Abstract
Around 3000 proteins are thought to bind zinc in vivo, which corresponds to ~10% of the human proteome. Zinc plays a pivotal role as a structural, catalytic, and signaling component that functions in numerous physiological processes. It is more widely used as a structural element in proteins than any other transition metal ion, is a catalytic component of many enzymes, and acts as a cellular signaling mediator. Thus, it is expected that zinc metabolism and homeostasis have sophisticated regulation, and elucidating the underlying molecular basis of this is essential to understanding zinc functions in cellular physiology and pathogenesis. In recent decades, an increasing amount of evidence has uncovered critical roles of a number of proteins in zinc metabolism and homeostasis through influxing, chelating, sequestrating, coordinating, releasing, and effluxing zinc. Metallothioneins (MT) and Zrt- and Irt-like proteins (ZIP) and Zn transporters (ZnT) are the proteins primarily involved in these processes, and their malfunction has been implicated in a number of inherited diseases such as acrodermatitis enteropathica. The present review updates our current understanding of the biological functions of MTs and ZIP and ZnT transporters from several new perspectives.
Keywords: zinc, metallothionein, ZIP and ZnT transporter, chaperone
1. Introduction
Following the uptake of zinc by cells, it is distributed within the cytoplasm (50%), nucleus (30%–40%), and cell membrane (10%) [1,2]. Cellular zinc is then available as four pools [2,3]. First, it can bind tightly to metalloproteins as a structural component or to metalloenzymes as a cofactor; Second, zinc binds metallothioneins (MTs) with a low affinity, which can occupy 5%–15% of the total cellular zinc pool [4]; Third, it can be compartmentalized into intracellular organelles and vesicles for zinc storage and as a supply for zinc-dependent proteins, which is mediated by zinc transporters [5,6]. As a result of the second and third functions, the fourth pool of cytosolic free zinc is maintained at a very low concentration (pM–low nM levels) [7,8,9]. MTs and two zinc transporter families, Zrt- and Irt-like proteins (ZIP, solute carrier 39A [SLC39A]) and Zn transporters (ZnT, SLC30A), play crucial roles to maintain this cellular zinc homeostasis [2,3,10,11,12] (Figure 1). In this review, we focus on recent progress to describe the physiological and biological functions of MTs and ZIP and ZnT transporters, and to provide a better understanding of zinc biology.
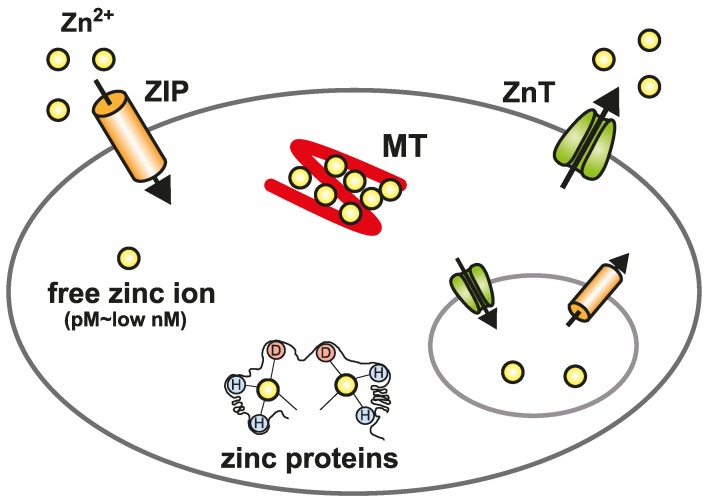
Figure 1.
Cellular zinc homeostasis is controlled by the cooperative function of metallothioneins (MT) and Zrt- and Irt-like proteins (ZIP) and Zn transporters (ZnT). The mobilization of zinc into or out of the cytosol is directed by two zinc transporter families, ZIP and ZnT. In the cytosol, MTs bind zinc to reserve, buffer, and chelate. Zinc is compartmentalized into or out of intracellular organelles and vesicles by ZnT and ZIP transporters. Because of the binding of zinc to many different proteins, the free zinc ion concentration in the cytosol is estimated to be well below pM–low nM levels.
2. Physiological and Cellular Functional Properties of Metallothioneins (MTs)
2.1. MT Isoforms
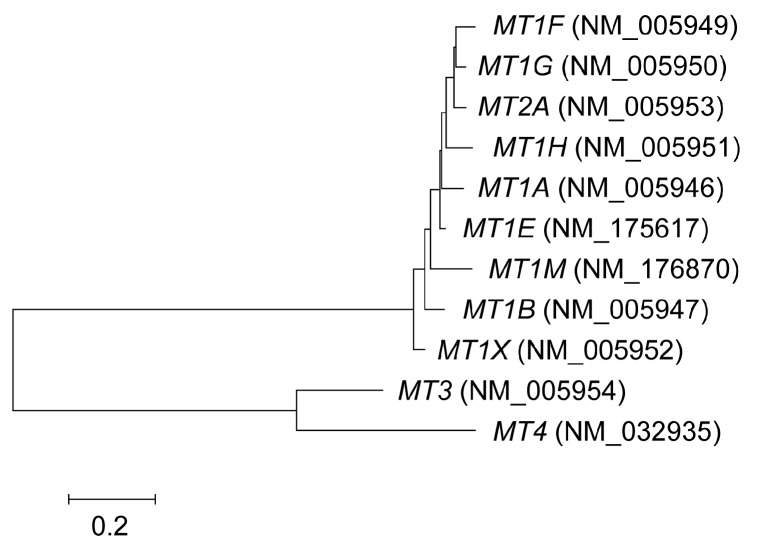
MTs are low-molecular-weight metal-binding proteins that lack disulfides and contain one-third cysteine residues. Human MTs have a total of 11 functional isoforms that can be divided into four classes, designated MT-1 to -4. These are encoded by eight active MT1 genes (MT1A, B, E, F, G, H, M, and X), and a single copy of MT2 (known as MT2A), MT3, and MT4. The human genome also contains five pseudo-MT1 genes derived from duplication and loss-of-function mutations of the original parental MT1. However, it is not clear whether the eight active MT1 genes have gained a new function. The mouse harbors single copies of MT1, 2, 3, and 4. A tree topology study suggested that two rounds of duplication have occurred in the MT family [13] (Figure 2). The neighbor-joining method of analysis of human MT genes using MEGA6 software (http://www.megasoftware.net/) (Figure 2) suggested that the ancestor of MT3/4 and MT1/2 diverged, after which MT3 and MT4 seem to have separated. Conversely, the Bayesian and maximum likelihood methods of analysis [13] indicated that divergence of the MT4 and MT1/2/3 ancestor was the first step. In either case, following duplication of these genes, gain-of-function mutations are likely to have occurred in MT1/2, MT3, and MT4. MT-3 and -4 show a restricted cell type-specific expression pattern, with MT-3 being expressed mainly in the brain and MT-4 most abundant in certain epithelial tissues. These isoforms show specific roles in these tissues [14].
Figure 2.
Phylogenetic tree of MT genes. The tree was constructed using coding sequences from NCBI RefSeq and the neighbor-joining method using MEGA6 software.
The ubiquitous MT isoforms MT-1 and -2 have been extensively investigated with regard to zinc metabolism. They are expressed in many cell types in various organs and tissues, as well as in most cultured cells, and their function is to maintain cellular zinc homeostasis and attenuate heavy metal-induced cytotoxicity by chelating these metals and lowering their intracellular concentrations. They also protect against several types of environmental stress through their radical scavenging properties [15].
It is unclear whether MT-1 and -2 have functional differences. Because of their similar amino acid sequences and inducibility in response to zinc and various stress conditions and compounds, most research studies of MT-1 and -2 have been done without separation. However, several lines of research show that the MT-1 and 2 isoforms have specific functions, which are summarized in Section 3.2. In the 1990s, two lines of MT1/2 double knockout (KO) mice were established to examine the functional properties of MT-1 and -2 [16,17]. MT1/2 KO mice were viable and reproduced normally when reared under standard laboratory conditions. The function of MT1/2 was shown not only in the protection against metals [16,17,18,19], oxidative stress [20], and carcinogens [21], but also in immune reactions and obesity (Table 1). MT1/2 KO mice have a high sensitivity to lipopolysaccharide (LPS) and LPS/d-galactosamine, so represent an acute hepatic failure model [22,23]. However, the protective mechanisms are unclear. MT1/2 KO mice also showed increased Helicobacter pylori (H. pylori)-induced gastric erosive lesions [24]. These lesions are associated with production of reactive oxygen species (ROS) from infiltrated macrophages and neutrophils. ROS scavenging activity of MT might be involved in the sensitization. A report detailing a decrease in interleukin (IL)-4 production in MT1/2 KO mice, which is mediated by FcεRI-induced calcineurin (CaN)/nuclear factor of activated T-cell (NFAT) signaling pathway, suggests that the MT-dependent control of zinc homeostasis regulates IL-4 production in basophil granulocytes [25]. Interestingly, zinc transporter KO mice such as Zip10 KO also showed an altered immune response [26,27]. We describe the KO phenotype of zinc transporters in Section 5.2 and Section 5.3.
Table 1.
Knockout (KO) phenotypes of MT1/2.
| Phenotype | Strain | References |
|---|---|---|
| Metal binding | ||
| Increased sensitivity to heavy metal toxicity | 129/Sv, C57BL/6 | [16,17,19] |
| Increased sensitivity to zinc deficiency and excess | C57BL/6 | [18] |
| Decreased FcεRI-induced IL-4 production, which is mediated by calcineurin (CaN)/nuclear factor of activated T-cell (NFAT) signaling, in basophil granulocytes | C57BL/6 | [25] |
| Reduced survival in Cu/Zn-superoxide dismutase (SOD1)-mutated (G93A) mice, which is a familial mouse model of amyotrophic lateral sclerosis (ALS) | 129/Sv | [30] |
| Reactive oxygen species (ROS) scavenging | ||
| Increased sensitivity to X-irradiation-induced bone marrow injury | C57BL/6 | [20] |
| Increased chemical and radiation-induced carcinogenesis | C57BL/6 | [21] |
| Increased Helicobacter pylori (H. pylori)-induced gastric erosive lesions with infiltration of leukocytes | C57BL/6 | [24] |
| Unknown mechanisms | ||
| Increased sensitivity to lipopolysaccharide (LPS)/d-galactosamine-induced lethality | 129/Sv | [22] |
| Increased coagulatory and fibrinolytic disturbance and multiple organ damage induced by LPS | C57BL/6 | [23] |
| High-fat-diet-induced obesity, increased plasma leptin and leptin mRNA in the white adipose tissue when fed the high-fat-die (a leptin-resistant state) | 129/Sv | [28] |
| Shorten the lifespan, exhibiting signs of weight loss, hunchbacked spines, lackluster fur and an absence of vigor in male living beyond the mean lifespan | 129/Sv | [29] |
As shown in Table 1, two strains of MT1/2 KO mice exist. The first is 129SvCPJ [16], while the second was originally developed on a mixed genetic background of OLA129 and C57BL/6 strains [17] then backcrossed with C57BL/6J Jcl. The KO phenotype shown in Table 1 might be strain-specific. Other phenotypes, such as high-fat diet-induced obesity [28] and a shortened lifespan [29], appeared through unknown mechanisms.
Mutations in the Cu/Zn-superoxide dismutase (SOD1) gene cause one form of familial amyotrophic lateral sclerosis (ALS), a progressive disorder of motor neurons leading to death. MT1/2 deficiency in mouse model of ALS involving mutated SOD1 (G93A SOD1) shows a reduction in survival compared with G93A SOD1 mice [30]. SOD1 is an enzyme that binds zinc, and abnormalities in this binding have been implicated in disease pathogenesis [31]. This study indicated that MT acts as a zinc chaperone for apo-SOD1. We discuss the possibility of MT being a zinc chaperone in more detail in Section 3.1 and Section 6.
