Abstract
Small G protein Rab27B is expressed in various secretory cell types and plays a role in mediating secretion. In pancreatic acinar cells, Rab27B was found to be expressed on the zymogen granule membrane and by overexpression to regulate the secretion of zymogen granules. However, the effect of Rab27B deletion on the physiology of pancreatic acinar cells is unknown. In the current study, we utilized the Rab27B KO mouse model to better understand the role of Rab27B in the secretion of pancreatic acinar cells. Our data show that Rab27B deficiency had no obvious effects on the expression of major digestive enzymes and other closely related proteins, e.g. similar small G proteins, such as Rab3D and Rab27A, and putative downstream effectors. The overall morphology of acinar cells was not changed in the knockout pancreas. However, the size of zymogen granules was decreased in KO acinar cells, suggesting a role of Rab27B in regulating the maturation of secretory granules. The secretion of digestive enzymes was moderately decreased in KO acini, compared with the WT control. These data indicate that Rab27B is involved at a different steps of zymogen granule maturation and secretion, which is distinct from that of Rab3D.
Keywords: Rab27B, pancreatic acinar cells, zymogen granules, secretion
INTRODUCTION
Rab proteins are a large family of Ras-like GTPases that are responsible for regulating the specificity of intracellular membrane trafficking. Rabs alternate between GTP-bound active and GDP-bound inactive forms and thereby trigger downstream signaling pathways. Within the large Rab family, Rab27 subfamily members, Rab27A and Rab27B have been reported to play roles in mediating secretion in various cell types, including pancreatic beta cells, neuroendocrine cells and neutrophils [1]. In our previous studies, we have shown that both Rab27A and Rab27B are present in pancreatic acinar cells and may regulate secretion of digestive enzymes [2,3,4]. Specifically, Rab27B is abundantly localized to zymogen granules in pancreatic acinar cells and are detected by mass spectrometry, western blot and immunofluorescence [2,3,4,5]. We have further identified EPI64B as a Rab27B specific GTPase-activating protein (GAP); EPI64B was also shown to affect secretion in acinar cells [6]. It has been suggested that Rab27B plays a positive role to mediate secretion in pancreatic acinar cells as well as in many other cell types, with most of the evidence provided by overexpression of constitutively active or dominant negative forms of Rab27B protein [5,7]. In some secretory cells, Rab27B has been shown to be functionally redundant to Rab27A and in others to regulate secretion differently than Rab27A [8,9,10,11]. Rab3GEP/MADD was shown to be a non-redundant GEF for Rab27A activation in melanocytes [12] as well as a GEF for all four Rab3 isoforms [13], indicating that Rab27 proteins are closely co-regulated with Rab3 proteins. Additionally, Rab3 isoforms are phylogenetically close to the Rab27 subfamily and share downstream effectors in some cell types [14]. It has been demonstrated that acute overexpression of Rab3D in pancreatic acini affects secretion, but Rab3D knockout does not affect amylase release in pancreatic acini, but rather increased the size of secretory granules, suggesting that Rab3D is not involved in the exocytosis, but exerts its function in the maturation of secretory granules [15]. It is currently unknown whether genetic deletion of Rab27B has any effects on ZG size or secretion and how Rab27B deficiency affects other GTPase activities in pancreatic acinar cells.
In the current study, we evaluated the effects of Rab27B on digestive enzyme secretion using Rab27B knockout mice. We found that Rab27B deficiency did not affect the expression of closely related small GTPases and major digestive enzymes, the intracellular localization of zymogen granules or the amount of Rab3D. However, CCK and carbachol-induced amylase secretion was moderately inhibited in KO acini. In addition, Rab27B deletion decreased the size of zymogen granules, which contrasts with the effect of Rab3D knockout, which increases the size of zymogen granules. Expression of Rab3D in zymogen granule membrane fractions was increased in Rab27B KO acini, suggesting that Rab27B and Rab3D are closely co-regulated in pancreatic acinar cells but may play different roles in the maturation of zymogen granules.
