Abstract
Objectives:
This study was primarily designed to determine the clinico-radiographic efficacy of platelet-rich fibrin (PRF) and beta-tri-calcium phosphate with collagen (β-TCP-Cl) in preserving extraction sockets.
Materials and Methods:
For Group I (PRF), residual sockets (n = 15) were filled with autologous PRF obtained from patients' blood; and for Group II (β-TCP-Cl), residual sockets (n = 15) were filled with β-TCP-Cl. For the sockets randomly selected for Group II (β-TCP-Cl), the reshaped Resorbable Tissue Replacement cone was inserted into the socket.
Results:
Clinically, there was a significantly greater decrease in relative socket depth, but apposition in midcrestal height in Group II (β-TCP-Cl) as compared to Group I (PRF), whereas more decrease in buccolingual width of Group I (PRF) than Group II (β-TCP-Cl) after 6 months. Radiographically, the mean difference in socket height, residual ridge, and width (coronal, middle, and apical third of socket) after 6 months was higher in Group I (PRF) as compared to Group II (β-TCP-Cl). The mean density (in Hounsfield Units) at coronal, middle, and apical third of socket was higher in Group I (PRF) as compared to Group II (β-TCP-Cl). There were statistically significant apposition and resorption for Group I (PRF) whereas nonsignificant resorption and significant apposition for Group II (β-TCP-Cl) in buccal and lingual/palatal cortical plate, respectively, at 6 months on computerized tomography scan.
Conclusion:
The use of either autologous PRF or β-TCP-Cl was effective in socket preservation. Results obtained from PRF were almost similar to β-TCP-Cl; therefore being autologous, nonimmune, cost-effective, easily procurable regenerative biomaterial, PRF proves to be an insight into the future biofuel for regeneration.
Keywords: Beta-tricalcium phosphate, extraction socket, platelet-rich fibrin, type I collagen
INTRODUCTION
Various bone-grafting and bone substitute materials have been used for ridge preservation procedures. The resorbable viable bone graft, beta tricalcium phosphate with type I collagen (β-TCP-Cl), has been utilized in orthopedic and other surgical specialties for almost 30 years. During breakdown and resorption of the graft, no cytotoxic compounds are released, and it disintegrate clinically, histologically, and radiographically. It provides sufficient period to place a dental implant in the grafted site.[1] It is commercially available as Resorbable Tissue Replacement (RTR) cone (Septodont, Saint-Maur-des-Fosses, France) for reconstruction of bone defects in maxillofacial and dental surgeries.[2] Synthetic graft biomaterials (β-TCP) stabilize the coagulum within the socket and avoid possible reduction of the hard tissue volume required for bone regeneration. Further, grafted material provides a scaffold for the in-growth of cellular and vascular components to form new bone of acceptable quality and quantity. β-TCP particles when mixed with the blood clot in the alveolar socket encircled by the bony walls, osteogenic cells (including undifferentiated mesenchymal stem cells) are stimulated by an adhesive glycoprotein, fibronectin, a component of the forming blood clot. They start migrating from the existing surrounding bone surface between and over the surface of β-TCP particles.[3,4] Moreover, type I collagen combined with β-TCP promotes osteogenesis by supporting osteoblastic differentiation and proliferation.[5,6] Brkovic et al.[2] exhibited that β-TCP-Cl composites could sufficiently maintain bone width and height for the preservation of the extraction socket.
Choukroun et al.[7] in 2001, first developed platelet-rich fibrin (PRF) which is a second-generation platelet concentrate. Since then, it has been used for the management of intrabony defects,[8,9] sinus lift techniques for implant placement and coverage of recession defects in the form of a membrane.[10] PRF is a viable and biocompatible autologous biologic material that can be used alone to maintain ridge dimension during preservation procedures while at the same time stimulating rapid osseous fill of the socket. Simon et al.[11] found that after 4 months of healing following ridge preservation using PRF, sockets were filled with a bone that appeared quite mature, and the sites did not exhibit discernable coronal invagination. Studies have reported that PRF could stimulate bone regeneration in situ without waiting for a normal body response.[12] PRF is a milieu of autologous fibrin, in which are embedded a large amount of platelets and leukocyte cytokines during centrifugation.[7] The intrinsic integration of cytokines within the fibrin mesh allows for their progressive release for 7–10 days, as the network of fibrin disintegrates. According to Simonpieri et al.,[13] the fibrin clot maintains and protects the grafted biomaterial, and PRF fragments serve as biological connectors between bone particles (mechanical role). The integration of this fibrin clot network into the regenerative site facilitates cellular migration, particularly for endothelial cells necessary for the neoangiogenesis, vascularization, and survival of the graft. Platelet cytokines (platelet-derived growth factor, transforming growth factor beta, insulin-like growth factor-1) are gradually released with the resorption of the fibrin matrix, thus generating prolong healing phenomenon. Leukocytes and cytokines present in the fibrin matrix may help in the self-regulation of inflammatory and infectious events within the grafted material.[13] Clinically, neovascularization forms through the PRF clot, and epithelial covering develops. Finally, in spite of the infection and inflammation of such sockets, rapid healing of the wound is observed without pain, dryness, or purulent complications. Dohan et al.[14] and He et al.[15] demonstrated that the PRF membrane stimulates its environment for a substantial period of remodeling and has a very significant slow unremitting release of critical growth factors for at least 7 days up to 28 days. The properties of this natural fibrin biomaterial thus offer a great potential for wound healing.
This study was primarily designed to determine the clinico-radiographic efficacy of PRF and β-TCP-Cl in preserving extraction sockets.
MATERIALS AND METHODS
This prospective, randomized, clinical trial was conducted in the Department of Periodontology from December 2012 to December 2014. The study design was approved by the Institutional Human Ethics Committee and written informed consents according to Helsinki's Declaration were obtained from all participants. For this study, a total of 26 (13 males/13 females) nonalcoholic, nonsmoker patients, within the age group of 19 years to 55 years (average age 31.22 ± 8.51 years) without any contributory medical history were selected from the outpatient Department of Periodontology [Figure 1].
Figure 1.
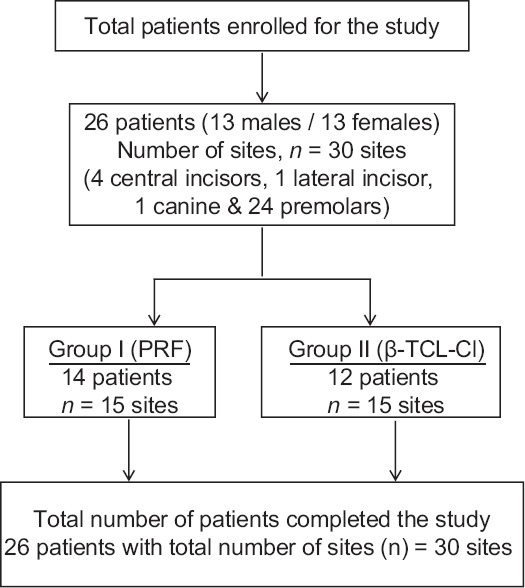
Flow chart: Study design
Inclusion criteria
(1) Patients aged more than 19 years who could read, understand, and were willing to comply with all study-related procedures after signing an informed consent statement. (2) Isolated alveolar sockets (a socket located between two sound teeth) of maxillary and mandibular monoradicular teeth, indicated for extraction, with at least 7 mm residual alveolar bone height as measured clinically and radiographically from periapical radiographs with intact socket walls. (3) Residual extraction sockets possessing intact bone in all dimensions (four-walled bony defects) and an occlusion suitable for the planned prosthodontic treatment. (4) The indications for tooth extraction were caries, endodontic complications (e.g., root fracture), periodontitis, and prosthetic reasons [Figure 2]. (5) Extractions next to saved teeth only and no multiple adjacent extractions included in the study.
Figure 2.
