Abstract
Study Objective:
To evaluate next-morning driving performance in adults younger than 65 years, after single and repeated doses of suvorexant 20 and 40 mg.
Design:
Double-blind, placebo-controlled, 4-period crossover study.
Setting:
Maastricht University, The Netherlands.
Participants:
28 healthy volunteers (15 females), aged 23 to 64 years.
Interventions:
Suvorexant (20 and 40 mg) for 8 consecutive nights; zopiclone 7.5 mg nightly on day 1 and 8; placebo.
Measurements:
Performance on day 2 and 9 (9 h after dosing) using a one-hour standardized highway driving test in normal traffic, measuring standard deviation of lateral position (SDLP). Drug-placebo changes in SDLP > 2.4 cm were considered to reflect meaningful driving impairment.
Results:
Mean drug-placebo changes in SDLP following suvorexant 20 and 40 mg were 1.01 and 1.66 cm on day 2, and 0.48 and 1.31 cm on Day 9, respectively. The 90% CIs of these changes were all below 2.4 cm. Symmetry analysis showed that more subjects had SDLP changes > 2.4 cm than < −2.4 cm following suvorexant 20 and 40 mg on day 2, and following suvorexant 40 mg on day 9. Four female subjects requested that a total of 5 driving tests—all following suvorexant—stop prematurely due to self-reported somnolence.
Conclusions:
As assessed by mean changes in standard deviation of lateral position (SDLP), there was no clinically meaningful residual effect of suvorexant in doses of 20 and 40 mg on next-morning driving (9 h after bedtime dosing) in healthy subjects < 65 years old. There may be some individuals who experience next-day effects, as suggested by individual changes in SDLP and prematurely stopped tests.
Clinical Trial Registration:
clinicaltrials.gov NCT01311882.
Citation:
Vermeeren A, Sun H, Vuurman EF, Jongen S, Van Leeuwen CJ, Van Oers AC, Palcza J, Li X, Laethem T, Heirman I, Bautmans A, Troyer MD, Wrishko R, McCrea J. On-the-road driving performance the morning after bedtime use of suvorexant 20 and 40 mg: a study in non-elderly healthy volunteers. SLEEP 2015;38(11):1803–1813.
Keywords: hypnotics, suvorexant, zopiclone, orexin antagonist, driving, memory, DSST, balance, plasma concentrations
INTRODUCTION
Residual daytime sleepiness and associated impairment of psychomotor and cognitive functioning the day after bedtime use constitutes one of the main concerns associated with the use of hypnotics. In car drivers this is of particular concern, since reduced alertness and slowed reactions may increase a person's risk to become involved in accidents. Currently available hypnotics are typically GABA agonists, i.e., benzodiazepines and benzodiazepine-like drugs. Results from epidemiological studies show that use of benzodiazepines is associated with increased risks of car accidents.1–3
In August 2014, suvorexant (MK-4305), a drug with a novel mechanism of action, was approved in the United States for adults with insomnia who have difficulty falling asleep and/or staying asleep.4 Suvorexant promotes sleep by blocking orexin receptors.5,6 Orexin (or hypocretin) is a peptide produced by neurons in the lateral hypothalamus that seems essential for stable waking, as shown by narcolepsy patients who suffer from a lack of orexin. In their recent review of orexin regulation of sleep and wakefulness, de Lecea and Huerta7 report that orexin neurons exhibit firing patterns that parallel those of monoaminergic neurons, i.e., most active during wakefulness, mild firing during NREM sleep, and silent during REM sleep. In line with this, orexin levels have been found to peak at the end of the active phase, and fall to about half their maximum levels during sleep. It was also found that phasic activity of orexin neurons during sleep increases the probability of awakening. Reduction of orexinergic tone seems therefore essential for initiation and maintenance of sleep.5,7 Blocking the orexin system during the night might reduce hyperarousal and thus improve sleep in insomnia patients.8,9
Suvorexant is a potent and selective antagonist at orexin-1 and orexin-2 receptors, i.e., a dual orexin receptor antagonist (DORA). Following oral administration it is well absorbed, with an average tmax occurring between 1.5 and 4 h after PM dosing and a half-life of about 12 h.10 Steady state is reached after 3 days of dosing.4 Clinical studies have shown that suvorexant in doses between 10 and 100 mg significantly improves subjective and objective measures of sleep.10–13 Suvorexant was found to decrease sleep latencies and wake time after sleep onset in healthy young volunteers and insomnia patients.10–13 A 1-year multicenter trial to determine the safety and efficacy of suvorexant 30 and 40 mg showed that suvorexant had sustained effects on subjective total sleep time up to 1 year.12 Furthermore, suvorexant was well tolerated and did not show rebound or withdrawal effects upon discontinuation. The most common adverse events associated with suvorexant 30 and 40 mg were primarily extensions of the drug's pharmacological activity, i.e., somnolence (13.2%), fatigue (6.5%), and dry mouth (5.0 %).12
Results from animal studies suggest that DORAs require a high level of orexin-2 receptor occupancy, i.e., 65% or more.14 When occupancy falls below this threshold, the sleep-promoting effects seem to disappear. Based on these findings it is hypothesized that orexin antagonists like suvorexant, with a pharmacokinetic profile such that occupancy falls below a sleep-promoting threshold by morning,10,14 are not likely to produce residual daytime sedation due to decreasing drug levels and increasing competition by endogenous orexin upon awakening. A key determinant for the extent of morning receptor occupancy is the dose used.
The recommended starting dose of suvorexant in the United States is 10 mg, which may be increased to a maximum of 20 mg.4 Clinical trials including exploratory evaluations of residual effects of suvorexant on performance, did not find consistent dose-dependent differences from placebo for doses up to 80 mg.10,11 A study in 19 young healthy male volunteers compared the effects of single bedtime doses of suvorexant 10, 50, and 100 mg, and placebo on simple reaction time, choice reaction, and digit symbol substitution test (DSST) performance the next morning, 10 h after intake.10 Statistically significant impairment was found after the 100 mg dose, but not after the 10 and 50 mg doses. A Phase 2B study in insomnia patients also evaluated residual effects of suvorexant 10, 20, 40, and 80 mg and placebo after the first dose and after 4 weeks of treatment, using a DSST and a digit symbol copying test.11 Results did not show consistent dose-dependent effects. The only significant differences from placebo were found after the first dose of 20 mg on DSST and after 4 weeks of treatment with 40 mg on digit symbol copying test. Exploratory findings of clinical trials therefore suggest that next-day effects of suvorexant are likely to be absent or minor after bedtime doses of 40 mg or less.
The primary objective of the present study was to evaluate the next-morning residual effects of suvorexant 20 and 40 mg on car driving, after single and repeated bedtime use in healthy volunteers younger than 65 years. Driving performance was assessed by the standard deviation of lateral position (SDLP in cm) in a standardized on-the-road driving test.15 The effects of suvorexant were to be compared to those of placebo by analysis of mean drug-placebo changes and by symmetry analysis of drug-placebo.16 Based on results from a previous calibration study with alcohol,17 a mean drug-placebo difference in SDLP with a 90% confidence interval < 2.4 cm was not considered clinically meaningful. A symmetry analysis was used to determine whether there was a statistically significant imbalance between the number of subjects with drug-placebo differences larger than the criterion of 2.4 cm (impairment) versus the number of subjects with drug-placebo differences less than −2.4 cm (improvement).
