Abstract
Mechanical thrombectomy is a novel treatment option for patients with acute ischemic stroke (AIS). Only a few studies have previously suggested strategies to categorize retrieved clots according to their histologic composition. However, these reports did not analyze potential biomarkers that are of importance in stroke-related inflammation. We therefore histopathologically investigated 37 intracerebral thrombi mechanically retrieved from patients with AIS, and focused on the composition of immune cells and platelets. We also conducted correlation analyses of distinctive morphologic patterns (erythrocytic, serpentine, layered, red, white, mixed appearance) with clinical parameters. Most T cells and monocytes were detected in erythrocytic and red clots, in which the distribution of these cells was random. In contrast, von Willebrand factor (vWF)-positive areas co-localized with regions of fibrin and collagen. While clots with huge amounts of vWF seem to be associated with a high National Institute of Health Stroke Scale score at admission, histologic findings could not predict the clinical outcome at discharge. In summary, we provide the first histologic description of mechanically retrieved intracerebral thrombi regarding biomarkers relevant for inflammation in ischemic stroke.
Keywords: immune cells, lymphocytes, mechanical thrombectomy, ischemic stroke, inflammation, thrombus formation
1. Introduction
After successful clinical trials in patients with occlusion of a major intracranial artery, mechanical thrombectomy (MT) with stent retrievers adds a novel therapeutic option in patients with acute ischemic stroke (AIS) [1,2,3]. As MT is only applicable in approximately 5%–10% of patients with ischemic stroke, intravenous thrombolysis (IVT) using recombinant tissue plasminogen activator is still the treatment of choice for most patients with AIS [4]. In IVT [5], but not MT with stent retrievers [6], the response to therapy regarding recanalization and subsequent clinical outcome depends on thrombus length. In contrast, recanalization rates and clinical outcomes are dependent on thrombus density in computed tomography (CT) imaging—and consequently dependent on histologic thrombus composition—in both therapeutic strategies, IVT [7,8] as well as MT [9]. Therefore, knowledge of the detailed clot composition could become helpful to assign patients to a distinct treatment strategy—at the latest when novel treatment options next to IVT and MT arise.
The first histopathologic evaluation of cerebral thrombi was carried out about 50 years ago using post-mortem material [10]. However, a timely assessment of thrombus material immediately after AIS occurrence has only been possible since the development of MT devices a few years ago. Since then, several researchers analyzed the histologic composition of retrieved cerebral clots and suggested different strategies for categorization [11,12,13,14,15]. In addition, an increasing number of imaging studies have been published trying to visualize clot composition with CT [16,17,18] and magnetic resonance imaging (MRI) [13,14,19]. A series of studies evaluating novel MRI contrast agents specific for activated platelets [20] or fibrin [21,22] have been published. In the near future, these imaging strategies could allow identification of individual patients who might profit the most from a therapeutic intervention in AIS, and—vice versa—of patients who would most likely not profit from a particular treatment strategy.
The important role of immune cells on stroke development [23,24,25,26] and the underlying mechanism of thromboinflammation [27] is well established in animal models of ischemic stroke. It has been shown that immune cells (e.g., cluster of differentiation (CD) 4+ T cells, CD68+ monocytes) interact with molecules that are of importance for platelet signaling (e.g., von Willebrand factor (vWF)) and contribute to thrombus formation [27]. Monocyte–platelet aggregates are increased in patients with acute thrombotic events [28,29]. A recent clinical trial provided the first evidence that a pharmacologically induced lymphocytopenia is also associated with beneficial effects in human AIS [30]. Further clinical stroke studies addressing immunologic targets are on the way (e.g., ClinicalTrials.gov: NCT01955707).
Until now, the published reports about the histologic characterization of intracranial thrombi mainly focused on coagulation, and a precise assessment of inflammation has not yet been reported. Therefore, we aimed for a detailed characterization of the retrieved cerebral thrombi regarding biomarkers that play major roles in stroke-related inflammation.
2. Results
2.1. Demographic and Clinical Characterization of Patients
We histologically analyzed 37 thrombi (retrieved between 2012 and 2015) from patients with AIS with a mean age of 66 ± 16 years. Forty-nine percent of patients were male. Twenty-six patients (70%) received IVT before clot retrieval. The mean National Institutes of Health Stroke Scale (NIHSS) score [31] was 17 ± 7 at admission and 7 ± 4 at discharge of the patients. Location of the vessel occlusion was the middle cerebral artery (MCA) in 22 cases, intracranial part of the internal carotid artery (“carotid-T”, C-T) in 10 cases, or the basilar artery (BA) in five cases (Table 1).
Table 1.
