Abstract
Objective:
To compare the fracture resistance of simulated immature teeth filled with an apical barrier of mineral trioxide aggregate (MTA), Biodentine, and calcium-enriched mixture (CEM).
Materials and Methods:
Fifty-two single-rooted human maxillary central incisors were used. For standardization, the teeth were sectioned 6 mm above and 9 mm below the cementoenamel junction to simulate immature apex. Simulations of roots into immature apices were carried out using 1.5 mm diameter drills. The specimens were then randomly divided into three experimental groups (n = 13) and one control group (n = 13). In experimental groups, MTA, Biodentine, and CEM were placed to apical 4 mm of the simulated immature roots. The samples were stored at 37° C and 100% humidity for 1 week. A load was applied on the crown of all teeth at 135° to their long axis until fracture. The data were analyzed using one-way analysis of variance and Tukey post-hoc tests.
Results:
No statistically significant differences were found among MTA, CEM, and Biodentine (P > 0.05), and these groups demonstrated higher fracture resistance than control group (P < 0.05).
Conclusions:
Using any of the MTA, Biodentine, and CEM as an apical plug and restoring with fiber post and composite resin increases the fracture resistance of immature teeth.
Keywords: Biodentine, calcium-enriched mixture, immature teeth, mineral trioxide aggregate, vertical root fracture
INTRODUCTION
Traumatic dental injuries are most frequent in young and adolescent age, and the maxillary central incisors are mostly affected by resulting pulp necrosis.[1] If the root development of these teeth is incomplete, the clinician should consider apexification treatment.[2] The treatment of immature teeth is a challenge due to the weak dentinal walls and has a high incidence of root fracture.[3,4]
Numerous materials have been recommended for the apexification of immature teeth up to date. Calcium hydroxide (CH) has been used for the induction of an apical barrier in immature teeth due to the high pH and antimicrobial activity for years.[5,6] However, CH has some disadvantages; the CH treatment may extend up to 1 year and this may decrease the fracture resistance of immature teeth due to the long-term CH treatment.[7] In addition, CH changes the organic matrix of the dentin and this may increase susceptibility to root fracture.[4,8] Mineral trioxide aggregate (MTA) is recommended as an alternative material to CH for the induction of apical barrier of immature teeth.[9,10] MTA contains tricalcium aluminate, dicalcium silicate, tricalcium silicate, tetracalcium aluminoferrite, and bismuth oxide.[11] MTA is a biocompatible material and has low solubility capacity, ability to set in a wet environment and presence of blood.[12,13] However, it has some disadvantages such as difficulty of handling, long setting time,[14] and has an effect on the fracture resistance of immature teeth after exposure more than 5 weeks.[15] A variety of new calcium silicate-based materials have been developed recently to overcome the shortcomings of MTA. One of these materials is Biodentine, which contains tricalcium silicate, calcium carbonate, and zirconium oxide. Biodentine is a biocompatible material, has good sealing ability, high compressive strength, short setting time, and biomineralization properties.[16,17] Another calcium silicate-based material is calcium-enriched mixture (CEM), which is a new material that contains various calcium combinations, including oxide sulfate, phosphate, carbonate, silicate, hydroxide, and chloride compounds. CEM has a shorter setting time, lower viscosity, and less film thickness compared to MTA.[18,19]
Endodontically treated immature teeth are more susceptible to root fracture than mature teeth because of it has thin dentinal walls.[20,21,22] White et al. stated that MTA reduced the fracture resistance of the bovine dentin by 33%.[15] Few studies in the literature have evaluated the fracture resistance of MTA-based materials.[15,20] MTA is the preferred product on the market, whereas Biodentine and CEM are new materials. No in vitro study in literature could be found that evaluated the effect on root fracture of immature teeth after apexification with Biodentine and CEM. The aim of this study was to evaluate and compare the fracture resistance of simulated immature teeth after using MTA, Biodentine, and CEM as an apical plug.
