Abstract
Tumor-associated macrophages (TAMs), the most abundant infiltrating immune cells in tumor microenvironment, have distinct functions in hepatocellular carcinoma (HCC) progression. CD68+ TAMs represent multiple polarized immune cells mainly containing CD86+ antitumoral M1 macrophages and CD206+ protumoral M2 macrophages. TAMs expression and density were assessed by immunohistochemical staining of CD68, CD86, and CD206 in tissue microarrays from 253 HCC patients. Clinicopathologic features and prognostic value of these markers were evaluated. We found that CD68+ TAMs were not associated with clinicopathologic characteristics and prognosis in HCC. Low presence of CD86+ TAMs and high presence of CD206+ TAMs were markedly correlated with aggressive tumor phenotypes, such as multiple tumor number and advanced tumor-node-metastasis (TNM) stage; and were associated with poor overall survival (OS) (p = 0.027 and p = 0.024, respectively) and increased time to recurrence (TTR) (p = 0.037 and p = 0.031, respectively). In addition, combined analysis of CD86 and CD206 provided a better indicator for OS (p = 0.011) and TTR (p = 0.024) in HCC than individual analysis of CD86 and CD206. Moreover, CD86+/CD206+ TAMs predictive model also had significant prognosis value in α-fetoprotein (AFP)-negative patients (OS: p = 0.002, TTR: p = 0.005). Thus, these results suggest that combined analysis of immune biomarkers CD86 and CD206 could be a promising HCC prognostic biomarker.
Keywords: hepatocellular carcinoma, tumor-associated macrophages, CD86, CD206, AFP, prognosis
1. Introduction
With an increasing incidence rate, hepatocellular carcinoma (HCC) is ranked the second cause of cancer-related deaths around the world [1]. Currently, surgical resection is the preferred treatment for HCC. However, large cohorts of HCC patients suffer from postoperative recurrence, and have a poor response to systemic chemotherapeutic treatments, with a five-year survival rate of only 30%–40% [2]. Still worse, current clinicopathologic factors, such as α-fetoprotein (AFP), tumor-node-metastasis (TNM) stage, and Barcelona clinic liver cancer (BCLC) stage, cannot accurately predict the outcome of HCC patients. Novel prognostic markers need to be developed for more customized HCC treatment. HCC is a typical inflammation-related cancer. Chronic inflammation provides a favorable surrounding to facilitate HCC progression [3,4]. Accumulating evidence indicates that tumor microenvironment plays a vital role in tumor progression and metastasis [5]. HCC microenvironment could be rich resources for identifying novel powerful prognostic biomarkers.
Macrophage is a main cellular ingredient in human tumor microenvironment, and is commonly known as tumor-associated macrophages (TAMs) [6]. Different types of macrophage have distinct functions in tumor progression. M1 macrophages, which activate tumor-killing mechanisms, as well as amplify Th1 immunocytes responses, provide a resistant role in tumorigenesis. On the other hand, M2 macrophages, via suppressing tumor-specific immune responses, mainly act to enhance tumor growth and metastasis [7].
Accumulating evidence indicates that CD68 was expressed in all macrophages, and is labeled as a pan-macrophage biomarker [8]. However, CD68 cannot effectively distinguish between M1 and M2 subtype macrophages. Previous study implied that M1 macrophages expressed high level of CD86 and tumor necrosis factor α (TNF-α), while M2 macrophages expressed relatively high level of CD206, CD163 and IL-10 [9,10]. Interestingly, Tan et al. reported that in HCC, M1 macrophages expressed increased level of CD86 relative to TNF-α and IL-12, while M2 macrophages expressed increased level of CD206 relative to IL-10, and transforming growth factor β (TGF-β) [11]. Recent studies have demonstrated that TAMs were associated with HCC progression, and may act as a promising prognostic factor and therapeutic target [12,13].
In this study, we showed that CD68+ TAMs alone had no prognostic value in HCC patients, indicating that total macrophages had no impact on HCC prognosis. Low presence of CD86+ and high presence of CD206+ TAMs were clearly correlated with aggressive tumor phenotypes, such as multiple tumor number and advanced TNM stage; and were associated with a poor prognosis in survival and recurrence. Furthermore, combined analysis of CD86 and CD206 provided a better prognostic indicator for HCC patients than individual analysis of CD86 and CD206. Furthermore, CD86+/CD206+ TAMs predictive model also showed strong prognosis value in AFP-negative patients.
2. Results
2.1. Characterization of Tumor-Associated Macrophages in Hepatocellular Carcinoma (HCC) Patients
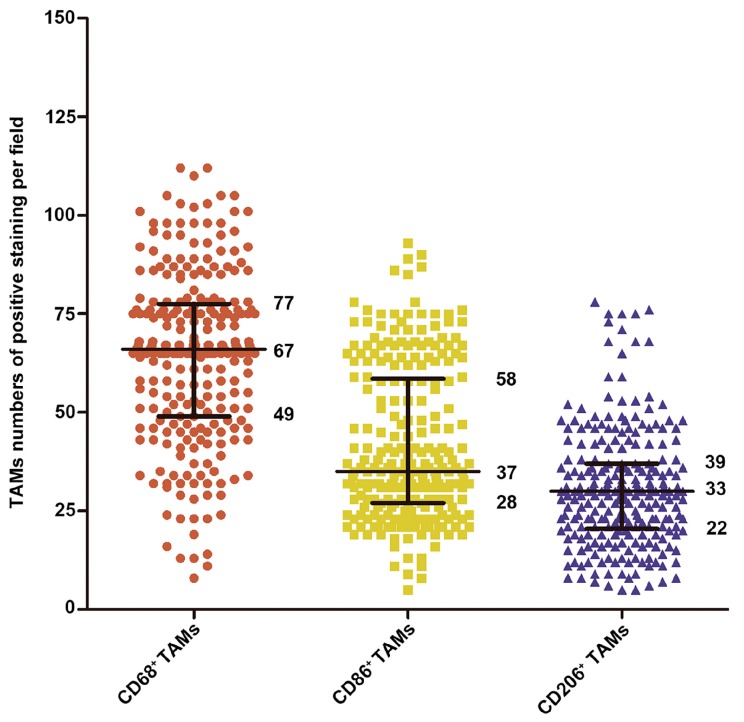
Immunohistochemistry was performed to assess the expression and presence of macrophages in tumor tissues from 253 HCC patients who had undergone curative resection. CD68, CD86, and CD206 positive staining were mainly located in the cytoplasm of macrophages (Figure 1). In tumor tissues, the number of CD68 positive cells (median, 67 cells/field) was higher than CD86 positive (median, 37 cells/field, p < 0.001) and CD206 positive cells (median, 33 cells/field, p < 0.001, Figure 2 and Table S1).
