Abstract
Background:
Ensuring stability of the disease process is essential for undertaking surgical intervention in vitiligo. However, there is no consensus regarding the minimum duration of stability or the relative importance of disease and lesional stability in selecting patients for vitiligo grafting.
Aim:
This multicentric study aims to assess the relative importance of lesional and disease stability on selecting patients for vitiligo grafting.
Materials and Methods:
One hundred seventy patients were recruited into the study and divided into two groups: Group A with lesional stability of >1 year but overall disease stability of only 6-11 months and Group B with overall disease stability of >1 year. Patients underwent either tissue or cellular vitiligo grafting on the selected lesions and the repigmentation achieved was scored from 0 (no repigmentation) to 6 (100% repigmentation). Repigmentation achieved on different sites of the body was compared between the two groups. Adverse effects at both the donor and the recipient sites were also compared.
Results:
Of the 170 patients who were enrolled, 82 patients were placed in Group A and 88 patients in Group B. Average repigmentation achieved (on scale of 0 to 6) was 3.8 and 4.04 in Group A and Group B, respectively. In Group A, ≥90% repigmentation was achieved in 36.6% (30/82) patients, while 37.5% (33/88) achieved similar results in Group B. Additionally, 47.6% (39/82) and 53.4% (47/88) of cases achieved partial repigmentation in Group A and Group B, respectively. Perigraft halo was the commonest adverse effect observed in both groups. Statistical analysis revealed no significant differences between the two groups with respect to the repigmentation achieved or adverse effects observed. Repigmentation achieved was the best on the face and neck area, while acral areas responded the least.
Conclusions:
Lesional stability seems to be as relevant as the overall disease stability in selecting patients for surgical intervention in vitiligo.
KEYWORDS: Duration of stability, lesional stability, stability, surgical treatment, vitiligo
INTRODUCTION
Vitiligo is one of the commonest acquired disorders of skin pigmentation and it affects about 1% of the world population.[1] Morbidity associated with vitiligo mainly stems from its psychological impact, which is even more severe in people with skin of color.[2,3] Majority of patients with vitiligo are managed by medical treatments, including phototherapy. However, there remains a subset of these cases where medical management fails to repigment the lesions. Many of these resistant cases are amenable to treatment by surgical methods such as vitiligo grafting.[4,5] The prime requisite for surgical management in vitiligo is the stability of the disease process.[6,7,8] Surgical intervention is recommended only in patients with a stable, nonprogressive vitiligo as repigmentation from the grafted tissue or cells is not likely to occur if the disease is still active.[6,7,8] However, the issue of “stability” of vitiligo has been debated and there are still no foolproof methods to characterize the disease as stable or unstable.[9,10,11] Additionally, there is no consensus in the world literature on the duration of stability needed in a vitiligo patient to qualify him/her for surgical intervention. In fact, there are different time periods of stability proposed by different authors for vitiligo surgery, ranging from as short as 3 months to even 4 years.[12,13,14,15] While there are certain dermatosurgeons who feel that a duration of 6 months of disease stability is enough to qualify for vitiligo surgery, there are others who feel that the disease should be stable for at least 3 years before any surgical intervention can be contemplated. The discordance of different authors on the issue of the ideal duration of disease stability can be easily visualized from the data given in Table 1.
Table 1.
Minimum duration of disease stability recommended by different authors

Interestingly, it is not only the overall duration of stability but also the relative importance of lesional versus disease stability that is debatable in the context of vitiligo surgery.[16] In vitiligo, it is not uncommon to see depigmentation at some sites with concurrent repigmentation of lesions at other sites in a single patient.[10,11] Even after grafting procedures, depigmentation at the recipient site with donor repigmentation is sometimes observed. On the other hand, there are reports of graft repigmentation with depigmentation the donor site at the same time. These reports have called into question whether the disease stability in vitiligo is applicable to the patient as a whole or if it is lesion-specific.
