Abstract
Background:
Lower extremity deep vein thrombosis (DVT) is a common illness with an annual incidence of 1 per 1000 adults. The major long-term complication of DVT is post-thrombotic syndrome (PTS) which occurs in up to 60% of patients within 2 years of an episode of DVT.
Aims:
We aim to evaluate the outcomes of catheter-directed treatment (CDT) for symptomatic acute or subacute lower extremity DVT.
Materials and Methods:
A retrospective 12-year study was conducted on the outcomes of CDT on 54 consecutive patients who presented with acute or subacute lower extremity DVT to our hospital.
Statistical Analysis:
Descriptive summary statistics and the Chi-square test were used to measure the outcomes of CDT.
Results:
Grade 3 thrombolysis was achieved in 25 (46.3%) patients, grade 2 thrombolysis in 25 (46.3%) patients, and grade 1 thrombolysis in 4 (7.4%) patients. Significant recanalization (grade 2 or 3 thrombolysis) was possible in 50 (92.6%) patients. There was no statistically significant difference in the percentage of significant recanalization that could be achieved between patients who underwent CDT before and after 10 days. There was no significant difference between the thrombolysis achieved between urokinase and r-tPA. PTS was seen in 33% of the patients. Major complications were seen in 5.5% of the patients.
Conclusion:
CDT is a safe and effective therapeutic technique in patients with acute and subacute lower extremity DVT, if appropriate patient selection is made.
Keywords: Catheter-directed treatment, deep vein thrombosis, thrombolysis
Introduction
Lower extremity deep vein thrombosis (DVT) is a common illness with an annual incidence of 1 per 1000 adults.[1] The major immediate outcomes of acute DVT include thrombus progression, pulmonary embolism (PE), phlegmasia alba dolens, phlegmasia cerulea dolens, venous gangrene, and death. The standard treatment of acute DVT is with oral anticoagulation which aims to prevent thrombus propagation and reduce the risk of PE; it, however, does not precipitate active thrombolysis.[2] The major long-term complication of DVT is post-thrombotic syndrome (PTS). PTS is a chronic debilitating illness characterized by limb swelling, pruritus, hyperpigmentation, pain, and ulcers. It is thought to result from chronic venous hypertension, which results from damage to the venous valves by thrombosis. PTS can occur in up to 60% of patients within 2 years of an episode of DVT.[3] It is traditionally managed conservatively with graded compression stockings and limb elevation. A Cochrane review showed that the risk of developing PTS reduced by a third when patients with DVT underwent catheter-directed treatment (CDT) against those who received standard anticoagulation treatment.[4] In this retrospective study, we aimed to evaluate the outcomes of CDT of symptomatic acute or subacute lower extremity thrombosis.
Materials and Methods
The Institutional Review Board of our institution approved this retrospective study. This was a 12-year retrospective study between 1 December 2002 and 30 November 2014 and data were collected from the online medical records department of the institution. All adult patients who underwent CDT for DVT of the popliteal or more proximal deep veins were included in the study. DVT was documented on Doppler ultrasonography (US) when there was a non-compressible deep vein with no color flow or by the presence of echogenic contents within the lumen of the vein. It was then confirmed on catheter venography where the extent of the DVT was also assessed. We performed CDT in patients up to 21 days from the onset of their symptoms, although in three patients, the exact time of onset of symptoms was less clear (documented as approximately 1 month in the medical record) and the decision to perform CDT was made based on imaging characteristics of the thrombus and other clinical determinants (e.g. young age, imminent venous gangrene, or Doppler US features of acute on chronic thrombosis).
