Abstract
Background and Purpose
Homoharringtonine (HHT) is a natural alkaloid isolated from various Cephalotaxus species. HHT has been used to treat acute myeloid leukaemia (AML), chronic myeloid leukaemia (CML), chronic lymphocyte leukaemia and myelodysplastic syndromes. Although HHT inhibits protein synthesis and promotes apoptosis of leukaemia cells in preclinical studies, its molecular target proteins remain unknown. The aim of this study was to identify target proteins of HHT.
Experimental Approach
We have synthesized a biotinylated affinity column and used it to identify targets of HHT and confirmed the results by MS and Western blots. We also examined the effects of HHT on the target protein and determined roles of the target protein in anti‐leukaemia activities of HHT through Western blots, flow cytometry and retrovirus transfection.
Key Results
Myosin‐9, a member of the myosin super‐family, was identified as a direct interactor of HHT. Furthermore, HHT up‐regulated the expression level of myosin‐9 in both AML and CML cell lines in a time‐dependent manner. Thus, HHT‐induced apoptosis of leukaemia cells begins in 6 h and continues to increase for 24 h. There is a positive correlation between up‐regulated myosin‐9 expression level and increased percentage of apoptotic cells mediated by HHT. Overexpression of myosin‐9 could increase the sensitivity of the leukaemia cells to the cytotoxicity of HHT and arrest cells in S and G2/M phases.
Conclusions and Implications
Our results indicated that myosin‐9 was the target protein of HHT and played an important role in the HHT‐induced apoptosis of leukaemia cells.
Abbreviations
- AML
acute myeloid leukaemia
- CML
chronic myeloid leukaemia
- GST
glutathione S‐transferase
- HHT
homoharringtonine
- NM II
non‐muscle myosin II
- NMHC‐IIA
non-muscle myosin heavy chain‐IIA; alternatively called myosin‐9
- TKI
TK inhibitor
Tables of Links
| TARGETS |
|---|
| Other proteins |
| Bcl‐2 |
| Myeloid cell leukaemia‐1(Mcl‐1) |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013).
Introduction
Homoharringtonine (HHT; omacetaxine mepesuccinate) is a cephalotaxine ester that was initially extracted from the evergreen tree Cephalotaxus harringtonia. The potent anti‐leukaemia effects of HHT were initially reported by Chinese investigators in the 1970s (Chin Med J (Engl), (1976; Chin Med J (Engl), (1977). To date, HHT‐based regimens have been widely used to treat haematopoietic malignant disorders, such as acute myeloid leukaemia (AML) and chronic myeloid leukaemia (CML). During the past decades, the results from a large number of clinical trials in patients with AML have indicated that HHT, given alone or in combination with other chemotherapy drugs, induces remission in patients with AML. Our group has also demonstrated that HHT in combination with cytarabine and aclarubicin resulted in a high complete remission rate in patients with AML (Jin et al., 2006; Jin et al., 2013; Yu et al., 2013). Furthermore, a series of studies conducted in Western countries also confirmed the promising effectiveness of HHT in treating patients with CML after TK inhibitor (TKI) failure (Quintas‐Cardama et al., 2007; Quintas‐Cardama et al., 2009; Cortes et al., 2012).
However, little is known about the mechanism underlying the anti‐leukaemia activities of HHT. According to the results available from preclinical studies, HHT exerts its anti‐leukaemia activities by inhibiting protein synthesis and inducing cell death in a variety of leukaemia cell lines. This natural compound blocked substrates from binding to the receptor site on the ribosome subunit, thereby impairing chain elongation and inhibiting protein synthesis (Fresno et al., 1977; Tujebajeva et al., 1989; Gurel et al., 2009). Our previous study also revealed that cell apoptosis induced by HHT was mediated by the up‐regulation of the proapoptotic protein Bax and the induction of caspase‐3‐mediated cleavage of PARP (Yinjun et al., 2004). Down‐regulation of myeloid cell leukaemia‐1, one of the antiapoptotic proteins of the Bcl‐2 family was observed in HHT‐treated leukaemia cells (Allan et al., 2011; Chen et al., 2011). To date, the molecular target and mechanisms of HHT against leukaemia remain unknown.
In this study, we first synthesized an effective HHT affinity reagent to identify its molecular target. Myosin‐9, the skeletal contractile protein, was identified as the direct interactor of HHT in leukaemia cells by the novel affinity column in combination with MS and Western blot analysis. Furthermore, the effect of HHT on the target protein was evaluated. We found that HHT up‐regulated myosin‐9 expression in both AML and CML cell lines. The increase in the target protein induced S and G2/M phase arrests of leukaemia cells, enhanced the sensitivity of leukaemia cells to HHT and was involved in cell apoptosis mediated by the anticancer drug. Taken together, these data provide insights into the mechanism underlying the anti‐leukaemia effects of HHT.
Methods
Affinity column preparation
A total of 50 mg of PFP‐biotin (pentafluorophenyl ester of biotin, Pierce, Rockford, IL, USA) was dissolved in 1 mL anhydrous dimethylformamide (DMF), and 250 mg of HHT dissolved in 1 mL DMF was added. The mixture was stirred at 60°C in a nitrogen atmosphere shielded from light. The production of HHT biotin ester was monitored with LC/MS (m/z 772 [M + H]+) and MS/MS (772 → 258).
