Abstract
The purpose of this review is to discuss ways to think about and study sex differences in preclinical animal models. We use the framework of addiction, in which animal models have excellent face and construct validity, to illustrate the importance of considering sex differences. There are four types of sex differences: qualitative, quantitative, population, and mechanistic. A better understanding of the ways males and females can differ will help scientists design experiments to characterize better the presence or absence of sex differences in new phenomena that they are investigating. We have outlined major quantitative, population, and mechanistic sex differences in the addiction domain using a heuristic framework of the three established stages of the addiction cycle: binge/intoxication, withdrawal/negative affect, and preoccupation/anticipation. Female rats, in general, acquire the self-administration of drugs and alcohol more rapidly, escalate their drug taking with extended access more rapidly, show more motivational withdrawal, and (where tested in animal models of “craving”) show greater reinstatement. The one exception is that female rats show less motivational withdrawal to alcohol. The bases for these quantitative sex differences appear to be both organizational, in that estradiol-treated neonatal animals show the male phenotype, and activational, in that the female phenotype depends on the effects of gonadal hormones. In animals, differences within the estrous cycle can be observed but are relatively minor. Such hormonal effects seem to be most prevalent during the acquisition of drug taking and less influential once compulsive drug taking is established and are linked largely to progesterone and estradiol. This review emphasizes not only significant differences in the phenotypes of females and males in the domain of addiction but emphasizes the paucity of data to date in our understanding of those differences.
I. Introduction
In this review, we discuss ways to think about and study sex differences using preclinical models. These are general guidelines or suggestions and are not meant to be exhaustive. The domain of addiction will be used as a framework because animal models of addiction have excellent face and construct validity.
When beginning to study both males and females, it is important to first consider why it is that males and females might respond differently to experimental conditions, such as the experimenter and housing conditions. Consider the nature of the trait as you think about your experimental paradigm. Is there anything in the evolutionary history and/or current social structures of the species under investigation that might cause males and females to be different? Such considerations are relevant even for studies where the dependent measure is a physiologic or neuroanatomical characteristic or response. Housing conditions, handling, and the experimenter may impact the animal’s response to a treatment or manipulation. Furthermore, males and females may be differentially affected.
This concept can also apply to the reproducibility of findings within one sex. Small changes in experimental conditions, the experimenter, and housing conditions can affect experimental outcomes, even when males and females are considered independently. This has been demonstrated elegantly by the work of Mogil and colleagues (Sorge et al., 2014).
II. Investigation of Sex Differences
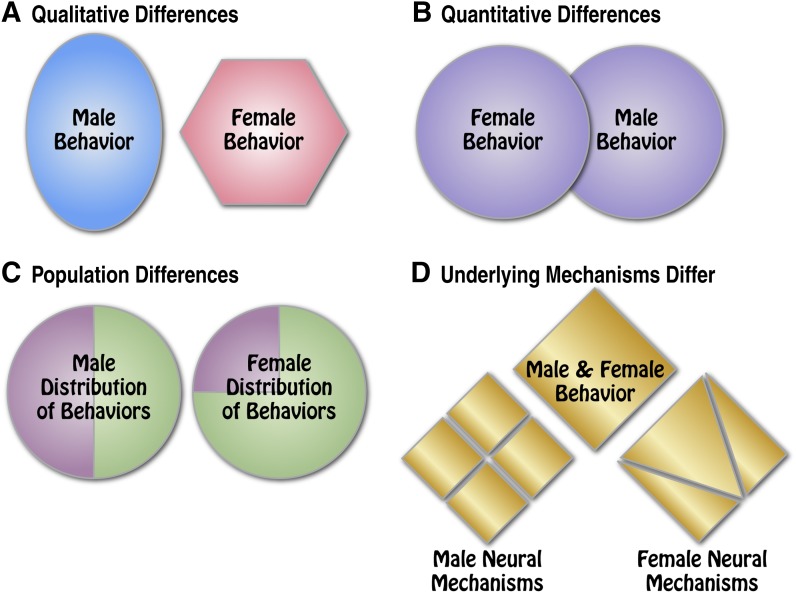
It can be confusing to start to investigate whether there is a sex difference because not all sex differences are alike. Some are easier than others to define and characterize, whereas others are the result of multiple factors. A number of authors have described various ways that males and females can be different (e.g., McCarthy et al., 2012). We describe four types of sex differences with the hope that understanding how males and females may differ will help scientists design experiments to better characterize the presence or absence of sex differences in new phenomena that they are investigating (Fig. 1).
Fig. 1.
Illustration of the four types of sex differences that can be observed in animal models: qualitative, quantitative, population, and mechanistic. Some sex differences are so dramatic that the traits that are exhibited by males and females do not look the same. These “qualitative differences” are exemplified by sexual behavior in the rat. Many sex differences are also “quantitative differences,” in which males and females differ in the magnitude of their response. Sex differences in the incidence or distribution of individual traits are called “population differences.” Finally, for some behaviors or processes, the expression of a trait may look the same for males and females, but there are sex differences in the neural “mechanisms” that mediate the behaviors.
As illustrated schematically in Fig. 1A, some sex differences are so dramatic that the traits that are exhibited by males and females do not look the same. These “qualitative differences” are exemplified by sexual behavior in the rat. During sexual interactions, the female exhibits lordosis behavior (dorsoflexion of the back with lateral diversion of the tail), and the male exhibits mounting with pelvic thrusting. These behaviors cannot be measured on the same scales, nor can they be directly compared.
On the other hand, many sex differences are “quantitative differences,” in which males and females differ in the magnitude of their response (Fig. 1B). The trait is the same, but one sex exhibits a greater response than the other. This can be seen in many dose-response studies. For example, female rats exhibit a greater locomotor response to psychomotor stimulants and more behavioral sensitization than do males (Robinson and Becker, 1986).
There can also be sex differences in the incidence or distribution of individual traits. We call these “population differences” (Fig. 1C). An example would be that more females (50%) choose cocaine over palatable pellets than males (16%), but the behaviors exhibited during cocaine taking are the same for males and females (Perry et al., 2013b, 2015). The population differences within a sex may be influenced by environmental events that interact with development, but these population differences are not necessarily attributable to the process of sexual differentiation. For example, more females than males acquire cocaine self-administration within three test sessions. Prenatal stress increases the percentage of males that acquire cocaine self-administration within three sessions, changing the distribution of males that rapidly acquire cocaine-taking behavior to be equivalent to females (Thomas et al., 2009).
It is important to note that for some behaviors or processes, the expression of a trait may look the same for males and females, but there are sex differences in the neural mechanisms that mediate the behaviors (Fig. 1D). Both males and females exhibit a corticotropin-releasing factor-1 (CRF1) receptor response to stimulation with CRF, for example, but there is a difference in the intracellular signaling pathways (Bangasser and Valentino, 2014). Both male and female prairie voles form pair-bonds, but the neural mechanisms that mediate pair-bond formation are different (Hammock and Young, 2006). In humans, such sex differences are also seen. Men and women exhibit an equivalent recall of emotional memories, but the response in the amygdala is different between sexes (Cahill, 2014).
More than one of these types of sex differences can contribute to a given trait, and there may also be effects of the estrous cycle and/or influences of gonadal hormones on a trait. Many people assume that they need to start by looking at the effects of the estrous cycle on a particular function or behavior. For some traits, this may be true, but for many, the presence or absence of sex differences is sufficiently robust that randomly cycling females can be studied without additional variability being introduced by the estrous cycle (e.g., Prendergast et al., 2014).
Thus, just as there are species differences in the brain and behavior, there are also sex differences. These differences have evolved because of the different ecological niches that males and females inhabit. Considering the perspective of the male’s or female’s natural ecology can help design tests that are valid for both sexes.
A. Behavioral Ecology of Male and Female Mammals
In the wild, male and female mammals experience the world through different lenses because females lactate and must provide nourishment to their young. This includes mammalian species with biparental care, in which the neural and behavioral mechanisms are different for mothers and fathers (Keverne, 2007). Evolutionary scholars theorize that even for humans, sex differences in brain and behavior evolved due to different demands related to child care for women and successful hunting and gathering strategies for men (Keverne, 2007).
For many species that do not exhibit biparental care, from rats to primates (but not mice), at puberty females remain in the natal unit and males disperse (Schultz and Lore, 1993). In species with this strategy, females tend to live in a social group that consists primarily of other related females and juveniles. The reproductive success of a female depends on successfully rearing their young. To this end, females of many species coordinate their menstrual or estrous cycles so that the care of offspring can be shared (McClintock, 1984). Social dominance among females means greater support in caring for offspring and even the suppression of fertility in nondominant females that care for the dominant female’s young (Kleiman, 2011).
For males, reproductive success depends on having access to females by maintaining dominance in a social group or defending a large territory. Therefore, males tend to be more aggressive toward other males than toward females. In seasonal breeders, males will form bachelor bands during the nonbreeding season when circulating testosterone is low but as soon as the hormones begin to surge, males become solitary and aggressive toward other males (Bonenfant et al., 2004). In some species, males will form coalitions in an attempt to entice a female away from her social group, but this tends to be a strategy that is used by young males (e.g., dolphins and baboons) that are not strong enough to risk challenging the dominant male for access to females (Smuts, 1992).
B. Behavioral Ecology of Rats and Mice
The social ecology of the mouse and rat are quite different, although both are small rodents. A short summary of the factors that may impact the study of sex differences in behavior follows. Rats and mice have a highly developed vomeronasal system and use ultrasonic communication extensively. Much information that is important in the world of the rat or mouse is lost to the investigator. These ultrasonic and chemical signals convey important information to other rats or mice about environmental dangers and how individuals differ from each other. Males and females will respond differently to the signals from conspecifics (e.g., the scent of a male rat might provoke an aggressive response in another male but induce sexual motivation in an estrous female).
In the wild, a female rat’s territory is the nest she shares with her female relations (e.g., sisters or mother/daughter), whereas males disperse at puberty (Schultz and Lore, 1993). Female rats in the nest engage in communal rearing of the young (Schultz and Lore, 1993). A male rat will defend a territory of ∼30 m in diameter, so the number of males a female encounters is limited by food availability and how far she must travel to forage (Barnett, 1963; Calhoun, 1963). Thus, a male rat may experience the least social stress if individually housed once sexually mature, whereas adult females are stressed least when housed with other females. It should be noted that social housing during adolescence is important for the normal social development of males (e.g., Inagaki et al., 2013). As adults, even the albino rat pays close attention to the activity of animals in adjacent cages when the cage walls are translucent (J.B. Becker, personal observation), so individually housed males each have their own territory but retain the company of other rats. Female rats that are housed individually exhibit chronic stress as indicated by adrenal hypertrophy, whereas individually housed males do not differ from pair-housed males on this measure (Westenbroek et al., 2005).
By contrast, the mouse is an opportunistic animal, and its social structure depends to a great extent on the environment where it finds itself and the availability of resources (Bronson 1979). Under the least stressful conditions for mice, with plentiful food availability and few predators, a dominant male defends a territory with multiple females living within the male’s territory, and each female has its own area. Both males and females scent-mark their respective territories, and urinary cues are thought to convey information about an individual’s identity to establish its territory, relay cues related to breeding status (e.g., male, female, sexual state, social status, etc.), and simultaneously prevent in-breeding.
Females are frequently found in groups with pups, but unlike rats in which communal nursing is common, the instance of communal nursing in mice is less than 33% (Weidt et al., 2014). There are both pheromonal and ultrasonic cues to prevent in-breeding, and unlike rats, both male and female mice disperse at puberty (Bronson, 1979; Asaba et al., 2014).
Finally, female mice that are reared in isolation from males tend to have disorganized reproductive cycles and are often anovulatory. This is attributable to olfactory/pheromonal influences of the urine of adult females, which inhibits puberty in prepubertal females, whereas male urine can accelerate puberty. Because the vomeronasal system is very important for reproduction in the mouse and other rodents, it is unsurprising that this neural system is sexually dimorphic (Guillamon and Segovia, 1997).
Thus, when studying the behavior of rodents, we need to be aware that they perceive information that humans do not detect with their senses, and these signals convey important information that can mean something different for males and females or interact with sex differences in behavior. For example, rodents are prey animals. The most salient information in their environment, other than conspecifics, is the presence of predators. Mogil and colleagues (Sorge et al., 2014) demonstrated that odors from male animals (humans and other species) induce a stress response that reduces the response to painful stimuli in rats and mice of both sexes, even when females have higher pain responses than males.
C. Implications for Working with Rats and Mice of Both Sexes
From the discussion above, sex and species differences in the sensitivity of males and females to pheromonal, ultrasonic, and social cues should also be considered when determining housing conditions, not just testing conditions. In particular, animal housing and husbandry conditions that reduce stress can be different for males and females (for discussion, see Bind et al., 2013).
The effects of housing can have real outcomes on experimental results. For example, we have found that housing conditions differentially affect the self-administration of cocaine in male and female rats. For female rats, pair housing reduces the rate of acquisition of cocaine self-administration and motivation to take cocaine, whereas pair housing does not affect the same behaviors in males (Westenbroek et al., 2013). For additional details and a discussion of the effects of species- and sex-specific effects of social conditions and the stress response, see Beery and Kaufer (2015).
Another consideration is how the data will be analyzed and whether there are sufficient numbers of animals to be tested to know whether there is a sex difference in the outcome. For an initial analysis, if the sex difference is a quantitative sex difference, then comparisons of the means are sufficient. For either qualitative differences or population differences, it is also important to look at whether there are differences in the distribution of males and females and a sufficient number of animals of each sex to obtain a valid index of the distribution. Some qualitative differences may be subtle and can be missed by automated equipment.
If the underlying mechanisms that mediate a trait differ, then males and females may look the same. It is important to keep in mind that although a trait does not differ for males and females, the mechanisms that mediate the trait can still be different, so both sexes need to be studied when mechanisms are subsequently investigated.
III. Sex Differences in Drug Abuse
Drug addiction can be heuristically framed by the stages through which users progress: binge/intoxication withdrawal/negative affect, and preoccupation/anticipation. These stages involve allostatic changes in the brain reward and stress systems. Both positive and negative reinforcement have been hypothesized to play a role in the processes associated with addiction. The binge/intoxication stage involves the facilitation of incentive salience and is mediated largely by neurocircuitry in the basal ganglia, with a focus on activation of the “reward” neurotransmitters dopamine and opioid peptides that bind to μ opioid receptors. The withdrawal/negative affect stage of addiction involves not only drug-induced specific “physical” withdrawal but also a common drug-induced “motivational” withdrawal that is characterized by dysphoria, malaise, irritability, sleep disturbances, and hypersensitivity to pain, mediated largely by recruitment of the brain stress systems in the extended amygdala, including the neurochemical systems of CRF and dynorphin (Carlezon et al., 2000; Koob and Le Moal, 2001) that are sexually dimorphic (reviewed in Becker et al., 2012). The preoccupation/anticipation (or “craving”) stage of the addiction cycle involves the dysregulation of executive control via dysregulation of prefrontal cortex circuits (Koob and Volkow, 2010).
