Abstract
Background and Purpose
Resveratrol exerts a range of beneficial actions in several areas of pathophysiology, including vascular biology. Here, we have investigated the effects of resveratrol on apolipoprotein M (apoM), a carrier and modulator of sphingosine 1‐phosphate (S1P), a vasoactive lipid mediator.
Experimental Approach
We used a hepatoma cell line (HepG2), human primary hepatocytes and C57BL/6 mice. We measured apoM, S1P and related enzymes, LDL receptors and sirtuin1 activity, using Western blotting, RT‐PCR and enzyme assays. We also used si‐RNA to knock‐down sirtuin1 in HepG2 cells.
Key Results
In cultures of HepG2 cells, resveratrol (1‐10 μM) increased intracellular apoM and S1P. High concentrations of resveratrol (100 μM) decreased extracellular (in the culture medium) apoM, whereas moderate concentrations of resveratrol (1–10 μM) increased extracellular apoM. High concentrations of resveratrol also increased LDL receptor expression, while all concentrations of resveratrol activated the histone deacetylase sirtuin1. In cultures of human primary hepatocytes, resveratrol, at all concentrations, increased both intra‐ and extracellular apoM. When wild‐type mice were fed a resveratrol‐containing chow (0.3% w/w) for 2 weeks, both the plasma and hepatic apoM and S1P levels were increased. However, the resveratrol diet did not affect hepatic LDL receptor levels in this in vivo study.
Conclusions and Implications
Resveratrol increased intra‐ and extracellular levels of apoM, along with intracellular S1P levels, while a high concentration of resveratrol reduced extracellular apoM. The present findings suggest that resveratrol has novel effects on the metabolic kinetics of S1P, a multi‐functional bioactive phospholipid.
Abbreviations
- apoM
apolipoprotein M
- c‐RSV
cis‐resveratrol
- S1P
sphingosine 1‐phosphate
- SIRT1
sirtuin 1
- SK
sphingosine kinase
- Spgl
S1P lyase
- t‐RSV
trans‐resveratrol
Tables of Links
| TARGETS |
|---|
| Enzymes a |
| SIRT1, sirtuin 1 |
| SK, sphingosine kinase |
| Spgl, S1P lyase |
| GPCRs b |
| S1P1 receptor |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a bAlexander et al., 2013a,2013b).
Introduction
Resveratrol has received much attention as a key component of red wine polyphenols, which might explain the ‘French paradox’ (Renaud and de Lorgeril, 1992). Resveratrol exhibits anti‐inflammatory, anti‐cancer and life‐prolonging properties (Baur et al., 2006; Orallo, 2008). In addition to these beneficial properties, resveratrol has also been suggested to possess cardiovascular protective effects (Wu et al., 2001; Das and Maulik, 2006). Although these beneficial effects are attributed to several bioactive effects of resveratrol reported in previous papers, such as anti‐oxidant properties (Leonard et al., 2003) and the activation of the histone deacetylase sirtuin 1 (SIRT1; Howitz et al., 2003; Borra et al., 2005; Baur and Sinclair, 2006), the potential beneficial properties of resveratrol have not yet been completely elucidated.
Among the possible beneficial biological activities of resveratrol, Lim et al. (2012) reported that resveratrol suppressed sphingosine kinase (SK) 1 in breast cancer cell lines, which might explain the proposed cancer‐prevention properties of resveratrol, because SK1 is abundantly expressed in many cancers (Pyne and Pyne, 2010) and sphingosine 1‐phosphate (S1P), the product of SK1, is known to exhibit cell‐proliferating properties (Hannun and Obeid, 2008; Takabe and Spiegel, 2014). Furthermore, Abdin (2013) recently reported that resveratrol improved experimentally induced ulcerative colitis in rats by inhibiting SK1.
Contrary to the possible harmful effects of S1P reported in the fields of cancer and inflammation, S1P also exerts beneficial effects in cardiovascular diseases, such as anti‐apoptosis (Goetzl, 2001), anti‐inflammation (Kimura et al., 2006), vasorelaxation through the induction of eNOS (Igarashi et al., 2001) and the preservation of vascular permeability (Garcia et al., 2001). Because the genetic deletion of SK1 decreased plasma S1P levels (Allende et al., 2004), resveratrol might decrease plasma S1P levels if it suppressed SK1 systemically. However, the effects of resveratrol on plasma S1P levels have not been previously investigated. Moreover, while local S1P levels might be relevant to its effects on cancer cells, the plasma S1P levels, especially the HDL‐linked S1P content, might be important in terms of atherosclerosis from both a basic perspective and a clinical perspective (Kimura et al., 2006; Argraves et al., 2011; Wilkerson et al., 2012). Among the various factors that may influence S1P levels, apolipoprotein M (apoM), a minor apolipoprotein, bound mainly to HDL (Xu and Dahlback, 1999), can substantially affect the blood levels of S1P, especially the S1P linked to HDL, and, in the circulation, S1P is carried mainly on HDL (~70%), followed by albumin (~30%) (Okajima, 2002; Rodriguez et al., 2009). Recently, S1P has been shown to be carried on HDL via apoM (Christoffersen et al., 2011). Moreover, apoM is not only a carrier of S1P but also a modulator of the levels of S1P, preventing its degradation and increasing the amount linked to HDL (Christoffersen et al., 2011; Kurano et al., 2013; Liu et al., 2014; Nojiri et al., 2014). Also, mature apoM is necessary for the formation of S1P‐enriched HDL (Liu et al., 2015) and apoM affects erythrocyte efflux and tubular reabsorption of S1P (Sutter et al., 2014).
In view of the proposed protective properties of resveratrol in the cardiovascular system and of S1P in the fields of vascular biology, we investigated, here, the effects of resveratrol on the plasma levels of apoM and S1P in cultured hepatocytes and in vivo in mice.
Methods
Cell experiments
HepG2 cells were obtained from the American Type Culture Collection (Manassas, VA, USA). The cells were cultured in DMEM (D5796; Sigma‐Aldrich Co., St. Louis, MO, USA) supplemented with 10% FBS (10099–141; Gibco BRL, Eggstein, Germany) and 1% penicillin/streptomycin (15070–063; Gibco BRL).
To examine the effects of resveratrol on apoM, cells were allowed to reach a confluency of 80–90% and were then washed with PBS three times before being incubated with various concentrations of DMSO (vehicle), cis‐resveratrol (c‐RSV) (10004235; Cayman Chemical Co., Ann Arbor, MI, USA) or trans‐resveratrol (t‐RSV) (70675, Cayman Chemical Co.) in FBS‐free DMEM for 16 h. The culture medium and cellular components were then analysed as described below.
To investigate the metabolism of S1P synthesized de novo, we pre‐treated cells with various concentrations of c‐RSV; after 16 h, we replaced the medium with one containing 10 μM of C17‐sphingosine (860654P; Avanti Polar Lipids, Alabaster, AL, USA). After 10 min, we then replaced the medium (by washing with PBS twice) with serum‐free medium. After an additional 30 min, we measured the C17‐S1P contents in the culture medium and the cells.
