Abstract
Objective
To determine whether first and second births among teenagers are associated with increased risk of adverse perinatal outcomes after confounding variables have been taken into account.
Design
Population based retrospective cohort study using routine discharge data for 1992-8.
Setting
Scotland.
Main outcome measures
Stillbirth, preterm delivery, emergency caesarean section, and small for gestational age baby among non-smoking mothers aged 15-19 and 20-29.
Results
The 110 233 eligible deliveries were stratified into first and second births. Among first births, the only significant difference in adverse outcomes by age group was for emergency caesarean section, which was less likely among younger mothers (odds ratio 0.5, 95% confidence interval 0.5 to 0.6). Second births in women aged 15-19 were associated with an increased risk of moderate (1.6, 1.2 to 2.1) and extreme prematurity (2.5, 1.5 to 4.3) and stillbirth (2.6, 1.3 to 5.3) but a reduced risk of emergency caesarean section (0.7, 0.5 to 1.0).
Conclusions
First teenage births are not independently associated with an increased risk of adverse pregnancy outcome and are at decreased risk of delivery by emergency caesarean section. However, second teenage births are associated with an almost threefold risk of preterm delivery and stillbirth.
What is already known on this topic
Teenage mothers are more likely to deliver prematurely and to have a perinatal death than older women
Teenage mothers are also more likely to smoke, be having a first baby, and live in adverse social circumstances
What this study adds
Non-smoking women aged 15-19 having a first birth were not at increased risk of adverse obstetric outcomes compared with women aged 20-29 after potential confounding variables were adjusted for
Non-smoking women aged 15-19 having a second birth were at significantly increased risk of both premature delivery and stillbirth compared with women aged 20-29
Introduction
Teenage pregnancy is an important public health problem as it often occurs in the context of poor social support and maternal wellbeing. Some studies have suggested that first teenage pregnancies have a higher frequency of adverse perinatal outcomes.1,2 However, there is argument about whether this is an independent association1,2 or explained by confounding factors.3–5 In general, the risk of adverse outcomes is lower in second pregnancies. However, longitudinal studies comparing outcomes in first and second pregnancies in teenagers have produced inconsistent results.6–9 Cross sectional studies comparing the outcome of second births in teenagers and older women have observed increased rates of preterm birth, low birth weight, and perinatal death10,11 but have failed to adjust for potential confounding factors such as smoking and socioeconomic deprivation.
Scotland is well placed to study the outcomes of teenage pregnancy. Teenage pregnancy rates in the United Kingdom are the highest in western Europe. Routine obstetric data have been collected on more than 99% of births in Scotland for over 20 years.12 Scotland has a population that is relatively homogeneous in terms of race, and health care is free at the point of access, including all medical, surgical, drug, and dental treatment during pregnancy. The aims of this study were to determine whether teenage pregnancy was associated with increased rates of adverse perinatal outcome, whether the association differed by parity, and whether any associations were independent of confounding factors.
Methods
Data collection and selection criteria
Throughout Scotland discharge data are routinely collected on all patients admitted to NHS maternity hospitals using the Scottish morbidity record 2 (SMR2).12 The SMR2 database has regular quality assurance studies. An analysis of 1414 records in 1996-7 showed that the database was free of major errors in more than 98% of records in all the fields used in the present analysis, with the exception of postcode (94.0%), height (96.2%), and estimated gestation (94.4%) (Jim Chalmers, Information and Statistics Division, NHS, Scotland, personal communication).
We used the SMR2 database to identify all singleton births resulting in a live or stillborn baby during 1992-8. Inclusion in the main study group was restricted to first or second births, gestation at birth of between 24 and 43 weeks, birth weight >500 g, maternal age between 15 and 29 years, and non-smoking mothers. We also selected a second cohort who fulfilled all the above criteria except that they were classified as smokers at the time of first attendance for antenatal care.
Definitions and denominators
First births were defined as births to women who had had no previous pregnancies or whose previous pregnancies had all ended in abortion. Second births were defined as having been preceded by only one pregnancy that did not result in abortion. Gestational age at birth was defined as the number of completed weeks of gestation based on the estimated delivery date in the clinical record. A small for gestational age baby was defined as a live baby who was less than the 5th percentile of birth weight for the given week of gestation, using percentiles derived from all Scottish singleton live births recorded in the SMR2 database with values for both birth weight and gestational age in 1992-8 (n=409 541). The denominator was all live births.
