Abstract
Background:
Bromeliads can be epiphytic, terrestrial or saxicolous and use strategies to allow water to be retained in their leaf axils, where various arthropods can be found. These include mosquitoes, whose larvae are the most abundant and commonly found organisms in the leaf axils. The objective of this study was to look for immature forms of mosquitoes (the larval and pupal stages) in bromeliads in municipal parks in São Paulo and to discuss the ecological and epidemiological importance of these insects.
Methods:
From October 2010 to July 2013, immature mosquitoes were collected from bromeliads in 65 municipal parks in the city of São Paulo, Brazil, using suction samplers. The immature forms were maintained until adult forms emerged, and these were then identified morphologically.
Results:
Two thousand forty-two immature-stage specimens belonging to the genera Aedes, Culex, Trichoprosopon, Toxorhynchites, Limatus and Wyeomyia were found in bromeliads in 15 of the 65 parks visited. Aedes albopictus was the most abundant species (660 specimens collected), followed by Culex quinquefasciatus (548 specimens) and Cx. (Microculex) imitator (444). The taxa with the most widespread distribution were Ae. aegypti and Toxorhynchites spp, followed by Ae. albopictus and Cx. quinquefasciatus.
Conclusion:
Bromeliads in urban parks are refuges for populations of native species of Culicidae and breeding sites for exotic species that are generally of epidemiological interest. Hence, administrators and surveillance and mosquito-control agencies must constantly monitor these microenvironments as the presence of these species endangers the health of park users and employees as well as people living near the parks.
Keywords: Mosquitoes, Bromeliads, Municipal Parks, Culicidae, Breeding sites, Immature, Brazil
Introduction
Members of the family Bromeliaceae can be epiphytic, terrestrial or saxicolous. The taxon is essentially Neotropical, with more than 3,100 described species distributed in 58 genera. In Brazil, where 40 % of these species are found, the highest diversity is in the Atlantic forest ( Givnish et al. 2011). Bromeliads use strategies that allow water and organic debris to be retained in their leaf axils, forming rosettes or tanks from which they acquire nutrients.
These structures also act as biotopes for various organisms. Central and lateral bromeliad tanks provide a permanent habitat for many terrestrial arthropods that have aquatic immature stages ( Frank et al. 2004), such as insects of the order Diptera, whose larvae are the most abundant and commonly found organisms in these biotopes ( Derraik and Heath 2005). Among the most common dipterans, the culicids include species whose immature forms are specialists in colonizing these microhabitats as well as species that are occasional visitors ( Frank and Lounibos 2009).
The family Culicidae currently has around 3,500 valid described species worldwide ( Harbach 2014). Many culicid species are of great importance in public health because the blood-feeding habits of the females are associated with allergies and the transmission of pathogens ( Consoli and Lourenço-de-Oliveira 1994, Forattini 2002). The genera Aedes, Anopheles and Culex include important vectors of diseases such as malaria, dengue and filariasis in various regions of the planet (WHO 2013a, Bhatt et al. 2013, WHO 2013b). In addition, a number of mosquito-borne diseases, such as the West Nile virus, Chikungunya and yellow fever were or are public health problems in many countries ( Weaver 2013).
Brazil has a great variety of culicids, 490 species having been described to date (WRBU 2014). In Brazil, malaria, which is transmitted by Anopheles, and dengue, which is transmitted by Ae. aegypti, are together responsible for thousands of cases of morbidity. Also worthy of note is lymphatic filariasis, which is transmitted by Cx. quinquefasciatus in some cities in the northeast of the country, as well as various arboviruses that circulate in natural environments and cause sporadic cases of epizootics or even human infections such as Saint Louis encephalitis, Mayaro encephalitis, equine encephalitis, Rocio encephalitis and wild yellow fever ( Forattini 2002, Oliveira-Ferreira et al. 2010, Viana and Ignotti 2013).
The city of São Paulo, located in southeastern Brazil, is the largest city in South America and has more than 11 million inhabitants. Mosquito-borne diseases in the city include dengue, outbreaks of which occur every year and which was responsible for 2,617 confirmed autochthonous cases in 2013 ( São Paulo 2014a). Malaria outbreaks due to Plasmodium vivax and P. malariae in the Atlantic Forest have been detected on the edge of the city of São Paulo ( Couto et al. 2010, Duarte et al. 2013). The Ilhéus virus was isolated in birds in the Tietê Ecological Park, in the municipality of São Paulo ( Pereira et al. 2001). In regions of the Atlantic Forest close to the city of São Paulo, antibodies for various arboviruses that cause different types of encephalitis were detected in birds ( Ferreira et al. 1994) and there have been sporadic cases of wild yellow fever in humans and primates, the virus responsible for the disease having been isolated in mosquitoes from the species Haemagogus leucocelaenus (Souza et al. 2009).