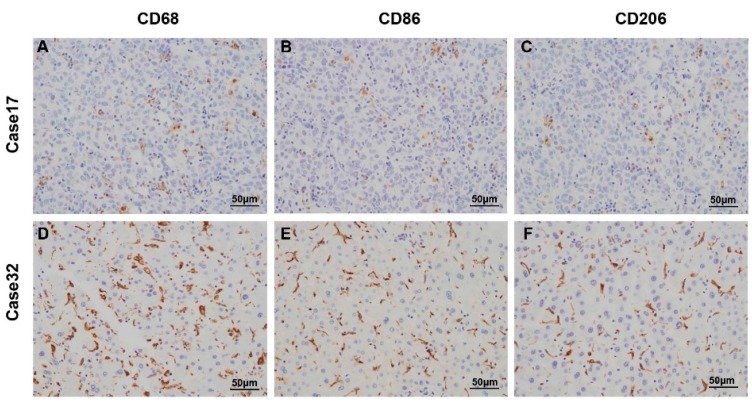
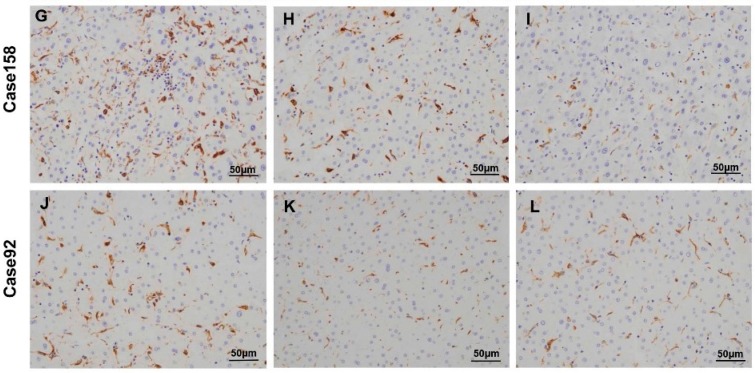
Figure 1.
Representative immunostaining images of CD68 (A,D,G,J); CD86 (B,E,H,K); and CD206 (C,F,I,L) in HCC tissue microarray sections were shown. Case 17 (A–C) showed low staining presence of CD68+, CD86+ and CD206+ macrophages; Case 32 (D–F) showed high staining presence of CD68+, CD86+ and CD206+ macrophages; Case 158 (G–I) showed high staining presence of CD68+ and CD86+ macrophages, but low staining presence of CD206+ macrophages; Case 92 (J–L) showed high staining presence of CD68+ and CD206+ macrophages, but low staining presence of CD86+ macrophages.
Figure 2.
The number distribution of CD68+, CD86+ and CD206+ macrophages in the cohort (n = 253). Lines indicated 25th, 50th, and 75th percentiles. TAMs: Tumor-associated macrophages.
2.2. Association between Macrophage Markers Presence (CD68, CD86 and CD206) and Clinicopathologic Characteristics in HCC Patients
We next investigated the association between macrophage markers (CD68, CD86 and CD206) and patients’ clinicopathologic characteristics. The 253 patients were divided into two groups (low and high) based on the median value of CD68, CD86, and CD206 staining cells, respectively. As summarized in Table 1, CD68 positive staining count in tumor had no relationship with any clinicopathologic features. However, lower infiltration of CD86+ TAMs was associated with aggressive tumor phenotypes, such as multiple tumor number (p = 0.006), high-grade TNM stage (p = 0.001) and elevated alaninetransaminase (ALT) (p = 0.020). Interestingly, higher infiltration of CD206+ TAMs was also positively correlated with multiple tumor number (p = 0.038), presence of vascular invasion (p = 0.011), appearance of tumor capsulation (p = 0.004), and advanced TNM stage (p = 0.005).
Table 1.
Correlation between immunohistochemical variables and clinicopathologic features of HCC patients in the cohort (n = 253).