MATERIALS AND METHODS
Mice and Reagents
The Rab27B−/− C57BL/6 mice were bred from Rab27a/b double knockout mice, which were provided by Dr. Miguel Seabra [6] and crossed with C57BL/6 from Taconic (Hudson, NY). By subsequent breeding of pups, Rab27a−/−, Rab27b−/− and C57BL/6 lines were established and bred as homozygotes. Genotyping of Rab27 was performed as previously described [8]. Rab27a KO mice showed reduced fertility but Rab27b KO mice bred normally. Collagenase NB8 from C. histolyticum Broad Range was purchased from SERVA (Heidelberg, Germany); rabbit polyclonal anti-Rab27B and anti-Rab27A antibodies were from Synaptic Systems (Goettingen, Germany); anti-Rab3D antiserum was a gift from Dr. Mark McNiven (Mayo Clinic, Rochester, MN); rabbit polyclonal anti-elastase and anti-ribonuclease antibodies were from Rockland Immunochemicals (Gilbertsville, PA); rabbit polyclonal anti-chymotrypsin and mouse monoclonal anti-lipase antibodies from Santa Cruz (Dallas, TX); rabbit polyclonal anti-amylase antibody from Sigma.
Isolation of pancreatic acini and analysis of amylase secretion
Pancreatic acini from 6-8 week old male WT C57BL/6 or Rab27B KO mice were isolated by enzymatic digestion with collagenase followed by mechanical shearing as previously described [16,17]. After filtration through a 150 micron Nitex mesh, the mixture of acini and islets were purified by sedimentation in centrifugation buffer containing 4% BSA.
Freshly prepared acini were used for amylase release after pre-incubation at 37 °C for 30 min. After incubation in DMEM plus 0.1% BSA and 0.01% soybean trypsin inhibitor containing cholecystokinin (CCK) or carbachol of various indicated concentrations for 30 min, the acinar suspension was then centrifuged for 20 s in a microcentrifuge and the supernatant was assayed for amylase activity using Phadebas reagent (Amersham Biosciences and Upjohn) as previously described [16,18]. The pellets were collected and lysed for DNA content measurement using Qubit 2.0 fluorometer and Qubit dsDNA HS Assay Kit (Life Technologies, Eugene, OR). Secretion was expressed both as a percentage of initial acinar amylase total content or as units of amylase per milligram DNA.
Assessment of Pancreatic Morphology and Ultrastructure
Immunofluorescence was performed as described previously [19]. Briefly, pancreatic tissues were fixed for 30 min in 4% formaldehyde (freshly prepared from paraformaldehyde) in PBS, cryoprotected as described [19], embedded in a 1:1 mixture of OCT and 20% sucrose and then 6 μm sections were cut with a CM 1950 Leica Cryostat. The primary polyclonal anti-amylase antibody, and Oregon Green 488 Phalloidin which stains F-actin were diluted 1:1,000, and 1:50, respectively. Secondary antibody anti-rabbit-Alexa 594 was diluted 1:500. Prolong Gold with 4,6-diamino-2-phenylindole (DAPI) was added to mounting medium to counterstain nuclei. Other tissue was fixed with 4% formaldehyde overnight, embedded in paraffin and cut in 5μm sections. H&E stained images were taken with an x40 objective on an Olympus BX-51 microscope. Fluorescence images were taken with an Olympus FluoView 500 confocal microscope using a x60 water immersion objective. Images were processed in Photoshop. Electron microscopy was performed as described previously [2,20]. Briefly, pancreas was minced with razor blade and fixed for 2 hours with a mixture of 2% glutaraldehyde and 2% formaldehyde in phosphate-buffered saline (PBS), post-fixed for 45 minutes with 1% OsO4, and then dehydrated and embedded in Epon. Ultrathin sections were stained with uranyl acetate and lead citrate; images were recorded digitally using a Philips CM-100 electron microscope. Metamorph software (Molecular Devices, Sunnyvale, CA) was used to quantify diameters of zymogen granules from the electron micrographs.
