Abstract
A growing body of evidence shows that female birds use male plumage coloration as an important criterion in mate choice. In the field, however, males with brighter coloration may both compete better for high quality territories and be the object of female choice. Positive associations between territory quality, male-male competitive ability, and female preferences can make it difficult to determine whether females actively choose the most ornamented males. Male eastern bluebirds (Sialia sialis) display brilliant ultraviolet (UV)-blue plumage coloration on their heads, backs, wings, and tails, and chestnut coloration on their breasts which is positively correlated with condition, reproductive effort, and reproductive success. We tested the hypothesis that female bluebirds prefer males that display brighter and more chromatic coloration by widowing males in the field and allowing replacement females to choose partners. We controlled for the influence of territory quality on female choice by widowing dyads of males with adjacent territories. We found no evidence that UV-blue or chestnut plumage coloration, body size, or body condition predicted the male with which females would pair. We found no support for the hypothesis that the coloration of male eastern bluebirds functions as a criterion in female mate choice.
Keywords: Eastern bluebird, female preferences, male-male competition, mate choice, plumage coloration, Sialia sialis, sexual selection
It is generally thought that the bright plumage coloration displayed by males of many bird species evolved in response to selection from female choice for elaborate displays (Hill 2006). Indicator models of sexual selection propose that the expression of sexually selected traits, such as plumage coloration, reliably signal individual condition (Andersson 1994; Hill 2002). These traits can act as honest signals of an individual’s phenotypic or genetic quality if only exceptionally fit individuals in a population achieve maximum expression of such traits (Zahavi 1975; Hamilton & Zuk 1982; Kodric-Brown & Brown 1984). Indicator models predict that individuals that display more-exaggerated traits compete better for mates and thus experience higher reproductive success. There is now substantial experimental and correlational evidence that, in some species of birds, females discriminate between potential mates by assessing colourful plumages (reviewed in Hill 2006).
Studies of female mate choice in birds range from weak correlative field studies and uncontrolled aviary observations to carefully controlled experimental tests in both the field and the laboratory. Laboratory experiments can be useful because they allow researchers to manipulate coloration and disassociate colour traits from other correlated traits. Eliminating the confounding influence of male-male competition, for example, can be very helpful (Wagner 1998). However, eliminating male-male competitive interactions usually necessitates that males are housed individually and thus reduces physical access to females. When females do not have physical access to potential mates and cannot copulate with males, researchers generally use association time as a proxy for mate choice. Association time, however, may not always be an accurate and consistent measure of mate preference (Hill 2006). Females also may not make mate choice decisions in the relatively unnatural environment of the laboratory. Finally, in laboratory- based mate choice trials, it becomes difficult for researchers to investigate the fitness consequences of mating decisions (Hill 2006). For example, for most species in the laboratory, it is not possible to examine how the chosen male contributes to the female’s breeding success. Contributions to reproductive success include direct benefits (territory quality and parental care) and indirect benefits (fertilization rate and genetic quality) provided by males. Controlled tests of female preferences conducted in the field provide an alternative to laboratory-based trials and can allow researchers to quantify both female choice and the ramifications of those choices.
The eastern bluebird (Sialia sialis) is among the better studied bird species with respect to the function of ornamental plumage coloration, and yet the importance of male coloration as a criterion in female mate choice remains uncertain. Male bluebirds display a structural UV-blue coloration on the plumage of their backs, heads, wings and tails and chestnut melanin coloration on their breasts compared with the overall duller coloration in females. Although no studies have examined visual perception in eastern bluebirds, UV vision has been demonstrated in closely related species (Hart et al. 2000) and UV-blue coloration in passerine bird has been suggested to play an important role in signal communication (Hill 2006). A correlative field study of eastern bluebirds shows that male eastern bluebirds with brighter UV-blue plumage and darker melanin breast colour pair earlier in the season, feed chicks more often, and gain higher reproductive success (Siefferman & Hill 2003). Experimental manipulations of paternal investment demonstrate that UV-blue structural coloration is a condition-dependent trait in bluebirds (Siefferman & Hill 2005a). Although the field correlative study linking coloration earlier pairing and reproductive success suggests that females may use coloration to choose mates, experimental manipulations of structural coloration in an aviary show no consistent relationship between male UV-blue coloration and female association time (Liu et al. 2007).
Bluebirds are obligate cavity-nesting passerines and males that express more-ornamented UV-blue coloration are more successful competitors for limited nest sites (Siefferman & Hill 2005b). Thus, in the eastern bluebirds as in many species that defend key resources necessary for reproduction, female choice for male coloration is confounded by female choice for the superior resources defended by brighter males. Simple correlative studies cannot distinguish the relative influences of female choice of mate and male-male competition in driving the elaboration of plumage coloration.
To more directly test female preferences for ornamental plumage coloration in a wild population of breeding eastern bluebirds, we conducted a mate removal experiment. We removed the original mates from males to quantify the latency time for males to establish new pair bonds. We used this protocol to avoid any bias associated with prior pair bonds, because past relationships can confound mate choice in two ways. Pairs that have previous breeding experience at the same territory might experience advantages in acquiring nesting sites (Qvarnstrom & Forsgren 1998) and in reproductive success (Ligon 1999). We removed the mates of two males that held neighboring territories with the assumption that males with adjacent territories held territories of similar quality. This experiment was designed to measure female preference in the wild and to quantify the breeding success of the subsequent mate selection on a platform of equal quality territories.