Many concepts have been proposed over the years to settle the issue of disease stability for surgical intervention in vitiligo. These are the Koebner phenomenon, vitiligo disease activity (VIDA) score, and the “Minigraft” test proposed by Falabella.[17,18] None of these concepts have been shown to be foolproof and accurate in predicting either disease stability or the response after vitiligo surgery.[19]
MATERIALS AND METHODS
This study was conducted in eight dermatological centers distributed in different regions of India, in the year 2012. The study was given the name of the “ASSIST” study, which stands for the ACSI Study on Stability In Surgical Treatment of vitiligo.[9] Under the project, nonsegmental vitiligo patients were recruited from all the eight participating centers and were divided into two groups on the basis of duration of disease and lesional stability. Patients with a disease stability of 6-11 months and a lesional stability of >1 year were included in Group A, while Group B included all those patients with a disease stability of >1 year. Exclusion criteria included any keloidal tendency, age less than 15 years, segmental vitiligo, pregnancy, immunosuppressant therapy, and any other contraindication to surgery. After obtaining informed consent, all the recruited cases underwent either tissue or cellular grafting procedure on the selected vitiligo lesion(s). The surgical procedures included miniature punch grafting (MPG), ultra-thin and split-thickness skin grafting (UTSG and STSG, respectively), and nonculture epidermal cell suspension technique (NCES). Each center was encouraged to give a fair and equal representation to both the groups while recruiting patients for the study. All the recruited patients were followed up for a minimum duration of 6 months after the surgery and the repigmentation achieved as well as any adverse effects were noted down over the follow-up period.
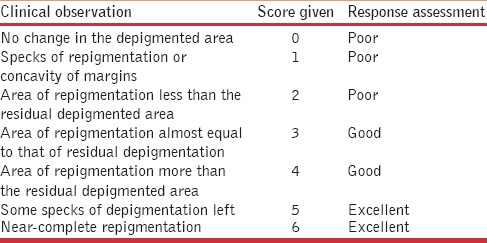
Repigmentation achieved on the grafted lesions was assessed and scored from 0 (no repigmentation) to 6 (complete repigmentation), as given in Table 2. The response was termed as excellent if the score was 5 or 6 (90-100% repigmentation), good if the score was 3 or 4 (40-75% repigmentation), and poor when the score was <3 (<40% repigmentation). In addition, any complications at the donor site, such as depigmentation and scarring, were noted down. Complications monitored at the recipient sites included depigmentation of grafts, perigraft halo of depigmentation, scarring, nonuptake of grafts, and aggravation of vitiligo. Repigmentation achieved as well as the adverse effects were thus compared between the two groups and differences, if any, were tested for their statistical significance. The overall cosmetic results achieved were also correlated with the surgical procedure performed as well as the site of the grafted lesion.
Table 2.
Scoring system used to assess repigmentation
RESULTS
A total of 170 cases were enrolled for the study, out of whom 82 belonged to Group A, while there were 88 cases in Group B. The mean age of these enrolled patients in Group A was 25.98 ± 8.01 years (range 13-52 years), while in Group B the mean age of enrolled cases was 26.4 ± 8.81 years (range 14-61 years); P = 0.746.
In Group A, there were 23 males and 59 females, while the corresponding figures in Group B were 33 and 55, respectively; P = 0.190. Grafting procedures that were performed included MPG in 75 patients, STSG/UTSG in 64 patients, and NCES in 31 patients.
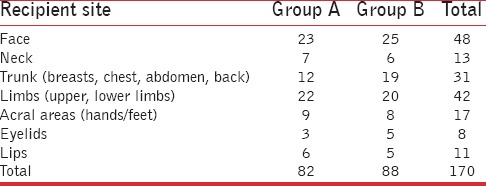
Grafted lesions were located on different sites on the body in both the groups, as shown in Table 3. As can be understood from the table, the distribution of grafted lesions was almost similar in both the groups. For the purpose of classifying lesions according to site, acral lesions were grouped separately from limb lesions as they are known to respond less favorably to vitiligo grafting. Similarly, the lip and eyelid lesions were grouped separately from facial lesions as these sites are classified as difficult sites for vitiligo surgery.
Table 3.
Distribution of grafted lesions according to site
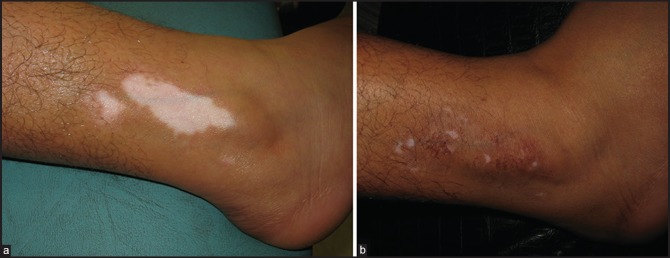
Average repigmentation score (on the scale of 0-6) was 3.8 in Group A, while the corresponding figure in Group B was 4.04 (80%). Out of 82 patients in Group A, there were 69 responders (84.1%) who showed partial or complete repigmentation after the grafting procedure, while 13 cases (15.9%) responded poorly. Among the responders, 36.6% cases (30/82) achieved excellent results in the form of near-complete (90-100%) repigmentation [Figure 1a and b], while good results (50-75% repigmentation) were achieved in 47.6% cases (39/82).
