Moderator analyses did not find a significant difference in SMDs between studies that used intention-to-treat analysis and those that did not for any outcome (p =.064 to.558).
Studies with smaller sample sizes reported slightly larger SMDs for knowledge (p < .001), function (p = .037), and symptoms (p = .003). Although knowledge and quality of life results were not linked to project funding, function and symptoms SMDs were. Unfunded projects reported larger SMDs than funded projects for function (0.355 vs. 0.087, p = .021) and symptoms (0.362 vs 0.123, p = .036).
Funnel plot asymmetry suggestive of publication bias was observed in the distribution of SMDs for some variables. Begg's test confirmed statistically significant asymmetry in plots of quality of life, physical function, and energy/vitality. Begg's test failed to detect significant asymmetry in plots of knowledge, pain, symptoms related to respiratory disease or cardiovascular disease, depression, or anxiety. Funnel plots are presented in the electronic supplementary materials.
Additional analyses were conducted to calculate overall SMDs based on published comparisons only, with unpublished comparisons excluded. The SMDs were similar regardless of whether unpublished studies were included. The overall SMD for knowledge when four unpublished comparisons were excluded was 0.417 (k: 56; CI: 0.312, 0.523; I2: 70.509). The overall SMD for quality of life when five unpublished comparisons were excluded was 0.130 (k: 81; CI: 0.069, 0.191; I2: 51.529). For physical function, the overall SMD when three unpublished comparisons were excluded was 0.150 (k: 26; CI: 0.046, 0.255; I2: 62.788). For depressive symptoms, the SMD was 0.207 (k: 24; CI: 0.096, 0.319; I2: 42.662) when seven unpublished comparisons were excluded. For anxiety symptoms, the overall SMD was 0.019 (k: 9; CI: −0.162, 0.199; I2: 29.887) when one unpublished comparison was excluded. The SMD for vitality/energy was 0.167 (k: 23; CI: 0.029, 0.305; I2: 80.358) with two unpublished comparisons excluded. For pain, the SMD when two unpublished comparisons were excluded was 0.123 (k: 24; CI: 0.034, 0.213; I2: 46.434). No unpublished comparisons were included in the respiratory symptom calculations.
Conclusions
This report is the first comprehensive systematic review and meta-analysis of patient-centered outcomes reported in studies testing interventions to increase medication adherence. Synthesis of SMDs across 141 studies with 176 eligible treatments vs. control comparisons determined that patients receiving medication adherence interventions on average exhibited small-to-moderate but statistically significant improvements in their knowledge of medications, quality of life, physical function, and symptoms relative to untreated controls. Most of these outcomes were also improved when interventions were delivered within specific disease populations.
The largest SMDs were found for knowledge, likely reflecting the frequent emphasis in medication adherence interventions on educating patients about their medications. Increasing knowledge may be a necessary component of increasing patients’ skills in managing medications but is not in itself sufficient to ensure medication adherence [31]. Nevertheless, increased knowledge of medications may enhance the tolerability of medications and thereby indirectly enhance adherence. For example, if a patient understands that it is best to take a medication at bedtime because the medication can cause drowsiness, then the patient is less likely to stop taking the medication due to this side effect. Knowledge may also positively impact drug efficacy. A patient who is knowledgeable about the factors that may increase or decrease absorption of a medication (e.g., the need to avoid taking a medication with certain foods or to separate a medication from H2-blockers), then such behaviors will likely lead to greater medication efficacy. Overall, informed patients may be better partners with providers in achieving health goals. Future primary research should examine the role that medication knowledge may play in mediating positive outcomes of medication adherence interventions.
Smaller, but nonetheless significant, SMDs were found for quality of life, physical function, and disease symptoms following medication adherence interventions. The SMDs found for quality of life were comparable to those reported for other interventions such as exercise [32] and nonpharmacological treatments for pain [33, 34]. One plausible explanation for the observed positive results is that medications directly impact these outcomes, so improvements inevitably occur as patients become more adherent. For example, physical function may increase because improved medication adherence reduces or eliminates debilitating symptoms. Alternatively, the impacts of interventions may occur through indirect means. For example, outcomes such as quality of life and mood, which are more subjective in nature, may improve because patients believe they are actively contributing to management of their diseases by consuming prescribed medications.
The relatively modest SMDs suggest that multiple other factors may be directly or indirectly influencing the variables analyzed in this report. Also, because the primary goal in the studies was to improve medication adherence, the interventions may not have included content specifically directed at improving patient-centered outcomes. Further, the instruments used to measure patient-centered outcomes may not have had adequate sensitivity to detect improvements [35]. It is also possible some patients experienced new symptoms or decreased quality of life as a result of taking the medications to treat their conditions, thereby offsetting positive effects of interventions.
Quality of life in particular is an especially challenging construct to evaluate. A multiplicity of factors and circumstances can impact quality of life, and unreported negative influences in some patient samples may have offset any improvements resulting from the intervention itself. Moreover, patients may not be readily able to identify improvements in their quality of life in relation to their disease condition. This may be true especially for patients whose health conditions have few symptoms, such as hypertension. For silent diseases such as hypertension, even though medications will reduce complications from the disease, they may have short-term detrimental effects on symptoms or quality of life outcomes due to side effects. Longer follow-up times after interventions might be necessary to permit patients to more accurately assess whether their quality of life has improved as a result of increased medication adherence. Quality of life increasingly is recognized as an important component of health care [36]. One strategy that health care providers can use to motivate their patients to become more medication adherent is pointing out improved quality of life as an important benefit of taking medications as prescribed.
This meta-analysis found that depressive symptoms, vitality/energy, pain, and respiratory and cardiac symptoms were improved among treatment subjects compared to controls following interventions. These findings were statistically significant for most subsamples. Some symptoms such as depressed mood are associated with poor medication adherence, so the results serve as further evidence of the link between patient symptoms and self-management behaviors. Because patients experience disease-related symptoms on a daily basis, awareness that they feel better when they are adherent may be an important aspect in their decision-making process about whether or not to take medications.
Findings from this meta-analysis indicate that improved patient-centered outcomes are associated with medication adherence interventions even in cases where the intervention is not specifically designed to address these variables. In general, medication adherence interventions that have attempted to change health care provider behavior as a means of improving patient medication adherence have not focused on patient-centered outcomes and have resulted in only minor improvements in adherence [37]. Practicing clinicians tend to emphasize to patients the importance of taking medications in order to improve clinical outcomes such as reducing blood pressure or improving laboratory values. Perhaps greater clinician focus on patient-centered benefits of taking medications would be a more effective approach to increasing medication adherence. This approach might be most useful for those medications that have few negative side effects.
The limitations inherent in meta-analyses and specific to this project must be acknowledged. One limitation was the dearth of primary studies with patient-centered outcomes. Although the outcomes had overall positive SMDs, the relatively small number of primary studies limits confidence in the findings. Incomplete descriptions of intervention content and dose along with insufficient reporting of treatment fidelity and follow-up times in primary studies imposed further limitations on the meta-analysis. This project's focus was on widely recognized patient-centered outcomes including quality of life, symptoms, and physical function. However, empirical research needs to be conducted to confirm which outcomes are most important to patients depending on their life circumstances and the illnesses for which they are being treated.