First, by removing original mates early in the breeding season, we caused pairs to reconstruct the early breeding season processes of mate choice and pairing. Territories in which females had been removed represented available males. Second, we investigated the characteristics of the males and determined which traits predicted male attractiveness to females. We tested the hypothesis that female eastern bluebirds prefer to pair with males that display the more-ornamented plumage coloration. Additionally, by monitoring the behaviour of the newly formed pairs throughout the breeding season, we quantified reproductive success.
METHODS
General methods
Breeding cavities are likely the key resource that limits reproduction in eastern bluebirds (Gowaty & Plissner 1998). Non-breeding birds (floaters) are present in suitable habitats, are sexually mature, and display breeding condition (Liu, pers. obs.). When breeding opportunities became available (the original box owners died or disappeared from a territory), these floaters frequently assumed the territory and bred with widowed birds.
This study was conducted from March to August 2005 and 2006 in two field sites, located in Southeastern Alabama, USA, with a total of 200 nest boxes. The distance between nest boxes was approximately 100 m. The habitat consisted mainly of pastures and hay fields surrounded by fragmented pine forests.
In this long-term research population, the birds were captured and banded in the early breeding season (March1-15), before the experiment began. At each field site, we also monitored all other unmanipulated territories. To capture birds, we lured pairs into mist nets by playing recordings of eastern bluebird calls. Each bird was individually marked with unique combination of three coloured bands and one U.S. Fish and Wildlife Service aluminum band. We measured body mass (accuracy = 0.1 g), and the length of tarsi, wings, and tails (accuracy = 0.5 mm). We used principle component analysis (PCA) to reduce our morphological measurements (tarsus, wing, tail) into PC scores. Principal Component 1 explained 62.5% of the variation in morphological measurements. Body condition was calculated as the residuals of a regression of body mass on PC1 of body size (Jacob et al. 1996).
At the time of capture, we carefully plucked 8-10 rump (blue) and breast (chestnut) feathers. Feathers were placed in envelops and stored in a temperature and humidity controlled laboratory before analysis. We taped feathers onto black paper (Canson® Cat: #425 Stygian black) using an overlapping fashion to mimic how feathers naturally occur on the birds. We measured the plumage reflectance using an Ocean Optics S2000 spectrometer and deuterium tungsten halogen light source (range 250-880nm). We used a fiber optic cord equipped with a black rubber cap on the metal probe to exclude ambient light. The distance between the probe and feather was set to 5mm to create a measurement diameter of approximately 2mm. The probe was perpendicular to the feather surface. We took all colour measurements at the same setting and the same person (ML) processed all data. We generated reflectance data relative to a white standard (Labsphere, Inc.®) using OOIBase software (Ocean Optics®). We measured each feather sample five times and used the mean to generate colour scores for each male.
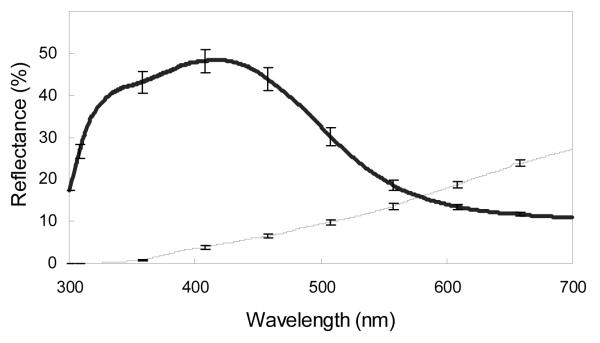
For chestnut coloration (Fig. 1), we summarized reflectance data by calculating two standard descriptors of reflectance spectra: brightness and chroma. Mean brightness was calculated as the mean summed reflectance (R300-700nm). Red chroma was calculated as the proportion of the total reflectance (R300-700nm) in the red part of the spectrum (R575-700nm). For UV-blue colour (Fig. 1), we calculated three standard descriptors of reflectance spectra: brightness, chroma, and hue. Mean brightness was calculated as the mean summed reflectance (R300-700nm). UV chroma was calculated as the proportion of the total reflectance (R300-700nm) in the UV part of the spectrum (R300-400nm). Blue chroma was calculated as the proportion of the total reflectance (R300-700nm) in the blue part of the spectrum (R400-512nm). Hue was calculated as the wavelength at peak reflectance. Calculations taken from the same spectral curves are correlated in eastern bluebirds such that the most ornamented UV-blue males display brighter coloration, greater UV chroma, and hues with wavelengths shifted towards the shorter wavelengths (Siefferman et al. 2005). Moreover, the most ornamented chestnut males display darker coloration (lower brightness) and greater red chroma.
Figure 1.
Mean (±1SE) reflectance spectra of the UV-blue of rumps (thick line) and the chestnut coloration of breasts (thin line) male eastern bluebirds (n=16).
