Abstract
Sexual identity is continuously maintained in specific differentiated cell types long after sex determination occurs during development. In the adult Drosophila testis, the putative transcription factor Chronologically inappropriate morphogenesis (Chinmo) acts with the canonical male sex determinant DoublesexM (DsxM) to maintain the male identity of somatic cyst stem cells and their progeny. Here we find that ectopic expression of chinmo is sufficient to induce a male identity in adult ovarian somatic cells, but it acts through a DsxM-independent mechanism. Conversely, the feminization of the testis somatic stem cell lineage caused by loss of chinmo is enhanced by expression of the canonical female sex determinant DsxF, indicating that chinmo acts in parallel with the canonical sex determination pathway to maintain the male identity of testis somatic cells. Consistent with this finding, ectopic expression of female sex determinants in the adult testis disrupts tissue morphology. The miRNA let-7 downregulates chinmo in many contexts, and ectopic expression of let-7 in the adult testis is sufficient to recapitulate the chinmo loss-of-function phenotype, but we find no apparent phenotypes upon removal of let-7 in the adult ovary or testis. Our finding that chinmo is necessary and sufficient to promote a male identity in adult gonadal somatic cells suggests that the sexual identity of somatic cells can be reprogrammed in the adult Drosophila ovary as well as in the testis.
KEY WORDS: Jak-STAT signaling, Ovary, Sex maintenance, Stem cell, Testis, Niche
Highlighted article: Manipulating expression of the transcription factor Chinmo in Drosophila gonads shows that sexual identity can be reprogrammed, and must be actively maintained throughout life.
INTRODUCTION
The phenotypic difference between males and females, or sexual dimorphism, arises from a variety of genetic or environmental mechanisms across animal species. In many organisms, male versus female cell fate decisions established during development were thought to be unalterable; however, recent work has shown that the sexual identity of cells is actively maintained, and that transdifferentiation from one cell fate to another can occur even in adult tissues. For example, in adult mouse testes, loss of the transcription factor Doublesex and mab3-related (DMRT1) causes differentiated somatic Sertoli cells to transdifferentiate to their female counterparts (granulosa cells) (Matson et al., 2011). Similarly, in adult mouse ovaries, loss of the female regulator Forkhead box protein L2 (FOXL2) in granulosa cells triggers their conversion to Sertoli cells (Uhlenhaut et al., 2009). DMRT1 is not only necessary but also sufficient to specify male cell fate in mice; ectopic expression of DMRT1 is sufficient to silence FOXL2 expression and masculinize the ovary (Lindeman et al., 2015).
We recently found that sex maintenance extends to adult Drosophila; loss of the transcription factor Chronologically inappropriate morphogenesis (Chinmo) causes somatic stem cells in the adult testis to adopt female cell fates and produce daughter cells that resemble ovarian somatic cells (Ma et al., 2014). Chinmo maintains the male identity of testis somatic stem cells in part by promoting the expression of the male sex determinant and DMRT1 homologue DoublesexM (DsxM) in these cells, and forced expression of DsxM can partially rescue the chinmo sex transformation phenotype. Thus, Chinmo is an essential regulator of sex maintenance in the testis and DsxM is one of its targets. However, whether Chinmo and DsxM are sufficient to determine a male sexual identity in gonadal cells is not known.
Stem cell niches in the adult Drosophila ovary and testis are well defined (de Cuevas and Matunis, 2011; Eliazer and Buszczak, 2011; Sahai-Hernandez et al., 2012). In the testis (Fig. 1A), sperm-producing germline stem cells (GSCs) and somatic cyst stem cells adhere to a cluster of quiescent somatic cells called the hub. Two cyst stem cells wrap around each GSC and support its self-renewal and differentiation. Both types of stem cells are maintained by the Janus kinase-Signal Transducer and Activator of Transcription (Jak-STAT) pathway, which is activated locally by the ligand Unpaired (Upd) that is secreted from the hub (Kiger et al., 2001; Tulina and Matunis, 2001). In addition to its role in maintaining the male sexual identity of cyst stem cells, chinmo is a target of Jak-STAT signaling and is required in cyst stem cells for their self-renewal (Flaherty et al., 2010). In the ovary (Fig. 1B), egg-producing GSCs and transit-amplifying germ cells are supported by somatic terminal filament, cap and escort cells. Jak-STAT signaling is not required directly in ovarian GSCs, but it is required in adjacent somatic cells to maintain the GSCs, and overexpression of Upd in these cells is sufficient to promote GSC and escort cell proliferation (Decotto and Spradling, 2005; López-Onieva et al., 2008). Two somatic follicle stem cells, located posterior to the GSCs and transit-amplifying germ cells, produce follicle precursor cells that differentiate into follicle cells or stalk cells (Margolis and Spradling, 1995). Follicle cells surround clusters of differentiating germ cells, forming egg chambers that are linked together by chains of stalk cells. The morphology and behavior of somatic stem cells and their descendants in the adult ovary and testis are distinct: male cyst stem cells produce squamous cyst cells, which are quiescent, whereas female follicle stem cells produce columnar epithelial cells that continue to proliferate as the egg chamber grows. Although the Jak-STAT signaling pathway is active in both the ovary and testis, it is not clear if Chinmo has any functions in the ovary, and relatively little is known about the regulation of sex maintenance in either tissue.
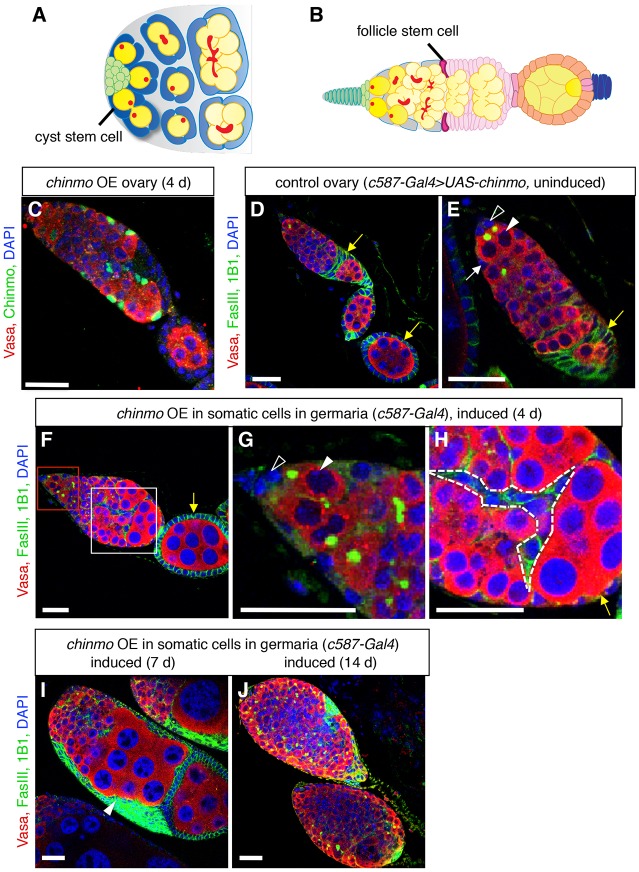
Fig. 1.
