Abstract
Background
The US Food and Drug Administration mandated that enriched grain products be fortified with folic acid by 1998. We evaluated whether intake of folic acid from supplements and diet was associated with a reduction in spina bifida in the setting of folic acid fortification.
Methods
Data were collected as part of the Slone Birth Defects Study from 1998 to 2008. Mothers of infants with and without birth defects were interviewed within 6 months of delivery about pregnancy exposures, including details of diet and vitamin intake. Dietary natural folate and synthetic folic acid from fortification were combined into a single, weighted measure—dietary folate equivalent. Periconceptional folic acid supplementation and dietary folate consumption were compared between 205 mothers of spina bifida cases and 6357 mothers of nonmalformed controls. Relative risks of a spina bifida-affected birth were estimated with odds ratios (ORs) and 95% confidence intervals (CIs).
Results
Spina bifida was not associated with regular folic acid supplementation (≥4 days per week) either around the time of conception (adjusted OR = 1.1 [95% CI = 0.74 –1.7]) or initiated in early pregnancy (0.79 [0.54–1.2]). After adjustment for confounders, a 13% reduced odds of spina bifida was estimated for each 100-µg increase in daily dietary folate equivalent consumed.
Conclusions
In the setting of folic acid fortification of grains, our data suggest that folic acid supplementation does not appear to offer further benefit for reducing risk of spina bifida. Rather, the folate-associated benefit on spina bifida risk was found with increasing amounts of dietary folic acid consumed, regardless of folic acid supplementation level.
Since 1998, when the US Food and Drug Administration mandated that enriched grain products be fortified with folic acid, the estimated number of pregnancies affected by neural tube defects (NTDs) has declined by approximately 27%.1,2 However, a greater decline was predicted,3 raising the question of whether at least some of the remaining cases can be prevented through increased periconceptional supplementation or dietary folic acid intake.
Mosely and colleagues4 recently investigated this issue, using data collected from the National Birth Defects Prevention Study. The authors found that neither periconceptional folic acid supplementation nor dietary folic acid intake was associated with a reduction in the risk of NTDs, including spina bifida. The objective of our study was to examine risks of spina bifida in relation to maternal folic acid supplementation and dietary intake in another large case-control study, focusing on the time period since folic acid fortification began.
METHODS
The Slone Birth Defects Study was initiated by the Slone Epidemiology Center in 1976. Infants with birth defects were identified by the study staff from discharge records of participating hospitals serving the areas surrounding Boston, MA; Philadelphia, PA; San Diego, CA; and Toronto, Canada; in addition, cases have been identified through birth-defect registries in Massachusetts and parts of New York State. Nonmalformed controls have been randomly selected each month from study hospitals’ discharge lists or from statewide birth records. Malformed live-born infants, therapeutic abortions after 12 weeks’ gestation, and fetal deaths after 20 weeks’ gestation were eligible as cases for our study; however, ascertainment of non–live-born cases has not been routine. Only live-born nonmalformed infants were eligible as controls for our study. The Birth Defects Study has been approved by the institutional review boards of Boston University and relevant participating hospitals and centers.
Mothers of eligible cases and controls were telephoned within 6 months of delivery by a research nurse to conduct the computer-assisted interview. After obtaining informed consent, interviews were conducted in either English or Spanish and lasted for approximately 45–60 minutes.
The interview included questions on the following topics: pregnancy intention, medical and obstetric history, illness and medication history during the period 2 months before the last menstrual period (LMP) through the end of the pregnancy, weight and diet before pregnancy, and behavioral risk factors (such as alcohol consumption and smoking) during pregnancy. In addition, the interview obtained demographic information and family history of birth defects. In the medication portion of the interview, mothers were asked specifically about the use of prenatal vitamins, multivitamins, and folic acid supplements before and during the pregnancy.
Using the Slone Drug Dictionary, we reviewed all reports of vitamins for folic acid content. As neural tube closure occurs 3–4 weeks postconception, we focused on regular use of folic acid during an exposure window defined as the 2 lunar months before (LM −2, LM −1) through the 2 lunar months after (LM 1, LM 2) the last menstrual period. During this period, we considered those women as “nonusers” who reported no folic acid supplementation, use <1 day per month, or use only during LM −2. Women who reported folic acid use ≥4 days per week during at least 2 of the 3 periconceptional months (LM −2, LM −1, LM 1) were considered “consistent users.” Women who reported use ≥4 days per week beginning in LM 1 or LM 2 were considered “early-pregnancy initiators.” Women with all other use patterns were considered “inconsistent users.”