DVT was treated with a standard CDT protocol as follows:
Venous access was obtained using an US-guided puncture and secured with a vascular sheath. The site of venous access varied through the years of the study. In the initial few years of the study, superficial or common femoral vein access was obtained in some patients with iliac or ilio-femoral DVT. Later, the preferred site of access was the popliteal vein (PV) irrespective of the extent of DVT. The thrombus was then laced with a hydrophilic-coated wire to improve subsequent drug penetration into the thrombus. This was followed by suction thrombectomy using a 6 French FasGuide catheter (Boston Scientific, Marlborough, MA, USA) [Figure 1A and B] and mechanical thrombus maceration using an angioplasty balloon. Pharmacological thrombolysis was done via a multi-side-hole infusion catheter with an end occluder (Fountain; Merit Medical, Galway, Ireland) [Figure 2] placed within the thrombus. The thrombolytic drug used was either urokinase at a dose rate of 500,000 to 1,000,000 units per 12 h or recombinant-tissue plasminogen activator (r-tPA) at a dose rate of 5–10 mg per 12 h, depending on the subjective thrombus load. A repeat catheter venogram was done after the initial thrombolysis and if there was inadequate thrombolysis, thrombolytic infusion continued at the same or reduced rate depending on the thrombus burden for a maximum of 72 h. The technical treatment endpoint was when (a) >90% thrombus load reduction was achieved on venography; or (b) maximum thrombolytic drug dosage was reached (1,000,000 units over a 12 h period for urokinase and 10 mg over a 12 h period for r-tPA); or (c) when the patient developed complications such as significant bleeding or pulmonary embolism. In patients with residual stenosis of the iliac or femoral veins, balloon venoplasty was performed (n = 45 [83%]) followed by stenting of the iliac veins (n = 11 [20%]) [Figure 3A–F]. In patients with a high risk for PE, either due to the presence of free floating thrombus or thrombus in the inferior vena cava (IVC), or in patients who developed DVT while already on systemic anticoagulation, an IVC filter was placed through the contralateral limb [(n = 8 ([15%]). Systemic heparin was administered during and after the procedure at the discretion of the physician, and warfarin was started prior to discharge with advice to continue it for at least 6 months, although no strict anticoagulation protocol was followed.
Figure 1 (A and B).
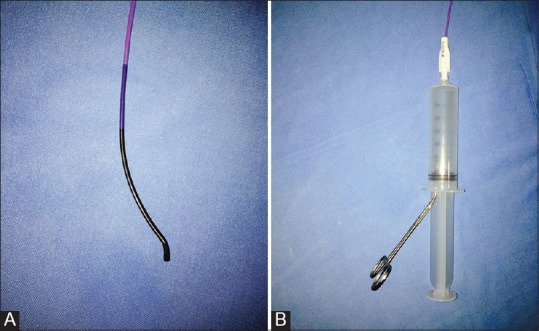
(A) Fasguide catheter (Boston Scientific, USA) used to perform suction thrombectomy. (B) Continuous suction applied using a 50 cc syringe with plunger withdrawn and clamped with a towel clamp
Figure 2.
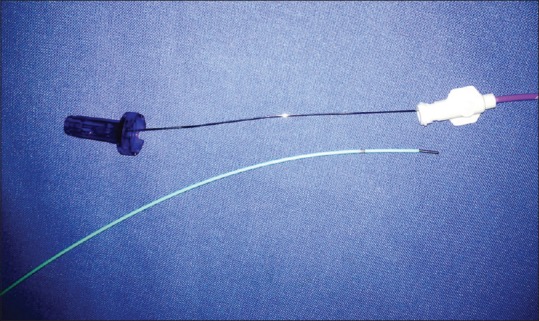
Fountain thrombolysis infusion catheter (Merit Medical, Galway, Ireland) with multiple side holes and end occluder
Figure 3 (A-F).
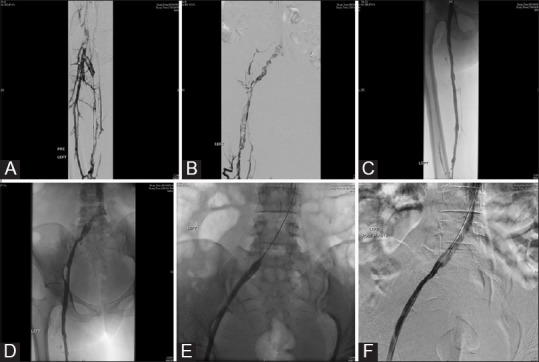
A 55-year-old female with acute left lower limb swelling and pain: (A) Popliteal venogram shows extensive filling defects within the SFV and CFV consistent with acute DVT; (B) iliac venogram shows extensive filling defects within the external and common iliac veins with no antegrade flow into the IVC; (C) and (D) post-thrombolysis venograms show no residual filling defects in the superficial and common femoral veins with some residual thrombus within the external and common iliac veins; (E) balloon plasty of the residual thrombus in the iliac veins; (F) check venogram post-balloon plasty shows grade 3 thrombolysis
Follow-up and measures of outcome
Patients were then followed up in the vascular surgery out-patient department and underwent US screening when clinically indicated. The average follow-up period was 29 months. We graded the success of thrombolysis using a well-known scale based on the percentage of thrombolysis achieved.[5] For the purpose of calculating the percentage of thrombolysis achieved, the venous system was divided into seven segments [Figure 4]:
Figure 4.
