ABSTRACT
Ciliary axonemes and basal bodies were present in the last eukaryotic common ancestor and play crucial roles in sensing and responding to environmental cues. Peptidergic signaling, generally considered a metazoan innovation, is essential for organismal development and homeostasis. Peptidylglycine α-amidating monooxygenase (PAM) is crucial for the last step of bioactive peptide biosynthesis. However, identification of a complete PAM-like gene in green algal genomes suggests ancient evolutionary roots for bioactive peptide signaling. We demonstrate that the Chlamydomonas reinhardtii PAM gene encodes an active peptide-amidating enzyme (CrPAM) that shares key structural and functional features with the mammalian enzyme, indicating that components of the peptide biosynthetic pathway predate multicellularity. In addition to its secretory pathway localization, CrPAM localizes to cilia and tightly associates with the axonemal superstructure, revealing a new axonemal enzyme activity. This localization pattern is conserved in mammals, with PAM present in both motile and immotile sensory cilia. The conserved ciliary localization of PAM adds to the known signaling capabilities of the eukaryotic cilium and provides a potential mechanistic link between peptidergic signaling and endocrine abnormalities commonly observed in ciliopathies.
KEY WORDS: Neuropeptide, Chlamydomonas, Amidation, Monooxygenase, Cuproenzyme, Axoneme
Highlighted Article: Bioactive peptide amidation is catalyzed by peptidylglycine α-amidating monooxygenase (PAM). Functional PAM is present in Chlamydomonas and cilia, organelles that play key roles in signaling.
INTRODUCTION
A key feature of eukaryotic cells is extensive compartmentalization; an example that is conserved in many eukaryotic cells is the cilium, a microtubule-based extension that projects from the cell surface (Singla and Reiter, 2006; Fliegauf et al., 2007). Cilia and the basal bodies that give rise to their axonemal structure are ubiquitously distributed across major eukaryotic groups, with the exception of flowering plants, most fungi and amoebae (Johnson and Leroux, 2010; Carvalho-Santos et al., 2011). Although the membrane surrounding the microtubule-based axonemal core is continuous with the plasma membrane, the ciliary lipidome and proteome are distinct (Nachury et al., 2010; Hsiao et al., 2012). Among the proteins targeted to the cilium are signaling molecules and receptors involved in the response to environmental signals. Components of the Hedgehog and Wnt pathways, along with several G-protein-coupled receptors, are selectively trafficked into the cilium by the motor-protein-driven intraflagellar transport (IFT) system (Huangfu et al., 2003; Berbari et al., 2008; Wallingford and Mitchell, 2011; Mukhopadhyay and Rohatgi, 2014). The importance of cilia-based signaling is demonstrated by the wide array of cilia-related disorders that occur in vertebrates (Waters and Beales, 2011; Brown and Witman, 2014). Only motile cilia can generate motive force, but both motile and primary cilia perform sensory and signaling roles (Shah et al., 2009; Bloodgood, 2010; Warner et al., 2014).
Accumulating evidence suggests that there is a link between cilia and peptidergic signaling, an ancient metazoan pathway. Neuropeptides regulate ciliary beating and influence larval swimming behavior in marine annelids (Conzelmann et al., 2011) and melanin-concentrating hormone regulates ciliary beat frequency in the ependymal cells that move cerebrospinal fluid in the ventricles (Conductier et al., 2013). In addition, the G-protein-coupled receptors for neuropeptide Y, somatostatin and kisspeptin localize to neuronal cilia (Loktev and Jackson, 2013). Interestingly, neuropeptide precursors have been identified in ciliated metazoans lacking a nervous system, suggesting that regulation of ciliary motility was an ancestral function of peptide-based signaling (Jekely, 2011, 2013).
In eukaryotes, neuropeptides are synthesized from precursors that have a signal sequence, confining subsequent processing steps to the secretory pathway lumen. Secretory pathway enzymes convert inactive precursors into smaller bioactive peptides. Many bioactive metazoan peptides have an amidated C-terminus (Prigge et al., 2000). This modification requires a bifunctional enzyme, peptidylglycine α-amidating monooxygenase (PAM) (Fig. 1A). The peptidylglycine α-hydroxylating monooxygenase (PHM) domain hydroxylates the α-carbon of peptides with a C-terminal glycine; peptidyl-α-hydroxyglycine α-amidating lyase (PAL) subsequently cleaves the C–N bond, producing glyoxylate and an α-amidated product (Fig. 1A). Amidation, often essential for bioactivity, can reduce peptide turnover and increase receptor affinity. PAM is essential in metazoans; mice and flies lacking PAM die at mid-gestation or mid-larval stages, respectively (Kolhekar et al., 1997b; Czyzyk et al., 2005). PAM missense alleles have been associated with metabolic disorders (Huyghe et al., 2013; Steinthorsdottir et al., 2014).
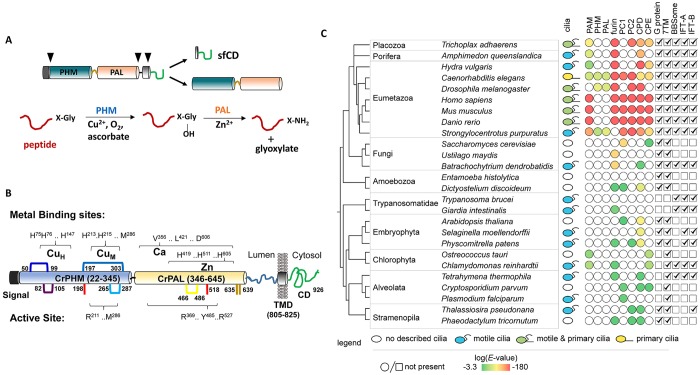
Fig. 1.
CrPAM protein. (A) Schematic of mammalian PAM2 protein and the amidation reaction. Sequential processing of glycine-extended peptide by PHM and PAL and co-factor requirements are shown. Black arrowheads point to sites cleaved in the endoplasmic reticulum, secretory granules and endocytic pathway, removing the signal sequence and separating the enzymatic domains from the transmembrane domain or generating a soluble cytoplasmic domain fragment (sfCD), respectively. (B) Domains of CrPAM and predicted key catalytic residues are highlighted; predicted disulfide linkages are shown and cysteine residues unique to CrPAM are highlighted in red or brown. (C) Identification of protein homologs and co-occurrence with described cilia. Type of cilia (or absence thereof) for each organism is based on a literature search (Carvalho-Santos et al., 2011; van Dam et al., 2013). Proteins with similarity to human PAM, furin, PC1 (also known as PCSK1), PC2 (also known as PCSK2), CPD and CPE were determined using NCBI Blastp (default parameters; Table S2 lists Uniprot IDs). The presence of a bifunctional ortholog of human PAM (PHM and PAL domains in a single protein) is indicated in the PAM column. If the Blastp hit is a monofunctional protein, it is indicated in the columns labeled PHM or PAL, as appropriate. An E-value of 1×10−3 was used as a cutoff; only the top non-redundant protein sequence was reported (a hit is represented as a filled circle; the color represents the E-value between the hit and the human query sequence). The presence of heterotrimeric G proteins (α, β and γ subunits) and seven-pass transmembrane domain receptors based on a literature search is indicated with a check mark. BBSome, IFT-A and IFT-B components were taken from van Dam et al., 2013; organisms with even one component received a check mark.
The identification of PAM-like genes in ciliated green algal genomes and in Amphimedon, the simplest animal lacking a nervous system, suggests that it was present in the last eukaryotic common ancestor (Attenborough et al., 2012). Like PAM, amidated peptides have not been found in yeast or higher plants (Jekely, 2013). Thus, there is a striking correlation between organisms with cilia and organisms expressing PAM. Although its enzymatic functions in the secretory pathway lumen do not require a transmembrane or cytosolic domain, these features are conserved in algal PAM. The steady-state localization of vertebrate PAM in the trans-Golgi network, secretory granules and endosomes reflects secretion of soluble PAM and endocytic trafficking of membrane PAM (Milgram et al., 1997). Examination of Pam+/− mice and neuroendocrine cells engineered for inducible Pam expression have revealed its signaling role. Following exocytosis, active membrane PAM appears on the cell surface; after endocytosis, membrane PAM can be returned to granules or degraded. In the endocytic pathway, γ-secretase-mediated intramembrane cleavage can release a soluble fragment of the PAM cytosolic domain, which then translocates into the nucleus, leading to altered gene expression (Ciccotosto et al., 1999; Francone et al., 2010). With its requirement for copper (the ions Cu+ and Cu2+) and oxygen, PAM might long have played a role in coordinating events in the luminal compartment, cytosol and surrounding environment.
The intriguingly similar co-occurrence of PAM and cilia led us to investigate its properties in organisms where amidated peptides have not yet been described. We used Chlamydomonas reinhardtii, one of the premier organisms for studying ciliary (flagellar) function, to explore the significance of the correlation between PAM expression and the presence of cilia.
RESULTS
Identification of PAM gene in Chlamydomonas
The PAM-like sequence previously predicted for Chlamydomonas lacked key residues in the PAL domain (Attenborough et al., 2012). Our analysis of sequenced Chlamydomonas expressed sequence tags (ESTs) and the subsequent release of an improved gene model revealed an additional exon, resulting in a domain organization for Cre03.g152850 (hereafter referred to as CrPAM) that is very similar to the mammalian PAM2 isoform (Fig. 1B). We confirmed this by sequencing CrPAM cDNA (Genbank KT033716). Two signal peptide prediction tools, SignalP (Petersen et al., 2011) and PredAlgo (Tardif et al., 2012), identified a 21-residue signal peptide, in agreement with the localization of PAM in the secretory pathway. We found no evidence of CrPAM alternative splicing in EST libraries and RNA-Seq data. Like mammalian PAM, CrPAM is predicted to contain a transmembrane domain followed by a cytosolic domain (Fig. 1B). Alignment of the CrPAM amino acid sequence with several metazoan PAM sequences revealed that all residues essential for the catalytic activities of PHM and PAL were conserved, with two copper-binding sites in PHM and sites for Zn2+ and Ca2+ in PAL (Fig. 1B; Figs S1 and S2). Four potential N-linked glycosylation sites were identified, two in CrPHM and two between CrPAL and the transmembrane domain. Single Cys residues in the CrPHM and CrPAL domains lack homologs in mammalian PAM and could remain free or participate in disulfide bond formation (red and brown in Fig. 1B).
