Abstract
Objective
Posttraumatic stress disorder (PTSD) has been linked to chronic inflammation, a condition that poses a risk for cardiovascular disease. Attenuated vagal activity has been proposed as a potential mediator of PTSD and inflammation, although associated behavioral health risks—namely cigarette smoking and alcohol dependence—might also account for that link.
Methods
Inflammation was quantified by fasting serum concentrations of C-reactive protein (CRP), tumor necrosis factor (TNF)-α, interleukin (IL)-10, and thymus- and activation-regulated chemokine (TARC)/CCL17 collected from 85 participants with PTSD and 82 without PTSD. Latent variable modeling was used to assess the relationship between PTSD symptom severity and inflammation along with potential mediators vagal activity (respiratory sinus arrhythmia; RSA), smoking status, and lifetime alcohol dependence.
Results
PTSD symptom severity was associated with increased inflammation (β = .18, p = .02). However, this association was reduced in models that adjusted for RSA, smoking status, and lifetime alcohol dependence. Independent mediation effects were deemed significant via bootstrapping analyses. Together, RSA, smoking status, and lifetime alcohol dependence accounted for 95% of the effect of PTSD symptom severity on inflammation.
Conclusion
Although RSA accounted for a modest proportion of the association between posttraumatic stress and pro-inflammatory responses, behavioral factors – specifically cigarette smoking and alcohol dependence – proved to be larger mediators. The benefits of PTSD treatment may be enhanced by additional interventions aimed at modifying these health behaviors.
Keywords: posttraumatic stress disorder, inflammation, cytokines, cigarette smoking, alcohol dependence, vagal tone
Introduction
Posttraumatic stress disorder (PTSD) is a chronic condition precipitated by exposure to a traumatic event. It is characterized by intrusive re-experiencing of the traumatic event, avoidance of stimuli evocative of that event, negative alterations in cognitions and mood, and hyperarousal (1). PTSD also frequently conveys physical health symptoms, perhaps most notably cardiovascular disease (2). For instance, PTSD has been prospectively associated with coronary heart disease (3) and cardiovascular mortality (4). Although the pathway from posttraumatic stress to cardiovascular risk is not well understood, emerging evidence suggests that inflammation may play a key role (5).
Under conditions of heightened threat or stress, the sympathetic nervous system activates a “fight or flight” response, characterized by increased cardiovascular and metabolic activity. The immune system also responds in kind, presumably to stave off infections resulting from injuries sustained during such fight or flight. The initial immune response is fast and generalized, during which numbers of phagocytes, including neutrophils and macrophages, are mobilized. Macrophages in turn release pro-inflammatory communication factors (cytokines), including interleukin (IL)-1, IL-6, C-reactive protein (CRP), and tumor necrosis factor alpha (TNF-α), which cause fever and inflammation while contributing to healing. A second, more specific immune response is also initiated in which lymphocytes become activated upon attaching to chemically matched pathogens, thereby initiating lymphocyte expansion and cytokine release. These cytokines include the pro-inflammatory IL-2 and interferon gamma (IFN-γ) as well as the anti-inflammatory IL-4, IL-10, and thymus- and activation-regulated chemokine (TARC/CLL17), which regulate lymphocyte activity.
Over the past two decades, a number of studies have found that psychological stress is associated with elevated cytokine levels, reflecting heightened inflammation (6). For instance, several studies have found that exposure to trauma in childhood (7–9) and in adulthood (10) is subsequently predictive of increased inflammation. One study even found that increased cytokine levels post-trauma were predictive of later development of PTSD (11). In fact, with the exception of a few studies (12–15), PTSD is generally associated with increased cytokine levels (16–22), even above and beyond the effect of trauma exposure (23, 24).
The link between PTSD and inflammation is complex but may be partially explained by behavioral risk factors associated with PTSD (22). For instance, individuals with PTSD are more likely than those without PTSD to smoke and do so heavily (25), be obese (26), and abuse alcohol (27). Each of these risk factors is independently associated with inflammation (28–30). Autonomic dysfunction may also partially account for the association between PTSD and inflammation. Individuals with PTSD exhibit suppressed heart-rate variability (HRV) (31–33), which is likely due to attenuated vagal regulation of sympathetic arousal (34). Given the central role of the vagus nerve in inhibiting generalized immune response (35–37), vagal dysregulation has been proposed as a pathway by which PTSD is associated with chronic inflammation (38).
Although behavioral risk factors and depressed vagal activity have been suggested as potential mechanisms linking PTSD and inflammation, no research has verified this let alone compared their relative mediation effects. Thus, the purpose of the present study was to determine whether the association between PTSD symptom severity and inflammation is partially mediated by vagal activity, smoking status, and history of alcohol dependence, and, if so, which mediator accounts for the largest portion of that association. As such, fasting serum concentrations of CRP, TNF-α, IL-10, and TARC were assayed from a sample of young (i.e., < 40 years of age), largely trauma-exposed adults. Latent variable modeling was used to model inflammation via the four cytokines en route to testing three sets of hypotheses: 1) PTSD symptom severity is positively associated with inflammation; 2) PTSD symptom severity is associated with reduced vagal activity, greater smoking, and higher rates of lifetime alcohol dependence; and 3) vagal activity, smoking status, and lifetime alcohol dependence partially mediate the association between PTSD symptom severity and inflammation.
Material and methods
Participants
Participants were 167 young adults (18–39 years old; 80 women), including 63 U.S. military veterans, who were recruited via fliers displayed in hospital clinics and waiting rooms as well as online ads such as Craigslist to complete a study of the metabolic, cardiovascular, and neuroimmunological risk factors associated with trauma exposure. Criteria for exclusion from the study included presence of a) organic mental disorder, b) schizophrenia, c) bipolar I mixed state or bipolar II, d) lifetime PTSD without current PTSD, e) current substance abuse/dependence, f) current major depressive disorder (MDD) without PTSD, g) pregnancy, h) AIDS or HIV, and i) uncontrolled medical condition (e.g., liver failure). Eleven women using birth control and three participants using statins were additionally omitted from the present analysis in light of the potential effects of these drugs on cytokine levels. The study was approved by both the Durham Veterans Affairs and Duke University Medical Center Institutional Review Boards. All patients gave written informed consent before participation. Data were collected between August 2008 and September 2013. Previous findings from this sample include linkages between PTSD and orthostatic hypotension (39), decreased HRV (32), and dyslipidemia (40).
