Abstract
African trypanosomes are tsetse fly transmitted protozoan parasites responsible for human African trypanosomiasis, a disease characterized by a plethora of neurological symptoms and death. How the parasites under microvascular shear stress (SS) flow conditions in the brain cross the blood–brain barrier (BBB) is not known. In vitro studies using static models comprised of human brain microvascular endothelial cells (BMEC) show that BBB activation and crossing by trypanosomes requires the orchestration of parasite cysteine proteases and host calcium-mediated cell signaling. Here, we examine BMEC barrier function and the activation of extracellular signal-regulated kinase (ERK)1/2 and ERK5, mitogen-activated protein kinase family regulators of microvascular permeability, under static and laminar SS flow and in the context of trypanosome infection. Confluent human BMEC were cultured in electric cell-substrate impedance sensing (ECIS) and parallel-plate glass slide chambers. The human BMEC were exposed to 2 or 14 dyn/cm2 SS in the presence or absence of trypanosomes. Real-time changes in transendothelial electrical resistance (TEER) were monitored and phosphorylation of ERK1/2 and ERK5 analyzed by immunoblot assay. After reaching confluence under static conditions human BMEC TEER was found to rapidly increase when exposed to 2 dyn/cm2 SS, a condition that mimics SS in brain postcapillary venules. Addition of African trypanosomes caused a rapid drop in human BMEC TEER. Increasing SS to 14 dyn/cm2, a condition mimicking SS in brain capillaries, led to a transient increase in TEER in both control and infected human BMEC. However, no differences in ERK1/2 and ERK5 activation were found under any condition tested. African trypanosomiasis alters BBB permeability under low shear conditions through an ERK1/2 and ERK5 independent pathway.
Keywords: African trypanosomes, blood–brain barrier, brain microvascular endothelial cells, trypanosoma brucei rhodesiense, shear stress, MAPK
African trypanosomes are tsetse fly transmitted pathogens of humans that play an important, and sometimes devastating, role in the health and welfare of people throughout sub-Saharan Africa. Two subspecies of Trypanosoma brucei, T. b. rhodesiense and T. b. gambiense, cause human African trypanosomiasis (HAT; aka sleeping sickness). Death is inevitable if a patient is untreated.1 African trypanosomes replicate at the tsetse fly bite site before disseminating from the skin through the hemolymphatic system to infect various organ systems (stage 1). The parasites that cross the blood–brain barrier (BBB) produce central nervous system (CNS) disease (stage 2).2 Once CNS disease is established, the parasites are shielded from most trypanocidal drugs, the majority of which do not cross the BBB.3 4 Indeed, the brain is probably the source for relapses.3 Recent intravital and vibratome brain imaging studies in the mouse by us5 and others6 7 have demonstrated that the parasites enter the meninges and the parenchyma of the cerebral cortex and cerebellum soon after infection. In fact, some stage-1 drugs may kill trypanosomes localized in the meninges.6 Neurological features recently reported in T. b. rhodesiense sleeping sickness patients with stage-1 disease8 provide further clinical support for the potential for early brain entry.
The BBB, comprised brain microvascular endothelial cells (BMEC), separates the brain interstitial fluid from the blood,9 maintains the stable environment that allows for effective neuronal function, and protects neurons from circulating neurotransmitters, toxic substances, and pathogens. Shear stress (SS) plays an important, dynamic role in regulating the structure and function of BMEC involved in BBB signaling and ultimately neurological function.10 11 12 13 14 When exposed to low SS conditions found in brain postcapillary venules (PCV) and high SS found in brain capillaries, BMEC in vitro, respectively, develop low and high transendothelial electrical resistances (TEERs) that are characteristic of brain PCV and capillaries, respectively.15 This is important because trypanosomes accumulate and cross the BBB at the level of PCVs but not of capillaries.16 17
Consistent with a possible function in the extracellular milieu, T. brucei C1 (papain) family cysteine proteases cathepsin B-like and cathepsin L-like (TbCatL) enzymes18 19 retain activity and stability at physiological pH19 and are partially resistant to serum-derived inhibitors.20 Using established static in vitro BBB models based on human BMEC, our studies lead to the hypothesis that mobile trypanosomes and cysteine proteases secreted by the parasites can trigger protease-activated receptors (PARs). Working alone, or in concert with other BMEC receptors/ion channels, PARs induce rises of intracellular Ca2+ ([Ca2+]i) within BMECs. These Ca2+ events permit trypanosomes to cross the BBB endothelium paracellularly and initiate precursory inflammatory events that are the signature of CNS HAT. In cardiomyocytes TbCatL has been shown to increase the propensity for spontaneous [Ca2+]i release (Ca2+ waves) via ryanodine receptors.21 Overall, trypanosome disruption of Ca2+ handling by host cells defines a common pathogenic mechanism used by the parasites and might identify potential therapeutic HAT targets.