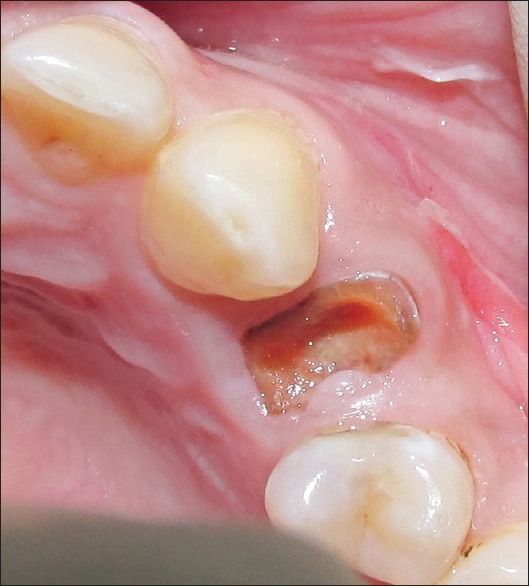
Root stump indicated for extraction
Platelet-rich fibrin
PRF was prepared as per suggested protocol of Choukroun et al.[7] by means of REMI Laboratories (India) table top centrifuge using whole venous blood (around 10 ml) in sterile dry glass test-tube without anticoagulant collected from the antecubital region of the forearm [Figure 3].[8] The centrifuge machine was positioned close to the dental chair, and all efforts were made to reduce the time period between the procurement of PRF and its placement in the extraction socket.[8,16]
Figure 3.
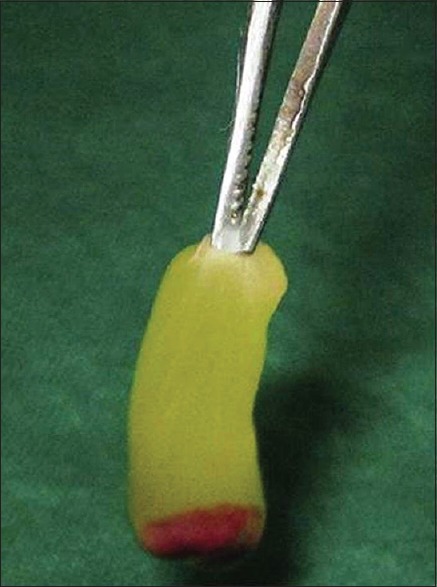
Platelet-rich fibrin obtained
Beta-tricalcium phosphate with type I collagen
β-TCP-Cl, RTR bone graft cones (β-TCP-Cl, Septodont, Saint-Maur-des-Fosses, France) is an osteoconductive material which has a chemical composition of natural bone (Ca8.3[PO4]4.3[HPO4, CO3]1.7[OH, CO3]0.3) that releases ions to favor bone regeneration. Tricalcium phosphate, a calcium phosphate system, exists mainly in alpha and beta phases. Both forms are resorbed simultaneously with new bone formation when they are used to promote healing of osseous defects.[17] Commercial RTR bone graft material is available as RTR syringes, RTR cones, and RTR 2cc granules. RTR cones used for the study are available as 0.3 cm3 cone (diameter 6 mm, length 10 mm) consisting of β-TCP-Cl in a sterile plastic bag and blister pack.[18]
Initial therapy
Patients fulfilling the selection criteria initially underwent emergency, if required, and phase I periodontal therapy that included oral hygiene instructions (patient's motivation and education), scaling and root planing, temporary/permanent restoration as per requirement to enhance patient's compliance, reduce bacterial load, and to avoid any embedment of calculus into the socket during extraction. Compliant patients were then recruited for surgical phase.
Surgical therapy
Compliant patients were appointed for surgical therapy. Before the start of the surgical procedure, operated sites were randomly assigned for desired treatment modality. The patients were then prepared for surgery using standard surgical protocol. The extraoral surface was mopped with betadine, and 0.2% chlorhexidine was used as the preprocedural rinse. After administration of local anesthesia (2% Xylocaine with 1:80000 adrenaline), extraction was performed for indicated tooth/root stump using periotomes, while minimizing trauma to the hard and soft tissues around the tooth being extracted.
Using #15 blade, intrasulcular incisions were made to elevate the adjacent interdental papillae and marginal gingiva. The periosteal elevator was used to reflect the flap. This resulted in the exposure of crestal bone around the sockets that allowed the direct visualization and measurement of the crestal bone level. Periotome was used for incising periodontal ligament (PDL) apical to alveolar crest and for wedging the tooth against the opposing cribriform plate. The initial use of a periotome helps in creating a better access point for the subsequent use of the luxator. The luxators were used to widen the PDL space and get some mobility of the tooth root being extracted [Figures 4 and 5]. Once the tooth/root stump is luxated, a traditional dental forceps may be used judiciously without harming the socket walls for pulling it out of the socket. The socket was completely debrided using surgical spoon curette. Copious irrigation of the socket was done and examined for any breach in the socket wall with the explorer/probe.
Figure 4.
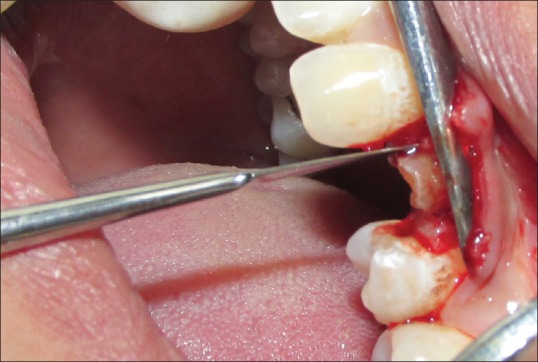
Periotome in position (on mesial side) for extraction
Figure 5.
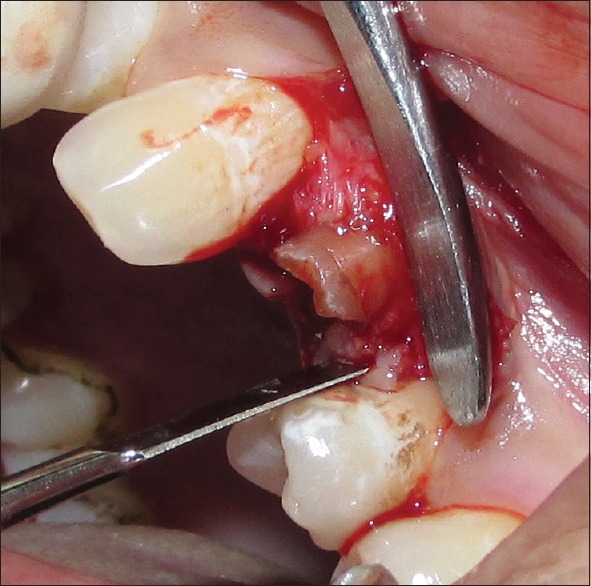
Periotome in position (on distal side) for extraction
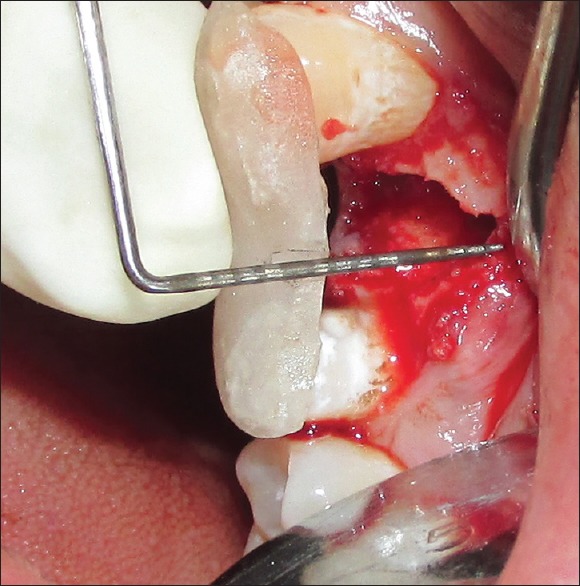
Residual sockets are then randomly treated for either of the experimental materials. For the Group I (PRF), residual sockets were filled with autologous PRF obtained from patients' blood [Figure 6]; and for Group II (β-TCP-Cl), residual sockets were filled with β-TCP-Cl. For the sockets randomly selected for Group II (β-TCP-Cl), the reshaped β-TCP-Cl was inserted into the socket [Figure 7]. To retain the grafted materials (PRF or β-TCP-Cl) and to close the wound, cross-mattress sutures were given for both the groups to avoid tension [Figure 8].
Figure 6.
