Abstract
There are several established lipid-modifying agents, including statins, fibrates, niacin, and ezetimibe, that have been shown in randomized clinical outcome trials to reduce the risk of having an atherosclerotic cardiovascular event. However, in many people, the risk of having an event remains unacceptably high despite treatment with these established agents. This has stimulated the search for new therapies designed to reduce residual cardiovascular risk. New approaches that target atherogenic lipoproteins include: 1) inhibition of proprotein convertase subtilisin/kexin type 9 to increase removal of atherogenic lipoproteins from plasma; 2) inhibition of the synthesis of apolipoprotein (apo) B, the main protein component of atherogenic lipoproteins; 3) inhibition of microsomal triglyceride transfer protein to block the formation of atherogenic lipoproteins; 4) inhibition of adenosine triphosphate citrate lyase to inhibit the synthesis of cholesterol; 5) inhibition of the synthesis of lipoprotein(a), a factor known to cause atherosclerosis; 6) inhibition of apoC-III to reduce triglyceride-rich lipoproteins and to enhance high-density lipoprotein (HDL) functionality; and 7) inhibition of cholesteryl ester transfer protein, which not only reduces the concentration of atherogenic lipoproteins but also increases the level and function of the potentially antiatherogenic HDL fraction. Other new therapies that specifically target HDLs include infusions of reconstituted HDLs, HDL delipidation, and infusions of apoA-I mimetic peptides that mimic some of the functions of HDLs. This review describes the scientific basis and rationale for developing these new therapies and provides a brief summary of established therapies.
I. Introduction
Relationships between plasma lipids and lipoproteins and the risk of having an atherosclerotic cardiovascular disease (ASCVD) event have been observed in human population studies for many years. Furthermore, there is an overwhelming body of evidence showing that interventions that target plasma lipids and lipoproteins have the potential to reduce ASCVD risk. It was shown 40 years ago that treatment with niacin reduced the risk of having an ASCVD event in high-risk men (Coronary Drug Project Research Group, 1975). It is more than 30 years since publication of the Coronary Primary Prevention Trial, which showed that reducing the concentration of low-density lipoprotein cholesterol (LDL-C) by treatment with cholestyramine significantly reduced the risk of having a coronary event (Lipid Research Clinics, 1984). It is 28 years since the Helsinki Heart Study, which was conducted in men with increased levels of atherogenic lipoproteins, revealed a significant reduction in ASCVD events after treatment with the fibrate, gemfibrozil (Frick et al., 1987). Finally, it is more than 20 years since publication of the Scandinavian Simvastatin Survival Study, which showed that treatment with simvastatin reduced ASCVD morbidity and mortality in men with elevated levels of LDL-C (Scandinavian Simvastatin Survival Study Group, 1994).
However, despite the ability of these agents to lower LDL-C and reduce ASCVD risk, many high-risk people have levels of LDL-C that remain unacceptably high despite being treated with maximally tolerated doses of statins and other lipid-lowering agents. A residual risk of having an ASCVD event in statin-treated people may also relate to other lipid abnormalities, such as elevated levels of triglyceride-rich lipoproteins, elevated levels of lipoprotein(a) [Lp(a)], and low levels of high-density lipoproteins (HDLs). The presence of residual risk has fueled the search for additional approaches to target plasma lipids and lipoproteins to further reduce the risk of having an ASCVD event.
The first part of this review summarizes current knowledge of established lipid-modifying agents. The remainder of the review focuses on the development of novel agents with the potential to reduce the residual ASCVD risk that persists in many people who are treated with established lipid-modifying agents.
II. Established Therapies
A. Inhibitors of 3-Hydroxy-3-Methylglutaryl -Coenzyme A Reductase
Statins inhibit 3-hydroxy-3-methylglutaryl-CoA reductase, the rate-limiting enzyme in cholesterol synthesis. Inhibition of cell cholesterol synthesis by statins transiently reduces the concentration of cholesterol in cells, which activates the sterol regulatory element binding protein (SREBP)-2. This leads to increased expression of the low-density protein (LDL) receptor on the cell surface, a consequent increase in the uptake of LDLs by the cell, and thus a decrease in the plasma concentration of LDL-C.
The availability of statins as agents to lower the level of LDL-C has revolutionized the management of people at risk of having an ASCVD event. Treatment with statins reduces the concentration of LDL-C by up to 50% and has been shown in many randomized, double-blind clinical outcome trials to reduce the risk of having a future ASCVD event (Baigent et al., 2010).
However, many people continue to have levels of LDL-C that are higher than desirable despite taking maximal tolerated doses of statins. These people remain at an unacceptably high risk of having an ASCVD event, indicating that there is a need for using additional LDL-C–lowering agents in many statin-treated people. This need is met to some extent by the currently available agents as outlined below.
B. Inhibitors of Cholesterol Absorption
Niemann-Pick C1-Like 1 (NPC1L1) is a protein in intestinal cells that promotes the absorption of cholesterol. Mutations of the NPC1L1 gene that result in loss of function of NPC1L1 are associated with lower concentrations of LDL-C and a significantly reduced risk of having an ASCVD event (Stitziel et al., 2014).
Inhibition of NPC1L1 by ezetimibe reduces the intestinal absorption of cholesterol, lowers the concentration of LDL-C, and reduces ASCVD events (Cannon et al., 2015).
C. Fibrates
Fibrates (Staels et al., 1998) have been in use for more than 50 years. They target and activate the hormone-activated nuclear receptor, peroxisome proliferator–activated receptor α (PPARα). This increases the oxidation of free fatty acids in the liver and reduces the hepatic synthesis of triglyceride. Activation of PPARα also induces expression of lipoprotein lipase (LPL), the enzyme responsible for hydrolyzing triglycerides and phospholipids in very low-density lipoproteins (VLDLs) and chylomicrons (Heller and Harvengt, 1983). Thus, activators of PPARα reduce the level of plasma triglyceride by the combined effects of reducing its synthesis and increasing its rate of hydrolysis. Activation of PPARα also inhibits synthesis of apolipoprotein (apo) C-III (Staels et al., 1995), an apolipoprotein that delays the catabolism of triglyceride-rich lipoproteins. Fibrates also increase the concentration of high-density lipoprotein cholesterol (HDL-C) and increase synthesis of both apoA-I and apoA-II, the two main HDL apolipoproteins, by a mechanism that is not fully understood.
The results of clinical outcome trials of fibrates have varied. Positive results were obtained for gemfibrozil in primary prevention in the Helsinki Heart Study (Frick et al., 1987) and in secondary prevention in the Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial (Rubins et al., 1999). A neutral result was obtained for bezafibrate in secondary prevention in the Bezafibrate Infarction Prevention Study (Bezafibrate Infarction Prevention Study Group, 2000) and for fenofibrate in people with diabetes in the Fenofibrate Intervention and Event Lowering in Diabetes (Keech et al., 2005) and Action to Control Cardiovascular Risk in Diabetes (Ginsberg et al., 2010) studies. It should be noted that in the Fenofibrate Intervention and Event Lowering in Diabetes trial, there was a significantly greater use of statin therapy in patients allocated placebo than in those taking fenofibrate; in the Action to Control Cardiovascular Risk in Diabetes trial, patients in both the placebo and fenofibrate groups were taking a statin. Post hoc analyses of the fibrate trials have consistently shown that people with high levels of plasma triglyceride and low levels of HDL-C derive a disproportionately large benefit when treated with these agents in terms of a reduction in cardiovascular events. These analyses indicate that there is a clear need for a clinical cardiovascular outcome trial using fibrates in such individuals.
New selective PPARα agonists that are in current development are discussed below.
D. Bile Acid Sequestering Agents
Bile acid sequestering agents have been used to reduce the concentration of LDL-C for many years. They act by binding to bile acids in the intestine, thus preventing their reabsorption. This results in increased formation of bile acids from cholesterol in hepatocytes, which transiently reduces cellular cholesterol levels and increases synthesis of LDL receptors. This increases the hepatic uptake of LDL from plasma and reduces the plasma concentration of LDL-C.
Treatment with the bile acid sequestering agent, cholestyramine, resulted in a significant reduction in clinical cardiovascular events in the Coronary Primary Prevention Trial (Lipid Research Clinics, 1984).
E. Niacin
Niacin has been used to modify plasma lipid levels for more than 50 years. When given in pharmacological doses, niacin reduces the level of plasma triglyceride by about 35%, reduces LDL-C levels by 10%–15%, and increases the concentration of HDL-C by up to 25%. The precise mechanism of these effects remains uncertain. The reduction in plasma triglycerides may be the consequence of the ability of niacin to inhibit the release of nonesterified fatty acids (NEFAs) from adipose tissue. This results in a decrease in the plasma concentration of NEFAs, reduced hepatic uptake of NEFAs, and reduced formation of triglycerides in the liver. The mechanism by which niacin decreases LDL-C levels and increases HDL-C levels is not known.
In a trial conducted in the prestatin era, treatment with niacin significantly reduced ASCVD events (Coronary Drug Project Research Group, 1975). However, in two recent large, randomized clinical outcome trials conducted in people taking statins, treatment with niacin did not reduce ASCVD events (Boden et al., 2011; Landray et al., 2014).
F. Omega-3 Fatty Acids
Omega-3 fatty acids decrease the hepatic synthesis and secretion of VLDLs (Madsen et al., 1999). They also inhibit lipogenesis (Davidson, 2006) and increase LPL activity (Weintraub et al., 1988), which reduces the concentration of plasma triglycerides. There is also evidence that omega-3 fatty acids have anti-inflammatory effects that may improve the stability of atherosclerotic plaques (von Schacky et al., 1999; Thies et al., 2003). Omega-3 fatty acids are also antiarrhythmic (Billman et al., 1994).
An open-label study from Japan showed that treatment with omega-3 fatty acids reduced the risk of having an ASCVD event (Yokoyama et al., 2007). The effect of omega-3 fatty acids on ASCVD risk is currently being evaluated in two ongoing randomized clinical outcome trials.
III. New Therapies
Agents in current development will be presented under two headings: 1) those that target atherogenic lipoproteins and 2) those that target the potentially cardioprotective HDLs.
A. Therapies That Target Atherogenic Lipoproteins
Atherogenic lipoproteins include LDLs and the catabolic remnants of triglyceride-rich lipoproteins. Novel agents that decrease plasma levels of these lipoprotein fractions include proprotein convertase subtilisin/kexin type-9 (PCSK9) inhibitors, microsomal triglyceride transfer protein (MTP) inhibitors, and cholesteryl ester transfer protein (CETP) inhibitors. Other agents that inhibit the synthesis of apoB, adenosine triphosphate citrate lyase (ACL), apoC-III, and apo(a) have also been developed.
1. Proprotein Convertase Subtilisin/Kexin Type 9 Inhibition
a. Rationale
PCSK9 is an enzyme that degrades the LDL receptor. It decreases the number of LDL receptors on the surface of liver cells and thus increases the plasma concentration of LDL-C and apoB. Gain-of-function mutations in the PCSK9 gene increase the plasma concentration of LDL-C and apoB, whereas loss-of-function mutations decrease LDL-C and apoB levels and reduce ASCVD risk. Overexpression of PCSK9 in mice increases susceptibility to atherosclerotic lesion development. Inhibition of PCSK9 in humans results in a major reduction in the plasma concentrations of LDL-C and apoB.
b. What Is Proprotein Convertase Subtilisin/Kexin Type 9?
The existence of PCSK9 was first reported by Seidah et al. (2003), when it was identified as proprotein convertase neural apoptosis-regulated convertase 1. Since then, there has been an explosion of information about PCSK9, with more than 1000 articles being published, including several comprehensive reviews of its function, regulation, and the consequences of its inhibition (Horton et al., 2009; Catapano and Papadopoulos, 2013; Poirier and Mayer, 2013; Urban et al., 2013; Seidah et al., 2014; Stawowy, 2015).
PCSK9 is a 692–amino acid proteinase K–like serine protease that is expressed mainly in the liver but also in the small intestine and kidney (Zaid et al., 2008). After cleavage of its signal peptide (amino acids 1–30) in the endoplasmic reticulum, the resulting proPCSK9 (amino acids 31–692) cleaves itself to release the mature enzyme (amino acids 153–692). Mature PCSK9 remains noncovalently bound to its inhibitory prosegment (amino acids 32–152), forming an inactive complex that is secreted from the cell (Benjannet et al., 2004; Cunningham et al., 2007). This PCSK9 complex binds to target proteins, including the LDL receptor, which are then degraded in intracellular compartments. One target of PCSK9 is the LDL receptor on the surface of hepatocytes (Benjannet et al., 2004; Maxwell and Breslow, 2004; Park et al., 2004).
c. Function of Proprotein Convertase Subtilisin/Kexin Type 9
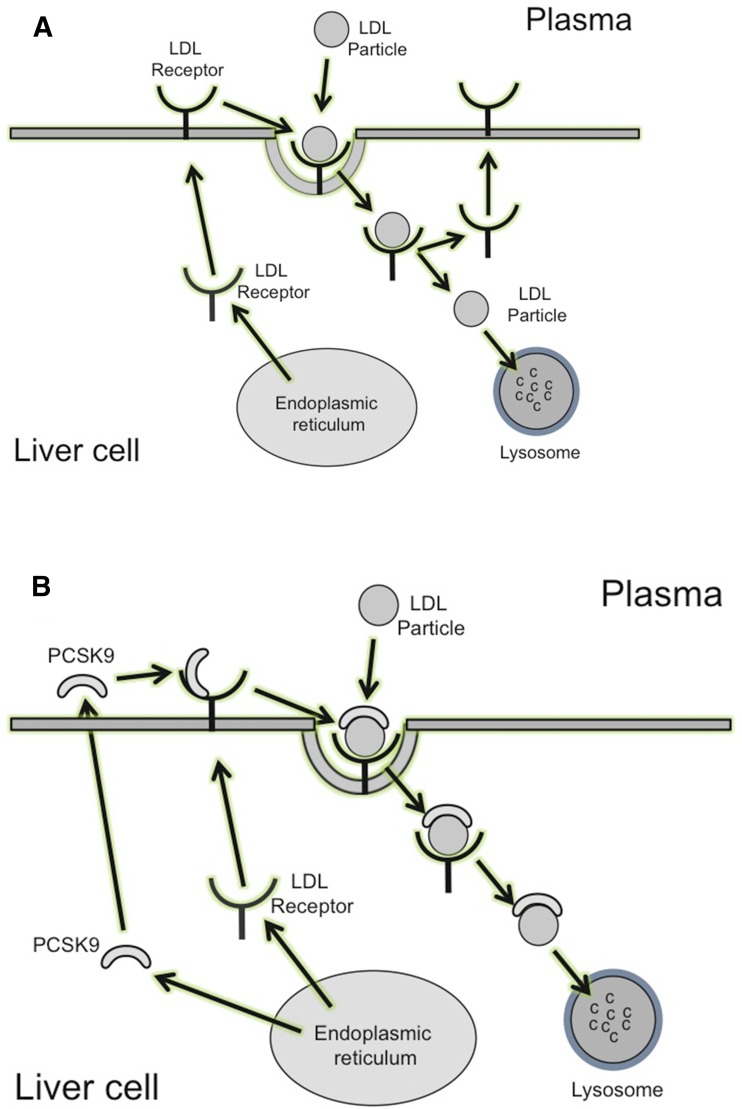
The major pathway for removal of LDLs from plasma is via LDL receptors residing on the surface of hepatocytes (Dietschy et al., 1993). LDLs in plasma bind to LDL receptors to form complexes that are endocytosed into the cell. The lower intracellular pH dissociates LDLs from the LDL receptor, leaving the receptor to recycle back to the cell surface. The dissociated LDLs enter the lysosomal compartment where they are degraded. The cholesterol that is released during LDL degradation is metabolized by the cell (Huang et al., 2010) (Fig. 1A). It has been estimated that each LDL receptor recycles between intracellular compartments and the cell surface about 150 times (Goldstein et al., 1985).
