Abstract
Study Objectives:
Insomnia is a debilitating comorbidity of chronic pain. This study evaluated the effect of nonpharmacological sleep treatments on patient-reported sleep quality, pain, and well-being in people with long-term cancer and non-cancer (e.g., back pain, arthritis, fibromyalgia) pain conditions.
Design:
We systematically searched Cochrane CENTRAL, MEDLINE, Embase, and PsychINFO for relevant studies. Search period was set to inception of these databases to March 2014. Studies were included if they were: original randomized controlled trials (RCTs); testing a nonpharmacological intervention; that targets sleep; in adults; with painful health conditions; that has a control group; includes a measure of sleep quality; and at least one other health and well-being outcome.
Measurement and Findings:
Means and standard deviations of sleep quality, pain, fatigue, depression, anxiety, physical and psychological functioning were extracted for the sleep treatment and control groups at baseline, posttreatment and final follow-up. Methodological details concerning the treatment, participants, and study design were abstracted to guide heterogeneity and subgroup analyses. Eleven RCTs involving 1,066 participants (mean age 45–61 years) met the criteria for the meta-analysis. There was no systematic evidence of publication bias. Nonpharmacological sleep treatments in chronic pain patients were associated with a large improvement in sleep quality (standardized mean difference = 0.78, 95% Confidence Interval [0.42, 1.13]; P < 0.001), small reduction in pain (0.18 [0, 0.36] P < 0.05), and moderate improvement in fatigue (0.38 [0.08, 0.69]; P < 0.01) at posttreatment. The effects on sleep quality and fatigue were maintained at follow-up (up to 1 year) when a moderate reduction in depression (0.31, [0.09, 0.53]; P < 0.01) was also observed. Both cancer and non-cancer pain patients benefited from nonpharmacological sleep treatments. Face-to-face treatments achieved better outcomes than those delivered over the phone/internet.
Conclusions:
Although the body of evidence was small, nonpharmacological sleep interventions may represent a fruitful avenue for optimizing treatment outcomes in patients with chronic pain.
Registration:
PROSPERO registration: CRD42013004131.
Citation:
Tang NK, Lereya T, Boulton H, Miller MA, Wolke D, Cappuccio FP. Nonpharmacological treatments of insomnia for long-term painful conditions: a systematic review and meta-analysis of patient-reported outcomes in randomized controlled trials. SLEEP 2015;38(11):1751–1764.
Keywords: insomnia, chronic pain, meta-analysis, nonpharmacological treatment, health, sleep, systematic review
INTRODUCTION
Poor sleep is a potential cause of ill-health. Self-reported short and long habitual sleep duration, difficulties initiating or maintaining sleep, non-restorative sleep, and the use of hypnotic drugs are significant predictors of obesity, diabetes, widespread pain, stroke, coronary heart disease (CHD), and even mortality.1–6 Insomnia also increases the risk of subsequent onset of depression, anxiety disorders and substance misuse in otherwise healthy individuals.7–10 These findings, assuming they reflect causality, highlight sleep as a plausible therapeutic target for preventing a range of long-term conditions.
Insomnia is a major problem to many people living with chronic pain that lasts longer than 3–6 months.11 Chronic pain has been ranked the top cause of quality-adjusted life-year loss in primary care, ahead of recognized sources of burden of disease such as depression, anxiety disorders, diabetes, respiratory conditions, high blood pressure and CHD.12 It is estimated that 50% to 90% of chronic pain patients report insomnia of a severity that warrants clinical attention.13–16 In experimental studies, the introduction of sleep disruption can trigger pro-inflammatory responses, reduce endogenous pain inhibitory control, amplify pain experience, lower pain tolerance, and increase somatic symptoms.17–20 These findings are in line with the idea of a reciprocal, rather than unidirectional, relationship between sleep and pain.21–25 Recently, there has been a surge of interest in applying established nonpharmacological sleep interventions to treat chronic pain patients with comorbid insomnia. At odds with the hypothesized reciprocal relationship, results have been inconsistent. While some studies observed no change in pain post-intervention,26–29 others found a significant reduction in pain intensity after sleep improvement.30–32 It remains unclear whether better sleep could lead to less pain and better health and well-being.
The current meta-analysis aimed to evaluate the efficacy of nonpharmacological sleep interventions for people with long-term cancer and non-cancer painful conditions. We were interested in the effect of these interventions on sleep and their broader impact on health and well-being as indicated by pain, fatigue, depression, anxiety, physical and psychosocial functioning. We restricted our evaluation to nonpharmacological sleep interventions only, because pharmacological sleep interventions were not recommended for the protracted type of insomnia experienced by patients with chronic pain.33 Based on the similarities in presentation and underpinning mechanisms between primary and pain-related insomnia,34,35 it was hypothesized that nonpharmacological sleep interventions would have a beneficial impact on sleep. However, the meta-analysis was exploratory with regards to the effect of these interventions on the aforementioned health and well-being outcomes.
METHODS
Data Sources and Searches
Our data sources were original randomized controlled trials (RCTs) testing the utility of nonpharmacological treatments for insomnia in adults with long-term painful conditions. To identify these, we performed systematic searches in 4 electronic databases; Cochrane CENTRAL, MEDLINE, Embase, and PsychINFO. The search duration was between the inception of each database and March 2014. No language restriction was applied. Abstracts/articles written in foreign languages were translated for review.
Search terms used (Appendix, supplemental material) were decided a priori by the review team after consulting published systematic reviews/meta-analyses36,37 and conducting a series of pilot searches. A methodological filter (e.g., random* in Trials) was used in combination with search keywords that reflected the treatment approach (e.g., nonpharma*, psychologic*), treatment content (e.g., sleep, insomnia) and population (e.g., chronic next pain*, cancer, musculo*, arthritis*) of interest. We took a transdiagnostic approach to amalgamate a range of malignant and non-malignant conditions presented with chronic pain.38 This we hoped would reflect the increasing application of nonpharmacological sleep interventions beyond primary insomnia39 and offer an opportunity to compare the effectiveness of these treatments between diagnostic subgroups.
The searches and subsequent screening were independently carried out by two of the authors (STL and HB). Disagreements between reviewers were resolved via discussion with the review team. Reference lists of included studies and relevant review articles were hand-searched to ensure comprehensive coverage. Gray literature (e.g., conference abstracts and PhD theses) was also consulted to reduce the risk of publication bias.
Study Selection
Figure 1 depicts the searches and screening process. The searches yielded a total of 1,887 records. After 604 duplicates between databases were removed, 1,283 titles and abstracts were screened. In the instance of foreign language, abstracts were translated into English for a judgment to be made.40 Seventy-two articles were selected for full-text screening, which was aided with a checklist developed by NKYT and MAM according to the inclusion criteria: original RCT; testing a nonpharmacological intervention; that targets sleep; in adults (aged 18 years); with painful health conditions (e.g., musculoskeletal pain, arthritis, fibromyalgia, headache, cancer); that has a control group; includes an outcome measure of sleep; and at least one other health and well-being outcome.
Figure 1.
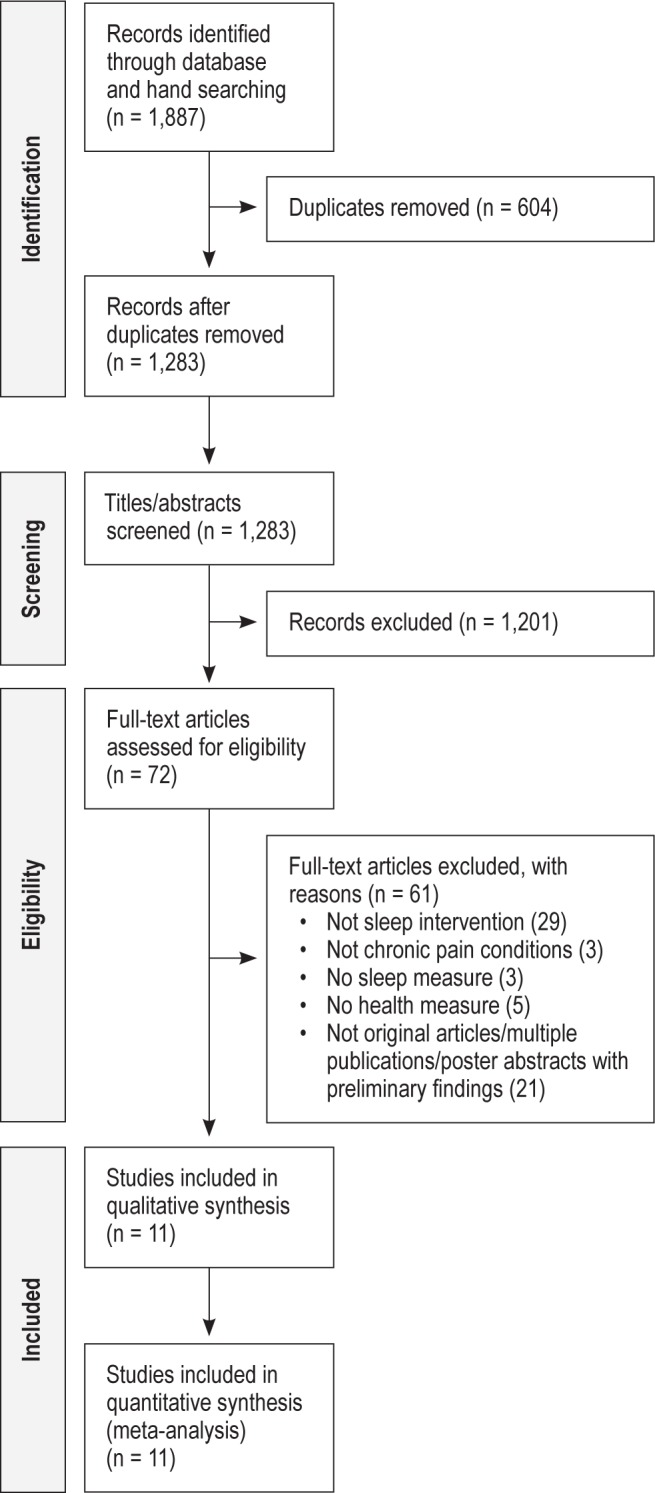
Flow diagram.