Female removal
In both years, we conducted the experiment within 3 weeks of the first egg date in the population (2005: 1 March – 15 March; 2006: 15 March - 1 April). For each removal, we choose two adjacent territories (within 200m of each other) such that each territory had a resident pair and the two pairs were synchronized in their breeding cycles (the females began laying eggs on the same date (± 2 days)). Within 5 days of the date that the females of two adjacent pairs began laying eggs, we captured males and females of both pairs. On the morning of the capture, we released males on their original territory within 30 min of capturing them and placed each female in captivity. Upon capture, the eggs and nest materials were collected from the nest boxes. In each year, the manipulated dyads of territories were geographically scattered at two study sites and occurred nearby or interspersed with other eastern bluebird territories where breeding pairs remained unmanipulated. Each day, we removed no more than two dyads of females.
We monitored the behaviour of the experimentally widowed males twice daily and determined the date in which they attracted a new female to their territory. After each male had secured a new mate, we monitored the breeding activities of the newly-formed pair throughout the breeding season. To reduce disturbance, replacement females were captured during the late incubation or early nestling stages.
Behavioral observations
We began behavioural observations of focal males within 12 hrs of the time of female removal. Each day following the removal, we monitored the behaviour of each focal male in the morning (60 min) to determine whether the male had attracted a replacement mate. During morning observations, we recorded male song rate (defined as number of songs per min) for approximately 30 min. The open grassland habitat and propensity of bluebirds to perch in the open, near their nest boxes, allowed for detection of new females. We determined that a new female had arrived if the female was seen with the male for a minimum of 30 continuous mins and if the female was seen with the focal male for two consecutive days. We digitally photographed pairs to confirm the identity of the newly arrived female. After one of the males from each dyad had successfully attracted a new female, we reduced our observation frequency to once every 2-3 days and continued to monitor all the territories until the end of the breeding season. For all subsequent nests, we recorded the date of the first laid egg of each brood, the clutch size, the brood size and the fledging success.
After a female was removed from the original territory, widowed males sang and exhibited display behaviours on or near the nest boxes (perched on boxes, repeatedly looked into nest holes, waved wings, and carried nesting material). We recorded the song rates of males during morning observations in 2006. Because some males attracted females quickly, and because song rate and display behaviour were altered by the presence of the new females, we were able to record singing behaviour of only 10 males. Song rate did not correlate with plumage colour (all P > 0.1), so we eliminated song as a variable in subsequent analyses.
When newly arrived females were visible, we recorded behaviours indicative of mating interest including when females foraged in the males’ territory, perched on boxes, repeatedly looked into nest holes, followed (or was followed by) the male of the territory. Mate-guarding behaviours are un-ambiguous in this species (pairs are usually observed within 20 m of each other during courtship and egg laying) and we are confident that we could accurately determine when a male paired with a female (LS, personal observation). We used two measurements to describe the males’ success in attracting new females: 1) the latency period between female removal and attraction of new mate (days), (latency to attract, LA1), and 2) the time lapse between the date of the original female’s removal and the date that the replacement female commenced egg laying (latency to attract, LA2). We considered each dyad of widowed males to represent one trial. During the study, most of the males (N = 40) were only used once. However, 4 males were used in both 2005 and 2006 but all males were paired with new mates, occupied new territories, and were placed in dyads with unique males. In each trial, the male ‘won’ if he attracted a female to his territory before the other male in the dyad (‘winning’ males exhibited shorter LA1 than ‘losing’ males). In two cases, we missed the exact date that the winner attracted his female, but they were assigned by the first egg date of their mates.
Ethical note
This study was conducted with approval from IACUC (PRN: 2003-0448) at Auburn University. After capture, females were housed in outdoor flight cages of a permanent aviary at Auburn University (for details see Liu et al. 2007). Birds were given ad libitum water, and live mealworms and crickets. Our aviary setting effectively reduced the cage stress; we bred a pair of eastern bluebirds successfully in such an aviary cage in 2004. All birds were maintained in the aviary for approximately 4-6 weeks and then released to their original territories in the breeding season (mid-May) after the experiment. Because most bluebird pairs initiate second broods after mid-May, we expected that any females that could find a mate at this time had the opportunity to breed later in the season. We did observe that a released female bred successfully with a new male in June of 2006. Additionally, we recorded the return rate of the original (17.3%) versus replacement females (21.7%) in 2007. Captivity did not influence the likelihood that females would return to breed at the field site in the following year (Fisher’s exact test: P = 0.73).
Statistical analyses
We used SPSS 11.5 (SPSS Inc. 2003) for all analyses. We used paired t-tests to compare male characteristics within each dyad. Additionally, we combined all data and used backwards and forward regression models to examine whether male characteristics predicted female arrival time, time to breed, and total seasonal reproductive success (number of offspring fledged). All data conformed to normality (Shapiro-Wilks’ tests), and parametric tests were used. Blue brightness differed by year, thus we standardized these data for year (mean = 0, SD = 1). All significance levels were two-tailed. Samples sizes vary between some variables because we failed to collect feathers and morphological measurements of 3 males, and we did not collect plumage data from 10 females.
