Abstract
Paraneoplastic encephalitis is a multifocal inflammatory disorder of the central nervous system (CNS) that is associated with remote neoplasias. The most common malignancy associated with it is bronchial carcinoma, typically small cell carcinoma of lung. It has never been described in association with intracranial neoplasm. We present and discuss the clinical, radiological, and histopathological findings of paraneoplastic encephalitis with intracranial space-occupying lesions (SOLs) in a 55-year-old man. He was thoroughly investigated and biopsy revealed presence of astrocytoma with changes of paraneoplastic encephalitis.
Keywords: Astrocytoma, limbic encephalitis, paraneoplastic encephalitis
Introduction
Paraneoplastic neurologic disorders (PND) are a heterogeneous group of neurologic disorders associated with systemic cancer and caused by mechanisms other than metastases, metabolic and nutritional deficits, infections, coagulopathy, or side effects of cancer treatment.[1] These syndromes may affect any part of the nervous system from the cerebral cortex to the neuromuscular junction and muscle, either damaging one or multiple areas.
Multiple malignancies are associated with paraneoplastic disorders like bronchial carcinoma, typically small cell carcinoma of the lung, testicular carcinoma, carcinoma of breast, immature teratoma of ovaries, etc. Case reports of thymoma with PND have also been described.[2]
We describe the first case of paraneoplastic encephalitis with astrocytoma. Our case is unique for two reasons. Firstly, paraneoplastic encephalitis has never been described in the presence of intracranial SOL. It has always been described with extracranial remote malignancy without any intracranial SOLs. Secondly, it is the first case describing features of paraneoplastic encephalitis in a case of astrocytoma with histolopathological evidence of both.
Case Report
A 55-year-old gentleman presented with memory lapses, insidious onset giddiness, gait disturbances, and imbalance since 6 months with a few episodes of convulsions. He underwent MRI four times in 6 months. His first MRI brain revealed two focal mass lesions in the right temporal and occipital lobes with multiple asymmetrical focal non-enhancing cortical hyperintensities involving bilateral temporal and fronto-parietal lobes. These were reported as granulomas with changes of focal cerebritis. Patient was treated with steroids. No antibacterial/antituberculous treatment (ATT) was started. Patient continued to be symptomatic. Follow-up second MRI brain revealed increase in size of lesions in the right temporo-occipital lobes. He was started on empirical ATT for suspected tuberculomas. Follow-up third MRI brain after 2 months of starting ATT showed persistent lesions. Steroids were added to ATT; however, patient had an episode of convulsion 1 month after his last MRI study.
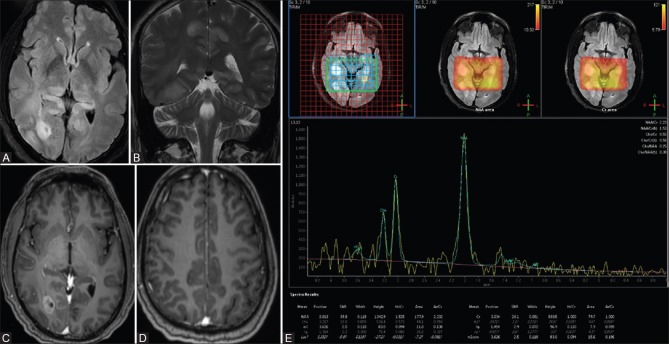
His subsequent MRI brain with MR spectroscopy was performed at our center. T2-weighted coronal images and fluid-attenuated inversion recovery (FLAIR) axial images revealed two well-defined focal mass lesions in the right postero-medial temporal lobe and occipital lobe without any perilesional edema [Figure 1A and B]. Diffusion-weighted images (DWI) revealed no significant restriction of diffusion in the mass lesions [Figure 1C and D]. Post-contrast study revealed heterogeneous enhancement in the lesions [Figure 2A and B]. MR spectroscopy revealed mild elevation of choline in the lesions, with choline to creatinine ratio in the range of 1.3-1.9 [Figure 2C]. There was mild reduction in N-acetylaspartate (NAA, with choline to NAA ratio in the range of 1.2-1.5. Choline to contralateral choline was also elevated. There was mild elevation of choline and mildly elevated choline to creatine ratio (1.1) beyond the apparent margins of the lesion; however, choline to NAA ratio was maintained.