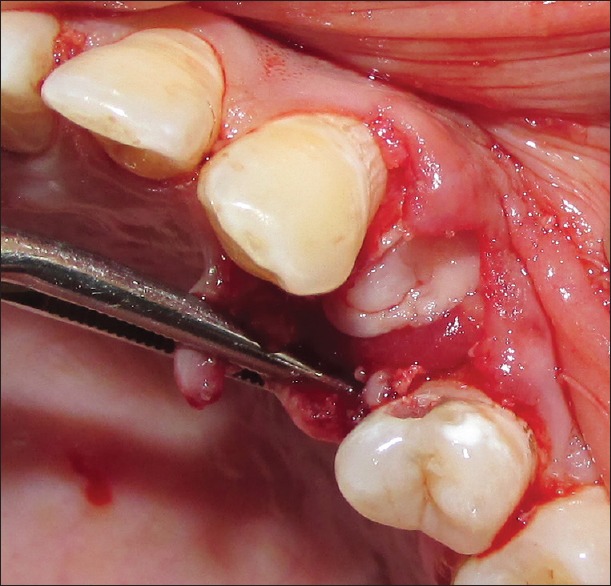
Platelet-rich fibrin in extraction socket
Figure 7.
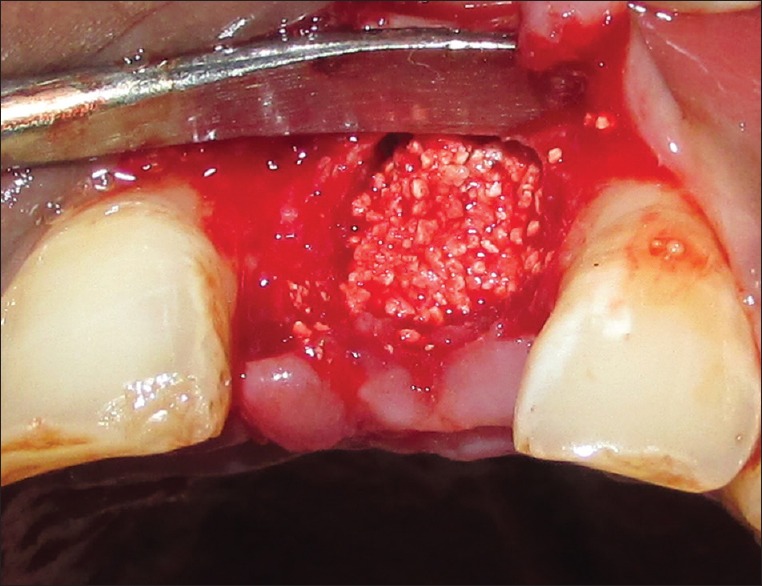
Beta-tricalcium phosphate with collagen (β- TCP-Cl) in extraction socket
Figure 8.
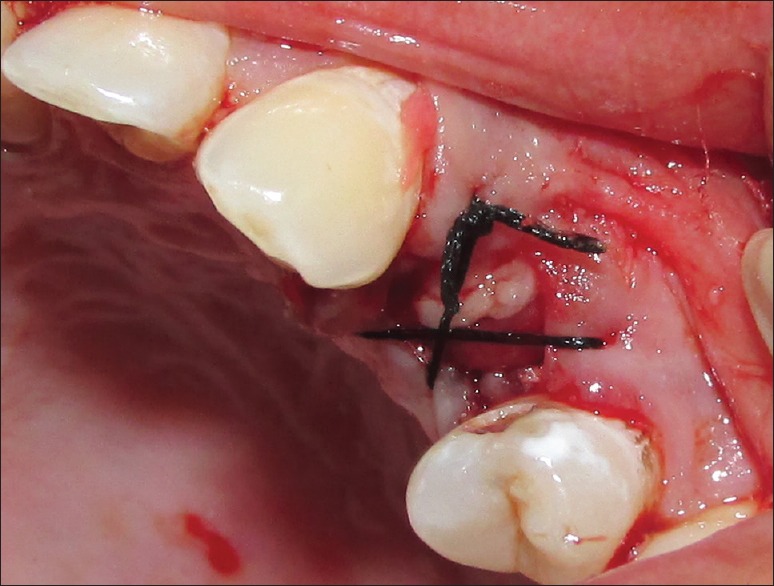
Crisscross suture on socket
Postoperative care
Written postoperative instructions were given. Patients were instructed to rinse with 0.2% chlorhexidine digluconate twice daily for a 2-week period. Amoxicillin, 500 mg, 3 times daily was given for 7 days, and analgesic medication (ibuprofen, 500 mg) was prescribed in case of postoperative pain. The sutures were removed 10 days following the surgical procedure.
Parameters recorded
All the periodontal health, clinical, and radiographic parameters were recorded at the baseline and 6 months.
Clinical parameters
To ascertain patient motivation and compliance following periodontal health, parameters were recorded on Ramfjord index teeth taken for the study. Clinical parameters were obtained at baseline and after 6 months.
To standardize the measurement of clinical parameters, acrylic stents were fabricated on the cast models of the dentition prepared during the treatment planning appointment of all the patients using self-cure clear acrylic resin. The surgical site was blocked with a layer of wax to avoid any impingement of the stent on the soft tissue. Acrylic stents were prepared up to 1/3rd of the crown covering one tooth adjacent on either side of the surgical site. A hole corresponding to the central part of the alveoli was made in the prepared acrylic resin stent, and grooves were prepared on the midbuccal and midpalatal/midlingual aspect of the stent corresponding to the respective cortical plates [Figure 9]. The stent allowed for accurate replications of clinical measurements from baseline at the surgical appointment to 6 months follow-up. On the cast model, two perpendicular lines were drawn through the center of the alveoli, one in the mesiodistal direction (line k) and another one in the buccolingual direction (line l). The distance of intersection of buccolingual line on mesiodistal line was recorded for reproducibility [Figure 10].
Figure 9.
Clinical parameter recording
Figure 10.
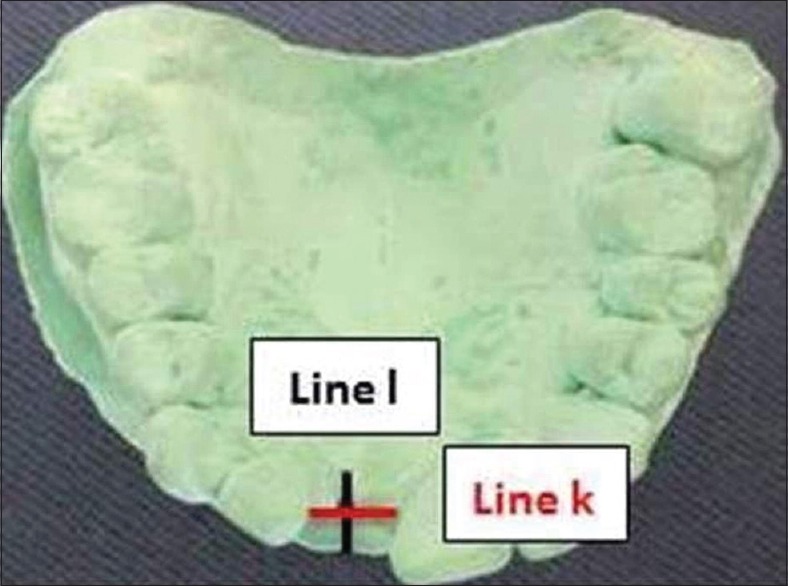
Cast model and line l and k for clinical measurement
Clinical horizontal dimensions (buccolingual width)
Buccolingual width was measured corresponding with the line (line l), 2 mm apical to coronal most point on the socket/residual ridge using Ridge Mapping Caliper (GDC, Punjab, India). Local infiltration was used while measuring buccolingual dimension at 6 months follow-up.
Clinical vertical dimensions
Mid-buccal crestal height
Corresponds to the distance from the fixed reference point (FRP) on the acrylic stent to the most coronal mid-buccal crestal point on the buccal cortical plate using a UNC-15 probe.
Mid-palatal/lingual crestal height
Corresponds to the distance from the FRP on the acrylic stent to the most coronal-mid palatal/lingual crestal point on the palatal/lingual cortical plate using a UNC-15 probe.
Relative socket depth
Corresponds to the distance measured through the central hole on the acrylic stent to the most apical end of the socket/ridge using a spreader (20 mm) with a stopper.