Fig. 1.
(A) Removal of LDL from plasma. LDLs bind to LDL receptors on the surface of cells to form a complex containing an LDL particle bound to an LDL receptor. This complex is taken up by the cell, where the low pH dissociates the LDL receptor from the complex. The LDL enters a lysosome, where it is degraded, leaving the LDL receptor to recycle to the cell surface to bind another LDL particle. (B) Effects of PCSK9 on LDL receptor activity. PCSK9 is synthesized mainly in the liver and is secreted into plasma, where it binds to LDL receptors on the surface of cells. When LDL binds to an LDL receptor to which PCSK9 is already bound, a complex consisting of the LDL receptor, LDL, and PCSK9 is formed. When this complex enters the cell, PCSK9 blocks the dissociation of the LDL receptor from the complex, which is directed in its entirety to lysosomes for degradation. This prevents recycling of the LDL receptor to the cell.
Binding of PCSK9 to the LDL receptor at the cell surface prevents recycling of the LDL receptor (Fig. 1B). When an LDL particle interacts with an LDL receptor to which PCSK9 is already bound, a complex consisting of PCSK9, an LDL receptor, and an LDL particle is formed. This complex enters the cell, where PCSK9 prevents LDL from dissociating from the LDL receptor and the entire complex (including the LDL receptor) is directed toward lysosomes for degradation (Benjannet et al., 2004; Nassoury et al., 2007). This prevents the LDL receptor from recycling back to the cell surface, thus reducing the number of cell surface LDL receptors, decreasing the removal of LDLs from plasma, and increasing the plasma concentration of LDL-C.
d. Proprotein Convertase Subtilisin/Kexin Type 9 Gene Mutations and Atherosclerotic Cardiovascular Disease
In 2003, three families were identified with autosomal dominant hypercholesterolemia and premature coronary heart disease that was caused by gain-of-function mutations in the PCSK9 gene (Abifadel et al., 2003). Subsequent studies in humans identified loss-of-function mutations in the PCSK9 gene that were associated with reduced levels of LDL-C (Cohen et al., 2005; Horton et al., 2007) and a significant reduction in ASCVD risk (Cohen et al., 2007; Benn et al., 2010). These loss-of-function mutations in the PCSK9 gene were associated with a 15%–28% reduction in LDL-C levels and a risk of having a coronary heart disease event that was 47%–88% lower than in people without the mutations.
e. Regulation of Proprotein Convertase Subtilisin/Kexin Type 9
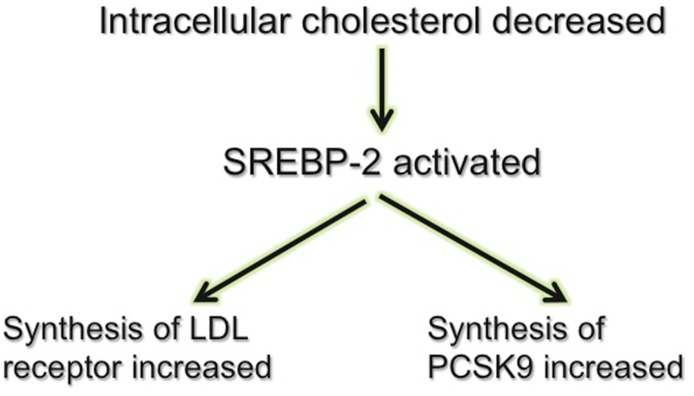
PCSK9 is regulated by SREBPs (Horton et al., 2007) (Fig. 2). SREBPs are transcription factors that regulate transcription of enzymes involved in sterol biosynthesis. The SREBP isoform, SREBP-2, specifically regulates genes involved in cholesterol metabolism (Yang et al., 1994). Suppression of SREBP-2 by cholesterol feeding reduces hepatic PCSK9 mRNA levels in mice (Jeong et al., 2008). Conversely, decreasing the level of intracellular cholesterol by treatment with a statin activates SREBP-2, an effect that increases the expression of not only the LDL receptor but also PCSK9 (Careskey et al., 2008; Welder et al., 2010). This statin-induced increase in PCSK9 expression opposes the statin-induced increase in LDL receptor expression on the cell surface and limits the ability of statins to reduce the concentration of LDL-C. Inhibitors of PCSK9 therefore not only reduce LDL-C in their own right but also have the potential to enhance the LDL-C–lowering efficacy of statins.
Fig. 2.
Regulation of PCSK9. The transcription factor, SREBP-2, is activated by a low intracellular cholesterol concentration. This increases synthesis of the LDL receptor and increases the number of LDL receptors on the cell surface. It also increases synthesis of PCSK9, an effect that reduces the number of cell surface LDL receptors.
Inhibition of PCSK9 is thus an extremely attractive strategy for reducing the concentration of LDL-C, whether given as monotherapy or as add-on therapy for people taking statins.
f. Development of Inhibitors of Proprotein Convertase Subtilisin/Kexin Type 9
Several approaches have been used to develop inhibitors of PCSK9 for use as antiatherosclerotic agents in humans. These include anti-PCSK9 monoclonal antibodies that bind to and neutralize PCSK9 in plasma, mimetic peptides and adnectins that act as competitive inhibitors of PCSK9, and gene silencing that reduces PCSK9 gene transcription.
The development of anti-PCSK9 monoclonal antibodies is much more advanced than any of the other approaches that target atherogenic lipoproteins, with multiple phase I, phase II, and phase III clinical trials now reported. Several large randomized clinical outcome trials designed to assess the effect of anti-PCSK9 monoclonal antibodies on the risk of having an ASCVD event are now underway.
g. Anti–Proprotein Convertase Subtilisin/Kexin Type 9 Monoclonal Antibodies
Monoclonal antibodies have been used as therapeutic agents to treat a number of conditions, including cancers and autoimmune diseases. One advantage of monoclonal antibodies over other therapeutic approaches is the specificity of the antibody for its target antigen. Administration of murine antibodies to humans tends to induce the formation of antimouse antibodies with a consequent loss of efficacy. Administration of murine monoclonal antibodies to humans can also lead to hypersensitivity reactions. This problem has been largely overcome by the use of fully human or humanized monoclonal antibodies that have reduced immunogenicity and are mostly free of the problems encountered with murine antibodies. Fully human and humanized monoclonal antibodies are generally well tolerated, with adverse events tending to be target related rather than related to the antibody itself.
To date, results of the effects of three anti-PCSK9 monoclonal antibodies in humans have been reported. Evolocumab and alirocumab are fully human monoclonal antibodies, whereas bococizumab is a humanized monoclonal antibody. When administered subcutaneously at two to four weekly intervals, these agents are well tolerated and reduce the plasma concentration of PCSK9 by up to 80%, with concentrations of LDL-C and apoB being reduced by up to 60% (Giugliano et al., 2012; Koren et al., 2012, 2014; McKenney et al., 2012; Raal et al., 2012; Roth et al., 2012; Stein et al., 2012; Sullivan et al., 2012; Ballantyne et al., 2015; Zhang et al., 2015). These agents also reduce the concentration of Lp(a) (Stein et al., 2014) by an unknown mechanism.
h. Effects of Anti–Proprotein Convertase Subtilisin/Kexin Type 9 Monoclonal Antibodies on Atherosclerotic Cardiovascular Disease Risk in Humans
Post hoc, exploratory analyses of studies with evolocumab (Sabatine et al., 2015) and alirocumab (Robinson et al., 2015) have added a degree of confidence to the proposition that treatment with anti-PCSK9 monoclonal antibodies not only markedly reduce the concentration of LDL-C but will also reduce the risk of having a clinical ASCVD event. The results of large-scale randomized clinical outcome studies with evolocumab (ClinicalTrials.gov NCT01764633), alirocumab (ClinicalTrials.gov NCT01663402), and bococizumab (ClinicalTrials.gov NCT01975389 and NCT01975376) are awaited with great interest.
i. Inhibition of Proprotein Convertase Subtilisin/Kexin Type 9 Synthesis by Gene Silencing
Agents that inhibit the synthesis of PCSK9 in mice include a PCSK9 antisense oligonucleotide (ASO) that reduces plasma cholesterol levels by 53% (Graham et al., 2013), and a locked nucleic acid ASO that decreases PCSK9 mRNA levels and increases LDL receptor expression (Gupta et al., 2010). A PCSK9 locked nucleic acid ASO that reduces plasma LDL-C and hepatic cholesterol levels in nonhuman primates is in development (Lindholm et al., 2012) and a PCSK9 small interfering RNA that reduces LDL-C levels in nonhuman primates has also been reported (Frank-Kamenetsky et al., 2008). Results of trials in which these agents have been used in humans are awaited with great interest.
2. Inhibition of Apolipoprotein B Synthesis
a. Rationale
ApoB is an essential component of both VLDLs and LDLs. Familial hypobetalipoproteinemia (FHBL) is a rare disorder caused by an autosomal codominant mutation in the gene for apoB that results in the formation of a truncated form of apoB and very low levels of apoB-containing lipoproteins in plasma. Inhibition of apoB synthesis decreases the plasma concentration of all apoB-containing lipoproteins. Inhibition of apoB synthesis has the potential to reduce the concentration of apoB-containing lipoproteins in people with homozygous familial hypercholesterolemia (hoFH) who lack functional LDL receptors and thus do not respond to agents such as statins and PCSK9 inhibitors that lower the level of apoB-containing lipoproteins by increasing the number of LDL receptors on the cell surface.
b. What Is Apolipoprotein B?
ApoB is the main protein component of all atherogenic lipoproteins. In its absence, VLDLs and their catabolic products, LDLs, cannot be formed. Inhibition of the synthesis of apoB is thus a logical therapeutic target for reducing the concentration of apoB-containing lipoproteins, especially in people who do not have functioning LDL receptors.
c. Familial Hypobetalipoproteinemia
FHBL is a rare disorder caused by an autosomal, codominant mutation in the gene for apoB, which results in the synthesis of a truncated form of apoB. People who are homozygous for this condition have very low plasma concentrations of LDL-C and apoB (Cefalù et al., 2015). Affected individuals also have fat malabsorption, retinitis pigmentosa, and acanthocytosis. Those with severe homozygous FHBL also have coagulopathy, bone abnormalities, fatty liver, and neurologic deficits (Lee and Hegele, 2014). Heterozygotes have decreased levels of LDL-C and apoB and are usually asymptomatic.
d. Inhibition of Apolipoprotein Synthesis
Mipomersen is an ASO that inhibits apoB-100 synthesis in the liver. A 300-mg dose of mipomersen reduced apoB levels by 33%–54%, LDL-C levels by 34%–52%, and Lp(a) levels by 24% (Kastelein et al., 2006; Akdim et al., 2010a,b; Raal et al., 2010). However, mipomersen administration has been associated with frequent adverse effects in those receiving the agent, including severe injection site reactions in 72%–97% of subjects, flu-like reactions in 25%–29%, headache in 15%–31%, and liver enzyme elevation in 3%–17% (Kastelein et al., 2006; Akdim et al., 2010a,b; Raal et al., 2010).
3. Microsomal Triglyceride Transfer Protein Inhibition
a. Rationale
MTP is the protein responsible for transferring triglyceride to apoB in the liver and intestine to form apoB-containing lipoproteins. A genetic deficiency of MTP (abetalipoproteinemia) prevents the formation of apoB-containing lipoproteins. MTP inhibition has the potential to reduce the concentration of apoB-containing lipoproteins in people with hoFH who lack functional LDL receptors and thus do not respond to agents such as statins and PCSK9 inhibitors.
b. What Is Microsomal Triglyceride Transfer Protein and What Does It Do?
MTP is localized in the endoplasmic reticulum. It catalyzes the transfer of triglyceride to apoB in hepatocytes and intestinal cells in the initial step in the formation of VLDLs and chylomicrons. Given that intermediate-density lipoproteins (IDLs) and LDLs are catabolic products of VLDLs, it follows that there will be a deficiency not only of VLDLs but also of IDLs and LDLs in the absence of MTP.
c. Microsomal Triglyceride Transfer Protein Gene Mutations
Loss-of-function mutations in the gene encoding for MTP result in abetalipoproteinemia, a rare autosomal recessive genetic disorder in which there is a virtual absence of circulating apoB-containing lipoproteins (VLDLs, IDLs, LDLs, and chylomicrons) (Wetterau et al., 1992; Sharp et al., 1993).
