Abstract
Recent studies have revealed IL-33 as a key factor in promoting antiviral T-cell responses. However, it is less clear as to how IL-33 regulates innate immunity. In this study, we infected wild-type (WT) and IL-33−/− mice with lymphocytic choriomeningitis virus (LCMV) and demonstrated an essential role of infection-induced IL-33 expression for robust innate IFN-γ production in the liver. We first shown that IL-33 deficiency resulted in a marked reduction in the number of IFN-γ+ γδ T and NK cells, but an increase in that of IL-17+ γδ T cells at 16 hours post-infection. Recombinant IL-33 (rIL-33) treatment could reverse such deficiency via increasing IFN-γ-producing γδ T and NK cells, and inhibiting IL-17+ γδ T cells. We also found that rIL-33-induced type 2 innate lymphoid cells (ILC2) were not involved in T-cell responses and liver injury, since the adoptive transfer of ILC2s neither affected the IFN-γ and TNF-α production in T cells, nor liver transferase levels in LCMV-infected mice. Interestingly, we found that while IL-33 was not required for costimulatory molecule expression, it was critical for DC proliferation and cytokine production. Together, this study highlights an essential role of IL-33 in regulating innate IFN-γ-production and DC function during viral hepatitis.
Keywords: IL-33, dendritic cell, LCMV, viral hepatitis, IFN-γ, γδ T cell, innate lymphoid cell
Introduction
The innate immune system is the first line of defense against invading microorganisms, followed by the adaptive immune responses which provide antigen-specific immunity to infection [1]. Engagement of pathogen-associated molecular pattern (PAMP) molecules in innate cells triggers their phagocytic activities and cytokine production, inducing innate immune responses [2]. In the case of autoimmune diseases, where PAMP signals do not exist, strong immune responses are initiated and perpetuated by damage-associated molecular pattern (DAMP) molecules, which are directly released by necrotic cells in injured tissues. DAMPs are considered endogenous alarmins and synergistically regulate innate and adaptive immune responses in various kinds of infectious and autoimmune diseases [3].
IL-33 is a newly discovered DAMP molecule [4] and is crucial for the innate immunity [5]. Recent reports highlight the essential role of IL-33 in boosting antiviral immunity in both chronic and acute viral infection. For example, IL-33 drives the antiviral CD8+ T-cell responses in the infection of lymphocytic choriomeningitis virus (LCMV) [6]. Exogenous IL-33 can reduce inflammatory cytokine production and attenuate adenovirus (Ad)-induced hepatitis [7]. However, the mechanism of IL-33-dependent T-cell function is not clear, and it is unclear how IL-33 contributes to the innate immunity of viral hepatitis.
Among the innate cell types, γδ T cells are an important subset of lymphocytes, capable of responding rapidly to microbial infection [8]. γδ T cells can provide an innate source of cytokines, such as IFN-γ and IL-17, and influence the ensuing adaptive responses [9]. NK cells are another type of innate cells and are important in the control of viral infection by producing antiviral cytokines and cytotoxic molecules [10]. Dendritic cells, as the potent antigen-presenting cell (APC), are crucial for priming T-cell activation and enhancing CTL responses by upregulation of costimulatory molecules (signal 2, e.g. CD80, CD86, MHC II) and the secretion of cytokines (signal 3, e.g. IL-12) [11, 12]. All of these cells are involved in the first-line of antiviral responses, and also able to educate the adaptive antiviral T-cell responses. Recent study revealed that type 2 innate lymphoid cells (ILCs), which could be induced by IL-33, are emerging as important effectors of innate immunity and play a central role in tissue remodeling [13, 14]. Currently, however, the role of IL-33 in innate immunity against viral infection is not totally understood.
In this study, we demonstrated that IL-33 can inhibit the proliferation of IL-17+ γδ T cells, but promote IFN-γ production in CD27+ γδ T cells in the liver at the early stages of viral hepatitis. IL-33 deficiency led to reduced numbers of liver NK cells, as well as decreased levels of IFN-γ expression. In addition, ILC2 was not involved in antiviral T-cell responses and hepatoprotection in LCMV-induced hepatitis. Mechanistically, IL-33 was not required for costimulatory molecule expression. However, it is critical for DC proliferation and cytokine production, which was necessary for T-cell activation and proliferation. This study indicates an essential role of IL-33 in regulating innate IFN-γ-production and DC function through signal 3 in viral hepatitis, highlighting IL-33 as a potential immunomodulating agent in viral hepatitis treatment.
Results
Hepatic IL-33 expression is upregulated in the liver of LCMV-infected mice
The innate immune system responds quickly to viral infection and regulates the following antiviral adaptive immune responses. We found that the numbers of NK cells, dendritic cells and γδ T cells were all significantly increased in the liver as early as 1 dpi (Fig. 1A). As shown in Fig. 1B, IL-33 expression was significantly upregulated at 1 dpi. The expression of IL-33, which was mainly from hepatocytes [15], was further increased at 6 dpi, which coincided with ST2 expression. The pro-inflammatory cytokines IFN-γ and TNF-α were increased at the early innate responding stage (1 dpi) and reached their highest level at 6 dpi, when T effector cells were recruited into the liver. Chemokines CXCL9 and CXCL10, which controlled NK and T-cell recruitment, were sharply increased at 1 and 6 dpi. These data demonstrated the correlation of IL-33 with other pro-inflammatory cytokines and chemokines, suggesting that IL-33 may play a role in both innate and adaptive immune responses in viral hepatitis.
Figure 1. Hepatic IL-33 expression is upregulated in the livers of LCMV-infected mice.
WT mice were infected and sacrificed at the indicated time points and liver tissues were collected. (A) The numbers of intrahepatic lymphocytes, dendritic cells, NK cells and γδ T cells were enumerated by flow cytometry. (B) RNA was extracted and real-time RT-PCR analysis of hepatic IL-33, ST2, IFN-γ, CXCL9, CXCL10, and TNF- α mRNA in infected mice compared with that of uninfected mice was performed. The data are shown as mean + SEM of 3–5 mice from a single representative experiment. The experiment was repeated twice independently. One-way ANOVA was used for statistical analysis. * P<0.05; ** P<0.01.
