Abstract
GATA4 and GATA6 are zinc finger transcription factors that have important functions in several mesodermal and endodermal organs, including heart, liver and pancreas. In humans, heterozygous mutations of either factor are associated with pancreatic agenesis; however, homozygous deletion of both Gata4 and Gata6 is necessary to disrupt pancreas development in mice. In this study, we demonstrate that arrested pancreatic development in Gata4fl/fl; Gata6fl/fl; Pdx1:Cre (pDKO) embryos is accompanied by the transition of ventral and dorsal pancreatic fates into intestinal or stomach lineages, respectively. These results indicate that GATA4 and GATA6 play essential roles in maintaining pancreas identity by regulating foregut endodermal fates. Remarkably, pancreatic anlagen derived from pDKO embryos also display a dramatic upregulation of hedgehog pathway components, which are normally absent from the presumptive pancreatic endoderm. Consistent with the erroneous activation of hedgehog signaling, we demonstrate that GATA4 and GATA6 are able to repress transcription through the sonic hedgehog (Shh) endoderm-specific enhancer MACS1 and that GATA-binding sites within this enhancer are necessary for this repressive activity. These studies establish the importance of GATA4/6-mediated inhibition of hedgehog signaling as a major mechanism regulating pancreatic endoderm specification during patterning of the gut tube.
KEY WORDS: GATA4, GATA6, Pancreas, Foregut endoderm, Hedgehog, Mouse
Summary: Gata4 and Gata6 regulate mouse foregut endoderm patterning via control of hedgehog signaling. In their absence, the pancreatic anlage is respecified to intestinal and stomach fates.
INTRODUCTION
The pancreas is a vital organ that functions to regulate digestive processes and glucose homeostasis (Hegyi and Petersen, 2013; Mastracci and Sussel, 2012). In adults, the three major diseases associated with the pancreas are pancreatitis, pancreatic cancer and diabetes. In addition, congenital genetic defects contribute to defective organ development, such as annular pancreas, and pathological conditions, including pancreatic agenesis and neonatal diabetes (Etienne et al., 2012; Rubio-Cabezas and Ellard, 2013).
Pancreas development is initiated in two distinct regions of the foregut endoderm in response to signals derived from adjacent tissues (Chen et al., 2004; Kim and MacDonald, 2002; Kumar et al., 2003; Zaret and Grompe, 2008; Zaret et al., 2008). In particular, several studies have shown that a major function of these signals is to repress hedgehog signaling in the presumptive pancreatic endoderm. In the prospective dorsal pancreatic endoderm, notochord-derived activin and fibroblast growth factor (FGF) signals are necessary for the inhibition of sonic hedgehog (Shh) to allow the induction of bud morphogenesis and the pancreatic transcriptional program (Apelqvist et al., 1997; Hebrok et al., 1998). In the prospective ventral pancreatic endoderm, signals secreted by the lateral plate mesoderm, cardiac mesoderm and septum transversum play important roles in inducing pancreatic versus liver fates; the inhibition of WNT and bone morphogenetic protein (BMP) signaling is necessary for ventral pancreas induction, and FGF induces the local expression of Shh to inhibit pancreatic fates in favor of liver lineages (Deutsch et al., 2001; Zaret and Grompe, 2008). As development proceeds, the dorsal and ventral pancreatic buds merge, pancreatic cell specification is initiated and the diverse pancreatic cell types differentiate and proliferate to form the mature functional organ (reviewed by Jorgensen et al., 2007; Pan and Wright, 2011; Pictet and Rutter, 1972).
The GATA regulatory proteins belong to a highly conserved six-member family of zinc finger transcription factors that play essential distinct and overlapping roles during embryonic development, including germ layer specification, organ formation and cell lineage determination. GATA1, GATA2 and GATA3 are important for hematopoiesis, whereas GATA4, GATA5 and GATA6 are important for the development of mesoderm- and endoderm-derived organs, including heart, liver and pancreas (Zhou et al., 2012). Gata4 null mice die at around embryonic day (E) 9.5 owing to defects in heart morphogenesis (Kuo et al., 1997; Molkentin et al., 1997; Narita et al., 1997) and Gata6 null mice die before E7.5 due primarily to defects in extra-embryonic endoderm (Koutsourakis et al., 1999; Morrisey et al., 1998). In the pancreas, Gata4 and Gata6 have overlapping expression in the pancreatic endoderm, but gradually become expressed in separate domains: Gata4 becomes restricted to the exocrine compartment and Gata6 is predominantly expressed in the endocrine compartment (Decker et al., 2006; Ketola et al., 2004). Using a Cre-lox approach, we previously demonstrated that simultaneous deletion of Gata4 and Gata6 from pancreatic progenitor cells leads to pancreatic agenesis in newborn mice (Carrasco et al., 2012; Xuan et al., 2012). The importance of GATA4 and GATA6 in human pancreas development has also been highlighted in reports of genetic cases of human pancreatic agenesis: GATA6 haploinsufficiency contributes to the majority of pancreatic agenesis cases (Lango Allen et al., 2012), and GATA4 haploinsufficiency has been documented in a small number of patients with pancreatic agenesis (Shaw-Smith et al., 2014).
To characterize the molecular changes underlying GATA4/6-mediated pancreas development, we performed global transcriptome analysis on pancreatic buds isolated from E12.5 embryos from control and Gata4fl/fl; Gata6fl/fl; Pdx1:Cre (pDKO); R26R:Tomato mice. Consistent with impaired pancreas development, the majority of downregulated genes were pancreatic progenitor markers. Surprisingly, however, many of the upregulated genes included a large number of intestinal and gastric transcription factors. Furthermore, genetic lineage tracing of cells expressing the pancreatic progenitor marker Pdx1 indicated that the pancreatic lineages were converted to stomach and intestinal cell fates. In addition, there was a notable upregulation of many components of the hedgehog pathway, suggesting that the major mechanism of GATA4/6-regulated pancreas development is through suppression of the hedgehog pathway. Previous studies have demonstrated that GATA factors can negatively regulate Shh expression in the stomach, limb bud, and somites (Daoud et al., 2014; Jacobsen et al., 2005; Kozhemyakina et al., 2014). Our studies suggest that GATA4 and GATA6 pattern the foregut endoderm through the repression of hedgehog signaling.