Average diet during the 6 months before pregnancy was assessed by a modified, semi-quantitative 58-item Willett Food Frequency Questionnaire,5 which included questions on types of breakfast cereal. Micrograms (µg) of dietary synthetic folic acid and natural folate for individual food items were obtained from the Harvard University Food Composition Database 2003 and 2007 versions, which were partly derived from the US Department of Agriculture database release versions 13 and 18.6,7 Average daily dietary synthetic folic acid and natural folate were summed separately for each subject based on her reported frequency of eating each food. As synthetic folic acid has a higher bioavailability than natural folate, a weighted dietary folate equivalent was calculated for each subject by summing her daily natural folate intake with 1.7 times her daily dietary synthetic folic acid intake.
Because blood folate levels were already increasing in the US population by mid-1997,8,9 study subjects were confined to women whose pregnancies were conceived after June 1997. Cases included 205 fetuses or infants with spina bifida aperta and no known chromosomal anomaly. Controls were 6357 infants without any known major malformations. Of the spina bifida cases, 168 were isolated and 37 had additional etiologically distinct or possibly antecedent birth-defect diagnoses, including 5 cloacal exstrophy, 2 amniotic band, and 1 body wall complex case(s).
Statistical Analysis
Maternal demographic and behavioral factors were tabulated for spina bifida cases and controls separately. Logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for spina bifida at each level of folic acid supplementation. When 5 or more cases were exposed to a given level of supplementation, multivariable regression was used to adjust for race (white non-Hispanic, black non-Hispanic, Hispanic, other), body mass index (BMI [kg/m2]; <18.5, 18.5–<24.9, 25–<30, 30+, missing), pregnancy intention (“wanted pregnancy” then vs. “wanted pregnancy later,” “didn’t want pregnancy,” or “didn’t know”), and study center. Other factors, such as maternal age, education, family income, smoking, and alcohol consumption did not substantially further change OR estimates and, therefore, were not included in multivariable models. All women were included in supplementation level models, even if dietary information was not available.
ORs for folic acid supplementation and spina bifida were also estimated for subgroups of women according to race/ethnicity, BMI (<30 vs. ≥30 kg/m2), and dietary folate equivalent intake (<400 vs. ≥400 µg per day).
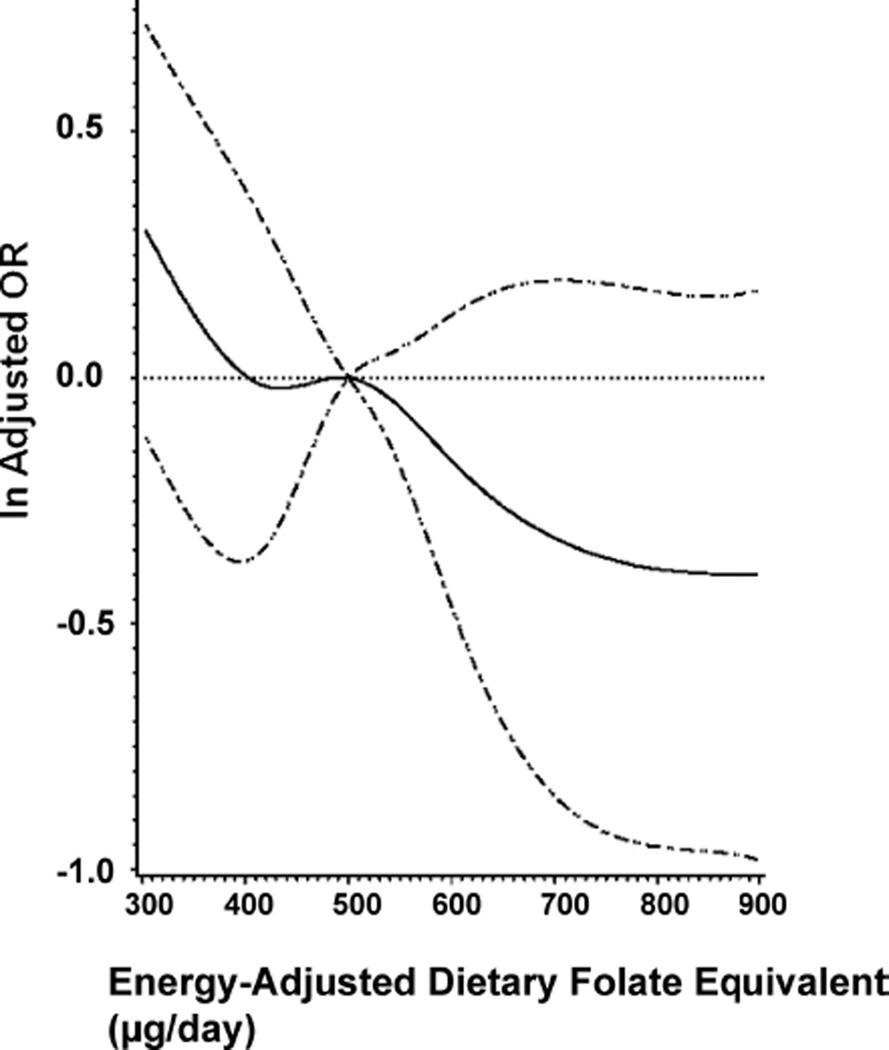