As urbanization continues apace, many cities around the world strive to preserve their green areas ( Koh and Sodhi 2004). The construction, preservation and rehabilitation of green areas in parks or plazas is intended to provide leisure facilities for the population, preserve remnants of flora and fauna and reduce the environmental and social stress caused by urbanization ( McIntyre 2000, McKinney 2008). The city of São Paulo currently has a hundred public parks, many of which contain fragments of the Atlantic Forest and representatives of native or exotic flora and fauna (São Paulo 2014b). The diversity of culicids found in some of these green fragments has been investigated in a variety of studies ( Urbinatti et al. 2001, Taipe-Lagos and Natal 2003, Montes 2005, Ribeiro et al. 2012). A recent preliminary survey in fifty-nine urban parks in the city of São Paulo found that some medically important species of culicids, such as Ae. albopictus, Ae. fluviatilis, Ae. scapularis, Cx. nigripalpus, and Cx. quinquefasciatus, are quite common in these environments ( Medeiros-Sousa et al. 2013). These and other species maintain their populations using a variety of natural or artificial containers found in these green fragments that allow their immature forms to develop. Bromeliads can act as breeding grounds for many species of epidemiologic interest in the city as they are very common in parks, where they occur naturally and in some cases are planted as part of the landscape.
The aim of this study was to investigate the species composition and ecological aspects of mosquitoes collected in bromeliads in urban parks in São Paulo to determine how important these plants are for the maintenance of populations of native or exotic culicid species, with a particular emphasis on species of epidemiological interest.
Materials and Methods
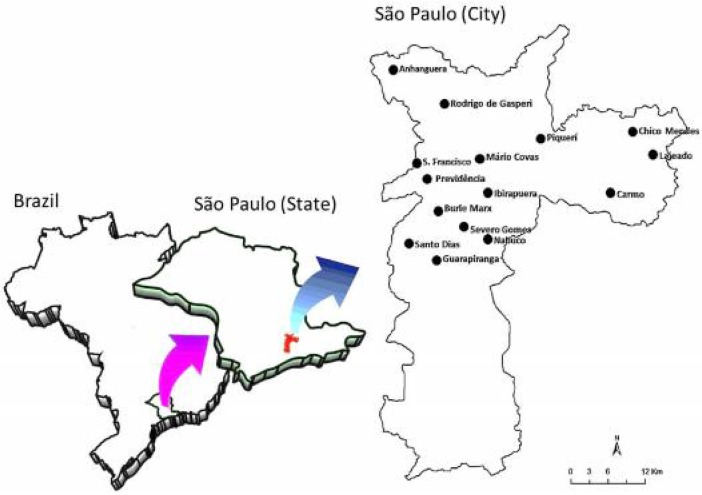
The city of São Paulo (23.54° W 46.63° S) is located in the Atlantic Forest in the southeast of Brazil at an altitude of 750 m above sea level and with a mean relative humidity of 78 % (Fig. 1). The climate is considered temperate tropical, and there is reduced rainfall in the winter. Mean annual temperature is 20.7 °C, winters are mild (15 to 21 °C) and summer temperatures are moderately high (22 to 27 °C). Mean annual rainfall is 1,376 mm, and most of the rainfall is concentrated in the summer months (IBGE 2014).
Fig. 1.
Location of the fifteen urban parks in the city of São Paulo, Brazil, where immature stages of culicids were found in bromeliads between October 2010 and July 2013
The species composition of immature culicids in fifteen parks with bromeliads in the city of São Paulo was investigated over thirty-four months. Data collected by Medeiros-Sousa et al. (2013) between October 2010 and February 2011 were used, together with data from collections made between March 2011 and July 2013. Bromeliads planted in the ground (terrestrial bromeliads) and bromeliads on tree trunks (epiphytic bromeliads) at heights ranging from 1.0 to 5.0 m were sampled. Immature specimens were actively sought out in the central and lateral leaf axils of bromeliads using suction samplers (Fig. 2). The extracted contents were then transferred to small recipients (400ml) and analyzed for the presence of mosquito larvae and pupae. The larvae and pupae collected were taken to the Public Health Entomology Laboratory at the Faculty of Public Health, University of São Paulo, where they were maintained until the emergence of adult forms, which were identified morphologically based on Lane (1953), Consoli and Lourenço-de-Oliveira (1994) and Forattini (2002), as well as by comparing them with standard specimens in the collection at the Faculty of Public Health, University of São Paulo. The abbreviations for genera and subgenera used here follow the standardization proposed by Reinert (2009).
Fig. 2.