Clinical characteristics of patients and categorization of thrombus histology.
| No. | Sex | Age, Years |
Smoker | Vascular Site |
Lysis | NIHSS Admission |
NIHSS Discharge |
Thrombus Histology |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 54 | Yes | Right MCA | Yes | 15 | 8 | e/red |
| 2 | F | 72 | No | Right C-T | No | 4 | 8 | e/red |
| 3 | F | 83 | No | Right C-T | Yes | 17 | 7 | e/red |
| 4 | F | 57 | No | Left MCA | Yes | 18 | 2 | s/red |
| 5 | M | 61 | Yes | Left MCA | Yes | 16 | 4 | l/mixed |
| 6 | M | 42 | No | Left C-T | Yes | 18 | 10 | l/white |
| 7 | M | 50 | Yes | Right MCA | Yes | 24 | 3 | s/mixed |
| 8 | F | 72 | No | BA | No | 23 | 9 | l/red |
| 9 | F | 74 | No | BA | Yes | 28 | 10 | l/red |
| 10 | F | 59 | No | Left MCA | No | 23 | 7 | s/white |
| 11 | F | 80 | No | Left C-T | Yes | 23 | 14 | l/white |
| 12 | M | 56 | Yes | Right MCA | Yes | 8 | 8 | e/red |
| 13 | M | 54 | No | Right MCA | Yes | 30 | 11 | l/white |
| 14 | F | 83 | Yes | BA | Yes | 31 | Deceased | s/mixed |
| 15 | F | 56 | No | Left MCA | Yes | 17 | 10 | l/mixed |
| 16 | M | 58 | No | Left MCA | Yes | 18 | 8 | s/red |
| 17 | M | 57 | No | Left MCA | Yes | 10 | 4 | l/white |
| 18 | F | 84 | No | Left MCA | Yes | 15 | 9 | s/white |
| 19 | F | 75 | No | Left C-T | Yes | 19 | 7 | l/mixed |
| 20 | M | 61 | Yes | BA | Yes | 4 | 0 | l/white |
| 21 | M | 67 | No | Left C-T | No | 13 | 15 | l/white |
| 22 | M | 75 | No | Left MCA | Yes | 13 | 3 | l/white |
| 23 | F | 82 | No | Right MCA | Yes | 22 | 5 | l/white |
| 24 | F | 83 | No | Right MCA | No | 17 | 7 | e/red |
| 25 | F | 80 | No | Right MCA | Yes | 5 | 2 | s/mixed |
| 26 | F | 69 | No | Left MCA | Yes | 18 | 13 | e/red |
| 27 | M | 43 | No | Left MCA | Yes | 18 | 6 | s/red |
| 28 | F | 93 | No | Right MCA | Yes | 11 | 4 | l/white |
| 29 | M | 40 | No | Right MCA | Yes | 10 | 1 | l/white |
| 30 | M | 77 | No | Left C-T | Yes | 20 | 13 | s/white |
| 31 | M | 28 | No | Right C-T | Yes | 12 | 0 | s/mixed |
| 32 | F | 63 | No | Left MCA | No | 25 | 10 | s/red |
| 33 | F | 84 | No | Right MCA | No | 17 | 9 | l/white |
| 34 | M | 47 | No | Right C-T | No | 11 | 1 | e/red |
| 35 | M | 95 | n.d. | Left MCA | No | 13 | Deceased | l/red |
| 36 | M | 58 | Yes | Left C-T | No | 3 | 12 | l/mixed |
| 37 | M | 60 | Yes | BA | No | 23 | 3 | l/mixed |
BA, basilar artery; C-T, intracranial part of the internal carotid artery (“carotid-T”); F, female; M, male; MCA, middle cerebral artery; thrombus histology: e, erythrocytic; l, layered; s, serpentine.
2.2. Categorization of Retrieved Clots
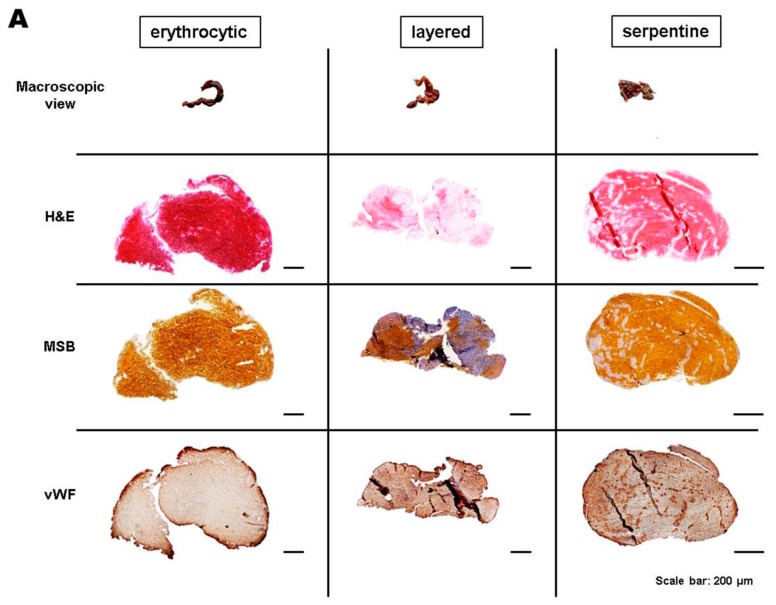
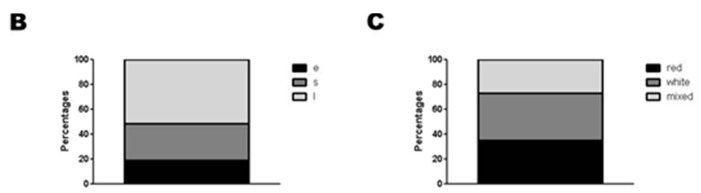
After clinical characterization of patients, we histopathologically categorized the extracted thrombi according to distinctive patterns into erythrocytic (19%), layered (30%), and serpentine (51%) [11], and according to the content of the red blood cells (RBCs) and fibrin/collagen as red (35%), white (38%), or mixed (27%) [12] (Table 1, Figure 1A).