Radiographic parameters
For radiographic analysis multi-slice computerized tomography-computed (CT) scan was used. Multi-slice helical CT is considered one of the current modalities for measuring the bone density in Hounsfield units (HU). 64 slice CT Scan (Philips Brilliance 64 CT scanner, Italy, Rome) with DICOM software image viewer (RadiAnt DICOM Viewer 1.9.4 [32 bits]) installed at the Department of Radio-diagnosis, Dr. Ram Manohar Lohia Institute of Medical Sciences, Lucknow, India was used for the present study. Data were captured in high resolution of 0.5 mm voxel size with an exposure time of 3.5 s at 30 mA current, 120 kV, 200 mA s, CTDIvol 12.90 mGy, DLP 279.7 mGy-cm at phantom type body (32 cm) with field of view (FOV) of 180 mm. The digital data from the CT scan was transformed to a computer for processing using vision software RadiAnt DICOM Viewer 1.9.4 (32 bits). CT scan images with the constant slice thickness of 0.5 mm cross-section with a spacing of 1 mm were analyzed and recorded by a single trained technician at baseline and 6 months. The voltage, current, exposure time, and field of view were kept constant for each patient at both the time of exposure.
Radiographic (dental CT) parameters were recorded at baseline and after 6 months. For baseline data collection, dental CT scans were taken within 24 h after the surgery. Analysis of dental CT scan images was done using Dentascan software and images were marked and traced in panoramic, cross-sectional, axial, and sagittal view [Figure 11a]. Three-dimensional reconstruction images were also analyzed between baseline and 6 months [Figure 11b]. For measuring the radiographic parameters, same reference points and lines were used both at baseline and at 6 months as given below [Figure 12]:
Figure 11.
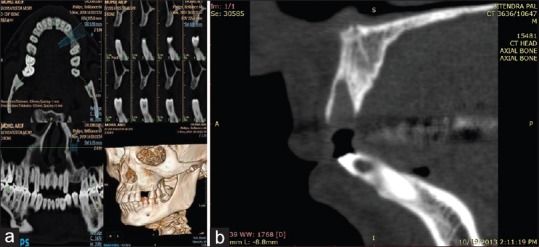
(a) Computerized tomography scan image analysis, (b) preoperative computerized tomography scan image
Figure 12.
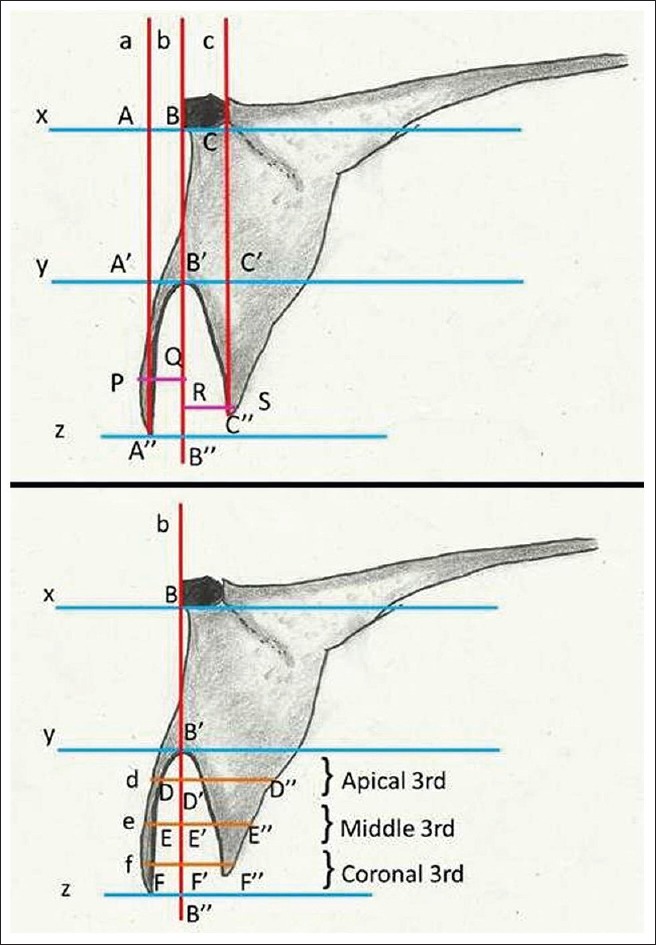
Radiographic landmarks
Line x: Drawn parallel to the horizontal plane at FRP on basal bone or a tangent drawn at FRP;
Line y: Drawn parallel to the horizontal plane at the most apical end of the socket;
Line z: Drawn parallel to the horizontal plane at the most coronal point on the residual socket/ridge;
Line a: A perpendicular drop from “line x” up to the most coronal point on the buccal plate and intersect “line x” at point A, “line y” at point A', and “line z” at point A”;
Line b: A perpendicular drop from “FRP” on “line x” to “line z” and intersect “line x” at point B, “line y” at point B', and “line z” at point B”;
Line c: A perpendicular drop from “line x” up to the most coronal point on the palatal/lingual plate and intersect “line x” at point C, “line y” at point C', and “line z” at point C”;
Line d: The horizontal line at middle of apical third of the socket and intersect “line b” at point D';
Line e: The horizontal line at middle of middle third of the socket and intersect “line b” at point E';
Line f: The horizontal line at the middle of coronal third of the socket and intersect “line b” at point F'.
Point D, E, and F represent the buccal most point at apical, middle, and coronal third of the socket and point D”, E”, F” represent the palatal/lingual most point at apical, middle, and coronal third of the socket correspond to “line d,” “e,” and “f” respectively.
Radiographic vertical dimensions
VD of basal bone (BB')
The vertical distance from the “line x” to the “line y” depicting the vertical dimension of basal bone.
Buccal cortical height (A'A”)
The vertical distance from the “line y” to the coronal-most point on the buccal cortical plate depicting the vertical distance of the buccal cortical plate.
Palatal/lingual cortical height (C'C”)
The vertical distance from the “line y” to the coronal-most point on the palatal/lingual cortical plate depicting the vertical distance of the palatal/lingual cortical plate.
Socket depth (B'B”)
The vertical distance from the “line y” to the “line z” depicting the vertical dimension of the socket/residual ridge.
Radiographic horizontal dimensions
Horizontal width at coronal 3rd (FF”)
The width of residual socket/ridge at coronal third.
Horizontal width at middle 3rd (EE”)
The width of residual socket/ridge at middle third.
Horizontal width at Apical 3rd (DD”)
The width of residual socket/ridge at apical third.
Buccal plate width (line PQ)
Distance of the most coronal-buccal point on the buccal cortical plate from “line b”.
Lingual/palatal plate width (line RS)
The distance of the most coronal-palatal/lingual point on the palatal/lingual cortical plate from “line b”.
Point D', E,' and F' on “line b” at the intersection of “line d,” “line e,” and “line f,” respectively, were recorded at baseline for reproducibility at follow-up.
Density in Hounsfield unit
The density was recorded in reconstructed sagittal CT scan images using Radiant DICOM image software. The density was recorded at the center of the socket at the apical, middle, and coronal third, as well as at the buccal, palatal/lingual wall of the socket. To standardize and reproduce the site, all the baseline and 6 months, follow-up measurements were recorded on “line b” at coronal 3rd, at middle 3rd, and apical 3rd.
Histological examination
At the end of 6 months, bone biopsies were procured from the preserved socket under local anesthesia after raising full-thickness flap in selected patients two from Group I (PRF) and two from Group II (β-TCP-Cl). A surgical trephine (GDC, Punjab, India) with the external diameter of 3 mm was used for harvesting 2 mm × 6 mm of newly formed bone from the center part of the preexisting socket. The osteotomy sites obtained after harvesting core biopsy were then enlarged and deepened to receive endosseous dental implants. Tissue biopsy harvested at osteotomy sites were sent for histological examination.
Specimens obtained were immediately fixed in 10% neutral buffered formalin and were subjected to acid decalcification using 10% formal formic acid. The decalcified tissues were washed overnight in running water and processed for embedding in paraffin wax. Briefly, the tissues were dehydrated in ascending grades of alcohol, cleared in two changes of xylene, and impregnated with paraffin wax. Five micron thick sections were prepared using automatic rotary microtome (York, Scientific Industry Pvt Ltd., Sahibabad, India). The sections were stained with routine (hematoxylin and eosin) (H and E) stain and analyzed in bright field microscopy using Olympus BX51 microscope.