For the experiments with siRNA, we administered siRNA to HepG2 cells using reverse transfection and lipofectamine RNAiMAX (13778075; Invitrogen Co. Carlsbad, CA, USA), according to the manufacturer's protocol. After 48 h, the cells were washed with PBS three times, and the culture medium was replaced with FBS‐free medium with or without c‐RSV. The following siRNAs were utilized: siRNA control A (sc‐37007; Santa Cruz Biotechnology, Dallas, TX, USA), siRNA against human LDL receptor (sc‐35802; Santa Cruz Biotechnology) and siRNA against SIRT1 (sc‐40986; Santa Cruz Biotechnology).
Human primary hepatocytes (HP‐NP2, lot number: ZBH2063‐P10) were obtained from Zen‐Bio Inc. (NC, USA) and plated in hepatocyte plating medium (HM‐1, Zen‐Bio Inc.), and after 6 h, the medium was replaced with hepatocyte‐maintaining medium (HM‐2, Zen‐Bio Inc.) according to the manufacturer's protocol. On the next day, human primary hepatocytes were treated with various concentrations of DMSO or c‐RSV and investigated for the modulation of the medium and cellular apoM, in the same way as the HepG2 cells.
SK activity assay
We measured the SK activity as described previously (Kurano et al., 2013; Nojiri et al., 2014). Briefly, we homogenized cells or liver tissues in 20 mM Tris‐HCl (pH 7.4), 20% glycerol, 1 mM β‐mercaptoethanol, 15 mM NaF, 1 mM EDTA, 1 mM Na3VO4, 1 mM PMSF and protease inhibitor cocktail (Roche, Mannheim, Germany). The reaction was initiated by adding 25 μL of 200 μM C17‐sphingosine and 25 μL of 20 mM ATP (Sigma‐Aldrich Co.) to 300 µg of protein in a final volume of 500 μL. After incubation at 37 °C for 20 min, we terminated the reaction by adding 50 μL of 1 M HCl and 3 mL of methanol and chloroform (v/v = 2:1), followed by the C17‐S1P measurement.
Animal experiments
All animal care and experimental procedures were conducted in accordance with the guidelines for Animal Care and were approved by the Animal Committee of the University of Tokyo. Studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
C57BL/6 mice were purchased from CLEA Japan (Tokyo, Japan). Chow containing 0.3% t‐RSV (w/w) was prepared by adding resveratrol to chow diet using carboxymethyl cellulose as a vehicle. Ten‐week‐old male mice were divided into two groups: one group was fed with the 0.3% t‐RSV‐containing chow, and the other group was fed with chow containing the vehicle alone for 2 weeks. We utilized t‐RSV instead of c‐RSV because of a previous report that t‐RSV was more stable than c‐RSV (Trela and Waterhouse, 1996). The mice were then subjected to a 6 h fast, and blood samples were subsequently collected.
Analyses of total cholesterol levels in plasma
The levels of total cholesterol and triglycerides were measured using enzymic methods (439‐17501 and 432‐40201; WAKO Pure Chemical Industries, Osaka, Japan). To fractionate the lipoproteins with fast protein LC, the plasma samples were pooled together and then separated using fast protein LC, with a Superose 6 column. To separate HDL and lipoprotein‐depleted fraction, the HDL fraction was collected with a standard ultracentrifugation method from 250 μL of murine plasma, and the residue was collected as the lipoprotein‐depleted fraction.
Measurement of S1P, dihydro‐S1P and C17‐S1P contents
The S1P, dihydro‐S1P and C17‐S1P contents in the plasma, medium and liver were determined using a two‐step lipid extraction process followed by HPLC separation, as described previously (Yatomi, 2008). Briefly, samples were sonicated in 3 mL of methanol/chloroform (2:1) with an internal standard for 30 min. After adding 2 mL of chloroform, 2.1 mL of 1 mM KCl and 100 μL of 3 N NaOH, the samples were centrifuged, and the alkaline upper phase (3.8 mL) was collected into new tubes, to which 4 mL of chloroform and 200 μL of concentrated HCl were added. The resultant lower chloroform phases (3.5 mL) formed under these new acidic conditions were collected and evaporated under nitrogen gas and then resolved in methanol, followed by HPLC separation using a TSKgel ODS‐80TM column (0017202; Tosoh, Tokyo, Japan). For the measurement of the S1P content, we used C17‐S1P (860641P; Avanti Polar Lipids) as an internal standard, while for the C17‐S1P content, we used FTY720‐phosphate (10006408; Cayman Chemical).
Measurement of plasma resveratrol
The resveratrol concentration in murine plasma was measured as described previously (Katsagonis et al., 2005; Chen et al., 2007). Briefly, 100 μL of plasma was mixed with 100 μL of caffeine (C1778, Sigma‐Aldrich Co.), which was dissolved in methanol at 1 mg mL−1 and used as the internal standard. To this plasma mixture, 200 μL of phosphate buffer (pH 6.0) prepared with 56.8 mM NaH2PO4 was added. Resveratrol was extracted with 1 mL of ethyl acetate twice and then evaporated under nitrogen gas. The residue was dissolved in 200 μL of the mobile phase solvent [acetonitrile/phosphate buffer (pH 4.9) prepared with 30 mM NaH2PO4 at a ratio of 25:75 v/v], followed by HPLC separation using a Waters Nova Pack C18 (150 × 3.9 mm; Milford, MA, USA), a 4 mm particle size chromatography column (WAT036975, Waters Co.). Caffeine and resveratrol were detected at the retention times of 1.69 and 3.77 min, respectively, with UV detection at 310 nm (Figure S1).
Western blotting
Cellular proteins and liver proteins were extracted using RIPA [25 mM Tris‐HCl (pH 7.6), 1% NP‐40, 0.1% SDS, 150 mM NaCl, 0.02% sodium deoxycholate, 1 mM orthovanadate, 1 mM PMSF and protease inhibitor cocktail (11836153001; Roche)]. Western blotting was performed using 30 µg of the cellular or liver proteins according to a standard method. To analyse the proteins in the media, the amount of medium subjected to Western blotting was adjusted according to the cellular protein level. For the plasma analysis, a volume corresponding to 0.2 μL of plasma was used for the western blotting analysis. The following antibodies were used: anti‐human apoM antiserum (developed in a previously reported paper, Kurano et al., 2013), anti‐mouse apoM antibody (A00954; GenScript Co, Piscataway, NJ, USA), anti‐mouse albumin antibody (sc‐46293; Santa Cruz Biotechnology), anti‐LDL receptor antibody (10007665; Cayman Chemical Co.), anti‐SIRT1 antibody (D739; Cell Signaling Technology, Beverly, MA, USA), anti‐human albumin antibody (E80‐129A; Bethyl Laboratories, Inc., Montgomery, TX, USA), anti‐apoA‐I antibody (AB740; Chemicon International Inc., Temecula, CA, USA) and anti‐β‐actin antibody (PM053; MBL, Nagoya, Japan). The intensities of the bands were measured using Image J (from the NIH), and the bands from different gels were compared by normalizing to the same sample placed on both gels as an internal standard. All data were normalized to the mean value of the control group.