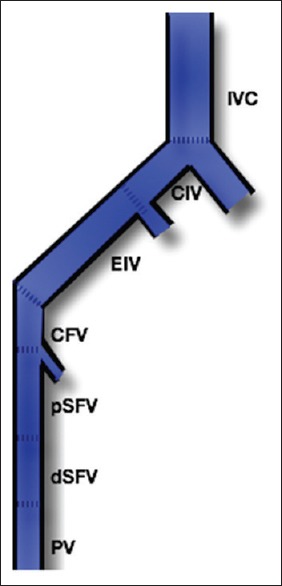
Segmental division of the venous system of the lower extremity. IVC: Inferior vena cava, CIV: Common iliac vein, EIV: External iliac vein, CFV: Common femoral vein, pSFV: Proximal superficial femoral vein, dSFV: Distal superficial femoral vein, PV: Popliteal vein
IVC
Common iliac vein (CIV)
External iliac vein (EIV)
Common femoral vein (CFV)
Proximal superficial femoral vein (pSFV)
Distal superficial femoral vein (dSFV)
Popliteal vein (PV).
Based on the assessment of the initial catheter venogram, a score of 0, 1, or 2 was assigned to each segment depending on whether there was no thrombus, partial thrombosis, or total occlusion, respectively. A thrombus load score was calculated as the sum of the score of the seven segments. Pre-treatment and post-treatment thrombus load scores were documented and the percentage thrombolysis was calculated as:

The thrombolysis was then graded based on the % of thrombolysis achieved [Table 1].
Table 1.
Grading of thrombolysis

Early patency of the deep veins was considered to have been achieved in patients with grade 2 or grade 3 thrombolysis on the immediate post-CDT venogram. We studied the association between the grade of thrombolysis achieved and the timing of CDT from patients’ initial symptoms. We studied the association between the grade of thrombolysis and the thrombolytic agent used. Intermediate and late patency rates of the venous system were studied at 6 months and 2 years wherever feasible. The presence or absence of PTS was noted at 1 year or later based on the clinical findings of limb swelling, skin induration or discoloration from venous stasis, dilated veins, or pain on calf compression.