Secondary or exploratory objectives were to compare the residual effects of suvorexant with those of placebo on performance in tests of word learning, digit symbol substitution and postural balance, and to determine the relation between suvorexant blood concentrations (obtained at 11 h post dose) and driving performance. Zopiclone 7.5 mg was selected as an active control, because it was consistently found to have moderate impairing effects on driving the morning after bedtime administration.16,18–20
METHODS
Subjects
Twenty-eight subjects (13 men, 15 women) were recruited via advertisements placed in local newspapers. Healthy volunteers between 21 and 64 years of age (inclusive) were eligible to enroll if they possessed a valid driver's license, had driving experience ≥ 5,000 km/year on average within the last 3 years, body mass index between 18 and 30 kg/m2 (inclusive), and normal vision (corrected or uncorrected). Subjects were required to be in good health, as confirmed by their medical history, physical examination, vital sign measurement, electrocardiogram, and laboratory safety tests (blood chemistry and hematology). Women of childbearing potential had to agree not to become pregnant and be willing to use double barrier methods of birth control.
Subjects who met any of the following criteria were excluded from the study: history or present evidence of any clinically significant physical, neurological, psychiatric or sleep disorders, alcoholism or drug abuse; pregnancy or breastfeeding; use of medication known to affect driving performance or hepatic drug metabolism; estimated creatinine clearance ≤ 80 mL/min based on the Cockroft-Gault equation; major surgery, blood donation or participation in any other clinical trial within 4 weeks prior to screening; smoking > 6 cigarettes per week; alcohol consumption > 3 drinks per day; caffeine consumption > 6 servings per day. All subjects were tested for drug use (amphetamines, benzodiazepines, cannabis, cocaine, ecstasy, and opiates), and females for pregnancy at prestudy screening and at the start of each test session.
During participation subjects were required to abstain from prescription and non-prescription medication, and grapefruits or grapefruit products. They also had to refrain from smoking and/or consuming caffeine and alcohol from the time of arrival at the site on treatment days until the completion of all tests the next day. In addition, alcoholic drinks, fruit juice, caffeine, and food were not permitted from 48, 12, 10, and 4 h prior to arrival, respectively. Furthermore, subjects were required not to drive their own vehicles from intake of the first dose until 24 h after the last dose of each treatment period.
Ethical approval was obtained from the Medical Ethics Committee of Maastricht University and all volunteers provided written informed consent prior to enrollment. The study was carried out in accordance with the principles of good clinical practice.
Design
The study (Merck protocol 035) was conducted from June to October 2011 according to a randomized, double-blind, placebo, and active drug controlled, 4-period crossover design. Each treatment period lasted for 8 days and residual effects were assessed in the mornings of day 2 and 9. Treatments were bedtime doses of suvorexant 20 mg, suvorexant 40 mg, and placebo for 8 consecutive days, and zopiclone 7.5 mg as an active control on day 1 and 8 only, with placebo given for the 6 days in between (day 2 to 7). Order of treatment conditions was balanced over subjects. Washout between treatment periods was at least 7 days. The study was registered at clinicaltrials. gov (NCT01311882).
Assessments
Highway Driving Test
Residual effects were assessed using a standardized highway driving test,15 which assesses SDLP as a measure of driver vehicle control (Figure 1).
Figure 1.
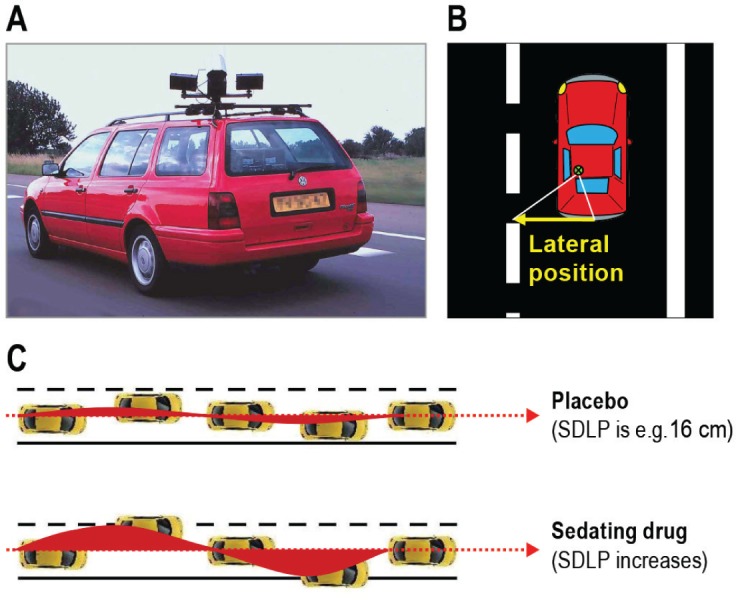
Highway driving test. (A) Subjects drive a specially instrumented vehicle for about 1 hour over a 100 km primary highway circuit, accompanied by a licensed driving instructor having access to dual controls. The subjects' task is to drive with a steady lateral position between the delineated boundaries of the slower (right) traffic lane, while maintaining a constant speed of 95 km/h. (B) A camera on top of the car continuously registers the lateral position of the car on the road with respect to the left lane delineation. (C) The standard deviation of lateral position (SDLP in cm) is an index of road tracking error or “weaving.” SDLP scores increase compared to placebo after the use of many sedating drugs including low doses of alcohol.
In this test, subjects operate a specially instrumented vehicle for about 1 h over a 100-km (61-mile) primary highway circuit, accompanied by a licensed driving instructor having access to dual controls (brakes and accelerator). The subjects' task is to drive with a steady lateral position between the delineated boundaries of the slower (right) traffic lane, while maintaining a constant speed of 95 km/h (58 mph). Subjects may deviate from those instructions only to pass a slower vehicle, and to leave and re-enter the highway at the turnaround points. During the drive, the vehicle's speed and lateral distance to the left lane-line are continuously recorded. These signals are digitized at a rate of 4 Hz and stored on an on-board computer disk file for later preprocessing and analysis. Preprocessing consists of off-line visual inspection of all data by trained processors to mark data segments that reveal signal loss or disturbances such as passing maneuvers and turn-around points. The preprocessed dataset is then used to calculate means and variances of lateral position and speed of clean (unmarked) data. The primary outcome variable is SDLP in cm, and the secondary outcome variable is standard deviation of speed (SDS in km/h). Performance in this test of healthy volunteers and insomnia patients has repeatedly been found sensitive to residual effects of zopiclone 7.5 mg.16,18,21
Word Learning Test
In the Word Learning Test22 measuring verbal memory, a sequence of 15 monosyllabic nouns is shown on a computer display at a rate of 1 per 2 seconds. Immediately thereafter the subject is required to verbally recall as many words as possible. The sequence is repeated on 4 more trials, and the sum of separate trial scores is the Immediate Recall Score. After a delay ≥ 30 min, the subject is again required to recall as many words as possible without prompting. The total number of words correctly recalled is the Delayed Recall Score. Finally, the subject is shown a sequence of 30 words on the computer display, including 15 words from the original set and 15 new words in random order. The subject has to indicate as quickly as possible whether a word originates from the original set or not by pressing a corresponding buttons. The number and speed of correct responses are recorded as the Recognition Score and the Recognition Reaction Time (in ms), respectively. Nine parallel lists were used, with a different list for each of the 8 testing days and the training. Lists were balanced across treatments. Performance in this test has repeatedly been found sensitive to residual effects of zopiclone 7.5 mg.23–26
Body Sway
The ability of subjects to maintain a balanced body posture was evaluated by measures of body sway during quiet stance maintenance using a portable AccuSwayPlus force platform (Advanced Mechanical Technology Inc., Watertown, MA). In this test, subjects are instructed to stand as still as possible on the platform in an upright position with bare feet placed parallel at hip width, arms relaxed along the body and facing forward. The system measures ground reacting force and movement in 3 orthogonal directions, providing the center of foot pressure (CoP) coordinates. These data are used to calculate the extent of movement of the CoP during each recording. Dependent variables are the path length of the CoP (in cm) and the surface area of 95% confidence ellipse enclosing the CoP (A95 in cm2). A95 is the primary measure, as it has shown to be a more sensitive measure of postural stability.27–29 Each test consisted of six 40-second trial recordings comprising 2 task conditions. In the first 3 trials, subjects were instructed to keep their eyes open and fixated on a point 50 cm in front of them at eye level. In the following 3 trials, subjects were instructed to keep their eyes closed. A95 and CoP scores were averaged over each set of 3 trials. Foot position is standardized between trials by markings on the platform. A recent study has shown that postural balance as measured with this test is dose-dependently sensitive to the acute effects of low and moderate doses of alcohol.30
Digit Symbol Substitution Test
The DSST measures processing speed and working memory. It is a computerized version31 of the original paper and pencil test taken from the Wechsler Adults Intelligence Scale. The subject is shown an encoding scheme consisting of a row of squares at the top of a touch-screen, wherein nine digits are randomly associated with particular symbols. The same symbols are presented in a fixed sequence at the bottom of the screen as a row of separate response buttons. The encoding scheme and the response buttons remain visible while the subject is shown successive presentations of a single digit at the center of the screen. The subject is required to match each digit with a symbol from the encoding list as rapidly as possible by touching the corresponding symbol on the touch-screen. The number of digits correctly encoded within 3 minutes is the performance measure. Performance in this test has previously been found sensitive to residual effects of zopiclone 7.5 mg.25
Subjective Ratings
Before starting the cognitive tests, subjects indicated their subjective feelings using the Bond and Lader32 16-item mood scale for providing three factor analytically defined summary scores: “alertness,” “contentedness,” and “calmness.” The scale for alertness summary score ranges from 0 to 27, where 0 corresponds to most alert. The driving instructors used 2 similar 0–100 visual-analog scales for describing the subject's driving quality and apparent sedation at the conclusion of the driving test, where 0 corresponds to best driving quality and lowest sedation.