Real‐time PCR
The total RNA extracted from the murine livers using the GenElute Mammalian Total RNA Miniprep kit (RTN‐70; Sigma‐Aldrich) was subjected to reverse transcription with ReverTra Ace qPCR RT Master Mix (FSQ‐201; TOYOBO Co., Ltd, Osaka, Japan). Quantitative PCR was performed using an ABI 7300 Real‐Time PCR System (Applied Biosystems, Foster City, CA, USA) for human apoM (Hs01597780_g1), human LDLr (Hs01092524_m1), human SK1 (Hs01116530_g1), human SK2 (Hs01016543_g1), human S1P lyase (Spgl) (Hs00187407_m1), human β‐actin (Hs01060665_g1), mouse apoM (Mm00444525_m1), mouse LDLr (Mm01177349_m1), mouse SK1 (Mm00448841_g1), mouse SK2 (Mm00445021_m1), mouse Spgl (Mm00486079_m1) and β‐actin (Mm00607939_s1). The expression levels of the genes of interest were adjusted to that of endogenous β‐actin mRNA, which was used as a control.
SIRT1 activity assay
SIRT1 activity was determined with universal SIRT activity assay kit (ab156915; Abcam Inc., Boston, MA, USA), utilizing nuclear extracts prepared as described previously (Kurano et al., 2011).
Data analysis
The results are expressed as the mean ± SEM. Differences between two groups were evaluated using the Student's t‐test when F‐test validated homogeneity of variance or the Welch t‐test when inhomogeneity of variance existed. Differences among more than two groups were assessed using a one‐way anova, followed by multiple comparison tests when Bartlett test validated homogeneity of variance or Kruskal–Wallis test followed by Steel–Dwass test when inhomogeneity of variance existed. P < 0.05 was deemed as statistically significant.
Results
Resveratrol increased cellular apoM but had biphasic effects on medium apoM
Resveratrol is composed of two principal isomers, c‐RSV and t‐RSV, and the functional difference between these isomers has not yet been established completely. First, we treated HepG2 cells, a human hepatoma cell line, with a range of concentrations of c‐RSV (1‐100 μM). As shown in Figure 1A–D, c‐RSV increased the cellular apoM levels in a concentration‐dependent manner, while c‐RSV increased the medium apoM level when administered at a concentration of 1–10 μM but decreased the medium apoM level when administered at a concentration of 100 μM. We also examined the effects of resveratrol on apoM using t‐RSV, the second principal isomer of resveratrol, and obtained similar results (Figure 1E–H), although its effects on the induction of apoM seemed slightly weaker than those of c‐RSV. Activation of SIRT1 by c‐RSV (Figure 1I) was greater than that by t‐RSV only at 1 μM.
Figure 1.
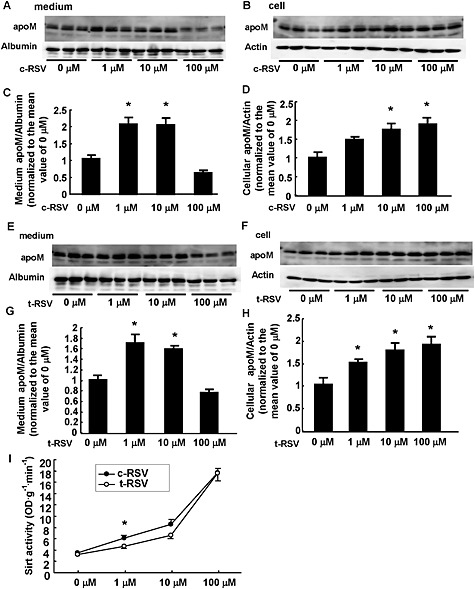
Effects of resveratrol on the levels of apoM in cells or culture medium of HepG2 cells. HepG2 cells were treated with FCS‐free medium containing various concentrations of c‐RSV (A–D) or t‐RSV (E–H), and after 16 h, the medium and the cells were collected and analysed by Western blotting. The intensity of the bands was quantified using Image J. For the medium apoM, albumin was utilized as a control; for the cellular apoM, β‐actin was utilized as a control. (A–D) Effects of c‐RSV. (A, C) Medium apoM levels. *P < 0.05, significantly different from 0 or 100 μM. (B, D) Cellular apoM levels. *P < 0.05, significantly different from 0 μM (n = 6 each group). (E–H) Effects of t‐RSV. (E, G) Medium apoM levels. *P < 0.05, significantly different from 0 or 100 μM. (F, H) Cellular apoM levels. *P < 0.05, significantly different from 0 μM (n = 6 each group). The data were normalized to the mean of the control group. (I) Nuclear extracts of HepG2 cells treated with various concentrations of c‐RSV or t‐RSV were analysed for SIRT activity (n = 6 each group). *P < 0.05, significantly different from t‐RSV at 1 μM.
A high concentration of resveratrol increased cellular S1P content
ApoM is not only a carrier (Christoffersen et al., 2011) but also a modulator of S1P metabolism, increasing S1P levels in liver and retarding S1P degradation in the circulation (Kurano et al., 2013). Therefore, we next investigated the ability of resveratrol to modulate S1P metabolism, using a high concentration of c‐RSV. As shown in Figure 2A, incubation of HepG2 cells with c‐RSV (100 μM) increased the cellular content of S1P but did not affect the S1P in the culture medium. We also examined the de novo synthesis of S1P, from C17‐sphingosine (a labelled sphingosine and a precursor of C17S1P) and observed that c‐RSV increased cellular C17S1P content, but not that in the culture medium (Figure 2B).
Figure 2.
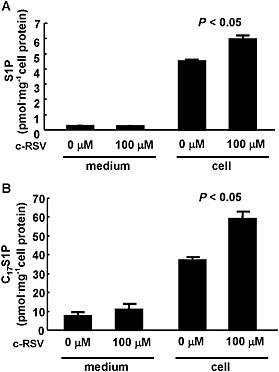
Effects of resveratrol on cellular and medium S1P levels in HepG2 cells. (A) HepG2 cells in FCS‐free medium were treated with c‐RSV at a concentration of 100 μM or the vehicle alone. After 16 h, the medium and the cells were collected, and the S1P contents were measured, after adjustments for the cellular protein level. *P < 0.05, significantly different from 0 μM (n = 5 each group). (B) HepG2 cells in FCS‐free medium were treated with c‐RSV at a concentration of 100 μM or the vehicle alone. After 16 h, the cells were treated with 10 μM of C17‐sphingosine for 10 min. Then, the medium was replaced with the FCS‐free medium, and another 30 min later, the medium and the cells were collected, and the C17S1P contents were measured, after adjustments for the cellular protein level. *P < 0.05, significantly different from 0 μM (n = 4 each group).
Resveratrol did not modulate the key enzymes involved in S1P metabolism
The metabolism of S1P in hepatocytes is affected by several factors other than apoM, including SK (SK1 and SK2) which forms S1P from sphingosine and Spgl, which degrades S1P irreversibly. In HepG2 cells, resveratrol did not modulate the expression of these key enzymes (Figure 3A), but it did significantly increase the mRNA for apoM (Figure 3B). We also confirmed that resveratrol did not enhance SK activity (Figure 3C).