MATERIALS AND METHODS
The study was approved by the Institutional Review Board of Şifa University (Protocol number 211–58), Izmir, Turkey. Fifty-two extracted, intact, human maxillary central incisors with single root and straight root canals were selected and stored in distilled water. For standardization, the teeth were sectioned 6 mm above and 9 mm below the cementoenamel junction using a diamond-coated bur under water cooling. The teeth were examined with a stereomicroscope under × 10 magnification (Zeiss, Oberkochen, Germany) to exclude any roots with open apices, root caries, cracks, or fractures. The buccolingual (BL) and mesiodistal (MD) dimensions of the root canals were measured using a digital caliper (Teknikel, Istanbul, Turkey). The weights of the roots were measured with a sensitive precision balance (Kern, Balingen, Germany). We equally splitted the roots to each group in an active sense based on their weights and the homogeneity of the groups. The roots were distributed into three experimental groups and one control group (n = 13). Then, the root canals were shaped with ProTaper Universal rotary files (Dentsply, Ballaigues, Switzerland) up to F5 size. Simulation of roots into immature apices was carried out using size 4 green 1.5 mm diameter UniCore® postdrills (Ultradent Products, Inc., USA). The smear layer was removed using 3 ml 17% ethylenediaminetetraacetic acid followed by 3 ml 5.25% NaOCl and 5 ml distilled water.
Group 1: The method followed was a modification of the technique described previously by Brito-Júnior et al.[23] White MTA mixed at a powder to liquid ratio of 3:1, MTA (Angelus, Londrina, PR, Brazil-M24929) was placed into the simulated immature roots and condensed with a hand plugger as a 4-mm apical plug. The apical height, homogeneity, and the thickness of apical plug were confirmed with radiograph [Figure 1]. The samples were stored at 37°C and 100% humidity for 1 week. The glass-fiber post UniCore® size 4 (Ultradent Products, Inc., USA) was cemented into the root canals with a self-adhesive resin cement (Bifix SE; Voco, Cuxhaven, Germany). The mixed resin cement was placed into the root canal, postinserted and cured with light for 20 s [Figure 2]. The fiber post was sectioned 3 mm above the cementoenemal junction and the remaining space was filled with a Grandio SO (Voco GmbH, Cuxhaven, Germany) composite resin and cured with light for 40 s.
Figure 1.
The apical height, homogeneity, and the thickness of mineral trioxide aggregate plug
Figure 2.
Mineral trioxide aggregate plug and backfilled with fiber post and composite resin
Group 2: Five doses of liquid and powder of Biodentine (Septodont, Saint-Maur-des-Fosses Cedex, France-B08889) was supplied for 30 s. With a mixed amalgamator, a 4-mm apical plug with Biodentine was placed into the simulated immature roots and condensed with hand plugger. And then, the same method of Group 1 was done for this group.
Group 3: The powder with the liquid of CEM (Yektazist Dandan, Tehran, Iran-C100501) was mixed for 15–30 s, and a 4-mm apical plug with CEM was placed into the simulated immature roots and condensed with hand plugger. And then, the same method of Group 1 was done for this group.
Group 4 (control): The apical roots of 4 mm were not filled with any of the MTA, Biodentine, and CEM. And then, the same method for postplacement procedure was applied as in the Group 1 for this group.
Periodontal ligament simulation
Periodontal ligament (PDL) simulation of the teeth was performed by the method described by Soares et al.[24] The teeth were immersed in wax with 0.2–0.3 mm thickness and 2.0 mm below the cementoenamel junction. Then, the teeth were embedded with 45° angled perpendicularly to the surface of self-cured acrylic resin blocks (Imicryl, Konya, Turkey) with dimensions of 25 mm high and 10 mm in diameter. After polymerization, the teeth were removed from the resin blocks; the wax was removed from root surface using warm water. The resin cylinders were filled with C-type silicone-based impression material (Zeta Plus, Zhermack, Badia Polesine Rovigo, Italy) and the teeth were inserted again into the resin blocks and excess impression material was removed. The roots were kept wet with a wet towel to prevent dehydration until they were ready for fracture testing.
Fracture strength test
The universal testing machine (Shimadzu Corporation, Kyoto, Japan) was used at a speed of 1 mm/min and a load was applied on the crown of all teeth at 135° to their long axis until fracture [Figure 3]. The values measured at the moment of fracture were recorded in Newton.
Figure 3.

The universal testing machine
Statistical analysis
The SPSS v11.5 (IBM, Chicago, IL, USA) software was used for statistical analysis. The BL and MD dimensions and weights were subjected to Kolmogorov–Smirnov statistical test to test the normality of these continuous variables. One-way analysis of variance test was used to evaluate the difference among the BL and MD dimensions and the weight of the samples. After completing the fracture test, the data were subjected to statistical analysis using one-way analysis of variance with Tukey post-hoc test for multiple comparisons. The testing was performed at the 95% level of confidence (P < 0.05).
RESULTS
The statistical analysis approved the standardization of roots among the groups according to weight, BL, and MD diameter.
