Abstract
Objective:
This study was conducted to evaluate the effect of incorporation of silica particles with different concentrations on some properties of resin-modified glass ionomer cement (RMGIC): Microleakage, compressive strength, tensile strength, water sorption, and solubility.
Materials and Methods:
Silica particle was incorporated into RMGIC powder to study its effects, one type of RMGIC (Type II visible light-cured) and three concentrations of silica particles (0.06, 0.08, and 0.1% weight) were used. One hundred and twenty specimens were fabricated for measuring microleakage, compressive strength, tensile strength, water sorption, and solubility.
Statistical Analysis:
One-way analysis of variance and Tukey's tests were used for measuring significance between means where P ≤ 0.05.
Results:
RMGIC specimens without any additives showed significantly highest microleakage and lowest compressive and tensile strengths.
Conclusion:
Silica particles added to RMGIC have the potential as a reliable restorative material with increased compressive strength, tensile strength, and water sorption but decreased microleakage and water solubility.
Keywords: Class V, glass-ionomer, microleakage, silica particle, teeth restoration
INTRODUCTION
Incorporation of photopolymerizable components into conventional acid-base mixture leads to formation of hybrid materials named resin-modified glass ionomer. Composition of this material is complex and formed from many components include poly or modified poly (acrylic acid), photocurable monomer such as hydroxyethyl methacrylate or side chain grafted onto the poly (acrylic acid) and further photopolymerizable molecule used in composite resin such as bisphenol A-glycidyl methacrylate and an ion-leachable glass and water.[1]
Resin-modified glass-ionomer cements (RMGICs) formed of both GICs and resin composites. RMGICs acquire chemical adhesion and antibacterial properties from GICs, but from resin composites, RMGICs acquire many properties such as setting behavior, good mechanical properties, and wear resistance.[2] Comparison between GICs and RMGICs found that RMGICs have been characterized by longer working time and fast setting, translucency appearance, higher strength bonding to tooth structure,[3] and less microleakage.[4]
One positive effect of water absorption for filling materials is that it provides a mechanism for the potential compensation of polymerization shrinkage and the relaxation of stress.[5]
Silica filler (SF) addition on RMGIC gives good results after 24 h as compressive strength, diametral tensile strength, and flexural strength are increased, and water uptake,[6,7] marginal gaps in tooth cavities, and setting shrinkage decreased. So that addition of SF in a spherical shape to RMGIC powder improves flowability and workability of the cement based.[8,9]
In this study, the first hypothesis was that the addition of SF to RMGIC would reduce microleakage in Class V cavities and water solubility. The second hypothesis was that addition of SF would significantly increase the mechanical properties and water sorption of RMGIC since these properties correlate with interfacial gap formation.
Hence, the objective of this study was to evaluate the effect of incorporation of silica particles with different concentrations on some properties of RMGIC: Microleakage, compressive strength, tensile strength, water sorption, and solubility.
MATERIALS AND METHODS
A total of 120 specimens were used in this study. The specimens of RMGIC were divided into four main groups (30 each); Group A (RMGIC, control group), Group B (RMGIC powder with 0.06 weight % silica), Group C (RMGIC powder with 0.08 weight % silica), and Group D (RMGIC powder with 0.1 weight % silica). They were added separately to powder of RMGIC with concentrations 0.06, 0.08, and 0.1% weight every group was further subdivided into three subgroups (10 each) according to the type of test (microleakage, compressive and tensile strength, water sorption, and solubility).
Preparation of silica
A certain amount of sodium silicate was diluted by up to 400 ml of deionized water added to 200 ml of ethylene glycol to the above mixture. Precipitation of silica gel was performed by titration with formic acid while pH stayed below five. After centrifugation and washing for several times, the resulted ppt. was collected and dried at 40°C for 6 days. The obtained solid was calcined at 600°C (i.e., 100°C every 40 min). The fluoro-alumino-silicate glass powder and silica were hand mixed before the addition of the liquid was provided. The recommended powder–liquid ratio was used in all of the prepared specimens.
Microleakage
Forty caries-free freshly extracted human molar teeth (which had been extracted for periodontal disease or orthodontic treatment reasons) were collected; standardized Class V cavity was prepared on the buccal surface of each tooth. The dimensions of cavity were 2 mm occlusocervical, 3 mm mesiodistal width at occlusal margin, 2 mm mesiodistal width at gingival margin, and 2 mm axial depth.[10] The cavity was filled with RMGIC using and cured with light emitting diodes (LEDs) for 40 s.