In this condition, apoB is not lipidated in the endoplasmic reticulum of hepatocytes and enterocytes. This inhibits the formation of chylomicrons and VLDLs. Although affected individuals have normal synthesis of apoB, all of their nonlipidated apoB is degraded in proteasomes. Since IDLs and LDLs are catabolic products of VLDLs, the failure to form VLDLs also prevents the formation of IDLs and LDLs. Affected individuals have the same problems as those with homozygous FHBL, including fat malabsorption, retinitis pigmentosa, acanthocytosis, coagulopathy, bone abnormalities, fatty liver, and neurologic deficits (Lee and Hegele, 2014)
d. Inhibition of Microsomal Triglyceride Transfer Protein
Lomitapide is a small molecule that inhibits lipid transfer to apoB by binding to MTP. Inhibition of MTP by treatment with lomitapide in Watanabe heritable hyperlipidemic rabbits that do not have LDL receptors reduces plasma cholesterol levels (Shiomi and Ito, 2001). Treatment with lomitapide also reduces the concentration of LDL-C in humans (Cuchel et al., 2013).
In a small 78-week study of patients with hoFH who lack functioning LDL receptors, treatment with lomitapide reduced the concentration of LDL-C by 40%–50% (Cuchel et al., 2013). As predicted, adverse effects were observed in most of the patients, including gastrointestinal symptoms, elevations of liver transaminases, and an increase in liver fat. Despite these adverse effects, lomitapide has been approved in the United States for treatment of people with hoFH.
4. Adenosine Triphosphate Citrate Lyase Inhibition
a. Rationale
ACL catalyzes the cleavage of citrate to oxaloacetate and acetyl-CoA. Acetyl-CoA is a substrate for both fatty acid and cholesterol synthesis. Inhibition of ACL decreases the synthesis of cholesterol. Inhibition of ACL in humans reduces the concentration of LDL-C and apoB.
b. What Is Adenosine Triphosphate Citrate Lyase and What Does It Do?
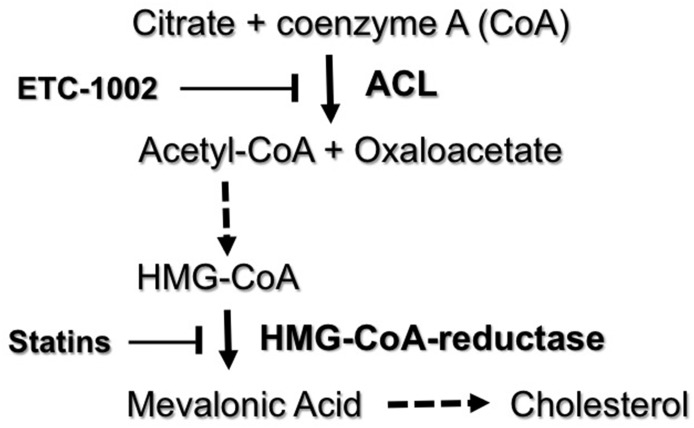
ACL is a cytosolic enzyme expressed in liver and adipose tissue (Elshourbagy et al., 1990). ACL catalyzes a reaction in which citrate and CoA are converted into acetyl-CoA and oxaloacetate. Acetyl-CoA is an essential substrate in the pathway of cholesterol synthesis that is upstream from 3-hydroxy-3-methylglutaryl-CoA, the molecular target of statins (Fig. 3).
Fig. 3.
Inhibition of ACL by ETC-1002. ACL catalyzes the conversion of citrate plus CoA to acetyl-CoA plus oxaloacetate. Acetyl-CoA is then converted in a series of steps to HMG-CoA, which is converted by HMG-CoA reductase into mevalonic acid. This is the rate-limiting step in the formation of cholesterol. Mevalonic acid is subsequently converted in a number of additional steps into cholesterol. ETC-1002 inhibits ACL, whereas statins inhibit HMG-CoA reductase. HMG, 3-hydroxy-3-methylglutaryl.
c. Inhibition of Adenosine Triphosphate Citrate Lyase
Pharmacological inhibition of ACL by treatment with ETC-1002 (8-hydroxy-2,2,14,14-tetramethylpentadecanedioic acid) reduces cytosolic acetyl-CoA (Pinkosky et al., 2013). This decreases hepatic sterol synthesis, upregulates the LDL receptor, and reduces plasma LDL-C levels (Ballantyne et al., 2013). ETC-1002 also activates AMP, which has the potential to favorably affect glucose homeostasis (Pinkosky et al., 2013).
In animal models, ETC-1002 decreases plasma levels of both cholesterol and triglycerides, decreases liver fat, improves glycemic control, and reduces atherosclerosis (Cramer et al., 2004; Pinkosky et al., 2013). ETC-1002 is well tolerated in humans, with no apparent adverse effects (Ballantyne et al., 2013; Thompson et al., 2015). When given at dosages of 40, 80, and 120 mg/d, ETC-1002 reduces LDL-C levels by 18%, 25%, and 27%, respectively (Ballantyne et al., 2013). It also dose-dependently reduces levels of non–HDL-C and apoB as well as LDL particle number. Administration of ETC-1002 to humans has beneficial effects on insulin levels, C-reactive protein, and blood pressure (Ballantyne et al., 2013).
The long-term tolerability and safety of ACL inhibition and its effects on ASCVD risk will not be known until results of randomized clinical outcome trials are reported.
5. Inhibition of Lipoprotein(a) Synthesis
a. Rationale
Elevated plasma levels of Lp(a) are associated with an increased risk of having an ASCVD event. Polymorphisms of the gene that encodes for Lp(a), LPA, that increase plasma levels of Lp(a) are associated with an increased risk of having an ASCVD event. This is indicative of a causal relationship. Interventions that reduce the level of Lp(a) in plasma are therefore predicted to reduce ASCVD risk.
b. What Is Lipoprotein(a) and What Does It Do?
Lp(a) is a plasma lipoprotein consisting of an LDL particle to which an apo(a) molecule is attached via a disulfide bond. Evidence that Lp(a) is an independent, causal risk factor for myocardial infarction, stroke, peripheral arterial disease, and calcific aortic valve stenosis has come from epidemiologic, genetic association, and Mendelian randomization studies (Clarke et al., 2009; Erqou et al., 2009; Thanassoulis et al., 2013; Kamstrup et al., 2014). The mechanisms by which Lp(a) contributes to ASCVD are uncertain but may relate to known effects of apo(a), which is prothrombotic, impairs fibrinolysis, activates endothelial cells, and transports proinflammatory, oxidized phospholipids (Bergmark et al., 2008; Spence and Koschinsky, 2012; Kronenberg and Utermann, 2013).
The evidence that Lp(a) causes ASCVD has stimulated a search for therapies that reduce the level of Lp(a) in plasma. Inhibitors of both PCSK9 and CETP reduce plasma Lp(a) by approximately 30%. An almost 80% reduction in plasma levels of Lp(a) has been achieved with ASOs that inhibit apo(a) synthesis. Apo(a) ASOs also reduce the levels of oxidized phospholipids that are associated with apoB-100 and apo(a) (Tsimikas et al., 2015).
Time will tell whether a therapeutic reduction in plasma levels of Lp(a) translates into a reduction in ASCVD events.
6. Targeting Peroxisome Proliferator–Activated Receptor α
Currently available PPARα agonists have an efficacy that is limited by dose-related adverse effects that include aminotransferase elevations, muscle-associated adverse effects (especially when gemfibrozil is coadministered with a statin), and elevations of serum creatinine and homocysteine. This problem has been addressed by the development of potent and selective PPARα modulators that separate the benefits of PPARα agonists from the unwanted side effects of existing fibrates (Fruchart, 2013; Khera et al., 2015). One of these is pemafibrate [previously known as K-877 or (2R)-2-[3-({(1,3-benzoxazol-2-yl)[3-(4- methoxyphenoxy)propyl]amino}methyl)phenoxy] butanoic acid], a selective PPARα modulator that has greater PPARα-activating efficacy compared with fenofibrate and achieves greater reductions in plasma triglyceride and greater increases in HDL-C levels but has a reduced incidence of adverse effects (Fruchart, 2013). Plans to conduct the Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Diabetic Patients trial have recently been announced. This will be a multicenter cardiovascular clinical outcomes trial to be conducted in approximately 10,000 statin-treated patients with diabetes who have elevated levels of plasma triglyceride and low levels of HDL-C. The results of this trial are awaited with great interest and anticipation.
7. Cholesteryl Ester Transfer Protein Inhibition
a. Rationale
CETP transfers cholesteryl esters from the nonatherogenic HDL fraction to potentially proatherogenic non-HDL fractions. Inhibition of CETP in humans decreases the concentration of LDL-C while increasing levels of HDL-C. CETP gene polymorphisms that associate with decreased CETP activity in humans are accompanied by reduced ASCVD risk. Inhibition of CETP in rabbits inhibits development of atherosclerosis.
b. What Is Cholesteryl Ester Transfer Protein and What Does It Do?
Inhibition of CETP as a strategy to reduce ASCVD risk remains under investigation despite the failure of human clinical outcome trials with three different CETP inhibitors. CETP inhibitors increase the concentration of HDL-C by up to 180%. They also reduce the level of LDL-C by 0%–45% and apoB by 0%–34% over and above what can be achieved with a statin. The mechanism by which CETP inhibitors lower the plasma level of LDL-C and apoB is not known with certainty. At the simplest level, the lowering of LDL-C by CETP inhibitors may be the consequence of a decreased transfer of cholesteryl esters from the HDL fraction into the LDL fraction. However, there is also evidence of an increased rate of removal of LDLs from plasma (Millar et al., 2015), which suggests that the number of LDL receptors in the liver may be increased. There have been reports of some CETP inhibitors also inhibiting the synthesis of PCSK9 in what appears to be an effect that is unrelated to the inhibition of CETP (van der Tuin et al., 2015). Neither the mechanism nor the clinical significance of this observation is known.
CETP inhibition as a strategy to reduce ASCVD risk by increasing HDL-C levels is described in detail below in the section on agents that target HDLs.
8. Inhibition of Apolipoprotein C-III Synthesis
a. Rationale
ApoC-III is a proinflammatory protein that increases the concentration of triglyceride-rich lipoproteins and their atherogenic, catabolic remnants. An elevated level of apoC-III in apoB-containing lipoproteins is an independent predictor of the risk of having an ASCVD event. Overexpression of human apoC-III in transgenic mice increases plasma triglyceride levels and susceptibility to atherosclerosis. An increased concentration of HDLs that do not contain apoC-III is associated with a decreased risk of having an ASCVD event, whereas HDLs that do contain apoC-III are associated with an increased risk of having an ASCVD event. Loss-of-function mutations in the APOC3 gene are associated with a significantly reduced risk of having an ASCVD event.
b. What Is Apolipoprotein C-III and What Does It Do?
ApoC-III is synthesized mainly in the liver and circulates in plasma as a component of all lipoprotein fractions (Jong et al., 1999). It inhibits LPL (Wang et al., 1985; Larsson et al., 2013), which reduces the lipolysis of triglyceride-rich lipoproteins and increases the concentration of plasma triglyceride. It also inhibits hepatic lipase (Kinnunen and Ehnolm, 1976) and thus reduces the catabolism of triglyceride-rich lipoprotein remnants. ApoC-III also inhibits the hepatic uptake of atherogenic triglyceride-rich lipoprotein remnants (Windler and Havel, 1985). The plasma concentration of apoC-III is increased in people with metabolic syndrome and insulin resistance (Olivieri et al., 2003; Altomonte et al., 2004).
ApoC-III, whether as a free apolipoprotein or as a component of VLDLs, activates the master regulator of inflammation, nuclear factor-κB, which in turn upregulates vascular cell adhesion molecule-1 expression and increases the adhesion of monocytes to vascular endothelial cells (Kawakami et al., 2006a). ApoC-III also affects HDL function. Although HDLs without apoC-III inhibit the adhesion of monocytes to endothelial cells, HDLs that contain apoC-III do not have this effect (Kawakami et al., 2006b). This may explain the observation that, in contrast with HDLs that do not contain apoC-III, higher concentrations of HDLs that contain apoC-III are associated with an increased ASCVD risk (Jensen et al., 2012).
c. Genetic Variants of Apolipoprotein C-III and Atherosclerotic Cardiovascular Disease Risk
The case for developing inhibitors of apoC-III synthesis has been strengthened by the results of two large genetic studies, one conducted in Denmark in people of mainly European origin (Jørgensen et al., 2014) and the other conducted in the United States in people of European and African origin (Crosby et al., 2014). In both studies, people with loss-of-function mutations in the APOC3 gene had a significantly reduced risk of having an ASCVD event.
d. Therapies That Reduce Plasma Levels of Apolipoprotein C-III
Fibrates reduce the concentration of apoC-III (Staels et al., 1995), an effect that may contribute to the ability of these agents to reduce ASCVD events in people with hypertriglyceridemia (Barter and Rye, 2008). However, the fact that fibrates have many other effects makes it difficult to draw any firm conclusions about their effect on apoC-III.
A more targeted approach to reducing plasma levels of apoC-III has been provided by the development of volanesorsen, an antisense inhibitor of apoC-III synthesis (Graham et al., 2013). When people with severe hypertriglyceridemia, including those with a deficiency of LPL, are treated with this inhibitor, the resulting decrease in apoC-III plasma levels is accompanied by a major reduction in plasma triglyceride levels and a substantial increase in concentration of HDL-C (Gaudet et al., 2014, 2015).
The effects of inhibiting apoC-III synthesis on ASCVD risk remain to be established.
B. Therapies That Target High-Density Lipoprotein
The concentration of HDL-C is a robust inverse predictor of the risk of having an ASCVD event (Di Angelantonio et al., 2009). Increasing the concentration of HDL in plasma with HDL infusions, or by overexpressing apoA-I, reduces atherosclerosis in mice (Rubin et al., 1991; Plump et al., 1994) and rabbits (Badimon et al., 1990; Duverger et al., 1996).
In a proof-of-concept study in humans, infusion of a preparation of reconstituted high-density lipoproteins (rHDLs) reduced coronary atheroma burden as assessed by intravascular ultrasonography (Nissen et al., 2003). HDLs also have several potentially cardioprotective properties (Rye and Barter, 2014), including promotion of the efflux of cholesterol from macrophages and inhibition of vascular inflammation. HDLs also have antioxidant and antithrombotic effects. In addition, they increase the synthesis and secretion of insulin in pancreatic β cells, improve insulin sensitivity, enhance endothelial function, and promote angiogenesis.
