Abstract
Study Objectives:
The obstructive sleep apnea syndrome (OSAS) involves recurrent sleep-related upper airways (UA) collapse. UA mechanical properties and neural control are altered, imposing a mechanical load on inspiration. UA collapse does not occur during wakefulness, hence arousal-dependent compensation. Experimental inspiratory loading in normal subjects elicits respiratory-related cortical activity. The objective of this study was to test whether awake OSAS patients would exhibit a similar cortical activity.
Design:
Descriptive physiology study.
Setting:
Sleep laboratory in a large university affiliated tertiary hospital.
Patients:
26 patients with moderate OSAS according to polysomnography (5 < apnea-hypopnea index [AHI] ≤ 30, n = 14) or severe OSAS (AHI > 30, n = 12); 13 non-OSAS patients for comparison.
Interventions:
None.
Measurements:
Respiratory time-locked electroencephalographic segments ensemble averaged and analyzed for slow premotor potentials preceding inspiration (“pre-inspiratory potentials” [PIPs]).
Results:
PIPs were present in 1/13 controls and 11/26 patients (P = 0.0336; 4/14 “moderate” and 7/12 “severe” patients). Awake OSAS patients therefore exhibit respiratory-related cortical activity during quiet breathing significantly more frequently than non-OSAS individuals. The corresponding PIPs resemble those observed during prepared voluntary inspirations and in response to experimental inspiratory loads in normal subjects, which involve a cortical network comprising the supplementary motor area.
Conclusions:
A respiratory-related cortical activity could contribute to the increased neural drive to upper airway and to inspiratory muscles that has previously been described in obstructive sleep apnea, and could therefore contribute to the arousal-dependent compensation of upper airway abnormalities. Whether or not such cortical compensatory mechanisms have cognitive consequences remains to be determined.
Citation:
Launois C, Attali V, Georges M, Raux M, Morawiec E, Rivals I, Arnulf I, Similowski T. Cortical drive to breathe during wakefulness in patients with obstructive sleep apnea syndrome. SLEEP 2015;38(11):1743–1749.
Keywords: sleep apnea syndrome, control of breathing, cerebral cortex, pre-inspiratory potentials
INTRODUCTION
The obstructive sleep apnea syndrome (OSAS) is a common disorder characterized by recurrent sleep-related upper airway collapse. The resulting apneas and hypopneas disrupt sleep, leading to daytime sleepiness and sleepiness-related accidents. They also induce repeated bursts of sympathetic activity and an elevated sympathetic tone that are considered to be responsible for the OSAS-associated increase in cardiovascular risk.1 The pathophysiology of OSAS is complex and has not been fully elucidated. It involves anatomical factors responsible for upper airway narrowing and neural factors pertaining to upper airway control.2,3 Indeed, during wakefulness, OSAS patients present dysfunctional neuromechanical coupling of upper airway muscles and impaired upper airway stabilizing forces.4 They can exhibit increased upper airway resistance and increased upper airway collapsibility.5,6 Stabilizing the upper airway by means of positive pressure or mandibular advancement can prevent upper airway collapse and constitute efficient treatments.
Remarkably, OSAS patients do not snore and do not experience upper airway collapse when they are awake. This suggests that arousal-dependent neural mechanisms compensate for the constraints imposed on inspiration by upper airway abnormalities. Increased activity of upper airway dilator muscles has been described in awake OSAS patients compared to controls,7 and loss of this activity at sleep onset is considered to be responsible for upper airway collapse.8 Similarly, a transcranial magnetic stimulation study found enhanced genioglossus responses in awake OSAS patients compared to controls.9 The brain substrate for the arousal-dependent compensation of upper airway abnormalities in OSAS remains unknown.
In healthy subjects, experimental inspiratory loading engages cortical networks.10 Electroencephalographic evidence of this activation has been obtained,11 in the form of slow preinspiratory potentials that resemble the premotor potentials typical of movement preparation and that partly originate in the supplementary motor area (SMA).12,13 We therefore hypothesized that the “intrinsic” inspiratory resistive loading characteristic of OSAS could result in a similar pattern of cortical activation. We tested this hypothesis by looking for pre-inspiratory potentials on electroencephalographic recordings (EEG) performed in awake OSAS patients, in comparison to non-OSAS individuals in whom such an activity is not expected during resting breathing (as reviewed by Tremoureux et al.14).