Figure 1.
(A) Macroscopic view, hematoxylin and eosin (H&E), Martius scarlet blue (MSB), and von Willebrand factor (vWF) staining of three representative thrombi that show erythrocytic, layered, or serpentine morphology; (B,C) Bar graph of all thrombi after categorization by morphologic subtypes. e, erythrocytic; s, serpentine; l, layered.
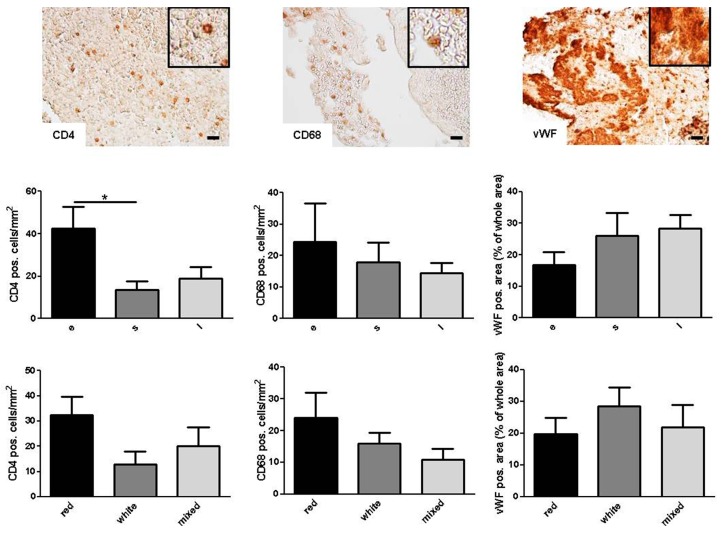
In the next step, we correlated the histopathologic thrombus subgroups (Figure 1B,C) with the number of immune cells (CD4+ T cells, CD68+ monocytes) and the fraction of vWF+ areas. Importantly, a significant accumulation of CD4+ T cells (p < 0.05) and a trend for CD68+ monocytes (p > 0.05) was detected in erythrocytic when compared with serpentine thrombi (Figure 2). The CD4+ and CD68+ cells were randomly distributed within the clot. In contrast, white thrombi (79% layered and 21% serpentine) showed higher percentages of vWF+ areas that are co-localized with the regions of fibrin/collagen (p > 0.05) (Figure 2).
Figure 2.
Histologic quantification of T cells (CD4+), monocytes (CD68+), and platelets (vWF+) in all thrombi by morphologic subgroups. e, erythrocytic; s, serpentine; l, layered. Scale bar: 20 µm. Magnification: 20-fold. Inserts show high magnification of representative staining. * p < 0.05.
2.3. Correlation of Histologic Results with Clinical Parameters
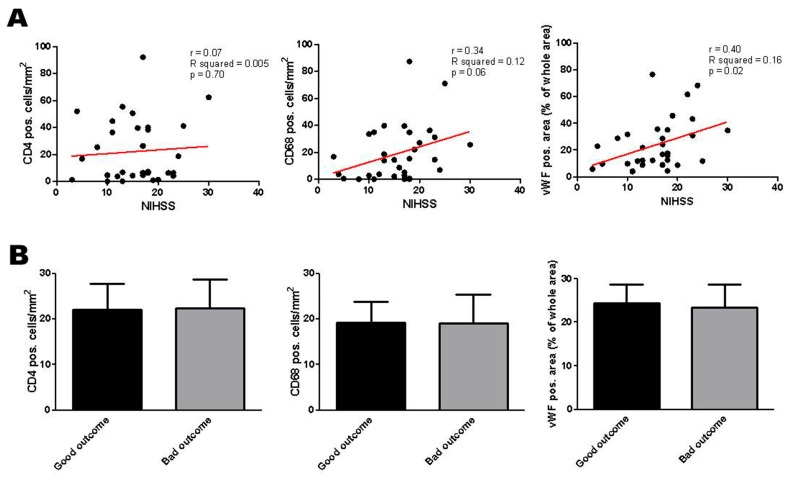
Finally, linear regression analysis was performed to correlate the number of immune cells or vWF+ areas within the thrombi with the NIHSS score as a marker for AIS symptom severity. The number of CD68+ monocytes and vWF+ platelets—but hardly the number of CD4+ T cells—displayed a clear trend to correlate with a high NIHSS score at admission (Figure S1). To reduce sample inhomogenities, we statistically re-evaluated our findings after exclusion of basilar artery occlusions. Here, the associations between CD68+ monocytes (p = 0.06) or vWF+ platelets (p = 0.02) and NIHSS score were even higher (Figure 3). In contrast, neither the number of CD4+ and CD68+ nor the area of vWF+ cells was predictive of the clinical outcome at discharge (Figure 3B). In a multivariate linear regression model, neither age nor sex significantly influenced these results (data not shown). Also Trial of Org 10172 in Acute Stroke Treatment (TOAST) [32] criteria and thrombolysis in cerebral infarction (TICI) scores [33] (Table S1) had no impact on the fraction or distribution of immune cells or platelets in the clots (data not shown).