| Characteristics | CD68+ TAMs | CD86+ TAMs | CD206+ TAMs | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Low | High | p # | Low | High | p # | Low | High | p # | |
| Age (years) | |||||||||
| ≤51 | 50 | 81 | 0.522 | 86 | 45 | 0.602 | 80 | 51 | 0.796 |
| >51 | 52 | 70 | - | 76 | 46 | - | 77 | 45 | - |
| Gender | |||||||||
| Female | 17 | 16 | 0.184 | 142 | 78 | 0.699 | 21 | 12 | 1.000 |
| Male | 85 | 135 | - | 20 | 13 | - | 136 | 84 | - |
| HBsAg | |||||||||
| Negative | 4 | 8 | 0.767 | 8 | 4 | 1.000 | 8 | 4 | 1.000 |
| Positive | 98 | 143 | - | 154 | 87 | - | 149 | 92 | - |
| HCVAb | |||||||||
| Negative | 101 | 146 | 0.406 | 158 | 89 | 1.000 | 153 | 94 | 1.000 |
| Positive | 1 | 5 | - | 4 | 2 | - | 4 | 2 | - |
| AFP (ng/mL) | |||||||||
| ≤20 | 44 | 55 | 0.296 | 61 | 38 | 0.592 | 58 | 41 | 0.426 |
| >20 | 58 | 96 | - | 101 | 53 | - | 99 | 55 | - |
| ALT (U/L) | |||||||||
| ≤75 | 88 | 137 | 0.309 | 150 | 75 | 0.020 | 141 | 84 | 0.680 |
| >75 | 14 | 14 | - | 12 | 16 | - | 16 | 12 | - |
| γ-GT (U/L) | |||||||||
| ≤54 | 47 | 64 | 0.606 | 67 | 44 | 0.294 | 72 | 39 | 0.436 |
| >54 | 55 | 87 | - | 95 | 47 | - | 85 | 57 | - |
| Liver cirrhosis | |||||||||
| NO | 15 | 18 | 0.570 | 23 | 10 | 0.561 | 22 | 11 | 0.701 |
| YES | 87 | 133 | - | 139 | 81 | - | 135 | 85 | - |
| Tumor size (cm) | |||||||||
| ≤5 | 59 | 81 | 0.522 | 86 | 54 | 0.359 | 90 | 50 | 0.436 |
| >5 | 43 | 70 | - | 76 | 37 | - | 67 | 46 | - |
| Tumor number | |||||||||
| Single | 82 | 118 | 0.753 | 137 | 63 | 0.006 | 131 | 69 | 0.038 |
| Multiple | 20 | 33 | - | 25 | 28 | - | 26 | 27 | - |
| Vascular invasion | |||||||||
| No | 65 | 95 | 1.000 | 106 | 54 | 0.345 | 109 | 51 | 0.011 |
| Yes | 37 | 56 | - | 56 | 37 | - | 48 | 45 | - |
| Tumor encapsulation | |||||||||
| None | 45 | 66 | 1.000 | 73 | 38 | 0.692 | 80 | 31 | 0.004 |
| Complete | 57 | 85 | - | 89 | 53 | - | 77 | 65 | - |
| Tumor differentiation | |||||||||
| I + II | 71 | 105 | 1.000 | 113 | 63 | 1.000 | 106 | 70 | 0.400 |
| III + IV | 81 | 46 | - | 49 | 28 | - | 51 | 26 | - |
| TNM stage | |||||||||
| I | 65 | 92 | 0.693 | 113 | 49 | 0.001 | 108 | 49 | 0.005 |
| III + IV | 37 | 59 | - | 44 | 47 | - | 49 | 47 | - |
HCC, hepatocellular carcinoma; HBsAg, hepatitis B surface antigen; HCVAb, hepatitis C virus antibody; AFP, α-fetoprotein; ALT, alanine transaminase; γ-GT, γ-glutamyltransferase; TNM, tumor-node-metastasis. #: The Pearson Chi square test was applied.
2.3. Analysis of Macrophages Immune Marker (CD68, CD86 and CD206) Prognostic Value in HCC Patients
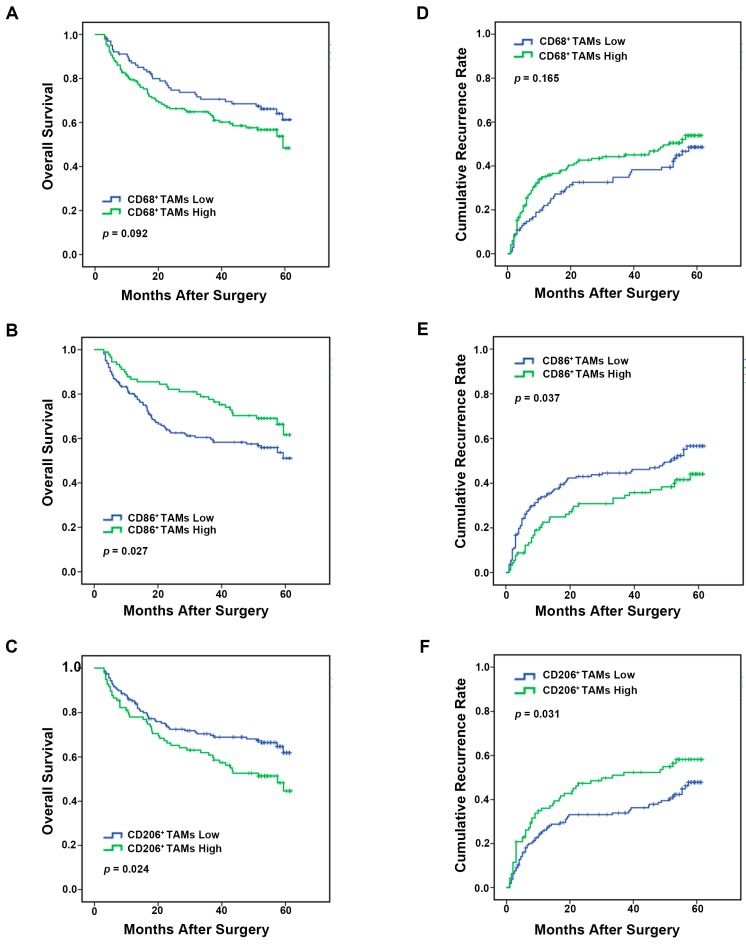
We further investigated the clinic prognosis value of TAMs markers in this cohort of 253 patients (Figure 2). CD68+ TAMs had no prognostic value in HCC patients (Figure 3A,D). Patients with low CD86+ TAMs staining cells had a significantly shorter median overall survival (OS) and time to recurrence (TTR) (OS, 41.3 months; TTR, 36.3 months) than those with high staining cells (OS, 49.1 months, p = 0.027; TTR, 43.2 months, p = 0.037) (Figure 3B,E). Conversely, low presence of CD206+ TAM group had a markedly longer median OS and TTR (OS, 46.2 months; TTR, 41.7 months) when compared with the high presence group (OS, 40.1 months, p = 0.024; TTR, 34.0 months, p = 0.031) (Figure 3C,F).