For cavity preparation, a No. 2 round bur (Tungsten Carbide Bur, England) was used to gain access through the enamel, and then cavity preparation was completed using a No. 2 inverted cone bur followed by fissure carbide bur by high-speed hand piece with water coolant.
Teeth were evaluated by sealing root apices with sticky wax except the restorations and 1 mm from margins, other surfaces were coated with two layers of nail varnish to avoid dye penetration. Teeth were stored in distilled water for 48 h, then subjected to 500 thermal cycles between 5°C and 55°C of water baths, dwell time was 1 min with 10 s transmission time between baths.
After thermocycling, teeth were immersed in 3% methylene blue solution for 24 h. Then, all teeth were sectioned longitudinally into two half-buccolingual dimensions with low-speed diamond disc under water coolant.
Dye penetration was measured under stereomicroscope at ×10 magnification. Linear dye penetration was measured along gingival floor and axial wall. The ratio of linear dye penetration of gingival floor or axial wall was calculated, and percentage of dye penetration was obtained per each individual specimen.
Compressive strength
A specially designed Teflon mold of 4 mm in diameter and 6 mm in height was fabricated according to the International Standards Organization No. 9917 (2000).
Each group was mixed with glass-ionomer cement liquid on a glass slab using plastic cement spatula, then cement was condensed in Teflon mold, which was placed on a glass plate. Specimens were covered with celluloid strips and pressed with another glass plate. All specimens were exposed to LED for 40 s from both sides. The specimens were removed from the mold and stored in distilled water for 24 h before testing. Curing radiometer equipment was used to ensure light intensity through polymerization of all specimens.
Specimens were loaded on the Lloyd mechanical testing machine (Lloyd instrument, LRX plus PI, England) to measure at crosshead speed of 0.5 mm/min.
The load was applied until the specimen was crushed; the fracture of each specimen was recorded.
Diametric tensile strength
RMGIC is brittle material, therefore, measuring the tensile strength of RMGIC is done using an indirect tensile test.
A compressive load is placed on the diameter of cylindrical specimen. Compressive stress induces a tensile stress in the plan of application of the force. In such a situation, the tensile stress is directly proportional to compressive load.
Water sorption and solubility
A specially designed split Teflon mold was fabricated onto disc specimens with dimensions of 10 mm in diameter and 2 mm in thickness. These dimensions were performed according to Toledano et al.,[11] each group was mixed as described before for the compressive strength.
The discs were conditioned by placing in desiccator containing calcium sulfate at 37°C until a constant weight had been achieved (m0).
For weight measurements, an electronic analytical balance (PS.3A, Advanced Technology, Egypt) was used, and they were made to 0.0001 g; the complete device was mounted on an antivibration table.[11] The discs were placed in a glass vial containing 100 ml of distilled water. The vials were wrapped in aluminum foil to exclude light and placed in an incubator at 37°C at intervals (1 h, 6 h, 24 h, and subsequently at 2 days) removed, blot dried, and weighed, then returned to water; this was continued until a constant weight had been achieved (m1) and weight after 48 h then returned to be reweighed after 1 week.[12,13]
The discs were removed from water and replaced in a desiccator containing calcium sulfate at 37°C until a constant weight had been achieved.
They were subsequently dried by placing in a vacuum oven at 60°C for 24 h and reweighed for last time (m2).
These steps were carried out to evaluate water sorption (A) and water solubility (S) according to Oysaed and Ruyter[14] formula: A = m1 − m2/V S = m0 − m2/V Where m0 is the sample weight in mg before immersion, m1 is the sample weight in mg after immersion, m2 is the sample weight in mg after immersion and desiccation, and V is the specimen volume in mm3.
The recorded values were collected, tabulated, and statistically analyzed. One-way analysis of variance and Tukey's tests were used for testing the significance between means of tested properties of all tested materials when statistically significant when the P ≤ 0.05.
RESULTS
Microleakage
Group A (control group) showed the significantly highest mean microleakage followed by Group B then Group C. Group D showed the significantly lowest mean microleakage [Table 1].
Table 1.
Comparison between mean microleakage (%) groups of resin-modified glass ionomer cement
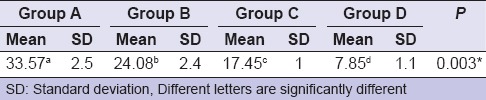
Compressive and tensile strengths
All groups of RMGICs (B, C, and D) showed compressive strength values higher than that of control group (A). RMGIC specimen (Group D) showed significantly highest mean compressive and tensile strengths followed by the RMGIC specimen (Group C) then RMGIC specimen (Group B). RMGIC specimens without any additives showed significantly lowest mean compressive and tensile strengths [Table 2].