In this review, we focus primarily on the neurobiological bases for sex differences in addiction because those are the aspects we can best study in animal models. It should be noted that for humans our culture also plays an important role in shaping behavior. From the first day of life, infants are categorized by whether they are male or female. The clothes and colors that are worn, the toys infants are given to play with, whether the mother and father keep a toddler close or allow him or her to run farther away are all influenced by the perceived sex of the child. Some of these adult behaviors are based on the child’s preference, such as toy choice, but many are biased by the adults’ perception of what it means to be male or female (for discussion, see Fausto-Sterling, 2012). Thus, whether girls and boys engage in risky behaviors, such as experimentation with drugs of abuse, is shaped by their culture in addition to their biology.
It should also be noted that as our culture has been changing in the United States, with greater opportunities and access for girls and women, the gap has been closing between boys and girls in terms of drug use during adolescence since 2000 (Substance Abuse and Mental Health Services Administration, 2007). Most of the data we have about the numbers of individuals who use drugs of abuse are cross-sectional surveys of use. We will not know the actual impact of the equal opportunity for adolescents to use drugs on the use of drugs by women and men for many years. There are data that we discuss herein where it seems the animal models are at odds with the human situation, but this may not be the case. It may simply reflect the prior sex biases in experimenting with drugs of abuse.
Although no animal model of addiction fully emulates the human condition, animal models do permit the investigation of specific elements of the drug addiction process. Thus, animal models are most likely to have construct or predictive validity when the model mimics only the specific signs or symptoms associated with the psychopathological condition (Geyer and Markou, 1995). Such elements can be defined by models of different systems, models of psychologic constructs such as positive and negative reinforcement, models of actual symptoms of addiction outlined by psychiatric nosology, and models of different stages of the addiction cycle (Koob and Le Moal, 1997; Koob et al., 1998).
In the present review, the animal models are organized by the stage of the addiction cycle that they most likely represent. However, it is critical to note that the particular behavior that is being studied in an animal model may or may not be symptomatic of the disorder and must be defined objectively and observed reliably. Indeed, the specific behavior may be found in both pathologic and nonpathological states but still have predictive validity. A good example of such a situation is the widespread use of drug reward as an animal model of the addiction process. Drug reward does not necessarily lead to addiction (e.g., the social drinking of alcohol), but the self-administration of alcohol has major predictive validity for the initiation and maintenance of drug use or the binge/intoxication stage of addiction, and it is difficult to imagine addiction without alcohol reward. A summary of the research results on sex differences in these stages of addiction for humans and rodents is found in Table 1. A discussion of the studies that contributed to the results that are presented in Table 1 follows.
TABLE 1.
Sex differences in addiction to different classes of drugsa
| Drug |
Species |
Stage of Addiction Cycle | ||
|---|---|---|---|---|
| Binge/Intoxication |
Withdrawal/Negative Affect |
Preoccupation/Anticipation |
||
| Alcohol | Humans | • Escalation of use F > M (qualitative) | • Negative affect F > M (qualitative) | • Stress or anxiety-induced relapse F > M (qualitative) |
| • Amount of intake M > F (quantitative) | ||||
| • Incidence M > F (population) | ||||
| Rodents | • Amount of intake F > M (quantitative) | • Withdrawal symptoms M > F (quantitative) | • Stress-induced reinstatement F > M (qualitative) | |
| • In some studies intake does not differ F = M | ||||
| Cocaine | Humans | • Escalation of use F > M (qualitative) | • Negative affect F > M (qualitative) | • Stress-induced relapse F > M (qualitative) |
| • Amount of intake F > M (quantitative) | • Cue-induced relapse F > M (qualitative) | |||
| • Incidence M > F (population) | • Cue-induced craving F > M (qualitative) | |||
| Rodents | • Escalation of use F > M (qualitative) | • Withdrawal symptoms F > M (quantitative) | • Stress-induced reinstatement F > M (qualitative) | |
| • Amount of intake and motivation F > M (quantitative) | • Cue-induced reinstatement F > M (qualitative) | |||
| • Incidence F > M (population) | ||||
| Opiates | Humans | • Incidence M > F (population) | ||
| Rodents | • Reward/conditioned place preference F > M (quantitative) | • Symptoms/duration of symptoms M > F (quantitative) | ||
| • Acquisition of self-administration F faster than M (qualitative) | • Acoustic startle as withdrawal index M = F (quantitative) | |||
| • Motivation F > M (quantitative) | ||||
| Nicotine | Humans | • Stress promotes initiation in women (qualitative) | • Negative affect F > M (qualitative) | • Higher cortisol predicts relapse in women (qualitative) |
| • Women acquire self-administration at lower doses than men (quantitative) | • Lower cortisol and craving predict relapse in men (qualitative) | |||
| • Amount of intake F > M (quantitative) | • Stress promotes relapse in women (qualitative) | |||
| Rodents | • Stress promotes initiation in females and not males (qualitative) | • Stress and anxiety F > M (quantitative) | • No sex difference in stress-induced reinstatement | |
| • Greater physical signs of withdrawal F > M (quantitative) | • Stress promotes relapse in females (qualitative) | |||
| Cannabinoids | Humans | • Women report enhanced subjective ratings in response to smoked cannabis and progress from use to disordered use more rapidly than men (qualitative) | ||
| Rodents | • F acquire faster than M (quantitative) | • Anxiogenic symptoms F > M (qualitative); Anxiolytic symptoms M > F (qualitative); No sex differences in precipitated withdrawal | • Cue-induced reinstatement F > M (qualitative) | |
| • Amount of intake F > M (quantitative) | ||||
For summarizing information presented in this article, see text for references.
A. Sex Differences in the Binge/Intoxication Stage
1. Clinical Evidence.
Historically, men drink larger amounts of alcohol than women, but the data indicate that consumption for men and women is becoming more similar and has been for the last several decades (Keyes et al., 2008). Indeed, Keyes et al. (2008) found that among the oldest cohort of women (born in 1913–1932), only 2% reported a lifetime prevalence of frequent binge drinking, whereas in the youngest cohort of women (born in 1968–1984), 16% reported a lifetime prevalence of frequent binge drinking. Furthermore, among the oldest cohort, the odds of binge drinking in men was 10.55-times higher than in women, but for the youngest cohort, the odds of binge drinking in men was 2.66-times higher than in women (Keyes et al., 2008).
Differences in the drinking patterns of women and men continued to narrow between 2002 and 2012. Among respondents aged 12 and older, there were increases in the percentages of females but not males who were current drinkers and binge drinkers. Drinking days per month increased for females and decreased for males (Keyes et al., 2010). Sex differences in the patterns of alcohol drinking behavior in humans emerge by the end of adolescence (around 17 years of age) and persist across adulthood (Substance Abuse and Mental Health Services Administration, 2007).
Both social factors and biologic mechanisms have been argued to contribute to sex differences in alcohol drinking patterns that emerge at puberty (for review, see Witt, 2007). For nicotine, the evidence indicates that stress plays a major role in the initiation of use in women but not in men (Torres and O’Dell, 2015).
For other drugs of abuse, more men use and are addicted to opiates (Lee and Ho, 2013) as well as other drugs (Substance Abuse and Mental Health Services Administration, 2007). However, clinical reports suggest that for all substances, women who become addicted progress through the landmark stages from initial use to dependence at a faster rate than men (Kosten et al., 1993; Brady and Randall, 1999). For cannabis, women report enhanced subjective ratings compared with men, and this may contribute to the more rapid progression to disordered cannabis use (Cooper and Haney, 2014). This phenomenon has been termed “telescoping,” and it was first described for alcoholism in women (Piazza et al., 1989) but has also been described for cocaine (reviewed in Becker and Hu, 2008) as well as cannabis use (Cooper and Haney, 2014).
We note that whether women exhibit greater telescoping for alcoholism than men remains controversial (Keyes et al., 2010; Sharrett-Field et al., 2013), but this highlights the need for a better understanding of what it means for there to be sex differences in “telescoping.” The initial report of telescoping considered the onset and time course of alcohol dependence in men and women in a treatment program. This has been replicated a number of times, although not all studies have found evidence of greater telescoping in a treatment-seeking population of women compared with men (for a summary, see Diehl et al., 2007).
Indeed, a recent analysis of evidence of telescoping using data from two United States national surveys found no evidence that women exhibit a shorter period of time from first use to dependence in the general population, but at the same time they reported a convergence in the overall hazard of alcohol dependence for men and women (Keyes et al., 2010). The discrepancy between an analysis of the general population and a treatment-seeking population can be explained by considering the mechanisms that influence the expression of this sex difference. There is evidence for 1) population differences in the susceptibility to alcoholism, in which more men than women are at risk, and 2) sex differences in the rate of progression from use to addiction within the most vulnerable population, with women progressing more rapidly. When the general population is considered, the effect of the larger population difference can be hypothesized to obscure the qualitative difference in the rate of progression to addiction.
The multiple types of sex differences can also obfuscate the differences between men and women. This points out the importance of carefully considering how a study is designed and what question is being asked. In addiction, we know that for both men and women only a subgroup is likely to end up being addicted. For questions of treatment and prevention, it is this at-risk population that needs to be studied.
2. Animal Models.
Animal models of the binge/intoxication stage of addiction can be considered as measuring acute drug reward/reinforcement, as well as the animal’s motivation to take the drug. A reward can be defined as a positive reinforcer with some added positive hedonic value, such as pleasure. Positive reinforcement is represented by any event that increases the probability of an operant response. Animal models of positive reward and reinforcement are extensive and well validated and include intravenous or intracranial drug self-administration, place conditioning, and states of lower brain reward thresholds (Shippenberg and Koob, 2002). Included here are measures such as the rate of acquisition of drug taking, escalation of drug taking, and motivation to self-administer drugs.
Some models also examine the preference for drugs compared with other natural rewards, such as food, or the rate of escalation of motivation for drugs (Roberts et al., 1989; Perry et al., 2013b, 2015). Brain reward thresholds are measured by intracranial self-stimulation methodology, in which animals press a lever to obtain electrical stimulation of the medial forebrain bundle.
a. Ethanol.
Nonhuman primates show similar sex differences as humans. Male cynomolgus monkeys that are given limited access and unlimited access to alcohol drink more than females and attain higher blood alcohol levels (Vivian et al., 2001). However, female rodents drink more than males (Eriksson and Pikkarainen, 1968; Hutchins et al., 1981; Li and Lumeng, 1984; Lancaster and Spiegel, 1992; Lancaster et al., 1996; Almeida et al., 1998; Juárez and Barrios de Tomasi, 1999; Blanchard et al., 1993; Walker et al., 2008; Maldonado-Devincci et al., 2010; Gamsby et al., 2013).
Studies of sex differences in the acquisition of ethanol self-administration in rats have only been possible in selectively bred rats that prefer ethanol. In these rats, females exhibited greater intake than males during the first 10 days, but after that time, total intake was not different between males and females (Moore and Lynch, 2015). In the Wistar rat, there are also sex differences in the rewarding versus aversive properties of ethanol that are dose dependent, with females displaying enhanced sensitivity to the rewarding effects of ethanol relative to males (Torres et al., 2014).
Typically, studies of alcohol consumption in rats have used the two-bottle choice method to examine preference for alcohol over a 24-hour period. Female rats of various strains show greater alcohol intake and preference in two-bottle choice testing (Li and Lumeng, 1984). When measuring the pattern of responding, these early studies also showed that females did not decrease intake as much as males when the alcohol concentration was increased (Lancaster and Spiegel, 1992; Meliska et al., 1995). Note though that there are some studies that do not see a sex difference in ethanol intake in adult rats (van Haaren and Anderson, 1994; Schramm-Sapyta et al., 2014; Varlinskaya and Spear, 2015).
Developmentally in rodents, there is some evidence of a female/male difference during the period of early postpuberty in rats, in which males drink more alcohol than females (Lancaster et al., 1996; Varlinskaya et al., 2015). In mice, the emergence of greater alcohol consumption in adult females was not limited to a specific developmental period but was present across adolescence and adulthood (Tambour et al., 2008).
The neonatal estrogenization of females, which effectively confers a male phenotype on a genetically female brain (Patchev et al., 1995), reduced ethanol intake compared with intact female rats and resulted in patterns of drinking that were similar to those displayed by intact male rats (Almeida et al., 1998). These results indicate that sex differences in alcohol drinking and alcohol drinking patterns may be at least partially attributable to biologic differences in the female rat brain that arise because of the developmental hormonal milieu.
b. Cocaine and other stimulants.
Female rodents escalate drug use more rapidly than do males, consistent with what is reported in humans (Becker and Hu, 2008; Anker and Carroll, 2011; Becker et al., 2012). Female rats acquire cocaine taking more rapidly than males, progress to addictive-like behavior more rapidly than males, and are more highly motivated to take cocaine than are male rats (Becker and Hu, 2008; Perry et al., 2013b, 2015). In both males and females, the formation of a preference for cocaine over tasty pellets is associated with reduced cocaine-induced dopamine overflow in the nucleus accumbens (Perry et al., 2015). Furthermore, there are also population differences in rats, but in this case, more females than males are likely to develop a preference for cocaine over tasty pellets (Perry et al., 2013b, 2015). Whether this is a difference between alcohol and cocaine (or tasty pellets) or a species difference remains to be determined.
As reviewed recently (Becker et al., 2012), female rats develop a conditioned place preference (CPP) for lower doses of cocaine than do males, whereas both sexes show equivalent CPP at higher doses of cocaine. The reinstatement of CPP is also more pronounced in females at higher cocaine doses.
Female rats also “binge” longer and take more cocaine when allowed access for 7 days than do males (Lynch and Taylor, 2004). However, once females develop an “addicted” phenotype, their behavior looks very similar to that of males (Ramôa et al., 2013; Perry et al., 2015).
c. Opiates.
There are sex differences and strain differences in the rewarding properties of morphine, but female rodents generally also show more opioid reward than male rodents. In Wistar rats, low doses of morphine are more rewarding to females than to males (Karami and Zarrindast, 2008). In Sprague-Dawley rats, however, there is no difference in morphine-induced CPP between males and females at lower doses, but females continue to display CPP at high doses of morphine that males do not prefer (Cicero et al., 2000). Females acquire morphine and heroin self-administration faster and show a higher motivation to self-administer morphine and heroin than do males (Lynch and Carroll, 1999; Cicero et al., 2003; Roth et al., 2004). Thus, sex differences in the rewarding value of morphine vary according to the strain of rat and dose that is used to test CPP.
d. Nicotine.
As was seen in women, stress promotes the initiation of nicotine intake in female rats (Torres and O’Dell, 2015). Furthermore, female rats acquire nicotine self-administration at lower doses than do males and display higher rates of self-administration then males (reviewed in Torres and O’Dell, 2015).
e. Cannabinoids.
In one study, the cannabinoid-1 (CB1) receptor agonist WIN55,212-2 was tested in three strains of rats. Female Long-Evans and Lister Hooded rats but not Sprague-Dawley rats acquired stable responding more rapidly and took more WIN55,212-2 than did male rats of the same strains (Fatttore et al., 2007). Thus, in two of the three strains, females exhibited greater intake of the CB1 agonist.
