Abstract
Background:
Mosquitoes lay eggs in a wide range of habitats with different physicochemical parameters. Ecological data, including physicochemical factors of oviposition sites, play an important role in integrated vector management. Those data help the managers to make the best decision in controlling the aquatic stages of vectors especially using source reduction.
Methods:
To study some physicochemical characteristics of larval habitat waters, an investigation was carried out in Qom Province, central Iran, during spring and summer 2008 and 2009. Water samples were collected during larval collection from ten localities. The chemical parameters of water samples were analyzed based on mg/l using standard methods. Water temperature (°C), turbidity (NTU), total dissolved solids (ppm), electrical conductivity (μS/cm), and acidity (pH) were measured using digital testers. Thermotolerant coliforms of water samples were analyzed based on MPN/100ml. Data were assessed by Kruskal-Wallis test and Spearman Correlation analysis.
Results:
In total, 371 mosquito larvae were collected including 14 species representing four genera. Some physicochemical parameters of water in Emamzadeh Esmail, Qomrood, Qom City, and Rahjerd showed significant differences among localities (P< 0.05). The physicochemical and microbial parameters did not show any significant differences among different species (P> 0.05). There was no significant correlation between the abundance of larvae and the different physicochemical and microbial parameters (P> 0.05).
Conclusion:
The means of EC, TDS, and phosphate of localities and species were remarkably higher than those of the previous studies. Other parameters seem to be in the range of other investigations.
Keywords: Anopheles, Culex, Culiseta, Ochlerotatus, larvae, oviposition site
Introduction
Mosquitoes (Diptera: Culicidae) lay eggs in a wide range of habitats with different physicochemical characteristics. The temperature of larval habitat water has a great influence on the development of the aquatic stages of mosquitoes ( Muirhead-Thomson 1951). While some subarctic aedine species larvae are able to develop at 1.1°C, the development of many mosquito species critically decrease below 14–16 °C. On the other hand, few species are able to breed and survive at 42 °C in tropical areas, while the temperature above 30 °C decreases larval development and 37–38 °C is fatal for many temperate species ( Muirhead-Thomson 1951, Clements 1992). Most of mosquito larvae develop in fresh water (a salinity of up to 2 parts per thousand). However, nearly 5 % live in brackish (a salinity between fresh water and sea water - 34.5 per thousand) or saline waters (very rich in soluble salts). Some species occur in both fresh and brackish waters, even closely related species may be found in different larval habitats for example fresh and brackish waters for the Maculipennis Group species and fresh, brackish, and saline habitats for An. gambiae Giles complex species ( Clements 1992). In nature different mosquito larvae species were found in a wide range of pH from 3.3 to 10.5 and some species were reared in pH from 2 to 9 in the laboratory. Many species larvae were found in both acid and alkaline habitats. Though pH effects on the distribution of some species, there is no evidence that pH is a limiting factor ( Clements 1992). Organic matter and pollution, for example ammonium ion, which has a range between 2 to 5 mM in sewage, can restrict larval breeding and few species survive in heavily polluted waters. Anopheline larvae mainly develop in clean water and seldom are found in polluted habitats, which seem to be favorable for some culicines ( Muirhead-Thomson 1951, Clements 1992).
Malaria is the most important mosquito-borne disease in Iran, especially in southern and southeastern areas, and seven species of the genus Anopheles Meigen are known as proven vectors in the country, An. culicifacies Giles s.l., An. dthali Patton, An. fluviatilis James s.l., An. maculipennis Meigen s.l., An. sacharovi Favre, An. stephensi Liston, and An. superpictus Grassi ( Hanafi-Bojd et al. 2011). The mosquito fauna of Iran includes seven genera and 64 species ( Azari-Hamidian 2007a).
Qom Province is located in the central plateau of Iran where the risk of malaria infection is lower than the southern and southeastern areas of the country. However, the situation of the province increases the risk of transmission, because many passengers/ pilgrims visit the province from different parts of the country including malarious areas ( Farzinnia et al. 2010). The province is classified in the stratum 2 (with the imported cases of malaria and potential transmission) of the National Malaria Strategy Plan for Malaria Control in Iran ( Raeisi et al. 2004). In total, 448 cases of malaria were recorded in Qom Province during 2001–2008. The trend of disease shows decline in the province like many other Iranian provinces. Total number of the cases has been decreased from 151 in 2001 to 22 in 2008. Though nearly all cases are imported from outside of the province with mostly non-Iranian origin, one indigenous case was found in 2004 ( Farzinnia et al. 2010). After 2008, the cases were 16, 24, 49, and 54 from 2009 to 2012, respectively (unpublished data from Department of Disease Control, Deputy of Health, Qom University of Medical Sciences).
