Abstract
The receptor deleted in colorectal cancer (DCC) mediates the attraction of growing axons to netrin-1 during brain development. In response to netrin-1 stimulation, DCC becomes a signaling platform to recruit proteins that promote axon outgrowth and guidance. The Ras GTPase-activating protein (GAP) p120RasGAP inhibits Ras activity and mediates neurite retraction and growth cone collapse in response to repulsive guidance cues. Here we show an interaction between p120RasGAP and DCC that positively regulates netrin-1-mediated axon outgrowth and guidance in embryonic cortical neurons. In response to netrin-1, p120RasGAP is recruited to DCC in growth cones and forms a multiprotein complex with focal adhesion kinase and ERK. We found that Ras/ERK activities are elevated aberrantly in p120RasGAP-deficient neurons. Moreover, the expression of p120RasGAP Src homology 2 (SH2)-SH3-SH2 domains, which interact with the C-terminal tail of DCC, is sufficient to restore netrin-1-dependent axon outgrowth in p120RasGAP-deficient neurons. We provide a novel mechanism that exploits the scaffolding properties of the N terminus of p120RasGAP to tightly regulate netrin-1/DCC-dependent axon outgrowth and guidance.
Keywords: neurite outgrowth, neurobiology, neurodevelopment, Ras protein, signal transduction, DCC, netrin-1
Introduction
Netrin-1 is one of the many extracellular cues that guide axons to their target during development of the CNS (1–3). It has the ability to attract or repel axons via several transmembrane receptors (4). The netrin-1 receptor deleted in colorectal cancer (DCC)4 is expressed in the spinal cord and forebrain of vertebrates and mediates the netrin-1-dependent attraction of neurons (5, 6). Deficiencies in netrin-1 or DCC expression result in the loss of cerebral and spinal commissures (7, 8). In humans, genetic variations within the dcc locus have been linked to neurological disorders such as congenital mirror movement (9, 10), schizophrenia (11), and Parkinson disease (12). DCC is phosphorylated on threonine, serine, and tyrosine residues in response to netrin-1 stimulation (13). Phosphorylation at C-terminal Tyr-1418 of DCC by Src family kinases is essential for netrin-1 to mediate axon outgrowth and guidance in vertebrates (13–16). The significance of Tyr-1418 phosphorylation in the netrin-1/DCC signaling pathway is highlighted by its position within the P3 motif, a highly conserved region of the DCC C-terminal tail that regulates the recruitment of several proteins, including focal adhesion kinase (FAK), Src, Fyn, ezrin, and Myosin X (13–21).
The neuronal growth cone is found at the distal periphery of an extending axon where the signals from guidance cues are integrated. The signaling cascades initiated by the receptors expressed on the surface of the growth cone produce a coordinated cellular response by regulating cytoskeletal rearrangements (22, 23). Rho GTPases are important mediators of the classic axon guidance cues netrins, slits, ephrins, and semaphorins during cytoskeletal reorganization in growth cones (1, 24). Netrin-1/DCC signal transduction activates Rac1 in neurons, whereas RhoA is inhibited (25–28). Ras GTPases are also regulated by ephrins, semaphorins, and neurotrophins during neuronal development, but their role in netrin-1/DCC signaling has not been explored (24, 29). ERK is activated downstream of netrin-1 and DCC and is required for netrin-1-dependent axon outgrowth and guidance (30–32), but it remains unclear whether Ras mediates ERK activation downstream of netrin-1 and DCC. Until now, the Ras GTPase-activating protein (GAP) p120RasGAP was considered only to be an inhibitor of axon outgrowth and guidance because of the activity of its C-terminal RasGAP domain (33–35). In addition to its C-terminal GAP domain, the N terminus of p120RasGAP, comprising one Src homology (SH) 3 and two SH2 domains, interacts with a wide variety of proteins to regulate cell survival, proliferation, and migration (36, 37). Here we identified p120RasGAP in an SH2 domain screen for proteins that interact with the phosphorylated Tyr-1418 residue of DCC. We show that p120RasGAP forms a signaling complex with DCC in netrin-1-stimulated cortical neurons. p120RasGAP is required to control the basal levels of Ras and ERK activities in neurons. Moreover, p120RasGAP is essential for the attractive response of axons to netrin-1 in cortical neurons, and the N terminus of p120RasGAP is sufficient to mediate netrin-1-mediated axon outgrowth. Together, these findings add another layer to the intricacy of the multiple and essential signaling pathways regulated by netrin-1 and DCC during axon extension and attraction.
Experimental Procedures
Antibodies and Reagents
The following antibodies were purchased: anti-GST, anti-RasGAP B4F8, and anti-DCC A-20 (Santa Cruz Biotechnology); anti-phosphotyrosine 4G10, anti-tubulin, and anti-DCC AF5 (Millipore); anti-DCC G92-13 and anti-FAK (BD Biosciences); anti-phospho-p44/42 MAPK (Erk1/2) (Thr-202/Tyr-204) and anti-p44/42 MAPK (Erk1/2) (Cell Signaling Technology); anti-FAK (Tyr(P)-861) and anti-FAK (Tyr(P)-397) (Life Technologies, Novex); anti-active Ras (NewEast Biosciences); anti-ezrin (provided by M. Arpin (38)); anti-DCC (Tyr(P)-1418) (polyclonal antibodies raised in rabbit against the peptide KPTEDPASVpYEQDDL (DCC- Tyr(P)-1418)); anti-mouse Alexa Fluor 488, anti-mouse and anti-rabbit Cy3, anti-rabbit Alexa Fluor 555 (Life Technologies, Molecular Probes); and anti-goat IgG Cy3 (Sigma). The following reagents were used. Recombinant chick netrin-1 and netrin-1 VI-V were produced and purified as described previously (39, 40). Glutamate was provided by D. Bowie (McGill University), and NGF was from Cedarlane.