B. Sex Differences in the Withdrawal/Negative Affect Stage
1. Clinical Evidence.
Women have greater sensitivity to ethanol- or ethanol withdrawal-induced harm compared with men (Hommer, 2003). In adolescent humans, high-dose drinking is associated with elevated negative affect, and such negative mood states may take longer to resolve for girls than for boys following heavy drinking episodes (Bekman et al., 2013).
Sex differences also exist with regard to both the nature of the neuroadaptations to ethanol during the development of dependence (as reflected in a withdrawal state) and the neurotoxic consequences of alcohol dependence. As noted above, women exhibit more rapid development of alcoholic liver disease and neurotoxicity (Norton et al., 1987; Lishman et al., 1987; Hommer, 2003; Mann et al., 2005).
Women exhibit greater symptoms on motivational measures of withdrawal in the withdrawal/negative affect stage with psychostimulant drugs. For example, women smokers report increased negative affect during withdrawal and have increased levels of cortisol (Hogle and Curtin, 2006). The self-report did not appear to result from experiencing an exacerbated initial negative response to stressors in their environment but instead appeared to reflect their overall affective experience relating to their ability to recover from or otherwise effectively regulate their negative affective response (Hogle and Curtin, 2006). These results suggest that it may be particularly important to overcome deficits in emotional regulation that occur during acute nicotine withdrawal in women (Hogle and Curtin, 2006). Female smokers may have less success in smoking cessation, thus explaining the narrowing of the male/female ratio of smokers in the 1990s (Perkins et al., 1999).
2. Animal Models.
The withdrawal/negative affect stage is measured in tests of anxiety-like responses, decreases in pain thresholds, elevations in reward thresholds, conditioned place aversion (versus CPP) to either spontaneous or precipitated withdrawal from chronic drug exposure, and extended access- or dependence-induced increases in drug-seeking behavior during withdrawal. Rodents will increase intravenous or oral self-administration of drugs with extended access to the drugs and during withdrawal from the dependent state, measured both by an increased amount of drug administration and working exponentially harder to obtain the drug in progressive-ratio schedules of reinforcement. Such increased self-administration in dependent animals has been observed with cocaine, methamphetamine, nicotine, heroin, and alcohol (Ahmed and Koob, 1998; Ahmed et al., 2000; Roberts et al., 2000; Breese et al., 2005; Kitamura et al., 2006; O’Dell et al., 2007).
a. Ethanol.
From the perspective of actual signs and symptoms of withdrawal, males generally show a greater withdrawal response and slower recovery from acute alcohol withdrawal (Varlinskaya and Spear, 2004; Reilly et al., 2009; Carroll and Anker, 2010). For example, male rats, compared with females, that were withdrawn from alcohol had greater physical signs of withdrawal, such as greater seizure susceptibility (Devaud and Chadda, 2001). Perhaps more relevant, male rats also showed greater motivational signs of withdrawal, such as anxiety-like responses in the social interaction test (Varlinskaya and Spear, 2004) and elevated plus maze after one cycle of exposure to a liquid diet (Overstreet et al., 2004). Males showed greater increases in acoustic startle responses than either intact female rats or ovariectomized female rats (Reilly et al., 2009), and males showed greater hangover-like anxiety-like responses (Varlinskaya and Spear, 2004). Chronic ethanol exposure/withdrawal results in modestly elevated ethanol intake in male HAP-2 mice. In contrast, HAP-2 females did not show changes in ethanol intake (Lopez et al., 2011).
Ethanol withdrawal increases plasma corticosterone in male but not female rats (Janis et al., 1998), whereas plasma and brain levels of 3α,5α-tetrahydroprogesterone (allopregnanolone) are markedly higher in female rats than in male rats (Purdy et al., 1991). Female rats are also more sensitive to the anxiolytic-like and anticonvulsant effects of allopregnanolone during withdrawal (Devaud et al., 1999, 2003).
Ethanol withdrawal has also been shown to be reduced in female versus male mice, and this may be related to the increased activity of progesterone and endogenous neurosteroids (Tanchuck-Nipper et al., 2015). Increased levels of progesterone and allopregnanolone are associated with less intense alcohol withdrawal symptoms (Martin-Garcia and Pallares, 2005). Thus, the reduced aversive effects observed during ethanol withdrawal in female versus male animals may be attributable to anxiolytic-like effects of progesterone and allopregnanolone (Carroll and Anker, 2010). Consistent with these observations, an increase in acute ethanol withdrawal severity in female Withdrawal Seizure-Prone and Withdrawal Seizure-Resistant mice after adrenalectomy and gonadectomy confirms evidence that withdrawal from a high dose of ethanol can be modulated by anticonvulsant steroids that are produced in the periphery (Strong et al., 2009). In contrast, in this study, adrenalectomy and gonadectomy did not alter withdrawal severity in male Withdrawal Seizure-Prone and Withdrawal Seizure-Resistant mice.
Male and female mice that are exposed to the same number of repeated cycles of ethanol administration and withdrawal also exhibit differences in the expression of seizures. Males show the typical sensitization of seizures or a kindling response, which has been reported clinically as well as in animal models, but females do not. The reason for the lack of seizure sensitization in female mice remains to be elucidated but may be related to sex differences in alcohol’s effects on excitatory/inhibitory neurotransmission rather than to blood alcohol level differences (Veatch et al., 2007).
Female rats also show differential responses to the development of alcohol withdrawal and recover more quickly than male rats (Devaud and Chadda, 2001; Walls et al., 2012). Female rats were slower to develop dependence as measured by ethanol withdrawal seizure sensitivity and were quicker to recover compared with male rats (Devaud and Chadda, 2001). The faster recovery for females than males also includes the expression of tolerance to the hypnotic effects of ethanol and may involve some differential neuroadaptations in glutamatergic signaling (Walls et al., 2012).
Neurochemical studies have shown notable differences in γ-aminobutyric acid (GABA)-ergic and glutamatergic neurotransmission with chronic ethanol, such as a decrease in GABA α1 receptor subunits in the cerebral cortex in male rats but not in female rats (Devaud et al., 2000). Female rats but not male rats showed differences in the GluN2B subunit of the N-methyl-d-aspartate (NMDA) receptor in the cortex (Devaud and Morrow, 1999; Devaud and Chadda, 2001; Devaud et al., 2003).
A comparison of hippocampal slice cultures that were derived from postnatal day 2 (PND2) or PND8 rats showed that at PND8, the female hippocampus may be more sensitive to polyamine (targeting GluN2 NMDA receptor subunits)-induced neurotoxicity than the male hippocampus (Barron et al., 2008). Chronic intermittent exposure to ethanol at a very high dose (100 mM) in organotypic hippocampal slices from PND8 rats similarly elicited a toxic response in the CA1 and dentate gyrus subregions of the hippocampus that was greater in females than in males, which was exacerbated by exposure to pentylenetetrazol (Walls et al., 2013). Altogether, these data show that there are sex differences in the response to the toxic effects of ethanol in an immature state and differences in GluN2 subunit sensitivity during alcohol withdrawal. These may prove to be molecular targets that can be exploited to moderate alcohol withdrawal in a sex-specific manner (Sharrett-Field et al., 2013).
b. Cocaine and other stimulants.
Female rats with extended access to cocaine showed greater intake during escalation and showed significantly greater running during a 14-day abstinence period than males given 2-hour/day access to a running wheel (Peterson et al., 2014). Female rats also showed an “addicted phenotype,” self-administering more cocaine with greater dysregulation of cocaine intake with 24-hour access and greater motivation for cocaine after forced abstinence than male rats (Lynch and Taylor, 2004). Female rats but not male rats showed impairment in an object placement task during withdrawal from chronic amphetamine (Bisagno et al., 2003). Altogether, the results suggest that females may have an enhanced vulnerability to the neuroadaptive changes that lead to the binge/intoxication/escalation of drug intake but may be similar to males once intake is well-established.
c. Opiates.
Consistent with the alcohol data, physical signs of opioid withdrawal are more pronounced in male than in female mice (Diaz et al., 2005). Additionally, the duration of withdrawal symptoms during spontaneous withdrawal from opioids is longer in male than in female rats (Cicero et al., 2002). When acoustic startle is used as an index of withdrawal, there was no sex difference after acute morphine withdrawal in rats (Radke et al., 2015).
d. Benzodiazepines.
Sex differences in withdrawal from other drugs of abuse suggest a different pattern from that of alcohol and perhaps opioids. From the perspective of actual signs and symptoms of withdrawal, females generally show a greater withdrawal response than males. For example, female rats show greater “physical” withdrawal than male rats from pentobarbital (Suzuki et al., 1992) and methaqualone (Suzuki et al., 1988). Additionally, diazepam-dependent female rats, compared with diazepam-dependent male rats, showed more intense flumazenil-precipitated physical withdrawal and motivational withdrawal (i.e., vocalizations; Sloan et al., 2000).
e. Nicotine.
In a mouse study, continuous nicotine exposure in the drinking water produced an anxiogenic-like response in the elevated plus maze in females but failed to produce an anxiogenic-like response in males during withdrawal (Caldarone et al., 2008). Female rats showed greater adrenocorticotropic hormone levels during nicotine withdrawal. Corticosterone increased only in females in response to nicotine after continuous nicotine exposure, and corticosterone responses were increased in females after the precipitation of withdrawal (Gentile et al., 2011). In other studies, female rats exhibited greater symptoms of nicotine withdrawal, including an exaggerated stress response, compared with males (Torres and O’Dell, 2015).
Another exception to the enhanced sensitivity of females to motivational withdrawal is the observation in nonhuman primates that males are more responsive to the aversive effects of phencyclidine withdrawal, measured by the suppression of operant responding (Perry et al., 2006), a result similar to the one observed with ethanol discussed above.
f. Cannabinoids.
In a study of adolescent rats that received chronic dosing with Δ9-tetrahydrocannabinol (THC) on PND35–41, females showed a greater motivational withdrawal response, manifested by significant locomotor depression in female THC-treated rats during the drug abstinence period than in male THC-treated rats. There was also a significant anxiogenic-like effect in females during drug abstinence but an anxiolytic-like effect in males during drug abstinence (Harte-Hargrove and Dow-Edwards, 2012). Previous studies have also reported no sex differences in precipitated withdrawal (Marusich et al., 2014).
To summarize, sex differences during withdrawal exist, and females tend to exhibit greater symptoms of motivational withdrawal (quantitative sex difference), with the exception of alcohol and possibly opioids, in which females show less withdrawal but greater negative affect than males.
C. Sex Differences in the Preoccupation/Anticipation Stage
1. Clinical Evidence.
The clinical literature indicates that women generally show a greater propensity to relapse for most drugs of abuse compared with men, and there are shorter periods of abstinence between relapses (Kosten et al., 1993; Hudson and Stamp, 2011). Various hypotheses have been proposed, from increased withdrawal responses (see above) to increased reactivity to internal (emotional) cues (negative reinforcement) to increased sensitivity to external (drug-associated) cues (positive reinforcement; Hudson and Stamp, 2011). For alcohol, cocaine, and nicotine, stress-induced relapse is a greater risk for women than for men (Kennedy et al., 2013; al’Absi et al., 2015; Torres and O’Dell, 2015). Women also show a greater incidence of cue-induced craving and relapse for cocaine (Sinha et al., 2007; Kennedy et al., 2013). In one study of smokers, lower cortisol with craving in men predicted relapse, whereas higher cortisol predicted relapse in women (al’Absi et al., 2015). No studies to our knowledge have investigated sex differences in the causes of relapse for opiates or cannabinoids.
2. Animal Models.
Most of the animal models discussed above have predictive validity for some components of the addiction cycle (e.g., compulsive use, withdrawal, or craving) and are highly reliable. Animal models of withdrawal can be focused on either motivational constructs of withdrawal or physical/somatic signs. Animal models of conditioned drug effects (e.g., reinstatement and place preference) are successful in predicting the potential for conditioned drug effects in humans. However, for such constructs as craving, predictive validity is more problematic, largely because of the inadequate formulation of the concept of “craving” in humans (Markou et al., 1993; Sayette et al., 2000; Tiffany et al., 2000). Clearly, much remains to be explored about the face validity and predictive validity of unconditioned positive and negative motivational states and particularly the conditioned positive and negative motivational states associated with drug use, withdrawal, and relapse.
In preclinical research, there is enhanced drug-, cue-, and stress-induced reinstatement in animals models of alcohol and drug seeking in females compared with males (Anker and Carroll, 2010; Hudson and Stamp, 2011). Animal models of craving, representing the preoccupation/anticipation stage of drug addiction, involve the reinstatement of drug-seeking behavior after extinction training and subsequent exposure to the drugs themselves, cues/contexts that are paired with drug self-administration, or stressors (Self, 1998; Shaham et al., 2003). The latency to reinitiate responding or the amount of responding on the previously extinguished lever is hypothesized to reflect the motivation for drug-seeking behavior.
Stress-induced reinstatement most often involves the application of acute stressors that reinitiate drug seeking, such as foot shock, although more natural stressors should be used (Miczek et al., 2008). In rats with a history of dependence, protracted abstinence can be defined as a period after acute withdrawal has disappeared and is often accompanied by elevations in drug intake or drug-seeking behavior (e.g., 2–8 weeks postwithdrawal from chronic drug self-administration in rats). Protracted abstinence has also been linked to elevations of brain reward thresholds, increased sensitivity to cues associated with withdrawal (conditioned place aversions to opioids), and increases in the sensitivity to anxiety-like behavior (alcohol) that have been shown to persist after acute withdrawal symptoms have subsided in animals with a history of dependence (Stinus et al., 2000; Valdez et al., 2002; Skjei and Markou, 2003; Niikura et al., 2013). The stress-induced reinstatement of drug seeking and stress-induced reinstatement of anxiety-like states during protracted abstinence represent models of the persistent preoccupation/anticipation (craving) stage of the addiction cycle.
Male and female rodents differ in their stress response. Females exhibit greater basal and stress-induced glucocorticoid secretion and a greater stress response in the paraventricular nucleus of the hypothalamus than do males (for a detailed review, see Handa and Weiser, 2014). Estradiol enhances the stress response during the estrous cycle in rats and monkeys, whereas progesterone tends to inhibit the response. Sex differences in the stress response are mediated by both adult levels of gonadal hormones and developmental programming of the stress axis (Handa and Weiser, 2014).
As stated in Bangasser and Valentino (2014):
“Preclinical data demonstrates sex differences in several cellular and molecular mechanisms, including cell signaling, peptide expression, hormone release, receptor trafficking, synaptogenesis, and dendritic remodeling. What is remarkable is that at each level of analysis, sex differences exist that can be linked to increased endocrine, emotional, and/or arousal responses to stress in females compared to males” (p. 313).
Thus, sex differences in the stress response may impact results at many levels, from the molecular results to the behavioral effects of a treatment. In rats, stress enhances the acquisition of self-administration (Haney et al., 1995) and the reinstatement of drug seeking for several types of drugs of abuse in both males and females (Feltenstein et al., 2011; Buffalari et al., 2012)
a. Ethanol.