METHODS
Participants
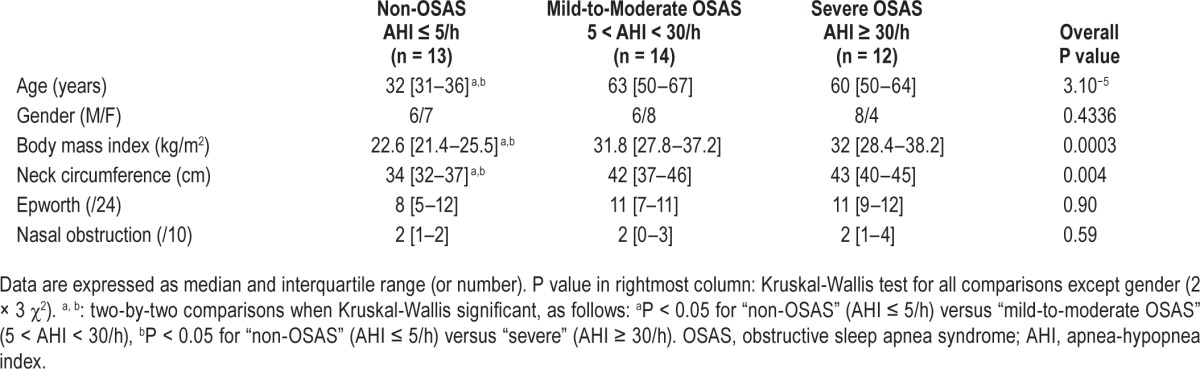
We studied OSAS patients in whom overnight polysomnography (PSG, details below) demonstrated an apnea-hypopnea index (AHI) > 5 events/h (“OSAS group,” n = 26, divided into a “moderate OSAS” group, AHI ≤ 30, n = 14, and a “severe OSAS” group, AHI > 30, n = 12; Table 1). These patients had no history of respiratory or neurological disorders and did not take any form of psychotropic treatment. A control group of non-OSAS individuals (6 healthy subjects and 7 patients referred for suspected parasomnia but with normal PSG) was also studied (“non-OSAS” group, n = 13, Table 1). The study complied with the recommendations of the Declaration of Helsinki and received ethical clearance from the appropriate authority (Comité de Protection des Personnes Ile-de-France 6, Pitié-Salpêtrière, France). The participants received detailed information and provided written participation consent. Demographic data, body mass index (BMI), neck circumference, Epworth Sleepiness Scale, nasal obstruction and respiratory discomfort (visual analogue scales [VAS]) were recorded.
Table 1.
Anthropometric and clinical characteristics of participants.
Polysomnography (PSG)
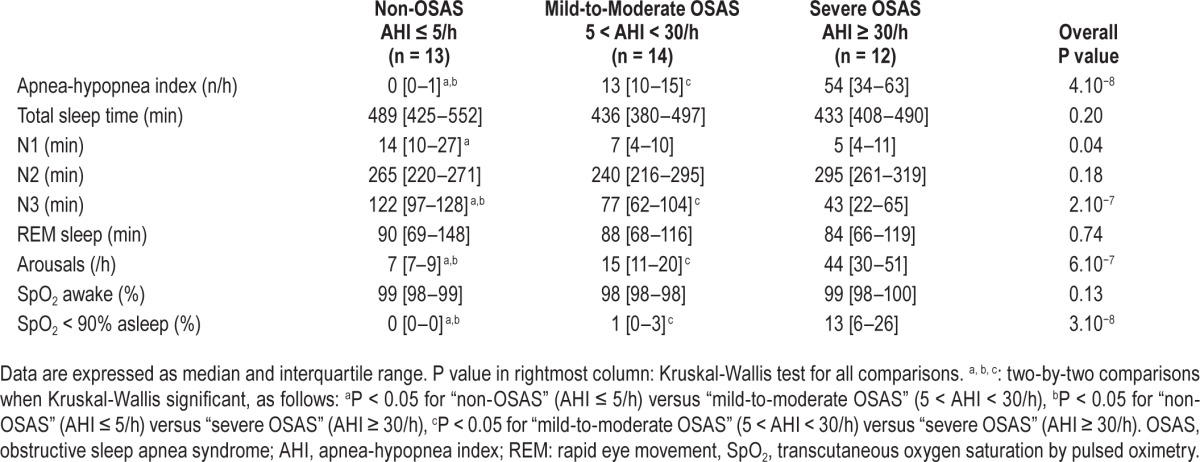
Participants underwent full-night standard polysomnography (PSG) (Medatec, France). PSG recordings were analyzed according to the 2013 update of the American Academy of Sleep Medicine rules for scoring respiratory events in sleep.15 Apnea was defined as absence of airflow ≥ 10 sec, hypopnea as reduction of airflow ≥ 30% associated with a decrease in oxygen saturation ≥ 3% or with a micro-awakening. The AHI was calculated as the number of apneas and hypopneas per hour of total sleep time (TST), including central and mixed apneas (that both accounted for less than 2% of apneas on average). The arousal index was defined as the number of electroencephalographic arousals per hour of TST.
