Abstract
Study Objective:
To test the hypothesis that respiratory event duration exhibits an endogenous circadian rhythm.
Design:
Within-subject and between-subjects.
Settings:
Inpatient intensive physiologic monitoring unit at the Brigham and Women's Hospital.
Participants:
Seven subjects with moderate/severe sleep apnea and four controls, age 48 (SD = 12) years, 7 males.
Interventions:
Subjects completed a 5-day inpatient protocol in dim light. Polysomnography was recorded during an initial control 8-h night scheduled at the usual sleep time, then through 10 recurrent cycles of 2 h 40 min sleep and 2 h 40 min wake evenly distributed across all circadian phases, and finally during another 8-h control sleep period.
Measurements and Results:
Event durations, desaturations, and apnea-hypopnea index for each sleep opportunity were assessed according to circadian phase (derived from salivary melatonin), time into sleep, and sleep stage. Average respiratory event durations in NREM sleep significantly lengthened across both control nights (21.9 to 28.2 sec and 23.7 to 30.2 sec, respectively). During the circadian protocol, event duration in NREM increased across the circadian phases that corresponded to the usual sleep period, accounting for > 50% of the increase across normal 8-h control nights. AHI and desaturations were also rhythmic: AHI was highest in the biological day while desaturations were greatest in the biological night.
Conclusions:
The endogenous circadian system plays an important role in the prolongation of respiratory events across the night, and might provide a novel therapeutic target for modulating sleep apnea.
Citation:
Butler MP, Smales C, Wu H, Hussain MV, Mohamed YA, Morimoto M, Shea SA. The circadian system contributes to apnea lengthening across the night in obstructive sleep apnea. SLEEP 2015;38(11):1793–1801.
Keywords: sleep disordered breathing, apnea hypopnea index, arousal, time of day, clock
INTRODUCTION
In obstructive sleep apnea (OSA), the upper airway collapses during sleep and prevents respiration. The ensuing asphyxia leads to a life-saving arousal, which enables breathing and gas exchange to temporarily recover, before sleep, apnea, and arousal cycles recur. Given these significant physiological challenges every night, it is not surprising that OSA is independently associated with serious cardiovascular events and even death.1 OSA severity is described by the average number of respiratory events per hour of sleep (apnea-hypopnea index, AHI).2,3 The durations of apneas are also important, as these are related to the extent of the hypoxemia, hypercapnia, and circulatory changes that accompany the asphyxia/arousal cycles.
Descriptive measures of OSA vary with time of night, but the causes are not known. For example, apnea duration systematically increases across the night for the same sleep stage.4–7 Whether AHI also varies with time of day or night for the same sleep stage is somewhat equivocal. AHI has been reported to be similar between day and night,8,9 as well as to differ between day and night.10,11 Within a night, AHI in NREM sleep has been reported to increase, but only in those with severe OSA.6
Most daily rhythms in physiology and behavior are coordinated by a central circadian (∼24 h) clock in the hypothalamus.12,13 Therefore, we tested whether the nocturnal changes in apnea/hypopnea duration and/or AHI could be caused by an effect of the endogenous circadian clock.
METHODS
To test for an endogenous circadian clock influence on apnea/hypopnea duration and AHI, we studied participants with and without OSA who slept at all phases of the internal circadian cycle over 5 days in the laboratory. The protocol was approved by the Institutional Review Board of Brigham and Women's Hospital and Partners Health Care.
Subjects
Men and women between 18 and 70 years of age, with BMI < 40, and with untreated OSA were recruited by advertisements on internet sources and local newspapers. Subjects with a high probability of having OSA, based on responses to the STOP questionnaire,14 also participated. Eleven subjects completed the study (Table 1). Medical suitability was determined by clinical history, physical examination, chemistry and hematology screening, and psychiatric interview. Exclusion criteria were other sleep disorders including insomnia, other untreated medical conditions including uncontrolled hyper-tension, diabetes, and cardiovascular disease, history of night shift work, or travel across time zones within the preceding 3 months. One subject had used continuous positive airway pressure (CPAP) for treatment of OSA but had stopped 3 months before participating. One subject had controlled hypertension (lisinopril 20 mg/day).
Table 1.
Anthropometric data for subjects.

Protocol
To stabilize circadian rhythms and sleep timing, subjects maintained a regular 8-h sleep period for ≥ 1 wk prior to the laboratory study. To determine compliance, subjects completed a sleep diary at home, telephoned a time-stamped voicemail box at each bedtime and wake-time, and wore a wrist actigraphy monitor throughout this pre-laboratory period (Actiwatch, Minimitter, Bend, OR). Subjects abstained from alcohol, nicotine, caffeine, and other medications or supplements (except for lisinopril, n = 1). Comprehensive toxicology screening of urine was performed on admission to the laboratory.
After admission, subjects stayed in individual laboratory suites and were closely monitored for 5 consecutive days and nights. The protocol consisted of an initial 8-h control night with sleep scheduled at each subject's usual time, a period when subjects slept across all phases of the internal circadian cycle (accomplished by 10 recurring 5 h 20 min “days” consisting of 2 h 40 min of wake and 2 h 40 min sleep opportunity), and finally, another 8-h control night at the usual time (Figure 1).15 This short-day protocol is termed “forced desynchrony” because the sleep/wake cycles are desynchronized from the endogenous clock.16 Room lights were dim (< 4 lux at the horizontal angle of gaze) during wake times to prevent light-induced phase shifts of the circadian clock17 and were off during scheduled sleep periods. In these conditions, the central circadian clock proceeds with an endogenous period of ∼24.1 h,18 and variables of interest can be measured at all circadian phases.
Figure 1.