Very little work has been done on sex differences in reinstatement with alcohol. One study in mice used CPP and showed that a dose of 2.5 mg/kg induced CPP in both males and females but with some age differences. This dose produced CPP in female mice at both early and late adolescence and in male mice only at early adolescence but also in males at a lower dose (1.25 mg/kg; Roger-Sanchez et al., 2012). In a study of the alcohol deprivation effect (ADE), in which forced abstinence results in a rebound increase in alcohol intake, in adult rats of both sexes that began alcohol consumption during preadolescence or adolescence, female but not male rats increased ethanol intake after the second and third deprivation (showing an ADE), regardless of the age of onset of alcohol drinking (Garcia-Burgos et al., 2010).
A similarly greater ADE in female versus male rats was observed in a strain with an inborn depressive-like phenotype (Vengeliene et al., 2005), but a study of Indiana alcohol-preferring P rats showed no difference between male and females in the ADE (McKinzie et al., 1998). Chronic circadian desynchrony that was induced by repeated light/dark phase shifts resulted in the sex-specific modulation of voluntary ethanol intake in High-Alcohol-Drinking-1 rats, reducing ethanol intake in males while slightly increasing intake in females (Clark et al., 2007). In summary, there are few studies that have compared ethanol relapse models in male and female rodents, but there is the suggestion of an enhanced ADE and enhanced response to a stressor in females.
b. Cocaine and other stimulants.
The reinstatement of cocaine seeking in rats is used as a model of relapse in humans. With limited access to intravenous cocaine self-administration, female rats show more nonreinforced responding and a prolongation of extinction (Lynch et al., 2005; Fuchs et al., 2005; Kosten and Zhang, 2008). Under similar conditions, female rats also responded more to cocaine-induced reinstatement (Lynch and Carroll, 2000; Anker et al., 2009). In studies that used prolonged access to cocaine (24-hour access in discrete trials), female rats also showed enhanced responding on a progressive-ratio schedule during reinstatement after abstinence without extinction compared with males (Lynch and Taylor, 2004).
Sex differences in the effects of stressors on the reinstatement of cocaine seeking are also seen in rats, with females presenting greater stress-induced reinstatement of cocaine seeking than males, as is seen in humans. For stress-induced reinstatement, females with limited access to cocaine self-administration showed greater yohimbine-induced reinstatement, and allopregnanolone blocked yohimbine-induced reinstatement in female but not male rats (Anker and Carroll, 2010). By using intracerebroventricular CRF administration as the stressor in rats with limited access to intravenous cocaine, female high-responder rats responded more for cocaine than males (Buffalari et al., 2012).
With limited access to methamphetamine, female rats also exhibited greater methamphetamine- and cue-induced reinstatement compared with males. Oxytocin suppressed methamphetamine-induced reinstatement in both sexes and cue-induced reinstatement in females only (Cox et al., 2013).
c. Opiates.
We could find no studies of sex differences in relapse to opiates.
d. Nicotine.
Although studies in rodents have found that stress promotes nicotine intake and relapse in female rats compared with male rats (Torres and O’Dell, 2015), no sex differences in stress-induced reinstatement have also been reported (Feltenstein et al., 2012).
e. Cannabinoids.
With limited access to a synthetic cannabinoid, female rats responded more to drug and exhibited greater cue-induced reinstatement than male rats (Fattore et al., 2010).
The most consistent finding across species is that females are more susceptible to stress- and cue-induced relapse. There may be variations in the extent of the sex difference, depending on the conditions and specific drug, but the role of stress and how it may differentially impact males and females during recovery from addiction is important to consider in treatment programs.
D. Effects of Hormones during the Reproductive Cycle in Females on Stages of Addiction
The menstrual cycle in humans and estrous cycle in rats and mice are not completely analogous (for a more complete discussion, see Becker et al., 2005). The human menstrual cycle consists of follicular, periovulatory, and luteal phases. During the 10- to 12-day follicular phase, estradiol is secreted from the ovary as the follicle develops, with concentrations of estradiol increasing day by day. During the 2- to 4-day periovulatory phase, there is a rapid increase in estradiol that triggers the luteinizing hormone surge that induces ovulation. The luteal phase lasts 10–12 days and is characterized by the release of relatively high concentrations of both estradiol and progesterone from the remnant of the follicle that is retained by the ovary (the corpus luteum). Menstruation occurs at the end of the luteal phase as hormone levels fall and overlaps with the beginning of the follicular phase (Table 2).
TABLE 2.
Equivalent phases of the reproductive cycle in women and rats/mice
| Women | ||
| Follicular |
Periovulatory |
Luteal |
| Estradiol secretion from ovaries as follicle develops |
Rapid increase in estradiol that triggers luteinizing hormone surge that induces ovulation |
Release of relatively high concentrations of both estradiol and progesterone from the corpus luteum |
| Rats/Mice | ||
| Metestrus/Diestrus |
Proestrus/Estrus |
No equivalent phase |
| Estradiol secretion from ovaries as follicle develops | Rapid increase in estradiol that triggers luteinizing hormone surge that induces progesterone release and ovulation | |
The female rat or mouse does not have a functional corpus luteum and so does not have a luteal phase. The estrous cycle consists of a 3- to 4-day follicular phase (termed metestrus or diestrus), followed by the periovulatory period (proestrus) when estradiol peaks, followed by an increase in progesterone. Behavioral receptivity occurs coincident with ovulation during estrus. By the onset of behavioral estrus, estradiol and progesterone levels have fallen. The estrous cycle terminates with ovulation after the brief increases in estradiol and progesterone that last less than 12 hours. The estrous cycle then begins again with the next follicular phase (Table 2). This means that it is not possible to find events during the estrous cycle that are analogous to the luteal or premenstrual phases because rats and mice do not have these phases. Nevertheless, the hormones estradiol and progesterone are the same for all mammalian species, and it is possible to model the effects of these hormones on drug taking in nonhuman animals.
1. Clinical Evidence.
Nonalcoholic women, in some studies, show changes in ethanol intake during the course of the menstrual cycle (Podolsky, 1963; Sutker et al., 1983; Harvey and Beckman, 1985; Mello et al., 1990; McLeod et al., 1994; Evans and Levin, 2011; Kiesner, 2012; DiMatteo et al., 2012) but not in other studies (Tate and Charette, 1991; Terner and de Wit, 2006). The most convincing data are found in studies that reported that higher premenstrual or menstrual distress and negative affective states were associated with higher alcohol intake (Table 3). The data support the hypothesis that women with premenstrual syndrome have higher levels of alcohol use/abuse (Mello et al., 1990; Svikis et al., 2006; Evans and Levin, 2011; Kiesner, 2012).
TABLE 3.
Effects of the reproductive cycle and ovarian hormones on drugs of abuse and addictiona
| Drug |
Species |
Stage of Addiction Cycle | |
|---|---|---|---|
| Binge/Intoxication |
Withdrawal/Negative Affect & Preoccupation/Anticipation |
||
| Alcohol | Humans | • Premenstrual syndrome-related negative affect increases intake | • Less stress-induced craving during luteal phase |
| • Enhanced positive effect in luteal phase | |||
| Rodents | • Intake greatest during diestrus | • Progesterone converted to allopregnanolone modulates withdrawal symptoms | |
| • Preference lowest during proestrus/estrus | |||
| • Ovariectomy or pregnancy decreases preference | |||
| Cocaine | Humans | • Subjective effects greatest during follicular phase | • High progesterone associated with less stress- and cue-induced craving than a low progesterone group |
| Rodents | • Ovariectomy attenuates self-administration | • Estradiol enhances cocaine-induced reinstatement and augments cocaine seeking | |
| • Estradiol enhances intake and motivation | • Females in estrus exhibit greater cocaine-induced responding | ||
| • Motivation for cocaine greater in proestrus/estrus than in diestrus | • Progesterone and allopregnanolone block cocaine-primed reinstatement | ||
| Opiates | Humans | • No studies on reinforcing effects of opiates across reproductive cycle | • No studies of withdrawal or relapse across reproductive cycle |
| Rodents | • Estradiol enhances intake and motivation | • No studies of withdrawal or relapse across reproductive cycle | |
| Nicotine | Humans | • Reduced craving during luteal phase | • Meta-analysis found greater withdrawal during the luteal phase than during the follicular phase |
| • Women with higher progesterone in luteal phase have blunted subjective pleasure from smoking | • Enhanced effect of nicotine cues during follicular phase | ||
| Rodents | • Estradiol is necessary for initiation of nicotine intake in females | • Estradiol is necessary for the effect of stress on relapse in females | |
| Cannabinoids | Humans | • No hormone effect on use or negative affect | • No information available |
| Rodents | • No information available | • Ovariectomy attenuates cue-induced reinstatement | |
Summarizing information presented in this article, see text for references.
In one study, women with a premenstrual syndrome-like pattern of affective responses reported (retrospectively) higher levels of distilled beverage alcohol use, a less negative affective response associated with alcohol use, and lower levels of sleep changes in relation to alcohol use, suggesting a form of negative reinforcement (Kiesner, 2012). In another study with family history-positive individuals, in which subjects with premenstrual syndrome were excluded, there was a modest increase in the positive subjective response to alcohol during the luteal phase, lending support to previous clinical studies and suggesting that some women drink more alcohol in the late luteal phase to alleviate dysphoric symptoms, particularly women with moderate to severe premenstrual symptoms (Evans and Levin, 2011).
The subjective effects of cocaine have been reported to be more intense during the follicular phase relative to the luteal phase of the menstrual cycle in women (Sofuoglu et al., 1999; Evans et al., 2002). Men and women have the same response to cocaine in terms of “good drug effect.” Only during the luteal phase are sex differences seen, when men have a greater positive response to smoked cocaine than women in the luteal phase (Evans and Foltin, 2010). Hormonal status also plays an important role during abstinence that can lead to relapse. Clinical studies have shown a relationship between higher plasma levels of progesterone and the blunting of stress-induced cocaine craving in women (Sinha et al., 2007).
In contrast, studies of the effect of the menstrual cycle on the subjective response to nicotine have reported reduced craving during the luteal phase (Allen et al., 2015). A related finding is that there was an enhanced effect of nicotine cues during the follicular phase (Franklin et al., 2015). In another study, women with higher progesterone in the luteal phase reported more blunted subjective pleasure from smoking than women with lower progesterone (Goletiani et al., 2015). For smoking cessation, greater withdrawal has been observed in human females during the luteal phase (O’Hara et al., 1989). In a recent meta-analysis of smoking cessation studies, greater withdrawal was found during the luteal phase compared with the follicular phase (Weinberger et al., 2015). Thus, the reduced subjective effect of nicotine during the luteal phase may contribute to less saliency of nicotine-related cues, but that does not necessarily correspond to reduced withdrawal symptoms.
For cannabinoids, no effect of the menstrual cycle was found on use or negative affect (Moran-Santa Maria et al., 2014). Further work is needed with both cannabinoids and opiates.
2. Animal Models.
a. Binge/intoxication.
i. Ethanol.
There is evidence of a reproductive cycle-dependent effect on alcohol intake in nonhuman species, but the hormonal relationships may be different from those in humans. In female Macaque monkeys, the animals self-administered significantly more alcohol at mid-cycle than during menstruation or during the luteal phase, with the lowest self-administration during menstruation (Mello et al., 1986). The authors concluded that the data did “not support the hypothesis, derived from clinical data, that alcohol self-administration increases at the premenstrum in comparison to other menstrual cycle phases” (Mello et al., 1986).
Animal models for ethanol consumption or preference usually rely on establishing the behavior with saccharin-sweetened solutions, so the rate of acquisition of ethanol consumption cannot be evaluated independently. The estrous cycle affects preference for ethanol in rodents, in which pregnant and lactating rats exhibited a reduced preference for ethanol, and preference was lowest during proestrus, but intact females had a greater preference for ethanol than ovariectomized rats (Forger and Morin, 1982).
In intact females, higher drinking was observed during the diestrous stage of the estrous cycle (Forger and Morin, 1982; Roberts et al., 1998; Witt, 2007). The highest level of ethanol intake occurred in diestrus in female rats that lever-pressed for ethanol with gonadotropin-releasing hormone-synchronized estrous cycles (Roberts et al., 1998). Ethanol bout sizes also were significantly greater during estrus and diestrus (when circulating estradiol and progesterone are lowest) compared with proestrus (when both hormones are elevated; Ford et al., 2002). Possibly consistent with these observations, allopregnanolone decreases ethanol drinking in male mice but has no effects in female mice (Sinnott et al., 2002). Additionally, as noted above, ovariectomized female rats presented drinking that is comparable to that of male rats, and estradiol or estradiol + progesterone replacement did not rescue the decrease in drinking (Almeida et al., 1998).
Altogether, the data suggest that there are sex differences in alcohol drinking in animal models. These differences are attributable to both organizational sex differences in the brain and hormonal status, possibly related to neurosteroids, albeit in a sex-specific way. Drinking can vary with the menstrual cycle in humans and the estrous cycle in animals, but the exact hormonal milieu that conveys this variability remains to be determined. Additionally, the effects of the estrous cycle in rodents, even when estrous cycles are synchronized, remain modest (in the range of 35% at maximum; Roberts et al., 1998), suggesting that in situations of free-running cycles, any variance that is attributable to the estrous cycle will be masked by overall sex differences.
ii. Cocaine and Other Stimulants.
Estradiol administration in ovariectomized females affects behaviors that are induced by psychomotor stimulants, including self-administration (Becker et al., 2012). For example, ovariectomy attenuates cocaine self-administration, and estradiol treatment is sufficient to reinstate the acquisition of cocaine self-administration in ovariectomized rats (Lynch et al., 2001; Becker et al., 2012). Furthermore, castration in male rats does not affect the acquisition of cocaine self-administration, and a dose of estradiol that enhances self-administration in female rats has no effect on cocaine self-administration in males (Jackson et al., 2006).
Female rats will work harder for cocaine during the estrous phase of the cycle than during other phases of the cycle. Estradiol enhances motivation in ovariectomized rats, and females work harder than males for cocaine at moderate doses (Roberts et al., 1989; Becker and Hu, 2008; Cummings et al., 2011). In other studies, estradiol treatment increases the initial binge period and enhances cocaine self-administration (Lynch and Taylor, 2005).
The strength of CPP in females also depends on ovarian hormones. Ovariectomized females show a shift in the dose-response curve for CPP for cocaine or amphetamine, and replacement with estradiol and progesterone is necessary for the reinstatement of CPP in rats (reviewed in Becker et al., 2012).
The finding that the motivation to self-administer cocaine is greater during the estrous phase of the cycle may be related to the finding that stimulant-induced dopamine release is enhanced during estrus relative to diestrus (Becker and Ramirez, 1980; Becker and Cha, 1989). In contrast, we recently reported that cocaine intake and cocaine-induced dopamine overflow in the nucleus accumbens do not vary with the estrous cycle when animals are administering cocaine daily in a choice paradigm, but their intake of palatable food pellets varies with the estrous cycle (in the same animals). These results suggest that estradiol may be involved in the initial acquisition of drug-taking behavior, but once the behavior is well-established, it is no longer under hormonal regulation (Perry et al., 2013a, 2015).
Estradiol enhances the initial motivation to self-administer drugs of abuse, such as cocaine, and this is clearly attributable to sexual differentiation of the brain during early life (Perry et al., 2013a). Importantly, once addiction-like behavior is established, estradiol does not continue to play as important a role (Perry et al., 2015). This is similar to the effect of estradiol in establishing maternal behavior, in which estradiol is necessary for the initial expression of the behavior, but once established, maternal behavior is maintained by cues from the pups. Thus, it seems likely that estradiol in females is involved in exacerbating the rate of escalation of drug taking by increasing the reinforcing effects of many drugs of abuse during the initial binge/intoxication stage.
iii. Opiates.