2.2. The Zinc-Responsive Transcription Factor MTF-1
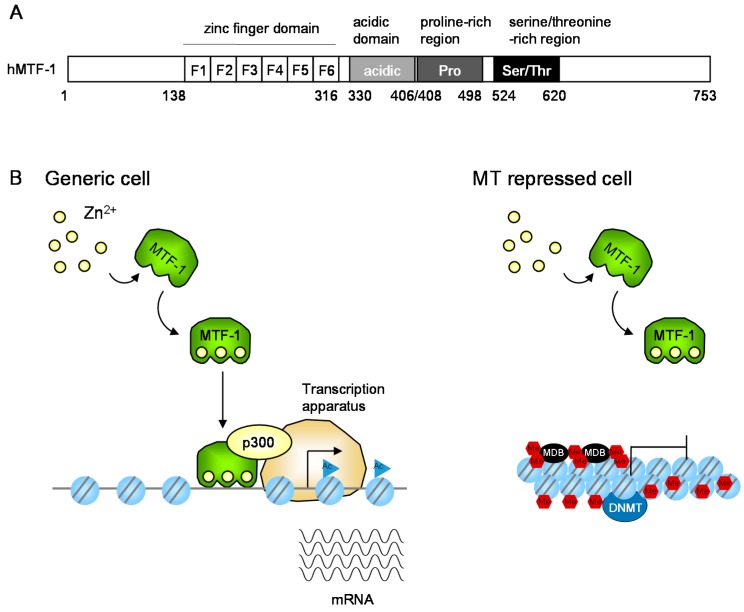
MT1/2 transcription is regulated by the metal response element-binding transcription factor-1 (MTF-1), which is a zinc finger transcription factor that regulates metal-responsive gene expression [32] (Figure 3). In MTF-1 KO cells, MT1/2 genes are silent. MTF-1 is an essential factor for basal and heavy metal-induced MT1/2 expression. It possesses six Cys2His2 zinc fingers and three transcriptional activation domains, namely an acidic domain, a proline-rich region, and a serine/threonine-rich region. A simple metalloregulatory model suggests that MTF-1 has a low intrinsic binding affinity for zinc, and only binds zinc under zinc excess conditions. Specific zinc fingers in MTF-1 were shown to have zinc-binding affinities in the nM to sub-µM range [33], while canonical Cys2His2 zinc fingers typically bind zinc with higher affinity (10−9–10−12 M). Because zinc affinities of all six fingers are similar in MTF-1 (within ~10–50-fold of each other), zinc-sensing by MTF-1 is suggested to occur within a 100-fold or less range of accessible zinc concentration [34].
Figure 3.
Expression regulation of MT gene expression. (A) Schematic representation of human metal response element-binding transcription factor-1 (MTF-1). The regions of the six-zinc fingers (F1–F6), acidic, proline-rich, and serine/threonine-rich domains are indicated by boxes and amino acid numbers; (B) Proposed molecular mechanisms in MT transcription in response to increases of intracellular free zinc. In generic cells, MTF-1 recruits the histone acetyltransferase p300 and increases MT transcription. In MT-repressed cells such as lymphosarcoma cells, and cancer cells, the promoter is highly methylated. DNA methyltransferase (DNMT) and methyl CpG binding proteins (MBD) are involved in the suppression. The epigenetic mechanism is described in Section 4.2. Ac, acetyl group; Me, methyl group; blue circle with two lines, nucleosome.
MTF-1 also regulates the zinc-responsive transcription of ZnT1 and ZnT2 [35,36] and represses the expression of Zip10 [37,38], indicating that it plays an important role in zinc homeostasis. The tumor suppressor phosphatase and tensin homolog modulates the MTF-1-mediated expression of ZnT1 and MT [39], suggesting a relationship between tumorigenesis and zinc homeostasis. Protein phosphatase 2A has also been reported to be involved in MT induction [40]. In addition to MTF-1, a recent finding revealed that another zinc finger transcription factor, ZNF658, is involved in the regulation of zinc transporter expression [41]. Thus, ZNF658 may cooperatively function as a transcriptional regulator with MTF-1 in the controlled cellular response to zinc availability.
3. Structural and Biochemical Functions of MTs
3.1. MT Functions in Physiological and Cellular Zinc Homeostasis
MT was first identified as a protein containing heavy metals such as cadmium and zinc [42]. MTs intracellularly bind these metals and lower their concentration at critical sites. A role for MT in cellular zinc homeostasis was predicted before being experimentally confirmed [4,14,43]. In one study, mouse fibroblasts were adapted to extreme zinc deprivation (<0.06 μM zinc vs. sub-μM levels in normal medium) by increasing MT-1 mRNA expression through MT1 amplification without MT protein accumulation. Apo-MT chelates zinc from the environment and increases intracellular zinc. When zinc levels were insufficient to stabilize the MT protein, the MT was rapidly proteolyzed. Zinc is then released by MT degradation, so the intracellular zinc concentration was kept constant. In contrast, when cells expressing MT3 were deprived of zinc, cell proliferation was arrested and MT-3 protein levels persisted. MT1/2 were shown to scavenge extracellular zinc, not to compete with essential zinc-requiring proteins, and to be degraded, while MT3 was shown to compete for zinc and to exacerbate the zinc deficiency [44].
MTs can also function as a “zinc buffer” through their low-affinity binding, providing labile zinc for use by target proteins/enzymes when zinc is limited [45,46]. Specifically, several zinc-requiring apoenzymes can be reactivated by the transfer of zinc from zinc-saturated MT, which binds seven zinc ions. One study proposed the model that zinc-saturated Zn7-MT and Zn6-MT are the primary zinc-donating species for apo-carbonic anhydrase, a zinc-requiring enzyme [47]. In particular, MT is a source of zinc ions under conditions of redox signaling through the modifications of zinc-thiolate coordination environments [48,49], which contribute to the functions of zinc in cellular signaling [50]. It has also been reported that apo-MT removes zinc from the zinc finger transcription factors Sp1 and transcription factor IIIA in vitro, and eliminates their DNA-binding ability [51,52]. Thus, MT appears to act as a chaperone for zinc proteins/enzymes. This role involves the transfer of zinc from MT to proteins/enzymes via ligand exchange in zinc-mediated protein–protein interactions in the absence of freely released zinc ions, which is known as “the associative mechanism” [46,53,54] as seen in copper metabolism [55,56,57]. In this similar metabolism, several copper chaperones play critical roles in transferring cytosolic copper to target proteins/enzymes [55,56,57].
3.2. Specific Functions of MT-1 and -2 Isoforms
Although MT-1 and 2 have been largely studied together, the specific functions of these proteins have only begun to be elucidated [58,59]. For instance, comparing the stability of rat zinc-saturated MT-1 and MT-2, MT-2 was shown to degrade more slowly than MT-1 [58]. More recently, zinc-saturated MT-2 was found not to exist under normal physiological conditions [60], although it is unclear whether stability differs among zinc-unsaturated isoforms.
Several studies of MT single nucleotide polymorphisms (SNPs) showed that MT2A but not MT1 SNPs are associated with an increased cancer risk [61,62,63] (Table 2). However, because the basal level of MT2A expression appears to be 5–10 times higher than that of MT1X, this association might be caused by MT2A expression levels. MT isoform-specific gene regulation mechanisms have also been reported. As an example, vascular endothelial growth factor was shown to induce human MT1G, but not MT2A, by regulating the E2F transcription factor [64], while bovine MT1A and 1E, but not MT2A, are induced through the Keap1-Nrf2 system [65]. Such isoform-specific functions may result from isoform-specific expression mechanisms.
Table 2.
Specific MT1/2 isoform functions.
| Isoform | Isoform Specific Function | Findings | Ref. |
|---|---|---|---|
| MT1A | Increase risk of lung cancer | Single nucleotide polymorphisms (SNPs) (rs7196890) | [68] |
| MT1A, 1G | Regulate myeloid differentiation | Negatively regulated by PU.1 in leukemia cells (in microarray analysis) Inhibition of retinoic acid-induced differentiation by MT1G overexpression. |
[69,70] |
| MT1X | Mediate cisplatin-induced apoptosis | Interacts with Akt and tongue cancer resistance-associated protein 1 (TCRP1) in oral squamous cell carcinoma (in microarray analysis) | [67] |
| Increased sensitivity to cisplatin through activation of phosphatidylinositol-3-kinase (PI3K)/Akt/nuclear factor-κB (NF-κB) signaling pathway by knockdown of MT1X with TCRP1 | |||
| MT2A | Regulate autophagy and apoptosis | Inhibition of intracellular free zinc elevation by knockdown of MT2A | [66] |
| Interacts with homeobox containing 1 (HMBOX1) (in yeast two-hybrid assay), overexpression of which increases intracellular free zinc | |||
| Inhibition of anti-apoptosis and pro-autophagy effects of HMBOX1 by zinc chelator, N,N,N',N'-Tetrakis(2-pyridylmethyl)ethylenediamine (TPEN) | |||
| MT2A | Increase risk of prostate cancer | SNPs (rs28366003) | [61,62] |
| MT2A | Increase risk of ductal breast cancer | SNPs (rs28366003) | [63] |
However, MT isoform-specific linkage has also been reported. MT2A interacts with homeobox-containing 1 (HMBOX1) in human umbilical vascular endothelial cells to increase intracellular free zinc concentrations [66]. Knockdown of MT2A decreases intracellular free zinc, while treatment with a zinc chelator inhibited HMBOX1-regulated apoptosis and promoted HMBOX1-regulated autophagy. The interaction between MT1X, Akt, and tongue cancer resistance-associated protein 1 (TCRP1) in oral squamous cell carcinoma [67] was also reported. In this report, MT1X knockdown increased cisplatin-induced apoptosis. Although these MT isoforms might have specific functions, their amino acid sequences have a high level of homology. Therefore, further investigations are needed to clarify the mechanisms of isoform-specific function.
4. Novel Regulation of MT Expression
4.1. SNPs in the MT Promoter
As described in Section 2.2, MT1/2 expression is regulated by the transcription factor MTF-1 (Figure 3). In MTF-1 KO cells, MT1/2 genes are silent, and therefore MTF-1 is essential for basal and heavy metal-induced MT1/2 expression, while other transcription factors are also involved in the expression [71]. For example, signal transducer and activator of transcription 3 (STAT3) and glucocorticoid receptor (GR) transcription factors are required for MT expression in response to immune response mediation by IL-6. The SNP-dependent decrease of MT2A expression was also reported in a Japanese study [72] in which 17.6% of 119 individuals had an A → G SNP (A/G: 16.8%, G/G: 0.8%) in the MT2A promoter near the TATA box (rs28366003). A reporter gene assay using HEK293 cells showed that replacement of A by G reduced MT2A expression to 30%–70% after zinc and cadmium treatment. In separate studies, Yoshida et al. reported increased cadmium levels in the renal cortex of individuals with (group A) and without (group B) MT accumulation [73,74]. Because MT is a cytoprotective factor against cadmium, group B individuals might be expected to be more sensitive to cadmium toxicity than group A. Although the genetic background of these groups was not examined, group B might be expected to have A/G or G/G genotypes. As shown in Table 2, this SNP is also positively associated with lung, prostate and ductal breast cancer [61,62,63,68]. Another possibility for the difference between groups A and B is the involvement of epigenetic regulation of MT expression.
4.2. Epigenetic Regulation of MT Expression
MT expression is also regulated through epigenetic mechanisms. Although MT1/2 is ubiquitously expressed, some cell lines do not express MTs (Figure 3). Jacob et al. reported that the suppression of MT1 expression was caused by promoter-specific DNA methylation [75,76]. Moreover, inhibitors of histone deacetylase (HDAC) and DNA methyltransferase (DNMT) synergistically activate its expression [77]. In MT1/2-expressing cells, the HDAC p300 is involved in a zinc-induced MTF-1-containing complex [78]. MTF-1 deletion mutant analysis revealed that this complex plays an essential role in the activation of MT1 transcription. Furthermore, zinc rapidly and locally disrupts the MT1 promoter chromatin structure by nucleosome removal. Binding of MTF-1 to the MT1 promoter was required to initiate histone exclusion, but was not necessary to maintain this exclusion, at least in the short term [79]. IL-2 transcription has been reported to occur earlier in zinc re-stimulated mouse T cells than at the first stimulation [80], and this is thought to reflect histone exclusion from the MT1 promoter, which acts as a memory of the first zinc exposure.