Very preterm delivery was defined as birth of a live baby at 24 to 32 weeks' gestation, and the denominator was all live births at or after 24 weeks' gestation. Moderately preterm delivery was defined as live births at 33 to 36 weeks' gestation, and the denominator was all live births at or after 33 weeks' gestation. Stillbirth was defined as delivery of a dead baby at or after 24 weeks' gestation, and the denominator was all births at or after 24 weeks' gestation. Neonatal death was defined as death of a liveborn baby in the first 28 days of life, and the denominator was all live births. Emergency caesarean section was defined as any unplanned caesarean delivery, and the denominator was all live births.
Maternal age was defined as the age of the mother in completed years at the time of birth because many of the outcomes were delivery related. Maternal height was measured in centimetres. Postcode of residence was used to derive Carstairs socioeconomic deprivation scores.13 These are based on 1991 census data on car ownership, unemployment, overcrowding, and social class within postcode sectors. The deprivation scores were then used to categorise women into quintiles based on the study population. Non-smoking was defined as never having smoked at the time of first attendance for antenatal care, and smokers were defined as women who were current smokers at the time of first attendance for antenatal care.
Statistical analyses
We did separate analyses for six dichotomous outcomes: delivery of a small for gestational age baby, moderately and extremely preterm delivery, stillbirth, neonatal death, and emergency caesarean section. For each outcome, we compared the risk between different groups using odds ratios and 95% confidence intervals. We tested the null hypothesis that the risk of adverse outcomes associated with maternal age 15 to 19 was the same for first and second births using the Mantel-Haenszel test of homogeneity.14
We calculated adjusted odds ratios by logistic regression analysis.15 Height category, maternal age category, socioeconomic deprivation quintile, and previous spontaneous and therapeutic abortion were entered into the model as dummy variables. We excluded cases with missing values for height from multivariate analysis.
We estimated the significance of main effects using the Wald test and the significance of interaction terms using the likelihood ratio test. We assessed goodness of fit of models using the Hosmer and Lemeshow test based on deciles of probability.15 Continuous variables were summarised by the mean and standard deviation, and we compared groups using analysis of variance. We used the Stata software package (Stata Corporation, Texas, USA), version 6.0, for all analyses.
Results
There were 411 553 singleton births in Scotland during 1992 to 1998. Data were missing on gestational age at birth for 691 (0.2%), on parity for 1012 (0.2%), on birth weight for 304 (0.1%), on maternal age for 12 (<0.1%) and on smoking status for 38 334 (9.3%). Data on all these variables were complete in 371 531 (90.3%) cases, and the main study group comprised the 110 233 non-smoking women aged between 15 and 29 years having a first or second birth between 24 and 43 weeks gestation of a baby weighing over 500 g.
Table 1 shows the demographic characteristics and the frequency of adverse outcomes in the study group. Within the study group there were missing values for mode of delivery in 24 (<0.1%) and for maternal height in 8201 (7.4%). Several women who had live births experienced multiple adverse outcomes (preterm birth, emergency caesarean section, being small for gestational age, or neonatal death): 1942 had two adverse outcomes, 159 had three, and four women had all four outcomes.
Table 1.