It should be emphasized that the cholesterol transported in HDLs does not protect against ASCVD. Rather, if HDLs do protect, it is probably a consequence of one or more of the functions of HDLs. It is possible that in some disease states, and after treatment with some HDL-raising agents, HDLs may be dysfunctional (Rosenson et al., 2016), in which case the concentration of HDL-C may no longer be a marker of HDL function.
Results from animal studies provide a compelling case for targeting the HDL fraction as a strategy to reduce ASCVD risk. However, there is still no evidence in humans that HDL-raising interventions translate into a reduction in the risk of having an ASCVD event.
1. Reconstituted High-Density Lipoprotein Infusions
a. Rationale
rHDLs have many potentially cardioprotective properties. Infusion of rHDLs into animals inhibits vascular inflammation and promotes regression of atherosclerosis. Infusion of rHDLs into humans promotes regression of coronary atheroma.
b. What Are Reconstituted High-Density Lipoproteins?
rHDLs are complexes of apoA-I and phospholipids. They are structurally similar to the discoidal, nascent HDLs that are secreted into plasma by the liver. rHDLs possess many of the functions of the mature, spherical HDLs that predominate in normal human plasma.
rHDLs are especially effective as acceptors of the cholesterol that is released from cells via the ATP binding cassette transporter, ABCA1 (Favari et al., 2009; Du et al., 2015). They also possess anti-inflammatory (Wu et al., 2013) and antioxidant (Tabet et al., 2011) properties, promote endothelial repair (Tso et al., 2006), and enhance endothelial function (Nieuwdorp et al., 2008). Most importantly, they also inhibit the development of atherosclerosis in animal models (Shah et al., 1998) and in humans (Nissen et al., 2003; Tardif et al., 2007).
Two forms of apoA-I have been used in rHDLs. One is monomeric apoA-I, which is present in the HDL fraction of most humans. The other is a dimeric form of apoA-I (apoA-IMilano) that may have an enhanced ability to promote the efflux of cholesterol from cells. People who carry the apoA-IMilano mutation were first identified in Limone sul Garda, a small town near Milan in Italy. People with the apoA-IMilano mutation have low plasma concentrations of HDL-C and apoA-I but are not at increased risk of ASCVD (Sirtori et al., 2001). Infusions of rHDLs containing apoA-IMilano promote regression of atheroma in animal models (Shah et al., 1998).
In a proof-of-concept study in humans, five weekly infusions of rHDLs containing apoA-IMilano resulted in significant regression of coronary atheroma as assessed by intravascular ultrasonography (Nissen et al., 2003). Another study of rHDLs containing monomeric apoA-I gave similar results (Tardif et al., 2007). A third rHDL preparation consisting of complexes of recombinant human apoA-I with two naturally occurring phospholipids (egg sphingomyelin and dipalmitoylphosphatidyl-glycerol) has been tested in humans, with evidence that it promotes coronary atheroma regression when infused at a low dose (Tardif et al., 2014).
Investigation of rHDLs as agents with the potential to promote regression of coronary atheroma is ongoing.
2. High-Density Lipoprotein Delipidation
a. Rationale
Selective delipidation of HDLs generates particles that are especially effective as acceptors of cholesterol from macrophages. In a proof-of-concept study conducted in humans, infusion of delipidated HDLs resulted in a trend toward regression of coronary atheroma volume.
b. What Is High-Density Lipoprotein Delipidation?
The concentration of pre–β-migrating HDL particles is markedly increased by a technique of plasma selective delipidation that converts α-migrating spherical HDL particles into lipid-poor, pre–β-migrating discoidal HDL particles that enhance reverse cholesterol transport (Sacks et al., 2009).
A small randomized placebo-controlled study was conducted to determine whether serial, autologous infusions of plasma in which the HDLs had been selectively delipidated were feasible and tolerated in patients with acute coronary syndrome (ACS) (Waksman et al., 2010). Twenty-eight patients with ACS were randomized to receive either seven weekly autologous infusions of plasma after selective delipidation of HDLs or control plasma. An intravascular ultrasonographic evaluation of the target coronary vessel revealed a trend toward regression in total atheroma volume in those treated with plasma containing delipidated HDL compared with an increase of atheroma volume in those treated with control plasma.
Further investigation of this approach is ongoing.
3. Apolipoprotein A-I Mimetic Peptides
a. Rationale
ApoA-I is responsible for many of the potentially cardioprotective properties of HDLs. ApoA-I mimetic peptides that have one or more of these potentially cardioprotective properties have been developed. ApoA-I mimetic peptides have the potential to reduce ASCVD risk.
b. What Are Apolipoprotein A-I Mimetic Peptides?
Synthetic peptides that mimic the primary and secondary structure of apoA-I have been developed. Some have sought to reproduce the cholesterol efflux function of apoA-I (Sviridov et al., 2011), whereas others have sought to replicate the anti-inflammatory properties of apoA-I (Tabet et al., 2010; Di Bartolo et al., 2011).
One apoA-I mimetic peptide (d-4F) was synthesized from d-amino acids to increase bioavailability after oral administration (Navab et al., 2002). d-4F inhibited atheroma development in mice (Navab et al., 2002) but has not been developed further for use in humans.
Development of mimetic peptides that mimic one or more of the potentially cardioprotective properties of HDL apolipoproteins are ongoing and the results in humans are awaited with interest.
4. Cholesteryl Ester Transfer Protein Inhibition
a. Rationale
CETP is a plasma protein that transfers cholesteryl esters from the nonatherogenic HDL fraction to potentially the proatherogenic non-HDL fraction. Inhibition of CETP blocks this transfer and decreases the concentration of cholesterol in potentially proatherogenic non-HDL fractions while increasing it in the nonatherogenic HDL fraction. CETP gene polymorphisms that reduce CETP activity are almost always associated with a decreased concentration of LDL-C, an increased concentration of HDL-C and a significant reduction in the risk of having an ASCVD event. Inhibition of CETP in rabbits prevents the development of diet-induced atherosclerosis.
b. What Is Cholesteryl Ester Transfer Protein and What Does It Do?
CETP is a glycoprotein that is synthesized mainly in the liver (Drayna et al., 1987; Hesler et al., 1987). Within plasma, CETP promotes bidirectional transfers of both cholesteryl esters and triglycerides between all plasma lipoprotein particles in processes that results in the equilibration of these lipids between lipoprotein fractions (Barter and Rye, 2012). Since most of the cholesteryl esters in plasma are formed by lecithin–cholesterol acyltransferase (LCAT) in a reaction that takes place primarily in HDLs, and most triglyceride enters plasma as a component of VLDLs or chylomicrons, the effect of the equilibration promoted by CETP is a net mass transfer of cholesteryl esters from the HDL fraction to the potentially proatherogenic non-HDL lipoproteins and a net mass transfer of triglyceride from triglyceride-rich VLDLs and chylomicrons into the HDL and LDL fractions.
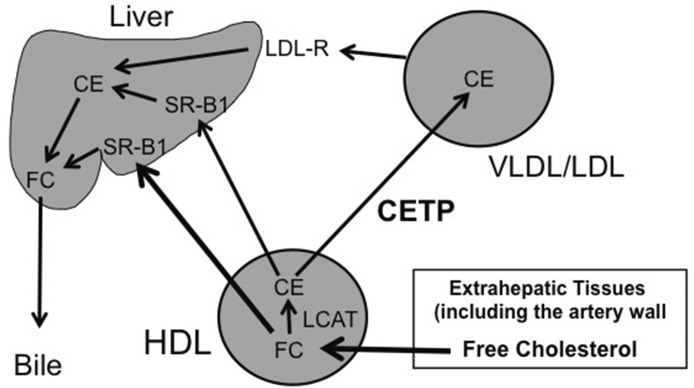
The role of CETP in plasma cholesterol transport is presented schematically in Fig. 4. Cholesterol in extrahepatic tissues is transferred to HDLs in the extracellular space. Once in HDLs, the cholesterol is esterified by activity of LCAT to form cholesteryl esters. HDL cholesteryl esters are subsequently delivered to the liver by either of two pathways: 1) a direct pathway that involves selective delivery to the liver in a process mediated by scavenger receptor B1 and 2) an indirect pathway in which HDL cholesteryl esters are transferred by CETP to the VLDL/LDL fraction and then taken up by the liver via binding of LDLs to hepatic LDL receptors.
Fig. 4.
Role of CETP in plasma cholesterol transport. Cells in extrahepatic tissues eliminate any cholesterol that is surplus to their needs by transferring it as free (unesterified) cholesterol to HDLs in the extracellular space. The free (unesterified) cholesterol in HDLs is then either delivered to the liver in a process dependent on hepatic scavenger receptor B1or is converted into cholesteryl esters by LCAT. The cholesteryl esters formed in HDLs are subsequently transported to the liver by either of two pathways: 1) a direct pathway that is scavenger receptor B1 dependent and 2) an indirect pathway in which HDL cholesteryl ester are first transferred to the VLDL/LDL fraction by CETP and then taken up by the liver after binding of LDLs to LDL receptors. CE, cholesteryl ester; FC, free (unesterified) cholesterol; LDL-R, low-density lipoprotein receptor; SR-B1, scavenger receptor B1.
In contrast with humans, nonhuman primates, rabbits, and hamsters (all of which have high activity of CETP), most other animal species are deficient in CETP (Ha and Barter, 1982).
c. Cholesteryl Ester Transfer Protein and Atherosclerosis in Rodents
Rodents lack CETP (Ha and Barter, 1982) and are naturally resistant to the development of atherosclerosis. Transgenic expression of the Cetp gene in mice and rats has generated conflicting and model-dependent results, with some studies supporting the proposition that CETP is proatherogenic (Marotti et al., 1993; Herrera et al., 1999; Plump et al., 1999; Westerterp et al., 2006; Hildebrand et al., 2010; Kühnast et al., 2015), whereas others suggest that CETP has no effect or is antiatherogenic (Hayek et al., 1995; Föger et al., 1999; Cazita et al., 2003; MacLean et al., 2003; Casquero et al., 2006).
Many studies have suggested that CETP is pro-atherogenic in rodents. Transgenic expression of the CETP gene was proatherogenic in mice fed an atherogenic diet (Marotti et al., 1993) as well as in apoE knockout mice (Plump et al., 1999), LDL receptor knockout mice (Plump et al., 1999), APOE*3Leiden mice (Westerterp et al., 2006), and hypertensive rats (Herrera et al., 1999). In another study of APOE*3Leiden CETP-expressing mice, treatment with the CETP inhibitor, anacetrapib, dose-dependently reduced atherosclerosis and improved lesion stability in effects that were mainly attributed to a reduction in the plasma concentration of non–HDL-C (Kühnast et al., 2015).
By contrast, other studies have shown that CEPT either has no effect or is antiatherogenic in rodents. For example, expression of CETP had no effect on atherosclerosis in a study of scavenger receptor class B type 1 knockout mice (Hildebrand et al., 2010). Expression of the CETP gene was antiatherogenic in obese, diabetic db/db mice (MacLean et al., 2003) as well as in LCAT transgenic mice (Föger et al., 1999), ovariectomized mice (Cazita et al., 2003), testosterone-deficient mice (Casquero et al., 2006), and hypertriglyceridemic mice transgenic for APOC3 (Hayek et al., 1995). In the last study, however, it was also reported that expression of the CETP gene was proatherogenic in normotriglyceridemic mice with normal levels of apoC-III (Hayek et al., 1995).
The relevance of these results in rodents to the situation in humans remains uncertain.
d. Evidence That Cholesteryl Ester Transfer Protein Is Proatherogenic in Rabbits
In contrast with rodents, rabbits have a high level of CETP activity and are also extremely susceptible to the development of diet-induced atherosclerosis. Inhibiting CETP in rabbits with a CETP ASO (Sugano et al., 1998), an anti-CETP vaccine (Rittershaus et al., 2000), or by administration of the small molecule CETP inhibitors dalcetrapib (Okamoto et al., 2000) and torcetrapib (Morehouse et al., 2007) reduces atherosclerosis. There are no reports of studies in rabbits to suggest that inhibition of CETP increases atherosclerosis. Although these studies do provide support for the hypothesis that CETP inhibition is antiatherogenic, the relevance to humans is again uncertain.
e. Genetic Variants of Cholesteryl Ester Transfer Protein and Atherosclerotic Cardiovascular Disease Risk in Humans
Overall, the results of human genetic studies have supported the proposition that CETP is proatherogenic and that its inhibition may reduce ASCVD risk. Although some small genetic studies have generated conflicting results, the larger studies have consistently shown that CETP gene variants that are associated with low CETP activity are accompanied by a significantly lower risk of having an ASCVD event.
Several large meta-analyses (Boekholdt et al., 2005; Thompson et al., 2008; Niu and Qi, 2015) and two large cohort studies (Ridker et al., 2009; Johannsen et al., 2012) have concluded that CETP gene polymorphisms that are associated with decreased CETP activity are accompanied by a significantly decreased risk of having a coronary event. The most compelling genetic evidence that activity of CETP is proatherogenic was provided by an analysis of the Copenhagen City Heart Study, in which 10,261 people were followed for up to 34 years (Johannsen et al., 2012). There were more than 3000 cardiovascular events and 3807 deaths in the cohort over this time. Two common CETP gene polymorphisms known to result in low CETP activity were associated with significant reductions in the risk of ischemic heart disease, myocardial infarction, ischemic cerebrovascular disease, and ischemic stroke. Furthermore, the greater the number of affected alleles, the greater the effect on ASCVD risk (Johannsen et al., 2012). People with these polymorphisms also had increased longevity, with no evidence of adverse effects.