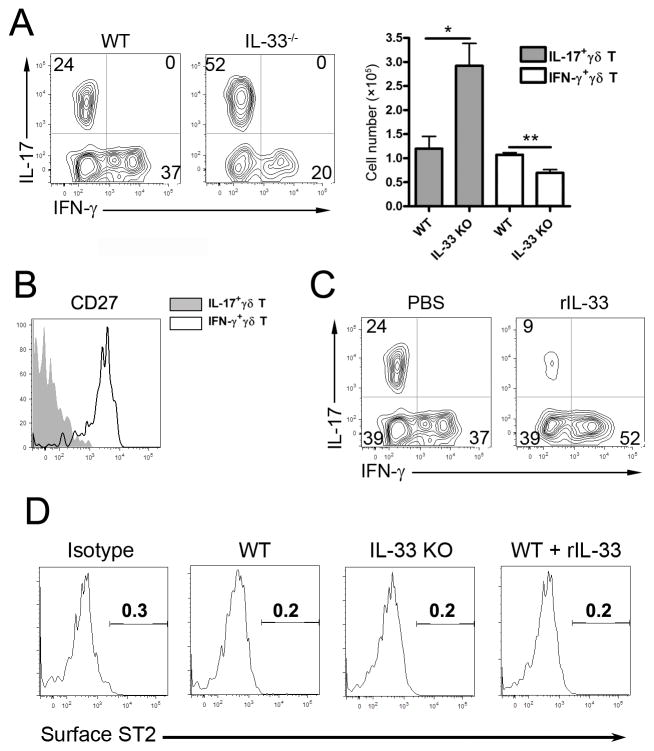
IL-33 promotes expansion of IFN-γ+ γδ T cells, but inhibits that of IL-17+ γδ T cells
In viral infection, γδ T cells are a group of critical innate immune cells, as they are capable of producing IFN-γ or IL-17 in the early stages of a viral infection [16]. To investigate whether IL-33 regulates γδ T-cell functions in the liver, we infected WT and IL-33−/− mice with LCMV and measured the cytokine production in γδ T cells at 16 hpi. We found that a similar number of hepatic γδ T cells of infected WT animals produced IFN-γ and IL-17 (Fig. 2A). On the other hand, IL-33−/− mice failed to stimulate the IFN-γ+ γδ T-cell population, but preferentially expanded their IL-17+ γδ T-cell compartment (Fig. 2A). IFN-γ+ γδ cells were mainly CD27+, whereas the IL-17+ γδ T ones were CD27−, which was consistent with a previous report [17] (Fig. 2B). To further investigate the role of IL-33 for IFN-γ production in γδ T cells, we injected the recombinant IL-33 (rIL-33) into WT mice at 2 hpi. We found that rIL-33 increased the percentage of IFN-γ+ γδ T cells, but markedly suppressed that of IL-17+ γδ T cells in the liver at 16 hpi (Fig. 2C). These results demonstrated that IL-33 can promote IFN-γ-producing γδ T cells in early viral infection. However, surface ST2 was non-detectable on γδ T cells (Fig. 2D). Although IL-17 can modulate DC function in intracellular bacterial and Ad infections [16, 18], blockage of IL-17 by neutralization Abs did not seem to hinder DC maturation, innate cytokine production and liver injury in LCMV infection (Fig. S1).
Figure 2. IL-33 rebalances IL-17/IFN-γ-producing γδ T cells in the livers of early LCMV-infected mice.
(A) WT and IL-33−/− mice were infected and sacrificed at 16 hours post-infection (hpi). IHLs were isolated for FACS analysis. CD3+γδ TCR + cells were first gated, which was followed by the detection of intracellular IL-17 and IFN-γ levels. The absolute numbers of hepatic IL-17- and IFN-γ-producing γδ T cells are also shown (right). Data are shown as mean + SEM of 3–4 mice/group from a single experiment representative of three experiments performed. (B) The hepatic IL-17- and IFN-γ-producing γδ T cells were gated for examining the surface CD27 expression. (C) The infected WT mice were i.p.-treated with PBS and rIL-33 (1 μg/mouse) respectively at 2 hpi, and sacrificed at 16 hpi. The hepatic IL-17- and IFN-γ-producing γδ T cells were analyzed. (D) Surface ST2 expression on γδ T cells. The data are from a single experiment representative of at least three experiments performed. One-way ANOVA was used for statistical analysis. * P<0.05; ** P<0.01.
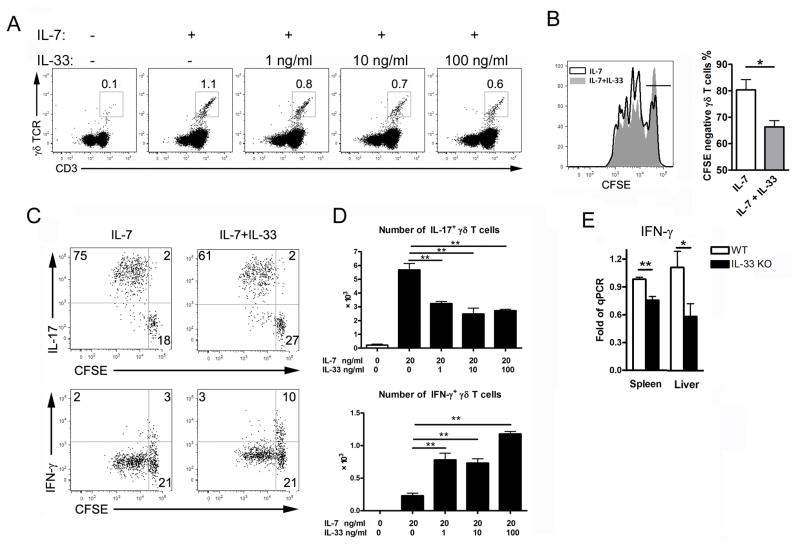
To investigate the mechanism of IL-33-dependent regulation of γδ T cells, we isolated LN cells from naïve mice and cultured them with hematopoietic growth factor IL-7. Compared to the medium controls, γδ T cells expanded 10 fold in the presence of IL-7 for 4 days, as previously described [19]. However, addition of rIL-33 reduced the percentage of γδ T cells, and the effect of IL-33 was dose-dependent (Fig. 3A). The results of the CFSE experiment led us to suggest a reduced proliferation of γδ T cells in the presence of IL-33 (Fig 3B). Since IL-17+ γδ T-cell expansion is IL-7- and IL-7R-dependent [16, 19], we speculated that IL-33 inhibited the proliferation of the IL-17+ γδ T cells, but not of the IFN-γ+ cells. Indeed, IL-33 only inhibited the proliferation of IL-17+ γδ T cells, but facilitated that of IFN-γ+ cells (Fig. 3C and D). Consistently, tissue IFN-γ expressions of IL-33−/− mice were reduced compared with those in WT mice at the innate stage of LCMV infection (Fig. 3E).
Figure 3. IL-33 inhibits IL-17+ γδ T-cell proliferation, but promotes IFN-γ+ γδ T cells in vitro.
(A) Cells from lymph node (LN) of naïve mice were cultured with various concentrations of rIL-33 in the presence of rIL-7 (20 ng/ml) for 4 days. The number of γδ T cells in the cultures was determined by gating on CD3+ γδ TCR+ cells. (B) LN cells of naïve mice were labeled with CFSE first and cultured with or without rIL-33 (100 ng/ml) in the presence of rIL-7 (20 ng/ml) for 4 days. Cellular proliferation was measured by CFSE dilution. (C) LN cells were cultured as in (B), then stimulated with PMA/Iono plus GolgiStop for the last 5 h of culture for intracellular staining of IL-17 and IFN-γ. (D) LN cells were cultured as in (A). The numbers of IL-17+ and IFN-γ+ γδ T cells as determined by flow cytometry are shown. LN of 5–6 naive mice were pooled for a single representative experiment. (E) WT and IL-33−/− mice were infected and sacrificed at 3 dpi. Gene transcript levels of IFN-γ were detected by qRT-PCR. 3–5 mice were used in each group for a single representative experiment. All data are shown as mean + SEM from single experiments representative of at least three experiments performed. One-way ANOVA and a two-tailed t test was used for statistical analysis. * P<0.05, ** P<0.01.