Statistical tools employed
The statistical analysis was done using Statistical Package for Social Sciences (SPSS) Version 15.0 (SPSS Inc., Chicago, IL, USA) Statistical Analysis Software. The values were represented in number (%) and mean ± standard deviation. As the sample size was small, hence a nonparametric evaluation plan was followed. Intergroup comparisons were prepared using the Mann–Whitney U-test, and intragroup-group change was studied using the Wilcoxon signed rank test. To test the significance of two means the Student's t-test and Fisher exact test for a cross-tabulation were used.
RESULTS
For this study, a total of 26 patients (13 males, 13 females) fulfilling the inclusion criteria were initially enrolled for the study. All patients completed the study uneventfully. Maximum numbers of patients were aged between 20 and 30 years. In Group I (PRF), 41.7% of patients were male and remained 58.3% were females whereas in Group II (β-TCP-Cl), 45.5% were male, and 54.5% were females. Statistically, there was no significant difference between two groups. The mean age of patients in Group I (PRF) was 30.25 ± 8.65 years as compared to 32.27 ± 8.64 years for patients in Group II (β-TCP-Cl). Statistically, this difference was not significant. Thus, the two groups were matched for age and gender. Upper left first premolar (24) was the most common site in Group I (PRF) (30.4%) while lower left first premolar (34) was the most common site in Group II (β-TCP-Cl) (30.4%).
Clinical findings
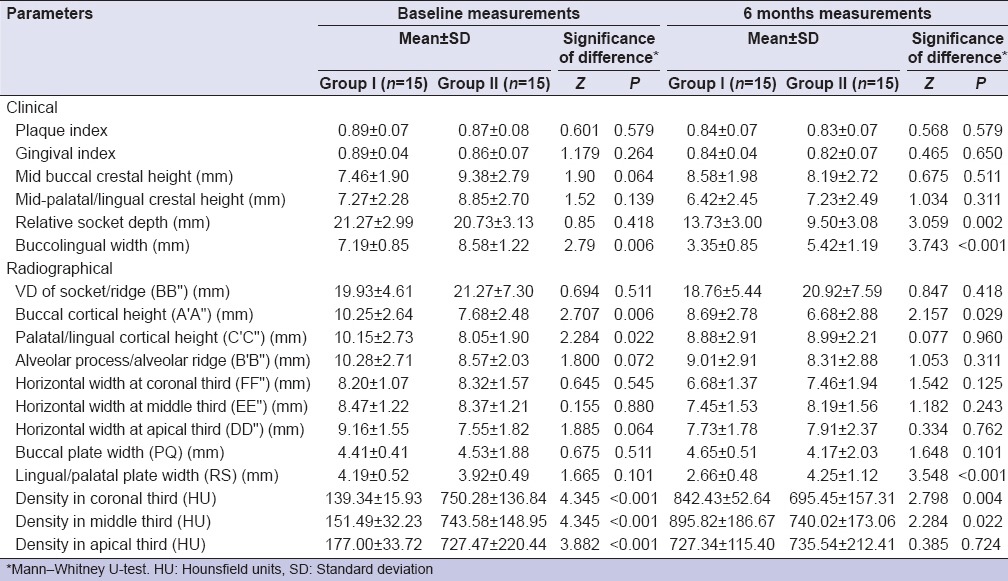
At baseline, all the clinical parameters except relative socket depth of Group II (β-TCP-Cl) were higher in contrast to that of Group I (PRF). At 6 months, except for mid-buccal crestal height for which an increase in Group I (PRF) was observed, for all the other parameters both the groups showed a reduction. The reduction was higher in Group II (β-TCP-Cl) as compared to Group I (PRF) for all the parameters and difference between two groups was also significant (P < 0.05) [Table 1].
Table 1.
Clinical and radiographic parameters at baseline and 6 months
Radiographic findings
At baseline, the radiological investigation revealed that statistically significant differences between the two groups were observed only for A'A” and C'C” (P < 0.05), for both the parameters, the mean value of Group I (PRF) was higher as compared to that of Group II (β-TCP-Cl) at baseline. Except for BB” and PQ, for all parameters baseline mean value of Group I (PRF) was higher as compared to that of Group II (β-TCP-Cl). For BB” and PQ, baseline value of Group II (β-TCP-Cl) was higher as compared to that of Group I (PRF) [Table 1].
For A'A”, B'B,” and PQ, at 6 months value of Group I (PRF) was higher as compared to that of Group II (β-TCP-Cl). Statistically significant differences between two groups were observed only for A'A” and RS (P < 0.05). For A'A”, values of Group I (PRF) were significantly higher as compared to that of Group II (β-TCP-Cl) and for RS, values of Group II (β-TCP-Cl) were significantly higher as compared to that of Group I (PRF). Statistically significant differences between two groups were observed for all the parameters except A'A”, EE,” and PQ [Table 1].
For density, for all the three locations, Group II (β-TCP-Cl) had significantly higher density as compared to Group I (PRF) (P < 0.001) at baseline. At 6 months interval density in Hounsfield, for coronal and middle third of the socket in Group I (PRF) had significantly higher mean value as compared to Group II (β-TCP-Cl) (P < 0.05). For apical third, the mean value of Group II (β-TCP-Cl) was higher as compared to that of Group I (PRF), but this difference was not significant statistically. In Group I (PRF), for all the three sites, an increase in mean value was observed whereas in Group II (β-TCP-Cl), except for a minor increase at apical third at both the other locations a decrease in mean value was observed [Table 1].
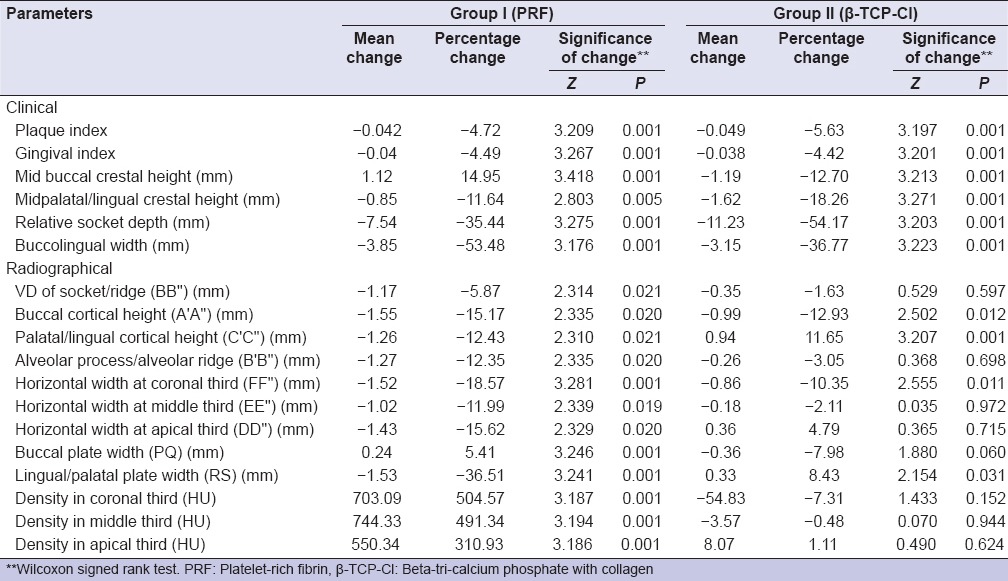
Within group, all the clinical and radiographic parameters, a statistically significant postintervention change was observed in Group I (PRF) at 6 months interval as compared to baseline values. In Group II (β-TCP-Cl), a significant change in mean values of plaque index (PI), gingival index (GI), mid-buccal crestal height, mid-palatal/lingual crestal height, relative socket depth, and buccolingual width was observed after 6 months postintervention. For radiographic parameters, though mean value changes were observed, however, the majority of these changes were not significant statistically [Table 2].
Table 2.
Mean change in clinical and radiographic parameters at baseline and 6 months
Histological findings
In Group I (PRF) the histological sections stained with H and E showed well-formed mature bone with cortical as well as cancellous bone. The bony trabeculae are well formed with adequate medullary spaces that are filled with fatty tissue. No evidence of inflammatory infiltrates seen [Figure 13].
Figure 13.