Collectively, these genetic studies lend strong support to the proposition that CETP inhibition may be antiatherogenic.
f. Effects of Cholesteryl Ester Transfer Protein Inhibitors in Humans
There have been reports of CETP inhibition by small molecule inhibitors, by an anti-CETP vaccine, and by an ASO approach. The small molecule inhibitors, which have been tested much more extensively than the other agents in humans, include torcetrapib, dalcetrapib, anacetrapib, evacetrapib, and TA-8995 (4-[2-[[3,5-bis(trifluoromethyl)phenyl]methyl-[(2R,4S)-1-ethoxycarbonyl-2-ethyl-6-(trifluoromethyl)-3,4-dihydro-2H-quinolin-4-yl]amino]pyrimidin-5-yl]oxybutanoic acid).
The mechanism of action of small molecule CETP inhibitors is uncertain, although there is evidence with torcetrapib and anacetrapib that these agents reduce the dissociation of CETP from HDL particles and thus prevent the shuttling of cholesteryl esters and triglycerides between lipoprotein particles (Clark et al., 2006). Dalcetrapib appears to act by a different mechanism, but this has yet to be confirmed (Niesor et al., 2010).
g. Effects of Cholesteryl Ester Transfer Protein Inhibition on Plasma Lipids and Lipoproteins in Humans
Treatment of humans with CETP inhibitors decreases the concentration of LDL-C and apoB, decreases the concentration of Lp(a), and increases the concentration of HDL-C and apoA-I (Table 1).
TABLE 1.
Effects of CETP inhibitors on plasma lipids and lipoproteins
Data are given as the effect on concentration with percentages in parentheses.
| CETP Inhibitor | HDL-C | ApoA-I | LDL-C | ApoB | Lp(a) |
|---|---|---|---|---|---|
| Torcetrapib | Increase (72) | Increase (25) | Decrease (24) | Decrease (13) | Not available |
| Dalcetrapib | Increase (30) | Increase (10) | No change | No change | Not available |
| Anacetrapib | Increase (140) | Increase (45) | Decrease (30) | Decrease (21) | Decrease (39) |
| Evacetrapib | Increase (130) | Increase (40) | Decrease (30) | Decrease (25) | Not available |
| TA-8995 | Increase (180) | Increase (60) | Decrease (45) | Decrease (35) | Decrease (35) |
Effects of CETP inhibition on HDL function have also been studied. HDLs isolated from people treated with torcetrapib (Yvan-Charvet et al., 2007), anacetrapib (Yvan-Charvet et al., 2010), and TA-8995 (Hovingh et al., 2015) have been shown in ex vivo studies to have a normal or enhanced ability to promote the efflux of cholesterol from macrophages. In a post hoc analysis of the Investigation of Lipid Level Management to Understand Its Impact in Atherosclerotic Events trial, the level of HDL-C achieved in the torcetrapib-treated patients was an inverse predictor of events (Barter, 2009), suggesting that the HDLs were functional. A post hoc analysis of the Investigation of Lipid Level Management Using Coronary Ultrasound to Assess Reduction of Atherosclerosis by CETP Inhibition and HDL Elevation trial also supported normal HDL function in people treated with torcetrapib in that there was significant regression of coronary atheroma in the torcetrapib-treated patients who achieved the highest on-treatment level of HDL-C (Nicholls et al., 2008).
Inhibition of CETP in rabbits with des-fluoro-anacetrapib, an active analog of the CETP inhibitor anacetrapib, enhanced endothelial repair and improved endothelial function (Wu et al., 2015b) and also promoted angiogenesis in animals with hind-limb ischemia (Wu et al., 2015a).
h. Clinical Outcome Trials of Cholesteryl Ester Transfer Protein Inhibition in Humans
Despite the favorable effect of CETP inhibition on plasma lipids and lipoproteins, three different CETP inhibitors [torcetrapib (Barter et al., 2007), dalcetrapib (Schwartz et al., 2012), and evacetrapib (ClinicalTrials.gov NCT01687998)] all failed to reduce ASCVD events when tested in humans in large, randomized clinical outcome trials. In the case of torcetrapib, the treatment caused harm. Precisely why these trials failed is not known.
The harm caused by torcetrapib may have been the consequence of known serious off-target adverse effects unrelated to inhibition of CETP (Forrest et al., 2008; Hu et al., 2009; Connelly et al., 2010; Simic et al., 2012), making it impossible to interpret the results of this trial. The failure of dalcetrapib may have been because it was tested in patients soon after an ACS event at a time when the HDL fraction is known to be dysfunctional (Besler et al., 2011). This possibility was supported by the observation that the concentration of HDL-C did not predict ASCVD events in the placebo group in this trial (Schwartz et al., 2012). The failure of evacetrapib may simply have been because the trial was too short to be able to detect benefit.
The future of CETP inhibition now depends on an ongoing study with anacetrapib. Randomized Evaluation of the Effects of Anacetrapib through Lipid Modification (REVEAL; ClinicalTrials.gov NCT01252953) is a phase III trial designed to determine whether treatment with anacetrapib given at a daily dose of 100 mg reduces the risk of a composite end point (coronary death, myocardial infarction, or coronary revascularization) in patients with circulatory problems who have their LDL-C optimally treated with a statin. More than 30,000 high-risk participants have been randomized to receive anacetrapib or placebo, with a planned follow-up of about 4 years. This study includes men and women with a history of myocardial infarction, cerebrovascular atherosclerotic disease, peripheral arterial disease, or diabetes mellitus with other evidence of symptomatic coronary heart disease.
The independent data monitoring committee for REVEAL met in early November 2015 and reviewed safety and efficacy data from the study. The data monitoring committee also conducted an analysis for futility and recommended that the trial continue as planned. The future of CETP inhibition as a strategy to reduce ASCVD risk will depend on what is found when the results of the REVEAL trial are reported.
IV. Conclusions
The use of established lipid-modifying drugs, especially statins, has translated into major reductions in the risk of having an ASCVD event. In many people, however, the risk of having an ASCVD event remains unacceptably high despite taking maximally tolerated doses of these established agents. This has fueled the development of a number of new agents designed to reduce levels of atherogenic lipoproteins and increase levels of the potentially protective HDL fraction. Some of these new agents have recently been approved for use in selected countries, whereas others are awaiting the results of randomized clinical outcome trials before decisions can be made about their place in clinical management. Several of these new agents have the potential to reduce ASCVD risk to very low levels.
Abbreviations
- ACL
adenosine triphosphate citrate lyase
- ACS
acute coronary syndrome
- apo
apolipoprotein
- ASCVD
atherosclerotic cardiovascular disease
- ASO
antisense oligonucleotide
- CETP
cholesteryl ester transfer protein
- ETC-1002
8-hydroxy-2,2,14,14-tetramethylpentadecanedioic acid
- FHBL
hypobetalipoproteinemia
- HDL
high-density lipoprotein
- HDL-C
high-density lipoprotein cholesterol
- hoFH
homozygous familial hypercholesterolemia
- IDL
intermediate-density lipoprotein
- K-877
(2R)-2-[3-({(1,3-benzoxazol-2-yl)[3-(4- methoxyphenoxy)propyl]amino}methyl)phenoxy] butanoic acid
- LCAT
lecithin–cholesterol acyltransferase
- LDL
low-density lipoprotein
- LDL-C
low-density lipoprotein cholesterol
- Lp(a)
lipoprotein(a)
- LPL
lipoprotein lipase
- MTP
microsomal triglyceride transfer protein
- NEFA
nonesterified fatty acid
- PCSK9
proprotein convertase subtilisin/kexin type 9
- PPARα
peroxisome proliferator–activated receptor α
- rHDL
reconstituted high-density lipoprotein
- SREBP
sterol regulatory element binding protein
- TA-8995
4-[2-[[3,5-bis(trifluoromethyl)phenyl]methyl-[(2R,4S)-1-ethoxycarbonyl-2-ethyl-6-(trifluoromethyl)-3,4-dihydro-2H-quinolin-4-yl]amino]pyrimidin-5-yl]oxybutanoic acid
- VLDL
very low-density lipoprotein
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Barter, Rye.
Footnotes
This research was supported by the Australian National Health and Medical Research Council [Program Grant RG124089]. P.J.B. has received research grants from Pfizer and Merck and honorariums for participation in Ad Boards or for lectures given for Amgen, AstraZeneca, Kowa, Lilly, Merck, Novartis, Pfizer, and Sanofi-Regeneron. K.-A.R. has received research grants from Merck and honorariums for participation as a consultant for CSL-Behring.
References
- Abifadel M, Varret M, Rabès JP, Allard D, Ouguerram K, Devillers M, Cruaud C, Benjannet S, Wickham L, Erlich D, et al. (2003) Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet 34:154–156. [DOI] [PubMed] [Google Scholar]
- Akdim F, Stroes ES, Sijbrands EJ, Tribble DL, Trip MD, Jukema JW, Flaim JD, Su J, Yu R, Baker BF, et al. (2010a) Efficacy and safety of mipomersen, an antisense inhibitor of apolipoprotein B, in hypercholesterolemic subjects receiving stable statin therapy. J Am Coll Cardiol 55:1611–1618. [DOI] [PubMed] [Google Scholar]
- Akdim F, Visser ME, Tribble DL, Baker BF, Stroes ES, Yu R, Flaim JD, Su J, Stein EA, Kastelein JJ. (2010b) Effect of mipomersen, an apolipoprotein B synthesis inhibitor, on low-density lipoprotein cholesterol in patients with familial hypercholesterolemia. Am J Cardiol 105:1413–1419. [DOI] [PubMed] [Google Scholar]
- Altomonte J, Cong L, Harbaran S, Richter A, Xu J, Meseck M, Dong HH. (2004) Foxo1 mediates insulin action on apoC-III and triglyceride metabolism. J Clin Invest 114:1493–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badimon JJ, Badimon L, Fuster V. (1990) Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J Clin Invest 85:1234–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, et al. Cholesterol Treatment Trialists’ (CTT) Collaboration (2010) Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantyne CM, Davidson MH, Macdougall DE, Bays HE, Dicarlo LA, Rosenberg NL, Margulies J, Newton RS. (2013) Efficacy and safety of a novel dual modulator of adenosine triphosphate-citrate lyase and adenosine monophosphate-activated protein kinase in patients with hypercholesterolemia: results of a multicenter, randomized, double-blind, placebo-controlled, parallel-group trial. J Am Coll Cardiol 62:1154–1162. [DOI] [PubMed] [Google Scholar]
- Ballantyne CM, Neutel J, Cropp A, Duggan W, Wang EQ, Plowchalk D, Sweeney K, Kaila N, Vincent J, Bays H. (2015) Results of bococizumab, a monoclonal antibody against proprotein convertase subtilisin/kexin type 9, from a randomized, placebo-controlled, dose-ranging study in statin-treated subjects with hypercholesterolemia. Am J Cardiol 115:1212–1221. [DOI] [PubMed] [Google Scholar]
- Barter P. (2009) Lessons learned from the Investigation of Lipid Level Management to Understand Its Impact in Atherosclerotic Events (ILLUMINATE) trial. Am J Cardiol 104 (Suppl):10E–15E. [DOI] [PubMed] [Google Scholar]
- Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, et al. ILLUMINATE Investigators (2007) Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 357:2109–2122. [DOI] [PubMed] [Google Scholar]
- Barter PJ, Rye KA. (2008) Is there a role for fibrates in the management of dyslipidemia in the metabolic syndrome? Arterioscler Thromb Vasc Biol 28:39–46. [DOI] [PubMed] [Google Scholar]
- Barter PJ, Rye KA. (2012) Cholesteryl ester transfer protein inhibition as a strategy to reduce cardiovascular risk. J Lipid Res 53:1755–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjannet S, Rhainds D, Essalmani R, Mayne J, Wickham L, Jin W, Asselin MC, Hamelin J, Varret M, Allard D, et al. (2004) NARC-1/PCSK9 and its natural mutants: zymogen cleavage and effects on the low density lipoprotein (LDL) receptor and LDL cholesterol. J Biol Chem 279:48865–48875. [DOI] [PubMed] [Google Scholar]
- Benn M, Nordestgaard BG, Grande P, Schnohr P, Tybjaerg-Hansen A. (2010) PCSK9 R46L, low-density lipoprotein cholesterol levels, and risk of ischemic heart disease: 3 independent studies and meta-analyses. J Am Coll Cardiol 55:2833–2842. [DOI] [PubMed] [Google Scholar]
- Bergmark C, Dewan A, Orsoni A, Merki E, Miller ER, Shin MJ, Binder CJ, Hörkkö S, Krauss RM, Chapman MJ, et al. (2008) A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J Lipid Res 49:2230–2239. [DOI] [PubMed] [Google Scholar]
- Besler C, Heinrich K, Rohrer L, Doerries C, Riwanto M, Shih DM, Chroni A, Yonekawa K, Stein S, Schaefer N, et al. (2011) Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J Clin Invest 121:2693–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezafibrate Infarction Prevention Study Group (2000) Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease. Circulation 102:21–27. [DOI] [PubMed] [Google Scholar]
- Billman GE, Hallaq H, Leaf A. (1994) Prevention of ischemia-induced ventricular fibrillation by omega 3 fatty acids. Proc Natl Acad Sci USA 91:4427–4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W, AIM-HIGH Investigators (2011) Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 365:2255–2267. [DOI] [PubMed] [Google Scholar]