Macan (1950) found An. multicolor Combouliu larvae in “Darya-i-Namak” (Namak Lake, Salt Lake, or Qom Lake). Macan (1950) also showed An. superpictus around Qom in the distributional maps. Farzinnia et al. (2010) reported An. claviger (Meigen) for the first time in the province. Saghafipour et al. (2012) found 14 species representing four genera in Qom Province including 12 new provincial records. In total, 15 species and 4 genera are found in the province.
There is little information about physicochemical characteristics of larval habitats in Iran. In the most of previous investigations, the collecting data of larval habitats included only water temperature and/or pH (eg Macan 1950, Lotfi 1976, Azari-Hamidian et al. 2004, Azari-Hamidian 2005, 2006, 2007b, 2011). Yaghoobi-Ershadi et al. (2001) investigated the bionomics of An. sacharovi in Ardebil Province, northwestern Iran, with a note on salinity, based on Calcium Bicarbonate and Sodium Sulfate, for the larval habitats of species. Ghanbari et al. (2005) studied some physical and chemical factors of oviposition sites including turbidity, electrical conductivity (EC), temperature, pH, total hardness, calcium, chloride, sulfate, nitrate, phosphate, and nitrite in Iranshahr, southeastern Iran, where eight anopheline species including An. culicifacies s.l., An. stephensi, An. dthali, An. hyrcanus (Pallas) (Most probably misidentification), An. superpictus, An. turkhudi Liston, An. multicolor, and An. pulcherrimus Theobald were collected. Hanafi-Bojd et al. (2012) investigated larval habitats, including the temperature, pH, total hardness, EC, and dry residue of waters, and biodiversity of anophelines in Bashagard, southern Iran.
Ecological data, such as physicochemical factors of oviposition sites, larval habitat characteristics, species composition, and active season play an important role in integrated vector management (IVM). Those data help the managers to make the best decision in controlling the aquatic stages of vectors especially using source reduction through environmental manipulation and modification in addition to chemical and biological controls. To study some physicochemical features of habitat water of mosquito larvae, this investigation was carried out in Qom Province, central Iran.
Materials and Methods
Study area
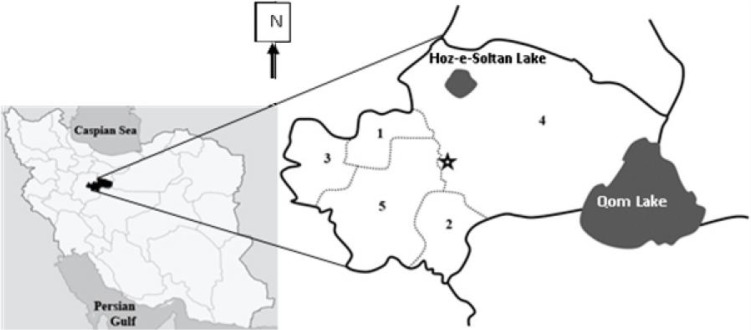
Qom Province is bounded by Tehran Province in the north, Isfahan Province in the south, Semnan Province in the east, and Markazi Province in the west with an area of approximately 11,240 square kilometers (0.68 % total area of Iran). The center of the province, Qom City, is almost 880 m above sea level. The province with arid climate has about 150 mm annual rainfall and is located between 34°09′–35°11′ N latitude and 50°06′–51°58′ E longitude and formally includes one county (Qom) and five districts, Jafarabad, Kahak, Khalajestan, Markazi (Qom), and Salafchegan (Fig. 1).
Fig. 1.
Map of Iran in which the position of Qom Province and its five districts is highlighted, 1. Jafarabad, 2. Kahak, 3. Khalajestan, 4. Markazi, and 5. Salafchegan (*Qom City)
Specimen and data collection
Larval collection was carried out from different habitats using dipping method (350 ml dipper) in ten localities of four districts, Kahak District (Emamzadeh Esmail and Dastgerd), Khalajestan District (Ahmadabad, Agholak, Dastjerd, and Ghahan), Markazi District (Qom City and Qomrood), and Salafchegan District (Ghal-e-cham and Rahjerd) during spring and summer 2008 and 2009. The larvae were preserved in lactophenol and the microscope slides of the preserved larvae were prepared using de Faure’s medium. The third-and fourth-instar larvae were identified using the key of Azari-Hamidian and Harbach (2009). The mosquito name abbreviations follow Reinert (2009).
Physicochemical analysis of water of larval habitats
The samples of water of larval habitats in ten aforementioned localities were collected and the chemical factors including alkalinity, total hardness (both based on CaCo3), calcium (Ca), chloride (Cl), fluoride (F), nitrite (NO2), nitrate (NO3), phosphate (PO4), and sulfate (SO4) were analyzed based on mg/l using standard methods ( Rice et al. 2012). Moreover, other physicochemical parameters which were tested, their units, and digital testers, are as follow: water temperature (°C) (Cyberscan, Singapore), turbidity (NTU) (Aqualytic, Germany), Total Dissolved Solids (TDS) (ppm) (Cyberscan, Singapore), Electrical Conductivity (EC) (μS/cm) (Cyberscan, Singapore), and acidity (pH) (Cyberscan, Singapore). Thermotolerant coliforms of water samples were analyzed as a biological (microbial) parameter that shows habitat pollution with human sewage and feces based on MPN (Most Probable Number)/100 ml.