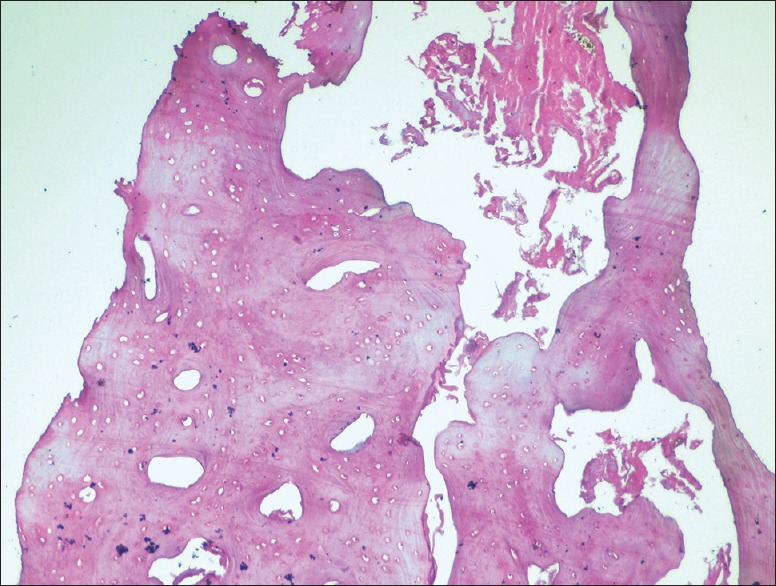
Six months histologic images at ×10 for Group I (platelet-rich fibrin)
In Group II (β-TCP-Cl), the histological sections stained with H and E showed predominantly cortical bone showing well-formed osteons with Haversian canals, lamellae, and osteocytes. Minimal medullary spaces are seen. Foci of amorphous, structureless, and eosinophilic deposits are evident, possibly remnants of graft material [Figure 14].
Figure 14.
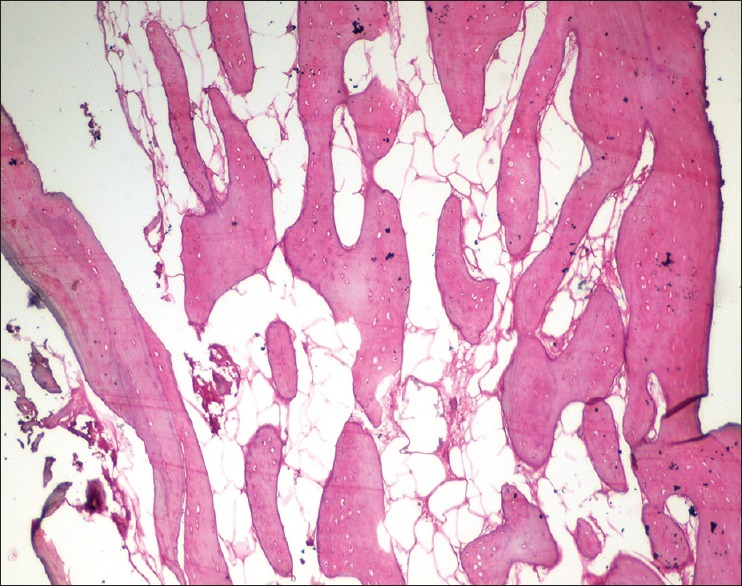
Six months histologic images at ×10 for Group II (β-tri-calcium phosphate with collagen)
DISCUSSION
With a rationale for achieving optimal residual ridge that reduces the need for future ridge augmentation to obtain optimal, functional, and esthetic results, this study was conducted to determine the efficacy of PRF and β-TCP-Cl in preserving extraction sockets.
The mean age difference between the Group I (PRF) and Group II (β-TCP-Cl) was nonsignificant. Although, there is no age or gender prediction for socket preservation procedures yet younger age group may be considered as a preferred group for regenerative surgeries as healing.[19] As the sample size was small and heterogeneous, hence a nonparametric evaluation plan was followed. Tomkins[20] suggested the application of the nonparametric test in health science research to analyze data from descriptive studies and small sample size studies.
At 6 months, there was a statistically significant reduction in PI and GI value for both groups I (PRF) and Group II (β-TCP-Cl). The mean reduction in PI and GI scores for the Group I (PRF) and Group II (β-TCP-Cl) showed statistically nonsignificant difference between two groups after 6 months. Overall good oral hygiene during the study may have resulted because of periodic recall visits in which the patients were regularly reinforced for oral hygiene maintenance and underwent supragingival scaling if required.[21]
All the clinical parameters were recorded from an FRP on the acrylic stent fabricated on the cast as advocated by Talwar et al.[22] Similar to their study,[22] in our study acrylic stent was not used for measuring the buccolingual width. Instead, a bone mapping caliper was used for socket width measurements clinically.[23] For reproducibility of buccolingual measurements, points on arbitrarily drawn perpendicular lines on the cast were utilized as explained in material and methods.
Intergroup comparison of clinical parameters between Group I (PRF) and Group II (β-TCP-Cl) of our study observed a decrease in mid-buccal crestal height in Group I (PRF) and buccolingual/palatal width of both the groups, whereas increase for all the other parameters in Group I (PRF) as well as Group II (β-TCP-Cl), as compared to baseline. The reduction was higher in Group II (β-TCP-Cl) as compared to Group I (PRF) for all the parameters and difference between two groups was also statistically significant (P < 0.05). Suttapreyasri and Leepong[12] reported resorption of 1.96 ± 1.10 mm and 1.59 ± 0.64 mm, respectively, in the buccal and lingual marginal bones 8 weeks after the tooth extraction. They also reported a significant change in buccolingual width concurrent with our study.[12] Brkovic et al.[24] reported a significant reduction of the horizontal dimension in β-TCP with type I collagen group 9 months after socket preservation, however, reported nonsignificant vertical resorption as compared to baseline.
CT image scan was used for the analysis and comparison of osseous changes and compared before and after surgical procedures. The socket/ridge height, buccal cortical height, and lingual/palatal cortical height were calculated by subtracting the distance of the coronal most point up to the apical end of the socket from the total distance measured from the FRP on the basal bone.[25]
In the present study, statistically significant apposition was observed in palatal/lingual cortical plate height as the radiopaque image, after 6 months. The mean difference of comparative postintervention change between the two groups was statistically significant for palatal/lingual cortical height and residual alveolar process/ridge, but nonsignificant for vertical dimension of socket/ridge and buccal cortical height. Suttapreyasri and Leepong[12] also reported accumulative radiographic resorption of marginal bone levels of 0.70 mm and 1.23 mm, respectively, at mesial and distal PRF grafted extraction sockets. Brkovic et al.[24] reported nonsignificant apposition in buccal and palatal/lingual vertical dimensions in β-TCP-Cl grafted extraction sockets in a clinico-histological study. To the best of author's knowledge, none of the publications has compared and reported efficacy of platelet-rich fibrin and/or beta-tricalcium phosphate in socket preservation using CT scan. Madan et al.[25] reported an increase in palatal/lingual cortical plate height in poly (lactic acid)-poly (glycolic acid) grafted site on CT scan.
There was a statistically significant greater reduction in the coronal third of alveolar ridge in Group I (PRF) as compared to Group II (β-TCP-Cl), but the reduction after 6 months postintervention was nonsignificant in the middle third. For horizontal width at apical third of alveolar ridge, we observed apposition in Group II (β-TCP-Cl), whereas resorption in Group I (PRF) and the difference was a statistically significant. A systematic review published by Hämmerle et al.[26] reported the alveolar ridge undergoes a mean horizontal reduction in width of 3.80 mm and an average vertical reduction in height of 1.24 mm within 6 months after tooth extraction without ridge preservation therapies. A case series by Suttapreyasri and Leepong[12] reported radiographic resorption on the horizontal dimension in socket orifice 8 weeks after PRF grating in extraction sockets.
For the 1st time, our study presents dimensional changes in the buccal plate and palatal/lingual plate position 6 months after socket preservation technique. To measure dimensional changes in the buccal and lingual plate, a reproducible perpendicular line was dropped from FRP on the basal bone. The distance of buccal/labial-most and palatal/lingual-most points on buccal and palatal/lingual plate were then measured from the perpendicular line drawn both at baseline and at 6 months as detailed in materials and methods.
The present study also determines the change in density of alveolar socket/ridge from baseline to 6 months using a CT scan. The density was recorded at the center of the socket both at apical, middle and coronal third both at baseline and at 6 months follow-up. It was observed that the mean density determined at 6 months were comparable to normal bone, as documented by Louis and Carl,[27] who reported the bone density values of >1250 HU, 850–1250 HU, 350–850 HU, 150–350 HU, and <150 HU in D1, D2, D3, D4, and D5, respectively.