- Boekholdt SM, Sacks FM, Jukema JW, Shepherd J, Freeman DJ, McMahon AD, Cambien F, Nicaud V, de Grooth GJ, Talmud PJ, et al. (2005) Cholesteryl ester transfer protein TaqIB variant, high-density lipoprotein cholesterol levels, cardiovascular risk, and efficacy of pravastatin treatment: individual patient meta-analysis of 13,677 subjects. Circulation 111:278–287. [DOI] [PubMed] [Google Scholar]
- Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, et al. IMPROVE-IT Investigators (2015) Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 372:2387–2397. [DOI] [PubMed] [Google Scholar]
- Careskey HE, Davis RA, Alborn WE, Troutt JS, Cao G, Konrad RJ. (2008) Atorvastatin increases human serum levels of proprotein convertase subtilisin/kexin type 9. J Lipid Res 49:394–398. [DOI] [PubMed] [Google Scholar]
- Casquero AC, Berti JA, Salerno AG, Bighetti EJ, Cazita PM, Ketelhuth DF, Gidlund M, Oliveira HC. (2006) Atherosclerosis is enhanced by testosterone deficiency and attenuated by CETP expression in transgenic mice. J Lipid Res 47:1526–1534. [DOI] [PubMed] [Google Scholar]
- Catapano AL, Papadopoulos N. (2013) The safety of therapeutic monoclonal antibodies: implications for cardiovascular disease and targeting the PCSK9 pathway. Atherosclerosis 228:18–28. [DOI] [PubMed] [Google Scholar]
- Cazita PM, Berti JA, Aoki C, Gidlund M, Harada LM, Nunes VS, Quintão EC, Oliveira HC. (2003) Cholesteryl ester transfer protein expression attenuates atherosclerosis in ovariectomized mice. J Lipid Res 44:33–40. [DOI] [PubMed] [Google Scholar]
- Cefalù AB, Norata GD, Ghiglioni DG, Noto D, Uboldi P, Garlaschelli K, Baragetti A, Spina R, Valenti V, Pederiva C, et al. (2015) Homozygous familial hypobetalipoproteinemia: two novel mutations in the splicing sites of apolipoprotein B gene and review of the literature. Atherosclerosis 239:209–217. [DOI] [PubMed] [Google Scholar]
- Clark RW, Ruggeri RB, Cunningham D, Bamberger MJ. (2006) Description of the torcetrapib series of cholesteryl ester transfer protein inhibitors, including mechanism of action. J Lipid Res 47:537–552. [DOI] [PubMed] [Google Scholar]
- Clarke R, Peden JF, Hopewell JC, Kyriakou T, Goel A, Heath SC, Parish S, Barlera S, Franzosi MG, Rust S, et al. PROCARDIS Consortium (2009) Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med 361:2518–2528. [DOI] [PubMed] [Google Scholar]
- Cohen J, Pertsemlidis A, Kotowski IK, Graham R, Garcia CK, Hobbs HH. (2005) Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet 37:161–165. [DOI] [PubMed] [Google Scholar]
- Cohen SY, Dubois L, Tadayoni R, Delahaye-Mazza C, Debibie C, Quentel G. (2007) Prevalence of reticular pseudodrusen in age-related macular degeneration with newly diagnosed choroidal neovascularisation. Br J Ophthalmol 91:354–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly MA, Parry TJ, Giardino EC, Huang Z, Cheung WM, Chen C, Cools F, Van der Linde H, Gallacher DJ, Kuo GH, et al. (2010) Torcetrapib produces endothelial dysfunction independent of cholesteryl ester transfer protein inhibition. J Cardiovasc Pharmacol 55:459–468. [DOI] [PubMed] [Google Scholar]
- Coronary Drug Project Research Group (1975) Clofibrate and niacin in coronary heart disease. JAMA 231:360–381. [PubMed] [Google Scholar]
- Cramer CT, Goetz B, Hopson KL, Fici GJ, Ackermann RM, Brown SC, Bisgaier CL, Rajeswaran WG, Oniciu DC, Pape ME. (2004) Effects of a novel dual lipid synthesis inhibitor and its potential utility in treating dyslipidemia and metabolic syndrome. J Lipid Res 45:1289–1301. [DOI] [PubMed] [Google Scholar]
- Crosby J, Peloso GM, Auer PL, Crosslin DR, Stitziel NO, Lange LA, Lu Y, Tang ZZ, Zhang H, Hindy G, et al. TG and HDL Working Group of the Exome Sequencing Project, National Heart, Lung, and Blood Institute (2014) Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med 371:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuchel M, Meagher EA, du Toit Theron H, Blom DJ, Marais AD, Hegele RA, Averna MR, Sirtori CR, Shah PK, Gaudet D, et al. Phase 3 HoFH Lomitapide Study investigators (2013) Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, open-label, phase 3 study. Lancet 381:40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham D, Danley DE, Geoghegan KF, Griffor MC, Hawkins JL, Subashi TA, Varghese AH, Ammirati MJ, Culp JS, Hoth LR, et al. (2007) Structural and biophysical studies of PCSK9 and its mutants linked to familial hypercholesterolemia. Nat Struct Mol Biol 14:413–419. [DOI] [PubMed] [Google Scholar]
- Davidson MH. (2006) Mechanisms for the hypotriglyceridemic effect of marine omega-3 fatty acids. Am J Cardiol 98:27i–33i. [DOI] [PubMed] [Google Scholar]
- Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, et al. Emerging Risk Factors Collaboration (2009) Major lipids, apolipoproteins, and risk of vascular disease. JAMA 302:1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Bartolo BA, Nicholls SJ, Bao S, Rye KA, Heather AK, Barter PJ, Bursill C. (2011) The apolipoprotein A-I mimetic peptide ETC-642 exhibits anti-inflammatory properties that are comparable to high density lipoproteins. Atherosclerosis 217:395–400. [DOI] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD, Spady DK. (1993) Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J Lipid Res 34:1637–1659. [PubMed] [Google Scholar]
- Drayna D, Jarnagin AS, McLean J, Henzel W, Kohr W, Fielding C, Lawn R. (1987) Cloning and sequencing of human cholesteryl ester transfer protein cDNA. Nature 327:632–634. [DOI] [PubMed] [Google Scholar]
- Du XM, Kim MJ, Hou L, Le Goff W, Chapman MJ, Van Eck M, Curtiss LK, Burnett JR, Cartland SP, Quinn CM, et al. (2015) HDL particle size is a critical determinant of ABCA1-mediated macrophage cellular cholesterol export. Circ Res 116:1133–1142. [DOI] [PubMed] [Google Scholar]
- Duverger N, Kruth H, Emmanuel F, Caillaud JM, Viglietta C, Castro G, Tailleux A, Fievet C, Fruchart JC, Houdebine LM, et al. (1996) Inhibition of atherosclerosis development in cholesterol-fed human apolipoprotein A-I-transgenic rabbits. Circulation 94:713–717. [DOI] [PubMed] [Google Scholar]
- Elshourbagy NA, Near JC, Kmetz PJ, Sathe GM, Southan C, Strickler JE, Gross M, Young JF, Wells TN, Groot PH. (1990) Rat ATP citrate-lyase. Molecular cloning and sequence analysis of a full-length cDNA and mRNA abundance as a function of diet, organ, and age. J Biol Chem 265:1430–1435. [PubMed] [Google Scholar]
- Erqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, White IR, Marcovina SM, Collins R, Thompson SG, Danesh J, Emerging Risk Factors Collaboration (2009) Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA 302:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favari E, Calabresi L, Adorni MP, Jessup W, Simonelli S, Franceschini G, Bernini F. (2009) Small discoidal pre-beta1 HDL particles are efficient acceptors of cell cholesterol via ABCA1 and ABCG1. Biochemistry 48:11067–11074. [DOI] [PubMed] [Google Scholar]
- Föger B, Chase M, Amar MJ, Vaisman BL, Shamburek RD, Paigen B, Fruchart-Najib J, Paiz JA, Koch CA, Hoyt RF, et al. (1999) Cholesteryl ester transfer protein corrects dysfunctional high density lipoproteins and reduces aortic atherosclerosis in lecithin cholesterol acyltransferase transgenic mice. J Biol Chem 274:36912–36920. [DOI] [PubMed] [Google Scholar]
- Forrest MJ, Bloomfield D, Briscoe RJ, Brown PN, Cumiskey AM, Ehrhart J, Hershey JC, Keller WJ, Ma X, McPherson HE, et al. (2008) Torcetrapib-induced blood pressure elevation is independent of CETP inhibition and is accompanied by increased circulating levels of aldosterone. Br J Pharmacol 154:1465–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank-Kamenetsky M, Grefhorst A, Anderson NN, Racie TS, Bramlage B, Akinc A, Butler D, Charisse K, Dorkin R, Fan Y, et al. (2008) Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc Natl Acad Sci USA 105:11915–11920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick MH, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P, Huttunen JK, Kaitaniemi P, Koskinen P, Manninen V, et al. (1987) Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med 317:1237–1245. [DOI] [PubMed] [Google Scholar]
- Fruchart JC. (2013) Selective peroxisome proliferator-activated receptor α modulators (SPPARMα): the next generation of peroxisome proliferator-activated receptor α-agonists. Cardiovasc Diabetol 12:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet D, Alexander VJ, Baker BF, Brisson D, Tremblay K, Singleton W, Geary RS, Hughes SG, Viney NJ, Graham MJ, et al. (2015) Antisense inhibition of apolipoprotein C-III in patients with hypertriglyceridemia. N Engl J Med 373:438–447. [DOI] [PubMed] [Google Scholar]
- Gaudet D, Brisson D, Tremblay K, Alexander VJ, Singleton W, Hughes SG, Geary RS, Baker BF, Graham MJ, Crooke RM, et al. (2014) Targeting APOC3 in the familial chylomicronemia syndrome. N Engl J Med 371:2200–2206. [DOI] [PubMed] [Google Scholar]
- Ginsberg HN, Elam MB, Lovato LC, Crouse JR, 3rd, Leiter LA, Linz P, Friedewald WT, Buse JB, Gerstein HC, Probstfield J, et al. ACCORD Study Group (2010) Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 362:1563–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giugliano RP, Desai NR, Kohli P, Rogers WJ, Somaratne R, Huang F, Liu T, Mohanavelu S, Hoffman EB, McDonald ST, et al. LAPLACE-TIMI 57 Investigators (2012) Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study. Lancet 380:2007–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JL, Brown MS, Anderson RG, Russell DW, Schneider WJ. (1985) Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol 1:1–39. [DOI] [PubMed] [Google Scholar]
- Graham MJ, Lee RG, Bell TA, 3rd, Fu W, Mullick AE, Alexander VJ, Singleton W, Viney N, Geary R, Su J, et al. (2013) Antisense oligonucleotide inhibition of apolipoprotein C-III reduces plasma triglycerides in rodents, nonhuman primates, and humans. Circ Res 112:1479–1490. [DOI] [PubMed] [Google Scholar]
- Gupta N, Fisker N, Asselin MC, Lindholm M, Rosenbohm C, Ørum H, Elmén J, Seidah NG, Straarup EM. (2010) A locked nucleic acid antisense oligonucleotide (LNA) silences PCSK9 and enhances LDLR expression in vitro and in vivo. PLoS One 5:e10682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha YC, Barter PJ. (1982) Differences in plasma cholesteryl ester transfer activity in sixteen vertebrate species. Comp Biochem Physiol B 71:265–269. [DOI] [PubMed] [Google Scholar]
- Hayek T, Masucci-Magoulas L, Jiang X, Walsh A, Rubin E, Breslow JL, Tall AR. (1995) Decreased early atherosclerotic lesions in hypertriglyceridemic mice expressing cholesteryl ester transfer protein transgene. J Clin Invest 96:2071–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller F, Harvengt C. (1983) Effects of clofibrate, bezafibrate, fenofibrate and probucol on plasma lipolytic enzymes in normolipaemic subjects. Eur J Clin Pharmacol 25:57–63. [DOI] [PubMed] [Google Scholar]
- Herrera VL, Makrides SC, Xie HX, Adari H, Krauss RM, Ryan US, Ruiz-Opazo N. (1999) Spontaneous combined hyperlipidemia, coronary heart disease and decreased survival in Dahl salt-sensitive hypertensive rats transgenic for human cholesteryl ester transfer protein. Nat Med 5:1383–1389. [DOI] [PubMed] [Google Scholar]
- Hesler CB, Swenson TL, Tall AR. (1987) Purification and characterization of a human plasma cholesteryl ester transfer protein. J Biol Chem 262:2275–2282. [PubMed] [Google Scholar]
- Hildebrand RB, Lammers B, Meurs I, Korporaal SJ, De Haan W, Zhao Y, Kruijt JK, Praticò D, Schimmel AW, Holleboom AG, et al. (2010) Restoration of high-density lipoprotein levels by cholesteryl ester transfer protein expression in scavenger receptor class B type I (SR-BI) knockout mice does not normalize pathologies associated with SR-BI deficiency. Arterioscler Thromb Vasc Biol 30:1439–1445. [DOI] [PubMed] [Google Scholar]
- Horton JD, Cohen JC, Hobbs HH. (2007) Molecular biology of PCSK9: its role in LDL metabolism. Trends Biochem Sci 32:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JD, Cohen JC, Hobbs HH. (2009) PCSK9: a convertase that coordinates LDL catabolism. J Lipid Res 50 (Suppl):S172–S177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovingh GK, Kastelein JJ, van Deventer SJ, Round P, Ford J, Saleheen D, Rader DJ, Brewer HB, Barter PJ. (2015) Cholesterol ester transfer protein inhibition by TA-8995 in patients with mild dyslipidaemia (TULIP): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet 386:452–460. [DOI] [PubMed] [Google Scholar]
- Hu X, Dietz JD, Xia C, Knight DR, Loging WT, Smith AH, Yuan H, Perry DA, Keiser J. (2009) Torcetrapib induces aldosterone and cortisol production by an intracellular calcium-mediated mechanism independently of cholesteryl ester transfer protein inhibition. Endocrinology 150:2211–2219. [DOI] [PubMed] [Google Scholar]
- Huang S, Henry L, Ho YK, Pownall HJ, Rudenko G. (2010) Mechanism of LDL binding and release probed by structure-based mutagenesis of the LDL receptor. J Lipid Res 51:297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MK, Rimm EB, Furtado JD, Sacks FM. (2012) Apolipoprotein C-III as a potential modulator of the association between HDL-cholesterol and incident coronary heart disease. J Am Heart Assoc 1:e000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong HJ, Lee HS, Kim KS, Kim YK, Yoon D, Park SW. (2008) Sterol-dependent regulation of proprotein convertase subtilisin/kexin type 9 expression by sterol-regulatory element binding protein-2. J Lipid Res 49:399–409. [DOI] [PubMed] [Google Scholar]