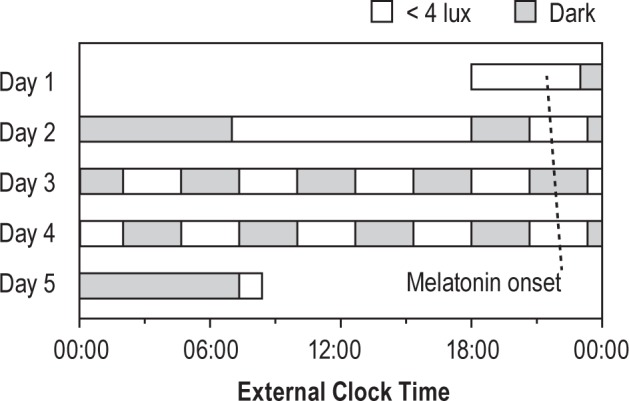
Forced desynchrony protocol. After a baseline 8-h control night, subjects completed 10 alternating 2:40 sleep and wake intervals, such that sleep opportunities were evenly spaced across the 24-h day. Before the first short sleep and during subsequent short wake periods, subjects ate an isocaloric meal beginning 90 min prior to lights-off. Melatonin onsets are shown for the same subject as in Figure 2 (period = 24.17 h).
To minimize postural changes that can affect AHI,19 subjects slept in a semi-recumbent position (20° head-up) with vertical pillows placed snugly on both sides of the head to ensure a supine head-up position. During each short wake-period, subjects completed cognitive tests on a computer and ate one isocaloric meal representing 22% of the 24-h total.
Measurements
Full polysomnography (PSG) was recorded during all sleep periods according to guidelines of the American Academy of Sleep Medicine (AASM),20 using a Vitaport 3 recorder (Temec Instruments, The Netherlands). PSG included electroencephalogram derivations F3/4, C3/4, and O1/2, referenced to M2/1, respectively, bilateral electro-oculogram, and submental and bilateral anterior tibialis electromyograms (Grass disc electrodes, Grass Technologies, Astro-Med Inc., West Warwick, RI). Cardiorespiratory data included oro-nasal airflow (Disposable BreathSensor, 4140118, Embla Systems, Buffalo, NY), intra-nasal pressure (Pro-tech Pro-Flow Plus, P1259, Respironics, Murrysville, PA), breathing movements by inductive plethysmography (Respibands, Temec), snoring (M92500, Temec), arterial oxygen saturation via pulse oximetry (SpO2; Ohmeda Flex II or Nonin Pulse Oximeter Flex measured on the non-dominant index finger), and 3-lead electrocardiogram. Body and head position were monitored with single axis accelerometers (M92600, Temec).
To assess endogenous circadian phases, saliva samples were collected approximately once every 60–90 min during wake periods. Salivary melatonin was assessed by radioimmunoassay (Buhlmann RK-DSM2, Alpco Diagnostics, Salem, NH: inter-assay and intra-assay coefficients of variation at ∼3.5 pg/mL were 8.9% and 4.1%, respectively, as reported by the manufacturer).
Analyses
Sleep Scoring
Sleep stages and sleep disordered breathing events (hypopneas, plus obstructive, central, and mixed apneas) were scored according to AASM criteria2,20 by one registered polysomnographic technician who was blind to circadian time and who scored all of the records together at the conclusion of the study (Sleep Strategies, Ontario, Canada). Special emphasis was placed on accurate measurement of respiratory event durations. Apneas were defined by > 90% loss of airflow as measured by the oro-nasal thermistor for ≥ 10 seconds. Duration was defined as the period between the end-expiratory trough preceding the absent or ineffective inhalation to the beginning of the first unobstructed inhalation. Hypopneas were defined by ≥ 30% reduction in nasal pressure with ≥ 4% desaturation,20 or with an EEG identified arousal2 in cases when oxygen saturation data were unreliable (19% of hypopneas). Hypopnea duration was defined as the period between the end-expiratory trough preceding the first breath with ≥ 30% diminished inhalation to the beginning of the first unobstructed inhalation. Events associated with and without arousals were not scored separately.
Circadian Phase Marker
Circadian phase (0–359°) was derived for each subject from the salivary melatonin concentrations.21 Melatonin data were fit by nonorthogonal spectral analysis (NOSA) with 3 harmonics (Figure 2).22 Dim light melatonin onset was used as the 0° reference: it was calculated as the interpolated time point when melatonin levels exceeded 25% of the fitted trough-to-peak amplitude.23,24
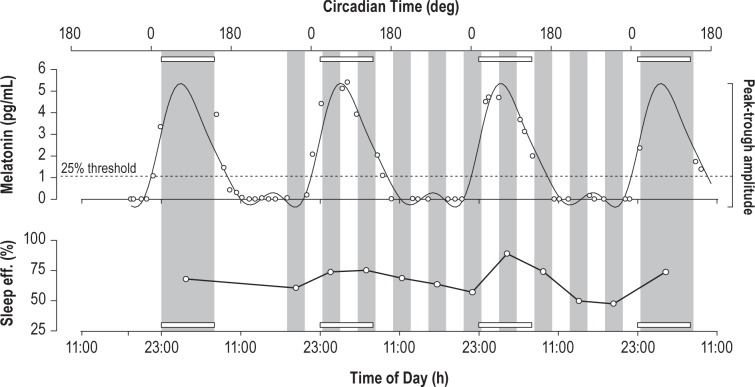
Figure 2.
Salivary melatonin (sample concentrations and best fit curve) and sleep efficiency for an example subject. Dim light melatonin onset is defined by the point at which the fitted melatonin curve crosses 25% of the trough-to-peak amplitude (dotted line). Sleep periods are shaded in gray; open rectangles demarcate the time of habitual sleep (23:00–07:00).
Statistics
To account for differences between subjects, all respiratory event durations were normalized within-subject and within-event type (hypopneas, obstructive apneas, central apneas, and mixed apneas) to the mean duration observed during the first control 8-h sleep period. Thus, these variables are reported as percentages of the mean.
Each respiratory event had an associated circadian phase, sleep stage, time-into-experiment, and time-into-sleep opportunity. Data were analyzed by mixed model cosinor analysis, including circadian time (4-term 2-harmonic parametrization),25 time-into-experiment, time-into-sleep opportunity, sleep stage, and subject as a random factor (JMP Pro 11, SAS Institute, Cary, NC). Adjusting for age, sex, and BMI did not alter the conclusions or significance of model terms, so these were not included. Sleep measures and melatonin data were also assigned to 60° (∼4 h) or 30° (∼2 h) circadian bins, and analyzed with repeated measures ANOVA or Student's t-test (Statview 5.0, SAS Institute, Cary, NC). For illustration purposes, means and standard errors for each 60° circadian bin were obtained by normalizing and binning within each subject, and then averaging across subjects (i.e., in these cases, standard errors are calculated with n = 7 for subjects with apnea or n = 4 for control subjects). Unless otherwise noted, data are reported as mean ± SE. Best-fit curves are plotted with 95% confidence intervals.