Ectopic expression of chinmo in somatic cells of adult germaria disrupts oogenesis. (A) Illustration of a wild-type Drosophila testis apex (adapted from de Cuevas and Matunis, 2011). Germline stem cells (GSCs, dark yellow) and somatic cyst stem cells (cyst stem cells, dark blue) adhere to the hub (green). GSCs, which contain spherical fusomes (red), produce differentiating male germ cells (spermatogonia, yellow), which contain branched fusomes. Approximately two somatic cyst stem cells flank each GSC; cyst stem cells produce squamous, quiescent cyst cells (light blue), which encase differentiating germ cells. (B) Illustration of a wild-type Drosophila germarium and egg chamber (adapted from Ma et al., 2014). Terminal filament cells (dark green) and cap cells (light green) support GSCs (dark yellow), which produce differentiating female germ cells (light yellow). Escort cells (gray) surround dividing germ cells in the anterior half of the germarium. Two somatic follicle stem cells (follicle stem cells, magenta) produce follicle precursor cells (light pink), which differentiate into follicle cells (orange) and stalk cells (blue). Each egg chamber contains a cluster of 16 germ cells surrounded by a monolayer of columnar epithelial follicle cells. Egg chambers are linked by chains of stalk cells. (C) Immunofluorescence detection of ectopic Chinmo protein (green) in an adult ovary. Chinmo is undetectable in wild-type ovaries (Fig. S1H), but after four days of ectopic chinmo overexpression (OE) in somatic cells in the adult germarium, Chinmo is easily detected in the chinmo-expressing cells. (D-J) Immunofluorescence detection in adult ovaries of FasIII (green at cell periphery) to visualize somatic cell membranes, and 1B1 (green in germ cells) to mark fusomes. Before ectopic chinmo expression, the adult ovariole (D) and germarium (E) look normal. GSCs (arrowheads in E,G) are attached to caps cells (open arrowheads in E,G). Escort cells (white arrow) associate with germ cells in the anterior portion of the germarium; follicle cells (yellow arrows), which express higher levels of FasIII, form a monolayer of columnar epithelial cells around germ cells at the posterior end of the germarium. After ectopic chinmo expression in adult somatic cells for four days (F-H), defects in egg chamber formation are apparent. The stem cell niche looks normal (F, magnified in G), but clusters of differentiating germ cells accumulate at the posterior end of the germarium (F, magnified in H). Somatic cells are evident between germ cells (dashed lines in H) and no longer form a regular columnar epithelial monolayer (yellow arrow in H). After ectopic chinmo expression for longer times (I-J), ovaries fail to form normal egg chambers, and germaria are filled with overproliferating early germ cells and somatic cells. In all panels, Vasa marks germ cells (red), DAPI marks nuclei (blue), and anterior is to the left. Scale bars: 20 μm.
Here, to explore the role of Chinmo in maintaining sexual identity, we asked if Chinmo is sufficient to induce a male sexual identity in the adult Drosophila ovary. Because Chinmo functions through DsxM to maintain the male fate of testis cyst stem cells, we also examined the role of the canonical sex determination pathway in adult testes and ovaries. We found that Chinmo and sex determinants are both necessary and sufficient for sex maintenance, but might function through overlapping but distinct mechanisms. We also found that microRNAs transcribed from the let-7-Complex (let-7-C), which regulate chinmo in the brain (Wu et al., 2012), can regulate chinmo in the adult testis.
RESULTS
Chinmo is not required in somatic cells in the adult Drosophila ovary
We began this study by asking if chinmo is required in the adult ovary. chinmo is essential during development (Flaherty et al., 2010; Zhu et al., 2006); therefore, to circumvent embryonic lethality, we allowed flies to develop to adulthood and then used cell type-specific RNA interference (RNAi) to knock down chinmo specifically in the adult ovary. Because the cells in the adult testis that undergo sex transformation upon loss of Chinmo are the somatic stem cells, we asked if chinmo is required in the somatic stem cells in the ovary, which are the follicle stem cells (Fig. 1B). Using the temperature-inducible Gal4/Gal80TS system (McGuire et al., 2004), we conditionally expressed two independent RNAi lines against chinmo in somatic cells within the germarium, which is the anteriormost region of the ovary where stem cells reside. Tools to manipulate gene expression exclusively in follicle stem cells do not exist; however, several Gal4 lines drive gene expression in small subsets of somatic cells that include the follicle stem cells. We used two Gal4 driver lines for this experiment: c587-Gal4, which is expressed specifically in escort cells, follicle stem cells and follicle precursor cells, and eyaA3-Gal4, which is expressed in a pattern that overlaps that of c587-Gal4 but also extends to follicle cells and stalk cells (Fig. S1A,B). We knocked down chinmo for up to 4 weeks in adult flies and then dissected, fixed and immunostained ovaries: experimental and control ovaries were morphologically indistinguishable (Fig. S1E,F). Both RNAi lines reduce Chinmo protein levels and recapitulate the chinmo loss-of-function phenotype when expressed in the testis (Ma et al., 2014), which confirms that they are functional. Ovaries from adult females bearing the partial loss-of-function allele chinmoSex Transformation (chinmoST) are also indistinguishable from wild-type ovaries (data not shown). Consistent with these results, in wild-type ovaries, chinmo is expressed only at very low levels (Gan et al., 2010). Furthermore, although Chinmo protein is detectable at very low and variable levels in a few germ cells at the anterior tip of the germarium, it is undetectable in somatic cells by immunostaining with anti-Chinmo antisera (Fig. S1G,H). Together, these data support the hypothesis that Chinmo is not required in somatic cells in the adult ovary.
Ectopic expression of Chinmo in adult ovarian somatic cells disrupts oogenesis
We next asked whether expressing chinmo ectopically in the adult ovary is sufficient to disrupt oogenesis. We used the same somatic Gal4 driver, c587-Gal4 with Gal80TS, to conditionally express ectopic chinmo in a subset of somatic cells in the adult ovary: escort cells, follicle stem cells and follicle precursor cells. Immunostaining ovaries with anti-Chinmo antisera confirmed that Chinmo was strongly upregulated in these cells upon four days of transgene induction (Fig. 1C). To analyze the phenotype of chinmo-overexpressing ovaries, we immunostained them with anti-Vasa, which marks germ cells; anti-Fasciclin 3 (Fas3, also known as FasIII), which highlights follicle cell membranes; and 4′,6-diamidino-2-phenylindole (DAPI), which marks nuclei in all cells. Without induction of ectopic chinmo expression, ovaries were phenotypically wild type, and clusters of germ cells at the posterior end of the germarium were surrounded by monolayers of follicle cells as in wild-type ovaries (Fig. 1D,E). However, after transgene induction, ovaries developed a progressive phenotype beginning in the germarium. After four days of induction (Fig. 1F-H), the somatic cells at the posterior end of the germarium no longer had a columnar morphology or formed regular monolayers around the clusters of germ cells as in wild type. Instead, the somatic cells were more irregular in shape, and clusters of germ cells appeared to pile up in the posterior half of the germarium with irregular layers of somatic cells between them (Fig. 1H). At this early timepoint, germline and somatic cells in the anterior portion of the germarium still resembled those in wild-type ovaries (Fig. 1G), as did egg chambers that had exited the germarium, which likely formed before induction of chinmo overexpression (Fig. 1F). After the period of ectopic chinmo expression was extended to one week, the phenotype became more severe (Fig. 1I). Large clumps of somatic cells, marked by high levels of Fasciclin 3, were found near the posterior end of the germarium, which was enlarged and filled with clusters of older germ cells. The failure of germ cell clusters to be surrounded by follicle cells and exit the germarium, and the accumulation of somatic cells at the posterior end of the germarium, suggests that chinmo-overexpressing somatic cells are not functioning properly and cannot package germ cells into egg chambers separated by distinct stalk cells. As the duration of ectopic chinmo induction increased to more than one week, ovaries developed a tumorous phenotype; germaria filled with early germ cells intermingled with somatic cells, and mature germ cells or normal egg chambers were no longer detected, indicating that oogenesis was completely disrupted (Fig. 1J). We confirmed these results by overexpressing chinmo with a different somatic driver, traffic jam-Gal4 (tj-Gal4) (Fig. S2A-C), which is expressed in escort cells, follicle stem cells, follicle cells and stalk cells in the adult ovary (Fig. S1C). We further confirmed these results by overexpressing chinmo using two other chinmo transgenes, chinmo-5′UTR and -3′UTR, which are identical to the full length transgene but lack the 3′ or 5′ untranslated regions (UTRs), respectively, that are present in the full length transgene (Zhu et al., 2006). After four days to seventeen days of induction with c587-Gal4, both transgenes produced ectopic Chinmo and resulted in a phenotype similar to that observed with the full length construct (Fig. S1J-N), suggesting that chinmo is not regulated via its 5′ or 3′UTR as in the brain (Zhu et al., 2006). By contrast, overexpressing chinmo conditionally in germ cells in the ovary (nos-Gal4>chinmo) yielded ovaries that were indistinguishable from wild-type control ovaries (Fig. S1D,I). Taken together, these results indicate that chinmo overexpression in the somatic cells of the germarium disrupts oogenesis. They also suggest that ectopic chinmo initially disrupts the functions of somatic cells in the germarium, and that germ cell differentiation is blocked as a secondary consequence.