Dietary folate measures were adjusted for daily caloric intake using the residual method.10 All dietary folate models were restricted to women who completed the food frequency questionnaire and reported plausible prepregnancy caloric intake (between 500 and 4000 calories/day). Logistic regression was used to model the effect of calorie-adjusted dietary folate equivalent, dietary synthetic folic acid, and dietary natural folate on spina bifida, by supplementation level and adjusted for maternal race, BMI, pregnancy intention, and study center. To accomplish this, we first examined the possible nonlinear relation between dietary folate measures and the odds of spina bifida with restricted cubic spline analysis (Fig.).4,11,12 Tests for nonlinearity used the likelihood ratio test, comparing an adjusted logistic regression model with only linear terms to one with both linear and cubic spline terms. These tests, performed on all women and within each supplementation level, failed to reject the null hypothesis that an association, if any, was linear for both dietary folate equivalent and natural folate. Therefore, dietary folate equivalent and natural folate were modeled as continuous variables with ORs and confidence intervals calculated per 100-µg increase. Dietary synthetic folic acid likelihood ratio test P values were 0.03 and 0.04, respectively, for all women and nonusers, and were above 0.29 for consistent users and early-pregnancy initiators. For ease of comparison, dietary synthetic folic acid was also modeled as a continuous variable using logistic regression, and estimates were calculated per 100-µg increase.
FIGURE.
Adjusted spline (solid line) of daily intake of dietary folate equivalent on risk of spina bifida for all women with diet history (n = 6099). Also shown is 95% CI (dashed line). Reference value is 500 µg/day dietary folate equivalent (50th percentile value in controls).
During the study period, approximately 6904 mothers of fetuses/infants with major malformations, which include spina bifida, were approached by study staff and 4566 (66%) agreed to be interviewed. At the same time, 12,008 mothers of control infants were approached and 6357 (53%) agreed to be interviewed and were reviewed. Dietary information was available for 87% of 205 spina bifida cases and 93% of the 6357 controls.
RESULTS
Participants
Demographic and behavioral characteristics of mothers of spina bifida cases and controls are presented in Table 1. Case mothers were more often black and Hispanic, had higher prepregnancy BMIs, and were less likely to have wanted the pregnancy at the time. One-fifth of spina bifida pregnancies were electively terminated.
TABLE 1.