Respiratory-Related Cortical Activity
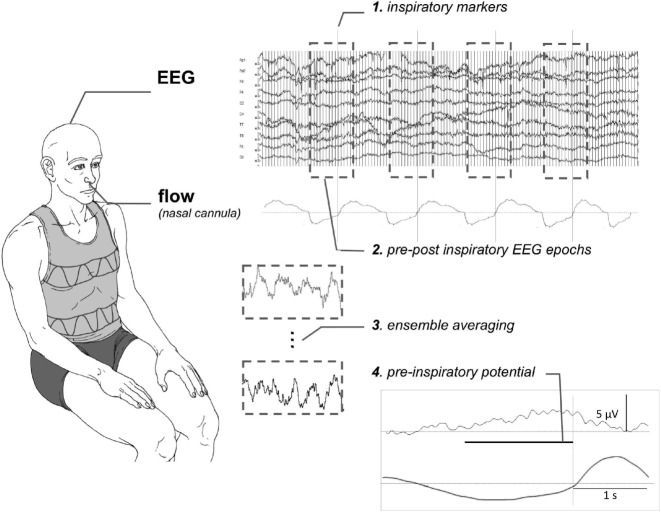
For this part of the study, the participants were comfortably seated in a chair in a quiet room. Fifteen-minute EEG recordings were performed with 14 scalp electrodes at Fz, FCz, Cz, Fp1, Fp2, F3, F4, C3, C4, T7, T8, A1, and A2 (Acticap, Brain Products, Germany). Electrode impedance was maintained below 2Ω. The electrodes were connected to an EEG preamplifier (V-Amp, Brain Products GmbH, Germany) from which the signals were digitized at 2000 Hz. Respiratory flow was measured with a nasal oxygen cannula (GT013-101A, Greetmed, China) connected to a differential pressure transducer (FE141 Spirometer ADInstruments, Australia), from which the signal was digitized at 200 Hz (Powerlab 16/30 ADInstruments, Colorado Springs, CO, USA). The EEG and respiratory signals were processed according to the method previously described.10,11 Briefly, the onset of inspiration was detected on the flow signal and the EEG signal was split into respiratory-synchronized segments starting 2.5 s before the beginning of inspiration and ending 1 s after its end. EEG segments with obvious artifacts (EEG gradient > 5 μV/ms; EEG amplitude > 50 μV; visible baseline fluctuations) were rejected. The remaining segments (≥ 72 for each subject) were ensemble averaged point-by-point. To identify pre-inspiratory potentials, the averaged EEG was visually inspected to detect negative inflections from baseline during the time window at which the potential was expected, namely 0.5–2 s before the onset of inspiration. This identification was conducted by a single investigator (CL) under blinded conditions. In the instance of a doubt, the recordings were examined by 2 other investigators, also under blinded conditions. A potential was considered present when the investigators reached a unanimous decision. Figure 1 summarizes the experimental setup and data processing.
Figure 1.
Schematic representation of the experimental setup and of the data analysis process. The pre-inspiratory potential represented in the bottom right corner of the figure has been recorded in one of the severe OSAS patient included in the study.
Statistical Analysis
All statistical analyses were performed using online university resources (biostatgv.sentiweb.fr). Data are expressed as median and interquartile range. Continuous variables (anthropometric and sleep data) were compared by a Kruskal-Wallis test followed by Dunn post hoc test for two-by-two comparisons. Differences between the 3 groups regarding pre-inspiratory potentials were tested with Fisher exact test (3 × 2 contingency table for global significance followed by 2 × 2 contingency tables for two-by-two group comparisons). The difference between the non-OSAS group and the OSAS group and the difference between participants classified into “obese” and “non-obese” (threshold 30 kg/m2) were also tested. Finally, all the participants were stratified according to the presence or absence of a pre-inspiratory potential. Univariate comparisons between those 2 groups were conducted using the Mann-Whitney test. In an exploratory manner, the association of explanatory variables with the presence of pre-inspiratory potentials were also evaluated using multivariate logistic regression (Matlab version 8.3.0.73043 -R2014a-; Statistics Toolbox version 9.0). Age and the AHI (or obstructive apnea index) were always forced into the models. Only models with a goodness of fit (χ2 statistic of comparison to the constant model) that was significant at the 0.05 level were considered, thus restricting the procedure to 3 or 4 variables models given the limited size of the study population. Two-tailed P values of the significance of their input variables were computed.