Ectopic Chinmo is sufficient to promote the expression of male-specific markers in the adult ovary
We speculated that ectopic Chinmo could be disrupting oogenesis by causing somatic cells in the adult ovary to adopt a male fate. To test this hypothesis, we looked at the expression of male-specific markers in chinmo-overexpressing ovaries, although few such markers exist. We identified an enhancer trap inserted in the escargot gene (Buszczak et al., 2007), referred to here as esg-GFP, which we introduced into flies conditionally overexpressing chinmo in adult somatic cells (esg-GFP, c587-Gal4>chinmo). Before induction of chinmo expression, this transgene expressed green fluorescent protein (GFP) robustly in early germ cells and somatic cells in testes (Fig. 2A) but was undetectable in ovaries (Fig. 2B). By contrast, after three days of ectopic chinmo expression, 10-20% of germaria developed the mild phenotype shown in Fig. 1F-H, and esg-GFP became apparent in a subset of their somatic cells (Fig. 2C). At this early timepoint, the esg-GFP-positive cells were usually located in the posterior half of the germarium. The anteriormost esg-GFP-positive cells were located in the area where follicle stem cells and their early progeny reside in wild-type germaria, but we cannot identify them unambiguously as follicle stem cells because markers that distinguish follicle stem cells from neighboring cells have not been identified. However, the female-specific somatic marker Castor was undetectable in these esg-GFP-positive cells, suggesting that female-specific Castor expression is lost as male markers become expressed (Fig. S2D-E). When the duration of ectopic chinmo expression was extended, the phenotype became more severe, as expected; after 1-2 weeks of ectopic chinmo expression, almost all germaria had esg-GFP-positive somatic cells, and more cells in each germarium were esg-GFP-positive, including some in the anterior portion of the germarium (Fig. 2D-F). We confirmed these results by repeating this experiment with the somatic driver tj-Gal4 in place of c587-Gal4, which yielded similar results, as expected (Fig. S2A-C). These observations support the hypothesis that ectopic expression of chinmo in ovarian somatic cells is sufficient to cause these cells to adopt a male fate.
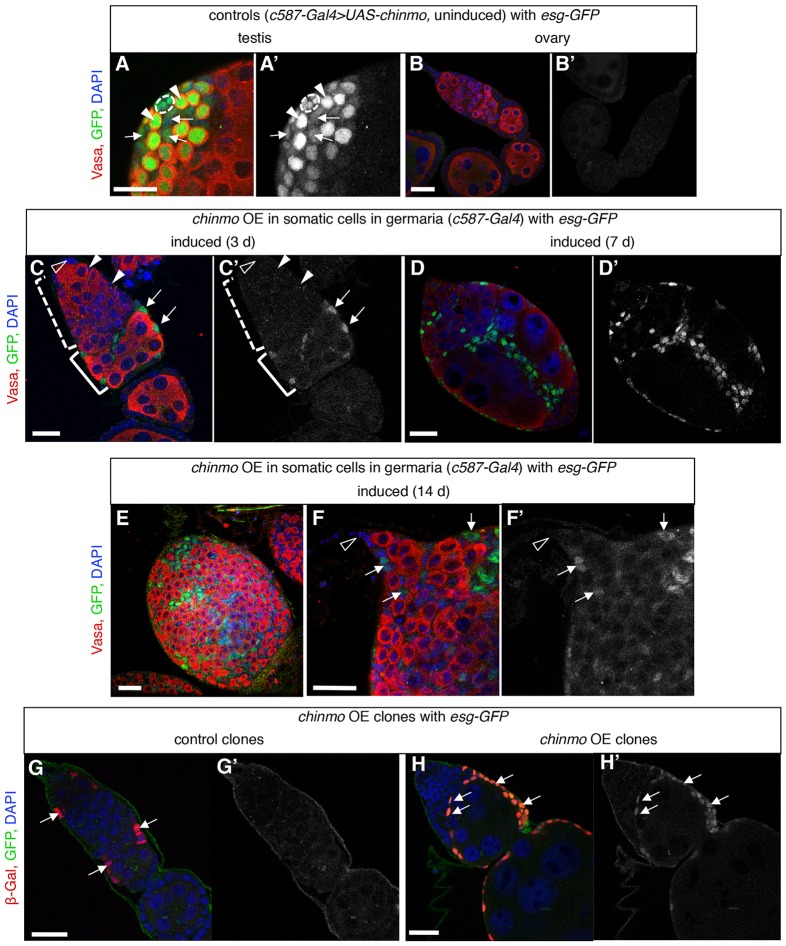
Fig. 2.
Somatic cells in the adult germarium express a male-specific marker when expressing ectopic chinmo. (A-F) Immunofluorescence detection in the adult testis (A,A′) and ovaries (B-F) of GFP (green in A-F; gray in A′-F′) to visualize expression of a male-specific enhancer trap esg-GFP and Vasa (red) to visualize germ cells. (A,B) Before ectopic chinmo expression, the adult testis and ovary look normal. In the testis, esg-GFP is expressed in the hub (dashed outline), early germ cells (arrowheads), and cyst stem cells and early cyst cells (arrows), but it is not expressed in the ovary. (C) After 3 days of ectopic chinmo overexpression (OE) in somatic cells in adult germaria, somatic cells (arrows) in the posterior portion of the germarium (bracket) start to express esg-GFP. esg-GFP is not expressed in escort cells (arrowheads) or cap cells (open arrowheads). (D-F) After ectopic expression of chinmo for longer times, the ovaries develop a more severe phenotype as described in Fig. 1, and esg-GFP is expressed in more somatic cells (arrows) including cells close to the stem cell niche. (G-H) Immunofluorescence detection in adult ovaries of GFP (green in G,H; gray in G′,H′) to visualize expression of male-specific esg-GFP and β-Gal (red) to visualize clones with chinmo overexpressed. A germarium with control clones (G, arrows), which lack the chinmo transgene (hs-FLP, esg-GFP, Ay-Gal4>lacZ), looks normal and does not express esg-GFP (G′) in any cells before or after clone induction. In chinmo overexpressing clones (H, arrows), by six days after clone induction, esg-GFP (H′) is expressed in many clone cells, and the germarium has a strong phenotype similar to global chinmo overexpression (compare with Fig. 1). DAPI marks nuclei (blue). Anterior is to the left in all panels. Scale bars: 20 μm.