This project was not focused on medication adherence behavior outcomes. Future comprehensive meta-analyses focused on medication adherence behavior outcomes that examine intervention characteristics such as content, dose, and interventionist in relationship to outcomes would be valuable. For example, the work of Kripalani, Yao, and Haynes should be updated with more recent studies, a more comprehensive search, and examination of more intervention characteristics [38]. The findings of such moderator analyses could be valuable for designing interventions that are effective to increase medication adherence behavior.
The diversity of studies included in the sample was both strength and a limitation. This diversity permitted investigation of whether medication adherence interventions as a whole can have any impact on outcomes of importance to patients. The SMDs were significantly heterogeneous, which was expected given the clinical patient and methodological variations among primary studies. While heterogeneity permits moderator analysis to identify those demographic, design, and intervention variables that are influencing outcomes, a meaningful analysis is possible only when a sufficient number of studies reporting variables of interest are available. In the present study, moderator analysis of intervention content was hindered because many of the studies in the meta-analysis sample failed to provide sufficiently detailed description of intervention content. Sample size also restricted analysis of the effects of interventions on specific clinical populations; patient-centered outcomes are not always reported in medication adherence trials that focus on patients with specific diseases. Because studies conducted in different countries were included in the sample, it is also possible that differences in health care systems, political environments, and cultures affected intervention implementation and therefore influenced outcomes. As more medication adherence intervention studies accrue that report patient-centered outcomes, future meta-analyses will be able to more fully explore heterogeneity via moderator analyses of both intervention and participant characteristics.
Sufficient numbers of studies were available for moderator analysis to assess whether study quality posed any risks for bias in the findings. Publication bias in the sample was problematic for some outcomes; in some cases, statistically significant studies were overrepresented in the sample. Given that the attention subjects receive when they participate in clinical trials can potentially impact outcomes, the paucity of attention control groups in the study sample is troubling. Confidence in findings of future studies would be strengthened by designs that incorporate components to reduce risks of bias.
Meta-analysis involving a survey of the literature is observational rather than experimental research, and the results of this synthesis therefore should be regarded as exploratory and hypothesis generating. Several directions for designing and reporting future research are evident from this analysis. Certainly more adherence intervention research is needed to examine outcomes of importance to patients. Design of such studies should minimize potential risks of bias such as use of attention control groups, masking of data collectors, and the like. Research designs should also incorporate measures of treatment fidelity to determine whether variations in intervention content or dose may be influencing results. Use of multiple follow-up times would permit assessment of the sustainability of outcomes. Reports of primary studies should include detailed information about intervention content and patient demographics including co-morbidities and the number and types of medications taken. Intervention studies linking adherence behavior with patient-centered outcomes would be informative.
Although the scope of this meta-analysis was limited by the number studies that examined patient-centered outcomes in relation to medication adherence interventions, it does suggest that these interventions can improve patient knowledge, increase quality of life, and decrease symptoms. More reporting of patient-centered outcomes in medication adherence research, especially in subsamples of patients having specific diseases, would permit more comprehensive examination of how these outcomes are impacted. Understanding the extent and basis for that impact will permit tailoring of interventions to meet the needs of particular patient populations.
Supplementary Material
Acknowledgments
Funding: The project was supported by Award Number R01NR011990 (Conn-principal investigator) from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding agency had no role in study design, data collection, data analysis, manuscript preparation, or publication decisions.
Appendix: Primary Study Reports
- 1.Abrahams N, Jewkes R, Lombard C, Mathews S, Campbell J, Meel B. Impact of telephonic psycho-social support on adherence to post-exposure prophylaxis (PEP) after rape. AIDS Care: Psychological and Socio-Medical Aspects of AIDS/HIV. 2010;22(10):1173–1181. doi: 10.1080/09540121003692185. [DOI] [PubMed] [Google Scholar]
- 2.Adamolekun B, Mielke JK, Ball DE. An evaluation of the impact of health worker and patient education on the care and compliance of patients with epilepsy in Zimbabwe. Epilepsia. 1999;40(4):507–511. doi: 10.1111/j.1528-1157.1999.tb00749.x. [DOI] [PubMed] [Google Scholar]
- 3.Al Mazroui NR, Kamal MM, Ghabash NM, Yacout TA, Kole PL, McElnay JC. Influence of pharmaceutical care on health outcomes in patients with type 2 diabetes mellitus. Br J Clin Pharmacol. 2009;67(5):547–557. doi: 10.1111/j.1365-2125.2009.03391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amado Guirado E, Pujol Ribera E, Pacheco Huergo V, Borras JM. Knowledge and adherence to antihypertensive therapy in primary care: Results of a randomized trial. Gac Sanit. 2011;25(1):62–67. doi: 10.1016/j.gaceta.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Anderson KO, Mendoza TR, Payne R, et al. Pain education for underserved minority cancer patients: A randomized controlled trial. J Clin Oncol. 2004;22:4918–4925. doi: 10.1200/JCO.2004.06.115. [DOI] [PubMed] [Google Scholar]
- 6.Anderson S. Evaluation of pharmacist discharge medication counselling. Australian Journal of Hospital Pharmacy. 1987;17(2):131–136. [Google Scholar]
- 7.Andres Rodriguez N, Fornos Perez JA, Andres Iglesias JC. Assessment of knowledge/compliance in a drug therapy follow-up program involving type 2 diabetic patients in community pharmacy: A randomized study. Pharmaceutical Care Espana. 2007;9(1):2–9. [Google Scholar]
- 8.Aoun S, Rosenberg M. Are rural people getting HeartSmart? Aust J Rural Health. 2004;12(2):81–88. doi: 10.1111/j.1038-5282.2004.00553.x. [DOI] [PubMed] [Google Scholar]