Pharmacokinetics
Blood samples (7 mL) for suvorexant and zopiclone determinations were collected after driving, at approximately 11 h post dose. Plasma samples were stored and frozen at −20°C and later analyzed.
The analytical methods for the determination of suvorexant were based on a liquid-liquid extraction of drug from human plasma. The drug and internal standard were separated using reverse-phase high-performance liquid chromatography (HPLC) and detected with tandem mass spectrometry (LC-MS/ MS), employing a heated nebulizer (HN) interface in the positive ion mode and multiple reaction monitoring (MRM) mode. The lower limit of quantitation (LLOQ) for this method was 1 ng/mL with a linear calibration range from 1 to 1,000 ng/mL. Samples were assayed by WuXi AppTec Co. (Shanghai, China).
The analytical methods for the determination of zopiclone were based on a liquid-liquid extraction of drug from human plasma. The drug and internal standard were separated using reverse-phase HPLC and detected with LC-MS/MS. The LLOQ for this method was 0.30 ng/mL with a linear calibration range from 0.30 to 150 ng/mL. Samples were assayed by PharmaNet Canada (Québec, Canada).
Procedure
Within two weeks before the first treatment period, subjects slept one night in the same facilities as during treatment conditions, to overcome possible sleep disturbances associated with sleeping in an unfamiliar environment. In the evening preceding their habituation night and the following morning, subjects were individually trained to perform all tests, including the driving test.
On days 1 and 8 of each treatment period, subjects arrived at the test facility at approximately 21:30, upon which their eligibility and compliance with study restrictions was verified by questioning, urine screens for drugs of abuse and pregnancy, breath testing for alcohol, and measurement of vital signs. A maximum of 4 subjects were treated on the same night and tested the following day with 5-min difference between their activities. At 23:25, the first subject was administered drug or placebo with 240 mL water in the presence of an investigator and retired to bed. At 07:25 the first subject was awakened and vital signs were measured. Following toilet and dress, subjects were provided a standardized light breakfast without caffeine and transported to the highway. The first driving test started at approximately 08:25, i.e., 9 h after bedtime dosing. After completion of the driving test subjects were transported to the university for further assessments. Approximately 11 h after dosing, a blood sample was taken and vital signs were measured. Subsequently subjects performed the immediate recall part of the Word Learning test, the DSST, the balance test, and the delayed recall and recognition parts of the Word Learning test, in fixed order. Upon completion of all tests at approximately 11:30, subjects were served a light snack and transported home.
During days 2 to 7 of all treatment periods, trial medication was taken by the subjects at their homes, within one hour of the scheduled times on days 1 and 8. Subjects recorded time of administration in a diary. On day 4 or 5 of each treatment period, subjects were contacted by telephone to check treatment compliance and possible adverse events.
Approximately 14 days after the last treatment period, subjects' health and well-being were confirmed by questioning them about adverse events, and by physical examination and laboratory tests (blood chemistry and hematology).
Statistical Analyses
The primary endpoint was mean SDLP. Secondary endpoints were symmetry analysis of individual changes from placebo in SDLP (see below) and mean word learning and body sway test scores. Mean DSST and subjective rating scores were exploratory endpoints. Sample size was determined based on power calculations to rule out a clinically relevant mean difference in SDLP between suvorexant and placebo, defined as the 90% confidence interval for the mean difference in SDLP falling below 2.4 cm. A mean increase in SDLP of 2.4 cm as compared to placebo corresponds to the effects previously found for alcohol while subjects drove with average blood alcohol concentrations of 0.5 g/L.17 Assuming a within-subject variance in SDLP of 3.55 cm2 based on a previous study,19 and a sample size of 24, the study would have a probability of at least 0.80 that the 90% CI would fall below 2.4 cm if the true mean difference was as high as 1.0 cm. To ensure 24 completers, 28 subjects were enrolled.
All performance parameters were analyzed using a linear mixed model ANOVA for repeated measures with fixed factors for Treatment (suvorexant 20 and 40 mg, zopiclone and placebo, abbreviated as S20, S40, ZOP, and PBO respectively), Day (D2 and D9), Period (1 to 4), and a Treatment by Day interaction and a random factor for Subject.
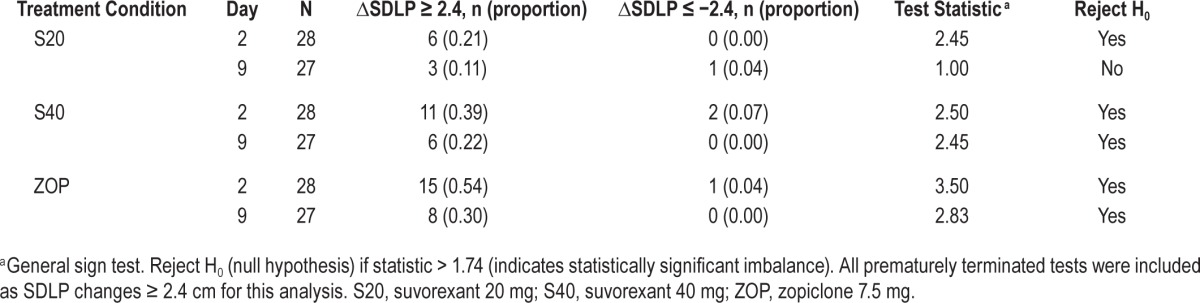
As a secondary analysis SDLP was also analyzed using symmetry analysis of individual changes from placebo in SDLP. To perform this symmetry analysis, generalized signs test were used for each treatment condition and treatment day separately to test whether the number of subjects with an increase in SDLP ≥ 2.4 cm (reflecting impairment) differed significantly from the number of subjects with a decrease in SDLP of −2.4 cm or more (reflecting improvement).