Maternal Demographic and Behavioral Characteristics of Spina Bifida Cases and Controls, Slone Birth Defects Study, 1998–2008
| Spina Bifida Cases | Controls | |
|---|---|---|
| (n = 205) | (n = 6357) | |
| Maternal race/ethnicity; % | ||
| White, non–Hispanic | 62 | 71 |
| Black, non–Hispanic | 11 | 7 |
| Hispanic | 19 | 14 |
| Other races | 8 | 7 |
| Missing data | 0 | 0 |
| Maternal age (years) at conception | ||
| Median | 28 | 30 |
| Percent distribution | ||
| <20 | 5 | 7 |
| 20–25 | 22 | 17 |
| 26–35 | 62 | 61 |
| ≥36 | 12 | 14 |
| Missing data | 0 | 0 |
| Maternal education (years); % | ||
| 2–11 | 16 | 9 |
| 12 | 21 | 18 |
| 13–15 | 25 | 24 |
| 16 | 19 | 25 |
| >16 | 20 | 24 |
| Missing data | 0 | 0 |
| Household income; % | ||
| <$10,000 | 9 | 5 |
| $10,000–$44,000 | 27 | 23 |
| ≥$45,000 | 52 | 64 |
| Missing data | 12 | 8 |
| Maternal smoking, LM 1a;% | ||
| Yes, smoked | 18 | 16 |
| No | 82 | 84 |
| Missing data | 0 | 0 |
| Maternal alcohol drinking, LM 1a;% | ||
| Yes, drank alcohol ≥once per month | 34 | 45 |
| No | 66 | 55 |
| Missing data | 0 | 0 |
| Maternal body mass index (kg/m2) | ||
| Median | 23.9 | 22.7 |
| Percent distribution | ||
| <18.5 | 3 | 6 |
| 18.5–<24.9 | 52 | 62 |
| 25–<29.9 | 22 | 19 |
| ≥30 | 18 | 11 |
| Missing data | 5 | 2 |
| Intended pregnancy; % | ||
| Yes, wanted to be pregnant then | 60 | 63 |
| No, all others | 40 | 37 |
| First or second degree family history of NTDs; % | ||
| Yes | 2 | 1 |
| No | 94 | 96 |
| Missing data | 4 | 3 |
| Singleton or multiple pregnancy; % | ||
| Singleton pregnancy | 97 | 97 |
| Multiples | 3 | 3 |
| Live–born; % | ||
| Therapeutic abortion | 20 | 0 |
| Neonatal death | 3 | 0 |
| Stillborn | 0 | 0 |
| Live–born, no death | 77 | 100 |
| Gestational age (weeks) | ||
| Median | 37 | 39 |
| Percent distribution | ||
| 12–20 | 13 | 0 |
| 21–25 | 6 | 0 |
| 26–30 | 2 | 0 |
| 31–35 | 8 | 3 |
| 36–37 | 25 | 10 |
| 38–42 | 45 | 86 |
| Missing data | 1 | 0 |
| Study center; % | ||
| Boston | 16 | 57 |
| Philadelphia | 33 | 17 |
| Toronto | 29 | 10 |
| San Diego | 13 | 12 |
| New York State | 13 | 12 |
LM 1 indicates first lunar month after last menstrual period.
Supplement Use
No periconceptional folic acid supplementation was reported by 29% of mothers of spina bifida cases and 23% of controls (Table 2). However, the proportion of mothers who reported consistent periconceptional use was the same for cases and controls (41%). Initiation in either LM 1 or LM 2 was less prevalent in case (29%) than control (36%) mothers. For consistent users and early-pregnancy initiators, the adjusted ORs for spina bifida were 1.1 (95% CI = 0.74 –1.7) and 0.79 (0.54 –1.2), respectively. In subanalyses confined to white women and nonobese women, spina bifida risk was also not associated with consistent supplementation during the periconceptional period (Table 3). Among Hispanic women, consistent use was associated with a possible increase in risk (adjusted OR = 2.2 [CI = 0.98–4.9]), but over 25 countries of origin were represented with no clear majority, hindering further interpretation. In women with a BMI of 30 kg/m2 or more, consistent users appeared at lower risk of spina bifida (0.52 [0.20 –1.3]) but a null effect could also not be ruled out. Early-pregnancy initiators’ OR estimates, by maternal race and BMI, did not differ from those for women overall. Stratification by dietary folic acid intake did not substantially change OR estimates for supplementation level.
TABLE 2.