RESULTS AND DISCUSSION
Hedgehog signaling is highly upregulated in E12.5 pDKO pancreata
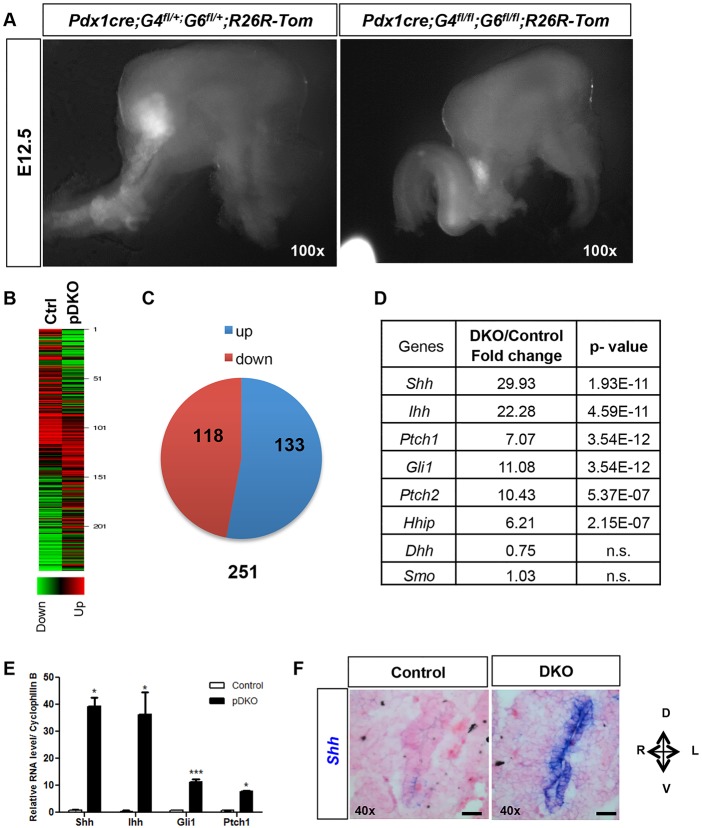
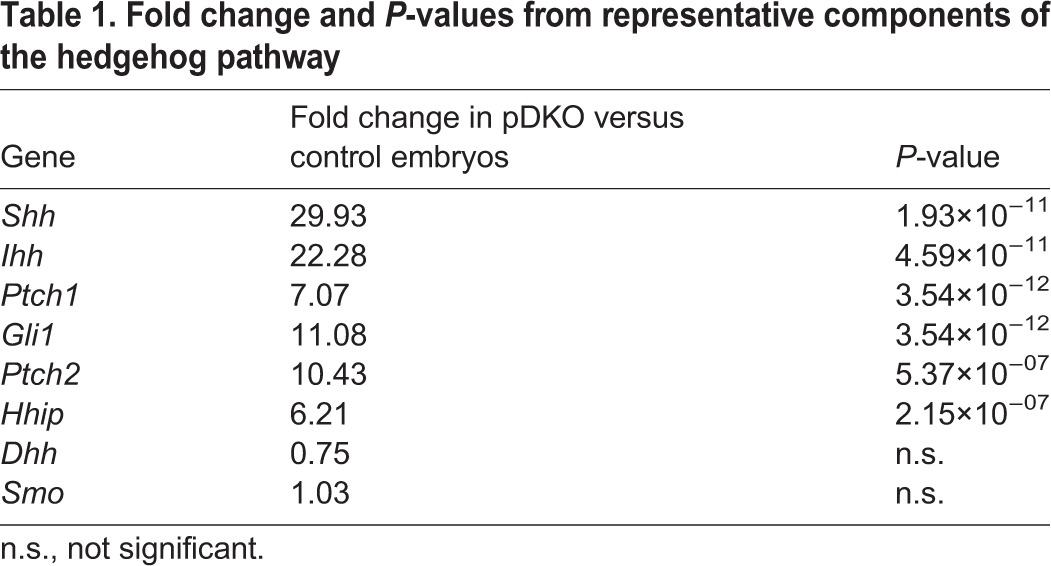
Simultaneous deletion of Gata4 and Gata6 in the pancreatic progenitor domain resulted in severely aplastic pancreatic buds (Carrasco et al., 2012; Xuan et al., 2012). To characterize the molecular pathways that function downstream of GATA4 and GATA6 to mediate the regulation of pancreatic differentiation and morphogenesis, we performed genome-wide transcriptome analysis of the arrested pancreatic rudiments from pDKO embryos compared with littermate control embryos. A R26R;Tomato fluorescent reporter was introduced into the strain to facilitate purification of the PDX1-derived lineages from pooled pancreata of each genotype (Fig. 1A). Quantitative real-time PCR (qPCR) was used to confirm deletion of Gata4 and Gata6, and validate the reduction in pancreatic progenitor markers Pdx1 and Ptf1a (Fig. S1). RNA-Seq analysis of pooled wild-type versus pDKO Tomato+ pancreata identified ∼251 genes that were significantly differentially expressed in the mutant embryos: 118 genes were downregulated and 133 genes were upregulated (Fig. 1B,C). Strikingly, hedgehog signaling was revealed to be the most affected pathway [using ingenuity pathway analysis (IPA) software], with many of the transcriptionally regulated components of the hedgehog pathway being dramatically upregulated (Table 1). qPCR confirmed a 30- to 50-fold upregulation of the two major hedgehog ligands Shh and Indian hedgehog (Ihh), and a 7- to 10-fold upregulation of the receptor patched homolog 1 (Ptch1) and the downstream transcriptional activator Gli1 (Fig. 1D). In situ hybridization for Shh on E10.5 embryos revealed the erroneous expression of Shh expression throughout the pDKO pancreatic domain (Fig. 1E). As inhibition of Shh expression by notochord-derived activin and FGF or cardiac mesoderm-derived FGF has been postulated to be an early event in pancreas induction (Deutsch et al., 2001; Hebrok et al., 1998), our findings suggest that repression of hedgehog signaling downstream of these mesodermal signals is mediated by GATA4 and GATA6 activity.
Fig. 1.
Transcriptome analysis of E12.5 pancreata revealed upregulation of hedgehog pathway in pDKO pancreatic anlage. (A) Representative brightfield images of E12.5 dissected visceral tissue from control and pDKO R26R:Tomato embryos. The fluorescent signal guided dissection of Pdx1-derived lineages. (B) Heatmap display of significant differentially expressed genes (P<0.0008). (C) Pie chart representation of 251 genes that are significantly altered in the pDKO embryos; 118 genes are downregulated and 133 genes are upregulated. (D) qRT-PCR confirmation of highly increased expression of several components in the hedgehog pathway. Error bars represent s.e.m. *P<0.05, ***P<0.001 (n=3). (E) Shh in situ hybridization of E10.5 pancreatic epithelium demonstrates increased Shh expression in pDKO pancreatic epithelium.