All statistical analyses were done by using the SAS statistical program version 9.3 (SAS Institute Inc. Cary, NC). No adjustments were made for multiple comparisons.
This study was not powered for the secondary/exploratory endpoints, nor were any adjustments for multiplicity made across these numerous endpoints and timepoints. Results should be interpreted with this in mind.
RESULTS
Demographic Data
All 28 enrolled subjects completed the study. Mean ± standard deviation (SD) age was 45.6 ± 13.2 y (range 23 to 64 y). Twenty-seven subjects were white, and one was Asian. Mean ± SD body mass index of 24.7 ± 3.0 kg/m2. Mean ± SD body weight was 81.7 ± 6.8 kg for the males (range 72 to 94 kg), and 68.1 ± 10.8 kg for the females (range 51 to 82 kg).
Missing Data
Data for 3 subjects were incomplete. For one subject, all performance data on D9 of PBO were missing because of adverse events: the subject reported severe anxiety and insomnia during the night (approx. 1:25 h post dosing) and withdrew from further testing. For a second subject, speed data during driving on D2 and D9 of S20 were missing due to technical problems. Finally, for a third subject the DSST score on D2 of PBO was missing, because the subject did not follow test instructions.
Driving
Four female subjects requested that a total of 5 driving tests be stopped prematurely because they felt too drowsy to continue safely: 2 subjects in S40, 1 subject in S20, and 1 subject in S20 and S40 (Table 1).
Table 1.
Driving tests terminated prematurely due to subjective feelings of drowsiness.
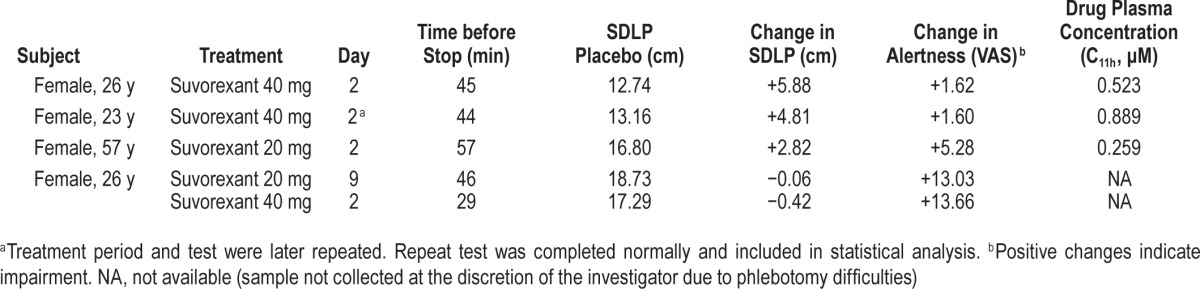
One of the subjects who stopped driving on D2 of S40 was not able to return for the D8 visit for personal reasons, and therefore repeated this S40 treatment period. The repeat driving test was completed as scheduled. Only the data from her repeat treatment period were used for analysis of performance assessments. Finally, one driving test on D2 of S20 had to be terminated after 20 minutes due to technical problems. SDLP scores for prematurely terminated tests were calculated from the data collected until termination of each ride. For symmetry analysis, all prematurely terminated tests were included as SDLP changes ≥ 2.4 cm in this analysis.
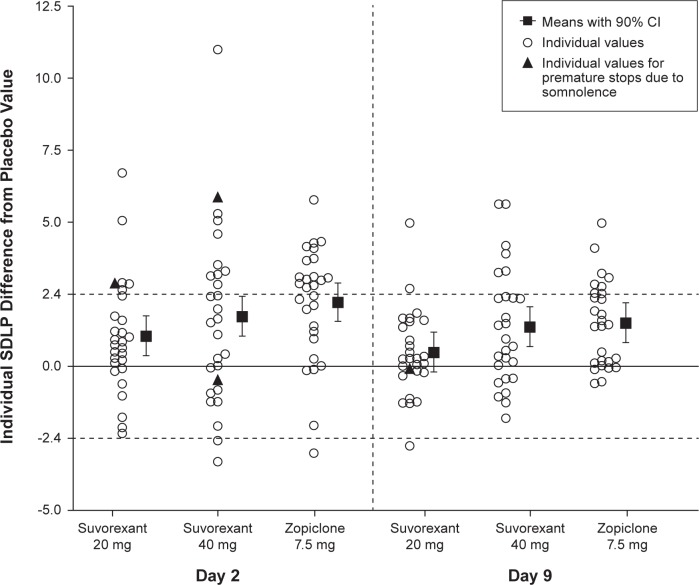
Mean SDLP scores per treatment and test day, and mean drug-placebo changes with 90% CI are shown in Table 2. Individual and mean changes in SDLP from placebo are shown in Figure 2.
Table 2.
Model-based mean performance scores, and mean drug-placebo changes (90% CI) at both test days in each treatment condition.
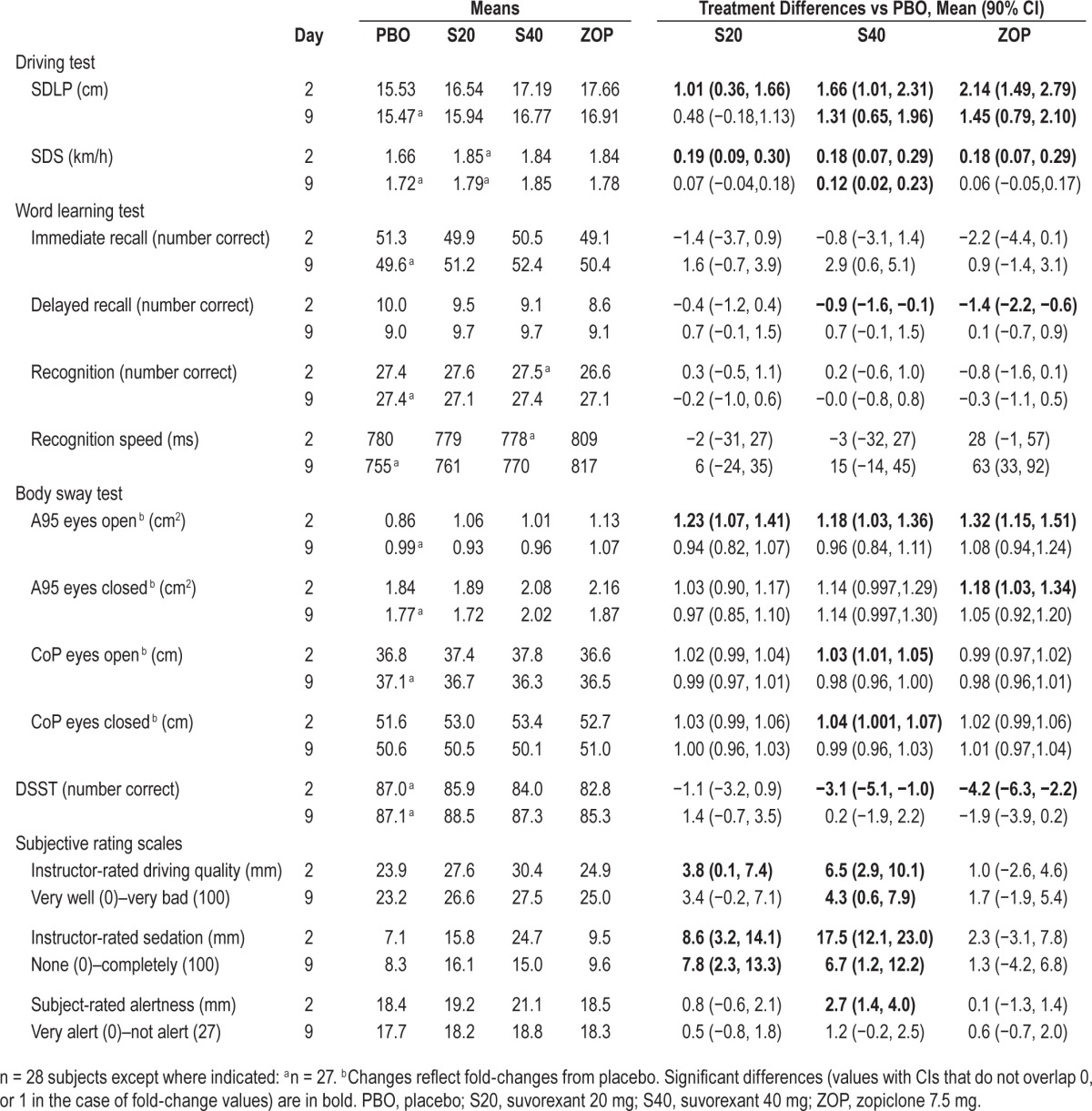
Figure 2.