Figure 1.
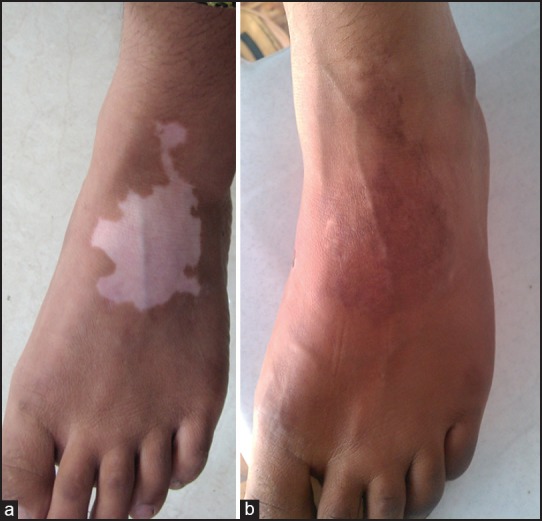
(a) Vitiligo on foot in Group B patient (b) Excellent response after UTSG
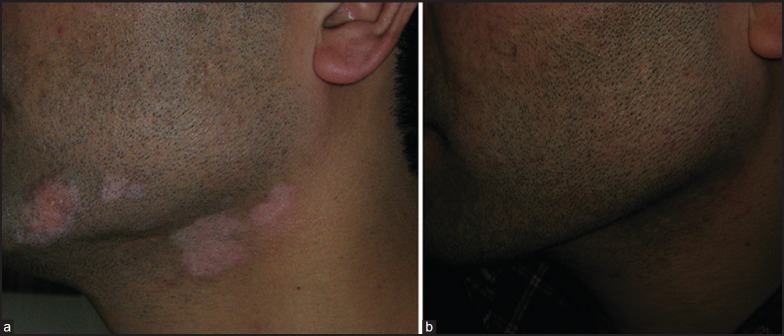
In Group B, out of a total of 88 patients, there were 80 responders (90.9%) who achieved partial or complete repigmentation of treated lesions. Among these, there were 37.5% patients (33/88) who achieved excellent results (90-100% repigmentation), while 53.4% patients (47/88) achieved good results [Figure 2a and b]. Only 8 cases (9.1%) in Group B responded poorly to the surgical intervention [Table 4].
Figure 2.
(a) Vitiligo on face in Group A patient (b) Complete repigmentation after UTSG
Table 4.
Comparison of results achieved in Group A and B

Repigmentation achieved in the two groups was compared statistically by means of chi-square test and the difference between the two groups was shown to be statistically not significant (P value of 0.393).
Adverse effects noticed in the study group included perigraft halo (15 cases), hyperpigmentation (9 cases), graft dislodgement (4 cases), cobblestoning (4 cases), textural irregularity (3 cases) keloid formation (1 case 0 and infection (1 case). No patient developed Koebnerization or depigmentation at the donor site in any of the groups. The incidence of adverse effects did not differ significantly between the two groups.
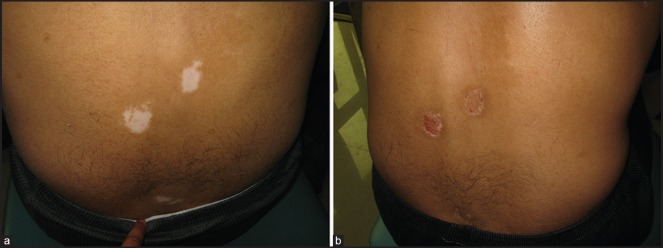
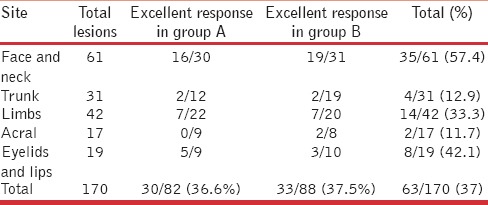
Response to grafting procedure was also correlated with the site of the recipient lesion as well as the procedure performed. The face and neck area was seen to respond most favorably to surgical intervention, with 51.6% lesions (16/31) and 55.9% lesions (19/34) achieving complete repigmentation in Group A and Group B, respectively [Figure 3a and b]. As expected, acral lesions were the worst responders with just 2 out of 17 lesions undergoing complete repigmentation at this site [Table 5]. The correlation of the response with the site of lesions was seen to be statistically significant by means of the chi-square test (P value < 0.001).