Table 1.
Fold change and P-values from representative components of the hedgehog pathway
Cell fate switching occurs in the pDKO pancreatic endoderm
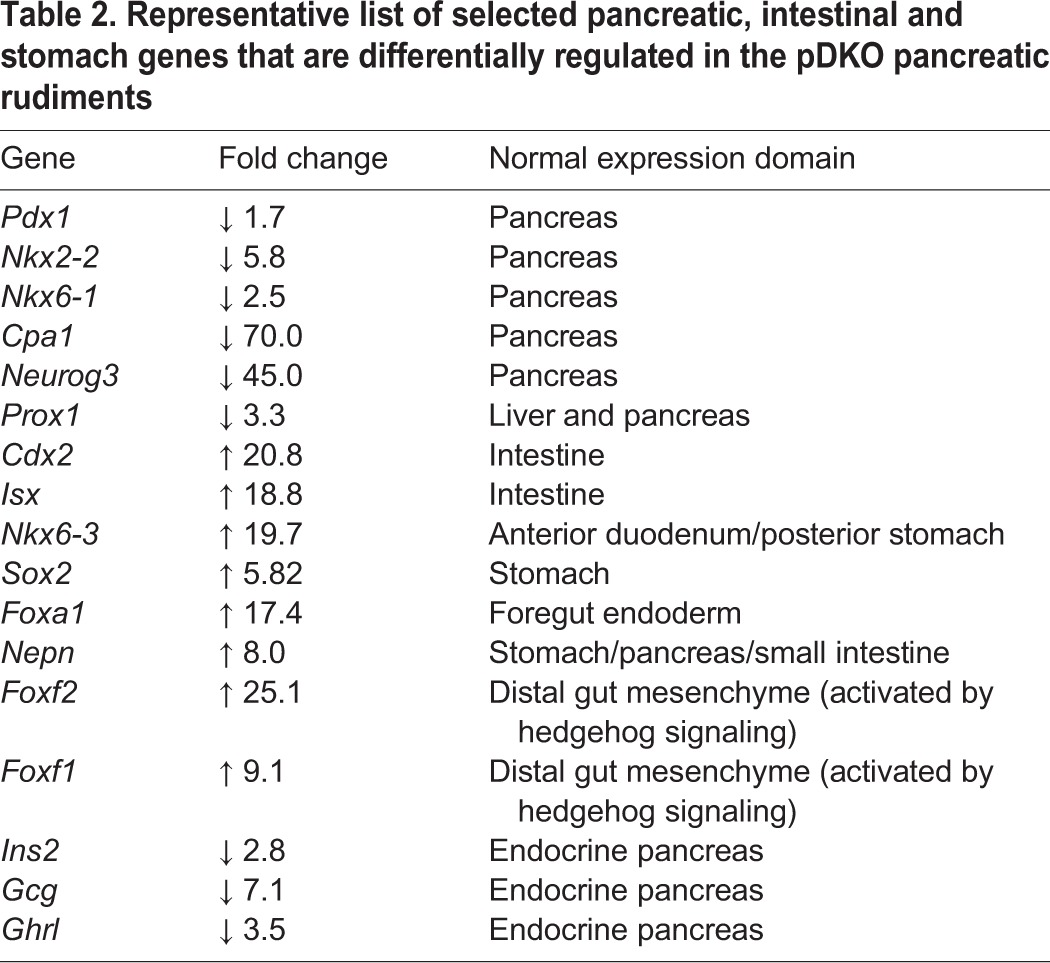
Previous studies have demonstrated that the absence of Shh expression in the early pancreatic domain is required for normal pancreas development (Hebrok et al., 2000). Furthermore, ectopic upregulation of Shh (Apelqvist et al., 1997; Haumaitre et al., 2005) is associated with diminished pancreas formation and expanded stomach or gut regionalization. Consistent with the elevation of hedgehog signaling in the pDKO pancreatic endoderm domain, there was a large reduction of pancreatic progenitor markers in the pDKO, whereas there was a notable increase in genes encoding stomach progenitor markers, such as Sox2 and Nkx6-3 (5.8- and 19.7-fold, respectively), and intestinal progenitor marker genes, such as Cdx2 and Isx (20-fold) (Table 2).
Table 2.