Plasmids, Sequence Alignment, and siRNAs
The plasmids pRK5, pRK5-DCC, pRK5-DCC-Y1418F, pRK5-DCC-Y1361F, pRK5-DCC 1–1327, and pRK5-DCC 1–1421 have been described previously (13, 25, 41). The pCDNA3-GAP and pCDNA3-GAP-N (human) constructs were provided by T. Pawson (42). The plasmids encoding GST and GST-human p120RasGAP (N-SH2, C-SH2, and SH2-SH3-SH2) were provided by L. Larose. pmaxGFP was purchased from Lonza. The following siRNAs were purchased: silencer negative control 1 siRNA (Life Technologies/Ambion) and 5′-GCAGGGAAATCTGGAAGCTACCTTA-3′ p120RasGAP siRNA as described previously (Dharmacon) (34).
Purification of GST Fusion Proteins
Production of GST and GST-p120RasGAP proteins was induced with 0.5 mm isopropyl 1-thio-β-d-galactopyranoside for 2 h at 37 °C. Bacterial pellets were resuspended in β-mercaptoethanol phosphate buffer (1:1000 β-mercaptoethanol, 1 mm PMSF, and 1 mm EDTA in PBS) supplemented with 0.5 mg/ml lysozyme and incubated for 30 min at 4 °C. Resuspended pellets were frozen in an ethanol/dry ice bath, thawed in warm water, sonicated, and incubated with 1% Triton X-100 for 10 min at 4 °C. Protein lysates obtained after centrifugation were incubated with glutathione-agarose beads (Sigma) for 2 h at 4 °C. The beads were washed three times in β-mercaptoethanol phosphate buffer and stored at 4 °C, or proteins were eluted with 5 mm glutathione buffer in 50 mm Tris-HCl (pH 8.0) and concentrated with a Nanosep 10K Omega column (PALL) and stored at 4 °C. Purity and concentration were determined by Coomassie Blue-stained SDS-PAGE.
Peptides, SH2 Domain Array, and Dot Blot Assays
The following peptides were purchased from the Keck mass spectrometry and Proteomics Resource: biotinylated TEDPASVpYEQDDLSE (DCC- Tyr(P)-1418). The SH2 domain-based RTK profiling kit (Signosis) was used according to the instructions of the manufacturer with some modifications. Streptavidin-HRP was added directly to samples of 50 or 100 nm of DCC- Tyr(P)-1418 without any primary antibodies. Absorbance was read spectrophotometrically at 450 nm. The absorbance -fold increase was calculated by normalizing the absorbance of each condition with the GST control absorbance. For the dot blots, 20 μg of DCC-Tyr-1418 and 300 μg of BSA were spotted onto nitrocellulose membranes. The membranes were incubated with freshly purified GST or GST-p120RasGAP proteins overnight at 4 °C. The membranes were immunoblotted with anti-GST and anti-DCC- Tyr(P)-1418 antibodies.
Cell Culture, Transfection, and Electroporation
The cell culture was maintained in a humidified incubator at 37 °C with 5% CO2. HEK293 cells were cultured in DMEM (Wisent Bioproducts) supplemented with 10% FBS and antibiotics. Cells were transfected overnight using PEI (PolySciences) according to the instructions of the manufacturer (16, 43). Cortical neurons from embryonic day (E) 18 rat embryos (Charles River Laboratories) were dissociated mechanically and plated on dishes treated with poly-d-lysine (0.1 mg/ml, Sigma-Aldrich) or glass coverslips treated with poly-l-lysine (0.1 mg/ml, Sigma-Aldrich). Neurons were cultured in 10% FBS DMEM for 4 h, and the medium was replaced with Neurobasal-A medium supplemented with 2% B27 and 1% l-glutamine (Invitrogen) (16). The Amaxa rat neuron nucleofector kit (Lonza) was used according to the instructions of the manufacturer to electroporate siRNAs and plasmids. Neurons were treated with the following reagents: purified recombinant netrin-1 or netrin-1 VI-V (200 or 500 ng/ml), glutamate (50 μm), and NGF (100 ng/ml).
GST Pulldowns
Transfected HEK293 cells were lysed in 1% Triton X-100 lysis buffer as described previously (28). Protein lysates (1 mg) were precleared with 30 μl of glutathione-agarose beads (Sigma-Aldrich) for 2 h at 4 °C and incubated with 10 or 20 μg of fresh GST or GST-p120RasGAP proteins coupled to glutathione-agarose beads for 3 h at 4 °C. Beads were washed three times in ice-cold lysis buffer and boiled in SDS sample buffer.
Immunoprecipitation
Cortical neuron (2 days in vitro (DIV)) lysates were prepared as described previously (16). Protein lysates (1 mg) were incubated with 4 μg of anti-p120RasGAP with protein G-Sepharose beads (GE Healthcare) for 3 h at 4 °C.
Immunoblotting and Quantitative Densitometry
Proteins were resolved by SDS-PAGE and transferred onto nitrocellulose membrane. Membranes were stained with Ponceau S (Sigma-Aldrich), immunoblotted with the indicated antibodies, and visualized using enhanced chemiluminescence (Millipore). Optical density was measured using Quantity One software (Bio-Rad). The following optical density ratios were calculated: co-immunoprecipitated DCC and p120RasGAP over immunoprecipitated p120RasGAP, Tyr(P) (p120RasGAP) over p120RasGAP, and p120RasGAP over ezrin. The optical density -fold change was calculated by normalizing the ratio of each condition with the control ratio.