Avidin agarose beads (2 mL; Pierce) were packed in a column and equilibrated with 10 mL 0.1 M PBS pH 7.4. Then, HHT biotin ester diluted with 0.1 M PBS pH 7.4 was added to the column. The column was plugged, sealed and then rotated for 2 h at room temperature. Then, the column was eluted with 20 mL of 0.1 M PBS pH 7.4 to wash out the free chemicals. To block the unbound avidin sites, 5 mL of 1 mM biotin was added to the column. After the biotin solution had flowed through the column, the column was plugged and incubated for 2 h at room temperature. After incubation, the column was eluted with 20 mL 0.1 M PBS pH 7.4. The control column was prepared by replacing the HHT biotin ester with 0.1 M PBS pH 7.4. The columns were stored at 4°C in 0.1 M PBS pH 7.4 with 0.02% sodium azide.
Affinity chromatography
Cells were washed with ice‐cold PBS and lysed in RIPA buffer containing protease inhibitors. Whole lysate of Kasumi‐1 cells was incubated with affinity matrix or control affinity matrix at 4°C for 2 h. After washing with 0.1 M PBS pH 7.4 buffer and 2.0 M NaCl in 0.1 M PBS pH 7.4 buffer, the affinity columns were eluted with 0.1 M PBS pH 7.4 buffer containing 1 mM HHT as a competitor, three times. The eluates were collected and dialyzed overnight at 4°C. After drying in a vacuum freeze drier, the samples were loaded and separated on 8–15% Tris‐Glycine SDS‐PAGE. The gels were stained with a Silver Stain Plus Kit (Bio‐Rad, Hercules, CA, USA) and a Colloidal Blue Staining Kit (Invitrogen, Waltham, MA, USA). The protein bands were excised and digested with trypsin. The purified peptides were dried under a vacuum, resuspended in 0.1% formic acid and 5% acetonitrile and then analysed by LC‐MS/MS in the positive ion mode with a Thermo Scientific LTQ Orbitrap Velos mass spectrometer coupled with a Waters nanoAcquity Ultra Performance LC system. The peptide masses obtained by LC‐MS/MS analysis were processed against the Human IPI database.
Western blot analysis
The protein samples were resuspended in loading buffer, denatured for 5 min at 95°C, subjected to SDS‐PAGE and then transferred onto nitrocellulose membranes. The membranes were blocked for 1 h at room temperature with 5% non‐fat dry milk in PBS‐Tween 20 (TBS‐T) and then immunoblotted overnight at 4°C with primary antibody against myosin‐9 (1:1000, ab24762, Abcam, Cambridge, UK). After washing with TBS‐T, the membranes were exposed to the HRP‐conjugated secondary antibody for 1 h at room temperature, and then the antigen–antibody complexes were allowed to react with the SuperSignal West Pico Chemiluminescent Substrate (Pierce).
Cell culture
The human AML cell lines Kasumi‐1 and HL‐60 (Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China) and the human CML cell lines KCL‐22 and imatinib‐resistant KCL‐22 M (PhD Rongzhen Xu (Cancer Institute, Zhejiang Univ. Hangzhou 310009,China)) with T315I mutant Bcr‐ Abl were cultured in RPMI‐1640 supplemented with 10% fetal calf serum (FCS). The cell culture medium DMEM containing 10% FCS was used to culture the Plat‐GP retrovirus packaging cell line (Cell Biolabs Inc., San Diego, CA, USA). All these cell lines were maintained in a 37°C humidified incubator with 5% CO2.
Cell proliferation assay
Cells were cultured in 96‐well plates and treated with HHT at various concentrations for 24 h. Cell proliferation was measured with an MTS assay (Promega, Madison, WI, USA) following the manufacturer's instructions.
Assessment of apoptosis
Apoptotic cells were evaluated by annexin V and propidium iodide (PI) staining. Briefly, samples of the cells (106 cells per sample) were washed once in PBS and resuspended in annexin‐binding buffer. Alexa Fluor 488 annexin V and PI working solution were added to the cell suspension, and the cells were incubated at room temperature for 15 min as instructed by the manufacturer (Invitrogen). The percentage of apoptotic cells was determined by flow cytometry.
Cell cycle assay
Cultured cells were trypsinized and fixed with ice‐cold 70% ethanol. For DNA content assay, PI was added at 40 g·mL−1, and cells were incubated in the presence of RNase at 100 g·mL−1 for 30 min at 37°C. DNA contents were determined in a Becton Dickinson FACAS. The percentage of cells in each of the phases of the cell cycles was calculated.