RESULTS
Demographic and Clinical Characteristics
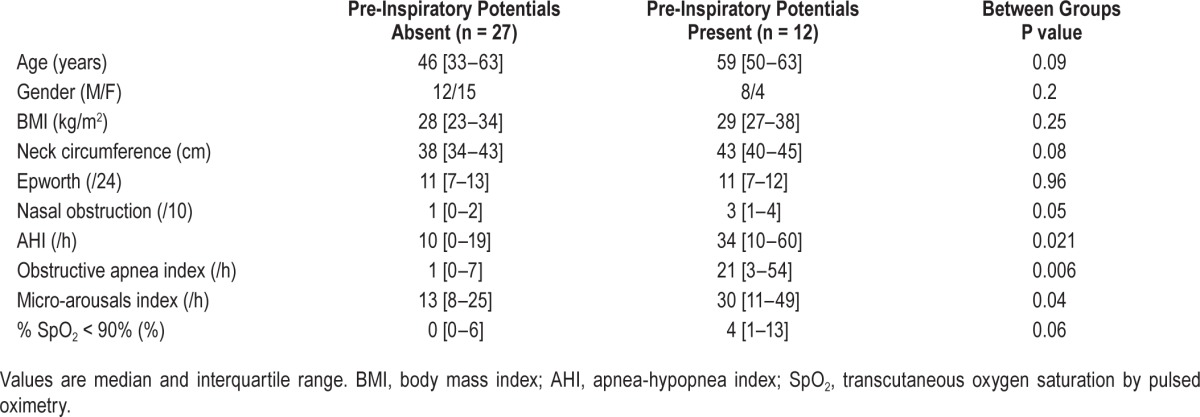
Non-OSAS individuals were younger and had a lower BMI and neck circumference than OSAS patients (Table 1). Patients with mild-to-moderate OSAS and patients with severe OSAS were comparable.
Sleep
The PSG results are shown in Table 2. Patients with severe OSAS had less N3 sleep, more arousals, and more severe oxygen desaturation than control individuals and patients with mild-to-moderate OSAS (P < 0.05).
Table 2.
Sleep characteristics.
Respiratory-Related Cortical Activity
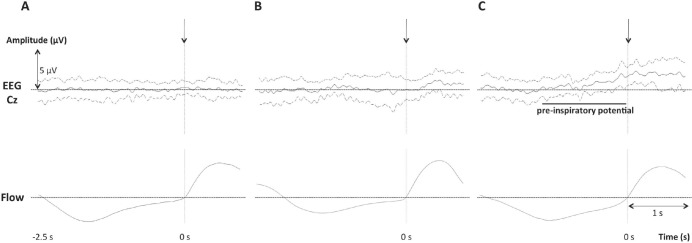
Pre-inspiratory potentials were present in one of the thirteen non-OSAS individuals, 4 of the 14 patients with mild-to-moderate OSAS, and 7 of the 12 patients with severe OSAS (Figures 2 and 3). A significant difference existed between the non-OSAS group and the severe OSAS group (P = 0.0112) and between the non-OSAS group and the OSAS group as a whole (P = 0.0336). No significant difference was observed between the non-OSAS group and the mild-to-moderate OSAS group (P = 0.3259) or between the mild-to-moderate OSAS group and the severe OSAS group (P = 0.2329). The latencies and amplitudes of the pre-inspiratory potentials, when present, were similar between groups (Table 3).
Figure 2.
Average EEG waveforms (mean ± 1 SD) obtained in: (A) All the non-OSAS patients in the study. (B) All OSAS patients not exhibiting a preinspiratory potential. (C) All OSAS patients exhibiting a pre-inspiratory potential. The vertical arrows correspond to inspiratory markers.
Figure 3.