Ras G-LISA Assay
Cortical neuron (2 DIV) lysates were prepared and processed according to the instructions of the manufacturer (Cytoskeleton). Absorbance was read spectrophotometrically at 492 nm. The optical density -fold increase was calculated by normalizing each condition with the optical density of the control.
Immunofluorescence
Cortical neurons (2 DIV) were fixed for 30 min with 3.7% formaldehyde in PBS containing 20% sucrose at 37 °C, quenched for 5 min in 0.1 m glycine at room temperature, permeabilized for 5 min in 0.25% Triton X-100, and blocked for 30 min with 3% BSA. Primary and secondary antibodies were incubated in 0.3% BSA. A 15-min fixation with 10% trichloroacetic acid in water was used for phospho-specific antibodies (44). Neurons were examined with an Olympus IX81 motorized inverted microscope (×40 U PLAN Fluorite and ×60 U PLAN S-APO oil objective lenses) with a CoolSnap 4K camera (Photometrics) and a Zeiss LSM780 confocal microscope (×63/1.40 oil Plan-Apochromat) with 488-nm argon and 561-nm DPSS lasers and a GaAsP detector. Pearson's correlation coefficient and fluorescence intensity quantification were measured with Metamorph software.
Axon Outgrowth and Dunn Chamber Assays
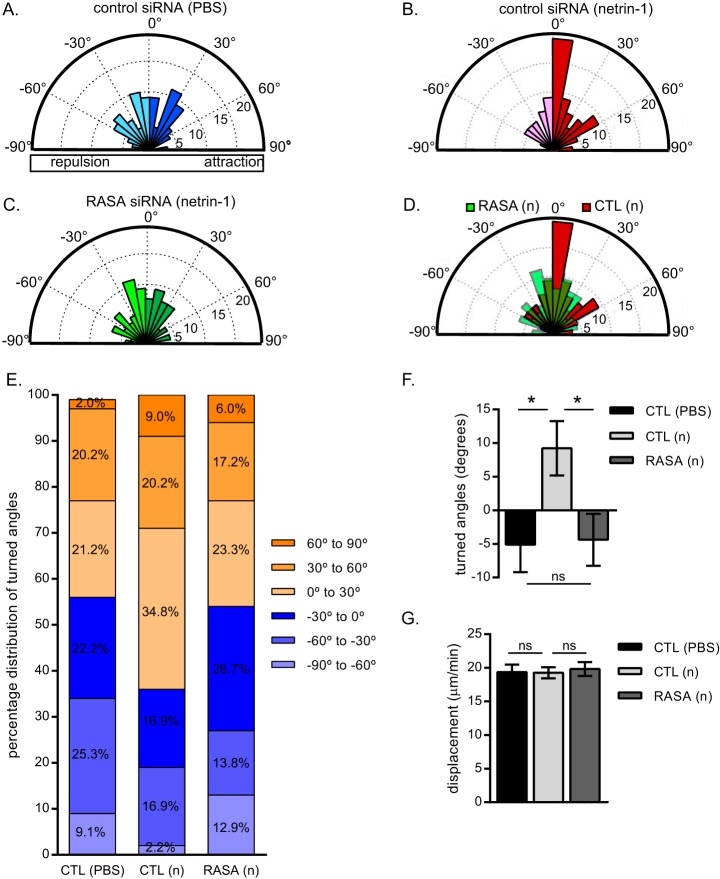
The axon length of GFP-expressing cortical neurons (2 DIV) was measured with Metamorph software. Cortical neurons (2 DIV) were plated on coverslips used for Dunn chamber assembly as described previously (45). Gradients were generated with purified netrin-1 VI-V (200 ng/ml) or buffer containing PBS in the outer well. Cell images were acquired every 3–4 min for at least 90 min on a temperature-controlled stage. Neurites of at least 10 μm in length were tracked in GFP-expressing neurons. The final position of the growth cone was used to determine the angle turned over 90 min relative to the gradient position. Measurements are presented in rose histograms in bins of 10°, with the length of each segment representing the frequency of measurements in percent. Percentage distribution of turned angles, average turned angle, and average displacement are also represented.
Statistical Analysis
Statistical analysis was performed with GraphPad Prism 6. The data are presented as mean ± S.E.
Results
The N-terminal SH2 Domain of p120RasGAP Interacts with DCC via the Phosphorylated Tyr-1418 Residue in Vitro
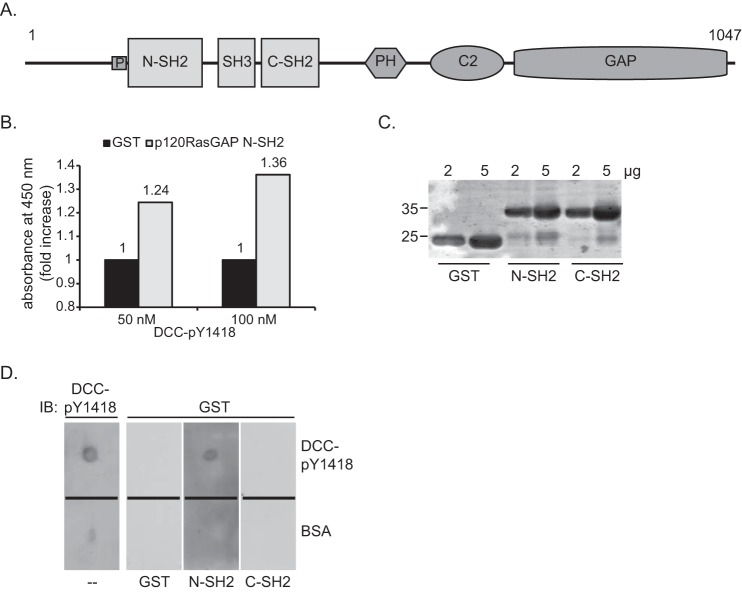
To identify SH2-containing proteins that bind to the phosphorylated Tyr-1418 residue of DCC, we screened an SH2 domain array using as bait a 15-amino acid synthetic DCC peptide comprising Tyr(P)-1418 (DCC- Tyr(P)-1418). Among an array of 46 SH2 domains, the N-terminal SH2 (N-SH2) domain of p120RasGAP (Fig. 1A) bound to the DCC- Tyr(P)-1418 peptide, as revealed by a colorimetric ELISA. The N-SH2 domain of p120RasGAP displayed 1.24- and 1.36-fold increases in absorbance relative to the GST control with 50 and 100 nm of DCC- Tyr(P)-1418 peptide, respectively (Fig. 1B).