Collection of immature mosquitoes in leaf axils of bromeliads using suction samplers in urban parks in the city of São Paulo, Brazil, between October 2010 and July 2013. (A) Terrestrial bromeliad, (B) Epiphytic bromeliad
After all the individuals collected had been identified morphologically, the number of individuals in terrestrial and epiphytic bromeliads in each park broken down by species was recorded in tabular form (Table 1). The frequency with which each species was found in terrestrial and epiphytic bromeliads was calculated to determine whether there was an association between culicid species and type of bromeliad (Table 2).
Table 1.
Number of immature mosquitoes collected by species for each urban park and type of bromeliad (terrestrial or epiphytic) collected between October 2010 and July 2013 in the city of São Paulo, Brazil
| Taxon | Anhanguera | Burle Marx | Carmo | Colinas de São Francisco | Chico Mendes | Guarapiranga | Ibirapuera | Lajeado | Mário Covas | Nabuco | Piqueri | Previdência | Rodrigo de Gasperi | Santo Dias | Severo Gomes | Total | (%) Frequency | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Terrestrial | Epiphytic | Terrestrial | Epiphytic | Terrestrial | Epiphytic | Terrestrial | Epiphytic | Terrestrial | Epiphytic | Terrestrial | Epiphytic | Terrestrial | Epiphytic | Terrestrial | Epiphytic | Terrestrial | Epiphytic | Terrestrial | Epiphytic | Terrestrial | Epiphytic | Terrestrial | Epiphytic | Terrestrial | Epiphytic | Terrestrial | Epiphytic | Terrestrial | Epiphytic | |||
| Aedes (Stegomyia) aegypti (Linnaeus, 1762) | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 72 | 19 | 2 | 2 | 0 | 7 | 0 | 13 | 0 | 0 | 122 | 5.97 |
| Aedes ( Stegomyia ) albopictus (Skuse, 1895) | 16 | 0 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 28 | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 90 | 467 | 2 | 7 | 0 | 0 | 3 | 21 | 0 | 0 | 660 | 32.3 |
| Aedes ( Ochlerotatus ) fluviatilis (Lutz, 1904) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0.3 |
| Culex ( Culex ) chidesteri Dyar, 1921 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.05 |
| Culex ( Culex ) quinquefasciatus Say, 1823 | 15 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 70 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 362 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 548 | 26.8 |
| Culex ( Microculex ) imitator imitator Theobald, 1903 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 78 | 362 | 0 | 0 | 444 | 21.7 |
| Culex ( Microculex .) Group Imitator (Theobald, 1903) | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 13 | 23 | 0 | 0 | 40 | 1.9 |
| Limatus durhami Theobald, 1901 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 0.4 |
| Trichoprosopon ( Trichoprosopon ) pallidiventer (Lutz, 1905) | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0.15 |
| Toxorhynchites bambusicola ( Lutz, Neiva, 1913 ) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 9 | 0.4 |
| Toxorhynchites solstitialis (Lutz, 1904) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.1 |
| Toxorhynchites spp. Theobald, 1901 | 9 | 0 | 29 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 5 | 0 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 8 | 0 | 0 | 1 | 3 | 0 | 0 | 66 | 3.2 |
| Wyeomyia ( Phoniomyia ) davisi (Lutz, 1904) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 52 | 0 | 0 | 59 | 2.9 |
| Wyeomyia ( Miamyia ) oblita (Lutz, 1904) | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0.15 |
| Wyeomyia serratoria (Dyar, Nunez Tovar, 1927) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 0.1 |
| Wyeomyia ( Phoniomyia ) cf galvaoi (Correa, Ramalho, 1956) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 51 | 0 | 0 | 64 | 3.1 |
| Wyeomyia . spp Theobald, 1901 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0.2 |
| Total no. of individuals | 49 | 0 | 41 | 0 | 0 | 3 | 1 | 2 | 0 | 7 | 14 | 0 | 119 | 17 | 1 | 0 | 102 | 0 | 0 | 1 | 533 | 486 | 6 | 19 | 0 | 7 | 106 | 526 | 0 | 2 | 2,042 | 100 |
| Total no. of species | 8 | 4 | 3 | 2 | 3 | 2 | 8 | 2 | 2 | 5 | 6 | 1 | 8 | 1 | ||||||||||||||||||
Table 2.