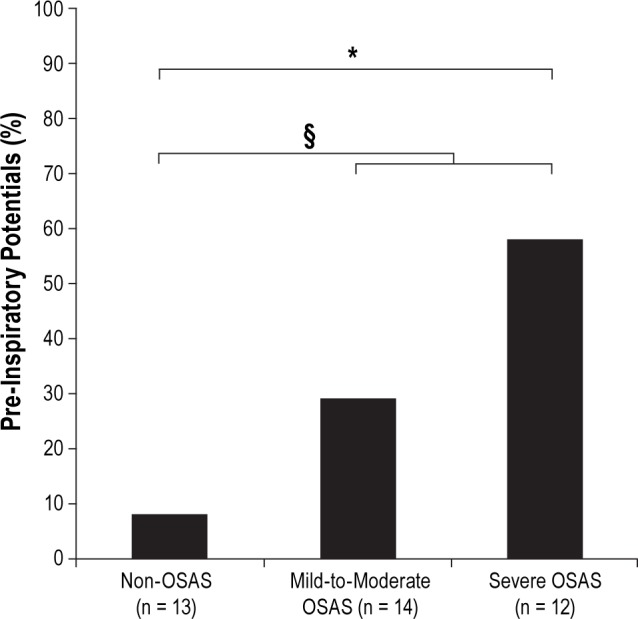
Percentages of individuals exhibiting pre-inspiratory potentials in the “non-OSAS” group, the “mild/moderate OSAS” group and the “severe OSAS” group. *p = 0.0112 between “non-OSAS” and “severe OSAS” §p = 0.0336 between “non-OSAS” and pooled “mild-to-moderate OSAS” and “severe OSAS.”
Table 3.
Characteristics of the pre-inspiratory potentials.

The incidence of pre-inspiratory potentials was not significantly different between obese and non-obese participants irrespective of OSAS.
Comparison of Participants with or without Pre-Inspiratory Potentials, Irrespective of OSAS Status
Univariate comparisons of these two groups are provided in Table 4. The groups were similar in terms of gender repartition and BMI. Those of the participants who did present a preinspiratory potential (n = 12) were older than those who did not present one (n = 27) but not significantly so; they had higher neck circumferences but also not significantly so; they reporter higher VAS ratings for nasal obstruction (P = 0.05), and had significantly higher values of AHI, obstructive apnea index, and micro-arousal index. Multivariate analysis showed that 3 variables models achieved significance regarding the goodness of fit criterion (P = 0.0356 for age + IAH + nasal obstruction; P = 0.0234 for age + severe OSAS category + nasal obstruction). Within these models, none of the input variables reached significance but “IAH” and “severe OSAS category” trended toward it (P = 0.1327 and P = 0.0743, respectively).
Table 4.
Comparison of patients according to the presence or absence of pre-inspiratory potentials.
DISCUSSION
In this study respiratory-related cortical activity was observed significantly more frequently during resting breathing in awake OSAS patients than in non-OSAS individuals, under the form of pre-inspiratory potentials resembling those observed during preparation of voluntary movements,12 preparation of voluntary inspiratory movements,16 and compensation of experimental inspiratory loads.11 This probably reflects activation of premotor cortical areas including the SMA, to which sensory17 and motor respiratory connections have been described.18,19 This is seemingly the first description of respiratory-related cortical activity during resting breathing in patients not suffering from defective automatic ventilatory control.14 In such patients, respiratory-related motor cortical activity has been interpreted as a cooperative wake-related mechanism supplementing the deficient homeostatic ventilatory drive.14 In OSAS patients, this brain activity could correspond to compensation of the intrinsic inspiratory load imposed by upper airway abnormalities. It could contribute to the increased inspiratory ventilatory drive described in the obstructive sleep apnea syndrome during wakefulness20 and could therefore contribute to the wakefulness-dependent compensation of upper airway dysfunctional neuromechanical coupling characteristics of OSAS.
Methodological Considerations
The non-OSAS subjects included in this study cannot be considered a true “control group,” as there was no actual age and BMI matching. However, studying this group was important to confirm that the experimental setup used in this study provided expected results in view of previous work (absence of respiratory-related premotor cortical activity in normal individuals). This was the case, as only one subject in the non-OSAS group presented pre-inspiratory potentials during resting breathing, a spurious finding that has been described before. In the absence of actual matching, the higher incidence of pre-inspiratory potentials in the OSAS group compared to the non-OSAS group could be due to a differences in age or in BMI (Table 1) rather than to the presence of OSAS. However, pre-inspiratory potentials were not more frequent among subjects with a BMI greater than 30 kg/m2 than in subjects with a lower BMI, regardless of the presence or absence of OSAS. Of note, pre-inspiratory potentials were more frequent in the severe OSAS group than in the mild-to-moderate OSAS group, although BMI was similar in these two groups. The univariate comparisons performed between the participants to the study who exhibited and did not exhibit pre-inspiratory potentials irrespectively of their OSAS status (Table 4) shows that the most important differences between those two groups were the AHI and the obstructive apnea index. There were other differences between these two groups (Table 4) which, even though they did not reach statistical significance, led us to conduct exploratory multivariate analyses. Although limited in power by the size of the study population, these analyses indicated that the obstructive apneas belonged to pre-inspiratory potentials explanatory variables. We believe that this study demonstrates, at least in a “proof of concept” manner, that OSAS is associated with an abnormally high incidence of pre-inspiratory potentials and therefore with specific brain “abnormalities.”