Figure 3.
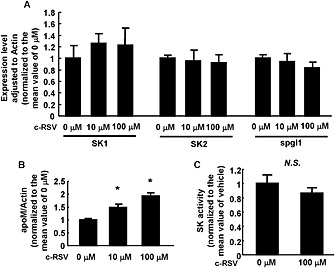
Effects of resveratrol on the expressions of proteins involved in S1P metabolism. HepG2 cells were treated with FCS‐free medium containing various concentrations of c‐RSV; after 16 h, the cells were collected and analysed by real‐time PCR (A, B) or an SK activity assay. β‐actin was utilized as the internal control. (A) Expression levels of SK1, SK2 and Spgl (n = 5 each group). (B) Expression level of apoM. *P < 0.05, significantly different from 0 μM (n = 5 each group). (C) SK activity assay (n = 6 each group). All the data were normalized to the mean of the control group.
Resveratrol decreased the apoM levels in the culture medium by increasing LDL receptor expression
The opposing effects of high concentrations of resveratrol (increasing cellular and decreasing medium apoM) could reflect changes in the clearance of apoM by the LDL receptor (Christoffersen et al., 2012; Kurano et al., 2015). Earlier work had shown that resveratrol increased expression and activity of LDL receptors (Yashiro et al., 2012). In our cultures of HepG2 cells, resveratrol did indeed increase the expression of LDL receptors, at both the mRNA and protein levels, but only at 100 μM (Figure 4A–C).
Figure 4.
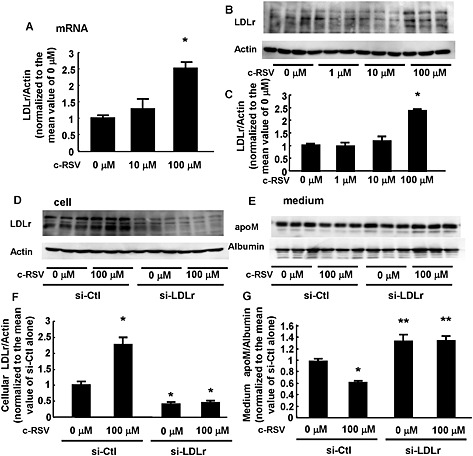
A high concentration of resveratrol decreased the medium apoM levels by increasing LDL receptor (LDLr) expression. (A–C) HepG2 cells were treated with FCS‐free medium containing various concentrations of c‐RSV; after 16 h, the cells were collected and analysed by real‐time PCR (A) or Western blotting (B, C). (A) Expression level of LDL receptors (n = 5 each group). β‐actin was utilized as the internal control. *P < 0.05, significantly different from other groups. (B, C) Protein level of LDLr. β‐actin was utilized as the internal control (n = 6 each group). *P < 0.05, significantly different from other groups. (D–G) HepG2 cells were treated with siRNA against LDLr (si‐LDLr) or control siRNA (si‐Ctl). After 48 h, the medium was replaced with the FCS‐free medium containing c‐RSV at a concentration of 100 μM or the vehicle alone for 16 h. Then, the medium and the cells were collected and analysed by Western blotting (n = 6 each group). (D, F) Cellular LDLreceptor level. β‐actin was utilized as the internal control. *P < 0.05, significantly different from si‐Ctl alone. (E, G) Medium apoM level. Albumin was utilized as an internal control. *P < 0.05, significantly different from si‐Ctl alone, **P < 0.05, significantly different from si‐Ctl alone and si‐Ctl with 100 μM resveratrol. All the data were normalized to the mean of the control group.
To examine further the involvement of LDL receptors in the effects of high concentrations of resveratrol on apoM in culture medium, we knocked down these receptors in HepG2 cells, using specific siRNA for LDL receptors. In these cells (Figure 4D and F), the levels of cellular LDL receptor protein were decreased and the effects of 100 μM c‐RSV on LDL receptors was abolished. Moreover, knock‐down of these receptors increased the apoM in culture medium, compared with the effects of control siRNA, and incubation with c‐RSV at 100 μM no longer decreased the medium apoM (Figure 4E and G).
Resveratrol increased the cellular apoM level through the SIRT1 pathway
We now looked for mechanisms underlying the resveratrol‐induced increase in cellular apoM (Figure 1B and D). As shown in Figure 3B, resveratrol increased the mRNA for apoM and resveratrol is known to activate the histone deacetylase SIRT1 (Howitz et al., 2003; Borra et al., 2005; Baur and Sinclair, 2006), so we pursued the possibility of SIRT1 involvement by suppressing its expression of SIRT1 with specific siRNA. As shown in Figure 5A, B and E, knock‐down of SIRT1 decreased cellular apoM at both the protein and mRNA levels and also the apoM in culture medium. Concordant with these effects on apoM, the knock‐down of SIRT1 also decreased cellular S1P (Figure 5C). However, knock‐down of SIRT1 did not affect the expression of any the key enzymes related to S1P (Figure 5E).
Figure 5.
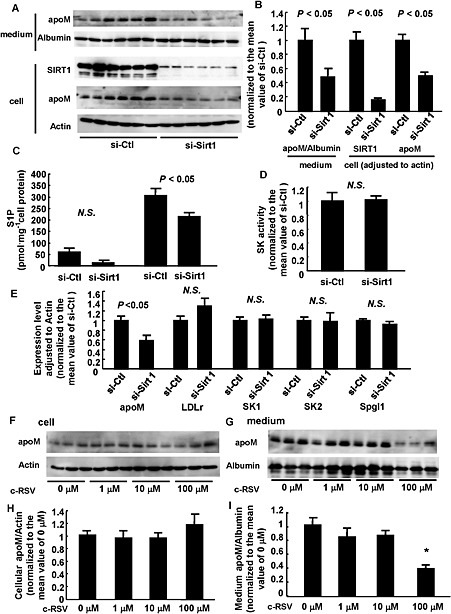
Resveratrol increased the cellular apoM level through the activation of SIRT1. (A–E) HepG2 cells were treated with siRNA against SIRT1 (si‐Sirt1) or control siRNA (si‐Ctl). After 48 h, the medium was replaced with FCS‐free medium for another 16 h. Then, the medium and the cells were collected. (A, B) Western blot analysis (n = 6 each group). *P < 0.05, significantly different from si‐Ctl. β‐actin was utilized as the internal control. (C) Cellular and medium S1P contents adjusted for the cellular protein level (n = 6 each group). *P < 0.05, significantly different from si‐Ctl. (D) SK activity assay (n = 6 each group). (E) Real‐time PCR (n = 5 each group). *P < 0.05, significantly different from si‐Ctl. β‐actin was utilized as the internal control. (F–I) HepG2 cells were treated with si‐SIRT1. After 48 h, the medium was replaced with FCS‐free medium containing various concentrations of c‐RSV for 16 h. Then, the medium and the cells were collected and analysed by Western blotting (n = 6 each group). (F, H) Cellular apoM level. β‐actin was utilized as the internal control. (G, I) Medium apoM level. Albumin was utilized as the internal control. *P < 0.05, significantly different from other groups. All the data except (C) were normalized to the mean of the control group.