Attenborough et al. (2012) attributed their identification of organisms with genes encoding bifunctional PAM, monofunctional PHM and monofunctional PAL (as in D. melanogaster and C. elegans) to an early gene duplication. We revisited the phylogenetic distribution and found a striking correlation between the presence of cilia and bifunctional PAM or monofunctional PHM and PAL (Fig. 1C). Our analysis included organisms analyzed previously for the presence of cilia and key ciliary trafficking components, IFT-A, IFT-B and BBSomes (Carvalho-Santos et al., 2011; van Dam et al., 2013). These evolutionarily conserved coat-like proteins play crucial roles in ciliogenesis and localization of signaling proteins required for ciliary function. Although not every ciliated organism in this analysis contains PAM, organisms that have PAM generally have cilia. Organisms lacking cilia such as red algae, most fungi, amoebae and land plants lack PAM. An exception was noted for the green alga Ostreococcus tauri that is purported not to have cilia, but has retained a PAM gene. Interestingly, several of the ciliated organisms lacking PAM have also lost one or more components of the IFT and Bardet–Biedl syndrome (BBS) subcomplexes (Fig. 1C).
We surveyed these same organisms for other peptide-processing pathway components, such as enzymes operating upstream of PAM (e.g. prohormone convertases and carboxypeptidases) and downstream targets of bioactive peptides (e.g. seven-pass transmembrane receptors and heterotrimeric G-proteins). Overall, genomes of organisms encoding a PAM-like protein also encode other putative components of the peptide biosynthetic pathway (Fig. 1C; Table S2). Whereas Chlamydomonas lacks heterotrimeric G proteins, other organisms expressing PAM encode both heterotrimeric G proteins and seven-pass transmembrane domain receptors. Collectively, this analysis strengthens the connection between the presence of cilia and peptide amidation.
Characterization of PAM activity in Chlamydomonas lysates
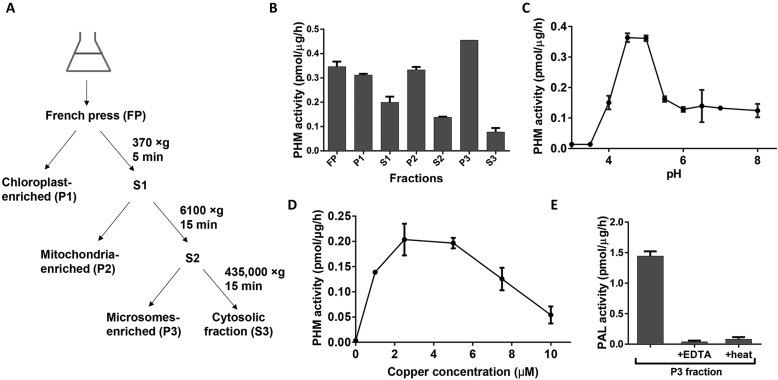
Our sequence analyses predicted an active PAM enzyme that localized to the secretory pathway in Chlamydomonas. To test this, we subjected Chlamydomonas homogenates to differential centrifugation (Fig. 2A). Fractions were tested for PHM activity using an in vitro assay that measures conversion of a synthetic tripeptide substrate (Acetyl-Tyr-Val-Gly) into amidated product (Acetyl-Tyr-Val-NH2). PHM activity was measurable in all fractions, with the highest specific activity in the particulate fraction containing small membrane fragments derived from multiple subcellular organelles (Fig. 2B) (Klein et al., 1983; El Meskini et al., 2000).
Fig. 2.
Chlamydomonas cell lysates contain active PAM. (A) Schematic of subcellular fractionation protocol for Chlamydomonas. (B) PHM activity was detected in all fractions. (C) Microsomal PHM activity plotted as a function of pH. (D) Microsomal PHM activity was greatest at 2.5–5 µM copper. (E) The microsomal fraction displayed PAL activity that was inhibited by a divalent metal ion chelator (EDTA) or heat (5 min at 100°C). Data shown are mean±s.d. (n=2 or 3). Graphs are from one of two experiments, which yielded similar results.
Mammalian PAM is optimally active at low pH, consistent with its presence in the secretory pathway lumen and mature secretory granules (luminal pH ∼5), but remains active at neutral pH (Husten and Eipper, 1994). We tested the effect of pH on PHM activity in Chlamydomonas lysates and found optimum activity at pH 4.5–5.0, with significant activity at pH 7 and above (Fig. 2C). CrPHM activity was also dependent on copper, with little activity observed in the absence of exogenous copper. As for the mammalian enzyme, higher concentrations of copper were inhibitory, presumably due to oxidation of the enzyme (Fig. 2D) (Eipper et al., 1983). PAL activity, which is dependent on Zn2+, was detected in the same microsomal fraction and was greatly reduced by addition of a divalent metal ion chelator; PAL activity was also eliminated by heating at 100°C for 5 min (Fig. 2E).
Expression of CrPAM in mammalian cells
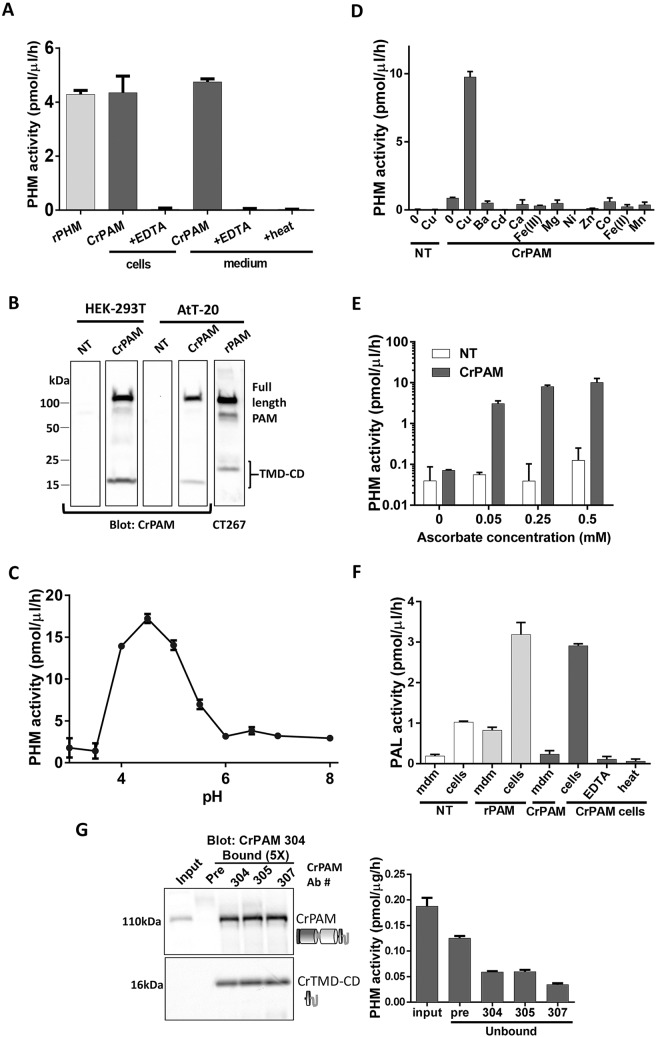
To assess the properties of CrPAM, we transiently expressed the full-length cDNA in HEK-293T cells, which produce very little endogenous PHM activity. PHM activity was then readily detected in cell lysates and spent medium (Fig. 3A); the latter observation indicates that luminal domain cleavage of CrPAM occurred, generating soluble enzyme that was secreted, as observed for mammalian PAM. To assess this possibility, we generated antibodies to the C-terminus of CrPAM, which will detect intact CrPAM and any cell-associated fragments generated by the proteolytic cleavages that must occur before active enzyme can be secreted. A cross-reactive band appeared in lysates from transfected HEK-293T and AtT-20 cells, a neuroendocrine cell line that stores the products of proopiomelanocortin cleavage in secretory granules (Fig. 3B); its apparent molecular mass, ∼117 kDa, suggested that some of the potential N-glycosylation sites were utilized (predicted mass for CrPAM lacking its signal peptide, 97.2 kDa). In addition, a 16-kDa fragment was detected in lysates prepared from both cell types; cleavage between CrPAL and the transmembrane domain (as occurs in mammalian PAM) could generate a C-terminal fragment of this mass (CrTMD-CD), along with soluble bifunctional enzyme, which would be secreted (Rajagopal et al., 2010). The products produced by AtT-20 cells expressing rat PAM1 (rPAM1) and detected with antibodies to its C-terminus are shown for comparison.
Fig. 3.
CrPAM encodes active PAM. (A) PHM activity was detected in spent medium and cell extracts of transiently transfected HEK-293T cells expressing CrPAM cDNA. EDTA or heat (100°C, 5 min) reduced activity in cell extracts and spent medium. (B) Immunoblot analysis of HEK-293T and AtT-20 cell lysates using an antibody against the CrPAM C-terminal domain (#304). Bands at 117-kDa and 16-kDa were detected only in transfected cell lysates. Lysate from AtT-20 cells expressing rPAM1 immunoblotted with an anti-C-terminal antibody (CT267) is shown for reference. (C) pH dependence of PHM activity in spent medium from HEK-293T cells transiently expressing CrPAM. NT, not transfected. (D) Spent medium from non-transfected (NT) or CrPAM-transfected HEK-293-T cells showed little or no PHM activity when a copper chelator (2 µM diethyldithiocarbamate) was added. The indicated metal salts (10 µM) were added to the reaction mixture in addition to the chelator. (E) CrPHM activity was dependent on inclusion of ascorbate (note the log scale); other single electron donors also support rPHM activity (Prigge et al., 2000). (F) PAL activity was detected in lysates of HEK-293T cells transiently expressing rPAM or CrPAM. PAL activity in lysates of HEK-293T cells expressing CrPAM was reduced by EDTA (5 mM) or heat. (G) Immunoprecipitation of CrPAM from Chlamydomonas cell lysates using affinity-purified C-terminal antibodies (Ab #304, #305 and #307) or pre-immune sera (Pre, as a control) showed enrichment of a 117-kDa band in the bound fractions, which were obtained from five-times more material than the input. A 16-kDa fragment (CrTMD-CD) was also seen in the bound fractions, indicating some cleavage of the full-length protein and separation of the enzymatic domains (which will not be bound by this antibody) from the cytosolic domain; the mid-region of the blot (with antibody heavy chain) is not shown. Immunoprecipitation reduced the amount of PHM activity in the unbound fractions. Data shown are mean±s.d. (n=2 or 3). Graphs are from one of two experiments, which yielded similar results.