A broad definition of nonpharmacological treatments for insomnia was adopted. These treatments might include the sole or combined use of components of cognitive behavior therapy for insomnia (CBT-I). Common components of CBT-I include psychoeducation, sleep hygiene, stimulus control therapy, sleep restriction therapy, sleep scheduling, relaxation, paradoxical intention, imagery, and cognitive therapy.33,41 Studies testing the utility of physiotherapies, exercise, yoga, qigong, mindfulness meditation, massage, acupuncture, hormone therapy, and hypnosis were included if the interventions being evaluated were designed to address insomnia specifically. If multiple publications were available for the same trial, only the article reporting the primary analysis with the most relevant information to the current meta-analysis was included.32,42–44 We did not automatically exclude non-inferiority trials from the meta-analysis if nonpharmacological sleep interventions were tested as the standard treatment control against which a novel treatment demonstrated non-inferiority.45
Following the full-text screening, 61 studies did not meet criteria for inclusion and 11 studies were selected for data extraction. High inter-rater agreement was noted for both the title/ abstract (κ = 0.90, P < 0.001) and the full-text screens (κ = 1.00, P < 0.001).
Data Extraction and Quality Assessment
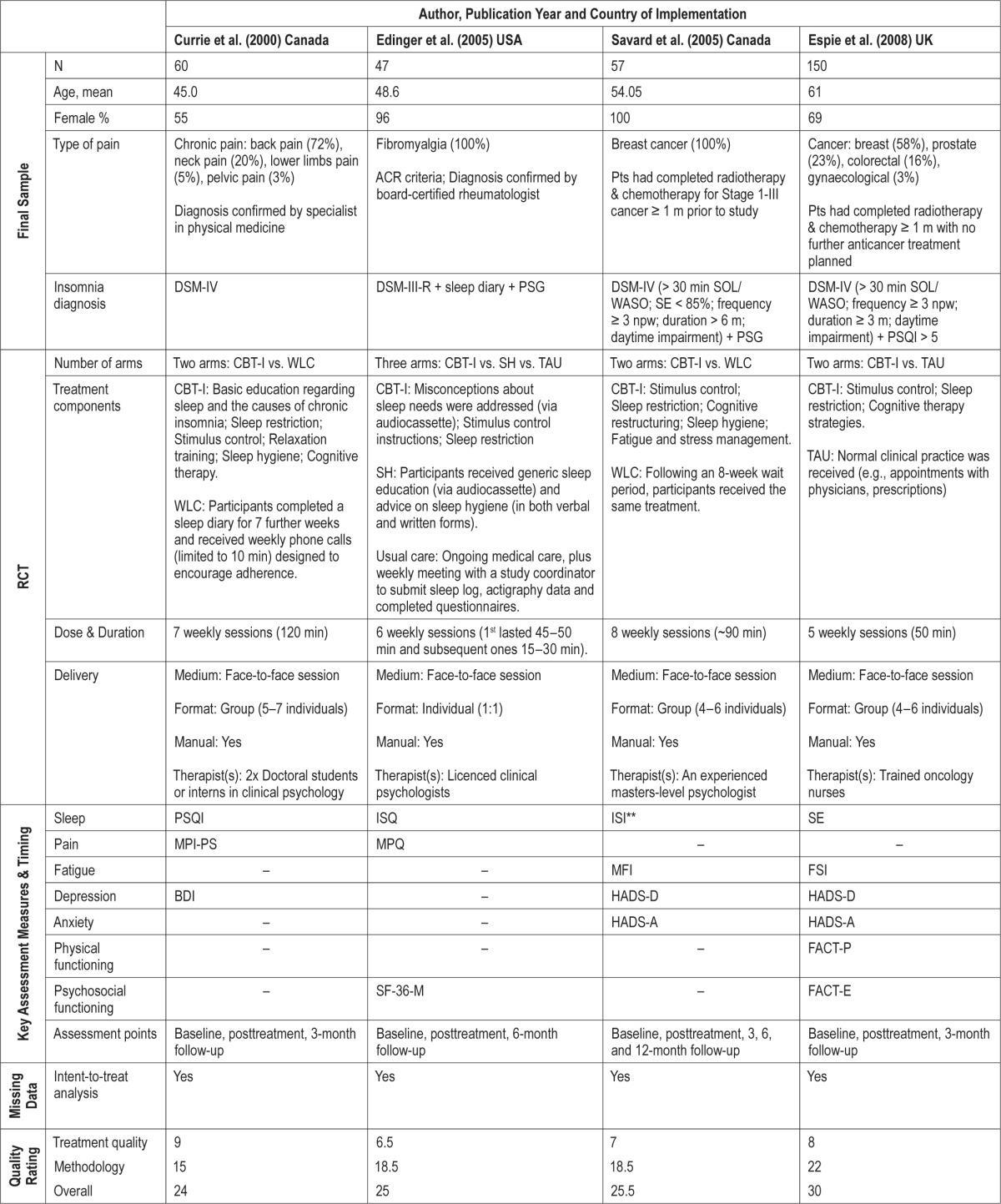
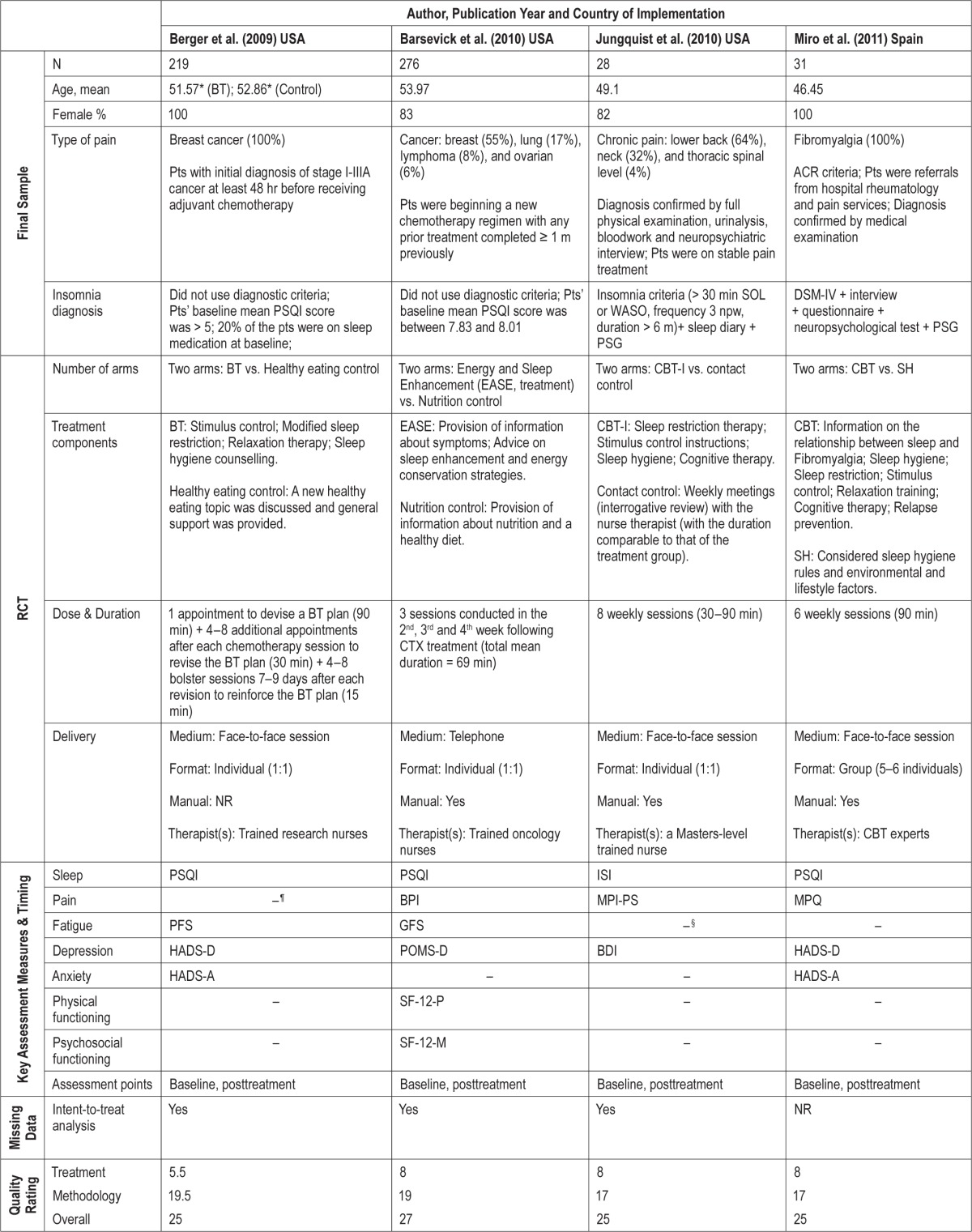
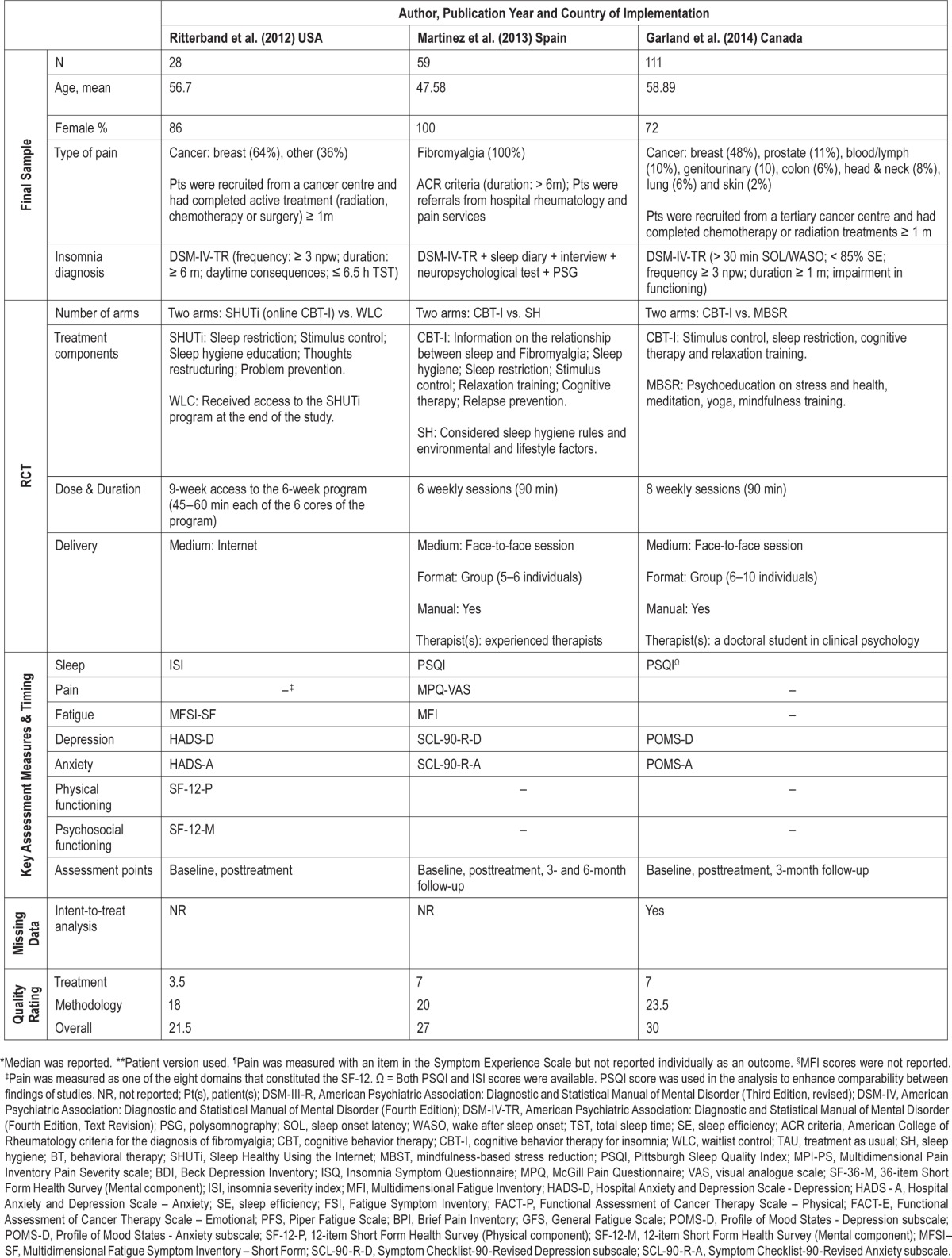
Data extraction was performed in duplicate to counteract human errors and individual biases (HB & STL). In addition to extracting relevant data on sleep, health, and well-being outcomes, information was gathered from individual studies to compose a study characteristics table (Table 1) which incorporated methodological details about the design of the trials (sample size, participants, number of arms), treatments tested (content, duration, method of delivery), outcome measures used, whether intention-to-treat analysis was applied, and their quality ratings. When data were not available in the published report, authors were contacted to provide information. The data extraction sheets were checked by the review team and differences between reviewers were resolved by discussion.
Table 1.
Study characteristics.
For the meta-analysis, means and standard deviations of relevant outcome measures were extracted for the sleep treatment and control group at baseline, posttreatment (i.e., immediately on completion of the sleep/control intervention), and the final follow-up (due to variability in assessment timing). For studies that used multiple measures to assess the same outcome, the most prevalent measure used across the final 11 studies was used to maximize comparability of the findings.
We assessed the risk of bias quantitatively using the quality rating scale developed by Yates and colleagues46 and qualitatively following the Cochrane guidance.47 The quality rating scale was designed to assess RCTs of nonpharmacological treatment for the quality of the treatment and the design and reporting of the trials. The scale has shown face, content, and construct validity, and good inter-rater reliability.46 The overall score of the scale ranges from 0 to 35 with higher scores indicating better quality. In the validation studies involving 17 RCTs of nonpharmacological treatments for chronic