Figure 3.
(a) Group B patient with vitiligo on back (b) Complete repigmentation
Table 5.
Correlation of repigmentation achieved with the site of lesion
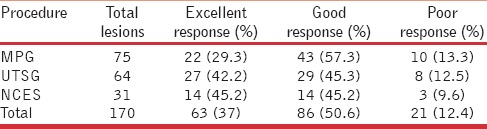
Among the different grafting procedures, UTSG/STSG and NCES gave the best possible results, with 42.2% (2764/) and 45.2% (14/31) patients achieving complete (>90%) repigmentation with these two techniques, respectively [Figure 4]. Poor response (<50% repigmentation) was seen in some cases with each of the grafting techniques, but the number of nonresponders (13.3%) was the highest in the MPG group [Table 6]. Statistical analysis by means of the chi-square test revealed a significant correlation of the response with procedure performed (P value of 0.002).
Figure 4.
(a) Group A patient with vitiligo on lower leg (b) Excellent response after grafting
Table 6.
Correlation of repigmentation achieved with the grafting technique employed
DISCUSSION
The etiopathogenesis of vitiligo is still not understood with clarity and there are many hypotheses to explain the patchy loss of pigment. While the majority of researchers view vitiligo as an immune-mediated disease, the exact origin and nature of this immune-mediated damage is still not known.[20] Other theories that have been put forward to explain the pathogenesis of vitiligo include the autocytotoxic theory, suggesting a build-up of toxic metabolic products in melanocytes and melanocytorrhagy, which suggests a detachment of the affected melanocytes from the epidermal surface.[21,22] Whatever the underlying pathogenic mechanisms, there are certain phenomena seen in vitiligo that none of these theories are able to explain. Spontaneous repigmentation at one site and progression at other sites is a well-known phenomenon seen in vitiligo. Similarly, spontaneous depigmentation at donor site with repigmentation at the recipient site after grafting is also a reality in this disease. Conversely, normal repigmentation of the donor site with depigmentation of grafts at the recipient site can also occur in vitiligo cases. Thus, is the disease process somehow related to the local immune system in and around the lesions in vitiligo? We still do not have an answer to this question.
For vitiligo grafting to be successful, stability of the disease process is thought to be the prime requisite. However, we do not have clear-cut guidelines about whether this stability refers to the lesion that needs to be surgically treated or to the patients as a whole. Additionally, as far as the overall duration of disease stability is concerned, a consensus still eludes us, with different authors proposing different durations ranging from 3 months to as long as 4 years.[19] Many attempts have been made over the years to reach to certain conclusions regarding this issue. However, none of these attempts or concepts has stood the test of time. There are clinical and experimental studies that have tried to assess the stability of a lesion on the basis of biochemical and immunological parameters. In a landmark study on the issue of stability, investigators have shown cluster of differentiation (CD)8+ T-cell counts to be associated with the stability of the disease process. Lesional and perilesional CD8+ T-cell count has been shown to have a positive correlation with disease activity in vitiligo.[23] Confocal microscopy has also been reported to be useful in predicting or establishing lesional stability in vitiligo. The bright rings normally seen at the dermoepidermal junction on confocal microscopy are usually lost in vitiligo-affected skin. Even the nonlesional skin in vitiligo cases shows some abnormalities of the dermoepidermal junction on confocal microscopy.[24] In addition, some biochemical markers such as the catecholamine levels in urine or plasma have been reported in active vitiligo patients.[25,26] In addition, the antioxidant status in an individual vitiligo patient can also be correlated with the stability of the disease process.[27] However, monitoring such biochemical markers and using such rarely available gadgets is not practically possible in routine dermatosurgery practice, where decisions about individuals’ suitability for surgical intervention are made on the basis of clinical parameters alone.