IL-33 deficiency impairs NK-cell expansion and IFN-γ production
In addition to γδ T cells, NK cells are highly enriched in the liver and are the main source of IFN-γ during viral hepatitis [10]. We found the decreased numbers of NK cells in the liver of IL-33−/− mice compared with WT mice at 3 dpi (Fig. 4A). The percentage of IFN-γ+ NK cells was significantly lower in IL-33−/− mice at both 1 and 3 dpi (Fig. 4B and S2). The levels of serum IFN-γ at 3 and 6 dpi were significantly lower in IL-33−/− mice. However, the levels of granzyme B proteins were similar between two groups (Fig. 4C). To further confirm the effect of IL-33 on NK-derived IFN-γ, we cultured LN cells in vitro with rIL-33, and found that IL-33 can induce high levels of IFN-γ expression, but not those of IL-17 in NK cells (Fig. 4D).
Figure 4. IL-33 deficiency leads to reduced NK-cell IFN-γ production during acute viral hepatitis.
WT and IL-33−/− mice were infected, and then sacrificed at 3 dpi. (A) The percentage of liver NK cells was determined by gating on CD3- NK1.1+ populations. (B) IHLs were isolated and stimulated with PMA and Ionomycin for 5 hours in the presence of GolgiStop, followed by intracellular staining of IFN-γ. The percentages of IFN-γ-producing NK cells are shown. Serum IFN-γ levels at 3 and 6 dpi are shown. (C) Granzyme B staining of NK cells (solid gray is isotype control; solid line is WT NK cell and dash line is IL-33−/− NK cells. (D) Cells from LN of naïve WT mice were cultured with rIL-33 (10 ng/ml) in the presence of rIL-7 (20 ng/ml) for 4 days. Cells were stimulated with PMA and Ionomycin in the presence of GolgiStop during the last 5 hours, and intracellular IFN-γ and IL-17 were detected. The data are shown as mean + SEM of n = 3–5 mice/group from single experiments representative of at least three experiments performed. A two-tailed t test was used for comparison between two groups. *P<0.05,***P<0.001.
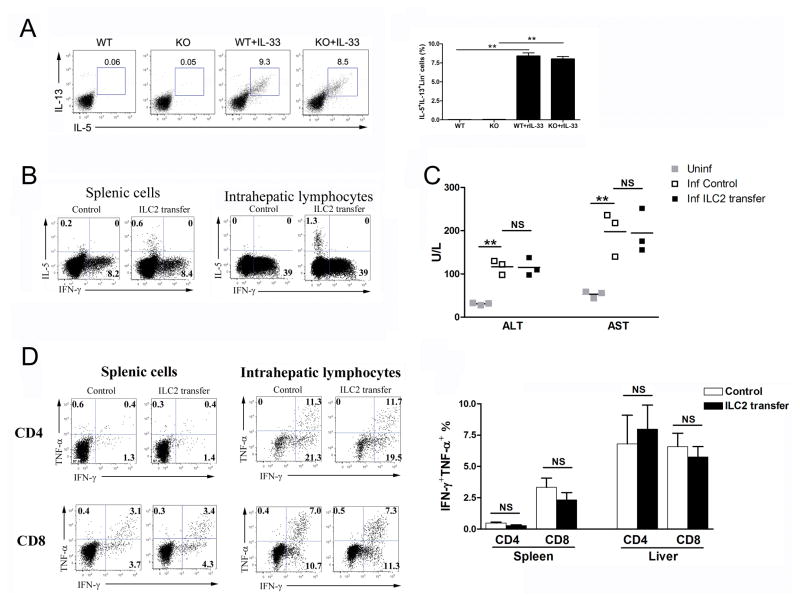
ILC2s do not influence antiviral T-cell responses and liver injury in LCMV infection
IL-33 can induce ILC2 and thus modulate Ad-induced hepatitis [7]; however, the role of ILC2 in LCMV infection remains unclear. Here, we found that IL-33 treatment strongly induced liver ILC2 in both WT and IL-33−/− mice (Fig. 5A). To determine the role of ILC2 in LCMV infection, we adoptively transferred these cells to infected WT mice at 1, 3 and 5 dpi (Figure S3) [7]. At 7 dpi, ILC2 could be detected in the spleen and liver (Fig. 5B). However, the liver injury and antiviral T-cell responses were comparable between ILC2-transferred and the control groups (Fig. 5C and D).
Figure 5. ILC2s are dispensable for anti-viral T-cell responses.
(A) WT and IL-33−/− mice were infected, followed by rIL-33 treatment (1 μg/mouse, PBS was used as a control) daily starting from 1 dpi. All mice were sacrificed at 7 dpi. IHLs were stimulated with PMA/Iono for 4 h in the presence of GolgiStop, followed by the surface staining of lineage markers (Lin) and intracellular IL-5 and IL-13. Lin- cells were gated out first and further analyzed for IL-5+IL-13+ populations. (B) 5×106 ILC2s were adoptively transferred into LCMV-infected WT mice at 1, 3 and 5 dpi (PBS was used as a control). Total live cells from spleens and livers were gated for detection of IL-5- and IFN-γ-producing cells. (C) Serum ALT and AST levels were evaluated by enzymatic reaction assay. Symbols represent individual mice and bars represent means. (D) Splenic and hepatic virus-specific T-cell responses were evaluated by intracellular IFN-γ and TNF-α staining. Data are shown as mean + SEM of 3–5 mice/group, from single experiments representative of at least three experiments performed. A two-tailed t test was used to compare the two groups. * P<0.05, ** P<0.01.