- 9.Armour C, Bosnic-Anticevich S, Brillant M, et al. Pharmacy Asthma Care Program (PACP) improves outcomes for patients in the community. Thorax. 2007;62(6):496–502. doi: 10.1136/thx.2006.064709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.AstraZenecaPharmaceuticals. Improved quality of the treatment and increased compliances in asthmatics through the dialog tool Soren - between patient and caregiver. 2009 Mar 2; [Google Scholar]
- 11.Bailey WC, Richards JM, Jr., Brooks CM, Soong SJ, Windsor RA, Manzella BA. A randomized trial to improve self-management practices of adults with asthma. Arch Intern Med. 1990;150(8):1664–1668. [PubMed] [Google Scholar]
- 12.Beaucage K, Lachance-Demers H, Ngo TT, et al. Telephone follow-up of patients receiving antibiotic prescriptions from community pharmacies. Am J Health Syst Pharm. 2006;63(6):557–563. doi: 10.2146/ajhp050177. [DOI] [PubMed] [Google Scholar]
- 13.Begley S, Livingstone C, Hodges N, Williamson V. Impact of domiciliary pharmacy visits on medication management in an elderly population. Int J Pharm Pract. 1997;5(3):111–121. [Google Scholar]
- 14.Berger S, Schad T, Von Wyl V, et al. Effects of cognitive behavioral stress management on HIV-1 RNA, CD4 cell counts and psychosocial parameters of HIV-infected persons. AIDS. 2008;22(6):767–775. doi: 10.1097/QAD.0b013e3282f511dc. [DOI] [PubMed] [Google Scholar]
- 15.Bernsten C, Bjorkman I, Caramona M, et al. Improving the well-being of elderly patients via community pharmacy-based provision of pharmaceutical care: A multicentre study in seven European countries. Drugs Aging. 2001;18(1):63–77. doi: 10.2165/00002512-200118010-00005. [DOI] [PubMed] [Google Scholar]
- 16.Bheekie A, Syce JA, Weinberg EG. Peak expiratory flow rate and symptom self-monitoring of asthma initiated from community pharmacies. J Clin Pharm Ther. 2001;26(4):287–296. doi: 10.1046/j.1365-2710.2001.00361.x. [DOI] [PubMed] [Google Scholar]
- 17.Bogner H, de Vries H. Integration of depression and hypertension treatment: A pilot, randomized controlled trial. Ann Fam Med. 2008;6(4):295–301. doi: 10.1370/afm.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bogner HR, de Vries HF. Integrating type 2 diabetes mellitus and depression treatment among African Americans: A randomized controlled pilot trial. Diabetes Educ. 2010;36(2):284–292. doi: 10.1177/0145721709356115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bogner HR, Morales KH, de Vries HF, Cappola AR. Integrated management of type 2 diabetes mellitus and depression treatment to improve medication adherence: A randomized controlled trial. Ann Fam Med. 2012;10(1):15–22. doi: 10.1370/afm.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouvy ML, Heerdink ER, Urquhart J, Grobbee DE, Hoes AW, Leufkens HG. Effect of a pharmacist-led intervention on diuretic compliance in heart failure patients: A randomized controlled study. J Card Fail. 2003;9(5):404–411. doi: 10.1054/s1071-9164(03)00130-1. [DOI] [PubMed] [Google Scholar]
- 21.Cabezas C, Martin A, Comin E, et al. Compliance of antibiotic treatment in primary health care. Value of the personalized prescription. Rev Clin Esp. 1989;185(7):366–369. [PubMed] [Google Scholar]
- 22.Carrico AW, Antoni MH, Duran RE, et al. Reductions in depressed mood and denial coping during cognitive behavioral stress management with HIV-positive gay men treated with HAART. Ann Behav Med. 2006;31(2):155–1164. doi: 10.1207/s15324796abm3102_7. [DOI] [PubMed] [Google Scholar]
- 23.Chalifoux ZL. An effectiveness analysis of the comparative impact of home-based versus clinic-based supervision in rural elders experiencing depressive symptomatology [Doctoral dissertation] 2001. [Google Scholar]
- 24.Chang M, Chang Y, Chiou J, Tsou T, Lin C. Overcoming patient-related barriers to cancer pain management for home care patients: A pilot study. Cancer Nurs. 2002;25(6):470–476. doi: 10.1097/00002820-200212000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Chun DS. An assessment of support group participation on depression and adherence in veterans with hepatitis C [Doctoral dissertation] 2002. [Google Scholar]
- 26.Cordasco KM, Asch SM, Bell DS, et al. A low-literacy medication education tool for safety-net hospital patients. Am J Prev Med. 2009;37(6 Suppl 1):S209–216. doi: 10.1016/j.amepre.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 27.Cross RK, Cheevers N, Rustgi A, Langenberg P, Finkelstein J. Randomized, controlled trial of home telemanagement in patients with ulcerative colitis. Inflamm Bowel Dis. 2011;18(6):1018–1025. doi: 10.1002/ibd.21795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dignass AU, Bokemeyer B, Adamek H, et al. Mesalamine once daily is more effective than twice daily in patients with quiescent ulcerative colitis. Clin Gastroenterol Hepatol. 2009;7(7):762–769. doi: 10.1016/j.cgh.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Dowse R, Ehlers M. Medicine labels incorporating pictograms: Do they influence understanding and adherence? Patient Educ Couns. 2005;58(1):63–70. doi: 10.1016/j.pec.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Edworthy SM, Devins GM. Improving medication adherence through patient education distinguishing between appropriate and inappropriate utilization. J Rheumatol. 1999;26(8):1793–1801. [PubMed] [Google Scholar]
- 31.Evers AW, Kraaimaat FW, van Riel PL, de Jong AJ. Tailored cognitive-behavioral therapy in early rheumatoid arthritis for patients at risk: A randomized controlled trial. Pain. 2002;100(1-2):141–153. doi: 10.1016/s0304-3959(02)00274-9. [DOI] [PubMed] [Google Scholar]
- 32.Falces C, Lopez-Cabezas C, Andrea R, Arnau A, Ylla M, Sadurni J. An educative intervention to improve treatment compliance and to prevent readmissions of elderly patients with heart failure. Med Clin (Barc) 2008;131(12):452–456. doi: 10.1157/13126954. [DOI] [PubMed] [Google Scholar]
- 33.Farmer AJ, Wade AN, French DP, et al. Blood glucose self-monitoring in type 2 diabetes: A randomised controlled trial. Health Technol Assess. 2009;13(15) doi: 10.3310/hta13150. [DOI] [PubMed] [Google Scholar]
- 34.Freedman D. The effects of group mental health intervention on adherence to medication, for people with HIV and AIDS [Doctoral dissertation] School of Social Work; 2007. [Google Scholar]
- 35.Gallefoss F, Bakke PS. How does patient education and self-management among asthmatics and patients with chronic obstructive pulmonary disease affect medication? Am J Respir Crit Care Med. 1999;160(6):2000–2005. doi: 10.1164/ajrccm.160.6.9901028. [DOI] [PubMed] [Google Scholar]