pain being assessed by two expert reviewers, the mean total scores were 22.70 (SD 1.95) for “excellent,” 18.71 (SD 2.25) for “average” and 12.10 (SD 3.17) for “poor” trials.46 Of the 5 suggested Cochrane “risk of bias” categories,47 we included random sequence generation (selection bias), allocation concealment (selection bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), and selective reporting (reporting bias). We excluded the option of “blinding participants and personnel” because, during the delivery of most nonpharmacological treatments, neither therapists nor patients can be (sufficiently) blinded to the type of treatment they deliver or receive.
Data Synthesis and Analysis
Comparisons were made between the “sleep treatment” and “control” groups with reference to the change from baseline at posttreatment and at follow-up for each of the outcome measures. Changes were calculated such that a positive difference represents an improvement, a negative difference a deterioration. Since different measures were used to assess the same outcome in different studies, the change scores were transformed into z scores to reduce heterogeneity and enhance comparability using the standard formula:
 |
where x = pretreatment − posttreatment change, x = mean change of all included studies, and s = pooled standard deviation. Standardized mean differences (SMD) between the effect of treatment and control were then estimated using a random effect model.
For each outcome measure, data from all trials were entered into a funnel plot. Asymmetry of the plot was visually examined to detect overt publication bias. None of the analyses demonstrated overt asymmetry that required follow-ups with Egger's regression test. Statistical heterogeneity among the included studies was assessed using the χ2 test and the I2 statistic, along with visual inspection of the forest plot. Comparisons with significant heterogeneity were followed up by a sensitivity analysis in which one study was omitted at a time to identify the possible source of heterogeneity; the study that resulted in the largest drop in heterogeneity was removed. If dropping the first study did not sufficiently reduce heterogeneity to a nonsignificant level, a second study was then removed. Subgroup analyses were also carried out to examine possible sources of heterogeneity attributable to the study characteristics. Two exploratory subgroup analyses were defined a priori to compare the effect of sleep treatment between those with cancer pain and those with non-cancer pain patients, and between those with an intervention delivered face-to-face or using the phone or internet. The former subgroup analysis should provide insights into the applicability of nonpharmacological interventions for sleep across patients with malignant and non-malignant pain, while the latter should show if the effect of nonpharmacological sleep interventions varied by treatment delivery method. The diverse components of the treatment packages precluded any subgroup analysis by type of treatment for the identification of active treatment ingredients.
All statistical analyses were performed using RevMan 5.
RESULTS
Characteristics of the Included RCTs
A total of 11 RCTs involving 1,066 participants (female: 55% to 100%; mean age: 45–61 years) from 4 different countries (Canada = 3, Spain = 2, UK = 1, US = 5) provided data for the meta-analysis (Table 1).