FIGURE 1.
The N-terminal SH2 domain of p120RasGAP interacts in vitro with DCC via phosphorylated Tyr-1418. A, p120RasGAP contains a proline-rich region (P) and N-SH2 and C-SH2, SH3, pleckstrin homology (PH), calcium-dependent phospholipid-binding (C2), and GAP domains. B, DCC- Tyr(P)-1418 was used as bait to screen a SH2 domain array by ELISA. Binding of p120RasGAP N-SH2 with 50 and 100 nm of DCC- Tyr(P)-1418 peptide is represented as the -fold increase in absorbance relative to the absorbance obtained with a GST control. C, 2 and 5 μg of purified GST, GST-p120RasGAP N-SH2, and C-SH2 were resolved by SDS-PAGE, and the proteins were stained with Coomassie Blue. D, the DCC- Tyr(P)-1418 peptide was spotted onto nitrocellulose membranes with BSA as a control, and each membrane was incubated with either purified GST, GST-p120RasGAP N-SH2, or C-SH2 (100 ng/ml), followed by immunoblotting (IB) with anti-GST antibodies. One membrane was immunoblotted with phospho-specific anti-DCC-pY1418 (DCC-pY1418) antibodies.
To validate the interaction between the N-SH2 domain of p120RasGAP and DCC- Tyr(P)-1418, purified GST fusion proteins of the individual N-SH2 and C-terminal SH2 (C-SH2) domains of p120RasGAP (Fig. 1, A and C) were tested for their ability to bind to immobilized DCC- Tyr(P)-1418 peptide in a dot blot assay (Fig. 1D). The N-SH2 domain was the sole domain capable of interacting with DCC- Tyr(P)-1418 and did not bind to a control spot of BSA (Fig. 1D). The GST control protein did not bind to the DCC peptide or to BSA (Fig. 1D), and tyrosine phosphorylation of DCC- Tyr(P)-1418 was confirmed with a phospho-specific antibody raised against DCC- Tyr(P)-1418 (Fig. 1D). Therefore, we conclude that the N-SH2 domain of p120RasGAP interacts directly with the synthetic DCC peptide via Tyr(P)-1418.
Netrin-1 Promotes the Association of p120RasGAP with DCC in Embryonic Cortical Neurons
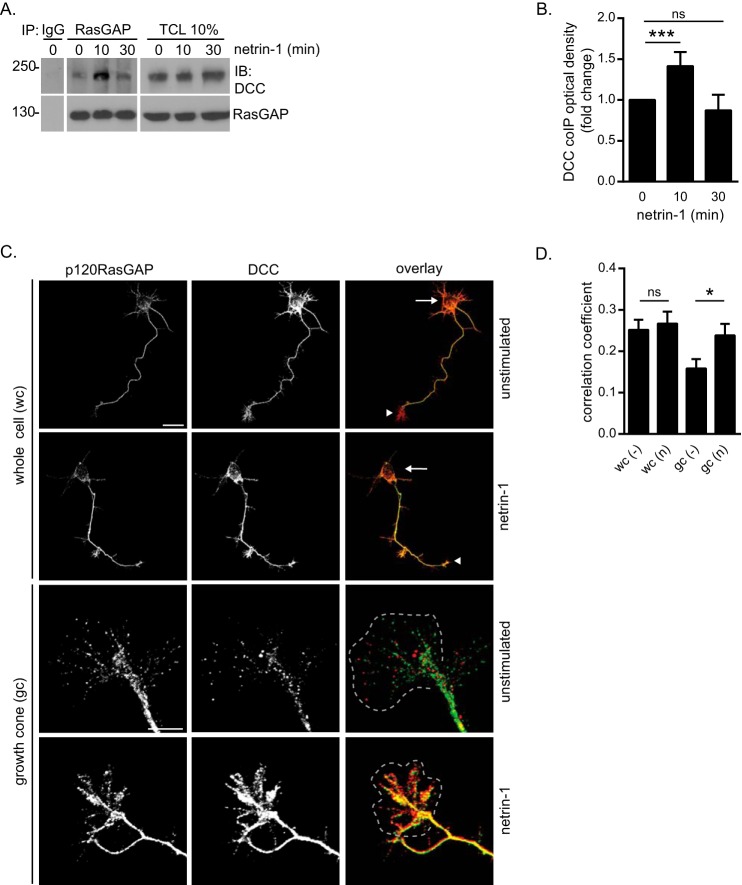
We next examined the interaction of p120RasGAP and DCC in dissociated E18 rat cortical neurons, which are a good cellular model to investigate netrin-1/DCC-induced signaling pathways in the context of axon outgrowth and guidance (6, 16, 28, 43, 46, 47). DCC and p120RasGAP co-immunoprecipitated, and the interaction peaked after 10 min of stimulation with netrin-1 (Fig. 2, A and B). Then we evaluated the localization of DCC and p120RasGAP by immunostaining cortical neurons following netrin-1 stimulation. Visualization by confocal microscopy revealed that p120RasGAP and DCC were both present in the cell bodies, axons, and growth cones of cortical neurons (Fig. 2C). Netrin-1 increased the fluorescence intensity of p120RasGAP and DCC in the axons and growth cones (Fig. 2C). Quantification of the mean Pearson's correlation coefficient (r) revealed that the correlation between p120RasGAP and DCC fluorescence intensity was increased significantly in growth cones after 10 min of netrin-1 stimulation (r = 0.24 versus 0.16, p = 0.028), whereas netrin-1 treatment resulted in no significant change of the co-association (r = 0.27 versus 0.24, p > 0.05) in whole cells (Fig. 2D). We identified p120RasGAP as a novel DCC binding partner in embryonic cortical neurons, and we demonstrated that netrin-1 promotes the recruitment of p120RasGAP to DCC preferentially in growth cones.