- 36.Gamble J, Stevenson M, Heaney LG. A study of a multi-level intervention to improve non-adherence in difficult to control asthma. Respir Med. 2011;105(9):1308–1315. doi: 10.1016/j.rmed.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 37.Gibbs S, Waters WE, George CF. The benefits of prescription information leaflets. Br J Clin Pharmacol. 1989;27(6):723–739. doi: 10.1111/j.1365-2125.1989.tb03434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gibbs S, Waters WE, George CF. Benefits of prescription information leaflets. Part 2. Br J Clin Pharmacol. 1989;28(3):345–351. doi: 10.1111/j.1365-2125.1989.tb05436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Golin CE, Earp J, Tien H, Stewart P, Porter C, Howie L. A 2-Arm, randomized,controlled trial of a motivational interviewing-based intervention to improve adherence to antiretroviral therapy (ART) among patients failing or initiating ART. J Acquir Immune Defic Syndr. 2006;42(1):42–51. doi: 10.1097/01.qai.0000219771.97303.0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goodyer LI, Miskelly F, Milligan P. Does encouraging good compliance improve patients' clinical condition in heart failure? The British Journal of Clinical Practice. 1995;49(4):173–176. [PubMed] [Google Scholar]
- 41.Gotsch AR, Liguori S. Knowledge, attitude, and compliance dimensions of antibiotic therapy with PPIs: A community pharmacy-based study. Med Care. 1982;20(6):581–595. doi: 10.1097/00005650-198206000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Gupta U, Sharma S, Sheth PD, Jha J, Chaudhury RR. Improving medicine usage through patient information leaflets in India. Trop Doct. 2005;35(3):164–166. doi: 10.1258/0049475054620644. [DOI] [PubMed] [Google Scholar]
- 43.Hanlon JT, Weinberger M, Samsa GP, et al. A randomized, controlled trial of a clinical pharmacist intervention to improve inappropriate prescribing in elderly outpatients with polypharmacy. Am J Med. 1996;100(4):428–437. doi: 10.1016/S0002-9343(97)89519-8. [DOI] [PubMed] [Google Scholar]
- 44.Herschorn S, Becker D, Miller E, Thompson M, Forte L. Impact of a health education intervention in overactive bladder patients. The Canadian Journal of Urology. 2004;11(6):2430–2437. [PubMed] [Google Scholar]
- 45.Hesselink AE, Penninx BWJH, van der Windt DAWM, et al. Effectiveness of an education programme by a general practice assistant for asthma and COPD patients: Results from a randomised controlled trial. Patient Educ Couns. 2004;55(1):121–128. doi: 10.1016/j.pec.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 46.Inui TS, Yourtee EL, Williamson JW. Improved outcomes in hypertension after physician tutorials. A controlled trial. Ann Intern Med. 1976;84(6):646–651. doi: 10.7326/0003-4819-84-6-646. [DOI] [PubMed] [Google Scholar]
- 47.Janson SL, Fahy JV, Covington JK, Paul SM, Gold WM, Boushey HA. Effects of individual self-management education on clinical, biological, and adherence outcomes in asthma. Am J Med. 2003;115(8):620–626. doi: 10.1016/j.amjmed.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 48.Janson SL, McGrath KW, Covington JK, Cheng S, Boushey HA. Individualized asthma self-management improves medication adherence and markers of asthma control. J Allergy Clin Immunol. 2009;123(4):840–846. doi: 10.1016/j.jaci.2009.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jarab AS, Alqudah SG, Khdour M, Shamssain M, Mukattash TL. Impact of pharmaceutical care on health outcomes in patients with COPD. Int J Clin Pharm. 2012;34(1):53–62. doi: 10.1007/s11096-011-9585-z. [DOI] [PubMed] [Google Scholar]
- 50.Jennings M, Auckland J, Franklin G, Giles R, Austin CA. Counselling does not improve compliance with drug therapy. Age Ageing. 1992;21(Suppl 2):13–14. [Google Scholar]
- 51.Jerant AF, Azari R, Martinez C, Nesbitt TS. A randomized trial of telenursing to reduce hospitalization for heart failure: Patient-centered outcomes and nursing indicators. Home Health Care Serv Q. 2003;22(1):1–20. doi: 10.1300/J027v22n01_01. [DOI] [PubMed] [Google Scholar]
- 52.Jiang X, Sit JW, Wong TK. A nurse-led cardiac rehabilitation programme improves health behaviours and cardiac physiological risk parameters: Evidence from Chengdu, China. J Clin Nurs. 2007;16(10):1886–1897. doi: 10.1111/j.1365-2702.2007.01838.x. [DOI] [PubMed] [Google Scholar]
- 53.Jones DL, Ishii M, LaPerriere A, et al. Influencing medication adherence among women with AIDS. AIDS Care. 2003;15(4):463–474. doi: 10.1080/0954012031000134700. [DOI] [PubMed] [Google Scholar]
- 54.Kane S, Huo D, Magnanti K. A pilot feasibility study of once daily versus conventional dosing mesalamine for maintenance of ulcerative colitis. Clin Gastroenterol Hepatol. 2003;1(3):170–173. doi: 10.1053/cgh.2003.50025. [DOI] [PubMed] [Google Scholar]
- 55.Kelly JM. Sublingual nitroglycerin: Improving patient compliance with a demonstration dose. J Am Board Fam Med. 1988;1(4):251–254. [PubMed] [Google Scholar]
- 56.Khdour M, Kidney JC, Smyth BM, McElnay JC. Clinical pharmacy-led disease and medicine management programme for patients with COPD. Br J Clin Pharmacol. 2009;68(4):588–598. doi: 10.1111/j.1365-2125.2009.03493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kotses H, Bernstein IL, Bernstein DI, et al. A self-management program for adult asthma. J Allergy Clin Immunol. 1995;95(2):529–540. doi: 10.1016/s0091-6749(95)70315-2. [DOI] [PubMed] [Google Scholar]
- 58.Krass I, Taylor SJ, Smith C, Armour CL. Impact on medication use and adherence of Australian pharmacists' diabetes care services. J Am Pharm Assoc (2003) 2005;45(1):33–40. doi: 10.1331/1544345052843093. [DOI] [PubMed] [Google Scholar]
- 59.Kritikos V, Armour CL, Bosnic-Anticevich SZ. Interactive small-group asthma education in the community pharmacy setting: A pilot study. J Asthma. 2007;44(1):57–64. doi: 10.1080/02770900601125755. [DOI] [PubMed] [Google Scholar]
- 60.Lerman I, Lopez-Ponce A, Villa AR, et al. Pilot study of two different strategies to reinforce self care behaviors and treatment compliance among type 2 diabetes patients from low income strata. Gac Med Mex. 2009;145(1):15–19. [PubMed] [Google Scholar]
- 61.Levensky ER. Further development and evaluation of an individualized intervention for increasing adherence to HIV medications [Doctoral dissertation]: Psychology. 2006. [Google Scholar]
- 62.Lin EHB, Katon W, Rutter C, et al. Effects of enhanced depression treatment on diabetes self-care. Ann Fam Med. 2006;4(1):46–53. doi: 10.1370/afm.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lipton HL, Bird JA. The impact of clinical pharmacists' consultations on geriatric patients' compliance and medical care use: A randomized controlled trial. Gerontologist. 1994;34(3):307–315. doi: 10.1093/geront/34.3.307. [DOI] [PubMed] [Google Scholar]
- 64.Lopez Cabezas C, Falces Salvador C, Cubi Quadrada D, et al. Randomized clinical trial of a postdischarge pharmaceutical care program vs regular follow-up in patients with heart failure. Farmacia Hospitalaria. 2006;30(6):328–342. doi: 10.1016/s1130-6343(06)74004-1. [DOI] [PubMed] [Google Scholar]
- 65.Lopez-Vina A, del Castillo-Arevalo E. Influence of peak expiratory flow monitoring on an asthma self-management education programme. Respir Med. 2000;94(8):760–766. doi: 10.1053/rmed.2000.0815. [DOI] [PubMed] [Google Scholar]
- 66.Lourenco LB, Rodrigues RCM, Gallani CBJ, Spana TM. Effectiveness of the combination of planning strategies in adhering to the drug therapy and health related quality of life among coronary heart disease outpatients.. International Nursing Intervention Conference; Montreal, Quebec, Canada. April, 2011. [Google Scholar]