Five of the RCTs tested the effect of nonpharmacological sleep treatments in patients with non-cancer chronic pain; 2 used a mixed variety of chronic pain patients (diagnosis confirmed by physicians),26,28 while the other 3 involved fibromyalgia patients meeting the American College of Rheumatology criteria48 only.27,29,49 Six of the RCTs tested the effect in cancer survivors; 2 of which comprised 100% breast cancer survivors,42,50 while 4 involved survivors of different types of cancer (e.g., lung, lymphoma ovarian, prostate, colorectal and gynecological) in addition to a majority of breast cancer patients.45,51–53 Cancer patients in most of these studies were in remission having completed active treatments (chemotherapy, radiation treatment, or surgery) at least one month prior to enrolling in the study, except in two studies where patients were enrolled as they began a new regimen of chemotherapy.50,51
All but two studies50,51 screened their participants' presenting sleep problems with reference to diagnostic criteria for insomnia disorder. The DSM diagnostic criteria (3rd edition, 4th edition, 4th edition text-revision54–56) were most commonly used as the core inclusion criteria, but there were variations between studies in terms of their specific frequency (e.g., ≥ 3 nights per week), severity (e.g., daytime impairment; Pittsburgh Sleep Quality Index Global Score > 5), and duration (e.g., ≥ 1 month, ≥ 3 months, or ≥ 6 months) cutoffs. The two studies that did not screen patients with reference to diagnostic criteria considered fatigue and poor sleep as known consequences in all phases of chemotherapy. In both of these studies, the mean Pittsburgh Sleep Quality Index Global Score at baseline were > 5, indicating the presence of significant sleep difficulties in these patients.57
As part of the assessment of clinical insomnia in accordance to the DSM diagnostic criteria,26–29,42,45,49,52,53 patients with a sleep disorder (e.g., sleep apnea) or a psychiatric Axis I disorder (e.g., psychosis, severe major depression, substance abuse disorder) that could better explain the insomnia were excluded. Some studies also specifically excluded patients who were receiving psychological treatment for insomnia, stress, anxiety, depression, or coping with pain and/or cancer outside of the RCT.26,29,42,45,49,51,53 Subsequently, samples of patients in the current meta-analysis presented moderate levels of anxiety and depression across studies, with most samples displaying sub-threshold symptoms,26,28,42,49–51 and a couple of samples exhibiting symptoms reaching or just crossing the suggested clinical thresholds adopted by validated questionnaires.29,52,53
The sleep treatments tested varied in their content, dose, duration, and delivery method. In terms of content, most treatment packages incorporated at least 1 component of CBT-I.33,41 Psychoeducation, sleep hygiene, stimulus control, sleep restriction, cognitive therapy, and relaxation were the most frequently used components. The treatments also differed in their dose and duration, with some offering just 3 telephone intervention sessions totalling an average of 69 minutes over 60 days51 and some offering 7 weekly sessions of 120-minute intervention.26 Regarding delivery method, most sleep treatments tested adopted a face-to-face approach, except 2 that delivered the intervention using the phone51 or internet.53 Of the 9 studies that involved face-to-face contact with health care professionals, 3 delivered the treatment individually,27,28,50 while 6 offered the treatment in groups.26,29,42,45,49,52 The control interventions generally consisted of passive control procedures (e.g., waitlist control, treatment as usual), although 4 studies used an active control procedure (e.g., sleep hygiene advice, healthy eating control, nutrition control) and 1 was, in fact, an RCT testing whether mindfulness-based stress reduction was non-inferior to CBT-I.45
All studies had data on sleep and at least 2 other health and well-being outcome measures at baseline and posttreatment. Six of the RCTs also reported follow-up data at 3–12 months (maximum follow-up period: 3 months: n = 3; 6 months: n = 2; 12 months: n = 1).
Risk of Bias in Included Studies
Using the scale of Yates et al.,46 the mean of quality score of the included RCTs was 26.00 (SD 2.58; range: 21.5–30.5), with a mean treatment quality subscore of 7.05 (SD 1.51; range: 3.5–9.0) and a mean method quality subscore of 18.91 (SD 2.36; range: 15.0–23.5) (Table 1).
Our qualitative assessment (Figure S1, supplemental material) identified a high risk of attrition bias in only 2 studies, both of which performed linear mixed model (LMM) analysis under the missing-at-random assumption to reduce biases. However, this approach was compromised when there was a pattern of missing data (due to attrition or differential attrition across groups) that could have been explained by confounding factors not controlled for, e.g., poorer health and patient's treatment preference.45,51
Effects of Interventions
Statistics of all analyses in this section are summarized in Table 2, with forest plots of the key analyses presented in Figure 2 and a panel of funnel plots in Figures S2–S8, supplemental material. To supplement the narrative, statistics of post hoc analyses are provided in the text.
Table 2.
Summary of findings from the main analysis, sensitivity analysis and subgroup analysis by patient type and treatment delivery method.
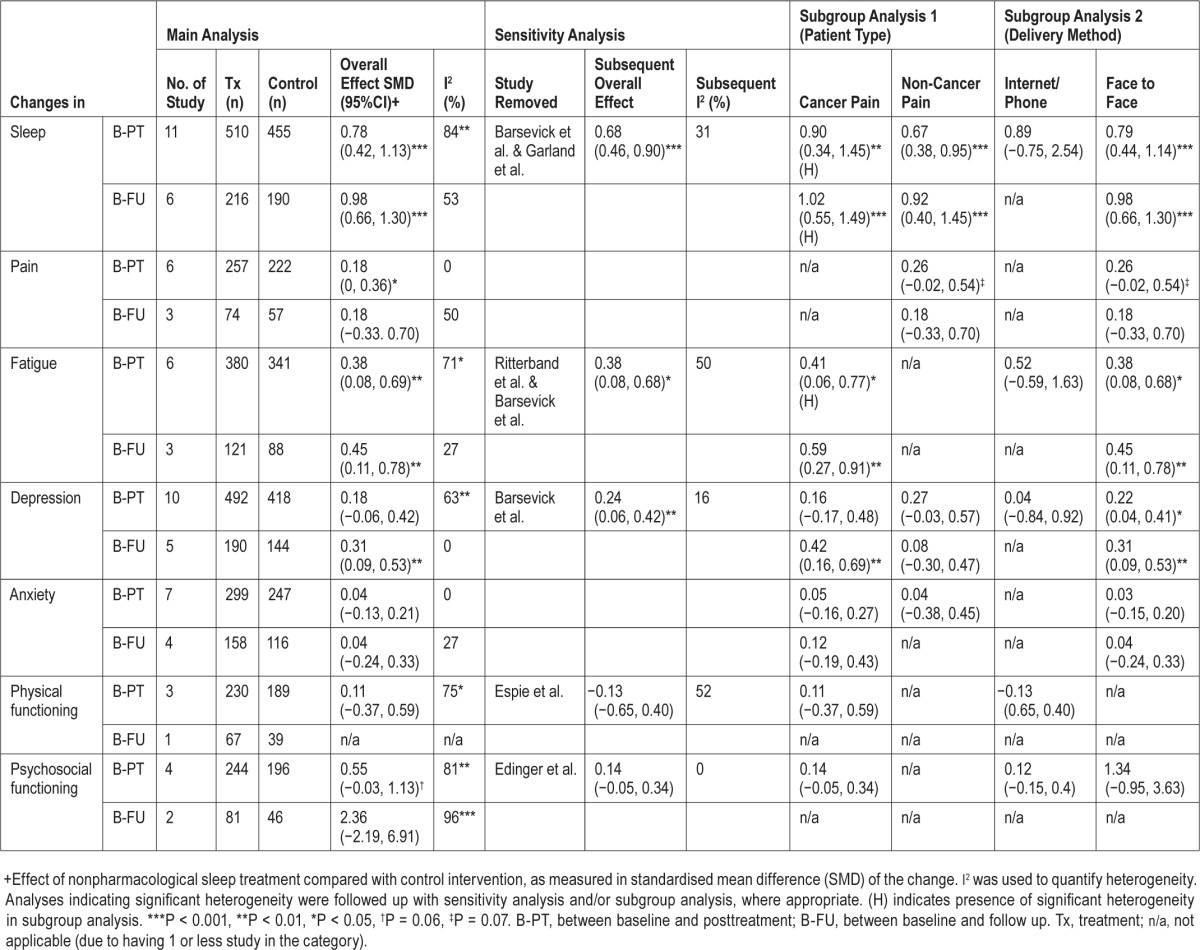
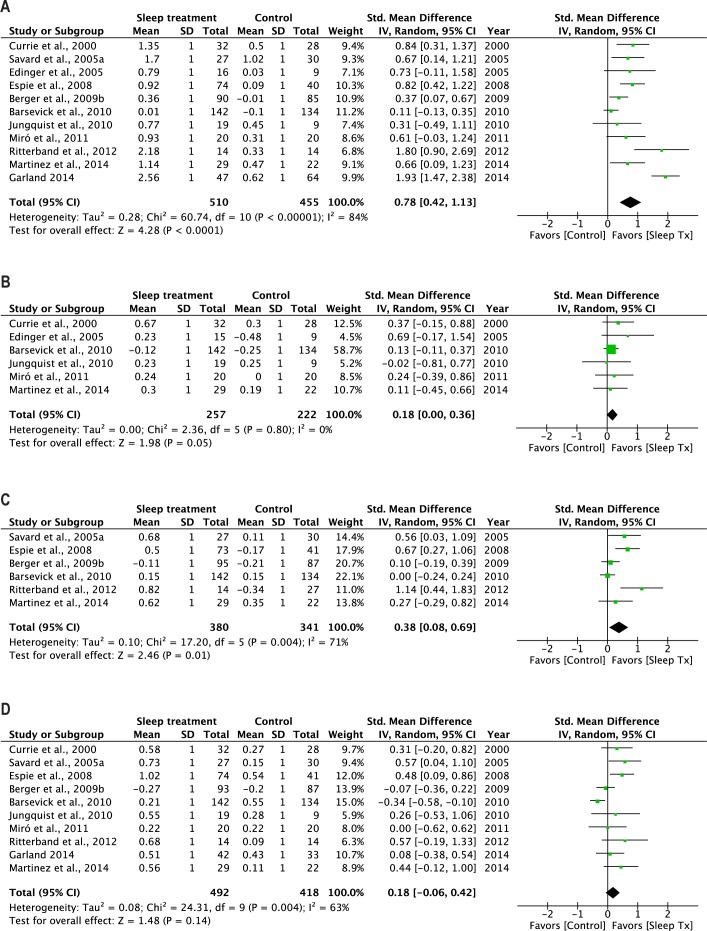
Figure 2.
Forest plots summarizing the posttreatment effects of CBT on (A) sleep, (B) pain, (C) fatigue, and (D) depression.