Statistical analysis
Descriptive summary statistics were presented as means (Standard Deviation) for continuous variables and as frequencies with percentages for categorical variables. Chi-square test was use to study the association between thrombolytic agent used and venous patency. All statistical analyses were performed using SPSS software.
Results
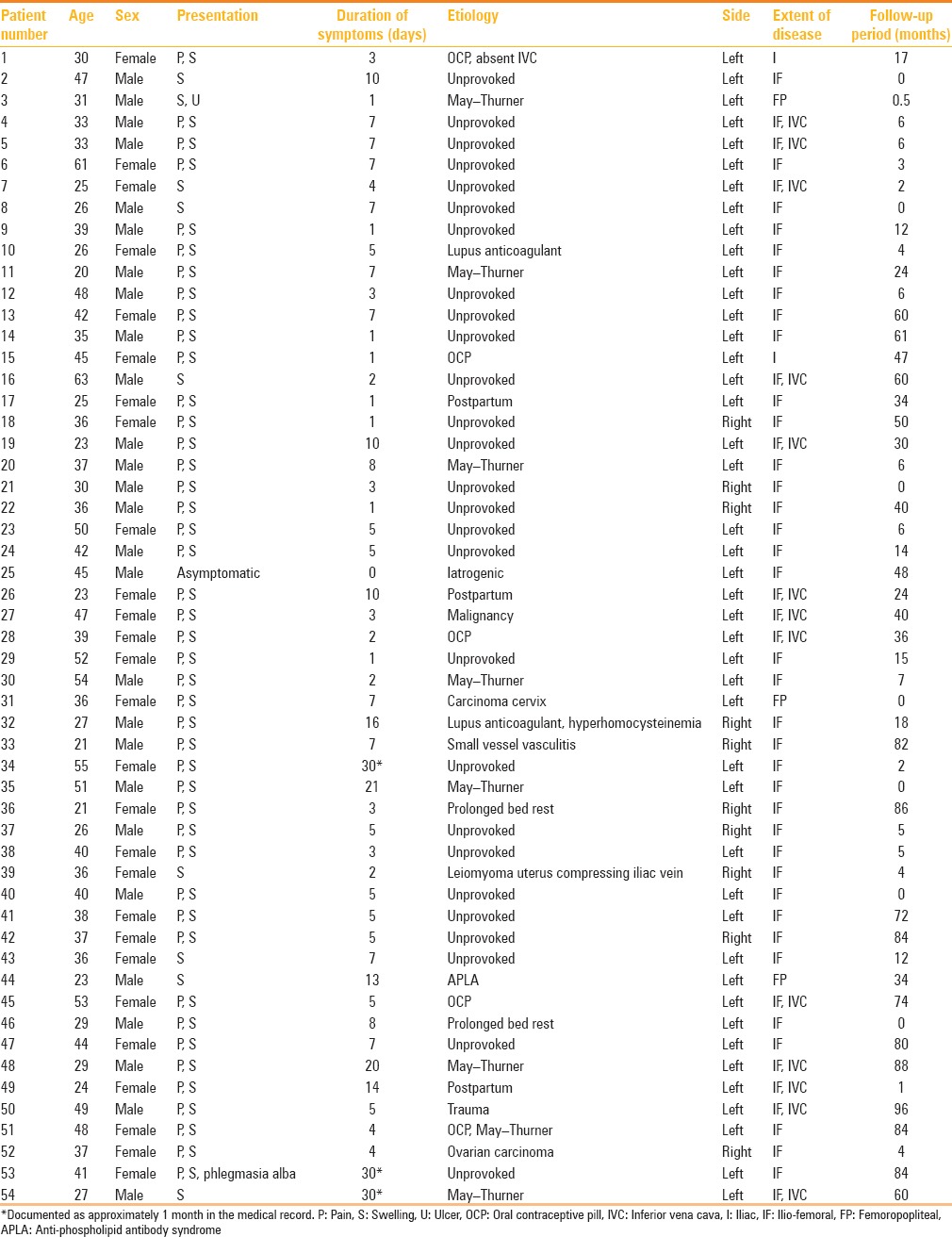
There were a total of 54 patients (27 male and 27 female; mean age, 37.2 years) included in the study. All patients had unilateral disease at the time of presentation with the left leg significantly involved more commonly (n = 44, 81%). The clinical details of patients included in this study are elaborated in Table 2. While 27 (n = 54, 50%) of the patients had no obvious etiology (unprovoked DVT), the commonest etiology for provoked DVT was May–Thurner syndrome (14.8%, 8/54) followed by oral contraceptive pill (OCP) intake (9.2%, 5/54). Acute symptoms (<10 days) were present in 46 patients and subacute symptoms (>10 days) in 8 patients. Seven of the patients who presented acutely underwent CDT after 10 days of presentation due to a delay in referral for medical reasons.
Table 2.
Clinical details of the patients included in the study
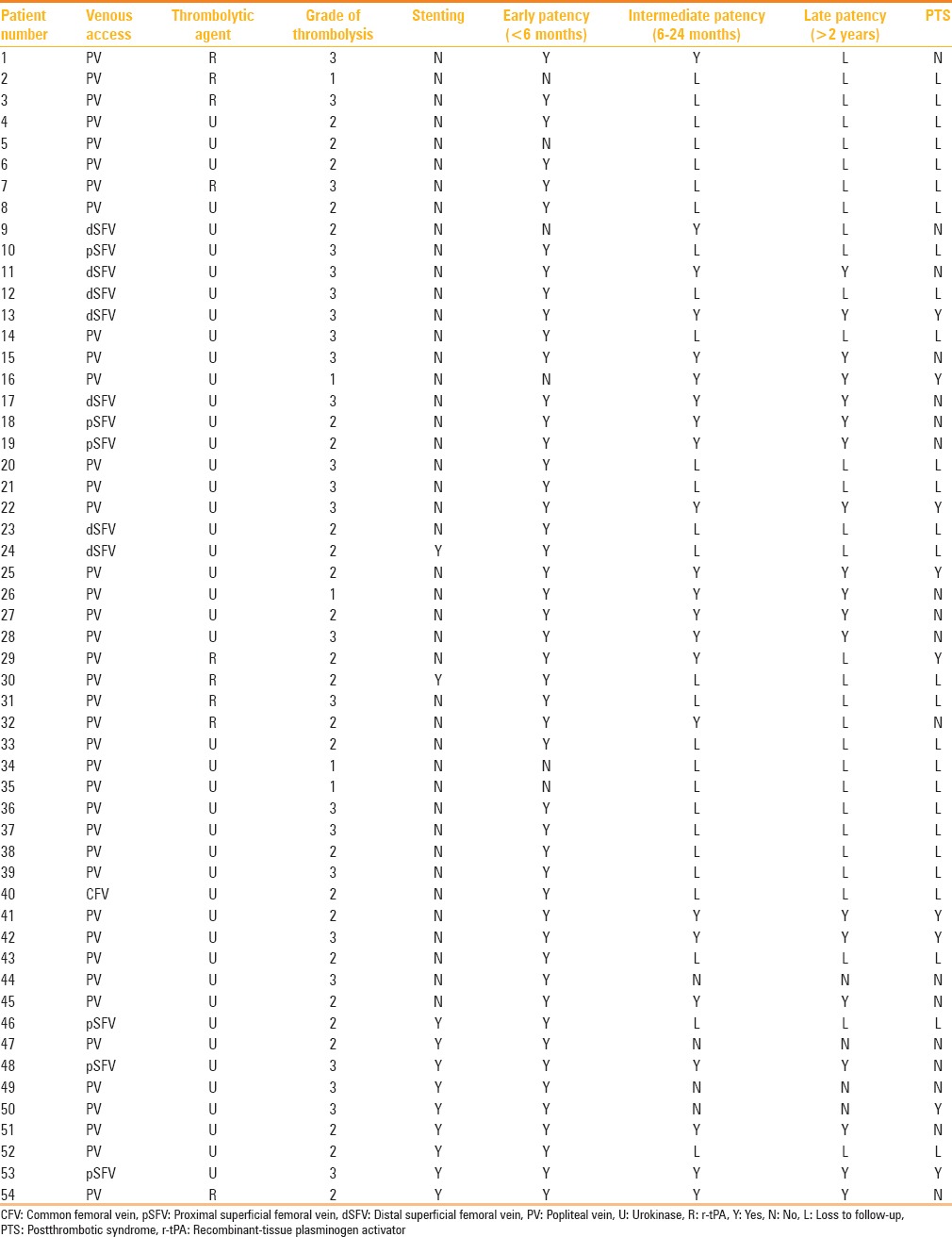
The procedural details and outcomes of the patients included in this study are elaborated in Table 3. The PV was the most common choice of access (74%, 40/54), irrespective of the extent of DVT. Urokinase was used in 45 patients (83.3%; mean dose 1,597,000 U) and r-tPA in 9 patients (16.7%; mean dose 31.25 mg) for a mean duration of 16.8 h.