- 67.Lowe CJ, Raynor DK, Purvis J, Farrin A, Hudson J. Effects of a medicine review and education programme for older people in general practice. Br J Clin Pharmacol. 2000;50(2):172–175. doi: 10.1046/j.1365-2125.2000.00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.MacDonald E, MacDonald JB, Phoenix M. Improving drug compliance after hospital discharge. Br Med J. 1977;2(6087):618–621. doi: 10.1136/bmj.2.6087.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maddigan SL, Majumdar SR, Guirguis LM, et al. Improvements in patient-reported outcomes associated with an intervention to enhance quality of care for rural patients with type 2 diabetes: Results of a controlled trial. Diabetes Care. 2004;27(6):1306–1312. doi: 10.2337/diacare.27.6.1306. [DOI] [PubMed] [Google Scholar]
- 70.Majumdar SR, Beaupre LA, Harley CH, et al. Use of a case manager to improve osteoporosis treatment after hip fracture: Results of a randomized controlled trial. Arch Intern Med. 2007;167(19):2110–2115. doi: 10.1001/archinte.167.19.2110. [DOI] [PubMed] [Google Scholar]
- 71.Majumdar SR, Johnson JA, McAlister FA, et al. Multifaceted intervention to improve diagnosis and treatment of osteoporosis in patients with recent wrist fracture: A randomized controlled trial. Can Med Assoc J. 2008;178(5):569–575. doi: 10.1503/cmaj.070981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mann M, Eliasson O, Patel K, ZuWallack RL. A comparison of the effects of bid and qid dosing on compliance with inhaled flunisolide. Chest. 1992;101(2):496–499. doi: 10.1378/chest.101.2.496. [DOI] [PubMed] [Google Scholar]
- 73.Mansoor LE, Dowse R. Medicines information and adherence in HIV/AIDS patients. J Clin Pharm Ther. 2006;31(1):7–15. doi: 10.1111/j.1365-2710.2006.00696.x. [DOI] [PubMed] [Google Scholar]
- 74.Mantzaris GJ, Petraki C, Petraki K, et al. Prospective, randomized study of seven versus fourteen days omeprazole quadruple therapy for eradication of Helicobacter pylori infection in patients with duodenal ulcer after failure of omeprazole triple therapy. Annals of Gastroenterology. 2005;18(3):330–335. [Google Scholar]
- 75.Martins N, Morris P, Kelly PM. Food incentives to improve completion of tuberculosis treatment: Randomised controlled trial in Dili, Timor-Leste. Br Med J. 2009;339(b4248) doi: 10.1136/bmj.b4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McKinnon RI. Regimen rejection--patient's decision. Study of the effects of education and motivation on compliance. Australian Journal of Hospital Pharmacy. 1984;14(4):149–154. [Google Scholar]
- 77.McManus JA, Craig A, McAlpine C, Langhorne P, Ellis G. Does behaviour modification affect post-stroke risk factor control? Three-year follow-up of a randomized controlled trial. Clin Rehabil. 2009;23(2):99–105. doi: 10.1177/0269215508095874. [DOI] [PubMed] [Google Scholar]
- 78.Mehuys E, Van Bortel L, De Bolle L, et al. Effectiveness of pharmacist intervention for asthma control improvement. Eur Respir J. 2008;31(4):790–799. doi: 10.1183/09031936.00112007. [DOI] [PubMed] [Google Scholar]
- 79.Mita Y, Dobashi K, Shimizu Y, et al. Levofloxacin 300 mg once-daily versus levofloxacin 100 mg three-times-daily in the treatment of respiratory tract infections in elderly patients. Kitakanto Medical Journal. 2003;53(3):251–255. [Google Scholar]
- 80.Moitra E, Herbert JD, Forman EM. Acceptance-based behavior therapy to promote HIV medication adherence. AIDS Care. 2011;23(12):1660–1667. doi: 10.1080/09540121.2011.579945. [DOI] [PubMed] [Google Scholar]
- 81.Mullan RJ, Montori VM, Shah ND, et al. The diabetes mellitus medication choice decision aid: A randomized trial. Arch Intern Med. 2009;169(17):1560–1568. doi: 10.1001/archinternmed.2009.293. [DOI] [PubMed] [Google Scholar]
- 82.Murphy DA, Lu MC, Martin D, Hoffman D, Marelich WD. Results of a pilot intervention trial to improve antiretroviral adherence among HIV-positive patients. J Assoc Nurses AIDS Care. 2002;13(6):57–69. doi: 10.1177/1055329002238026. [DOI] [PubMed] [Google Scholar]
- 83.Murphy DA, Marelich WD, Rappaport NB, Hoffman D, Farthing C. Results of an antiretroviral adherence intervention: STAR (Staying Healthy: Taking Antiretrovirals Regularly). Journal of the International Association of Physicians in AIDS Care. 2007;6(2):113–124. doi: 10.1177/1545109707301243. [DOI] [PubMed] [Google Scholar]
- 84.Murray MD, Harris LE, Overhage JM, et al. Failure of computerized treatment suggestions to improve health outcomes of outpatients with uncomplicated hypertension: Results of a randomized controlled trial. Pharmacotherapy. 2004;24(3):324–337. doi: 10.1592/phco.24.4.324.33173. [DOI] [PubMed] [Google Scholar]
- 85.Nazareth I, Burton A, Shulman S, Smith P, Haines A, Timberal H. A pharmacy discharge plan for hospitalized elderly patients--a randomized controlled trial. Age Ageing. 2001;30(1):33–40. doi: 10.1093/ageing/30.1.33. [DOI] [PubMed] [Google Scholar]
- 86.Ndekha MJ, Van Oosterhout JJG, Zijlstra EE, Manary M, Saloojee H, Manary MJ. Supplementary feeding with either ready-to-use fortified spread or corn-soy blend in wasted adults starting antiretroviral therapy in Malawi: Randomised, investigator blinded, controlled trial. Br Med J. 2009;338(7706):1309–1311. doi: 10.1136/bmj.b1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ngoh LN, Shepherd MD. Design, development, and evaluation of visual aids for communicating prescription drug instructions to nonliterate patients in rural Cameroon. Patient Educ Couns. 1997;30(3):257–270. doi: 10.1016/s0738-3991(96)00976-7. [DOI] [PubMed] [Google Scholar]
- 88.Nicoleau CA. Evaluation of a comprehensive cardiac rehabilitation program [Doctoral dissertation] 1985. [Google Scholar]
- 89.Nimpitakpong P. The effects of pharmacist interventions on patient adherence and rehospitalization in CHF patients in Thailand [Doctoral dissertation] 2002. [Google Scholar]
- 90.Nucifora G, Albanese MC, De Biaggio P, et al. Lack of improvement of clinical outcomes by a low-cost, hospital-based heart failure management programme. J Cardiovasc Med. 2006;7(8):614–622. doi: 10.2459/01.JCM.0000237910.34000.58. [DOI] [PubMed] [Google Scholar]
- 91.Oser M. Evaluation of a bibliotherapy intervention for improving patients' adherence to antihypertensive medications [Doctoral dissertation] 2008. [Google Scholar]
- 92.Park JJ, Kelly P, Carter BL, Burgess PP. Comprehensive pharmaceutical care in the chain setting. J Am Pharm Assoc. 1996;NS36(7):443–451. doi: 10.1016/s1086-5802(16)30099-7. [DOI] [PubMed] [Google Scholar]
- 93.Pierce JP, Watson DS, Knights S, Gliddon T, Williams S, Watson R. A controlled trial of health education in the physician's office. Prev Med. 1984;13(2):185–194. doi: 10.1016/0091-7435(84)90050-1. [DOI] [PubMed] [Google Scholar]