Study group characteristics and crude outcomes. Values are numbers (percentages) of women unless stated otherwise
| Women aged 15-19
|
Women aged 20-29
|
P value* | ||||
|---|---|---|---|---|---|---|
| First births (n=9699) | Second births (n=1225) | First births (n=59 315) | Second births (n=39 994) | |||
| Demographic characteristics | ||||||
| Mean (SD) height (cm) | 161.8 (6.3) | 160.9 (5.9) | 162.9 (6.5) | 162.3 (6.4) | <0.001 | |
| Socioeconomic deprivation quintile: | ||||||
| 1 | 698 (7.2) | 65 (5.3) | 11 411 (19.2) | 6 856 (17.1) | <0.001 | |
| 2 | 1299 (13.4) | 150 (12.2) | 11 890 (20.0) | 8 014 (20.0) | ||
| 3 | 1889 (19.5) | 265 (21.6) | 11 737 (19.8) | 8 141 (20.4) | ||
| 4 | 2340 (24.1) | 309 (25.2) | 11 719 (19.8) | 8 113 (20.3) | ||
| 5 | 3257 (33.6) | 411 (33.6) | 11 163 (18.8) | 7 969 (19.9) | ||
| Unclassified | 216 (2.2) | 25 (2.0) | 1 395 (2.4) | 901 (2.3) | ||
| Previous spontaneous abortions: | ||||||
| 0 | 9206 (94.9) | 1099 (89.7) | 52 647 (88.8) | 32 781 (82.0) | <0.001 | |
| 1 | 452 (4.7) | 119 (9.7) | 5 726 (9.7) | 5 954 (14.9) | ||
| >1 | 41 (0.4) | 7 (0.6) | 942 (1.6) | 1 259 (3.1) | ||
| Previous therapeutic abortions: | ||||||
| 0 | 9192 (94.8) | 1158 (94.5) | 54 581 (92.0) | 36 598 (91.5) | <0.001 | |
| 1 | 488 (5.0) | 66 (5.4) | 4 345 (7.3) | 3 053 (7.6) | ||
| >1 | 19 (0.2) | 1 (0.1) | 389 (0.7) | 343 (0.9) | ||
| Outcomes | ||||||
| Birth weight <5th percentile | 410 (4.2) | 24 (2.0) | 2 281 (3.8) | 832 (2.1) | <0.001 | |
| Delivery 24-32 weeks | 121 (1.2) | 17 (1.4) | 602 (1.0) | 220 (0.6) | <0.001 | |
| Delivery 33-36 weeks | 481 (5.0) | 56 (4.7) | 2 588 (4.4) | 1 173 (2.9) | <0.001 | |
| Stillbirth | 46 (0.5) | 9 (0.7) | 247 (0.4) | 121 (0.3) | 0.002 | |
| Neonatal death | 22 (0.2) | 3 (0.2) | 146 (0.2) | 67 (0.2) | 0.07 | |
| Emergency caesarean section | 831 (8.6) | 51 (4.2) | 8 346 (14.1) | 2 240 (5.6) | <0.001 | |
χ2 and analysis of variance tests applied to categorical and continuous data respectively.
We then compared the risk of adverse outcomes associated with maternal age 15-19 between first and second births using the Mantel-Haenszel test. This indicated that the risk of delivering a small for gestational age baby and of having an emergency caesarean section did not differ significantly by parity. However, when the risk of adverse obstetric outcomes associated with maternal age 15-19 was compared for first and second births, there were significant differences in the odds ratios of moderately premature birth (P=0.01), extremely premature birth (P=0.004), and stillbirth (P=0.03) (table 2). Therefore, the multivariate analyses were stratified by parity.
Table 2.
Univariate and multivariate logistic regression analysis of the risk of adverse perinatal outcomes among women aged 15 to 19 years of age compared with women aged 20-29 years (non-smokers)
| Outcomes | First births (n=63 565)
|
Second births (n=38 467)
|
|||
|---|---|---|---|---|---|
| Unadjusted odds ratio (95% CI) | Adjusted odds ratio† (95% CI) | Unadjusted odds ratio (95% CI) | Adjusted odds ratio† (95% CI) | ||
| Birth weight <5th percentile | 1.1 (1.0 to 1.2) | 1.0 (0.9 to 1.1) | 1.0 (0.7 to 1.5) | 0.9 (0.6 to 1.4) | |
| Delivery 24-32 weeks | 1.2 (0.9 to 1.4) | 1.1 (0.9 to 1.4) | 2.7 (1.6 to 4.6)** | 2.5 (1.5 to 4.3)*** | |
| Delivery 33-36 weeks | 1.1 (1.0 to 1.2)* | 1.1 (1.0 to 1.2) | 1.7 (1.2 to 2.2)*** | 1.6 (1.2 to 2.1)** | |
| Stillbirth | 1.2 (0.8 to 1.7) | 1.1 (0.8 to 1.6) | 2.7 (1.4 to 5.4)** | 2.6 (1.3 to 5.3)** | |
| Neonatal death | 0.8 (0.4 to 1.3) | 0.8 (0.5 to 1.4) | 1.2 (0.3 to 5.0) | 1.0 (0.2 to 4.3) | |
| Emergency caesarean section | 0.6 (0.5 to 0.6)*** | 0.5 (0.5 to 0.6)*** | 0.8 (0.6 to 1.0) | 0.7 (0.5 to 1.0)* | |
P<0.05, **P<0.01, ***P<0.001.
Adjusted for maternal height category, socioeconomic deprivation quintile, previous spontaneous and therapeutic abortions, and year (risks associated with second births also adjusted for previous perinatal death).