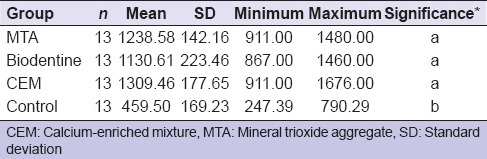
All the roots were fractured vertically that extended along the long axis of the root or fractures occurred in postmaterial conjunction [Table 1]. The mean, standard deviation, minimum, and maximum values of the groups were shown in Table 2. Statistically significant difference was found among the groups. According to the results of the present study, no statistically significant difference was found among MTA, CEM, and Biodentine (P > 0.05), and these groups demonstrated higher fracture resistance than control group (P < 0.05).
Table 1.
Fracture modes detected in all groups
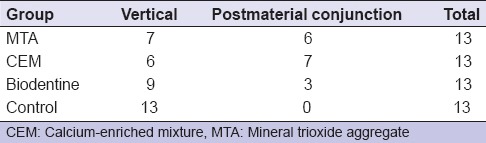
Table 2.
The mean, standard deviation, minimum, and maximum values of the groups
DISCUSSION
Standardization is an important factor in evaluating the fracture resistance of immature teeth. In the present study, BL, MD dimensions, and weights of the teeth were measured and no significant differences were found among groups. PDL is an important factor in the distribution of the stress in the root canals, and the fracture modes might be affected by the type of PDL simulation.[24] In the present study, the simulation of the PDL was performed using silicone-based impression material.
Immature teeth have a risk for root fracture because of it has weakened dentinal walls in root canals.[20,21,22] It has been suggested that using adhesive materials for filling immature root canals could improve the fracture resistance.[25,26] In addition, in another study, it has been suggested that using MTA for apical plug of the immature root canals and restoring with fiber post increases the fracture resistance of immature teeth[23] because elastic moduli of the fiber posts are similar with dentin and they can distribute loads in root canals by reducing the stress concentrations in root canals.[23] In the present study, we used MTA, Biodentine, and CEM for apical plug and fiber posts and composite resin for the restoration of immature teeth.
It has been reported that MTA increases the fracture resistance of teeth compared with CH.[20,27] Using MTA as a root canal filling material in immature teeth is an excellent choice due to its mechanical and biological properties.[2] Bortoluzzi et al.[2] stated in their study when MTA was used as apical plug and teeth restored with metallic posts, the fracture resistance of the teeth was four times higher than that of empty roots. According to the results of the present study, using MTA, Biodentine, or CEM as apical plug and teeth having restored with fiber posts, the fracture resistance of immature teeth was improved compared with control group. Control group had the lowest fracture strength among the groups. Using an apical plug with any of the MTA, Biodentine, and CEM has a major role to improve the fracture resistance of immature teeth.
Brito-Júnior et al.[23] evaluated the fracture resistance and stress distribution of simulated immature teeth after apexification with MTA and found that the teeth restored without fiber posts were more prone to root fracture in the cervical and middle thirds of the roots due to the stress concentration in these areas. They[23] stated that fiber posts were able to improve fracture resistance and stress distribution of immature teeth after apexification with MTA. In addition, according to the results of the present study, using any of the calcium silicate-based material and restoring immature teeth with fiber posts increased the fracture resistance which is similar to a study by Bortoluzzi et al.[2] and Brito-Júnior et al.[23]
The initial setting time of Biodentine was 9–12 min and final setting time was 45 min.[28] However, the setting time of MTA was 165 ± 5 min, which is a major drawback for MTA.[12,29] The initial setting time of CEM was 40 min and final setting time was 140 min,[30] which is longer than Biodentine. In addition, El-Ma'aita et al.[31] stated that particles of Biodentine are smaller than MTA and this provides Biodentine a better penetration capacity to dentine tubules. The differences between the setting time of the root and filling materials have a clinical significance.[30] According to the results of the present study, although there was no statistically significant difference among MTA, CEM, and Biodentine, the setting time and particle size gain an advantage to Biodentine compared with CEM and MTA. We might say that Biodentine is a good material for an apical plug in the apexification treatment of immature teeth due to good physical properties.
CONCLUSION
Within the limits of this study, it can be concluded that in the treatment of simulated immature teeth with open apices, using any of the MTA, Biodentine, and CEM as an apical plug and restoring with fiber post and composite resin increases the fracture resistance of immature teeth.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
Acknowledgment
The authors deny any conflicts of interest. I affirm that I/We have no financial affiliation (e.g., employment, direct payment, stock holdings, retainers, consultantships, patent licensing arrangements, or honoraria) or involvement with any commercial organization with direct financial interest in the subject or materials discussed in this manuscript, nor have any such arrangements existed in the past 3 years.