Figure 3.
(A) Cellular thrombus histology of all retrieved clots except from the basilar artery in correlation to NIHSS scores at admission and clinical outcome at discharge. Red line: linear regression curve; (B) Categorization of CD4+ T cells, CD68+ monocytes and vWF+ platelets into “Good outcome” (NIHSS score 0–4 or improvement >9 points) or “Bad outcome” at the time of discharge. NIHSS, National Institute of Health Stroke Scale. r = correlation coefficient. R squared = coefficient of determination. p = level of significance.
3. Discussion
In this study, we analyzed the histologic composition of cerebral clots retrieved by MT from patients with AIS. In contrast to previous publications [11,12,13,14], we here, for the first time, provide a detailed characterization of clots regarding the number and distribution of distinct immune cells known to play major roles in inflammatory mechanisms of experimental ischemic stroke in rodents [24,25,26,27,34] and which could also be highly relevant for the pathophysiology of human AIS.
The baseline categorization of cerebral thrombi regarding distinctive patterns (erythrocytic, layered, or serpentine) and the content of RBCs and fibrin/collagen (red, white, or mixed) is based on previous reports [11,12]. In contrast, the immunohistologic characterization of CD4+ T cells, CD68+ monocytes and vWF+ cells (considered to be platelets) adds valuable new pathomechanistic information for the understanding of the composition of cerebral clots in special and thrombus formation in the context of human AIS in general.
Immunohistologic work-up of the thrombi revealed that the number of CD4+ T cells and CD68+ monocytes was increased in erythrocytic and red clots compared with the other morphologic groups. In contrast, white thrombi comprised more vWF+ cells compared with red and mixed clots. Given the promising results of studies that tried to visualize cerebral clot composition using CT and MRI imaging [12,13], it might be feasible to indirectly predict immunologic clot composition by these routine imaging techniques. Moreover, the development of specific MRI contrast agents for immune cells [35] and platelets [36] could further pave the way for non-invasive, but sensitive and specific, novel imaging possibilities for in vivo characterization of intracranial clot composition regarding thromboinflammation.
There is increasing evidence that immune cells are not only biomarkers after AIS [37], but also potential therapeutic targets [38]. Nevertheless, the mechanisms by which immune cells contribute to AIS pathophysiology have not been understood in depth so far. Even though we did not observe unambiguous platelet-monocyte co-localizations within the retrieved clots, it appears plausible that the interaction of immune cells with platelets (e.g., via cluster of differentiation 40 (CD40) and CD40 ligand or P-selectin (CD62-P) and P-selectin glycoprotein ligand 1) [27,39] and endothelial cells (e.g., via intercellular adhesion molecule 1 and lymphocyte function-associated antigen 1) [24] also plays a role in human AIS. Based on this hypothesis, formation of platelet–leukocyte aggregates and leukocyte activation might not only contribute to vascular repair, but also to thrombus formation leading to AIS. While most of the preclinical studies dealing with the role of immune cells in AIS development focused on microcirculatory dysfunction [26] or pathomechanisms within the brain [40], the intracranial clots retrieved by MT reflect thrombus formation outside the brain, as most of the patients suffered AIS due to a cardioembolic or arterioarterial embolic event (see TOAST classification in Table S1). Nevertheless, also in the pathogenesis of peripheral (i.e., outside the central nervous system) macroangiopathic atherosclerosis, various immune cells are involved [41,42,43] and there is ongoing effort for in vivo visualization of atherosclerotic plaque composition [44,45]. Importantly, and in contrast to Niesten and co-workers [12] who reported AIS subtype-specific differences regarding the percentage of RBC infiltration, we found no differences in immune cell infiltration within the clots when having a detailed look at AIS subtypes according to TOAST criteria. Further clinical studies are needed to assess whether immune cell composition of clots—measured by non-invasive imaging—could predict the response to revascularization strategies, and, in the future, may influence the decision of which treatment option is most suitable in individual cases.
There are several limitations of this study that must be considered. First, the number of patients that could be recruited and consequently the power of statistical analyses are low. Reasons for this are the low number of MTs (only 5%–10% of all patients with AIS) and the difficulties in receiving informed consent from a patient severely affected by AIS. Therefore, patients who have suffered a severe stroke and/or have aphasia could be underrepresented in this study (compared with patients with milder symptoms) because neurologic deficits related to their condition may have prevented them from being capable of providing informed consent; Second, despite a recent publication showing that stent-retriever MT does not lead to significant intimal damage [46], it is possible that the procedure of MT itself might dislocate the original clot composition and thereby could influence the results of our histologic analysis; Furthermore, thrombi retrieved as multiple fragments could not be further processed for immunohistochemistry. Third, it was not possible to conduct a standardized follow-up of patients due to limited patient numbers and low response rates.