Representative list of selected pancreatic, intestinal and stomach genes that are differentially regulated in the pDKO pancreatic rudiments
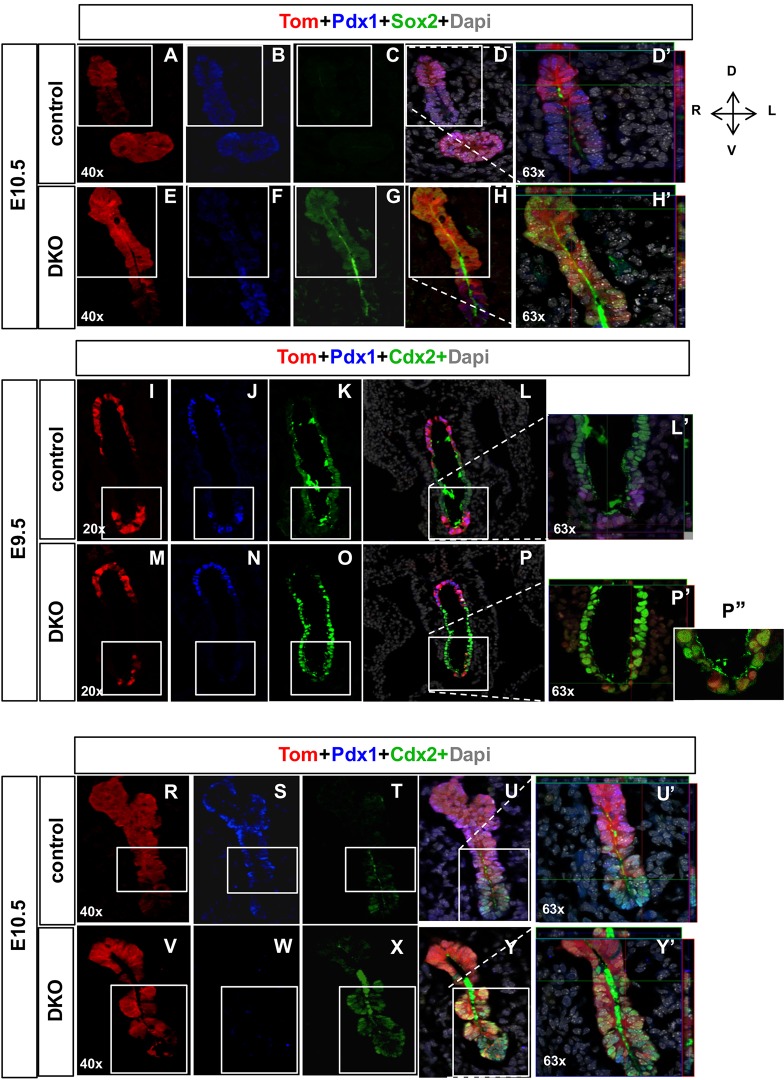
To determine whether the reciprocal changes in marker expression resulted from re-specification of the pancreatic endoderm in the pDKO embryos, we assessed pancreatic, stomach and intestinal lineage markers in the pDKO; R26R:Tomato mice using the lineage label to track the potentially re-specified pancreatic lineages. As expected, in control E10.5 pancreatic endoderm, Tomato-labeled pancreatic lineage cells in the dorsal pancreatic anlage express the pancreatic lineage marker Pdx1, but do not express the stomach marker gene Sox2 (Fig. 2A-D′). However, in the pDKO embryos, Tomato-labeled pancreatic lineage cells begin to express Sox2 in the place of Pdx1 (Fig. 2E-H′), indicating that pancreatic lineage cells in the dorsal pancreas have switched to a stomach identity. Similarly, in the ventral pancreatic endoderm, the pancreatic lineages appear to adopt intestinal cell fates (Fig. 2I-Y). In pDKO E9.5 embryos, a small number of pancreatic lineage cells begin to co-express Pdx1 and Cdx2 or express Cdx2 in place of Pdx1 (Fig. 2M-P″). By E10.5, there is an increasing number of Pdx1 lineage-labeled cells that express Cdx2 (Fig. 2V-Y′). At E18.5, conversion of the Pdx1 lineage to mature stomach fates is widespread and many of the Tomato lineage-labeled cells have become incorporated into the stomach epithelium as previously demonstrated (Xuan, et al., 2012) where they co-express mature stomach markers, including MUC5AC and ATP4B (Fig. S2). These results suggest that the erroneous upregulation of the hedgehog pathway in pDKO embryos results in re-specification of the dorsal and ventral pancreatic lineages to the adjacent stomach and intestinal fates, respectively.
Fig. 2.
Pancreatic lineage cells in pDKO embryos switch cell fates. (A-H′) Representative sections of immunofluorescence-stained pDKO dorsal pancreatic lineage cells from E10.5 embryos. In control embryos, pancreatic lineage cells express Pdx1 (blue) (B) but do not express Sox2 (green) (C). Merged images (D,D′) show overlapping Tomato-expressing (red) cells derived from the Pdx1 lineage and Pdx1-expressing cells (blue). In pDKO embryos (E-H′), Pdx1 lineage cells (E) do not express Pdx1 (F), but instead express Sox2 (G). Merged images (H,H′) show overlapping Tomato- and Sox2-expressing cells, suggesting that pancreatic-derived lineages are converted to a stomach identity. (I-P″) Representative sections of E9.5 pDKO foregut endoderm showing that pancreatic lineage cells express intestinal markers. In control embryos, Tomato+ pancreatic-derived lineages (I) express Pdx1 (J), but not Cdx2 (K), confirming their pancreatic identity. Images from merged channels (L,L′) show overlapping expression of Tomato+ cells and Pdx1+ cells. In the pDKO ventral pancreatic domain, a few pancreatic lineage cells (M) have lost their expression of Pdx1 (N) and start to express the intestinal cell marker Cdx2 (O), suggesting that these cells are converting into intestinal cells. Merged images are shown in P-P″. (R-Y′) At E10.5, control pancreatic lineage cells (R) continue to express Pdx1 (S), but not Cdx2 (T). Merged images are shown in U and U′. In pDKO, increasing numbers of Pdx1-derived Tomato+ lineage cells (V) do not express Pdx1 (W) and instead express Cdx2 (X). Merged channels (Y,Y′) demonstrate overlapping expression of Tomato and Cdx2, suggesting that these cells are switching to an intestinal cell fate. Boxes indicate regions shown at higher magnification in D′, H, L′, P′, U′ and Y′.