Statistical analysis
The means of physicochemical and microbial parameters of the water samples of the species were compared by Kruskal-Wallis test of nonparametric analysis and the relation of physicochemical and microbial parameters to abundance was assessed by Spearman Correlation analysis using SPSS software (Version 17 for windows, SPSS Inc. Chicago, IL).
Results
In total, 371 mosquito larvae were periodically collected from nine localities in four districts of Qom Province during 2008–2009 and morphologically identified including 14 species representing four genera (Table 1). No larva was collected from the river of Qom City. All larvae were collected from natural habitats including ground pools, stream edges, riverbeds, and river edges (Fig. 2). The physicochemical and microbial parameters of water of different localities were showed in Table 2 and 3. Phosphate in Emamzadeh Esmail, turbidity, EC, TDS, total hardness, Chloride, and Nitrate in Qomrood, EC, TDS, Sulfate in Qom City, and Nitrite in Rahjerd were significantly higher than other localities (P< 0.05), however temperature, pH, alkalinity, Calcium, and Fluoride did not show any significant difference among different localities (P> 0.05) (Table 2). The physicochemical and microbial parameters did not show significant differences among different species (P> 0.05) (Table 4 and 5). There was no significant correlation between the abundance of larvae and the different physicochemical and microbial parameters (P> 0.05) (Table 6). Most of physicochemical and microbial parameters for An. claviger and An. marteri Senevet and Prunnelle and all culicine species were presented for the first time in Iran (Table 4 and 5).
Table 1.
The distribution and composition of the mosquito larvae in nine localities of Qom Province, Iran, Spring– Summer 2008–2009
| Species | Locality | n | % | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Agholak | Ahmadabad | Dastgerd | Dastjerd | Emamzadeh Esmail | Ghahan | Ghal-e-cham | Qomrood | Rahjerd | |||
| An. claviger | 66 | 135 | - | 1 | - | 16 | 10 | - | - | 228 | 61.5 |
| An. marteri | - | - | - | - | - | - | - | - | 2 | 2 | 0.5 |
| An. superpictus | - | - | 12 | - | 20 | - | - | - | - | 32 | 8.6 |
| An. turkhudi | - | - | 7 | - | - | - | - | - | - | 7 | 1.9 |
| Cx. arbieeni | - | - | 1 | - | - | - | - | - | - | 1 | 0.3 |
| Cx. hortensis | 3 | 2 | 1 | - | - | 3 | - | - | - | 9 | 2.4 |
| Cx. mimeticus | - | - | 9 | - | 1 | 1 | - | - | - | 11 | 3.0 |
| Cx. modestus | - | - | - | - | - | - | - | 6 | - | 6 | 1.6 |
| Cx. pipiens | - | - | - | - | - | - | - | 13 | - | 13 | 3.5 |
| Cx. territans | 7 | 3 | - | - | - | - | - | - | - | 10 | 2.7 |
| Cx. theileri | - | 1 | - | - | 4 | - | - | 1 | - | 6 | 1.6 |
| Cs. longiareolata | - | 34 | - | - | - | 5 | - | - | 2 | 41 | 11.1 |
| Cs. subochrea | 1 | - | - | - | - | - | - | 1 | - | 2 | 0.5 |
| Oc. caspiuss.l. | - | - | - | - | - | - | - | 3 | - | 3 | 0.8 |
| Total | 77 | 175 | 30 | 1 | 25 | 25 | 10 | 24 | 4 | 371 | 100 |
Fig. 2.
Different types of larval habitats in Qom Province, Iran, Spring–Summer 2008–2009, a, b, and c) Ground pools, d) Stream edge, e) River bed, f) River edge (Original photos)
Table 2.