Estradiol facilitates the acquisition of opioid self-administration in ovariectomized rats (Lynch and Carroll, 1999; Cicero et al., 2003; Roth et al., 2004).
iv. Nicotine.
In female rats, estradiol is necessary for the initiation of nicotine intake. Estradiol also plays a role in the effect to trigger relapse in females. Intact female rats exhibit greater anxiety-like behaviors and stress responses during nicotine withdrawal compared with ovariectomized rats (Torres and O’Dell, 2015).
b. Withdrawal/negative affect.
There are also modulating effects of development and ovarian hormones, indicating mechanistic sex differences and possibly explaining less alcohol withdrawal in females. Progesterone, from peripheral sources, can be converted to allopregnanolone in the brain to modulate withdrawal symptoms in females (Kaufman et al., 2010).
i. Psychostimulants.
Ovariectomized female rats with extended access using a discrete trials procedure failed to show greater motivation to take cocaine after extended access, and this effect was reversed by estradiol (Ramôa et al., 2013). Estradiol facilitated responding in a discrete-trials procedure during escalation and the motivation to take cocaine during abstinence (Ramôa et al., 2013).
c. Preoccupation/anticipation.
i. Psychostimulants.
On the first day of extinction training, females in estrus show greater responding in the absence of reward and show greater cocaine-induced reinstatement of cocaine seeking compared with males and proestrous and diestrous females (for review, see Becker et al., 2012). Estradiol enhances cocaine-induced reinstatement in ovariectomized animals and augments cocaine seeking (Feltenstein et al., 2011).
Similarly, a combination of cue- and yohimbine-induced reinstatement in limited-access intravenous cocaine self-administration showed a greater response in female rats, with a higher level during proestrus of the estrous cycle (Feltenstein et al., 2011). Additionally, the estrous cycle modulates the effects of stress, with proestrous females displaying the greatest stress-induced reinstatement of cocaine seeking (Anker and Carroll, 2010; Feltenstein et al., 2011).
However, in another study, female rats with limited access to cocaine responded more on the cocaine-paired lever and more during extinction and also showed more cocaine-primed reinstatement during estrus of the estrous cycle (Kippin et al., 2005). More specifically, males and females in all estrous cycle phases exhibited significant dose-dependent reinstatement of cocaine-induced responding, with females in estrus showing higher levels of reinstatement following 10 mg/kg cocaine relative to both males and females in other cycle phases (Kippin et al., 2005). Curiously, these authors also reported no cue-induced reinstatement during estrus and argued that female rats attribute less motivational significance to the cue during this stage of the estrous cycle (Fuchs et al., 2005).
Progesterone and allopregnanolone (the levels of which are low during the estrous phase in rats) both selectively blocked cocaine-primed reinstatement only in female rats, and the effects of progesterone were blocked by finasteride, a drug that blocks the conversion of progesterone to allopregnanolone (Anker et al., 2009), suggesting a role for progesterone in drug-primed cocaine reinstatement. Estradiol, in contrast, increased cocaine-primed reinstatement in limited-access conditions (Larson and Carroll, 2007). Estradiol also facilitated escalation with extended-access intravenous cocaine self-administration, and progesterone inhibited escalation (Larson et al., 2007).
Thus, drug (cocaine)-primed reinstatement is greater during phases of the estrous cycle when estradiol is elevated, but cue-induced reinstatement is blunted. One hypothesis is that the state of mesocorticolimbic dopamine activity is greatest during estrus (linked to low levels of progesterone and high levels of estradiol), and this enhances greater drug responding and as such differentially affects drug- versus cue-induced reinstatement. Indeed, the mesocorticolimbic dopamine pathway, at least at the level of the dorsal striatum, shows a peak response to amphetamine during proestrus and estrus (Becker and Cha, 1989) and greater basal levels in estrus (Xiao and Becker, 1994).
ii. Cannabinoids.
With limited access to a synthetic cannabinoid, female rats responded more to drug and exhibited greater cue-induced reinstatement than ovariectomized female rats, implicating estradiol in cannabinoid intake (Fattore et al., 2010).
IV. Sex Differences in the Neurobiology of Addiction
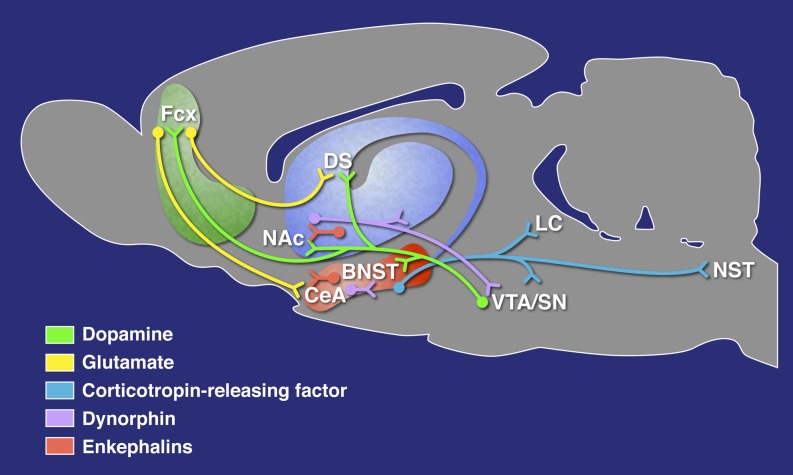
We will only provide a brief overview to provide a context for the sex differences described previously and refer the reader to recent reviews (e.g., Becker et al., 2012). The vast majority of research on the effects of abused drugs and neural changes that underlie dependence has focused on neurotransmission within the three stages of the addiction cycle and the functional domains therein. For the binge/intoxication stage, such neurotransmitters as dopamine and opioid peptides play key roles in the basal ganglia to facilitate drug reward, incentive salience, and habit formation (Fig. 2).
Fig. 2.
Sagittal section of a rodent brain showing the major neurotransmitter systems implicated in sex differences associated with drug addiction. The basal ganglia (blue) are implicated in the rewarding and incentive salience effects of the binge/intoxication stage, with inputs from the mesocorticolimbic dopamine system and opioid peptides. The extended amygdala (red) is implicated in the stress surfeit effects of the withdrawal/negative affect stage and contains the stress neurotransmitters corticotropin-releasing factor and dynorphin. The frontal cortex (green) is implicated in executive function deficits that are associated with the preoccupation/anticipation stage, with glutamatergic projections to the basal ganglia and extended amygdala. Fcx, frontal cortex; DS, dorsal striatum; NAc, nucleus accumbens; BNST, bed nucleus of the stria terminalis; CeA, central nucleus of the amygdala; LC, locus coeruleus; VTA, ventral tegmental area; SN, substantia nigra; NST, nucleus tractus solitarius.
Neurotransmission within the nucleus accumbens/dorsal striatum is heavily influenced by dopamine signaling that originates primarily from the ventral tegmental area and substantia nigra pars compacta. These brain regions contain multiple classes of local and projection neurons with diverse phenotypes, but we will focus herein on dopamine and endogenous opioids (e.g., enkephalins),.
As reviewed in Becker et al. (2012), the firing rate of dopamine neurons in the ventral tegmental area fluctuates over the course of the estrous cycle, with generally higher firing rates and more burst firing in estrous and diestrous female rats relative to those in proestrus. This is thought to be reflected in variations in extracellular dopamine concentrations that are seen with microdialysis across the estrous cycle (Xiao and Becker, 1994). Dopamine transporter expression and function also change over the reproductive cycle and after ovariectomy and hormone replacement, which may contribute to sex differences in extracellular dopamine concentrations.
Estradiol treatment in ovariectomized female rats enhances the cocaine-induced increase in dopamine in the dorsolateral striatum of females but not males (Cummings et al., 2014). There is a significantly lower cocaine-induced increase in dopamine in the nucleus accumbens in ovariectomized females compared with males with or without estradiol treatment (Cummings et al., 2014). A similar sex difference was reported for the dopamine response to amphetamine in the nucleus accumbens core (Gillies et al., 2014; Virdee et al., 2014) and dopamine response to nicotine in the ventral striatum in humans (Cosgrove et al., 2014). Munro et al. (2006) reported that men had greater dopamine release in the nucleus accumbens and presented greater subjective effects in response to amphetamine compared with women.
Male rats with lower nucleus accumbens dopamine were shown to be more impulsive and more likely to develop addiction-like behaviors (Belin and Everitt, 2008). Thus, the lower cocaine-induced dopamine response in the nucleus accumbens in females may play a role in the greater addiction-like behavior in female rats. Both male and female rats that developed addiction-like behavior exhibited a reduction of cocaine-induced dopamine in dialysate in the nucleus accumbens (Perry et al., 2015).
As discussed above, estradiol enhances the behavioral responses to and reinforcing effects of amphetamine and cocaine in female rats (reviewed in Becker and Hu, 2008). In addition to the effects of estradiol on dopamine release, although there are no sex differences in dopamine D2 receptor densities, estradiol rapidly downregulates D2 receptor binding in females but not males. In cell cultures, pretreatment with estradiol reduced the D2 receptor inhibition of adenylate cyclase activity and enhanced the D1 receptor activation of adenylate cyclase. Additionally, estradiol treatment decreased the expression of regulator of RGS9-2, a guanosine triphosphatase-activating protein associated with D2 receptor signaling (reviewed in Yoest et al., 2014).
For the withdrawal/negative affect stage, the stress neurotransmitters CRF and dynorphin play a key role in the extended amygdala to facilitate hormonal and behavioral responses to stressors. As noted above, female rodents exhibited greater basal and stress-induced glucocorticoid secretion and a greater stress response than male rodents. Although less studied than sex differences associated with the dopamine system, there is some evidence that CRF and dynorphin responses are greater in female rats than in male rats during acute withdrawal (Bradshaw et al., 2000; Blednov et al., 2006; Silva and Madeira, 2012; Torres et al., 2013; Garcia-Carmona et al., 2015). However, females are less sensitive to the analgesic effects of μ and κ opioids (Negus et al., 2002; Barrett et al., 2002).
For the preoccupation/anticipation stage, disruptions in glutamate or GABA neurotransmission play a key role in the disinhibition of both impulsivity (dorsal basal ganglia) and compulsivity (brain stress systems) via the medial prefrontal cortex, all leading to impairments in executive function. As such, there is some evidence of sex differences in these domains, with increased CRF-induced drug reinstatement (Buffalari et al., 2012), presumably reflecting a disinhibited CRF stress system that is particularly sensitive to κ opioid receptor antagonism (Deehan et al., 2012), and increased sensitivity to allopregnanolone in blocking methamphetamine-induced reinstatement (presumably reflecting changes in the sensitivity of GABAergic systems; Holtz et al., 2012). Notably, however, the decrease in ethanol withdrawal and facilitation of recovery from ethanol withdrawal appear to involve alterations in glutamatergic function (Devaud and Alele, 2004). Additionally, there is evidence that male rats may be more sensitive to a hypocretin-1 receptor antagonist in blocking cue-induced reinstatement (Zhou et al., 2012).
When using a cocaine self-administration/reinstatement model to examine neuronal activation with Fos expression in male and female rats, only Fos expression in the prelimbic cortex, nucleus accumbens shell, basolateral amygdala, and ventral subiculum were positively correlated with lever responding in response to conditioned cues across males and females. In contrast, females exhibited higher Fos expression than males in multiple brain regions, including the agranular insular cortex, dorsomedial caudate putamen, nucleus accumbens shell, ventral tegmental area, dorsal subiculum, and ventral CA1 and CA3 regions of the hippocampus. Although these findings indicate that cue-induced cocaine seeking and neuronal activation may be similar for males and females in key brain regions of the relapse circuit, sexually dimorphic Fos activation was shown to occur for the underlying neuronal substrates (Zhou et al., 2014). In light of this, particularly intriguing was a gene expression profile approach to the study of withdrawal seizure-prone and withdrawal seizure-resistant mice. A prominent common gene regulatory node in the medial prefrontal cortex that was activated during acute withdrawal for both sexes was the nuclear factor κB transcription factor, but the immune response was dramatically sexually dimorphic, with females presenting a proinflammatory gene expression profile and males presenting an anti-inflammatory gene expression profile (Wilhelm et al., 2014). The authors suggested that each stage of the addiction cycle is differentially influenced by sex versus genetic background, and they argued for the development of stage- and sex-specific therapies for alcohol withdrawal and relapse prevention. Altogether, these results suggest both quantitative and qualitative differences between males and females in key neurotransmitter systems associated with the stress component of the withdrawal/negative affect stage.
V. Summary
Significant sex differences in responses to drugs of abuse can be found in the human condition and in animal models. In humans, men engage in more drug taking and more alcohol consumption and present higher levels of misuse. However, the pronounced differences between men and women have been declining, and the misuse of drugs and alcohol in women is rapidly approaching that of men. The apparent population sex differences of addicted men and women that have been seen in the last century may be related more to cultural factors than to underlying biology. In adolescents, the sex difference in the use of drugs and alcohol has virtually disappeared. For some drugs, girls use them more than boys. This illustrates that drug use patterns in humans reflect both biological mechanisms and the social and legal constraints that our societies place on the use of drugs that can differentially affect men and women.
In animals, there are quantitative sex differences in the biological processes that are associated with addiction, and there may also be population and mechanistic sex differences. Female rats generally acquire the self-administration of drugs and alcohol more rapidly, escalate their drug taking with extended access more rapidly, show more motivational withdrawal, and where tested in animal models of “craving,” show greater reinstatement. The one exception is that female rats exhibit less motivational withdrawal from alcohol.
The basis for these quantitative sex differences appears to be organizational, in which estradiol-treated neonatal animals present the male phenotype and also depend on the activational effects of gonadal hormones, in which adult ovariectomized females resemble the male phenotype in most cases. For female humans and animals, differences within the menstrual cycle/estrous cycle can be observed but are relatively minor. In humans, there is some evidence of increased alcohol intake in females during the premenstrual stage of the menstrual cycle and increased withdrawal during the luteal phase of the menstrual cycle. For animals, there are modest effects of estrous cycle on drug- and alcohol-seeking behavior. Where observed, progesterone decreases drug-seeking behavior and estradiol increases drug-seeking behavior. These hormonal effects appear to be most prevalent during the acquisition of drug taking and less influential once compulsive drug taking is established. The decreased alcohol withdrawal response in females appears to be directly linked to the presence of progesterone and its metabolites in females and their actions on GABAergic neurotransmission. Little is known of the neurobiological bases for sex differences in animal models related to the three stages of the addiction cycle. Where there are data, however, the findings support roles for the dopaminergic system in the binge/intoxication stage and the brain stress systems in the withdrawal/negative affect and preoccupation/anticipation stages.
Thus, pronounced sex differences exist in animal models in the domain of drug addiction, paralleling those of humans with regard to mechanism. This review emphasizes both significant differences in the phenotypes of females and males in the addiction domain and notably the paucity of data that hampers our understanding of such differences.
