Abstract
In order to maximize their fitness, organisms in seasonal environments rely on external cues to optimally time their life‐history stages. One of the most important zeitgeber to time reproduction is the photoperiod, but further environmental cues are assessed to fine‐tune reproduction due to year‐to‐year variation in environmental conditions. However, in urbanized environments, the pervasive artificial light at night has altered the natural signal of light and darkness. Accordingly, artificial light at night was repeatedly shown to affect avian reproductive physiology and to advance seasonal reproduction in birds. However, these experiments were mainly conducted in the absence of further environmental cues to facilitate the investigation of the mechanisms which are still poorly understood. Here, we investigate whether the endocrine system of free‐ranging European blackbirds (Turdus merula) correlates with the amount of artificial light at night along a rural to urban gradient while the birds still encounter complementary environmental cues including seasonal variation in day length and temperature. Testosterone and estrone were assessed as metabolites in fecal samples and corticosterone in blood from mist‐netted blackbirds. We demonstrate that seasonal fluctuations in abiotic factors, individual conditions, but also light at night affect the reproductive and stress physiology of wild European blackbirds. Elevated artificial night light intensities were significantly positively correlated with corticosterone and negatively with female estrone levels. No effects of artificial light were found for testosterone levels. Our results suggest that female blackbirds in particular perceive even low levels of artificial light at night as a weak but chronic stressor that interacts with the hypothalamic‐pituitary‐gonadal axis and leads to a reduced secretion of reproductive hormones. These findings point out that the impacts of light pollution are diverse and we only slowly disentangle its multiple effects on physiology, ecology, and biodiversity.
Keywords: Circannual rhythmicity, endocrine disruption, light loggers, light pollution, timing of reproduction, urbanization
Introduction
In seasonal environments, organisms need to optimally time life‐history stages to maximize their fitness (Bradshaw and Holzapfel 2007). As most stages require preparations well in advance, proximate environmental cues are assessed to anticipate appropriate conditions in the future, while ultimate factors ensure further fine‐tuning according to year‐to‐year variations (Bradshaw and Holzapfel 2007; Ball and Ketterson 2008). The most important proximate cue is the photoperiod because it is easily assessed and highly reliable over evolutionary time scales (Bradshaw and Holzapfel 2007). In nontropical birds, for example, gonads are usually inactive and considerably regressed in size during the nonbreeding season (Dawson et al. 2001). The increasing photoperiod triggers a hormonal cascade via the hypothalamic–pituitary–gonadal (HPG) axis which stimulates the maturing of the reproductive organs (Dawson et al. 2001). Consequently, the gonads release sex steroids such as testosterone (T) and estrogens which initiate secondary sexual characteristics including a wide range of reproductive behaviors such as territory establishment, courtship display, and nest building (Ball 1993). Toward the end of the breeding season, long photoperiods no longer sustain reproduction, but induce photorefractoriness and birds rapidly regress their gonads (Dawson et al. 2001; Bradshaw and Holzapfel 2007). To break photorefractoriness and close the annual cycle, birds need to experience short days like in late autumn and early winter (Dawson et al. 2001; Bradshaw and Holzapfel 2007). Hence, avian reproduction is closely linked to the seasonal alternation of short and long days.
However, since the dawn of industrialization, an increasing amount of artificial light at night (LAN) scatters through the atmosphere and blurs the natural light signal. In developed areas, not only artificial lighting of roads, office and public buildings, but also private homes cause night skies to exceed the brightness of full‐moon nights (Cinzano et al. 2001; Kyba et al. 2011). Consequentially, the natural darkness decreases to a persistent twilight and the formerly reliable signal of day length loses its accurateness. This loss of the strong day/night signal affects organisms of many different taxa, including humans, due to the widespread interaction between the natural cycle of light and darkness and biological processes (Navara and Nelson 2007). Serious consequences range from disruption of behavior and physiology to changes in species interactions and community structures (Longcore and Rich 2004).
Urban settlements are the areas most affected by LAN and challenge organisms further by, for example, novel food resources, different microclimate, and anthropogenic noise (Partecke et al. 2005; Slabbekoorn and Ripmeester 2008). In particular, the latter seems to be challenging for urban birds as the noise interferes with avian acoustic communication, has the potential to change species composition, and may cause serious fitness consequences (Francis et al. 2009; González‐Oreja et al. 2012; Schroeder et al. 2012). Nevertheless, some species manage to settle and survive in these disturbed habitats. To cope with the new environmental conditions, urban organisms seem to adjust behavioral and physiological traits, as well as life‐history strategies. Becoming tamer, breeding in higher densities, extending the breeding period and daily activity patterns were considered as adaptations to urban life (e.g., Luniak and Mulsow 1988; Bergen and Abs 1997; Partecke et al. 2005; Fuller et al. 2007; Chamberlain et al. 2009; Atwell et al. 2012). These responses to the urban environment are mediated by the organism's endocrine system (Bonier 2012). For example, elevated baseline corticosterone (CORT) as well as reduced CORT response to handling in urban birds were seen as an adaptation to cope with the greater variety of challenges in the urban environment (Bonier et al. 2007; Zhang et al. 2011; Atwell et al. 2012). However, changes in endocrine patterns due to urban influences in general were not consistent between species, sexes, and life‐history stages (Bonier 2012).
A well‐studied example for urban–rural divergence is the European blackbird (Turdus merula). Laboratory experiments indicated that LAN has the potential to advance both seasonal reproduction and the onset of morning song activity of the blackbirds (Dominoni et al. 2013a,b). However, in field studies of blackbirds, these patterns were also attributed to better food supply, higher temperature and, regarding the onset of dawn song, also to urban noise (Cuthill and Macdonald 1990; Partecke et al. 2005; Nordt and Klenke 2013).
Despite the increasing amount of research examining the effects of LAN, it is still poorly understood how LAN interacts with the avian endocrinology under natural conditions. Here, we aim to find out whether variations in the release of reproductive hormones in free‐living European blackbirds correlate with LAN while the birds encounter environmental fluctuations in day length and temperature which affect the physiology of the birds in a complementary fashion. We investigated noninvasively the concentrations of T as fecal conjugate, and oestrogen as its main fecal metabolite estrone sulfate (E1S), to assess variations in these two sex steroids in the context of environmental, individual, and anthropogenic influences. Although we expected testosterone to show greater variations in males and, thus, be closer linked to male responses to environmental influences whereas E1S might represent female responses, both reproductive hormones were investigated in either sex of the blackbirds. A recent study indicated a positive correlation between LAN and baseline concentrations of the main avian glucocorticoid CORT in wild birds (Ouyang et al. 2015). Therefore, we also linked the reproductive hormones to the stress physiology by including plasma concentrations of CORT into the analysis.