Figure 3.
Kaplan–Meier curves for overall survival (A–C) and time to recurrence (D–F) of hepatocellular carcinoma (HCC) patients according to the staining presence of CD68+, CD86+ and CD206+ macrophages in the cohort (n = 253).
As summarized in Table 2, univariate analyses suggested that low CD86+ TAMs and high CD206+ TAMs were significantly associated with decreased survival and high risk of recurrence in HCC patients post curative resection. Furthermore, multivariate Cox’s regression analysis, after backward stepwise variable selections, suggested that apart from tumor size, tumor differentiation, and vascular invasion, infiltration of CD86+ and CD206+ TAMs remained as the independent prognostic factors in HCC patients for both OS (HR = 2.178, p = 0.040 and HR = 1.584, p = 0.027) and TTR (HR = 1.810, p = 0.006 and HR = 1.872, p = 0.030). Collectively, these data implied that CD86 and CD206 were valuable prognostic biomarkers in HCC patients.
Table 2.
Univariate and multivariate analysis of factors related to OS and TTR of HCC patients in the cohort (n = 253).
| Variables | OS | TTR | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| p | HR | 95% CI | p | p | HR | 95% CI | p | |
| Age, years (>51 vs. ≤51) | 0.624 | - | - | NA | 0.437 | - | - | NA |
| Gender (male vs. female) | 0.273 | - | - | NA | 0.990 | - | - | NA |
| HBsAg (positive vs. negative) | 0.594 | - | - | NA | 0.389 | - | - | NA |
| HCVAb (positive vs. negative) | 0.180 | - | - | NA | 0.307 | - | - | NA |
| Serum AFP, ng/mL (>20 vs. ≤20) | <0.001 | 2.095 | 1.323–3.318 | 0.002 | 0.027 | - | - | NS |
| Serum ALT, U/L (>75 vs. ≤75) | 0.945 | - | - | NA | 0.725 | - | - | NA |
| Serum γ-GT, U/L (>54 vs. ≤54) | <0.001 | 1.799 | 1.124–2.881 | 0.014 | 0.001 | 1.537 | 1.028–2.298 | 0.036 |
| Liver cirrhosis (yes vs. no) | 0.320 | - | - | NA | 0.751 | - | - | NA |
| Tumor size (cm) (>5 vs. ≤5) | <0.001 | 2.609 | 1.635–4.162 | <0.001 | <0.001 | 1.735 | 1.151–2.616 | 0.008 |
| Tumor multiplicity (multiple vs. single) | 0.362 | - | - | NA | 0.566 | - | - | NA |
| Tumor encapsulation (none vs. complete) | 0.493 | - | - | NA | 0.153 | - | - | NA |
| Tumor differentiation (poor vs. well) | <0.001 | 2.166 | 1.436–3.267 | <0.001 | 0.008 | - | - | NS |
| Vascular invasion (yes vs. no) | <0.001 | 1.756 | 1.139–2.709 | 0.011 | <0.001 | 1.696 | 1.136–2.531 | 0.016 |
| TNM stage (III-II vs. I b) | 0.028 | - | - | NS | 0.103 | - | - | NA |
| CD68+ TAMs (High vs. Low b) | 0.095 | - | - | NA | 0.169 | - | - | NA |
| CD86+ TAMs (Low vs. High b) | 0.029 | 2.178 | 1.333–3.558 | 0.002 | 0.040 | 1.810 | 1.181–2.776 | 0.006 |
| CD206+ TAMs (High vs. Low b) | 0.026 | 1.584 | 1.053–2.385 | 0.027 | 0.033 | 1.872 | 1.065–3.290 | 0.030 |
| CD86/CD206 signature a | 0.002 | - | - | 0.002 | 0.004 | - | - | 0.007 |
| II vs. I b | 0.020 | 2.343 | 1.173–4.681 | 0.016 | 0.033 | 1.872 | 1.065–3.290 | 0.029 |
| III vs. I b | 0.020 | 2.685 | 1.218–5.920 | 0.014 | 0.047 | 1.703 | 0.833–3.483 | 0.144 |
| IV vs. I b | 0.002 | 3.358 | 1.622–6.952 | 0.001 | 0.004 | 2.377 | 1.305–4.330 | 0.005 |
HCC, hepatocellular carcinoma; OS, overall survival; TTR, time to recurrence; HBsAg, hepatitis B surface antigen; HCVAb, hepatitis C virus antibody; AFP, alpha-fetoprotein; ALT, alanine transaminase; γ-GT, γ-glutamyltransferase; TNM, tumor-node-metastasis; HR, hazard ratio; CI, confidential interval; NA, not applicable; NS, not significant. Univariate analysis was performed by Kaplan–Meier method (log-rank test). Multivariate analysis was calculated using the Cox multivariate proportional hazard regression model with stepwise manner. a Patients were divided into four groups based on their staining densities of CD86 and CD206 positive TAMs: Group I, high expression of CD86 but low expression of CD206; Group II, both low expressions; Group III, both high expressions; and Group IV, low expression of CD86 but high expression of CD206; b Control group.