4. Materials and Methods
4.1. Patient Population and Study Design
We histopathologically studied a convenience sample of 37 occluding clots that were mechanically retrieved from large intracranial arteries of patients with AIS at the Department of Neurology, University Hospital of Würzburg, Germany. The study protocol was approved by the ethics committee of the Medical Faculty of the University of Würzburg, Germany (reference number 36/2012) and written informed consent was provided by all participants. Inclusion criteria were: patients with AIS ≥ 18 years with an occlusion of the proximal MCA, the C-T, or the BA, successful MT and informed consent of the patients or their legal representatives during the hospital treatment. The functional status of the patients was assessed using the NIHSS score [31] at hospital admission and again before discharge. Good neurologic outcome was defined as an NIHSS score of 0–4 or an NIHSS score improvement of >9 points [46]. The TICI score [33] was used to assess post-intervention vessel patency and has been performed by an independent investigator (Ignaz Gunreben) blinded to clinical and histologic outcome: (0) no perfusion; (1) penetration with minimal perfusion; (2) partial perfusion (2a, only partial filling (less than two-thirds) of the entire vascular territory is visualized); 2b, complete filling of all of the expected vascular territory is visualized, but the filling is slower than normal; (3) complete perfusion. The TOAST (Trial of Org 10172 in Acute Stroke Treatment) criteria [31] were applied to describe the assumed etiology of AIS: (1) cardioembolism; (2) large-artery atherosclerosis; (3) small-vessel occlusion; (4) other determined etiology; or (5) undetermined etiology.
4.2. Thrombectomy Procedure
MT was performed in accordance with local standard operating procedures and common recommendations [47]. Only stent retrievers were used. The MT was performed under general anesthesia in all of the patients. Participation in the study had no impact on the way the patients were treated.
4.3. Processing of Thrombi and Analysis
Immediately after clot retrieval, i.e., still within the catheter laboratory, thrombus material was fixed in phosphate-buffered formalin. The formalin-fixed specimens were embedded in paraffin (Leica, Wetzlar, Germany), cross-sectioned at 4-µm thickness and stained with hematoxylin and eosin (H&E), and Martius scarlet blue (MSB) (Atom Scientific, Cheshire, UK). Subsequently, based on H&E staining, thrombi were characterized according to their overall appearance into erythrocytic, layered, and serpentine [11]. Categorization was done by visual assignment of two independent investigators (Michael K. Schuhmann, Peter Kraft). In case of divergent results after first view, investigators independently re-categorized the clots and finally had to reach an agreement. Additionally, using MSB-stained sections, the content of RBCs and fibrin/collagen was quantitatively determined. Accordingly, thrombi were classified as red (RBCs outnumber fibrin/collagen ≥ 15%) or white (fibrin/collagen outnumber RBCs ≥ 15%). All others were classified as mixed [12]. To assess for thromboinflammation, all thrombi were stained immunohistochemically for CD4+ T cells (abcam; ab133616, Cambridge, UK), CD68+ monocytes (Acris; AM331235U-N, Herford, Germany) and von Willebrand factor (abcam; ab6994, Cambridge, UK).
4.4. Statistical Analysis
All results are presented as mean ± standard error of the mean. Distribution of data was evaluated using the Kolmogorov–Smirnov test. To test for significant differences between multiple groups, one-way analysis of variance with post hoc Bonferroni adjustment for p-values was used. The Pearson test was used to analyze the correlation between the number of immune cells or platelets and NIHSS at admission. To rule out potential confounders, we utilized a multivariate linear regression model adjusted for age and sex. In a second multivariate linear regression model we evaluated for TICI score and TOAST classification. p-values <0.05 were considered significant with * p < 0.05.
5. Conclusions
Intracranial thrombi retrieved by MT from patients with AIS comprise T cells, monocytes, and platelets as cellular components and potential inflammatory biomarkers that might also be relevant in the pathophysiology of human AIS. Our findings should stimulate further investigations to identify whether the immune cell composition of clots may influence the clinical response to IVT or MT.
Acknowledgments
We thank Andrea Sauer and Sabrina Braunschweig for excellent technical assistance as well as L. Solymosi and the Department of Neuroradiology, University Hospital Würzburg, Germany, for valuable support of this study.
Abbreviations
- AIS
acute ischemic stroke
- BA
basilar artery
- CD
cluster of differentiation
- C-T
intracranial part of the internal carotid artery
- CT
computed tomography
- H&E
hematoxylin and eosin
- IVT
intravenous thrombolysis
- MCA
middle cerebral artery
- MRI
magnetic resonance imaging
- MSB
Martius scarlet blue
- MT
mechanical thrombectomy
- NIHSS
National Institutes of Health Stroke Scale
- RBC
red blood cell
- TOAST
Trial of Org 10172 in Acute Stroke Treatment
- TICI
thrombolysis in cerebral infarction
- vWF
von Willebrand factor
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1422-0067/17/3/298/s1.
Author Contributions
Michael K. Schuhmann performed immunohistochemistry, analyzed data, and wrote the manuscript; Ignaz Gunreben recruited patients, analyzed data, and wrote the manuscript, Christoph Kleinschnitz conceived and funded the entire study and revised the manuscript; Peter Kraft recruited patients, analyzed data, and wrote the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. This work was supported by the Deutsche Forschungsgemeinschaft (individual research grant to Christoph Kleinschnitz).