To arrive at some conclusions regarding the minimum duration of disease stability and the relative importance of lesional versus disease stability, a multicentric study ASSIST was conducted on this issue. The participating investigators of this study were all expert dermatosurgeons and experienced teachers, with each of them having more than a decade of experience in dermatosurgery and vitiligo grafting. The results from each center were collected and the data were analyzed with relevant statistical tests. The most important of all the observations was that patients with a disease stability of just above 6 months but a lesional stability of >1 year achieved comparable results to those patients in whom the disease stability was of >1 year duration. These observations hold a lot of practical importance because any resistant vitiligo lesion stable over a period of 1 year becomes amenable to surgical treatment even if the total duration of disease stability in the patient is only 6-12 months. Thus lesional stability seems to be as important as disease stability in contemplating surgical intervention in vitiligo. These findings are really important in those cases who are desperate for surgical intervention for their nonresponding vitiligo lesions because of social or any other reasons. Such cases need not wait for surgical treatment just because their disease has not been stable for >1 year.
Vitiligo is a disease where progression of lesions can occur at any time, even after decades of stability, and in such cases, usually the older lesions retain their stable status even while the patient is developing fresh lesions on other parts of the body. In such cases also, the option of surgically treating the older stable lesions becomes a practical possibility after the disease has been controlled reasonably for about 6 months or longer. As the findings from this study suggest, the risk of the Koebner phenomenon at the donor site in such cases is really low.
While collecting data from all the participating centers it also came to the fore that tissue grafting techniques are still more popular than cellular grafting. In fact, cellular grafting was performed in only about 18% (31/170) of the cases enrolled in the present study. This is probably because the cost of reagents used in cellular grafting is still a prohibiting factor for Indian patients. Moreover, many of the practicing dermatosurgeons probably still prefer older tissue grafting techniques such as MPG and STSG over cellular grafting. It is important to note that none of the patients enrolled in this study was treated with melanocyte culture, as such facilities are still not commonly available.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Lerner AB. Vitiligo. J Invest Dermatol. 1959;32:285–310. [PubMed] [Google Scholar]
- 2.Ongenae K, Beelaert L, van Geel N, Naeyaert JM. Psychosocial effects of vitiligo. J Eur Acad Dermatol Venereol. 2006;20:1–8. doi: 10.1111/j.1468-3083.2005.01369.x. [DOI] [PubMed] [Google Scholar]
- 3.Mattoo SK, Handa S, Kaur I, Gupta N, Malhotra R. Psychiatric morbidity in vitiligo: Prevalence and correlates in India. J Eur Acad Dermatol Venereol. 2002;16:573–8. doi: 10.1046/j.1468-3083.2002.00590.x. [DOI] [PubMed] [Google Scholar]
- 4.Falabella R. Surgical treatment of vitiligo: Why, when and how. J Eur Acad Dermatol Venereol. 2003;17:518–20. doi: 10.1046/j.1468-3083.2003.00718.x. [DOI] [PubMed] [Google Scholar]
- 5.Falabella R. Repigmentation of segmental vitiligo by autologous minigrafting. J Am Acad Dermatol. 1983;9:514–21. doi: 10.1016/s0190-9622(83)70162-3. [DOI] [PubMed] [Google Scholar]
- 6.Parsad D, Gupta S. IADVL Dermatosurgery Task Force. Standard guidelines of care for vitiligo surgery. Indian J Dermatol Venereol Leprol. 2008;74(Suppl):S37–45. [PubMed] [Google Scholar]
- 7.Njoo MD, Westerhof W, Bos JD, Bossuyt PM. A systematic review of autologous transplantation methods in vitiligo. Arch Dermatol. 1998;134:1543–9. doi: 10.1001/archderm.134.12.1543. [DOI] [PubMed] [Google Scholar]
- 8.Gupta S, Kumar B. Epidermal grafting in vitiligo: Influence of age, site of lesion, and type of disease on outcome. J Am Acad Dermatol. 2003;49:99–104. doi: 10.1067/mjd.2003.415. [DOI] [PubMed] [Google Scholar]