Associations of Maternal Folic Acid Supplementation Level and Spina Bifida, Slone Birth Defects Study, 1998–2008
| Supplementation Level |
Cases (n = 205) No. (%) |
Controls (n = 6357) No. (%) |
Crude OR (95% CI) |
Adjusteda OR (95% CI) |
|---|---|---|---|---|
| Nonusersb | 59 (29) | 1438 (23) | 1.00 | 1.00 |
| Consistent users | 83 (41) | 2573 (40) | 0.79 (0.56–1.11) | 1.11 (0.74–1.65) |
| Early pregnancy initiators | 60 (29) | 2293 (36) | 0.64 (0.44–0.92) | 0.79 (0.54–1.16) |
| Inconsistent users | 3 (1) | 53 (1) | 1.38 (0.42–4.54) | 2.20 (0.64–7.62) |
Nonusers indicates no or very low use during LM −2 to LM 2; consistent users, use ≥4 days per week for ≥8 weeks during LM −2 to LM 1; early pregnancy initiators, use ≥4 days per week beginning in LM 1 or LM 2; inconsistent users, less frequent use.
The adjusted model includes the covariates maternal race (white non-Hispanic [referent], black non-Hispanic, Hispanic, other), body mass index (<18.5, 18.5–<25, 25–<30 [referent], 30+, missing), pregnancy intent (wanted vs. everything else), and study center (Boston [referent], Philadelphia, Toronto, San Diego, New York State).
Reference category.
TABLE 3.
Associations of Maternal Folic Acid Supplementation Level and Spina Bifida Within Selected Subgroups, Slone Birth Defects Study, 1998–2008
| Subgroup | Cases (n = 205) No. (%) |
Controls (n = 6357) No. (%) |
Crudea OR (95% CI) |
Adjustedb OR (95% CI) |
|---|---|---|---|---|
| White, non–Hispanic | (n = 128) | (n = 4535) | ||
| Supplementation level | ||||
| Nonusersc | 25 (20) | 684 (15) | 1.00 | 1.00 |
| Consistent users | 63 (49) | 2213 (49) | 0.78 (0.49–1.25) | 0.93 (0.56–1.54) |
| Early pregnancy initiators | 37 (29) | 1603 (35) | 0.63 (0.38–1.06) | 0.68 (0.40–1.16) |
| Black, non–Hispanic | (n = 22) | (n = 459) | ||
| Supplementation level | ||||
| Nonusersc | 11 (50) | 211 (46) | 1.00 | 1.00 |
| Consistent users | 4 (18) | 69 (15) | 1.11 (0.34–3.61) | NC |
| Early pregnancy initiators | 7 (32) | 175 (38) | 0.77 (0.29–2.02) | 0.86 (0.32–2.30) |
| Hispanic | (n = 39) | (n = 892) | ||
| Supplementation level | ||||
| Nonusersc | 18 (46) | 404 (45) | 1.00 | 1.00 |
| Consistent users | 12 (31) | 149 (17) | 1.81 (0.85–3.84) | 2.20 (0.98–4.92) |
| Early pregnancy initiators | 9 (23) | 330 (37) | 0.61 (0.27–1.38) | 0.74 (0.32–1.70) |
| BMI ≥30 kg/m2 | (n = 36) | (n = 690) | ||
| Supplementation level | ||||
| Nonusersc | 13 (36) | 187 (27) | 1.00 | 1.00 |
| Consistent users | 10 (28) | 240 (35) | 0.60 (0.26–1.39) | 0.52 (0.20–1.34) |
| Early pregnancy initiators | 13 (36) | 258 (38) | 0.73 (0.32–1.59) | 0.90 (0.38–2.09) |
| BMI <30 kg/m2 | (n = 158) | (n = 5535) | ||
| Supplementation level | ||||
| Nonusersc | 41 (26) | 1182 (21) | 1.00 | 1.00 |
| Consistent users | 71 (45) | 2312 (42) | 0.89 (0.60–1.31) | 1.24 (0.79–1.95) |
| Early pregnancy initiators | 43 (27) | 1994 (36) | 0.62 (0.40–0.96) | 0.74 (0.47–1.16) |
| <400 µg/day DFEd | (n = 52) | (n = 1291) | ||
| Supplementation level | ||||
| Nonusersc | 19 (37) | 381 (30) | 1.00 | 1.00 |
| Consistent users | 14 (27) | 392 (30) | 0.72 (0.35–1.45) | 1.03 (0.47–2.30) |
| Early pregnancy initiators | 19 (37) | 511 (40) | 0.75 (0.39–1.43) | 0.93 (0.47–1.85) |
| ≥400 µg/day DFEd | (n = 126) | (n = 4630) | ||
| Supplementation level | ||||
| Nonusersc | 31 (25) | 881 (19) | 1.00 | 1.00 |
| Consistent users | 57 (45) | 2065 (45) | 0.78 (0.50–1.20) | 0.96 (0.57–1.60) |
| Early pregnancy initiators | 35 (28) | 1642 (36) | 0.61 (0.37–0.99) | 0.68 (0.41–1.14) |
Crude models contained terms for consistent users, early pregnancy initiators, and inconsistent users; results for inconsistent users are not shown because too few women were observed in this group. If cases or controls had zero inconsistent users, then analysis excluded this group.