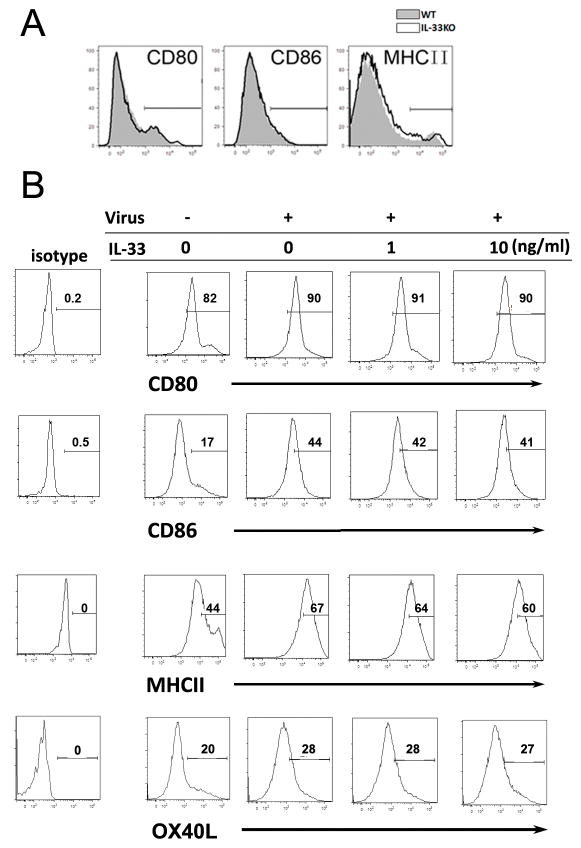
IL-33 promotes DC proliferation and cytokine signal 3
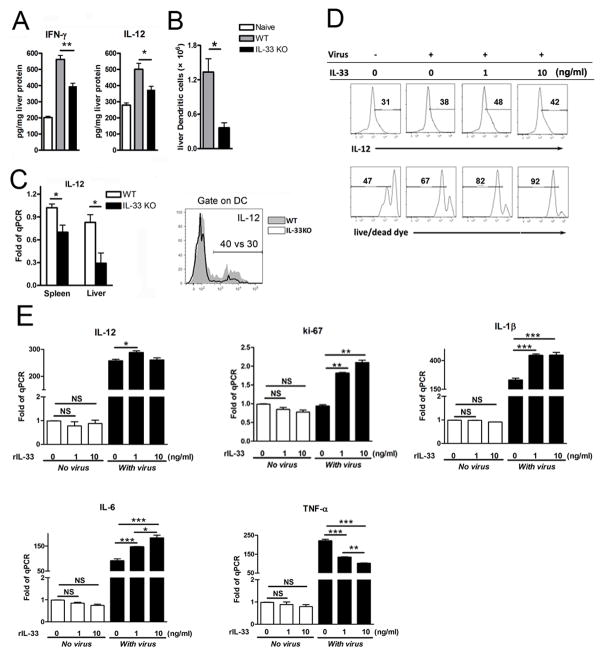
Activation of naïve T cells to undergo clonal expansion and develop effector function requires three signals: antigenic signal (signal 1), costimulation (signal 2) and cytokines (signal 3) [20]. The requirement for the signal 3, provided by IL-12 or other cytokines, to stimulate Ag-dependent proliferation is variable, making the greatest contribution when Ag levels are low [21]. To investigate whether IL-33 influences these signals on DC and how IL-33 affects T-cell function, we examined signals 1 and 2 on hepatic DC in WT and IL-33−/− mice with LCMV infection. We found that the expression of MHC II, CD80 and CD86 was comparable between the WT and IL-33−/− animals (Fig. 6A). To confirm these results, we cultured BMDC and stimulated them with LCMV and rIL-33. We found that the intensities of these molecules on the DC surface were not altered by IL-33 treatment (Fig. 6B), suggesting that IL-33 did not alter the signals 1 and 2 on DC surface. IL-12 is an important mediator of signal 3 that promotes Th1 and CTL development [21]. However, decreased IL-12 levels and DC numbers were observed in the livers of IL-33−/− mice in LCMV infection (Fig. 7A–C). Reduced signal 3 led to an impaired adaptive immune response as evidenced by lower levels of IFN-γ production (Fig. 7A), impaired Th1 and CTL responses, as well as compromised viral clearance in the livers of IL-33−/− mice (Fig. S4). To confirm the effect of IL-33 on DC signal 3, we stimulated BMDC with virus and rIL-33 in vitro and found that IL-33 significantly increased the number of live DCs in the presence of virus, as well as the expression of IL-12, Ki-67, IL-1β and IL-6, but not TNF-α (Fig. 7D and E).
Figure 6. Surface expression intensities of DC costimulatory molecules are not altered by IL-33.
(A) WT and IL-33−/− mice were infected for 3 days and IHLs were isolated and stimulated with LPS overnight. GolgiStop was added at the last 4 h. Intracellular IL-12 and surface CD80, CD86 and MHCII were detected on CD11b+CD11c+ cells by flow cytometry. (B) BMDCs were cultured with or without virus and rIL-33 for 2 days. Surface CD80, CD86, OX40L and MHCII were detected. The data are from a single representative experiment. The experiment was repeated three times independently, and representative graphs are shown (n = 3–5 mice/group).
Figure 7. IL-33 controls DC proliferation and IL-12 production in acute LCMV infection.
(A) WT and IL-33−/− mice were infected and then sacrificed at 6 dpi. Liver IFN-γ and IL-12 protein levels were measured by Bio-plex. (B) Numbers of DCs in the liver at 6 dpi was determined by flow cytometry. (C) WT and IL-33−/− mice were infected and then sacrificed at 3 dpi. Gene expressions of IFN-γ and IL-12 in liver tissues were detected by real-time PCR. IHLs were stimulated with LPS and followed by intracellular IL-12 detection in CD11b+CD11c+ cells. (D) BMDCs were cultured with or without virus and IL-33 for 3 days. Cells were stimulated with LPS at last 4 hours in the presence of GolgiStop. All CD11c+ cells were gated first, and Live/dead cell dyes were used for discriminating between live cells and dead cells. (E) Gene expression of IL-12, IL-1β, IL-6, TNF-α and Ki-67 by BMDCs were also detected. Data are shown as mean + SEM of 3–5 mice/group, from single experiments representative of at least three experiments performed. A two-tailed t test was used for comparison of two groups. * P<0.05, ** P<0.01, ***P<0.001.
Discussion
Early immune responses are crucial for a host defense against viral infection and determine the outcome of a persistent viral infection [1]. Innate immune cells activate rapidly during viral infections and modulate the following antiviral T-cell responses through cell-to-cell interaction and cytokine production. Recent clinical studies revealed high levels of serum IL-33 in patients with either hepatitis B or hepatitis C virus infections [22, 23]. These data suggest the potential role of IL-33 in human liver diseases. In a mouse model of chronic viral infection, the IL-33/ST2 axis was identified as a key factor in modulating CD8+ and CD4+ T-cell responses [6, 24]. Similar result was also found in our LCMV infection model by using IL-33−/− mice (data not shown). Interestingly, an increased expression of IL-33 was observed in the spleen of virus-infected mice as early as 36 h [6]. Although IL-33 seems to play a role in early immune responses, how IL-33 mediates antiviral T-cell responses in hepatitis is not entirely clear.
In this study, we examined the kinetics of IL-33 and ST2 expression in the liver during the acute phase (days 1–6) of LCMV infection. The biphasic elevation of IL-33 at 1 and 6 dpi, respectively seemed corresponded to innate and adaptive immune cell activation and infiltration in the liver (Fig. 1). By using knockout and add-back approaches, we demonstrated that IL-33 greatly enhances IFN-γ production among γδ T cells (Fig. 2), which can further promote DC maturation and prime the antigen-specific T cells in viral infection [25–27]. We revealed that IL-33 promoted IFN-γ production in CD27+ γδ T cells, but repressed IL-17+ γδ T cells both in vivo and in vitro (Figs. 2 and 3). However, surface ST2 was not expressed on γδ T cells, suggesting that IL-33 may regulate γδ T-cell response by modulating the cytokine production from other innate immune cells or by a ST2-independent way [28]. NK cells have been reported to express ST2 and require IL-12 signals, which are enhanced by IL-33-dependent signaling for proliferation and IFN-γ production [29, 30]. Herein, we found the decreased number of hepatic NK cells in IL-33−/− mice, suggesting that IL-33 is important for development and recruitment of NK cells in the liver. More importantly, IL-33−/− mice had reduced IFN-γ production by hepatic NK cells (Fig. 4A and S2), which is likely to hamper the control of viral replication at early infection. NK cells are an important source of innate IL-17 [31]; however, LCMV failed to induce NK cells to express IL-17 (data not shown). Rather, rIL-33-triggered NK cells expressed higher levels of IFN-γ, but not IL-17. More recently, NK cells have been reported to regulate antiviral immune responses by lysing the antiviral T cells [32]. However, we found that granzyme B expression was similar on hepatic NK cells in WT and IL-33−/− mice, indictating that IL-33 facilitated IFN-γ production by NK cells in viral hepatitis, but may not influence cytotoxicity.