Further experiments, using HepG2 cells with SIRT1 knock‐down, showed that low concentrations of c‐RSV (1‐10 μM) no longer increased cellular apoM or the medium apoM. However at the high concentration (100 μM), c‐RSV still decreased the apoM in culture medium levels, as observed in normal HepG2 cells.
Resveratrol increased both medium and cellular apoM levels even at a high concentration in human primary hepatocytes
In cultures of human primary hepatocytes, c‐RSV (1‐10 μM) increased both the medium and cellular apoM (Figure 6). In contrast to the results obtained with HepG2 cells, in human primary hepatocytes, the medium apoM was further increased by incubation with c‐RSV at 100 μM. The expression of LDL receptors tended to be increased by c‐RSV at 100 μM, although not statistically significant, in these human cells.
Figure 6.
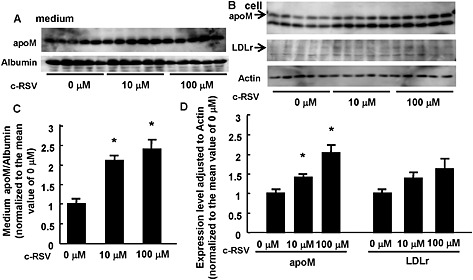
Resveratrol increased both the medium and cellular apoM levels, even at a high concentration in human primary hepatocytes. Human primary hepatocytes were treated with hepatocyte‐maintaining medium containing various concentrations of c‐RSV. After 16 h, the medium and the cells were collected and analysed by Western blotting (n = 5 each group). The intensity of the bands was quantified using Image J. For the medium apoM, albumin was utilized as a control; for the cellular protein, β‐actin was utilized as a control. (A, C) Medium apoM levels. (B, D) Cellular apoM and LDL receptor levels. *P < 0.05, significantly different from 0 μM. All the data were normalized to the mean of the control group.
Resveratrol increased both plasma and hepatic apoM and S1P contents in vivo
Finally, we investigated the effects of resveratrol on apoM and S1P levels in vivo. We fed wild‐type mice with resveratrol‐containing chow [0.3% (v/v)] or control chow for 2 weeks. In this model, treatment with resveratrol clearly increased the plasma resveratrol concentration to 0.34 ± 0.06 μM (Figure 7A) and decreased the plasma triglycerides (normal chow, 1.55 ± 0.19 g · L−1; resveratrol chow, 1.03 ± 0.09 g · L−1; P < 0.05) but did not affect total cholesterol (normal chow, 956 ± 25 mg · L−1; resveratrol chow, 990 ± 30 mg · L−1: P >0.05). As shown in Figure 7B and C, mice fed the resveratrol chow showed significantly increased levels of apoM and of S1P (Figure 7E and F), in both plasma and liver. However, resveratrol did not affect the expression of enzymes involved in S1P metabolism (data not shown), other than apoM (Figure 7D). The plasma concentration of dihydro‐S1P, which is considered to be another S1P receptor ligand, was also increased in mice fed with resveratrol, as expected (Kurano et al., 2013). Notably, the dietary resveratrol did not change either hepatic LDL receptor expression (Figure 7B and C) or LDL cholesterol levels (Figure 7G).
Figure 7.
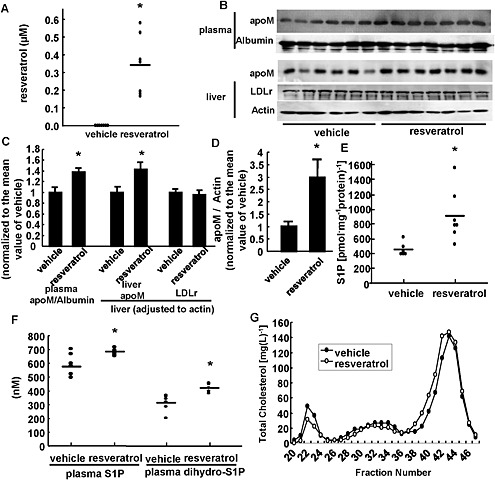
Resveratrol treatment in vivo increased both plasma and liver apoM and S1P contents, in mice. Ten‐week‐old male mice were fed with 0.3% resveratrol‐containing chow (resveratrol) or chow containing the vehicle (vehicle) for 2 weeks; then, the mice were subjected to a 6‐h fast, and blood and liver samples were subsequently collected (n = 6–7 each group). (A) Plasma resveratrol concentration. (B, C) Plasma apoM, hepatic apoM and LDL receptor (LDLr) levels. For the medium apoM, albumin was utilized as the control; for the cellular apoM, β‐actin was utilized as the control. (D) Hepatic apoM mRNA levels. β‐actin was utilized as the internal control. The data in (C) and (D) were normalized to the mean of the vehicle group. (E) Hepatic S1P levels adjusted for the hepatic protein level. (F) Plasma S1P and dihydro‐S1P levels. (G) Total cholesterol levels among the fractions separated using fast protein LC and a Superose 6 column. In A and C‐F, *P < 0.05, significant effect of resveratrol chow.
In plasma, S1P is bound to apoM in the HDL fraction or to albumin in the lipoprotein‐depleted fraction. Therefore, we measured the effects of dietary resveratrol on these two pools of plasma apoM. After two weeks of normal or resveratrol chow, blood samples were taken, plasma prepared and the plasma sample separated into the HDL fraction and the lipoprotein‐depleted fraction. As shown in Figure 8A, the S1P content was increased specifically in the HDL fraction, in samples from mice fed with resveratrol chow. Concordantly, the apoM content of the HDL fraction was also increased (Figure 8B and C).
Figure 8.
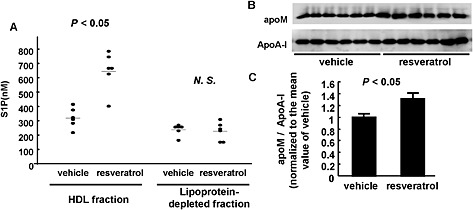
Resveratrol increased S1P contents only in the HDL fraction, but not in the lipoprotein‐depleted fraction. Ten‐week‐old male mice were fed with 0.3% resveratrol‐containing chow (resveratrol) or chow containing the vehicle (vehicle) for 2 weeks; then, the mice were subjected to a 6‐h fast, and plasma samples were collected, and HDL fraction and lipoprotein‐depleted fraction were separated with a ultracentrifugation method (n = 6 each group). (A) S1P contents in HDL fraction and lipoprotein‐depleted fraction. (B) apoM contents in HDL fraction. ApoA‐I was utilized as a control. The data were normalized to the mean of the vehicle group. In A and C, *P < 0.05, significant effect of resveratrol chow.
Discussion
Among the many proposed mechanisms underlying the numerous beneficial effects of resveratrol, such as its anti‐cancer and anti‐atherosclerotic properties (Baur et al., 2006; Orallo, 2008), resveratrol modulates S1P metabolism by suppressing SK1 and reducing S1P synthesis, changes which retarded the proliferation of cancer (Lim et al., 2012). Contrary to the harmful roles of S1P in oncology and immunology, S1P is believed to play protective roles in the field of vascular biology (Yatomi, 2006), and S1P was recently shown to be carried on apoM, which is itself carried by HDL (Christoffersen et al., 2011). Therefore, in this study, we investigated the effects of resveratrol on apoM and S1P, using both in vitro (HepG2 cells and human primary hepatocytes) and in vivo models.