Material and Methods
Study site and field techniques
Between April 2011 and May 2013, free‐ranging adult blackbirds (Fig. 1) were mist‐netted along a rural–urban gradient in the city of Leipzig, Germany (51°20′ 23″ N, 12°22′ 23″ E). This gradient ranged from an urban forest across several parks to the city center which is confined by a busy and brightly illuminated ring road (Fig. 2). For a detailed description of the study area, see Nordt and Klenke (2013). Captured individuals were banded with a metal ring and an alphanumeric coded color ring for later identification in the field. Birds were weighed to the nearest 0.1 g (TCB 200‐1; Kern, Balingen‐Frommern, Germany), and the tarsus was measured to the nearest 0.01 mm. To determine plasma CORT levels, blood samples (n = 168) were taken by puncturing the brachial vein with a 25‐g needle (Terumo, Eschborn, Germany) and collecting 200–500 μL of blood in a heparinized tube. The time between capture and completed blood sampling was taken for inclusion into further analysis. After bleeding was staunched, the bird was put into an opaque box for approximately 10–15 min to defecate and was released afterward. The box was lined with plastic foil to facilitate the collection of the fecal sample (n = 192) which consisted of liquid and solid components of the droppings and was further referred to as feces. Additional 447 fecal samples were obtained by observing ringed and naive adult blackbirds during activities at the ground until they defecated. Only those droppings with unambiguous identity were collected. Within 1–3 h of collection fecal samples were stored at −20°C. Blood samples were centrifuged (10 min, 500 g) to separate plasma from red blood cells and also stored at −20°C until further processing.
Figure 1.

Male European blackbird during the early breeding season. Photograph by A. Künzelmann, UFZ.
Figure 2.
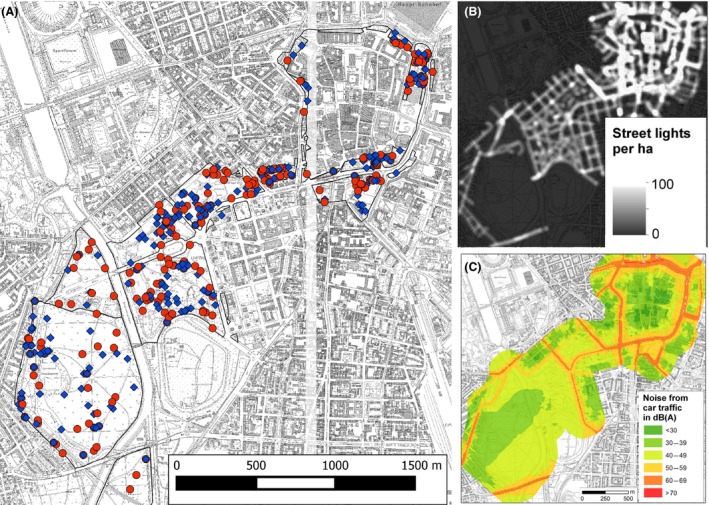
Overview of study area. Sampling of male (blue diamonds) and female (red circles) European blackbirds occurred along a rural to urban gradient (A) ranging from an urban forest in the southwest, across several larger parks to the city center in the northeast with adjacent tiny parks and green spaces. This gradient is also found in the distribution of artificial light (B) and anthropogenic noise from car traffic (C).
T and E1S hormone assays
Approximately one‐third of sampled feces were discarded because occasionally both methods of fecal sampling did not yield enough material, leaving 389 and 406 samples for the analyses of T and E1S, respectively. Of these samples, 54 % originated from male blackbirds and 46 % of females. Ninety‐three T samples (23.4 %) resulted from repeated sampling of 35 birds and 101 E1S samples (24.9 %) of 39 birds.
Preconditioning of feces was conducted as previously described elsewhere (Hahn et al. 2011). After lyophilization, the fecal samples were incubated with an enzyme solution (glucuronidase/arylsulfatase in citrate buffer, pH 4.8) to cleave conjugated steroids and thus to detect the total concentration of T and E1S. The extraction with diethylether was followed by vaporization of the supernatant and resuspension of the extracts in assay buffer (pH 7.2). The analysis of T and E1S was performed via specifically developed competitive enzyme immunoassays (Hahn et al. 2011; Weissmann et al. 2013). Ninety‐six well plates coated with sheep anti‐rabbit IgG and hormone‐specific antibodies (T: in‐house immunization; E1S: R522‐2, Clinical Endocrinology Laboratory, UC Davis, USA) as well as hormone‐enzyme conjugates (T‐HRP: Fitzgerald Industries International, North Acton, MA; E1S‐HRP: Clinical Endocrinology Laboratory) were used. The calibration curves ranged from 0.05 to 12.5 ng/mL (T) and 0.1 to 10 ng/mL (E1S), respectively. Sensitivities were 0.03 ng/mL (T) and 0.04 ng/mL (E1S), and recovery rates amounted to 78 % and 95 %, respectively. Interassay/intra‐assay variations were as follows: 4.4%/9.8% (T) and 8.5%/9.4% (E1S).
CORT hormone assay
Of the 168 blood samples, 52% were collected from males and 48% from female blackbirds. Thirty‐five of the samples (20.8 %) originated from repeated sampling of 17 recaptured individuals.
The plasma levels of CORT were quantified by a 3H‐radioimmunoassay (RIA) as previously described (Hahn et al. 2011). For sample preparation, disruptive proteins were eliminated by precipitation with ethanol. The supernatant solution was evaporated and resuspended in phosphate buffer. For the RIA, a hormone‐specific antibody (in‐house immunization), unmarked CORT (Serva Electrophoresis GmbH, Heidelberg, Germany) as a standard and [1,2,6,7‐3H]‐CORT (Perkin Elmer LAS GmbH, Rodgau Jügesheim, Germany) as a tracer were used. The hormone concentrations of the samples were recalculated by means of a calibration curve (0.1–5.0 ng/mL). The sensitivity of the assay amounted to 0.1 ng/mL. Intra‐assay and interassay coefficients of variation were 6.1 and 8.3%, respectively.
Environmental parameters
A detailed description of how LAN and anthropogenic noise was quantified can be found in Nordt and Klenke (2013). In brief, data of municipal street lighting were used as a proxy to quantify LAN at the mist‐netting and fecal sampling sites. The municipal traffic and works service, Leipzig, provided the light point data from which a lamp density index was calculated using kernel density estimation with a search radius of 50 m and a resolution of 10 m (ArcGIS 10; ESRI, Redlands, CA, USA). Validation of the lamp density index with illuminance measurements at 100 randomly selected points revealed a significant correlation. Thus, the lamp density index was regarded as an accurate indicator of LAN. The corresponding illuminance values (in lux) were calculated by means of this correlation and will be provided to facilitate comparability with other studies.
Traffic noise as another urban factor was considered to interfere with intraspecific acoustic communication and, thus, to stress the birds. Night‐time noise indices of car traffic and tramway were provided by the Environmental Protection Office Leipzig. They are based on separate counts of car and tramway traffic between 10 pm and 6 am, and provide standardized values of anthropogenic noise in dB(A) at a resolution of 10 by 10 m (Bundesministerium der Justiz 2006). Night‐time noise indices were included in the analyses because they overlap with periods of extensive blackbird dawn singing and also with the birds' exposure to LAN. Furthermore, night‐time noise indices are linearly correlated to daytime noise indices at 1000 random points (Pearson r car = 0.992, P < 0.001, r tramway = 0.996, P < 0.001) and can thus be used interchangeably.
Both noise and lamp density values were related to the mist‐netting and fecal sampling sites. Given the relatively coarse spatial resolution of noise and light indices and that resident birds were trapped and sampled at the nest site when rearing chicks or in their relatively small territories (0.74 ± 0.44 ha, A. Russ, unpublished data) when not breeding, we assume this approximation to provide an appropriate estimate of the birds' exposure to the anthropogenic factors traffic noise and LAN.