Previous studies with cultured cells have revealed the involvement of extracellular signal-regulated kinase (ERK)5 and ERK1/2 in many cellular responses, including cell proliferation.22 23 Recent genetic studies have identified that ERK5 is essential for cardiovascular development and neural differentiation, whereas ERK1/2 is important for mesoderm formation.23 ERK5 is ubiquitously expressed in numerous tissues and is activated by a variety of extracellular stimuli, such as cellular stresses and growth factors, to regulate processes such as cell proliferation and differentiation.24 The requirement of ERK5 in the maintenance of vascular integrity is highlighted by the fact that induced ablation of ERK5 in adult mice is lethal within 2 to 3 weeks as blood vessels become leaky due to EC apoptosis.
To achieve a proper understanding of how trypanosomes and their proteases trigger BBB and neurological dysfunction, we initiated studies that use BBB models that might mimic the dynamic fluid conditions in the brain during an active trypanosome infection. Using electric cell-substrate impedance sensing (ECIS) and a parallel plate human BMEC-based models under SS conditions found in brain PCVs and capillaries, we hypothesize that the trypanosomes would (1) increase BMEC barrier permeability precursory to paracellular crossing and (2) that barrier permeability will be regulated, in part, via phosphorylation of ERK1/2 and/or ERK5, members of the mitogen-activated protein kinase (MAPK) family containing the Thr-Glu-Try activation motif.
Methods
Human BMEC and Trypanosomes
Human BMEC (≤ passage 13) were maintained as previously described in medium M199 (Lonza, Walkersville, MD) containing 10% fetal bovine serum (Gemini Bio-Products, West Sacramento, CA).25 26 27 The T. b. rhodesiense used is a human cerebrospinal fluid and bloodstream form isolated from a Kenyan patient with sleeping sickness.25 27 28 This parasite was formerly classified as T. b. gambiense but has been reclassified as T. b. rhodesiense based on the presence of the SRA gene.28 The trypanosomes were maintained in culture in HMI-9 medium.29
Mechanical Stress Exposure
ECIS System
Human BMEC were seeded on fibronectin (BD Biosciences, Bedford, MA) coated 1F8 × 10E ECIS flow arrays (Applied Biophysics, Troy, NY). Although each array can only be used to monitor a single condition (e.g., with or without parasites; low or high SS), each of eight electrode groups will give an independent TEER measurement. After placing in the ECIS station, human BMEC were exposed to continuous unidirectional laminar flow (LF) by driving the perfusion medium with a computerized peristaltic pump (Cole-Parmer, Vernon Hills, IL) connected to a vented 25 mL reservoir and TEER monitored using an ECIS-Ζθ instrument (Fig. 1A).
Fig. 1.