Number and frequency of culicids in decreasing order of abundance in terrestrial and epiphytic bromeliads collected between October 2010 and July 2013 in urban parks in the city of São Paulo, Brazil
| Species | N. in terrestrial bromeliads | Frequency in terrestrial bromeliads | N. in epiphytic bromeliads | Frequency in epiphytic bromeliads | Total no. of specimens |
|---|---|---|---|---|---|
| Aedes (Stegomyia) albopictus (Skuse, 1895) | 146 | 0.22 | 514 | 0.77 | 660 |
| Culex (Culex) quinquefasciatus Say, 1823 | 547 | 0.99 | 1 | 0.002 | 548 |
| Culex (Microculex) imitator imitator Theobald, 1903 | 82 | 0.18 | 362 | 0.81 | 444 |
| Aedes (Stegomyia) aegypti (Linnaeus, 1762) | 80 | 0.65 | 42 | 0.34 | 122 |
| Toxorhynchites spp Theobald, 1901 | 52 | 0.78 | 14 | 0.21 | 66 |
| Wyeomyia (Phoniomyia) cf. galvaoi (Correa, Ramalho, 1956) | 13 | 0.20 | 51 | 0.79 | 64 |
| Wyeomyia (Phoniomyia) davisi (Lutz, 1904) | 7 | 0.11 | 52 | 0.88 | 59 |
| Culex (Microculex.) Group Imitator (Theobald, 1903) | 15 | 0.37 | 25 | 0.62 | 40 |
| Toxorhynchites bambusicola (Lutz, Neiva, 1913) | 4 | 0.44 | 5 | 0.55 | 9 |
| Limatus durhami Theobald, 1901 | 7 | 0.87 | 1 | 0.12 | 8 |
| Aedes (Ochlerotatus) fluviatilis (Lutz, 1904) | 6 | 1.0 | 0 | 0.0 | 6 |
| Wyeomyia spp Theobald, 1901 | 5 | 1.0 | 0 | 0.0 | 5 |
| Trichoprosopon (Trichoprosopon) pallidiventer (Lutz, 1905) | 3 | 1.0 | 0 | 0.0 | 3 |
| Wyeomyia (Miamyia) oblita (Lutz, 1904) | 3 | 1.0 | 0 | 0.0 | 3 |
| Toxorhynchites solstitialis (Lutz, 1904) | 1 | 0.5 | 1 | 0.5 | 2 |
| Wyeomyia serratoria (Dyar, Nunez Tovar, 1927) | 1 | 0.5 | 1 | 0.5 | 2 |
| Culex (Culex) chidesteri Dyar, 1921 | 0 | 0.0 | 1 | 1.0 | 1 |
| Total no. of individuals per type of bromeliad | 972 | 0.48 | 1,070 | 0.52 | 2,042 |
Results
Overall, 2,042 immature-stage specimens belonging to 6 genera (Aedes, Culex, Trichoprosopon, Toxorhynchites, Limatus and Wyeomyia) and 17 species were found in 15 of the 65 parks visited (Fig. 1, Table 1). Table 1 shows the number of specimens of each species collected and the type of bromeliad (epiphytic or terrestrial) in which collections were made. Sixty-six of the specimens collected were classified in the taxon Tx. spp and five in Wy. spp either because they died before the adult form emerged or because of limitations in the keys for morphological identification, which were out of date. The only clear association between culicid species and type of bromeliad (Table 2) was for Cx. quinquefasciatus, one of the most abundant species, which was found exclusively in terrestrial bromeliads.
The bromeliads found in the parks belonged to the genera Aechmea, Alcantarea, Edmundoa, Neoregelia, Nidularium, Quesnelia and Vriesea, all of which are common in the Atlantic Forest. Aedes albopictus was the most abundant mosquito species collected (660 specimens or 32.35% of the total), followed by Cx. quinquefasciatus (548 specimens or 26.86%) and Culex (Microculex) imitator (444 or 21.76%). Another epidemiologically important species collected was Ae. aegypti (122 or 5.98%). The other species together accounted for only 13.05% of the total number collected (Table 1). The taxa with the widest distributions were Ae. aegypti and Tx. spp., found in eight parks, followed by Ae. albopictus (seven parks) and Cx. quinquefasciatus (five parks).
The parks with the highest species richness were Anhanguera Park, Ibirapuera Park and Santo Dias Park, each of which had eight species, followed by Previdência Park with six species, Piqueri Park with five and Burle Marx Park with four. No more than three species were recorded for any of the other parks (Table 1).
The largest numbers of specimens independently of species were collected in Piqueri Park (1,019) and Santo Dias Park (632), which together accounted for almost three quarters of all the specimens collected and had the largest number of bromeliads. Ibirapuera Park and Park Mario Covas, where 136 and 102 individuals were collected, respectively, also accounted for a significant number of the sample population. The remaining parks together accounted for just 151 specimens.