Establishment of Kasumi‐1 cells stably overexpressing NMHC‐IIA
Approximately, 5 × 106 Plat‐GP retrovirus packaging cells were seeded onto a 100 mm culture dish without antibiotics, including puromycin and blasticidin, 1 day before transfection. When the culture was 70–80% confluent, pMxs‐non‐muscle myosin heavy chain‐IIA (NMHC‐IIA) or pMxs‐puro plasmid and the pCMV‐VSV‐G envelope vector (Cell biolabs, San Diego, CA, USA) were mixed with X‐treme GENE HP DNA Transfection Reagent (Roche Applied Science, Penzberg, Germany), and the plat‐GP cells were transfected with the DNA‐FuGENE complex. The retroviral supernatant was harvested 48 h after transfection. Kasumi‐1 cells were transduced by infection with retrovirus‐containing supernatants from Plat‐GP cells. At 24 h after transduction, these cells were plated in puromycin (0.5 mg·mL−1) selection medium. Resistant cells transduced by the recombinant retrovirus derived from pMXs‐NMHC‐IIA or pMxs‐puro were designated as Kasumi‐1/NMHC‐IIA or Kasumi‐1/puro cells respectively.
Data analysis
The optical densities of immunoblots were quantitatively analysed and compared by using QUANTITY ONE software (Bio‐Rad). To facilitate comparisons between groups, the band density values were normalized to the mean value for the control. Data are expressed as means ± SD. Differences were evaluated by a t‐test analysis of variance, and P < 0.05 was considered to be statistically significant.
Materials
Homoharringtonine was purchased from Zhejiang Institute of Drug Control (Zhejiang, China).
Results
HHT binds to myosin‐9
To identify the direct molecular target of HHT, a novel affinity reagent was used as a molecular probe. HHT was conjugated to biotin by the chemical reaction between HHT and PFP‐biotin (Figure 1). The biotinylated HHT was immobilized with avidin agarose beads, and the resulting beads were packed in a small column for affinity chromatography. Whole lysates of Kasumi‐1 cells were loaded onto the affinity column followed by washing with PBS buffer and 2.0 M NaCl in PBS buffer. The proteins retained in the column were eluted with an HHT‐containing solution. SDS‐PAGE analysis of the eluates revealed two protein bands at molecular masses of approximately 220 and 40 KDa (Figure 2A and B). The two proteins were identified as myosin‐9 (220 KDa) and actin (42 KDa) by MS‐based protein sequencing (data not shown), which was confirmed by immunoblot analysis (Figure 2C). It is known that myosin‐9 (alternatively called NMHC‐IIA) is an actin‐binding protein that interacts with actin filaments at its globular head domain. When bound to actin, myosin‐9 switches to its active form and exerts its functions in cells. To determine whether HHT binds directly to myosin‐9, additional pull down assays were performed. The recombinant proteins glutathione S‐transferase (GST)‐myosin‐9 and GST were individually incubated with biotinylated HHT, and the complexes were isolated with avidin agarose. Western blot analysis indicated that HHT specifically binds with myosin‐9 but not GST protein (Figure 2D). These results suggest that myosin‐9, the actin‐binding protein, is the target protein that directly interacts with HHT in leukaemia cells.
Figure 1.
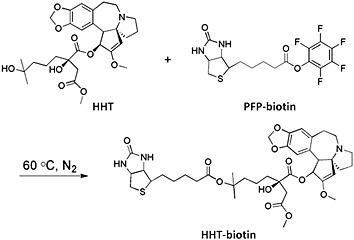
Structure of biotinylated HHT.
Figure 2.
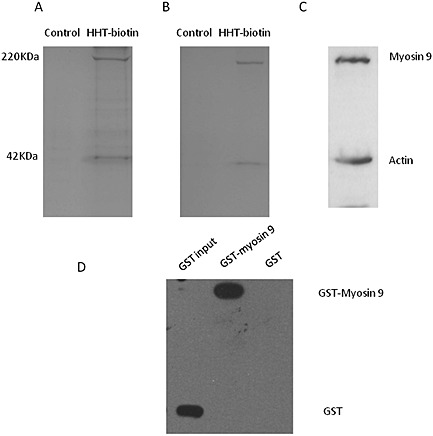
Identification of myosin‐9 as a HHT target protein. (A) Silver‐stained gel and (B) colloidal blue‐stained gel loaded with lysates of Kasumi‐1 cells eluted from biotin‐conjugated (control, lane 1) and HHT‐conjugated (lane 2) affinity columns. Two proteins with molecular masses of approximately 220 and 40 KDa were detected in the eluates. (C) The two bands were confirmed to be myosin‐9 and actin by immunoblot analysis with the corresponding antibodies. (D) HHT directly binds to the myosin‐9 protein. Purified GST‐myosin‐9 and GST proteins were individually incubated with biotinylated HHT, and the complexes were isolated with avidin agarose. Immunoblot analysis showed that HHT pulled down myosin‐9 protein but not GST protein.