Tissue Fractionation
Subcellular fractions were obtained from WT and Rab27B KO mouse pancreas as previously described [2]. The mouse pancreas was homogenized in buffer containing 0.25 M sucrose, 25 mM MES (2-Morpholinoethanesulfoni acid, monohydrate) pH 6.0, 0.1 mM MgSO4, 2 mM EGTA and 0.1 mM phenylmethylsulfonyl fluoride (PMSF). Unbroken cells and nuclei were removed by centrifugation at 200 g for 10 min at 4°C. Postnuclear supernatant was centrifuged at 2,000 g for 10 min to obtain a pellet enriched in zymogen granules (ZGs) and mitochondria. ZGs were further purified by Percoll gradient centrifugation [2,3,4]. The 2,000 g supernatant was centrifuged at 10,000 g for 10 min to produce a pellet enriched in mitochondria; the supernatant was further centrifuged at 100,000 g for 1 hr to separate microsomal and cytosolic fractions. The ZGs were washed with homogenization buffer and resuspended in ZG lysis buffer containing 150 mM sodium acetate, 10 mM MOPS, pH 7.0, 27 μg/ml Nigericin, 0.1 mM MgSO4, 0.1 mM PMSF, supplemented with protease inhibitors cocktail, and incubated at 37°C for 15 min. The lysate was centrifuged at 100,000 g for 1h in a Beckman ultracentrifuge using a Ti 70.1 rotor to pellet the ZG membrane. The supernatant was saved as ZG content. The ZG membrane pellet was sequentially washed with 250 mM KBr and 0.1 M Na2CO3 (pH 11.0). Equal amount of total protein from different fractions were separated on SDS-PAGE and blotted with indicated antibodies.
RESULTS
Rab27B deletion had no effect on the expression of Rab27A and Rab3D
To examine the effects of Rab27B deletion on the expression of other proteins in pancreatic acinar cells, pancreatic acini were isolated from WT and Rab27B KO mice. Rab27B deletion was confirmed by western blot, whereas the expression of closely related Rabs, Rab27A and Rab3D were not changed in Rab27B KO mouse acini (Figure 1A). The level of putative Rab27B downstream effector Slp1 was also not affected. The expression of major digestive enzymes--amylase, lipase, elastase, ribonuclease and chymotrypsin--was unaffected in Rab27B KO acini (Figure 1B). There was also no effect on the weight of the pancreas when normalized to body weight (data not shown).
Figure 1.
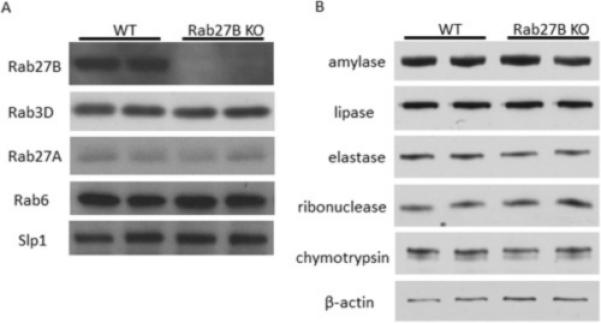
Rab27B deficiency does not affect the expression of closely related Rab proteins and digestive enzymes. Expression of Rab27A, Rab3D and Slp1(A) and major digestive enzymes (B) in isolated WT and Rab27B KO acini; each lane represents a sample from one mouse.
Rab27B deficiency caused increased expression of Rab3D in zymogen granule membrane fractions
Although the total expression of closely related Rab proteins Rab27A and Rab3D was not affected by Rab27B deletion, we sought to further test the effects of Rab27B deletion on the expression of Rab27A and Rab3D in different intracellular organelles. Subcellular fractions were obtained from WT and Rab27B KO pancreas and analyzed by western blot. The amount of Rab27A on zymogen granule membrane fraction was significantly decreased (Figure 2A), whereas the localization of Rab3D to ZG membrane was increased in Rab27B KO pancreas (Figure 2B). Consistent effects were not observed in mitochondrial or microsomal fractions.
Figure 2.