FIGURE 2.
p120RasGAP interacts with DCC in netrin-1-induced embryonic cortical neurons. E18 rat cortical neurons were stimulated with netrin-1 (500 ng/ml) for the indicated times after being cultured for 2 DIV. A, p120RasGAP was immunoprecipitated (IP) from cell lysates with anti-p120RasGAP antibodies or mouse IgG as a control. Immunoprecipitated proteins and total cell lysates (TCL) were resolved by SDS-PAGE and immunoblotted (IB) with antibodies against the indicated proteins. B, quantitative densitometry (mean ± S.E.) of DCC co-immunoprecipitated with p120RasGAP is represented as the -fold change relative to 0 min of netrin-1 stimulation for at least three independent experiments (unpaired Student's t test; ***, p < 0.005; ns, not significant). C, E18 embryonic rat cortical neurons (2 DIV) were incubated with 500 ng/ml netrin-1 or left unstimulated for 10 min, immunostained with antibodies against DCC and p120RasGAP, and imaged by confocal microscopy. Arrows indicate cell bodies, and arrowheads indicate growth cones. The gray dashed outlines represent growth cones. Scale bars = 50 μm (whole cells) and 20 μm (growth cones). D, the correlation between DCC and p120RasGAP fluorescence intensities in C was measured with Metamorph software using Pearson's correlation coefficient (mean ± S.E.) in whole cells (wc) and growth cones (gc) in three independent experiments (number of neurons = 65, 51, 54, and 40 from left to right; unpaired Student's t test; ns, not significant; *, p = 0.028). n, netrin-1; −, unstimulated.
p120RasGAP Associates with a DCC Multiprotein Signaling Complex in Netrin-1-stimulated Cortical Neurons
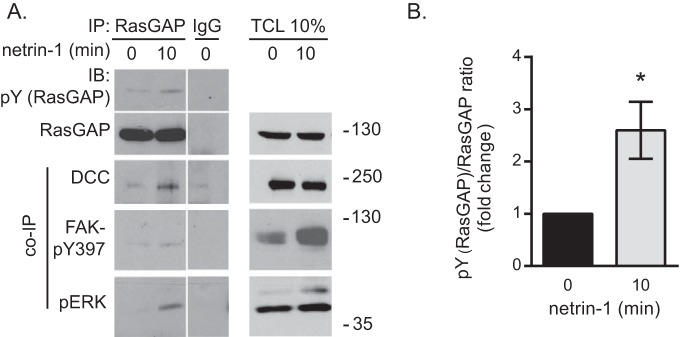
We next examined whether p120RasGAP is tyrosine-phosphorylated in response to netrin-1. We observed that p120RasGAP was tyrosine-phosphorylated in cortical neurons after 10 min of stimulation with netrin-1, concomitant with its association with DCC (Fig. 3, A and B). Moreover, activated ERK (pERK) and FAK (FAK- Tyr(P)-397) co-immunoprecipitated with p120RasGAP and DCC in response to netrin-1 stimulation (Fig. 3A). Together, these results show that the assembly of a DCC-p120RasGAP protein complex with ERK and FAK is induced by netrin-1 in dissociated rat cortical neurons.
FIGURE 3.
p120RasGAP associates with a DCC multiprotein complex in netrin-1-stimulated cortical neurons. A, cortical neurons were stimulated with netrin-1 for 10 min. p120RasGAP was immunoprecipitated from cell lysates with anti-p120RasGAP antibodies or mouse IgGs as a control. Immunoprecipitated proteins and total cell lysates (TCL) were resolved by SDS-PAGE and immunoblotted (IB) with antibodies against the indicated proteins (pY (RasGAP), anti-phosphotyrosine antibodies). Data were taken from the same film exposure. The white line between the RasGAP immunoprecipitation (IP) and IgG lanes indicates that irrelevant lanes were removed digitally from the original image. B, quantitative densitometry (mean ± S.E.) of Tyr(P) (the RasGAP)/RasGAP ratio is represented as the -fold change relative to 0 min of netrin-1 stimulation for at least three independent experiments; unpaired Student's t test; *, p < 0.05).