The pH optimum of PHM secreted by HEK-293T cells expressing CrPAM was similar to that observed in the microsomal fraction of Chlamydomonas lysates (Fig. 3C). A copper chelator was added to the reaction mixture containing spent medium from CrPAM-transfected and non-transfected cells and individual divalent metals were added in excess. CrPHM activity increased significantly only when copper was added to CrPAM spent medium along with the chelator; no other metal increased activity above baseline (marked ‘0’) (Fig. 3D). Similar to mammalian PHM, CrPAM secreted by HEK-293T cells required a single electron donor (ascorbate) for activity (Fig. 3E). PAL activity was also detected in these lysates and was eliminated by heat or EDTA. In contrast to PHM, PAL activity was not detected in HEK-293T cell medium; two potential endoprotease cleavage sites within the predicted CrPAL catalytic core (Fig. S2) might account for secretion of active PHM without secretion of active PAL (Fig. 3F).
To characterize endogenous CrPAM, we tested the ability of affinity-purified C-terminal CrPAM antibodies to immunoprecipitate cross-reactive protein and PHM activity from Chlamydomonas lysates. PHM activity and a 117-kDa protein were detected in lysates prepared using non-ionic detergent (Fig. 3G). To determine whether the 117-kDa band represented CrPAM, lysates were incubated with affinity-purified antibody to the C-terminus of CrPAM. Unbound fractions were assayed for PHM activity and bound fractions were fractionated by SDS-PAGE and visualized using one of the affinity-purified antibodies. PHM activity was partially removed from the unbound fraction; whereas our C-terminal anti-CrPAM antibody would be expected to bind intact CrPAM, any soluble proteins produced by its cleavage would not be recognized. A 117-kDa band was enriched in the bound fraction by all three affinity-purified antibodies (#304, #305 and #307) but not by pre-immune serum (Fig. 3G). In addition, a 16-kDa band, the mass expected for CrTMD-CD, was enriched by all three affinity-purified antibodies. These data are consistent with the conclusion that CrPAM, like the mammalian enzyme, was subject to endoproteolytic cleavage at a juxtamembrane luminal site.
Localization of CrPAM in mammalian neuroendocrine cells
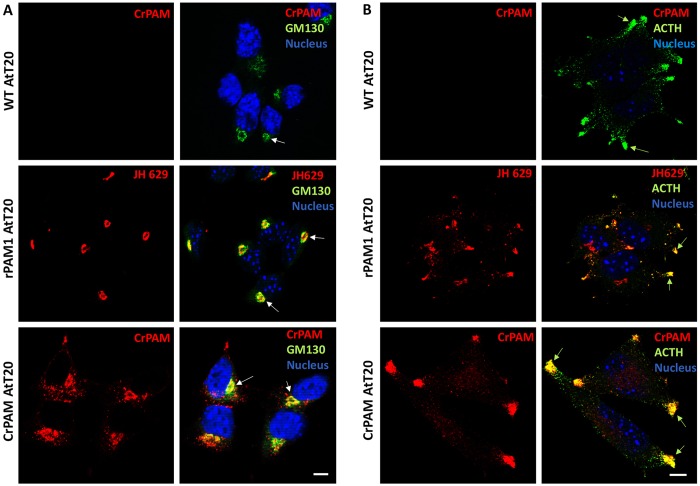
In specialized secretory cells, peptide hormones and their processing enzymes enter the regulated secretory pathway and are stored in secretory granules. Given that this pathway is well characterized in AtT-20 corticotrope tumor cells, we generated AtT-20 lines stably expressing CrPAM. In order to reduce signal from CrPAM diffusely distributed in the endoplasmic reticulum, wild-type AtT-20 cells and AtT-20 cells expressing CrPAM or rPAM1 were treated with cycloheximide for 1 h before fixation (Sobota et al., 2006). Localization of CrPAM to the region enriched in GM-130 (also known as GOLGA2), a cis-Golgi marker, was readily apparent (Fig. 4A; white arrows). As observed for PAM1, whose perinuclear localization reflects its presence in the TGN, immature secretory granules and endosomes (Milgram et al., 1997), staining for CrPAM was not coincident with GM-130. Secretory granules were visualized using an antibody specific for the C-terminus of the endogenous hormone ACTH, a product of the POMC precursor (Fig. 4B); granules were prevalent at the tips of cellular processes (green arrows). Using affinity-purified CrPAM and rPAM C-terminal antibodies, both proteins could be seen to colocalize with ACTH. Despite the evolutionary distance between rPAM and CrPAM, both proteins were similarly localized to multiple subcellular compartments in mouse corticotrope tumor cells.
Fig. 4.
Expression of CrPAM in mammalian neuroendocrine cells. Wild-type AtT-20 cells (WT) and stable lines expressing rPAM1 or CrPAM were stained for GM130 (A), a Golgi marker, or ACTH (B), a granule marker, using a FITC-tagged secondary antibody; cells were pretreated with 10 µM cycloheximide for 1 h (Sobota et al., 2006). WT and CrPAM-expressing cells were stained using an affinity-purified anti-CrPAM C-terminal antibody; no background was observed in WT AtT-20 cells (upper panel, A and B). rPAM1 AtT-20 cells were stained with antibody to the linker region of mammalian PAM (JH629). PAM staining was visualized using a Cy3-tagged secondary antibody. (A) Optical sections through the Golgi area showed perinuclear staining in rPAM1- and CrPAM-expressing cells that partially colocalized with GM130 (white arrows). (B) In optical sections near the bottom of each cell, rPAM1 and CrPAM were identified at the tips of cellular processes, where ACTH-containing granules accumulated (green arrows). Scale bars: 10 µm. Nuclei were visualized with Hoechst 33342 stain.
Expression and localization of endogenous CrPAM
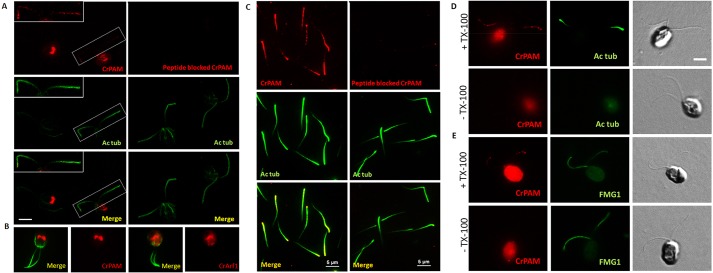
The localization of endogenous CrPAM was established using immunofluorescence microscopy; CrPAM localized to discrete vesicular structures in the cell body (Fig. 5A, left). To confirm staining specificity, we preincubated the antibody with the antigenic peptide; the fluorescence intensity was greatly reduced (Fig. 5A, right). Given that CrPAM expressed in mammalian cells displayed strong localization to the perinuclear area occupied by the Golgi complex, we hypothesized that the vesicular staining pattern obtained for CrPAM represented the Golgi complex. Indeed, the localization of CrPAM in Chlamydomonas strongly resembled that of Arf1, a Golgi-resident protein (Fig. 5B) (Dentler, 2013; Komsic-Buchmann et al., 2014). Chlamydomonas contains distinct Golgi stacks that are polarized and located close to the nucleus (Zhang and Robinson, 1986; Hummel et al., 2007). Treatment of cells with Brefeldin A, an inhibitor of ER-to-Golgi transport, disrupted the strong vesicular staining observed with the anti-CrPAM antibody (not shown), further confirming its Golgi localization. Unexpectedly, we also detected CrPAM staining in cilia, where it overlapped with staining for acetylated α-tubulin (Fig. 5A, left); ciliary CrPAM staining was also blocked by pre-incubation with the antigenic peptide (Fig. 5A, right). We obtained similar staining patterns with two other affinity-purified antibodies generated against the C-terminal domain of CrPAM (#304 and #305; data not shown).
Fig. 5.
CrPAM localizes to the Golgi and cilia in cell-wall-deficient CC4351 Chlamydomonas cells. (A) Confocal images of Chlamydomonas cells fixed, permeabilized and immunostained with affinity-purified antibody to CrPAM and acetylated tubulin (Ac tub, left) and the corresponding peptide-blocked control (right). CrPAM displayed tubulovesicular localization at the center of the cell body and punctate staining along the length of the cilia (white box and inset). Scale bar: 5 µm. The CrPAM signal was eliminated when antibody was pre-incubated with antigenic peptide. (B) CrPAM staining in the cell body of fixed and permeabilized cells resembled CrArf1 staining. (C) Cilia isolated from CC124 Chlamydomonas were fixed, permeabilized and immunostained for CrPAM and acetylated tubulin (left); cilia visualized using CrPAM antibody pre-incubated with antigenic peptide served as a specificity control (right). Scale bars: 5 µm. (D,E) Fixed Chlamydomonas were incubated with antibodies to CrPAM and acetylated tubulin (D) or FMG1 (E) in the presence (+TX-100) or absence (−TX-100) of detergent; images for both treatments were acquired under identical conditions. Differential interference contrast images (right panels) allowed identification of cilia. CrPAM and tubulin staining was only observed in TX-100-treated cells. Scale bar: 5 µm.
To confirm the presence of CrPAM in cilia, we stained detached cilia obtained from wild-type CC124 Chlamydomonas cells. We observed punctate localization of CrPAM along the length of the cilia (Fig. 5C, left). The signal was greatly reduced when the blocking peptide was used (Fig. 5C, right). To determine the topology of CrPAM in the ciliary membrane, we incubated cells with antibodies to CrPAM and acetylated tubulin or the major surface glycoprotein FMG1. Staining for CrPAM and acetylated tubulin was observed in cilia only after the cells were permeabilized with Triton X-100; staining for these antigens was not observed when detergent was omitted (Fig. 5D). As expected, staining for FMG1, a cell surface marker, was observed in both permeabilized and non-permeabilized cells (Fig. 5E). Our immunofluorescence data showed that the C-terminal domain of CrPAM was present in the ciliary matrix, suggesting that the catalytic domains were exposed on the ciliary surface.