References
- 1.Berkhemer O.A., Fransen P.S., Beumer D., van den Berg L.A., Lingsma H.F., Yoo A.J., Schonewille W.J., Vos J.A., Nederkoorn P.J., Wermer M.J., et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N. Engl. J. Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 2.Campbell B.C., Mitchell P.J., Investigators E.I. Endovascular therapy for ischemic stroke. N. Engl. J. Med. 2015;372:2365–2366. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 3.Jovin T.G., Chamorro A., Cobo E., de Miquel M.A., Molina C.A., Rovira A., San Roman L., Serena J., Abilleira S., Ribo M., et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N. Engl. J. Med. 2015;372:2296–2306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 4.Jauch E.C., Saver J.L., Adams H.P., Jr., Bruno A., Connors J.J., Demaerschalk B.M., Khatri P., McMullan P.W., Jr., Qureshi A.I., Rosenfield K., et al. Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 5.Riedel C.H., Zimmermann P., Jensen-Kondering U., Stingele R., Deuschl G., Jansen O. The importance of size: Successful recanalization by intravenous thrombolysis in acute anterior stroke depends on thrombus length. Stroke. 2011;42:1775–1777. doi: 10.1161/STROKEAHA.110.609693. [DOI] [PubMed] [Google Scholar]
- 6.Jindal G., Miller T., Shivashankar R., Mitchell J., Stern B.J., Yarbrough K., Gandhi D. Relationship of thrombus length to number of stent retrievals, revascularization, and outcomes in acute ischemic stroke. J. Vasc. Interv. Radiol. 2014;25:1549–1557. doi: 10.1016/j.jvir.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Puig J., Pedraza S., Demchuk A., Daunis I.E.J., Termes H., Blasco G., Soria G., Boada I., Remollo S., Banos J., et al. Quantification of thrombus hounsfield units on noncontrast CT predicts stroke subtype and early recanalization after intravenous recombinant tissue plasminogen activator. Am. J. Neuroradiol. 2012;33:90–96. doi: 10.3174/ajnr.A2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moftakhar P., English J.D., Cooke D.L., Kim W.T., Stout C., Smith W.S., Dowd C.F., Higashida R.T., Halbach V.V., Hetts S.W. Density of thrombus on admission CT predicts revascularization efficacy in large vessel occlusion acute ischemic stroke. Stroke. 2013;44:243–245. doi: 10.1161/STROKEAHA.112.674127. [DOI] [PubMed] [Google Scholar]
- 9.Mokin M., Morr S., Natarajan S.K., Lin N., Snyder K.V., Hopkins L.N., Siddiqui A.H., Levy E.I. Thrombus density predicts successful recanalization with Solitaire stent retriever thrombectomy in acute ischemic stroke. J. Neurointerv. Surg. 2015;7:104–107. doi: 10.1136/neurintsurg-2013-011017. [DOI] [PubMed] [Google Scholar]
- 10.Jorgensen L., Torvik A. Ischaemic cerebrovascular diseases in an autopsy series: Part 1. Prevalence, location and predisposing factors in verified thrombo-embolic occlusions, and their significance in the pathogenesis of cerebral infarction. J. Neurol. Sci. 1966;3:490–509. doi: 10.1016/0022-510X(66)90004-9. [DOI] [PubMed] [Google Scholar]
- 11.Marder V.J., Chute D.J., Starkman S., Abolian A.M., Kidwell C., Liebeskind D., Ovbiagele B., Vinuela F., Duckwiler G., Jahan R., et al. Analysis of thrombi retrieved from cerebral arteries of patients with acute ischemic stroke. Stroke. 2006;37:2086–2093. doi: 10.1161/01.STR.0000230307.03438.94. [DOI] [PubMed] [Google Scholar]
- 12.Niesten J.M., van der Schaaf I.C., van Dam L., Vink A., Vos J.A., Schonewille W.J., de Bruin P.C., Mali W.P., Velthuis B.K. Histopathologic composition of cerebral thrombi of acute stroke patients is correlated with stroke subtype and thrombus attenuation. PLoS ONE. 2014;9:298. doi: 10.1371/journal.pone.0088882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liebeskind D.S., Sanossian N., Yong W.H., Starkman S., Tsang M.P., Moya A.L., Zheng D.D., Abolian A.M., Kim D., Ali L.K., et al. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke. 2011;42:1237–1243. doi: 10.1161/STROKEAHA.110.605576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim S.K., Yoon W., Kim T.S., Kim H.S., Heo T.W., Park M.S. Histologic Analysis of Retrieved Clots in Acute Ischemic Stroke: Correlation with Stroke Etiology and Gradient-Echo MRI. Am. J. Neuroradiol. 2015;36:1756–1762. doi: 10.3174/ajnr.A4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boeckh-Behrens T., Schubert M., Forschler A., Prothmann S., Kreiser K., Zimmer C., Riegger J., Bauer J., Neff F., Kehl V., et al. The Impact of Histological Clot Composition in Embolic Stroke. Clin. Neuroradiol. 2014:1–9. doi: 10.1007/s00062-014-0347-x. [DOI] [PubMed] [Google Scholar]