The epigenetic mechanisms involved in MT transcription are not fully understood, although their disruption modifies MT transcription. Chromatin remodeling complexes such as the SWI–SNF complex are not required for cadmium-induced mouse MT1 transcription [81], while prenatal zinc deficiency affects cadmium-induced mouse MT2 transcription through epigenetic mechanisms [82]. Hexavalent chromium (Cr6+), a heavy metal known for over 100 years to be a human carcinogen, inhibits mouse MT1 transcription by modifying the transcription potential of p300 [83]. Cross-linking of HDAC1–DNMT1 complexes to chromatin might be involved in the inhibition of MT transcription [77,84,85]. These epigenetic mechanisms might influence the biochemical functions of MT.
5. Cellular Zinc Homeostasis Involving ZIP and ZnT Zinc Transporters
5.1. Zrt- and Irt-Like Proteins (ZIP) and Zn Transporters (ZnT) Transporters
In addition to the chelating and releasing by MTs, mobilization of zinc across biological membranes is important to maintain cellular and subcellular zinc homeostasis. Although zinc ions can cross biological membranes through various calcium channels, ZIP and ZnT transporter family proteins play crucial roles as transport routes [86,87] (Figure 4). ZIP transporters mobilize zinc from the extracellular space or intracellular organelles to the cytosol, while ZnT transporters function in zinc efflux and compartmentalization as cation diffusion proteins.
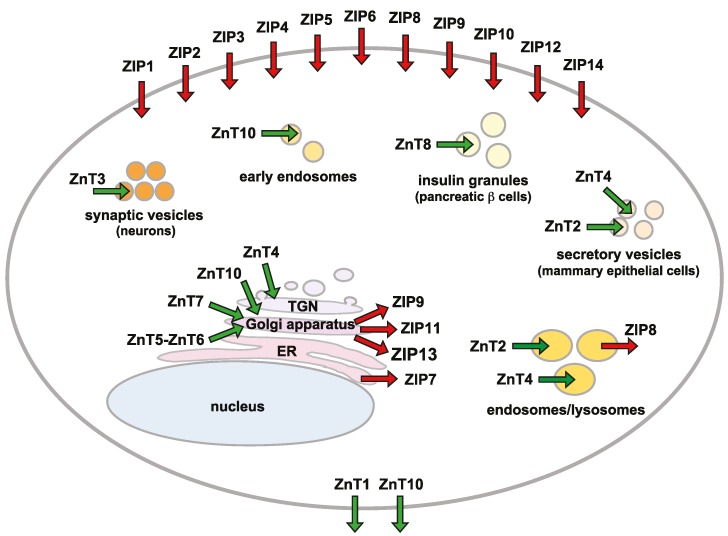
Figure 4.
The subcellular localization of ZIP and ZnT transporters. The primary localization of ZIP (red arrows) and ZnT (green arrows) transporters is shown according to available information. This schematic illustrates a static view of their localization. Cytosolic zinc is mobilized into or out of different subcellular compartments, including synaptic vesicles or insulin granules in a cell-specific manner. ER, endoplasmic reticulum; TGN, trans-Golgi network.
A total of 14 ZIP and nine ZnT transporter genes are encoded in mammalian genomes. Each of the zinc transporters shows a tissue-specific, developmental, stimulus-responsive expression pattern, and specific cellular and subcellular localization. Both transporters display specific changes in protein stability and cellular localization in response to various stimuli including zinc deficiency or excess [2,10,11,12]. Recent studies have indicated that epigenetic expression control occurs in a number of ZIP and ZnT transporters, as seen for MT genes (Section 4.1) [88,89]. Moreover, micro RNA-mediated expression control has also been revealed to control cellular zinc homeostasis [90,91]. Molecular information about both zinc transporters, including expression regulation at the protein level, discrimination of substrate metal, and transport mechanism, is extensively reviewed elsewhere [2,12,92], so is not covered here.
5.2. Overview of ZIP Transporter Knockout Animals and Human Diseases
Zip1, Zip2, and Zip3 KO mice are more likely to produce abnormal embryos when dietary zinc is deficient [93,94,95,96]. Zip3 KO mice also show zinc retention in the secreted milk pool [97], while Zip1 and Zip3 double KO mice reduce seizure-induced CA1 neurodegeneration [98], suggesting the involvement of ZIP3 in zinc reuptake from milk secreted from mammary glands and the involvement of ZIP1 and ZIP3 in neural degeneration induced by zinc entry. ZIP4 has been identified as the gene responsible for a rare autosomal-recessive inherited zinc deficiency, acrodermatitis enteropathica (AE) [99,100]. AE is caused by impaired intestinal absorption of zinc, and is characterized by eczematous dermatitis, diarrhea and alopecia [101,102]. The importance of ZIP4 in zinc absorption and intestinal integrity was also confirmed by intestine-specific conditional Zip4 KO mice [103] because complete Zip4 KO mice are embryonically unviable [104].
Missense and nonsense mutations of ZIP5 have been shown to be associated with nonsyndromic high myopia [105], although this has not been investigated using Zip5 KO mice. However, complete and tissue-specific Zip5 KO mice were used to show the participation of ZIP5 in the control of zinc excretion [106]. ZIP8 SNPs (such as rs13107325, resulting in A391T substitution) were associated with the circulation of high-density lipoprotein cholesterol [107] and blood pressure [108]. Hypomorphic Zip8 mice show utero and neonatal lethality because of multiple organ hypoplasia [109], while chondrocyte-specific conditional Zip8 KO mice suppress surgically induced osteoarthritis pathogenesis [110], indicating that ZIP8 induces the cartilage breakdown of osteoarthritis [110]. Recently, loss-of-function ZIP8 mutations have been associated with human diseases including intellectual disability, cerebellar atrophy, severe infantile spasms with hypsarrhythmia, disproportionate dwarfism, developmental delay and hypotonia, strabismus, and cranial asymmetry [111,112].
B-cell-specific conditional Zip10 KO mice show splenoatrophy with reduced peripheral B cell numbers and diminished immunoglobulin levels, indicating the involvement of ZIP10 in anti-apoptotic signaling in early B-cell survival [26]. Both T-cell-dependent and independent immune responses are attenuated in the mature B cells of Zip10 KO mice, revealing its importance in the modulation of B-cell receptor signaling [27]. Recent analysis using ZIP12 KO rats showed that ZIP12 regulates the pulmonary vascular response to chronic hypoxia [113], while ZIP13 mutations have been shown to cause the spondylocheiro dysplastic form of Ehlers–Danlos syndrome (SCD-EDS) [114,115], which is characterized by hard and connective tissue abnormalities. Zip13 KO mice show delayed growth, and skeletal and connective tissue abnormalities, which are phenotypes similar to those of SCD-EDS patients [115]. Finally, Zip14 KO mice exhibit dwarfism, impaired skeletogenesis [116], hypoglycemia, greater body fat, and higher insulin levels than wild-type [117]. Moreover, hepatocyte proliferation is decreased in Zip14 KO mice during liver regeneration [118]. The study using Zip14-KO mice revealed its involvement in the uptake of plasma non-transferrin bound iron by the liver and pancreas, and thus possibly in iron overload in hereditary hemochromatosis [119].
5.3. Overview of ZnT Transporter Knockout Animals and Human Diseases
KO mice of ZnT transporters also indicate their crucial roles in zinc-related pathophysiology. Znt1 KO mice are embryonically unviable from an early stage because of the impaired zinc transfer from the mother [120]. ZnT2 has been identified as the gene responsible for transient neonatal zinc deficiency (TNZD), which is caused by low zinc levels in breast milk [121,122,123,124,125]. The symptoms are similar to AE, but TNZD only develops in breast-fed infants, and does not reoccur after weaning. ZnT2 KO mice showed the importance of ZnT2 during lactation and mammary gland development [126], while ZnT3 KO mice show age-dependent defects in learning and memory such as spatial working and fear [127,128,129]. They also lack synaptic zinc [130] and display differences in protein and gene expression important in neurotransmission [127], suggesting modulation functions of ZnT3 in synaptic transmission and plasticity. Moreover, loss of ZnT3 function increases the risk of febrile seizures in humans [131].
A spontaneous Znt4 mutant mouse produces milk with reduced zinc levels, so-called lethal milk because pups nursed by these dams die before weaning [132]. Recently, the Znt4 mutant mouse was shown to have defects in mammary gland secretion and hallmarks of precocious involution during lactation [133]. Znt5 KO mice display poor growth, osteopenia, and male-specific sudden cardiac death [134], and also show cytokine production defects in mast cells, which is mediated by the high-affinity immunoglobulin E receptor [135]. Znt7 KO mice show poor growth, decreased adiposity, and mild zinc deficiency, while males also have high-fat diet induced-insulin resistance and glucose intolerance [136]. Nonsynonymous ZnT8 SNPs (rs13266634, resulting in R325W substitution) are known to increase the risk of type 2 diabetes [137,138,139,140], which is attributed to the lower zinc transport activity of the risk allele although the precise molecular mechanism of this requires further investigation because Znt8 KO mice phenotypes have been variable in sex and genetic background [2,141]. Nevertheless, all Znt8 KO mice have defects in the formation of zinc–insulin crystals [142,143,144,145,146]. Contrary to these findings, a recent finding showed that the single nucleotide variants causing the truncation of ZnT8 protect against type 2 diabetes in heterozygous individuals [147]. This discrepancy also needs further investigation from the viewpoint of ZnT8 zinc transport activity. The rs13266634 SNP is also a determinant of humoral autoreactivity to ZnT8 [148]. Finally, homozygous ZnT10 mutations are involved in Parkinsonism, which is characterized by hypermanganesemia, hepatic cirrhosis, polycythemia, and dystonia [149,150]. Recent molecular analysis indicates that ZnT10 is functional in the detoxification of cellular manganese [151].
We briefly summarize information about ZIP and ZnT transporter mutations in human genetic diseases in Table 3. Moreover, many SNPs have been reported in ZIP and ZnT transporter genes that are suggested to be associated with human diseases. This information is summarized elsewhere [2,152].
Table 3.
Zinc transporter mutations reported to be involved in inherited diseases.
| Gene | Disease | MIM No. | Clinical Features | Pattern of Inheritance | References |
|---|---|---|---|---|---|
| SLC39A4/ZIP4 | Acrodermatitis enteropathica (AE) | 201100 | Eczematous dermatitis on the perioral, perianal, and areas, alopecia, diarrhea, growth retardation because of decreased zinc absorption, Ameliorated with zinc supplementation. | Homozygous, Compound heterozygous, Dominant negative | [99,100,101,153,154] |
| SLC39A5/ZIP5 | Nonsymptomatic high myopia | 615946 | Refractive error, tigroid and focal atrophy of choroid. | Heterozygous | [105] |
| SLC39A8/ZIP8 | Cerebellar Atrophy Syndrome, a type II congenital disorder of glycosylation (CDG) | - | Intellectual disability, cerebellar atrophy, cranial asymmetry, dysproportionate dwarfism, severe infantile spasms with hypsarrhythmia, hypotonia, strabismus. | Homozygous, Compound heterozygous | [111,112] |
| SLC39A13/ZIP13 | spondylocheiro dysplastic Ehlers-Danlos syndrome (SCD-EDS) | 612350 | Postnatal growth retardation, skeletal and connective tissue abnormalities, finger contractures, joint hypermobility, protruding eyes with bluish sclera, decreased hydroxyl collagen levels. | Homozygous | [114,115] |
| SLC30A2/ZnT2 | Transient neonatal zinc deficiency (TNZD) | 608118 | Erosive dermatitis around the mouth, genital region, neck, and fingers, diarrhea, hair loss, alopecia, Ameliorated with zinc supplementation to infants. | Dominant negative, Heterozygous, Compound heterozygous | [121,122,123,124,125] |
| SLC30A3/ZnT3 | Increased risk of febrile seizures | - | Potentially a prelude to more severe epilepsy. | Heterozygous | [131] |
| SLC30A10/ZnT10 | Hypermanganesemia, syndrome of hepatic cirrhosis, dystonia, polycythemia | 613280 | Dysarthria, hypertonia, fine tremor, bradykinesia, spastic paraparesis, Improved by metal chelation therapy. | Homozygous | [149,150] |
6. Cooperative Functions of MT and ZnT Transporters in Cellular Events
6.1. Cooperative Regulation of MT and ZnT Transporters Controls Cytosolic Zinc Homeostasis
The maintenance mechanisms of cellular zinc homeostasis are known as “zinc buffering” and “zinc muffling” [60,155]. This buffering mechanism is important to maintain a zinc ion concentration in the cytosol in the pM range and is achieved by cytosolic zinc-binding proteins including MTs. The muffling mechanism is functional under non-steady conditions, in which transient changes in zinc ion concentrations in the cytosol are modulated by zinc-binding proteins such as MTs and zinc transporters through moving zinc ions into subcellular compartments or out of cells [60,155]. When free zinc ion concentrations in the cytosol are sufficiently high, MTF-1 induces the transcription of MT-1 and -2 and several ZnT transporters, and their cooperative expression contributes to the maintenance of cellular zinc homeostasis [32,34,35,36]. Thus, the cooperative regulation of MT and ZnT transporters is essential to control cellular zinc homeostasis over a variety of zinc levels.