HHT up‐regulates myosin‐9 expression in leukaemia cells
There are few studies regarding the importance of myosin‐9 in leukaemia, and no evidence indicating the myosin‐9 is involved in the mechanism underlying the antitumour activities of chemotherapeutic drugs. Therefore, we investigated the role of myosin‐9 in the anti‐leukaemia action of HHT. First, the effect of HHT on the expression of myosin‐9 in leukaemia cells was measured. Kasumi‐1 cells were treated with HHT (20 ng·mL−1) and collected at different time points. Then, the expression level of myosin‐9 was determined by immunoblot analysis. As shown in Figure 3A, a time‐dependent increase in myosin‐9 protein was found in the cells after exposure to HHT. An increase in myosin‐9 was detected as early as 6 h, and the expression level continued to increase in the following 6–18 h. Next, we treated the HL‐60 cell line, the U937 cell line, the THP‐1 cell line, the CML cell line KCL‐22 and imatinib‐resistant KCL‐22 M cells (T315I mutant Bcr‐Abl) with IC50 concentration of HHT for 6, 12 and 24 h and examined the myosin‐9 expression in these cells. Similar elevations in myosin‐9 expression were observed in these cell lines (Figure 3C–G). These results suggest that an up‐regulation in myosin‐9 expression occurs in not only AML but also in CML cells, as well as in imatinib‐resistant CML cells. In addition, we treated primary haematopoietic stem/progenitor cells (HSPCs) cells from three newly diagnosed adult AML patients with HHT and determined the myosin‐9 expression levels. The results showed that HHT treatment increased myosin‐9 expression in these primary cells (Figure 3H–J). Next, we determined whether the IC50 value of HHT correlated with the baseline expression of myosin‐9. As expected, baseline levels of myosin‐9 in leukaemia cells were detected. However, the myosin‐9 baselines did not show a good correlation with the IC50 values of HHT in these cells (Supporting Information).
Figure 3.
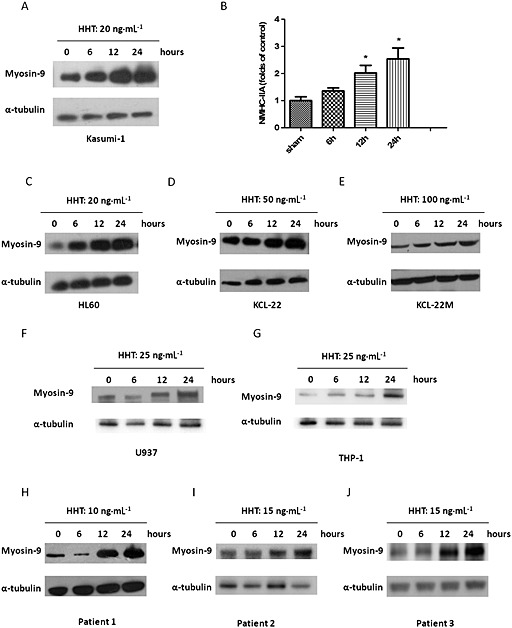
HHT increases myosin‐9 in leukaemia cells. (A) Expression of myosin‐9 protein. Kasumi‐1 cells were treated with HHT (20 ng·mL−1) for 6, 12 and 24 h, and the protein expression was determined by immunoblot analysis. α‐Tubulin expression was used as the internal standard. (B) Relative band densities of myosin‐9. Densities of protein bands were analysed and normalized to α‐tubulin. The data are expressed as percentage of sham‐operated control. Data shown are means ± SD, n = 3, *P < 0.05, significantly different from sham. (C–G) Levels of myosin‐9 expression in HL‐60 cells, U937 cells, THP‐1 cells, KCL‐22 cells and KCL‐22 M cells were evaluated after treatment with HHT. H–(J) Myosin‐9 expression levels in AML haematopoietic stem and progenitor cells after HHT treatment.
Importance of myosin‐9 for HHT‐induced cytotoxicity
To determine whether the HHT‐induced increase in myosin‐9 protein is associated with the apoptosis caused by this compound, we examined the apoptosis of leukaemia cells after HHT exposure. Kasumi‐1 cells were treated with HHT (20 ng·mL−1), and then cell apoptosis was evaluated by flow cytometry with annexin V/PI staining at the indicated time points. As shown in Figure 4A, apoptosis was observed at 6 h and peaked at 24 h after exposure to HHT. Early apoptotic cells (PI − AV+ cells) were increased more than four‐fold between 6 and 24h and late apoptotic cells (PI + AV+ cells) increased five‐fold over the same period. However the proportion of necrotic cells (PI + AV−) was unaffected. Analysis of corelations between induction of apoptosis and levels of myosin‐9 in the leukaemia cells treated with HHT (Figure 4B) demonstrated a clear association between the percentage of apoptotic cells and up‐regulation of myosin‐9 expression (R 2 = 0.959, P = 0.021), suggesting that the levels of myosin‐9 were involved in the cell apoptosis induced by HHT in leukaemia cells.
Figure 4.
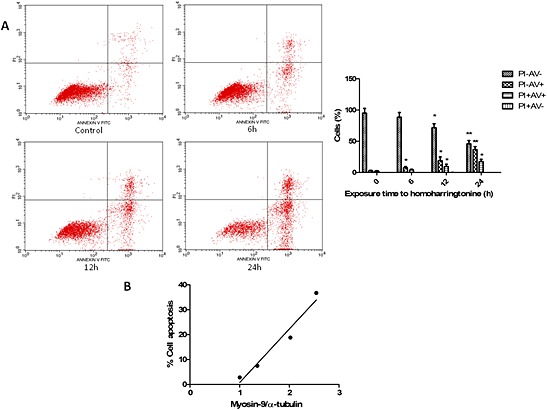
Correlation between myosin‐9 expression in leukaemia cells and HHT‐induced cell apoptosis and death. (A) Kasumi‐1 cells were seeded in six‐well plates and treated with HHT (20 ng·mL−1) for the indicated time points. Annexin V/PI staining was used to measure the apoptotic cells. Data presented are means ± SD of results from three independent experiments. *P < 0.05; **P < 0.01, significantly different from corresponding values at t=0. (B) Percentage of apoptotic cells correlated with levels of myosin‐9. Apoptosis in Kasumi‐1 cells, measured by annexin/PI at various time points, was correlated with the myosin‐9 expression level.