To look more closely at which somatic cells are adopting a male fate upon ectopic expression of chinmo, we used mosaic analysis with Gal4 (‘flip-out’ Gal4) to overexpress chinmo in only a few cells in adult ovaries carrying the male marker esg-GFP (hs-FLP, esg-GFP, Ay-Gal4>lacZ and chinmo). In control ovaries which lacked the chinmo transgene (hs-FLP, esg-GFP, Ay-Gal4>lacZ), we saw no expression of esg-GFP in any cells before or after clone induction, as expected (Fig. 2G). In the experimental flies, three days after clone induction, most germaria contained at least a few somatic cell clones (we were not able to recover germline clones with this system), but none expressed esg-GFP, and we did not see any obvious phenotypes associated with the clones (data not shown). By contrast, by six days after clone induction, 60% of germaria with clones (n=21/35) had esg-GFP-positive somatic cells (Fig. 2H), and many also displayed a strong phenotype similar to global chinmo overexpression in somatic cells (esg-GFP, c587-Gal4>chinmo) (Fig. 2C-F). All esg-GFP-positive somatic cells also expressed the clone marker (β-Galactosidase), and we did not see any obvious defects in germaria or regions of germaria without clones, which suggests that the chinmo-overexpression phenotype is cell-autonomous. Furthermore, we saw no obvious defects in germaria with clones only in the anterior half of the germarium (n=8) (Fig. S2I), and most esg-GFP-positive cells were located in clones in the posterior half of the germarium. These observations confirm and extend our results for global chinmo overexpression in somatic cells, where we detected esg-GFP first in somatic cells in the posterior half of the germarium (where FSCs reside). Based on these findings, we suggest that the first cells to become masculinized in ovaries with ectopic chinmo expression are follicle stem cells and/or their earliest progeny, and that escort cells are affected later, after longer periods of ectopic chinmo induction.
We next asked if the sexual fate of germ cells in ectopic chinmo-expressing ovaries could be changing as a result of their associated somatic cells becoming masculinized. To address this question, we expressed chinmo ectopically in ovarian somatic cells in adult flies carrying a different male-specific marker, M5-4 (M5-4, c587-Gal4>chinmo). This marker, which is also an enhancer trap insertion in the escargot gene but was derived independently from esg-GFP, has been used extensively in identifying a male identity in germ cells (Sheng et al., 2009; Wawersik et al., 2005) and marks early germ cells and hub cells (but not other somatic cells) in control testes (Fig. S2F). Importantly, it is not detected in control ovaries (Fig. S2G) (Ma et al., 2014; Tran et al., 2000). After a short period of ectopic chinmo expression (5 days), M5-4 remains undetectable in the ovary (data not shown); however, after 20 days, M5-4 marks a few early germ cells in the germarium (Fig. S2H). This finding suggests that ectopic expression of chinmo in somatic cells in the adult ovary is sufficient to alter the identity of neighboring germ cells, causing them to adopt a male fate. This conclusion is supported by previous work in Drosophila embryos, which showed that genetically female (XX) germ cells can become masculinized if the sex of their associated somatic cells is experimentally altered during development (Jinks et al., 2000; Wawersik et al., 2005; Hempel et al., 2008). Together, our data indicate that ectopic expression of chinmo in adult ovarian somatic cells is sufficient to cause these cells and their neighboring germ cells to adopt a male fate.
In the ovary, ectopic Chinmo does not act through the canonical sex determinant DsxM
Our previous work showed that Chinmo acts with the canonical sex determinant and DMRT1 homologue DsxM to maintain the male identity of cyst stem cells in the adult testis. Chinmo is required for dsxM expression, but loss of chinmo and loss of dsx yield distinct phenotypes, suggesting that Chinmo has additional targets besides dsx in the testis (Ma et al., 2014). In the developing ovary, a well-characterized alternative-splicing cascade mediated by the female determinants Sex lethal (Sxl) and TransformerF (TraF) splices dsx transcripts into the female-specific form DsxF (Whitworth et al., 2012), but whether these sex determinants play a role in somatic cells of the adult ovary is not clear. We speculated that ectopic Chinmo could be inducing a male fate at least partly by inducing the expression of DsxM; alternatively, it could be acting through a DsxM-independent mechanism. To distinguish between these possibilities, we asked if ectopic expression of DsxM could phenocopy ectopic chinmo expression in the adult ovary. We used the same strategy described above to conditionally express DsxM in somatic cells (Gal80TS, c587-Gal4>dsxM). As expected, testes overexpressing DsxM looked like wild-type testes, confirming that high levels of DsxM do not disrupt cell viability (Fig. S3A). Ovaries were also indistinguishable from wild-type ovaries both before and after one week of ectopic DsxM expression in an otherwise wild-type genetic background (Fig. 3A,B). In other genetic backgrounds, some ovaries displayed a range of phenotypes including degenerating germ cells and egg chambers filled with early germ cells or with a mix of early germ cells and older germ cells (Fig. S3B-F). Even in these ovaries, however, the somatic cells did not resemble those in ovaries with ectopic chinmo expression, as they could still form distinct layers around the germ cells, and egg chambers could still exit the germarium (compare Fig. 1F-J with Fig. S3B-F). Based on these results, we suggest that ectopic chinmo is not acting through DsxM to disrupt the morphology of the adult ovary. Consistent with this hypothesis, we did not detect DsxM protein in ovaries with ectopic chinmo expression (Fig. 3C,D), although we could detect it in control ovaries directly expressing ectopic DsxM (Fig. S3C). Together these data support the hypothesis that Chinmo induces a male somatic identity in ovaries through a DsxM-independent mechanism.
Fig. 3.
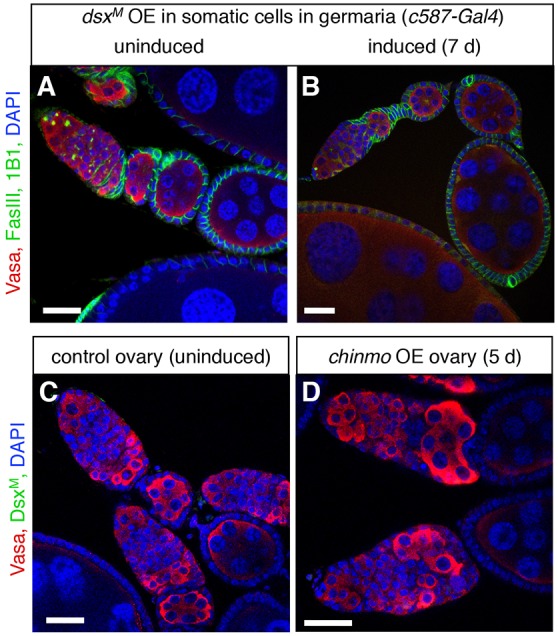
Chinmo is not acting through the male determinant DsxM to masculinize the ovary. (A,B) Immunofluorescence detection in adult ovaries of FasIII (green at cell periphery) to visualize somatic cell membranes, and 1B1 (green in germ cells) to mark fusomes. Before ectopic expression of DsxM, ovaries look normal (A). After ectopic overexpression (OE) of DsxM in somatic cells in adult germaria (B), ovaries are also indistinguishable from wild-type ovaries. (C,D) Immunofluorescence detection of the male-specific protein DsxM (green). DsxM is not expressed in control ovaries (C) or in chinmo overexpression ovaries after five days of ectopic chinmo expression in adult somatic cells (D). Vasa (red) marks germ cells and DAPI (blue) marks nuclei in all panels. Scale bars: 20 μm.