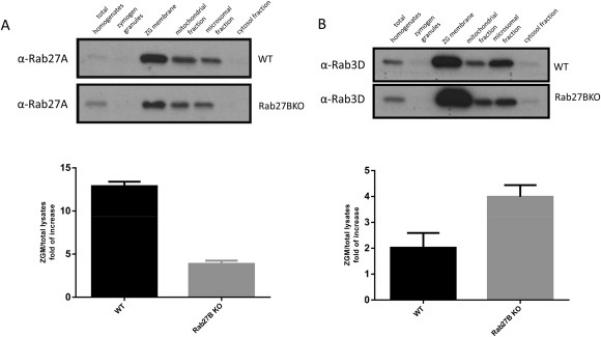
Localization of Rab3D and Rab27A to zymogen granule membrane was oppositely affected in Rab27B KO acinar cells. Cell fractionation samples from WT and Rab27B KO mouse pancreas were loaded at 10 μg/lane and separated by PAGE. Antibodies against Rab27A (A) and Rab3D (B) were used in western blots. Densitometry analysis of results from three independent experiments of anti-Rab27A (A) or Rab3D (B) blots are shown. The results are mean ± SE from three batches of samples of each genotype. *P < 0.05.
Rab27B deletion had no effect on the acinar cell morphology, but decreased the size of zymogen granules
Because Rab27B has been shown to be abundantly expressed on the zymogen granule membrane and to regulate secretion in pancreatic acinar cells [3,4], we examined the effects of Rab27B deficiency on overall acinar cell morphology and distribution of zymogen granules. As shown in Figure 3A and 3B, no obvious change in acinar cell morphology at the light microscopic level was observed in the Rab27B KO pancreas, compared with WT. Confocal immunofluorescence intensity and localization of amylase to zymogen granules in KO acinar cells were similar to that in WT pancreas (Figure 3C and 3D). Similarly, there was no change in filamentous actin labeled with phalloidin. Because it has been shown that the size of zymogen granules was increased in Rab3D knockout pancreas [15], we explored whether the lack of Rab27B had similar effects by analyzing the ultrastructure of pancreas using electronic microscopy. As indicated in Figure 3E and 3F, the zymogen granules appear to be smaller in Rab27B KO pancreas than in WT controls. For quantitation, approximately 4700 granules were randomly selected from 22 to 33 independent sections of each phenotype and the diameter of zymogen granules was measured using Metamorph software. As shown in Figure 3G, the size distribution curve from Rab27B KO pancreas shifted to smaller diameters by up to 300 nm (Figure 3G).
Figure 3.
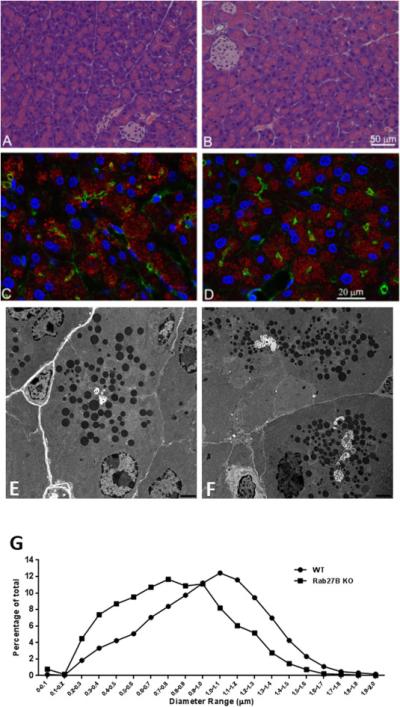
Rab27B deficiency did not affect the general morphology of pancreatic acinar cells and the localization of amylase, but decreased the size of zymogen granules. (A and B) Hematoxylin and Eosin staining was performed on paraffin sections from WT (A) or Rab27B KO (B) mouse pancreas. For immunofluorescence, WT(C) or Rab27B KO (D) pancreas tissues were cryosectioned at 10 μm, stained with phalloidin to label actin (green), DAPI to label nuclei (blue) and anti-amylase antibody (red), respectively. Images were obtained using confocal microscopy. (E and F) Pancreas tissues from WT (E) and Rab27B KO (F) mice were processed for electron microscopy as described in Material and Methods. Images were obtained at the magnification of 6000X. (G) The diameter of zymogen granules from WT and KO mouse pancreas was measured using images obtained from electron microscopy. Each data set represents a combination of values obtained from 7-12 sections from each of three mice. The values were normalized to the total number of granules.