Innate IL-17 is critical in initiating DC activation and immune priming in the early stages of Ad infection [16]. IL-33 is reported to repress IL-17 in an EAE model [33]. In our study, we found that IL-33 inhibited the proliferation of IL-17+ γδ T cells and that IL-17 signaling did not influence the ensuing antiviral T-cell response in LCMV infection (Fig. 3 and S1). This observation is consistent with a previous report, in which IL-7-induced IL-17 did not result in myeloid cell activation, leading to liver or systematic injury [34]. However, it was also reported that IL-17 plays a pro-inflammatory role in Ad infection or LCMV infection in FVB mice [35, 36]. The reason for the discrepancies may result from the different viral stocks and murine strains. In both cases, these viral infection elicited strong host responses and resulted in severe hepatic damage, as evidenced by high levels of serum ALT (e.g. 1,000–2,000 unit/L) and hepatic inflammation in WT and FBV mice. However, LCMV infection in WT mice results in low levels of ALT elevation (e.g. 200–300 unit/L) and few councilman bodies in the liver. These results suggest that IL-17 can only play a pro-inflammatory role under a selected host genetic background or cytokine microenviroment, but not in the model of LCMV Cl 13 infection in WT mice such as this one.
Recently, we have demonstrated that IL-33 can induce ILC2 in the liver [7]. However, it is not clear whether ILC2 are involved in IL-33-dependent T-cell responses in LCMV infection. To answer this question, we examined ILC2 in LCMV infection and found that ILC2 were not involved in LCMV infection. We observed comparable numbers of liver ILC2 in WT and IL-33−/− mice after rIL-33 treatment, indicating that the elicitation of ILC2 by rIL-33 was independent of endogenous IL-33 (Fig. 5A). Moreover, adoptive transfer of ILC2 did not affect T-cell responses and liver injury (Figs. 5B–D), indicating that the effect of IL-33 in promoting antiviral T-cell responses is independent of ILC2 in the early stages of LCMV-induced hepatitis. However, there is still a risk of liver fibrosis by long-term rIL-33 treatment in chronically affected hepatitis individuals, because of the pro-fibrosis effect of ILC2-derived IL-13[37]. In addition, rIL-33 treatment was unable to induce ILC3, which express IL-17 and IL-22 (data not shown) [38].
APCs need to deliver three kinds of signals for the expansion and differentiation of naïve T cells. Antigenic signal and costimulation are essential for cell activation and survival, while the third type, cytokine signal, is involved in T-cell differentiation into different subsets of effector T cells [21, 39]. In this study, we found the reduced antiviral T-cell responses in IL-33−/− mice (Fig. S4) and speculated that IL-33 deficiency may cause the impaired APC function. However, we found that MHC II, CD80 and CD86 of hepatic DC were comparably expressed in infected WT and IL-33−/− mice (Fig. 6A). In addition, BMDC did not alter the expression levels of these molecules by IL-33 treatment in vitro, suggesting that IL-33 is not capable of influencing signals 1 and 2 of DC in viral infection. DC-secreted signal 3 cytokines (e.g. IL-12) are the determinant for shaping adaptive T-cell responses [40, 41]. Interestingly, we observed that the absence of IL-33 led to low levels of IL-12, as well as a reduced number of DC (Figs. 7A–C), indicating the essential role of IL-33 on signal 3 of DC. IL-33 treatment slightly increased IL-12 levels on BMDC in vitro, possibly meaning that other cytokines may be required for synergism with IL-33 as others have reported in CD8+ T-cells [42]. IL-1β and IL-6 were also increased in response to IL-33 treatment and may play a role in T-cell activation and proliferation [43–46]. Interestingly, TNF-α decreased on DC following IL-33 treatment, consisting with our previous report that IL-33 inhibits TNF-α in the liver of infected animals [7]. These data demonstrated that IL-33 promoted DC’s signal 3 (cytokine signals), but not signal 2 (costimulatory molecule signals) in viral hepatitis. A previous report shows that instead of GM-CSF, IL-33 increases DC development in a BM culture system [47]. In our study, we found that IL-33 did not change the gene expression of uninfected DC. However, IL-33 significantly increased Ki-67 expression and the number of DCs in a dose-dependent manner (Fig. 7D and E). These results suggest that IL-33 can promote BMDC proliferation and maintain cell survival in viral infection.
In summary, our data revealed the key role of IL-33 in promoting IFN-γ production by γδ T cells and NK cells in viral hepatitis. This is the first report, illustrating that IL-33 promotes DC proliferation and cytokine signal production, which possibly enhances the pluri-functionality of antiviral T cells in viral infection. A better understanding of the mechanism of IL-33 action in viral infection will broaden our knowledge about how DAMP molecules regulate innate immune cells and reveal the potential of IL-33-related strategy in viral hepatitis.
Materials and methods
Animals, infection and treatment
C57BL/6 (B6) mice from the Jackson Laboratory and IL-33−/− mice of the B6 background [48] were bred and maintained under specific pathogen-free conditions in the UTMB animal care facility and used at 7–10 wk of age. All procedures were approved by UTMB’s Institutional Animal Care and Use Committee and performed according to the NIH Guidelines. In all experiments, LCMV strain Clone 13 (Cl 13, a gift from Dr. Maria Salvato at the University of Maryland) was used, and mice were i.v. injected with 2 × 106 PFU of viruses to induce hepatitis. Some infected mice were i.p. injected with rIL-33 (1 μg/mouse, Biolegend, San Diego, CA) or PBS, at indicated time points.
Propagation and quantitation of virus
The LCMV stocks were prepared and titrated according to a previous report [49]. Briefly, virus was incubated with baby hamster kidney (BHK) cells for 72 h. The culture fluid was centrifuged for 10 min at 350 g, 4°C, and the supernatant was stored at −70°C. For quantitation of the virus, Vero cells were cultured with a series of 10-fold virus dilutions for 90 min, followed by a 0.5% agarose overlay. After 4 days’ culture, immunocytochemistry was performed by using mouse anti-LCMV polyclonal antibody (gift from Dr. Robert Tesh, University of Texas Medical Branch) [35], and the numbers of positive clusters were counted, followed by the calculation of viral titers.
Recombinant cytokines and antibodies
Carrier-free rIL-33 was purchased from Biolegend (San Diego, CA), and rIL-7 was from Peprotech (Rocky Hill, NJ). The following Abs were from eBioscience (San Diego, CA): PE-anti-CD44 (IM7.8.1); PE-anti-CD45 (30-F11); Allophycocyanin-anti-CD86 (GL1); PE-anti-CD80 (16-10A1); Allophycocyanin-anti-CD25 (PC61.5); PE-anti-CD69 (H1.2F3); Allophycocyanin-anti-IL-17A (eBio17B7); FITC-anti-IFN-γ (XMG1.2); Allophycocyanin-anti-IFN-γ (XMG1.2); PE-anti-IL-12 (X17.8); Allophycocyanin-anti-TCRγδ (eBioGL3); PE-Cy7-anti-NK1.1 (PK136); PerCP-Cy5.5-anti-CD11b (M1/70); eFluor 450-anti-CD11c (N418); PE-Cy7-anti-CD27 (LG.7F9); PerCP-efluor 710-anti-TNF-α (MP6-XT22); PE-anti-Granzyme B (NGZB); PerCP-efluor 710-anti-ST2 (RMST2-2); PE-anti-IL-5 (TRFK5); Allophycocyanin-anti-IL-13 (ebio13A); and Fixable Viability Dye eFluor 506. For staining the lineage markers, we used FITC-anti-B220 (RA3-6B2); anti-CD11b (M1/70); anti-CD11c (N418); anti-Gr-1 (RB6-8C5); anti-Ter-119 (TER-119), anti-NK1.1 (PK136); anti-CD3 (145-2C11); anti-CD4 (GK1.5); and anti-CD8 (53-6.7); anti-OX40L (RM134L). The following Abs were purchased from Biolegend: PE-Cy7-anti-CD3 (17A2); Allophycocyanin-Cy7-anti-CD8 (53-6.7); Percp-Cy5.5-anti-ICOS (C398.4A); Allophycocyanin-Cy7-anti-SCA-1 (D7); Pacific blue-anti-CD4 (GK1.5); Dylight Alexa Fluor 488 Goat-anti-mouse IgG (Poly4055); and Purified anti-CD16/32 (2.4G2). The IL-17 neutralization Ab (17F3) was from Bio X Cell (West Lebanon, NH).