To better understand the involvement of myosin‐9 in the action of HHT against leukaemia, we established Kasumi‐1 cells that stably expressed a high level of myosin‐9 (Kasumi‐1/NMHC IIA) using pMxs‐NMHC‐IIA and a retrovirus expression system (Figure 5A) and then determined the effects of myosin‐9 overexpression on HHT‐induced leukaemia cell growth inhibition by MTS assay. As shown in Figure 5B, the myosin‐9‐overexpressing cells were found to be more susceptible to the cytotoxicity of HHT than the control cells. Taken together, these findings indicate that the HHT‐induced increase in myosin‐9 is involved in the effects of this anti‐leukaemia drug.
Figure 5.
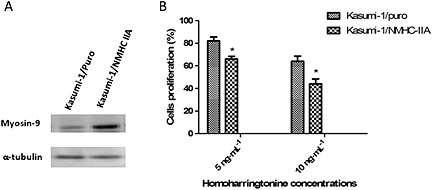
Up‐regulation in myosin‐9 expression increases the sensitivity of leukaemia cells to HHT. (A) The expression of myosin‐9 in Kasumi‐1/puro and Kasumi‐1/NMHC‐IIA cells was evaluated by immunoblot analysis. (B) Sensitivity of Kasumi‐1/puro and Kasumi‐1/NMHC‐IIA cells to HHT. Kasumi‐1/puro and Kasumi‐1/NMHC‐IIA cells were seeded in 96‐well plates and treated with HHT at concentrations of 5.0 and 10 ng·mL−1. Cell proliferation was measured by MTS assays. Data presented are the means ± SD of results from three independent experiments. *P < 0.05, significantly different from Kasumi‐1/puro values.
To examine the effect of the increased myosin‐9 on cell cycle of leukaemia cells, flow cytometry was used to measure the proportions of cells in various cell cycle phases. As shown in Figure 6, in the Kasumi‐1/NMHC‐IIA cells, the percentage of G0/G1 phase cells was lower and the percentage of cells in S phase was higher, relative to the corresponding values in Kasumi‐1/puro cells. The proportion of cells in G2/M phase was also relatively higher in the Kasumi‐1/NMHC‐IIA cells. These data indicate that myosin‐9 overexpression induced cell cycle arrest in S phase and G2 phase in Kasumi‐1 cells.
Figure 6.
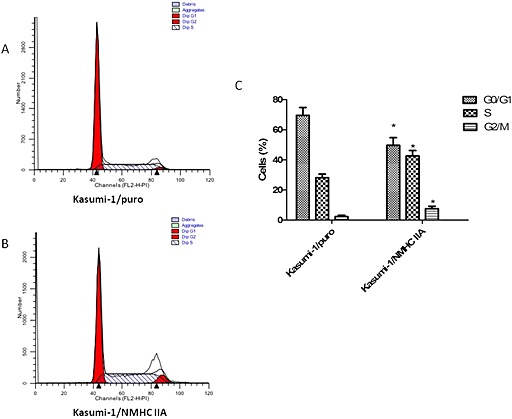
The effect of myosin‐9 overexpression on cell cycles in leukaemia cells. The cell cycle distribution of (A) Kasumi‐1/NMHC IIA and (B) Kasumi‐1/puro was analysed with flow cytometry. (C) The percentage of cells in each of the phases of the cell cycles was calculated. Data presented are the means ± SD of results from three independent experiments. *P < 0.05, significantly different from Kasumi‐1/puro values.
Discussion
Since the antitumour activities of HHT were initially reported in the 1970s, HHT has exhibited a good efficacy and safety profile in patients with AML and CML. The results from clinical studies have shown that the HHT‐based induction regimen is an effective and safe treatment option for patients with AML. Moreover, HHT has been shown to be a valuable option for patients who have imatinib‐resistant CML, including those who carry TKI‐insensitive mutations, such as the T315I mutation. Preclinical studies have shown that an interruption in protein synthesis and induction of cell apoptosis are responsible for the HHT‐mediated inhibition of cell proliferation in a variety of leukaemia cell lines. However, to date, the molecular mechanisms underlying the anti‐leukaemic activities of HHT have not yet been fully elucidated.