- 16.Liebeskind D.S., Jahan R., Nogueira R.G., Jovin T.G., Lutsep H.L., Saver J.L., Investigators S. Serial Alberta Stroke Program early CT score from baseline to 24 hours in Solitaire Flow Restoration with the Intention for Thrombectomy study: A novel surrogate end point for revascularization in acute stroke. Stroke. 2014;45:723–727. doi: 10.1161/STROKEAHA.113.003914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niesten J.M., van der Schaaf I.C., Biessels G.J., van Otterloo A.E., van Seeters T., Horsch A.D., Luitse M.J., van der Graaf Y., Kappelle L.J., Mali W.P., et al. Relationship between thrombus attenuation and different stroke subtypes. Neuroradiology. 2013;55:1071–1079. doi: 10.1007/s00234-013-1217-y. [DOI] [PubMed] [Google Scholar]
- 18.Simons N., Mitchell P., Dowling R., Gonzales M., Yan B. Thrombus composition in acute ischemic stroke: A histopathological study of thrombus extracted by endovascular retrieval. J. Neuroradiol. 2015;42:86–92. doi: 10.1016/j.neurad.2014.01.124. [DOI] [PubMed] [Google Scholar]
- 19.Minnerup J., Kleinschnitz C. Visualization of clot composition in ischemic stroke: Do we get what we see? Stroke. 2011;42:1193–1194. doi: 10.1161/STROKEAHA.110.612150. [DOI] [PubMed] [Google Scholar]
- 20.Von zur Muhlen C., von Elverfeldt D., Moeller J.A., Choudhury R.P., Paul D., Hagemeyer C.E., Olschewski M., Becker A., Neudorfer I., Bassler N., et al. Magnetic resonance imaging contrast agent targeted toward activated platelets allows in vivo detection of thrombosis and monitoring of thrombolysis. Circulation. 2008;118:258–267. doi: 10.1161/CIRCULATIONAHA.107.753657. [DOI] [PubMed] [Google Scholar]
- 21.Overoye-Chan K., Koerner S., Looby R.J., Kolodziej A.F., Zech S.G., Deng Q., Chasse J.M., McMurry T.J., Caravan P. EP-2104R: A fibrin-specific gadolinium-Based MRI contrast agent for detection of thrombus. J. Am. Chem. Soc. 2008;130:6025–6039. doi: 10.1021/ja800834y. [DOI] [PubMed] [Google Scholar]
- 22.Spuentrup E., Botnar R.M., Wiethoff A.J., Ibrahim T., Kelle S., Katoh M., Ozgun M., Nagel E., Vymazal J., Graham P.B., et al. MR imaging of thrombi using EP-2104R, a fibrin-specific contrast agent: Initial results in patients. Eur. Radiol. 2008;18:1995–2005. doi: 10.1007/s00330-008-0965-2. [DOI] [PubMed] [Google Scholar]
- 23.Yilmaz G., Arumugam T.V., Stokes K.Y., Granger D.N. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113:2105–2112. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- 24.Kleinschnitz C., Kraft P., Dreykluft A., Hagedorn I., Gobel K., Schuhmann M.K., Langhauser F., Helluy X., Schwarz T., Bittner S., et al. Regulatory T cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature. Blood. 2013;121:679–691. doi: 10.1182/blood-2012-04-426734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleinschnitz C., Schwab N., Kraft P., Hagedorn I., Dreykluft A., Schwarz T., Austinat M., Nieswandt B., Wiendl H., Stoll G. Early detrimental T-cell effects in experimental cerebral ischemia are neither related to adaptive immunity nor thrombus formation. Blood. 2010;115:3835–3842. doi: 10.1182/blood-2009-10-249078. [DOI] [PubMed] [Google Scholar]
- 26.Kraft P., Gob E., Schuhmann M.K., Gobel K., Deppermann C., Thielmann I., Herrmann A.M., Lorenz K., Brede M., Stoll G., et al. FTY720 ameliorates acute ischemic stroke in mice by reducing thrombo-inflammation but not by direct neuroprotection. Stroke. 2013;44:3202–3210. doi: 10.1161/STROKEAHA.113.002880. [DOI] [PubMed] [Google Scholar]
- 27.Nieswandt B., Kleinschnitz C., Stoll G. Ischaemic stroke: A thrombo-inflammatory disease? J. Physiol. 2011;589:4115–4123. doi: 10.1113/jphysiol.2011.212886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smout J., Dyker A., Cleanthis M., Ford G., Kesteven P., Stansby G. Platelet function following acute cerebral ischemia. Angiology. 2009;60:362–369. doi: 10.1177/0003319709332959. [DOI] [PubMed] [Google Scholar]
- 29.Htun P., Fateh-Moghadam S., Tomandl B., Handschu R., Klinger K., Stellos K., Garlichs C., Daniel W., Gawaz M. Course of platelet activation and platelet-leukocyte interaction in cerebrovascular ischemia. Stroke. 2006;37:2283–2287. doi: 10.1161/01.STR.0000236638.75591.61. [DOI] [PubMed] [Google Scholar]