Measures
Posttraumatic Stress Disorder
PTSD status was assessed using the Clinician Administered PTSD Scale (CAPS) (41), based on DSM-IV criteria (42). The 17-item structured interview was administered by a licensed clinical psychologist or by a trainee under the direct supervision of a licensed clinical psychologist. Interrater reliability among interviewers was high (Fleiss’ k = .94) across five training tapes. The CAPS interview has excellent reliability within multiple trauma populations and is widely accepted as the state-of-the-art method for PTSD assessment (43).
The 17-item self-report Davidson Trauma Scale (DTS) (44) was used to quantify PTSD symptom severity based on DSM-IV criteria. Each item measures the frequency (0, ―not at all,‖ to 4, “everyday”) and intensity (0, “not at all distressing,” to 4, “extremely distressing”) of corresponding symptoms. Total symptom severity scores were calculated by summing frequency and intensity ratings across all items.
Vagal Activity
One commonly used method for measuring vagal cardiac control, or activity, is via respiratory sinus arrhythmia (RSA) (45), which refers to the naturally occurring fluctuations in heart rate associated with breathing. To capture RSA, beat-by-beat blood pressure and heart rate data were measured continuously using the Finometer noninvasive blood-pressure monitor (Finapres Medical Systems, Amsterdam) under supine conditions. Following five minutes of calibration, one 5-minute file of continuous blood pressure and heart rate measurements was recorded for assessment of RSA while the participants breathed at their regular rate of breathing. Blood pressure waveforms were reviewed, and artifacts due to movement or abnormal heart beats was removed and replaced by the pulse interval values from the preceding beat(s). The beat-by-beat systolic pressure and heart rate data were linearly interpolated and resampled at a frequency of 4 Hz in order to generate an equally spaced time series for the variables. A fast Fourier transform was then applied to the interpolated data after detrending and application of a Hanning filtering window. Power spectra were derived using the Welch algorithm, which ensemble averages successive periodograms (46). The averaged spectrum was derived from the power spectra estimated from nine 60-second data segments, overlapping by half. For each 60-second segment, 256 points were analyzed, which included 240 sampled points with zero padding. Consistent with prior research (47–49), RSA was estimated from the R-R interval power summed across the high-frequency 0.13- to 0.50-Hz respiratory band. Raw RSA was log-transformed before analysis in order to normalize values.
Smoking Status
Smoking status was operationalized based on participants’ responses to the Fagerström Test for Nicotine Dependence (50). Non-smokers were assigned a value 0; past— but not present—smokers, 1; current smokers who consume 10 or fewer cigarettes per day, 2; and current smokers who consume more than 10 cigarettes per day, 3.
Lifetime Alcohol Dependence
The Structured Clinical Interview for the DSM-IV (SCID) (51) was used to assess Axis I disorders, including lifetime alcohol dependence. Study interviewers completed an extensive training program involving the rating of seven video-recorded interviews. Interviewers additionally participated in biweekly reliability meetings and were supervised by licensed clinical psychologists. Interrater reliability among interviewers for Axis I diagnoses was high (Fleiss’ k = .96).
Trauma Exposure
Trauma exposure was measured using the Traumatic Life Events Questionnaire (TLEQ) (52), a self-report questionnaire that documents 23 distinct types of traumas including the time of their occurrence. For the purposes of this study, trauma exposure was operationalized as the number of years since one’s initial exposure to a traumatic event resulting in feelings of fear, helplessness, and horror. Participants who were never exposed to such a trauma were assigned a trauma-exposure value of 0. We included years of trauma exposure, as opposed to number of types of traumatic experiences or childhood versus adulthood trauma exposure, in the present analysis as a covariate to minimize variance in allostatic load due to varying lengths of trauma exposure.
Procedure
Participants completed an initial interview and battery of questionnaires in person at intake, including the measures listed above. Health status and current medications were also recorded. Anthropometric measures were taken, including height and weight, from which body-mass index (BMI) was calculated. RSA was measured one week later.
Serum samples were collected at a subsequent session visit between 10am and 1pm to assess overnight fasting cytokine profile. Serum was separated by centrifugation, aliquoted, and stored at −70 F until time of assay. Plasma CRP, TNF-α, IL-10, and TARC were assayed in duplicate using ELISA technology (Meso Scale Discovery, Rockville, MD). The mean lower levels of detection (LLOD) were: CRP = 1.24 pg/ml, IL-10 = 0.23 pg/ml, TARC = 25.42 pg/ml, and TNF-α = 0.21 pg/ml.
A normal human control serum was run on each of the plates to assess plate-to-plate variability. The mean intra-assay coefficients of variation (CV) were: CRP = 1.2%, IL-10 = 4.9%, TARC = 3.2%, and TNF-α = 3.0%. Samples with intra-assay CV > 20% were deemed unreliable and treated as missing. As a result, IL-10 concentration was dropped for one participant with PTSD. Samples falling below the LLOD were replaced with ½ the LLOD. Two samples (n = 1 with PTSD) were replaced on account of low IL-10 concentrations. Samples were log-transformed to achieve normality and were similarly analyzed for extreme outliers (i.e., ≥ 5 SD above the mean). One participant with PTSD had an outlying IL-10 concentration that was dropped.
Analytic Plan
Latent variable modeling was used to test the hypothesis that PTSD symptom severity would be associated with increased inflammation, with subsequent models conducted to the test the mediation hypotheses. Initially, a latent variable representing inflammation was specified using log-transformed concentrations of CRP, IL-10, TARC, and TNF-α. The purpose of this was to minimize the measurement error in cytokine levels. The adequacy of the inflammation latent variable was determined prior to further modeling using standard fit criteria (53, 54): root mean square error of approximation (RMSEA) ≤ .05, comparative fit index (CFI) ≥ .90, and standardized root mean square residual (SRMR) < .05. The chi-squared test of model fit was also consulted, with non-significance indicative of good model fit.
Initially a direct-effects model was conducted to test the association between PTSD symptom severity and inflammation. In subsequent models, the indirect effect of PTSD symptoms on inflammation via RSA and associated behavioral risk factors was tested. To test the significance of mediation, bootstrapped confidence intervals around the indirect effects of PTSD symptoms on inflammation were generated using resampling. This method offers an advantage over conventional tests, such as Sobel’s z, because it takes into account the positive skew inherent to indirect effects. As such, bootstrapping methods are more powerful than conventional tests, with mediation deemed significant when the resulting confidence interval does not span 0.
In each of the models, age, BMI, and steroid use (e.g., prednisone) were covaried, given their associations with cytokine levels (28, 55, 56). Note that BMI was not tested as a potential mediator of PTSD symptom severity and inflammation in light of previously published analyses from this sample indicating that PTSD symptom severity was unassociated with obesity (32). Years since initial exposure to a traumatic event resulting in fear, helplessness, and horror was additionally controlled to assess the effect of PTSD status on inflammation independent of trauma exposure. All models were specified with PROC CALIS in SAS v9.2. Full information maximum likelihood estimation was used to handle missing data.