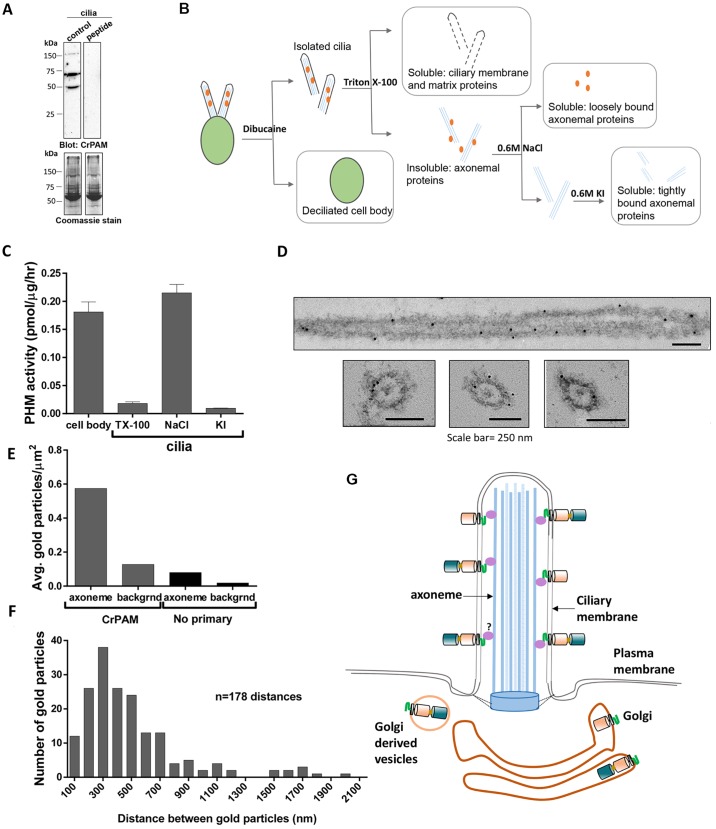
Knowing that endoproteolytic cleavages could separate the enzymatic domains from the C-terminus of CrPAM, we immunoblotted isolated cilia with our anti-CrPAM antibody (Fig. 6A). Three bands (150, 70 and 50 kDa) were detected with the C-terminal-specific antibody (control lane); none were present when the antibody was preincubated with blocking peptide (peptide lane). The two smaller fragments would be predicted to lack the PHM domain. Thus, ciliary CrPAM is a mixture of full-length and cleaved forms. The apparent masses observed suggest that CrPAM undergoes additional post-translational modifications (e.g. N- and O-linked glycosylation or phosphorylation), as documented for rPAM (Yun et al., 1995), before entering the cilium. Endoproteolytic cleavage might also generate membrane-anchored forms of PHM and PAL that lack the C-terminal domain and would not be detected by our antibodies (Fig. 6A).
Fig. 6.
CrPAM associates with the axoneme. (A) Isolated Chlamydomonas cilia extracted in SDS-lysis buffer were electrophoresed in a 10% polyacrylamide gel and immunoblotted with antibody against the CrPAM C-terminus (control) and antibody preincubated with a blocking peptide (peptide). Coomassie stain shows equal loading. The C-terminal antibody detected three specific bands. (B) Schematic of fractionation scheme used to enrich for proteins associated with different ciliary sub-structures. Wild-type CC124 Chlamydomonas cilia were sequentially treated with buffers containing 1% Triton X-100 (to solubilize ciliary membrane and matrix proteins), 0.6 M NaCl (to solubilize proteins associated with the axoneme) and 0.6 M KI (to solubilize the axoneme and tightly bound proteins). Deciliated cell bodies were solubilized in 1% Triton X-100. (C) PHM-specific activity in Triton X-100 (TX-100)- and salt-extracted fractions obtained from cilia and cell bodies with the fractionation scheme described in B. Data shown are mean±s.d. (n=2) from one of two experiments, which yielded similar results. (D) Immunoelectron microscopy images of CrPAM localization in detached cilia from wild-type CC124 Chlamydomonas. Gold particles were observed on or very close to the axonemal surface in both longitudinal and transverse sections. (E) The average density of gold particles in at least 50 axoneme and background regions was quantified for axonemes stained using the anti-CrPAM antibody and control (no primary antibody). Gold particles separated by more than 20 nm from the axoneme were considered background. The axonemal membrane is not visible because osmication was not performed. (F) To search for periodicity, the distance between adjacent gold particles on the same side of the axoneme was determined. The histogram was created using a 100-nm bin size; n=178 distances. (G) Model of CrPAM localization in C. reinhardtii Golgi and cilia. Multiple forms of CrPAM generated by proteolytic processing are present in the cilium and cell body (only the full-length form and CrPAL membrane-anchored forms are shown). The C-terminal domain resides in the cytoplasm in the cell body and in the ciliary matrix. The tight association of CrPAM with the axoneme could be indirectly mediated by unknown protein(s) depicted in purple.
To further explore the association of CrPAM with cilia, we turned to PHM assays; we sequentially treated detached cilia with Triton X-100 to solubilize ciliary membrane proteins and matrix components, followed by 0.6 M NaCl and then 0.6 M KI to extract proteins that were more tightly bound to the axoneme (Fig. 6B). Remarkably, CrPHM activity was recovered in the 0.6 M NaCl fraction, but not in the initial detergent-soluble fraction (Fig. 6C). This indicates that CrPAM either directly or indirectly associates with the axoneme; potentially both full-length CrPAM and cleaved forms containing the PHM domain but lacking the C-terminal domain could contribute to the activity measured. Thus, our results reveal the presence of a new axoneme-associated enzyme activity in cilia.
To further define the localization of CrPAM in cilia, we used immunoelectron microscopy. Isolated cilia were incubated with affinity-purified anti-CrPAM antibody, which was then visualized using secondary antibody tagged with 15-nm gold particles (Fig. 6D). In ultrathin sections of cilia stained with the anti-CrPAM antibody, gold particles were observed along the axoneme and were closely associated with outer doublet microtubules. Very few gold particles were observed either on cilia or in the background in the absence of primary antibody (Fig. 6E). Gold labeling suggested periodicity, with a peak inter-particle distance of ∼300 nm along the cilium (Fig. 6F). Based on these results, we conclude that CrPAM is a Golgi-localized protein that is also targeted to the cilium and associates closely with the ciliary axoneme (Fig. 6G).
Identification of endogenous PAM in motile and sensory mammalian cilia
Chlamydomonas cilia share many biochemical and structural characteristics with mammalian cilia. We therefore asked whether PAM localized to mammalian cilia. Notably, PAM gene expression has been reported in the ventricular layer of the brain, where the multiple motile cilia on ependymal cells move the cerebrospinal fluid (Schafer et al., 1992; Zhang et al., 1997). We isolated rodent ependymal cells and performed immunostaining with validated antibodies to several different domains of rPAM. Strikingly, punctate staining for PAM was apparent throughout the length of the cilia, which were co-stained for acetylated tubulin; similar patterns were observed with antibodies to the linker, CD and PHM domains of rPAM (Fig. 7A).
Fig. 7.
PAM localizes to motile cilia in mammalian cells. (A) Rat ependymal cells were immunostained with antibodies to acetylated tubulin (Ac tub) and mammalian PAM linker region (JH629), C-terminal domain (CT267) or PHM domain (JH1761); red brackets on PAM model show the antigen used to generate each antibody. Punctate PAM staining was observed along the length of the cilia using all three antibodies (white brackets). Scale bars: 10 µm. (B) Left, rat tracheal epithelial cells were immunostained with antibodies to acetylated tubulin and PAM (JH629). Staining for PAM was observed at the base of the cilia and foci of staining were observed in the cilia. Right, staining was greatly reduced in the presence of blocking peptide. Scale bar: 2 µm. (C) Tracheal epithelial cells were stained simultaneously for PAM (JH629) and γ-tubulin (Gam tub), a basal body marker. (D) Spermatozoa from wild-type mice were immunostained with antibodies to acetylated tubulin and mammalian PAM (CT267). Punctate localization of PAM along the length of the axoneme (white arrows), at the tip of the tail (asterisk) and in the acrosomal vesicle (white arrowhead) was observed. Scale bar: 20 µm.
To confirm the general presence of PAM in motile cilia, tracheal epithelial cells were isolated and stained simultaneously with antisera to PAM and acetylated tubulin (Fig. 7B, left). Confocal microscopy revealed punctate PAM staining at the base of the cilia and foci of staining distributed along the ciliary length. Staining specificity was verified by preincubating the anti-PAM antibody with blocking peptide (Fig. 7B, right). PAM staining at the base of the cilia in these multiciliated cells was adjacent to the basal bodies, which were revealed by staining for γ-tubulin (Fig. 7C).
Spermatozoa rely on a highly specialized flagellum for motility. PAM localization in this type of motile cilium was confirmed by immunostaining for PAM and acetylated tubulin (Fig. 7D). We observed a similar localization pattern with two other previously validated mammalian PAM antibodies (data not shown). Interestingly, we also observed PAM localization to the acrosomal vesicle, which houses proteolytic enzymes required for fertilization, and a stronger signal at the very tip of the tail, where the axoneme is not surrounded by the outer dense fibers.
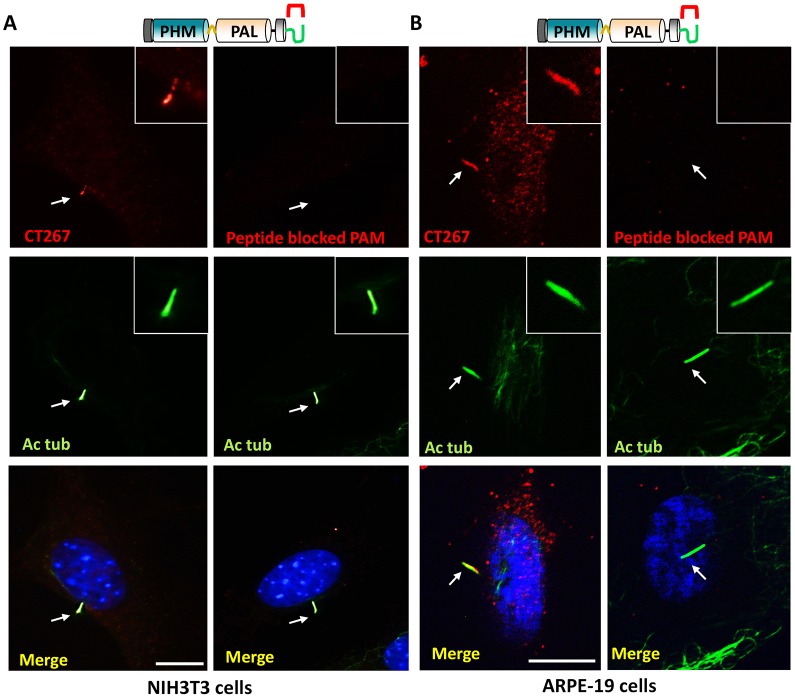
Most cell types possess immotile, primary cilia that play crucial roles in signaling and sensing environmental cues. We reasoned that the presence or absence of PAM in primary cilia could indicate whether PAM played a role in motility or a more general role in signaling. Primary cilia were induced in NIH3T3 fibroblast cells by serum starvation and stained with antibodies to acetylated tubulin and PAM (Fig. 8A, left). Primary cilia detected with acetylated tubulin staining also displayed PAM staining along their length (arrow marks the tip of the cilium); staining was greatly reduced by blocking peptide (Fig. 8A, right).
Fig. 8.