Table 2.
Comparison between mean compressive and tensile strengths in (MPa) of resin-modified glass-ionomer cement
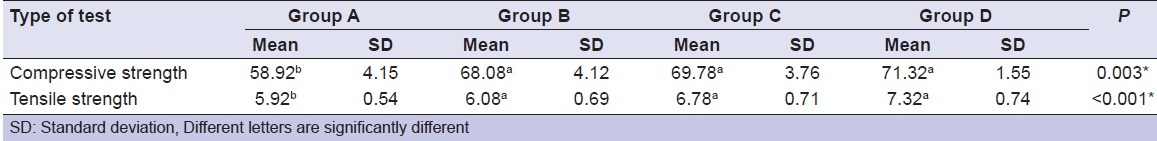
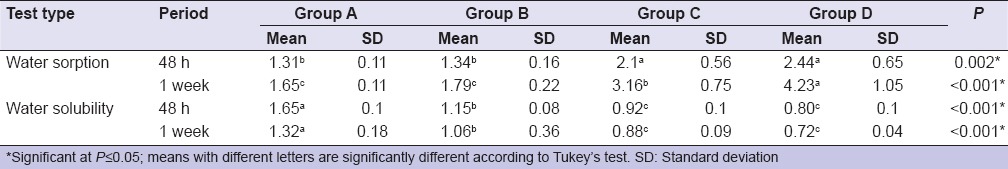
Water sorption and solubility
All specimens showed water sorption mean values higher than control group. RMGIC specimens (Group D) showed significantly highest mean water sorption followed (Group C) then (Group B). There was no statistically significant difference between control group (Group A) and RMGIC specimen (Group B). RMGIC specimens without any additives showed the significantly lowest mean water sorption rates.
There was no statistically significant difference between Group C and Group D in water solubility. The control group showed statistically significant highest mean solubility [Table 3].
Table 3.
Comparison between mean water sorption and solubility groups in (mg/mm3) of resin-modified glass ionomer cement
DISCUSSION
In this study, the addition of spherical SF significantly reduced interfacial gaps in RMGIC and water solubility but increased mechanical strength and water sorption.
Setting of resin-modified glass ionomer, especially in Class V cavities create leakage that contributes to failure of restoration and pulpal reaction.[15,16,17] The results of this study showed that addition of SF increases mechanical properties and the filler up to 0.1 weight % was most effective in improving of compressive and tensile strengths, these improvements occurred due to one or more of the following reasons:
First, SFs added to RMGIC was mixed with a higher powder/liquid ratio. It has been shown in previous studies that a higher powder/liquid ratio results in a smaller sum of interfacial gaps in the tooth cavity, and at the same time, imparts a greater mechanical strength to the GIC.
Second, SFs do not shrink, when the amount of fillers is increased this will lower the degree of shrinkage.[18,19]
Third, mechanical properties of RMGICs were improved using power/liquid ratios higher than recommended by the manufacturer. Addition of SF increased compressive strength, tensile strength, and flexural strength. This filler had the ability to adhere to the matrix by chemical bonding.[6]
Another reason for improved mechanical properties is silane-coupling agents that are used to reinforce adhesion of the filler to the matrix polymer.[20] The interaction between resin component of the matrix RMGIC and spherical SF was considered to be reinforced by silane-coupling agent.
RMGICs were shrinkage due to polymerization reaction of the monomers as well as the acid-base reaction.[21]
A filler-matrix coupling agent serves to enhance physical properties of RMGICs and allows for adequate wetting and dispersion of fillers within the resin matrices, although it does depend on the hydrophilicity of the silane-coupling agent.[22]
The fact of water solubility occurs due to most of the leachable materials come out of the composite within the first few hours,[23] days,[24] or 1 week[25] of soaking. This rapid release is due to ease mobility of low-molecular-weight species as well as further curing reaction were takes place after the initial light exposure and limiting number of molecules available to be leached.[24] The leaching was reduced by time due to the presence of unprotected filler at the surface after polishing; in this situation, this filler was leached at the beginning of exposed to the water immersion in the mouth.[10]
Basically, water sorption depends on the resin compositions,[26] in beginning glass-ionomer cement absorb water and disintegration of a surface layer as the main problem; glass particles, ions, and some of the organic materials can be found in the solvent.[27]
As setting reaction continued, and constant presence of water in the reaction of GICs lead to reduces solubility during the 1st h and days,[27] which the particles participate in the setting reaction leads to formation of a silica gel at the particle peripheries that link the filler core to the matrix phase.[28]
Reduction of water sorption values may have been due to the presence of the resin network, which leads to reduction of diffusion of water into the cement,[29] and not damage after water sorption.[30]
Data of the results of water sorption testing showed that reinforcing RMGIC with SF had effect on its water sorption rates. Addition of SF with different concentrations exhibited an increase of water sorption. This increase in water sorption may be attributed to the fact that SFs unsaturated the bonds of the polymer resin molecules or made imbalanced intermolecular force in the resin materials.[5] In agreement with these results are Tjandrawinata et al.[6] who found that water sorption of RMGIC was directly proportional to silica content and not to silica form or surface treatment.