Table 3.
Procedural details of the patients included in this study
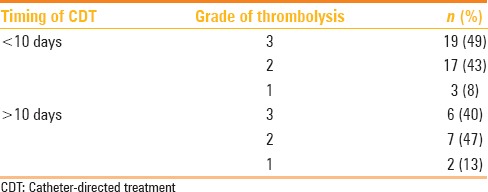
Early patency was graded based on the post-thrombolysis venogram. Grade 3 thrombolysis was achieved in 25 (n = 54, 46.3%) patients, grade 2 thrombolysis in 25 (n = 54, 46.3%) patients, and grade 1 thrombolysis in 4 (n = 54, 7.4%) patients. Significant recanalization (grade 2 or 3 thrombolysis) was possible in 50 (n = 54, 92.6%) patients. The association between the timing of CDT and grade of thrombolysis achieved is presented in Table 4. There was no statistically significant difference in the percentage of significant recanalization that could be achieved between patients undergoing CDT before and after 10 days.
Table 4.
Association between the timing of CDT from the onset of symptoms and the grade of thrombolysis achieved
Among the 11 patients who underwent stenting, all had adequate early patency (100%), although two patients developed partial in-stent thrombosis, one immediately after placement of the stent and another after 1 week. Both patients were successfully treated with bolus administration of 5000 U of unfractionated heparin and CDT with urokinase. The patency rate reduced to 63% at 6 months as four patients had developed complete occlusion of the stents, two of whom had short segment in-stent occlusions and underwent a failed attempt at endovascular recanalization. At 1 year of follow-up, one patient had an in-stent occlusion which was successfully treated with suction thrombectomy and venoplasty [Figure 5A–C]. At 2 years of follow-up, the patency rate remained stable at 63%.