- 94.Piette JD, Richardson C, Himle J, et al. A randomized trial of telephonic counseling plus walking for depressed diabetes patients. Med Care. 2011;49(7):641–648. doi: 10.1097/MLR.0b013e318215d0c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Piette JD, Weinberger M, McPhee SJ, Mah CA, Kraemer FB, Crapo LM. Do automated calls with nurse follow-up improve self-care and glycemic control among vulnerable patients with diabetes? Am J Med. 2000;108(1):20–27. doi: 10.1016/s0002-9343(99)00298-3. [DOI] [PubMed] [Google Scholar]
- 96.Pippalla RS. An impact assessment of pharmacist counseling on pharmaceutical care of hypertensives: Interrelationships of compliance, quality of life, and therapeutic outcomes, with some policy perspectives [Doctoral dissertation] 1994 [Google Scholar]
- 97.Ponnusankar S, Surulivelrajan M, Anandamoorthy N, Suresh B. Assessment of impact of medication counseling on patients' medication knowledge and compliance in an outpatient clinic in South India. Patient Educ Couns. 2004;54(1):55–60. doi: 10.1016/S0738-3991(03)00193-9. [DOI] [PubMed] [Google Scholar]
- 98.Powell LH, Calvin JE, Jr, Richardson D, et al. Self-management counseling in patients with heart failure: The heart failure adherence and retention randomized behavioral trial. J Am Med Assoc. 2010;304(12):1331–1338. doi: 10.1001/jama.2010.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Put C, van den Bergh O, Lemaigre V, Demedts M, Verleden G. Evaluation of an individualised asthma programme directed at behavioural change. Eur Respir J. 2003;21(1):109–115. doi: 10.1183/09031936.03.00267003. [DOI] [PubMed] [Google Scholar]
- 100.Ramaekers BLT, Janssen-Boyne JJ, Gorgels APM, Vrijhoef HJM. Adherence among telemonitored patients with heart failure to pharmacological and nonpharmacological recommendations. Telemed J E Health. 2009;15(6):517–524. doi: 10.1089/tmj.2009.0160. [DOI] [PubMed] [Google Scholar]
- 101.Rasmussen LM, Phanareth K, Nolte H, Backer V. Internet-based monitoring of asthma: A long-term, randomized clinical study of 300 asthmatic subjects. J Allergy Clin Immunol. 2005;115(6):1137–1142. doi: 10.1016/j.jaci.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 102.Raynor DK, Booth TG, Blenkinsopp A. Effects of computer generated reminder charts on patients' compliance with drug regimens. Br Med J. 1993;306(6886):1158–1161. doi: 10.1136/bmj.306.6886.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rich MW, Gray DB, Beckham V, Wittenberg C, Luther P. Effect of a multidisciplinary intervention on medication compliance in elderly patients with congestive heart failure. Am J Med. 1996;101(3):270–276. doi: 10.1016/s0002-9343(96)00172-6. [DOI] [PubMed] [Google Scholar]
- 104.Rimer B, Levy MH, Keintz MK, Fox L, Engstrom PF, MacElwee N. Enhancing cancer pain control regimens through patient education. Patient Educ Couns. 1987;10(3):267–277. doi: 10.1016/0738-3991(87)90128-5. [DOI] [PubMed] [Google Scholar]
- 105.Robinson JD, Segal R, Lopez LM, Doty RE. Impact of a pharmaceutical care intervention on blood pressure control in a chain pharmacy practice. Ann Pharmacother. 2010;44(1):88–96. doi: 10.1345/aph.1L289. [DOI] [PubMed] [Google Scholar]
- 106.Roden SM, Harvey PG, Mayer PP, Spence LI. Evaluation of two techniques to improve drug compliance in the elderly. Journal of Clinical and Experimental Gerontology. 1985;7(1):71–82. [Google Scholar]
- 107.Rozenfeld V, Pflomm JM, Singh KK, Bazil MK, Cheng JWM. Assessing the impact of medication consultations with a medication event monitoring system. Hosp Pharm. 1999;34(5):539–549, 559. [Google Scholar]
- 108.Rudd RE, Blanch DC, Gall V, et al. A randomized controlled trial of an intervention to reduce low literacy barriers in inflammatory arthritis management. Patient Educ Couns. 2009;75(3):334–339. doi: 10.1016/j.pec.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sacco WP, Malone JI, Morrison AD, Friedman A, Wells K. Effect of a brief, regular telephone intervention by paraprofessionals for type 2 diabetes. J Behav Med. 2009;32(4):349–359. doi: 10.1007/s10865-009-9209-4. [DOI] [PubMed] [Google Scholar]
- 110.Sadik A, Yousif M, McElnay JC. Pharmaceutical care of patients with heart failure. Br J Clin Pharmacol. 2005;60(2):183–193. doi: 10.1111/j.1365-2125.2005.02387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Santos D, Martins MC, Cipriano SL, Pinto RMC, Cukier A, Stelmach R. Pharmaceutical care for patients with persistent asthma: Assessment of treatment compliance and use of inhaled medications. Jornal Brasileiro de Pneumologia: Publicacao Oficial da Sociedade Brasileira de Pneumologia e Tisilogia. 2010;36(1):14–22. doi: 10.1590/s1806-37132010000100005. [DOI] [PubMed] [Google Scholar]
- 112.Schaffer SD, Tian L. Promoting adherence: Effects of theory-based asthma education. Clin Nurs Res. 2004;13(1):69–89. doi: 10.1177/1054773803259300. [DOI] [PubMed] [Google Scholar]
- 113.Simoni JM, Pantalone DW, Plummer MD, Huang B. A randomized controlled trial of a peer support intervention targeting antiretroviral medication adherence and depressive symptomatology in HIV-positive men and women. Health Psychol. 2007;26(4):488–495. doi: 10.1037/0278-6133.26.4.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Smith L, Bosnic-Anticevich SZ, Mitchell B, Saini B, Krass I, Armour C. Treating asthma with a self-management model of illness behaviour in an Australian community pharmacy setting. Soc Sci Med. 2007;64(7):1501–1511. doi: 10.1016/j.socscimed.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 115.Solomon DK, Portner TS, Bass GE, et al. Clinical and economic outcomes in the hypertension and COPD arms of a multicenter outcomes study. J Am Pharm Assoc. 1998;38(5):574–585. doi: 10.1016/s1086-5802(16)30371-0. [DOI] [PubMed] [Google Scholar]
- 116.Sorensen JL, Haug NA, Delucchi KL, et al. Voucher reinforcement improves medication adherence in HIV-positive methadone patients: A randomized trial. Drug Alcohol Depend. 2007;88(1):54–63. doi: 10.1016/j.drugalcdep.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stewart K, George J, Jackson SL, et al. Increasing community pharmacy involvement in the prevention of cardiovascular disease. 2007 [Google Scholar]
- 118.Taylor CT, Byrd DC, Krueger K. Improving primary care in rural Alabama with a pharmacy initiative. Am J Health Syst Pharm. 2003;60(11):1123–1129. doi: 10.1093/ajhp/60.11.1123. [DOI] [PubMed] [Google Scholar]
- 119.Tierney W, Overhage JM, Murray MD, et al. Effects of computerized guidelines for managing heart disease in primary care. J Gen Intern Med. 2003;18(12):967–976. doi: 10.1111/j.1525-1497.2003.30635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tierney WM, Overhage JM, Murray MD, et al. Can computer-generated evidence-based care suggestions enhance evidence-based management of asthma and chronic obstructive pulmonary disease? A randomized, controlled trial. Health Serv Res. 2005;40(2):477–497. doi: 10.1111/j.1475-6773.2005.00368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Udelson JE, Pressler SJ, Sackner-Bernstein J, et al. Adherence with once daily versus twice daily carvedilol in patients with heart failure: The Compliance And Quality of Life Study comparing once-daily controlled-release carvedilol CR and twice-daily immediate-release carvedilol IR in patients with heart failure (CASPER) Trial. J Card Fail. 2009;15(5):385–393. doi: 10.1016/j.cardfail.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 122.Uzma Q, Emmanuel F, Ather U, Zaman S. Efficacy of interventions for improving antiretroviral therapy adherence in HIV/AIDS cases at PIMS, Islamabad. Journal of the International Association of Physicians in AIDS Care. 2011;10(6):373–383. doi: 10.1177/1545109710383175. [DOI] [PubMed] [Google Scholar]