RESULTS
Paired comparisons
Over the course of two breeding seasons, we conducted 24 trials of female removals (females were removed from 48 territories). Forty-one widowed males attracted a new mate after mate removal and all of these newly formed pairs bred successfully later in the breeding season. Four males disappeared from their territories after their original mates were removed. One female escaped from the aviary and reunited with her original mate. Of 19 successful trials, two trials yielded “tie” results (both males attracted replacement mates on the same day), and were eliminated from the paired comparisons. The median days to attract a replacement female (LA1) was 3 days (range:0.5-58 days), and the median days from female removal until the replacement female laid her first egg (LA2) was 13 days (range: 7-63 days). LA1 and LA2 did not correlate (rs = 0.33, N = 31, P = 0.07).
Paired t-tests revealed that within dyads, there were no significant differences between the UV-blue coloration of the rumps of ‘winning’ and ‘losing’ males (Winners-losers; UV chroma: mean difference = −0.01 ± 0.03, t = 1.16, N = 15, P = 0.27; blue chroma: mean difference = −0.001 ± 0.02, t = 0.20, N = 15, P = 0.85; brightness (z): mean difference = 0.34 ± 1.34, t = 0.98, N = 15, P = 0.34; hue: mean difference = 3.94 ± 19.76, t = 0.77, N = 15, P = 0.45, Table 1). Nor did we find a significant difference in chestnut coloration of the breast of ‘winning’ and ‘losing’ males (red chroma: mean difference = −0.01, t = 1.04, N = 13, P = 0.32; brightness: mean difference = 0.38, t = 0.67, N = 13, P = 0.52; Table 1). Further, morphological measurements did not differ significantly between ‘winning’ and ‘losing’ males (body size: mean difference = 0.19 ± 1.23, t = 0.56, N = 13, P = 0.59; body condition: mean difference = 10.64 ± 135.6, t = 0.28, N = 13, P = 0.78, Table 1). Over the course of the breeding seasons, the ‘winning’ and ‘losing’ males fledged a similar number of chicks (mean difference = 1.08 ± 3.90, t = 0.96, N = 12, P = 0.36, Table 1).
Table 1.
Comparisons of characteristics of all males that gained a mate first (won) or second (lost), paired-t test.
| Trait | Outcome | Mean | SD | N | t | P |
|---|---|---|---|---|---|---|
| Tarsus (mm) | Won | 20.48 | 0.65 | 16 | −0.02 | 0.99 |
| Lost | 20.48 | 0.73 | 16 | |||
| Wing (mm) | Won | 101 | 2.27 | 16 | 0.25 | 0.81 |
| Lost | 100.78 | 2.89 | 16 | |||
| Tail (mm) | Won | 64.47 | 2.82 | 16 | 1.83 | 0.09 |
| Lost | 62.91 | 2.58 | 16 | |||
| Mass (g) | Won | 29.61 | 1.11 | 15 | 0.07 | 0.94 |
| Lost | 29.55 | 2.75 | 15 | |||
| Body Condition (z) | Won | 1.45 | 0.07 | 15 | 0.28 | 0.78 |
| Lost | 1.45 | 0.11 | 15 | |||
| Age (yr) | Won | 1.83 | 0.39 | 12 | 0.8 | 0.44 |
| Lost | 1.67 | 0.49 | 12 | |||
| Rump Brightness (z) | Won | 0.24 | 1.01 | 15 | 0.98 | 0.34 |
| Lost | −0.10 | 0.94 | 15 | |||
| Rump UV Chroma (z) | Won | 0.34 | 0.02 | 15 | −1.16 | 0.27 |
| Lost | 0.35 | 0.02 | 15 | |||
| Rump Blue Chroma (z) | Won | 0.40 | 0.01 | 15 | −0.2 | 0.85 |
| Lost | 0.40 | 0.01 | 15 | |||
| Rump Hue (z) | Won | 417.88 | 12.37 | 15 | 0.77 | 0.45 |
| Lost | 413.93 | 9.12 | 15 | |||
| Breast Brightness (z) | Won | 10.12 | 1.20 | 13 | 0.67 | 0.52 |
| Lost | 9.74 | 1.68 | 13 | |||
| Breast Red Chroma (z) | Won | 0.63 | 0.03 | 13 | −1.03 | 0.32 |
| Lost | 0.64 | 0.02 | 13 | |||
| LA 1 (days) | Won | 3.00 | 2.56 | 11 | −3.23* | 0.001 |
| Lost | 11.36 | 15.98 | 11 | |||
| LA 2 (days) | Won | 12.77 | 3.32 | 13 | −2.03* | 0.04 |
| Lost | 22.77 | 17.58 | 13 | |||
| Breeding success (young) | Won | 6.83 | 2.08 | 12 | 0.96 | 0.36 |
| Lost | 5.75 | 2.90 | 12 |
Because the manipulations that we conducted were technically difficult, our sample sizes were relatively small. Thus we used power analyses to determine whether our sample sizes were sufficient to find an effect if it existed. Previous studies that were laboratory based found that ≥80% of females preferred the males with the more-ornamented structurally-based plumage (Bennett et al. 1997, Hunt et al. 1999, Siitari et al. 2002). Given our sample sizes (N=17), we calculated that, if female bluebirds showed a similar preference to that of female bluetits and starlings, we had an 82% likelihood of finding an effect with our data set.