GATA4 and GATA6 suppress the activity of the Shh foregut endoderm enhancer MACS1
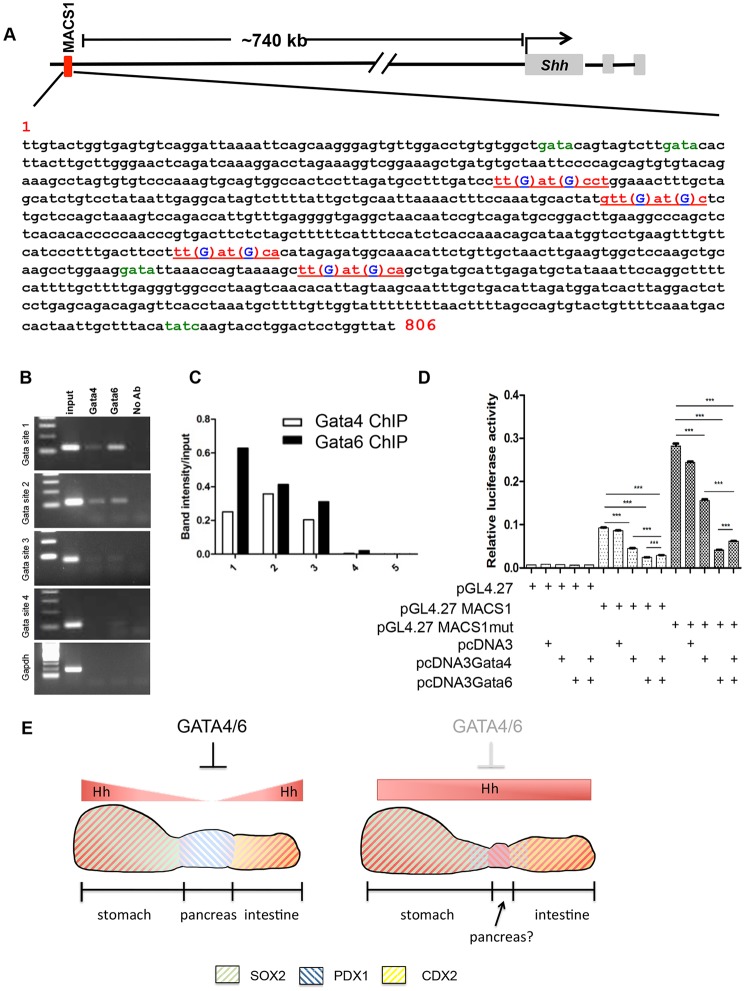
GATA regulation of Shh expression has been reported in several organ systems. During gastric development, Gata4 and Shh expression is mutually exclusive, suggesting that GATA4 might inhibit the expression of Shh (Jacobsen et al., 2005). In the limb bud, GATA6 was shown to inhibit Shh gene expression through a limb bud-specific enhancer of the Shh gene (Kozhemyakina et al., 2014), and in the somitic system, GATA4/5/6 were found to inhibit Shh signaling by inhibiting Gli1 expression, although direct inhibition of Shh was not required (Daoud et al., 2014). The upregulation of hedgehog pathway expression in the pDKO mice suggested that Shh might be repressed by GATA4 and/or GATA6. An 806-bp MACS1 enhancer located ∼740 kb upstream from the Shh transcriptional start site has been shown to be sufficient for driving Shh expression specifically in the foregut endoderm (Anderson et al., 2014; Sagai et al., 2009). Using position weight matrix analysis we identified four putative GATA consensus elements within the MACS1 enhancer (Fig. 3A, red text). Chromatin immunoprecipitation (ChIP) analysis of these sites revealed strong binding of GATA4 and GATA6 to the two most upstream GATA consensus sites (Gata site 1 and Gata site 2) and minimal, if any, binding to the 3′ sites (Gata site 3 and Gata site 4) (Fig. 3B,C).
Fig. 3.
GATA4 and GATA6 inhibit the activity of the Shh endoderm enhancer MACS1. (A) Schematic of the MACS1 enhancer element relative to the transcriptional start site of Shh. The MACS1 element is located ∼740 kb upstream of the Shh transcriptional start site. The MACS1 element is 806 base pairs, and is predicted to have four consensus GATA-binding sites (red text). Mutations in the GATA-binding sites are indicated by blue text. Four potential cryptic GATA-binding sites are designated with green text. (B) PCR analysis of E14.5 pancreata ChIP samples on four putative GATA-binding sites. The first two sites have strong binding for GATA4 and GATA6, whereas the third site has very weak binding. The fourth site has no detectable binding for GATA4 and GATA6. (C) Quantification of band intensities relative to their own inputs. Lane 1=Gata site 1 (GATA4 and GATA6 binding is about 25% and 63%, respectively); Lane 2=Gata site 2 (36% for GATA4 and 41% for GATA6); Lane 3=Gata site 3 (20% for GATA4 and 31% for GATA6); Lane 4=Gata site 4 (0.4% for GATA4 and 2.1% for GATA6); Lane 5=CPA1 site (no measurable binding). n=3. (D) Luciferase assay in α-TC6 cells. The pGL4.27-MACS1 plasmid has high luciferase activity in α-TC6 cells; this effect is suppressed by expression of GATA4 or GATA6. Co-transfection of GATA4 and GATA6 additively suppressed MACS1 expression. pGL4.27-MACS1mut refers to the MACS1 fragment that is mutated for the four GATA sites (red text in A). Error bars represent s.e.m. ***P<0.001. n=5. (E) Model summarizing the downstream consequences of a lack of GATA4 and GATA6 in the pancreatic epithelium. GATA4 and GATA6 expression in the presumptive pancreatic foregut endoderm represses hedgehog signaling to allow for the induction of Pdx1 expression (blue stripes) and initiation of pancreatic fates. In the absence of GATA4 and GATA6, hedgehog signaling is erroneously activated in the pre-pancreatic endoderm domain. As a result, the PDX1 domain is respecified into stomach fates expressing SOX2 (green stripes) and intestinal fates expressing CDX2 (yellow stripes). In the absence of GATA4 and GATA6, a small remnant of tissue co-expressing PDX1 and SOX2 or CDX2 can be detected. This domain transiently expresses pancreatic markers.
To determine whether GATA4 and GATA6 could inhibit Shh expression through the MACS1 enhancer element, we performed luciferase experiments in a Shh-expressing pancreatic αTC6 cell line that lacks endogenous Gata4 and Gata6 (Fig. S3A). Transfection of pGL4.27-MACS1 led to an approximately sixfold increase of luciferase activity compared with pGL4.27 vector alone, suggesting that MACS1 has high activity in these cells (Fig. 3C), which is consistent with previous in vivo expression analysis (Kawahira et al., 2005). Furthermore, co-transfection with either Gata4 or Gata6 reduced MACS1 activity by >50%, with GATA6 having the largest effect (∼75%). Furthermore, simultaneous transfection of half the amount each of Gata4 and Gata6, such that the total amount of GATA protein remained constant (Fig. S3B,C) demonstrated that GATA4 and GATA6 could function together to repress MACS1 activity (Fig. 3D). Surprisingly, however, overexpression of Gata4 and/or Gata6 was still able to partially repress a MACS1 fragment that contained mutations in the four putative Gata consensus sites (Fig. 3A, blue text; Fig. 3D, ‘MACS1mut’). This might indicate that overexpression of GATA factors can bind and activate through cryptic Gata sites (Fig. 3A, green text) present within the MACS1 enhancer or that GATA factors mediate MACS1 repression through an indirect mechanism, as previously described in the heart (Rivera-Feliciano et al., 2006).