Figure 1 (A-D).
FLAIR axial image showing two well-defined focal mass lesions in the right temporal and occipital lobes (A and B). DWI showing restriction of diffusion in the wall of the lesions in the right temporal/occipital lobe (C and D)
Figure 2 (A-C).
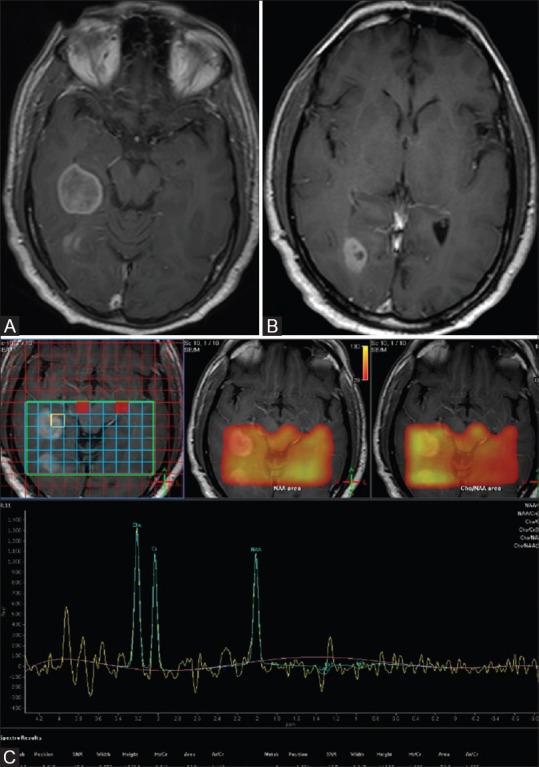
(A-C) Post-contrast T1 sagittal image shows heterogeneous enhancement of lesions in the right temporal and occipital lobes (A and B). Multivoxel intermediate TE MR spectroscopywith mild elevation of choline in mass lesions, with choline to creatine ratio range is 1.3-1.9 (C)
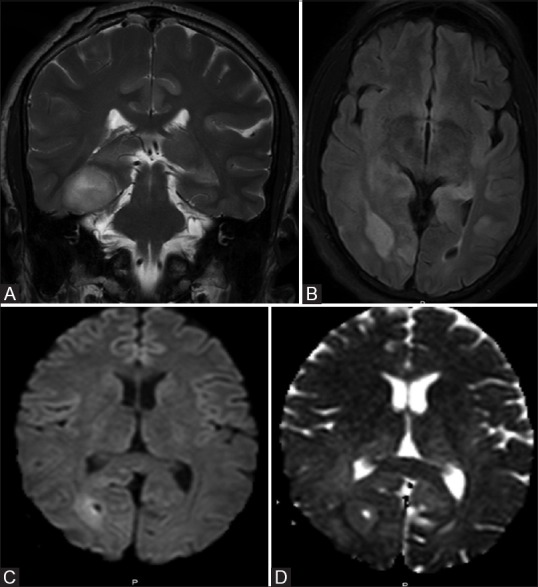
Also noted were multiple focal asymmetrical areas of altered signal intensities in bilateral hippocampi, dorsal thalami, and cingulate gyri [Figure 3A and B]. No restriction of diffusion was noted in bilateral hippocampi and left parietal lobe. There was no abnormal enhancement on post-contrast study [Figure 3C and D].
Figure 3 (A-E).
FLAIR and T2W images show multiple focal hyperintensities in bilateral hippocampi, dorsal thalami, and cingulate gyri (A and B), no enhancement was seen on post-contrast study in hippocampi and affected cortices (C and D). Multivoxel intermediate TE MR spectroscopy shows normal choline peak (E)
Spectroscopy of these lesions showed normal spectral pattern [Figure 3E].