RESULTS
Control Nights
Sleep apnea classification was based on the AHI on the first control night: 7 of 11 subjects had moderate to severe OSA (Table 1, AHI > 15), while 4 subjects did not have OSA (AHI < 5) and served as controls. During this first control night, across 56 h of scheduled sleep in the 7 subjects with OSA, there were only 16 respiratory events in stage N3 sleep. These 16 events were not consistently distributed across subjects or times, so we excluded N3 sleep events from subsequent analyses of event durations.
During the first 8-h control night, event duration lengthened significantly with time into the sleep opportunity (effect of time from lights-off: F1,1124 = 6.9, P < 0.01; NREM versus REM: F1,828 = 31.3, P < 0.001; interaction: F1,735 = 17.2, P < 0.001). On average, REM events were 14 % longer. The pattern was similar during the final 8-h control night (effect of time from lights off: F1,1310 = 9.5, P < 0.01; NREM versus REM: F1,1258 = 10.4, P < 0.001; interaction: F1,1307 = 5.5, P < 0.05; REM events 9% longer than NREM events). Stratifying by night and by sleep stage (NREM versus REM) revealed that event duration lengthened significantly within NREM on both nights (P < 0.001) but not during REM sleep on either night (P > 0.6 for both) (Figure 3).
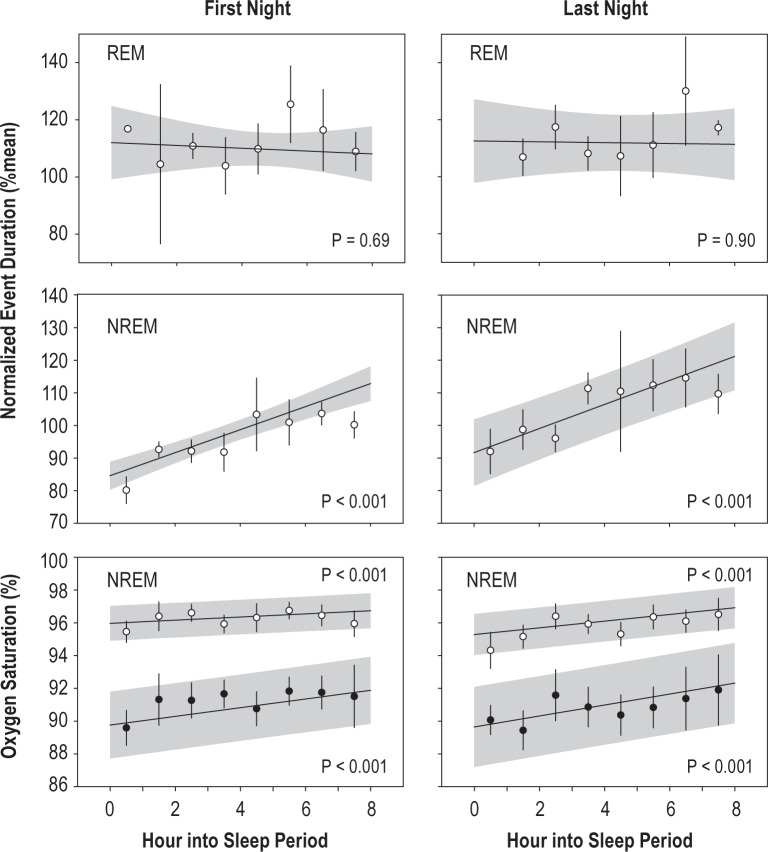
Figure 3.
Eight-hour control nights. Normalized event duration (% of mean duration within subject and event type) in REM (top) and NREM (middle), and pre-event and event-related minimum oxygen saturation (bottom, open and closed symbols, respectively), during the control 8-h sleep opportunities. Regression and shaded 95% confidence intervals show the model fit to all unbinned events. For illustration only, data are also binned by hour and plotted as mean and SE (NREM duration, n = 5–7 subjects per point; REM duration, n = 1–7 subjects per point; saturation, n = 3–7 subjects per point). Significance for the effect of time into night is shown in each panel.
The mean duration of all NREM respiratory events was 25.5 sec. Therefore, the increase of 25 percentage points over the baseline night in the fitted model from hours 1 to 8 was equivalent to 6.3 sec. This corresponded well to the 20 percentage point increase (5.1 sec) in the binned observations. The predicted change in the model for NREM was 6.6 sec over the last night, while predicted changes in REM were −0.9 sec and −0.3 sec for the first and last nights, respectively.
During the 8-h control nights, there were significant effects of time in bed on the pre-event saturation, the post-event minimum saturation, and the overall desaturation (Figure 3: effect of time from lights-off: First night [F1,799 > 17.6, P < 0.001]; Last night, [F1,925 > 11.1, P < 0.001]). Nevertheless, the trend was for desaturations to lessen from the beginning to the end of the night, opposite the expectation based on the lengthening of the event, implying that oxygen consumption declined across the night. Others have reported no time of night effect on desaturation during respiratory events.4,5,7,26
Forced Desynchrony Protocol
Melatonin Rhythms
Salivary melatonin concentrations displayed robust circadian rhythms (Figure 2). Those with and without OSA did not differ in their circadian period (OSA, 23.93 ± 0.09 h; Control, 23.78 ± 0.22 h; t-test, P = 0.50). Similarly, dim light melatonin onset relative to the first control night did not differ (OSA: 2.50 ± 0.36 h prior to habitual bedtime; Control: 2.04 ± 0.41 h prior to habitual bedtime; t-test, P = 0.44). These melatonin phase markers were equivalent to 20:30 for OSA and 21:00 for controls, given a 23:00 bedtime.