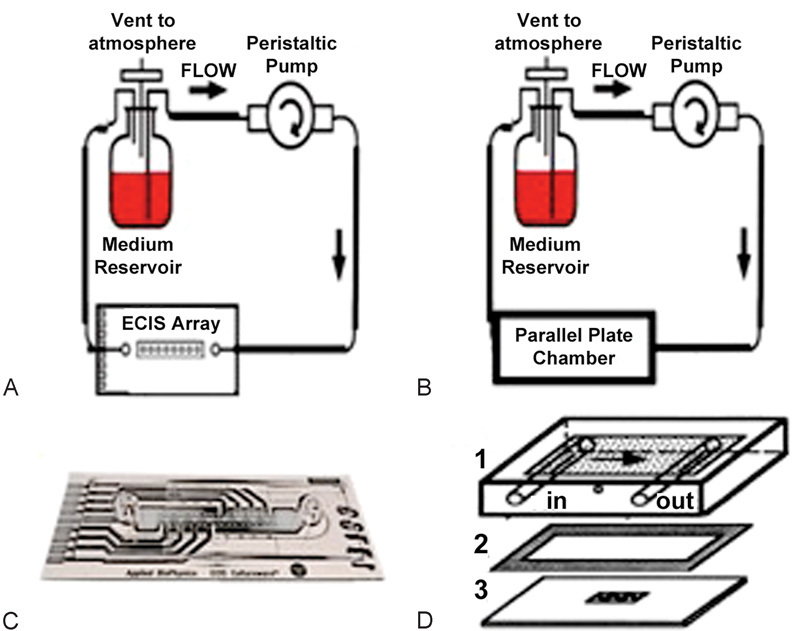
(A and B) Laminar flow setup using 25 mL reservoir and peristaltic pump to create continuous flow through ECIS array or parallel plate chambers. (C) ECIS 1F8 × 10E chamber with 8 sets of 10 active gold electrodes. Cells are grown on electrodes and flow is sent in through inlet on left side. (D) Parallel plate chamber setup: 1, Chamber is attached to laminar flow tubing and media flows on surface. 2, Gasket gives the chamber its height needed to create the correct shear stress. 3, Cells are grown on glass slide coated with fibronectin. 1, 2, and 3 are held together using two clamps (not shown).
Parallel-Plate Flow System30
Human BMEC seeded on fibronectin coated glass slides (75 × 38 mm; Fisher Scientific, Pittsburgh, PA) that were placed in a parallel-plate flow chamber system (Cytodyne, Sand Diego, CA) were exposed to LF driven by the hydrostatic pressure created by the vertical distance between the reservoirs and the flow chambers (Fig. 1B).
For both systems, the cells were kept at 37°C in a humidified 95% air/5% CO2 environment. The magnitude of SS was calculated as previously described.31 32 SSs of 2 or 14 dyn/cm2 were applied as flow.
Immunoblotting
After experimentation, human BMEC on the parallel plate flow system were washed in ice-cold PBS twice, scraped in RIPA lysis buffer 1X (Millipore, CA) and placed in an ice bath for 45 minutes. Cell extracts were sonicated and centrifuged at 15,000 g for 20 minutes at 4°C, and the supernatant was collected. Equal amounts of protein (30 μg per lane, BioRad protein assay system; BioRad Laboratories Inc, Hercules, CA) were separated by 10% sodium dodecylsulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane (BioRad Laboratories Inc). After blocking for 1 hour with tris-buffered saline containing 0.1% Tween 20 and 5% nonfat dry milk, the membrane was probed with primary antibody, either phospho-anti-ERK5, total anti-ERK5, phosphor anti-ERK1/2, or total anti-ERK1/2 antibody (Cell Signaling, Beverly, MA), and horseradish peroxidase-conjugated antirabbit polyclonal secondary antibody (Cell Signaling), before detection of immunoreactivity by enhanced chemiluminescence (Amersham). All blots were quantified with densitometry (BioImage, Ann Arbor, MI).
Real-Time TEER Measurements by ECIS
After human BMEC confluency on the ECIS flow array was reached under static conditions, 2 dyn/cm2 LF was applied to the human BMEC until stable TEERs under SS were again reached. A small volume of concentrated trypanosomes in medium was then added to one chamber so that a final parasite concentration of 5 × 106 parasites/mL was attained. Medium without parasites was added to the other array and used as the sham control and TEER measurements taken every 30 seconds. At a later time point, LF was increased to 14 dyn/cm2 and TEERs recorded.
Results
We have demonstrated that different types of SS regimens have different effects on ECs and may account for the variable response of ECs to hemodynamics in the circulation.30
Fig. 2 shows that the initial TEER values for the static confluent human BMEC monolayers were approximately 1,000 Ω. Upon application of 2 dyn/cm2 of LF to the ECIS flow arrays, TEER values increased approximately 60% and plateaued in about an hour. Addition of the trypanosomes under these SS conditions lead to a rapid 15% drop in BMEC TEER. Furthermore, when SS was increased to 14 dyn/cm2, transient increases in BMEC TEER were observed: the control BMEC responded by initially getting tighter, followed by a drop in TEER going back to the initial TEER values under the lower SS (2 dyn/cm2) condition. Remarkably, TEER values for the BMEC infected with trypanosomes also rapidly increased during this same time period and eventually reached control TEER values.