Among first births, women aged 15-19 years were not at increased risk of any of the six adverse outcomes studied compared with women aged 20-29 (table 2). However, among second births, mothers aged 15-19 were at significantly increased risk of moderately and extremely premature birth and stillbirth (table 2). The sizes of these associations were minimally attenuated by adjustment for socioeconomic deprivation quintile, height, year of delivery, previous abortions, and previous pregnancy resulting in a perinatal death. On multivariate analysis, emergency caesarean section was less likely among younger mothers at both first and second births. There were no significant interactions between maternal age at the time of delivery and socioeconomic deprivation quintile, height, year of delivery, or previous abortions for any of the outcomes for either first or second births.
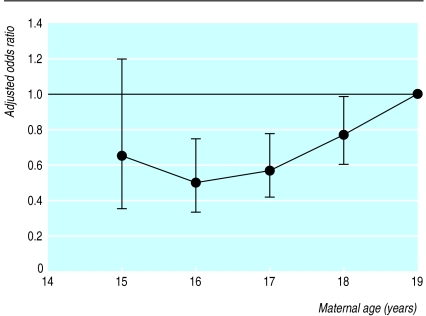
When the risk of adverse outcome was compared within the age range 15-19, there was no significant variation in the risk of moderately or extremely premature birth, stillbirth, neonatal death, or delivery by emergency caesarean section. However, compared with 19 year old women, the risk of delivering a baby weighing less than the fifth percentile for gestational age was significantly lower among women aged 16 to 18 (figure).
The proportion of women who were current smokers but fulfilled the other inclusion criteria at the time of first attendance for antenatal care varied by age and parity. Among women aged 15-19, 12 862 (47.5%) of first births and 2148 (54.8%) of second births were to smokers, whereas among women aged 20-29, 28 875 (27.4%) of first births and 26 120 (34.1%) of second births were to smokers (P<0.001).
When outcomes among 70 005 smokers were analysed, the risks associated with maternal age 15-19 again varied by parity. Among first births to smokers, there was a weak positive association between being aged 15-19 and moderately premature birth (table 3). Among second births to smokers, women aged 15-19 were at increased risk of moderately and extremely premature delivery and neonatal death. For both first and second births among smokers, being aged 15-19 was associated with a decreased risk of delivering a small for gestational age baby and being delivered by emergency caesarean section (table 3).
Table 3.
Adjusted odds ratios† (95% confidence intervals) for adverse outcomes in first and second births to women aged 15 to 19 compared with women aged 20-29 (smokers)
| Outcomes | First births (n=38 087) | Second births (n=25 992) |
|---|---|---|
| Birth weight <5th percentile | 0.8 (0.7 to 0.9)*** | 0.8 (0.6 to 1.0)* |
| Delivery 24-32 weeks | 1.1 (0.9 to 1.4) | 2.1 (1.5 to 2.9)*** |
| Delivery 33-36 weeks | 1.1 (1.0 to 1.3)** | 1.5 (1.2 to 1.8)*** |
| Stillbirth | 0.9 (0.7 to 1.2) | 0.5 (0.2 to 1.2) |
| Neonatal death | 1.4 (0.9 to 2.1) | 2.5 (1.3 to 4.8)** |
| Emergency caesarean section | 0.5 (0.5 to 0.6)*** | 0.7 (0.6 to 0.9)** |
P<0.05, **P<0.01, ***P<0.001. †Odds ratios adjusted for maternal height category, socioeconomic deprivation quintile, previous spontaneous and therapeutic abortions, and year (risks associated with second births also adjusted for previous perinatal death).
Discussion
Compared with older women, women who had a first birth during their teenage years were not at increased risk of any of the adverse outcomes studied and, indeed, were at significantly decreased risk of requiring emergency caesarean section. A previous study from the United States found that first teenage birth was independently associated with an increased risk of intrauterine growth restriction and of premature delivery,1 and a Swedish study observed that first teenage births were at increased risk of perinatal death.2 The main weakness of both studies was the failure to adjust for maternal smoking. Smoking is one of the strongest risk factors for adverse perinatal outcomes,16 and previous studies have shown that pregnant teenagers are more likely to smoke than pregnant older women.17,18 Our findings in non-smoking mothers suggest that the positive associations previously reported among first births might simply reflect inadequate adjustment for confounding variables. Indeed, when outcomes were compared within the age range 15-19, women aged 16-18 had a decreased risk of intrauterine growth retardation, which is consistent with a previous population based study from the United States.19
By contrast, we found that second births among women aged between 15 and 19 years were associated with an almost threefold risk of extremely premature birth and stillbirth compared with women aged between 20 and 29 years (table 2). A similar pattern was observed among women who smoked. However, the Scottish mortality record database does not include information on the number of cigarettes smoked a day or the duration of smoking. Both of these might be expected to vary systematically with age. Since there is a dose-effect relation between smoking and adverse outcomes,20 the findings among smokers should be interpreted with caution.