Within Group I (PRF), mean density values were highest in the middle third followed by coronal third and least in the apical third, whereas for Group II (β-TCP-Cl) mean density values were highest in the middle third followed by apical third and least in the coronal third at 6 months follow-up. In contrast to results obtained from earlier studies, where more evident density was observed in apical part of the socket/ridge as compared to the coronal and middle third.[25] As compared to baseline for mean density values, there was a significant increase in Group I (PRF) and there was the nonsignificant change for Group II (β-TCP-Cl). Although, there was a statistically significant difference between the two groups; however, while analyzing the results it should be noted that at baseline, the mean values of density for Group I (PRF) was attributed to PRF gel structure, whereas for Group II (β-TCP-Cl) the mean density values were attributed to the mineralized bone graft material (β-TCP-Cl). In Group II (β-TCP-Cl) at 6 months, there was the decrease in mean density value at middle third and the coronal third of the socket/ridge and increase at apical third as compared to baseline. The results obtained are in accordance with the already published report that documented the increase in density at apical third as compared to baseline.[25] Cardaropoli et al.[28] observed that bone formation initiated from the lateral and apical socket wall toward the center of the wound. Further, these density changes in Group II (β-TCP-Cl) may also be influenced by the resorption pattern of β-TCP-Cl. Published reports have suggested that β-TCP-Cl shows vary resorption pattern from almost 9 months to up to 12 months.[29]
In accordance with Simon et al.,[30] the present study also demonstrated improved rate and quality of bone formation in PRF treated sites during the histological evaluation at 6 months. Studies have demonstrated that after 4 months of healing, the sockets were filled with the bone that appears quite mature, and these sites did not exhibit discernable coronal invagination.[29] As documented in an experimental study in dogs by Takahashi et al.,[31] the present study also identified probable remnants of graft material in β-TCP-Cl treated sites. More recently, Muñoz-Corcuera et al.[32] reported that biomaterial (β-TCP-Cl) was not completely resorbed at 6 months. Histological findings in the present study are in accordance with the radiographic findings that reveal the relative density of the socket/ridge in HU in CT scan, which was equivalent to normal bone. However, due to a limited number of biopsies performed in the present study, these results must be carefully evaluated. In the present study, PRF was used alone without a membrane or any other graft material in extraction socket with intact walls. Bioresorbable or nonbioresorbable membrane for graft containment decreases the time required to perform socket preservation procedures. Further, it eliminates complications and reduction in ridge dimension with membrane exposure.[30] When used alone without any other graft material, better quality of bone in healed extraction sockets has been reported besides reducing the chances of inflammatory or foreign body reaction that may result in resorption of socket internal walls. Nonvital bone graft or alloplastic material may take longer time for resorption by macrophages, and residual grafted may weaken the host bone and/or create less than optimum bone next to dental implants.[33]
The combination of β-TCP-Cl in an integrated structure, such as an β-TCP-Cl as used in the study, has osteoconductive property, which facilitates bone formation.[34] Complete bone healing was observed with β-TCP in animals after 3 months, whereas defects in the control group took 5 months to fill with new bone. A significant resorption of the β-TCP granules is expected 3–6 months after placement.[29] However, the small residual amount of β-TCP graft did not compromise placement of the osseointegrated dental implant at 9 months after alveolar socket preservation, Biodegradation of β-TCP occurs by both osteoclastic activity and chemical dissolution by tissue fluids.[35] For the present study, instead of placing a barrier membrane or covering alveolar postextraction socket with mucoperiosteal flap, β-TCP-Cl was left uncovered to healing spontaneously.[36] At 7 days, the process of epithelialization was complete, and the socket was covered without clinical complications. Different mechanisms may explain the apparent blockade of fibrous tissue ingrowth into the porous structure of the β-TCP granules. These include inhibition of fibroblastic proliferation by β-TCP and its metabolites during the dissolution of β-TCP particles;[37] a local decrease in pH during dissolution of material; or direct bonding of β-TCP with a bone through a chemical reaction between calcium ions in the β-TCP particle and carboxyl groups in the collagen polypeptide chains.[4] Thus, a cone of β-TCP-Cl biomaterial can prevent alveolar crest resorption following tooth extraction without the use of a mucoperiosteal flap or a barrier membrane. Formation of the new bone of acceptable quality and quantity permits the placement of an osseointegrated dental implant.[36]
Limitation of the study
The absence of the control group, a small sample size of 30 extraction sockets in 26 patients and follow-up up to 6 months were the shortcomings of the present study. Another limitation of the study was heterogeneous sampling (in terms of site selection and reasons for tooth extraction) that may have influenced the results due to different healing pattern of extraction sockets in the maxillary and mandibular arches.
CONCLUSION
Although this randomized clinical trial was a single centered study, nevertheless, results of this study demonstrated that the use of autologous PRF and β-TCP-Cl were effective in socket preservation. Results obtained from PRF were almost similar to β-TCP-Cl; therefore being autologous, nonimmune, cost-effective, easily procurable regenerative biomaterial, PRF proves to be an insight into the future biofuel for regeneration. Additionally, the presence of residual β-TCP-Cl graft material in histological specimens at 6 months may preclude, although not contraindicate, early placement of the implant, and wait up to 9–12 months may be required to achieve complete healing. However, the availability of PRF material in huge quantity for large extraction sockets may limit its use.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
Acknowledgment
The authors thank Saraswati Dental College, Lucknow, India for providing infrastructural support for the conduct of the study. The authors also thank Dr. Vineet Raj, Ex-Reader, Department of Oral Pathology & Microbiology, Saraswati Dental College, Lucknow for conducting histologic evaluation.
REFERENCES
- 1.Tadic D, Epple M. A thorough physicochemical characterisation of 14 calcium phosphate-based bone substitution materials in comparison to natural bone. Biomaterials. 2004;25:987–94. doi: 10.1016/s0142-9612(03)00621-5. [DOI] [PubMed] [Google Scholar]
- 2.Brkovic BM, Prasad HS, Rohrer MD, Konandreas G, Agrogiannis G, Antunovic D, et al. Beta-tricalcium phosphate/type I collagen cones with or without a barrier membrane in human extraction socket healing: Clinical, histologic, histomorphometric, and immunohistochemical evaluation. Clin Oral Investig. 2012;16:581–90. doi: 10.1007/s00784-011-0531-1. [DOI] [PubMed] [Google Scholar]
- 3.Zerbo IR, Bronckers AL, de Lange GL, van Beek GJ, Burger EH. Histology of human alveolar bone regeneration with a porous tricalcium phosphate. A report of two cases. Clin Oral Implants Res. 2001;12:379–84. doi: 10.1034/j.1600-0501.2001.012004379.x. [DOI] [PubMed] [Google Scholar]
- 4.Zerbo IR, Bronckers AL, de Lange G, Burger EH. Localisation of osteogenic and osteoclastic cells in porous beta-tricalcium phosphate particles used for human maxillary sinus floor elevation. Biomaterials. 2005;26:1445–51. doi: 10.1016/j.biomaterials.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Güngörmüs M, Kaya O. Evaluation of the effect of heterologous type I collagen on healing of bone defects. J Oral Maxillofac Surg. 2002;60:541–5. doi: 10.1053/joms.2002.31852. [DOI] [PubMed] [Google Scholar]
- 6.Ignatius A, Blessing H, Liedert A, Schmidt C, Neidlinger-Wilke C, Kaspar D, et al. Tissue engineering of bone: Effects of mechanical strain on osteoblastic cells in type I collagen matrices. Biomaterials. 2005;26:311–8. doi: 10.1016/j.biomaterials.2004.02.045. [DOI] [PubMed] [Google Scholar]
- 7.Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, Dohan SL, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part V: Histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:299–303. doi: 10.1016/j.tripleo.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Gupta SJ, Jhingran R, Gupta V, Bains VK, Madan R, Rizvi I. Efficacy of platelet-rich fibrin vs. enamel matrix derivative in the treatment of periodontal intrabony defects: A clinical and cone beam computed tomography study. J Int Acad Periodontol. 2014;16:86–96. [PubMed] [Google Scholar]
- 9.Mathur A, Bains VK, Gupta V, Jhingran R, Singh GP. Evaluation of intrabony defects treated with platelet-rich fibrin or autogenous bone graft: A comparative analysis. Eur J Dent. 2015;9:100–8. doi: 10.4103/1305-7456.149653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta V, Bains VK, Singh GP, Mathur A, Bains R. Regenerative potential of platelet rich fibrin in dentistry: Literature review. Asian J Oral Health Allied Sci. 2011;1:23–8. [Google Scholar]
- 11.Simon BI, Gupta P, Tajbakhsh S. Quantitative evaluation of extraction socket healing following the use of autologous platelet-rich fibrin matrix in humans. Int J Periodontics Restorative Dent. 2011;31:285–95. [PubMed] [Google Scholar]
- 12.Suttapreyasri S, Leepong N. Influence of platelet-rich fibrin on alveolar ridge preservation. J Craniofac Surg. 2013;24:1088–94. doi: 10.1097/SCS.0b013e31828b6dc3. [DOI] [PubMed] [Google Scholar]
- 13.Simonpieri A, Del Corso M, Sammartino G, Dohan Ehrenfest DM. The relevance of Choukroun's platelet-rich fibrin and metronidazole during complex maxillary rehabilitations using bone allograft. Part II: Implant surgery, prosthodontics, and survival. Implant Dent. 2009;18:220–9. doi: 10.1097/ID.0b013e31819b5e3f. [DOI] [PubMed] [Google Scholar]
- 14.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e45–50. doi: 10.1016/j.tripleo.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 15.He L, Lin Y, Hu X, Zhang Y, Wu H. A comparative study of platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:707–13. doi: 10.1016/j.tripleo.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal SK, Jhingran R, Bains VK, Srivastava R, Madan R, Rizvi I. Patient-centered evaluation of microsurgical management of gingival recession using coronally advanced flap with platelet-rich fibrin or amnion membrane: A comparative analysis. Eur J Dent. 2015;9:(In press). doi: 10.4103/1305-7456.175686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamada M, Shiota M, Yamashita Y, Kasugai S. Histological and histomorphometrical comparative study of the degradation and osteoconductive characteristics of alpha- and beta-tricalcium phosphate in block grafts. J Biomed Mater Res B Appl Biomater. 2007;82:139–48. doi: 10.1002/jbm.b.30715. [DOI] [PubMed] [Google Scholar]
- 18. [Last accessed on 2016 Jan 5]. Available from: http://www.septodont.com.hr/pdf/rtr_apolonia.pdf .