The adjusted model includes the covariates maternal race (white non-Hispanic [referent], black non-Hispanic, Hispanic, other), body mass index (<18.5, 18.5–<25, 25–<30 [referent], 30+, missing), pregnancy intent (wanted vs. everything else), and study center (Boston [referent], Philadelphia, Toronto, San Diego, New York State). Models stratified by BMI do not include BMI categories as terms in the model.
Reference category.
Excludes women with outlier calories (<500, >4000) and missing diet information.
NC indicates not calculated due to too few (<5) exposed cases or controls to perform adjusted analysis.
When analyses were restricted to isolated spina bifida cases, subjects without a family history of NTDs, cases without fetal or neonatal death, or subjects from the study center that assessed 87% of the terminated cases, ORs did not differ appreciably from those overall. Too few women reported family history of NTDs to perform an analysis restricted to this group.
Dietary Folate
Among control mothers, the respective 25th, 50th, and 75th percentile values for noncalorie-adjusted natural folate were 180, 245, and 321 µg; dietary synthetic folic acid were 87, 138, and 207 µg; and dietary folate equivalent were 374, 493, and 655 µg, respectively.
Overall, for each 100-µg increase in calorie-adjusted dietary folate equivalent, the adjusted odds of spina bifida decreased by 13% (5%–21%) (Table 4). Corresponding adjusted ORs were 0.85 among nonusers (0.70 –1.03) and also among early-pregnancy initiators (0.71–1.01). For consistent users, the adjusted OR was 0.90 (0.78 –1.0). Similar patterns of adjusted ORs were observed for 100-µg increases in dietary synthetic folic acid and natural folate.
TABLE 4.
Association Between Spina Bifida and Indices of Dietary Folic Acid Intake, Overall and Stratified by Folic Acid Supplementation Level, Slone Birth Defects Study, 1998–2008
| Supplementation Level | Index of Folic Acid Intake (Per 100 µg)a |
Cases No. |
Controls No. |
Crude OR (95% CI) |
Adjustedb OR (95% CI) |
|---|---|---|---|---|---|
| Overall | 178 | 5921 | |||
| Dietary folate equivalent | 0.85 (0.78–0.93) | 0.87 (0.79–0.95) | |||
| Synthetic folic acid | 0.78 (0.66–0.93) | 0.82 (0.69–1.00) | |||
| Natural folate | 0.79 (0.67–0.94) | 0.81 (0.68–0.96) | |||
| Nonusers | 50 | 1262 | |||
| Dietary folate equivalent | 0.84 (0.71–0.99) | 0.85 (0.70–1.03) | |||
| Synthetic folic acid | 0.76 (0.52–1.10) | 0.85 (0.57–1.26) | |||
| Natural folate | 0.80 (0.60–1.06) | 0.78 (0.58–1.06) | |||
| Consistent users | 71 | 2457 | |||
| Dietary folate equivalent | 0.87 (0.76–1.00) | 0.90 (0.78–1.04) | |||
| Synthetic folic acid | 0.80 (0.62–1.04) | 0.85 (0.65–1.11) | |||
| Natural folate | 0.86 (0.64–1.16) | 0.89 (0.66–1.21) | |||
| Early pregnancy initiators | 54 | 2153 | |||
| Dietary folate equivalent | 0.85 (0.72–0.99) | 0.85 (0.71–1.01) | |||
| Synthetic folic acid | 0.79 (0.57–1.09) | 0.82 (0.58–1.16) | |||
| Natural folate | 0.77 (0.57–1.05) | 0.75 (0.54–1.04) | |||
| Inconsistent users | 3 | 49 | |||
| Dietary folate equivalent | 0.71 (0.28–1.80) | NC | |||
| Synthetic folic acid | 0.85 (0.21–3.50) | NC | |||
| Natural folate | 0.23 (0.03–2.06) | NC |
Excludes women with outlier calories (<500, >4000) and missing diet information.