The physicochemical parameters of water in Qom Province, Iran, Spring–Summer 2008–2009, *no larva was collected, a values in a row which are significantly different in habitats where include larvae (n=9) and b in all samples (n=10) (at P=0.05)
| Physicochemical parameters | Locality | Mean±SD (Larval habitats, n=9) | Mean±SD (Total, n=10) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Agholak | Ahmadabad | Dastgerd | Dastjerd | Emamzadeh Esmail | Ghahan | Ghal-e-cham | Qomrood | Qom City* | Rahjerd | |||
| Temperature (°C) | 20.4 | 17.5 | 21.8 | 17.5 | 22.0 | 20.4 | 22.7 | 22.7 | 15.3 | 20.4 | 20.60±1.98 | 20.07±2.51 |
| Acidity (pH) | 7.5 | 7.3 | 7.7 | 7.3 | 7.7 | 7.5 | 7.3 | 7.1 | 7.7 | 7.0 | 7.37±0.24 | 7.41±0.25 |
| Turbidity (NTU) | 1.0 | 0.1 | 0.3 | 0.1 | 1.9 | 1.0 | 0.2 | 6.0a | 0.5 | 0.2 | 1.20±1.89 | 1.13±1.80 |
| Electrical conductivity (μS/cm) | 2070 | 1827 | 1005 | 1827 | 815 | 2070 | 2240 | 4240a | 8820b | 392 | 1831.77±1110.74 | 2530.60±2445.44 |
| Total dissolved solids (ppm) | 1030 | 914 | 502 | 914 | 407 | 1030 | 1120 | 2120a | 4380b | 197 | 914.88±555.05 | 1261.40±1214.31 |
| Alkalinity (mg/l) | 300 | 540 | 230 | 540 | 250 | 300 | 530 | 410 | 300 | 210 | 367.77±138.90 | 361.00±132.70 |
| Total hardness (mg/l) | 799.6 | 428.4 | 238.0 | 428.4 | 266.5 | 799.6 | 534.7 | 1294.7a | 875.8 | 180.8 | 552.30±357.58 | 584.65±352.31 |
| Calcium (mg/l) | 323.6 | 649.5 | 30.4 | 649.5 | 30.4 | 323.6 | 620.8 | 220.8 | 83.7 | 26.6 | 319.46±267.23 | 295.89±262.74 |
| Chloride (mg/l) | 154.9 | 364.8 | 229.9 | 364.8 | 129.9 | 154.9 | 389.8 | 1479.5a | 329.8 | 24.9 | 365.93±435.91 | 362.32±411.14 |
| Fluoride (mg/l) | 0.96 | 1.05 | 0.28 | 1.05 | 0.41 | 0.96 | 0.95 | 0.75 | 1.23 | 0.17 | 0.731±0.349 | 0.781±0.365 |
| Nitrite (mg/l) | 0.003 | 0.008 | 0.001 | 0.008 | 0.006 | 0.003 | 0.004 | 0.004 | 0.004 | 0.012a | 0.0054±0.0033 | 0.0053±0.0032 |
| Nitrate (mg/l) | 0.0 | 1.0 | 2.2 | 1.0 | 1.2 | 0.0 | 1.8 | 9.8a | 0.4 | 0.0 | 1.88±3.07 | 1.74±2.93 |
| Phosphate (mg/l) | 0.43 | 0.39 | 1.53 | 0.39 | 2.74a | 0.43 | 0.48 | 0.48 | 0.23 | 0.12 | 0.776±0.834 | 0.722±0.805 |
| Sulfate (mg/l) | 784 | 400 | 134 | 400 | 184 | 784 | 832 | 832 | 1445b | 37 | 487.44±325.67 | 583.20±431.24 |
Table 3.
The microbial parameter of water in Qom Province, Iran, Spring–Summer 2008–2009 (*no larva was collected, N= not determined)
| Microbial parameter | Locality | Mean±SD(Larval habitats, n=8) | Mean±SD (Total, n=9) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Agholak | Ahmadabad | Dastgerd | Dastjerd | Emamzadeh Esmail | Ghahan | Ghal-e-cham | Qomrood | Qom City* | Rahjerd | |||
| Thermotalerant Coliforms (MPN/100ml) | N | 0 | 75 | 0 | 1100 | 15 | 0 | 1100 | 0 | 93 | 297.87±496.36 | 264.77±474.80 |
Table 4.