Isolation of lymphocytes from tissues
Spleen cells were prepared by using red blood cell lysing buffer (Sigma, St. Louis, MO). Intrahepatic lymphocytes (IHL) were isolated according to our previous method [35]. Briefly, the liver was perfused and mashed in complete RPMI-1640 followed by digestion with collagenase IV (0.05%, Roche Applied Science, Indianapolis, IN) at 37°C for 30 min. After digestion, cell suspensions were passed through a 70-μm nylon cell strainer to yield single-cell suspensions. IHL were purified by centrifugation (400 g) at room temperature (RT) for 30 min over a 30/70% discontinuous Percoll gradient (Sigma, St. Louis, MO). The total numbers of IHL per liver were counted. The relative percentages of cell subpopulations were measured by flow cytometry, and the absolute numbers were calculated according to their percentages and the IHL numbers.
Flow cytometry
After incubation, cells were collected, stained with fixable viability dye, blocked with FcγR blocker (CD16/32), and stained for specific surface molecules. For intracellular staining of IFN-γ, TNF-α and IL-17, cells were incubated for 4 h with PMA (50 ng/ml) and ionomycin (750 ng/ml) in the presence of GolgiStop (BD Bioscience). GP33 and GP61 peptides (5 μg/ml) were used to stimulate virus-specific CD8+ and CD4+ T-cell responses. For IL-12 intracellular staining, IHL were incubated with LPS (1 μg/ml, Sigma) for 18 h. DC were cultured with viruses and cytokines for 3 days with a low dose of LPS (1 ng/ml) for the last 6 h. GolgiStop was added at the last 4 h. For intracellular granzyme B staining, cells do not need stimulation. After surface staining, cells were fixed, permeabilized and stained for intracellular cytokines by using IC fixation buffer (eBioscience). Samples were processed on an LSRII FACSFortessa (Becton Dickinson, San Jose, CA) and analyzed by using FlowJo software (TreeStar, Ashland, OR).
Quantitative RT- PCR
We extracted total RNA from frozen liver tissues with an RNeasy Mini kit (Qiagen) and digested it with DNase I (Ambion). cDNA was prepared from 1 μg of RNA by using an iScriptTM Reverse Transcription Kit (Bio-Rad). The quantitative RT-PCR (qRT-PCR) assays were performed with iQ SYBR Green Supermix and a CFX96 Touch Real-Time PCR Detection System (Bio-Rad). The PCR assays were denatured for 10 min at 95°C, followed by 40 cycles of 15 s at 95°C, and 60 s at 60°C. Melt-curve analysis was also used to check the specificity of the amplification reaction. Relative quantity of mRNA expression was calculated by using the 2−ΔΔCT method. The standard curve was generated by viral stock (5×107 PFU/ml). The primers are listed below. GAPDH, Forward 5′-TGGAAAGCTGTGGCGTGAT-3′ and Reverse 5′-TGCTTCACCACCTTCTTGAT-3′; IFN-γ, Forward 5′-ATGAACGCTACACACTGCATC-3′ and Reverse 5′-CCATCCTTTTGCCAGTTCCTC-3′; TNF-α, Forward 5′-ATAGCTCCCAGAAAAGCAAGC -3′ and Reverse 5′-CACCCCGAAGTTCAGTAGACA-3′; IL-33, Forward 5′-TCCAACTCCAAGATTTCCCCG-3′ and Reverse 5′-CATGCAGTAGACATGGCAGAA-3′; IL-12, Forward 5′-CAATCACGCTACCTCCTCTTTT-3′ and Reverse 5′-CAGCAGTGCAGGAATAATGTTTC -3′; ST2, Forward 5′-TGTATTTGACAGTTACGGAGGGC-3′ and Reverse 5′-ACTTCAGACGATCTCTTGAGACA-3′; CXCL9, Forward 5′-GGAGTTCGAGGAACCCTAGTG-3′ and Reverse 5′-GGGATTTGTAGTGGATCGTGC-3′; IL-1β, Forward 5′-GCAACTGTTCCTGAACTCAACT-3′ and Reverse 5′-ATCTTTTGGGGTCCGTCAACT-3′; IL-6, Forward 5′-TAGTCCTTCCTACCCCAATTTCC-3′ and Reverse 5′-TTGGTCCTTAGCCACTCCTTC-3′; CXCL10, Forward 5′-CCAAGTGCTGCCGTCATTTTC-3′and Reverse 5′-GGCTCGCAGGGATGATTTCAA-3′; and finally Ki-67, Forward 5′-CCTTTGCTGTCCCCGAAGA-3′ and Reverse 5′-GGCTTCTCATCTGTTTCCT-3′.
Bio-Plex assay
Liver cytokine profiles were characterized by using a ProcartaPlex Mouse Cytokine Panel (eBioscience). Briefly, liver protein was extracted by using RIPA (Cell Signaling Technology) plus Protease Inhibitor Cocktails (Sigma). The concentration of protein was determined by a Pierce™ BCA™ Protein Assay kit (Thermo Scientific). Colored magnetic beads coated with different antigens were mixed together with liver protein samples and then allowed to incubate overnight at 2–8°C. After three wash cycles, detection antibody was added and allowed to incubate for 1 h at room RT, followed by incubation with Streptavidin-Phycoerythrin for 30 min at RT. After removal of excess conjugate, 150 μl of sheath fluid was added to each well. The beads were read on a Bio-Rad Bio-Plex 200 System. Raw data were measured as the relative fluorescence intensity and then converted to the concentration according to the standard curve.
Cell culture
Lymph node (LN) cells were isolated from the inguinal LN of naïve WT mice and seeded at 1 × 106/ml in 24-well plates in complete RPMI medium for 4 days at 37°C with 5% CO2. rIL-7 (20 ng/ml) was used to promote γδ T-cell proliferation, as previously described [19]. rIL-33 of various concentrations (1, 10, 100 ng/ml) was added at the beginning of the culture to examine its effect on γδ T cells. CFSE were used to monitor lymphocyte proliferation, as previously described [50].