Female sex determination factors might feminize male somatic cells in parallel with Chinmo
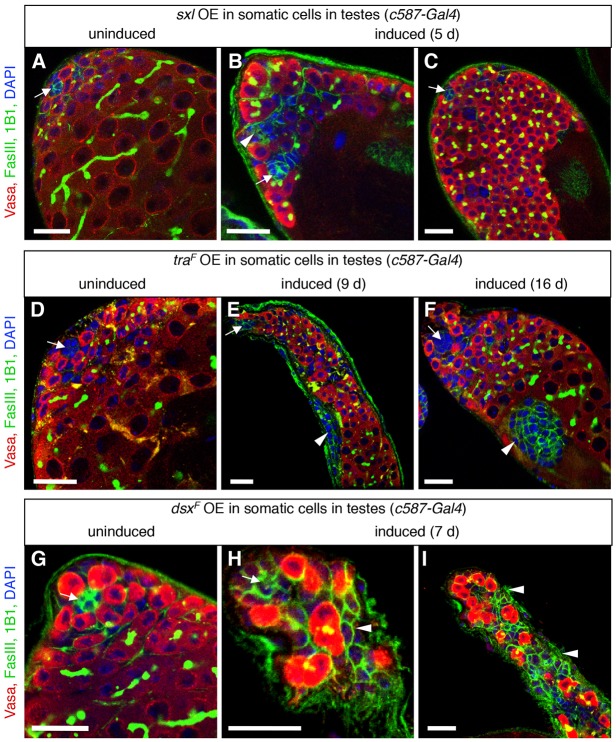
We next asked if ectopic expression of female sex determinants could disrupt the adult testis. We showed previously that the female sex determinants Sxl, TraF and DsxF are not upregulated in testes lacking chinmo (Ma et al., 2014), but it remained possible that these factors could phenocopy loss of chinmo when they are expressed ectopically on their own. Therefore, we conditionally expressed Sxl, TraF or DsxF specifically in the cyst stem cell lineage of adult testes (using c587-Gal4 and Gal80TS). Before transgene induction, testes were phenotypically indistinguishable from wild-type testes (Fig. 4A,D,G), and overexpressing these transgenes in the adult ovary did not affect oogenesis, confirming that high levels of these proteins do not disrupt cell viability (Fig. S4A-C). By contrast, after overexpression of each of these female sex determinants in adult testes, testes displayed a range of phenotypes including the formation of small aggregates of somatic cells and accumulation of early germ cells (Fig. 4B,C,E,F,H,I). These phenotypes are similar to the phenotypes we observed in testes lacking dsx and in young chinmoST mutant testes (Ma et al., 2014). In testes expressing ectopic female sex determinants, however, the aggregates of somatic cells did not expand into layers of follicle-like cells that line the testis periphery, as in older chinmoST mutant testes. We conclude that ectopic expression of female sex determinants in adult testes disrupts spermatogenesis, but phenocopies the loss of chinmo only partially.
Fig. 4.
Ectopic expression of female sex determinants in the adult testis disrupts testis morphology. (A-I) Immunofluorescence detection of FasIII (green at cell periphery) and 1B1 (green in germ cell fusomes) to visualize testis morphology before or after ectopic expression of female sex determinants in cyst stem cells and early cyst cells in adult testes. Vasa (red) marks germ cells; DAPI (blue) marks nuclei; arrows mark the hub. Before expression of sxl (A), traF (D) or dsxF (G), testes look normal. After ectopic overexpression (OE) of sxl (B,C) or traF (E,F), aggregates of FasIII+ somatic cells (arrowheads) and overproliferating early germ cells accumulate at the testis apex. After ectopic expression of dsxF (H,I), germ cells fail to differentiate and aggregates of somatic cells accumulate at the testis apex. Scale bars: 20 μm.
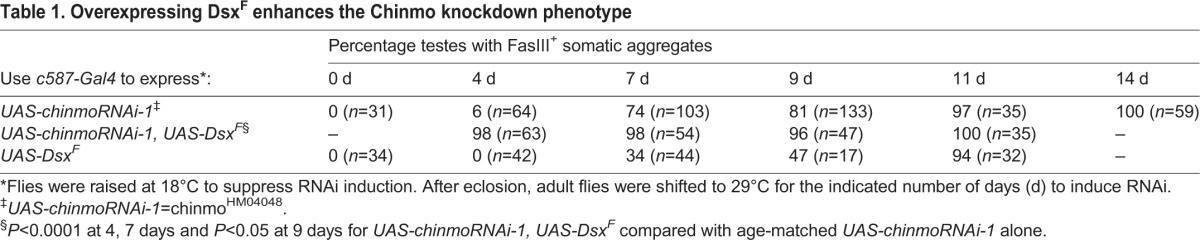
To further explore the roles of chinmo and dsx in sex maintenance, we then ectopically expressed dsxF and knocked down expression of chinmo at the same time in adult testes, thereby more closely mimicking the expression pattern that is found normally in developing ovaries (dsxF ‘on’, chinmo and dsxM ‘off’). Specifically, we asked if ectopic DsxF could enhance the chinmo mutant phenotype, and we found that this is indeed the case (Table 1). After co-expressing ectopic DsxF and chinmo RNAi in somatic cells in adult testes for 4 days (using c587-Gal4 and Gal80TS), we found that 98.4% of testes had the early chinmo mutant phenotype (the appearance of somatic cell aggregates), compared with 6.3% of control testes expressing only chinmo RNAi (n=64) and 0% of testes expressing only DsxF (n=42). We conclude that ectopic expression of DsxF greatly accelerates the feminization of the adult cyst stem cell lineage cells caused by loss of chinmo. Together, these data indicate that ectopic expression of female determinants is sufficient to disrupt male fate and promote the female fate of somatic cells in the adult testis, but is not sufficient to fully convert these cells into follicle-like cells as in testes lacking chinmo.
Table 1.
Overexpressing DsxF enhances the Chinmo knockdown phenotype
Ectopic expression of miRNA let-7 in testis somatic cells is sufficient to downregulate Chinmo and cause the sex conversion phenotype
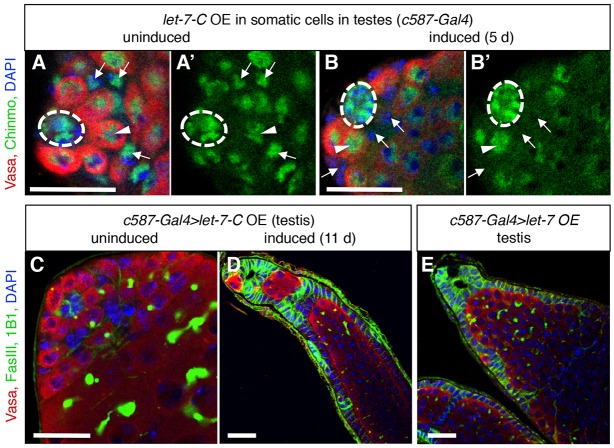
chinmo transcripts are directly regulated by microRNAs transcribed from the let-7-Complex (let-7-C) in the developing Drosophila brain, and ectopic expression of let-7-C is sufficient to downregulate Chinmo protein levels in mushroom body neurons (Wu et al., 2012). To ask if ectopic let-7-C expression could phenocopy loss of chinmo in the adult testis, we conditionally overexpressed let-7-C in the adult cyst stem cell lineage (using c587-Gal4 and Gal80TS) and immunostained testes with anti-Chinmo antisera. Before let-7-C overexpression, Chinmo was expressed as expected in hub cells, cyst stem cell lineage cells, and at lower levels in germ cells (Fig. 5A), and testes were phenotypically wild type (Fig. 5C). After five days of let-7-C transgene induction, Chinmo protein levels decreased in the cyst stem cells and cyst cells but not in the hub or germ cells (Fig. 5B), which was expected as we did not express the transgene in the latter two cell types. After the period of ectopic let-7-C expression was extended for several more days, most testes acquired follicle-like cells, strikingly phenocopying the chinmo somatic sex transformation phenotype (Fig. 5D). Immunostaining for Chinmo at this later timepoint indicated that the somatic aggregates and the follicle-like cells lacked Chinmo, whereas the hub cells and germ cells still expressed Chinmo (Fig. S5A,B). We confirmed this result using two additional cyst stem cell lineage Gal4 drivers (eyaA3-Gal4 and tj-Gal4), which gave similar results (Fig. S5C,D). We also obtained the same phenotype by overexpressing a single let-7-C miRNA, miRNA let-7, further confirming this result (Fig. 5E). Finally, as expected because chinmo is not required in testis germ cells, overexpressing let-7 in the germ cells yielded no obvious phenotypes (Fig. S5E). Together, these data indicate that ectopic let-7 is sufficient to downregulate Chinmo in the adult cyst stem cell lineage, and the resulting decrease in Chinmo levels is severe enough to feminize the testis.