2.4. Integrated Analysis of Immune Markers CD86 and CD206 Provides More Powerful Prognostic Value in HCC Patients
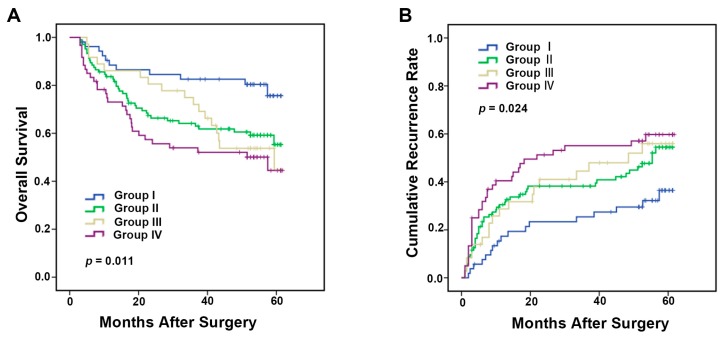
As important tumor microenvironment components, antitumoral M1 and protumoral M2 immunophenotype macrophages both influence the tumor development and progression. Thus, we hypothesized that combined analysis of CD86 and CD206 may better predict the prognosis of HCC patients by evaluating both M1 and M2 immunophenotype macrophages. Based on CD86+ and CD206+ TAMs presence, patients were classified into four groups: Group I, CD86high and CD206low; Group II, CD86low and CD206low; Group III, CD86high and CD206high; and Group IV, CD86low and CD206high. The median OS for Groups I, II, III, and IV were 52.5, 43.0, 45.2 and 37.8 months, respectively (Figure 4A). The median TTR for Groups I, II, III, and IV were 47.4 months, 38.7, 37.4 and 31.9 months, respectively (Figure 4B). Significant differences in OS and TTR were found among the four groups (OS: p = 0.011, TTR: p = 0.024, Figure 4A,B). Collectively, these data evidently suggested that combined analysis of CD86 and CD206 served as a better indicator of survival and recurrence in HCC patients than analyzing individual factors.
Figure 4.
Kaplan–Meier curves for overall survival (A) and time to recurrence (B) according to the comprehensive analysis of the staining presence of CD86+ and CD206+ macrophages in the above cohort (n = 253). Group I, high staining presence of CD86+ but low CD206+ macrophages; Group II, both low staining presence; Group III, both high staining presence; and Group IV, low staining presence of CD86+ but high CD206+ macrophages.
2.5. TAMs Predictive Model for α-Fetoprotein (AFP) Negative HCC Patients
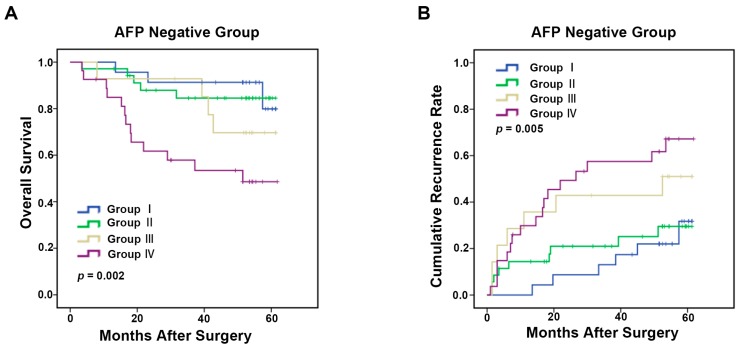
Several clinical studies demonstrated that preoperative serum AFP level was a promising predictor for HCC prognosis [14,15,16]. Low AFP level is generally associated with favorable prognosis. Nevertheless, some patients with negative AFP developed rapidly. Still worse, there is no reliable biomarker to differentiate the prognosis of AFP− HCC patients. To test the prognostic value of TAMs marker in AFP− patients (cut-off point 20 ng/mL) [17,18], 99 patients were selected from as the abovementioned cohort. In this AFP− cohort, patients with CD86low and CD206high status had the worse OS and TTR (OS: 40.5 months, p =0.002, TTR: 32.6 months, p = 0.005, Figure 5A,B) compared with three other groups (Group I, CD86high but CD206low, OS: 57.1 months, TTR: 54.1 months; Group II, CD86low and CD206low , OS: 54.8 months, TTR: 48.8 months; Group III, CD86high but CD206high , OS: 52.8 months, TTR: 37.4 months).
Figure 5.
Kaplan–Meier curves for overall survival (A) and time to recurrence (B) of HCC patients with negative α-fetoprotein (AFP) (AFP ≤ 20 ng/mL) based on the CD86+/CD206+ TAMs predictive model in the cohort (n = 99).
3. Discussion
Tumor milieu consists of many cell types. Cross-talks between tumor cells and the ambient microenvironment plays pivotal role in tumor progression and metastasis. In particular, TAM is a major cellular component that infiltrates most tumors, establishing a bridge between tumor cells and immune microenvironment [19]. Accumulating evidence indicated that the subtypes of macrophages, M1 and M2 phenotypes, performed exactly opposite roles in tumor progression and metastasis [10,20,21]. M1 acted as a proinflammatory factor against microorganisms and tumor cells [22], while M2 played an immunosuppressive role to promote tissue repair and tumor progression [23]. In this study, CD68 was used as pan macrophages immune marker; while CD86 and CD206 as marker for M1 and M2, respectively. Other molecules like CD11c and CD163 are also, respectively, expressed in M1 and M2 macrophages, but are at lower level compared with the former two in HCC. This makes CD86 and CD206 promising candidates as prognostic biomarkers compared with other macrophage biomarkers.
This study indicated that the presence of CD68+ TAMs had no impact on HCC prognosis. This may attribute to functional counterbalance regulated by M1 and M2 macrophages. Previous studies in HCC, colon cancer and gastric cancer implied that the abundance of CD68+ TAMs infiltrated in tumor tissue was not associated with patient prognosis after curative cancer tissue resection [24,25,26]. However, other research reported that CD68+ TAMs in tumor stroma was an independent prognostic factor for poor OS and TTR in breast cancer, cholangiocarcinoma and Hodgkin lymphoma [27,28,29]. The relationship between CD68+ TAMs and tumor prognosis is not clear-cut. Different CD68+ TAMs polarization status may indicate different prognosis.
In contrast to CD68+ TAMs infiltration, the low presence of CD86+ TAMs and high presence of CD206+ TAMs markedly correlated with poor HCC prognosis. Although plentiful studies described CD86 and CD206 as a cell surface immune marker of M1 and M2 macrophage respectively, only a few reports emphasized the clinic significance of CD86+ and CD206+ TAMs in tumors. In colorectal cancer, the infiltration of CD86+ TAMs indicated a favorable prognosis [30]. In multiple myeloma patients, CD86+ TAMs did not show correlation with tumor progression [31]. In prostate adenocarcinoma, the high presence of CD206+ TAMs infiltration was associated with poor prognosis [32]. Similarly, Xu et al. reported that CD206+ TAMs was a promising indicator for poor survival in renal cell carcinoma patients [33]. In gastric cancer, infiltration of polarized CD206+ TAMs in tumor indicated poor survival after surgical resection [26]. These results, as well as findings from this study, indicated that polarized M1 and M2 play a vital role in tumor prognosis.