p120RasGAP Is Required to Maintain Basal Ras and ERK Activities in Cortical Neurons
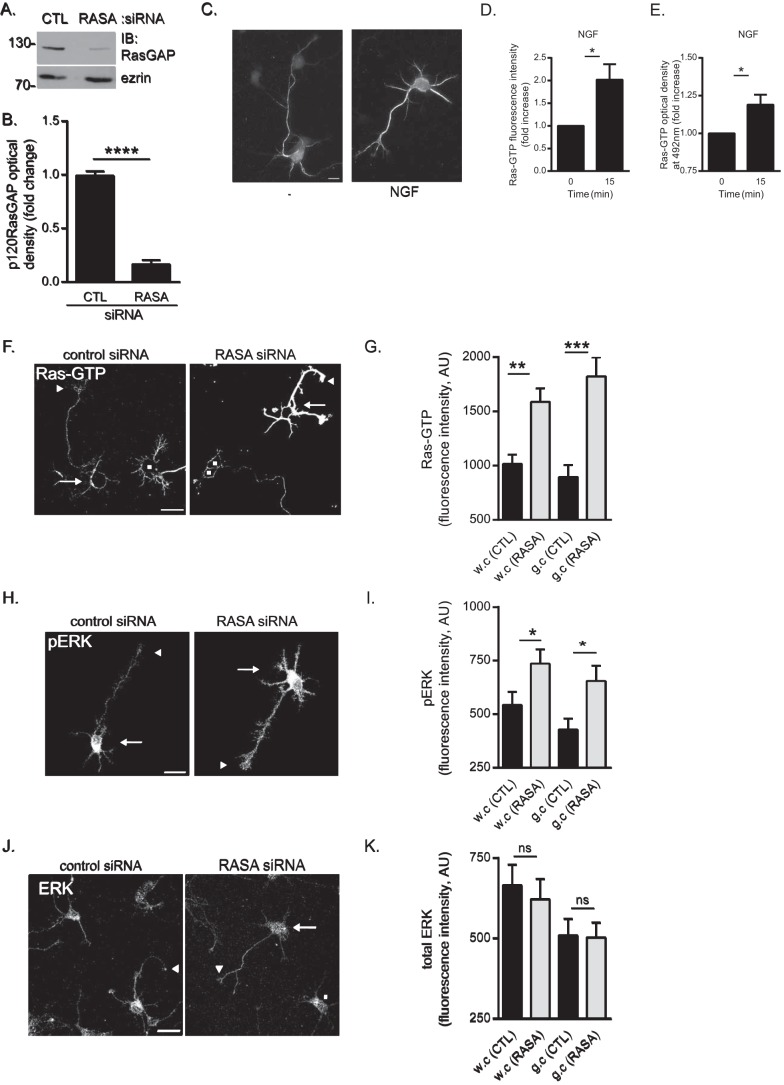
To further characterize the function of p120RasGAP in cortical neurons, endogenous p120RasGAP expression was down-regulated in E18 rat cortical neurons by electroporating synthetic siRNA targeting the 5′ end of p120RasGAP mRNA (RASA) (34), which led to a significant decrease of p120RasGAP expression compared with control siRNA (Fig. 4, A and B). Then we investigated the role of p120RasGAP, a negative regulator of Ras through its C-terminal GAP domain (37), in Ras activation in neurons by immunofluorescence using anti-Ras-GTP antibodies and confocal microscopy. To validate the anti-Ras-GTP antibodies, we first monitored the levels of Ras-GTP in cortical neurons stimulated with NGF, a well established activator of Ras in neurons (48, 49). In cortical neurons stimulated with NGF for 15 min, we observed a significant 2-fold (p < 0.05) increase in Ras-GTP fluorescence intensity (Fig. 4, C and D) that was comparable with the activation of Ras detected by G-LISA assay in NGF-treated cortical neuron lysates (Fig. 4E). In unstimulated p120RasGAP-deficient cortical neurons, Ras-GTP levels showed significant 1.56-fold (p < 0.005) and 2.03-fold (p < 0.001) increases in whole cells and growth cones, respectively (Fig. 4, F and G). Next we monitored the phosphorylation levels of ERK, a major signaling pathway activated downstream of Ras, by immunofluorescence and confocal microscopy in p120RasGAP-deficient neurons (Fig. 4H). We quantified the average fluorescence intensity of pERK at the plasma membrane of cortical neurons. In p120RasGAP-depleted neurons, pERK was increased significantly 1.35-fold (p < 0.05) and 1.53-fold (p < 0.05) in whole cells and growth cones, respectively (Fig. 4I). No change in total ERK fluorescence intensity was detected in p120RasGAP-deficient neurons (Fig. 4, J and K). Overall, p120RasGAP depletion in cortical neurons caused aberrant activation of Ras and ERK in whole cells and in neuronal growth cones. Therefore, these results demonstrate the involvement of p120RasGAP for the proper regulation of basal Ras and ERK activities in cortical neurons.
FIGURE 4.