6.2. Cooperative Regulation of MT and ZnT Transporters for the Activation of Zinc-Dependent Ectoenzymes
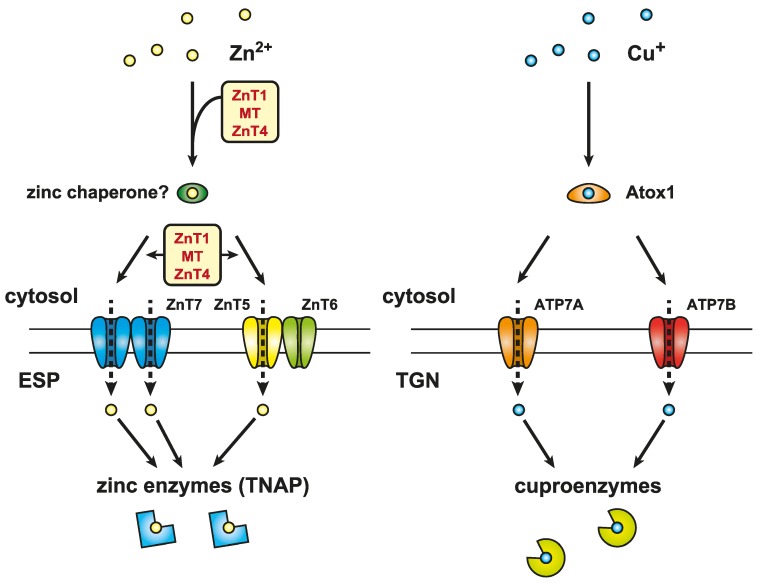
In addition to the maintenance of cellular zinc homeostasis, the cooperative functions of MTs and zinc transporters likely contribute to various biological events. However, the molecular evidence for this is limited. We recently studied the activation process of a zinc-requiring ectoenzyme, tissue non-specific alkaline phosphatase (TNAP), to more thoroughly investigate this [156]. The activation of TNAP needs ZnT5–ZnT6 heterodimers and ZnT7 homodimers of the early secretory pathway as a zinc entry route into the lumen of the pathway [157,158,159]. Unexpectedly, cells lacking MT, ZnT1, and ZnT4 (ZnT1−/−MT−/−ZnT4−/−) also show significantly reduced TNAP activity, in spite of normal operation of ZnT5–ZnT6 heterodimers and ZnT7 homodimers and increased cytosolic zinc levels [123,156]. Interestingly, the impairment of TNAP activation in ZnT1−/−MT−/−ZnT4−/− cells is reversed by excess zinc supplementation [156], strongly suggesting that the transfer of cytosolic zinc to ZnT5–ZnT6 heterodimers and ZnT7 homodimers may be facilitated under the cooperative control of ZnT1, MT, and ZnT4 (Figure 5).
Figure 5.
Cooperative function of MT, ZnT1, and ZnT4 in the activation of zinc-requiring ectoenzymes. The facilitated transfer of cytosolic zinc to ZnT5–ZnT6 heterodimers and ZnT7 homodimers may function under cooperative control of ZnT1, MT, and ZnT4 (left). ZnT1MTZnT4 KO cells exhibit significantly reduced TNAP activity (left), which is reminiscent of the phenotypes of cytosolic copper chaperone Atox1-deficient cells (right). Atox1 plays a crucial role as a copper chaperone in transferring cytosolic copper to two copper-transporting P-type ATPases, ATP7A and ATP7B, located in the trans-Golgi network (TGN). This therefore contributes to the activation of copper-requiring ectoenzymes (cuproenzymes). Considering the high level of analogy between ZnT1MTZnT4 KO and Atox1-deficient cells, a putative zinc chaperone under the cooperative control of ZnT1, MT, and ZnT4 is hypothesized to play a crucial role in facilitating the transfer of cytosolic zinc to ZnT5–ZnT6 heterodimers and ZnT7 homodimers (not shown) located in the early secretory pathway (ESP). This then contributes to the proper activation of zinc-requiring ectoenzymes such as TNAP (left).
The phenotypes of ZnT1−/−MT−/−ZnT4−/− cells are somewhat similar to those of cytosolic copper chaperone Atox1-deficient cells, in which intracellular copper levels are increased [160,161] but the activity of secretory cuproenzymes is significantly reduced [162,163]. However, the impairment of cuproenzyme activation is recovered by excess copper supplementation to the cells [162,163]. Atox1 plays a crucial role as a copper chaperone in the facilitated transfer of cytosolic copper to copper-transporting ATPases (ATP7A and ATP7B) to activate secretory cuproenzymes in the trans-Golgi network [55,56,164]. Considering the phenotypic analogies between ZnT1−/−MT−/−ZnT4−/− cells and Atox1-deficient cells, it is interesting to hypothesize the presence of cytosolic zinc chaperone proteins, which conduct the facilitated transfer of cytosolic zinc to ZnT5–ZnT6 heterodimers and ZnT7 homodimers. Both ZnT1 and ZnT4 are membrane proteins, while MT is a cytosolic protein. Thus, MT may be functional as a cytosolic chaperone through its cooperative operation with ZnT1 and ZnT4, although there is no experimental evidence as yet for this. This type of zinc chaperone protein has been speculated based on the molecular modeling of bacterial ZnT homologs, which may have an Atox1-like structure, and appears to dock to the intracellular cavity between transmembrane domains and carboxyl-terminal cytoplasmic domains of ZnT transporters [165].
7. Perspectives
The field of zinc metabolism and homeostasis has undergone a dramatic expansion indicating in recent decades, which has revealed that zinc plays important roles in a variety of biological processes. However, the molecular basis underlying these mechanisms has only relatively recently been identified. Many questions remain to be answered with respect to zinc metabolism and its involvement in the divergent array of physiological and pathophysiological processes. These include how zinc ions are sensed and recognized by proteins including MTF-1, MT, and ZIP and ZnT transporters. Moreover, how do MT and ZIP and ZnT transporters operate correctly during zinc transfer to target proteins/enzymes through zinc release via ligand-centered reactions in zinc-thiolate coordination in MT, or zinc mobilization across the biological membranes by ZIP and ZnT transporters? How are these processes activated and controlled in terms of both timing and location? In connection with this, is a zinc chaperone operative in cellular zinc metabolism? Furthermore, how are MT, ZIP and ZnT transporter gene expression controlled epigenetically? Finally, why are so many MTs present in humans and do they have an isoform-specific function? Answers to these questions should provide an important direction for future work on zinc as well as an understanding of the roles of zinc in health and disease.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research (B) (KAKENHI, Grant No. 15H04501 to Taiho Kambe), and Scientific Research (C) (KAKENHI, Grant No. 25460179 to Tomoki Kimura) from the Japan Society for the Promotion of Science.
Abbreviations
| AE | acrodermatitis enteropathica |
| ALS | amyotrophic lateral sclerosis |
| CDG | congenital disorder of glycosylation |
| CaN | calcineurin |
| DNMT | DNA methyltransferase |
| ER | endoplasmic reticulum |
| ESP | early secretory pathway |
| GR | glucocorticoid receptor |
| HDAC | histone deacetylase |
| HMBOX1 | homeobox-containing 1 |
| IL | interleukin |
| KO | knockout |
| LPS | lipopolysaccharide |
| MT | metallothionein |
| MBD | methyl CpG binding protein |
| MTF-1 | metal response element-binding transcription factor-1 |
| NFAT | nuclear factor of activated T-cell |
| NF-κB | nuclear factor-κB |
| PI3K | phosphatidylinositol-3-kinase |
| SCD-EDS | spondylocheiro dysplastic form of Ehlers–Danlos syndrome |
| SLC | solute carrier |
| SNPs | polymorphisms |
| SOD | superoxide dismutase |
| STAT3 | signal transducer and activator of transcription |
| TCRP1 | tongue cancer resistance-associated protein 1 |
| TGN | trans-Golgi network |
| TNAP | tissue non-specific alkaline phosphatase |
| TNZD | transient neonatal zinc deficiency |
| TPEN | N,N,N',N'-Tetrakis(2-pyridylmethyl)ethylenediamine |
| ZIP | Zrt- and Irt-like protein |
| ZnT | Zn transporter |
| ZNF | zinc finger transcription factor |
Author Contributions
Tomoki Kimura and Taiho Kambe wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Thiers R.E., Vallee B.L. Distribution of metals in subcellular fractions of rat liver. J. Biol. Chem. 1957;226:911–920. [PubMed] [Google Scholar]
- 2.Kambe T., Tsuji T., Hashimoto A., Itsumura N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol. Rev. 2015;95:749–784. doi: 10.1152/physrev.00035.2014. [DOI] [PubMed] [Google Scholar]
- 3.Kambe T., Yamaguchi-Iwai Y., Sasaki R., Nagao M. Overview of mammalian zinc transporters. Cell. Mol. Life Sci. 2004;61:49–68. doi: 10.1007/s00018-003-3148-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coyle P., Philcox J.C., Carey L.C., Rofe A.M. Metallothionein: The multipurpose protein. Cell. Mol. Life Sci. 2002;59:627–647. doi: 10.1007/s00018-002-8454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hennigar S.R., Kelleher S.L. Zinc networks: The cell-specific compartmentalization of zinc for specialized functions. Biol. Chem. 2012;393:565–578. doi: 10.1515/hsz-2012-0128. [DOI] [PubMed] [Google Scholar]
- 6.Kambe T. An overview of a wide range of functions of ZnT and ZIP zinc transporters in the secretory pathway. Biosci. Biotechnol. Biochem. 2011;75:1036–1043. doi: 10.1271/bbb.110056. [DOI] [PubMed] [Google Scholar]
- 7.Qin Y., Dittmer P.J., Park J.G., Jansen K.B., Palmer A.E. Measuring steady-state and dynamic endoplasmic reticulum and Golgi Zn2+ with genetically encoded sensors. Proc. Natl. Acad. Sci. USA. 2011;108:7351–7356. doi: 10.1073/pnas.1015686108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Outten C.E., O’Halloran T.V. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science. 2001;292:2488–2492. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- 9.Vinkenborg J.L., Nicolson T.J., Bellomo E.A., Koay M.S., Rutter G.A., Merkx M. Genetically encoded fret sensors to monitor intracellular Zn2+ homeostasis. Nat. Methods. 2009;6:737–740. doi: 10.1038/nmeth.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eide D.J. Zinc transporters and the cellular trafficking of zinc. Biochim. Biophys. Acta. 2006;1763:711–722. doi: 10.1016/j.bbamcr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Lichten L.A., Cousins R.J. Mammalian zinc transporters: Nutritional and physiologic regulation. Annu. Rev. Nutr. 2009;29:153–176. doi: 10.1146/annurev-nutr-033009-083312. [DOI] [PubMed] [Google Scholar]
- 12.Fukada T., Kambe T. Molecular and genetic features of zinc transporters in physiology and pathogenesis. Metallomics. 2011;3:662–674. doi: 10.1039/c1mt00011j. [DOI] [PubMed] [Google Scholar]
- 13.Moleirinho A., Carneiro J., Matthiesen R., Silva R.M., Amorim A., Azevedo L. Gains, losses and changes of function after gene duplication: Study of the metallothionein family. PLoS ONE. 2011;6:336. doi: 10.1371/journal.pone.0018487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vasak M., Meloni G. Chemistry and biology of mammalian metallothioneins. J. Biol. Inorg. Chem. 2011;16:1067–1078. doi: 10.1007/s00775-011-0799-2. [DOI] [PubMed] [Google Scholar]
- 15.Lazo J.S., Kondo Y., Dellapiazza D., Michalska A.E., Choo K.H., Pitt B.R. Enhanced sensitivity to oxidative stress in cultured embryonic cells from transgenic mice deficient in metallothionein I and II genes. J. Biol. Chem. 1995;270:5506–5510. doi: 10.1074/jbc.270.10.5506. [DOI] [PubMed] [Google Scholar]