Figure 5 (A-C).
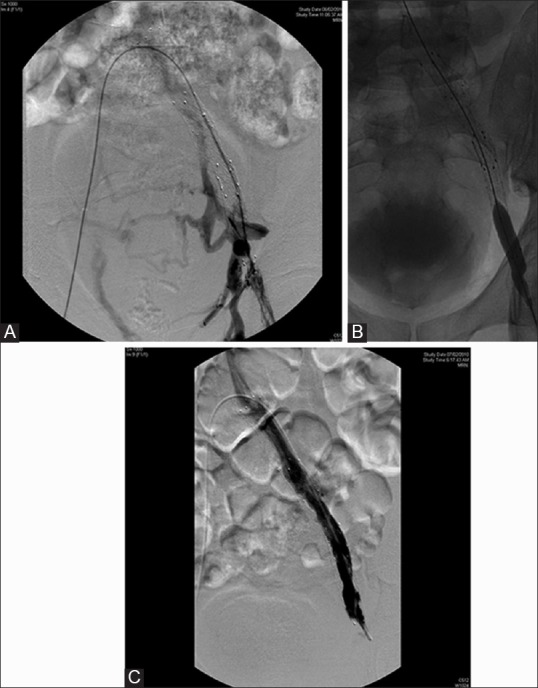
A 48-year-old female with May–Thurner-induced left lower limb ilio-femoral DVT, status post-CDT and stenting at 2-year follow-up: (A) iliac venogram showing complete occlusion of the stent; (B) post-suction thrombectomy balloon venoplasty of the in-stent stenosis; (C) post-venoplasty venogram showing complete recanalization
The IVC filter which was placed in eight patients was successfully removed in three patients. While four patients were lost to follow-up, in one patient it was not technically possible to remove the filter as the filter was embedded in the vena caval wall.
In patients with at least 6 months of follow-up, intermediate and late patency rates were 85% (23/27) and 82.6% (19/23), respectively. During follow-up, one patient who had previously been classified as grade 1 thrombolysis on the immediate post-CDT venogram was initiated on systemic anticoagulation subsequently and had adequate recanalization on follow-up with Doppler US 6 months later. All patients who had intermediate or late loss of patency had had iliac venous stents inserted.
Comparison of the early, intermediate, and late patency rates between thrombolysis achieved with urokinase and r-tPA showed that there was no significant difference between the two thrombolytic agents [Table 5].
Table 5.
Comparison of thrombolysis achieved between urokinase and r-tPA in terms of early, intermediate, and late venous patency

The incidence of PTS was studied in patients with at least 1 year of follow-up. PTS was seen in 33% (9/27) of the patients. The extent of initial DVT had no association with the occurrence of PTS.
Overall, there were four major complications seen in three patients (3/54, 5.5%). These included one patient with uterine fibroids who had major bleeding per vagina requiring blood transfusion and another patient who developed PE. One death was attributed to PE and presumed tumoral hemorrhage in a patient with an underlying esophageal malignancy.
Discussion
CDT has become one of the first-line treatments in the management of acute lower extremity DVT, especially in ilio-femoral and more proximal DVT.[6] Apart from achieving lysis of the thrombus using lower doses of anticoagulants, by means of CDT, the etiology that triggered the thrombosis can be treated in many patients with May–Thurner syndrome. Moreover, IVC filter placement during CDT can prevent recurrent PE in patients who are unsuitable candidates for long-term anticoagulation. CDT by itself does not increase the risk of PE; so, IVC filters should not be routinely placed during CDT. Patients who might benefit from IVC filter placement include those with standard indications like a contraindication to systemic anticoagulation, recurrence of DVT in patients already on effective anticoagulation, and in patients with free floating thrombus or thrombus in the IVC. We recommend that the decision to place an IVC filter be made on a case-to-case basis after discussion in a multi-disciplinary team meeting. We also recommend the placement of retrievable filters which must be removed once the indication for filter placement has passed.