Individual SDLP (cm) differences from placebo (distinguishing observations from subjects whose driving tests were prematurely stopped due to somnolence), mean and 90% confidence interval by Treatment and Day, following bedtime administration of suvorexant 20 mg, 40 mg single dose (Day 2) and multiple doses (Day 9), and single dose of zopiclone 7.5 mg (Day 2 and Day 9). n = 28 on Day 2, n = 27 on Day 9. Horizontal dashed lines indicate thresholds for impairment (> 2.4 cm) and improvement (< −2.4 cm). Only the SDLP data used for the statistical analysis are shown, which includes 4 prematurely terminated tests (black triangles).
On D2, mean changes from placebo in SDLP scores were 1.01 cm (90% CI: 0.36 to 1.66) in S20 and 1.66 cm (90% CI: 1.01 to 2.31) in S40. On D9, the differences were 0.48 (90% CI: −0.18 to 1.13) in S20 and 1.31 (90% CI: 0.65 to 1.96) in S40. The upper limits of the 90% CI of these changes all fell below the criterion of 2.4 cm, indicating that the residual effects of suvorexant on driving were not clinically relevant. The lower limits of the 90% CI, however, fell above 0 cm on both days in S40, and on D2 in S20, indicating that the mean change was statistically significantly different from placebo. Plots of individual SDLP changes from placebo by gender for suvorexant are shown in Figure S1 (supplemental material). There did not appear to be a marked gender difference in the distribution of scores.
In ZOP mean SDLP was increased by 2.14 (90% CI: 1.49 to 2.79) cm and 1.45 (90% CI: 0.79 to 2.10) cm on D2 and D9, respectively. These results confirm assay sensitivity, and show effects of zopiclone on driving were clinically relevant on D2, but not on D9.
Symmetry analysis of individual changes in SDLP showed that significantly more subjects showed an increase in SDLP ≥ 2.4 cm than a decrease in SDLP of the same magnitude on both treatment days in ZOP and S40, and on D2 in S20 (Table 3).
Table 3.
Symmetry analysis numbers of subjects whose SDLP increased more than 2.4 cm (indicating impairment) and numbers of subjects whose SDLP decreased more than 2.4 cm (indicating improvement).
Variability in speed during driving, as measured by SDS, was significantly increased compared to placebo in S20, S40, and ZOP on D2, and in S40 on D9, as shown by the lower limits of the 90% CIs above zero (Table 2).
Word Learning
No statistically significant impairment was observed in immediate recall. Delayed recall was significantly impaired in S40 and ZOP on D2, but not on D9 (Table 2). Finally, speed, but not accuracy, of word recognition was significantly impaired in ZOP on D9. No significant impairment was observed in S20.
Body Sway
Body sway was statistically significantly increased in S20, S40 and ZOP on D2, but not on D9 (Table 2). On D2, surface area of CoP (A95) with eyes open was increased by all active treatments compared to PBO. In addition, A95 with eyes closed was increased in ZOP, and path length of CoP was increased in S40, both with eyes open and eyes closed.
DSST
Performance in the DSST was statistically significantly impaired in S40 and ZOP on D2. No significant impairment was observed in S20, or on D9 (Table 2).
Subjective Evaluations
Subjects judged themselves as statistically significantly less alert on D2 in S40 as compared to placebo. The driving instructors judged the subjects as appearing significantly more sedated on both treatment days in S20 and S40 as compared to placebo. They judged the subjects' driving quality as significantly worse than placebo on both days in S40 and on D2 in S20.
Blood Samples
On D2 mean (range) plasma concentrations of suvorexant (C11h) were 0.290 (0.167 to 0.434) and 0.482 (0.299 to 0.889) μM, for 20 and 40 mg doses, respectively. On D9 mean C11h increased to 0.400 (0.216 to 0.673) and 0.552 (0.200 to 1.032) μM, respectively. Mean (range) plasma concentrations (C11h) of zopiclone were 15.62 ng/mL (8.73 to 23.79) on D2 and 15.82 ng/mL (8.72 to 26.41) on D9. The correlations between plasma concentrations of suvorexant (pooled over dose) and SDLP were weak, i.e., r = 0.21 on D2 and r = 0.32 on D9. Plots of individual SDLP difference from placebo scores versus suvorexant plasma concentration by gender are shown in Figure S2 (supplemental material). The weak relationship between SDLP and suvorexant plasma concentration appeared to be similar for women and men.
Safety
Table 4 presents a summary of adverse events reported by ≥ 5% of the subjects after any treatment.
Table 4.
Summary of AEs reported by at least 5% of the subjects after any treatment (N = 28).
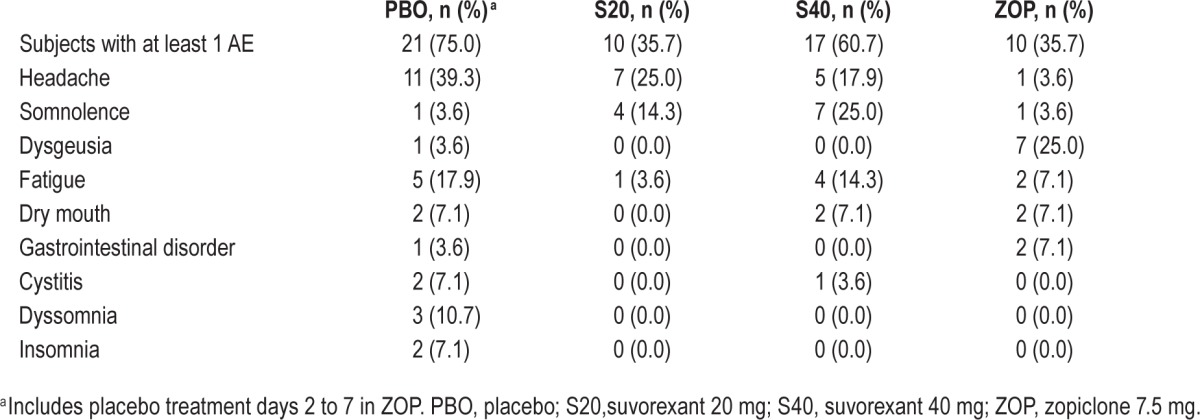
After suvorexant, the most common AEs were headache (17.9% with 40 mg; 25.0% with 20 mg), somnolence (25.0% with 40 mg; 14.3% with 20 mg), and fatigue (14.3% with 40 mg; 3.6% with 20 mg). Four subjects reported 5 occurrences of somnolence with moderate intensity that resulted in a prematurely stopped car driving test (Table 1).
After zopiclone, dysgeusia was the most common AE (25.0%). The remaining AEs were reported by no more than two (7.1%) subjects.