- 16.Masters B.A., Kelly E.J., Quaife C.J., Brinster R.L., Palmiter R.D. Targeted disruption of metallothionein I and II genes increases sensitivity to cadmium. Proc. Natl. Acad. Sci. USA. 1994;91:584–588. doi: 10.1073/pnas.91.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michalska A.E., Choo K.H. Targeting and germ-line transmission of a null mutation at the metallothionein I and II loci in mouse. Proc. Natl. Acad. Sci. USA. 1993;90:8088–8092. doi: 10.1073/pnas.90.17.8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly E.J., Quaife C.J., Froelick G.J., Palmiter R.D. Metallothionein I and II protect against zinc deficiency and zinc toxicity in mice. J. Nutr. 1996;126:1782–1790. doi: 10.1093/jn/126.7.1782. [DOI] [PubMed] [Google Scholar]
- 19.Klaassen C.D., Liu J. Metallothionein transgenic and knock-out mouse models in the study of cadmium toxicity. J. Toxicol. Sci. 1998;23(Suppl. 2):97–102. doi: 10.2131/jts.23.SupplementII_97. [DOI] [PubMed] [Google Scholar]
- 20.Shibuya K., Suzuki J.S., Kito H., Naganuma A., Tohyama C., Satoh M. Protective role of metallothionein in bone marrow injury caused by X-irradiation. J. Toxicol. Sci. 2008;33:479–484. doi: 10.2131/jts.33.479. [DOI] [PubMed] [Google Scholar]
- 21.Fujiwara Y., Satoh M. Protective role of metallothionein in chemical and radiation carcinogenesis. Curr. Pharm. Biotechnol. 2013;14:394–399. doi: 10.2174/1389201011314040002. [DOI] [PubMed] [Google Scholar]
- 22.Kimura T., Itoh N., Takehara M., Oguro I., Ishizaki J.I., Nakanishi T., Tanaka K. Sensitivity of metallothionein-null mice to LPS/d-galactosamine-induced lethality. Biochem. Biophys. Res. Commun. 2001;280:358–362. doi: 10.1006/bbrc.2000.4085. [DOI] [PubMed] [Google Scholar]
- 23.Inoue K., Takano H., Shimada A., Wada E., Yanagisawa R., Sakurai M., Satoh M., Yoshikawa T. Role of metallothionein in coagulatory disturbance and systemic inflammation induced by lipopolysaccharide in mice. FASEB J. 2006;20:533–535. doi: 10.1096/fj.05-3864fje. [DOI] [PubMed] [Google Scholar]
- 24.Mita M., Satoh M., Shimada A., Okajima M., Azuma S., Suzuki J.S., Sakabe K., Hara S., Himeno S. Metallothionein is a crucial protective factor against Helicobacter pylori-induced gastric erosive lesions in a mouse model. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;294:G877–G884. doi: 10.1152/ajpgi.00251.2007. [DOI] [PubMed] [Google Scholar]
- 25.Ugajin T., Nishida K., Yamasaki S., Suzuki J., Mita M., Kubo M., Yokozeki H., Hirano T. Zinc-binding metallothioneins are key modulators of IL-4 production by basophils. Mol. Immunol. 2015;66:180–188. doi: 10.1016/j.molimm.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Miyai T., Hojyo S., Ikawa T., Kawamura M., Irie T., Ogura H., Hijikata A., Bin B.H., Yasuda T., Kitamura H., et al. Zinc transporter SLC39A10/ZIP10 facilitates antiapoptotic signaling during early B-cell development. Proc. Natl. Acad. Sci. USA. 2014;111:11780–11785. doi: 10.1073/pnas.1323549111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hojyo S., Miyai T., Fujishiro H., Kawamura M., Yasuda T., Hijikata A., Bin B.H., Irie T., Tanaka J., Atsumi T., et al. Zinc transporter SLC39A10/ZIP10 controls humoral immunity by modulating B-cell receptor signal strength. Proc. Natl. Acad. Sci. USA. 2014;111:11786–11791. doi: 10.1073/pnas.1323557111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato M., Kawakami T., Kondoh M., Takiguchi M., Kadota Y., Himeno S., Suzuki S. Development of high-fat-diet-induced obesity in female metallothionein-null mice. FASEB J. 2010;24:2375–2384. doi: 10.1096/fj.09-145466. [DOI] [PubMed] [Google Scholar]
- 29.Kadota Y., Aki Y., Toriuchi Y., Mizuno Y., Kawakami T., Sato M., Suzuki S. Deficiency of metallothionein-1 and -2 genes shortens the lifespan of the 129/Sv mouse strain. Exp. Gerontol. 2015;66:21–24. doi: 10.1016/j.exger.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Puttaparthi K., Gitomer W.L., Krishnan U., Son M., Rajendran B., Elliott J.L. Disease progression in a transgenic model of familial amyotrophic lateral sclerosis is dependent on both neuronal and non-neuronal zinc binding proteins. J. Neurosci. 2002;22:8790–8796. doi: 10.1523/JNEUROSCI.22-20-08790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elliott J.L. Zinc and copper in the pathogenesis of amyotrophic lateral sclerosis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2001;25:1169–1185. doi: 10.1016/S0278-5846(01)00185-3. [DOI] [PubMed] [Google Scholar]
- 32.Kimura T., Itoh N., Andrews G.K. Mechanisms of heavy metal sensing by metal response element-binding transcription factor-1. J. Health Sci. 2009;55:484–494. doi: 10.1248/jhs.55.484. [DOI] [Google Scholar]
- 33.Potter B.M., Feng L.S., Parasuram P., Matskevich V.A., Wilson J.A., Andrews G.K., Laity J.H. The six zinc fingers of metal-responsive element binding transcription factor-1 form stable and quasi-ordered structures with relatively small differences in zinc affinities. J. Biol. Chem. 2005;280:28529–28540. doi: 10.1074/jbc.M505217200. [DOI] [PubMed] [Google Scholar]
- 34.Laity J.H., Andrews G.K. Understanding the mechanisms of zinc-sensing by metal-response element binding transcription factor-1 (MTF-1) Arch. Biochem. Biophys. 2007;463:201–210. doi: 10.1016/j.abb.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 35.Guo L., Lichten L.A., Ryu M.S., Liuzzi J.P., Wang F., Cousins R.J. STAT5-glucocorticoid receptor interaction and MTF-1 regulate the expression of ZnT2 (Slc30a2) in pancreatic acinar cells. Proc. Natl. Acad. Sci. USA. 2010;107:2818–2823. doi: 10.1073/pnas.0914941107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langmade S.J., Ravindra R., Daniels P.J., Andrews G.K. The transcription factor MTF-1 mediates metal regulation of the mouse ZnT1 gene. J. Biol. Chem. 2000;275:34803–34809. doi: 10.1074/jbc.M007339200. [DOI] [PubMed] [Google Scholar]
- 37.Wimmer U., Wang Y., Georgiev O., Schaffner W. Two major branches of anti-cadmium defense in the mouse: MTF-1/metallothioneins and glutathione. Nucleic Acids Res. 2005;33:5715–5727. doi: 10.1093/nar/gki881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lichten L.A., Ryu M.S., Guo L., Embury J., Cousins R.J. MTF-1-mediated repression of the zinc transporter ZIP10 is alleviated by zinc restriction. PLoS ONE. 2011;6:336. doi: 10.1371/journal.pone.0021526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin M.C., Liu Y.C., Tam M.F., Lu Y.J., Hsieh Y.T., Lin L.Y. PTEN interacts with metal-responsive transcription factor 1 and stimulates its transcriptional activity. Biochem. J. 2012;441:367–377. doi: 10.1042/BJ20111257. [DOI] [PubMed] [Google Scholar]
- 40.Chen L., Ma L., Bai Q., Zhu X., Zhang J., Wei Q., Li D., Gao C., Li J., Zhang Z., et al. Heavy metal-induced metallothionein expression is regulated by specific protein phosphatase 2A complexes. J. Biol. Chem. 2014;289:22413–22426. doi: 10.1074/jbc.M114.548677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogo O.A., Tyson J., Cockell S.J., Howard A., Valentine R.A., Ford D. The zinc finger protein ZNF658 regulates the transcription of genes involved in zinc homeostasis and affects ribosome biogenesis through the zinc transcriptional regulatory element. Mol. Cell. Biol. 2015;35:977–987. doi: 10.1128/MCB.01298-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kagi J.H., Valee B.L. Metallothionein: A cadmium- and zinc-containing protein from equine renal cortex. J. Biol. Chem. 1960;235:3460–3465. [PubMed] [Google Scholar]
- 43.Suhy D.A., Simon K.D., Linzer D.I., O’Halloran T.V. Metallothionein is part of a zinc-scavenging mechanism for cell survival under conditions of extreme zinc deprivation. J. Biol. Chem. 1999;274:9183–9192. doi: 10.1074/jbc.274.14.9183. [DOI] [PubMed] [Google Scholar]
- 44.Palmiter R.D. Constitutive expression of metallothionein-III (MT-III), but not MT-I, inhibits growth when cells become zinc deficient. Toxicol. Appl. Pharmacol. 1995;135:139–146. doi: 10.1006/taap.1995.1216. [DOI] [PubMed] [Google Scholar]
- 45.Krezel A., Maret W. Thionein/metallothionein control Zn(II) availability and the activity of enzymes. J. Biol. Inorg. Chem. 2008;13:401–409. doi: 10.1007/s00775-007-0330-y. [DOI] [PubMed] [Google Scholar]
- 46.Costello L.C., Fenselau C.C., Franklin R.B. Evidence for operation of the direct zinc ligand exchange mechanism for trafficking, transport, and reactivity of zinc in mammalian cells. J. Inorg. Biochem. 2011;105:589–599. doi: 10.1016/j.jinorgbio.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pinter T.B., Stillman M.J. Kinetics of zinc and cadmium exchanges between metallothionein and carbonic anhydrase. Biochemistry. 2015;54:6284–6293. doi: 10.1021/acs.biochem.5b00912. [DOI] [PubMed] [Google Scholar]
- 48.Maret W. Redox biochemistry of mammalian metallothioneins. J. Biol. Inorg. Chem. 2011;16:1079–1086. doi: 10.1007/s00775-011-0800-0. [DOI] [PubMed] [Google Scholar]
- 49.Aras M.A., Aizenman E. Redox regulation of intracellular zinc: Molecular signaling in the life and death of neurons. Antioxid. Redox Signal. 2011;15:2249–2263. doi: 10.1089/ars.2010.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fukada T., Kambe T. Zinc Signals in Cellular Functions and Disorders. Springer; Tokyo, Japan: 2014. [Google Scholar]
- 51.Zeng J., Heuchel R., Schaffner W., Kagi J.H. Thionein (apometallothionein) can modulate DNA binding and transcription activation by zinc finger containing factor Sp1. FEBS Lett. 1991;279:310–312. doi: 10.1016/0014-5793(91)80175-3. [DOI] [PubMed] [Google Scholar]
- 52.Zeng J., Vallee B.L., Kagi J.H. Zinc transfer from transcription factor IIIA fingers to thionein clusters. Proc. Natl. Acad. Sci. USA. 1991;88:9984–9988. doi: 10.1073/pnas.88.22.9984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maret W., Li Y. Coordination dynamics of zinc in proteins. Chem. Rev. 2009;109:4682–4707. doi: 10.1021/cr800556u. [DOI] [PubMed] [Google Scholar]
- 54.Maret W. Metals on the move: Zinc ions in cellular regulation and in the coordination dynamics of zinc proteins. Biometals. 2011;24:411–418. doi: 10.1007/s10534-010-9406-1. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y., Hodgkinson V., Zhu S., Weisman G.A., Petris M.J. Advances in the understanding of mammalian copper transporters. Adv. Nutr. 2011;2:129–137. doi: 10.3945/an.110.000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kambe T., Weaver B.P., Andrews G.K. The genetics of essential metal homeostasis during development. Genesis. 2008;46:214–228. doi: 10.1002/dvg.20382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim B.E., Nevitt T., Thiele D.J. Mechanisms for copper acquisition, distribution and regulation. Nat. Chem. Biol. 2008;4:176–185. doi: 10.1038/nchembio.72. [DOI] [PubMed] [Google Scholar]