Rab27B deficiency caused a modest decrease in amylase release from acinar cells
To test the effects of Rab27B deletion on amylase release, acini were isolated from WT orRab27B KO mice and stimulated with a range of cholecystokinin (CCK) or carbachol (CCh) concentrations. The CCK or CCh induced dose response curves, calculated as percentage of total amylase release, were moderately decreased in KO acini, compared with WT acini; at lower concentrations (CCK 10 pM and CCh 300 nM), KO acini exhibited significantly lower secretion (Figure 4A and 4B). When plotted in units/mg DNA, both secretagogue induced curves demonstrated similar pattern (data not shown). Thus, overall there is a modest decrease in stimulated amylase release in acini after deletion of Rab27B.
Figure 4.
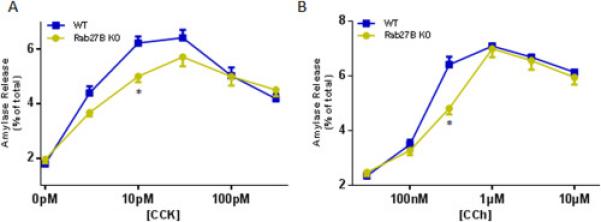
Rab27B deficiency caused moderate reduction in amylase release from isolated acini. Freshly prepared acini were incubated with indicated concentrations of CCK (A) or carbachol (CCh, B) for 30 min. Amylase release results were expressed as percentage of total acinar amylase content. All results are mean ± SE from five independent experiments, respectively. One WT and one Rab27B KO mouse were used in each experiment. Each value was compared to the WT group at each concentration point of the indicated secretagogue. *P < 0.05.
DISCUSSION
In our previous studies, Rab27B was shown to be abundantly expressed on the zymogen granule membrane [3,4]. However, its function in pancreatic acinar cells has not been clearly elucidated. Rab27B KO mouse model was used in the current study. Although some studies have demonstrated Rab27B plays a positive role in multiple secretory cell types using the Rab27B KO mouse model, pancreatic acinar cells were not included in these investigations. It has been shown in the lacrimal gland acinar cells that, Rab27B deficiency caused a decrease in the number of secretory vesicles and the distention of ER [7]. In contrast, we did not detect any obvious effects of Rab27B deletion on the morphology and size of other subcellular organelles, but observed a decreased size of zymogen granules and moderately decreased digestive enzyme secretion in Rab27B KO pancreatic acinar cells (Figures 3G, 4A and 4B).
Given that Rab3D is closely related to Rab27B in terms of structure and functions, we examined the effects of Rab27B deletion on Rab3D. Although we did not observe changes in total Rab3D levels (Figure 1A), or activation of basal levels of Rab3D (data not shown) in Rab27B KO pancreas, Rab3D was shown to be more densely enriched in the zymogen granule membrane fraction in KO pancreas, compared with WT (Figure 2B). This effect could be caused by a smaller granule size, as shown in Figure 3E-G, and resultant increased surface area of zymogen granule membrane, given the fact that total digestive enzyme level was not affected in KO pancreas (Figure 1B). Unlike Rab3D knockout acini, which showed no obvious changes in secretion and an increased zymogen granule size [15], Rab27B KO acini showed moderately decreased secretion and decreased granule size, suggesting that Rab27B may play a different role in the maturation and secretion of zymogen granules than Rab3D.
Our previous immunofluorescence localization observation showed that Rab27B exhibits a more diffuse subcellular localization than Rab3D (data not shown), suggesting that Rab27B could also be present on other organelles, for example the trans-Golgi network region and regulate the biogenesis of zymogen granules. Alternatively, because Rab27B has been shown to be predominantly localized on secretory vesicles and to mediate the docking and tethering to the plasma membrane, lack of Rab27B could prohibit granule fusion with the plasma membrane as a feedback, and even further the ZG-ZG fusion. As a result, this could lead to an increased number of smaller granules. Furthermore, the increased granules of small size could also contribute to the downregulation in secretion, since theoretically each fusion event would release less ZG contents.