Many reports highlighted the prognostic values of individual M1 or M2 in cancers, but did not perform a combined analysis of M1 and M2. It should be noted that M1 and M2 macrophage polarization are the two ends of macrophages. Most macrophages take on a mixed M1/M2 phenotype [34,35]. Combined analysis of M1/M2 phenotype TAMs seems to be more appropriate in cancer patients. In the present study, patients from CD86low/CD206low and CD86high/CD206high groups had the intermediate OS and TTR, possibly attributed to functional counterbalance regulated by CD86 and CD206. CD86high/CD206low group implied an immune profile of M1 macrophage polarization and served as a favorable prognostic factor in OS and TTR. On the other hand, CD86low/CD206high group showed an immune profile of M2 macrophage polarization, and was associated with a poor HCC prognosis. Our results further emphasized the opposite functions of M1 and M2 macrophages in HCC prognosis.
Previous reports revealed that about 40% early-stage HCC patients and 15%–20% late-stage HCC patients were AFP-negative [36]. Generally, HCC patients with negative AFP were associated with favorable prognosis. However, some AFP− patients progressed rapidly, with poor prognosis. Thus far, there is no satisfactory prognostic factor for AFP− patients. It is urgent to develop a novel prognostic factor for patients with negative AFP. In our study, when applied to preoperative serum AFP-negative patients, CD86low but CD206high TAMs status could effectively differentiate patients with poor prognosis. Thus, to some extent, this predictive model could be a powerful tool for making rational treatment decision in AFP-negative patients.
In summary, this study indicated that CD86high/CD206low group, implying M1 immunophenotype macrophages, and CD86low/CD206high group, implying M2 immunophenotype macrophages, provides favorable and poor prognosis of HCC, respectively. Combined analysis of immune biomarkers, CD86 and CD206, may serve as a better prognostic indicator than individual analysis for HCC survival and recurrence, especially in AFP negative patients.
4. Experimental Section
4.1. Patients
Human HCC tissues were obtained from surgical specimens of liver cancer patients in Fudan University affiliated Zhongshan Hospital (Shanghai, China). We retrospectively collected 253 patients undergoing liver cancer surgical resection between October 2009 and February 2011. Collection of clinical data and post-operation follow-up were consistent with harmonized standard [37]. Overall survival (OS) was defined as the time from the date of surgery to death. Time to recurrence (TTR) was defined as the time from the date of operation to recurrence. However, if recurrence was not observed or patients were still alive at the last follow-up (April 2015), data were censored. This study was approved by ethics committee of Fudan University affiliated Zhongshan Hospital (permission date, 26 February 2015; permission code, 20150102), and informed consent was obtained from each patient.
4.2. Immunohistochemistry
Paraffin-embedded tissue microarray (TMA) sections were deparaffinized in xylene and rehydrated in a reducing ethanol concentration series diluted with distilled water. Antigen retrieval was conducted in a preheating antigen retrieval buffer (citrate buffer, pH = 6.0) for 20 min. The TMA sections were stained with a rabbit monoclonal anti-CD68 (1:100, Abcam, Cambridge, MA, USA), a rabbit monoclonal anti-CD86 (1:100, Abcam, Cambridge, MA, USA) and a rabbit monoclonal anti-CD206 (1:100, Abcam, Cambridge, MA, USA) overnight at 4 °C. After being washed with phosphate-buffered saline (PBS) containing 0.1% Tween 20 (PBST), the sections were incubated with secondary antibodies for 1 h at room temperature. The slides were colorated with DAB (3,3′-diaminobenzidine tetrahydrochloride), and redyed with haematoxylin. The TMA sections were observed under the CKX31 microscope (Olympus, Osaka, Japan). Positive staining cells were counted every field under high magnification (200×). Positive staining cells were defined as those stained with brown regardless of the color depth. For each patient, the mean number of three random fields was used for data analysis. The positive staining cells were counted by Image Pro Plus6.0 analysis software (Media Cybernetics, Bethesda, MD, USA), and the detailed procedure is described elsewhere [38]. Based on the median value of CD68, CD86, and CD206 positive staining cells, patients were divided into high and low group [39]. The assessment ware carried out by two independent researchers, blinded of patient outcome. The intra-observer reproducibility was tested by obtaining the widely used statistical κ-scores [40].
4.3. Statistical Analysis
Analysis of the association between macrophage markers (CD68, CD86, and CD206) expression and clinicopathological characteristics was carried out using Pearson’s Chi-square test. Kaplan–Meier analysis (log-rank test) was utilized for OS and TTR curves. Variables related to OS and TTR were analyzed using univariable Cox’s regression analysis. Significant factors were further analyzed using a multivariable Cox’s regression analysis model. SPSS software (20.0; SPSS, Inc., Chicago, IL, USA) and Graph Pad Prism 5.0 software (Graph Pad-Prism Software, San Diego, CA, USA) were used for data analyses. p value <0.05 was considered significant.
Acknowledgments
This study was supported by the fund of Shanghai Science and Technology Commission (15410710100) and National Natural Science Foundation of China (81371268, 81100344, 81573423, 81572308, 81000968, and 81172273).
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1422-0067/17/3/320/s1.