Here, we showed that myosin‐9 and actin were putative target proteins for HHT in myeloid cells using affinity chromatography, MS and immunoblot analysis. Myosin‐9, an actin‐binding protein, is the heavy‐chain subunit of non‐muscle myosin IIA (Mermall et al., 1998; Conti and Adelstein, 2008). Previous studies have shown that non‐muscle myosin II belongs to the myosin super‐family and is involved in cell migration, adhesion and cell shape maintenance (Even‐Ram et al., 2007; Vicente‐Manzanares et al., 2009). Non‐muscle myosin II consists of two heavy chains and two pairs of light chains (Richards and Cavalier‐Smith, 2005). The globular head region of the heavy chains contains the ATP binding sites and their motor domain interacts with actin filaments. When myosin heavy chains bind to actin, their catalytic sites with ATPase activity are activated, causing the hydrolysis of ATP to generate energy. Then, the heavy chains, combined with myosin light chains and actin filaments, participate in a series of cellular functions (Even‐Ram et al., 2007; Vicente‐Manzanares et al., 2009). In this study, the result of pull‐down assays confirmed that myosin‐9 interacted directly with HHT. Currently, three isoforms of non‐muscle myosin II (NM IIA, NM IIB and NM IIC) have been described in humans based on the isoforms of the heavy chains (NMHC IIA, IIB and IIC), which are encoded by three different genes, MYH9, MYH10 and MYH14 (Simons et al., 1991; Golomb et al., 2004). It is interesting that myosin‐9 is the only non‐muscle myosin heavy chain expressed in human haematopoietic cells, such as platelets, lymphocytes, monocytes and neutrophils (Maupin et al., 1994). However, the role of myosin‐9 in the haematopoietic system is poorly understood. An earlier study showed that myosin‐9 functioned as a herpes simplex virus 1 entry receptor (Arii et al., 2010). It is known that many mutations in the MHY‐9 gene are highly associated with a group of inherited haematologic diseases, MYH9 disorders (Seri et al., 2003; Althaus and Greinacher, 2009; Kunishima and Saito, 2010). An increase in the expression of myosin‐9 was found during the course of myeloid differentiation (Sagara et al., 1982; Toothaker et al., 1991). In addition, myosin‐9 expression was decreased in familial platelet disorder/AML megakaryocytes (Bluteau et al., 2012). These data suggest that myosin‐9 may play an essential role in haematopoietic cells and is possibly associated with haematopoietic disease. Here, our findings demonstrate that an HHT‐affinity column binds directly to the protein. This is the first example of a known anti‐leukaemia drug interacting with the cytoskeletal contractile protein myosin‐9.
We then evaluated the effects of HHT on the expression of myosin‐9. The results of the Western blot analysis showed that HHT treatment increased myosin‐9 expression in Kasumi‐1 cells and in other AML cell lines, such as HL‐60, THP‐1 and U937 cells. In addition, HHT treatment enhanced the expression of myosin‐9 in human primary HSPCs. Moreover, we found a similar up‐regulation in the CML cell line KCL‐22 and imatinib‐resistant KCL‐22 M cells that carry the T315i‐mutated BCR/ABL. This finding may explain why HHT is effective in the treatment of not only AML but also CML. Imatinib, which acts on the ATP binding site of BCR/ABL1 kinase, has been the standard treatment for CML patients. However, this TKI appears to be ineffective in CML with BCR/ABL mutations. In recent years, in vitro studies have shown that HHT inhibits the proliferation of CML cells regardless of whether the cells have an unmutated BCR/ABL or imatinib‐resistant mutations (Scappini et al., 2002). HHT‐based therapy has also produced encouraging results in patients who have failed TKI treatment (Chen et al., 2006; Cortes et al., 2012). Our findings suggest that the up‐regulated myosin‐9 expression may be partly responsible for the action of HHT against imatinib resistance.
Our group further examined whether myosin‐9 is involved in the course of HHT‐induced apoptosis in leukaemia cells. A time‐dependent increase in apoptosis is observed in Kasumi‐1 cells after exposure to the drug and the HHT‐mediated cell apoptosis was positively associated with increased target protein. Furthermore, the sensitivity to HHT in NMHC IIA‐transgenic cells that stably expressed high levels of myosin‐9 was higher than that in the control.
It is well known that the accumulation of mutations and chromosomal translocations are strongly associated with tumourigenesis. In normal cells, when DNA damage occurs, cell cycle checkpoint control will arrest cell cycle progression and start to repair any DNA defects detected. If repair fails, cell will initiate the process of programmed cell death to ensure unrepaired DNA damage is not transmitted to the next generation. However, one feature of cancer is uncontrolled proliferation due to dysfunction of normal cell cycle control, causing increased genomic instability. A number of antitumour agents induce G0/G1, S and G2/M phase arrests and promote cell apoptosis. Thus, the cell cycle distribution of Kasumi‐1/NMHC IIA and Kasumi‐1/puro cells was analysed with flow cytometry. The results showed overexpression of myosin‐9‐induced S and G2/M phase arrest, which may provide an opportunity for leukaemia cells to undergo apoptotic progression. Moreover, HHT in combination with cytarabine has been widely used in the treatment of AML in China. The antitumour action of cytarabine is specific for cells in S phase of cell cycle through inhibiting the synthesis of DNA. The S phase arrest may partly contribute to the synergism of HHT with cytarabine or other chemotherapy drugs acting on cancer cells in the S and G2/M phases of the cell cycle. These findings indicated that myosin‐9 was a critical target of HHT and that the HHT‐induced increase in myosin‐9 could be responsible for its anti‐leukaemia activity. Needless to say, the functions of myosin‐9 in leukaemia cells and the role of myosin‐9 in the cytotoxicities induced by chemotherapeutic agents are complicated and need to be further studied.