The physicochemical parameters of habitat water of mosquito larvae in Qom Province, Iran, Spring–Summer 2008–2009
| Physicochemical parameters (Mean±SD) | Species (Number of occurrence) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| An. claviger (5) | An. marteri (1) | An. superpictus (2) | An. turkhudi (1) | Cx. arbieeni (1) | Cx. hortensis (4) | Cx. mimeticus (3) | Cx. modestus (1) | Cx. pipiens (1) | Cx. territans (2) | Cx. theileri (3) | Cs. longiareolata (3) | Cs. subochrea (2) | Oc. caspius s.l. (1) | |
| Temperature (°C) | 19.7±2.2 | 20.4 | 21.9±0.1 | 21.8 | 21.8 | 20.0±1.8 | 21.4±8.8 | 22.7 | 22.7 | 18.9±2.0 | 20.7±2.8 | 19.4±1.6 | 21.5±1.6 | 22.7 |
| Acidity (pH) | 7.3±0.1 | 7.0 | 7.7±0.0 | 7.7 | 7.7 | 7.5±0.1 | 7.6±0.1 | 7.1 | 7.1 | 7.4±0.1 | 7.3±0.3 | 7.2±0.2 | 7.3±0.2 | 7.1 |
| Turbidity (NTU) | 0.4±0.4 | 0.2 | 1.1±1.1 | 0.3 | 0.3 | 0.6±0.4 | 1.0±0.8 | 6.0 | 6.0 | 0.5±0.6 | 2.6±3.0 | 0.4±0.4 | 3.5±3.5 | 6.0 |
| Electrical conductivity(μS/cm) | 2006.8±178.2 | 392 | 910.0±134.3 | 1005 | 1005 | 1743.0±505.1 | 1296.6±676.4 | 4240 | 4240 | 1948.5±171.8 | 2294.0±1759.6 | 1429.6±906.8 | 3155.0±1534.4 | 4240 |
| Total dissolved solids (ppm) | 1001.6±88.0 | 197 | 454.5±67.1 | 502 | 502 | 869.0±259.7 | 335.6±193.7 | 2120 | 2120 | 972.0±82.0 | 1147.0±879.9 | 713.6±451.1 | 1575.0±770.7 | 2120 |
| Alkalinity (mg/l) | 442.0±129.6 | 210 | 240.0±14.1 | 230 | 230 | 342.5±135.7 | 260.0±36.0 | 410 | 410 | 420.0±169.7 | 400.0±145.2 | 416.6±120.1 | 355.0±77.7 | 410 |
| Total hardness (mg/l) | 598.1±188.9 | 180.8 | 252.2±20.1 | 238.0 | 238.0 | 566.4±280.2 | 434.7±316.3 | 1294.7 | 1294.7 | 614.0±262.4 | 663.2±552.8 | 469.6±311.4 | 1047.1±350.0 | 1294.7 |
| Calcium (mg/l) | 513.4±173.6 | 26.6 | 30.4±0.0 | 30.4 | 30.4 | 331.7±259.9 | 128.1±169.2 | 220.8 | 220.8 | 486.5±230.4 | 300.2±317.1 | 333.2±311.5 | 272.2±72.6 | 220.8 |
| Chloride (mg/l) | 285.8±119.9 | 24.9 | 179.9±70.7 | 229.9 | 229.9 | 226.1±98.9 | 171.5±52.0 | 1479.5 | 1479.5 | 259.8±148.4 | 658.0±721.0 | 181.5±171.5 | 817.2±936.6 | 1479.5 |
| Fluoride (mg/l) | 0.99±0.05 | 0.17 | 0.34±0.09 | 0.28 | 0.28 | 0.81±0.35 | 0.55±0.36 | 0.75 | 0.75 | 1.00±0.06 | 0.73±0.32 | 0.72±0.48 | 0.85±0.14 | 0.75 |
| Nitrite (mg/l) | 0.005±0.002 | 0.012 | 0.003±0.003 | 0.001 | 0.001 | 0.003±0.002 | 0.003±0.002 | 0.004 | 0.004 | 0.005±0.003 | 0.006±0.002 | 0.007±0.004 | 0.003±0.000 | 0.004 |
| Nitrate (mg/l) | 0.7±0.7 | 0.0 | 1.7±0.7 | 2.2 | 2.2 | 0.8±1.0 | 1.1±1.1 | 9.8 | 9.8 | 0.5±0.7 | 4.0±5.0 | 0.3±0.5 | 4.9±6.9 | 9.8 |
| Phosphate (mg/l) | 0.42±0.03 | 0.12 | 2.13±0.85 | 1.53 | 1.53 | 0.69±0.55 | 1.56±1.15 | 0.48 | 0.48 | 0.41±0.02 | 1.20±1.33 | 0.31±0.16 | 0.45±0.03 | 0.48 |
| Sulfate (mg/l) | 640.0±219.9 | 37 | 159.0±35.3 | 134 | 134 | 525.5±317.6 | 367.3±361.7 | 832 | 832 | 592.0±271.5 | 472.0±329.9 | 407.0±373.5 | 808.0±33.9 | 832 |
Table 5.
The microbial parameter of habitat water of mosquito larvae in Qom Province, Iran, Spring–Summer 2008–2009
| Microbial parameter (Mean±SD and range) | Species (Number of occurrence) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| An. claviger (4) | An. marteri (1) | An. superpictus (2) | An. turkhud (1) | Cx. arbieeni (1) | Cx. hortensis (3) | Cx. mimeticus (3) | Cx. modestus (1) | Cx. pipiens (1) | Cx. territans (1) | Cx. theileri (3) | Cs. longiareolata (3) | Cs. subochrea (1) | Oc. caspius s.l. (1) | |
| Thermotalerant Coliforms (MPN/100ml) | 3.75±7.50 (0–15) | 93 | 587.50±724.78 (75–1100) | 75 | 75 | 30.00±39.68 (0–75) | 396.66±609.84 (15–1100) | 1100 | 1100 | 0 | 733.33±635.08 (0–1100) | 36.00±49.92 (0–93) | 1100 | 1100 |
Table 6.