CONCLUSION
On the basis of this study, we concluded that addition of silica particles to RMGICs increased the compressive strength, tensile strength, and water sorption rates but decreased microleakage and water solubility.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
REFERENCES
- 1.Forss H, Widström E. From amalgam to composite: Selection of restorative materials and restoration longevity in Finland. Acta Odontol Scand. 2001;59:57–62. doi: 10.1080/000163501750157090. [DOI] [PubMed] [Google Scholar]
- 2.Meyer JM, Cattani-Lorente MA, Dupuis V. Compomers: Between glass-ionomer cements and composites. Biomaterials. 1998;19:529–39. doi: 10.1016/s0142-9612(97)00133-6. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi M, Kon M, Miyai K, Asaoka K. Strengthening of glass-ionomer cement by compounding short fibres with CaO-P2O5-SiO2-Al2O3 glass. Biomaterials. 2000;21:2051–8. doi: 10.1016/s0142-9612(00)00096-x. [DOI] [PubMed] [Google Scholar]
- 4.Irie M. Suzuki K: Water storage effect on the marginal seal of resinmodified glass-ionomer restorations. Oper Dent. 1999;24:272–78. [PubMed] [Google Scholar]
- 5.Feilzer AJ, Kakaboura AI, de Gee AJ, Davidson CL. The influence of water sorption on the development of setting shrinkage stress in traditional and resin-modified glass ionomer cements. Dent Mater. 1995;11:186–90. doi: 10.1016/0109-5641(95)80016-6. [DOI] [PubMed] [Google Scholar]
- 6.Tjandrawinata R, Irie M, Yoshida Y, Suzuki K. Effect of adding spherical silica filler on physico-mechanical properties of resin modified glass-ionomer cement. Dent Mater J. 2004;23:146–54. doi: 10.4012/dmj.23.146. [DOI] [PubMed] [Google Scholar]
- 7.Tjandrawinata R, Irie M, Suzuki K. Marginal gap formation and fluoride release of resin-modified glass-ionomer cement: Effect of silanized spherical silica filler addition. Dent Mater J. 2004;23:305–13. doi: 10.4012/dmj.23.305. [DOI] [PubMed] [Google Scholar]
- 8.Forsten L. Fluoride release from a glass ionomer cement. Scand J Dent Res. 1977;85:503–4. doi: 10.1111/j.1600-0722.1977.tb00586.x. [DOI] [PubMed] [Google Scholar]
- 9.Nicholson JW, Braybrook JH, Wasson EA. The biocompatibility of glass-poly(alkenoate) (Glass-Ionomer) cements: A review. J Biomater Sci Polym Ed. 1991;2:277–85. doi: 10.1163/156856291x00179. [DOI] [PubMed] [Google Scholar]
- 10.von Fraunhofer JA, Adachi EI, Barnes DM, Romberg E. The effect of tooth preparation on microleakage behavior. Oper Dent. 2000;25:526–33. [PubMed] [Google Scholar]
- 11.Toledano M, Osorio R, Osorio E, Fuentes V, Prati C, Garcia-Godoy F. Sorption and solubility of resin-based restorative dental materials. J Dent. 2003;31:43–50. doi: 10.1016/s0300-5712(02)00083-0. [DOI] [PubMed] [Google Scholar]
- 12.Musanje L, Shu M, Darvell BW. Water sorption and mechanical behaviour of cosmetic direct restorative materials in artificial saliva. Dent Mater. 2001;17:394–401. doi: 10.1016/s0109-5641(00)00097-x. [DOI] [PubMed] [Google Scholar]
- 13.Ortengren U, Wellendorf H, Karlsson S, Ruyter IE. Water sorption and solubility of dental composites and identification of monomers released in an aqueous environment. J Oral Rehabil. 2001;28:1106–15. doi: 10.1046/j.1365-2842.2001.00802.x. [DOI] [PubMed] [Google Scholar]
- 14.Oysaed H, Ruyter I. Composites for use in posterior teeth: Mechanical properties tested under dry and wet conditions. J Biomed Mater Res. 1986;20:261–71. doi: 10.1002/jbm.820200214. [DOI] [PubMed] [Google Scholar]