Data of temperature and precipitation were obtained from the German Weather Service' online Weather Request and Distribution System (https://werdis.dwd.de) to calculate running means per decade of the daily mean temperature (T 10 in °C) and running sums per decade of the daily precipitation (Prec10 in mm). These short‐term averages were expected to have a stronger influence on gonadal growth and regression than the highly variable daily mean temperature and sum of precipitation (Dawson 2008).
Statistical analyses
Whether the two fecal sampling methods revealed equal distributions of the concentrations of T and E1S was tested by a Wilcoxon rank‐sum test. Because the test indicated no differences between the two sampling methods (T: U = 12078, P = 0.24, E1S: U = 12385, P = 0.08), it was not differentiated between the sampling methods in subsequent analyses. Collinearity between potential explanatory variables was assessed using Pearson correlation. All pairwise correlations were far from being collinear (¦r¦ ≪ 0.8) and a variance inflation factor below 2 for all explanatory variables further indicated independence of the predictor variables (Zuur et al. 2009).
Three separate analyses were conducted to evaluate the relationship between the avian endocrine physiology and potential predictor variables, one analysis for each hormone response. We used general additive mixed models (GAMMs) to identify the major environmental predictors and their interactions that determine individual levels of reproductive and stress hormones. GAMMs were fitted in the statistical software system R (version 3.1.2, R Development Core Team 2013) using the packages mgcv (Wood 2004) and nlme (Pinheiro et al. 2013). We log10(x + 1)‐transformed T and E1S concentrations and the lamp density, and centered and standardized all continuous explanatory variables to allow comparison of the size of estimated coefficients (Schielzeth 2010).
The initial models for the responses of T and E1S contained the year and the sex of the individual as fixed factors. Cubic regression spline smoothers were constructed for the continuous explanatory variables day‐of‐year, sampling time, T 10, and lamp density, each separately for males and females, while cubic regression spline smoothers of Prec 10 were included for each year. Two‐way interactions between day‐of‐year and lamp density for each sex and year, as well as between day‐of‐year and T 10, for each sex were modeled as full tensor product smooths. To separate the influence of sampling time from the season, the sampling time was related to the length of day, starting with morning civil twilight (when the sun is 6° below the horizon) and ending with evening civil twilight when the sun is, again, 6° below the horizon.
In addition to the explanatory variables used in T and E1S models, the initial model of CORT included cubic regression spline smoothers of the elapsed time between capture and blood sampling (handling time), the body condition, the car noise and the tramway noise, the latter two separately for both males and females. The body condition was calculated as sex‐specific residuals from an ordinary least‐squares linear regression between body mass and tarsus length (Schulte‐Hostedde et al. 2005). As bird handling times ranged between 4 and 45 min (median = 12 min), which makes it difficult to differentiate between baseline CORT levels and a stress response, we restricted this analysis to samples for which the handling time did not exceed 10 min. This constraint reduced the sample size to N = 69 with 29 male and 40 female samples.
Because residuals showed heterogeneity between study years, different residual variances per year were included into the individual models of T, E1S, and CORT using the varIdent function (Zuur et al. 2009). To account for repeated sampling of birds, the individual was included as a random intercept in all models. The gonadal hormones T and E1S were further tested whether they correlate with CORT levels in individuals where both blood (taken within 10 min) and fecal samples were taken at the same day (N = 29). We built linear models with the log‐transformed T or E1S concentrations as response variable, included the linear and second‐order polynomial of day‐of‐year, as well as the sex, year, CORT, and T10 as fixed effects.
Model selection was based on Akaike's information criterion corrected for small sample sizes (AICc) (Wood 2006), an information‐theoretic approach (Burnham and Anderson 2002). All models with a ΔAICc < 4 of the respective best model were considered to receive competitive support and were included to calculate Akaike weights ω i (Burnham and Anderson 2002).
Results
Exposure to light at night and traffic noise
Along the rural to urban gradient, the lamp density at sampling sites ranged from 0 to 46.3 lamps per hectare, with a median of 6.3, which corresponds to an illuminance of 0.17 lux (range 0.07–61.7 lux). Anthropogenic noise ranged from 35 to 63 dB(A) (mean ± standard deviation, 47.5 ± 6.5 dB(A)) and from 25 to 64 dB(A) (42.4 ± 9.7 dB(A)) for night‐time car and tramway traffic, respectively.
T levels
Over the whole study period, T levels ranged from 0.2 to 1505.2 ng/g dry matter with an overall median of 20.5 ng/g. The best model (edf = 14.5, ω i = 0.69) indicated no effect of the lamp density on blackbird T concentrations but confirmed common patterns like significantly lower T levels for females (Table 1) as well as a clear annual cycle in male T levels with lowest concentrations in December and January and a steep increase in February (around day‐of‐year 50, Fig. 3). In addition to the annual cycle, male T concentrations were also significantly correlated to the mean temperature T 10. T increased with rising mean temperatures, peaked around 7°C, and decreased above 10°C. In contrast, female T levels showed a continuous increase with rising mean temperatures (Fig. 3). There was a strong year effect which mirrored to some extent the unequal sampling effort, that is, in 2013, samples were almost exclusively collected during the breeding period when T concentrations are elevated, whereas in 2011, the nonbreeding season was overrepresented. Apart from this, the winter 2012/2013 was extraordinary mild but subzero temperatures occurred in February and March 2013 (month means −1.5 and −5.9°C below long‐term average, respectively). T levels were significantly negatively correlated to the precipitation in 2013 only (Table 1), and hence, Prec10 contributed to a better model fit, especially its interaction with year (Prec10 × year: F = 7.21, P < 0.001). No further model received statistical support.
Table 1.