Fig. 2.
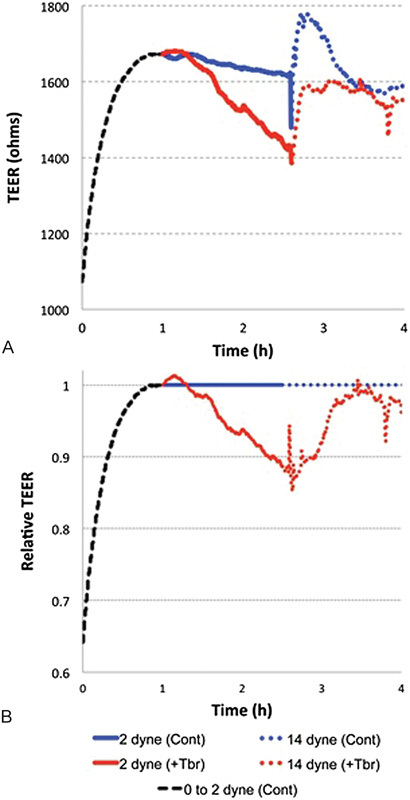
(A) Effect of shear stress and trypanosomes on BMEC TEER. At confluency under static conditions, BMEC TEER was around 1,100 Ω. This value increases to 60% when exposed to 2 dyn/cm2 laminar flow (black dashed line) as might occur in PCVs. When trypanosomes were added, a rapid decrease in TEER followed (red) relative to the control (blue). Shear stress was then increased to 14 dyn/cm2 causing a rapid but transient increase (red dashed) in BMEC TEER compared with the uninfected control (blue dash). Again, after a short time, TEER dropped and a second wave of barrier dysfunction followed. (B) Effects of shear stress and trypanosomes on BMEC normalizing control (blue) to 1. Representative of tao experiments and based on the mean of TEER readings from 7 to 8 independent electrodes, analysis of variance was determined by Student's t-test, 2-tails, and 2-sample equal variance. Statistical significance for data from 1 to 3 hours incubation was p < 0.05.
Phosphorylation of ERK1/2 and ERK5 were used as a measure of their activation.33 Fig. 3 shows that when human BMEC were exposed to LF at 2 and 14 dyn/cm2 for 4 hours, P-ERK5 did not significantly increase under any of the flow conditions in the presence or absence of trypanosomes. ERK1/2 levels also did not show they were affected by the parasites but may have been affected by different types of flow and SS. These findings support a previous study showing trypanosome crossing of human BMEC grown on Transwell (Corning, Tewksbury, MA) inserts is impaired by inhibitors of PI3K (phosphatidylinositol 3-kinase) and p38 MAPK, but not ERK1/2.34
Fig. 3.
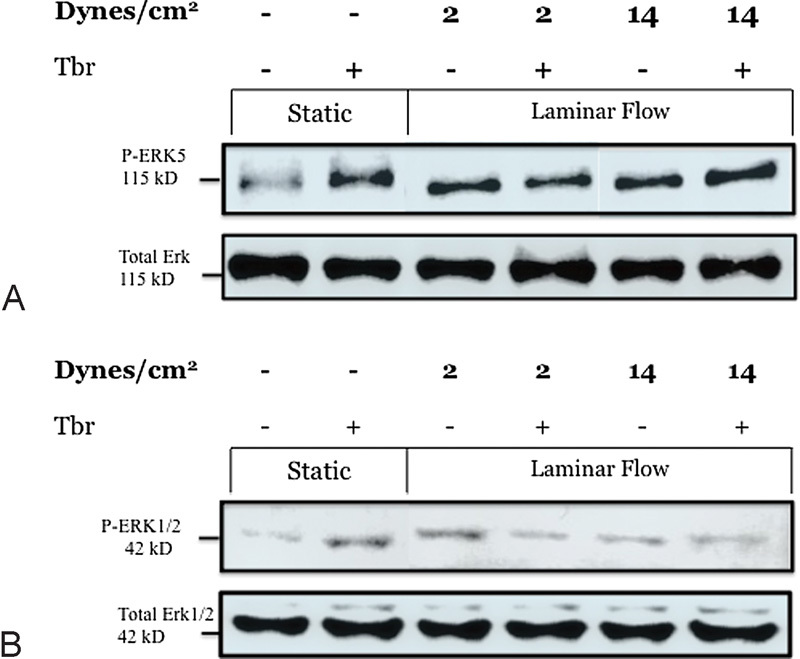
(A) ERK5 phosphorylation under static and laminar flow conditions with and without the addition of trypanosomes. P-ERK5 showed elevated levels in static cultures with the addition of trypanosomes or when exposed to laminar flow at 2 and 14 dyn/cm2 relative to the control. Exposure to both flow and trypanosomes did not further increase ERK5 activation, indicating that either a maximum level of phosphorylation was reached or that the two properties are not additive. (B) ERK1/2 showed a similar trend to ERK5 activation. Exposure to flow or static addition of trypanosomes increased levels of phosphorylation. Again, it appears that the two stimuli, flow and trypanosomes, did not further increase activation of ERK1/2.