Fig. 5.
Overexpression of let-7-C miRNAs in the adult testis phenocopies loss of chinmo. (A,B) Immunofluorescence detection of Chinmo (green) in testes before or after let-7-C overexpression in adult cyst stem cells and early cyst cells. (A) Before let-7-C overexpression (OE), Chinmo is expressed in cyst stem cell lineage cells (arrows), germ cells (arrowheads), and hub cells (dashed circles). (B) After five days of let-7-C overexpression, Chinmo is depleted from cyst stem cell lineage cells (arrows) but is still expressed in germ cells and hub cells. (C-E) Immunofluorescence detection of FasIII (green at cell periphery) and 1B1 (green in germ cell fusomes) to visualize the morphology of adult testes before or after overexpression of let-7-C or let-7 in adult cyst stem cells and early cyst cells. (C) Before let-7-C overexpression, the testis looks normal. (D) After overexpression of let-7-C, 63% of testes (n=30) contain monolayers of follicle-like cells and overproliferating germ cells, similar to chinmo mutant testes (Ma et al., 2014). (E) Overexpression of let-7 alone produces a similar phenotype in 70% of testes (n=23). Vasa (red) marks germ cells and DAPI (blue) marks nuclei in all panels. Scale bars: 20 μm.
let-7 might not repress Chinmo in the ovary
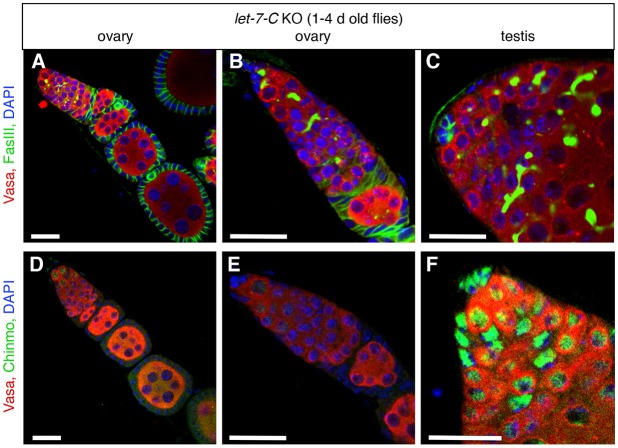
Because ectopic let-7 is sufficient to downregulate Chinmo in the testis and cause the testis sex conversion phenotype, we hypothesized that let-7 might be required to maintain Chinmo at undetectable levels in the wild-type ovary, thereby having an important role in preventing the female to male conversion of somatic cells. However, it is also possible that the regulation of Chinmo occurs at the transcriptional rather than the post-transcriptional level. Therefore, we examined ovaries from adult female flies carrying multiple different let-7-C deletions. We did not find any obvious ovary phenotypes (Fig. 6A,B). Consistent with this observation, we also did not detect any ectopically expressed Chinmo in these ovaries by immunostaining (Fig. 6D,E). Testes from adult male flies carrying let-7-C deletions expressed Chinmo as expected (Fig. 6C,F). We conclude that let-7 is not required to repress chinmo expression in the adult ovary.
Fig. 6.
let-7 does not repress Chinmo in the ovary. (A-C) Immunofluorescence detection of FasIII (green at cell periphery) and 1B1 (green in germ cell fusomes) to visualize the morphology of adult ovaries and testes in let-7-C knockout flies. Both ovaries (A,B) and testes (C) look normal. (D-F) Immunofluorescence detection of Chinmo (green) in ovaries and testes in let-7-C knockout flies. Chinmo is not ectopically expressed in somatic cells in let-7-C knockout ovaries (D,E) but is expressed in somatic and germline cells in let-7-C knock-out testes (F) in a pattern similar to control testes (compare with Fig. 5A). Vasa (red) marks germ cells and DAPI (blue) marks nuclei in all panels. Scale bars: 20 μm.
DISCUSSION
We have found that loss of chinmo from testes (Ma et al., 2014) or ectopic expression of chinmo in adult ovaries (this study) causes gonadal somatic cells to undergo a sex transformation. Therefore, Chinmo is both necessary and sufficient to induce a male fate in somatic cells of adult Drosophila gonads. A similar role is played in adult mice by the transcription factor DMRT1, which is necessary for maintaining the male identity of testis Sertoli cells (Matson et al., 2011), and also sufficient to induce a male identity in ovarian somatic cells (Lindeman et al., 2015). When expressed ectopically in the mouse ovary, DMRT1 acts by silencing the gene Foxl2, which is required for maintaining a female fate, and loss of FOXL2 by itself causes a similar female-to-male sex transformation (Uhlenhaut et al., 2009; Lindeman et al., 2015). Thus, sexual cell fates are actively maintained in the adult gonad in male and female mice, and loss of key maintenance factors results in a sex transformation. In Drosophila, a factor that maintains the female identity of somatic cells in the adult ovary has not yet been identified; however, the ability of these cells to switch their sexual identity in response to ectopic expression of chinmo suggests that adult female fate can be reprogrammed, as in the adult Drosophila testis and mouse ovary and testis. Other questions that remain for future studies are what factor(s) normally repress chinmo expression in the ovary, and whether Chinmo homologues play a role in sex maintenance in mammals or other vertebrates.
In mice, the sex-determining region of Chr Y (Sry) gene in the fetal gonad triggers male sex determination by activating the male determinant SOX9 (Matson and Zarkower, 2012). Dmrt1 is not required for the initial sex determination, but it is required for maintaining the male identity of Sertoli cells in postnatal male mice. In female mice, transgenic overexpression of Dmrt1 in somatic gonadal cells causes transdifferentiation of adult granulosa cells to Sertoli cells, which indicates that DMRT1 is sufficient to convert fully differentiated female somatic cells into functional male somatic cells. Interestingly, this somatic sex conversion does not depend on the male sex determinant SOX9 (Lindeman et al., 2015; Zhao et al., 2015). Similarly, in adult Drosophila females, we have found that ectopic expression of chinmo masculinizes the ovary independently of the male sex determinant DsxM. By contrast, Chinmo maintains the male fate of somatic cells in the adult Drosophila testis in part by promoting the expression of dsxM (Ma et al., 2014). Other downstream effectors of Chinmo in the wild-type testis or masculinized ovary are not known. Some might induce the expression of male-specific factors or shut down the expression of female-specific factors; others are likely to control cell morphology, altering the arrangement of somatic cells from a regular columnar epithelium that surrounds the germ cells and forms distinct egg chambers (in the absence of Chinmo) to squamous cells that pile up in the germarium (in the presence of Chinmo). Comparing genes that are expressed in wild-type testes to those in chinmo mutant testes, or in wild-type ovaries to those in chinmo overexpressing ovaries, could be informative.
miRNAs are thought to work by coordinately fine-tuning the expression of many target genes (Ebert and Sharp, 2012). In the Drosophila testis, an age-related increase in levels of let-7 is known to downregulate IGF-II mRNA-binding protein (Imp) in the hub, which in turn mediates a slight age-related decrease in GSC number (Toledano et al., 2012). We showed that overexpressing let-7 in somatic cells downregulates Chinmo enough to produce a phenotype that is indistinguishable from a strong loss-of-function chinmo allele. This finding prompted us to speculate whether let-7-C could normally downregulate Chinmo levels in the ovary to prevent masculinization of the somatic cells. However, we did not detect ectopic Chinmo or any obvious phenotypes similar to chinmo overexpression in ovaries from flies carrying deletions of let-7, even though the flies themselves displayed the neurological phenotypes characteristic of let-7-C deficiency (Sokol et al., 2008), indicating that the deletions were as expected. In a previous study, knocking out let-7 was shown to give a mild phenotype, in which the number of early germ cells increased from around four to seven (König and Shcherbata, 2015). However, the authors of that study did not report any phenotypes similar to the ones we observed in the ovary after ectopic expression of chinmo. Identifying the factors that keep Chinmo switched off in the ovary and prevent masculinization of adult ovarian somatic cells is a goal for future studies.