Male Comparisons
We treated each territory as an independent event and investigated the potential relationships between male characteristics, measures of mate attraction, and breeding success. We found no significant effects of year on either the time to attract a mate (t-test: LA1: t = 1.51, N = 15, 22, P > 0.1) or time for the new mate to commence egg laying (LA2: t = 1.20, N = 15, 24, P > 0.1).
All territories were manipulated in a relatively short period (three weeks), and we found no influence of removal date on the speed at which males attracted new mates (LA1, 2005: rs = 0.37, N = 15, P = 0.17; 2006: rs = 0.16, N = 22, P = 0.49), nor the speed at which the new female laid her first eggs (LA2, 2005: rs = − 0.10, N = 15, P = 0.72; 2006: rs = 0.1, N = 24, P = 0.65). Additionally, because males of similar quality were more likely to occupy neighboring territories, we calculated the average colour of males within dyads to test whether the average dyad coloration predicted average time to attract new females. We found no significant relationship in any comparison (Table 2.). Finally, we used a mixed-model analysis with dyad ID as a random factor, we found no significant relationship between male coloration and female arrival latency (UV-blue rump brightness: F1,33 = 1.85, P = 0.18; UV chroma: F1,33 = 0.29, P = 0.60; blue chroma: F1,33 = 0.19, P = 0.67; hue: F1,33 = 0.19, P = 0.67. Chestnut breast brightness: F1,24 = 2.91, P = 0.10; red chroma: F1,25 = 0.88, P = 0.36).
Table 2.
Correlations between the mean plumage coloration of males in each dyad and the mean latency to attracted a mate for each dyad (LA1).
| Trait | rs | N | P |
|---|---|---|---|
| Mean Rump Brightness (z) | 0.44 | 16 | 0.09 |
| Mean Rump UV Chroma (%) | −0.13 | 16 | 0.63 |
| Mean Rump Blue Chroma (%) | 0.27 | 16 | 0.31 |
| Mean Breast Brightness (%) | −0.34 | 15 | 0.22 |
| Mean Breast Red Chroma (%) | 0.24 | 15 | 0.39 |
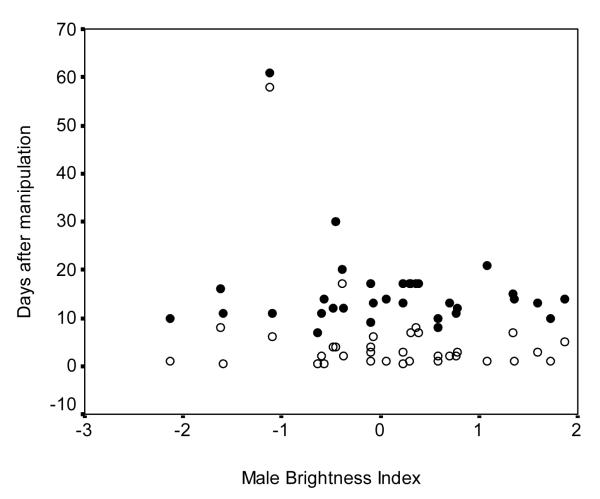
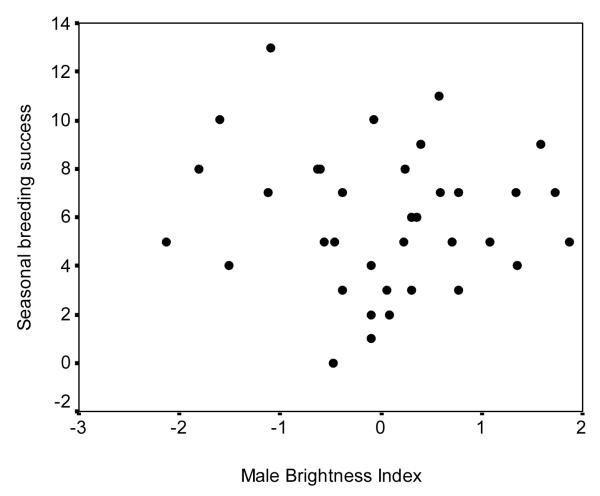
We used backwards multiple regression models to determine the influence of male colour measurements and morphology on 1) the latency to attract (LA1), 2) the latency between female removal and the day that the new female began laying eggs (LA2), and 3) the total offspring number of fledged. We found no significant influence of male coloration, body size, or body condition on latency to attract new females (Model included UV-blue brightness, chestnut brightness, and body size: R2 adj = 0.05, F3, 21 = 1.41, P = 0.27, Fig. 2), latency for new females to begin breeding (Model included chestnut red chroma, body condition, and body size: R2 adj = 0.04, F3, 24 = 1.41, P = 0.27, Fig. 2), or total number of offspring fledged in that season (Model included: UV-blue hue; chestnut brightness; chestnut red chroma: R2 adj = 0.07, F3, 24 = 1.69, P = 0.20, Fig. 3).
Figure 2.
Relationship between brightness of UV-blue rump coloration of the male eastern bluebirds and 1) time to acquire a new mate (open circles), and 2) time to begin breeding with new mate (filled circles). Colour data are standardized for year.