Discussion
Our results demonstrate that African trypanosomes are able to induce BBB opening precursory to trypanosome transmigration into the brain under SS conditions found at PCVs, a known BBB entry site for the parasites. Our study also lend support to recent in vivo intravital and vibratome brain imaging studies in mice by us and others that show that African trypanosomes can enter the brain soon after entering the systemic circulation.
The results from the Western blot analysis reveal an increase in ERK1/2 and ERK5 activation with the addition of flow. Under static conditions, the addition of trypanosomes activated both ERK1/2 and ERK5. Similarly, exposure of BMECs to 2 and 14 dyn/cm2 of LF also showed elevated levels of phosphorylated ERKs. Under LF conditions, there appears to be an increase in expression levels in BMEC as SS under low PCV conditions (a putatively permissive BBB crossing site) is increased to the higher high SS conditions found in brain capillaries, a less permissive crossing site. Addition of trypanosomes did not change this profile.
While ERK5 was activated, our results indicate that activation by flow and trypanosomes are not additive processes. Confluent BMECs exposed to trypanosomes had similar levels of activation as those exposed to high and low SS. However, both the presence of flow and trypanosomes did not further elevate levels of P-ERK1/2 and P-ERK5. These data are in concurrence with our previous data using ERK5 silencing in BMECs. Addition of these knock out genes did not prevent a reduction in permeability in the presence of trypanosomes or flow. While ERKs may play a role in the trypanosomes mechanism of crossing the BBB, there appear to be other proteins and pathways involved.
In summary, we present a novel in vitro methodology for studying BBB function under dynamic flow conditions analogous to the in vivo environment. We demonstrate that by using an ECIS apparatus configured with LF, we can assess changes in human BMEC permeability. Our findings demonstrate that African trypanosomes cause changes in BBB permeability through an ERK1/2 and ERK5 independent pathway. Future studies to assess the role of other putative signal molecules that regulate permeability are warranted and will be facilitated using this in vitro model.
Acknowledgments
This research was supported in part by grants from the National Institutes of Health (NIH) (5R01AI082695) and Johns Hopkins Swami Institute for International Medical Education (SIIME-02) to DJ Grab, and from the University of Malaya-Ministry of Higher Education (UM-MOHE) (UM.C/625/1/HIR/MOHE/H-20001-00-E000053) to SD Sekaran.
Footnotes
Conflict of Interest The authors have declared that no conflict of interest exists.