Results
Participant characteristics and intercorrelations between study variables are presented in Table 1. Eighty-five participants (51%) met criteria for current PTSD. Only 10 participants reported no exposure to a traumatic event resulting in fear, helplessness, and horror. Incidentally, the sample mean on the DTS, 45.72, fell directly between the means from a normative sample (57) for subthreshold PTSD with impairments (M = 20.5) and threshold PTSD (M = 67.1). As hypothesized, PTSD symptom severity was negatively associated with RSA and positively associated with smoking and history of alcohol dependence. However, the bivariate correlations between PTSD symptom severity and unadjusted cytokine levels failed to reach significance.
Table 1.
Participant Characteristics and Intercorrelations
| Intercorrelations (r-values) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Means (SD)/Freq (%) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10)a | (11)a | (12)a | (13)a |
| Age (1) | 29.78 (5.53) | −.03 | .07 | .09 | .61** | .22** | .05 | .22** | −.28** | .09 | .01 | −.12 | −.10 |
| Sex, female (2) | 80 (48%) | .10 | .11 | .06 | −.08 | −.18* | −.31** | .14† | .04 | −.16* | −.12 | −.06 | |
| BMI (3) | 29.83 (6.69) | .11 | .06 | .08 | .05 | .07 | −.01 | .50** | .22** | .04 | .17* | ||
| Steroid use (4) | 7 (4%) | .07 | .05 | −.16* | −.04 | −.07 | .18** | .01 | −.00 | −.19* | |||
| Trauma exposure, years (5) | 17.89 (9.65) | .44** | .15* | .28** | −.17* | .07 | −.09 | −.02 | −.01 | ||||
| DTS total (6) | 45.72 (37.78) | .39** | .31** | −.17* | .04 | .06 | .15† | .03 | |||||
| Smoking status (7) | 0.77 (0.83) | .34** | −.15† | .01 | .13 | .06 | .13 | ||||||
| LT alcohol dependence (8) | 53 (32%) | −.22** | .10 | .16* | −.04 | .06 | |||||||