- 123.van Servellen G, Nyamathi A, Carpio F, et al. Effects of a treatment adherence enhancement program on health literacy, patient-provider relationships, and adherence to HAART among low-income HIV-positive Spanish-speaking Latinos. AIDS Patient Care STDS. 2005;19(11):745–759. doi: 10.1089/apc.2005.19.745. [DOI] [PubMed] [Google Scholar]
- 124.Varma S, McElnay JC, Hughes CM, Passmore AP, Varma M. Pharmaceutical care of patients with congestive heart failure: Interventions and outcomes. Pharmacotherapy. 1999;19(7):860–869. doi: 10.1592/phco.19.10.860.31565. [DOI] [PubMed] [Google Scholar]
- 125.Vivian EM. Improving blood pressure control in a pharmacist-managed hypertension clinic. Pharmacotherapy. 2002;22(12):1533–1540. doi: 10.1592/phco.22.17.1533.34127. [DOI] [PubMed] [Google Scholar]
- 126.Vollmer W, Krishner M, Peters D, Drane A, Stibolt T, Hickey T. Use and impact of an automated telephone outreach system for asthma in a managed care setting. Am J Manag Care. 2006;12(12):725–733. [PubMed] [Google Scholar]
- 127.Volume CI, Farris KB, Kassam R, Cox CE, Cave A. Pharmaceutical care research and education project: patient outcomes. J Am Pharm Assoc. 2001;41(3):411–420. doi: 10.1016/s1086-5802(16)31255-4. [DOI] [PubMed] [Google Scholar]
- 128.Wakefield B, Holman JE, Ray A, et al. Outcomes of a home telehealth intervention for patients with heart failure. J Telemed Telecare. 2009;15(1):46–50. doi: 10.1258/jtt.2008.080701. [DOI] [PubMed] [Google Scholar]
- 129.Wang H, Zhou J, Huang L, Li X, Fennie KP, Williams AB. Effects of nurse-delivered home visits combined with telephone calls on medication adherence and quality of life in HIV-infected heroin users in Hunan of China. J Clin Nurs. 2010;19(3-4):380–388. doi: 10.1111/j.1365-2702.2009.03048.x. [DOI] [PubMed] [Google Scholar]
- 130.Wang KY, Chian CF, Lai HR, Tarn YH, Wu CP. Clinical pharmacist counseling improves outcomes for Taiwanese asthma patients. Pharm World Sci. 2010;32(6):721–729. doi: 10.1007/s11096-010-9427-4. [DOI] [PubMed] [Google Scholar]
- 131.Watakakosol R. Telephone-administered intervention to improve medication adherence in HIV-infected rural persons: A pilot randomized clinical trial [Doctoral dissertation] 2010 [Google Scholar]
- 132.Waters BM, Jensen L, Fedorak RN. Effects of formal education for patients with inflammatory bowel disease: A randomized controlled trial. Can J Gastroenterol. 2005;19(4):235–244. doi: 10.1155/2005/250504. [DOI] [PubMed] [Google Scholar]
- 133.Watson PB, Town GI, Holbrook N, Dwan C, Toop LJ, Drennan CJ. Evaluation of a self-management plan for chronic obstructive pulmonary disease. Eur Respir J. 1997;10(6):1267–1271. doi: 10.1183/09031936.97.10061267. [DOI] [PubMed] [Google Scholar]
- 134.Webel AR. Testing a peer-based symptom management intervention for women living with HIV/AIDS. AIDS Care. 2010;22(9):1029–1040. doi: 10.1080/09540120903214389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Weinberger M, Murray MD, Marrero DG, et al. Effectiveness of pharmacist care for patients with reactive airways disease: A randomized controlled trial. J Am Med Assoc. 2002;288(13):1594–1602. doi: 10.1001/jama.288.13.1594. [DOI] [PubMed] [Google Scholar]
- 136.Winfield AJ, Owen CW. Information leaflets: Means of improving patient compliance. British Journal of Pharmaceutical Practice. 1990;12(June):206, 208–209. [Google Scholar]
- 137.Wolever RQ, Dreusicke M, Fikkan J, et al. Integrative health coaching for patients with type 2 diabetes: A randomized clinical trial. Diabetes Educ. 2010;36(4):629–639. doi: 10.1177/0145721710371523. [DOI] [PubMed] [Google Scholar]
- 138.Wolfe SC, Schirm V. Medication counseling for the elderly: Effects on knowledge and compliance after hospital discharge. Geriatric Nursing. 1992;13(3):134–138. doi: 10.1016/s0197-4572(07)81022-6. [DOI] [PubMed] [Google Scholar]
- 139.Wong FK, Chow SK, Chan TM. Evaluation of a nurse-led disease management programme for chronic kidney disease: A randomized controlled trial. Int J Nurs Stud. 2010;47(3):268–278. doi: 10.1016/j.ijnurstu.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 140.Zhao Y. Effects of a discharge planning intervention for elderly patients with coronary heart disease in Tianjin, China: A randomized controlled trial [Doctoral dissertation] 2004. [Google Scholar]
- 141.Zuyderduin JR, Ehlers VJ, Dm The impact of a buddy system on the self-care behaviours of women living with HIV/AIDS in Botswana. Health SA Gesondheid. 2008;13(4):4–15. [Google Scholar]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Vicki S. Conn, School of Nursing, University of Missouri, S317 Sinclair Building, Columbia MO 65211 USA.
Todd M. Ruppar, School of Nursing, University of Missouri, Columbia, MIO, USA.
Maithe Enriquez, School of Nursing, University of Missouri, Columbia, MIO, USA.
Pamela S. Cooper, School of Nursing, University of Missouri, Columbia, MIO, USA.
References
- 1.National Council on Patient Information and Education . Enhancing prescription medicine adherence: a national action plan. National Council on Patient Information and Education; Rockville, MD: 2007. [Google Scholar]
- 2.World Health Organization . Adherence to long-term therapies: evidence for action. World Health Organization; Geneva, Switzerland: 2003. [Google Scholar]
- 3.Christiansen A. Patient Adherence to Medical Treatment Regimens. Yale University Press; New Haven, CT: 2004. [Google Scholar]
- 4.McDonald HP, Garg AX, Haynes R. Interventions to enhance patient adherence to medication prescriptions: scientific review. JAMA. 2002;288:2868–79. doi: 10.1001/jama.288.22.2868. [DOI] [PubMed] [Google Scholar]
- 5.Urquhart J. Pharmionics: research on what patients do with prescription drugs. Pharmacoepidemiol Drug Saf. 2004;13:587–90. doi: 10.1002/pds.1004. [DOI] [PubMed] [Google Scholar]
- 6.Kuntz JL, Safford M, Singh J, et al. Patient-centered interventions to improve medication management and adherence: A qualitative review of research findings. Patient Educ Couns. 2014;97:310–26. doi: 10.1016/j.pec.2014.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patient-Centered Outcomes Research Institute [July 14, 2015];Establishing the definition of patient-centered outcomes research. Available from: http://www.pcori.org/establishing-definition-patient-centered-outcomes-research.