References
- 1.Molyneux D H, Pentreath V, Doua F. London: Saunders WB; 1996. African trypanosomiasis in man. [Google Scholar]
- 2.Barrett M P, Burchmore R J, Stich A. et al. The trypanosomiases. Lancet. 2003;362(9394):1469–1480. doi: 10.1016/S0140-6736(03)14694-6. [DOI] [PubMed] [Google Scholar]
- 3.Jennings F W, Gray G D. Relapsed parasitaemia following chemotherapy of chronic T. brucei infections in mice and its relation to cerebral trypanosomes. Contrib Microbiol Immunol. 1983;7:147–154. [PubMed] [Google Scholar]
- 4.Jennings F W, Hunter C A, Kennedy P G, Murray M. Chemotherapy of Trypanosoma brucei infection of the central nervous system: the use of a rapid chemotherapeutic regimen and the development of post-treatment encephalopathies. Trans R Soc Trop Med Hyg. 1993;87(2):224–226. doi: 10.1016/0035-9203(93)90502-h. [DOI] [PubMed] [Google Scholar]
- 5.Frevert U, Movila A, Nikolskaia O V. et al. Early invasion of brain parenchyma by African trypanosomes. PLoS ONE. 2012;7(8):e43913. doi: 10.1371/journal.pone.0043913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myburgh E Coles J A Rodgers J et al. In vivo models of African trypanosomes to support drug discovery programs Proceeding of the XXXVII Annual Meeting of the Brazilian Society of Protozoology; September 19–21, 2011; Foz do Iguaçu
- 7.MacLean L, Myburgh E, Rodgers J, Price H P. Imaging African trypanosomes. Parasite Immunol. 2013;35(9–10):283–294. doi: 10.1111/pim.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacLean L M, Odiit M, Chisi J E, Kennedy P G, Sternberg J M. Focus-specific clinical profiles in human African Trypanosomiasis caused by Trypanosoma brucei rhodesiense. PLoS Negl Trop Dis. 2010;4(12):e906. doi: 10.1371/journal.pntd.0000906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abbott N J, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 10.Tarbell J M. Shear stress and the endothelial transport barrier. Cardiovasc Res. 2010;87(2):320–330. doi: 10.1093/cvr/cvq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neuwelt E, Abbott N J, Abrey L. et al. Strategies to advance translational research into brain barriers. Lancet Neurol. 2008;7(1):84–96. doi: 10.1016/S1474-4422(07)70326-5. [DOI] [PubMed] [Google Scholar]
- 12.Neuwelt E A, Bauer B, Fahlke C. et al. Engaging neuroscience to advance translational research in brain barrier biology. Nat Rev Neurosci. 2011;12(3):169–182. doi: 10.1038/nrn2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krizanac-Bengez L, Mayberg M R, Janigro D. The cerebral vasculature as a therapeutic target for neurological disorders and the role of shear stress in vascular homeostasis and pathophysiology. Neurol Res. 2004;26(8):846–853. doi: 10.1179/016164104X3789. [DOI] [PubMed] [Google Scholar]
- 14.Cucullo L, Hossain M, Puvenna V, Marchi N, Janigro D. The role of shear stress in blood-brain barrier endothelial physiology. BMC Neurosci. 2011;12(40):40. doi: 10.1186/1471-2202-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cucullo L, Hossain M, Tierney W, Janigro D. A new dynamic in vitro modular capillaries-venules modular system: cerebrovascular physiology in a box. BMC Neurosci. 2013;14:18. doi: 10.1186/1471-2202-14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kristensson K, Nygård M, Bertini G, Bentivoglio M. African trypanosome infections of the nervous system: parasite entry and effects on sleep and synaptic functions. Prog Neurobiol. 2010;91(2):152–171. doi: 10.1016/j.pneurobio.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Owens T, Bechmann I, Engelhardt B. Perivascular spaces and the two steps to neuroinflammation. J Neuropathol Exp Neurol. 2008;67(12):1113–1121. doi: 10.1097/NEN.0b013e31818f9ca8. [DOI] [PubMed] [Google Scholar]
- 18.Caffrey C R, Steverding D. Kinetoplastid papain-like cysteine peptidases. Mol Biochem Parasitol. 2009;167(1):12–19. doi: 10.1016/j.molbiopara.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Caffrey C R, Hansell E, Lucas K D. et al. Active site mapping, biochemical properties and subcellular localization of rhodesain, the major cysteine protease of Trypanosoma brucei rhodesiense. Mol Biochem Parasitol. 2001;118(1):61–73. doi: 10.1016/s0166-6851(01)00368-1. [DOI] [PubMed] [Google Scholar]