- Johannsen TH, Frikke-Schmidt R, Schou J, Nordestgaard BG, Tybjærg-Hansen A. (2012) Genetic inhibition of CETP, ischemic vascular disease and mortality, and possible adverse effects. J Am Coll Cardiol 60:2041–2048. [DOI] [PubMed] [Google Scholar]
- Jong MC, Hofker MH, Havekes LM. (1999) Role of ApoCs in lipoprotein metabolism: functional differences between ApoC1, ApoC2, and ApoC3. Arterioscler Thromb Vasc Biol 19:472–484. [DOI] [PubMed] [Google Scholar]
- Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjærg-Hansen A. (2014) Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med 371:32–41. [DOI] [PubMed] [Google Scholar]
- Kamstrup PR, Tybjærg-Hansen A, Nordestgaard BG. (2014) Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol 63:470–477. [DOI] [PubMed] [Google Scholar]
- Kastelein JJ, Wedel MK, Baker BF, Su J, Bradley JD, Yu RZ, Chuang E, Graham MJ, Crooke RM. (2006) Potent reduction of apolipoprotein B and low-density lipoprotein cholesterol by short-term administration of an antisense inhibitor of apolipoprotein B. Circulation 114:1729–1735. [DOI] [PubMed] [Google Scholar]
- Kawakami A, Aikawa M, Alcaide P, Luscinskas FW, Libby P, Sacks FM. (2006a) Apolipoprotein CIII induces expression of vascular cell adhesion molecule-1 in vascular endothelial cells and increases adhesion of monocytic cells. Circulation 114:681–687. [DOI] [PubMed] [Google Scholar]
- Kawakami A, Aikawa M, Libby P, Alcaide P, Luscinskas FW, Sacks FM. (2006b) Apolipoprotein CIII in apolipoprotein B lipoproteins enhances the adhesion of human monocytic cells to endothelial cells. Circulation 113:691–700. [DOI] [PubMed] [Google Scholar]
- Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, Forder P, Pillai A, Davis T, Glasziou P, et al. FIELD Study Investigators (2005) Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 366:1849–1861. [DOI] [PubMed] [Google Scholar]
- Khera AV, Millar JS, Ruotolo G, Wang MD, Rader DJ. (2015) Potent peroxisome proliferator-activated receptor-α agonist treatment increases cholesterol efflux capacity in humans with the metabolic syndrome. Eur Heart J 36:3020–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnunen PK, Ehnolm C. (1976) Effect of serum and C-apoproteins from very low density lipoproteins on human postheparin plasma hepatic lipase. FEBS Lett 65:354–357. [DOI] [PubMed] [Google Scholar]
- Koren MJ, Giugliano RP, Raal FJ, Sullivan D, Bolognese M, Langslet G, Civeira F, Somaratne R, Nelson P, Liu T, et al. OSLER Investigators (2014) Efficacy and safety of longer-term administration of evolocumab (AMG 145) in patients with hypercholesterolemia: 52-week results from the Open-Label Study of Long-Term Evaluation Against LDL-C (OSLER) randomized trial. Circulation 129:234–243. [DOI] [PubMed] [Google Scholar]
- Koren MJ, Scott R, Kim JB, Knusel B, Liu T, Lei L, Bolognese M, Wasserman SM. (2012) Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 as monotherapy in patients with hypercholesterolaemia (MENDEL): a randomised, double-blind, placebo-controlled, phase 2 study. Lancet 380:1995–2006. [DOI] [PubMed] [Google Scholar]
- Kronenberg F, Utermann G. (2013) Lipoprotein(a): resurrected by genetics. J Intern Med 273:6–30. [DOI] [PubMed] [Google Scholar]
- Kühnast S, van der Tuin SJ, van der Hoorn JW, van Klinken JB, Simic B, Pieterman E, Havekes LM, Landmesser U, Lüscher TF, Willems van Dijk K, et al. (2015) Anacetrapib reduces progression of atherosclerosis, mainly by reducing non-HDL-cholesterol, improves lesion stability and adds to the beneficial effects of atorvastatin. Eur Heart J 36:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, Wallendszus K, Craig M, Jiang L, Collins R, et al. HPS2-THRIVE Collaborative Group (2014) Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med 371:203–212. [DOI] [PubMed] [Google Scholar]
- Larsson M, Vorrsjö E, Talmud P, Lookene A, Olivecrona G. (2013) Apolipoproteins C-I and C-III inhibit lipoprotein lipase activity by displacement of the enzyme from lipid droplets. J Biol Chem 288:33997–34008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Hegele RA. (2014) Abetalipoproteinemia and homozygous hypobetalipoproteinemia: a framework for diagnosis and management. J Inherit Metab Dis 37:333–339. [DOI] [PubMed] [Google Scholar]
- Lindholm MW, Elmén J, Fisker N, Hansen HF, Persson R, Møller MR, Rosenbohm C, Ørum H, Straarup EM, Koch T. (2012) PCSK9 LNA antisense oligonucleotides induce sustained reduction of LDL cholesterol in nonhuman primates. Mol Ther 20:376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipid Research Clinics (1984) The Lipid Research Clinics Coronary Primary Prevention Trial results. I. Reduction in incidence of coronary heart disease. JAMA 251:351–364. [DOI] [PubMed] [Google Scholar]
- MacLean PS, Bower JF, Vadlamudi S, Osborne JN, Bradfield JF, Burden HW, Bensch WH, Kauffman RF, Barakat HA. (2003) Cholesteryl ester transfer protein expression prevents diet-induced atherosclerotic lesions in male db/db mice. Arterioscler Thromb Vasc Biol 23:1412–1415. [DOI] [PubMed] [Google Scholar]
- Madsen L, Rustan AC, Vaagenes H, Berge K, Dyrøy E, Berge RK. (1999) Eicosapentaenoic and docosahexaenoic acid affect mitochondrial and peroxisomal fatty acid oxidation in relation to substrate preference. Lipids 34:951–963. [DOI] [PubMed] [Google Scholar]
- Marotti KR, Castle CK, Boyle TP, Lin AH, Murray RW, Melchior GW. (1993) Severe atherosclerosis in transgenic mice expressing simian cholesteryl ester transfer protein. Nature 364:73–75. [DOI] [PubMed] [Google Scholar]
- Maxwell KN, Breslow JL. (2004) Adenoviral-mediated expression of Pcsk9 in mice results in a low-density lipoprotein receptor knockout phenotype. Proc Natl Acad Sci USA 101:7100–7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenney JM, Koren MJ, Kereiakes DJ, Hanotin C, Ferrand AC, Stein EA. (2012) Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J Am Coll Cardiol 59:2344–2353. [DOI] [PubMed] [Google Scholar]
- Millar JS, Reyes-Soffer G, Jumes P, Dunbar RL, deGoma EM, Baer AL, Karmally W, Donovan DS, Rafeek H, Pollan L, et al. (2015) Anacetrapib lowers LDL by increasing ApoB clearance in mildly hypercholesterolemic subjects. J Clin Invest 125:2510–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morehouse LA, Sugarman ED, Bourassa PA, Sand TM, Zimetti F, Gao F, Rothblat GH, Milici AJ. (2007) Inhibition of CETP activity by torcetrapib reduces susceptibility to diet-induced atherosclerosis in New Zealand White rabbits. J Lipid Res 48:1263–1272. [DOI] [PubMed] [Google Scholar]
- Nassoury N, Blasiole DA, Tebon Oler A, Benjannet S, Hamelin J, Poupon V, McPherson PS, Attie AD, Prat A, Seidah NG. (2007) The cellular trafficking of the secretory proprotein convertase PCSK9 and its dependence on the LDLR. Traffic 8:718–732. [DOI] [PubMed] [Google Scholar]
- Navab M, Anantharamaiah GM, Hama S, Garber DW, Chaddha M, Hough G, Lallone R, Fogelman AM. (2002) Oral administration of an Apo A-I mimetic peptide synthesized from D-amino acids dramatically reduces atherosclerosis in mice independent of plasma cholesterol. Circulation 105:290–292. [DOI] [PubMed] [Google Scholar]
- Nicholls SJ, Tuzcu EM, Brennan DM, Tardif JC, Nissen SE. (2008) Cholesteryl ester transfer protein inhibition, high-density lipoprotein raising, and progression of coronary atherosclerosis: insights from ILLUSTRATE (Investigation of Lipid Level Management Using Coronary Ultrasound to Assess Reduction of Atherosclerosis by CETP Inhibition and HDL Elevation). Circulation 118:2506–2514. [DOI] [PubMed] [Google Scholar]
- Niesor EJ, Magg C, Ogawa N, Okamoto H, von der Mark E, Matile H, Schmid G, Clerc RG, Chaput E, Blum-Kaelin D, et al. (2010) Modulating cholesteryl ester transfer protein activity maintains efficient pre-β-HDL formation and increases reverse cholesterol transport. J Lipid Res 51:3443–3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwdorp M, Vergeer M, Bisoendial RJ, op ’t Roodt J, Levels H, Birjmohun RS, Kuivenhoven JA, Basser R, Rabelink TJ, Kastelein JJ, et al. (2008) Reconstituted HDL infusion restores endothelial function in patients with type 2 diabetes mellitus. Diabetologia 51:1081–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, Lauer MA, Sheldon WS, Grines CL, et al. (2003) Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA 290:2292–2300. [DOI] [PubMed] [Google Scholar]
- Niu W, Qi Y. (2015) Circulating cholesteryl ester transfer protein and coronary heart disease: mendelian randomization meta-analysis. Circ Cardiovasc Genet 8:114–121. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Yonemori F, Wakitani K, Minowa T, Maeda K, Shinkai H. (2000) A cholesteryl ester transfer protein inhibitor attenuates atherosclerosis in rabbits. Nature 406:203–207. [DOI] [PubMed] [Google Scholar]
- Olivieri O, Bassi A, Stranieri C, Trabetti E, Martinelli N, Pizzolo F, Girelli D, Friso S, Pignatti PF, Corrocher R. (2003) Apolipoprotein C-III, metabolic syndrome, and risk of coronary artery disease. J Lipid Res 44:2374–2381. [DOI] [PubMed] [Google Scholar]
- Park SW, Moon YA, Horton JD. (2004) Post-transcriptional regulation of low density lipoprotein receptor protein by proprotein convertase subtilisin/kexin type 9a in mouse liver. J Biol Chem 279:50630–50638. [DOI] [PubMed] [Google Scholar]
- Pinkosky SL, Filippov S, Srivastava RA, Hanselman JC, Bradshaw CD, Hurley TR, Cramer CT, Spahr MA, Brant AF, Houghton JL, et al. (2013) AMP-activated protein kinase and ATP-citrate lyase are two distinct molecular targets for ETC-1002, a novel small molecule regulator of lipid and carbohydrate metabolism. J Lipid Res 54:134–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plump AS, Masucci-Magoulas L, Bruce C, Bisgaier CL, Breslow JL, Tall AR. (1999) Increased atherosclerosis in ApoE and LDL receptor gene knock-out mice as a result of human cholesteryl ester transfer protein transgene expression. Arterioscler Thromb Vasc Biol 19:1105–1110. [DOI] [PubMed] [Google Scholar]
- Plump AS, Scott CJ, Breslow JL. (1994) Human apolipoprotein A-I gene expression increases high density lipoprotein and suppresses atherosclerosis in the apolipoprotein E-deficient mouse. Proc Natl Acad Sci USA 91:9607–9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier S, Mayer G. (2013) The biology of PCSK9 from the endoplasmic reticulum to lysosomes: new and emerging therapeutics to control low-density lipoprotein cholesterol. Drug Des Devel Ther 7:1135–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raal F, Scott R, Somaratne R, Bridges I, Li G, Wasserman SM, Stein EA. (2012) Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) randomized trial. Circulation 126:2408–2417. [DOI] [PubMed] [Google Scholar]
- Raal FJ, Santos RD, Blom DJ, Marais AD, Charng MJ, Cromwell WC, Lachmann RH, Gaudet D, Tan JL, Chasan-Taber S, et al. (2010) Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet 375:998–1006. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Paré G, Parker AN, Zee RY, Miletich JP, Chasman DI. (2009) Polymorphism in the CETP gene region, HDL cholesterol, and risk of future myocardial infarction: genomewide analysis among 18 245 initially healthy women from the Women’s Genome Health Study. Circ Cardiovasc Genet 2:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittershaus CW, Miller DP, Thomas LJ, Picard MD, Honan CM, Emmett CD, Pettey CL, Adari H, Hammond RA, Beattie DT, et al. (2000) Vaccine-induced antibodies inhibit CETP activity in vivo and reduce aortic lesions in a rabbit model of atherosclerosis. Arterioscler Thromb Vasc Biol 20:2106–2112. [DOI] [PubMed] [Google Scholar]
- Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, El Shahawy M, et al. ODYSSEY LONG TERM Investigators (2015) Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 372:1489–1499. [DOI] [PubMed] [Google Scholar]
- Rosenson RS, Brewer HB, Jr, Ansell BJ, Barter P, Chapman MJ, Heinecke JW, Kontush A, Tall AR, Webb NR. (2016) Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat Rev Cardiol 13:48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth EM, McKenney JM, Hanotin C, Asset G, Stein EA. (2012) Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia. N Engl J Med 367:1891–1900. [DOI] [PubMed] [Google Scholar]
- Rubin EM, Krauss RM, Spangler EA, Verstuyft JG, Clift SM. (1991) Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature 353:265–267. [DOI] [PubMed] [Google Scholar]
- Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, Faas FH, Linares E, Schaefer EJ, Schectman G, et al. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group (1999) Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. N Engl J Med 341:410–418. [DOI] [PubMed] [Google Scholar]
- Rye KA, Barter PJ. (2014) Cardioprotective functions of HDLs. J Lipid Res 55:168–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, Ballantyne CM, Somaratne R, Legg J, Wasserman SM, et al. Open-Label Study of Long-Term Evaluation against LDL Cholesterol (OSLER) Investigators (2015) Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 372:1500–1509. [DOI] [PubMed] [Google Scholar]