- 15.Kouros P, Koliniotou-Koumpia E, Koulaouzidou E, Helvatjoglu-Antoniades M, Tziafas D. Pulp response to dentine adhesives: A study on mature human pulps. Eur J Dent. 2013;7(Suppl 1):S26–32. doi: 10.4103/1305-7456.119060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karaman E, Yazici AR, Aksoy B, Karabulut E, Ozgunaltay G, Dayangac B. Effect of operator variability on microleakage with different adhesive systems. Eur J Dent. 2013;7(Suppl 1):S60–5. doi: 10.4103/1305-7456.119075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irie M, Tjandrawinata R, Suzuki K. Effect of delayed polishing periods on interfacial gap formation of class V restorations. Oper Dent. 2003;28:552–9. [PubMed] [Google Scholar]
- 18.Arita K, Lucas ME, Nishino M. The effect of adding hydroxyapatite on the flexural strength of glass ionomer cement. Dent Mater J. 2003;22:126–36. doi: 10.4012/dmj.22.126. [DOI] [PubMed] [Google Scholar]
- 19.Hatanaka K, Irie M, Tjandrawinata R, Suzuki K. Effect of spherical silica filler addition on immediate interfacial gap-formation in class V cavity and mechanical properties of resin-modified glass-ionomer cement. Dent Mater J. 2006;25:415–22. doi: 10.4012/dmj.25.415. [DOI] [PubMed] [Google Scholar]
- 20.Halvorson RH, Erickson RL, Davidson CL. The effect of filler and silane content on conversion of resin-based composite. Dent Mater. 2003;19:327–33. doi: 10.1016/s0109-5641(02)00062-3. [DOI] [PubMed] [Google Scholar]
- 21.Davidson C, Mjör I. Advances in Glass-ionomer Cements. Chicago: Quintessence Publishing Co. Inc.; 1999. [Google Scholar]
- 22.Sakaguchi RL, Cross M, Douglas WH. A simple model of crack propagation in dental restorations. Dent Mater. 1992;8:131–6. doi: 10.1016/0109-5641(92)90068-n. [DOI] [PubMed] [Google Scholar]
- 23.Ferracane JL, Condon JR. Post-cure heat treatments for composites: Properties and fractography. Dent Mater. 1992;8:290–5. doi: 10.1016/0109-5641(92)90102-i. [DOI] [PubMed] [Google Scholar]
- 24.Pearson GJ. Long term water sorption and solubility of composite filling materials. J Dent. 1979;7:64–8. doi: 10.1016/0300-5712(79)90041-1. [DOI] [PubMed] [Google Scholar]
- 25.Braden M, Causton EE, Clarke RL. Diffusion of water in composite filling materials. J Dent Res. 1976;55:730–2. doi: 10.1177/00220345760550050501. [DOI] [PubMed] [Google Scholar]
- 26.Zui S, Arai K. A study on visible light-cured composite resins: The influence of long-term water immersion on physical and mechanical properties. Dent Mater J. 1986;5:602–15. [Google Scholar]
- 27.Um CM, Oilo G. The effect of early water contact on glass-ionomer cements. Quintessence Int. 1992;23:209–14. [PubMed] [Google Scholar]
- 28.Gladys S, Van Meerbeek B, Braem M, Lambrechts P, Vanherle G. Comparative physico-mechanical characterization of new hybrid restorative materials with conventional glass-ionomer and resin composite restorative materials. J Dent Res. 1997;76:883–94. doi: 10.1177/00220345970760041001. [DOI] [PubMed] [Google Scholar]
- 29.Peutzfeldt A, Asmussen E. Influence of ketones on selected mechanical properties of resin composites. J Dent Res. 1992;71:1847–50. doi: 10.1177/00220345920710111601. [DOI] [PubMed] [Google Scholar]
- 30.Davidson CL. Glass-ionomer bases under posterior composites. J Esthet Dent. 1994;6:223–4. doi: 10.1111/j.1708-8240.1994.tb00863.x. [DOI] [PubMed] [Google Scholar]