Neurophysiological Considerations
A respiratory-related EEG pattern was observed in some of our OSAS patients, which, to date, has only been described during resting breathing in healthy subjects in response to experimental inspiratory loading11 or in a very specific group of patients with defective autonomic breathing control.14 Inasmuch as OSAS patients do not present this type of defect, it is reasonable to hypothesize that OSAS-related upper airway dysfunction behaves like a natural inspiratory load and activates the same cortical networks as those activated by experimental loading in healthy subjects. The exact nature of this load is unclear and not elucidated by our study. Obesity per se was not associated with pre-inspiratory potentials. Of note, self-ratings of nasal obstruction were higher in the participants to the study who exhibited pre-inspiratory potentials than in the others (Table 4) and the association with pre-inspiratory potentials was suggested by the multivariate analysis. However, we did not measure upper airway mechanical properties, or precisely assess craniofacial morphological abnormalities or edematous infiltration, nor did we conduct dynamic studies of upper airway stability and control.21 Specific study designs are therefore necessary to identify the determinants of OSAS-associated respiratory-related premotor cortical activity.
The significance of the respiratory-related EEG activity observed in OSAS patients remains to be elucidated. Augmented ventilatory drive respective to controls has been reported in OSAS patients during resting breathing, with higher than normal ventilation, high occlusion pressure values20 and increased neural respiratory drive.22 During wakefulness, ventilatory drive depends on the sum and interactions of the various excitatory inputs received by spinal motoneurons.23 From our results (and even though we acknowledge as a limitation the fact that we did not assess ventilatory drive in our patients), it can therefore be hypothesized that the augmented ventilatory drive in OSAS results from corticospinal facilitation, with descending cortical inputs increasing the responsiveness of spinal respiratory motoneurons to bulbospinal drive.24 We postulate that certain cases of OSAS are characterized by an increase in the tonic facilitatory role of the SMA on phrenic motoneurons that exists in healthy humans,18 and that this activation relates to the wakefulness-related compensation of upper airway dysfunction phenomenologically characteristic of OSAS (see introduction). Several arguments support this hypothesis. Firstly, the ventilatory drive to breathe is distributed to an ensemble of motoneurons that encompasses upper airway dilator muscles and “pump” inspiratory muscles.25 A nonspecific increase in ventilatory drive resulting from cortico-subcortical cooperation (as discussed above) should lead to increased upper airway dilator muscle activity. This type of increased activity is actually present in awake OSAS patients7,26 and reflects the severity of upper airway anomalies.27 Secondly, the cortico-subcortical networks involved in the control of upper airway dilator muscles and pump inspiratory muscles are intimately interrelated28 in line with the extensive need for coordination of these muscle groups during breathing, swallowing, and speech. This coupling could be modified in OSAS as suggested by a transcranial magnetic stimulation study comparing OSAS patients to controls9 that showed that coupled electromyographic responses of the genioglossus and diaphragm occurred more frequently than dissociated responses in OSAS patients than in controls. In the same study, an inspiratory maneuver facilitated the genioglossus response to transcranial magnetic stimulation in patients, but had no effect in controls.9 Of note, stimulating the corticomotor pathway during sleep in OSAS patients improves airway dynamics.29
CONCLUSIONS AND PERSPECTIVES
We believe that our observations provide a neurophysiological substrate for one of the arousal-dependent compensatory mechanisms that prevent OSAS patients from experiencing airway collapse while awake. Because pre-inspiratory potentials were not consistently found in our patients, other arousal-dependent compensations must exist. Nevertheless, our findings open new research avenues. For example, it would be interesting to study the effects of inhibitory repetitive transcranial magnetic stimulation (rTMS) over the supplementary motor area on upper airway dynamics in awake OSAS patients exhibiting pre-inspiratory potentials. According to our hypothesis, inhibition of the connection between the supplementary motor area and the primary motor cortex by rTMS (which somehow would mimic the sleep-related breakdown in cortical connectivity described by Massimni et al.30) would be expected to increase upper airway resistance or collapsibility. It would also be important to study the prevalence of respiratory-related cortical activity in OSAS patients on a larger scale and with more sensitive EEG analysis; the factors associated with this activity; and the putative impact of this activity on OSAS-related alterations on cognitive performances. Indeed, it has been hypothesized that the “need” to mobilize cortical resources to breathe could have a negative impact on cognitive performance in patients with central congenital hypoventilation syndrome,14 an hypothesis supported by psycho-physiological and functional magnetic resonance imaging data acquired in one such patient.31 Whether or not this mechanism contribute to OSAS-related cognitive abnormalities should be elucidated.