After placebo (which includes placebo treatment days 2 to 7 in ZOP), 21 subjects reported at least one adverse experience, including the only adverse experiences which were rated as severe (anxiety and insomnia). All other AEs were rated as mild or moderate in intensity. Headache was the most frequently reported AE after placebo (39.3%), followed by fatigue (17.9%), and dyssomnia (10.7%).
No serious AEs and events of clinical interest, such as cataplexy, were reported in this study, and there were no consistent treatment-related changes in laboratory, vital signs, or ECG safety parameters.
DISCUSSION
The primary objective of this study was to assess the residual effects of suvorexant 20 and 40 mg on car driving as measured by SDLP, 9 hours after single and repeated bedtime use in healthy non-elderly (< 65 years old) volunteers. Driving impairment was evaluated by comparing means and 90% CIs of drug-placebo changes in SDLP to a predefined criterion of 2.4 cm, and by symmetry analyses of the numbers of subjects showing drug-placebo changes in SDLP exceeding 2.4 cm or −2.4 cm. Results showed that mean SDLP following suvorexant 40 mg increased by +1.66 cm on day 2, and by +1.31 cm on day 9 as compared to placebo. Following the first dose of suvorexant 20 mg mean SDLP increased by +1.01 cm. CIs of these changes lay above zero, but below the criterion of 2.4 cm, indicating that the effects were statistically significant, but not clinically meaningful. Following 8 consecutive nights of suvorexant 20 mg the difference from placebo in SDLP was 0.48 cm with a 90% CI less than 2.4 and including zero, indicating that driving performance was not clinically nor statistically different from placebo. Symmetry analyses showed a similar pattern of results. There was a statistically significant imbalance in the number of subjects who showed SDLP changes suggestive of impairment rather than improvement after both doses of suvorexant on day 2, and after the highest dose on day 9.
The effects of suvorexant on SDLP are supported by data from driving speed. Standard deviations of speed increased after both doses on day 2, and after the highest dose on day 9. Consistent with these results, driving tests that were prematurely stopped by the subjects due to feelings of drowsiness, were mostly on day 2 (4/5) and after suvorexant 40 mg (3/5).
Effects of zopiclone 7.5 mg clearly demonstrated assay-sensitivity in the current study. Zopiclone significantly increased mean SDLP by 2.14 cm on day 2, and by 1.45 cm on day 9. CIs of these changes lay well above zero, and included the 2.4 cm criterion on day 2, but not on day 9. Symmetry analysis indicated significantly more impairment than improvement on both days. The results are consistent with those of a number of studies that assessed the residual effects of zopiclone in healthy non-elderly volunteers using the same standardized highway driving test.16,18–20,25 All of these studies demonstrated that bedtime doses of zopiclone 7.5 mg produced residual driving impairment in the morning, comparable to a BAC between 0.5 and 0.8 g/L. In the present study, impairment after zopiclone confirms the sensitivity of test and procedures for sedative drug effects.
In line with objective measures of driving performance, instructors judged subjects' driving quality as slightly worse than placebo after both suvorexant doses on day 2, and after the highest dose on day 9. They also rated subjects as appearing slightly more sedated after both suvorexant doses and on both days. The rating results did not discriminate, however, between zopiclone and placebo. The latter is in line with results from previous studies showing that the instructors do not consistently judge driving quality and sedation to be worse after zopiclone.23,24 Subjects' ratings of alertness only differed significantly from placebo after the first dose of suvorexant 40 mg. It should be noted that these ratings do not necessarily reflect the subjects feeling at the time of the driving test, because the scales were filled out approximately 45 minutes later, at the start of the laboratory test battery.
Results of the secondary or exploratory psychomotor and memory tests suggested that suvorexant 20 mg had no significant effects on DSST and memory. The first doses of suvorexant 40 mg, however, and zopiclone appeared to have residual impairing effects as measured by the DSST and word learning test. These effects were not apparent on repeat testing after one week. On average the effects of single doses of suvorexant 40 mg were less severe than those of zopiclone in both tasks. The effects of zopiclone in these tests were comparable to those found in a previous study.25 The effects of suvorexant 40 mg on DSST are in contrast to those from a Phase 2B clinical trial in insomnia patients that found no residual effects on DSST, although there was some evidence of residual effects on a similar task (digit symbol copying) after 4 weeks of treatment with 40 mg.11 There was also some evidence of residual effects after a single dose of suvorexant 100 mg on simple and choice reaction time in a previous study in healthy subjects.10
With regard to the assessment of body sway, for the “eyes open” evaluation, results showed increased body sway after the first doses of suvorexant 20 and 40 mg, and zopiclone. The effects of suvorexant appeared on average less pronounced than those of zopiclone. For the “eyes closed” evaluation, suvorexant 20 mg had no effects, suvorexant 40 mg increased path length, and zopiclone increased sway surface area. Effects on body sway were not apparent after one week of suvorexant dosing.
Residual effects of suvorexant on SDLP were increased for the higher dose. Associated plasma concentrations increased dose-dependently. Plasma levels of suvorexant peak at approximately 2 hours after dosing and fall to levels below those predicted to provide sufficient receptor occupancy for sleep promotion by approximately 8 hours after dosing for the 20 mg dose.10,14 Although the suvorexant concentrations at 11 hours in the present study are consistent with data in other studies, residual effects are not only determined by plasma concentrations, as these concentrations were found to have a weak correlation with SDLP. It is possible that this weak correlation is influenced by increasing competition from rising levels of endogenous orexin on awakening, in the presence of declining suvorexant plasma levels. The finding that residual effects of hypnotics on driving correlate poorly with drug plasma concentrations is supported by similar results in previous studies.21,33
Results showed that residual effects of suvorexant decreased with repeated dosing. This suggests that tolerance may have developed, at least partially, for the residual effects. Data from zopiclone treatment in the present study also showed that the residual effects decreased from D2 to D9. These were assessed after two single doses, separated by one week of placebo treatment. The findings could therefore be due to chance variability. If not due to chance then the findings cannot easily be explained by development of physiological tolerance to residual effects. Possibly a single exposure to the effects of zopiclone may have induced behavioral tolerance. Subjects may have learned to perform the task by developing behavioral strategies to compensate for the drug effects, as seen with alcohol, for example.34 Simple learning effects are not likely, as shown by stable performance in the placebo condition.
Four female volunteers requested to have their driving prematurely stopped following suvorexant, due to feelings of drowsiness. There was no apparent relationship with driving performance or plasma concentrations of suvorexant in these volunteers, which is consistent with the literature.33,35 Previous studies show that it is not unusual that driving tests are terminated prematurely. The overall percentage of tests terminated in the present study is 2% (5 of 225 tests), which is comparable to that found in other studies.16,35 The SDLP difference from placebo during prematurely stopped driving tests did not always exceed the pre-specified cutoff for impaired driving performance (2.4 cm). The lack of relationship between SDLP and prematurely stopped driving is consistent with that reported in the literature.36 Contrary to previous studies, the decision to stop driving in the present study was made by the volunteers, instead of by the driving instructor. In previous studies assessing the residual effects of hypnotics on on-the-road driving, when the tests were stopped it was nearly always by the driving instructors judging the subjects to be too drowsy to continue safely.15,16,23–26,37–39 This discrepancy may be due to differences in the mechanism of action of the drugs, and the associated effects on mood and cognition. Benzodiazepines and benzodiazepine-like hypnotics have sedative as well as anxiolytic effects, which may alter subjects' judgment and reduce the likelihood that they will decide to terminate a driving test. Orexin antagonists, such as suvorexant, have no known anxiolytic effects. Subjects may therefore not only be more aware of the drug's effects on performance, but also sooner decide to stop driving (i.e., be more careful).