Figure 3.
Relationship between brightness of UV-blue rump coloration of the male and the seasonal reproductive success (total number of nestlings fledged per year) of eastern bluebirds. Colour data are standardized for year.
To further investigate these relationships, we used a series of forward multiple regression models to explore the potential influences of male characteristics on 1) the latency to attract (LA1) and 2) the latency between female removal and the day that the new female began laying eggs (LA2). The first model only included the six measurements of male plumage coloration as predictors variables and we found that coloration did not influence latency to attract new female (LA1, Full model: R2 adj = 0.12, F526 = 0.71, P = 0.62) or the timing to commence laying eggs (LA2, Full model: R2 adj = 0.12, F528 = 0.74, P = 0.6). In the next model, we only included male body size and body condition and, again, found no influence of male morphological characteristics on latency to attract a new female (LA1, Full model: R2 adj = 0.10, F225 = 1.42, P = 0.26), or timing to commence laying eggs (LA2, Full model: R2 adj = 0.10, F225 = 1.58, P = 0.22). Finally, a large model which included all measures of colour and morphology, found no influence on latency to attract a new female (LA1, full model: R2 adj = −0.05, F526 = 0.71, P = 0.62), or timing of new female to commence egg laying (LA2, full model: R2 adj = −0.04, F528 = 0.74, P = 0.60).
Finally, we also used discriminate function analyses (DFA) to identify any unique characteristics of ‘winning’ and ‘losing’ males. We combined all colour variables and morphological traits into these models. We found no significant difference between winning and losing group (Wilks’ lambda F7,27 = 5.19, P = 0.64). Based on previous studies of the same population (Siefferman & Hill 2005b), we selected three predictors (UV-blue brightness, UV chroma, and body size) for another DFA analysis. Again, we found that the overall model was not significant (Wilks’ lambda F3,28 = 3.54, P = 0.32) indicating that there was no significant differentiation in morphology or coloration of winning and losing males.
Assortative mating
Because pairs may mate assortatively for coloration, we tested whether the mated pairs’ plumage and body size characteristics were more similar than random using Pearson’s correlations. We found no significant relationships between the UV blue color (brightness r = 0.02, N = 35, P = 0.91; UV chroma r = − 0.03, N = 35, P = 0.85; blue chroma r = 0.02, N = 35, P = 0.93; hue r = 0.13, N = 35, P = 0.47), the chestnut color (brightness r = 0.02, N = 32, P = 0.93, red chroma r = 0.03, N = 32, P = 0.89,) or the body size (r = 0.06, N = 38, P = 0.72) of the original female and her mate. Nor did we find any significant relationships between the UV-blue (UV-blue rump: brightness r = − 0.13, N = 25, P = 0.55; UV chroma r = 0.09, N = 25, P = 0.67; blue chroma r = − 0.16, N = 25, P = 0.43; hue r = 0.01, N = 25 , P = 0.95) the chestnut color (brightness r = 0.29, N = 24, P = 0.17; red chroma r = 0.02, N = 24, P = 0.93) or body size (r = 0.06, N = 25, P = 0.78) of the replacement female and her mate.
DISCUSSION
Contrary to our hypothesis that female bluebirds prefer to pair with males that display the most-ornamented structural coloration, we found no evidence of mate choice based on plumage coloration. When we removed females and created experimentally widowed males, unmated females quickly settled in the opened territories. We found no evidence that females preferentially settled with the more-ornamented males. In comparisons of dyads of males that were carefully matched for territory quality and for timing of mate removal, the more-ornamented and larger males did not attract females more quickly compared to the less-ornamented and smaller males. Although we did not find evidence for a female preference, it is possible that a much larger sample size might have shown a trend.
The observation from our field manipulation that female eastern bluebirds do not use male coloration as a criterion in mate choice is consistent with aviary-based mate choice trials in which female eastern bluebirds chose males without regard to either manipulated UV-blue plumage coloration or natural variation in UV-blue plumage coloration (Liu et al. 2007). The lack of influence of male body size, body condition, or chestnut coloration on female mate-choice decisions is also consistent with results of the aviary-based trials (Liu et al. 2007, M. Liu, L. Siefferman, G. Hill, unpublished data).
Our experimental design differs from most approaches to mate choice in three important ways. First, unlike most field-based tests of mate choice, we matched pairs of widowed males on adjacent territories. This design was employed to control for territory quality as we assume that males, in the relatively homogenous grassland habitat of eastern bluebirds, with adjacent territories will have territories of similar quality. Second, unlike most field-based research of mate choice, we removed original females thus eliminating any influence of the pair’s prior breeding experience. Third, unlike most laboratory-based experiments of mate choice, our design is unique because females were free to interact with males, and to reproduce, and we quantified subsequent reproductive success.