DISCLOSURE STATEMENT
This was not an industry supported study. This research was supported by the French Government “Investissement d'Avenir ANR-10-AIHU 06” programme,” the “Legs Poix” programme of the “Chancellerie de l'Université de Paris,” the non-profit association “Association pour le Développement et l'Organisation de la Recherche en Pneumologie et sur le Sommeil,” and the “Fonds de Dotation Recherche en Santé Respiratoire” (Claire Launois). Dr. Arnulf has participated in speaking engagements and has consulted for UCB Pharma. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors are grateful to Felix Kindler and Lysandre Tremoureux for technical help during preliminary experiments and would like to thank Anthony Saul for editing English style and grammar.
REFERENCES
- 1.Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;383:736–47. doi: 10.1016/S0140-6736(13)60734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwab RJ, Pasirstein M, Pierson R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168:522–30. doi: 10.1164/rccm.200208-866OC. [DOI] [PubMed] [Google Scholar]
- 3.Hudgel DW, Harasick T. Fluctuation in timing of upper airway and chest wall inspiratory muscle activity in obstructive sleep apnea. J Appl Physiol. 1990;69:443–50. doi: 10.1152/jappl.1990.69.2.443. [DOI] [PubMed] [Google Scholar]
- 4.Series F, Cote C, St Pierre S. Dysfunctional mechanical coupling of upper airway tissues in sleep apnea syndrome. Am J Respir Crit Care Med. 1999;159:1551–5. doi: 10.1164/ajrccm.159.5.9804124. [DOI] [PubMed] [Google Scholar]
- 5.Lin CC, Wu KM, Chou CS, Liaw SF. Oral airway resistance during wakefulness in eucapnic and hypercapnic sleep apnea syndrome. Respir Physiol Neurobiol. 2004;139:215–24. doi: 10.1016/j.resp.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Verin E, Tardif C, Portier F, Similowski T, Pasquis P, Muir JF. Evidence for expiratory flow limitation of extrathoracic origin in patients with obstructive sleep apnoea. Thorax. 2002;57:423–8. doi: 10.1136/thorax.57.5.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism) J Clin Invest. 1992;89:1571–9. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mezzanotte WS, Tangel DJ, White DP. Influence of sleep onset on upper-airway muscle activity in apnea patients versus normal controls. Am J Respir Crit Care Med. 1996;153:1880–7. doi: 10.1164/ajrccm.153.6.8665050. [DOI] [PubMed] [Google Scholar]
- 9.Series F, Wang W, Similowski T. Corticomotor control of the genioglossus in awake OSAS patients: a transcranial magnetic stimulation study. Respir Res. 2009;10:74. doi: 10.1186/1465-9921-10-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raux M, Tyvaert L, Ferreira M, et al. Functional magnetic resonance imaging suggests automatization of the cortical response to inspiratory threshold loading in humans. Respir Physiol Neurobiol. 2013;189:571–80. doi: 10.1016/j.resp.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Raux M, Straus C, Redolfi S, et al. Electroencephalographic evidence for pre-motor cortex activation during inspiratory loading in humans. J Physiol. 2007;578:569–78. doi: 10.1113/jphysiol.2006.120246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shibasaki H. Cortical activities associated with voluntary movements and involuntary movements. Clin Neurophysiol. 2012;123:229–43. doi: 10.1016/j.clinph.2011.07.042. [DOI] [PubMed] [Google Scholar]
- 13.Ball T, Schreiber A, Feige B, Wagner M, Lucking CH, Kristeva-Feige R. The role of higher-order motor areas in voluntary movement as revealed by high-resolution EEG and fMRI. Neuroimage. 1999;10:682–94. doi: 10.1006/nimg.1999.0507. [DOI] [PubMed] [Google Scholar]
- 14.Tremoureux L, Raux M, Hudson AL, Ranohavimparany A, Straus C, Similowski T. Does the supplementary motor area keep patients with Ondine's curse syndrome breathing while awake? PLoS One. 2014;9:e84534. doi: 10.1371/journal.pone.0084534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berry RB, Brooks R, Gamaldo CE, et al. Darien, IL: American Academy of Sleep Medicine; 2013. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Version 2.0.2. www.aasmnet.org. [Google Scholar]