Model results for the variation in T, E 1 S, and CORT. Models are general additive mixed models (GAMMs). The response variables T and E1S were log‐transformed. Reference level for year is 2011 and for sex is male
| A) testosterone concentration | ||||
|---|---|---|---|---|
| Parameter | Level | Estimate ± SE | t | P |
| Intercept | 0.89 ± 0.07 | 12.8 | <0.001 | |
| year | 2012 | 0.18 ± 0.06 | 2.88 | 0.004 |
| 2013 | 1.12 ± 0.08 | 13.5 | <0.001 | |
| Sex | Female | −0.23 ± 0.05 | −4.63 | <0.001 |
| Prec10 | 0.06 ± 0.06 | 0.96 | 0.338 | |
| Prec10 × year | 2012 | 0.04 ± 0.06 | 0.70 | 0.484 |
| 2013 | −0.16 ± 0.08 | −2.07 | 0.040 | |
| Smooth terms | Level | edf | F | P |
|---|---|---|---|---|
| Day‐of‐year by sex | Male | 2.94 | 2.00 | <0.001 |
| Female | 0.00 | 0.00 | 0.976 | |
| T10 by sex | Male | 3.54 | 6.31 | <0.001 |
| Female | 1.00 | 42.1 | <0.001 |
| B) estrone sulfate concentration | ||||
|---|---|---|---|---|
| Parameter | Level | Estimate ± SE | t | P |
| Intercept | 1.19 ± 0.09 | 12.8 | <0.001 | |
| Year | 2012 | 0.06 ± 0.09 | 0.63 | 0.527 |
| 2013 | 0.03 ± 0.11 | 2.79 | 0.005 | |
| Sex | Female | 0.05 ± 0.04 | 1.42 | 0.157 |
| Lamp density | 0.05 ± 0.03 | 1.61 | 0.108 | |
| T10 | 0.10 ± 0.04 | 2.48 | 0.014 | |
| Lamp density × sex | Female | −0.10 ± 0.04 | −2.68 | 0.008 |
| Smooth terms | Level | edf | F | P |
|---|---|---|---|---|
| Day‐of‐year | 4.76 | 3.76 | <0.001 | |
| Prec10 by year | 2011 | 1.00 | 4.44 | 0.036 |
| 2012 | 1.00 | 1.51 | 0.220 | |
| 2013 | 4.22 | 6.62 | <0.001 |
| C) corticosterone concentration | ||||
|---|---|---|---|---|
| Parameter | Level | Estimate ± SE | t | P |
| Intercept | 35.34 ± 4.47 | 7.91 | <0.001 | |
| Year | 2012 | −10.92 ± 3.37 | −3.24 | 0.002 |
| 2013 | −23.7 ± 4.10 | −5.80 | <0.001 | |
| Sex | Female | −4.19 ± 1.91 | −2.20 | 0.033 |
| Body condition | −3.66 ± 0.95 | −3.86 | <0.001 | |
| Handling time | −8.00 ± 3.28 | −2.44 | 0.019 | |
| Smooth terms | Level | edf | F | P |
|---|---|---|---|---|
| Day‐of‐year | 4.43 | 6.06 | <0.001 | |
| Lamp density by sex | Male | 1.00 | 5.01 | 0.030 |
| Female | 2.10 | 10.25 | <0.001 | |
| T10 by sex | Male | 2.27 | 55.91 | <0.001 |
| Female | 2.02 | 7.83 | 0.001 | |
| Tram noise by sex | Male | 2.87 | 5.36 | 0.003 |
| Female | 1.00 | 16.96 | <0.001 |
Figure 3.
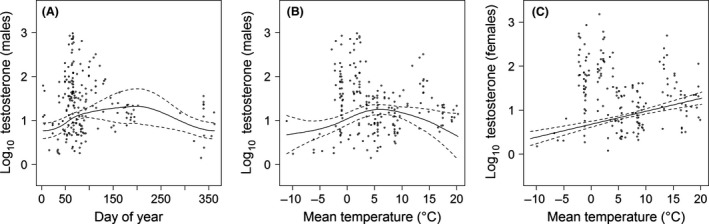
Smoothers related to day‐of‐year (A) and the mean temperature for males (B) and females (C) for the logarithmized testosterone concentration of blackbirds as predicted by the general additive mixed model (GAMM) with point wise 95 % confidence bands. The points indicate the distribution of the original data.
E1S levels
The median concentration of E1S in fecal samples was 23.2 ng/g (range 0.6–604.5 ng/g) over the study period. The best ranked model (edf = 10.7, ω i = 0.52) revealed equal E1S concentrations between the sexes. Also, E1S concentrations showed a significant seasonal pattern, they increased in February and March, declined thereafter until the end of May and showed intermediate values until the end of the year (Fig. 4). As in T concentrations, E1S levels differed between the years (F = 17.02, P < 0.001) with low levels in 2011 and 2012, whereas in 2013, significantly higher concentrations were measured, probably because most samples in 2013 were collected during the breeding season and the year effect is therefore partly confounded with seasonal variation. Mean temperature T 10 had a weak but significant effect on E1S concentrations (Table 1), but the precipitation did not influence the E1S concentrations and was therefore excluded from the best model. Furthermore, the model indicated that the sexes differ in their response of E1S concentrations to the lamp density. A marginally significant negative effect is predicted for female E1S, indicating decreasing E1S with increasing lamp densities, while there is no effect on male E1S concentrations. Further candidate models with statistic support included smoothers of Prec10 per year (edf = 17.8, ω i = 0.40) and excluded the temperature from the set of predictor variables (edf = 17.8, ω i = 0.08).
Figure 4.
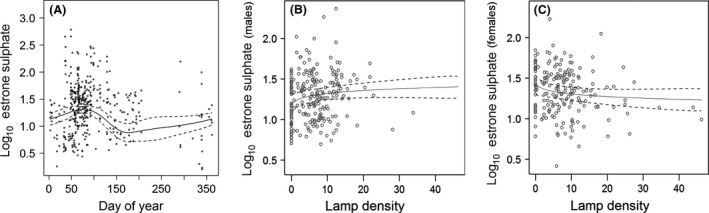
Smoother related to day‐of‐year (A) and lamp density for males (B) and females (C) for the logarithmized estrone sulfate concentration of blackbirds as predicted by the general additive mixed model (GAMM) with pointwise 95 % confidence bands. The points indicate the distribution of the original data.
CORT levels
Basal CORT levels during the study period averaged 29.5 ± 12.2 ng CORT/mL blood plasma (mean ± SD, range 7.9 – 55.6 ng/mL). Females had, on average, 12 % lower CORT levels than males, but both sexes showed equally decreasing CORT with higher body condition. The best model (edf = 21.7, ω i = 1.00) indicated also clear differences between years (F = 17.66, P < 0.001) with the lowest CORT concentrations in 2013 (Table 1). During breeding, blackbirds exhibited elevated CORT levels, which peaked at the height of breeding in April and May and decreased toward the end of the reproductive period in July (Fig. 5A). Lowest CORT was predicted for autumn and a second CORT peak occurred in winter, but the base data are very limited for this time of the year. The precipitation had no effect while the CORT decreased with increasing mean temperature but the extent differed between the sexes (Table 1, Fig. 5F,G). Furthermore, there was no daytime (sampling time) effect but CORT levels decreased significantly with higher body condition of the birds and, paradoxically, also marginally significant with longer bird handling times (Table 1). The noise from car traffic did not affect the CORT concentrations and was excluded from the best model, while tramway traffic was negatively correlated with CORT levels in females and showed a unimodal pattern in male blackbirds (Fig. 5D,E). Additionally, both sexes showed a significant correlation with artificial LAN: the higher the lamp density the higher the CORT level (Fig. 5B,C). No other candidate CORT model received statistical support according to their AIC value.
Figure 5.
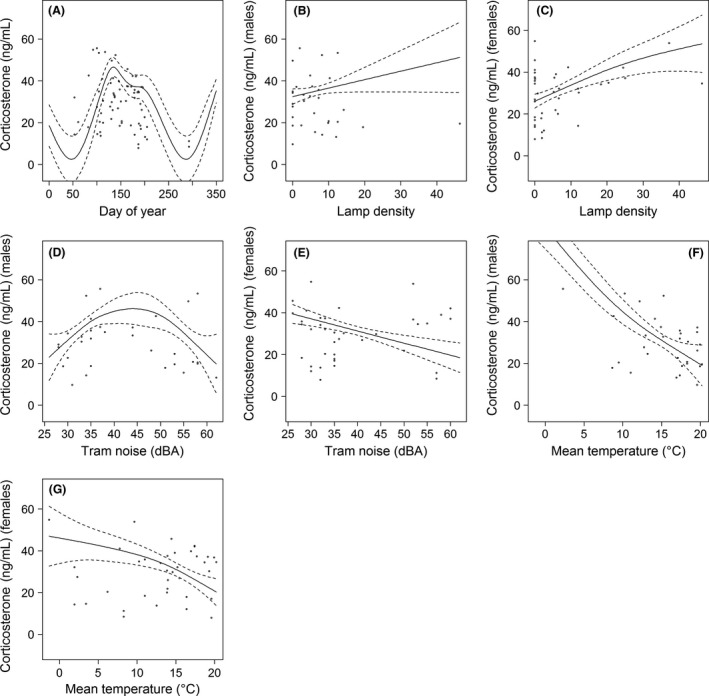
Smoother related to day‐of‐year (A), lamp density, tram noise, and mean temperature for plasma CORT of male (B, D, F) and female blackbirds (C, E, G) as predicted by the general additive mixed model (GAMM) with pointwise 95 % confidence bands. The points indicate the distribution of the original data.