PAM localizes to sensory cilia in mammalian cells. (A) Left, serum-starved NIH3T3 cells immunostained with antibody to acetylated tubulin and the C-terminus of mammalian PAM (CT267) displayed PAM staining along the length of the cilium (white arrow points to the cilium). Scale bar: 10 µm. Right, PAM protein could not be detected when the antibody was preincubated with the antigenic peptide. (B) Left, human retinal pigment epithelial cells immunostained with antibodies to the C-terminus of mammalian PAM (CT267) and acetylated tubulin (Ac tub) displayed a similar localization of PAM in the cilium (white arrow points to the cilium). Right, peptide blocking decreased the signal observed in the cilium. Scale bar: 10 µm.
Retinal pigment epithelial cell lines, with well-characterized sensory cilia, produce neuropeptide precursors and mature amidated neuropeptides such as neuropeptide Y (Ammar et al., 1998). We used a human retinal pigment epithelial cell line (ARPE-19) to determine whether PAM was targeted to these sensory cilia. PAM again localized to the cilium, where it overlapped with acetylated tubulin staining (arrow marks the tip of the cilium) (Fig. 8B, left); the signal was reduced when blocking peptide was added (Fig. 8B, right).
DISCUSSION
The Chlamydomonas genome encodes an active prohormone-processing enzyme
A previous phylogenetic study identified PAM-like genes in several green algal genomes including Chlamydomonas reinhardtii, Volvox carteri and Ostreococcus tauri (Attenborough et al., 2012). We report here the characterization of an active peptide- amidating monooxygenase in Chlamydomonas (Cre03.g152850). The CrPHM and CrPAL proteins exhibit 37% and 32% identity to the catalytic cores of human PHM and PAL, respectively (Figs S1 and S2). As expected, CrPAM exhibited a stringent requirement for ascorbate and copper. Supplying ascorbate and copper to the lumen of the secretory pathway requires transporters. The copper-handling machinery in Chlamydomonas is similar to that in all eukaryotic cells (Merchant et al., 2006; Page et al., 2009; Blaby-Haas and Merchant, 2012). Cytosolic chaperones deliver the copper they receive from plasma membrane transporters to two highly conserved copper-transporting P-type ATPases. Chlamydomonas, which synthesizes ascorbate, uses cytochrome b561 to regenerate reduced ascorbate in the secretory pathway lumen (Urzica et al., 2012). Optimal PAM activity occurs under acidic conditions, which are created by the vacuolar ATPase (V-ATPase), which is also highly conserved in Chlamydomonas (Table S1).
The presence of PAM in Chlamydomonas and metazoans suggests its presence in a shared ancestor and thus ancient evolutionary roots for its functions. Previous studies have demonstrated conservation of the exon–intron organization of PAM in metazoans, with similar intron phasing and exon start sites in rat, Drosophila and Cnidaria (Kolhekar et al., 1997a,b; Williamson et al., 2000). As expected, the gene encoding CrPAM had fewer and smaller introns than in rPAM. Furthermore, we found essentially no alignment of exon–intron junctions between Chlamydomonas and metazoans.
Remarkably, despite the evolutionary distance separating mammals and green algae, CrPAM was trafficked and processed much like mammalian PAM when expressed in a murine neuroendocrine cell line. The specific activity observed for PHM in Chlamydomonas and neuroendocrine cell lysates was comparable (Ciccotosto et al., 1999). The active sites of mammalian and Chlamydomonas PHM appear to be very similar (Prigge et al., 1997); any penultimate residue in a peptide substrate can be accommodated. Classically, prohormone processing involves the cleavage of secretory pathway proteins by subtilisin-like prohormone convertases, which recognize pairs of basic residues, followed by removal of C-terminal Lys and Arg residues by carboxypeptidase B-like enzymes (CPE and CPD), all of which have been identified in Chlamydomonas (Fig. 1C; Table S1).
The ancestral form of PAM was membrane tethered
The ability of PAM to produce amidated peptides only requires its luminal domains (Milgram et al., 1994). The identification of a membrane-tethered bifunctional PAM protein with a cytosolic domain in Chlamydomonas suggests that the ancestral protein had this topology and that it was important for function. The transmembrane and cytosolic domains of rPAM play an essential role in its trafficking in the biosynthetic and endocytic pathways. When the cytosolic domain is truncated, rPAM is largely localized to the plasma membrane (Milgram et al., 1993). Phosphorylation at multiple sites in the cytosolic domain of PAM controls its entry into the intraluminal vesicles of multivesicular bodies and its return to the trans-Golgi network (Bäck et al., 2010).
In mammals and Chlamydomonas, PAM exists as a mixture of full-length and N-terminally truncated forms in which the luminal catalytic domains have been separated from the transmembrane-cytosolic domain fragments. In AtT-20 cells, the transmembrane-cytosolic domain fragments of rPAM are subject to γ-secretase-mediated cleavage, generating a soluble fragment of the cytosolic domain that accumulates in the nucleus (Francone et al., 2010; Rajagopal et al., 2010). AtT-20 cells engineered to allow doxycycline-inducible expression of rPAM demonstrated that increased expression of PAM caused changes in cytoskeletal organization and gene expression (Ciccotosto et al., 1999; Francone et al., 2010). These observations imply an ancient signaling role for PAM; its sensitivity to molecular oxygen, pH, copper, ascorbate and amino acids suggest several fundamental signaling pathways in which PAM could function.
Studies of PAM-knockout mice also suggest that its role extends beyond its ability to produce amidated peptides. Although PAM-knockout mice fail to survive past mid-gestation, PAM heterozygotes (Pam+/−) survive to adulthood; with half the normal level of PAM protein and activity, levels of amidated product peptides declined only slightly, suggesting that there is usually an excess of PAM activity (Bousquet-Moore et al., 2009). Nevertheless, Pam+/− mice exhibit an inability to vasoconstrict in a cold environment, increased adiposity, increased anxiety-like behavior, defects in copper homeostasis and altered synaptic transmission (Bousquet-Moore et al., 2009, 2010; Gaier et al., 2010, 2014). Mice maintained on a low-copper diet mirrored some of the defects observed in PAM+/− mice; several deficits were reversed by a copper-supplemented diet (Bousquet-Moore et al., 2009; Gaier et al., 2010, 2014). These observations are consistent with a role for PAM in communicating information about secretory pathway function to cytosolic effectors and to the nucleus.
PAM is included in a conserved set of Golgi and cilia proteins
The identification of PAM in cilia, an organelle dedicated to signaling and sensing environmental cues, also supports its signaling role. Localization of PAM to the cilia of several different mammalian cell types and Chlamydomonas highlights the importance of its presence in this cellular compartment. Although studies have focused on its neuroendocrine functions, PAM is expressed in tissues where cilia play essential roles (e.g. heart and olfactory epithelium) (Schafer et al., 1992; Zhang et al., 1997; Koefoed et al., 2014).
Also noteworthy is the widespread distribution of PAM in ciliated organisms and PAM gene loss in non-ciliated higher plants and fungi (Fig. 1C). Thus, it is possible that the last common ancestor of plants and animals contained both a PAM gene and cilia, with present day green algae maintaining PAM coincident with the presence of cilia. This correlation is not perfect, as Ostreococcus tauri contains a PAM-like protein, but lacks cilia. However, the occurrence of axonemal inner dynein arm heavy chain genes in Ostreococcus has been reported, raising the possibility that loss of ciliary components is incomplete and that remnants of a cilium might still exist (Wickstead and Gull, 2007). Alternatively, as suggested for Chlorella, which encodes nearly all ciliary outer dynein arm components (and a PAM gene), some green algae might have cilia at specific developmental stages that have not yet been observed; indeed, many cilia-related genes that are absent in land plants are conserved in these algae (Blanc et al., 2010).
The anomalies in the correlation of PAM and cilia extend to ciliated organisms such as the embryophyte, Physcomitrella patens, the chytrid fungus, Batrachochytrium dedrobatidis, alveolates such as Tetrahymena thermophila and trypanosomes such as Trypanosoma brucei. One explanation for the lack of a PAM gene in several of these ciliated organisms is their apparent use of cilia primarily for gamete or organismal motility rather than in a sensory or signaling capacity. Second, loss of PAM in some of these organisms (such as Thalassiosira pseudonana) closely parallels loss of some or all of the intraflagellar transport proteins, especially the BBSome subunits (Fig. 1C) (van Dam et al., 2013). Furthermore, these ciliated organisms are closely related to species (such as Cryptosporidium parvum and Phaeodactylum tricornutum) where complete loss of the IFT system and cilium have occurred. This correlation led to the proposal of a step-wise gain and loss of ciliary coat protein components, with the BBSome being the most recently acquired and thus, the first in line to be lost in lineages that subsequently lost the cilium completely (van Dam et al., 2013).
Several studies show that the Golgi complex and cilia are intricately linked; like most membrane proteins, those destined for the cilium must traverse the Golgi complex, where they are sorted into cilia-bound vesicles. It is important to note that the majority of the PAM protein in Chlamydomonas was found in the Golgi complex, with only a small fraction localized to the cilium. A similar distribution has been noted for IFT20, the Golgi-localized component of the intraflagellar transport pathway required for proper ciliogenesis and trafficking of cilia localized proteins such as polycystin-2 (Follit et al., 2006).
Also intriguing is the association of CrPAM with the axonemal superstructure. The topology of CrPAM in the cilium places its C-terminal domain in the ciliary matrix or cilioplasm, suggesting that this domain is involved in a direct or indirect interaction with microtubules. Although most membrane proteins (e.g. FMG1) and trafficking proteins (e.g. BBSome and IFT components) appear in the membrane and matrix fraction, the inner and outer dynein arms are solubilized by 0.6 M NaCl and the radial spokes require an even stronger lyotrope (0.6 M KI) for solubilization (Huang et al., 2007; King, 2013). CrPKD2, the Chlamydomonas homolog of polycystin-2, a six-pass transmembrane domain ion channel, exhibits a similar axonemal association (Huang et al., 2007). Like CrPAM, CrPKD2 and several other ciliary proteins are subject to proteolytic processing, generating fragments with distinct roles. Full-length CrPKD2 is found in the cell body, but not in the cilium, where two lower molecular mass fragments are observed (Huang et al., 2007).
What was the ancestral function of PAM?