Sleep
Subjects were able to sleep in most of the 10 scheduled short sleep opportunities, though sleep efficiency was lower during the daytime (Figures 2, 4). Expected circadian rhythms of NREM and REM sleep duration occurred in this short forced desynchrony protocol (Figure 4), as have been observed in longer protocols.27 By cosinor analysis, significant main effects of circadian time were detected for total sleep time (TST), NREM duration, and REM duration (for each test, F1,102 > 11.0, P < 0.01). TST, NREM, and REM duration, shortened by 15.4, 12, and 3.4 min/day, respectively (F1,102 > 4.4, P < 0.05), suggesting that the subjects may have been carrying a slight sleep “debt” before entry into the laboratory and that the increased sleep opportunity in the circadian protocol (50% of time devoted to sleep versus 33.3% when at home and on the control nights) was sufficient to meet the subjects' need for sleep. Also, as expected,28,29 sleep latency to stage 1 also varied with circadian phase with a peak around 0° (F1,95 = 17.0, P < 0.001). There was neither a group effect (OSA versus control) nor a circadian time × group effect on TST, NREM and REM duration, or sleep latency.
Figure 4.
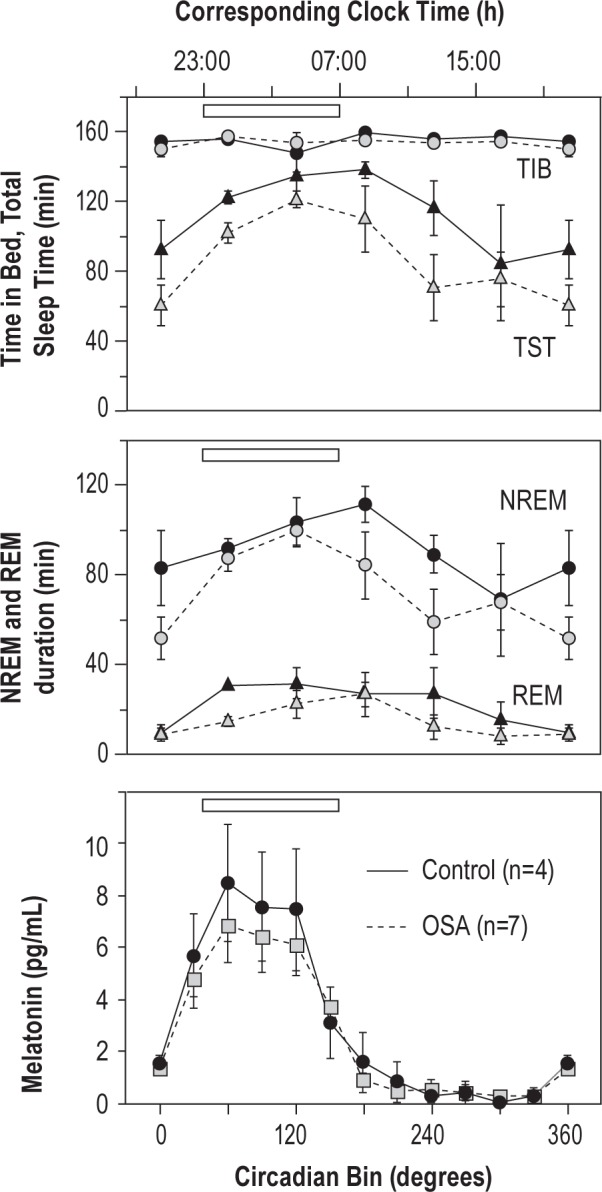
Circadian patterns of sleep in forced desynchrony conditions. Sleep measures were organized into 60° bins according to their midpoints. Corresponding clock time shown above assumes a bedtime of 23:00. Sleep duration is longest during the time of habitual sleep period (open bar) and early morning. Circadian rhythms of salivary melatonin secretion (30° bins) exhibited a peak during the biological night in both OSA (gray symbols, dotted lines) and control groups (black symbols, solid lines).
Respiratory Event Duration
During the forced desynchrony, only 74 respiratory events occurred in 205.3 person-hours of N3 sleep. Thus, as for the control night, we excluded N3 sleep events from subsequent analyses of event durations. Because event durations in REM did not change across 8-h nights, and because REM sleep is heavily dependent on circadian phase,27 REM events also were not analyzed further. The final data set included 68 central apneas, 1,527 hypopneas, 250 mixed apneas, and 882 obstructive apneas that occurred in stages N1 or N2 sleep. In all sleep stages, as well as in NREM only, the relative proportion of apneas compared to hypopneas did not vary with sleep period (All sleep, F10,86 = 0.7, P = 0.7; NREM, F10,83 = 0.7, P = 0.7).
There was a highly significant circadian rhythm of respiratory event duration (first harmonic (∼24h): F1,2715 = 16.9, P < 0.001; second harmonic (∼12h): F1,2715 = 22.5, P < 0.001). Additionally, there were significant main effects of time in bed (F1,2719 = 23.0, P < 0.001), and sleep stage (F1,2715 = 24.0, P < 0.001), but not of time into experiment (F1,2715 = 2.0, P > 0.1). The circadian components of the model are plotted in Figure 5. The circadian rhythm had a peak-to-trough amplitude of 15.1% (3.9 sec); duration was shortest at 30° and longest at 135°, corresponding to times of 22:30 and 05:30 for a subject with a 23:00 bedtime (Figure 5).
Figure 5.
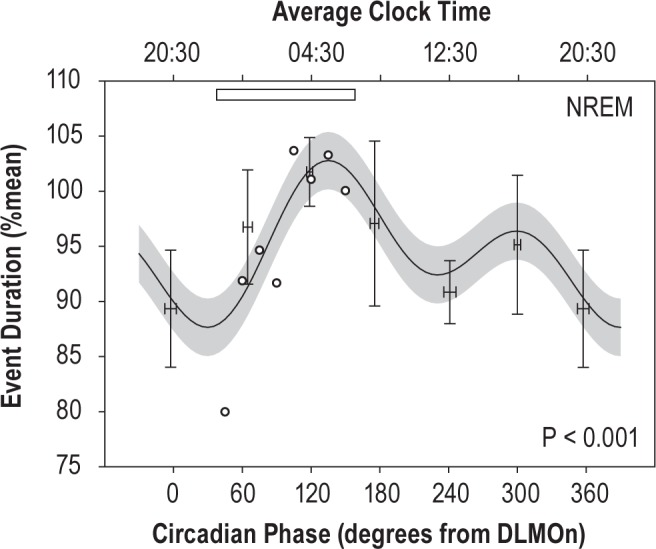
Normalized event duration (% of mean duration within subject and event type) during forced desynchrony conditions. Cosinor model of the circadian rhythm in duration is shown with 95% confidence interval, as evaluated at the mean time into experiment and time in bed and controlling for the proportion of events in stage N1 (28%) versus N2 (72%). Though analyzed by phase, the average corresponding clock times are shown at the top, together with an open bar representing the time of habitual sleep. Statistical analysis utilized unbinned event phases. For comparison with the model fit, the observed means for event duration by circadian bin are plotted as mean and SE (n = 7, mean and SE of phase within a bin is also plotted). To estimate how much of the change in event duration across the control night could be attributed to the circadian system, the mean durations during NREM sleep on the first control night are superimposed (open circles, from Figure 3) on the circadian model fit. DLMOn, dim light melatonin onset.