| RSA, logged (9) | 6.49 (1.25) | −.07 | −.11 | −.11 | −.04 | ||||||||
| Cytokine levels, pg/ml | |||||||||||||
| CRP (10) | 4628.92 (6919.20) | .27** | .23** | .10 | |||||||||
| TNF-α (11) | 3.20 (1.17) | .33** | .15* | ||||||||||
| IL-10 (12) | 1.19 (1.79) | .13† | |||||||||||
| TARC (13) | 902.16 (760.15) | ||||||||||||
Note. Correlations between sex, steroid use, and LT alcohol dependence are tetrachoric. BMI = body-mass index; DTS total = Davidson Trauma Scale total score; LT Alcohol Dependence = lifetime alcohol dependence; RSA, logged = respiratory sinus arrhythmia (logged values); CRP = C-reactive protein; TNF-α = tumor necrosis factor-α; IL-10 = interleukin-10; TARC = thymus activation-regulated chemokine.
Spearman rank-order correlations reported given pronounced positive skew of cytokine levels.
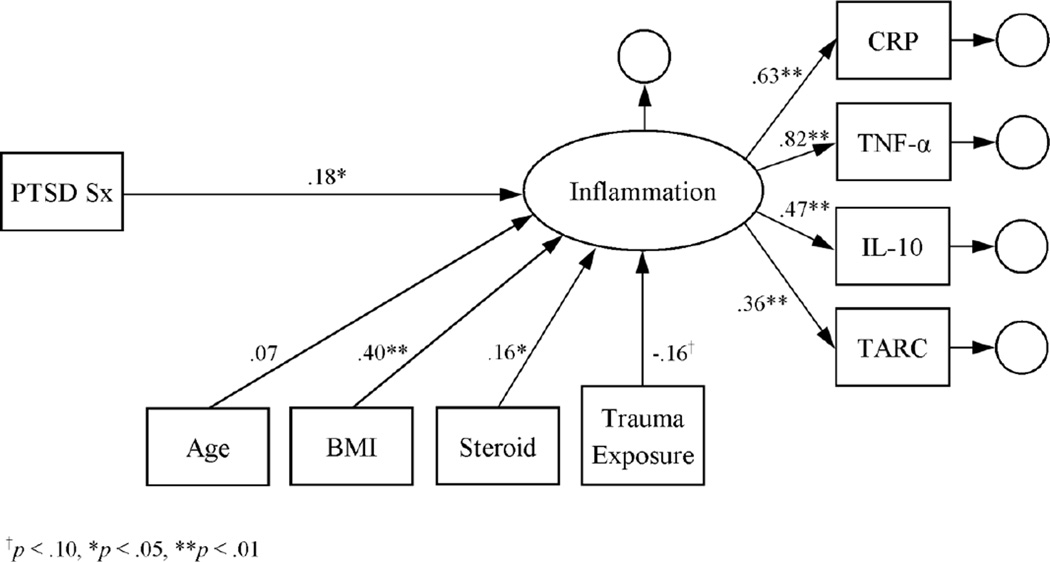
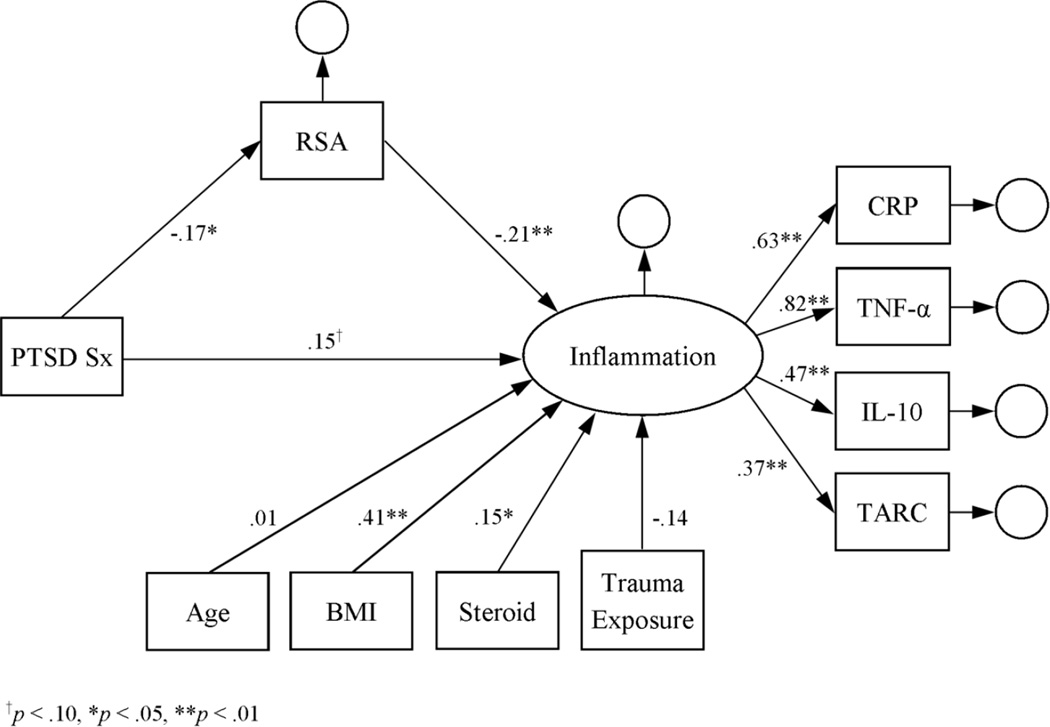
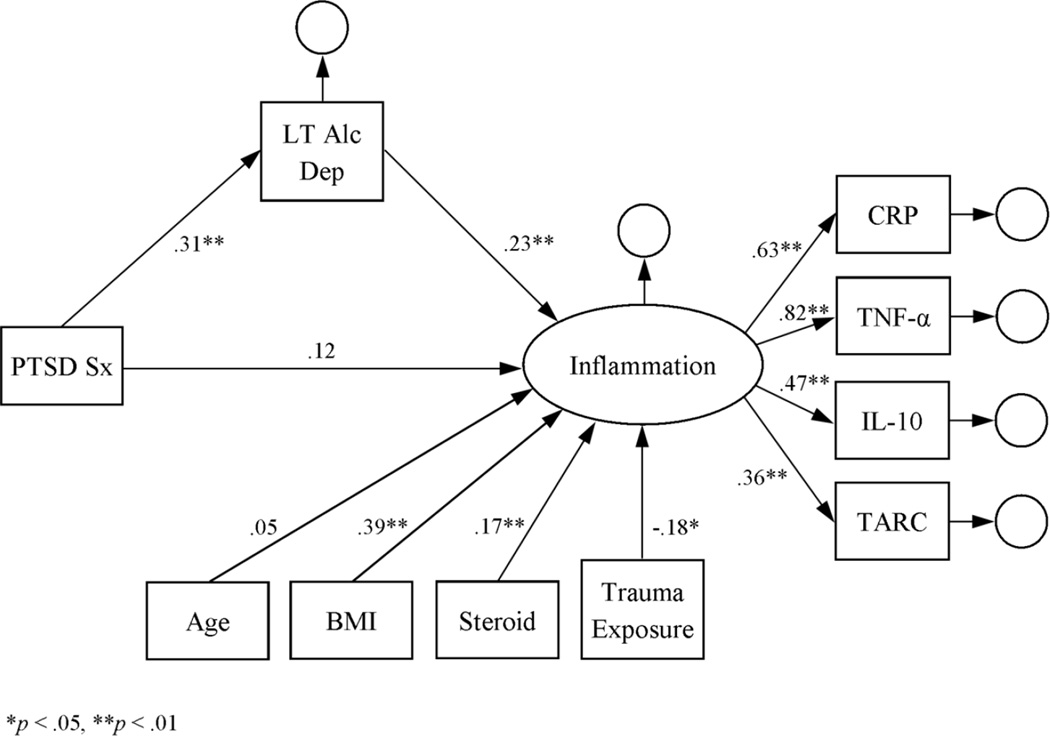
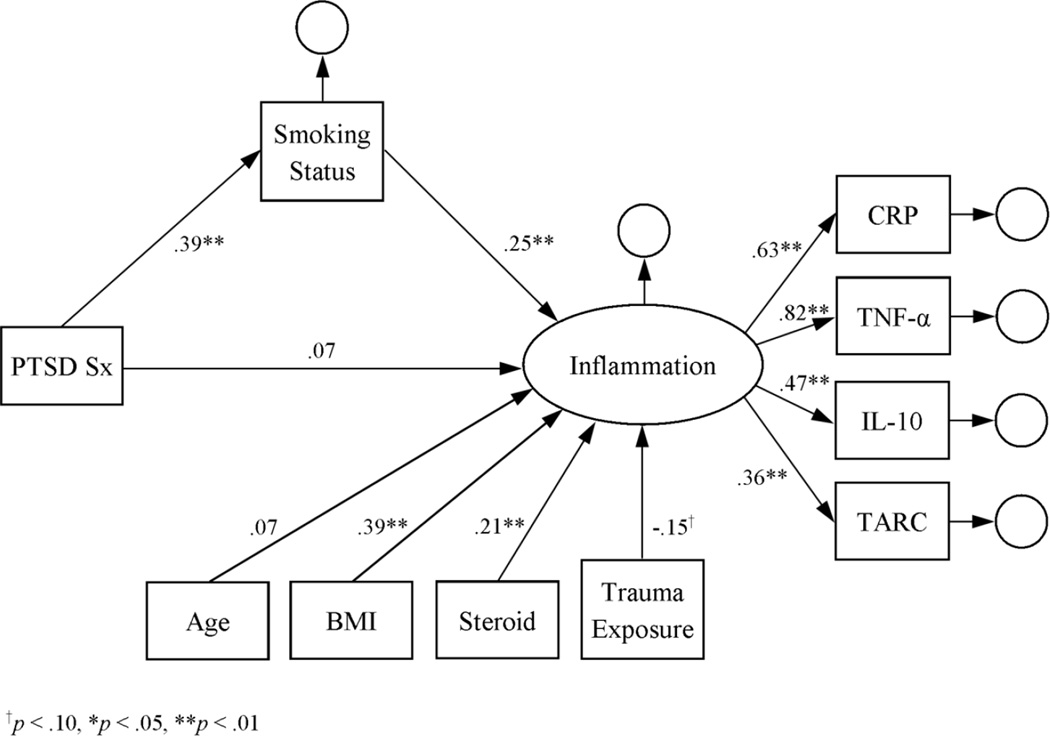
The initial measurement model of the inflammation latent variable indicated that it was a good fit for the log-transformed cytokine data: RMSEA = 0.004, CFI = 1.000, SRMR = 0.005, and X2(1) = 0.07, p = .79. According to a main-effects latent-variable model, inflammation was positively associated with BMI and steroid use (see Figure 1). Inflammation was also positively associated with PTSD symptom severity, thus supporting the first hypothesis. Subsequent modeling further indicated that the associations of RSA, smoking status, and lifetime alcohol dependence with inflammation were significant in the latent-variable models (see Figures 2–4). Moreover, the direct effect of PTSD symptom severity on inflammation was attenuated in the presence of each, suggesting mediation.