The linear regression between the two reproductive hormones and CORT indicated a significant negative correlation between CORT and T levels (−0.017 ± 0.006, F = 15.84, P < 0.001), but not for E1S and CORT (F = 2.47, P = 0.129).
Discussion
This study demonstrates that the reproductive physiology of wild European blackbirds is closely correlated with seasonal fluctuations in abiotic factors, whereas the stress physiology is also linked to individual condition, and anthropogenic factors.
Among the abiotic factors, photoperiod and increasing temperatures play a crucial role in determining the accurate time to start breeding in temperate birds (Dawson et al. 2001; Wingfield et al. 2003; Visser et al. 2009) which is often conveyed by elevated levels of reproductive hormones during spring (e. g. Smith et al. 1997). Here, the correlation of day‐of‐year with T and E1S levels confirm that the photoperiod provides a general cue of favorable conditions for breeding, whereas temperature and probably also precipitation allow fine‐tuning of the timing of reproduction due to year‐to‐year variations. A clear seasonal pattern was found for E1S levels but less definite in T levels. This might reason in T levels being less closely linked to photoperiod but stronger affected by year‐to‐year variations of abiotic or biotic factors, which is also indicated by the higher year effect and its interaction with the precipitation in T compared to E1S. However, in this study the predicted effect of year has to be handled with caution due to the unbalanced sampling effort in the three years of the study which impedes differentiating between effects of the year and the season. Regarding biotic factors, intraspecific aggression such as fighting territory intruders can elevate plasma T in males (reviewed in Wingfield et al. 1990; Parisot et al. 2005). Actually, these aggressive interactions might occur over almost the entire year, because a high percentage of the urban blackbirds refrain from migrating but remain in their breeding habitat, partly even defend territories and start breeding attempts in winter (Stephan 1999; Partecke and Gwinner 2007). Permanently elevated T, though, is disadvantageous for the individual as it may simultaneously suppress the immune function (Casto et al. 2001; Martin et al. 2008). Therefore, T may be converted into estrogens to mitigate the immunosuppression by reducing the concentration of circulating plasma T (reviewed in Hau 2007). Another possible but not mutually exclusive explanation lies in the extended breeding period of urban birds. Organisms with a relatively long breeding season may exhibit low to intermediate T levels over most of the reproductive season, which is only elevated during short phases of courtship and mating (Hau et al. 2000; Wingfield et al. 2001). We possibly missed those short events of drastically increased T by (1) catching blackbirds during the breeding season primarily while rearing offspring, hence, when peak T has already attenuated to provide parental care (reviewed in Hau 2007), and by (2) using fecal samples to determine T concentrations. In contrast to plasma T, fecal T levels show less variation and rarely reflect acute responses, but provide a cumulative measure over a prolonged period of time (Ninnes et al. 2010). The rate at which T is excreted in feces depends on the overall metabolism and defecation frequency of the organism (Palme 2005), but a reasonable estimate is 24–48 h (Tell 1997). Therefore, we do not find a daily pattern in T levels although there is evidence that it exists in plasma T (Kempenaers et al. 2008).
Nevertheless, despite applying the same methodology to obtain T and E1S concentrations, we find a strong correlation of E1S with the photoperiod. This strengthens our view that T levels are more susceptible to further environmental influences while E1S is more closely linked to the photoperiod. In line with this hypothesis, we find an effect of the lamp density for E1S but not for T levels. The marginal but significant effect of the lamp density on female E1S levels can be interpreted as perceived prolongation of the photoperiod. According to the annual cycle of reproduction and photorefractoriness (Dawson et al. 2001), the effect of a prolonged photoperiod might differ with the season and reproductive state of the bird. During late winter and early spring, while preparing for breeding, prolonged days should enhance the reproductive hormone levels, whereas a negative effect is expected during late breeding and molting in summer. However, our results indicate that higher lamp densities lower female E1S levels irrespective of the seasons (Table 1). This contradiction might erase from restricted accuracy of the chosen methods, particularly the light index included in our study. However, the corresponding night light intensities (mean 0.17 lux) fit well to those measured with light loggers attached to free‐ranging blackbirds (range 0–2.2 lux) and light treatment (0.3 lux) used in subsequent experiments to investigate the effects of LAN (Dominoni et al. 2013a).
Alternatively, the effect of the lamp density on E1S levels and the lack of a correlation in T levels can also be interpreted by the complex interrelationship of the endocrine system. An environmental cue which is perceived as a chronic stressor can trigger a stress response and affect the bird's reproductive physiology. CORT, the main avian glucocorticoid, mediates the endocrine stress response which downregulates the reproductive physiology at several levels of the HPG axis (Schoech et al. 2009). CORT decreases the expression of gonadotropin‐releasing hormone and the pituitary responsiveness to this neurohormone, which in turn suppresses the release of luteinizing hormone (LH), retards gonadal growth rates, and finally inhibits the release of sex steroids such as T and estradiol (E2) (rewied in Schoech et al. 2009; Goutte et al. 2010). Additionally, CORT acts via the gonadotropin‐inhibitory hormone (GnIH), which further downregulates the HPG axis (Schoech et al. 2009; Goutte et al. 2010). Although we did not find a significant correlation between E1S levels and CORT concentrations which might be due to the limited sample size and variations in environmental conditions, here, female CORT increased with higher lamp densities suggesting that LAN might act as such a chronic stressor and via the described pathways cause the observed decreasing female E1S levels. Likewise, in males, baseline CORT levels were positively correlated with the lamp density and the T levels in turn decreased with higher CORT levels. This interaction might have extenuated the seasonal variation in T levels although we did not find a direct correlation between T and the lamp density. Further support for this hypothesis comes, for example, from western scrub‐jays (Aphelocoma californica). Exposed to LAN, the scrub‐jays had lower LH, E2, and T levels than the dark controls (Schoech et al. 2013). Campo et al. (2007) reports that domestic chickens were significantly stressed under continuous light conditions, while diurnal rats exposed to dim LAN had elevated corticosterone levels, too, and showed an increased immune function (Fonken et al. 2012). However, why we do not find any effects in males and on T concentrations despite contrasting results in other studies (Partecke et al. 2005; Schoech et al. 2013) remains speculative but may be linked to the high variability seen in T levels.