Sleep Quality
All 11 RCTs measured improvement in sleep at posttreatment and contributed data to the pooled analysis involving 965 patients (Figure 2A). The most prevalent patient-reported outcome measure of sleep quality was the Pittsburgh Sleep Quality Index.57 Sleep treatment was associated with a significant improvement in sleep quality at posttreatment. There was no evidence of publication bias. However, there was significant heterogeneity across the studies. A sensitivity analysis identified 2 studies, Barsevick et al.51 and Garland et al.,45 as potential sources of the heterogeneity. By omitting these studies from the analysis, I2 reduced from 84% to 31% and the overall effect of sleep treatment on sleep quality decreased from 0.78 to 0.68. An effect size of 0.68 suggested that an average responder to nonpharmacological treatments of insomnia would report better sleep quality than approximately 76% in the control group. This interpretation of the effect size assumed normality in the data distribution and described the overlap between the sleep treatment and control group in terms of a comparison of percentiles.
The first subgroup analysis indicated that the effectiveness of sleep treatment was significant for both cancer42,45,50–53 and non-cancer pain patients.26–29,49 The second subgroup analysis indicated that the effectiveness of sleep treatment was significant for studies delivering the treatment face-to-face,26–29,42,45,49,50,52 but not for those that offered the treatment using the phone or internet.51,53
Six studies provided data on sleep quality at follow-up from 406 patients (range of follow-up: 3–12 months).26,27,42,45,49,52 We found a significant overall effect of sleep treatment, which was comparable to the effect achieved by the same 6 studies at posttreatment (SMD = 0.96 [95% CI: 0.53, 1.40]; Z = 4.34, P < 0.001). Heterogeneity between studies was nonsignificant, and there was no evidence of publication bias. Since all four studies included in this analysis delivered the treatment face-to-face, subgroup analysis was only carried out for patient type. Significant sleep treatment effect was found at follow-up for RCTs using both cancer pain patients42,45,52 and non-cancer pain patients.26,27,49
Pain
Six of the RCTs measured improvement in pain at post-treatment and contributed to the pooled analysis involving 479 patients.26–29,49,51 The most prevalent measure of pain was the McGill Pain Questionnaire.58 Sleep treatment was associated with a marginally significant improvement in pain at post-treatment (Figure 2B). The overall effect size was 0.18, which suggested that an average responder to nonpharmacological treatments of insomnia would report less pain than approximately 58% in the control group. There was no evidence of publication bias and heterogeneity across studies.
All but one RCT included in this analysis were conducted with non-cancer pain patients using the face-to-face approach.26–29,49 The effects of both subgroup analyses were nonsignificant (P = 0.07).
Three RCTs reported pain improvement in 131 patients at follow-up (range: 3–6 months).26,27,49 There was no significant improvement in pain. Neither was there evidence of publication bias nor heterogeneity between the two studies.
Fatigue
Six of the RCTs assessed improvement in fatigue at post-treatment and contributed to the pooled analysis involving 721 patients.42,49–53 The most prevalent measure of fatigue was the Multidimensional Fatigue Scale.59 The overall effect of sleep treatment on fatigue was significant (Figure 2C). There was no evidence of publication bias, but significant heterogeneity was detected. A sensitivity analysis revealed that by removing the studies of Ritterband et al.53 and Barsevick et al.,51 I2 dropped from 71% to 50% without attenuating the effect of sleep treatment on fatigue. The overall effect size following the sensitivity analysis was 0.38, which suggested that an average responder to nonpharmacological treatments of insomnia would report less fatigue than approximately 66% in the control group.
All but one RCT included in this analysis were conducted with cancer pain patients; a significant treatment effect on fatigue was observed in this group of patients.42,50–53 By delivery method, a significant effect of sleep treatment on fatigue was found for those studies delivered face-to-face42,49,50,52 but not via the phone or internet.51,53
Three RCTs assessed fatigue in 209 patients at follow-up (range: 3–12 months).42,49,52 The overall effect of sleep treatment was statistically significant and was comparable to the effect achieved by the same 3 studies at posttreatment (SMD = 0.54 [95% CI: 0.27, 0.82]; Z = 3.86, P < 0.001). There was no evidence of publication bias or heterogeneity among the three studies.
Depression
Ten of the RCTs measured depression at posttreatment and contributed to the pooled analysis involving 910 patients.26,28,29,42,45,49–53 The most prevalent measure of depression was the Hospital Anxiety and Depression Scale.60 No significant effect was found for the sleep treatment on depression (Figure 2D). There was no evidence of publication bias, but significant heterogeneity was detected. A sensitivity analysis revealed a drop in I2 from 63% to 16% following the omission of the study of Barsevick et al.51 The overall effect of sleep treatment on depression became statistically significant after the omission. The effect size was 0.24, suggesting that an average responder to nonpharmacological treatments of insomnia would report a lower level of depression than approximately 58% in the control group.
When the studies were analyzed by patient type (cancer42,50–53 vs. non-cancer pain patients26,28,29,45,49), the effect of sleep treatment on depression was nonsignificant for both subgroups. When the studies were analyzed by their delivery method, the effect of sleep treatment on depression was significant for those studies that delivered the treatment face-to-face,26,28,29,42,45,49,50,52 but nonsignificant for those that delivered the treatment using the phone or internet.51,53
Five RCTs measured depression in 334 patients at follow-up (range: 3–12 months).26,42,45,49,52 A significant effect of sleep treatment was found, and the effect was comparable to that achieved by the same 5 studies at posttreatment (SMD = 0.37 [95% CI: 0.16, 0.58]; Z = 3.41, P < 0.001). There was no evidence of publication bias or heterogeneity. All studies delivered the sleep treatment face-to-face. A subgroup analysis by patient type revealed a significant effect of sleep treatment on depression in cancer pain patients,42,45,52 but not in non-cancer pain patients.26,49
Anxiety, Physical Functioning, and Psychosocial Functioning
Sleep treatment effects were not significant for anxiety, physical functioning, and psychosocial functioning. Respectively, the most prevalent measures of anxiety, physical and psychosocial functioning were the Hospital Anxiety and Depression Scale60 and the 12-item Short-Form Health Survey.61
DISCUSSION
Summary of Findings
The current study offers the first meta-analysis of the effect of nonpharmacological sleep interventions in conditions with chronic pain, extending two previous systematic reviews that provided narrative evaluations for the use of CBT-I for cancer62 and non-cancer chronic pain.63 With enhanced statistical power from the bigger aggregate sample size, our findings indicate that these sleep treatments were moderately to strongly effective in improving sleep quality in patients with cancer and non-cancer chronic pain, with a durability of up to 12-month posttreatment. A caveat is that the sleep interventions appeared to be only effective when delivered face-to-face. Future research is required to elucidate how information technology could be usefully applied to effectively deliver these interventions to the masses. A previous meta-analysis that compared the effect of telemedicine against face-to-face patient care on health outcomes found “little evidence of clinical benefits” for patient care delivered using telecommunication technologies.64 Consistently, another recent meta-analysis evaluating the utility of computerized CBT-I for adults with primary insomnia only found a mild to moderate effect over the short term for insomnia.65 The authors concluded that computerized CBT-I, at least for the time being, should be considered as a form of “low-intensity therapy in the stepped care model for insomnia.” That said, the current meta-analysis only captured two early RCTs that used the phone or the internet to deliver sleep interventions. The small sample size might explain the nonsignificant effects in the subgroup analysis. The jury is still out on the capability of newer generations of fully automated and media-rich internet sleep treatments66,67 and on the most cost-effective model of sleep intervention delivery.68
In addition to the positive effect on sleep quality, we were able to detect a mild to moderate therapeutic impact on pain immediately after nonpharmacological sleep treatments. This analgesic effect of improved sleep has not been consistently documented in individual trials, which in isolation were probably underpowered to do so. We were also able to detect a therapeutic effect of improved sleep on fatigue and depression. This observation integrates well with the broader primary insomnia literature, where we saw in a recent trial of CBT-I with older adults significant improvements in fatigue and depression at posttreatment and at 16-month follow-up.69 The temporal association of better sleep with less pain and better mood mirrors the findings from longitudinal studies that identified untreated insomnia as a risk factor of adverse physical and mental health outcomes.1,2,5–8,70–73 Such temporality can be interpreted as evidence for a cause role of better sleep in shaping physical and mental health. It also highlights the value of treating insomnia comorbid with chronic pain early.