- Sacks FM, Rudel LL, Conner A, Akeefe H, Kostner G, Baki T, Rothblat G, de la Llera-Moya M, Asztalos B, Perlman T, et al. (2009) Selective delipidation of plasma HDL enhances reverse cholesterol transport in vivo. J Lipid Res 50:894–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandinavian Simvastatin Survival Study Group (1994) Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 344:1383–1389. [PubMed] [Google Scholar]
- Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, et al. dal-OUTCOMES Investigators (2012) Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med 367:2089–2099. [DOI] [PubMed] [Google Scholar]
- Seidah NG, Awan Z, Chrétien M, Mbikay M. (2014) PCSK9: a key modulator of cardiovascular health. Circ Res 114:1022–1036. [DOI] [PubMed] [Google Scholar]
- Seidah NG, Benjannet S, Wickham L, Marcinkiewicz J, Jasmin SB, Stifani S, Basak A, Prat A, Chretien M. (2003) The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc Natl Acad Sci USA 100:928–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah PK, Nilsson J, Kaul S, Fishbein MC, Ageland H, Hamsten A, Johansson J, Karpe F, Cercek B. (1998) Effects of recombinant apolipoprotein A-I(Milano) on aortic atherosclerosis in apolipoprotein E-deficient mice. Circulation 97:780–785. [DOI] [PubMed] [Google Scholar]
- Sharp D, Blinderman L, Combs KA, Kienzle B, Ricci B, Wager-Smith K, Gil CM, Turck CW, Bouma ME, Rader DJ, et al. (1993) Cloning and gene defects in microsomal triglyceride transfer protein associated with abetalipoproteinaemia. Nature 365:65–69. [DOI] [PubMed] [Google Scholar]
- Shiomi M, Ito T. (2001) MTP inhibitor decreases plasma cholesterol levels in LDL receptor-deficient WHHL rabbits by lowering the VLDL secretion. Eur J Pharmacol 431:127–131. [DOI] [PubMed] [Google Scholar]
- Simic B, Hermann M, Shaw SG, Bigler L, Stalder U, Dörries C, Besler C, Lüscher TF, Ruschitzka F. (2012) Torcetrapib impairs endothelial function in hypertension. Eur Heart J 33:1615–1624. [DOI] [PubMed] [Google Scholar]
- Sirtori CR, Calabresi L, Franceschini G, Baldassarre D, Amato M, Johansson J, Salvetti M, Monteduro C, Zulli R, Muiesan ML, et al. (2001) Cardiovascular status of carriers of the apolipoprotein A-I(Milano) mutant: the Limone sul Garda study. Circulation 103:1949–1954. [DOI] [PubMed] [Google Scholar]
- Spence JD, Koschinsky M. (2012) Mechanisms of lipoprotein(a) pathogenicity: prothrombotic, proatherosclerotic, or both? Arterioscler Thromb Vasc Biol 32:1550–1551. [DOI] [PubMed] [Google Scholar]
- Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC. (1998) Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation 98:2088–2093. [DOI] [PubMed] [Google Scholar]
- Staels B, Vu-Dac N, Kosykh VA, Saladin R, Fruchart JC, Dallongeville J, Auwerx J. (1995) Fibrates downregulate apolipoprotein C-III expression independent of induction of peroxisomal acyl coenzyme A oxidase. A potential mechanism for the hypolipidemic action of fibrates. J Clin Invest 95:705–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawowy P. (2015) Proprotein convertases in atherogenesis. Curr Opin Lipidol 26:338–344. [DOI] [PubMed] [Google Scholar]
- Stein EA, Gipe D, Bergeron J, Gaudet D, Weiss R, Dufour R, Wu R, Pordy R. (2012) Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial. Lancet 380:29–36. [DOI] [PubMed] [Google Scholar]
- Stein EA, Giugliano RP, Koren MJ, Raal FJ, Roth EM, Weiss R, Sullivan D, Wasserman SM, Somaratne R, Kim JB, et al. PROFICIO Investigators (2014) Efficacy and safety of evolocumab (AMG 145), a fully human monoclonal antibody to PCSK9, in hyperlipidaemic patients on various background lipid therapies: pooled analysis of 1359 patients in four phase 2 trials. Eur Heart J 35:2249–2259. [DOI] [PubMed] [Google Scholar]
- Stitziel NO, Won HH, Morrison AC, Peloso GM, Do R, Lange LA, Fontanillas P, Gupta N, Duga S, Goel A, et al. Myocardial Infarction Genetics Consortium Investigators (2014) Inactivating mutations in NPC1L1 and protection from coronary heart disease. N Engl J Med 371:2072–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugano M, Makino N, Sawada S, Otsuka S, Watanabe M, Okamoto H, Kamada M, Mizushima A. (1998) Effect of antisense oligonucleotides against cholesteryl ester transfer protein on the development of atherosclerosis in cholesterol-fed rabbits. J Biol Chem 273:5033–5036. [DOI] [PubMed] [Google Scholar]
- Sullivan D, Olsson AG, Scott R, Kim JB, Xue A, Gebski V, Wasserman SM, Stein EA. (2012) Effect of a monoclonal antibody to PCSK9 on low-density lipoprotein cholesterol levels in statin-intolerant patients: the GAUSS randomized trial. JAMA 308:2497–2506. [DOI] [PubMed] [Google Scholar]
- Sviridov DO, Ikpot IZ, Stonik J, Drake SK, Amar M, Osei-Hwedieh DO, Piszczek G, Turner S, Remaley AT. (2011) Helix stabilization of amphipathic peptides by hydrocarbon stapling increases cholesterol efflux by the ABCA1 transporter. Biochem Biophys Res Commun 410:446–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabet F, Lambert G, Cuesta Torres LF, Hou L, Sotirchos I, Touyz RM, Jenkins AJ, Barter PJ, Rye KA. (2011) Lipid-free apolipoprotein A-I and discoidal reconstituted high-density lipoproteins differentially inhibit glucose-induced oxidative stress in human macrophages. Arterioscler Thromb Vasc Biol 31:1192–1200. [DOI] [PubMed] [Google Scholar]
- Tabet F, Remaley AT, Segaliny AI, Millet J, Yan L, Nakhla S, Barter PJ, Rye KA, Lambert G. (2010) The 5A apolipoprotein A-I mimetic peptide displays antiinflammatory and antioxidant properties in vivo and in vitro. Arterioscler Thromb Vasc Biol 30:246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif JC, Ballantyne CM, Barter P, Dasseux JL, Fayad ZA, Guertin MC, Kastelein JJ, Keyserling C, Klepp H, Koenig W, et al. Can HDL Infusions Significantly QUicken Atherosclerosis REgression (CHI-SQUARE) Investigators (2014) Effects of the high-density lipoprotein mimetic agent CER-001 on coronary atherosclerosis in patients with acute coronary syndromes: a randomized trial. Eur Heart J 35:3277–3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif JC, Grégoire J, L’Allier PL, Ibrahim R, Lespérance J, Heinonen TM, Kouz S, Berry C, Basser R, Lavoie MA, et al. Effect of rHDL on Atherosclerosis-Safety and Efficacy (ERASE) Investigators (2007) Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA 297:1675–1682. [DOI] [PubMed] [Google Scholar]
- Thanassoulis G, Campbell CY, Owens DS, Smith JG, Smith AV, Peloso GM, Kerr KF, Pechlivanis S, Budoff MJ, Harris TB, et al. CHARGE Extracoronary Calcium Working Group (2013) Genetic associations with valvular calcification and aortic stenosis. N Engl J Med 368:503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thies F, Garry JM, Yaqoob P, Rerkasem K, Williams J, Shearman CP, Gallagher PJ, Calder PC, Grimble RF. (2003) Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial. Lancet 361:477–485. [DOI] [PubMed] [Google Scholar]
- Thompson A, Di Angelantonio E, Sarwar N, Erqou S, Saleheen D, Dullaart RP, Keavney B, Ye Z, Danesh J. (2008) Association of cholesteryl ester transfer protein genotypes with CETP mass and activity, lipid levels, and coronary risk. JAMA 299:2777–2788. [DOI] [PubMed] [Google Scholar]
- Thompson PD, Rubino J, Janik MJ, MacDougall DE, McBride SJ, Margulies JR, Newton RS. (2015) Use of ETC-1002 to treat hypercholesterolemia in patients with statin intolerance. J Clin Lipidol 9:295–304. [DOI] [PubMed] [Google Scholar]
- Tsimikas S, Viney NJ, Hughes SG, Singleton W, Graham MJ, Baker BF, Burkey JL, Yang Q, Marcovina SM, Geary RS, et al. (2015) Antisense therapy targeting apolipoprotein(a): a randomised, double-blind, placebo-controlled phase 1 study. Lancet 386:1472–1483. [DOI] [PubMed] [Google Scholar]
- Tso C, Martinic G, Fan WH, Rogers C, Rye KA, Barter PJ. (2006) High-density lipoproteins enhance progenitor-mediated endothelium repair in mice. Arterioscler Thromb Vasc Biol 26:1144–1149. [DOI] [PubMed] [Google Scholar]
- Urban D, Pöss J, Böhm M, Laufs U. (2013) Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis. J Am Coll Cardiol 62:1401–1408. [DOI] [PubMed] [Google Scholar]
- van der Tuin SJ, Kühnast S, Berbée JF, Verschuren L, Pieterman EJ, Havekes LM, van der Hoorn JW, Rensen PC, Jukema JW, Princen HM, et al. (2015) Anacetrapib reduces (V)LDL cholesterol by inhibition of CETP activity and reduction of plasma PCSK9. J Lipid Res 56:2085–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Schacky C, Angerer P, Kothny W, Theisen K, Mudra H. (1999) The effect of dietary omega-3 fatty acids on coronary atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 130:554–562. [DOI] [PubMed] [Google Scholar]
- Waksman R, Torguson R, Kent KM, Pichard AD, Suddath WO, Satler LF, Martin BD, Perlman TJ, Maltais JA, Weissman NJ, et al. (2010) A first-in-man, randomized, placebo-controlled study to evaluate the safety and feasibility of autologous delipidated high-density lipoprotein plasma infusions in patients with acute coronary syndrome. J Am Coll Cardiol 55:2727–2735. [DOI] [PubMed] [Google Scholar]
- Wang CS, McConathy WJ, Kloer HU, Alaupovic P. (1985) Modulation of lipoprotein lipase activity by apolipoproteins. Effect of apolipoprotein C-III. J Clin Invest 75:384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub MS, Zechner R, Brown A, Eisenberg S, Breslow JL. (1988) Dietary polyunsaturated fats of the W-6 and W-3 series reduce postprandial lipoprotein levels. Chronic and acute effects of fat saturation on postprandial lipoprotein metabolism. J Clin Invest 82:1884–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welder G, Zineh I, Pacanowski MA, Troutt JS, Cao G, Konrad RJ. (2010) High-dose atorvastatin causes a rapid sustained increase in human serum PCSK9 and disrupts its correlation with LDL cholesterol. J Lipid Res 51:2714–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerterp M, van der Hoogt CC, de Haan W, Offerman EH, Dallinga-Thie GM, Jukema JW, Havekes LM, Rensen PC. (2006) Cholesteryl ester transfer protein decreases high-density lipoprotein and severely aggravates atherosclerosis in APOE*3-Leiden mice. Arterioscler Thromb Vasc Biol 26:2552–2559. [DOI] [PubMed] [Google Scholar]
- Wetterau JR, Aggerbeck LP, Bouma ME, Eisenberg C, Munck A, Hermier M, Schmitz J, Gay G, Rader DJ, Gregg RE. (1992) Absence of microsomal triglyceride transfer protein in individuals with abetalipoproteinemia. Science 258:999–1001. [DOI] [PubMed] [Google Scholar]
- Windler E, Havel RJ. (1985) Inhibitory effects of C apolipoproteins from rats and humans on the uptake of triglyceride-rich lipoproteins and their remnants by the perfused rat liver. J Lipid Res 26:556–565. [PubMed] [Google Scholar]
- Wu BJ, Chen K, Shrestha S, Ong KL, Barter PJ, Rye KA. (2013) High-density lipoproteins inhibit vascular endothelial inflammation by increasing 3β-hydroxysteroid-Δ24 reductase expression and inducing heme oxygenase-1. Circ Res 112:278–288. [DOI] [PubMed] [Google Scholar]
- Wu BJ, Shrestha S, Ong KL, Johns D, Dunn LL, Hou L, Barter PJ, Rye KA. (2015a) Increasing HDL levels by inhibiting cholesteryl ester transfer protein activity in rabbits with hindlimb ischemia is associated with increased angiogenesis. Int J Cardiol 199:204–212. [DOI] [PubMed] [Google Scholar]
- Wu BJ, Shrestha S, Ong KL, Johns D, Hou L, Barter PJ, Rye KA. (2015b) Cholesteryl ester transfer protein inhibition enhances endothelial repair and improves endothelial function in the rabbit. Arterioscler Thromb Vasc Biol 35:628–636. [DOI] [PubMed] [Google Scholar]
- Yang J, Sato R, Goldstein JL, Brown MS. (1994) Sterol-resistant transcription in CHO cells caused by gene rearrangement that truncates SREBP-2. Genes Dev 8:1910–1919. [DOI] [PubMed] [Google Scholar]
- Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, et al. Japan EPA Lipid Intervention Study (JELIS) Investigators (2007) Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 369:1090–1098. [DOI] [PubMed] [Google Scholar]
- Yvan-Charvet L, Kling J, Pagler T, Li H, Hubbard B, Fisher T, Sparrow CP, Taggart AK, Tall AR. (2010) Cholesterol efflux potential and antiinflammatory properties of high-density lipoprotein after treatment with niacin or anacetrapib. Arterioscler Thromb Vasc Biol 30:1430–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yvan-Charvet L, Matsuura F, Wang N, Bamberger MJ, Nguyen T, Rinninger F, Jiang XC, Shear CL, Tall AR. (2007) Inhibition of cholesteryl ester transfer protein by torcetrapib modestly increases macrophage cholesterol efflux to HDL. Arterioscler Thromb Vasc Biol 27:1132–1138. [DOI] [PubMed] [Google Scholar]
- Zaid A, Roubtsova A, Essalmani R, Marcinkiewicz J, Chamberland A, Hamelin J, Tremblay M, Jacques H, Jin W, Davignon J, et al. (2008) Proprotein convertase subtilisin/kexin type 9 (PCSK9): hepatocyte-specific low-density lipoprotein receptor degradation and critical role in mouse liver regeneration. Hepatology 48:646–654. [DOI] [PubMed] [Google Scholar]
- Zhang XL, Zhu QQ, Zhu L, Chen JZ, Chen QH, Li GN, Xie J, Kang LN, Xu B. (2015) Safety and efficacy of anti-PCSK9 antibodies: a meta-analysis of 25 randomized, controlled trials. BMC Med 13:123. [DOI] [PMC free article] [PubMed] [Google Scholar]