REFERENCES
- 1.Ravn JJ. Dental injuries in Copenhagen schoolchildren, school years 1967-1972. Community Dent Oral Epidemiol. 1974;2:231–45. doi: 10.1111/j.1600-0528.1974.tb01658.x. [DOI] [PubMed] [Google Scholar]
- 2.Bortoluzzi EA, Souza EM, Reis JM, Esberard RM, Tanomaru-Filho M. Fracture strength of bovine incisors after intra-radicular treatment with MTA in an experimental immature tooth model. Int Endod J. 2007;40:684–91. doi: 10.1111/j.1365-2591.2007.01266.x. [DOI] [PubMed] [Google Scholar]
- 3.Hemalatha H, Sandeep M, Kulkarni S, Yakub SS. Evaluation of fracture resistance in simulated immature teeth using Resilon and Ribbond as root reinforcements – An in vitro study. Dent Traumatol. 2009;25:433–8. doi: 10.1111/j.1600-9657.2009.00804.x. [DOI] [PubMed] [Google Scholar]
- 4.Cvek M. Prognosis of luxated non-vital maxillary incisors treated with calcium hydroxide and filled with gutta-percha. A retrospective clinical study. Endod Dent Traumatol. 1992;8:45–55. doi: 10.1111/j.1600-9657.1992.tb00228.x. [DOI] [PubMed] [Google Scholar]
- 5.Kontakiotis E, Nakou M, Georgopoulou M. In vitro study of the indirect action of calcium hydroxide on the anaerobic flora of the root canal. Int Endod J. 1995;28:285–9. doi: 10.1111/j.1365-2591.1995.tb00317.x. [DOI] [PubMed] [Google Scholar]
- 6.Mohammadi Z, Dummer PM. Properties and applications of calcium hydroxide in endodontics and dental traumatology. Int Endod J. 2011;44:697–730. doi: 10.1111/j.1365-2591.2011.01886.x. [DOI] [PubMed] [Google Scholar]
- 7.Andreasen JO, Farik B, Munksgaard EC. Long-term calcium hydroxide as a root canal dressing may increase risk of root fracture. Dent Traumatol. 2002;18:134–7. doi: 10.1034/j.1600-9657.2002.00097.x. [DOI] [PubMed] [Google Scholar]
- 8.Fuss Z, Lustig J, Katz A, Tamse A. An evaluation of endodontically treated vertical root fractured teeth: Impact of operative procedures. J Endod. 2001;27:46–8. doi: 10.1097/00004770-200101000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Torabinejad M, Pitt Ford TR, McKendry DJ, Abedi HR, Miller DA, Kariyawasam SP. Histologic assessment of mineral trioxide aggregate as a root-end filling in monkeys. J Endod. 1997;23:225–8. doi: 10.1016/S0099-2399(97)80051-9. [DOI] [PubMed] [Google Scholar]
- 10.Torabinejad M. Clinical applications of mineral trioxide aggregate. Alpha Omegan. 2004;97:23–31. [PubMed] [Google Scholar]
- 11.Lee SJ, Monsef M, Torabinejad M. Sealing ability of a mineral trioxide aggregate for repair of lateral root perforations. J Endod. 1993;19:541–4. doi: 10.1016/S0099-2399(06)81282-3. [DOI] [PubMed] [Google Scholar]
- 12.Parirokh M, Torabinejad M. Mineral trioxide aggregate: A comprehensive literature review – Part I: Chemical, physical, and antibacterial properties. J Endod. 2010;36:16–27. doi: 10.1016/j.joen.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Fridland M, Rosado R. MTA solubility: A long term study. J Endod. 2005;31:376–9. doi: 10.1097/01.don.0000140566.97319.3e. [DOI] [PubMed] [Google Scholar]
- 14.Parirokh M, Torabinejad M. Mineral trioxide aggregate: A comprehensive literature review – Part III: Clinical applications, drawbacks, and mechanism of action. J Endod. 2010;36:400–13. doi: 10.1016/j.joen.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 15.White JD, Lacefield WR, Chavers LS, Eleazer PD. The effect of three commonly used endodontic materials on the strength and hardness of root dentin. J Endod. 2002;28:828–30. doi: 10.1097/00004770-200212000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Koubi G, Colon P, Franquin JC, Hartmann A, Richard G, Faure MO, et al. Clinical evaluation of the performance and safety of a new dentine substitute, Biodentine, in the restoration of posterior teeth – A prospective study. Clin Oral Investig. 2013;17:243–9. doi: 10.1007/s00784-012-0701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laurent P, Camps J, About I. Biodentine (TM) induces TGF-ß1 release from human pulp cells and early dental pulp mineralization. Int Endod J. 2012;45:439–48. doi: 10.1111/j.1365-2591.2011.01995.x. [DOI] [PubMed] [Google Scholar]