The analgesic and mood-enhancing effect of improved sleep may lie with the mechanisms in the central nervous system that are shared for the regulation of arousal, pain sensitivity, mood and other related functions; candidate mechanisms proposed include the serotoninergic74 and mesolimbic dopamine75 systems. Improved sleep may also reduce pain and increase well-being through modulating inflammatory responses. Using the aforementioned trial of CBT-I in older adults with primary insomnia69 as an example again, remission of insomnia was associated with a significant reduction of C-reactive protein (CRP), a clinically relevant marker of inflammation in rheumatic diseases and is prospectively linked to the development of diabetes, hypertension, and cardiovascular disease. More experimental studies are required to confirm these hypothesized mechanisms and explore other physiological and cognitive-behavioral pathways through which improved sleep impacts on pain and mood regulation. A handful of daily process studies with chronic pain patients have revealed that nights of better sleep quality predict less attention to pain, reports of lower pain intensity in the first half of the next day, higher level of physical activity in the second half of the day, and reports of great pain in the evening.31,73,76 Future research may wish to further investigate the role of attention and physical activity in mediating the sleep-pain relationship. Meanwhile, two treatment approaches may be pursued to capitalize on these bi-directional links. First, we could develop hybrid interventions that simultaneously address sleep and pain to optimize the treatment effects. Initial trials of such interventions have produced promising results over no treatment and the standard pain-specific treatment.77–79 Second, it may be beneficial to deploy insomnia treatment as a preventive, health-promoting measure for a range of long-term conditions that do not have an immediate cure. More research with larger sample size and longer-term follow-up is required to determine the speed, feasibility, and cost-effectiveness of these treatment strategies.80,81
Limitations
Although the PRISMA guidelines were closely adhered to when conducting and reporting this meta-analysis,47,82 the breadth and quality of the data pooled for analysis were limited by the quantity, design, and implementation of the original studies. Despite the general absence of methodological and publication biases, the above findings should be viewed with healthy scepticism as only 11 RCTs were included (we are aware of new RCTs being published since the completion of our review, e.g., Smith et al.83), and significant heterogeneity were found in some of the analyses. Heterogeneity was considerably reduced to a nonsignificant level when one or two individual studies were removed during the sensitivity analysis. The source of heterogeneity could be traced to variations in sample populations and treatment delivery method, as illuminated by the subgroup analyses. It could also be traced to the variations in treatment duration, dosage, and content, although most included RCTs named their intervention “CBT-I.” Qualitatively, we note that some trials employed treatment components that have been independently scrutinized for their clinical certainty, e.g., stimulus control, sleep restriction therapy,33,41 while some used methods that await empirical evaluation, e.g., sleep enhancement and energy conservation advice.51 In the current meta-analysis these interventions were evaluated as multi-component treatment packages and random effect model was used for the estimation of treatment effect, which assumed the effect being estimated in different studies were not identical. Future research may find value in evaluating the relative merits of individual components. To this end, single-case experimental designs may be a cost-effective methodology that offers greater flexibility. Of course, within the context of RCTs, more refined subgroup analyses by treatment dosage, duration, and delivery method would also help pinpoint the sources of heterogeneity.
Sleep, pain, health, and well-being are multidimensional constructs. The current meta-analysis focused on patient-reported outcome measures (PROMS), which provided unique insights into the patients' perception of their health and the impact of the treatments they received.84 These are subjective measures susceptible to recall and reporting biases. It would be informative if future trials would diversify the assessment methods with a broader range of subjective and objective outcome measures. However, with the exception of sleep for which polysomnography and actigraphy could provide established objective estimates,85,86 it is debatable what constitutes a valid and reliable objective measurement of pain, fatigue, mood, physical, and psychosocial functioning. Related to this, we saw variations in the selection of patient-reported outcome measures across the included RCTs. We opted to use the most prevalent measure to maximize comparability. The current study did not attempt to evaluate all aspects of sleep experience because there were appreciable differences in the sleep assessment methods in terms of the technology used (sleep diary, actigraphy, or polysomnography), procedure adopted (in lab or at home; number, frequency, duration and timing of assessment) and the reporting approach (specific parameters chosen for reporting; within-group vs. between-group comparisons). We considered the possibility of aggregating data by various sleep parameters but had decided against it for concerns of high heterogeneity and practicality. Future initiatives developing consensus and recommendations for core outcome measures to be used in RCTs of nonpharmacological sleep treatments may be a way forward.87
CONCLUSION AND RECOMMENDATIONS
The current meta-analysis found aggregate evidence to support the use of nonpharmacological sleep interventions in cancer and non-cancer pain patients with comorbid insomnia. The evidence substantiates and extends the initial conclusion drawn in the 2006 American Academy of Sleep Medicine review on the benefit of insomnia-specific treatment in individuals with chronic pain.41 Although the broader physical and psychological health benefits of these sleep interventions were moderate in magnitude and gradual in timing, they highlight the causal role of sleep and raise the possibility that more pro-active sleep treatment is a fruitful avenue for optimizing treatment outcomes in patients living with chronic painful conditions and for preventing the onset of adverse health outcomes. Aside from sleep researchers, these results are of particular interest to primary care physicians and allied health professionals, who are taking up an increasingly important role in preventing and managing long-term conditions. More research is now required to establish the feasibility, clinical utility, sustainability, and cost-effectiveness of such endeavors.
DISCLOSURE STATEMENT
This was not an industry supported study. All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declared that the study was funded by a Research Development Fund award from the University of Warwick, UK. The funder has no role in the study design, data collection, analysis, or interpretation of data; writing of the reporting or the decision to submit the article for publication; Dr. Tang's research is funded by the National Institute for Health Research, Department of Health, UK. She has no commercial conflicts of interest to declare; Prof. Cappuccio holds the Cephalon Chair, an endowed post at Warwick Medical School, the result of a donation from the company. The appointment to the Chair was made entirely independently of the company and the postholder is free to devise his own program of research. Cephalon does not have any stake in IP associated with the postholder, and the Chair has complete academic independence from the company. He has no commercial conflicts of interest to declare; no other relationships or activities that could appear to have influenced the submitted work. The other authors have indicated no financial conflicts of interest. Authors' contributions: Dr. Tang was the principal investigator of this project, responsible for the conception and design of the study. She coordinated the data search and extraction processes, which were carried out in duplicate by Drs. Lereya and Boulton. Dr. Tang led the data analysis, drafting and final editing of the paper. Prof. Cappuccio, Prof. Wolke, and Dr. Miller were co-investigators of the project. They were involved in the design of the study and the data extraction and analysis processes. All authors contributed to the writing and final editing of the manuscript. Ethics/PRISMA statement: Ethical approval was not required for this systematic review and meta-analysis. Reporting of this manuscript closely followed the PRISMA guideline.
ACKNOWLEDGMENTS
The authors thank Dr. Clemencia Rodas-Perez for her assistance in abstract translation and Dr. Helen Parsons for her statistical comments. Thanks also go to the anonymous reviewers for their helpful comments.
SUPPLEMENTAL MATERIAL
Appendix: Search Terms and Strategies by Database
1 Cochrane Library search strategy
#1 random* in Trials
#2 nonpharma*
#3 psychologic*
#4 behavi*
#5 cognitive
#6 relax*
#7 stimulus control*
#8 sleep
#9 insomnia*
#10 sleep near disorder*
#11 sleep near problem*
#12 sleep near difficult*
#13 sleep near disturb*
#14 sleep near pattern*
#15 wake*
#16 chronic next pain*
#17 cancer
#18 malignan*
#19 musculo*
#20 arthritis
#21 osteoarth*
#22 osteo*
#23 fibro*
#24 headache
#25 migraine
#26 neurop*
#27 neuralgi*
#28 rheuma*
#29 dysmenorrhea
#30 #2 or #3 or #4 or #5 or #6 or #7
#31 #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15
#32 #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29
#33 #1 and #30 and #31 and #32
2 MEDLINE search strategy
#1 random*
#2 nonpharma*
#3 psychologic*
#4 behavi*
#5 cognitive
#6 relax*
#7 stimulus control*
#8 sleep
#9 insomnia*
#10 sleep near disorder*
#11 sleep near problem*
#12 sleep near difficult*
#13 sleep near disturb*
#14 sleep near pattern*
#15 wake*
#16 cancer
#17 malignan*
#18 musculo*
#19 arthritis
#20 osteoarth*
#21 fibromyalg*
#22 fibros*
#23 headache
#24 migraine
#25 neuropath*
#26 neuralgi*
#27 rheuma*
#28 dysmenorrhea
#29 #2 or #3 or #4 or #5 or #6 or #7
#30 #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15
#31 #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28
#32 #1 and #29 and #30 and #31
3 EMBASE search strategy
#1 random*
#2 nonpharma*
#3 psychologic*
#4 behavi*
#5 cognitive
#6 relax*
#7 stimulus control*
#8 sleep
#9 insomnia*
#10 sleep near disorder*
#11 sleep near problem*
#12 sleep near difficult*
#13 sleep near disturb*
#14 sleep near pattern*
#15 wake*
#16 chronic next pain*
#17 cancer
#18 malignan*
#19 musculo*
#20 arthritis
#21 osteoarth*
#22 osteo*
#23 fibro*
#24 headache
#25 migraine
#26 neurop*
#27 neuralgi*
#28 rheuma*
#29 dysmenorrhea
#30 #2 or #3 or #4 or #5 or #6 or #7
#31 #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15
#32 #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29
#33 #1 and #30 and #31 and #32
4 PsycINFO search strategy
#1 random*
#2 nonpharma*
#3 psychologic*
#4 behavi*
#5 cognitive
#6 relax*
#7 stimulus control*
#8 sleep
#9 insomnia*
#10 sleep near disorder*
#11 sleep near problem*
#12 sleep near difficult*
#13 sleep near disturb*
#14 sleep near pattern*
#15 wake*
#16 chronic next pain*
#17 cancer
#18 malignan*
#19 musculo*
#20 arthritis
#21 osteoarth*
#22 osteo*
#23 fibro*
#24 headache
#25 migraine
#26 neurop*
#27 neuralgi*
#28 rheuma*
#29 dysmenorrhea
#30 #2 or #3 or #4 or #5 or #6 or #7
#31 #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15
#32 #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29
#33 #1 and #30 and #31 and #32
Qualitative risk assessment results: review authors' judgments about each risk of bias item presented as (A) visual summary for each study and (B) percentages across all included studies.
Funnel Plots for All Pooled Analyses by Outcome Measures and Assessment Time Points (Figures S2–S8)
Sleep. (A) Posttreatment. (B) Follow-up.