Past research with bluebirds demonstrates that males that display more exaggerated blue and chestnut coloration pair earlier and feed offspring more often (Siefferman & Hill 2003), suggesting that female choice of more- elaborately ornamented males would be adaptive. Further, Siefferman & Hill (2005b) found that UV-blue structural coloration is a good indicator of the ability of males to secure limited nest boxes, suggesting that males with more elaborate UV-blue coloration have advantages in competitive interactions for breeding sites. In this population, however, older male birds also tend to display brighter UV-blue coloration and duller chestnut coloration compared to younger males (Siefferman et al. 2005). The positive correlations between plumage coloration and reproductive success in eastern bluebirds could be a consequence of older pairs experiencing greater reproductive success because of increased mate familiarity and reproductive experience (Black 1996). Indeed, in this population, pairs mate assortatively by age and older females tend to breed earlier and experience greater seasonal reproductive success (Siefferman & Hill 2005c).
The newly-formed pairings that resulted from our experiment might have disentangled the past interrelationships between length of pair bond, age of birds, male plumage coloration, and reproductive success (Siefferman & Hill 2003). Perhaps females prefer to nest with males with which they have had prior nesting or social experience and those males tend to express greater UV-blue coloration because they tend to be older. The lack of effect of colour on timing of attraction of replacement mates suggests that females are not actively seeking more colorful males.
Several studies have found that pair duration and previous breeding experience are important determinants of reproductive success in socially monogamy species (Ligon 1999) and past pair bonds are likely important in this population of bluebirds. The majority of the males that were experimentally widowed successfully attracted females. In addition to experimentally manipulated pairs, we banded and monitored the nesting attempts of all bluebirds that bred on our study site (80 pairs) and found that no females left their original mates to pair with experimentally widowed males. Moreover, all replacement females arrived on our field site without bands, suggesting that those females came from the floating pool of birds.
Our data are consistent with research suggesting that the cost of leaving a mate is generally greater than the benefits gained from pairing with a higher-quality mate (Ligon 1999; but see Otter & Ratcliffe 1996). In general, females that left their mates experience the costs of delayed breeding (Black et al. 1996). Because species that nest exclusively in secondary cavities have historically experienced very limited nest sites, females that chose to leave their original mates face the risk of forsaking all breeding opportunities in that breeding season (Gowaty 1981). In our population, most pairs breed from March to August and are able to produce two or three successful broods. Pairs that initiate egg laying earlier in the season experience greater seasonal reproductive success (Siefferman & Hill 2005c). Indeed, in swans, mate familiarity has been shown to reduce the costs of courtship and facilitate earlier breeding (Rees et al. 1996).
Our experimental design helped us disentangle the confounding effects of coloration, prior pairing history, and reproductive success that may naturally occur in eastern bluebirds. We found no evidence that female eastern bluebirds seek out more colourful males as mates. We also found no evidence that pairs mate assortatively for coloration or body size, again this is consistent with field correlational evidence (Siefferman & Hill 2005c). It is possible, however, that female bluebirds may not all display similar mate choice preferences. Several researchers have demonstrated that female mating preference might vary depending on environment, prior experience, and body condition (reviewed in Qvarnstrom 2001). It is also possible that coloration functions in male-male interactions and signals male condition. Further, it is possible that females use male plumage coloration to choose extra-pair mates, as has been found in the sister species, mountain bluebirds (Sialia currucoides; Balenger et al. 2009). Future research should investigate individual variation in mate preferences and the signaling function of male coloration and genetic quality in eastern bluebirds (Mays & Hill 2004; Mays et al. 2008).
Acknowledgments
We are grateful to B. Rolek, and M. Rios for excellent help with data collection, and R. Montgomerie for the use of his spectral processing program (ColouR v1.7). We thank S. Balenger, C. Guyer, W. Hood, K. Huggins, R. Ligon, J. Randall, B. Rolek, J. Steffen, M. Wooten, D. Osorio, N. Hart and two anonymous reviewers for improvements to the manuscript. The National Science Foundation grants to GEH (IBN 0235778, and DEB 0077804), National Institute of Health/ National Science Foundation program in the Ecology of Infectious Diseases R01-AI49724 to GEH funded this research. This research was conducted according to an animal use permit from Auburn University, banding permits to GEH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson M. Sexual Selection. Princeton University Press; Princeton, NJ: 1994. [Google Scholar]