The success rate of early recanalization which we achieved, of 46.3% each of grade 3 and grade 2 thrombolysis, is comparable to that of other studies. In a randomized control trial of 103 patients, Enden et al. reported a grade 3 thrombolysis in 48% and grade 2 thrombolysis in 40% of patients who presented within 21 days of DVT. In their study, they compared the patency at 6 months in patients who underwent CDT versus those who were treated with oral anticoagulation alone and found that the patency improved from 36% to 64% with the addition of CDT.[7]
The success of recanalization is influenced by the timing of CDT: Fresh thrombus lyses more readily than chronic thrombus. There are no standard guidelines establishing a cut-off time for intervention using CDT for lower limb DVT with regards to onset of symptoms. In our institute, we use a cut-off time interval of 21 days from the onset of symptoms, although different trials have used varying cut-off time intervals: 14 days in the ATTRACT trial and 21 days in the CaVenT trial for performing CDT,[8,9] Interestingly, in our study, successful recanalization was achieved in a significant number of patients who presented even after 10 days of symptoms. This is similar to the success rates of recanalization achieved in a study by Mewissen et al.[5] This possibly indicates that the age of the thrombus cannot be accurately predicted based merely upon the duration of the patient's symptoms.
In patients with May–Thurner syndrome, primary stent placement can be performed after CDT. This improves short-term patency rates, with one study showing rethrombosis in all patients who were treated with only CDT without stent placement.[10] The long-term benefit of venous stent placement is less clear as the stents themselves can be thrombogenic with low luminal flow rates. Hence, we recommend frequent Doppler US follow-up in all patients who have undergone venous stenting.
The long-term patency achieved with CDT is excellent, with one of the largest long-term prospective studies demonstrating patency rates without reflux of up to 82% of patients at 6 years of follow-up.[11] This is higher than the 63% long-term patency that was achieved in our study, possibly because of differences in case selection. These rates compare favorably to the 45% rate of long-term patency achieved through systemic fibrinolytics.[12]
The occurrence of PTS did not have an association with the extent of DVT at initial presentation. This finding is similar to that of a study by Prandoni et al. who studied the long-term outcomes of DVT in 528 patients.[13] But there are other studies which show that the incidence of PTS is more common in patients with proximal or ilio-femoral DVT.[14] This may be because collateral venous channels that help divert blood from the lower limb to the IVC usually drain into the deep femoral vein. The obstruction of the iliac and common femoral vein prevents the effective formation of these collaterals as compared to patients with isolated calf or popliteal DVT.
Similar to the findings in our study, in a retrospective study of CDT for DVT by Grunwald and Hofmann, no significant difference was noted between urokinase and r-tPA with regards to successful recanalization or major complications.[15] But there is a considerable difference in the cost of the various thrombolytic agents and this directs the choice of agent at our institute. The recommended dose ranges for the commonly used thrombolytic agents are 80,000–120,000 IU/h of urokinase or 0.2–0.5 mg/h of r-tPA.[16]
Complication rates in CDT can be reduced by careful patient selection and using the lowest dose of thrombolytic drug. The most important major complication which can occur is hemorrhage. In patients with known malignancies, intracranial metastases should be excluded before proceeding with CDT, as intracranial hemorrhage can be fatal. One mortality in our study was of a patient with a non-metastatic esophageal malignancy who developed pulmonary embolism following CDT and had presumed tumoral bleeding which complicated the thrombolytic treatment. Careful patient selection could have prevented this complication.
The limitations of our study were its retrospective nature and loss to follow-up of some patients after the early treatment period.