Oxygen Desaturation
There were 2,442 events in stages N1 and N2 sleep during the forced desynchrony for which desaturations were obtained. Here, longer duration events were associated with greater de-saturation (P < 0.001). Oxygen saturation measures exhibited significant circadian rhythms (Figure 6: pre-event, F1,2429 = 24.6, P < 0.001; event-related minimum, F1,2428 = 19.0, P < 0.001). Event-related desaturation was also rhythmic (F1,2430 = 11.0, P < 0.001, not plotted). The peak-to-trough amplitude of the desaturation rhythm was 17% of the mean, though only 1% in absolute saturation %. Unlike during the baseline night, the greatest desaturations occurred at the same phase as the longest events. Desaturation was greatest during the biological night when events were longest (95° ∼02:50) and least during the day (210° ∼10:30).
Figure 6.
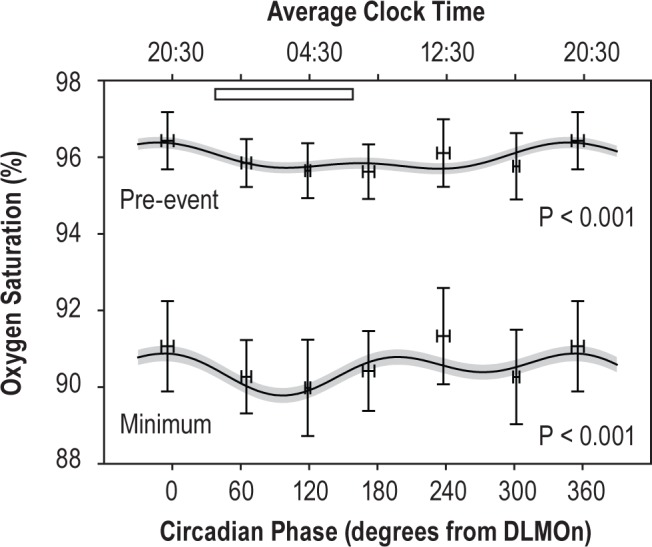
Blood oxygen saturation during forced desynchrony. The pre-event and event-related minimum saturations are shown. Data only include events in N1 and N2 sleep. Plotting conventions as in Figure 5. DLMOn, dim light melatonin onset.
Apnea-Hypopnea Index
To test whether respiratory event incidence varied with circadian phase, AHI was assessed for each sleep period during the forced desynchrony. Each sleep period was assigned a circadian phase according to its midpoint. Across all sleep stages, there was a significant effect of circadian phase on AHI (F1,56 = 4.2, P < 0.05) (Figure 7). This was due to a significant circadian rhythm in the NREM AHI (F1,55 = 5.8, P < 0.05; stages 1–3); in contrast, there was no rhythm in REM AHI (F1,37 = 2.3, P > 0.1). Neither time-into-experiment nor any interaction was significant. NREM AHI had a peak to trough amplitude of 19.3 events/h (61% of mean), with an afternoon peak (315° ∼17:30), and a nighttime trough (70° ∼01:20).
Figure 7.
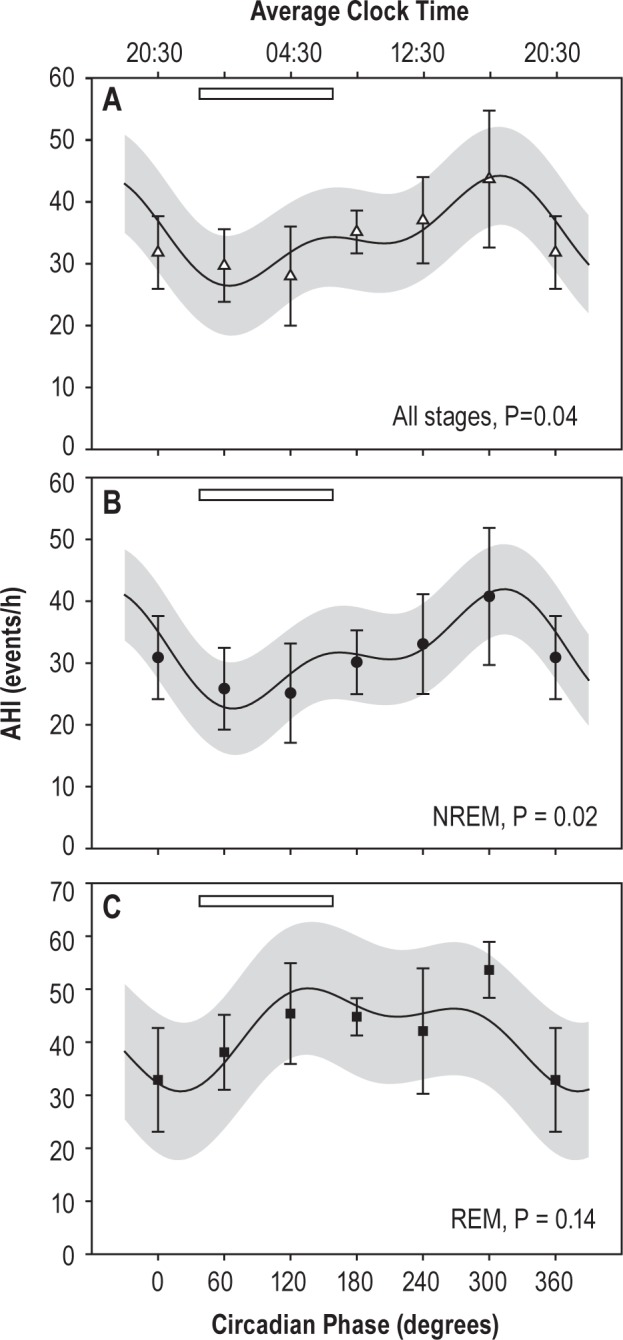
AHI is highest in NREM during the biological day. The circadian pattern and means of the observed AHI are plotted for all sleep stages (A), NREM sleep alone (B), and REM sleep alone (C). There was no significant rhythm in REM AHI. Open bar indicates habitual sleep time. Significance of the fundamental circadian frequency in the fit is shown for each panel.