Anthropogenic noise also possibly bears a stressful component as it was repeatedly shown to interfere with avian acoustic communication in urban environments (reviewed in Brumm and Slabbekoorn 2005). However, noise from car traffic did not affect the CORT in our analyses. Birds seem to be capable to avoid masking noise by changing temporal and acoustic traits of their song (e.g., Brumm 2004; Slabbekoorn and den Boer‐Visser 2006; Fuller et al. 2007) without perceiving noise as a stressor. Paradoxically, the noise from tramway traffic was predicted to have a mitigating effect on blackbird CORT (Fig. 5). However, this effect is difficult to interpret because tramway noise differs in its characteristics from traffic noise which is rather permanent. Tramway noise increases intermittently every time a tram passes by but between these noise peaks short fractions of time would allow acoustic communication between the birds. Since Rowan's first experiments on photoperiod and avian reproduction (Rowan 1925), numerous studies report a stimulating effect of experimentally increased day length or LAN on gonad recrudescence (e.g., Farner and Wilson 1957; Dominoni et al. 2013a). This seems to contrast with our results, but we do not exclude an advancing effect of LAN on the gonadal growth of the blackbirds in our study. Although we did not investigate the timing of gonad maturation, we found city blackbirds to start breeding earlier than their forest conspecifics (A. Russ, unpublished data) despite the moderate inhibition of E1S levels under high street lamp densities. Therefore, decreased E1S and possibly also T levels due to elevated LAN do not necessarily indicate suppressed reproduction. In contrast, reproduction or gonadal maturation can still be advanced, as observed in several studies, where overall T levels remained lower in light‐treated birds although they developed their gonads earlier than birds in dark controls (Partecke et al. 2005; Dominoni et al. 2013a; Zhang et al. 2014).
By now, the question persists why our results differ from those of laboratory experiments. An important aspect to consider in laboratory experiments is the comprehensive limitation to one or few environmental cues to assess the factor(s) of interest (Calisi and Bentley 2009). This restriction might also constrain the organism's plasticity because additional environmental cues certainly act in a complementary fashion. In the experiments of photoperiod and gonad recrudescence, birds are usually held under constant conditions of temperature and ad libitum food supply to measure the sole effect of photoperiod or LAN on gonad maturation. However, in nature, temperature and food availability underlie seasonal fluctuations like the photoperiod. In particular, the food availability plays an important role as supplemental factor in the decision to reproduce (reviewed in Ball and Ketterson 2008). Reproduction needs to be optimally timed so that food is available in sufficient amounts not only for adults, but also for the offspring during chick rearing (Dawson et al. 2001). Scarce food availability is perceived as stressful, and birds in an inferior body condition exhibit elevated CORT levels (Lanctot et al. 2003; Kitaysky et al. 2007), as our results also clearly indicate. Furthermore, males are more stressed than females probably due to intraspecific aggression, song production, or the maintenance of elevated T levels (Buchanan et al. 2001; Wingfield et al. 2001). As mentioned above, the release of CORT during stress responses shifts the trade‐off between reproduction and self‐maintenance toward the latter by redirecting energy toward physiological and behavioral adjustments that enhance the possibility to survive (Wingfield and Sapolsky 2003; Goutte et al. 2010). Hence, in an experimental setting, where food is provided ad libitum, the birds might overcome the stress triggered by LAN by ingesting additional food. In the wild, complying with higher energy demands might be a considerably selective pressure, especially in the context of additional stressors such as predation, intraspecific competition, or challenging weather conditions which are excluded in laboratory experiments. Hence, LAN might be perceived as longer or increasing photoperiod and, thus, stimulate gonadal recrudescence when further breeding requirements such as food availability, temperature, and nesting sites are not limiting.
In conclusion, our results suggest that LAN has the potential to interfere with the physiology of urban blackbirds. As we base these conclusions on observational findings, the causation remains to be tested because the found effects may be related to other influences which coincide with the presence of LAN. However, we suggest that LAN is perceived as a weak but chronic stressor that interacts with the HPG axis and leads to a reduced secretion of reproductive hormones. This interference might not have detrimental effects under optimal conditions or in species that appear to cope well with a life in urban areas such as the European blackbird, but may constitute an additional threat when environmental conditions deteriorate or in less stress‐tolerant species. As LAN rapidly increases by approximately 6 % per annum (Hölker et al. 2010a), the percentage of species that perceive LAN no further as a weak stressor but as a serious threat will certainly increase. The impacts of LAN are diverse, and we only slowly disentangle its multiple negative effects on ecology, biodiversity, and human health (Longcore and Rich 2004; Navara and Nelson 2007; Hölker et al. 2010b).
Data Accessibility
The data sets supporting this article can be assessed at the Dryad Digital Repository as Russ et al. (2015) doi: 10.5061/dryad.kv32b (http://dx.doi.org/10.5061/dryad.kv32b).
Conflict of Interest
None declared.
Acknowledgments
The authors acknowledge funding from the Federal Ministry of Education and Research, Germany (BMBF, FKZ 033L038E), the Helmholtz Impulse and Networking Fund through Helmholtz Interdisciplinary Graduate School for Environmental Research and the European Union's Seventh Programme for research, technological development and demonstration under grant agreement No 226852. The authors are thankful to all field assistants supporting us in sample collection and mist‐netting: Terézia Lučeničová, Sarah Effertz, Rebecca Thier‐Lange, Diana Höhlig, Simon Dietzel, Tobias Köhler, Alexandra Erbach, and Daniela Dunger. The comments of two anonymous reviewers considerably improved the manuscript. All procedures were carried out under licences of the appropriate German authorities (bird ringing 01/04‐MH, Environmental Protection Office Leipzig; blood sampling A 05/11, state directorate Leipzig).