In conclusion, the present study indicates that myosin‐9 is a direct molecular target of HHT in leukaemia. Furthermore, HHT up‐regulated myosin‐9 expression in both AML and CML cell lines, and the increase in the target protein was associated with HHT‐mediated cell apoptosis and induced S and G2/M cell cycle arrests. Taken together, these data provide insights into the mechanism underlying the anti‐leukaemia activity of HHT. To our knowledge, this is the first report that myosin‐9, a cytoskeletal protein, is a direct target protein of anticancer agents and that it plays an essential role in the development of tumour apoptosis and cell death. This finding reveals new pathways that should be investigated regarding the molecular mechanisms underlying antitumour effects.
Author contributions
T. Z. conceptualized the project, designed and performed the research, analysed the data and wrote the manuscript; S. S. performed experiments, discussed results and analysed data; Z. Z. , S. L. and X. Y. performed experiments J. Z. conceptualized the project, directed the experimental design, data analysis and manuscript writing. J. J. designed the work, supervised experiments and discussed results.
Conflict of interest
The authors have no competing interests.
Supporting information
Table S1 The baseline expression of myosin‐9 and IC50 values of HHT in AML cells.
Supporting info item
Acknowledgement
We thank Yasushi Kawaguchi (The Institute of Medical Science, The University of Tokyo) for the generous gift of plasmid pMxs‐NMHC‐IIA. This work was supported by the National Natural Science Foundation of China (81370643‐H0812), Zhejiang Provincial Key Innovation Team (2011R50015) and National Public Health Grand Research Foundation (201202017).
Zhang, T. , Shen, S. , Zhu, Z. , Lu, S. , Yin, X. , Zheng, J. , and Jin, J. (2016) Homoharringtonine binds to and increases myosin‐9 in myeloid leukaemia. British Journal of Pharmacology, 173: 212–221. doi: 10.1111/bph.13359.
References
- (1976). Cephalotaxine esters in the treatment of acute leukemia. A preliminary clinical assessment. Chin Med J (Engl) 2: 263–272. [PubMed] [Google Scholar]
- (1977). Harringtonine in acute leukemias. Clinical analysis of 31 cases. Chin Med J (Engl) 3: 319–324. [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, McGrath JC et al. (2013). The Concise Guide to PHARMACOLOGY 2013/14: Overview. Br J Pharmacol 170: 1449–1458.24528237 [Google Scholar]
- Allan EK, Holyoake TL, Craig AR, Jorgensen HG (2011). Omacetaxine may have a role in chronic myeloid leukaemia eradication through downregulation of Mcl‐1 and induction of apoptosis in stem/progenitor cells. Leukemia 25: 985–994. [DOI] [PubMed] [Google Scholar]
- Althaus K, Greinacher A (2009). MYH9‐related platelet disorders. Semin Thromb Hemost 35: 189–203. [DOI] [PubMed] [Google Scholar]
- Arii J, Goto H, Suenaga T, Oyama M, Kozuka‐Hata H, Imai T et al. (2010). Non‐muscle myosin IIA is a functional entry receptor for herpes simplex virus‐1. Nature 467: 859–862. [DOI] [PubMed] [Google Scholar]
- Bluteau D, Glembotsky AC, Raimbault A, Balayn N, Gilles L, Rameau P et al. (2012). Dysmegakaryopoiesis of FPD/AML pedigrees with constitutional RUNX1 mutations is linked to myosin II deregulated expression. Blood 120: 2708–2718. [DOI] [PubMed] [Google Scholar]
- Chen R, Gandhi V, Plunkett W (2006). A sequential blockade strategy for the design of combination therapies to overcome oncogene addiction in chronic myelogenous leukemia. Cancer Res 66: 10959–10966. [DOI] [PubMed] [Google Scholar]
- Chen R, Guo L, Chen Y, Jiang Y, Wierda WG, Plunkett W (2011). Homoharringtonine reduced Mcl‐1 expression and induced apoptosis in chronic lymphocytic leukemia. Blood 117: 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti MA, Adelstein RS (2008). Nonmuscle myosin II moves in new directions. J Cell Sci 121: 11–18. [DOI] [PubMed] [Google Scholar]
- Cortes J, Lipton JH, Rea D, Digumarti R, Chuah C, Nanda N et al. (2012). Phase 2 study of subcutaneous omacetaxine mepesuccinate after TKI failure in patients with chronic‐phase CML with T315I mutation. Blood 120: 2573–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Even‐Ram S, Doyle AD, Conti MA, Matsumoto K, Adelstein RS, Yamada KM (2007). Myosin IIA regulates cell motility and actomyosin‐microtubule crosstalk. Nat Cell Biol 9: 299–309. [DOI] [PubMed] [Google Scholar]
- Fresno M, Jimenez A, Vazquez D (1977). Inhibition of translation in eukaryotic systems by harringtonine. Eur J Biochem 72: 323–330. [DOI] [PubMed] [Google Scholar]
- Golomb E, Ma X, Jana SS, Preston YA, Kawamoto S, Shoham NG et al. (2004). Identification and characterization of nonmuscle myosin II‐C, a new member of the myosin II family. J Biol Chem 279: 2800–2808. [DOI] [PubMed] [Google Scholar]