In view of the imaging findings and spectroscopy data, possibility of intracranial metastases with paraneoplastic encephalitis was raised.
Subsequently, patient underwent whole-body positron emission tomography (PET) study which revealed focal fluorodeoxyglucose (FDG) avidity only in two intracranial mass lesions detected on MRI in the right temporo-occipital lobes without any FDG avid lesion in the rest of the body [Figure 4A and B]. Other cortical areas of signal abnormality did not reveal any FDG avidity.
Figure 4 (A and B).
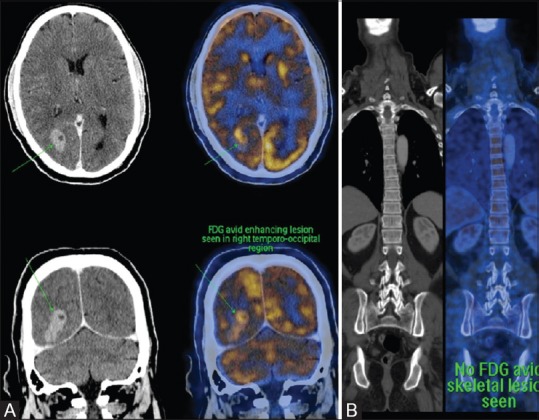
PET CT showing avid FDG uptake in intracranial masses and no abnormal FDG avid activity in the rest of the body (A and B)
Patient underwent surgery for the excision of SOLs. Biopsy from the affected cortex with suspicion of paraneoplastic encephalitis was also taken. Mass lesion histopathology revealed pleomorphic astrocytes with interspersed proliferated blood vessels having heaped up endothelial cells (glomeruloid structures) [Figure 5A]. Ki67 immunohistochemistry showed proliferation index of 30% [Figure 5B]. Immunohistochemistry also showed glial fibrillary acidic protein (GFAP) positivity in the tumor cells [Figure 5C]. This was suggestive of high-grade astrocytoma.
Figure 5 (A-D).
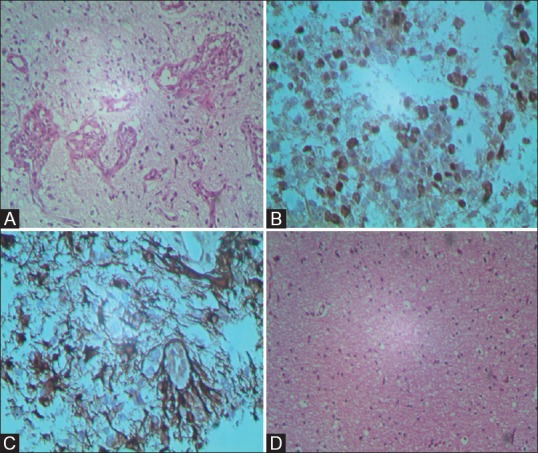
200× H and E and immunohistochemistry slide shows multiple neoplastic astrocytes with high mitotic index (A-D). 200× H and E slide shows reactive gliosis with interstitial and perivascular infiltrates of lymphocytes, a feature of paraneoplastic encephalitis (D)
Biopsy of cortical area showing non-neoplastic signal abnormality revealed reactive gliosis with interstitial and perivascular infiltrates of lymphocytes, a feature of paraneoplastic encephalitis [Figure 5D]. No tumor was detected.
No paraneoplastic antibody (Ab) screening was done in our patient due to financial constraints.
Follow-up postoperative and postradiotherapy MRI done 6 months after surgery showed significant resolution of non-neoplastic cortical lesions, possibly representing regression of paraneoplastic encephalitis after removal of the primary [Figure 6].
Figure 6 (A-D).