Our demonstration of an active component of the peptide biosynthetic pathway in Chlamydomonas raises the question of whether this organism uses bioactive peptides for signaling; as noted above, genes encoding other essential processing enzymes as well as potential neuropeptide precursors are present (Fig. 1C). Although heterotrimeric G-proteins have not been found in Chlamydomonas, atypical GPCRs that employ distinct mechanisms of downstream signaling are present (Urano and Jones, 2013, 2014). Based on its localization and processing, CrPAM could produce amidated products in the lumen of the secretory pathway or on the ciliary surface. Active PAM has been identified on the surface of mammalian cells (Tausk et al., 1992) and in serum. Both CrPHM and CrPAL activities were detected in spent medium of mammalian cells expressing CrPAM. The topology of the protein in the cilium places the enzymatic domains in the extracellular milieu. Chlamydomonas reinhardtii grows above pH 3 and exhibits maximal growth rates in the range pH 5.5–8.5, conditions where both PHM and PAL can be active (Messerli et al., 2005). Extracellular environments containing oxygen and single electron donors might well support PAM enzyme activity.
A key question is the functional advantage of trafficking a peptide-processing enzyme to the ciliary compartment. Characterization of Pam+/− mice has suggested a role for PAM in copper homeostasis. The ability of cells to amidate peptides has been recently shown to be responsive to physiologically relevant changes in oxygen tension, leading to the suggestion of PAM-mediated hypoxia signaling mechanisms (Simpson et al., 2015). PAM could act as a copper and/or oxygen sensor in the cilium and transmit that information to the nucleus. Certainly, the ability of the soluble C-terminal domain of PAM to signal to the nucleus coupled with the ability to generate bioactive peptides positions it as a potential sensor for these key environmental signals that are crucial for organismal survival.
MATERIALS AND METHODS
Cell culture
Chlamydomonas reinhardtii strains CC124 mt- and CC4351 (cw15 arg7-8) (Chlamydomonas Resource Center) were cultured in R medium and Tris-acetate-phosphate (TAP) medium supplemented with arginine as necessary (Witman, 1986). Cells were grown under a 12-h-light–12-h-dark cycle at 22°C and aerated with 95% air and 5% CO2. NIH-3T3 and ARPE-19 cells were maintained in DMEM/F12 containing 10% fetal calf serum (Hyclone), 100 units/ml penicillin-streptomycin and 25 mM Hepes, pH 7.4 at 37°C in a 5% CO2 incubator. AtT-20 mouse corticotrope tumor cells and pEAK-RAPID HEK-293T cells were maintained as described previously (Bonnemaison et al., 2014).
Cloning and molecular biology
Oligonucleotide dT-primed template cDNA was prepared from wild-type CC124 mt- Chlamydomonas genomic DNA using Superscript III (Life Technologies). The full-length CrPAM coding region (accession number KT033716) was subcloned into pCI-neo (Promega) and verified by sequencing. For generating antibodies, a codon-optimized DNA fragment encoding the C-terminal domain of CrPAM (CrCD) was ligated into pGEX-6P3 (GST-CrCD).
Transient transfections were performed using Lipofectamine 2000 (Invitrogen) or TransIT-2020 (Mirus Bio). Stable cell lines were generated as described previously (Vishwanatha et al., 2014). For analyzing spent medium, cells were incubated overnight in complete serum-free medium. Cell lysates were prepared in low ionic strength buffer containing 1% Triton-X-100 and protease inhibitors; soluble fractions (18,000 g, 10 min) were used for enzyme assays. For immunoblot analyses, cells collected in serum-free medium were solubilized in SDS lysis buffer. Samples (equal protein) were analyzed using standard electrophoretic and immunoblotting techniques.
Chlamydomonas cilia isolation and fractionation
CC124 mt- cells were deciliated with dibucaine (Witman, 1986; King, 1995). Isolated cilia in HMS (10 mM Hepes, pH 7.4, 5 mM MgSO4 and 4% sucrose) were sequentially extracted with NaTES-mannitol buffer containing 1% Triton X-100, 0.6 M NaCl and 0.6 M KI. Samples were concentrated and desalted using Amicon concentrators (10 kDa cut-off) for determination of enzyme activity. For immunostaining, isolated cilia were placed onto polylysine-coated coverslips and processed as described below. Isolated cilia were pelleted and mixed with equal volumes of DTT in sodium bicarbonate buffer (0.1 M DTT, 0.1 M NaHCO3 containing protease inhibitors) and SDS and sucrose buffer (5% SDS and 30% sucrose) for western blotting. Peptide blocking experiments were performed by preincubating primary antibody with excess antigenic peptide.
Immunofluorescence
Chlamydomonas (strain CC4351) were mixed with an equal volumes of 4% paraformaldehyde in 30 mM Hepes, 5 mM EGTA, 5 mM MgSO4, 25 mM KCl, 4% sucrose, pH 7.0 for 30 min at room temperature, placed onto polylysine-coated multichamber slides (Labtek) and permeabilized with PBS containing 0.5% Triton X-100 (this step was omitted in cells labeled ‘–TX-100’ in Fig. 5D,E). Cells were blocked with 3% fish skin gelatin, 1% BSA, 0.1% Tween 20 in PBS for 1 h at room temperature. Primary antibodies were prepared in blocking buffer and cells incubated overnight at 4°C or 1 h at room temperature for the topology experiment. Cells were incubated in secondary antibodies (Jackson ImmunoResearch) prepared in blocking buffer for 1–2 h at room temperature.
Mammalian cells were fixed, permeabilized and stained as described previously (Bonnemaison et al., 2014). Tracheal cells (Pedersen et al., 2007) and ependymal cells were isolated from adult rats (Grondona et al., 2013). Adult mouse sperm were obtained from the cauda epididymis. Primary cells fixed in solution were allowed to adhere to polylysine-coated coverslips and stained as described above. Protocols were approved by the UCHC Institutional Animal Care and Use Committees, in accordance with National Institutes of Health and ARRIVE guidelines.
Images were obtained using a Zeiss Axiovert 200M microscope with 40×, 63× and 100× oil objectives and AxioVision software; optical sections were collected with the ApoTome module. Confocal images were collected using the Zeiss LSM 510-Meta with an oil immersion 63× or 100× Plan Apochromat objective (NA 1.4). Detached cilia were imaged using a Nikon TE300 epifluorescence microscope. Antibodies were against CrPAM-CD (1:500–1:1000; see below for details), CrArf1 (1:4000, Agrisera), acetylated tubulin clone 6-11B-1 (1:2000, Santa Cruz Biotechnology), rPAM1 exon 16, JH629 (1:1000–1:3000) (Yun et al., 1995), rPAM1 PHM, JH1761 (1:1000) (Milgram et al., 1997), rPAM1 C-terminus, CT267 (1:400 - 1:1000) (Rajagopal et al., 2009), ACTH(1-39) C-terminus (1:100, Novocastra), FMG1 (1:200) (Bloodgood et al., 1986) and GM130 (1:1000, BD Biosciences). Hoechst 33342 nuclear stain (1:1000, Invitrogen) was used where indicated.
Subcellular fractionation of Chlamydomonas cells
Washed wild-type CC124 mt- Chlamydomonas were resuspended in HMN buffer (30 mM Hepes pH 7.4, 5 mM MgSO4 and 100 mM NaCl) containing a protease inhibitor cocktail and PMSF. The concentrated cell suspension was disrupted by passage through a French press. Fractions were obtained using the differential centrifugation protocol outlined in Fig. 2A (Klein et al., 1983); pellets were solubilized in 20 mM NaTES, 10 mM mannitol, pH 7.4, 1% Triton X-100 with protease inhibitors before use in enzyme assays.
Antibody generation and validation
Purified CrCD (1.5 mg) mixed with CrPAM C-terminal peptide (KRSTAEVEAARERERLLRSGP; 1.5 mg; Biomatik) was conjugated to keyhole limpet hemocyanin (4 mg; Enzo Life Sciences), and used to immunize three rabbits (#304, 305 and 307; Covance). Affinity-purification was performed using Affi-Gel-10 conjugated to CrCD or synthetic peptide. For immunoprecipitation, affinity-purified antibodies and preimmune sera were incubated with Chlamydomonas lysates overnight at 4°C; clarified supernatants were incubated with protein-A–agarose beads. Bound proteins were eluted with Laemmli sample buffer; unbound fractions were used to determine PHM activity.
Enzyme assays
PHM and PAL assays were carried out as described previously (Kolhekar et al., 1997a) using trace amounts of [125I]-labeled-Ac-Tyr-Val-Gly (PHM substrate) or [125I]-labeled-Ac-Tyr-Val-(OH)-Gly and the corresponding unlabeled peptide (0.5 µM).
Immunogold electron microscopy
Detached cilia were fixed in 4% electron microscopy grade paraformaldehyde for 20 min at room temperature, washed in PBS, dehydrated in ethanol at −20°C and embedded in LR gold. Thin sections (70–80 nm) were mounted on formvar-coated mesh nickel grids, and incubated for 30 min at room temperature in blocking buffer (1% BSA and 0.05% Triton X-100 in PBS).
Sections were incubated overnight at 4°C in primary antibody (affinity-purified CT307, 1:10) in blocking buffer. Washed sections were incubated for 60 min in goat anti-rabbit-IgG antibody conjugated to 15-nm gold particles (1:10, EM Sciences). Rinsed grids were post-stained with 3% uranyl acetate and lead acetate. Sections were imaged using a Hitachi H-7650 transmission electron microscope. Gold particle density and spacing were quantified using Metamorph. For determination of gold particle density, 50 longitudinal cilia sections and background were manually traced and the number of gold particles and area calculated for both. Periodicity measurements were performed using the manual distance measurement tool in Metamorph.
Acknowledgements
We thank Dr Andrew Taylor, Boston University, for ARPE-19 cells, Dr Branch Craige, UMass Medical School, for CrArf1 antibody, Dr Robert Bloodgood, University of Virginia, for FMG1 antibody and Dr Laurinda Jaffe, UConn Health Center for tracheal tissue. Special thanks to Dr Daniela Strenkert, University of California, Los Angeles for sharing buffer recipes. Many thanks to Yanping Wang, Darlene D'Amato and Ramila Patel-King for laboratory assistance and other Neuropeptide Laboratory members for their support.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
D.K., R.E.M., S.M.K. and B.A.E. designed and performed experiments; C.E.B.-H. and S.S.M. performed bioinformatic analyses. All authors contributed to writing the manuscript and approved the final version.