p120RasGAP is required to maintain basal Ras and ERK activities in cortical neurons. Control (CTL) or p120RasGAP (RASA) siRNA was electroporated in neurons at 0 DIV with pGFP as a transfection reporter plasmid. A, total cell lysates were resolved by SDS-PAGE and immunoblotted (IB) with antibodies against p120RasGAP and ezrin as a loading control. B, quantitative densitometry of A represented as the -fold change (mean ± S.E.) relative to control siRNA measured in 10 independent experiments (unpaired Student's t test; ****, p < 0.0001). C, cortical neurons were stimulated with NGF (100 ng/ml) or left unstimulated (−) for 15 min. Neurons were immunostained with anti-Ras-GTP antibodies. Scale bar = 10 μm. D, Ras-GTP fluorescence intensity and the -fold increase (mean ± S.E.) relative to unstimulated control neurons was measured in at least three independent experiments (n > 50 neurons/condition; unpaired Student's t test; *, p < 0.05). E, the levels of Ras-GTP in each cell lysate were evaluated by G-LISA assay by measuring the absorbance at 492 nm, which is represented as the -fold change (mean ± S.E.) relative to the unstimulated lysate (0 min) in at least three independent experiments (unpaired Student's t test; *, p < 0.05). F, neurons were immunostained with anti-Ras-GTP antibodies. Arrows indicate cell bodies, arrowheads indicate growth cones of GFP-expressing neurons, and squares represent untransfected neurons. Scale bar = 50 μm. G, the Ras-GTP fluorescence intensity (arbitrary units (AU), mean ± S.E.) of GFP-expressing neurons in F was measured in whole cells (w.c) and growth cones (g.c) in three independent experiments (n = 31, 32, 31, and 32 neurons; two-way ANOVA, Fisher's least significant difference post test; **, p < 0.0004; ***, p < 0.0001). H, neurons were immunostained with antibodies against pERK. Arrows indicate cell bodies, and arrowheads indicate growth cones. Scale bar = 50 μm. I, pERK fluorescence intensity (mean ± S.E.) of GFP-expressing neurons in H was measured in whole cells and growth cones in three independent experiments (n = 35, 36, 30, and 35 neurons; two-way ANOVA, Fisher's least significant difference post test; *, p < 0.05). J, neurons were immunostained with antibodies against ERK. Arrows and arrowheads indicate cell bodies and growth cones of GFP-expressing neurons, respectively, and squares indicate untransfected neurons. Scale bar = 50 μm. K, the total ERK fluorescence intensity (mean ± S.E.) of GFP-expressing neurons in J was measured in whole cells and growth cones (n = 41, 38, 41, and 38 neurons) in three independent experiments (two-way ANOVA, Tukey's post test; ns, not significant).
p120RasGAP Is Required for Netrin-1-dependent Attraction of Embryonic Cortical Neurons
To determine whether p120RasGAP regulates netrin-1-dependent chemoattraction, we evaluated the effect of p120RasGAP depletion on cortical growth cone turning in response to a netrin-1 gradient using a Dunn chamber turning assay (45, 50, 51). The growth cones of cortical neurons electroporated with control siRNA turned randomly with no particular preference for any direction when exposed to a control PBS gradient (Fig. 5A) but were attracted to a netrin-1 gradient (Fig. 5B). The introduction of p120RasGAP siRNA inhibited the attractive response to netrin-1, and growth cones reverted to turning randomly (Fig. 5, C and D). 64% of the control growth cones were attracted to the netrin-1 gradient, whereas the percentage that turned toward the netrin-1 gradient in p120RasGAP-deficient neurons (46%) was similar to the percentage that turned toward PBS in control neurons (43%) (Fig. 5E). In fact, the turned angle of growth cones in response to netrin-1 (9.22° ± 4.03°, mean angle turned ± S.E.) was reduced significantly when p120RasGAP was depleted (−4.39° ± 3.86°) (Fig. 5F). p120RasGAP down-regulation did not have an effect on displacement rates during the time the growth cones were imaged (Fig. 5G). These results demonstrate that p120RasGAP is required for netrin-1-dependent chemoattraction.
FIGURE 5.