In HepG2 cells, the levels of apoM in the culture medium were increased by lower concentrations of resveratrol (1–10 μM) but decreased by 100 μM resveratrol. The mechanism underlying this decrease in the medium apoM in HepG2 cell cultures is the increased expression of LDL receptors by the high concentration of resveratrol (Figure 4), as expected from earlier work (Christoffersen et al., 2012; Yashiro et al., 2012; Kurano et al., 2015). The high concentration of resveratrol did not decrease the S1P in the medium when the medium apoM level was decreased. This finding suggests that resveratrol increased S1P in the medium by enhancing S1P secreted from HepG2 cells, while resveratrol decreased the medium S1P level by up‐regulating the LDL receptor‐mediated clearance of apoM‐associated S1P from the medium. Such opposing effects might have offset each other in this experiment.
In contrast to its effects on apoM in the medium, resveratrol increased the cellular apoM levels at all concentrations tested, through the activation of SIRT1 (Figures 1 and 5). To our knowledge, this is the first report to demonstrate that resveratrol and SIRT1 increase apoM expression. Concordant with the modulation of the cellular apoM expression level, the cellular S1P levels were increased by resveratrol and decreased with the knockdown of SIRT1 (Figures 2A and 5C), which agrees with our previous reports that hepatic apoM and S1P are increased in a synchronized manner (Kurano et al., 2013; Nojiri et al., 2014). The result that resveratrol did not increase the cellular apoM in SIRT1‐knockdown HepG2 cells supported the importance of SIRT1 in the resveratrol‐mediated up‐regulation of apoM expression. In terms of the possible mechanisms involved in the resveratrol‐mediated regulation of apoM through the histone deacetylase SIRT1, a range of nuclear factors have been reported to regulate apoM expression. For instance, LXR (Zhang et al., 2008), Foxa2 (Wolfrum et al., 2008) and PPARs (Luo et al., 2014) suppressed apoM expression, while LRH‐1 (Venteclef et al., 2008) and HNF‐4 (Mosialou et al., 2010) increased apoM expression. Among these candidates, resveratrol suppressed the activity of PPARα/γ (Picard et al., 2004; Calleri et al., 2014), although Luo et al. reported that PPARβ/δ is an especially important inhibitory nuclear factor in apoM expression. Another aspect of the regulation of apoM derived from the present study is the lack of a negative feedback regulating apoM because the mRNA level of apoM was not decreased in the HepG2 cells treated with resveratrol at 100 μM, which increased the uptake of apoM from the medium into the cells through the up‐regulation of LDL receptors. Further studies are necessary to investigate the regulation of apoM expression.
As HepG2 cells are hepatoma cells and their reaction to resveratrol might be different from that of normal hepatocytes, we assessed the effects of resveratrol on the apoM level in human primary hepatocytes. Interestingly, in these hepatocytes, although resveratrol increased the cellular apoM level, as in HepG2 cells, the high concentration of resveratrol (100 μM) did not decrease, but increased the medium apoM level (Figure 6), which was not the case with HepG2 cells (Figure 1). This difference might result from the different extent of LDL receptor induction by the high concentration of resveratrol in the two cell types, as 100 μM resveratrol clearly increased expression of LDL receptors in HepG2cells, but not in human primary hepatocytes.
Finally, we also investigated the effects of resveratrol on apoM and S1P in vivo using wild‐type mice. Resveratrol chow increased hepatic apoM expression, together with S1P content, and also increased the plasma apoM and S1P levels (Figure 7). Because the plasma concentrations of resveratrol in mice fed resveratrol‐containing chow were very much lower than 100 μM (Figure 7A), it is reasonable to suggest that the plasma apoM level was not decreased and that hepatic LDL receptor expression was not increased in this in vivo experiment. Taking this in vivo result into consideration together with the results from the human primary hepatocytes, the apoM and S1P levels would not be changed in humans taking normal doses of resveratrol.
Regarding the modulation of SK, resveratrol did not increase SK expression or activity in HepG2 cells or murine liver (Figure 3A and C and data not shown), while previous reports demonstrated that resveratrol inhibited the expression and activity of SK in MCF‐7 breast cancer cells (Lim et al., 2012) and in colons of a rat model for ulcerative colitis (Abdin, 2013). Although the reason for this discrepancy remains to be elucidated, the inhibitory effect of resveratrol on SK might depend on the cell type or the cell status. If this apparent selectivity of the inhibitory property of resveratrol on SK is a reliable finding, resveratrol might be utilized safely for the treatment of specific diseases as an SK inhibitor, because S1P is an important biological lipid for all cells other than the target cells.
The results from the present study suggest that the anti‐atherosclerotic properties of resveratrol might be attributed at least partly to the induction of apoM and S1P, especially HDL‐linked S1P, which is consistent with the previous reports that S1P shares several cardio‐protective properties with resveratrol, such as anti‐apoptosis (Goetzl, 2001; Ungvari et al., 2007), anti‐inflammation (Csiszar et al., 2006; Kimura et al., 2006), induction of vasorelaxation (Igarashi et al., 2001; Hung et al., 2004; Klinge et al., 2008) and maintenance of vascular permeability (Garcia et al., 2001; Jing et al., 2010). Further studies are needed to elucidate the links between resveratrol and S1P, especially in terms of vascular diseases. Along with the anti‐atherosclerotic effects, the induction of apoM by resveratrol suggests further benefits of resveratrol in other fields. In diabetes, apoM increased in streptozotocin‐induced diabetic mice (Nojiri et al., 2014)and apoM induced insulin secretion (Kurano et al., 2014). In infectious diseases, decreased apoM is associated with plasma leakage and systemic inflammatory responses (Kumaraswamy et al., 2012; Michels et al., 2015). Moreover, because recent elegant studies have demonstrated that apoM‐bound S1P acts exclusively on the S1P1 receptor (Blaho et al., 2015; Galvani et al., 2015), resveratrol could exert several beneficial effects in many other diseases in which this S1P receptor is involved.
In summary, resveratrol increased the apoM and S1P contents in the plasma and liver by activating SIRT1, while a high concentration of resveratrol can decrease apoM and S1P in the circulation through the up‐regulation of LDL receptors.
Conflict of interest
Authors declare that they have not any conflict of interest.
Supporting information
Figure S1 Measurement of plasma resveratrol with HPLC method.
Supporting info item
Acknowledgements
This work was supported by JSPS KAKENHI grant number 25253040 (Y. Y.) and grant number 25860740 (M. K.) and by CREST from the JST.
Kurano, M. , Hara, M. , Nojiri, T. , Ikeda, H. , Tsukamoto, K. , and Yatomi, Y. (2016) Resveratrol exerts a biphasic effect on apolipoprotein M. British Journal of Pharmacology, 173: 222–233. doi: 10.1111/bph.13360.