Figure 1.
Latent variable model of inflammation as a function of PTSD symptom severity. Estimates represent standardized coefficients. N = 164.
Figure 2.
Latent variable model of inflammation as a function of PTSD symptom severity and RSA. Estimates represent standardized coefficients. N = 164.
Figure 4.
Latent variable model of inflammation as a function of PTSD symptom severity and lifetime alcohol dependence. Estimates represent standardized coefficients. N = 164.
To test significance of RSA, smoking-status, and alcohol-dependence mediation effects, bootstrapped confidence intervals (CI) around the indirect effects of PTSD symptom severity on inflammation were generated from 500 re-samples. In support of the third hypothesis, the indirect effects for RSA (bootstrapped 95% CI of standardized effect: .00 – .09), smoking status (bootstrapped 95% CI of standardized effect: .04 – .17), and lifetime alcohol dependence (bootstrapped 95% CI of standardized effect: .03 – .16) were each significant in separate models, independently accounting for 23%, 57%, and 51% of the effect of PTSD symptom severity on inflammation. In combination, the three mediators accounted for 95% of the effect of PTSD symptom severity on inflammation (bootstrapped 95% CI of combined standardized effects: .06 −.22), with both health risks, but not RSA (bootstrapped 95% CI for standardized effect: −.00 −.07), maintaining significance as independent mediators (bootstrapped 95% CI for standardized effect of smoking status: .02 – .14; bootstrapped 95% CI for standardized effect of lifetime alcohol dependence: .01 – .11).
Given evidence that smoking (58) and drinking (59) are associated with vagal activity, mediation of the association between PTSD symptom severity and RSA by smoking status and lifetime alcohol dependence was further investigated. However, the total effect of PTSD symptom severity on RSA after controlling for age, BMI, steroid use, and trauma exposure was not significant (β = −.14, p = .10), thus ruling out mediation.
Discussion
The present study examined the association of PTSD symptoms with serum cytokine levels along with potential psychophysiological and behavioral health mediators within a sample of young adults. Consistent with previous work (23, 24), PTSD symptom severity was positively associated with inflammation (i.e., higher cytokine levels) independent of trauma exposure. A novel finding was that this association was partially mediated by attenuated vagal activity as well as smoking status and lifetime alcohol dependence. The behavioral risk variables each explained twice as much of the covariance between PTSD symptom severity and inflammation as vagal activity. Together, the three mediators accounted for the entire effect of PTSD symptom severity on inflammation.
These findings represent an important contribution to the growing literature linking PTSD with inflammation. Although short-term inflammation may be beneficial for staving off infection, long-term inflammation is well-known to increase the risk of chronic medical illness. For one, elevated concentrations of circulating pro-inflammatory cytokines activate inflammatory processes within the cells of blood vessel walls, promoting apoptosis, the adhesion of macrophages to the wall surfaces, and the formation of smooth muscle fibers over the resulting lesions (60). These processes accelerate atherosclerosis and potentiate thrombosis, increasing risk of myocardial infarction and stroke (61). Chronic inflammation has also been implicated in the development of insulin resistance. Elevated levels of circulating proinflammatory cytokines, primarily TNF-α, induce inflammatory signaling within insulin target cells, thereby disrupting normal insulin-receptor signalizing (62). Thus, inflammation may mark an important pathway by which PTSD conveys cardiovascular and metabolic risk.
In turn, the association between PTSD and inflammation may be largely due to risky health behaviors. Although the hypothesis that attenuated vagal activity partially explains under-regulation of pro-inflammatory processes in PTSD was substantiated, the mediation effect was small, particularly in comparison to those for smoking status and lifetime alcohol dependence. Moreover, the mediation effect for vagal activity was rendered non-significant in a model controlling for smoking status and lifetime alcohol dependence. Considering that smoking (58) and drinking (59) are both known to depress vagal activity, to some extent they may have driven the association between PTSD symptom severity and RSA. In fact, in a recent study based on a subset of the present sample, smoking and drinking accounted for the majority of the association between PTSD symptom severity and HRV, which is influenced by RSA (32). In the present sample, however, there was no support for mediation of PTSD symptom severity and RSA by either behavioral health risk.
It is significant that smoking and drinking accounted for so much of the association between PTSD symptom severity and inflammation. The detection of these effects among young adults reveals just how rapidly PTSD-related health problems may arise due to poor health behaviors. As such, our results emphasize the importance of efforts to reduce nicotine and alcohol consumption among individuals with PTSD. Individuals with PTSD are at least twice as likely as non-affected individuals to smoke (63, 64) and 1.5 times as likely to meet criteria for alcohol abuse or dependence (65). These behaviors pose substantial health risks. As mentioned above, smoking and drinking accounted for a large portion of the covariance between PTSD and HRV in a subset of this sample (32). In two additional sets of analyses from the same sample, smoking and drinking were similarly implicated in linking PTSD with orthostatic hypotension (39) and dyslipidemia (40). Thus, efforts to intervene upon these health risk behaviors could potentially pose a huge impact on the health outlook of trauma-exposed individuals.
Of course, the present findings must be taken into the context of several limitations. For one, the cross-sectional design limits our interpretation of the directionality of the associations. Moreover, mediation detected in cross-sectional data cannot always be generalized to a longitudinal process (66). Despite these limitations, our interpretation is consistent with the conventional view that PTSD engenders excessive use of nicotine and alcohol, and that these health risks are known to convey chronic inflammation (25, 27). Future work testing the prospective effect of PTSD symptom severity on health risks and subsequent inflammation is clearly warranted.
Another limitation was the exclusion of individuals with current alcohol abuse or dependence and the dichotomous nature of the lifetime-alcohol-dependence variable. These likely diminished the sensitivity of that construct. Nevertheless, its influence on inflammation was sufficient in the present study to demonstrate a significant mediation effect. In any case, future research in this area should make use of higher-resolution measures of alcohol consumption and misuse.