DC generation and treatment
Bone marrow-derived DCs (BMDCs) were generated from B6 mice by using complete RPMI-1640 medium containing 10% FBS (Hyclone, Logan, UT), supplemented with 20 ng/ml rGM-CSF (Peprotech), as described previously [51]. The culture medium was replaced with fresh GM-CSF-containing medium at days 3 and 6, respectively. DC were harvested at day 8 and adjusted to 1× 106/ml for experiments. DC were cultured with Cl 13 (MOI: 1) and IL-33 for 48 h, followed by flow cytometry and PCR detection.
Adoptive transfer of ILC2
Purification of ILC2 was performed, as previously described [52]. B6 mice were i.p. injected with rIL-33 (1 μg/mouse) for 5 days. Lineage marker negative cells (Lin-) were isolated from lymphoid organs (spleens, draining LN and mesenteric LN) by a lineage cell depletion kit (Miltenyi Biotec, San Diego, CA) and cultured in 24-well plates with rIL-7 and rIL-33 (both at 10 ng/ml). Cells from 2–6 days’ culture were analyzed by flow cytometry and used for adoptive transfer experiments as reported [7]. The expanded ILC2 (5 × 106 cells in 200 μl PBS) were i.v. transferred into mice at 1, 3 and 5 dpi (PBS was used as a control). All of the mice were sacrificed at 6 dpi.
ELISA
Levels of serum IFN-γ were assessed by using an ELISA kit (eBioscience) according to the manufacturer’s instructions.
Statistical analyses
Data were shown as mean ± SEM and analyzed by using the two-tailed Student’s T-test when compared between two groups. One-way ANOVA was used for statistical analysis of two more groups. *, ** or *** means p-value < 0.05, < 0.01 or < 0.001, respectively. Statistical analyses were operated by GraphPad Prism software 5.0 (GraphPad Software Inc., San Diego, CA).
Supplementary Material
Acknowledgments
We thank Dr. Robert Tesh for providing mouse anti-LCMV polyclonal antibody, Lihong Zhang for providing BHK and Vero cell lines, Kyle Wilson and Ms. Mardelle Susman for assistance with manuscript preparation. The authors wish to express gratitude to other members of the UTMB Joint Immunology Working Group (Drs. Soong, Cong, Stephens, Rajsbaum, and Sun and their trainees), for many helpful discussions. This work was supported in part by grants from the NIH (AI109100 to JS). Wei Wang was a visiting scientist partially supported by the Natural Science Foundation of Jiangsu Province (BK20141105, GW2014-3) and by a Jiangsu Government Scholarship for Overseas Studies (JS-2012-130). Zakari Kwota was a recipient of a summer internship from NIAID T35 training grant (AI078878 to LS), as well as the Stjepcevich Scholarship from the UTMB School of Medicine.
Abbreviations
- Ad
adenovirus
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- DAMP
damage-associated molecular pattern
- γδ
gamma delta
- IHL
intrahepatic lymphocyte
- ILC
innate lymphoid cell
- ILC2
group 2 innate lymphoid cell
- LCMV
lymphocytic choriomeningitis virus
- Lin
Lineage
- rIL
recombinant interleukin
Footnotes
Conflict of interest
The authors declare no financial or commercial conflict of interest.
References
- 1.Medzhitov R, Janeway CA., Jr Innate immune recognition and control of adaptive immune responses. Semin Immunol. 1998;10:351–353. doi: 10.1006/smim.1998.0136. [DOI] [PubMed] [Google Scholar]
- 2.Saito T, Owen DM, Jiang F, Marcotrigiano J, Gale M., Jr Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature. 2008;454:523–527. doi: 10.1038/nature07106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 4.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Oboki K, Ohno T, Kajiwara N, Arae K, Morita H, Ishii A, Nambu A, et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci U S A. 2010;107:18581–18586. doi: 10.1073/pnas.1003059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonilla WV, Frohlich A, Senn K, Kallert S, Fernandez M, Johnson S, Kreutzfeldt M, et al. The alarmin interleukin-33 drives protective antiviral CD8 T cell responses. Science. 2012;335:984–989. doi: 10.1126/science.1215418. [DOI] [PubMed] [Google Scholar]
- 7.Liang Y, Jie Z, Hou L, Aguilar-Valenzuela R, Vu D, Soong L, Sun J. IL-33 induces nuocytes and modulates liver injury in viral hepatitis. J Immunol. 2013;190:5666–5675. doi: 10.4049/jimmunol.1300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonneville M, O’Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol. 2010;10:467–478. doi: 10.1038/nri2781. [DOI] [PubMed] [Google Scholar]
- 9.Hayday AC. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity. 2009;31:184–196. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Jost S, Altfeld M. Control of human viral infections by natural killer cells. Annu Rev Immunol. 2013;31:163–194. doi: 10.1146/annurev-immunol-032712-100001. [DOI] [PubMed] [Google Scholar]
- 11.Probst HC, van den Broek M. Priming of CTLs by lymphocytic choriomeningitis virus depends on dendritic cells. J Immunol. 2005;174:3920–3924. doi: 10.4049/jimmunol.174.7.3920. [DOI] [PubMed] [Google Scholar]
- 12.Bhardwaj N, Seder RA, Reddy A, Feldman MV. IL-12 in conjunction with dendritic cells enhances antiviral CD8+ CTL responses in vitro. J Clin Invest. 1996;98:715–722. doi: 10.1172/JCI118843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.KleinJan A, Klein Wolterink RG, Levani Y, de Bruijn MJ, Hoogsteden HC, van Nimwegen M, Hendriks RW. Enforced expression of Gata3 in T cells and group 2 innate lymphoid cells increases susceptibility to allergic airway inflammation in mice. J Immunol. 2014;192:1385–1394. doi: 10.4049/jimmunol.1301888. [DOI] [PubMed] [Google Scholar]
- 14.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arshad MI, Piquet-Pellorce C, L’Helgoualc’h A, Rauch M, Patrat-Delon S, Ezan F, Lucas-Clerc C, et al. TRAIL but not FasL and TNFalpha, regulates IL-33 expression in murine hepatocytes during acute hepatitis. Hepatology. 2012;56:2353–2362. doi: 10.1002/hep.25893. [DOI] [PubMed] [Google Scholar]
- 16.Hou L, Jie Z, Desai M, Liang Y, Soong L, Wang T, Sun J. Early IL-17 production by intrahepatic T cells is important for adaptive immune responses in viral hepatitis. J Immunol. 2013;190:621–629. doi: 10.4049/jimmunol.1201970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, Girardi M, et al. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat Immunol. 2009;10:427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bai H, Cheng J, Gao X, Joyee AG, Fan Y, Wang S, Jiao L, et al. IL-17/Th17 promotes type 1 T cell immunity against pulmonary intracellular bacterial infection through modulating dendritic cell function. J Immunol. 2009;183:5886–5895. doi: 10.4049/jimmunol.0901584. [DOI] [PubMed] [Google Scholar]