References
- Atwell, J. W. , Cardoso G. C., Whittaker D. J., Campbell‐Nelson S., Robertson K. W., and Ketterson E. D.. 2012. Boldness behavior and stress physiology in a novel urban environment suggest rapid correlated evolutionary adaptation. Behav. Ecol. 23:960–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball, G. F. 1993. The neural integration of environmental information by seasonally breeding birds. Am. Zool. 33:185–199. [Google Scholar]
- Ball, G. F. , and Ketterson E. D.. 2008. Sex differences in the response to environmental cues regulating seasonal reproduction in birds. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363:231–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen, F. , and Abs M.. 1997. Verhaltensökologische Studie zur Gesangsaktivität von Blaumeise (Parus caeruleus), Kohlmeise (Parus major) und Buchfink (Fringilla coelebs) in einer Großstadt. J. Ornithol. 138:451–467. [Google Scholar]
- Bonier, F. 2012. Hormones in the city: endocrine ecology of urban birds. Horm. Behav. 61:763–772. [DOI] [PubMed] [Google Scholar]
- Bonier, F. , Martin P. R., Sheldon K. S., Jensen J. P., Foltz S. L., and Wingfield J. C.. 2007. Sex‐specific consequences of life in the city. Behav. Ecol. 18:121–129. [Google Scholar]
- Bradshaw, W. E. , and Holzapfel C. M.. 2007. Evolution of animal photoperiodism. Annu. Rev. Ecol. Evol. Syst. 38:1–25. [Google Scholar]
- Brumm, H. 2004. The impact of environmental noise on song amplitude in a territorial bird. J. Anim. Ecol. 73:434–440. [Google Scholar]
- Brumm, H. , and Slabbekoorn H.. 2005. Acoustic communication in noise Pp. 151–209 in Slater P. J. B., Snowdon C. T., Roper T. J., Brockmann H. J. and Naguib M., eds. Advances in the Study of Behavior. Academic Press, San Diego. [Google Scholar]
- Buchanan, K. L. , Evans M. R., Goldsmith A. R., Bryant D. M., and Rowe L. V.. 2001. Testosterone influences basal metabolic rate in male house sparrows: a new cost of dominance signalling? Proc. R. Soc. B 268:1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundesministerium der Justiz (2006) Bekanntmachung der vorläufigen Berechnungsverfahren für den Umgebungslärm nach § 5 Abs. 1 der Verordnung über die Lärmkartierung (34. BImSchV). Bundesanzeiger Verlagsgesellschaft mbH, Bonn. [Google Scholar]
- Burnham, K. P. , and Anderson D. R.. 2002. Model Selection and Multimodel Interference: A Practical Information‐Theoretic Approach, 2nd edn Springer, New York. [Google Scholar]
- Calisi, R. M. , and Bentley G. E.. 2009. Lab and field experiments: are they the same animal? Horm. Behav. 56:1–10. [DOI] [PubMed] [Google Scholar]
- Campo, J. L. , Gil M. G., Davila S. G., and Munoz I.. 2007. Effect of lighting stress on fluctuating asymmetry, heterophil‐to‐lymphocyte ratio, and tonic immobility duration in eleven breeds of chickens. Poult. Sci. 86:37–45. [DOI] [PubMed] [Google Scholar]
- Casto, J. M. , Nolan V. Jr, and Ketterson E. D.. 2001. Steroid hormones and immune function: experimental studies in wild and captive dark‐eyed Juncos (Junco hyemalis). Am. Nat. 157:408–420. [DOI] [PubMed] [Google Scholar]
- Chamberlain, D. E. , Cannon A. R., Toms M. P., Leech D. I., Hatchwell B. J., and Gaston K. J.. 2009. Avian productivity in urban landscapes: a review and meta‐analysis. Ibis 151:1–18. [Google Scholar]
- Cinzano, P. , Falchi F., and Elvidge C. D.. 2001. The first World Atlas of the artificial night sky brightness. Mon. Not. R. Astron. Soc. 328:689–707. [Google Scholar]
- Cuthill, I. C. , and Macdonald W. A.. 1990. Experimental manipulation of the dawn and dusk chorus in the blackbird Turdus merula . Behav. Ecol. Sociobiol. 26:209–216. [Google Scholar]
- Dawson, A. 2008. Control of the annual cycle in birds: endocrine constraints and plasticity in response to ecological variability. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363:1621–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson, A. , King V. M., Bentley G. E., and Ball G. F.. 2001. Photoperiodic control of seasonality in birds. J. Biol. Rhythms 16:365–380. [DOI] [PubMed] [Google Scholar]
- Dominoni, D. , Quetting M., and Partecke J.. 2013a. Artificial light at night advances avian reproductive physiology. Proc. R. Soc. B 280:20123017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominoni, D. M. , Helm B., Lehmann M., Dowse H. B., and Partecke J.. 2013b. Clocks for the city: circadian differences between forest and city songbirds. Proc. R. Soc. B 280:20130593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farner, D. S. , and Wilson A. C.. 1957. A quantitative examination of testicular growth in the White‐crowned sparrow. Biol. Bull. 113:254–267. [DOI] [PubMed] [Google Scholar]
- Fonken, L. K. , Haim A., and Nelson R. J.. 2012. Dim light at night increases immune function in Nile grass rats, a diurnal rodent. Chronobiol. Int. 29:26–34. [DOI] [PubMed] [Google Scholar]
- Francis, C. D. , Ortega C. P., and Cruz A.. 2009. Noise pollution changes avian communities and species interactions. Curr. Biol. 19:1415–1419. [DOI] [PubMed] [Google Scholar]
- Fuller, R. A. , Warren P. H., and Gaston K. J.. 2007. Daytime noise predicts nocturnal singing in urban robins. Biol. Lett. 3:368–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González‐Oreja, J. A. , De La Fuente‐Díaz‐Ordaz A. A., Hernández‐Santín L., Bonache‐Regidor C., and Buzo‐Franco D.. 2012. Can human disturbance promote nestedness? Songbirds and noise in urban parks as a case study. Landsc. Urban Plan. 104:9–18. [Google Scholar]
- Goutte, A. , Angelier F., Chastel C. C., Trouvé C., Moe B., Bech C., et al. 2010. Stress and the timing of breeding: glucocorticoid‐luteinizing hormones relationships in an arctic seabird. Gen. Comp. Endocrinol. 169:108–116. [DOI] [PubMed] [Google Scholar]
- Hahn, A. , Reitemeier S., Gottschalk J., Haense M., Schmidt V., Steinbach‐Sobiraj K., et al. 2011. Endokrinologische Untersuchung männlicher Papageienvögel zur Beurteilung ihres Reproduktionsstatus. Tieraerztl Prax K H 39:249–257. [PubMed] [Google Scholar]
- Hau, M. 2007. Regulation of male traits by testosterone: implications for the evolution of vertebrate life histories. BioEssays 29:133–144. [DOI] [PubMed] [Google Scholar]
- Hau, M. , Wikelski M., Soma K. K., and Wingfield J. C.. 2000. Testosterone and year‐round territorial aggression in a tropical bird. Gen. Comp. Endocrinol. 117:20–33. [DOI] [PubMed] [Google Scholar]
- Hölker, F. , Moss T., Griefahn B., Kloas W., Voigt C. C., Henckel D., et al. 2010a. The dark side of light: a transdisciplinary research agenda for light pollution policy. Ecol. Soc. 15:13. [Google Scholar]
- Hölker, F. , Wolter C., Perkin E. K., and Tockner K.. 2010b. Light pollution as a biodiversity threat. Trends Ecol. Evol. 25:681–682. [DOI] [PubMed] [Google Scholar]
- Kempenaers, B. , Peters A., and Foerster K.. 2008. Sources of individual variation in plasma testosterone levels. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363:1711–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaysky, A. , Piatt J., and Wingfield J.. 2007. Stress hormones link food availability and population processes in seabirds. Mar. Ecol. Prog. Ser. 352:245–258. [Google Scholar]
- Kyba, C. C. M. , Ruhtz T., Fischer J., and Hölker F.. 2011. Cloud coverage acts as an amplifier for ecological light pollution in urban ecosystems. PLoS One 6:e17307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanctot, R. B. , Hatch S. A., Gill V. A., and Eens M.. 2003. Are corticosterone levels a good indicator of food availability and reproductive performance in a kittiwake colony? Horm. Behav. 43:489–502. [DOI] [PubMed] [Google Scholar]