It cannot be eliminated that Rab27B deficiency could potentially be compensated by the upregulation of other closely related proteins, e.g. other small G proteins or downstream effectors. However, the other isoform of Rab27 subfamily, Rab27A, seems less likely to play a redundant role to Rab27B, given that total Rab27A level was not affected by the lack of Rab27B. Surprisingly, the localization of Rab27A to zymogen granule membrane was decreased in Rab27B KO pancreas (Figure 2A), suggesting that Rab27B could be effective in the regulation of Rab27A in a physiological setting.
In summary, we have shown that Rab27B deficiency had a modest negative effect on digestive enzyme secretion in pancreatic acinar cells and also caused a reduction in average granule size. Compared with Rab3D, Rab27B could also play an important but different role in the maturation of zymogen granules. Further studies should be focused on the effects of Rab27B knockout on the function and regulation of its upstream regulators and downstream effectors.
Supplementary Material
● First report on the phenotype of Rab27B genetic deletion in pancreatic acinar cells
● Discovered that Rab27B deletion decreases size of zymogen granules
● Demonstrated that Rab27B deletion moderately decreases amylase release
Acknowledgements
This work was supported by NIH grant R37DK041122 to JAW. We thank Dr. Miguel Seabra at Imperial College of London for providing the Rab27A/B double knockout mice. We thank Brad Nelson for assistance with immunofluorescence and electron microscopy, and Dr. Edward Stuenkel for his constructive suggestions. We thank the Protein Identification and Localization Core of the Michigan Gastrointestinal Peptide Research Center (supported by grant P30DK34933) and the Morphology and Image Analysis Core of the Michigan Diabetes Research Center (supported by grant P30DK020572 from the National Institute of Diabetes and Digestive and Kidney Diseases) for their assistance with imaging techniques and support in the use of equipment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- 1.Hou Y, Chen X, Ernst SA, Williams JA. Rab27, Pancreapedia: Exocrine Pancreas Knowledge Base. 2015 DOI: 10.3998/panc.2015.12. [Google Scholar]
- 2.Hou Y, Ernst SA, Stuenkel EL, Lentz SI, Williams JA. Rab27A Is Present in Mouse Pancreatic Acinar Cells and Is Required for Digestive Enzyme Secretion. PLoS One. 2015;10:e0125596. doi: 10.1371/journal.pone.0125596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen X, Ulintz PJ, Simon ES, Williams JA, Andrews PC. Global topology analysis of pancreatic zymogen granule membrane proteins. Mol Cell Proteomics. 2008;7:2323–2336. doi: 10.1074/mcp.M700575-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen X, Walker AK, Strahler JR, Simon ES, Tomanicek-Volk SL, Nelson BB, Hurley MC, Ernst SA, Williams JA, Andrews PC. Organellar proteomics: analysis of pancreatic zymogen granule membranes. Mol Cell Proteomics. 2006;5:306–312. doi: 10.1074/mcp.M500172-MCP200. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Li C, Izumi T, Ernst SA, Andrews PC, Williams JA. Rab27b localizes to zymogen granules and regulates pancreatic acinar exocytosis. Biochem Biophys Res Commun. 2004;323:1157–1162. doi: 10.1016/j.bbrc.2004.08.212. [DOI] [PubMed] [Google Scholar]