- 19.Shirota T, Donath K, Ohno K, Matsui Y, Michi K. Effect of age and radiation on bone healing adjacent to hydroxyapatite placed in the tibia of rats. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:285–94. doi: 10.1016/s1079-2104(05)80221-5. [DOI] [PubMed] [Google Scholar]
- 20.Tomkins CC. An introduction to non-parametric statistics for health scientists. Univ Alberta Health Sci J. 2006;3:20–6. [Google Scholar]
- 21.Pinipe J, Mandalapu NB, Manchala SR, Mannem S, Gottumukkala NV, Koneru S. Comparative evaluation of clinical efficacy of ß-tri calcium phosphate (Septodont-RTR)™ alone and in combination with platelet rich plasma for treatment of intrabony defects in chronic periodontitis. J Indian Soc Periodontol. 2014;18:346–51. doi: 10.4103/0972-124X.134573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talwar N, Singh BP, Chand P, Pal US. Use of diagnostic and surgical stent: A simplified approach for implant placement. J Indian Prosthodont Soc. 2010;10:234–9. doi: 10.1007/s13191-010-0036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson DJ. Ridge mapping for determination of alveolar ridge width. Int J Oral Maxillofac Implants. 1989;4:41–3. [PubMed] [Google Scholar]
- 24.Brkovic BM, Prasad HS, Rohrer MD, Konandreas G, Agrogiannis G, Antunovic D, et al. Beta-tricalcium phosphate/type I collagen cones with or without a barrier membrane in human extraction socket healing: Clinical, histologic, histomorphometric, and immunohistochemical evaluation. Clin Oral Investig. 2012;16:581–90. doi: 10.1007/s00784-011-0531-1. [DOI] [PubMed] [Google Scholar]
- 25.Madan R, Mohan R, Bains VK, Gupta V, Singh GP, Madan M. Analysis of socket preservation using polylactide and polyglycolide (PLA-PGA) sponge: A clinical, radiographic, and histologic study. Int J Periodontics Restorative Dent. 2014;34:e36–42. doi: 10.11607/prd.1375. [DOI] [PubMed] [Google Scholar]
- 26.Hämmerle CH, Araújo MG, Simion M. Osteology Consensus Group. Evidence-based knowledge on the biology and treatment of extraction sockets. Clin Oral Implants Res. 2012;23(Suppl 5):80–2. doi: 10.1111/j.1600-0501.2011.02370.x. [DOI] [PubMed] [Google Scholar]
- 27.Louis TK, Carl EM. In: Diagnostic Imaging and Techniques, Contemporary Implant Dentistry. 2nd ed. Carl EM, editor. St. Louis: Mosby; 1999. pp. 73–87. [Google Scholar]
- 28.Cardaropoli G, Araújo M, Hayacibara R, Sukekava F, Lindhe J. Healing of extraction sockets and surgically produced – Augmented and non-augmented – Defects in the alveolar ridge. An experimental study in the dog. J Clin Periodontol. 2005;32:435–40. doi: 10.1111/j.1600-051X.2005.00692.x. [DOI] [PubMed] [Google Scholar]
- 29.Artzi Z, Weinreb M, Givol N, Rohrer MD, Nemcovsky CE, Prasad HS, et al. Biomaterial resorption rate and healing site morphology of inorganic bovine bone and beta-tricalcium phosphate in the canine: A 24-month longitudinal histologic study and morphometric analysis. Int J Oral Maxillofac Implants. 2004;19:357–68. [PubMed] [Google Scholar]
- 30.Simon BI, Zatcoff AL, Kong JJ, O'Connell SM. Clinical and histological comparison of extraction socket healing following the use of autologous platelet-rich fibrin matrix (PRFM) to ridge preservation procedures emploting demineralized freeze dried bone allograft material and membrane. Open Dent J. 2009;3:92–9. doi: 10.2174/1874210600903010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi Y, Marukawa E, Omura K. Application of a new material (ß-TCP/collagen composites) in extraction socket preservation: An experimental study in dogs. Int J Oral Maxillofac Implants. 2013;28:444–52. doi: 10.11607/jomi.2794. [DOI] [PubMed] [Google Scholar]
- 32.Muñoz-Corcuera M, Bascones-Martínez A, Ripollés-de Ramón J. Post-extraction application of beta tricalcium phosphate in alveolar socket. J Osseointegration. 2015;7:8–14. [Google Scholar]
- 33.Becker W, Becker BE, Caffesse R. A comparison of demineralized freeze-dried bone and autologous bone to induce bone formation in human extraction sockets. J Periodontol. 1994;65:1128–33. doi: 10.1902/jop.1994.65.12.1128. [DOI] [PubMed] [Google Scholar]
- 34.Zou C, Weng W, Deng X, Cheng K, Liu X, Du P, et al. Preparation and characterization of porous beta-tricalcium phosphate/collagen composites with an integrated structure. Biomaterials. 2005;26:5276–84. doi: 10.1016/j.biomaterials.2005.01.064. [DOI] [PubMed] [Google Scholar]
- 35.Lu JX, Gallur A, Flautre B, Anselme K, Descamps M, Thierry B, et al. Comparative study of tissue reactions to calcium phosphate ceramics among cancellous, cortical, and medullar bone sites in rabbits. J Biomed Mater Res. 1998;42:357–67. doi: 10.1002/(sici)1097-4636(19981205)42:3<357::aid-jbm3>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 36.Brkovic BM, Prasad HS, Konandreas G, Milan R, Antunovic D, Sándor GK, et al. Simple preservation of a maxillary extraction socket using beta-tricalcium phosphate with type I collagen: Preliminary clinical and histomorphometric observations. J Can Dent Assoc. 2008;74:523–8. [PubMed] [Google Scholar]
- 37.Pioletti DP, Takei H, Lin T, Van Landuyt P, Ma QJ, Kwon SY, et al. The effects of calcium phosphate cement particles on osteoblast functions. Biomaterials. 2000;21:1103–14. doi: 10.1016/s0142-9612(99)00250-1. [DOI] [PubMed] [Google Scholar]