The adjusted model includes the covariates maternal race (white non-Hispanic [referent], black non-Hispanic, Hispanic, other), body mass index (<18.5, 18.5–<25, 25–<30 [referent], 30+, missing), pregnancy intent (wanted vs. everything else), and study center (Boston [referent], Philadelphia, Toronto, San Diego, New York State).
DISCUSSION
In the setting of folic acid fortification, we found that folic acid supplementation does not appear to offer further benefit for reducing spina bifida risk. In particular, women who reported taking folic acid supplements at least 4 days per week during the months before neural tube closure did not have a decreased risk of spina bifida compared with women who reported no supplementation. The lack of a protective relationship was observed for white and nonobese women, and for women with high and low dietary intakes of folic acid. We observed a possible increased risk among consistent users of Hispanic ethnicity and a suggestion of decreased risk in women with high BMI, but these findings may be due to chance. Using data from the National Birth Defects Prevention Study, Mosley and colleagues also did not identify a benefit of supplement use.4 However, we did observe a 10%–15% decrease in spina bifida risk associated with each 100 µg of dietary folate equivalent consumed, whereas Mosely and colleagues found no benefit from corresponding dietary intakes.
Both our study and the study by Mosley and colleagues4 are case-control studies that relied on maternal report for exposure and covariate measurement. Awareness of the protective effect of folic acid supplementation on NTDs (observed before dietary fortification) has increased among reproductive-age women.13 Women with a spina bifida-affected pregnancy are especially likely to be aware of this protective effect, even if they were unaware prior to their pregnancy, which could influence their reporting accuracy. The impact that inaccurate recall might have on OR estimation in these studies is difficult to predict, but the possibility cannot be ruled out that a true protective effect is being masked—especially if mothers of spina bifida cases are systematically falsely reporting periconceptional folic acid supplementation. Our finding of no protective effect of supplement use even among women with low dietary folate intake could provide indirect evidence for this type of recall bias.
Dietary intake of folic acid may be less vulnerable to recall bias because knowledge of which foods are high in folic acid may not be well-disseminated. Our observed reduction in spina bifida risk associated with increasing dietary folic acid intake is expected and supports the benefit of the folic acid fortification mandate. The distribution of synthetic folic acid and natural folate intake among control mothers was higher in the Birth Defects Study than in the National Birth Defects Prevention Study, but both studies found dietary folic acid intake similar to national data estimates.14,15
Our study’s OR estimates were virtually unchanged after excluding women who reported pregestational diabetes, folic acid antagonist medication use, multifetal gestations, or a family history of NTDs; thus, these women were retained in the final sample. The 205 spina bifida cases in our study represent a relatively small sample, which was further reduced when analyses were stratified by maternal characteristics. In light of these small numbers, our findings could be due to chance.
Birth Defects Study participation rates are slightly higher among mothers of malformed cases than controls, which could introduce bias if study participation were also associated with folic acid intake differentially for cases and controls; however, we have no evidence to support this association. Dietary information was available for slightly more controls than spina bifida cases, yet a subanalysis of supplement use on risk of spina bifida among women with dietary information yielded similar results to women overall, indicating that there was little selection bias with regard to available dietary information. Terminated spina bifida cases, resulting from prenatal diagnosis, are likely to be under-ascertained in our study and could result in a selection bias. Such cases were available primarily from one study center; however, findings did not change substantially when we restricted analysis to that study center.
The possibility of error introduced through folic acid intake misclassification might suggest that cohort studies with prospective measurement of exposure would be preferable, but such studies are difficult to conduct due to the rarity of spina bifida. In addition, case-control studies conducted before fortification that found a protective effect of folic acid supplementation on the risk of NTDs, used exposure assessment similar to that in our current study. What does differ between the early studies showing a benefit of supplementation and recent null studies is fortification of the food supply and wider knowledge of purported benefit of folic acid supplement.