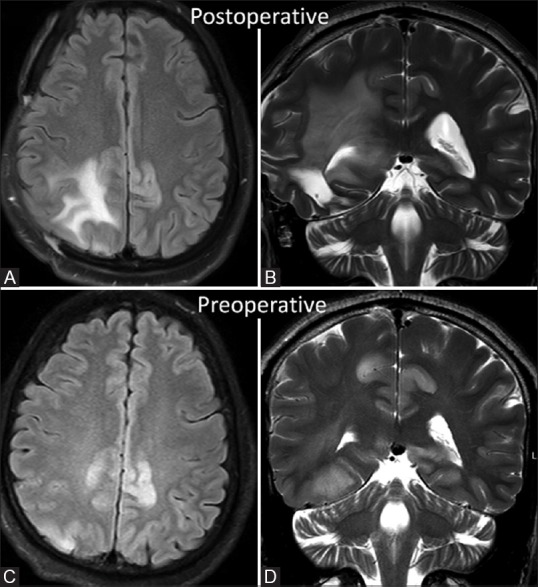
FLAIR axial and T2W coronal images in follow-up MRI done after 6 months reveal significant decrease in cortical hyperintensity in bilateral cigulate gyri when compared with preoperative initial MRI images (C and D)
Discussion
PND cover a wide range of diseases and involve both central nervous system (CNS) and peripheral nervous system. PND comprise several diseases such as paraneoplastic cerebellar degeneration (PCD), limbic encephalitis (LE), paraneoplastic encephalomyelitis (PEM), brainstem encephalitis, opsomyoclonus syndrome, in addition to other even less frequently occurring entities.
LE is now regarded as a more frequent disorder than it was earlier thought to be, as a result of improved neuroimaging and ability to detect associated Abs.
In the past few decades, several autoantibodies have been described in association with LE. These encompass the classical “onconeuronal” Abs such as Hu, Yo, Ri, and others, and, in addition, the Abs toward either ion channels or surface antigens like AMPA, GABAB, and NMDA receptors. Autoantibodies not to neurons, but to glial cells, i.e. anti-glial nuclear antibody, have also been detected in patients with paraneoplastic LE associated with lung cancer.[3,4]
Common malignancies associated with LE are small cell lung cancer, thymoma, germ cell tumors of the testis, breast cancer, and ovarian teratoma. However, astrocytoma is not listed as a possible cause of LE. There is a case report of spinal cord astrocytomas with possible paraneoplastic encephalitis; however, neither it had histological confirmation nor were the immunological markers found positive.[5]
LE is characterized by personality changes, irritability, depression, seizures, confusion, loss of recent memory, and dementia. The symptoms are due to dysfunction of the limbic system (hippocampus, amygdala, hypothalamus, insular and cingulated cortex). The diagnosis of LE is often difficult clinically because LE may precede detection of tumor and in patients with known malignancy, similar symptoms can be attributed to many other malignancy-related complications, including brain metastases, metabolic encephalopathies, infections (especially with herpes simplex encephalitis) and toxic encephalopathy secondary to the side effects of chemotherapeutic agents, etc.
Imaging plays a crucial role in such circumstances. MR imaging can aid in establishing the diagnosis of paraneoplastic LE, especially in those cases in which no characterized antineuronal antibody can be detected in serum or CSF. T2-weighted imaging and FLAIR imaging techniques are useful.
Gultekin et al.[6] mentioned that typical MRI findings of paraneoplastic encephalitis include unilateral or bilateral mesial temporal lobe abnormalities that are best seen on FLAIR and T2-weighted images. On T1 sequences, the temporal-limbic regions may be hypointense and atrophic, and may sometimes enhance with contrast injection. They also mentioned that 64% of their patients with paraneoplastic encephalopathy had abnormal MRI studies, which showed the changes indicated above in 89% of the cases. Contrast enhancement is very rare with only a few cases reported.[7]
In our patient, imaging differential of seizure-induced changes in the presence of intracranial SOLs was excluded. There was no resolution or interval change in cortical abnormalities on sequential MRIs when compared to seizure-induced cortical changes which show resolution on follow-up MRI.