Funding
This work was supported by the National Institutes of Health (NIH) [grant numbers DK032949 to B.A.E., GM051293 to S.M.K., GM100753 to C.E.B.-H. and GM042143 to S.S.M.]. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.177410/-/DC1
References
- Ammar D. A., Hughes B. A. and Thompson D. A. (1998). Neuropeptide Y and the retinal pigment epithelium: receptor subtypes, signaling, and bioelectrical responses. Invest. Ophthalmol. Vis. Sci. 39, 1870-1878. [PubMed] [Google Scholar]
- Attenborough R. M. F., Hayward D. C., Kitahara M. V., Miller D. J. and Ball E. E. (2012). A “neural” enzyme in nonbilaterian animals and algae: preneural origins for peptidylglycine alpha-amidating monooxygenase. Mol. Biol. Evol. 29, 3095-3109. 10.1093/molbev/mss114 [DOI] [PubMed] [Google Scholar]
- Bäck N., Rajagopal C., Mains R. E. and Eipper B. A. (2010). Secretory granule membrane protein recycles through multivesicular bodies. Traffic 11, 972-986. 10.1111/j.1600-0854.2010.01066.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbari N. F., Johnson A. D., Lewis J. S., Askwith C. C. and Mykytyn K. (2008). Identification of ciliary localization sequences within the third intracellular loop of G protein-coupled receptors. Mol. Biol. Cell 19, 1540-1547. 10.1091/mbc.E07-09-0942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaby-Haas C. E. and Merchant S. S. (2012). The ins and outs of algal metal transport. Biochim. Biophys. Acta 1823, 1531-1552. 10.1016/j.bbamcr.2012.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc G., Duncan G., Agarkova I., Borodovsky M., Gurnon J., Kuo A., Lindquist E., Lucas S., Pangilinan J., Polle J. et al. (2010). The Chlorella variabilis NC64A genome reveals adaptation to photosymbiosis, coevolution with viruses, and cryptic sex. Plant Cell 22, 2943-2955. 10.1105/tpc.110.076406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodgood R. A. (2010). Sensory reception is an attribute of both primary cilia and motile cilia. J. Cell Sci. 123, 505-509. 10.1242/jcs.066308 [DOI] [PubMed] [Google Scholar]
- Bloodgood R. A., Woodward M. P. and Salomonsky N. L. (1986). Redistribution and shedding of flagellar membrane glycoproteins visualized using an anti-carbohydrate monoclonal antibody and concanavalin A. J. Cell Biol. 102, 1797-1812. 10.1083/jcb.102.5.1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnemaison M., Bäck N., Lin Y., Bonifacino J. S., Mains R. and Eipper B. (2014). AP-1A controls secretory granule biogenesis and trafficking of membrane secretory granule proteins. Traffic 15, 1099-1121. 10.1111/tra.12194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousquet-Moore D., Ma X. M., Nillni E. A., Czyzyk T. A., Pintar J. E., Eipper B. A. and Mains R. E. (2009). Reversal of physiological deficits caused by diminished levels of peptidylglycine alpha-amidating monooxygenase by dietary copper. Endocrinology 150, 1739-1747. 10.1210/en.2008-1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousquet-Moore D., Prohaska J. R., Nillni E. A., Czyzyk T., Wetsel W. C., Mains R. E. and Eipper B. A. (2010). Interactions of peptide amidation and copper: novel biomarkers and mechanisms of neural dysfunction. Neurobiol. Dis. 37, 130-140. 10.1016/j.nbd.2009.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. M. and Witman G. B. (2014). Cilia and diseases. Bioscience 64, 1126-1137. 10.1093/biosci/biu174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho-Santos Z., Azimzadeh J., Pereira-Leal J. B. and Bettencourt-Dias M. (2011). Evolution: tracing the origins of centrioles, cilia, and flagella. J. Cell Biol. 194, 165-175. 10.1083/jcb.201011152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccotosto G. D., Schiller M. R., Eipper B. A. and Mains R. E. (1999). Induction of integral membrane PAM expression in AtT-20 cells alters the storage and trafficking of POMC and PC1. J. Cell Biol. 144, 459-471. 10.1083/jcb.144.3.459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conductier G., Brau F., Viola A., Langlet F., Ramkumar N., Dehouck B., Lemaire T., Chapot R., Lucas L., Rovère C. et al. (2013). Melanin-concentrating hormone regulates beat frequency of ependymal cilia and ventricular volume. Nat. Neurosci. 16, 845-847. 10.1038/nn.3401 [DOI] [PubMed] [Google Scholar]
- Conzelmann M., Offenburger S.-L., Asadulina A., Keller T., Munch T. A. and Jekely G. (2011). Neuropeptides regulate swimming depth of Platynereis larvae. Proc. Natl. Acad. Sci. USA 108, E1174-E1183. 10.1073/pnas.1109085108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czyzyk T. A., Ning Y., Hsu M.-S., Peng B., Mains R. E., Eipper B. A. and Pintar J. E. (2005). Deletion of peptide amidation enzymatic activity leads to edema and embryonic lethality in the mouse. Dev. Biol. 287, 301-313. 10.1016/j.ydbio.2005.09.001 [DOI] [PubMed] [Google Scholar]
- Dentler W. (2013). A role for the membrane in regulating Chlamydomonas flagellar length. PLoS ONE 8, e53366 10.1371/journal.pone.0053366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eipper B. A., Glembotski C. C. and Mains R. E. (1983). Bovine intermediate pituitary alpha-amidation enzyme: preliminary characterization. Peptides 4, 921-928. 10.1016/0196-9781(83)90091-8 [DOI] [PubMed] [Google Scholar]
- El Meskini R., Mains R. E. and Eipper B. A. (2000). Cell type-specific metabolism of peptidylglycine alpha-amidating monooxygenase in anterior pituitary. Endocrinology 141, 3020-3034. [DOI] [PubMed] [Google Scholar]
- Fliegauf M., Benzing T. and Omran H. (2007). When cilia go bad: cilia defects and ciliopathies. Nat. Rev. Mol. Cell Biol. 8, 880-893. 10.1038/nrm2278 [DOI] [PubMed] [Google Scholar]
- Follit J. A., Tuft R. A., Fogarty K. E. and Pazour G. J. (2006). The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol. Biol. Cell 17, 3781-3792. 10.1091/mbc.E06-02-0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francone V. P., Ifrim M. F., Rajagopal C., Leddy C. J., Wang Y., Carson J. H., Mains R. E. and Eipper B. A. (2010). Signaling from the secretory granule to the nucleus: Uhmk1 and PAM. Mol. Endocrinol. 24, 1543-1558. 10.1210/me.2009-0381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaier E. D., Rodriguiz R. M., Ma X.-M., Sivaramakrishnan S., Bousquet-Moore D., Wetsel W. C., Eipper B. A. and Mains R. E. (2010). Haploinsufficiency in peptidylglycine alpha-amidating monooxygenase leads to altered synaptic transmission in the amygdala and impaired emotional responses. J. Neurosci. 30, 13656-13669. 10.1523/JNEUROSCI.2200-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaier E. D., Rodriguiz R. M., Zhou J., Ralle M., Wetsel W. C., Eipper B. A. and Mains R. E. (2014). In vivo and in vitro analyses of amygdalar function reveal a role for copper. J. Neurophysiol. 111, 1927-1939. 10.1152/jn.00631.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grondona J. M., Granados-Durán P., Fernández-Llebrez P. and López-Ávalos M. D. (2013). A simple method to obtain pure cultures of multiciliated ependymal cells from adult rodents. Histochem. Cell Biol. 139, 205-220. 10.1007/s00418-012-1008-2 [DOI] [PubMed] [Google Scholar]
- Hsiao Y.-C., Tuz K. and Ferland R. J. (2012). Trafficking in and to the primary cilium. Cilia 1, 4 10.1186/2046-2530-1-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K., Diener D. R., Mitchell A., Pazour G. J., Witman G. B. and Rosenbaum J. L. (2007). Function and dynamics of PKD2 in Chlamydomonas reinhardtii flagella. J. Cell Biol. 179, 501-514. 10.1083/jcb.200704069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D., Liu A., Rakeman A. S., Murcia N. S., Niswander L. and Anderson K. V. (2003). Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426, 83-87. 10.1038/nature02061 [DOI] [PubMed] [Google Scholar]
- Hummel E., Schmickl R., Hinz G., Hillmer S. and Robinson D. G. (2007). Brefeldin A action and recovery in Chlamydomonas are rapid and involve fusion and fission of Golgi cisternae. Plant Biol. 9, 489-501. 10.1055/s-2006-924759 [DOI] [PubMed] [Google Scholar]
- Husten E. J. and Eipper B. A. (1994). Purification and characterization of PAM-1, an integral membrane protein involved in peptide processing. Arch. Biochem. Biophys. 312, 487-492. 10.1006/abbi.1994.1336 [DOI] [PubMed] [Google Scholar]
- Huyghe J. R., Jackson A. U., Fogarty M. P., Buchkovich M. L., Stančáková A., Stringham H. M., Sim X., Yang L., Fuchsberger C., Cederberg H. et al. (2013). Exome array analysis identifies new loci and low-frequency variants influencing insulin processing and secretion. Nat. Genet. 45, 197-201. 10.1038/ng.2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jekely G. (2011). Origin and early evolution of neural circuits for the control of ciliary locomotion. Proc. R. Soc. B Biol. Sci. 278, 914-922. 10.1098/rspb.2010.2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jekely G. (2013). Global view of the evolution and diversity of metazoan neuropeptide signaling. Proc. Natl. Acad. Sci. USA 110, 8702-8707. 10.1073/pnas.1221833110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J.-L. F. and Leroux M. R. (2010). cAMP and cGMP signaling: sensory systems with prokaryotic roots adopted by eukaryotic cilia. Trends Cell Biol. 20, 435-444. 10.1016/j.tcb.2010.05.005 [DOI] [PubMed] [Google Scholar]
- King S. M. (1995). Large-scale isolation of Chlamydomonas flagella. Methods Cell Biol. 47, 9-12. 10.1016/S0091-679X(08)60783-9 [DOI] [PubMed] [Google Scholar]
- King S. M. (2013). Biochemical and physiological analysis of axonemal dyneins. Methods Enzymol. 524, 123-145. 10.1016/B978-0-12-397945-2.00008-1 [DOI] [PubMed] [Google Scholar]
- Klein U., Chen C., Gibbs M. and Platt-Aloia K. A. (1983). Cellular fractionation of Chlamydomonas reinhardii with emphasis on the isolation of the chloroplast. Plant Physiol. 72, 481-487. 10.1104/pp.72.2.481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koefoed K., Veland I. R., Pedersen L. B., Larsen L. A. and Christensen S. T. (2014). Cilia and coordination of signaling networks during heart development. Organogenesis 10, 108-125. 10.4161/org.27483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolhekar A. S., Mains R. E. and Eipper B. A. (1997a). Peptidylglycine alpha-amidating monooxygenase: an ascorbate-requiring enzyme. Methods Enzymol. 279, 35-43. 10.1016/S0076-6879(97)79007-4 [DOI] [PubMed] [Google Scholar]