A last consideration is the reliability of cytokine levels as an indicator of chronic inflammation. The extant literature on PTSD and inflammation is far from definitive, with a number of studies demonstrating at least mixed evidence that PTSD is associated with decreased, rather than increased, levels of pro-inflammatory cytokines (12, 13, 15) or no association at all (14). In the present analysis, PTSD symptom severity was associated with increases in both pro-and anti-inflammatory cytokines, which stands in contrast to other research demonstrating a negative association between PTSD and anti-inflammatory cytokine levels (12, 13, 22). However, a compensatory anti-inflammatory response likely accounts for the present findings of increased pro- and anti-inflammatory cytokine levels associated with PTSD symptom severity (67). Regardless, the temporal reliability of the four cytokines selected for the present study is moderate to high, with 2-year intra-class correlations (ICC) of .76 for CRP, .69 for TNF-α, and .75 for IL-10 (68), and a 3-year ICC of .52 for TARC (69). These suggest that a single measurement of each should provide a fair indication of inflammation. Moreover, our use of latent-variable modeling served to minimize further measurement error by modeling an unobserved, theoretical construct (i.e., inflammation) believed to influence each of the cytokine serum concentrations. These steps likely aided our efforts to gather precise, reliable findings in support of our hypotheses. That the bivariate associations between PTSD symptom severity and cytokine levels failed to reach significance—only the association between symptom severity and IL 10 approached significance—highlights the complexity of these cytokines and the large number of factors that could potentially influence their levels. The amount of noise inherent to their quantification likely underlies the variability in findings associating PTSD with cytokine levels. The use of latent variable modeling to construct an empirically derived composite score based on each of the cytokines represents a potentially useful technique for quantifying inflammation in future research.
In sum, study results further emphasize the deleterious effects of PTSD on health, even among younger adults. In the present study, PTSD symptom severity was significantly associated with inflammation, which is a known cardiovascular and metabolic risk. Moreover, major contributors to this linkage were smoking and alcohol dependence. These findings complement evidence from three additional findings based on the same sample indicating that PTSD is also associated with increased risk of orthostatic hypotension (39), attenuated HRV (32), and dyslipidemia (40). In those analyses, smoking and alcohol dependence were also identified as potential mechanisms for cardiovascular risk. Together, these findings underscore the extent to which interventions for individuals with PTSD aimed at smoking and alcohol cessation could yield meaningful, long-term benefits, both psychiatric and cardiovascular.
Figure 3.
Latent variable model of inflammation as a function of PTSD symptom severity and smoking status. Estimates represent standardized coefficients. N = 164.
Highlights.
Fasting serum cytokine levels of young adults with and without PTSD were assessed.
PTSD symptom severity was positively associated with CRP, TNF-α, IL-10, and TARC.
Smoking, alcohol dependence, and RSA each independently mediated that association.
The effects of smoking and alcohol dependence were larger than that of RSA.
All three mediators accounted for 95% of the link between PTSD and cytokine levels.
Acknowledgments
Preparation of this work was supported by the National Institute of Mental Health (2R01MH062482), the National Institute on Drug Abuse (5K24DA016388), the Durham, NC Veterans Affairs Medical Center, and the Department of Veterans Affairs office of Research and Development Clinical Science.
The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Department of Veterans Affairs, or the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors have no competing interests to report.
Statement of Ethics
All procedures followed were in accordance with the ethical standards of the Duke University Medical Center and Durham Veterans Affairs Institutional Review Boards and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for their inclusion in the study.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 2.Buckley TC, Green BL, Schnurr PP, Trauma PTSD. Assessing psychological trauma and PTSD. New York: Guilford Press; 2004. physical health; pp. 441–465. [Google Scholar]
- 3.Kubzansky LD, Koenen KC, Spiro A, Vokonas PS, Sparrow D. Prospective study of posttraumatic stress disorder symptoms and coronary heart disease in the Normative Aging Study. Arch Gen Psychiatry. 2007;64:109–116. doi: 10.1001/archpsyc.64.1.109. [DOI] [PubMed] [Google Scholar]
- 4.Boscarino JA. A prospective study of PTSD and early-age heart disease mortality among Vietnam veterans: Implications for surveillance and prevention. Psychosom Med. 2008;70:668–676. doi: 10.1097/PSY.0b013e31817bccaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plantinga L, Bremner JD, Miller AH, Jones DP, Veledar E, Goldberg J, Vaccarino V. Association between posttraumatic stress disorder and inflammation: A twin study. Brain Behav Immun. 2013;30:125–132. doi: 10.1016/j.bbi.2013.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Segerstrom SC, Miller GE. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carpenter LL, Gawuga CE, Tyrka A, Lee A, Anderson GM, Price LH. Association between plasma IL-6 response to acute stress and early-life adversity in heathy adults. Noeropsychopharmacology. 2010;35:2617–2623. doi: 10.1038/npp.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiecolt-Glaser JK, Gouin J-P, Weng N-p, Malarkey WB, Beversdorf DQ, Glaser R. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosom Med. 2011;73:16–22. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Donovan A, Neylan TC, Metzler T, Cohen BE. Lifetime exposure to traumatic psychological stress is associated with elevated inflammation in the Heart and Soul Study. Brain Behav Immun. 2012;26:642–649. doi: 10.1016/j.bbi.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pervanidou P, Kolaitis G, Charitaki S, Margeli A, Ferentinos S, Bakoula C, Lazaropoulou C, Papassotiriou I, Tsiantis J, Chrousos GP. Elevated morning serum interleukin (IL)-6 or evening salivary cortisol concentrations predict posttraumatic stress disorder in children and adolescents six months after a motor vehicle accident. Psychoneuroendocrinology. 2007;32:991–999. doi: 10.1016/j.psyneuen.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Ironson G, Wynings C, Schneiderman N, Baum A, Rodriguez M, Greenwood D, Benight C, Antoni M, LaPerriere A, Huang H-S. Posttraumatic stress symptoms, intrusive thoughts, loss, and immune function after Hurricane Andrew. Psychosom Med. 1997;59:128–141. doi: 10.1097/00006842-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Kawamura N, Kim Y, Asukai N. Suppression of cellular immunity in men with a past history of posttraumatic stress disorder. Am J Psychiatry. 2001;158:484–486. doi: 10.1176/appi.ajp.158.3.484. [DOI] [PubMed] [Google Scholar]