The use of healthy volunteers instead of patients could be considered as a limitation of this study. An important reason to study the effects of suvorexant in healthy volunteers is to facilitate comparisons to previous driving studies, which were virtually all conducted with normal volunteers.3,16,18–20,25 More importantly, a recent study comparing the effects of zopiclone on driving in patients with insomnia and healthy volunteers suggests that healthy volunteers may be more sensitive to the residual effects.40 Thus, studying drug effects in healthy volunteers minimizes the risk of failing to detect clinically relevant impairment associated with use of a drug. Additional studies in patients may help to determine the interaction of these effects with the diagnosis of insomnia and other comorbid disorders, or concomitant medication. The present study was intended to determine the impairment potential of suvorexant alone, as compared with that of other drugs such as zopiclone 7.5 mg at bedtime.
Another point for discussion relates to the cut point used for individual driving impairment in the symmetry analysis. It is based on known average effects of alcohol, the only drug for which widely accepted limits in blood concentrations during driving and accident statistics are available17,41,42 No other widely accepted criterion for driving impairment is available. The residual effects of zopiclone 7.5 mg are increasingly used as reference in studies evaluating residual effects of hypnotics, because they are reliable and comparable in magnitude to those of alcohol at BACs of 0.5 g/L.
In conclusion, as assessed by overall group mean changes in SDLP, there was no clinically meaningful residual effect of suvorexant doses of 20 and 40 mg on next-morning driving performance (9 hours or more after bedtime dosing) in healthy subjects younger than 65 years. Measurable impairment did occur as compared to placebo, but the effects were less severe than those of alcohol in blood concentrations of 0.5 g/L, which is the limit for driving in most countries. For the 20 mg dose of suvorexant, the maximum approved daily dose in the United States, driving impairment was not apparent in most subjects after one week of daily dosing. There may be some individuals who experience next-day effects, as suggested by the symmetry analysis of individual changes in SDLP and the prematurely stopped driving tests due to somnolence.
DISCLOSURE STATEMENT
This study was financially supported by Merck & Co. Inc. Maastricht University received financial support from Merck & Co. Inc., to conduct this study. Drs. Vermeeren, Vuurman, Van Leeuwen, Mr. Jongen, and Ms. Van Oers are employees of Maastricht University. The remaining authors are current or former employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co. Inc., Kenilworth, NJ, USA, and own or owned stock/stock options in Merck. Dr. Sun is currently affiliated with Amgen in Thousand Oaks, CA. Drs. Vermeeren and Sun contributed equally to the work and are joint first authors.
ACKNOWLEDGMENTS
The authors thank Anique van Dorp, MD, for medical supervision of the subjects; Henk Brauers, Jo Gorissen, Hans Sleebe, Bert Adriaens, and Eef Gorissen for ensuring safety of the subjects during driving; Irma Brauers, Sander Huisman, Lizzy Vuurman, ZsaZsa Weerts, Camiel Zeijen, Eveline Beurskens, Marit van der Sande, Vivian Vernimmen, Noortje Wismans, Sam Kirsch, Marc Leclerc, and Jan Schmitt for assistance during data collection; Christopher Lines from Merck for assistance in editing the manuscript; and Sheila Erespe from Merck for assistance in formatting the manuscript.
ABBREVIATIONS
- A95
area of 95%
- AE
adverse event
- BAC
blood alcohol concentration
- CI
confidence interval
- CoP
center of pressure
- D2
day 2
- D9
day 9
- DORA
dual orexin receptor antagonist
- DSST
digit symbol substitution test
- GABA
gamma amino butyric acid
- h
hour(s)
- HPLC
high performance liquid chromatography
- km
kilometers
- LC-MS/MS
tandem mass spectrometry
- LLOQ
lower limit of quantification
- PBO
placebo
- S20
suvorexant 20 mg
- S40
suvorexant 40 mg
- SD
standard deviation
- SDLP
standard deviation of lateral position
- SDS
standard deviation speed
- ZOP
zopiclone 7.5 mg
SUPPLEMENTAL MATERIAL
Suvorexant SDLP Differences from Placebo by Gender and Day for Suvorexant 40 mg (A) and Suvorexant 20 mg (B).
Plots of Individual SDLP Differences from Placebo versus Suvorexant Plasma Concentrations by Gender at Day 2 (A) and Day 9 (B).
REFERENCES
- 1.Dassanayake T, Michie P, Carter G, Jones A. Effects of benzodiazepines, antidepressants and opioids on driving: a systematic review and meta-analysis of epidemiological and experimental evidence. Drug Saf. 2011;34:125–56. doi: 10.2165/11539050-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Smink BE, Egberts AC, Lusthof KJ, Uges DR, de Gier JJ. The relationship between benzodiazepine use and traffic accidents: a systematic literature review. CNS Drugs. 2010;24:639–53. doi: 10.2165/11533170-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Vermeeren A. Residual effects of hypnotics: epidemiology and clinical implications. CNS Drugs. 2004;18:297–328. doi: 10.2165/00023210-200418050-00003. [DOI] [PubMed] [Google Scholar]
- 4.Merck & Co. Inc. Whitehouse Station, NJ: Merck & Co., Inc.; 2014. BELSOMRA (suvorexant) prescribing information. [Google Scholar]
- 5.Winrow CJ. Promotion of sleep by suvorexant-a novel dual orexin receptor antagonist. J Neurogenet. 2011;25:52–61. doi: 10.3109/01677063.2011.566953. [DOI] [PubMed] [Google Scholar]
- 6.Cox CD, Breslin MJ, Whitman DB, et al. Discovery of the dual orexin receptor antagonist [(7R)-4-(5-chloro-1,3-benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl][5-methyl-2-(2H -1,2,3-triazol-2-yl)phenyl] methanone (MK-4305) for the treatment of insomnia. J Med Chem. 2010;53:5320–32. doi: 10.1021/jm100541c. [DOI] [PubMed] [Google Scholar]
- 7.de Lecea L, Huerta R. Hypocretin (orexin) regulation of sleep-to-wake transitions. Front Pharmacol. 2014;5:16. doi: 10.3389/fphar.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riemann D, Spiegelhalder K, Feige B, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14:19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Riemann D, Spiegelhalder K. Orexin receptor antagonists: a new treatment for insomnia? Lancet Neurol. 2014;13:441–3. doi: 10.1016/S1474-4422(13)70311-9. [DOI] [PubMed] [Google Scholar]
- 10.Sun H, Kennedy WP, Wilbraham D, et al. Effects of suvorexant, an orexin receptor antagonist, on sleep parameters as measured by polysomnography in healthy men. Sleep. 2013;36:259–67. doi: 10.5665/sleep.2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herring WJ, Snyder E, Budd K, et al. Orexin receptor antagonism for treatment of insomnia: a randomized clinical trial of suvorexant. Neurology. 2012;79:2265–74. doi: 10.1212/WNL.0b013e31827688ee. [DOI] [PubMed] [Google Scholar]
- 12.Michelson D, Snyder E, Paradis E, et al. Safety and efficacy of suvorexant during 1-year treatment of insomnia with subsequent abrupt treatment discontinuation: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2014;13:461–71. doi: 10.1016/S1474-4422(14)70053-5. [DOI] [PubMed] [Google Scholar]