Author Contributions
She Chen and Ruyi Xue conceived and designed the experiments; Pingping Dong and Longzi Liu performed the experiments; Lijie Ma and Guangxi Zhao analyzed the data; Si Zhang and Ling Dong contributed reagents/materials/analysis tools; and Pingping Dong and Lijie Ma draft the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Maluccio M., Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J. Clin. 2012;62:394–399. doi: 10.3322/caac.21161. [DOI] [PubMed] [Google Scholar]
- 2.Li X., Yao W., Yuan Y., Chen P., Li B., Li J., Chu R., Song H., Xie D., Jiang X., et al. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut. 2015 doi: 10.1136/gutjnl-2015-310514. [DOI] [PubMed] [Google Scholar]
- 3.Xue T.C., Jia Q.A., Ge N.L., Zhang B.H., Wang Y.H., Ren Z.G., Ye S.L. The platelet-to-lymphocyte ratio predicts poor survival in patients with huge hepatocellular carcinoma that received transarterial chemoembolization. Tumour Biol. 2015;36:6045–6051. doi: 10.1007/s13277-015-3281-x. [DOI] [PubMed] [Google Scholar]
- 4.Capece D., Fischietti M., Verzella D., Gaggiano A., Cicciarelli G., Tessitore A., Zazzeroni F., Alesse E. The inflammatory microenvironment in hepatocellular carcinoma: a pivotal role for tumor-associated macrophages. Biomed. Res. Int. 2013;2013:187204. doi: 10.1155/2013/187204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin L.X. Inflammatory immune responses in tumor microenvironment and metastasis of hepatocellular carcinoma. Cancer Microenviron. 2012;5:203–209. doi: 10.1007/s12307-012-0111-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmieder A., Michel J., Schonhaar K., Goerdt S., Schledzewski K. Differentiation and gene expression profile of tumor-associated macrophages. Semin. Cancer Biol. 2012;22:289–297. doi: 10.1016/j.semcancer.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Murray P.J., Wynn T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falini B., Flenghi L., Pileri S., Gambacorta M., Bigerna B., Durkop H., Eitelbach F., Thiele J., Pacini R., Cavaliere A., et al. PG-M1: A new monoclonal antibody directed against a fixative-resistant epitope on the macrophage-restricted form of the CD68 molecule. Am. J. Pathol. 1993;142:1359–1372. [PMC free article] [PubMed] [Google Scholar]
- 9.Biswas S.K., Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 10.Olsson A., Nakhle J., Sundstedt A., Plas P., Bauchet A.L., Pierron V., Bruetschy L., Deronic A., Torngren M., Liberg D., et al. Tasquinimod triggers an early change in the polarization of tumor associated macrophages in the tumor microenvironment. J. Immunother. Cancer. 2015;3:53. doi: 10.1186/s40425-015-0098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan H.Y., Wang N., Man K., Tsao S.W., Che C.M., Feng Y. Autophagy-induced RelB/p52 activation mediates tumour-associated macrophage repolarisation and suppression of hepatocellular carcinoma by natural compound baicalin. Cell Death Dis. 2015;6:e1942. doi: 10.1038/cddis.2015.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shirabe K., Mano Y., Muto J., Matono R., Motomura T., Toshima T., Takeishi K., Uchiyama H., Yoshizumi T., Taketomi A., et al. Role of tumor-associated macrophages in the progression of hepatocellular carcinoma. Surg. Today. 2012;42:1–7. doi: 10.1007/s00595-011-0058-8. [DOI] [PubMed] [Google Scholar]
- 13.Ding T., Xu J., Wang F., Shi M., Zhang Y., Li S.P., Zheng L. High tumor-infiltrating macrophage density predicts poor prognosis in patients with primary hepatocellular carcinoma after resection. Hum. Pathol. 2009;40:381–389. doi: 10.1016/j.humpath.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Ma W.J., Wang H.Y., Teng L.S. Correlation analysis of preoperative serum α-fetoprotein (AFP) level and prognosis of hepatocellular carcinoma (HCC) after hepatectomy. World J. Surg. Oncol. 2013;11 doi: 10.1186/1477-7819-11-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park H., Park J.Y. Clinical significance of AFP and PIVKA-II responses for monitoring treatment outcomes and predicting prognosis in patients with hepatocellular carcinoma. Biomed. Res. Int. 2013;2013:310427. doi: 10.1155/2013/310427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang S.H., Kim D.Y., Jeon S.M., Ahn S.H., Park J.Y., Kim S.U., Kim J.K., Lee K.S., Chon C.Y., Han K.H. Clinical characteristics and prognosis of hepatocellular carcinoma with different sets of serum AFP and PIVKA-II levels. Eur. J. Gastroenterol. Hepatol. 2012;24:849–856. doi: 10.1097/MEG.0b013e3283535c34. [DOI] [PubMed] [Google Scholar]
- 17.Toyoda H., Kumada T., Tada T., Sone Y., Kaneoka Y., Maeda A. Tumor Markers for Hepatocellular Carcinoma: Simple and Significant Predictors of Outcome in Patients with HCC. Liver Cancer. 2015;4:126–136. doi: 10.1159/000367735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toyoda H., Kumada T., Kiriyama S., Sone Y., Tanikawa M., Hisanaga Y., Yamaguchi A., Isogai M., Kaneoka Y., Washizu J. Prognostic significance of simultaneous measurement of three tumor markers in patients with hepatocellular carcinoma. Clin. Gastroenterol. Hepatol. 2006;4:111–117. doi: 10.1016/S1542-3565(05)00855-4. [DOI] [PubMed] [Google Scholar]
- 19.Fukuda K., Kobayashi A., Watabe K. The role of tumor-associated macrophage in tumor progression. Front. Biosci. Sch. Ed. 2012;4:787–798. doi: 10.2741/S299. [DOI] [PubMed] [Google Scholar]