Conclusion
Catheter-directed thrombolysis is a safe and effective therapeutic technique in patients with acute and subacute DVT. The rate of successful recanalization can be high even in patients who present with more than 10 days of symptoms. The incidence of PTS can be reduced with the appropriate usage of CDT, especially in patients with ilio-femoral and more proximal DVT. There is no significant difference in the efficacy or complications between r-tPA and urokinase. Complication rates are low and can be further reduced with appropriate case selection and use of low doses of thrombolytic agents.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I4–8. doi: 10.1161/01.CIR.0000078468.11849.66. [DOI] [PubMed] [Google Scholar]
- 2.Büller HR, Agnelli G, Hull RD, Hyers TM, Prins MH, Raskob GE. Antithrombotic therapy for venous thromboembolic disease: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):401S–28S. doi: 10.1378/chest.126.3_suppl.401S. [DOI] [PubMed] [Google Scholar]
- 3.Brandjes DP, Büller HR, Heijboer H, Huisman MV, de Rijk M, Jagt H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet. 1997;349:759–62. doi: 10.1016/S0140-6736(96)12215-7. [DOI] [PubMed] [Google Scholar]
- 4.Watson L, Broderick C, Armon MP. Thrombolysis for acute deep vein thrombosis. Cochrane Database Syst Rev. 2014;1:CD002783. doi: 10.1002/14651858.CD002783.pub3. [DOI] [PubMed] [Google Scholar]
- 5.Mewissen MW, Seabrook GR, Meissner MH, Cynamon J, Labropoulos N, Haughton SH. Catheter-directed thrombolysis for lower extremity deep venous thrombosis: Report of a national multicenter registry. Radiology. 1999;211:39–49. doi: 10.1148/radiology.211.1.r99ap4739. [DOI] [PubMed] [Google Scholar]
- 6.Sousa Nanji L, Torres Cardoso A, Costa J, Vaz-Carneiro A. Analysis of the cochrane review: Thrombolysis for acute deep vein thrombosis. Cochrane Database Syst Rev 2014;1:CD002783. Acta Med Port Jan-Feb. 2015;28(1):12–14. doi: 10.20344/amp.6286. [DOI] [PubMed] [Google Scholar]
- 7.Enden T, Kløw NE, Sandvik L, Slagsvold CE, Ghanima W, Hafsahl G, et al. Catheter-directed thrombolysis vs.anticoagulant therapy alone in deep vein thrombosis: Results of an open randomized, controlled trial reporting on short-term patency. J Thromb Haemost. 2009;7:1268–75. doi: 10.1111/j.1538-7836.2009.03464.x. [DOI] [PubMed] [Google Scholar]
- 8.Comerota AJ. The ATTRACT trial: Rationale for early intervention for iliofemoral DVT. Perspect Vasc Surg Endovasc Ther. 2009;21:221–4. doi: 10.1177/1531003509359311. [DOI] [PubMed] [Google Scholar]
- 9.Enden T, Sandvik L, Kløw NE, Hafsahl G, Holme PA, Holmen LO, et al. Catheter-directed Venous thrombolysis in acute iliofemoral vein thrombosis – the CaVenT study: Rationale and design of a multicenter, randomized, controlled, clinical trial ( NCT00251771) Am Heart J. 2007;154:808–14. doi: 10.1016/j.ahj.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Kim JY, Choi D, Guk Ko Y, Park S, Jang Y, Lee do Y. Percutaneous treatment of deep vein thrombosis in May-Thurner syndrome. Cardiovasc Intervent Radiol. 2006;29:571–5. doi: 10.1007/s00270-004-0165-7. [DOI] [PubMed] [Google Scholar]
- 11.Baekgaard N, Broholm R, Just S, Jørgensen M, Jensen LP. Long-term results using catheter-directed thrombolysis in 103 lower limbs with acute iliofemoral venous thrombosis. Eur J Vasc Endovasc Surg. 2010;39:112–7. doi: 10.1016/j.ejvs.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Comerota AJ, Aldridge SC. Thrombolytic therapy for deep venous thrombosis: A clinical review. Can J Surg. 1993;36:359–64. [PubMed] [Google Scholar]
- 13.Prandoni P, Villalta S, Bagatella P, Rossi L, Marchiori A, Piccioli A, et al. The clinical course of deep-vein thrombosis. Prospective long-term follow-up of 528 symptomatic patients. Haematologica. 1997;82:423–8. [PubMed] [Google Scholar]
- 14.Tick LW, Kramer MH, Rosendaal FR, Faber WR, Doggen CJ. Risk factors for post-thrombotic syndrome in patients with a first deep venous thrombosis. J Thromb Haemost. 2008;6:2075–81. doi: 10.1111/j.1538-7836.2008.03180.x. [DOI] [PubMed] [Google Scholar]
- 15.Grunwald MR, Hofmann LV. Comparison of urokinase, alteplase, and reteplase for catheter-directed thrombolysis of deep venous thrombosis. J Vasc Interv Radiol. 2004;15:347–52. doi: 10.1097/01.rvi.0000121407.46920.15. [DOI] [PubMed] [Google Scholar]
- 16.Swischuk JL, Smouse HB. Differentiating pharmacologic agents used in catheter-directed thrombolysis. Semin Intervent Radiol. 2005;22:121–9. doi: 10.1055/s-2005-871867. [DOI] [PMC free article] [PubMed] [Google Scholar]