Discussion
This study, the first to investigate immature mosquitoes in bromeliads in municipal parks in the city of São Paulo, Brazil, found seventeen species or groups from the genera Aedes, Culex, Trichoprosopon, Toxorhynchites, Limatus and Wyeomyia in these plants. The findings were similar to those of previous studies except that we failed to find species from the subgenus Kerteszia of Anopheles, a group of mosquitoes found almost exclusively in bromeliads, some species of which are considered vectors of “bromeliad-malaria” in the Atlantic Forest of South and Southeast Brazil ( Consoli and Lourenço-de-Oliveira 1994, Marques et al. 2012, Ribeiro et al. 2012). This absence could be due to the urban or peri-urban nature of the parks visited, as this subgenus is considered a bioindicator of preserved environments ( Dorvillé 1996).
Of the Sabethini tribe, which includes mosquito species with diurnal habits usually associated with less anthropic environments, only species of the genera Limatus, Trichoprosopon and Wyeomyia were found. Of these species, Limatus durhami tends to be adapted to anthropic environments ( Consoli and Lourenço de Oliveira 1994), and immature forms of this species have been reported in artificial breeding sites such as waste tires near houses ( Zequi et al. 2005). Like most other Culicidae, the Sabethini are potential vectors of several arboviruses and have been found carrying the Pixuna, Bussuquara, Wyeomyia, Ilhéus and yellow fever viruses ( Forattini 2002).
In our survey, we recorded Ae. aegypti and Ae. albopictus in eight and seven parks, respectively. These species are the main vehicles of dengue and Chikungunya virus in various parts of the world but are considered exotic species in Brazil ( Bhatt et al. 2013). They use the same breeding sites in the urban environment, although Ae. albopictus is also often found inhabiting rural and natural environments.
The finding of Ae. albopictus and Ae. aegypti in bromeliads demonstrates the high ecological valence of both species. According to Crovello and Hacker (1972), the use of bromeliads by Ae. aegypti may be considered a remnant of primitive behavior of the species, as outside its original environment (Northwest of Africa) this species is found strictly only in synanthropic environments, is highly dependent on clean water in containers associated with intradomicile environments and exhibits anthropophilic behavior ( Forattini 2002). Aedes albopictus is considered the wilder and more exophilic of the two species, with the potential to disperse through various habitats, and is often found breeding in phytotelmata, such as those in bromeliads and bamboos ( Natal et al. 1997). Our results indicate that in urban parks Ae. albopictus is better able to colonize bromeliads, a finding that may be related to the fact that these environments possess similar characteristics to those of rural and wild environments despite being embedded in an urban landscape.
Another Aedeini registered was Ae. fluviatilis, a suspected vector in the transmission of arboviruses to humans that is found in wild or modified environments and is associated with small, usually temporary rupiculous breeding sites with little shade ( Forattini 2002). In the present study, only six specimens were found (in Ibirapuera Park), showing that, although not their preferred breeding sites, bromeliad phytotelmata may be used by this species for the development of its immature form.
Culex quinquefasciatus is a recognized vector of the worm that causes Bancroftian filariasis infection, Wuchereria bancrofti. This cosmopolitan mosquito species is also associated with the transmission of West Nile virus in the United States, Canada and Mexico ( Murray et al. 2010). The species is adapted to environments that have been changed by anthropogenic activities, mainly in areas where the disorderly use of urban spaces is associated with a lack of infrastructure and failures in sewage and drainage systems, resulting in the accumulation of dirty still waters, which favor the proliferation of this culicid ( Morais et al. 2006). Although the species is characteristic of large breeding sites, it has high ecological valence and may occupy small natural and artificial containers, such as bromeliads, tree holes, bamboo and tires ( Consoli and Lourenço de Oliveira 1994, Forattini 2002). Our results suggest that this species is found more abundantly in terrestrial bromeliads in parks in the city of São Paulo.
The subgenus Microculex Theobald, 1907 of Culex displays a strong preference for breeding in natural containers, especially bromeliads and tree hollows, the former typically used by species Cx. (Mcx) imitator ( Muller and Marcondes 2006). In the present study, Cx. imitator was recorded in five parks, but in high abundance only in Santo Dias Park, where it was found in terrestrial and epiphytic bromeliads.
The genus Toxorhynchites includes species that typically develop in bromeliads, tree holes and bamboo internodes and rarely in artificial breeding sites such as tin cans, barrels and tires ( Lopes 1997). The adults are exclusively phytophagous and are considered to be the only culicid that is harmless to man and other vertebrates. Their larvae are equipped with powerful jaws adapted to predation of smaller immature aquatic arthropods, including other culicids. For this reason, species of this genus have been suggested as tools for biological control in the context of integrated pest management ( Nyamah et al. 2011).