Spearman Correlation coefficient between physicochemical and microbial parameters of habitat waters and larvae abundance in Qom Province, Iran, Spring–Summer 2008–2009, Sig. (2-tailed), n=9 (for Thermotalerant Coliforms, n=8) (P> 0.05)
| Physicochemical and microbial parameters | Abundance |
|---|---|
| Temperature (°C) | −0.137 |
| 0.725 | |
| Acidity (pH) | 0.532 |
| 0.140 | |
| Turbidity (NTU) | 0.174 |
| 0.655 | |
| Electrical conductivity (μS/cm) | 0.030 |
| 0.940 | |
| Total dissolved solids (ppm) | 0.030 |
| 0.940 | |
| Alkalinity (mg/l) | −0.030 |
| 0.940 | |
| Total hardness (mg/l) | 0.131 |
| 0.737 | |
| Calcium (mg/l) | 0.076 |
| 0.845 | |
| Chloride (mg/l) | −0.080 |
| 0.838 | |
| Fluoride (mg/l) | 0.190 |
| 0.625 | |
| Nitrite (mg/l) | −0.453 |
| 0.220 | |
| Nitrate (mg/l) | −0.051 |
| 0.896 | |
| Phosphate (mg/l) | 0.233 |
| 0.546 | |
| Sulfate (mg/l) | −0.004 |
| 0.991 | |
| Thermotalerant Coliforms (MPN/100ml) | 0.056 |
| 0.896 | |
Discussion
In the present study, 371 mosquito larvae representing 14 species in four genera were collected from Qom Province. All species except for An. claviger and An. superpictus were collected for the first time in the province ( Saghafipour et al. 2012) (Table 1). Anopheles multicolor, recorded in Qom Province already ( Macan 1950), was not found in the present investigation.
Some physicochemical parameters of water in Emamzadeh Esmail (Phosphate), Qom City (EC, TDS, and Sulfate), and Rahjerd (Nitrite) showed significant differences among localities, however Qomrood included much more significantly different parameters (turbidity, EC, TDS, total hardness, Chloride, and Nitrate) (P< 0.05) (Table 2). It is noteworthy that no larva was found in the river of Qom City where three parameters (EC=8,820 μS/cm, TDS=4,380 ppm, and Sulfate=1,445 mg/l) were significantly higher than those of other localities (P< 0.05) (Table 2). Some aforementioned water parameters have a public health importance and are used as an indicator of drinking-water quality (WHO 2008).
Khamala (1971) found that sedimentary solids, suspended solids, dissolved organic matter, total nitrogen, and pH did not have significant correlation with the density of Mansonia africana (Theobald) and Ma. uniformis (Theobald) larvae in Kenya, while Sasikumar et al. (1986) showed that Na, K, and pH had significant correlation with the density of Ma. uniformis and Ma. annulifera (Theobald) larvae, however Ca, Mg, and rainfall showed no significant relation in India. In Iran, Ghanbari et al. (2005) showed the significant correlation of seven physicochemical factors including pH, total hardness, Nitrate, phosphate, EC, calcium, and sulfate with five species An. culicifacies s.l., An. stephensi, An. superpictus, An. turkhudi, An. multicolor. Though some of aforementioned factors were found to be predictor species, none of them was predictor for all anopheline species. That might show biological differences of the species. Hanafi-Bojd et al. (2012) noted the temperature, pH, total hardness, EC, and dry residue of larval habitats in Bashagard where eight species An. culicifacies s.l., An. dthali, An. stephensi, An. superpictus, An. fluviatilis s.l., An. turkhudi, An. moghulensis Christophers, and An. apoci Marsh were collected, however they did not calculated any correlation. Both Ghanbari et al. (2005) and Hanafi-Bojd et al. (2012) mentioned the figures of habitats in general and none of them provided the exact values (means) of physicochemical features for each species. Piyaratne et al. (2005) found a positive correlation of An. culicifacies s.l. and An. varuna Iyengar abundances only to temperature and Calcium, respectively. Surendran and Ramasamy (2005) observed a significant correlation of An. culicifacies species E abundance to dissolved oxygen. Abdel-Hamid et al. (2011) found that there is a positive correlation between larval density and temperature for total collected species including three more prevalent ones: Cx. antennatus (Becker), Cx. pipiens Linnaeus, and Cx. perexiguus Theobald and a negative correlation between density and pH for total collected species and for Cx. pipiens. However the correlation was positive for Cx. antennatus and Cx. perexiguus in El Gharbia Governorate, Egypt. Ibrahim et al. (2011) found that temperature, Amonia, and Nitrate are the best predictor for larval density in Qalyubiya Governorate, Egypt, where they collected nine species in which Cx. pipiens was the most prevalent (64.7%). However, no correlation was found between larval density, pH, and dissolved oxygen.