- Longcore, T. , and Rich C.. 2004. Ecological light pollution. Front. Ecol. Environ. 2:191–198. [Google Scholar]
- Luniak, M. , and Mulsow R.. 1988. Ecological parameters in urbanization of the European Blackbird Pp. 1787–1793 in Ouellet H., ed. Acta XIX Congressus Internationalis Ornithologici. University of Ottawa Press, Ottawa. [Google Scholar]
- Martin, L. B. , Weil Z. M., and Nelson R. J.. 2008. Seasonal changes in vertebrate immune activity: mediation by physiological trade‐offs. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363:321–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navara, K. J. , and Nelson R. J.. 2007. The dark side of light at night: physiological, epidemiological, and ecological consequences. J. Pineal Res. 43:215–224. [DOI] [PubMed] [Google Scholar]
- Ninnes, C. , Waas J. R., Ling N., Nakagawa S., Banks J. C., Bell D. G., et al. 2010. Comparing plasma and faecal measures of steroid hormones in Adelie penguins Pygoscelis adeliae . J. Comp. Physiol. B. 180:83–94. [DOI] [PubMed] [Google Scholar]
- Nordt, A. , and Klenke R.. 2013. Sleepless in town – drivers of the temporal shift in dawn song in urban European blackbirds. PLoS One 8:e71476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang, J. Q. , de Jong M., Hau M., Visser M. E., van Grunsven R. H. A., and Spoelstra K.. 2015. Stressful colours: corticosterone concentrations in a free‐living songbird vary with the spectral composition of experimental illumination. Biol. Lett., 11:20150517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palme, R. 2005. Measuring fecal steroids: guidelines for practical application. Ann. N. Y. Acad. Sci. 1046:75–80. [DOI] [PubMed] [Google Scholar]
- Parisot, M. , Tanvez A., Lacroix A., Vallet E., Beguin N., and Leboucher G.. 2005. Social competition and plasma testosterone profile in domesticated canaries: an experimental test of the challenge hypothesis. Horm. Behav. 48:225–232. [DOI] [PubMed] [Google Scholar]
- Partecke, J. , and Gwinner E.. 2007. Increased sedentariness in European blackbirds following urbanization: a consequence of local adaptation? Ecology 88:882–890. [DOI] [PubMed] [Google Scholar]
- Partecke, J. , Van't Hof T. J., and Gwinner E.. 2005. Underlying physiological control of reproduction in urban and forest‐dwelling European blackbirds Turdus merula . J. Avian Biol., 36:295–305. [Google Scholar]
- Pinheiro, J. , Bates D., DebRoy S., Sarkar D., and R Development Core Team (2013) nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1‐109. http://cran.r-project.org/package=nlme. [Google Scholar]
- R Development Core Team (2013) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Rowan, W. 1925. Relation of light to bird migration and developmental changes. Nature 115:494–495. [Google Scholar]
- Russ, A. , Reitemeier S., Weissmann A., Gottschalk J., Einspanier A., and Klenke R.. 2015. Data from: Seasonal and urban effects on the endocrinology of a wild passerine. Dryad Digital Repository, http://dx.doi.org/10.5061/dryad.kv32b. [DOI] [PMC free article] [PubMed]
- Schielzeth, H. 2010. Simple means to improve the interpretability of regression coefficients. Methods Ecol. Evol. 1:103–113. [Google Scholar]
- Schoech, S. J. , Rensel M. A., Bridge E. S., Boughton R. K., and Wilcoxen T. E.. 2009. Environment, glucocorticoids, and the timing of reproduction. Gen. Comp. Endocrinol. 163:201–207. [DOI] [PubMed] [Google Scholar]
- Schoech, S. J. , Bowman R., Hahn T. P., Goymann W., Schwabl I., and Bridge E. S.. 2013. The effects of low levels of light at night upon the endocrine physiology of Western scrub‐jays (Aphelocoma californica). J. Exp. Zool. A Ecol. Genet. Physiol. 319:527–538. [DOI] [PubMed] [Google Scholar]
- Schroeder, J. , Nakagawa S., Cleasby I. R., and Burke T.. 2012. Passerine birds breeding under chronic noise experience reduced fitness. PLoS One 7:e39200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte‐Hostedde, A. I. , Zinner B., Millar J. S., and Hickling G. J.. 2005. Restitution of mass‐size residuals: Validating body condition indices. Ecology 86:155–163. [Google Scholar]
- Slabbekoorn, H. , and den Boer‐Visser A.. 2006. Cities change the songs of birds. Curr. Biol. 16:2326–2331. [DOI] [PubMed] [Google Scholar]
- Slabbekoorn, H. , and Ripmeester E. A. P.. 2008. Birdsong and anthropogenic noise: Implications and applications for conservation. Mol. Ecol. 17:72–83. [DOI] [PubMed] [Google Scholar]
- Smith, G. T. , Brenowitz E. A., Beecher M. D., and Wingfield J. C.. 1997. Seasonal Changes in Testosterone, Neural Attributes of Song Control Nuclei, and Song Structure in Wild Songbirds. J. Neurosci. 17:6001–6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan, B. (1999) Die Amsel. Die Neue Brehm‐Bücherei Bd. 95, 2nd edn Westarp Wissenschaften, Hohenwarsleben. [Google Scholar]
- Tell, L. A. 1997. Excretion and metabolic fate of radiolabeled estradiol and testosterone in the cockatiel (Nymphicus hollandicus). Zoo Biol. 16:505–518. [Google Scholar]
- Visser, M. E. , Holleman L. J. M., and Caro S. P.. 2009. Temperature has a causal effect on avian timing of reproduction. Proc. R. Soc. B 276:2323–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann, A. , Reitemeier S., Hahn A., Gottschalk J., and Einspanier A.. 2013. Sexing domestic chicken before hatch: A new method for in ovo gender identification. Theriogenology 80:199–205. [DOI] [PubMed] [Google Scholar]
- Wingfield, J. C. , and Sapolsky R. M.. 2003. Reproduction and resistance to stress: When and how. J. Neuroendocrinol. 15:711–724. [DOI] [PubMed] [Google Scholar]
- Wingfield, J. C. , Hegner R. E., Dufty A. M. Jr, and Ball G. F.. 1990. The “challenge hypothesis”: Theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am. Nat. 136:829–846. [Google Scholar]
- Wingfield, J. C. , Lynn S., and Soma K. K.. 2001. Avoiding the ‘costs’ of testosterone: Ecological bases of hormone‐behavior interactions. Brain Behav. Evol. 57:239–251. [DOI] [PubMed] [Google Scholar]
- Wingfield, J. C. , Hahn T. P., Maney D. L., Schoech S. J., Wada M., and Morton M. L.. 2003. Effects of temperature on photoperiodically induced reproductive development, circulating plasma luteinizing hormone and thyroid hormones, body mass, fat deposition and molt in mountain white‐crowned sparrows, Zonotrichia leucophrys oriantha . Gen. Comp. Endocrinol. 131:143–158. [DOI] [PubMed] [Google Scholar]
- Wood, S. N. 2004. Stable and efficient multiple smoothing parameter estimation for generalized additive models. J. Am. Stat. Assoc. 99:673–686. [Google Scholar]
- Wood, S. N. (2006) Generalized Additive Models: An Introduction with R. Chapman & Hall/CRC, Boca Raton. [Google Scholar]
- Zhang, S. , Lei F., Liu S., Li D., Chen C., and Wang P.. 2011. Variation in baseline corticosterone levels of Tree Sparrow (Passer montanus) populations along an urban gradient in Beijing, China. J. Ornithol. 152:801–806. [Google Scholar]
- Zhang, S. , Chen X., Zhang J., and Li H.. 2014. Differences in the reproductive hormone rhythm of tree sparrows (Passer montanus) from urban and rural sites in Beijing: The effect of anthropogenic light sources. Gen. Comp. Endocrinol. 206:24–29. [DOI] [PubMed] [Google Scholar]
- Zuur, A. F. , Ieno E. N., Walker N. J., Saveliev A. A., and Smith G. M.. 2009. Mixed Effects Models and Extensions in Ecology with R. Springer, New York. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets supporting this article can be assessed at the Dryad Digital Repository as Russ et al. (2015) doi: 10.5061/dryad.kv32b (http://dx.doi.org/10.5061/dryad.kv32b).