- 30.Zhu Z., Fu Y., Tian D., Sun N., Han W., Chang G., Dong Y., Xu X., Liu Q., Huang D., et al. Combination of the Immune Modulator Fingolimod With Alteplase in Acute Ischemic Stroke: A Pilot Trial. Circulation. 2015;132:1104–1112. doi: 10.1161/CIRCULATIONAHA.115.016371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muir K.W., Weir C.J., Murray G.D., Povey C., Lees K.R. Comparison of neurological scales and scoring systems for acute stroke prognosis. Stroke. 1996;27:1817–1820. doi: 10.1161/01.STR.27.10.1817. [DOI] [PubMed] [Google Scholar]
- 32.Adams H.P., Jr., Bendixen B.H., Kappelle L.J., Biller J., Love B.B., Gordon D.L., Marsh E.E., 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.STR.24.1.35. [DOI] [PubMed] [Google Scholar]
- 33.Higashida R.T., Furlan A.J., Roberts H., Tomsick T., Connors B., Barr J., Dillon W., Warach S., Broderick J., Tilley B., et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34:e109–e137. doi: 10.1161/01.STR.0000082721.62796.09. [DOI] [PubMed] [Google Scholar]
- 34.Benakis C., Garcia-Bonilla L., Iadecola C., Anrather J. The role of microglia and myeloid immune cells in acute cerebral ischemia. Front. Cell. Neurosci. 2014;8:298. doi: 10.3389/fncel.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahrens E.T., Bulte J.W. Tracking immune cells in vivo using magnetic resonance imaging. Nat. Rev. Immunol. 2013;13:755–763. doi: 10.1038/nri3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacobin-Valat M.J., Laroche-Traineau J., Lariviere M., Mornet S., Sanchez S., Biran M., Lebaron C., Boudon J., Lacomme S., Cerutti M., et al. Nanoparticles functionalised with an anti-platelet human antibody for in vivo detection of atherosclerotic plaque by magnetic resonance imaging. Nanomedicine. 2015;11:927–937. doi: 10.1016/j.nano.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 37.Kraft P., Drechsler C., Schuhmann M.K., Gunreben I., Kleinschnitz C. Characterization of Peripheral Immune Cell Subsets in Patients with Acute and Chronic Cerebrovascular Disease: A Case-Control Study. Int. J. Mol. Sci. 2015;16:25433–25449. doi: 10.3390/ijms161025433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fu Y., Liu Q., Anrather J., Shi F.D. Immune interventions in stroke. Nat. Rev. Immunol. 2015;11:524–535. doi: 10.1038/nrneurol.2015.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rinder H.M., Bonan J.L., Rinder C.S., Ault K.A., Smith B.R. Dynamics of leukocyte-platelet adhesion in whole blood. Blood. 1991;78:1730–1737. [PubMed] [Google Scholar]
- 40.Jin R., Yang G., Li G. Inflammatory mechanisms in ischemic stroke: Role of inflammatory cells. J. Leukoc. Biol. 2010;87:779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pende A., Artom N., Bertolotto M., Montecucco F., Dallegri F. Role of Neutrophils in Atherogenesis: An Update. Eur. J. Clin. Investig. 2016;46:252–263. doi: 10.1111/eci.12566. [DOI] [PubMed] [Google Scholar]
- 42.Idzkowska E., Eljaszewicz A., Miklasz P., Musial W.J., Tycinska A.M., Moniuszko M. The Role of Different Monocyte Subsets in the Pathogenesis of Atherosclerosis and Acute Coronary Syndromes. Scand. J. Immunol. 2015;82:163–173. doi: 10.1111/sji.12314. [DOI] [PubMed] [Google Scholar]
- 43.Zernecke A. Dendritic cells in atherosclerosis: Evidence in mice and humans. Arterioscler. Thromb. Vasc. Biol. 2015;35:763–770. doi: 10.1161/ATVBAHA.114.303566. [DOI] [PubMed] [Google Scholar]
- 44.Makris G.C., Teng Z., Patterson A.J., Lin J.M., Young V., Graves M.J., Gillard J.H. Advances in MRI for the evaluation of carotid atherosclerosis. Br. J Radiol. 2015;88:298. doi: 10.1259/bjr.20140282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alie N., Eldib M., Fayad Z.A., Mani V. Inflammation, Atherosclerosis, and Coronary Artery Disease: PET/CT for the Evaluation of Atherosclerosis and Inflammation. Clin. Med. Insights Cardiol. 2014;8(Suppl. 3):S13–S21. doi: 10.4137/CMC.S17063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh P., Doostkam S., Reinhard M., Ivanovas V., Taschner C.A. Immunohistochemical analysis of thrombi retrieved during treatment of acute ischemic stroke: Does stent-retriever cause intimal damage? Stroke. 2013;44:1720–1722. doi: 10.1161/STROKEAHA.113.000964. [DOI] [PubMed] [Google Scholar]
- 47.Mohlenbruch M., Stampfl S., Behrens L., Herweh C., Rohde S., Bendszus M., Hametner C., Nagel S., Ringleb P.A., Pham M. Mechanical thrombectomy with stent retrievers in acute basilar artery occlusion. Am. J. Neuroradiol. 2014;35:959–964. doi: 10.3174/ajnr.A3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.