VI. What We Do Not Know
We have identified ways to think about and study sex differences using preclinical animal models. We identified clear qualitative, quantitative, population, and mechanistic sex differences using an addiction domain framework. Nonetheless, much remains to be understood. Largely unknown are the brain changes that occur that convey the female phenotype of faster acquisition of self-administration of drugs and alcohol, the more rapid escalation of drug taking with extended access, the manifestation of more motivational withdrawal, and greater reinstatement.
Although the basis for these quantitative sex differences appears to be organizational, they also depend on the activational effects of gonadal hormones. How are these organizational and activational effects mediated? There are sexually dimorphic brain regions, such as the dorsal striatum, prefrontal cortex, nucleus accumbens, and medial extended amygdala, that contain gonadal hormone receptors that should be explored. Modern brain techniques for molecular, cellular, and circuitry analysis should be applied.
To date, differences within the estrous cycle can be observed but are relatively minor, but they may be relevant if they can be linked to human conditions in which menstrual cycle effects are evident, such as in the role of negative emotional states in withdrawal and relapse. Few studies have analyzed data with regard to uncovering evidence of population differences within male and female rodents. The responsibility for filling the gaps in knowledge may lie more in the human framework of diagnosis than in the animal models domain (Piazza and Deroche-Gamonet, 2013, 2014; Badiani, 2014; George et al., 2014; Ahmed, 2014; Kalivas and Gipson, 2014; Tiffany, 2014; Litten et al., 2015).
Finally, all drugs of abuse are not identical with regard to how they impact the addiction cycle, and alcohol in particular appears to produce some unique sex differences in the withdrawal/negative affect stage. Further work is needed in this domain. The gaps in our knowledge emphasize the significant differences in the phenotypes of females and males in the addiction domain and the exciting challenge of understanding a major part of individual differences in our search for the keys to understanding human biology.
Acknowledgments
The authors thank Mr. Michael Arends for assistance with manuscript preparation and Dr. Ellen Witt of the Division of Neuroscience and Behavior at the National Institute on Alcohol Abuse and Alcoholism for scholarly comments on the manuscript.
Abbreviations
- ADE
alcohol deprivation effect
- CPP
conditioned place preference
- CRF
corticotropin-releasing factor
- GABA
γ-aminobutyric acid
- NMDA
N-methyl-d-aspartate
- PND
postnatal day
- THC
Δ9-tetrahydrocannabinol
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Becker, Koob.
Footnotes
This work was supported by the National Institutes of Health, National Institute on Drug Abuse [Grant 5R21DA032747].
References
- Ahmed SH. (2014) A redescription of data does not count as a general theory. Psychopharmacology (Berl) 231:3909–3910. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. (1998) Transition from moderate to excessive drug intake: change in hedonic set point. Science 282:298–300. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Walker JR, Koob GF. (2000) Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology 22:413–421. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Nakajima M, Allen S, Lemieux A, Hatsukami D. (2015) Sex differences in hormonal responses to stress and smoking relapse: a prospective examination. Nicotine Tob Res 17:382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen AM, Lunos S, Heishman SJ, al’Absi M, Hatsukami D, Allen SS. (2015) Subjective response to nicotine by menstrual phase. Addict Behav 43:50–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida OF, Shoaib M, Deicke J, Fischer D, Darwish MH, Patchev VK. (1998) Gender differences in ethanol preference and ingestion in rats. The role of the gonadal steroid environment. J Clin Invest 101:2677–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. (2010) Sex differences in the effects of allopregnanolone on yohimbine-induced reinstatement of cocaine seeking in rats. Drug Alcohol Depend 107:264–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. (2011) Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr Top Behav Neurosci 8:73–96. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Holtz NA, Zlebnik N, Carroll ME. (2009) Effects of allopregnanolone on the reinstatement of cocaine-seeking behavior in male and female rats. Psychopharmacology (Berl) 203:63–72. [DOI] [PubMed] [Google Scholar]
- Asaba A, Hattori T, Mogi K, Kikusui T. (2014) Sexual attractiveness of male chemicals and vocalizations in mice. Front Neurosci 8:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiani A. (2014) Is a ‘general’ theory of addiction possible? A commentary on: a multistep general theory of transition to addiction. Psychopharmacology (Berl) 231:3923–3927. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Valentino RJ. (2014) Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front Neuroendocrinol 35:303–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett SA. (1963) The Rat: A Study in Behaviour, Aldine, Chicago. [Google Scholar]
- Barrett AC, Smith ES, Picker MJ. (2002) Sex-related differences in mechanical nociception and antinociception produced by μ- and κ-opioid receptor agonists in rats. Eur J Pharmacol 452:163–173. [DOI] [PubMed] [Google Scholar]
- Barron S, Mulholland PJ, Littleton JM, Prendergast MA. (2008) Age and gender differences in response to neonatal ethanol withdrawal and polyamine challenge in organotypic hippocampal cultures. Alcohol Clin Exp Res 32:929–936. [DOI] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, et al. (2005) Strategies and methods for research on sex differences in brain and behavior. Endocrinology 146:1650–1673. [DOI] [PubMed] [Google Scholar]
- Becker JB, Cha JH. (1989) Estrous cycle-dependent variation in amphetamine-induced behaviors and striatal dopamine release assessed with microdialysis. Behav Brain Res 35:117–125. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. (2008) Sex differences in drug abuse. Front Neuroendocrinol 29:36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Perry AN, Westenbroek C. (2012) Sex differences in the neural mechanisms mediating addiction: a new synthesis and hypothesis. Biol Sex Differ 3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J, Ramirez VD. (1980) Dynamics of endogenous catecholamine release from brain fragments of male and female rats. Neuroendocrinology 31:18–25. [DOI] [PubMed] [Google Scholar]
- Beery AK, Kaufer D. (2015) Stress, social behavior, and resilience: insights from rodents. Neurobiol Stress 1:116–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekman NM, Winward JL, Lau LL, Wagner CC, Brown SA. (2013) The impact of adolescent binge drinking and sustained abstinence on affective state. Alcohol Clin Exp Res 37:1432–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Everitt BJ. (2008) Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron 57:432–441. [DOI] [PubMed] [Google Scholar]
- Bind RH, Minney SM, Rosenfeld S, Hallock RM. (2013) The role of pheromonal responses in rodent behavior: future directions for the development of laboratory protocols. J Am Assoc Lab Anim Sci 52:124–129. [PMC free article] [PubMed] [Google Scholar]
- Bisagno V, Ferguson D, Luine VN. (2003) Chronic D-amphetamine induces sexually dimorphic effects on locomotion, recognition memory, and brain monoamines. Pharmacol Biochem Behav 74:859–867. [DOI] [PubMed] [Google Scholar]
- Blanchard BA, Steindorf S, Wang S, Glick SD. (1993) Sex differences in ethanol-induced dopamine release in nucleus accumbens and in ethanol consumption in rats. Alcohol Clin Exp Res 17:968–973. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Martinez M, Harris RA. (2006) Reduced alcohol consumption in mice lacking preprodynorphin. Alcohol 40:73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonenfant C, Loe LE, Mysterud A, Langvatn R, Stenseth NC, Gaillard JM, Klein F. (2004) Multiple causes of sexual segregation in European red deer: enlightenments from varying breeding phenology at high and low latitude. Proc Biol Sci 271:883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw H, Miller J, Ling Q, Malsnee K, Ruda MA. (2000) Sex differences and phases of the estrous cycle alter the response of spinal cord dynorphin neurons to peripheral inflammation and hyperalgesia. Pain 85:93–99. [DOI] [PubMed] [Google Scholar]
- Brady KT, Randall CL. (1999) Gender differences in substance use disorders. Psychiatr Clin North Am 22:241–252. [DOI] [PubMed] [Google Scholar]
- Breese GR, Chu K, Dayas CV, Funk D, Knapp DJ, Koob GF, Lê DA, O’Dell LE, Overstreet DH, Roberts AJ, et al. (2005) Stress enhancement of craving during sobriety: a risk for relapse. Alcohol Clin Exp Res 29:185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson FH. (1979) The reproductive ecology of the house mouse. Q Rev Biol 54:265–299. [DOI] [PubMed] [Google Scholar]
- Buffalari DM, Baldwin CK, Feltenstein MW, See RE. (2012) Corticotrophin releasing factor (CRF) induced reinstatement of cocaine seeking in male and female rats. Physiol Behav 105:209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L. (2014) Equal ≠ the same: sex differences in the human brain. Cerebrum 2014:5. [PMC free article] [PubMed] [Google Scholar]
- Caldarone BJ, King SL, Picciotto MR. (2008) Sex differences in anxiety-like behavior and locomotor activity following chronic nicotine exposure in mice. Neurosci Lett 439:187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun JB. (1963) The Ecology and Sociology of the Norway Rat, U.S. Department of Health, Education, and Welfare, Bethesda. [Google Scholar]
- Carlezon WA, Jr, Nestler EJ, Neve RL. (2000) Herpes simplex virus-mediated gene transfer as a tool for neuropsychiatric research. Crit Rev Neurobiol 14:47–67. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ. (2010) Sex differences and ovarian hormones in animal models of drug dependence. Horm Behav 58:44–56. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Aylward SC, Meyer ER. (2003) Gender differences in the intravenous self-administration of mu opiate agonists. Pharmacol Biochem Behav 74:541–549. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Ennis T, Ogden J, Meyer ER. (2000) Gender differences in the reinforcing properties of morphine. Pharmacol Biochem Behav 65:91–96. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Nock B, Meyer ER. (2002) Gender-linked differences in the expression of physical dependence in the rat. Pharmacol Biochem Behav 72:691–697. [DOI] [PubMed] [Google Scholar]
- Clark JW, Fixaris MC, Belanger GV, Rosenwasser AM. (2007) Repeated light-dark phase shifts modulate voluntary ethanol intake in male and female high alcohol-drinking (HAD1) rats. Alcohol Clin Exp Res 31:1699–1706. [DOI] [PubMed] [Google Scholar]
- Cooper ZD, Haney M. (2014) Investigation of sex-dependent effects of cannabis in daily cannabis smokers. Drug Alcohol Depend 136:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Wang S, Kim SJ, McGovern E, Nabulsi N, Gao H, Labaree D, Tagare HD, Sullivan JM, Morris ED. (2014) Sex differences in the brain’s dopamine signature of cigarette smoking. J Neurosci 34:16851–16855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BM, Young AB, See RE, Reichel CM. (2013) Sex differences in methamphetamine seeking in rats: impact of oxytocin. Psychoneuroendocrinology 38:2343–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JA, Gowl BA, Westenbroek C, Clinton SM, Akil H, Becker JB. (2011) Effects of a selectively bred novelty-seeking phenotype on the motivation to take cocaine in male and female rats. Biol Sex Differ 2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JA, Jagannathan L, Jackson LR, Becker JB. (2014) Sex differences in the effects of estradiol in the nucleus accumbens and striatum on the response to cocaine: neurochemistry and behavior. Drug Alcohol Depend 135:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deehan GA, Jr, McKinzie DL, Carroll FI, McBride WJ, Rodd ZA. (2012) The long-lasting effects of JDTic, a kappa opioid receptor antagonist, on the expression of ethanol-seeking behavior and the relapse drinking of female alcohol-preferring (P) rats. Pharmacol Biochem Behav 101:581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaud LL, Alele P. (2004) Differential effects of chronic ethanol administration and withdrawal on γ-aminobutyric acid type A and NMDA receptor subunit proteins in male and female rat brain. Alcohol Clin Exp Res 28:957–965. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Alele P, Ritu C. (2003) Sex differences in the central nervous system actions of ethanol. Crit Rev Neurobiol 15:41–59. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Chadda R. (2001) Sex differences in rats in the development of and recovery from ethanol dependence assessed by changes in seizure susceptibility. Alcohol Clin Exp Res 25:1689–1696. [PubMed] [Google Scholar]
- Devaud LL, Matthews DB, Morrow AL. (1999) Gender impacts behavioral and neurochemical adaptations in ethanol-dependent rats. Pharmacol Biochem Behav 64:841–849. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Morrow AL. (1999) Gender-selective effects of ethanol dependence on NMDA receptor subunit expression in cerebral cortex, hippocampus and hypothalamus. Eur J Pharmacol 369:331–334. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Morrow AL, Nguyen UT. (2000) Ovariectomy has minimal effects on neuroadaptations associated with ethanol dependence in female rats. Neurochem Int 37:433–442. [DOI] [PubMed] [Google Scholar]
- Diaz SL, Kemmling AK, Rubio MC, Balerio GN. (2005) Morphine withdrawal syndrome: involvement of the dopaminergic system in prepubertal male and female mice. Pharmacol Biochem Behav 82:601–607. [DOI] [PubMed] [Google Scholar]
- Diehl A, Croissant B, Batra A, Mundle G, Nakovics H, Mann K. (2007) Alcoholism in women: is it different in onset and outcome compared to men? Eur Arch Psychiatry Clin Neurosci 257:344–351. [DOI] [PubMed] [Google Scholar]
- DiMatteo J, Reed SC, Evans SM. (2012) Alcohol consumption as a function of dietary restraint and the menstrual cycle in moderate/heavy (“at-risk”) female drinkers. Eat Behav 13:285–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson K, Pikkarainen PH. (1968) Differences between the sexes in voluntary alcohol consumption and liver ADH-activity in inbred strains of mice. Metabolism 17:1037–1042. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. (2010) Does the response to cocaine differ as a function of sex or hormonal status in human and non-human primates? Horm Behav 58:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. (2002) The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 159:397–406. [DOI] [PubMed] [Google Scholar]
- Evans SM, Levin FR. (2011) Response to alcohol in women: role of the menstrual cycle and a family history of alcoholism. Drug Alcohol Depend 114:18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Altea S, Angius F, Fadda P, Fratta W. (2007) Cannabinoid self-administration in rats: sex differences and the influence of ovarian function. Br J Pharmacol 52:795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Altea S, Fadda P, Fratta W. (2010) Drug- and cue-induced reinstatement of cannabinoid-seeking behaviour in male and female rats: influence of ovarian hormones. Br J Pharmacol 160:724–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausto-Sterling A. (2012) Sex/Gender: Biology in a Social World, Routledge, New York. [Google Scholar]
- Feltenstein MW, Ghee SM, See RE. (2012) Nicotine self-administration and reinstatement of nicotine-seeking in male and female rats. Drug Alcohol Depend 121:240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, Henderson AR, See RE. (2011) Enhancement of cue-induced reinstatement of cocaine-seeking in rats by yohimbine: sex differences and the role of the estrous cycle. Psychopharmacology (Berl) 216:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Eldridge JC, Samson HH. (2002) Microanalysis of ethanol self-administration: estrous cycle phase-related changes in consumption patterns. Alcohol Clin Exp Res 26:635–643. [PubMed] [Google Scholar]