- 20.Locatelli L., Cadamuro M., Spirli C., Fiorotto R., Lecchi S., Maria M.C., Popov Y., Scirpo R., de Matteis M., Amenduni M., et al. Macrophage recruitment by fibrocystin-defective biliary epithelial cells promotes portal fibrosis in congenital hepatic fibrosis. Hepatology. 2015 doi: 10.1002/hep.28382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Den Breems N.Y., Eftimie R. The re-polarisation of M2 and M1 macrophages and its role on cancer outcomes. J. Theor. Biol. 2015;390:23–39. doi: 10.1016/j.jtbi.2015.10.034. [DOI] [PubMed] [Google Scholar]
- 22.Martinez F.O., Gordon S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014;6 doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solinas G., Germano G., Mantovani A., Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J. Leukoc. Biol. 2009;86:1065–1073. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 24.Kong L.Q., Zhu X.D., Xu H.X., Zhang J.B., Lu L., Wang W.Q., Zhang Q.B., Wu W.Z., Wang L., Fan J., et al. The clinical significance of the CD163+ and CD68+ macrophages in patients with hepatocellular carcinoma. PLoS ONE. 2013;8:320. doi: 10.1371/journal.pone.0059771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernandez C., Barrachina M.D., Cosin-Roger J., Ortiz-Masia D., Alvarez A., Terradez L., Nicolau M.J., Alos R., Esplugues J.V., Calatayud S. Progastrin represses the alternative activation of human macrophages and modulates their influence on colon cancer epithelial cells. PLoS ONE. 2014;9:320. doi: 10.1371/journal.pone.0098458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang H., Wang X., Shen Z., Xu J., Qin J., Sun Y. Infiltration of diametrically polarized macrophages predicts overall survival of patients with gastric cancer after surgical resection. Gastric Cancer. 2015;18:740–750. doi: 10.1007/s10120-014-0422-7. [DOI] [PubMed] [Google Scholar]
- 27.Medrek C., Ponten F., Jirstrom K., Leandersson K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 2012;12 doi: 10.1186/1471-2407-12-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atanasov G., Hau H.M., Dietel C., Benzing C., Krenzien F., Brandl A., Wiltberger G., Matia I., Prager I., Schierle K., et al. Prognostic significance of macrophage invasion in hilar cholangiocarcinoma. BMC Cancer. 2015;15 doi: 10.1186/s12885-015-1795-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tzankov A., Matter M.S., Dirnhofer S. Refined prognostic role of CD68-positive tumor macrophages in the context of the cellular micromilieu of classical Hodgkin lymphoma. Pathobiology. 2010;77:301–308. doi: 10.1159/000321567. [DOI] [PubMed] [Google Scholar]
- 30.Kinouchi M., Miura K., Mizoi T., Ishida K., Fujibuchi W., Ando T., Yazaki N., Saito K., Shiiba K., Sasaki I. Infiltration of CD14-positive macrophages at the invasive front indicates a favorable prognosis in colorectal cancer patients with lymph node metastasis. Hepatogastroenterology. 2011;58:352–358. [PubMed] [Google Scholar]
- 31.Beider K., Bitner H., Leiba M., Gutwein O., Koren-Michowitz M., Ostrovsky O., Abraham M., Wald H., Galun E., Peled A., et al. Multiple myeloma cells recruit tumor-supportive macrophages through the CXCR4/CXCL12 axis and promote their polarization toward the M2 phenotype. Oncotarget. 2014;5:11283–11296. doi: 10.18632/oncotarget.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu W., Qian Y., Yu F., Liu W., Wu Y., Fang X., Hao W. Alternatively activated macrophages are associated with metastasis and poor prognosis in prostate adenocarcinoma. Oncol. Lett. 2015;10:1390–1396. doi: 10.3892/ol.2015.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu L., Zhu Y., Chen L., An H., Zhang W., Wang G., Lin Z., Xu J. Prognostic value of diametrically polarized tumor-associated macrophages in renal cell carcinoma. Ann. Surg. Oncol. 2014;21:3142–3150. doi: 10.1245/s10434-014-3601-1. [DOI] [PubMed] [Google Scholar]
- 34.Bosca L., Gonzalez-Ramos S., Prieto P., Fernandez-Velasco M., Mojena M., Martin-Sanz P., Alemany S. Metabolic signatures linked to macrophage polarization: from glucose metabolism to oxidative phosphorylation. Biochem. Soc. Trans. 2015;43:740–744. doi: 10.1042/BST20150107. [DOI] [PubMed] [Google Scholar]
- 35.Galvan-Pena S., O’Neill L.A. Metabolic reprograming in macrophage polarization. Front. Immunol. 2014;5:420. doi: 10.3389/fimmu.2014.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He X., Wang Y., Zhang W., Li H., Luo R., Zhou Y., Liao C.L., Huang H., Lv X., Xie Z., et al. Screening differential expression of serum proteins in AFP-negative HBV-related hepatocellular carcinoma using iTRAQ -MALDI-MS/MS. Neoplasma. 2014;61:17–26. doi: 10.4149/neo_2014_001. [DOI] [PubMed] [Google Scholar]
- 37.Masuda T., Beppu T., Okabe H., Nitta H., Imai K., Hayashi H., Chikamoto A., Yamamoto K., Ikeshima S., Kuramoto M., et al. Predictive factors of pathological vascular invasion in hepatocellular carcinoma within 3 cm and 3 nodules without radiological vascular invasion. Hepatol. Res. 2015 doi: 10.1111/hepr.12637. [DOI] [PubMed] [Google Scholar]
- 38.Alvaro T., Lejeune M., Salvado M.T., Bosch R., Garcia J.F., Jaen J., Banham A.H., Roncador G., Montalban C., Piris M.A. Outcome in Hodgkin’s lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T cells. Clin. Cancer Res. 2005;11:1467–1473. doi: 10.1158/1078-0432.CCR-04-1869. [DOI] [PubMed] [Google Scholar]
- 39.Kuang D.M., Zhao Q., Wu Y., Peng C., Wang J., Xu Z., Yin X.Y., Zheng L. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J. Hepatol. 2011;54:948–955. doi: 10.1016/j.jhep.2010.08.041. [DOI] [PubMed] [Google Scholar]
- 40.Landis J.R., Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.