References
- Abdin AA (2013). Targeting sphingosine kinase 1 (SphK1) and apoptosis by colon‐specific delivery formula of resveratrol in treatment of experimental ulcerative colitis in rats. Eur J Pharmacol 718: 145–153. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M et al. (2013a). The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol 170: 1797–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M et al. (2013b). The Concise Guide to PHARMACOLOGY 2013/14: G Protein‐Coupled Receptors. Br J Pharmacol 170: 1459–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allende ML, Sasaki T, Kawai H, Olivera A, Mi Y, van Echten‐Deckert G et al. (2004). Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J Biol Chem 279: 52487–52492. [DOI] [PubMed] [Google Scholar]
- Argraves KM, Sethi AA, Gazzolo PJ, Wilkerson BA, Remaley AT, Tybjaerg‐Hansen A et al. (2011). S1P, dihydro‐S1P and C24:1‐ceramide levels in the HDL‐containing fraction of serum inversely correlate with occurrence of ischemic heart disease. Lipids Health Dis 10: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A et al. (2006). Resveratrol improves health and survival of mice on a high‐calorie diet. Nature 444: 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA (2006). Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 5: 493–506. [DOI] [PubMed] [Google Scholar]
- Blaho VA, Galvani S, Engelbrecht E, Liu C, Swendeman SL, Kono M et al. (2015). HDL‐bound sphingosine‐1‐phosphate restrains lymphopoiesis and neuroinflammation. Nature 523: 342–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borra MT, Smith BC, Denu JM (2005). Mechanism of human SIRT1 activation by resveratrol. J Biol Chem 280: 17187–17195. [DOI] [PubMed] [Google Scholar]
- Calleri E, Pochetti G, Dossou KS, Laghezza A, Montanari R, Capelli D et al. (2014). Resveratrol and its metabolites bind to PPARs. Chembiochem 15: 1154–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, He H, Wang G, Yang B, Ren W, Ma L et al. (2007). Stereospecific determination of cis‐ and trans‐resveratrol in rat plasma by HPLC: application to pharmacokinetic studies. Biomed Chromatogr 21: 257–265. [DOI] [PubMed] [Google Scholar]
- Christoffersen C, Benn M, Christensen PM, Gordts PL, Roebroek AJ, Frikke‐Schmidt R et al. (2012). The plasma concentration of HDL‐associated apoM is influenced by LDL receptor‐mediated clearance of apoB‐containing particles. J Lipid Res 53: 2198–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffersen C, Obinata H, Kumaraswamy SB, Galvani S, Ahnstrom J, Sevvana M et al. (2011). Endothelium‐protective sphingosine‐1‐phosphate provided by HDL‐associated apolipoprotein M. Proc Natl Acad Sci U S A 108: 9613–9618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A, Ungvari Z (2006). Resveratrol attenuates TNF‐alpha‐induced activation of coronary arterial endothelial cells: role of NF‐kappaB inhibition. Am J Physiol Heart Circ Physiol 291: H1694–1699. [DOI] [PubMed] [Google Scholar]
- Das DK, Maulik N (2006). Resveratrol in cardioprotection: a therapeutic promise of alternative medicine. Mol Interv 6: 36–47. [DOI] [PubMed] [Google Scholar]
- Galvani S, Sanson M, Blaho VA, Swendeman SL, Conger H, Dahlback B et al. (2015). HDL‐bound sphingosine 1‐phosphate acts as a biased agonist for the endothelial cell receptor S1P1 to limit vascular inflammation. Sci Signal 8 ra79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT et al. (2001). Sphingosine 1‐phosphate promotes endothelial cell barrier integrity by Edg‐dependent cytoskeletal rearrangement. J Clin Invest 108: 689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl EJ (2001). Pleiotypic mechanisms of cellular responses to biologically active lysophospholipids. Prostaglandins 64: 11–20. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM (2008). Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 9: 139–150. [DOI] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG et al. (2003). Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425: 191–196. [DOI] [PubMed] [Google Scholar]
- Hung LM, Su MJ, Chen JK (2004). Resveratrol protects myocardial ischemia‐reperfusion injury through both NO‐dependent and NO‐independent mechanisms. Free Radic Biol Med 36: 774–781. [DOI] [PubMed] [Google Scholar]
- Igarashi J, Bernier SG, Michel T (2001). Sphingosine 1‐phosphate and activation of endothelial nitric‐oxide synthase: differential regulation of Akt and MAP kinase pathways by EDG and bradykinin receptors in vascular endothelial cells. J Biol Chem 276: 12420–12426. [DOI] [PubMed] [Google Scholar]
- Jing YH, Chen KH, Yang SH, Kuo PC, Chen JK (2010). Resveratrol ameliorates vasculopathy in STZ‐induced diabetic rats: role of AGE‐RAGE signalling. Diabetes Metab Res Rev 26: 212–222. [DOI] [PubMed] [Google Scholar]
- Katsagonis A, Atta‐Politou J, Koupparis MA (2005). HPLC method with UV detection for the determination of trans‐resveratrol in plasma. J Liq Chromatogr R T 28: 1393–1405. [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). NC3Rs Reporting Guidelines Working Group. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Tomura H, Mogi C, Kuwabara A, Damirin A, Ishizuka T et al. (2006). Role of scavenger receptor class B type I and sphingosine 1‐phosphate receptors in high density lipoprotein‐induced inhibition of adhesion molecule expression in endothelial cells. J Biol Chem 281: 37457–37467. [DOI] [PubMed] [Google Scholar]
- Klinge CM, Wickramasinghe NS, Ivanova MM, Dougherty SM (2008). Resveratrol stimulates nitric oxide production by increasing estrogen receptor alpha‐Src‐caveolin‐1 interaction and phosphorylation in human umbilical vein endothelial cells. FASEB J 22: 2185–2197. [DOI] [PubMed] [Google Scholar]
- Kumaraswamy SB, Linder A, Akesson P, Dahlback B (2012). Decreased plasma concentrations of apolipoprotein M in sepsis and systemic inflammatory response syndromes. Crit Care 16: R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurano M, Hara M, Tsuneyama K, Sakoda H, Shimizu T, Tsukamoto K et al. (2014). Induction of insulin secretion by apolipoprotein M, a carrier for sphingosine 1‐phosphate. Biochim Biophys Acta 1841: 1217–1226. [DOI] [PubMed] [Google Scholar]
- Kurano M, Iso ON, Hara M, Ishizaka N, Moriya K, Koike K et al. (2011). LXR agonist increases apoE secretion from HepG2 spheroid, together with an increased production of VLDL and apoE‐rich large HDL. Lipids Health Dis 10: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurano M, Tsukamoto K, Hara M, Ohkawa R, Ikeda H, Yatomi Y (2015). LDL receptor and ApoE are involved in the clearance of ApoM‐associated sphingosine 1‐phosphate. J Biol Chem 290: 2477–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurano M, Tsukamoto K, Ohkawa R, Hara M, Iino J, Kageyama Y et al. (2013). Liver involvement in sphingosine 1‐phosphate dynamism revealed by adenoviral hepatic overexpression of apolipoprotein M. Atherosclerosis 229: 102–109. [DOI] [PubMed] [Google Scholar]