- 13.Herring WJ, Connor KM, Ivgy-May N, et al. Suvorexant in patients with insomnia: results from two 3-month randomized controlled clinical trials. Biol Psychiatry. 2014 Oct 23; doi: 10.1016/j.biopsych.2014.10.003. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Gotter AL, Winrow CJ, Brunner J, et al. The duration of sleep promoting efficacy by dual orexin receptor antagonists is dependent upon receptor occupancy threshold. BMC Neurosci. 2013;14:90. doi: 10.1186/1471-2202-14-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Hanlon JF. Driving performance under the influence of drugs: rationale for, and application of, a new test. Br J Clin Pharmacol. 1984;18(Suppl 1):121S–129S. doi: 10.1111/j.1365-2125.1984.tb02590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vermeeren A, Vuurman EF, Leufkens TR, et al. Residual effects of low-dose sublingual zolpidem on highway driving performance the morning after middle-of-the-night use. Sleep. 2014;37:489–96. doi: 10.5665/sleep.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louwerens JW, Gloerich ABM, De Vries G, Brookhuis KA, O'Hanlon JF. The relationship between drivers' blood alcohol concentration (BAC) and actual driving performance during high speed travel. In: Noordzij PC, Roszbach R, editors. International Congress on Alcohol, Drugs and Traffic Safety. Amsterdam: Exerpta Medica; 1987. pp. 183–6. [Google Scholar]
- 18.Leufkens TR, Vermeeren A. Zopiclone's residual effects on actual driving performance in a standardized test: a pooled analysis of age and sex effects in 4 placebo-controlled studies. Clin Ther. 2014;36:141–50. doi: 10.1016/j.clinthera.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Ramaekers JG, Conen S, de Kam PJ, et al. Residual effects of esmirtazapine on actual driving performance: overall findings and an exploratory analysis into the role of CYP2D6 phenotype. Psychopharmacology (Berl) 2011;215:321–32. doi: 10.1007/s00213-010-2149-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mets MA, de Vries JM, Senerpont Domis LM, Volkerts ER, Olivier B, Verster JC. Next-day effects of ramelteon (8 mg), zopiclone (7.5 mg), and placebo on highway driving performance, memory functioning, psychomotor performance, and mood in healthy adult subjects. Sleep. 2011;34:1327–34. doi: 10.5665/SLEEP.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leufkens TR, Ramaekers JG, de Weerd AW, Riedel WJ, Vermeeren A. Residual effects of zopiclone 7.5 mg on highway driving performance in insomnia patients and healthy controls: a placebo controlled crossover study. Psychopharmacology (Berl) 2014;231:2785–98. doi: 10.1007/s00213-014-3447-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rey A. L'examen clinique en psychologie. Paris: Presses Universitaire de France; 1964. [Google Scholar]
- 23.Vermeeren A, Danjou PE, O'Hanlon JF. Effects of evening and middle of the night doses of zaleplon 10 and 20 mg on memory and actual driving performance. Hum Psychopharmacol. 1998;13:S98–107. [Google Scholar]
- 24.Vermeeren A, Riedel WJ, van Boxtel MP, Darwish M, Paty I, Patat A. Differential residual effects of zaleplon and zopiclone on actual driving: a comparison with a low dose of alcohol. Sleep. 2002;25:224–31. [PubMed] [Google Scholar]
- 25.Leufkens TR, Lund JS, Vermeeren A. Highway driving performance and cognitive functioning the morning after bedtime and middle-of-the-night use of gaboxadol, zopiclone and zolpidem. J Sleep Res. 2009;18:387–96. doi: 10.1111/j.1365-2869.2009.00746.x. [DOI] [PubMed] [Google Scholar]
- 26.Leufkens TR, Vermeeren A. Highway driving in the elderly the morning after bedtime use of hypnotics: a comparison between temazepam 20 mg, zopiclone 7.5 mg, and placebo. J Clin Psychopharmacol. 2009;29:432–8. doi: 10.1097/JCP.0b013e3181b57b43. [DOI] [PubMed] [Google Scholar]
- 27.Boyle J, Danjou P, Alexander R, et al. Tolerability, pharmacokinetics and night-time effects on postural sway and critical flicker fusion of gaboxadol and zolpidem in elderly subjects. Br J Clin Pharmacol. 2009;67:180–90. doi: 10.1111/j.1365-2125.2008.03331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Norris JA, Marsh AP, Smith IJ, Kohut RI, Miller ME. Ability of static and statistical mechanics posturographic measures to distinguish between age and fall risk. J Biomech. 2005;38:1263–72. doi: 10.1016/j.jbiomech.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 29.Otmani S, Metzger D, Guichard N, et al. Effects of prolonged-release melatonin and zolpidem on postural stability in older adults. Hum Psychopharmacol. 2012;27:270–6. doi: 10.1002/hup.2219. [DOI] [PubMed] [Google Scholar]
- 30.Jongen S, Vuurman E, Ramaekers J, Vermeeren A. Alcohol calibration of tests measuring skills related to car driving. Psychopharmacology (Berl) 2014;231:2435–47. doi: 10.1007/s00213-013-3408-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramaekers JG, Louwerens JW, Muntjewerff ND, et al. Psychomotor, Cognitive, extrapyramidal, and affective functions of healthy volunteers during treatment with an atypical (amisulpride) and a classic (haloperidol) antipsychotic. J Clin Psychopharmacol. 1999;19:209–21. doi: 10.1097/00004714-199906000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Bond A, Lader M. The use of analogue scales in rating subjective feelings. Br J Med Psychol. 1974;47:211–8. [Google Scholar]
- 33.Verster JC, Roth T. Blood drug concentrations of benzodiazepines correlate poorly with actual driving impairment. Sleep Med Rev. 2013;17:153–9. doi: 10.1016/j.smrv.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Vogel-Sprott M. Is behavioral tolerance learned? Alcohol Health Res World. 1997;21:161–8. [PMC free article] [PubMed] [Google Scholar]
- 35.Verster JC, Roth T. Drivers can poorly predict their own driving impairment: a comparison between measurements of subjective and objective driving quality. Psychopharmacology (Berl) 2012;219:775–81. doi: 10.1007/s00213-011-2400-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verster JC, Roth T. The prevalence and nature of stopped on-the-road driving tests and the relationship with objective performance impairment. Accid Anal Prev. 2012;45:498–506. doi: 10.1016/j.aap.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Vermeeren A, O'Hanlon JF, DeClerck AC, KL Acute effects of zolpidem and flunitrazepam on sleep, memory and driving performance, compared to those of partial sleep deprivation and placebo. Acta Ther. 1995;21:47–64. [Google Scholar]
- 38.Vermeeren A, Ramaekers JG, VLC, O'Hanlon JF. Residual effects an actual car driving of evening dosing of chlorpheniramine 8 and 12 mg when used with terfenadine 60 mg in the morning. Hum Psychopharmacol. 1998;13:S79–86. [Google Scholar]
- 39.Volkerts ER, van Laar MW, van Willigenburg APP. A comparative study of on-the-road and simulated driving performance after nocturnal treatment with lormetazepam 1 mg and oxazepam 50 mg. Hum Psychopharmacol. 1992;7:297–309. [Google Scholar]
- 40.Leufkens TRM, Ramaekers JG, De Weerd AL, Riedel WJ, Vermeeren A. On-the-road driving performance and driving related skills in older untreated insomnia patients and chronic users of hypnotics. Psychopharmacology. 2014;231:2851–65. doi: 10.1007/s00213-014-3455-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borkenstein RF, Crowther RF, Shumate RP, Zeil WB, Zylman R. The role of the drinking driver in traffic accidents. Bloomington, IN: Indiana University; 1964. [Google Scholar]
- 42.Krüger HP, Vollrath M. The alcohol-related accident risk in Germany: procedure, methods and results. Accid Anal Prev. 2004;36:125–33. doi: 10.1016/s0001-4575(02)00134-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suvorexant SDLP Differences from Placebo by Gender and Day for Suvorexant 40 mg (A) and Suvorexant 20 mg (B).
Plots of Individual SDLP Differences from Placebo versus Suvorexant Plasma Concentrations by Gender at Day 2 (A) and Day 9 (B).