- Kolhekar A. S., Roberts M. S., Jiang N., Johnson R. C., Mains R. E., Eipper B. A. and Taghert P. H. (1997b). Neuropeptide amidation in Drosophila: separate genes encode the two enzymes catalyzing amidation. J. Neurosci. 17, 1363-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komsic-Buchmann K., Wostehoff L. and Becker B. (2014). The contractile vacuole as a key regulator of cellular water flow in Chlamydomonas reinhardtii. Eukaryotic Cell 13, 1421-1430. 10.1128/EC.00163-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loktev A. V. and Jackson P. K. (2013). Neuropeptide Y family receptors traffic via the Bardet-Biedl syndrome pathway to signal in neuronal primary cilia. Cell Rep. 5, 1316-1329. 10.1016/j.celrep.2013.11.011 [DOI] [PubMed] [Google Scholar]
- Merchant S. S., Allen M. D., Kropat J., Moseley J. L., Long J. C., Tottey S. and Terauchi A. M. (2006). Between a rock and a hard place: trace element nutrition in Chlamydomonas. Biochim. Biophys. Acta 1763, 578-594. 10.1016/j.bbamcr.2006.04.007 [DOI] [PubMed] [Google Scholar]
- Messerli M. A., Amaral-Zettler L. A., Zettler E., Jung S.-K., Smith P. J. S. and Sogin M. L. (2005). Life at acidic pH imposes an increased energetic cost for a eukaryotic acidophile. J. Exp. Biol. 208, 2569-2579. 10.1242/jeb.01660 [DOI] [PubMed] [Google Scholar]
- Milgram S. L., Mains R. E. and Eipper B. A. (1993). COOH-terminal signals mediate the trafficking of a peptide processing enzyme in endocrine cells. J. Cell Biol. 121, 23-36. 10.1083/jcb.121.1.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgram S. L., Eipper B. A. and Mains R. E. (1994). Differential trafficking of soluble and integral membrane secretory granule-associated proteins. J. Cell Biol. 124, 33-41. 10.1083/jcb.124.1.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgram S. L., Kho S. T., Martin G. V., Mains R. E. and Eipper B. A. (1997). Localization of integral membrane peptidylglycine alpha-amidating monooxygenase in neuroendocrine cells. J. Cell Sci. 110, 695-706. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S. and Rohatgi R. (2014). G-protein-coupled receptors, Hedgehog signaling and primary cilia. Semin. Cell Dev. Biol. 33, 63-72. 10.1016/j.semcdb.2014.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachury M. V., Seeley E. S. and Jin H. (2010). Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annu. Rev. Cell Dev. Biol. 26, 59-87. 10.1146/annurev.cellbio.042308.113337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M. D., Kropat J., Hamel P. P. and Merchant S. S. (2009). Two Chlamydomonas CTR copper transporters with a novel cys-met motif are localized to the plasma membrane and function in copper assimilation. Plant Cell 21, 928-943. 10.1105/tpc.108.064907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen L. B., Rompolas P., Christensen S. T., Rosenbaum J. L. and King S. M. (2007). The lissencephaly protein Lis1 is present in motile mammalian cilia and requires outer arm dynein for targeting to Chlamydomonas flagella. J. Cell Sci. 120, 858-867. 10.1242/jcs.03374 [DOI] [PubMed] [Google Scholar]
- Petersen T. N., Brunak S., von Heijne G. and Nielsen H. (2011). SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods 8, 785-786. 10.1038/nmeth.1701 [DOI] [PubMed] [Google Scholar]
- Prigge S. T., Kolhekar A. S., Eipper B. A., Mains R. E. and Amzel L. M. (1997). Amidation of bioactive peptides: the structure of peptidylglycine alpha-hydroxylating monooxygenase. Science 278, 1300-1305. 10.1126/science.278.5341.1300 [DOI] [PubMed] [Google Scholar]
- Prigge S. T., Mains R. E., Eipper B. A. and Amzel L. M. (2000). New insights into copper monooxygenases and peptide amidation: structure, mechanism and function. Cell. Mol. Life Sci. 57, 1236-1259. 10.1007/PL00000763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal C., Stone K. L., Francone V. P., Mains R. E. and Eipper B. A. (2009). Secretory granule to the nucleus: role of a multiply phosphorylated intrinsically unstructured domain. J. Biol. Chem. 284, 25723-25734. 10.1074/jbc.M109.035782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal C., Stone K. L., Mains R. E. and Eipper B. A. (2010). Secretion stimulates intramembrane proteolysis of a secretory granule membrane enzyme. J. Biol. Chem. 285, 34632-34642. 10.1074/jbc.M110.145334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer M. K., Stoffers D. A., Eipper B. A. and Watson S. J. (1992). Expression of peptidylglycine alpha-amidating monooxygenase (EC 1.14.17.3) in the rat central nervous system. J. Neurosci. 12, 222-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A. S., Ben-Shahar Y., Moninger T. O., Kline J. N. and Welsh M. J. (2009). Motile cilia of human airway epithelia are chemosensory. Science 325, 1131-1134. 10.1126/science.1173869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson P. D., Eipper B. A., Katz M. J., Gandara L., Wappner P., Fischer R., Hodson E. J., Ratcliffe P. J. and Masson N. (2015). Striking oxygen sensitivity of the Peptidylglycine alpha-Amidating Monooxygenase (PAM) in neuroendocrine cells. J. Biol. Chem. 290, 24891-24901. 10.1074/jbc.M115.667246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla V. and Reiter J. F. (2006). The primary cilium as the cell's antenna: signaling at a sensory organelle. Science 313, 629-633. 10.1126/science.1124534 [DOI] [PubMed] [Google Scholar]
- Sobota J. A., Ferraro F., Bäck N., Eipper B. A. and Mains R. E. (2006). Not all secretory granules are created equal: partitioning of soluble content proteins. Mol. Biol. Cell 17, 5038-5052. 10.1091/mbc.E06-07-0626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinthorsdottir V., Thorleifsson G., Sulem P., Helgason H., Grarup N., Sigurdsson A., Helgadottir H. T., Johannsdottir H., Magnusson O. T., Gudjonsson S. A. et al. (2014). Identification of low-frequency and rare sequence variants associated with elevated or reduced risk of type 2 diabetes. Nat. Genet. 46, 294-298. 10.1038/ng.2882 [DOI] [PubMed] [Google Scholar]
- Tardif M., Atteia A., Specht M., Cogne G., Rolland N., Brugiere S., Hippler M., Ferro M., Bruley C., Peltier G. et al. (2012). PredAlgo: a new subcellular localization prediction tool dedicated to green algae. Mol. Biol. Evol. 29, 3625-3639. 10.1093/molbev/mss178 [DOI] [PubMed] [Google Scholar]
- Tausk F. A., Milgram S. L., Mains R. E. and Eipper B. A. (1992). Expression of a peptide processing enzyme in cultured cells: truncation mutants reveal a routing domain. Mol. Endocrinol. 6, 2185-2196. [DOI] [PubMed] [Google Scholar]
- Urano D. and Jones A. M. (2013). “Round up the usual suspects”: a comment on nonexistent plant G protein-coupled receptors. Plant Physiol. 161, 1097-1102. 10.1104/pp.112.212324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano D. and Jones A. M. (2014). Heterotrimeric G protein-coupled signaling in plants. Annu. Rev. Plant Biol. 65, 365-384. 10.1146/annurev-arplant-050213-040133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urzica E. I., Adler L. N., Page M. D., Linster C. L., Arbing M. A., Casero D., Pellegrini M., Merchant S. S. and Clarke S. G. (2012). Impact of oxidative stress on ascorbate biosynthesis in Chlamydomonas via regulation of the VTC2 gene encoding a GDP-L-galactose phosphorylase. J. Biol. Chem. 287, 14234-14245. 10.1074/jbc.M112.341982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam T. J. P., Townsend M. J., Turk M., Schlessinger A., Sali A., Field M. C. and Huynen M. A. (2013). Evolution of modular intraflagellar transport from a coatomer-like progenitor. Proc. Natl. Acad. Sci. USA 110, 6943-6948. 10.1073/pnas.1221011110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishwanatha K., Bäck N., Mains R. E. and Eipper B. A. (2014). A histidine-rich linker region in peptidylglycine alpha-amidating monooxygenase has the properties of a pH sensor. J. Biol. Chem. 289, 12404-12420. 10.1074/jbc.M113.545947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford J. B. and Mitchell B. (2011). Strange as it may seem: the many links between Wnt signaling, planar cell polarity, and cilia. Genes Dev. 25, 201-213. 10.1101/gad.2008011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner J. F., McCarthy A. M., Morris R. L. and McClay D. R. (2014). Hedgehog signaling requires motile cilia in the sea urchin. Mol. Biol. Evol. 31, 18-22. 10.1093/molbev/mst176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters A. M. and Beales P. L. (2011). Ciliopathies: an expanding disease spectrum. Pediatr. Nephrol. 26, 1039-1056. 10.1007/s00467-010-1731-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstead B. and Gull K. (2007). Dyneins across eukaryotes: a comparative genomic analysis. Traffic 8, 1708-1721. 10.1111/j.1600-0854.2007.00646.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson M., Hauser F. and Grimmelikhuijzen C. J. (2000). Genomic organization and splicing variants of a peptidylglycine alpha-hydroxylating monooxygenase from sea anemones. Biochem. Biophys. Res. Commun. 277, 7-12. 10.1006/bbrc.2000.3629 [DOI] [PubMed] [Google Scholar]
- Witman G. B. (1986). Isolation of Chlamydomonas flagella and flagellar axonemes. Methods Enzymol. 134, 280-290. 10.1016/0076-6879(86)34096-5 [DOI] [PubMed] [Google Scholar]
- Yun H.-Y., Milgram S. L., Keutmann H. T. and Eipper B. A. (1995). Phosphorylation of the cytosolic domain of peptidylglycine alpha-amidating monooxygenase. J. Biol. Chem. 270, 30075-30083. 10.1074/jbc.270.50.30075 [DOI] [PubMed] [Google Scholar]
- Zhang Y.-H. and Robinson D. G. (1986). The Endomembranes of Chlamydomonas reinhardii: a comparison of the wildtype with the wall mutants CW 2 and CW 15. Protoplasma 133, 186-194. 10.1007/BF01304634 [DOI] [Google Scholar]
- Zhang J., Zheng M., Eipper B. A. and Pintar J. E. (1997). Embryonic and uterine expression patterns of peptidylglycine alpha-amidating monooxygenase transcripts suggest a widespread role for amidated peptides in development. Dev. Biol. 192, 375-391. 10.1006/dbio.1997.8750 [DOI] [PubMed] [Google Scholar]