- Forger NG, Morin LP. (1982) Reproductive state modulates ethanol intake in rats: effects of ovariectomy, ethanol concentration, estrous cycle and pregnancy. Pharmacol Biochem Behav 17:323–331. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Jagannathan K, Wetherill RR, Johnson B, Kelly S, Langguth J, Mumma J, Childress AR. (2015) Influence of menstrual cycle phase on neural and craving responses to appetitive smoking cues in naturally cycling females. Nicotine Tob Res 17:390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Mehta RH, Case JM, See RE. (2005) Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology (Berl) 179:662–672. [DOI] [PubMed] [Google Scholar]
- Gamsby JJ, Templeton EL, Bonvini LA, Wang W, Loros JJ, Dunlap JC, Green AI, Gulick D. (2013) The circadian Per1 and Per2 genes influence alcohol intake, reinforcement, and blood alcohol levels. Behav Brain Res 249:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Burgos D, Manrique Zuluaga T, Gallo Torre M, González Reyes F. (2010) [Sex differences in the alcohol deprivation effect in rats]. Psicothema 22:887–892. [PubMed] [Google Scholar]
- García-Carmona JA, Baroja-Mazo A, Milanés MV, Laorden ML. (2015) Sex differences between CRF1 receptor deficient mice following naloxone-precipitated morphine withdrawal in a conditioned place aversion paradigm: implication of HPA axis. PLoS One 10:e0121125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile NE, Andrekanic JD, Karwoski TE, Czambel RK, Rubin RT, Rhodes ME. (2011) Sexually diergic hypothalamic-pituitary-adrenal (HPA) responses to single-dose nicotine, continuous nicotine infusion, and nicotine withdrawal by mecamylamine in rats. Brain Res Bull 85:145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Koob GF, Vendruscolo LF. (2014) Negative reinforcement via motivational withdrawal is the driving force behind the transition to addiction. Psychopharmacology (Berl) 231:3911–3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Markou A. (1995) Animal models of psychiatric disorders, in Psychopharmacology: The Fourth Generation of Progress (Bloom FE, Kupfer DJ, eds) pp 787–798, Raven Press, New York. [Google Scholar]
- Gillies GE, Virdee K, McArthur S, Dalley JW. (2014) Sex-dependent diversity in ventral tegmental dopaminergic neurons and developmental programing: A molecular, cellular and behavioral analysis. Neuroscience 282C:69–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goletiani NV, Siegel AJ, Lukas SE, Hudson JI. (2015) The effects of smoked nicotine on measures of subjective States and hypothalamic-pituitary-adrenal axis hormones in women during the follicular and luteal phases of the menstrual cycle. J Addict Med 9:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillamón A, Segovia S. (1997) Sex differences in the vomeronasal system. Brain Res Bull 44:377–382. [DOI] [PubMed] [Google Scholar]
- Hammock EA, Young LJ. (2006) Oxytocin, vasopressin and pair bonding: implications for autism. Philos Trans R Soc Lond B Biol Sci 361:2187–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa RJ, Weiser MJ. (2014) Gonadal steroid hormones and the hypothalamo-pituitary-adrenal axis. Front Neuroendocrinol 35:197–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Maccari S, Le Moal M, Simon H, Piazza PV. (1995) Social stress increases the acquisition of cocaine self-administration in male and female rats. Brain Res 698:46–52. [DOI] [PubMed] [Google Scholar]
- Harte-Hargrove LC, Dow-Edwards DL. (2012) Withdrawal from THC during adolescence: sex differences in locomotor activity and anxiety. Behav Brain Res 231:48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey SM, Beckman LJ. (1985) Cyclic fluctuation in alcohol consumption among female social drinkers. Alcohol Clin Exp Res 9:465–467. [DOI] [PubMed] [Google Scholar]
- Hogle JM, Curtin JJ. (2006) Sex differences in negative affective response during nicotine withdrawal. Psychophysiology 43:344–356. [DOI] [PubMed] [Google Scholar]
- Holtz NA, Lozama A, Prisinzano TE, Carroll ME. (2012) Reinstatement of methamphetamine seeking in male and female rats treated with modafinil and allopregnanolone. Drug Alcohol Depend 120:233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommer DW. (2003) Male and female sensitivity to alcohol-induced brain damage. Alcohol Res Health 27:181–185. [PMC free article] [PubMed] [Google Scholar]
- Hudson A, Stamp JA. (2011) Ovarian hormones and propensity to drug relapse: a review. Neurosci Biobehav Rev 35:427–436. [DOI] [PubMed] [Google Scholar]
- Hutchins JB, Allen DL, Cole-Harding LS, Wilson JR. (1981) Behavioral and physiological measures for studying ethanol dependence in mice. Pharmacol Biochem Behav 15:55–59. [DOI] [PubMed] [Google Scholar]
- Inagaki H, Kuwahara M, Tsubone H, Mori Y. (2013) The effect of post-weaning individual housing on 50-kHz calls emitted from male rats to sexually receptive female rats. Physiol Behav 110-111:30–33. [DOI] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. (2006) Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology 31:129–138. [DOI] [PubMed] [Google Scholar]
- Janis GC, Devaud LL, Mitsuyama H, Morrow AL. (1998) Effects of chronic ethanol consumption and withdrawal on the neuroactive steroid 3α-hydroxy-5α-pregnan-20-one in male and female rats. Alcohol Clin Exp Res 22:2055–2061. [PubMed] [Google Scholar]
- Juárez J, Barrios de Tomasi E. (1999) Sex differences in alcohol drinking patterns during forced and voluntary consumption in rats. Alcohol 19:15–22. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Gipson CD. (2014) “Mourning” a lost opportunity. Psychopharmacology (Berl) 231:3921–3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karami M, Zarrindast MR. (2008) Morphine sex-dependently induced place conditioning in adult Wistar rats. Eur J Pharmacol 582:78–87. [DOI] [PubMed] [Google Scholar]
- Kaufman KR, Tanchuck MA, Strong MN, Finn DA. (2010) Replacement with GABAergic steroid precursors restores the acute ethanol withdrawal profile in adrenalectomy/gonadectomy mice. Neuroscience 166:5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AP, Epstein DH, Phillips KA, Preston KL. (2013) Sex differences in cocaine/heroin users: drug-use triggers and craving in daily life. Drug Alcohol Depend 132:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keverne EB. (2007) Genomic imprinting and the evolution of sex differences in mammalian reproductive strategies. Adv Genet 59:217–243. [DOI] [PubMed] [Google Scholar]
- Keyes KM, Grant BF, Hasin DS. (2008) Evidence for a closing gender gap in alcohol use, abuse, and dependence in the United States population. Drug Alcohol Depend 93:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Martins SS, Blanco C, Hasin DS. (2010) Telescoping and gender differences in alcohol dependence: new evidence from two national surveys. Am J Psychiatry 167:969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesner J. (2012) Affective response to the menstrual cycle as a predictor of self-reported affective response to alcohol and alcohol use. Arch Women Ment Health 15:423–432. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, Mehta RH, Case JM, Parker MP, Bimonte-Nelson HA, See RE. (2005) Potentiation of cocaine-primed reinstatement of drug seeking in female rats during estrus. Psychopharmacology (Berl) 182:245–252. [DOI] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. (2006) Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology (Berl) 186:48–53. [DOI] [PubMed] [Google Scholar]
- Kleiman DG. (2011) Canid mating systems, social behavior, parental care and ontogeny: are they flexible? Behav Genet 41:803–809. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. (1997) Drug abuse: hedonic homeostatic dysregulation. Science 278:52–58. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. (2001) Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 24:97–129. [DOI] [PubMed] [Google Scholar]
- Koob GF, Sanna PP, Bloom FE. (1998) Neuroscience of addiction. Neuron 21:467–476. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238 erratum: 35: 1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Zhang XY. (2008) Sex differences in non-reinforced responding for cocaine. Am J Drug Alcohol Abuse 34:473–488. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ. (1993) Gender differences in cocaine use and treatment response. J Subst Abuse Treat 10:63–66. [DOI] [PubMed] [Google Scholar]
- Lancaster FE, Brown TD, Coker KL, Elliott JA, Wren SB. (1996) Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period. Alcohol Clin Exp Res 20:1043–1049. [DOI] [PubMed] [Google Scholar]
- Lancaster FE, Spiegel KS. (1992) Sex differences in pattern of drinking. Alcohol 9:415–420. [DOI] [PubMed] [Google Scholar]
- Larson EB, Carroll ME. (2007) Estrogen receptor beta, but not alpha, mediates estrogen’s effect on cocaine-induced reinstatement of extinguished cocaine-seeking behavior in ovariectomized female rats. Neuropsychopharmacology 32:1334–1345. [DOI] [PubMed] [Google Scholar]
- Larson EB, Anker JJ, Gliddon LA, Fons KS, Carroll ME. (2007) Effects of estrogen and progesterone on the escalation of cocaine self-administration in female rats during extended access. Exp Clin Psychopharmacol 15:461–471. [DOI] [PubMed] [Google Scholar]
- Lee CW, Ho IK. (2013) Sex differences in opioid analgesia and addiction: interactions among opioid receptors and estrogen receptors. Mol Pain 9:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TK, Lumeng L. (1984) Alcohol preference and voluntary alcohol intakes of inbred rat strains and the National Institutes of Health heterogeneous stock of rats. Alcohol Clin Exp Res 8:485–486. [DOI] [PubMed] [Google Scholar]
- Lishman WA, Jacobson RR, Acker C. (1987) Brain damage in alcoholism: current concepts. Acta Med Scand Suppl 717:5–17. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Ryan ML, Falk DE, Reilly M, Fertig JB, Koob GF. (2015) Heterogeneity of alcohol use disorder: understanding mechanisms to advance personalized treatment. Alcohol Clin Exp Res 39:579–584. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Grahame NJ, Becker HC. (2011) Development of ethanol withdrawal-related sensitization and relapse drinking in mice selected for high- or low-ethanol preference. Alcohol Clin Exp Res 35:953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. (1999) Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl) 144:77–82. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. (2000) Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacology (Berl) 148:196–200. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Mickelberg JL, Carroll ME. (2001) Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol Biochem Behav 68:641–646. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Taylor JR. (2004) Sex differences in the behavioral effects of 24-h/day access to cocaine under a discrete trial procedure. Neuropsychopharmacology 29:943–951. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Taylor JR. (2005) Decreased motivation following cocaine self-administration under extended access conditions: effects of sex and ovarian hormones. Neuropsychopharmacology 30:927–935. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Mangini LD, Taylor JR. (2005) Neonatal isolation stress potentiates cocaine seeking behavior in adult male and female rats. Neuropsychopharmacology 30:322–329. [DOI] [PubMed] [Google Scholar]
- Maldonado-Devincci AM, Alipour KK, Michael LA, Kirstein CL. (2010) Repeated binge ethanol administration during adolescence enhances voluntary sweetened ethanol intake in young adulthood in male and female rats. Pharmacol Biochem Behav 96:476–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K, Ackermann K, Croissant B, Mundle G, Nakovics H, Diehl A. (2005) Neuroimaging of gender differences in alcohol dependence: are women more vulnerable? Alcohol Clin Exp Res 29:896–901. [DOI] [PubMed] [Google Scholar]
- Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, Koob GF. (1993) Animal models of drug craving. Psychopharmacology (Berl) 112:163–182. [DOI] [PubMed] [Google Scholar]
- Martin-Garcia E, Pallares M. (2005) Effects of intrahippocampal nicotine and neurosteroid administration on withdrawal in voluntary and chronic alcohol-drinking rats. Alcohol Clin Exp Res 29:1654–1663. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Lefever TW, Antonazzo KR, Craft RM, Wiley JL. (2014) Evaluation of sex differences in cannabinoid dependence. Drug Alcohol Depend 137:20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ. (2012) Sex differences in the brain: the not so inconvenient truth. J Neurosci 32:2241–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock MK. (1984) Estrous synchrony: modulation of ovarian cycle length by female pheromones. Physiol Behav 32:701–705. [DOI] [PubMed] [Google Scholar]
- McKinzie DL, Nowak KL, Yorger L, McBride WJ, Murphy JM, Lumeng L, Li TK. (1998) The alcohol deprivation effect in the alcohol-preferring P rat under free-drinking and operant access conditions. Alcohol Clin Exp Res 22:1170–1176. [PubMed] [Google Scholar]
- McLeod DR, Foster GV, Hoehn-Saric R, Svikis DS, Hipsley PA. (1994) Family history of alcoholism in women with generalized anxiety disorder who have premenstrual syndrome: patient reports of premenstrual alcohol consumption and symptoms of anxiety. Alcohol Clin Exp Res 18:664–670. [DOI] [PubMed] [Google Scholar]
- Meliska CJ, Bartke A, McGlacken G, Jensen RA. (1995) Ethanol, nicotine, amphetamine, and aspartame consumption and preferences in C57BL/6 and DBA/2 mice. Pharmacol Biochem Behav 50:619–626. [DOI] [PubMed] [Google Scholar]
- Mello NK, Bree MP, Mendelson JH. (1986) Alcohol and food self-administration by female Macaque monkeys as a function of menstrual cycle phase. Physiol Behav 36:959–966. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Lex BW. (1990) Alcohol use and premenstrual symptoms in social drinkers. Psychopharmacology (Berl) 101:448–455. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Yap JJ, Covington HE., 3rd (2008) Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther 120:102–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CF, Lynch WJ. (2015) Alcohol preferring (P) rats as a model for examining sex differences in alcohol use disorder and its treatment. Pharmacol Biochem Behav 132:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran-Santa Maria MM, Flanagan J, Brady K. (2014) Ovarian hormones and drug abuse. Curr Psychiatry Rep 16:511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro CA, McCaul ME, Wong DF, Oswald LM, Zhou Y, Brasic J, Kuwabara H, Kumar A, Alexander M, Ye W, et al. (2006) Sex differences in striatal dopamine release in healthy adults. Biol Psychiatry 59:966–974. [DOI] [PubMed] [Google Scholar]
- Negus SS, Zuzga DS, Mello NK. (2002) Sex differences in opioid antinociception in rhesus monkeys: antagonism of fentanyl and U50,488 by quadazocine. J Pain 3:218–226. [DOI] [PubMed] [Google Scholar]
- Niikura K, Zhou Y, Ho A, Kreek MJ. (2013) Proopiomelanocortin (POMC) expression and conditioned place aversion during protracted withdrawal from chronic intermittent escalating-dose heroin in POMC-EGFP promoter transgenic mice. Neuroscience 236:220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton R, Batey R, Dwyer T, MacMahon S. (1987) Alcohol consumption and the risk of alcohol related cirrhosis in women. Br Med J (Clin Res Ed) 295:80–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Chen SA, Smith RT, Specio SE, Balster RL, Paterson NE, Markou A, Zorrilla EP, Koob GF. (2007) Extended access to nicotine self-administration leads to dependence: Circadian measures, withdrawal measures, and extinction behavior in rats. J Pharmacol Exp Ther 320:180–193. [DOI] [PubMed] [Google Scholar]