- 19.Michel ML, Pang DJ, Haque SF, Potocnik AJ, Pennington DJ, Hayday AC. Interleukin 7 (IL-7) selectively promotes mouse and human IL-17-producing gammadelta cells. Proc Natl Acad Sci U S A. 2012;109:17549–17554. doi: 10.1073/pnas.1204327109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6:476–483. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- 21.Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J Exp Med. 2003;197:1141–1151. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Cai Y, Ji H, Feng J, Ayana DA, Niu J, Jiang Y. Serum IL-33 levels are associated with liver damage in patients with chronic hepatitis B. J Interferon Cytokine Res. 2012;32:248–253. doi: 10.1089/jir.2011.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Zhao P, Guo H, Sun X, Jiang Z, Xu L, Feng J, et al. Serum IL-33 levels are associated with liver damage in patients with chronic hepatitis C. Mediators Inflamm. 2012;2012:819636. doi: 10.1155/2012/819636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baumann C, Bonilla WV, Frohlich A, Helmstetter C, Peine M, Hegazy AN, Pinschewer DD, et al. T-bet- and STAT4-dependent IL-33 receptor expression directly promotes antiviral Th1 cell responses. Proc Natl Acad Sci U S A. 2015;112:4056–4061. doi: 10.1073/pnas.1418549112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toka FN, Kenney MA, Golde WT. Rapid and transient activation of gammadelta T cells to IFN-gamma production, NK cell-like killing, and antigen processing during acute virus infection. J Immunol. 2011;186:4853–4861. doi: 10.4049/jimmunol.1003599. [DOI] [PubMed] [Google Scholar]
- 26.Wang T, Scully E, Yin Z, Kim JH, Wang S, Yan J, Mamula M, et al. IFN-gamma-producing gamma delta T cells help control murine West Nile virus infection. J Immunol. 2003;171:2524–2531. doi: 10.4049/jimmunol.171.5.2524. [DOI] [PubMed] [Google Scholar]
- 27.Fang H, Welte T, Zheng X, Chang GJ, Holbrook MR, Soong L, Wang T. gammadelta T cells promote the maturation of dendritic cells during West Nile virus infection. FEMS Immunol Med Microbiol. 2010;59:71–80. doi: 10.1111/j.1574-695X.2010.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luzina IG, Pickering EM, Kopach P, Kang PH, Lockatell V, Todd NW, Papadimitriou JC, et al. Full-length IL-33 promotes inflammation but not Th2 response in vivo in an ST2-independent fashion. J Immunol. 2012;189:403–410. doi: 10.4049/jimmunol.1200259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bourgeois E, Van LP, Samson M, Diem S, Barra A, Roga S, Gombert JM, et al. The pro-Th2 cytokine IL-33 directly interacts with invariant NKT and NK cells to induce IFN-gamma production. Eur J Immunol. 2009;39:1046–1055. doi: 10.1002/eji.200838575. [DOI] [PubMed] [Google Scholar]
- 30.Nabekura T, Girard JP, Lanier LL. IL-33 Receptor ST2 Amplifies the Expansion of NK Cells and Enhances Host Defense during Mouse Cytomegalovirus Infection. J Immunol. 2015 doi: 10.4049/jimmunol.1500424. pii: 1500424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pandya AD, Al-Jaderi Z, Hoglund RA, Holmoy T, Harbo HF, Norgauer J, Maghazachi AA. Identification of human NK17/NK1 cells. PLoS One. 2011;6:e26780. doi: 10.1371/journal.pone.0026780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waggoner SN, Cornberg M, Selin LK, Welsh RM. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 2011;481:394–398. doi: 10.1038/nature10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang HR, Milovanovic M, Allan D, Niedbala W, Besnard AG, Fukada SY, Alves-Filho JC, et al. IL-33 attenuates EAE by suppressing IL-17 and IFN-gamma production and inducing alternatively activated macrophages. Eur J Immunol. 2012;42:1804–1814. doi: 10.1002/eji.201141947. [DOI] [PubMed] [Google Scholar]
- 34.Pellegrini M, Calzascia T, Toe JG, Preston SP, Lin AE, Elford AR, Shahinian A, et al. IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell. 2011;144:601–613. doi: 10.1016/j.cell.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Jie Z, Liang Y, Hou L, Dong C, Iwakura Y, Soong L, Cong Y, et al. Intrahepatic innate lymphoid cells secrete IL-17A and IL-17F that are crucial for T cell priming in viral infection. J Immunol. 2014;192:3289–3300. doi: 10.4049/jimmunol.1303281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schnell FJ, Sundholm S, Crumley S, Iversen PL, Mourich DV. Lymphocytic choriomeningitis virus infection in FVB mouse produces hemorrhagic disease. PLoS Pathog. 2012;8:e1003073. doi: 10.1371/journal.ppat.1003073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y, Munker S, Mullenbach R, Weng HL. IL-13 Signaling in Liver Fibrogenesis. Front Immunol. 2012;3:116. doi: 10.3389/fimmu.2012.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 39.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 40.Walsh KP, Mills KH. Dendritic cells and other innate determinants of T helper cell polarisation. Trends Immunol. 2013;34:521–530. doi: 10.1016/j.it.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 41.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Freeman BE, Hammarlund E, Raue HP, Slifka MK. Regulation of innate CD8+ T-cell activation mediated by cytokines. Proc Natl Acad Sci U S A. 2012;109:9971–9976. doi: 10.1073/pnas.1203543109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nambu A, Nakae S, Iwakura Y. IL-1beta, but not IL-1alpha, is required for antigen-specific T cell activation and the induction of local inflammation in the delayed-type hypersensitivity responses. Int Immunol. 2006;18:701–712. doi: 10.1093/intimm/dxl007. [DOI] [PubMed] [Google Scholar]
- 44.Ben-Sasson SZ, Hu-Li J, Quiel J, Cauchetaux S, Ratner M, Shapira I, Dinarello CA, et al. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc Natl Acad Sci U S A. 2009;106:7119–7124. doi: 10.1073/pnas.0902745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bottcher JP, Schanz O, Garbers C, Zaremba A, Hegenbarth S, Kurts C, Beyer M, et al. IL-6 trans-signaling-dependent rapid development of cytotoxic CD8+ T cell function. Cell Rep. 2014;8:1318–1327. doi: 10.1016/j.celrep.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 46.Besnard AG, Togbe D, Guillou N, Erard F, Quesniaux V, Ryffel B. IL-33-activated dendritic cells are critical for allergic airway inflammation. Eur J Immunol. 2011;41:1675–1686. doi: 10.1002/eji.201041033. [DOI] [PubMed] [Google Scholar]
- 47.Mayuzumi N, Matsushima H, Takashima A. IL-33 promotes DC development in BM culture by triggering GM-CSF production. Eur J Immunol. 2009;39:3331–3342. doi: 10.1002/eji.200939472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Louten J, Rankin AL, Li Y, Murphy EE, Beaumont M, Moon C, Bourne P, et al. Endogenous IL-33 enhances Th2 cytokine production and T-cell responses during allergic airway inflammation. Int Immunol. 2011;23:307–315. doi: 10.1093/intimm/dxr006. [DOI] [PubMed] [Google Scholar]
- 49.Welsh RM, Seedhom MO. Lymphocytic choriomeningitis virus (LCMV): propagation, quantitation, and storage. Curr Protoc Microbiol. 2008;Chapter 15(Unit 15A):11. doi: 10.1002/9780471729259.mc15a01s8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quah BJ, Parish CR. The use of carboxyfluorescein diacetate succinimidyl ester (CFSE) to monitor lymphocyte proliferation. J Vis Exp. 2010 doi: 10.3791/2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vargas-Inchaustegui DA, Xin L, Soong L. Leishmania braziliensis infection induces dendritic cell activation, ISG15 transcription, and the generation of protective immune responses. J Immunol. 2008;180:7537–7545. doi: 10.4049/jimmunol.180.11.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.