DISCUSSION
In this forced desynchrony protocol, we observed rhythms in NREM respiratory event duration and incidence (AHI), while controlling experimentally for time in bed, wake-time behaviors, posture, and food intake. These data suggest that the circadian system plays an important role in apnea/hypopnea initiation and termination mechanisms. At a circadian phase corresponding to early morning, apnea and hypopnea durations were longest and AHI was low. In contrast, at circadian phases corresponding to late afternoon and early evening, event durations were shortest and AHI was high.
To what degree does the circadian rhythm explain normal nocturnal increases in NREM event duration? On the first control night from 23:30 to 06:30, event durations increased on average by 5.1 sec in the binned observations or by 6.3 sec in the model fit (Figure 3). The peak-to-trough circadian amplitude was 3.9 sec (Figure 5): of this, 3.5 sec mapped to the same 23:30–06:30 interval (45–150°). Thus, the endogenous circadian effect explains much of the increase observed during normal nights, as is evident in Figure 5 with these effects superimposed. The circadian rhythm, peaking late in the biological night, may explain the early lengthening and later plateau in event durations that we observed in this study, and that others have reported.7,26
Nocturnal changes in duration have been attributed to a number of potential factors; principal among these are: (1) an increase in arousal threshold to respiratory stimuli such as hypoxemia, hypercapnia, and mechanoreceptive cues related to respiratory effort; (2) slowing of metabolism over the night; and (3) factors associated with time in bed and prior apneas including inflammation and edema of the airway.5,7,26,30,31 The influence of the endogenous clock on these factors will be considered in turn.
Apnea duration correlates well with the end-apnea maximum respiratory effort; late in the night, arousal occurs at greater inspiratory pressures, suggesting a higher threshold for arousal.5,7,31 Circadian rhythms of elements of arousal have been documented in vigilance, sleep inertia of cognitive performance and sleep propensity.28,29,32–34 Circadian rhythms in arousability from sleep on the other hand have not been studied. Louder tones are necessary to wake subjects late in the night compared to early in the night, suggesting rhythmicity.35 However, these results may be confounded by the effect of prior time asleep, and so the conclusions could be impacted by homeostatic sleep pressure and dissipation.
Late night apneas are associated with greater hypoxia and hypercapnia.7,31 Nevertheless, chemosensory pathways probably play an indirect role in the night-time changes in duration. Experimentally altering oxygen and carbon dioxide concentrations can change apnea duration, but in these conditions, arousal remains tied closely to measures of effort, e.g., diaphragmatic tension-time index.31 Hypoxia and hypercapnia can increase effort, and these may slow late in the night because of reduced metabolic rate, inferred from the slower desaturation rates during apneas.4 Finally, although the hypercapnic ventila-tory response exhibits a circadian rhythm,36 this would predict gradually increasing responsiveness at the end of the night, opposite what we observed.
For both mechanosensory and chemosensory pathways, it is not known whether the circadian system acts on peripheral receptor sensitivity or central sensitivity to those afferents. The arousal-promoting ascending reticular formation receives sensory input, and is also a target of the brain's clock in the suprachiasmatic nucleus. The locus coeruleus receives projections from the suprachiasmatic nucleus and in turn modulates arousal state,37,38 indicating a potential central site of action.
Our data shed no light on the portion of variation not explained by the circadian clock. Other factors that could be sensitive to time in bed and that could explain longer apneas include airway edema (from snoring vibration and repetitive airway closure) that can blunt airway sensory responsiveness,4 fatigue of the respiratory muscles which could lengthen the time to achieve a threshold4,39 and rostral fluid shift.40 It will be important to delineate how factors that are sensitive to circadian time and to time in bed interact.
We observed a circadian rhythm of AHI suggesting that the airway is more prone to collapse in the biological day. Patency of the airway depends critically on the dynamic balance between activation of respiratory pump muscles (diaphragm and intercostals) and upper airway dilator muscles.41,42 Rhythms in AHI could be caused by circadian rhythms in the dynamic balance of these muscle groups. Such considerations may be of value to those interested in stimulating the upper airway dilator muscles as a therapy for sleep apnea.43
The efficacy of daytime naps for the diagnosis of OSA has been examined in several contexts. The specificity and sensitivity of these tests vary with nap duration, predominance of REM-related events, and prior sleep deprivation.8–11,44 In the current protocol, daytime naps had a consistent history of prior wakefulness and food intake: the data show that the circadian system also contributes to day-night differences by elevating NREM AHI during the day. Daytime PSG may therefore be a sensitive test for OSA based on NREM events. Our data suggest that REM AHI does not vary with time of day, but the sparse presentation of REM during the day, itself attributable to the circadian clock, continues to hinder daytime estimates of REM-dependent OSA in this and other protocols.
Strengths and Weaknesses of Our Protocol
Strengths of the current protocol are the use of a highly controlled forced desynchrony protocol to assess circadian rhythms that are independent of 24-h cycles of waking/sleeping and of eating/fasting. To minimize positional effects on AHI, we controlled body position during sleep. Though some small changes in neck position are unavoidable, these are unlikely to influence the primary duration results, because event lengthening is independent of position.45 Weaknesses of the current protocol include the small sample size, inability to separately measure the 8-h evolution of respiratory event duration at all circadian phases, lack of esophageal or intrathoracic pressure measures to gauge inspiratory effort, and heterogeneous presentation of apneas and hypopneas. The confirmation of event duration lengthening across the night reported by others nevertheless provides reassurance that we were measuring biologically and clinically relevant parameters. The experiment was not powered to detect differences between those with and without OSA. The lack of group differences that we report for sleep parameters are therefore susceptible to type II error. They are presented here as secondary measures to illustrate the general similarity in sleep in forced desynchrony conditions between the groups. It will be important to replicate and confirm these results in future studies.