In the era of dietary folic acid fortification, our study findings raise the possibility that supplementation with folic acid during the months immediately preceding neural tube closure does not offer further benefit in reducing the risk of a spina-bifida-affected pregnancy. This lack of benefit was observed even among women consuming low amounts of dietary folic acid. However, further study of the association between supplementation and spina bifida among subgroups of women at high risk of an NTD-affected pregnancy is needed. As expected, dietary folic acid intake does appear to decrease the risk of spina bifida, regardless of whether folic acid supplements were taken at the time of conception.
Acknowledgments
Supported by a grant from the Centers for Disease Control and Prevention, Center on Birth Defects and Developmental Disabilities.
We thank Dawn Jacobs, Fiona Rice, Rita Krolak, Kathleen Sheehan, Clare Coughlin, Moira Quinn, Nancy Rodriguez, Carolina Tejedor Meyers, and Nastia Dynkin, for their assistance in data collection and computer programming. We also thank all the mothers who participated in the study.
REFERENCES
- 1.Department of Health and Human Services, Food and Drug Administration. Food standards: amendment of standards of identity for enriched grain products to require addition of folic acid. Fed Regist. 1996;61:8781–8807. [Google Scholar]
- 2.Mersereau P, Kilker K, Carter H, et al. Spina bifida and anencephaly before and after folic acid mandate—United States, 1995–1996 and 1999–2000. Morb Mortal Wkly Rep. 2004;53:362–365. [PubMed] [Google Scholar]
- 3.Centers for Disease Control. Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. Morb Mortal Wkly Rep. 1992;42(RR-14):1–7. [PubMed] [Google Scholar]
- 4.Mosley B, Cleves M, Siega-Riz A, et al. Neural tube defects and maternal folate intake among pregnancies conceived after folic acid fortification in the United States. Am J Epidemiol. 2009;169:9. doi: 10.1093/aje/kwn331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nurses’ Health Study. Questionnaire. 1998 Available at: http://www.channing.harvard.edu/nhs/questionnaires/pdfs/NHSI/1998.PDF.
- 6.US Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 13. Nutrient Data Laboratory; 2000. Available at: http://www.nal.usda.gov/fnic/foodcomp/Data/SR13/sr13_doc.pdf. [Google Scholar]
- 7.US Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 18. Nutrient Data Laboratory; 2005. Available at http://www.nal.usda.gov/fnic/foodcomp/Data/SR13/sr13.html. [Google Scholar]
- 8.Jacques P, Selhub J, Bostom A, Wilson P, Rosenberg I. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N Engl J Med. 1999;340:1449. doi: 10.1056/NEJM199905133401901. [DOI] [PubMed] [Google Scholar]
- 9.Lawrence J, Bpetitti D, Watkins M, Umekubo M. Trends in serum folate after food fortification. Lancet. 1999;354:915–916. doi: 10.1016/s0140-6736(99)03227-4. [DOI] [PubMed] [Google Scholar]
- 10.Willett W, Stampfer M. Monographs in Epidemiology and Biostatistics. New York: Oxford University Press; 1998. Implications of total energy intake for epidemiologic analysis; pp. 273–301. [Google Scholar]
- 11.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 12.Li R, Hertzmark E, Louie M, Chen L, Spiegelman D. The SAS lgtphcurv8 Macro Version 9. 2004 Available at: http://www.hsph.harvard.edu/faculty/spiegelman/lgtphcurv8/lgtphcurv8.pdf.
- 13.Gallup Organization and March of Dimes Foundation. Improving preconception health: women’s knowledge and use of folic acid. White Plains, NY: March of Dimes; 2008. [Google Scholar]
- 14.Dietrich M, Brown C, Block G. The effect of folate fortification of cereal-grain products on blood folate status, dietary folate intake, and dietary folate sources among adult non-supplement users in the United States. J Am Coll Nutr. 2005;24:266. doi: 10.1080/07315724.2005.10719474. [DOI] [PubMed] [Google Scholar]
- 15.Tinker S, Cogswell M, Devine O, Berry R. Folic acid intake among US women aged 15–44 years, National Health and Nutrition Examination Survey, 2003–2006. Am J Prev Med. 2010;38:534–542. doi: 10.1016/j.amepre.2010.01.025. [DOI] [PubMed] [Google Scholar]