- 8.Viswanathan M, Golin CE, Jones CD, et al. Closing the quality gap: revisiting the state of the science (vol. 4: medication adherence interventions: comparative effectiveness). Evid Rep/Technol Assess. 2012;208.4:1–685. [PMC free article] [PubMed] [Google Scholar]
- 9.Coleman CI, Limone B, Sobieraj DM, et al. Dosing frequency and medication adherence in chronic disease. Journal of Managed Care Pharmacy. 2012;18:527–39. doi: 10.18553/jmcp.2012.18.7.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petry NM, Rash CJ, Byrne S, et al. Financial reinforcers for improving medication adherence: findings from a meta-analysis. Am J Med. 2012;125:888–96. doi: 10.1016/j.amjmed.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cutrona SL, Choudhry NK, Fischer MA, et al. Targeting cardiovascular medication adherence interventions. J Am Pharm Assoc (2003) 2012;52:381–97. doi: 10.1331/JAPhA.2012.10211. [DOI] [PubMed] [Google Scholar]
- 12.Aspry KE, Furman R, Karalis DG, et al. Effect of health information technology interventions on lipid management in clinical practice: a systematic review of randomized controlled trials. J Clin Lipidol. 2013;7:546–60. doi: 10.1016/j.jacl.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Bonner K, Mezochow A, Roberts T, et al. Viral load monitoring as a tool to reinforce adherence: a systematic review. J Acquir Immune Defic Syndr. 2013;64:74–8. doi: 10.1097/QAI.0b013e31829f05ac. [DOI] [PubMed] [Google Scholar]
- 14.Clarkesmith DE, Pattison HM, Lane DA. Educational and behavioural interventions for anticoagulant therapy in patients with atrial fibrillation. Cochrane Database Syst Rev. 2013;6:CD008600. doi: 10.1002/14651858.CD008600.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Free C, Phillips G, Galli L, et al. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: a systematic review. PLoS Med. 2013;10:e1001362. doi: 10.1371/journal.pmed.1001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahana SY, Rohan J, Allison S, et al. A meta-analysis of adherence to antiretroviral therapy and virologic responses in HIV-infected children, adolescents, and young adults. AIDS Behav. 2013;17:41–60. doi: 10.1007/s10461-012-0159-4. [DOI] [PubMed] [Google Scholar]
- 17.Han S, Iglay K, Davies MJ, et al. Glycemic effectiveness and medication adherence with fixed-dose combination or coadministered dual therapy of antihyperglycemic regimens: a meta-analysis. Curr Med Res Opin. 2012;28:969–77. doi: 10.1185/03007995.2012.684045. [DOI] [PubMed] [Google Scholar]
- 18.Cooper H, Hedges LV, Valentine JC. The Handbook of Research Synthesis and Meta-analysis. 2nd ed. Russell Sage Foundation; New York: 2009. [Google Scholar]
- 19.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Borenstein M, Hedges L, Higgins JPT, et al. Introduction to Meta-Analysis. John Wiley & Sons, Ltd.; West Sussex, England: 2009. [Google Scholar]
- 21.Rothstein HR, Hopewell S. Grey literature. In: Cooper H, Hedges L, Valentine J, editors. The Handbook of Research Synthesis and Meta-Analysis. 2nd ed. Russell Sage Foundation; New York: 2009. [Google Scholar]
- 22.Dickersin K. How important is publication bias? A synthesis of available data. AIDS Educ Prev. 1997;9:15–21. [PubMed] [Google Scholar]
- 23.Hopewell S, Loudon K, Clarke MJ, et al. Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database Syst Rev. 2009:MR000006. doi: 10.1002/14651858.MR000006.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White H. Scientific communication and literature retrieval. In: Cooper H, Hedges L, Valentine J, editors. The Handbook of Research Synthesis and Meta-Analysis. 2nd ed. Russell Sage Foundation; New York: 2009. [Google Scholar]
- 25.Wilson D. Systematic coding. In: Cooper H, Hedges L, Valentine J, editors. The Handbook of Research Synthesis and Meta-Analysis. 2nd ed. Russell Sage Foundation; New York: 2009. [Google Scholar]
- 26.Hedges L, Olkin I. Statistical Methods for Meta-Analysis. Academic Press; Orlando, FL: 1985. [Google Scholar]
- 27.Raudenbush S. Random effects models. In: Cooper H, Hedges L, Valentine J, editors. The Handbook of Research Synthesis and Meta-Analysis. 2nd ed. Russell Sage Foundation; New York: 2009. [Google Scholar]
- 28.Borenstein M, Hedges LV, Higgins JPT, et al. A basic introduction to fixed-effect and random-effects models for meta-analysis. Research Synthesis Methods. 2010;1:97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 29.Valentine J. Judging the quality of primary research. In: Cooper H, Hedges L, Valentine J, editors. The Handbook of Research Synthesis and Meta-Analysis. 2nd ed. Russell Sage Foundation; New York: 2009. [Google Scholar]
- 30.Sutton AJ. Publication bias. In: Cooper H, Hedges L, Valentine J, editors. The Handbook of Research Synthesis and Meta-Analysis. 2nd ed. Russell Sage Foundation; New York: 2009. [Google Scholar]
- 31.Conn VS, Hafdahl AR, Cooper PS, et al. Interventions to improve medication adherence among older adults: meta-analysis of adherence outcomes among randomized controlled trials. Gerontologist. 2009;49:447–62. doi: 10.1093/geront/gnp037. [DOI] [PubMed] [Google Scholar]
- 32.van Tol BA, Huijsmans RJ, Kroon DW, et al. Effects of exercise training on cardiac performance, exercise capacity and quality of life in patients with heart failure: a meta-analysis. Eur J Heart Fail. 2006;8:841–50. doi: 10.1016/j.ejheart.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 33.Barker AL, Talevski J, Morello RT, et al. Effectiveness of aquatic exercise for musculoskeletal conditions: a meta-analysis. Arch Phys Med Rehabil. 2014;95:1776–86. doi: 10.1016/j.apmr.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Manyanga T, Froese M, Zarychanski R, et al. Pain management with acupuncture in osteoarthritis: a systematic review and meta-analysis. BMC Complement Altern Med. 2014;14:312. doi: 10.1186/1472-6882-14-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lipsey MW. Design Sensitivity: Statistical Power for Experimental Research. Sage Publications; Newbury Park, CA: 1990. [Google Scholar]
- 36.Osaba D, King M. Meaningful differences. In: Fayers P, Hays R, editors. Assessing Quality of Life in Clinical Trials. Oxford Press; New York, NY: 2005. [Google Scholar]
- 37.Conn V, Ruppar T, Enriquez M, et al. Health care provider targeted interventions to improve medication adherence: systematic review and meta-analysis. Int J Clin Pract. doi: 10.1111/ijcp.12632. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions: a systematic review. Arch Intern Med. 2007;167:540–50. doi: 10.1001/archinte.167.6.540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.