- O’Hara P, Portser SA, Anderson BP. (1989) The influence of menstrual cycle changes on the tobacco withdrawal syndrome in women. Addict Behav 14:595–600. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. (2004) Similar anxiety-like responses in male and female rats exposed to repeated withdrawals from ethanol. Pharmacol Biochem Behav 78:459–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patchev VK, Hayashi S, Orikasa C, Almeida OF. (1995) Implications of estrogen-dependent brain organization for gender differences in hypothalamo-pituitary-adrenal regulation. FASEB J 9:419–423. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Donny E, Caggiula AR. (1999) Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nicotine Tob Res 1:301–315. [DOI] [PubMed] [Google Scholar]
- Perry AN, Westenbroek C, Becker JB. (2013a) Impact of pubertal and adult estradiol treatments on cocaine self-administration. Horm Behav 64:573–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry AN, Westenbroek C, Becker JB. (2013b) The development of a preference for cocaine over food identifies individual rats with addiction-like behaviors. PLoS One 8:e79465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry AN, Westenbroek C, Jagannathan L, Becker JB. (2015) The roles of dopamine and α1-adrenergic receptors in cocaine preferences in female and male rats. Neuropsychopharmacology 40:2696–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Normile LM, Morgan AD, Carroll ME. (2006) Sex differences in physical dependence on orally self-administered phencyclidine (PCP) in rhesus monkeys (Macaca mulatta). Exp Clin Psychopharmacol 14:68–78. [DOI] [PubMed] [Google Scholar]
- Peterson AB, Hivick DP, Lynch WJ. (2014) Dose-dependent effectiveness of wheel running to attenuate cocaine-seeking: impact of sex and estrous cycle in rats. Psychopharmacology (Berl) 231:2661–2670. [DOI] [PubMed] [Google Scholar]
- Piazza NJ, Vrbka JL, Yeager RD. (1989) Telescoping of alcoholism in women alcoholics. Int J Addict 24:19–28. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonet V. (2013) A multistep general theory of transition to addiction. Psychopharmacology (Berl) 229:387–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonet V. (2014) A general theory of transition to addiction it was and a general theory of transition to addiction it is: reply to the commentaries of Ahmed, Badiani, George & Koob, Kalivas & Gipson, and Tiffany. Psychopharmacology (Berl) 231:3929–3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky E. (1963) The woman alcoholic and premenstrual tension. J Am Med Womens Assoc 18:816–818. [PubMed] [Google Scholar]
- Prendergast BJ, Onishi KG, Zucker I. (2014) Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev 40:1–5. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Moore PH, Jr, Morrow AL, Paul SM. (1991) The 3α-hydroxy ring-A reduced metabolites of progesterone and deoxycortisone: natural ligands of central GABAA receptors, in Neurosteroids and Brain Function (Costa E, Paul SM, eds) pp 95–101, Thieme Medical, New York. [Google Scholar]
- Radke AK, Gewirtz JC, Carroll ME. (2015) Effects of age, but not sex, on elevated startle during withdrawal from acute morphine in adolescent and adult rats. Behav Pharmacol 26:485–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramôa CP, Doyle SE, Naim DW, Lynch WJ. (2013) Estradiol as a mechanism for sex differences in the development of an addicted phenotype following extended access cocaine self-administration. Neuropsychopharmacology 38:1698–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly W, Koirala B, Devaud LL. (2009) Sex differences in acoustic startle responses and seizure thresholds between ethanol-withdrawn male and female rats. Alcohol Alcohol 44:561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. (2000) Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology 22:581–594. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Smith AD, Weiss F, Rivier C, Koob GF. (1998) Estrous cycle effects on operant responding for ethanol in female rats. Alcohol Clin Exp Res 22:1564–1569. [PubMed] [Google Scholar]
- Roberts DCS, Bennett SA, Vickers GJ. (1989) The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology (Berl) 98:408–411. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. (1986) Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res 396:157–198. [DOI] [PubMed] [Google Scholar]
- Roger-Sánchez C, Aguilar MA, Rodríguez-Arias M, Aragon CM, Miñarro J. (2012) Age- and sex-related differences in the acquisition and reinstatement of ethanol CPP in mice. Neurotoxicol Teratol 34:108–115. [DOI] [PubMed] [Google Scholar]
- Roth ME, Cosgrove KP, Carroll ME. (2004) Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci Biobehav Rev 28:533–546. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Shiffman S, Tiffany ST, Niaura RS, Martin CS, Shadel WG. (2000) The measurement of drug craving. Addiction 95 (Suppl 2):S189–S210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Francis R, MacDonald A, Keistler C, O’Neill L, Kuhn CM. (2014) Effect of sex on ethanol consumption and conditioned taste aversion in adolescent and adult rats. Psychopharmacology (Berl) 231:1831–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz LA, Lore RK. (1993) Communal reproductive success in rats (Rattus norvegicus): effects of group composition and prior social experience. J Comp Psychol 107:216–222. [DOI] [PubMed] [Google Scholar]
- Self DW. (1998) Neural substrates of drug craving and relapse in drug addiction. Ann Med 30:379–389. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. (2003) The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 168:3–20. [DOI] [PubMed] [Google Scholar]
- Sharrett-Field L, Butler TR, Reynolds AR, Berry JN, Prendergast MA. (2013) Sex differences in neuroadaptation to alcohol and withdrawal neurotoxicity. Pflugers Arch 465:643–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Koob GF. (2002) Recent advances in animal models of drug addiction and alcoholism, in Neuropsychopharmacology: The Fifth Generation of Progress (Davis KL, Charney D, Coyle JT, Nemeroff C, eds) pp 1381–1397, Lippincott Williams and Wilkins, Philadelphia. [Google Scholar]
- Silva SM, Madeira MD. (2012) Effects of chronic alcohol consumption and withdrawal on the response of the male and female hypothalamic-pituitary-adrenal axis to acute immune stress. Brain Res 1444:27–37. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox H, Hong KI, Sofuoglu M, Morgan PT, Bergquist KT. (2007) Sex steroid hormones, stress response, and drug craving in cocaine-dependent women: implications for relapse susceptibility. Exp Clin Psychopharmacol 15:445–452. [DOI] [PubMed] [Google Scholar]
- Sinnott RS, Phillips TJ, Finn DA. (2002) Alteration of voluntary ethanol and saccharin consumption by the neurosteroid allopregnanolone in mice. Psychopharmacology (Berl) 162:438–447. [DOI] [PubMed] [Google Scholar]
- Skjei KL, Markou A. (2003) Effects of repeated withdrawal episodes, nicotine dose, and duration of nicotine exposure on the severity and duration of nicotine withdrawal in rats. Psychopharmacology (Berl) 168:280–292. [DOI] [PubMed] [Google Scholar]
- Sloan JW, Wala EP, Jing X, Holtman JR., Jr (2000) The pharmacodynamics of PK 11195 in diazepam-dependent male and female rats. Pharmacol Biochem Behav 66:751–764. [DOI] [PubMed] [Google Scholar]
- Smuts B. (1992) Male aggression against women : An evolutionary perspective. Hum Nat 3:1–44. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. (1999) Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol 7:274–283. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Martin LJ, Isbester KA, Sotocinal SG, Rosen S, Tuttle AH, Wieskopf JS, Acland EL, Dokova A, Kadoura B, et al. (2014) Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods 11:629–632. [DOI] [PubMed] [Google Scholar]
- Stinus L, Caille S, Koob GF. (2000) Opiate withdrawal-induced place aversion lasts for up to 16 weeks. Psychopharmacology (Berl) 149:115–120. [DOI] [PubMed] [Google Scholar]
- Strong MN, Kaufman KR, Crabbe JC, Finn DA. (2009) Sex differences in acute ethanol withdrawal severity after adrenalectomy and gonadectomy in Withdrawal Seizure-Prone and Withdrawal Seizure-Resistant mice. Alcohol 43:367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (2007) Results from the 2006 National Survey on Drug Use and Health: National Findings (Office of Applied Statistics, NSDUH Series H-32, DHHS publication no. SMA 07-4293), Substance Abuse and Mental Health Services Administration, Rockville. [Google Scholar]
- Sutker PB, Libet JM, Allain AN, Randall CL. (1983) Alcohol use, negative mood states, and menstrual cycle phases. Alcohol Clin Exp Res 7:327–331. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Koike Y, Misawa M. (1988) Sex differences in physical dependence on methaqualone in the rat. Pharmacol Biochem Behav 30:483–488. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Koike Y, Yanaura S, George FR, Meisch RA. (1992) Sex differences in physical dependence on pentobarbital in four inbred strains of rats. Gen Pharmacol 23:487–492. [DOI] [PubMed] [Google Scholar]
- Svikis DS, Miles DR, Haug NA, Perry B, Hoehn-Saric R, McLeod D. (2006) Premenstrual symptomatology, alcohol consumption, and family history of alcoholism in women with premenstrual syndrome. J Stud Alcohol 67:833–836. [DOI] [PubMed] [Google Scholar]
- Tambour S, Brown LL, Crabbe JC. (2008) Gender and age at drinking onset affect voluntary alcohol consumption but neither the alcohol deprivation effect nor the response to stress in mice. Alcohol Clin Exp Res 32:2100–2106. [DOI] [PubMed] [Google Scholar]
- Tanchuck-Nipper MA, Ford MM, Hertzberg A, Beadles-Bohling A, Cozzoli DK, Finn DA. (2015) Sex differences in ethanol’s anxiolytic effect and chronic ethanol withdrawal severity in mice with a null mutation of the 5α-reductase type 1 gene. Behav Genet 45:354–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate DL, Charette L. (1991) Personality, alcohol consumption, and menstrual distress in young women. Alcohol Clin Exp Res 15:647–652. [DOI] [PubMed] [Google Scholar]
- Terner JM, de Wit H. (2006) Menstrual cycle phase and responses to drugs of abuse in humans. Drug Alcohol Depend 84:1–13. [DOI] [PubMed] [Google Scholar]
- Thomas MB, Hu M, Lee TM, Bhatnagar S, Becker JB. (2009) Sex-specific susceptibility to cocaine in rats with a history of prenatal stress. Physiol Behav 97:270–277. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. (2014) Consideration of a comprehensive animal model of addiction: the limitations of modeling a counterfeit condition. Psychopharmacology (Berl) 231:3919–3920. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Carter BL, Singleton EG. (2000) Challenges in the manipulation, assessment and interpretation of craving relevant variables. Addiction 95 (Suppl 2):S177–S187. [DOI] [PubMed] [Google Scholar]
- Torres OV, O’Dell LE. (2015) Stress is a principal factor that promotes tobacco use in females. Prog Neuropsychopharmacol Biol Psychiatry, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Gentil LG, Natividad LA, Carcoba LM, O’Dell LE. (2013) Behavioral, biochemical, and molecular indices of stress are enhanced in female versus male rats experiencing nicotine withdrawal. Front Psychiatry 4:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Walker EM, Beas BS, O’Dell LE. (2014) Female rats display enhanced rewarding effects of ethanol that are hormone dependent. Alcohol Clin Exp Res 38:108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. (2002) Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res 26:1494–1501. [DOI] [PubMed] [Google Scholar]
- van Haaren F, Anderson K. (1994) Sex differences in schedule-induced alcohol consumption. Alcohol 11:35–40. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. (2004) Acute ethanol withdrawal (hangover) and social behavior in adolescent and adult male and female Sprague-Dawley rats. Alcohol Clin Exp Res 28:40–50. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. (2015) Social consequences of ethanol: Impact of age, stress, and prior history of ethanol exposure. Physiol Behav 148:145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Truxell EM, Spear LP. (2015) Ethanol intake under social circumstances or alone in sprague-dawley rats: impact of age, sex, social activity, and social anxiety-like behavior. Alcohol Clin Exp Res 39:117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch LM, Wright TM, Randall CL. (2007) Only male mice show sensitization of handling-induced convulsions across repeated ethanol withdrawal cycles. Alcohol Clin Exp Res 31:477–485. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Vollmayr B, Henn FA, Spanagel R. (2005) Voluntary alcohol intake in two rat lines selectively bred for learned helpless and non-helpless behavior. Psychopharmacology (Berl) 178:125–132. [DOI] [PubMed] [Google Scholar]
- Virdee K, McArthur S, Brischoux F, Caprioli D, Ungless MA, Robbins TW, Dalley JW, Gillies GE. (2014) Antenatal glucocorticoid treatment induces adaptations in adult midbrain dopamine neurons, which underpin sexually dimorphic behavioral resilience. Neuropsychopharmacology 39:339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivian JA, Green HL, Young JE, Majerksy LS, Thomas BW, Shively CA, Tobin JR, Nader MA, Grant KA. (2001) Induction and maintenance of ethanol self-administration in cynomolgus monkeys (Macaca fascicularis): long-term characterization of sex and individual differences. Alcohol Clin Exp Res 25:1087–1097. [PubMed] [Google Scholar]
- Walker BM, Walker JL, Ehlers CL. (2008) Dissociable effects of ethanol consumption during the light and dark phase in adolescent and adult Wistar rats. Alcohol 42:83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls SA, Macklin ZL, Devaud LL. (2012) Ethanol-induced loss-of-righting response during ethanol withdrawal in male and female rats: associations with alterations in Arc labeling. Alcohol Clin Exp Res 36:234–241. [DOI] [PubMed] [Google Scholar]
- Walls SA, Rosenwasser AM, Devaud LL. (2013) Sex and regional differences in effects of chronic intermittent ethanol exposure on subsequent excitotoxic challenges in hippocampal slice cultures. Neurosci Lett 550:6–11. [DOI] [PubMed] [Google Scholar]
- Weidt A, Lindholm AK, König B. (2014) Communal nursing in wild house mice is not a by-product of group living: females choose. Naturwissenschaften 101:73–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, Smith PH, Allen SS, Cosgrove KP, Saladin ME, Gray KM, Mazure CM, Wetherington CL, McKee SA. (2015) Systematic and meta-analytic review of research examining the impact of menstrual cycle phase and ovarian hormones on smoking and cessation. Nicotine Tob Res 17:407–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenbroek C, Perry AN, Becker JB. (2013) Pair housing differentially affects motivation to self-administer cocaine in male and female rats. Behav Brain Res 252:68–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenbroek C, Snijders TA, den Boer JA, Gerrits M, Fokkema DS, Ter Horst GJ. (2005) Pair-housing of male and female rats during chronic stress exposure results in gender-specific behavioral responses. Horm Behav 47:620–628. [DOI] [PubMed] [Google Scholar]
- Wilhelm CJ, Hashimoto JG, Roberts ML, Sonmez MK, Wiren KM. (2014) Understanding the addiction cycle: a complex biology with distinct contributions of genotype vs. sex at each stage. Neuroscience 279:168–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt ED. (2007) Puberty, hormones, and sex differences in alcohol abuse and dependence. Neurotoxicol Teratol 29:81–95. [DOI] [PubMed] [Google Scholar]
- Yoest KE, Cummings JA, Becker JB. (2014) Estradiol, dopamine and motivation. Cent Nerv Syst Agents Med Chem 14:83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Becker JB. (1994) Quantitative microdialysis determination of extracellular striatal dopamine concentration in male and female rats: effects of estrous cycle and gonadectomy. Neurosci Lett 180:155–158. [DOI] [PubMed] [Google Scholar]
- Zhou L, Ghee SM, Chan C, Lin L, Cameron MD, Kenny PJ, See RE. (2012) Orexin-1 receptor mediation of cocaine seeking in male and female rats. J Pharmacol Exp Ther 340:801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Pruitt C, Shin CB, Garcia AD, Zavala AR, See RE. (2014) Fos expression induced by cocaine-conditioned cues in male and female rats. Brain Struct Funct 219:1831–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]