- 58.Miles A.T., Hawksworth G.M., Beattie J.H., Rodilla V. Induction, regulation, degradation, and biological significance of mammalian metallothioneins. Crit. Rev. Biochem. Mol. Biol. 2000;35:35–70. doi: 10.1080/10409230091169168. [DOI] [PubMed] [Google Scholar]
- 59.Miura N., Koizumi S. [Heavy metal responses of the human metallothionein isoform genes] Yakugaku Zasshi. 2007;127:665–673. doi: 10.1248/yakushi.127.665. [DOI] [PubMed] [Google Scholar]
- 60.Colvin R.A., Holmes W.R., Fontaine C.P., Maret W. Cytosolic zinc buffering and muffling: Their role in intracellular zinc homeostasis. Metallomics. 2010;2:306–317. doi: 10.1039/b926662c. [DOI] [PubMed] [Google Scholar]
- 61.Forma E., Krzeslak A., Wilkosz J., Jozwiak P., Szymczyk A., Rozanski W., Brys M. Metallothionein 2A genetic polymorphisms and risk of prostate cancer in a polish population. Cancer Genet. 2012;205:432–435. doi: 10.1016/j.cancergen.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 62.Krzeslak A., Forma E., Chwatko G., Jozwiak P., Szymczyk A., Wilkosz J., Rozanski W., Brys M. Effect of metallothionein 2A gene polymorphism on allele-specific gene expression and metal content in prostate cancer. Toxicol. Appl. Pharmacol. 2013;268:278–285. doi: 10.1016/j.taap.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 63.Krzeslak A., Forma E., Jozwiak P., Szymczyk A., Smolarz B., Romanowicz-Makowska H., Rozanski W., Brys M. Metallothionein 2A genetic polymorphisms and risk of ductal breast cancer. Clin. Exp. Med. 2014;14:107–113. doi: 10.1007/s10238-012-0215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Joshi B., Ordonez-Ercan D., Dasgupta P., Chellappan S. Induction of human metallothionein 1G promoter by VEGF and heavy metals: Differential involvement of E2F and metal transcription factors. Oncogene. 2005;24:2204–2217. doi: 10.1038/sj.onc.1208206. [DOI] [PubMed] [Google Scholar]
- 65.Fujie T., Segawa Y., Kimura T., Fujiwara Y., Yamamoto C., Satoh M., Naka H., Kaji T. Induction of metallothionein isoforms by copper diethyldithiocarbamate in cultured vascular endothelial cells. J. Toxicol. Sci. 2016 doi: 10.2131/jts.41.225. in press. [DOI] [PubMed] [Google Scholar]
- 66.Ma H., Su L., Yue H., Yin X., Zhao J., Zhang S., Kung H., Xu Z., Miao J. Hmbox1 interacts with MT2A to regulate autophagy and apoptosis in vascular endothelial cells. Sci. Rep. 2015;5 doi: 10.1038/srep15121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peng B., Gu Y., Xiong Y., Zheng G., He Z. Microarray-assisted pathway analysis identifies MT1X & NFκB as mediators of TCRP1-associated resistance to cisplatin in oral squamous cell carcinoma. PLoS ONE. 2012;7:336. doi: 10.1371/journal.pone.0051413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakane H., Hirano M., Ito H., Hosono S., Oze I., Matsuda F., Tanaka H., Matsuo K. Impact of metallothionein gene polymorphisms on the risk of lung cancer in a Japanese population. Mol. Carcinog. 2015;54(Suppl. 1):E122–E128. doi: 10.1002/mc.22198. [DOI] [PubMed] [Google Scholar]
- 69.Imoto A., Okada M., Okazaki T., Kitasato H., Harigae H., Takahashi S. Metallothionein-1 isoforms and vimentin are direct PU.1 downstream target genes in leukemia cells. J. Biol. Chem. 2010;285:10300–10309. doi: 10.1074/jbc.M109.095810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hirako N., Nakano H., Takahashi S. A PU.1 suppressive target gene, metallothionein 1G, inhibits retinoic acid-induced NB4 cell differentiation. PLoS ONE. 2014;9:336. doi: 10.1371/journal.pone.0103282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kimura T., Itoh N. Function of metallothionein in gene expression and signal transduction: Newly found protective role of metallothionein. J. Health Sci. 2008;54:251–260. doi: 10.1248/jhs.54.251. [DOI] [Google Scholar]
- 72.Kita K., Miura N., Yoshida M., Yamazaki K., Ohkubo T., Imai Y., Naganuma A. Potential effect on cellular response to cadmium of a single-nucleotide A → G polymorphism in the promoter of the human gene for metallothionein IIA. Hum. Genet. 2006;120:553–560. doi: 10.1007/s00439-006-0238-6. [DOI] [PubMed] [Google Scholar]
- 73.Yoshida M., Ohta H., Yamauchi Y., Seki Y., Sagi M., Yamazaki K., Sumi Y. Age-dependent changes in metallothionein levels in liver and kidney of the Japanese. Biol. Trace Elem. Res. 1998;63:167–175. doi: 10.1007/BF02778875. [DOI] [PubMed] [Google Scholar]
- 74.Miura N. Individual susceptibility to cadmium toxicity and metallothionein gene polymorphisms: With references to current status of occupational cadmium exposure. Ind. Health. 2009;47:487–494. doi: 10.2486/indhealth.47.487. [DOI] [PubMed] [Google Scholar]
- 75.Majumder S., Ghoshal K., Li Z., Bo Y., Jacob S.T. Silencing of metallothionein-I gene in mouse lymphosarcoma cells by methylation. Oncogene. 1999;18:6287–6295. doi: 10.1038/sj.onc.1203004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ghoshal K., Majumder S., Li Z., Dong X., Jacob S.T. Suppression of metallothionein gene expression in a rat hepatoma because of promoter-specific DNA methylation. J. Biol. Chem. 2000;275:539–547. doi: 10.1074/jbc.275.1.539. [DOI] [PubMed] [Google Scholar]
- 77.Ghoshal K., Datta J., Majumder S., Bai S., Dong X., Parthun M., Jacob S.T. Inhibitors of histone deacetylase and DNA methyltransferase synergistically activate the methylated metallothionein I promoter by activating the transcription factor MTF-1 and forming an open chromatin structure. Mol. Cell. Biol. 2002;22:8302–8319. doi: 10.1128/MCB.22.23.8302-8319.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li Y., Kimura T., Huyck R.W., Laity J.H., Andrews G.K. Zinc-induced formation of a coactivator complex containing the zinc-sensing transcription factor MTF-1, p300/CBP, and Sp1. Mol. Cell. Biol. 2008;28:4275–4284. doi: 10.1128/MCB.00369-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Okumura F., Li Y., Itoh N., Nakanishi T., Isobe M., Andrews G.K., Kimura T. The zinc-sensing transcription factor MTF-1 mediates zinc-induced epigenetic changes in chromatin of the mouse metallothionein-I promoter. Biochim. Biophys. Acta. 2011;1809:56–62. doi: 10.1016/j.bbagrm.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 80.Rao S., Procko E., Shannon M.F. Chromatin remodeling, measured by a novel real-time polymerase chain reaction assay, across the proximal promoter region of the IL-2 gene. J. Immunol. 2001;167:4494–4503. doi: 10.4049/jimmunol.167.8.4494. [DOI] [PubMed] [Google Scholar]
- 81.De La Serna I.L., Carlson K.A., Hill D.A., Guidi C.J., Stephenson R.O., Sif S., Kingston R.E., Imbalzano A.N. Mammalian SEI-SNF complexes contribute to activation of the hsp70 gene. Mol. Cell. Biol. 2000;20:2839–2851. doi: 10.1128/MCB.20.8.2839-2851.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kurita H., Ohsako S., Hashimoto S., Yoshinaga J., Tohyama C. Prenatal zinc deficiency-dependent epigenetic alterations of mouse metallothionein-2 gene. J. Nutr. Biochem. 2013;24:256–266. doi: 10.1016/j.jnutbio.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 83.Kimura T., Li Y., Okumura F., Itoh N., Nakanishi T., Sone T., Isobe M., Andrews G.K. Chromium(VI) inhibits mouse metallothionein-I gene transcription by preventing the zinc-dependent formation of an MTF-1-p300 complex. Biochem. J. 2008;415:477–482. doi: 10.1042/BJ20081025. [DOI] [PubMed] [Google Scholar]
- 84.Majumder S., Kutay H., Datta J., Summers D., Jacob S.T., Ghoshal K. Epigenetic regulation of metallothionein-I gene expression: Differential regulation of methylated and unmethylated promoters by DNA methyltransferases and methyl CpG binding proteins. J. Cell. Biochem. 2006;97:1300–1316. doi: 10.1002/jcb.20738. [DOI] [PubMed] [Google Scholar]
- 85.Schnekenburger M., Talaska G., Puga A. Chromium cross-links histone deacetylase 1-DNA methyltransferase 1 complexes to chromatin, inhibiting histone-remodeling marks critical for transcriptional activation. Mol. Cell. Biol. 2007;27:7089–7101. doi: 10.1128/MCB.00838-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang L., Tepaamorndech S. The SLC30 family of zinc transporters—A review of current understanding of their biological and pathophysiological roles. Mol. Asp. Med. 2013;34:548–560. doi: 10.1016/j.mam.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 87.Jeong J., Eide D.J. The SLC39 family of zinc transporters. Mol. Asp. Med. 2013;34:612–619. doi: 10.1016/j.mam.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fujishiro H., Okugaki S., Yasumitsu S., Enomoto S., Himeno S. Involvement of DNA hypermethylation in down-regulation of the zinc transporter ZIP8 in cadmium-resistant metallothionein-null cells. Toxicol. Appl. Pharmacol. 2009;241:195–201. doi: 10.1016/j.taap.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 89.Kumar L., Michalczyk A., McKay J., Ford D., Kambe T., Hudek L., Varigios G., Taylor P.E., Ackland M.L. Altered expression of two zinc transporters, SLC30A5 and SLC30A6, underlies a mammary gland disorder of reduced zinc secretion into milk. Genes Nutr. 2015;10 doi: 10.1007/s12263-015-0487-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang Y., Yang J., Cui X., Chen Y., Zhu V.F., Hagan J.P., Wang H., Yu X., Hodges S.E., Fang J., et al. A novel epigenetic CREB-miR-373 axis mediates ZIP4-induced pancreatic cancer growth. EMBO Mol. Med. 2013;5:1322–1334. doi: 10.1002/emmm.201302507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weaver B.P., Andrews G.K. Regulation of zinc-responsive Slc39a5 (Zip5) translation is mediated by conserved elements in the 3′-untranslated region. Biometals. 2012;25:319–335. doi: 10.1007/s10534-011-9508-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kambe T. Zinc Transport: Regulation. Encycl. Inorg. Bioinorg. Chem. 2013 doi: 10.1002/9781119951438.eibc2135. [DOI] [Google Scholar]
- 93.Dufner-Beattie J., Huang Z.L., Geiser J., Xu W., Andrews G.K. Mouse ZIP1 and ZIP3 genes together are essential for adaptation to dietary zinc deficiency during pregnancy. Genesis. 2006;44:239–251. doi: 10.1002/dvg.20211. [DOI] [PubMed] [Google Scholar]
- 94.Dufner-Beattie J., Huang Z.L., Geiser J., Xu W., Andrews G.K. Generation and characterization of mice lacking the zinc uptake transporter ZIP3. Mol. Cell. Biol. 2005;25:5607–5615. doi: 10.1128/MCB.25.13.5607-5615.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peters J.L., Dufner-Beattie J., Xu W., Geiser J., Lahner B., Salt D.E., Andrews G.K. Targeting of the mouse Slc39a2 (Zip2) gene reveals highly cell-specific patterns of expression, and unique functions in zinc, iron, and calcium homeostasis. Genesis. 2007;45:339–352. doi: 10.1002/dvg.20297. [DOI] [PubMed] [Google Scholar]