Study design and confounding factors
Previous longitudinal studies of first and second births among teenagers have produced conflicting results. Some have described consistently worse outcomes in the second birth,6 others have reported better outcomes,8 and others have described reduced risk of intrauterine growth retardation but increased risk of preterm birth.9 The weakness of longitudinal studies is that, overall, first births are at a greater risk of intrauterine growth retardation, preterm birth, and stillbirth than subsequent births.21,22 The cross sectional design of our study allows the normal protective effect of second birth to be taken into account.
The strength of the association between second teenage birth and adverse outcomes was virtually unaltered by adjusting for confounding variables. This suggests that there may be a causal relation between second teenage birth and these outcomes. Alternatively, the persistence of the association in the multivariate analysis might reflect incomplete adjustment for social deprivation. Although not perfect, deprivation scores based on postcode sector have been shown to be strongly associated with deprivation related diseases.13 Furthermore, other methods of adjusting for deprivation used in previous studies have greater weaknesses. Some studies have used marital status as a measure of deprivation.1,19 However, the relation between preterm delivery and marriage varies with maternal age.10 Some studies used the woman's highest eventual educational attainment as an index of social deprivation.17,23 It is difficult to distinguish between cause and effect with this indicator because childbearing in teenage years and complications of pregnancy may both adversely affect a woman's chance of completing higher education.
Conclusions
Our findings suggest a causal relation between second teenage birth and adverse pregnancy outcome. It is unlikely that the association can be explained by differences in the interval between pregnancies among teenage and older mothers since the associations observed were much greater than those previously reported for short intervals between pregnancies.24 Furthermore, teenage mothers were not at increased risk of a small for gestational age baby, which is known to be more common after a short interval between pregnancies.24 A biological cause could be confirmed or refuted only by access to more detailed socioeconomic information at the individual level. This would require prospective collection of data.
Figure.
Adjusted odds ratios and 95% confidence intervals for delivering a small for gestational age baby (less than the 5th percentile for gestational age) associated with maternal age among first teenage births to non-smokers. Odds ratios were adjusted for maternal height category, socioeconomic deprivation quintile, previous spontaneous and therapeutic abortions, and year. The reference category was women giving birth aged 19
Acknowledgments
We thank Jim Chalmers, consultant in public health medicine, at the Information and Statistics Division for providing the crude Scottish morbidity record (SMR2) data.
Footnotes
Funding: None.
Competing interests: None declared.
References
- 1.Fraser AM, Brockert JE, Ward RH. Association of young maternal age with adverse reproductive outcomes. N Engl J Med. 1995;332:1113–1117. doi: 10.1056/NEJM199504273321701. [DOI] [PubMed] [Google Scholar]
- 2.Olausson PO, Cnattingius S, Haglund B. Teenage pregnancies and risk of late fetal death and infant mortality. Br J Obstet Gynaecol. 1999;106:116–121. doi: 10.1111/j.1471-0528.1999.tb08210.x. [DOI] [PubMed] [Google Scholar]
- 3.Strobino DM, Ensminger ME, Kim YJ, Nanda J. Mechanisms for maternal age differences in birth weight. Am J Epidemiol. 1995;142:504–514. doi: 10.1093/oxfordjournals.aje.a117668. [DOI] [PubMed] [Google Scholar]
- 4.Berenson AB, Wiemann CM, McCombs SL. Adverse perinatal outcomes in young adolescents. J Reprod Med. 1997;42:559–564. [PubMed] [Google Scholar]