Repression of the hedgehog pathway in the presumptive ventral and dorsal pancreatic endoderm in response to signals from adjacent tissues has been well documented (Apelqvist et al., 1997; Chung and Stainier, 2008; diIorio et al., 2007; Haumaitre et al., 2005; Hebrok et al., 1998; Roy et al., 2001). However, the transcriptional regulators that mediate these mesodermal signals upstream of the hedgehog pathway have not yet been identified. In this study, we demonstrate the importance of GATA4 and GATA6 in repressing hedgehog pathway components within the pancreatic anlage. Furthermore, our in vitro data suggest GATA-mediated repression of Shh occurs partially through the endoderm-specific MACS1 enhancer. Consistent with a crucial role for GATA4 and GATA6 in repressing hedgehog signaling in the pancreatic endoderm, there is a conversion of pancreatic lineages to stomach or intestinal cell fates when GATA function is absent. The timing of Shh activation relative to the observed fate changes supports a model in which GATA-mediated repression of hedgehog signaling is necessary to delineate and maintain foregut endoderm fates (Fig. 3E). These findings also reinforce the proposed role for GATA4 and GATA6 as pioneer factors that are positioned at the top of the gene regulatory cascade that patterns tissue-specific gene expression pathways (Zaret et al., 2008).
Recently, it has been reported that haploinsufficiency of GATA4 or GATA6 genes account for more than half of all human pancreatic agenesis cases (Lango Allen et al., 2012), suggesting their important role in human pancreas development. Our discovery that GATA4 and GATA6 are essential for maintaining repression of hedgehog signaling provides important implications for the pathways regulated by the GATA factors during human pancreas development and could lead to novel strategies to detect and/or prevent pancreatic agenesis before birth.
MATERIALS AND METHODS
RNA-Seq and bioinformatics analysis
Fluorescently labeled cells from E12.5 control and pDKO pancreata were manually dissected using a Leica MZ16F fluorescence dissecting microscope. Six controls and 12 pDKO pancreata were pooled for RNA-Seq (n=1 pools per genotype). RNA was generated (RNeasy Micro kit; Qiagen) and tested for quality (RIN values >8; Agilent Bioanalyzer 2100). RNA-Seq was performed on the Illumina HISEQ 2000V3 Instrument (Columbia Genome Center) at a depth of 25-30 million 100-bp single-end reads. FPKM values were used to measure RNA expression level and 23,700 genes were compared between control and mutant samples. DEseq analysis (DESeq2, R software package) was performed to identify differentially expressed genes (P<0.0008). RNA-Seq data have been deposited in Gene Expression Omnibus under accession number GSE77083.
Quantitative real-time PCR
RNA from E12.5 pancreata was isolated (RNeasy Micro, Qiagen) to generate cDNA (Invitrogen). qRT-PCR was performed using Taqman AOD probe sets or SYBR green Premix (Bio-Rad). Taqman AODs: Pdx1, Mm00435565-ml; Ptf1a, Mm04203788_gl; Gata4, Mm00484689_ml; Gata6, Mm00802636_ml; Cdx2, Mm01212280_m1; Sox2, Mm03053820_s1 (Open Biosystems-Thermo Scientific). Specific primers: Shh ex1 F, 5′-GGAGCAGACCGGCTGATGAC-3′; Shh ex2 R, 5′-TCGGTCACTCGCAGCTTCAC-3′; Ihh ex1 F, 5′-TCTTCAAGGACGAGGAGAACACG 3′; Ihh ex2 R, 5′-CACCCGCAGTTTCACACCAG-3′; Gli1 F, 5′TGGTACCATGAGCCCTTCTT 3′; Gli1 R, 5′-GTGGTACACAGGGCTGGACT-3′; Ptch1 F, 5′ATCTCGAGACCAACGTGGAG 3′; Ptch1 R, 5′-GCCTCTTCTCCTATCTTCTGACG-3′; Gata4 F, 5′-TAGTCTGGCAGTTGGCACAG-3′; Gata4 R, 5′-ACGGGACACTACCTGTGCAA-3′; Gata6 F, 5′-AGTTTTCCGGCAGAGCAGTA-3′; Gata6 R, 5′-AGTCAAGGCATCCACTGTC-3′.
In situ hybridization and immunofluorescence analysis
RNA in situ hybridization was performed as previously described (Prado et al., 2004). Shh antisense riboprobe was prepared from a pBSK-Shh plasmid containing a full-length Shh cDNA. T3 RNA polymerase was used to transcribe a HindIII-linearized plasmid. Brightfield images were acquired using a Leica DM5500 microscope.
All immunofluorescence analysis was performed on frozen sections as previously described (Xuan et al., 2012). Primary antibodies were: rabbit anti-Pdx1 (1:1000; 07-696, Millipore), mouse anti-Sox2 (1:250; MAB4343, Santa Cruz), mouse anti-Cdx2 (1:80; CDX2-88, BioGenex), mouse anti-Muc5AC (1:500; ab3649, Abcam) and rabbit anti-ATP4b (1:1000; MA3-923, Thermo Fisher Scientific). Secondary antibodies were Alexa Fluor 488-, 594- or 697- conjugates (1:500; Jackson ImmunoResearch). Fluorescence and confocal images were acquired using a Zeiss LSM 710 confocal microscope. Images and z-stack images were analyzed using ZEN software (Zeiss).
Western blot analysis
Cell lysates from a-TC cells were analyzed using mouse anti-Gata4 (1:1000; SC-25310, Santa Cruz), rabbit anti-Gata6 (1:200; SC-9055, Santa Cruz ) and rabbit anti-Gapdh (1:1000; ab9845, Abcam). Quantification of the relative intensity was performed using ImageJ software.
Chromatin immunoprecipitation
E14.5 pancreata were manually dissected. Chromatin was prepared from these tissues as reported previously (Xuan et al., 2012). The following primers were used for PCR analysis: MACS1 5′ primers: forward 5′-GTGTACAGAAAGCCTAGTGTGTC-3′, reverse 5′-GCAGCAATAAAAGACTATGCCTC-3′; MACS1 3′ primers: forward 5′-ACATTCTGTTGCTAACTTGAAGTG-3′, reverse 5′-AAGCCTGGAATTTATAGCATCTCA-3′; Gata site 2 primers: forward 5′-GAGGCATAGTCTTTTATTGCTGC-3′, reverse 5′-GCATCTGACGGATTGTTAGCCT-3′; Gata site 3 primers: forward 5′-ATGGTCCTGAAGTTTGTTCATCC-3′, reverse 5′-CACTTCAAGTTAGCAACAGAATGT-3′; CPA1exF, CGGAGCTAGTAGCAACCCCT; CPA1exR, CAGGAGCTGGTTCTGATGTG.