Pain. (A) Posttreatment. (B) Follow-up.
Fatigue. (A) Posttreatment. (B) Follow-up.
Depression. (A) Posttreatment. (B) Follow-up.
Anxiety. (A) Posttreatment. (B) Follow-up.
Physical functioning. (A) Posttreatment. (B) Follow-up.
Psychosocial functioning. (A) Posttreatment. (B) Follow-up.
REFERENCES
- 1.Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32:1484–92. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 2.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33:585–92. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cappuccio FP, Miller MA. A new challenge to widely held views on the role of sleep. Ann Int Med. 2012;157:593–4. doi: 10.7326/0003-4819-157-8-201210160-00016. [DOI] [PubMed] [Google Scholar]
- 4.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 5.Mork PJ, Nilsen TI. Sleep problems and risk of fibromyalgia: longitudinal data on an adult female population in Norway. Arthritis Rheum. 2012;64:281–4. doi: 10.1002/art.33346. [DOI] [PubMed] [Google Scholar]
- 6.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity. 2008;16:643–53. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell P, Tang NK, McBeth J, et al. The role of sleep problems in the development of depression among those with chronic pain: a prospective cohort study. Sleep. 2013;36:1693–8. doi: 10.5665/sleep.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders: an opportunity for prevention? JAMA. 1989;262:1479–84. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 9.Johnson EO, Roth T, Breslau N. The association of insomnia with anxiety disorders and depression: exploration of the direction of risk. J Psychiatr Res. 2006;40:700–8. doi: 10.1016/j.jpsychires.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Morphy H, Dunn KM, Lewis M, Boardman HF, Croft PR. Epidemiology of insomnia: a longitudinal study in a UK population. Sleep. 2007;30:274–80. [PubMed] [Google Scholar]
- 11.Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10:287–333. doi: 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Fernández A, Saameño JÁB, Pinto-Meza A, et al. Burden of chronic physical conditions and mental disorders in primary care. Br J Psychiatry. 2010;196:302–9. doi: 10.1192/bjp.bp.109.074211. [DOI] [PubMed] [Google Scholar]
- 13.Atkinson JH, Ancoli-Israel S, Slater MA, Garfin SR, Gillin C. Subjective Sleep Disturbance in Chronic Back Pain. Clin J Pain. 1988;4:225–32. [Google Scholar]
- 14.Bigatti SM, Hernandez AM, Cronan TA, Rand KL. Sleep disturbances in fibromyalgia syndrome: relationship to pain and depression. Arthritis Care Res. 2008;59:961–7. doi: 10.1002/art.23828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang NKY, Wright KJ, Salkovskis PM. Prevalence and correlates of clinical insomnia co-occurring with chronic back pain. J Sleep Res. 2007;16:85–95. doi: 10.1111/j.1365-2869.2007.00571.x. [DOI] [PubMed] [Google Scholar]
- 16.McCracken LM, Williams JL, Tang NKY. Psychological flexibility may reduce insomnia in persons with chronic pain: a preliminary retrospective study. Pain Med. 2011;12:904–12. doi: 10.1111/j.1526-4637.2011.01115.x. [DOI] [PubMed] [Google Scholar]
- 17.Haack M, Mullington JM. Sustained sleep restriction reduces emotional and physical well-being. Pain. 2005;119:56–64. doi: 10.1016/j.pain.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Irwin MR, Olmstead R, Carrillo C, et al. Sleep loss exacerbates fatigue, depression, and pain in rheumatoid arthritis. Sleep. 2012;35:537–43. doi: 10.5665/sleep.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moldofsky H, Scarisbrick P. Induction of neurasthenic musculoskeletal pain syndrome by selective sleep stage deprivation. Psychosom Med. 1976;38:35–44. doi: 10.1097/00006842-197601000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Smith M, Edwards R, McCann U, Haythornthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30:494–505. doi: 10.1093/sleep/30.4.494. [DOI] [PubMed] [Google Scholar]
- 21.Moldofsky H. Sleep and pain. Sleep Med Rev. 2001;5:385–96. doi: 10.1053/smrv.2001.0179. [DOI] [PubMed] [Google Scholar]
- 22.Roehrs T, Roth T. Sleep and pain: interaction of two vital functions. Semin Neurol. 2005;25:106–16. doi: 10.1055/s-2005-867079. [DOI] [PubMed] [Google Scholar]
- 23.Smith M, Quartana P, Okonkwo R, Nasir A. Mechanisms by which sleep disturbance contributes to osteoarthritis pain: a conceptual model. Curr Sci. 2009;13:447–54. doi: 10.1007/s11916-009-0073-2. [DOI] [PubMed] [Google Scholar]
- 24.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8:119–32. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 25.Tang NKY. Cognitive-behavioral therapy for sleep abnormalities of chronic pain patients. Curr Rheum Rep. 2009;11:451–60. doi: 10.1007/s11926-009-0066-5. [DOI] [PubMed] [Google Scholar]
- 26.Currie SR, Wilson KG, Pontefract AJ, deLaplante L. Cognitive-behavioral treatment of insomnia secondary to chronic pain. J Consult Clin Psychol. 2000;68:407–16. doi: 10.1037//0022-006x.68.3.407. [DOI] [PubMed] [Google Scholar]
- 27.Edinger JD, Wohlgemuth WK, Krystal AD, Rice JR. Behavioral insomnia therapy for fibromyalgia patients: a randomized clinical trial. Arch Intern Med. 2005;165:2527–35. doi: 10.1001/archinte.165.21.2527. [DOI] [PubMed] [Google Scholar]
- 28.Jungquist CR, O'Brien C, Matteson-Rusby S, et al. The efficacy of cognitive-behavioral therapy for insomnia in patients with chronic pain. Sleep Med. 2010;11:302–9. doi: 10.1016/j.sleep.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miró E, Lupiáñez J, Martínez MP, et al. Cognitive-behavioral therapy for insomnia improves attentional function in fibromyalgia syndrome: a pilot, randomized controlled trial. J Health Psychol. 2011;16:770–82. doi: 10.1177/1359105310390544. [DOI] [PubMed] [Google Scholar]
- 30.Goforth H, Preud'homme X, Krystal A. A randomized, double-blind, placebo-controlled trial of eszopiclone for the treatment of insomnia in patients with chronic low back pain. Sleep. 2014;37:1053–60. doi: 10.5665/sleep.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Affleck G, Urrows S, Tennen H, Higgins P, Abeles M. Sequential daily relations of sleep, pain intensity, and attention to pain among women with fibromyalgia. Pain. 1996;68:363–8. doi: 10.1016/s0304-3959(96)03226-5. [DOI] [PubMed] [Google Scholar]
- 32.Vitiello MV, Rybarczyk B, Von Korff M, Stepanski EJ. Cognitive behavioral therapy for insomnia improves sleep and decreases pain in older adults with co-morbid insomnia and osteoarthritis. J Clin Sleep Med. 2009;5:355–62. [PMC free article] [PubMed] [Google Scholar]
- 33.Morgenthaler T, Kramer M, Alessi C, et al. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An American Academy of Sleep Medicine report. Sleep. 2006;29:1415–9. [PubMed] [Google Scholar]
- 34.Okura K, Lavigne GJ, Huynh N, Manzini C, Fillipini D, Montplaisir JY. Comparison of sleep variables between chronic widespread musculoskeletal pain, insomnia, periodic leg movements syndrome and control subjects in a clinical sleep medicine practice. Sleep Med. 2008;9:352–61. doi: 10.1016/j.sleep.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 35.Tang NKY, Goodchild CE, Hester J, Salkovskis PM. Pain-related insomnia versus primary insomnia: a comparison study of sleep pattern, psychological characteristics, and cognitive-behavioral processes. Clin J Pain. 2012;28:428–36. doi: 10.1097/AJP.0b013e31823711bc. [DOI] [PubMed] [Google Scholar]
- 36.Eccleston C, Williams A, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2009:2. doi: 10.1002/14651858.CD007407.pub2. [DOI] [PubMed] [Google Scholar]
- 37.Montgomery P, Dennis J. Cognitive behavioural interventions for sleep problems in adults aged 60+ Cochrane Database Syst Rev. 2003;1:CD003161. doi: 10.1002/14651858.CD003161. [DOI] [PubMed] [Google Scholar]
- 38.Harvey AG, Watkins E, Mansell W, Shafran R. Oxford: Oxford University Press; 2004. Cognitive behavioural processes across psychological disorders: a transdiagnostic approach to research and treatment. [Google Scholar]
- 39.Smith MT, Huang MI, Manber R. Cognitive behavior therapy for chronic insomnia occurring within the context of medical and psychiatric disorders. Clin Psychol Rev. 2005;25:559–92. doi: 10.1016/j.cpr.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Arcos-Carmona IM, Castro-Sanchez AM, Mataran-Penarrocha GA, Gutierrez-Rubio AB, Ramos-Gonzalez E, Moreno-Lorenzo C. [Effects of aerobic exercise program and relaxation techniques on anxiety, quality of sleep, depression, and quality of life in patients with fibromyalgia: a randomized controlled trial] Med Clin (Barc) 2011;137:398–401. doi: 10.1016/j.medcli.2010.09.045. [DOI] [PubMed] [Google Scholar]
- 41.Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004) Sleep. 2006;29:1398–414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- 42.Savard J, Simard S, Ivers H, Morin CM. Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part I: sleep and psychological effects. J Clin Oncol. 2005;23:6083–96. doi: 10.1200/JCO.2005.09.548. [DOI] [PubMed] [Google Scholar]
- 43.Savard J, Simard S, Ivers H, Morin CM. Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part II: immunologic effects. J Clin Oncol. 2005;23:6097–106. doi: 10.1200/JCO.2005.12.513. [DOI] [PubMed] [Google Scholar]
- 44.Rybarczyk B, Stepanski E, Fogg L, Lopez M, Barry P, Davis A. A placebo-controlled test of CBT for co-morbid insomnia in older adults. J Consult Clin Psychol. 2005;73:1164–74. doi: 10.1037/0022-006X.73.6.1164. [DOI] [PubMed] [Google Scholar]