- 96.Kambe T., Geiser J., Lahner B., Salt D.E., Andrews G.K. Slc39a1 to 3 (subfamily II) Zip genes in mice have unique cell-specific functions during adaptation to zinc deficiency. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294:R1474–R1481. doi: 10.1152/ajpregu.00130.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kelleher S.L., Lopez V., Lonnerdal B., Dufner-Beattie J., Andrews G.K. Zip3 (Slc39a3) functions in zinc reuptake from the alveolar lumen in lactating mammary gland. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;297:R194–R201. doi: 10.1152/ajpregu.00162.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Qian J., Xu K., Yoo J., Chen T.T., Andrews G., Noebels J.L. Knockout of Zn transporters Zip-1 and Zip-3 attenuates seizure-induced ca1 neurodegeneration. J. Neurosci. 2011;31:97–104. doi: 10.1523/JNEUROSCI.5162-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kury S., Dreno B., Bezieau S., Giraudet S., Kharfi M., Kamoun R., Moisan J.P. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat. Genet. 2002;31:239–240. doi: 10.1038/ng913. [DOI] [PubMed] [Google Scholar]
- 100.Wang K., Zhou B., Kuo Y.M., Zemansky J., Gitschier J. A novel member of a zinc transporter family is defective in acrodermatitis enteropathica. Am. J. Hum. Genet. 2002;71:66–73. doi: 10.1086/341125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schmitt S., Kury S., Giraud M., Dreno B., Kharfi M., Bezieau S. An update on mutations of the SLC39A4 gene in acrodermatitis enteropathica. Hum. Mutat. 2009;30:926–933. doi: 10.1002/humu.20988. [DOI] [PubMed] [Google Scholar]
- 102.Kambe T., Hashimoto A., Fujimoto S. Current understanding of ZIP and ZnT zinc transporters in human health and diseases. Cell. Mol. Life Sci. 2014;71:3281–3295. doi: 10.1007/s00018-014-1617-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Geiser J., Venken K.J., De Lisle R.C., Andrews G.K. A mouse model of acrodermatitis enteropathica: Loss of intestine zinc transporter ZIP4 (Slc39a4) disrupts the stem cell niche and intestine integrity. PLoS Genet. 2012;8:336. doi: 10.1371/journal.pgen.1002766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dufner-Beattie J., Weaver B.P., Geiser J., Bilgen M., Larson M., Xu W., Andrews G.K. The mouse acrodermatitis enteropathica gene Slc39a4 (Zip4) is essential for early development and heterozygosity causes hypersensitivity to zinc deficiency. Hum. Mol. Genet. 2007;16:1391–1399. doi: 10.1093/hmg/ddm088. [DOI] [PubMed] [Google Scholar]
- 105.Guo H., Jin X., Zhu T., Wang T., Tong P., Tian L., Peng Y., Sun L., Wan A., Chen J., et al. SLC39A5 mutations interfering with the BMP/TGF-β pathway in non-syndromic high myopia. J. Med. Genet. 2014;51:518–525. doi: 10.1136/jmedgenet-2014-102351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Geiser J., De Lisle R.C., Andrews G.K. The zinc transporter Zip5 (Slc39a5) regulates intestinal zinc excretion and protects the pancreas against zinc toxicity. PLoS ONE. 2013;8:336. doi: 10.1371/journal.pone.0082149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Waterworth D.M., Ricketts S.L., Song K., Chen L., Zhao J.H., Ripatti S., Aulchenko Y.S., Zhang W., Yuan X., Lim N., et al. Genetic variants influencing circulating lipid levels and risk of coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2010;30:2264–2276. doi: 10.1161/ATVBAHA.109.201020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ehret G.B., Munroe P.B., Rice K.M., Bochud M., Johnson A.D., Chasman D.I., Smith A.V., Tobin M.D., Verwoert G.C., Hwang S.J., et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–109. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Galvez-Peralta M., He L., Jorge-Nebert L.F., Wang B., Miller M.L., Eppert B.L., Afton S., Nebert D.W. ZIP8 zinc transporter: Indispensable role for both multiple-organ organogenesis and hematopoiesis in utero. PLoS ONE. 2012;7:336. doi: 10.1371/journal.pone.0036055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim J.H., Jeon J., Shin M., Won Y., Lee M., Kwak J.S., Lee G., Rhee J., Ryu J.H., Chun C.H., et al. Regulation of the catabolic cascade in osteoarthritis by the zinc-ZIP8-MTF1 axis. Cell. 2014;156:730–743. doi: 10.1016/j.cell.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 111.Park J.H., Hogrebe M., Gruneberg M., DuChesne I., von der Heiden A.L., Reunert J., Schlingmann K.P., Boycott K.M., Beaulieu C.L., Mhanni A.A., et al. SLC39A8 deficiency: A disorder of manganese transport and glycosylation. Am. J. Hum. Genet. 2015;97:894–903. doi: 10.1016/j.ajhg.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Boycott K.M., Beaulieu C.L., Kernohan K.D., Gebril O.H., Mhanni A., Chudley A.E., Redl D., Qin W., Hampson S., Kury S., et al. Autosomal-recessive intellectual disability with cerebellar atrophy syndrome caused by mutation of the manganese and zinc transporter gene SLC39A8. Am. J. Hum. Genet. 2015;97:886–893. doi: 10.1016/j.ajhg.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhao L., Oliver E., Maratou K., Atanur S.S., Dubois O.D., Cotroneo E., Chen C.N., Wang L., Arce C., Chabosseau P.L., et al. The zinc transporter ZIP12 regulates the pulmonary vascular response to chronic hypoxia. Nature. 2015;524:356–360. doi: 10.1038/nature14620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Giunta C., Elcioglu N.H., Albrecht B., Eich G., Chambaz C., Janecke A.R., Yeowell H., Weis M., Eyre D.R., Kraenzlin M., et al. Spondylocheiro dysplastic form of the ehlers-danlos syndrome—An autosomal-recessive entity caused by mutations in the zinc transporter gene SLC39A13. Am. J. Hum. Genet. 2008;82:1290–1305. doi: 10.1016/j.ajhg.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fukada T., Civic N., Furuichi T., Shimoda S., Mishima K., Higashiyama H., Idaira Y., Asada Y., Kitamura H., Yamasaki S., et al. The zinc transporter SLC39A13/ZIP13 is required for connective tissue development; its involvement in BMP/TGF-β signaling pathways. PLoS ONE. 2008;3:336. doi: 10.1371/annotation/a6c35a12-e8eb-43a0-9d00-5078fa6da1bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hojyo S., Fukada T., Shimoda S., Ohashi W., Bin B.H., Koseki H., Hirano T. The zinc transporter SLC39A14/ZIP14 controls G-protein coupled receptor-mediated signaling required for systemic growth. PLoS ONE. 2011;6:336. doi: 10.1371/journal.pone.0018059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Beker Aydemir T., Chang S.M., Guthrie G.J., Maki A.B., Ryu M.S., Karabiyik A., Cousins R.J. Zinc transporter ZIP14 functions in hepatic zinc, iron and glucose homeostasis during the innate immune response (endotoxemia) PLoS ONE. 2012;7:336. doi: 10.1371/journal.pone.0048679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Aydemir T.B., Sitren H.S., Cousins R.J. The zinc transporter Zip14 influences c-Met phosphorylation and hepatocyte proliferation during liver regeneration in mice. Gastroenterology. 2012;142:1536–1546. doi: 10.1053/j.gastro.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jenkitkasemwong S., Wang C.Y., Coffey R., Zhang W., Chan A., Biel T., Kim J.S., Hojyo S., Fukada T., Knutson M.D. SLC39A14 is required for the development of hepatocellular iron overload in murine models of hereditary hemochromatosis. Cell Metab. 2015;22:138–150. doi: 10.1016/j.cmet.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Andrews G.K., Wang H., Dey S.K., Palmiter R.D. Mouse zinc transporter 1 gene provides an essential function during early embryonic development. Genesis. 2004;40:74–81. doi: 10.1002/gene.20067. [DOI] [PubMed] [Google Scholar]
- 121.Chowanadisai W., Lonnerdal B., Kelleher S.L. Identification of a mutation in SLC30A2 (ZnT-2) in women with low milk zinc concentration that results in transient neonatal zinc deficiency. J. Biol. Chem. 2006;281:39699–39707. doi: 10.1074/jbc.M605821200. [DOI] [PubMed] [Google Scholar]
- 122.Lasry I., Seo Y.A., Ityel H., Shalva N., Pode-Shakked B., Glaser F., Berman B., Berezovsky I., Goncearenco A., Klar A., et al. A dominant negative heterozygous G87R mutation in the zinc transporter, ZnT-2 (SLC30A2), results in transient neonatal zinc deficiency. J. Biol. Chem. 2012;287:29348–29361. doi: 10.1074/jbc.M112.368159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Itsumura N., Inamo Y., Okazaki F., Teranishi F., Narita H., Kambe T., Kodama H. Compound heterozygous mutations in SLC30A2/ZnT2 results in low milk zinc concentrations: A novel mechanism for zinc deficiency in a breast-fed infant. PLoS ONE. 2013;8:336. doi: 10.1371/journal.pone.0064045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Miletta M.C., Bieri A., Kernland K., Schoni M.H., Petkovic V., Fluck C.E., Eble A., Mullis P.E. Transient neonatal zinc deficiency caused by a heterozygous G87R mutation in the zinc transporter ZnT-2 (SLC30A2) gene in the mother highlighting the importance of Zn2+ for normal growth and development. Int. J. Endocrinol. 2013;2013 doi: 10.1155/2013/259189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lova Navarro M., Vera Casano A., Benito Lopez C., Fernandez Ballesteros M.D., Godoy Diaz D.J., Crespo Erchiga A., Romero Brufau S. Transient neonatal zinc deficiency due to a new autosomal dominant mutation in gene SLC30A2 (ZnT-2) Pediatr. Dermatol. 2014;31:251–252. doi: 10.1111/pde.12257. [DOI] [PubMed] [Google Scholar]
- 126.Lee S., Hennigar S.R., Alam S., Nishida K., Kelleher S.L. Essential role for zinc transporter 2 (ZnT2)-mediated zinc transport in mammary gland development and function during lactation. J. Biol. Chem. 2015;290:13064–13078. doi: 10.1074/jbc.M115.637439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Adlard P.A., Parncutt J.M., Finkelstein D.I., Bush A.I. Cognitive loss in zinc transporter-3 knock-out mice: A phenocopy for the synaptic and memory deficits of Alzheimer’s disease? J. Neurosci. 2010;30:1631–1636. doi: 10.1523/JNEUROSCI.5255-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sindreu C., Palmiter R.D., Storm D.R. Zinc transporter ZnT-3 regulates presynaptic ERK1/2 signaling and hippocampus-dependent memory. Proc. Natl. Acad. Sci. USA. 2011;108:3366–3370. doi: 10.1073/pnas.1019166108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Martel G., Hevi C., Friebely O., Baybutt T., Shumyatsky G.P. Zinc transporter 3 is involved in learned fear and extinction, but not in innate fear. Learn. Mem. 2010;17:582–590. doi: 10.1101/lm.1962010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cole T.B., Wenzel H.J., Kafer K.E., Schwartzkroin P.A., Palmiter R.D. Elimination of zinc from synaptic vesicles in the intact mouse brain by disruption of the ZnT3 gene. Proc. Natl. Acad. Sci. USA. 1999;96:1716–1721. doi: 10.1073/pnas.96.4.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hildebrand M.S., Phillips A.M., Mullen S.A., Adlard P.A., Hardies K., Damiano J.A., Wimmer V., Bellows S.T., McMahon J.M., Burgess R., et al. Loss of synaptic Zn2+ transporter function increases risk of febrile seizures. Sci. Rep. 2015;5 doi: 10.1038/srep17816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Huang L., Gitschier J. A novel gene involved in zinc transport is deficient in the lethal milk mouse. Nat. Genet. 1997;17:292–297. doi: 10.1038/ng1197-292. [DOI] [PubMed] [Google Scholar]