Bromeliads are considered highly selective breeding sites, and some species of culicids, such as those of the genera Wyeomiya and Trichoprosopon, are closely associated with these plants ( Mocellin et al. 2009). The spatial distribution of these culicids is therefore quite restricted. The bromeliads found in parks may represent ecological refuges for these species, which may have been introduced in these green areas unintentionally when these plants were used for ornamentation.
Previous studies have shown that various species of culicids are present in conservation areas and public parks in the city of São Paulo ( Urbinatti et al. 2001, Taipe-Lagos and Natal 2003, Montes 2005, Ribeiro et al. 2012, Medeiros-Sousa et al. 2013). Contributing to this body of knowledge, our study has shown that bromeliads in urban parks in the city of São Paulo not only act as refuges for populations of native species of culicids, but are also used for breeding by exotic and epidemiologically important species such as Ae. aegypti, Ae. albopictus and Cx. quinquefasciatus, which have a high ecological valence, are synanthropic and play an important role as vectors of human diseases. These green areas constitute highly favorable niches, as they increase the supply of resources (food and shelter), provide protection against natural enemies and physical changes in the environment (such as changes in temperature and rainfall) and may improve the survival of species that can exploit small and isolated remnants of native habitats and therefore tolerate a greater degree of urban development than species that are more sensitive to the same transformations in environments occupied by humans ( McIntyre 2000).
Conclusion
The bromeliads present in urban parks of the city of São Paulo not only act as refuges for populations of native species of culicids but also allow exotic and epidemiological important species such as Ae. aegypti, Ae. albopictus and Cx. quinquefasciatus, to use these habitats for proliferation of their populations. It is important for administrators and mosquito control and surveillance agencies to constantly monitor this microenvironment, since the presence of these species endangers the health of park users and employees as well as people living near the parks.
Acknowledgements
We would like to thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (BIOTA Program, Project 2010/51230-8) for providing financial support. We are also grateful to the field team from the Department of Epidemiology in the Faculty of Public Health, University of São Paulo; to the staff at the Office of Parks and Green Areas, Department of the Environment and Green Areas, São Paulo, and to the staff at the Zoonosis Control Center, São Paulo City Hall, who helped collect and identify the specimens. We extend our special thanks to M Bicudo de Paula for assistance with the identification of the specimens and to Dr PR Urbinatti for assistance with the fieldwork and for the pictures. The authors declare that there is no conflict of interests.
References
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. (2013) The global distribution and burden of dengue. Nature. 496( 7446): 504– 507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consoli RAG, Lourenço-de-Oliveira R. (1994) Principais mosquitos de importância sanitária no Brasil. FIOCRUZ, Rio de Janeiro, p. 225. [Google Scholar]
- Couto RDA, Latorre MDRDD, Di Santi SM, Natal D. (2010) Autochthonous malaria notified in the State of São Paulo: clinical and epidemiological characteristics from 1980 to 2007. Rev Soc Bras Med Trop. 43( 1): 52– 58. [DOI] [PubMed] [Google Scholar]
- Crovello TJ, Hacker CS. (1972) Evolutionary strategies in life table characteristics among feral and urban strains of Aedes aegypti (L). Evol. 26( 2): 185– 196. [DOI] [PubMed] [Google Scholar]
- Derraik JGB, Heath ACG. (2005) Immature Diptera (excluding Culicidae) inhabiting phytotelmata in the Auckland and Wellington regions. New Zealand J Marine Freshw Res. 39( 4): 981– 987. [Google Scholar]
- Dorvillé LFM. (1996) Mosquitoes as bioindicators of forest degradation in Southeastern Brazil, a statistical evaluation of published data in the literature. Stud Neotrop Fauna Env. 31( 2): 68– 78. [Google Scholar]
- Duarte AMR, Pereira DM, de Paula MB, Fernandes A, Urbinatti PR, Ribeiro AF, Homem de Mello MHSH, Matos Jr MO, Mucci LF, Fernandes LN, Natal D, Malafronte RS. (2013) Natural infection in anopheline species and its implications for autochthonous malaria in the Atlantic forest in Brazil. Parasit Vectors. 6( 1): 50– 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira IB, Pereira LE, Rocco IM, Marti AT, de Souza LT, Iversson LB. (1994) Surveillance of arbovirus infections in the Atlantic Forest Region, State of São Paulo, Brazil. I. Detection of hemagglutination-inhibiting antibodies in wild birds between 1978 and 1990. Rev Inst Med Trop São Paulo. 36( 3): 265– 274. [DOI] [PubMed] [Google Scholar]
- Forattini OP. (2002) Culicidologia Médica. Vol. 2 Edusp, São Paulo, p. 864. [Google Scholar]
- Frank JH, Sreenivasan S, Benshoff PJ, Deyrup MA, Edwards GB, Halbert SE, Hamon AB, Lowman MD, Mockford EL, Scheffrahn RH, Steck GJ, Thomas MC, Walker TJ, Welbourn WC. (2004) Invertebrate animals extracted from native Tillandsia (Bromeliales: Bromeliaceae) in Sarasota County, Florida. Florida Entomol. 87( 2): 176– 185. [Google Scholar]
- Frank JH, Lounibos LP. (2009) Insects and allies associated with bromeliads: a review. Terr Arthropod Rev. 1( 2): 125– 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givnish TJ, Barffus MHJ, Van EB, Riina R, Schulte K, Horres R, Gonsiska PA, Jabaily RS, Crayn DM, Smith JAC, Inverno K, Brown GK, Evans TM, Holst BK, Luther H, Até W, Zizka G, Barry PE, Sytsma KJ. (2011) Phylogeny, adaptative radiation, and historical biogeography in Bromeliaceae: insights from an eight-locus plastid phylogeny. Am J Botany. 98( 5): 872– 895. [DOI] [PubMed] [Google Scholar]
- Harbach RE. (2014) Mosquito Taxonomic Inventory. Available at: http://mosquito-taxonomic-inventory.info/.