- 18.Asgary S, Shahabi S, Jafarzadeh T, Amini S, Kheirieh S. The properties of a new endodontic material. J Endod. 2008;34:990–3. doi: 10.1016/j.joen.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Asgary S, Eghbal MJ, Parirokh M, Ghoddusi J, Kheirieh S, Brink F. Comparison of mineral trioxide aggregate's composition with Portland cements and a new endodontic cement. J Endod. 2009;35:243–50. doi: 10.1016/j.joen.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Tuna EB, Dinçol ME, Gençay K, Aktören O. Fracture resistance of immature teeth filled with BioAggregate, mineral trioxide aggregate and calcium hydroxide. Dent Traumatol. 2011;27:174–8. doi: 10.1111/j.1600-9657.2011.00995.x. [DOI] [PubMed] [Google Scholar]
- 21.Katebzadeh N, Dalton BC, Trope M. Strengthening immature teeth during and after apexification. J Endod. 1998;24:256–9. doi: 10.1016/s0099-2399(98)80108-8. [DOI] [PubMed] [Google Scholar]
- 22.Nur BG, Ok E, Altunsoy M, Tanriver M, Capar ID. Fracture strength of roots instrumented with three different single file systems in curved root canals. Eur J Dent. 2015;9:189–93. doi: 10.4103/1305-7456.156804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brito-Júnior M, Pereira RD, Veríssimo C, Soares CJ, Faria-e-Silva AL, Camilo CC, et al. Fracture resistance and stress distribution of simulated immature teeth after apexification with mineral trioxide aggregate. Int Endod J. 2014;47:958–66. doi: 10.1111/iej.12241. [DOI] [PubMed] [Google Scholar]
- 24.Soares CJ, Pizi EC, Fonseca RB, Martins LR. Influence of root embedment material and periodontal ligament simulation on fracture resistance tests. Braz Oral Res. 2005;19:11–6. doi: 10.1590/s1806-83242005000100003. [DOI] [PubMed] [Google Scholar]
- 25.Lawley GR, Schindler WG, Walker WA, 3rd, Kolodrubetz D. Evaluation of ultrasonically placed MTA and fracture resistance with intracanal composite resin in a model of apexification. J Endod. 2004;30:167–72. doi: 10.1097/00004770-200403000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Wilkinson KL, Beeson TJ, Kirkpatrick TC. Fracture resistance of simulated immature teeth filled with resilon, gutta-percha, or composite. J Endod. 2007;33:480–3. doi: 10.1016/j.joen.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Cauwels RG, Pieters IY, Martens LC, Verbeeck RM. Fracture resistance and reinforcement of immature roots with gutta percha, mineral trioxide aggregate and calcium phosphate bone cement: A standardized in vitro model. Dent Traumatol. 2010;26:137–42. doi: 10.1111/j.1600-9657.2010.00869.x. [DOI] [PubMed] [Google Scholar]
- 28.Grech L, Mallia B, Camilleri J. Investigation of the physical properties of tricalcium silicate cement-based root-end filling materials. Dent Mater. 2013;29:e20–8. doi: 10.1016/j.dental.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Torabinejad M, Hong CU, McDonald F, Pitt Ford TR. Physical and chemical properties of a new root-end filling material. J Endod. 1995;21:349–53. doi: 10.1016/S0099-2399(06)80967-2. [DOI] [PubMed] [Google Scholar]
- 30.Chng HK, Islam I, Yap AU, Tong YW, Koh ET. Properties of a new root-end filling material. J Endod. 2005;31:665–8. doi: 10.1097/01.don.0000157993.89164.be. [DOI] [PubMed] [Google Scholar]
- 31.El-Ma'aita AM, Qualtrough AJ, Watts DC. The effect of smear layer on the push-out bond strength of root canal calcium silicate cements. Dent Mater. 2013;29:797–803. doi: 10.1016/j.dental.2013.04.020. [DOI] [PubMed] [Google Scholar]