The Drosophila testis and ovary are highly tractable genetic systems for studying how somatic sexual identity is actively maintained in adult gonads. Some human gonadal cancers, such as granulosa cell tumors, are thought to arise from mutations in genes that mediate somatic sexual identity (Hanson and Ambaye, 2011), and sexual dimorphism might also be actively maintained in other mammalian organs. Analyzing Chinmo and Dsx/DMRT1-mediated sex maintenance pathways in Drosophila could elucidate the mechanisms underlying these processes and, more generally, transdifferentiation of adult somatic cells in vivo.
MATERIALS AND METHODS
Fly stocks and cultures
Fly stocks were raised at 25°C on standard molasses/yeast medium unless otherwise indicated. The following fly stocks were used: UAS-FL-chinmo, UAS-5′UTR-chinmo and UAS-3′UTR-chinmo (Zhu et al., 2006); eyaA3-Gal4 (Leatherman and DiNardo, 2008); M5-4 (Gönczy and DiNardo, 1996); esg-GFP (CB02017, Buszczak et al., 2007); c587-Gal4 (Kai and Spradling, 2003); nanos-Gal4-VP16 (Van Doren et al., 1998); tj-Gal4 (Drosophila Genetic Resource Center); UAS-Sxl (Horabin et al., 2003); UAS-dsxF (from Baker Lab, HHMI/Janelia Research Campus, USA); UAS-dsxM (Lee et al., 2002); let-7-CGKI, let-7-CKO1, UAS-let-7-C and UAS-let-7 (Sokol et al., 2008). y w flies were used as control flies unless otherwise indicated. Other fly stocks were from the Bloomington Drosophila Stock Center or Vienna Drosophila Resource Center.
Immunostaining
Testes and ovaries were dissected, fixed and stained as described previously (Matunis et al., 1997). Tyramide signal amplification (Invitrogen) was used to increase sensitivity of rat anti-DsxM (Hempel and Oliver, 2007; 1:500 dilution). The following antibodies were also used: rabbit anti-Vasa (d-260) and goat anti-Vasa (dN-13) (sc-30210 and sc-26875, Santa Cruz Biotechnology; 1:400); rabbit anti-GFP (TP401, Torrey Pines Biolabs; 1:10,000); chicken anti-GFP (ab13970, Abcam; 1:10,000); mouse anti-β-Galactosidase (Z378A, Promega; 1:1000); mouse anti-adducin-related protein (1B1; 1:25) and mouse anti-Fasciclin 3 (7G10 anti-Fas III; 1:50), both from Developmental Studies Hybridoma Bank, University of Iowa; rat-anti-Chinmo (Wu et al., 2012; 1:500); and guinea pig anti-Tj (Li et al., 2003; 1:4000). Alexa Fluor-conjugated secondary IgG (H+L) antibodies were diluted at 1:200 for 568 and 633 conjugates and 1:400 for 488 conjugates. Secondary antisera were: goat anti-rat 488, goat anti-rabbit 488 and 568, goat anti-mouse 488 and 568, goat anti-chick 488, and goat anti-guinea pig 568 (A11006, A11078, A11011, A11001, A11004, A11039 and A11075, Molecular Probes/Invitrogen). DNA was stained with 4,6-diamidino-2-phenylindole (DAPI; Sigma) at 1 mg/ml.
Conditional gene expression
To overexpress or knock down genes in a temporal and cell-specific manner, cell type-specific Gal4 drivers were used in combination with a temperature-sensitive allele of the Gal4 repressor (Gal80TS) to conditionally express transgenic RNAi or overexpression constructs of different genes. To induce transgene expression only in adult flies but not during development, flies were grown at the permissive temperature of 18°C in which Gal4 expression is repressed, and shifted to the restrictive temperature of 29°C or 31°C after eclosion to induce gene expression for various lengths of time.
Mosaic analysis
Adult flies with the genotype Ay-Gal4 UAS-lacZ/esg-GFP; hs-FLP/UAS-chinmo (or control flies lacking UAS-chinmo) were heat shocked in a 37°C water bath once for 2 h or three times for 30 min, with 30 min breaks in between, and placed at 29°C after heat shock. Experimental flies that were placed at 29°C but not heat shocked did not contain any clones (data not shown).
Microscopy and image analysis
Fixed testes were mounted in Vectashield (Vector Labs), imaged with a Zeiss LSM 5 Pascal or LSM 510 Meta, and analyzed using the Zeiss LSM Image Browser software. All panels are single confocal sections unless stated otherwise.
Acknowledgements
We thank Leah Greenspan and Geraldine Seydoux for comments on the manuscript, and Nick Sokol, Brian Oliver, Jamila Horabin, Allan Spradling, Steve DiNardo, Dorothea Godt, Tzumin Lee, Gyanghee Lee, Bruce Baker, Bloomington Drosophila Stock Center, Vienna Drosophila Resource Center, Drosophila Genetic Resource Center, and Developmental Studies Hybridoma Bank at the University of Iowa for flies or antisera. We also thank two anonymous reviewers for insightful comments and suggestions.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Q.M. and M.d.C. performed the experiments; Q.M., M.d.C. and E.L.M. designed the experiments, analyzed the data, and prepared the manuscript.