- 45.Garland SN, Carlson LE, Stephens AJ, Antle MC, Samuels C, Campbell TS. Mindfulness-based stress reduction compared with cognitive behavioral therapy for the treatment of insomnia comorbid with cancer: a randomized, partially blinded, noninferiority trial. J Clin Oncol. 2014;32:449–57. doi: 10.1200/JCO.2012.47.7265. [DOI] [PubMed] [Google Scholar]
- 46.Yates SL, Morley S, Eccleston C, de C Williams AC. A scale for rating the quality of psychological trials for pain. Pain. 2005;117:314–25. doi: 10.1016/j.pain.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 47.Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration. 2011 [Google Scholar]
- 48.Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Arthritis Rheum. 1990;33:160–72. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 49.Martínez MP, Miró E, Sánchez AI, et al. Cognitive-behavioral therapy for insomnia and sleep hygiene in fibromyalgia: a randomized controlled trial. J Behav Med. 2013:1–15. doi: 10.1007/s10865-013-9520-y. [DOI] [PubMed] [Google Scholar]
- 50.Berger AM, Kuhn BR, Farr LA, et al. Behavioral therapy intervention trial to improve sleep quality and cancer-related fatigue. PsychoOncology. 2009;18:634–46. doi: 10.1002/pon.1438. [DOI] [PubMed] [Google Scholar]
- 51.Barsevick A, Beck SL, Dudley WN, et al. Efficacy of an intervention for fatigue and sleep disturbance during cancer chemotherapy. J Pain Symptom Manag. 2010;40:200–16. doi: 10.1016/j.jpainsymman.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Espie CA, Fleming L, Cassidy J, et al. Randomized controlled clinical effectiveness trial of cognitive behavior therapy compared with treatment as usual for persistent insomnia in patients with cancer. J Clin Oncol. 2008;26:4651–8. doi: 10.1200/JCO.2007.13.9006. [DOI] [PubMed] [Google Scholar]
- 53.Ritterband LM, Bailey ET, Thorndike FP, Lord HR, Farrell-Carnahan L, Baum LD. Initial evaluation of an Internet intervention to improve the sleep of cancer survivors with insomnia. PsychoOncology. 2012;21:695–705. doi: 10.1002/pon.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd ed. Washington, DC: American Psychiatric Association; 1980. [Google Scholar]
- 55.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 56.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000. text revision. [Google Scholar]
- 57.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatr Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 58.Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1:277–99. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- 59.Smets E, Garssen B, Bonke Bd, De Haes J. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315–25. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 60.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 61.Ware JE, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 62.Garland S, Johnson J, Savard J, et al. Sleeping well with cancer: a systematic review of cognitive behavioral therapy for insomnia in cancer patients. Neuropsychiatr Dis Treat. 2014;10:1113–24. doi: 10.2147/NDT.S47790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bohra MH, Espie CA. Is cognitive behavioural therapy for insomnia effective in treating insomnia and pain in individuals with chronic non-malignant pain? Br J Pain. 2013 doi: 10.1177/2049463713489384. 2049463713489384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Currell R, Urquhart C, Wainwright P, Lewis R. Telemedicine versus face to face patient care: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2000;(2):CD002098. doi: 10.1002/14651858.CD002098. [DOI] [PubMed] [Google Scholar]
- 65.Cheng SK, Dizon J. Computerised cognitive behavioural therapy for insomnia: a systematic review and meta-analysis. Psychother Psychosom. 2012;81:206–16. doi: 10.1159/000335379. [DOI] [PubMed] [Google Scholar]
- 66.Ritterband L, Thorndike F. The further rise of internet interventions. Sleep. 2012;35:737–8. doi: 10.5665/sleep.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Espie C, Kyle S, Williams C, et al. A randomized, placebo-controlled trial of online cognitive behavioral therapy for chronic insomnia disorder delivered via an automated media-rich web application. Sleep. 2012;35:769–81. doi: 10.5665/sleep.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Siversten B, Vedaa Ø, Nordgreen T. The future of insomnia treatment— the challenge of implementation. Sleep. 2013;36:303–4. doi: 10.5665/sleep.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Irwin MR, Olmstead R, Carrillo C, et al. Cognitive behavioral therapy vs. Tai Chi for late life insomnia and inflammatory risk: a randomized controlled comparative efficacy trial. Sleep. 2014;37:1543–52. doi: 10.5665/sleep.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoevenaar-Blom MP, Spijkerman AM, Kromhout D, van den Berg JF, Verschuren WM. Sleep duration and sleep quality in relation to 12-year cardiovascular disease incidence: the MORGEN study. Sleep. 2011;34:1487–92. doi: 10.5665/sleep.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lou P, Chen P, Zhang L, et al. Relation of sleep quality and sleep duration to type 2 diabetes: a population-based cross-sectional survey. BMJ Open. 2012;2:e000956. doi: 10.1136/bmjopen-2012-000956. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Neckelmann D, Mykletun A, Dahl AA. Chronic insomnia as a risk factor for developing anxiety and depression. Sleep. 2007;30:873–80. doi: 10.1093/sleep/30.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tang NKY, Goodchild CE, Sanborn AN, Howard J, Salkovskis PM. Deciphering the temporal link between pain and sleep in a heterogeneous chronic pain patient sample: a multilevel daily process study. Sleep. 2012;35:675–87. doi: 10.5665/sleep.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carver CS, Johnson SL, Joormann J. Serotonergic function, two-mode models of self-regulation, and vulnerability to depression: what depression has in common with impulsive aggression. Psychol Bull. 2008;134:912–43. doi: 10.1037/a0013740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Finan PH, Smith MT. The comorbidity of insomnia, chronic pain, and depression: dopamine as a putative mechanism. Sleep Med Rev. 2013;17:173–83. doi: 10.1016/j.smrv.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tang NKY, Sanborn AN. Better quality sleep promotes daytime physical activity in patients with chronic pain? a multilevel analysis of the within-person relationship. PLoS One. 2014;9:e92158. doi: 10.1371/journal.pone.0092158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pigeon WR, Moynihan J, Matteson-Rusby S, et al. Comparative effectiveness of CBT interventions for co-morbid chronic pain and insomnia: a pilot study. Behav Res Ther. 2012;50:685–9. doi: 10.1016/j.brat.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tang NKY, Goodchild CE, Salkovskis PM. Hybrid cognitive-behaviour therapy for individuals with insomnia and chronic pain: a pilot randomised controlled trial. Behav Res Ther. 2012;50:814–21. doi: 10.1016/j.brat.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 79.Vitiello MV, McCurry SM, Shortreed SM, et al. Cognitive-behavioral treatment for comorbid insomnia and osteoarthritis pain in primary care: the Lifestyles Randomized Controlled Trial. J Am Geriatr Soc. 2013;61:947–56. doi: 10.1111/jgs.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martínez-García MÁ, Soler-Cataluña JJ, Ejarque-Martínez L, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med. 2009;180:36–41. doi: 10.1164/rccm.200808-1341OC. [DOI] [PubMed] [Google Scholar]
- 81.Campos-Rodriguez F, Martinez-Garcia MA, de la Cruz-Moron I, Almeida-Gonzalez C, Catalan-Serra P, Montserrat JM. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatmenta cohort study. Ann Intern Med. 2012;156:115–22. doi: 10.7326/0003-4819-156-2-201201170-00006. [DOI] [PubMed] [Google Scholar]
- 82.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 83.Smith MT, Finan PH, Buenaver LF, et al. Cognitive-behavioral therapy for insomnia in knee osteoarthritis: a randomized, double-blind, active placebo-controlled clinical trial. Arthritis Rheumatol. 2015;67:1221–33. doi: 10.1002/art.39048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Black N. Patient reported outcome measures could help transform healthcare. BMJ (Clin Res Ed) 2013;346:f167. doi: 10.1136/bmj.f167. [DOI] [PubMed] [Google Scholar]
- 85.Morgenthaler T, Alessi C, Friedman L, et al. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30:519–29. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- 86.Kushida CA, Littner MR, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28:499–521. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- 87.Turk DC, Dworkin RH, Revicki D, et al. Identifying important outcome domains for chronic pain clinical trials: an IMMPACT survey of people with pain. Pain. 2008;137:276–85. doi: 10.1016/j.pain.2007.09.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Qualitative risk assessment results: review authors' judgments about each risk of bias item presented as (A) visual summary for each study and (B) percentages across all included studies.
Sleep. (A) Posttreatment. (B) Follow-up.
Pain. (A) Posttreatment. (B) Follow-up.
Fatigue. (A) Posttreatment. (B) Follow-up.
Depression. (A) Posttreatment. (B) Follow-up.
Anxiety. (A) Posttreatment. (B) Follow-up.
Physical functioning. (A) Posttreatment. (B) Follow-up.
Psychosocial functioning. (A) Posttreatment. (B) Follow-up.