Clinical Perspective
Longer duration events have recently been linked to mortality in a case-control study.46 Duration also has effects on the event-associated arterial blood pressure rise.47 We are currently assessing the effects of apnea duration using the National Sleep Research Resource.48,49 Apnea duration holds important information regarding arousability and individual event severity, both of which may have health consequences.
CONCLUSIONS
As we learn more about the mechanisms that modify initiation and termination of respiratory events, novel therapeutic targets for sleep-related breathing disorders, such as the circadian system, may emerge. Our findings suggest an important contribution of the circadian system in determining the duration of apneas and hypopneas, potentially accounting for over half of the lengthening observed during normal overnight sleeps. Circadian oscillation is observed in all tissues throughout the body, and these influence organ function throughout the day. Our data show that circadian processes, both central and peripheral, must be considered in the mechanisms underlying the initiation and termination of apneas, and thus their severity.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest. Financial Support: K24-HL76446, R21-HL092407, T32-HL007901, 1UL1-RR025758, 8UL1-TR000170.
ACKNOWLEDGMENTS
The authors thank Pamela N. DeYoung and Natalie Morin for expert sleep scoring, Frank A.J.L. Scheer, Atul Malhotra, Christopher J. Morris, Scott Beckett, Joanna Garcia, Jessica Yang, and the Center for Clinical Investigation staff for expert advice and assistance, and the Harvard Catalyst Clinical and Translational Science Center for support.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- NREM
non-rapid eye movements
- CPAP
continuous positive airway pressure
- DLMOn
dim light melatonin onset
- NOSA
nonorthagonal spectral analysis
- OSA
obstructive sleep apnea
- PSG
polysomnography
- REM
rapid eye movements
- TST
total sleep time
REFERENCES
- 1.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health) Circulation. 2008;118:1080–111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 2.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 3.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd edition. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 4.Charbonneau M, Marin JM, Olha A, Kimoff RJ, Levy RD, Cosio MG. Changes in obstructive sleep apnea characteristics through the night. Chest. 1994;106:1695–701. doi: 10.1378/chest.106.6.1695. [DOI] [PubMed] [Google Scholar]
- 5.Montserrat JM, Kosmas EN, Cosio MG, Kimoff RJ. Mechanism of apnea lengthening across the night in obstructive sleep apnea. Am J Respir Crit Care Med. 1996;154:988–93. doi: 10.1164/ajrccm.154.4.8887596. [DOI] [PubMed] [Google Scholar]
- 6.Lavie P, Halperin E, Zomer J, Alroy G. Across-night lengthening of sleep apneic episodes. Sleep. 1981;4:279–82. doi: 10.1093/sleep/4.3.279. [DOI] [PubMed] [Google Scholar]
- 7.Sforza E, Krieger J, Petiau C. Nocturnal evolution of respiratory effort in obstructive sleep apnoea syndrome: influence on arousal threshold. Eur Respir J. 1998;12:1257–63. doi: 10.1183/09031936.98.12061257. [DOI] [PubMed] [Google Scholar]
- 8.Series F, Cormier Y, La Forge J. Validity of diurnal sleep recording in the diagnosis of sleep apnea syndrome. Am Rev Respir Dis. 1991;143:947–9. doi: 10.1164/ajrccm/143.5_Pt_1.947. [DOI] [PubMed] [Google Scholar]
- 9.Miyata S, Noda A, Nakata S, et al. Daytime polysomnography for early diagnosis and treatment of patients with suspected sleep-disordered breathing. Sleep Breath. 2007;11:109–15. doi: 10.1007/s11325-006-0091-9. [DOI] [PubMed] [Google Scholar]
- 10.Persson HE, Svanborg E. Sleep deprivation worsens obstructive sleep apnea. Comparison between diurnal and nocturnal polysomnography. Chest. 1996;109:645–50. doi: 10.1378/chest.109.3.645. [DOI] [PubMed] [Google Scholar]
- 11.Van Keimpema AR, Rutgers SR, Strijers RL. The value of one hour daytime sleep recording in the diagnosis of the sleep apnoea syndrome. J Sleep Res. 1993;2:257–9. doi: 10.1111/j.1365-2869.1993.tb00097.x. [DOI] [PubMed] [Google Scholar]
- 12.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–62. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–49. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 14.Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812–21. doi: 10.1097/ALN.0b013e31816d83e4. [DOI] [PubMed] [Google Scholar]
- 15.Pavlova MK, Shea SA, Scheer FA, Bromfield EB. Is there a circadian variation of epileptiform abnormalities in idiopathic generalized epilepsy? Epilepsy Behav. 2009;16:461–7. doi: 10.1016/j.yebeh.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson MP, Duffy JF, Dijk DJ, Ronda JM, Dyal CM, Czeisler CA. Short-term memory, alertness and performance: a reappraisal of their relationship to body temperature. J Sleep Res. 1992;1:24–9. doi: 10.1111/j.1365-2869.1992.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 17.St Hilaire MA, Gooley JJ, Khalsa SB, Kronauer RE, Czeisler CA, Lockley SW. Human phase response curve to a 1 h pulse of bright white light. J Physiol. 2012;590:3035–45. doi: 10.1113/jphysiol.2012.227892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duffy JF, Cain SW, Chang AM, et al. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci U S A. 2011;108(Suppl 3):15602–8. doi: 10.1073/pnas.1010666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Kesteren ER, van Maanen JP, Hilgevoord AA, Laman DM, de Vries N. Quantitative effects of trunk and head position on the apnea hypopnea index in obstructive sleep apnea. Sleep. 2011;34:1075–81. doi: 10.5665/SLEEP.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iber C, Ancoli-Israel S, Chesson A, Quan S for the American Academy of Sleep Medicine. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 21.Klerman EB, Gershengorn HB, Duffy JF, Kronauer RE. Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythms. 2002;17:181–93. doi: 10.1177/074873002129002474. [DOI] [PubMed] [Google Scholar]