- 133.McCormick N.H., Lee S., Hennigar S.R., Kelleher S.L. ZnT4 (SLC30A4)-null (“lethal milk”) mice have defects in mammary gland secretion and hallmarks of precocious involution during lactation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015;310:R33–R40. doi: 10.1152/ajpregu.00315.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Inoue K., Matsuda K., Itoh M., Kawaguchi H., Tomoike H., Aoyagi T., Nagai R., Hori M., Nakamura Y., Tanaka T. Osteopenia and male-specific sudden cardiac death in mice lacking a zinc transporter gene, Znt5. Hum. Mol. Genet. 2002;11:1775–1784. doi: 10.1093/hmg/11.15.1775. [DOI] [PubMed] [Google Scholar]
- 135.Nishida K., Hasegawa A., Nakae S., Oboki K., Saito H., Yamasaki S., Hirano T. Zinc transporter Znt5/Slc30a5 is required for the mast cell-mediated delayed-type allergic reaction but not the immediate-type reaction. J. Exp. Med. 2009;206:1351–1364. doi: 10.1084/jem.20082533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Huang L., Yu Y.Y., Kirschke C.P., Gertz E.R., Lloyd K.K. Znt7 (Slc30a7)-deficient mice display reduced body zinc status and body fat accumulation. J. Biol. Chem. 2007;282:37053–37063. doi: 10.1074/jbc.M706631200. [DOI] [PubMed] [Google Scholar]
- 137.Sladek R., Rocheleau G., Rung J., Dina C., Shen L., Serre D., Boutin P., Vincent D., Belisle A., Hadjadj S., et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 138.Saxena R., Voight B.F., Lyssenko V., Burtt N.P., de Bakker P.I., Chen H., Roix J.J., Kathiresan S., Hirschhorn J.N., Daly M.J., et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- 139.Zeggini E., Weedon M.N., Lindgren C.M., Frayling T.M., Elliott K.S., Lango H., Timpson N.J., Perry J.R., Rayner N.W., Freathy R.M., et al. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316:1336–1341. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Scott L.J., Mohlke K.L., Bonnycastle L.L., Willer C.J., Li Y., Duren W.L., Erdos M.R., Stringham H.M., Chines P.S., Jackson A.U., et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.O’Halloran T.V., Kebede M., Philips S.J., Attie A.D. Zinc, insulin, and the liver: A menage a trois. J. Clin. Investig. 2013;123:4136–4139. doi: 10.1172/JCI72325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pound L.D., Sarkar S.A., Benninger R.K., Wang Y., Suwanichkul A., Shadoan M.K., Printz R.L., Oeser J.K., Lee C.E., Piston D.W., et al. Deletion of the mouse Slc30a8 gene encoding zinc transporter-8 results in impaired insulin secretion. Biochem. J. 2009;421:371–376. doi: 10.1042/BJ20090530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nicolson T.J., Bellomo E.A., Wijesekara N., Loder M.K., Baldwin J.M., Gyulkhandanyan A.V., Koshkin V., Tarasov A.I., Carzaniga R., Kronenberger K., et al. Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants. Diabetes. 2009;58:2070–2083. doi: 10.2337/db09-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lemaire K., Ravier M.A., Schraenen A., Creemers J.W., van de Plas R., Granvik M., van Lommel L., Waelkens E., Chimienti F., Rutter G.A., et al. Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice. Proc. Natl. Acad. Sci. USA. 2009;106:14872–14877. doi: 10.1073/pnas.0906587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wijesekara N., Dai F.F., Hardy A.B., Giglou P.R., Bhattacharjee A., Koshkin V., Chimienti F., Gaisano H.Y., Rutter G.A., Wheeler M.B. Beta cell-specific Znt8 deletion in mice causes marked defects in insulin processing, crystallisation and secretion. Diabetologia. 2010;53:1656–1668. doi: 10.1007/s00125-010-1733-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tamaki M., Fujitani Y., Hara A., Uchida T., Tamura Y., Takeno K., Kawaguchi M., Watanabe T., Ogihara T., Fukunaka A., et al. The diabetes-susceptible gene SLC30A8/ZnT8 regulates hepatic insulin clearance. J. Clin. Investig. 2013;123:4513–4524. doi: 10.1172/JCI68807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Flannick J., Thorleifsson G., Beer N.L., Jacobs S.B., Grarup N., Burtt N.P., Mahajan A., Fuchsberger C., Atzmon G., Benediktsson R., et al. Loss-of-function mutations in SLC30A8 protect against type 2 diabetes. Nat. Genet. 2014;46:357–363. doi: 10.1038/ng.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wenzlau J.M., Juhl K., Yu L., Moua O., Sarkar S.A., Gottlieb P., Rewers M., Eisenbarth G.S., Jensen J., Davidson H.W., et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc. Natl. Acad. Sci. USA. 2007;104:17040–17045. doi: 10.1073/pnas.0705894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Quadri M., Federico A., Zhao T., Breedveld G.J., Battisti C., Delnooz C., Severijnen L.A., di Toro Mammarella L., Mignarri A., Monti L., et al. Mutations in SLC30A10 cause parkinsonism and dystonia with hypermanganesemia, polycythemia, and chronic liver disease. Am. J. Hum. Genet. 2012;90:467–477. doi: 10.1016/j.ajhg.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tuschl K., Clayton P.T., Gospe S.M., Jr., Gulab S., Ibrahim S., Singhi P., Aulakh R., Ribeiro R.T., Barsottini O.G., Zaki M.S., et al. Syndrome of hepatic cirrhosis, dystonia, polycythemia, and hypermanganesemia caused by mutations in SLC30A10, a manganese transporter in man. Am. J. Hum. Genet. 2012;90:457–466. doi: 10.1016/j.ajhg.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Leyva-Illades D., Chen P., Zogzas C.E., Hutchens S., Mercado J.M., Swaim C.D., Morrisett R.A., Bowman A.B., Aschner M., Mukhopadhyay S. SLC30A10 is a cell surface-localized manganese efflux transporter, and parkinsonism-causing mutations block its intracellular trafficking and efflux activity. J. Neurosci. 2014;34:14079–14095. doi: 10.1523/JNEUROSCI.2329-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Marger L., Schubert C.R., Bertrand D. Zinc: An underappreciated modulatory factor of brain function. Biochem. Pharmacol. 2014;91:426–435. doi: 10.1016/j.bcp.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 153.Maverakis E., Fung M.A., Lynch P.J., Draznin M., Michael D.J., Ruben B., Fazel N. Acrodermatitis enteropathica and an overview of zinc metabolism. J. Am. Acad. Dermatol. 2007;56:116–124. doi: 10.1016/j.jaad.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 154.Andrews G.K. Regulation and function of Zip4, the acrodermatitis enteropathica gene. Biochem. Soc. Trans. 2008;36:1242–1246. doi: 10.1042/BST0361242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Krezel A., Maret W. Zinc-buffering capacity of a eukaryotic cell at physiological pZn. J. Biol. Inorg. Chem. 2006;11:1049–1062. doi: 10.1007/s00775-006-0150-5. [DOI] [PubMed] [Google Scholar]
- 156.Fujimoto S., Itsumura N., Tsuji T., Anan Y., Tsuji N., Ogra Y., Kimura T., Miyamae Y., Masuda S., Nagao M., et al. Cooperative functions of ZnT1, metallothionein and ZnT4 in the cytoplasm are required for full activation of TNAP in the early secretory pathway. PLoS ONE. 2013;8:336. doi: 10.1371/journal.pone.0077445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ishihara K., Yamazaki T., Ishida Y., Suzuki T., Oda K., Nagao M., Yamaguchi-Iwai Y., Kambe T. Zinc transport complexes contribute to the homeostatic maintenance of secretory pathway function in vertebrate cells. J. Biol. Chem. 2006;281:17743–17750. doi: 10.1074/jbc.M602470200. [DOI] [PubMed] [Google Scholar]
- 158.Fukunaka A., Suzuki T., Kurokawa Y., Yamazaki T., Fujiwara N., Ishihara K., Migaki H., Okumura K., Masuda S., Yamaguchi-Iwai Y., et al. Demonstration and characterization of the heterodimerization of ZnT5 and ZnT6 in the early secretory pathway. J. Biol. Chem. 2009;284:30798–30806. doi: 10.1074/jbc.M109.026435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fukunaka A., Kurokawa Y., Teranishi F., Sekler I., Oda K., Ackland M.L., Faundez V., Hiromura M., Masuda S., Nagao M., et al. Tissue nonspecific alkaline phosphatase is activated via a two-step mechanism by zinc transport complexes in the early secretory pathway. J. Biol. Chem. 2011;286:16363–16373. doi: 10.1074/jbc.M111.227173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hamza I., Faisst A., Prohaska J., Chen J., Gruss P., Gitlin J.D. The metallochaperone Atox1 plays a critical role in perinatal copper homeostasis. Proc. Natl. Acad. Sci. USA. 2001;98:6848–6852. doi: 10.1073/pnas.111058498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Miyayama T., Suzuki K.T., Ogra Y. Copper accumulation and compartmentalization in mouse fibroblast lacking metallothionein and copper chaperone, Atox1. Toxicol. Appl. Pharmacol. 2009;237:205–213. doi: 10.1016/j.taap.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 162.Jeney V., Itoh S., Wendt M., Gradek Q., Ushio-Fukai M., Harrison D.G., Fukai T. Role of antioxidant-1 in extracellular superoxide dismutase function and expression. Circ. Res. 2005;96:723–729. doi: 10.1161/01.RES.0000162001.57896.66. [DOI] [PubMed] [Google Scholar]
- 163.El Meskini R., Culotta V.C., Mains R.E., Eipper B.A. Supplying copper to the cuproenzyme peptidylglycine α-amidating monooxygenase. J. Biol. Chem. 2003;278:12278–12284. doi: 10.1074/jbc.M211413200. [DOI] [PubMed] [Google Scholar]
- 164.Lutsenko S., Barnes N.L., Bartee M.Y., Dmitriev O.Y. Function and regulation of human copper-transporting ATPases. Physiol. Rev. 2007;87:1011–1046. doi: 10.1152/physrev.00004.2006. [DOI] [PubMed] [Google Scholar]
- 165.Cherezov V., Hofer N., Szebenyi D.M., Kolaj O., Wall J.G., Gillilan R., Srinivasan V., Jaroniec C.P., Caffrey M. Insights into the mode of action of a putative zinc transporter CzrB in thermus thermophilus. Structure. 2008;16:1378–1388. doi: 10.1016/j.str.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]