- Leonard SS, Xia C, Jiang BH, Stinefelt B, Klandorf H, Harris GK et al. (2003). Resveratrol scavenges reactive oxygen species and effects radical‐induced cellular responses. Biochem Biophys Res Commun 309: 1017–1026. [DOI] [PubMed] [Google Scholar]
- Lim KG, Gray AI, Pyne S, Pyne NJ (2012). Resveratrol dimers are novel sphingosine kinase 1 inhibitors and affect sphingosine kinase 1 expression and cancer cell growth and survival. Br J Pharmacol 166: 1605–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Allegood J, Zhu X, Seo J, Gebre AK, Boudyguina E et al. (2015). Uncleaved ApoM signal peptide is required for formation of large ApoM/sphingosine 1‐phosphate (S1P)‐enriched HDL particles. J Biol Chem 290: 7861–7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Seo J, Allegood J, Bi X, Zhu X, Boudyguina E et al. (2014). Hepatic apolipoprotein M (apoM) overexpression stimulates formation of larger apoM/sphingosine 1‐phosphate‐enriched plasma high density lipoprotein. J Biol Chem 289: 2801–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G, Shi Y, Zhang J, Mu Q, Qin L, Zheng L et al. (2014). Palmitic acid suppresses apolipoprotein M gene expression via the pathway of PPARbeta/delta in HepG2 cells. Biochem Biophys Res Commun 445: 203–207. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, Kilkenny C, Wainwright C (2010). Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol 160: 1573–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels M, Japtok L, Alisjahbana B, Wisaksana R, Sumardi U, Puspita M et al. (2015). Decreased plasma levels of the endothelial protective sphingosine‐1‐phosphate are associated with dengue‐induced plasma leakage. J Infect 71: 480–487. [DOI] [PubMed] [Google Scholar]
- Mosialou I, Zannis VI, Kardassis D (2010). Regulation of human apolipoprotein m gene expression by orphan and ligand‐dependent nuclear receptors. J Biol Chem 285: 30719–30730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojiri T, Kurano M, Tokuhara Y, Ohkubo S, Hara M, Ikeda H et al. (2014). Modulation of sphingosine‐1‐phosphate and apolipoprotein M levels in the plasma, liver and kidneys in streptozotocin‐induced diabetic mice. J Diabetes Investig 5: 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okajima F (2002). Plasma lipoproteins behave as carriers of extracellular sphingosine 1‐phosphate: is this an atherogenic mediator or an anti‐atherogenic mediator? Biochim Biophys Acta 1582: 132–137. [DOI] [PubMed] [Google Scholar]
- Orallo F (2008). Trans‐resveratrol: a magical elixir of eternal youth? Curr Med Chem 15: 1887–1898. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP et al. (2014). The IUPHAR/BPS Guide to PHARMACOLOGY: an expert‐driven knowledge base of drug targets and their ligands. Nucl Acids Res 42 (Database Issue): D1098–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard F, Kurtev M, Chung N, Topark‐Ngarm A, Senawong T, Machado De Oliveira R et al. (2004). Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR‐gamma. Nature 429: 771–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne NJ, Pyne S (2010). Sphingosine 1‐phosphate and cancer. Nat Rev Cancer 10: 489–503. [DOI] [PubMed] [Google Scholar]
- Renaud S, de Lorgeril M (1992). Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 339: 1523–1526. [DOI] [PubMed] [Google Scholar]
- Rodriguez C, Gonzalez‐Diez M, Badimon L, Martinez‐Gonzalez J (2009). Sphingosine‐1‐phosphate: A bioactive lipid that confers high‐density lipoprotein with vasculoprotection mediated by nitric oxide and prostacyclin. Thromb Haemost 101: 665–673. [PubMed] [Google Scholar]
- Sutter I, Park R, Othman A, Rohrer L, Hornemann T, Stoffel M et al. (2014). Apolipoprotein M modulates erythrocyte efflux and tubular reabsorption of sphingosine‐1‐phosphate. J Lipid Res 55: 1730–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabe K, Spiegel S (2014). Export of sphingosine‐1‐phosphate and cancer progression. J Lipid Res 55: 1839–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trela BC, Waterhouse AL (1996). Resveratrol: isomeric molar absorptivities and stability. J Agr Food Chem 44: 1253–1257. [Google Scholar]
- Ungvari Z, Orosz Z, Rivera A, Labinskyy N, Xiangmin Z, Olson S et al. (2007). Resveratrol increases vascular oxidative stress resistance. Am J Physiol Heart Circ Physiol 292: H2417–2424. [DOI] [PubMed] [Google Scholar]
- Venteclef N, Haroniti A, Tousaint JJ, Talianidis I, Delerive P (2008). Regulation of anti‐atherogenic apolipoprotein M gene expression by the orphan nuclear receptor LRH‐1. J Biol Chem 283: 3694–3701. [DOI] [PubMed] [Google Scholar]
- Wilkerson BA, Grass GD, Wing SB, Argraves WS, Argraves KM (2012). Sphingosine 1‐phosphate (S1P) carrier‐dependent regulation of endothelial barrier: high density lipoprotein (HDL)‐S1P prolongs endothelial barrier enhancement as compared with albumin‐S1P via effects on levels, trafficking, and signaling of S1P1. J Biol Chem 287: 44645–44653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfrum C, Howell JJ, Ndungo E, Stoffel M (2008). Foxa2 activity increases plasma high density lipoprotein levels by regulating apolipoprotein M. J Biol Chem 283: 16940–16949. [DOI] [PubMed] [Google Scholar]
- Wu JM, Wang ZR, Hsieh TC, Bruder JL, Zou JG, Huang YZ (2001). Mechanism of cardioprotection by resveratrol, a phenolic antioxidant present in red wine (Review). Int J Mol Med 8: 3–17. [DOI] [PubMed] [Google Scholar]
- Xu N, Dahlback B (1999). A novel human apolipoprotein (apoM). J Biol Chem 274: 31286–31290. [DOI] [PubMed] [Google Scholar]
- Yashiro T, Nanmoku M, Shimizu M, Inoue J, Sato R (2012). Resveratrol increases the expression and activity of the low density lipoprotein receptor in hepatocytes by the proteolytic activation of the sterol regulatory element‐binding proteins. Atherosclerosis 220: 369–374. [DOI] [PubMed] [Google Scholar]
- Yatomi Y (2006). Sphingosine 1‐phosphate in vascular biology: possible therapeutic strategies to control vascular diseases. Curr Pharm Des 12: 575–587. [DOI] [PubMed] [Google Scholar]
- Yatomi Y (2008). Plasma sphingosine 1‐phosphate metabolism and analysis. Biochim Biophys Acta 1780: 606–611. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhu Z, Luo G, Zheng L, Nilsson‐Ehle P, Xu N (2008). Liver X receptor agonist downregulates hepatic apoM expression in vivo and in vitro. Biochem Biophys Res Commun 371: 114–117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Measurement of plasma resveratrol with HPLC method.
Supporting info item