- 14.McCanlies EC, Araia SK, Joseph PN, Mnatsakanova A, Andrew ME, Burchfiel CM, Violanti JM. C-reactive protein, interleukin-6, and posttraumatic stress disorder symptomology in urban police officers. Cytokine. 2011;55:74–78. doi: 10.1016/j.cyto.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 15.Sondergaard HP, Hansson LO, Theorell T. The inflammatory markers C-reactive protein and serum amyloid A in refugees with and without posttraumatic stress disorder. International Journal of Clinical Chemistry. 2004;342:93–98. doi: 10.1016/j.cccn.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 16.Guo M, Liu T, Guo J-C, Jiang X-L, Chen F, Gao Y-S. Study on serum cytokine levels in posttraumatic stress disorder patients. Asian Pacific Journal of Tropical Medicine. 2012;5:323–325. doi: 10.1016/S1995-7645(12)60048-0. [DOI] [PubMed] [Google Scholar]
- 17.Hoge E, Brandstetter K, Moshier S, Pollack M, Wong K, Simon N. Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress Anxiety. 2009;26:447–455. doi: 10.1002/da.20564. [DOI] [PubMed] [Google Scholar]
- 18.Maes M, Lin AH, Delmeire L, Van Gastel A, Kenis G, De Jongh R, Bosmans E. Elevated serum interleukin-6 (IL-6) and IL-6 receptor concentrations in posttraumatic stress disorder following accidental man-made traumatic events. Biol Psychiatry. 1999;45:833–839. doi: 10.1016/s0006-3223(98)00131-0. [DOI] [PubMed] [Google Scholar]
- 19.Miller R, Sutherland A, Hutchison J, Alexander D. C-reactive protein and interleukin 6 receptor in post-traumatic stress disorder: A pilot study. Cytokine. 2001;13:253–255. doi: 10.1006/cyto.2000.0825. [DOI] [PubMed] [Google Scholar]
- 20.Rohleder N, Joksimovic L, Wolf JM, Kirschbaum C. Hypocortisolism and increased glucocorticoid sensitivity of pro-Inflammatory cytokine production in Bosnian war refugees with posttraumatic stress disorder. Biol Psychiatry. 2004;55:745–751. doi: 10.1016/j.biopsych.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 21.Spivak B, Shohat B, Mester R, Avraham S, Gil-Ad I, Bleich A, Valevski A, Weizman A. Elevated levels of serum interleukin-1β in combat-related posttraumatic stress disorder. Biol Psychiatry. 1997;42:345–348. doi: 10.1016/S0006-3223(96)00375-7. [DOI] [PubMed] [Google Scholar]
- 22.Von Kanel R, Hepp U, Kraemer B, Traber R, Keel M, Mica L, Schnyder U. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J Psychiatr Res. 2007;41:744–752. doi: 10.1016/j.jpsychires.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 23.Lindqvist D, Wolkowitz OM, Mellon S, Yehuda R, Flory JD, Henn-Haase C, Bierer LM, Abu-Amara D, Coy M, Neylan TC. Proinflammatory milieu in combat-related PTSD is independent of depression and early life stress. Brain Behav Immun. 2014;42:81–88. doi: 10.1016/j.bbi.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Spitzer C, Barnow S, Völzke H, Wallaschofski H, John U, Freyberger HJ, Löwe B, Grabe HJ. Association of posttraumatic stress disorder with low-grade elevation of C-reactive protein: Evidence from the general population. J Psychiatr Res. 2010;44:15–21. doi: 10.1016/j.jpsychires.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Beckham JC, Roodman AA, Shipley RH, Hertzberg MA, Cunha GH, Kudler HS, Levin ED, Rose JE, Fairbank JA. Smoking in Vietnam combat veterans with posttraumatic stress disorder. J Trauma Stress. 1995;8:461–472. doi: 10.1007/BF02102970. [DOI] [PubMed] [Google Scholar]
- 26.Pagoto SL, Schneider KL, Bodenlos JS, Appelhans BM, Whited MC, Ma Y, Lemon SC. Association of post-traumatic stress disorder and obesity in a nationally representative sample. Obesity. 2012;20:200–205. doi: 10.1038/oby.2011.318. [DOI] [PubMed] [Google Scholar]
- 27.McFarlane AC. Epidemiological evidence about the relationship between PTSD and alcohol abuse: The nature of the association. Addict Behav. 1998;23:813–825. doi: 10.1016/s0306-4603(98)00098-7. [DOI] [PubMed] [Google Scholar]
- 28.Fantuzzi G. Adipose tissue, adipokines, and inflammation. Journal of Allergy and Clinical Immunology. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 29.Imhof A, Froehlich M, Brenner H, Boeing H, Pepys MB, Koenig W. Effect of alcohol consumption on systemic markers of inflammation. Lancet. 2001;357:763–767. doi: 10.1016/S0140-6736(00)04170-2. [DOI] [PubMed] [Google Scholar]
- 30.Kuschner W, D’Alessandro A, Wong H, Blanc P. Dose-dependent cigarette smoking-related inflammatory responses in healthy adults. Eur Respir J. 1996;9:1989–1994. doi: 10.1183/09031936.96.09101989. [DOI] [PubMed] [Google Scholar]
- 31.Cohen H, Kotler M, Matar MA, Kaplan Z, Miodownik H, Cassuto Y. Power spectral analysis of heart rate variability in posttraumatic stress disorder patients. Biol Psychiatry. 1997;41:627–629. doi: 10.1016/s0006-3223(96)00525-2. [DOI] [PubMed] [Google Scholar]
- 32.Dennis PA, Watkins L, Calhoun PS, Oddone A, Sherwood A, Dennis MF, Rissling MB, Beckham JC. Posttraumatic stress disorder, heart-rate variability, and the mediating role of behavioral health risks. Psychosom Med. 2014;76:629–637. doi: 10.1097/PSY.0000000000000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shah AJ, Lampert R, Goldberg J, Veledar E, Bremner JD, Vaccarino V. Posttraumatic stress disorder and impaired autonomic modulation in male twins. Biol Psychiatry. 2013;73:1103–1110. doi: 10.1016/j.biopsych.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jensen CF, Keller TW, Peskind ER, McFall ME, Veith RC, Martin D, Wilkinson CW, Raskind MA. Behavioral and neuroendocrine responses to sodium lactate infusion in subjects with posttraumatic stress disorder. Am J Psychiatry. 1997;154:266–268. doi: 10.1176/ajp.154.2.266. [DOI] [PubMed] [Google Scholar]
- 35.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 36.Pavlov V, Tracey K. Controlling inflammation: The cholinergic anti-inflammatory pathway. Biochemical Society Transactions. 2006;34:1037–1040. doi: 10.1042/BST0341037. [DOI] [PubMed] [Google Scholar]
- 37.Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ. The cholinergic anti-inflammatory pathway: A missing link in neuroimmunomodulation. Molecular Medicine. 2003;9:125–134. [PMC free article] [PubMed] [Google Scholar]
- 38.Rohleder N, Karl A. Role of endocrine and inflammatory alterations in comorbid somatic diseases of post-traumatic stress disorder. Minerva Endocrinologica. 2006;31:273–288. [PubMed] [Google Scholar]