Funding
This work was funded by the National Institutes of Health [HD040307, HD052937 to E.L.M.]. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.129627/-/DC1
References
- Buszczak M., Paterno S., Lighthouse D., Bachman J., Planck J., Owen S., Skora A. D., Nystul T. G., Ohlstein B., Allen A. et al. (2007). The Carnegie protein trap library: a versatile tool for Drosophila developmental studies. Genetics 175, 1505-1531. 10.1534/genetics.106.065961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cuevas M. and Matunis E. L. (2011). The stem cell niche: lessons from the Drosophila testis. Development 138, 2861-2869. 10.1242/dev.056242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decotto E. and Spradling A. C. (2005). The Drosophila ovarian and testis stem cell niches: similar somatic stem cells and signals. Dev. Cell 9, 501-510. 10.1016/j.devcel.2005.08.012 [DOI] [PubMed] [Google Scholar]
- Ebert M. S. and Sharp P. A. (2012). Roles for microRNAs in conferring robustness to biological processes. Cell 149, 515-524. 10.1016/j.cell.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliazer S. and Buszczak M. (2011). Finding a niche: studies from the Drosophila ovary. Stem Cell Res. Ther. 2, 45 10.1186/scrt86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty M. S., Salis P., Evans C. J., Ekas L. A., Marouf A., Zavadil J., Banerjee U. and Bach E. A. (2010). chinmo is a functional effector of the JAK/STAT pathway that regulates eye development, tumor formation, and stem cell self-renewal in Drosophila. Dev. Cell 18, 556-568. 10.1016/j.devcel.2010.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan Q., Chepelev I., Wei G., Tarayrah L., Cui K., Zhao K. and Chen X. (2010). Dynamic regulation of alternative splicing and chromatin structure in Drosophila gonads revealed by RNA-seq. Cell Res. 20, 763-783. 10.1038/cr.2010.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gönczy P. and DiNardo S. (1996). The germ line regulates somatic cyst cell proliferation and fate during Drosophila spermatogenesis. Development 122, 2437-2447. [DOI] [PubMed] [Google Scholar]
- Hanson J. A. and Ambaye A. B. (2011). Adult testicular granulosa cell tumor: a review of the literature for clinicopathologic predictors of malignancy. Arch. Pathol. Lab. Med. 135, 143-146. 10.1043/2009-0512-RSR.1 [DOI] [PubMed] [Google Scholar]
- Hempel L. U. and Oliver B. (2007). Sex-specific DoublesexM expression in subsets of Drosophila somatic gonad cells. BMC Dev. Biol. 7, 113 10.1186/1471-213X-7-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel L. U., Kalamegham R., Smith J. E. III and Oliver B. (2008). Drosophila germline sex determination: integration of germline autonomous cues and somatic signals. Curr. Top. Dev. Biol. 83, 109-150. 10.1016/S0070-2153(08)00404-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horabin J. I., Walthall S., Vied C. and Moses M. (2003). A positive role for Patched in Hedgehog signaling revealed by the intracellular trafficking of Sex-lethal, the Drosophila sex determination master switch. Development 130, 6101-6109. 10.1242/dev.00865 [DOI] [PubMed] [Google Scholar]
- Jinks T. M., Polydorides A. D., Calhoun G. and Schedl P. (2000). The JAK/STAT signaling pathway is required for the initial choice of sexual identity in Drosophila melanogaster. Mol. Cell 5, 581-587. 10.1016/S1097-2765(00)80451-7 [DOI] [PubMed] [Google Scholar]
- Kai T. and Spradling A. (2003). An empty Drosophila stem cell niche reactivates the proliferation of ectopic cells. Proc. Natl. Acad. Sci. 100, 4633-4638. 10.1073/pnas.0830856100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiger A. A., Jones D. L., Schulz C., Rogers M. B. and Fuller M. T. (2001). Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science 294, 2542-2545. 10.1126/science.1066707 [DOI] [PubMed] [Google Scholar]
- König A. and Shcherbata H. R. (2015). Soma influences GSC progeny differentiation via the cell adhesion-mediated steroid-let-7-Wingless signaling cascade that regulates chromatin dynamics. Biol. Open 4, 285-300. 10.1242/bio.201410553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leatherman J. L. and DiNardo S. (2008). Zfh-1 controls somatic stem cell self-renewal in the Drosophila testis and nonautonomously influences germline stem cell self-renewal. Cell Stem Cell 3, 44-54. 10.1016/j.stem.2008.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G., Hall J. C. and Park J. H. (2002). Doublesex gene expression in the central nervous system of Drosophila melanogaster. J. Neurogenet. 16, 229-248. 10.1080/01677060216292 [DOI] [PubMed] [Google Scholar]
- Li M. A., Alls J. D., Avancini R. M., Koo K. and Godt D. (2003). The large Maf factor Traffic Jam controls gonad morphogenesis in Drosophila. Nat. Cell Biol. 5, 994-1000. 10.1038/ncb1058 [DOI] [PubMed] [Google Scholar]
- Lindeman R. E., Gearhart M. D., Minkina A., Krentz A. D., Bardwell V. J. and Zarkower D. (2015). Sexual cell-fate reprogramming in the ovary by DMRT1. Curr. Biol. 25, 764-771. 10.1016/j.cub.2015.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Onieva L., Fernandez-Minan A. and Gonzalez-Reyes A. (2008). Jak/Stat signalling in niche support cells regulates dpp transcription to control germline stem cell maintenance in the Drosophila ovary. Development 135, 533-540. 10.1242/dev.016121 [DOI] [PubMed] [Google Scholar]
- Ma Q., Wawersik M. and Matunis E. L. (2014). The Jak-STAT target Chinmo prevents sex transformation of adult stem cells in the Drosophila testis niche. Dev. Cell 31, 474-486. 10.1016/j.devcel.2014.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis J. and Spradling A. (1995). Identification and behavior of epithelial stem cells in the Drosophila ovary. Development 121, 3797-3807. [DOI] [PubMed] [Google Scholar]
- Matson C. K. and Zarkower D. (2012). Sex and the singular DM domain: insights into sexual regulation, evolution and plasticity. Nat. Rev. Genet. 13, 163-174. 10.1038/nrg3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson C. K., Murphy M. W., Sarver A. L., Griswold M. D., Bardwell V. J. and Zarkower D. (2011). DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature 476, 101-104. 10.1038/nature10239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matunis E., Tran J., Gonczy P., Caldwell K. and DiNardo S. (1997). punt and schnurri regulate a somatically derived signal that restricts proliferation of committed progenitors in the germline. Development 124, 4383-4391. [DOI] [PubMed] [Google Scholar]
- McGuire S. E., Mao Z. and Davis R. L. (2004). Spatiotemporal gene expression targeting with the TARGET and Gene-Switch systems in Drosophila. Sci. Signal. 2004, pl6 10.1126/stke.2202004pl6 [DOI] [PubMed] [Google Scholar]
- Sahai-Hernandez P., Castanieto A. and Nystul T. G. (2012). Drosophila models of epithelial stem cells and their niches. Wiley Interdiscip. Rev. Dev. Biol. 1, 447-457. 10.1002/wdev.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng X. R., Posenau T., Gumulak-Smith J. J., Matunis E., Van Doren M. and Wawersik M. (2009). Jak-STAT regulation of male germline stem cell establishment during Drosophila embryogenesis. Dev. Biol. 334, 335-344. 10.1016/j.ydbio.2009.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol N. S., Xu P., Jan Y.-N. and Ambros V. (2008). Drosophila let-7 microRNA is required for remodeling of the neuromusculature during metamorphosis. Genes Dev. 22, 1591-1596. 10.1101/gad.1671708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledano H., D'Alterio C., Czech B., Levine E. and Jones D. L. (2012). The let-7-Imp axis regulates ageing of the Drosophila testis stem-cell niche. Nature 485, 605-610. 10.1038/nature11061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran J., Brenner T. J. and DiNardo S. (2000). Somatic control over the germline stem cell lineage during Drosophila spermatogenesis. Nature 407, 754-757. 10.1038/35037613 [DOI] [PubMed] [Google Scholar]
- Tulina N. and Matunis E. (2001). Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science 294, 2546-2549. 10.1126/science.1066700 [DOI] [PubMed] [Google Scholar]
- Uhlenhaut N. H., Jakob S., Anlag K., Eisenberger T., Sekido R., Kress J., Treier A.-C., Klugmann C., Klasen C., Holter N. I. et al. (2009). Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell 139, 1130-1142. 10.1016/j.cell.2009.11.021 [DOI] [PubMed] [Google Scholar]
- Van Doren M., Williamson A. L. and Lehmann R. (1998). Regulation of zygotic gene expression in Drosophila primordial germ cells. Curr. Biol. 8, 243-246. 10.1016/S0960-9822(98)70091-0 [DOI] [PubMed] [Google Scholar]
- Wawersik M., Milutinovich A., Casper A. L., Matunis E., Williams B. and Van Doren M. (2005). Somatic control of germline sexual development is mediated by the JAK/STAT pathway. Nature 436, 563-567. 10.1038/nature03849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth C., Jimenez E. and Van Doren M. (2012). Development of sexual dimorphism in the Drosophila testis. Spermatogenesis 2, 129-136. 10.4161/spmg.21780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.-C., Chen C.-H., Mercer A. and Sokol N. S. (2012). Let-7-complex microRNAs regulate the temporal identity of Drosophila mushroom body neurons via chinmo. Dev. Cell 23, 202-209. 10.1016/j.devcel.2012.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Svingen T., Ng E. T. and Koopman P. (2015). Female-to-male sex reversal in mice caused by transgenic overexpression of Dmrt1. Development 142, 1083-1088. 10.1242/dev.122184 [DOI] [PubMed] [Google Scholar]
- Zhu S., Lin S., Kao C.-F., Awasaki T., Chiang A.-S. and Lee T. (2006). Gradients of the Drosophila Chinmo BTB-zinc finger protein govern neuronal temporal identity. Cell 127, 409-422. 10.1016/j.cell.2006.08.045 [DOI] [PubMed] [Google Scholar]