- 16.Macefield G, Gandevia SC. The cortical drive to human respiratory muscles in the awake state assessed by premotor cerebral potentials. J Physiol. 1991;439:545–58. doi: 10.1113/jphysiol.1991.sp018681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Logie ST, Colrain IM, Webster KE. Source dipole analysis of the early components of the RREP. Brain Topogr. 1998;11:153–64. doi: 10.1023/a:1022210723257. [DOI] [PubMed] [Google Scholar]
- 18.Laviolette L, Nierat MC, Hudson AL, Raux M, Allard E, Similowski T. The supplementary motor area exerts a tonic excitatory influence on corticospinal projections to phrenic motoneurons in awake humans. PLoS One. 2013;8:e62258. doi: 10.1371/journal.pone.0062258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raux M, Xie H, Similowski T, Koski L. Facilitatory conditioning of the supplementary motor area in humans enhances the corticophrenic responsiveness to transcranial magnetic stimulation. J Appl Physiol. 2010;108:39–46. doi: 10.1152/japplphysiol.91454.2008. [DOI] [PubMed] [Google Scholar]
- 20.Radwan L, Maszczyk Z, Koziorowski A, et al. Control of breathing in obstructive sleep apnoea and in patients with the overlap syndrome. Eur Respir J. 1995;8:542–5. [PubMed] [Google Scholar]
- 21.Series F, Demoule A, Marc I, Sanfacon C, Derenne JP, Similowski T. Inspiratory flow dynamics during phrenic nerve stimulation in awake normals during nasal breathing. Am J Respir Crit Care Med. 1999;160:614–20. doi: 10.1164/ajrccm.160.2.9812036. [DOI] [PubMed] [Google Scholar]
- 22.Steier J, Jolley CJ, Seymour J, et al. Increased load on the respiratory muscles in obstructive sleep apnea. Respir Physiol Neurobiol. 2010;171:54–60. doi: 10.1016/j.resp.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Butler JE. Drive to the human respiratory muscles. Respir Physiol Neurobiol. 2007;159:115–26. doi: 10.1016/j.resp.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Mehiri S, Straus C, Arnulf I, et al. Responses of the diaphragm to transcranial magnetic stimulation during wake and sleep in humans. Respir Physiol Neurobiol. 2006;154:406–18. doi: 10.1016/j.resp.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Saboisky JP, Butler JE, Fogel RB, et al. Tonic and phasic respiratory drives to human genioglossus motoneurons during breathing. J Neurophysiol. 2006;95:2213–21. doi: 10.1152/jn.00940.2005. [DOI] [PubMed] [Google Scholar]
- 26.Saboisky JP, Stashuk DW, Hamilton-Wright A, et al. Neurogenic changes in the upper airway of patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2012;185:322–9. doi: 10.1164/rccm.201106-1058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pierce R, White D, Malhotra A, et al. Upper airway collapsibility, dilator muscle activation and resistance in sleep apnoea. Eur Respir J. 2007;30:345–53. doi: 10.1183/09031936.00063406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corfield DR, Murphy K, Josephs O, et al. Cortical and subcortical control of tongue movement in humans: a functional neuroimaging study using fMRI. J Appl Physiol. 1999;86:1468–77. doi: 10.1152/jappl.1999.86.5.1468. [DOI] [PubMed] [Google Scholar]
- 29.Melo-Silva CA, Gakwaya S, Rousseau E, Series F. Consecutive transcranial magnetic stimulation twitches reduce flow limitation during sleep in apnoeic patients. Exp Physiol. 2013;98:1366–75. doi: 10.1113/expphysiol.2013.073072. [DOI] [PubMed] [Google Scholar]
- 30.Massimini M, Ferrarelli F, Huber R, Esser SK, Singh H, Tononi G. Breakdown of cortical effective connectivity during sleep. Science. 2005;309:2228–32. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- 31.Sharman M, Gallea C, Lehongre K, et al. The cerebral cost of breathing: an FMRI case-study in congenital central hypoventilation syndrome. PLoS One. 2014;9:e107850. doi: 10.1371/journal.pone.0107850. [DOI] [PMC free article] [PubMed] [Google Scholar]