- 6.Hou Y, Chen X, Tolmachova T, Ernst SA, Williams JA. EPI64B Acts as a GTPase-activating Protein for Rab27B in Pancreatic Acinar Cells. J Biol Chem. 2013;288:19548–19557. doi: 10.1074/jbc.M113.472134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiang L, Ngo J, Schechter JE, Karvar S, Tolmachova T, Seabra MC, Hume AN, Hamm-Alvarez SF. Rab27b regulates exocytosis of secretory vesicles in acinar epithelial cells from the lacrimal gland. Am J Physiol Cell Physiol. 2011;301:C507–521. doi: 10.1152/ajpcell.00355.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barral DC, Ramalho JS, Anders R, Hume AN, Knapton HJ, Tolmachova T, Collinson LM, Goulding D, Authi KS, Seabra MC. Functional redundancy of Rab27 proteins and the pathogenesis of Griscelli syndrome. J Clin Invest. 2002;110:247–257. doi: 10.1172/JCI15058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westbroek W, Lambert J, De Schepper S, Kleta R, Van Den Bossche K, Seabra MC, Huizing M, Mommaas M, Naeyaert JM. Rab27b is up-regulated in human Griscelli syndrome type II melanocytes and linked to the actin cytoskeleton via exon F-Myosin Va transcripts. Pigment Cell Res. 2004;17:498–505. doi: 10.1111/j.1600-0749.2004.00173.x. [DOI] [PubMed] [Google Scholar]
- 10.Singh RK, Furze RC, Birrell MA, Rankin SM, Hume AN, Seabra MC. A role for Rab27 in neutrophil chemotaxis and lung recruitment. BMC Cell Biol. 2014;15:39. doi: 10.1186/s12860-014-0039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh RK, Liao W, Tracey-White D, Recchi C, Tolmachova T, Rankin SM, Hume AN, Seabra MC. Rab27a-mediated protease release regulates neutrophil recruitment by allowing uropod detachment. J Cell Sci. 2012;125:1652–1656. doi: 10.1242/jcs.100438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Figueiredo AC, Wasmeier C, Tarafder AK, Ramalho JS, Baron RA, Seabra MC. Rab3GEP is the non-redundant guanine nucleotide exchange factor for Rab27a in melanocytes. J Biol Chem. 2008;283:23209–23216. doi: 10.1074/jbc.M804134200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wada M, Nakanishi H, Satoh A, Hirano H, Obaishi H, Matsuura Y, Takai Y. Isolation and characterization of a GDP/GTP exchange protein specific for the Rab3 subfamily small G proteins. J Biol Chem. 1997;272:3875–3878. doi: 10.1074/jbc.272.7.3875. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda M. Regulation of secretory vesicle traffic by Rab small GTPases. Cell Mol Life Sci. 2008;65:2801–2813. doi: 10.1007/s00018-008-8351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riedel D, Antonin W, Fernandez-Chacon R, Alvarez de Toledo G, Jo T, Geppert M, Valentijn JA, Valentijn K, Jamieson JD, Sudhof TC, Jahn R. Rab3D is not required for exocrine exocytosis but for maintenance of normally sized secretory granules. Mol Cell Biol. 2002;22:6487–6497. doi: 10.1128/MCB.22.18.6487-6497.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams JA. Isolation of rodent pancreatic acinar cells and acini by collagenase digestion., The Pancreapedia: Exocrine Pancreas Knowledge Base. 2010 DOI: 10.3998/panc.2010.18. [Google Scholar]
- 17.Williams JA, Korc M, Dormer RL. Action of secretagogues on a new preparation of functionally intact, isolated pancreatic acini. Am J Physiol. 1978;235:517–524. doi: 10.1152/ajpendo.1978.235.5.E517. [DOI] [PubMed] [Google Scholar]
- 18.Hou Y, Ernst SA, Heidenreich K, Williams JA. Glucagon-like peptide-1 receptor is present in pancreatic acinar cells and regulates amylase secretion through cAMP. Am J Physiol Gastrointest Liver Physiol. 2016;310:G26–33. doi: 10.1152/ajpgi.00293.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sans MD, Sabbatini ME, Ernst SA, D'Alecy LG, Nishijima I, Williams JA. Secretin is not necessary for exocrine pancreatic development and growth in mice. Am J Physiol Gastrointest Liver Physiol. 2011;301:G791–798. doi: 10.1152/ajpgi.00245.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crozier SJ, D'Alecy LG, Ernst SA, Ginsburg LE, Williams JA. Molecular mechanisms of pancreatic dysfunction induced by protein malnutrition. Gastroenterology. 2009;137:1093–1101. 1101, e1091–1093. doi: 10.1053/j.gastro.2009.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