- 5.Lao TT, Ho LF. The obstetric implications of teenage pregnancy. Hum Reprod. 1997;12:2303–2305. doi: 10.1093/humrep/12.10.2303. [DOI] [PubMed] [Google Scholar]
- 6.Elster AB. The effect of maternal age, parity, and prenatal care on perinatal outcome in adolescent mothers. Am J Obstet Gynecol. 1984;149:845–847. doi: 10.1016/0002-9378(84)90602-1. [DOI] [PubMed] [Google Scholar]
- 7.Hellerstedt WL, Pirie PL, Alexander GR. Adolescent parity and infant mortality, Minnesota, 1980 through 1988. Am J Public Health. 1995;85:1139–1142. doi: 10.2105/ajph.85.8_pt_1.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sweeney PJ. A comparison of low birth weight, perinatal mortality, and infant mortality between first and second births to women 17 years old and younger. Am J Obstet Gynecol. 1989;160:1361–1367. doi: 10.1016/0002-9378(89)90856-9. [DOI] [PubMed] [Google Scholar]
- 9.Blankson ML, Cliver SP, Goldenberg RL, Hickey CA, Jin J, Dubard MB. Health behavior and outcomes in sequential pregnancies of black and white adolescents. JAMA. 1993;269:1401–1403. [PubMed] [Google Scholar]
- 10.Lumley J. The epidemiology of preterm birth. Baillieres Clin Obstet Gynaecol. 1993;7:477–498. doi: 10.1016/s0950-3552(05)80445-6. [DOI] [PubMed] [Google Scholar]
- 11.McCormick MC, Shapiro S, Starfield B. High-risk young mothers: infant mortality and morbidity in four areas in the United States, 1973-1978. Am J Public Health. 1984;74:18–23. doi: 10.2105/ajph.74.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole SK. Scottish maternity and neonatal records. In: Chalmers I, McIlwaine GM, editors. Perinatal audit and surveillance. London: Royal College of Obstetricians and Gynaecologists; 1980. pp. 39–51. [Google Scholar]
- 13.McLoone P, Boddy FA. Deprivation and mortality in Scotland, 1981 and 1991. BMJ. 1994;309:1465–1470. doi: 10.1136/bmj.309.6967.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rothman KJ, Greenland S. Modern epidemiology. Philadelphia: Lippincott-Raven; 1998. [Google Scholar]
- 15.Hosmer DW, Lemeshow S. Applied logistic regression. New York: Wiley; 1989. [Google Scholar]
- 16.DiFranza JR, Lew RA. Effect of maternal cigarette smoking on pregnancy complications and sudden infant death syndrome. J Fam Pract. 1995;40:385–394. [PubMed] [Google Scholar]
- 17.Olausson PM, Cnattingius S, Goldenberg RL. Determinants of poor pregnancy outcomes among teenagers in Sweden. Obstet Gynecol. 1997;89:451–457. doi: 10.1016/s0029-7844(97)00009-4. [DOI] [PubMed] [Google Scholar]
- 18.Lao TT, Ho LF. Obstetric outcome of teenage pregnancies. Hum Reprod. 1998;13:3228–3232. doi: 10.1093/humrep/13.11.3228. [DOI] [PubMed] [Google Scholar]
- 19.Lee KS, Ferguson RM, Corpuz M, Gartner LM. Maternal age and incidence of low birth weight at term a population study. Am J Obstet Gynecol. 1988;158:84–89. doi: 10.1016/0002-9378(88)90783-1. [DOI] [PubMed] [Google Scholar]
- 20.Kyrklund-Blomberg NB, Cnattingius S. Preterm birth and maternal smoking: risks related to gestational age and onset of delivery. Am J Obstet Gynecol. 1998;179:1051–1055. doi: 10.1016/s0002-9378(98)70214-5. [DOI] [PubMed] [Google Scholar]
- 21.Kramer MS. Determinants of low birth weight methodological assessment and meta- analysis. Bull World Health Org. 1987;65:663–738. [PMC free article] [PubMed] [Google Scholar]
- 22.Smith GCS. Life-table analysis of the risk of perinatal death at term and post term in singleton pregnancies. Am J Obstet Gynecol. 2001;184:489–496. doi: 10.1067/mob.2001.109735. [DOI] [PubMed] [Google Scholar]
- 23.Astolfi P, Zonta LA. Risks of preterm delivery and association with maternal age, birth order, and fetal gender. Hum Reprod. 1999;14:2891–2894. doi: 10.1093/humrep/14.11.2891. [DOI] [PubMed] [Google Scholar]
- 24.Zhu BP, Rolfs RT, Nangle BE, Horan JM. Effect of the interval between pregnancies on perinatal outcomes. N Engl J Med. 1999;340:589–594. doi: 10.1056/NEJM199902253400801. [DOI] [PubMed] [Google Scholar]