- Balenger SL, Johnson LS, Masters BS. Sexual selection in a socially monogamous bird: male color predicts paternity success in the mountain bluebirds, Sialia currucoides. Behavioral Ecology & Sociobiology. 2009;63:403–411. [Google Scholar]
- Bennett ATD, Cuthill IC, Partridge JC, Lunau K. Ultraviolet plumage colors predict mate preferences in starlings. Proceedings of the National Academy of Science USA. 1997;94:8618–8621. doi: 10.1073/pnas.94.16.8618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JM. Partnerships in birds: the study of monogamy. Oxford University Press; Oxford: 1996. [Google Scholar]
- Black MJ, Choudhury S, Owen M. Do barnacle geese benefit from lifelong monogamy? In: Black JM, editor. Partnerships in birds. Oxford University Press; Oxford: 1996. pp. 91–117. [Google Scholar]
- Gowaty PA. An extension of the Orians-Willson model to account for mating systems besides polygyny. American Naturalist. 1981;118:851–859. [Google Scholar]
- Gowaty PA, Plissner JH. Eastern Bluebird. In: Poole A, Gill F, editors. Birds of North America. The Birds of North America Inc.; Philadelphia, PA: 1998. [Google Scholar]
- Hamilton WD, Zuk M. Heritable true fitness and bright birds: a role for parasites? Science. 1982;218:384–386. doi: 10.1126/science.7123238. [DOI] [PubMed] [Google Scholar]
- Hart NS, Partridge JC, Cuthill IC, Bennett ATD. Visual pigments, oil droplets, ocular media and cone receptor distribution in two species of passerine bird: the blue tit (Parus caeruleus L.) and the blackbird (Turdus merula L.) Journal of Comparative Physiology A. 2000;186:375–387. doi: 10.1007/s003590050437. [DOI] [PubMed] [Google Scholar]
- Hill GE. A red bird in a brown bag: the function and evolution of ornamental plumage coloration in the House Finch. Oxford University Press; New York, NY: 2002. [Google Scholar]
- Hill GE. Female mate choice for ornamental coloration. In: Hill GE, McGraw KJ, editors. Bird Coloration Vol II, Function and evolution. Harvard University Press; Cambridge, MA: 2006. pp. 137–200. [Google Scholar]
- Hunt S, Cuthill IC, Bennett ATD, Griffiths R. Preferences for ultraviolet partners in the blue tit. Animal Behaviour. 1999;58:809–815. doi: 10.1006/anbe.1999.1214. [DOI] [PubMed] [Google Scholar]
- Jakob EM, Marshall SD, Uetz GW. Estimating fitness: a comparison of body condition indices. Oikos. 1996;77:61–67. [Google Scholar]
- Kodric-Brown A, Brown JH. Truth in advertising: the kinds of traits favored by sexual selection. American Naturalist. 1984;124:309–323. [Google Scholar]
- Ligon JD. The evolution of avian breeding systems. Oxford University Press; Oxford: 1999. [Google Scholar]
- Liu M, Siefferman L, Hill EG. An experimental test of female choice relative to male structural coloration in eastern bluebirds. Behavioral Ecology and Sociobiology. 2007;61:623–630. doi: 10.1007/s00265-007-0416-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays HL, Albrecht T, Liu M, Hill GE. Female choice for genetic complementarity in birds: a review. Genetica. 2008;134:147–158. doi: 10.1007/s10709-007-9219-5. [DOI] [PubMed] [Google Scholar]
- Mays HL, Hill GE. Choosing mates: good genes versus genes that are a good fit. Trends in Ecology & Evolution. 2004;19:554–559. doi: 10.1016/j.tree.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Otter K, Ratcliffe L. Female initiated divorce in a monogamous songbird: abandoning mates for males of higher quality. Proceedings of the Royal Society of London B. 1996;263:351–354. [Google Scholar]
- Qvarnstrom A. Context-dependent genetic benefits from mate choice. Trends in Ecology & Evolution. 2001;16:5–7. doi: 10.1016/s0169-5347(00)02030-9. [DOI] [PubMed] [Google Scholar]
- Qvarnstrom A, Forsgren S. Should females prefer dominant males? Trends in Ecology & Evolution. 1998;13:498–501. doi: 10.1016/s0169-5347(98)01513-4. [DOI] [PubMed] [Google Scholar]
- Rees CE, Lievesley P, Pettifor AR, Perrins C. Mate fidelity in Swans: an interspecific comparison. In: Jeffrey BM, editor. Partnerships in birds. Oxford University Press; Oxford: 1996. pp. 118–137. [Google Scholar]
- Siefferman L, Hill GE. Structural and melanin coloration indicate parental effort and reproductive success in male eastern bluebirds. Behavioral Ecology. 2003;14:855–861. [Google Scholar]
- Siefferman L, Hill GE. Male eastern bluebirds trade future ornamentation for current reproductive investment. Biology Letters. 2005a;1:208–211. doi: 10.1098/rsbl.2004.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siefferman L, Hill GE. UV-blue structural coloration and competition for nestboxes in male eastern bluebirds. Animal Behaviour. 2005b;69:67–72. [Google Scholar]
- Siefferman L, Hill GE. Evidence for sexual selection on structural plumage coloration in female eastern bluebirds. Evolution. 2005c;59:1819–1828. [PubMed] [Google Scholar]
- Siefferman L, Hill GE, Dobson FS. Ornamental plumage coloration and condition are dependent on age in eastern bluebirds Sialia sialis. Journal of Avian Biology. 2005;36:428–435. [Google Scholar]
- Siitari H, Honkavaara J, Huhta E, Viitala J. Ultraviolet reflection and female mate choice in the pied flycatcher, Ficedula hypoleuca. Animal Behaviour. 2002;63:97–102. [Google Scholar]
- SPSS_Inc. SPSS for Windows. 11.5 edn. SPSS Inc.; Chicago, IL: 2003. Rel. [Google Scholar]
- Wagner WE. Measuring female mating preferences. Animal Behaviour. 1998;55:1029–1042. doi: 10.1006/anbe.1997.0635. [DOI] [PubMed] [Google Scholar]
- Zahavi A. Mate selection - a selection for a handicap. Journal of Theoretical Biology. 1975;53:205–214. doi: 10.1016/0022-5193(75)90111-3. [DOI] [PubMed] [Google Scholar]