Among the physicochemical parameters of the present investigation, the means of EC, TDS, and Phosphate of localities (1,831.77, 914.88, and 0.77, respectively) (Table 2) and species (Table 4) were remarkably higher than those of Surendran and Ramasamy (2005) (EC: 715.7), Piyaratne et al. (2005) (EC: 534.7 and 828.8, TDS: 265.3 and 407.7, Phosphate: 0.03 and 0.22), and Ghanbari et al. (2005) (EC: 256, Phosphate: 0.07). Other parameters seem to be in the range of other investigations ( Muirhead-Thomson 1951, Khamala 1971, Sasikumar et al. 1986, Clements 1992, Ghanbari et al. 2005, Piyaratne et al. 2005, Surendran and Ramasamy 2005, Abdel-Hamid et al. 2011, Ibrahim et al. 2011, Hanafi-Bojd et al. 2012).
As it is obvious, some available data are contradictory and there is not enough information about physicochemical parameters of larval habitats for many mosquito species. In addition to the biological differences of different species, the same species has a range of tolerance and sometimes show different correlation with physicochemical parameters. Thus, the present data is too basic for a general assessment and conclusion.
The most important limitation of the present study is the limited numbers and occurrences of different species because of the arid climate of Qom Province. This phenomenon caused that the distribution of some parameters were not normal (Table 2) and the occurrences of the species ranged from only one to five (Tables 4 and 5). That is why Kruskal-Wallis test and Spearman Correlation coefficient of nonparametric analysis were used to assess data. Though according to the present physicochemical and microbial parameters, there is no significant difference among different species (Tables 4 and 5) and the parameters did not show correlation with the abundance of larvae (Table 6), they may change with more sampling and using parametric analysis.
Conclusion
In the present investigation, the means of EC, TDS, and Phosphate of localities and species were remarkably higher than those of the previous studies. Other parameters seem to be in the range of other investigations. More samplings of habitat waters (with or without larvae for comparing) in different climates and topographical regions (to collect more diversified species) are recommended.
Acknowledgements
The authors are grateful to Dr A Akbari, MH Babakhani, F Abedi-Astaneh, Qom Provincial Health Center, Qom University of Medical Sciences, Qom, for their kind collaborations. The authors also thank Dr SM Omrani, Department of Medical Parasitology, School of Medicine, Shahrekord University of Medical Sciences, Shahrekord, and Shadi Azari-Hamidian for reviewing the manuscript. This study was financially supported by the Institute of Public Health Research, Academic Pivot for Education and Research, Tehran University of Medical Sciences: project No.: 241.83.77. This investigation also was financially supported by Qom University of Medical Sciences. The authors declare that there is no conflict of interests.
References
- Abdel-Hamid YM, Mostafa AA, Allam KM, Kenawy MA. (2011) Mosquitoes (Diptera: Culicidae) in El Gharbia Governorate, Egypt: their spatial distribution, abundance and factors affecting their breeding related to the situation of lymphatic filariasis. Egypt Acad J Biolog Sci. 3: 9– 16. [Google Scholar]
- Azari-Hamidian S, Joeafshani MA, Mosslem M, Rassaei AR. (2004) Notes on Coquillettidia richiardii and Uranotaenia unguiculata (Diptera: Culicidae) in Guilan Province. J Med Fac Guilan Univ Med Sci. 13( 51: 1– 9 (in Persian). [Google Scholar]
- Azari-Hamidian S. (2005) Larval habitat characteristics of mosquitoes of the genus Culiseta Felt, 1904 (Diptera: Culicidae) in the Caspian Sea littoral, Iran. Zool Middle East. 36: 59– 66. [Google Scholar]
- Azari-Hamidian S. (2006) On the ecology of the tribe Aedini (Diptera: Culicidae) larvae in the Caspian Sea littoral, Iran. The 11th International Congress of Parasitology, 6–11 August 2006, Glasgow, Scotland, UK. [Google Scholar]
- Azari-Hamidian S. (2007a) Checklist of Iranian mosquitoes (Diptera: Culicidae). J Vector Ecol. 32: 235– 242. [DOI] [PubMed] [Google Scholar]
- Azari-Hamidian S. (2007b) Larval habitat characteristics of mosquitoes of the genus Culex (Diptera: Culicidae) in Guilan Province, Iran. Iran J Arthropod-Borne Dis. 1: 9– 20. [PMC free article] [PubMed] [Google Scholar]