Luciferase assay
The 806-bp MACS1 genomic fragment was PCR amplified (5′ GGTACCTTGTACTGGTGAGTGT, 3′ AGATCTATAACCAGGAGTCCAGG) and cloned into pGL4.27 luciferase reporter plasmid. A DNA fragment containing the 806-bp MACS1mut with point mutations in the four putative Gata sites was synthesized by Integrated DNA Technologies. Full-length cDNAs of mouse Gata4 and Gata6 were cloned into the pcDNA3 vector. These plasmids were transfected along with Renilla luciferase construct into α-TC6 cells using X-treme (Roche) transfection reagent according to the manufacturer's instructions. Forty-eight hours after transfection, cell lysates were collected and luciferase samples were prepared using the Dual Luciferase Reporter Assay System (Promega). Luciferase activities were measured using an Orion II Luminometer and luciferase activity was normalized to Renilla activity.
Image quantification
ImageJ software was used to quantify the band intensities in the images for PCR gel and western blotting.
Statistical analysis
All values are expressed as mean±s.e.m. Statistical analysis was performed using a two-tailed Student's t-test. Results were considered significant at P<0.05.
Acknowledgements
We would like to thank Ms Irene Yu for assistance with the model illustration and Ms Jayne Martin (Leibel lab, Columbia University) for providing hedgehog real-time PCR primers. We also thank Matthew Borok and the other members of the Sussel lab for helpful discussions and critical reading of the manuscript. We thank Ms Katie Sinagoga and Dr James Wells for their assistance with whole-mount immunofluorescent staining and 3D imaging of the gut tube, which was not included in the final manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
L.S. and S.X. designed experiments and interpreted results. S.X. performed the experiments. L.S. and S.X. wrote the manuscript.
Funding
This work was supported by the National Institutes of Health [RO1 DK087711 to L.S.]. Additional core facility support was provided from the Columbia Diabetes Research Center (DRC) [P30 DK063608]. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.127217/-/DC1
References
- Anderson E., Devenney P. S., Hill R. E. and Lettice L. A. (2014). Mapping the Shh long-range regulatory domain. Development 141, 3934-3943. 10.1242/dev.108480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apelqvist Å., Ahlgren U. and Edlund H. (1997). Sonic hedgehog directs specialised mesoderm differentiation in the intestine and pancreas. Curr. Biol. 7, 801-804. 10.1016/S0960-9822(06)00340-X [DOI] [PubMed] [Google Scholar]
- Carrasco M., Delgado I., Soria B., Martín F. and Rojas A. (2012). GATA4 and GATA6 control mouse pancreas organogenesis. J. Clin. Invest. 122, 3504-3515. 10.1172/JCI63240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Pan F. C., Brandes N., Afelik S., Sölter M. and Pieler T. (2004). Retinoic acid signaling is essential for pancreas development and promotes endocrine at the expense of exocrine cell differentiation in Xenopus. Dev. Biol. 271, 144-160. 10.1016/j.ydbio.2004.03.030 [DOI] [PubMed] [Google Scholar]
- Chung W.-S. and Stainier D. Y. R. (2008). Intra-endodermal interactions are required for pancreatic beta cell induction. Dev. Cell 14, 582-593. 10.1016/j.devcel.2008.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daoud G., Kempf H., Kumar D., Kozhemyakina E., Holowacz T., Kim D.-W., Ionescu A. and Lassar A. B. (2014). BMP-mediated induction of GATA4/5/6 blocks somitic responsiveness to SHH. Development 141, 3978-3987. 10.1242/dev.111906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker K., Goldman D. C., Grasch C. L. and Sussel L. (2006). Gata6 is an important regulator of mouse pancreas development. Dev. Biol. 298, 415-429. 10.1016/j.ydbio.2006.06.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch G., Jung J., Zheng M., Lora J. and Zaret K. S. (2001). A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development 128, 871-881. [DOI] [PubMed] [Google Scholar]
- diIorio P., Alexa K., Choe S.-K., Etheridge L. and Sagerström C. G. (2007). TALE-family homeodomain proteins regulate endodermal sonic hedgehog expression and pattern the anterior endoderm. Dev. Biol. 304, 221-231. 10.1016/j.ydbio.2006.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne D., John A., Menias C. O., Ward R., Tubbs R. S. and Loukas M. (2012). Annular pancreas: a review of its molecular embryology, genetic basis and clinical considerations. Ann. Anat. 194, 422-428. 10.1016/j.aanat.2012.04.006 [DOI] [PubMed] [Google Scholar]
- Haumaitre C., Barbacci E., Jenny M., Ott M. O., Gradwohl G. and Cereghini S. (2005). Lack of TCF2/vHNF1 in mice leads to pancreas agenesis. Proc. Natl. Acad. Sci. USA 102, 1490-1495. 10.1073/pnas.0405776102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebrok M., Kim S. K. and Melton D. A. (1998). Notochord repression of endodermal Sonic hedgehog permits pancreas development. Genes Dev. 12, 1705-1713. 10.1101/gad.12.11.1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebrok M., Kim S. K., St Jacques B., McMahon A. P. and Melton D. A. (2000). Regulation of pancreas development by hedgehog signaling. Development 127, 4905-4913. [DOI] [PubMed] [Google Scholar]
- Hegyi P. and Petersen O. H. (2013). The exocrine pancreas: the acinar-ductal tango in physiology and pathophysiology. Rev. Physiol. Biochem. Pharmacol. 165, 1-30. 10.1007/112_2013_14 [DOI] [PubMed] [Google Scholar]
- Jacobsen C. M., Mannisto S., Porter-Tinge S., Genova E., Parviainen H., Heikinheimo M., Adameyko I. I., Tevosian S. G. and Wilson D. B. (2005). GATA-4:FOG interactions regulate gastric epithelial development in the mouse. Dev. Dyn. 234, 355-362. 10.1002/dvdy.20552 [DOI] [PubMed] [Google Scholar]
- Jorgensen M. C., Ahnfelt-Rønne J., Hald J., Madsen O. D., Serup P. and Hecksher-Sørensen J. (2007). An illustrated review of early pancreas development in the mouse. Endocr. Rev. 28, 685-705. 10.1210/er.2007-0016 [DOI] [PubMed] [Google Scholar]