- 20.Troeberg L, Pike R N, Morty R E, Berry R K, Coetzer T H, Lonsdale-Eccles J D. Proteases from Trypanosoma brucei brucei. Purification, characterisation and interactions with host regulatory molecules. Eur J Biochem. 1996;238(3):728–736. doi: 10.1111/j.1432-1033.1996.0728w.x. [DOI] [PubMed] [Google Scholar]
- 21.Elliott E B, McCarroll D, Hasumi H. et al. Trypanosoma brucei cathepsin-L increases arrhythmogenic sarcoplasmic reticulum-mediated calcium release in rat cardiomyocytes. Cardiovasc Res. 2013;100(2):325–335. doi: 10.1093/cvr/cvt187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikeda M, Takei T, Mills I, Kito H, Sumpio B E. Extracellular signal-regulated kinases 1 and 2 activation in endothelial cells exposed to cyclic strain. Am J Physiol. 1999;276(2, Pt 2):H614–H622. doi: 10.1152/ajpheart.1999.276.2.h614. [DOI] [PubMed] [Google Scholar]
- 23.Nishimoto S, Nishida E. MAPK signalling: ERK5 versus ERK1/2. EMBO Rep. 2006;7(8):782–786. doi: 10.1038/sj.embor.7400755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nithianandarajah-Jones G N, Wilm B, Goldring C E, Müller J, Cross M J. ERK5: structure, regulation and function. Cell Signal. 2012;24(11):2187–2196. doi: 10.1016/j.cellsig.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Grab D J, Nikolskaia O, Kim Y V. et al. African trypanosome interactions with an in vitro model of the human blood-brain barrier. J Parasitol. 2004;90(5):970–979. doi: 10.1645/GE-287R. [DOI] [PubMed] [Google Scholar]
- 26.Kim Y V, Di Cello F, Hillaire C S, Kim K S. Differential Ca2+ signaling by thrombin and protease-activated receptor-1-activating peptide in human brain microvascular endothelial cells. Am J Physiol Cell Physiol. 2004;286(1):C31–C42. doi: 10.1152/ajpcell.00157.2003. [DOI] [PubMed] [Google Scholar]
- 27.Nikolskaia O V, de A Lima A P, Kim Y V. et al. Blood-brain barrier traversal by African trypanosomes requires calcium signaling induced by parasite cysteine protease. J Clin Invest. 2006;116(10):2739–2747. doi: 10.1172/JCI27798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nikolskaia O V. Expression of concern. J Clin Invest. 2008;118(5):1974. doi: 10.1172/JCI8707EX1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirumi H, Hirumi K. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J Parasitol. 1989;75(6):985–989. [PubMed] [Google Scholar]
- 30.Kadohama T, Nishimura K, Hoshino Y, Sasajima T, Sumpio B E. Effects of different types of fluid shear stress on endothelial cell proliferation and survival. J Cell Physiol. 2007;212(1):244–251. doi: 10.1002/jcp.21024. [DOI] [PubMed] [Google Scholar]
- 31.Azuma N, Akasaka N, Kito H. et al. Role of p38 MAP kinase in endothelial cell alignment induced by fluid shear stress. Am J Physiol Heart Circ Physiol. 2001;280(1):H189–H197. doi: 10.1152/ajpheart.2001.280.1.H189. [DOI] [PubMed] [Google Scholar]
- 32.Frangos J A Eskin S G McIntire L V Ives C L Flow effects on prostacyclin production by cultured human endothelial cells Science 1985227(4693):1477–1479. [DOI] [PubMed] [Google Scholar]
- 33.Rochier A, Nixon A, Yamashita N, Abe R, Madri J A, Sumpio B E. Laminar shear, but not orbital shear, has a synergistic effect with thrombin stimulation on tissue factor expression in human umbilical vein endothelial cells. Journal of vascular surgery: official publication, the Society for Vascular Surgery [and] International Society for Cardiovascular Surgery North American Chapter. J Vasc Surg. 2011;54(2):480–488. doi: 10.1016/j.jvs.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Patuzzi G Santos C C Grab D J Coombs G H Mottram J C Lima A PCA, eds. ICP regulation of Trypanosoma brucei cysteine peptidases: influence on parasite traversal of the brain barrier Second Kinetoplastid Molecular Cell Biology Meeting; 2007; Marine Biological Laboratory, Woods Hole, MA