- IBGE (2014) Instituto Brasileiro de Geografia e Estatística. Cidade de São Paulo. Available at: http://cidades.ibge.gov.br/xtras/home.php.
- Koh LP, Sodhil NS. (2004) Importance of reserves, fragments, and parks for butterfly conservation in tropical urban landscape. Ecol Appl. 14( 6): 1698– 1708. [Google Scholar]
- Lane J. (1953) Neotropical Culicidae. Vol. 2 Universidade de São Paulo, São Paulo, Brazil: pp. 565. [Google Scholar]
- Lopes J. (1997) Mosquito (Diptera: Culicidae) ecology in natural and artificial rural breeding places in northern Parana, Brazil. V. Larvae collection in artificial containers installed in ciliary Forest. Rev Saude Publ. 31( 4): 370– 377. [DOI] [PubMed] [Google Scholar]
- Marques TC, Bourke BP, Laporta GZ, Sallum MAM. (2012) Mosquito (Diptera: Culicidae) assemblages associated with Nidularium and Vriesea bromeliads in Serra do Mar, Atlantic Forest, Brazil. Parasit Vectors. 5( 1): 1– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre NE. (2000) Ecology of urban arthropods: a review and a call to action. An Entomol Soc Am. 93( 4): 825– 835. [Google Scholar]
- McKinney ML. (2008) Effects of urbanization on species richness: a review of plants and animals. Urban Ecosyst. 11( 2): 161– 176. [Google Scholar]
- Medeiros-Sousa AR, Ceretti W, Jr, Urbinatti PR, Carvalho GC, Bicudo De Paula M, Fernandes A, Matos MO, JR, Orico LD, Araujo AB, Nardi MS, Marrelli MT. (2013) Mosquito fauna in Municipal parks of São Paulo City, Brazil: A preliminary survey. J Am Mosq Control Assoc. 29( 3): 275– 279. [DOI] [PubMed] [Google Scholar]
- Mocellin MG, Simões TC, Nascimento TFS, Teixeira MLFT, Lounibos LP, Oliveira RP. (2009) Bromeliad-inhabiting mosquitoes in an urban botanical garden of dengue endemic Rio de Janeiro. Are bromeliads productive habitats for the invasive vectors Aedes aegypti and Aedes albopictus?. Mem Inst Oswaldo Cruz. 104( 8): 1171– 1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montes J. (2005) Culicidae fauna of Serra da Cantareira, Sao Paulo, Brazil. Rev Saúde Pública. 39( 4): 578– 584. [DOI] [PubMed] [Google Scholar]
- Morais AS, Marrelli MT, Natal D. (2006) Aspects of the distribution of Culex (Culex) quinquefasciatus Say (Diptera: Culicidae) in the region of the Pinheiros River, in the city of São Paulo, State of São Paulo, Brazil. Rev Bras Entomol. 50( 3): 413– 418. [Google Scholar]
- Muller GA, Marcondes CB. (2006) Bromeliad-associated mosquitoes from Atlantic forest in Santa Catarina Island, southern Brazil (Diptera, Culicidae), with new records for the State of Santa Catarina. Iheringia Sér Zool. 96 ( 3): 315– 319. [Google Scholar]
- Murray KO, Mertens E, Desprès P. (2010) West Nile virus and its emergence in the United States of America. Vet Res. 41( 6): 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natal D, Urbinatti PR, Taipe-Lagos CB, Ceretti W, Jr, Diederich ATB, Souza RG, Souza RP. (1997) Aedes (Stegomyia) albopictus (Skuse) breeding in Bromeliaceae in the outskirts of an urban area of the city of São Paulo, Brazil. Rev Saude Publ. 31( 5): 517– 518. [DOI] [PubMed] [Google Scholar]
- Nyamah MA, Sulaiman S, Omar B. (2011) Field observation on the efficacy of Toxorhynchites splendens (Wiedemann) as a biocontrol agent against Aedes albopictus (Skuse) larvae in a cemetery. Trop Biomed. 28( 2): 312– 319. [PubMed] [Google Scholar]