- Kawahira H., Scheel D. W., Smith S. B., German M. S. and Hebrok M. (2005). Hedgehog signaling regulates expansion of pancreatic epithelial cells. Dev. Biol. 280, 111-121. 10.1016/j.ydbio.2005.01.008 [DOI] [PubMed] [Google Scholar]
- Ketola I., Otonkoski T., Pulkkinen M.-A., Niemi H., Palgi J., Jacobsen C. M., Wilson D. B. and Heikinheimo M. (2004). Transcription factor GATA-6 is expressed in the endocrine and GATA-4 in the exocrine pancreas. Mol. Cell. Endocrinol. 226, 51-57. 10.1016/j.mce.2004.06.007 [DOI] [PubMed] [Google Scholar]
- Kim S. K. and MacDonald R. J. (2002). Signaling and transcriptional control of pancreatic organogenesis. Curr. Opin. Genet. Dev. 12, 540-547. 10.1016/S0959-437X(02)00338-6 [DOI] [PubMed] [Google Scholar]
- Koutsourakis M., Langeveld A., Patient R., Beddington R. and Grosveld F. (1999). The transcription factor GATA6 is essential for early extraembryonic development [corrected and republished with original paging, article originally printed in Development 1999 Feb;126(4):723-32]. Development 126, 723-732. [PubMed] [Google Scholar]
- Kozhemyakina E., Ionescu A. and Lassar A. B. (2014). GATA6 is a crucial regulator of Shh in the limb bud. PLoS Genet. 10, e1004072 10.1371/journal.pgen.1004072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Jordan N., Melton D. and Grapin-Botton A. (2003). Signals from lateral plate mesoderm instruct endoderm toward a pancreatic fate. Dev. Biol. 259, 109-122. 10.1016/S0012-1606(03)00183-0 [DOI] [PubMed] [Google Scholar]
- Kuo C. T., Morrisey E. E., Anandappa R., Sigrist K., Lu M. M., Parmacek M. S., Soudais C. and Leiden J. M. (1997). GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 11, 1048-1060. 10.1101/gad.11.8.1048 [DOI] [PubMed] [Google Scholar]
- Lango Allen H., Flanagan S. E., Shaw-Smith C., De Franco E., Akerman I., Caswell R., the International Pancreatic Agenesis Consortium, Ferrer J., Hattersley A. T. and Ellard S. (2012). GATA6 haploinsufficiency causes pancreatic agenesis in humans. Nat. Genet. 44, 20-22. 10.1038/ng.1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastracci T. L. and Sussel L. (2012). The endocrine pancreas: insights into development, differentiation, and diabetes. Wiley Interdiscip. Rev. Dev. Biol. 1, 609-628. 10.1002/wdev.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molkentin J. D., Lin Q., Duncan S. A. and Olson E. N. (1997). Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev. 11, 1061-1072. 10.1101/gad.11.8.1061 [DOI] [PubMed] [Google Scholar]
- Morrisey E. E., Tang Z., Sigrist K., Lu M. M., Jiang F., Ip H. S. and Parmacek M. S. (1998). GATA6 regulates HNF4 and is required for differentiation of visceral endoderm in the mouse embryo. Genes Dev. 12, 3579-3590. 10.1101/gad.12.22.3579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita N., Bielinska M. and Wilson D. B. (1997). Cardiomyocyte differentiation by GATA-4-deficient embryonic stem cells. Development 124, 3755-3764. [DOI] [PubMed] [Google Scholar]
- Pan F. C. and Wright C. (2011). Pancreas organogenesis: from bud to plexus to gland. Dev. Dyn. 240, 530-565. 10.1002/dvdy.22584 [DOI] [PubMed] [Google Scholar]
- Pictet R. and Rutter W. J. (1972). Development of the Embryonic Endocrine Pancreas. Washington, DC: Williams and Wilkins. [Google Scholar]
- Prado C. L., Pugh-Bernard A. E., Elghazi L., Sosa-Pineda B. and Sussel L. (2004). Ghrelin cells replace insulin-producing beta cells in two mouse models of pancreas development. Proc. Natl. Acad. Sci. USA 101, 2924-2929. 10.1073/pnas.0308604100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Feliciano J., Lee K.-H., Kong S. W., Rajagopal S., Ma Q., Springer Z., Izumo S., Tabin C. J. and Pu W. T. (2006). Development of heart valves requires Gata4 expression in endothelial-derived cells. Development 133, 3607-3618. 10.1242/dev.02519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S., Qiao T., Wolff C. and Ingham P. W. (2001). Hedgehog signaling pathway is essential for pancreas specification in the zebrafish embryo. Curr. Biol. 11, 1358-1363. 10.1016/S0960-9822(01)00402-X [DOI] [PubMed] [Google Scholar]
- Rubio-Cabezas O. and Ellard S. (2013). Diabetes mellitus in neonates and infants: genetic heterogeneity, clinical approach to diagnosis, and therapeutic options. Horm. Res. Paediatr. 80, 137-146. 10.1159/000354219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagai T., Amano T., Tamura M., Mizushina Y., Sumiyama K. and Shiroishi T. (2009). A cluster of three long-range enhancers directs regional Shh expression in the epithelial linings. Development 136, 1665-1674. 10.1242/dev.032714 [DOI] [PubMed] [Google Scholar]
- Shaw-Smith C., De Franco E., Lango Allen H., Batlle M., Flanagan S. E., Borowiec M., Taplin C. E., van Alfen-van der Velden J., Cruz-Rojo J., Perez de Nanclares G. et al. (2014). GATA4 mutations are a cause of neonatal and childhood-onset diabetes. Diabetes 63, 2888-2894. 10.2337/db14-0061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan S., Borok M. J., Decker K. J., Battle M. A., Duncan S. A., Hale M. A., Macdonald R. J. and Sussel L. (2012). Pancreas-specific deletion of mouse Gata4 and Gata6 causes pancreatic agenesis. J. Clin. Invest. 122, 3516-3528. 10.1172/JCI63352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaret K. S. and Grompe M. (2008). Generation and regeneration of cells of the liver and pancreas. Science 322, 1490-1494. 10.1126/science.1161431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaret K. S., Watts J., Xu J., Wandzioch E., Smale S. T. and Sekiya T. (2008). Pioneer factors, genetic competence, and inductive signaling: programming liver and pancreas progenitors from the endoderm. Cold Spring Harb. Symp. Quant. Biol. 73, 119-126. 10.1101/sqb.2008.73.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., He A. and Pu W. T. (2012). Regulation of GATA4 transcriptional activity in cardiovascular development and disease. Curr. Top. Dev. Biol. 100, 143-169. 10.1016/B978-0-12-387786-4.00005-1 [DOI] [PubMed] [Google Scholar]