- 9.Malakar S, Lahiri K, Malakar RS. How unstable is the concept of stability in surgical repigmentation of vitiligo? Dermatology. 2000;201:182–3. doi: 10.1159/000018449. [DOI] [PubMed] [Google Scholar]
- 10.Lahiri K, Malakar S, Banerjee U, Sarma N. Clinico-cellular stability of vitiligo in surgical repigmentation: An unexplored frontier. Dermatology. 2004;209:170–1. doi: 10.1159/000079612. [DOI] [PubMed] [Google Scholar]
- 11.Lahiri K. Stability in vitiligo? What's that? J Cutan Aesthet Surg. 2009;2:38–40. doi: 10.4103/0974-2077.53100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das SS, Pasricha JS. Punch grafting as a treatment for residual lesions in vitiligo. Indian J Dermatol Venereol Leprol. 1992;58:315–9. [Google Scholar]
- 13.Boersma BR, Westerhof W, Bos JD. Repigmentation in vitiligo vulguris by autologous minigrafting: Results in nineteen patients. J Am Acad Dermatol. 1995;33:990–5. doi: 10.1016/0190-9622(95)90292-9. [DOI] [PubMed] [Google Scholar]
- 14.Singh KG, Bajaj AK. Autologous miniature skin punch grafting in vitiligo. Indian J Dermatol Venereol Leprol. 1995;61:77–80. [PubMed] [Google Scholar]
- 15.Falabella R, Escobar C, Borrero I. Treatment of refractory and stable vitiligo by transplantation of in vitro cultured epidermal autografts bearing melanocytes. J Am Acad Dermatol. 1992;26:230–6. doi: 10.1016/0190-9622(92)70032-b. [DOI] [PubMed] [Google Scholar]
- 16.Lahiri K, Malakar S. The concept of stability of vitiligo: A reappraisal. Indian J Dermatol. 2012;57:83–9. doi: 10.4103/0019-5154.94271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falabella R, Arrunategui A, Barona MI, Alzate A. The minigrafting test for vitiligo: Detection of stable lesions for melanocyte transplantation. J Am Acad Dermatol. 1995;32:228–32. doi: 10.1016/0190-9622(95)90131-0. [DOI] [PubMed] [Google Scholar]
- 18.Njoo MD, Das PK, Bos JD, Westerhof W. Association of Köbner phenomenon with disease activity and therapeutic responsiveness in vitiligo vulgaris. Arch Dermatol. 1999;135:407–13. doi: 10.1001/archderm.135.4.407. [DOI] [PubMed] [Google Scholar]
- 19.Sahni K, Parsad D. Stability in vitiligo: Is there a perfect way to predict it? J Cutan Aesthet Surg. 2013;6:75–82. doi: 10.4103/0974-2077.112667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilhar A, Zelickson B, Ulman Y, Etzioni A. In vivo destruction of melanocytes by the IgG fraction of serum from patients with vitiligo. J Invest Dermatol. 1995;105:683–6. doi: 10.1111/1523-1747.ep12324456. [DOI] [PubMed] [Google Scholar]
- 21.Dell’anna ML, Picardo M. A review and a new hypothesis for non-immunological pathogenetic mechanisms in vitiligo. Pigment Cell Res. 2006;19:406–11. doi: 10.1111/j.1600-0749.2006.00333.x. [DOI] [PubMed] [Google Scholar]
- 22.Gauthier Y, Cario Andre M, Taïeb A. A critical appraisal of vitiligo etiologic theories. Is melanocyte loss a melanocytorrhagy? Pigment Cell Res. 2003;16:322–32. doi: 10.1034/j.1600-0749.2003.00070.x. [DOI] [PubMed] [Google Scholar]
- 23.Rao A, Gupta S, Dinda AK, Sharma A, Sharma VK, Kumar G, et al. Study of clinical, biochemical and immunological factors determining stability of disease in patients with generalized vitiligo undergoing melanocyte transplantation. Br J Dermatol. 2012;136:1230–6. doi: 10.1111/j.1365-2133.2012.10886.x. [DOI] [PubMed] [Google Scholar]
- 24.Ardigo M, Malizewsky I, Dell’anna ML, Berardesca E, Picardo M. Preliminary evaluation of vitiligo using in vivo reflectance confocal microscopy. J Eur Acad Dermatol Venereol. 2007;21:1344–50. doi: 10.1111/j.1468-3083.2007.02275.x. [DOI] [PubMed] [Google Scholar]
- 25.Morrone A, Picardo M, de Luca C, Terminali O, Passi S, Ippolito F. Catecholamines and vitiligo. Pigment Cell Res. 1992;5:65–9. doi: 10.1111/j.1600-0749.1992.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 26.Cucchi ML, Frattini P, Santagostino G, Preda S, Orecchia G. Catecholamines increase in the urine of non-segmental vitiligo especially during its active phase. Pigment Cell Res. 2003;16:111–6. doi: 10.1034/j.1600-0749.2003.00015.x. [DOI] [PubMed] [Google Scholar]
- 27.Ines D, Sonia B, Riadh BM, Amel el G, Slaheddine M, Hamida T, et al. A comparative study of oxidant-antioxidant status in stable and active vitiligo patients. Arch Dermatol Res. 2006;298:147–52. doi: 10.1007/s00403-006-0680-2. [DOI] [PubMed] [Google Scholar]