- Azari-Hamidian S, Harbach RE. (2009) Keys to the adult females and fourth-instar larvae of the mosquitoes of Iran (Diptera: Culicidae). Zootaxa. 2078: 1– 33. [Google Scholar]
- Azari-Hamidian S. (2011) Larval habitat characteristics of the genus Anopheles (Diptera: Culicidae) and a checklist of mosquitoes in Guilan Province, northern Iran. Iran J Arthropod-Borne Dis. 5: 37– 53. [PMC free article] [PubMed] [Google Scholar]
- Clements AN. (1992) The Biology of Mosquitoes Volume 1. Development, Nutrition and Reproduction. Chapman and Hall, London. [Google Scholar]
- Farzinnia B, Saghafipour A, Abai MR. (2010) Malaria situation and anopheline mosquitoes in Qom Province, central Iran. Iran J Arthropod-Borne Dis. 4: 61– 67. [PMC free article] [PubMed] [Google Scholar]
- Ghanbari MR, Rakhsh Khorshid A, Salehi M, Hassanzehi A. (2005) The study of physical and chemical factors affecting breeding places of Anopheles in Iran-shahr. Tabib-e-Shargh, J Zahedan Univ Med Sci. 7: 221– 227 (in Persian). [Google Scholar]
- Hanafi-Bojd AA, Azari-Hamidian S, Vatandoost H, Charrahy Z. (2011) Spatio-temporal distribution of malaria vectors (Diptera: Culicidae) across different climatic zones of Iran. Asian Pac J Trop Med. 4: 498– 504. [DOI] [PubMed] [Google Scholar]
- Hanafi-Bojd AA, Vatandoost H, Oshaghi MA, Charrahy Z, Haghdoost AA, Sedaghat MM, Abedi F, Soltani M, Raeisi A. (2012) Larval habitats and biodiversity of anopheline mosquitoes (Diptera: Culicidae) in a malarious area of southern Iran. J Vector Borne Dis. 49: 91– 100. [PubMed] [Google Scholar]
- Ibrahim AA, El-Monairy OM, El-Sayed YA, Baz MM. (2011) Mosquito breeding sources in Qalyubiya Governorate, Egypt. Egypt Acad J Biolog Sci. 3: 25– 39. [Google Scholar]
- Khamala PMC. (1971) The biting flies of Kano plains, Kenya: Part II. Larval habitats of common mosquito species (Diptera: Culicidae). Bull Entomol Res. 61: 299– 307. [Google Scholar]
- Lotfi MD. (1976) Key to Culicinae of Iran, genus Culex and their biology (Diptera: Culicidae). Iran J Public Health. 5: 71– 84. [Google Scholar]
- Macan TT. (1950) Anopheles and Malaria in the Near East. Part III. The Anopheline Mosquitoes of Iraq and North Persia. London School of Hygiene and Tropical Medicine Research Memoir No 7, HK and Lewis Co Ltd, London. [Google Scholar]
- Muirhead-Thomson RC. (1951) Mosquito Behaviour in Relation to Malaria Transmission and Control in the Tropics. Edward Arnold and Co, London. [Google Scholar]
- Piyaratne MK, Amerasinhe FP, Amerasinghe PH, Konradsen F. (2005) Physicochemical characteristics of Anopheles culicifacies and Anopheles varuna breeding water in a dry zone stream in Sri Lanka. J Vector Borne Dis. 42: 61– 67. [PubMed] [Google Scholar]
- Raeisi A, Shahbazi A, Ranjbar M, Shoghli A, Vatandoost H, Faraji L. (2004) National Strategy Plan for Malaria Control (I.R. Iran 2004–2008). Ministry of Health and Medical Education (Iran), Seda Publishing Center, Tehran. [Google Scholar]
- Reinert JF. (2009) List of abbreviations for currently valid generic-level taxa in family Culicidae (Diptera). Eur Mosq Bull. 27: 68– 76. [Google Scholar]
- Rice EW, Baird RB, Eaton AD, Clesceri LS. (2012) Standard Methods for the Examination of Water and Wastewater. 22nd Edition American Public Health Association, American Water Works Association, Water Environment Federation, Cenveo Publisher Services, Richmond. [Google Scholar]
- Saghafipour A, Abai MR, Farzinnia B, Nafar R, Ladonni H, Azari-Hamidian S. (2012) Mosquito (Diptera: Culicidae) fauna of Qom Province, Iran. J Arthropod-Borne Dis. 6: 54– 61. [PMC free article] [PubMed] [Google Scholar]
- Sasikumar PS, Suryanarayanan P, Thomas C, Kalyanaraman K, Prasad RS. (1986) Influence of certain physic-chemical factors upon the larval population of Mansonia mosquitoes (Culicidae: Diptera) in Trivandrum City, India. Proc Indian Acad Sci (Anim Sci). 95: 549– 555. [Google Scholar]
- Surendran SN, Ramasamy R. (2005) Some characteristics of the larval breeding sites of Anopheles culicifacies species B and E in Sri Lanka. J Vector Borne Dis. 42: 39– 44. [PubMed] [Google Scholar]
- WHO (2008) Guidelines for Drinking-Water Quality. Second Addendum to Third Edition. Vol. 1. Recommendations. World Health Organization, Geneva. [Google Scholar]
- Yaghoobi-Ershadi MR, Namazi J, Piazak N. (2001) Bionomics of Anopheles sacharovi in Ardebil Province, northwestern Iran during a larval control program. Acta Trop. 78: 207– 215. [DOI] [PubMed] [Google Scholar]