- Gurel G, Blaha G, Moore PB, Steitz TA (2009). U2504 determines the species specificity of the A‐site cleft antibiotics: the structures of tiamulin, homoharringtonine, and bruceantin bound to the ribosome. J Mol Biol 389: 146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Jiang DZ, Mai WY, Meng HT, Qian WB, Tong HY et al. (2006). Homoharringtonine in combination with cytarabine and aclarubicin resulted in high complete remission rate after the first induction therapy in patients with de novo acute myeloid leukemia. Leukemia 20: 1361–1367. [DOI] [PubMed] [Google Scholar]
- Jin J, Wang JX, Chen FF, Wu DP, Hu J, Zhou JF et al. (2013). Homoharringtonine‐based induction regimens for patients with de‐novo acute myeloid leukaemia: a multicentre, open‐label, randomised, controlled phase 3 trial. Lancet Oncol 14: 599–608. [DOI] [PubMed] [Google Scholar]
- Kunishima S, Saito H (2010). Advances in the understanding of MYH9 disorders. Curr Opin Hematol 17: 405–410. [DOI] [PubMed] [Google Scholar]
- Maupin P, Phillips CL, Adelstein RS, Pollard TD (1994). Differential localization of myosin‐II isozymes in human cultured cells and blood cells. J Cell Sci 107 (Pt 11): 3077–3090. [DOI] [PubMed] [Google Scholar]
- Mermall V, Post PL, Mooseker MS (1998). Unconventional myosins in cell movement, membrane traffic, and signal transduction. Science 279: 527–533. [DOI] [PubMed] [Google Scholar]
- Quintas‐Cardama A, Kantarjian H, Cortes J (2009). Homoharringtonine, omacetaxine mepesuccinate, and chronic myeloid leukemia circa 2009. Cancer 115: 5382–5393. [DOI] [PubMed] [Google Scholar]
- Quintas‐Cardama A, Kantarjian H, Garcia‐Manero G, O'Brien S, Faderl S, Estrov Z et al. (2007). Phase I/II study of subcutaneous homoharringtonine in patients with chronic myeloid leukemia who have failed prior therapy. Cancer 109: 248–255. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP et al. (2014). The IUPHAR/BPS Guide to PHARMACOLOGY: an expert‐driven knowledge base of drug targets and their ligands. Nucl. Acids Res. 42 (Database Issue): D1098–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards TA, Cavalier‐Smith T (2005). Myosin domain evolution and the primary divergence of eukaryotes. Nature 436: 1113–1118. [DOI] [PubMed] [Google Scholar]
- Sagara J, Nagata K, Ichikawa Y (1982). Changes in myosin during differentiation of myeloid leukemia cells. J Biochem 91: 1363–1372. [DOI] [PubMed] [Google Scholar]
- Scappini B, Onida F, Kantarjian HM, Dong L, Verstovsek S, Keating MJ et al. (2002). In vitro effects of STI 571‐containing drug combinations on the growth of Philadelphia‐positive chronic myelogenous leukemia cells. Cancer 94: 2653–2662. [DOI] [PubMed] [Google Scholar]
- Seri M, Pecci A, Di Bari F, Cusano R, Savino M, Panza E et al. (2003). MYH9‐related disease: May–Hegglin anomaly, Sebastian syndrome, Fechtner syndrome, and Epstein syndrome are not distinct entities but represent a variable expression of a single illness. Medicine (Baltimore) 82: 203–215. [DOI] [PubMed] [Google Scholar]
- Simons M, Wang M, McBride OW, Kawamoto S, Yamakawa K, Gdula D et al. (1991). Human nonmuscle myosin heavy chains are encoded by two genes located on different chromosomes. Circ Res 69: 530–539. [DOI] [PubMed] [Google Scholar]
- Toothaker LE, Gonzalez DA, Tung N, Lemons RS, Le Beau MM, Arnaout MA et al. (1991). Cellular myosin heavy chain in human leukocytes: isolation of 5' cDNA clones, characterization of the protein, chromosomal localization, and upregulation during myeloid differentiation. Blood 78: 1826–1833. [PubMed] [Google Scholar]
- Tujebajeva RM, Graifer DM, Karpova GG, Ajtkhozhina NA (1989). Alkaloid homoharringtonine inhibits polypeptide chain elongation on human ribosomes on the step of peptide bond formation. FEBS Lett 257: 254–256. [DOI] [PubMed] [Google Scholar]
- Vicente‐Manzanares M, Ma X, Adelstein RS, Horwitz AR (2009). Non‐muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol 10: 778–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yinjun L, Jie J, Weilai X, Xiangming T (2004). Homoharringtonine mediates myeloid cell apoptosis via upregulation of pro‐apoptotic Bax and inducing caspase‐3‐mediated cleavage of poly(ADP‐ribose) polymerase (PARP). Am J Hematol 76: 199–204. [DOI] [PubMed] [Google Scholar]
- Yu W, Mao L, Qian J, Qian W, Meng H, Mai W et al. (2013). Homoharringtonine in combination with cytarabine and aclarubicin in the treatment of refractory/relapsed acute myeloid leukemia: a single‐center experience. Ann Hematol 92: 1091–1100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 The baseline expression of myosin‐9 and IC50 values of HHT in AML cells.
Supporting info item