Other differential that needs consideration with such imaging findings would be herpes encephalitis. However, clinical course of herpes encephalitis is acute. In our case, clinical course was subacute. Presence of mass lesions could not be explained with herpes encephalitis.
In cases of glial tumors, sometimes differentiation between malignant tumors, including dissemination or gliomatosis cerebri and paraneoplastic LE, becomes difficult. Nagata et al. reported a patient with secondary gliomatosis cerebri that mimicked the clinico-radiological features of LE.[8] Gliomatosis cerebri might present a variety of MRI images. Thus, the possibility of gliomatosis cerebri in the bilateral temporal lobes could not be fully excluded in their case. Furthermore, a case with bilateral temporal lobe glioma presenting as paraneoplastic LE had been reported by Deramecourt et al.[9]
To the best of our knowledge, this case proves to be the first of its kind where intracranial astrocytic neoplasm is associated with paraneoplastic encephalitis. Also, direct histological evidence of both these entities makes it unique.
Conclusion
In conclusion, possibility of paraneoplastic LE should be thought of in cases of astrocytomas when intracranial findings cannot be explained by tumoral invasion. Differences in the imaging appearances and spectroscopy data between tumor and other areas of cortical signal abnormality may aid in suspecting this rare entity.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Dalmau J, Rosenfeld MR. ParaneoplasticsyndromesoftheCNS. Lancet Neurol. 2008;7:327–40. doi: 10.1016/S1474-4422(08)70060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antoine JC, Honnorat J, Anterion CT, Aguera M, Absi L, Fournel P, et al. Limbic encephalitis and immunological perturbations in two patients with thymoma. J NeurolNeurosurg Psychiatry. 1995;58:706–10. doi: 10.1136/jnnp.58.6.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grisold W, Giometto B, Vitaliani R, Oberndorfer S. Current approaches to the treatment of paraneoplastic encephalitis. TherAdvNeurolDisord. 2011;4:237–48. doi: 10.1177/1756285611405395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graus F, Vincent A, Pozo-Rosich P, Sabater L, Saiz A, Lang B, et al. Anti-glial nuclear antibody: Marker of lung cancer-related paraneoplastic neurological syndromes. J Neuroimmunol. 2005;165:166–71. doi: 10.1016/j.jneuroim.2005.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seki M, Suzuki S, Ishii K, Izawa Y, Takahashi S, Toyama Y, et al. Differential diagnosis between intracranial dissemination of spinal cord astrocytoma and paraneoplastic limbic encephalitis. Intern Med. 2012;51:321–4. doi: 10.2169/internalmedicine.51.5568. [DOI] [PubMed] [Google Scholar]
- 6.Gultekin SH, Rosenfeld MR, Voltz R, Eichen J, Posner JB, Dalmau J. Paraneoplastic limbic encephalitis: Neurological symptoms, immunological findings and tumor association in 50 patients. Brain. 2000;123:1481–94. doi: 10.1093/brain/123.7.1481. [DOI] [PubMed] [Google Scholar]
- 7.Thuerl C, Müller K, Laubenberger J, Volk B, Langer M. MR imaging of autopsy-proved paraneoplastic limbic encephalitis in non-Hodgkin lymphpma. AJNR Am J Neuroradiol. 2003;24:507–11. [PMC free article] [PubMed] [Google Scholar]
- 8.Nagata R, Ikeda K, Nakamura Y, Ishikawa Y, Miura K, Sato R, et al. A case of gliomatosiscerebri mimicking limbic encephalitis: Malignant transformation to glioblastoma. Intern Med. 2010;49:1307–10. doi: 10.2169/internalmedicine.49.3278. [DOI] [PubMed] [Google Scholar]
- 9.Deramecourt V, Bombois S, Debette S, Delbeuck X, Ramirez C, Reyns N, et al. Bilateral temporal gliomapresenting as a paraneoplasticlimbicencephalitiswith pure cognitive impairment. Neurologist. 2009;15:208–11. doi: 10.1097/NRL.0b013e31818fc022. [DOI] [PubMed] [Google Scholar]