- 39.Oddone A, Dennis PA, Calhoun PS, Watkins L, Sherwood A, Dedert EA, Green KT, Stein JN, Dennis MF, Beckham JC. Orthostatic hypotension in younger individuals with and without posttraumatic stress disorder. Psychological Trauma: Theory, Research, Practice, and Policy. 2015;7:229–233. doi: 10.1037/a0036716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dennis PA, Ulmer CS, Calhoun PS, Sherwood A, Watkins LL, Dennis MF, Beckham JC. Behavioral health mediators of the link between posttraumatic stress disorder and dyslipidemia. J Psychosom Res. 2014;77:45–50. doi: 10.1016/j.jpsychores.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blake DD, Weathers FS, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. Journal of Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 42.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, text revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 43.Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: A review of the first ten years of research. Depress Anxiety. 2001;13:132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- 44.Davidson JRT, Book SW, Colket JT, Tupler LA, Roth S, David D, Hertzberg MA, Mellman T, Beckham JC, Smith RD, Davidson RM, Katz R, Feldman ME. Assessment of a new self-rating scale for posttraumatic stress disorder: The Davidson Trauma Scale. Psychol Med. 1997;27:153–160. doi: 10.1017/s0033291796004229. [DOI] [PubMed] [Google Scholar]
- 45.Shannon DC, Carley DW, Benson H. Aging of modulation of heart rate. Am J Physiol. 1987;253:H874–H877. doi: 10.1152/ajpheart.1987.253.4.H874. [DOI] [PubMed] [Google Scholar]
- 46.Welch PD. The use of fast Fourier transform for the estimation of power spectra: A method based on time averaging over short, modified periodograms. IEEE Transactions on Audio and Electroacoustics. 1967;15:70–73. [Google Scholar]
- 47.Grossman P, Watkins LL, Wilhelm FH, Manolakis D, Lown B. Cardiac vagal control and dynamic responses to psychological stress among patients with coronary artery disease. Am J Cardiol. 1996;78:1424–1427. doi: 10.1016/s0002-9149(97)89295-8. [DOI] [PubMed] [Google Scholar]
- 48.Rottenberg J, Wilhelm FH, Gross JJ, Gotlib IH. Respiratory sinus arrhythmia as a predictor of outcome in major depressive disorder. J Affect Disord. 2002;71:265–272. doi: 10.1016/s0165-0327(01)00406-2. [DOI] [PubMed] [Google Scholar]
- 49.Blechert J, Lajtman M, Michael T, Margraf J, Wilhelm FH. Identifying anxiety states using broad sampling and advanced processing of peripheral physiological information. Biomed Sci Instrum. 2006;42:136–141. [PubMed] [Google Scholar]
- 50.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 51.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for the DSM-IV Axis I Disorders. New York, NY: Biometrics Research, New York State Psychiatric Institute; 1996. [Google Scholar]
- 52.Kubany ES, Haynes SN, Leisen MB, Owens JA, Kaplan AS, Watson SB, Burns K. Development and preliminary validation of a brief broad-spectrum measure of trauma exposure: The Traumatic Life Events Questionnaire. Psychol Assess. 2000;12:210–224. doi: 10.1037//1040-3590.12.2.210. [DOI] [PubMed] [Google Scholar]
- 53.Hoyle RH. Structural equation modeling: Concepts, issues, and applications. Thousand Oaks, CA: Sage Publications; 1995. [Google Scholar]
- 54.Hu Lt, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- 55.Brattsand R, Linden M. Cytokine modulation by glucocorticoids: mechanisms and actions in cellular studies. Alimentary Pharmacology and Therapeutics. 1996;10:81–90. doi: 10.1046/j.1365-2036.1996.22164025.x. [DOI] [PubMed] [Google Scholar]
- 56.Miller RA. The aging immune system: Primer and prospectus. Science. 1996;273:70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- 57.Davidson JR, Tharwani HM, Connor KM. Davidson Trauma Scale (DTS): Normative scores in the general population and effect sizes in placebo-controlled SSRI trials. Depress Anxiety. 2002;15:75–78. doi: 10.1002/da.10021. [DOI] [PubMed] [Google Scholar]
- 58.Niedermaier ON, Smith ML, Beightol LA, Zukowska-Grojec Z, Goldstein DS, Eckberg DL. Influence of cigarette smoking on human autonomic function. Circulation. 1993;88:562–571. doi: 10.1161/01.cir.88.2.562. [DOI] [PubMed] [Google Scholar]
- 59.Reed SF, Porges SW, Newlin DB. Effect of alcohol on vagal regulation of cardiovascular function: Contributions of the polyvagal theory to the psychophysiology of alcohol. Exp Clin Psychopharmacol. 1999;7:484–492. doi: 10.1037//1064-1297.7.4.484. [DOI] [PubMed] [Google Scholar]
- 60.Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochemical Pharmacology. 2009;78:539–552. doi: 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tousoulis D, Antoniades C, Koumallos N, Stefanadis C. Pro-inflammatory cytokines in acute coronary syndromes: From bench to bedside. Cytokine and Growth Factor Reviews. 2006;17:225–233. doi: 10.1016/j.cytogfr.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 62.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annual Review of Physiology. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 63.Hapke U, Schumann A, Rumpf HJ, John U, Konerding U, Meyer C. Association of smoking and nicotine dependence with trauma and posttraumatic stress disorder in a general population sample. J Nerv Ment Dis. 2005;193:843–846. doi: 10.1097/01.nmd.0000188964.83476.e0. [DOI] [PubMed] [Google Scholar]
- 64.Kalman D, Morissette SB, George TP. Co-morbidity of smoking in patients with psychiatric and substance use disorders. Am J Addict. 2005;14:106–123. doi: 10.1080/10550490590924728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pietrzak RH, Gold RB, Southwick SM, Grant BF. Prevalence and Axis I comorbidity of full and partial posttraumatic stress disorder in the United States: Results from wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. J Anxiety Disord. 2011;25:456–465. doi: 10.1016/j.janxdis.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maxwell SE, Cole DA. Bias in cross-sectional analyses of longitudinal mediation. Psychol Methods. 2007;12:23–44. doi: 10.1037/1082-989X.12.1.23. [DOI] [PubMed] [Google Scholar]
- 67.Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117:1162–1172. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- 68.Clendenen TV, Arslan AA, Lokshin AE, Idahl A, Hallmans G, Koenig KL, Marrangoni AM, Nolen BM, Ohlson N, Zeleniuch-Jacquotte A. Temporal reliability of cytokines and growth factors in EDTA plasma. BMC Research Notes. 2010;3:302–310. doi: 10.1186/1756-0500-3-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Agalliu I, Xue X, Cushman M, Cornell E, Hsing AW, Kaplan RC, Anastos K, Rajpathak S, Ho GY. Detectability and reproducibility of plasma levels of chemokines and soluble receptors. Results in Immunology. 2013;3:79–84. doi: 10.1016/j.rinim.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]