- 22.Czeisler CA, Duffy JF, Shanahan TL, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–81. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 23.Lewy AJ, Sack RL, Miller LS, Hoban TM. Antidepressant and circadian phase-shifting effects of light. Science. 1987;235:352–4. doi: 10.1126/science.3798117. [DOI] [PubMed] [Google Scholar]
- 24.Lewy AJ, Sack RL. The dim light melatonin onset as a marker for circadian phase position. Chronobiol Int. 1989;6:93–102. doi: 10.3109/07420528909059144. [DOI] [PubMed] [Google Scholar]
- 25.Hu K, Scheer FA, Laker M, Smales C, Shea SA. Endogenous circadian rhythm in vasovagal response to head-up tilt. Circulation. 2011;123:961–70. doi: 10.1161/CIRCULATIONAHA.110.943019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sforza E, Krieger J, Petiau C. Arousal threshold to respiratory stimuli in OSA patients: evidence for a sleep-dependent temporal rhythm. Sleep. 1999;22:69–75. [PubMed] [Google Scholar]
- 27.Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15:3526–38. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dijk DJ, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci Lett. 1994;166:63–8. doi: 10.1016/0304-3940(94)90841-9. [DOI] [PubMed] [Google Scholar]
- 29.Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am J Physiol. 1999;277:R1152–63. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]
- 30.Cala SJ, Sliwinski P, Cosio MG, Kimoff RJ. Effect of topical upper airway anesthesia on apnea duration through the night in obstructive sleep apnea. J Appl Physiol. 1996;81:2618–26. doi: 10.1152/jappl.1996.81.6.2618. [DOI] [PubMed] [Google Scholar]
- 31.Kimoff RJ, Cheong TH, Olha AE, et al. Mechanisms of apnea termination in obstructive sleep apnea. Role of chemoreceptor and mechanoreceptor stimuli. Am J Respir Crit Care Med. 1994;149:707–14. doi: 10.1164/ajrccm.149.3.8118640. [DOI] [PubMed] [Google Scholar]
- 32.Carskadon MA, Dement WC. Multiple sleep latency tests during the constant routine. Sleep. 1992;15:396–9. doi: 10.1093/sleep/15.5.396. [DOI] [PubMed] [Google Scholar]
- 33.Darwent D, Ferguson SA, Sargent C, et al. Contribution of core body temperature, prior wake time, and sleep stages to cognitive throughput performance during forced desynchrony. Chronobiol Int. 2010;27:898–910. doi: 10.3109/07420528.2010.488621. [DOI] [PubMed] [Google Scholar]
- 34.Scheer FA, Shea TJ, Hilton MF, Shea SA. An endogenous circadian rhythm in sleep inertia results in greatest cognitive impairment upon awakening during the biological night. J Biol Rhythms. 2008;23:353–61. doi: 10.1177/0748730408318081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lammers WJ, Badia P, Hughes R, Harsh J. Temperature, time-of-night of testing, and responsiveness to stimuli presented while sleeping. Psychophysiology. 1991;28:463–7. doi: 10.1111/j.1469-8986.1991.tb00732.x. [DOI] [PubMed] [Google Scholar]
- 36.Spengler CM, Czeisler CA, Shea SA. An endogenous circadian rhythm of respiratory control in humans. J Physiol. 2000;526(Pt 3):683–94. doi: 10.1111/j.1469-7793.2000.00683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aston-Jones G, Chen S, Zhu Y, Oshinsky ML. A neural circuit for circadian regulation of arousal. Nat Neurosci. 2001;4:732–8. doi: 10.1038/89522. [DOI] [PubMed] [Google Scholar]
- 38.Carter ME, Yizhar O, Chikahisa S, et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13:1526–33. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Griggs GA, Findley LJ, Suratt PM, Esau SA, Wilhoit SC, Rochester DF. Prolonged relaxation rate of inspiratory muscles in patients with sleep apnea. Am Rev Respir Dis. 1989;140:706–10. doi: 10.1164/ajrccm/140.3.706. [DOI] [PubMed] [Google Scholar]
- 40.White LH, Bradley TD. Role of nocturnal rostral fluid shift in the pathogenesis of obstructive and central sleep apnoea. J Physiol. 2013;591:1179–93. doi: 10.1113/jphysiol.2012.245159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akahoshi T, White DP, Edwards JK, Beauregard J, Shea SA. Phasic mechanoreceptor stimuli can induce phasic activation of upper airway muscles in humans. J Physiol. 2001;531:677–91. doi: 10.1111/j.1469-7793.2001.0677h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strohl KP, Hensley MJ, Hallett M, Saunders NA, Ingram RH., Jr Activation of upper airway muscles before onset of inspiration in normal humans. J Appl Physiol Respir Environ Exerc Physiol. 1980;49:638–42. doi: 10.1152/jappl.1980.49.4.638. [DOI] [PubMed] [Google Scholar]
- 43.Strollo PJ, Jr., Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370:139–49. doi: 10.1056/NEJMoa1308659. [DOI] [PubMed] [Google Scholar]
- 44.Sergi M, Rizzi M, Greco M, et al. Validity of diurnal sleep recording performed by an ambulatory device in the diagnosis of obstructive sleep apnoea. Respir Med. 1998;92:216–20. doi: 10.1016/s0954-6111(98)90098-1. [DOI] [PubMed] [Google Scholar]
- 45.Oksenberg A, Khamaysi I, Silverberg DS. Apnoea characteristics across the night in severe obstructive sleep apnoea: influence of body posture. Eur Respir J. 2001;18:340–6. doi: 10.1183/09031936.01.00038101. [DOI] [PubMed] [Google Scholar]
- 46.Muraja-Murro A, Kulkas A, Hiltunen M, et al. The severity of individual obstruction events is related to increased mortality rate in severe obstructive sleep apnea. J Sleep Res. 2013;22:663–9. doi: 10.1111/jsr.12070. [DOI] [PubMed] [Google Scholar]
- 47.Alex R, Manchikatla S, Machiraju K, et al. Effect of apnea duration on apnea induced variations in cerebral blood flow velocity and arterial blood pressure. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:270–3. doi: 10.1109/EMBC.2014.6943581. [DOI] [PubMed] [Google Scholar]
- 48.Redline S, et al. “Sleep Heart Health Study.”. National Sleep Research Resource. Web. [cited 2014]; Available from: http://sleepdata.org/datasets/shhs.
- 49.Redline S, Sanders MH, Lind BK, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21:759–67. [PubMed] [Google Scholar]