- Oliveira-Ferreira J, Lacerda MV, Brasil P, Ladislau JL, Tauil PL, Daniel-Ribeiro CT. (2010) Malaria in Brazil: an overview. Mal J. 9: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira LE, Suzuki A, Coimbra TLM, Souza RD, Chamelet ELB. (2001) Ilheus arbovirus in wild birds (Sporophila caerulescens and Molothrus bonariensis). Rev Saúde Pública. 35 ( 2): 119– 123. [DOI] [PubMed] [Google Scholar]
- Reinert JF. (2009) List of abbreviations for currently valid generic-level taxa in family Culicidae (Diptera). Eur Mos Bull. 27: 68– 76. [Google Scholar]
- Ribeiro AF, Urbinatti PR, de Castro Duarte AM, de Paula MB, Pereira DM, Mucci LF, Fernandes A, de Mello MH, de Matos Júnior MO, de Oliveira RC, Natal D, dos Santos Malafronte R. (2012) Mosquitoes in degraded and preserved areas of the Atlantic Forest and potential for vector-borne disease risk in the municipality of São Paulo, Brazil. J Vector Ecol. 37( 2): 316– 324. [DOI] [PubMed] [Google Scholar]
- São Paulo (2014a) Guia dos Parques Municipais de São Paulo. Prefeitura Municipal de São Paulo, SP, Brazil. 4th ed Secretaria do Verde e Meio Ambiente, São Paulo, p. 193. [Google Scholar]
- São Paulo (2014b) Informe sobre Dengue. Secretaria de Saúde do Município de São Paulo, SP, Brazil. Available at: http://www.prefeitura.sp.gov.br/cidade/secretarias/upload/chamadas/dengue6_1424709250.pdf.
- Souza RPD, Petrella S, Coimbra TLM, Maeda AY, Rocco IM, Bisordi I, Silveira VR, Pereira LE, Suzuki A, Silva SJS, Silva FG, Salvador FS, Tubaki RM, Menezes RT, Pereira M, Bergo ES, Hoffmann RC, Spinola RMF, Tengan CH, Siciliano MM. (2011) Isolation of yellow fever virus (YFV) from naturally infected Haemagogus (Conopostegus) leucocelaenus (Dipteral: Culicidae) in São Paulo State, Brazil, 2009. Rev Inst Med Trop São Paulo. 53( 3): 133– 139. [DOI] [PubMed] [Google Scholar]
- Taipe-Lagos CB, Natal D. (2003) Abundância de culicídeos em área metro-politana preservada e suas implicações epidemiológicas. Rev Saúde Pública. 37( 3): 275– 279. [DOI] [PubMed] [Google Scholar]
- Urbinatti PR, Sendacz S, Natal D. (2001) Imaturos de mosquitos (Diptera: Culicidae) em parque de área metropolitana aberto à visitação pública. Rev Saúde Pública. 35( 5): 261– 266. [DOI] [PubMed] [Google Scholar]
- Viana DV, Ignotti EA. (2013) Ocorrência da dengue e variações meteorológicas no Brasil: revisão sistemática. Rev Bras Epidemiol. 16( 2): 240– 256. [DOI] [PubMed] [Google Scholar]
- WRBU (2014) Walter Reed Bio-systematics Unit. Systematic catalog of Culicidae. Smithsonian Institution, Washington DC, USA: Available at: http://www.mosquitocatalog.org. [Google Scholar]
- Weaver SC. (2013) Urbanization and geographic expansion of zoonotic arboviral diseases: mechanisms potential strategies for prevention. Trends Microbiol. 21( 8): 360– 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2013a) World Health Organization; Malaria entomology and vector control: Guide for participants. Geneva, Switzerland. [Google Scholar]
- WHO (2013b) World Health Organization; Lymphatic filariasis: managing morbidity and preventing disability: an aide-mémoire for national programme managers. Geneva, Switzerland. [Google Scholar]
- Zequi AC, Lopes J, Medri IM. (2005) Imaturos de Culicidae (Diptera) encontrados em recipientes instalados em mata residual no município de Londrina, Paraná, Brasil. Rev Bras Zool. 22( 3): 656– 661. [Google Scholar]