p120RasGAP is required for netrin-1-dependent attraction. Control (CTL) or p120RasGAP (RASA) siRNA was electroporated with pGFP as a transfection reporter plasmid in E18 rat cortical neurons at 0 DIV. At 2 DIV, the neurons were exposed to a control PBS or a 200 ng/ml netrin-1 VI-V (n) gradient for 90 min. A–C, rose histograms representing the distribution of turned angles of cortical growth cones when exposed to a control PBS (A) or a netrin-1 gradient (B and C). Responses of individual neurons were clustered in 10° bins, and the percentage of total neurons per bin is represented by the radius of each segment (n = 99, 89, and 116 neurons, respectively). D, overlay of the rose histograms in B and C comparing the response to netrin-1 of control and p120RasGAP-deficient neurons. E, the turned angle percentage distribution of cortical growth cones in A–C. F, the mean turned angle (±S.E.) toward the gradient was measured in degrees for each condition. G, the mean displacement ± S.E. for a 90-min netrin-1 treatment was calculated. F and G, one-way ANOVA, Fisher's least significant difference post test; ns, not significant; *, p < 0.05.
The N Terminus of p120RasGAP Is Sufficient to Mediate Netrin-1-dependent Cortical Axon Outgrowth
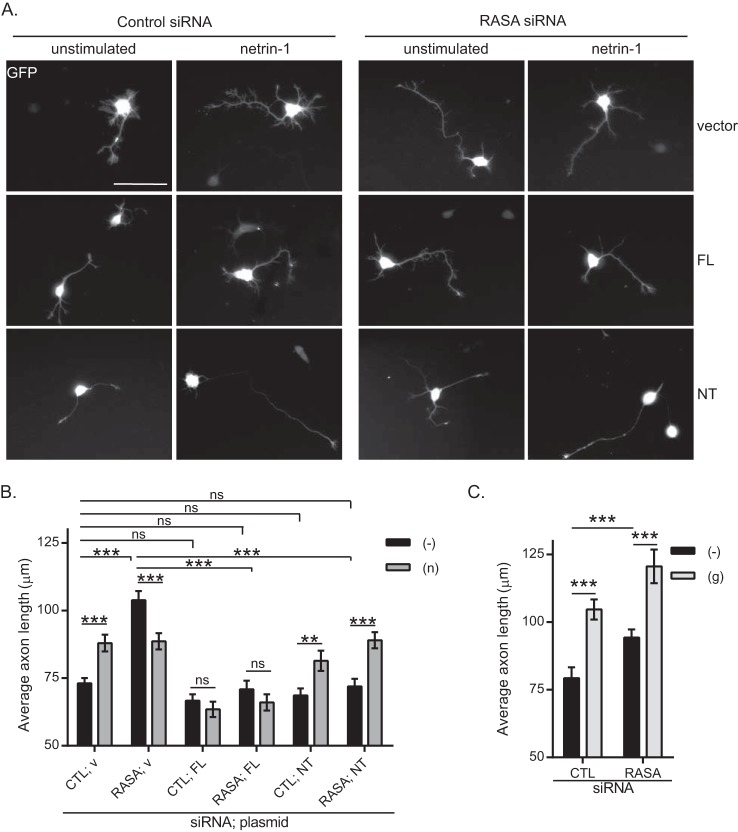
We then explored the role of p120RasGAP in netrin-1-induced axon outgrowth in cortical neurons (16, 28). p120RasGAP siRNA was electroporated together with GFP cDNA as a reporter to visualize the neurons. We measured the average axon length of GFP-expressing neurons after 24 h of incubation with netrin-1. Netrin-1 significantly increased the average axon length compared with unstimulated control neurons (Fig. 6, A, top panels, and B), as described previously (16, 28, 43). In unstimulated neurons, depletion of p120RasGAP resulted in significantly longer axons (103.8 μm, p < 0.0001) (Fig. 6, A, top panels, and B), which correlated well with the higher levels of activated Ras and ERK detected in p120RasGAP-depleted neurons (Fig. 4). However, netrin-1 decreased the length of p120RasGAP-deficient neurons (88.6 μm, p = 0.0051) (Fig. 6, A, top panels, and B). To assess the specificity of the p120RasGAP effects on axon extension, we analyzed the glutamate response in p120RasGAP-deficient neurons. Consistent with previous studies, glutamate stimulation increased axon outgrowth in cortical neurons, and the effect was independent of p120RasGAP (16, 28) (Fig. 6C). Therefore, the lack of stimulation in axon outgrowth of p120RasGAP-deficient neurons was specific to netrin-1, suggesting that p120RasGAP is required for netrin-1 to positively regulate axon extension.
FIGURE 6.
The N terminus of p120RasGAP is sufficient to mediate netrin-1-dependent cortical axon outgrowth. Control (CTL) or p120RasGAP (RASA) siRNA was electroporated in E18 rat cortical neurons at 0 DIV with pGFP as a transfection reporter plasmid. Neurons at 1 DIV were incubated with 200 ng/ml netrin-1 (n) or 50 μm glutamate (g) or left unstimulated (−) for 24 h, and axon outgrowth was assessed in GFP-expressing neurons. A, control vector (v), full-length (FL), and NT p120RasGAP were co-expressed with control or p120RasGAP siRNA and pGFP in cortical neurons. Scale bar = 50 μm. B and C, axon outgrowth was measured and expressed as the average axon length (micrometer, mean ± S.E.) in at least three independent experiments (n in B = 381, 224, 272, 191, 228, 180, 146, 162, 222, 165, 179, and 182 neurons, and n in C = 299, 210, 381, and 190 neurons; two-way ANOVA, Fisher's least significant difference post test; ns, not significant; **, p < 0.005; ***, p < 0.001).
To determine the domains within p120RasGAP responsible for the response to netrin-1, we expressed siRNA-resistant human full-length or the N terminus (NT) domain of p120RasGAP in control or p120RasGAP-deficient neurons (Fig. 6, A and B, and supplemental Fig. S1). Re-expression of p120RasGAP full-length restored basal axon length in p120RasGAP-deficient neurons, whereas it inhibited netrin-1-induced axon outgrowth in both control and p120RasGAP-depleted neurons (Fig. 6, A and B). In contrast, re-expression of the NT of p120RasGAP lacking the GAP domain was sufficient to rescue netrin-1-induced cortical axon extension (p < 0.001) in p120RasGAP-depleted neurons (Fig. 6, A and B). Therefore, the N-terminal scaffolding SH2-SH3-SH2 domains mediate the positive regulation of netrin-1-dependent axon outgrowth by p120RasGAP, whereas overexpression of p120RasGAP blocks the response of axons to netrin-1. This is in agreement with our findings showing that p120RasGAP is necessary to maintain basal Ras-GTP and pERK levels (Fig. 4), which is a requisite for axon extension (34).
The N Terminus of p120RasGAP Interacts with the C Terminus Tail of DCC
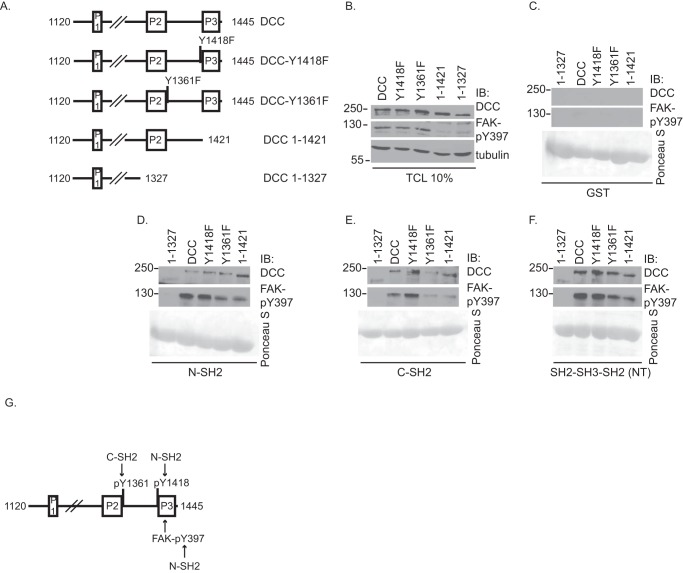
Because the N terminus of p120RasGAP mediates netrin-1-induced axon outgrowth, we next investigated the molecular interaction between the SH2-SH3-SH2 domains of p120RasGAP and DCC. GST-p120RasGAP domains were incubated with protein lysates from HEK293 cells overexpressing either wild-type DCC or DCC mutant proteins (Fig. 7, A and B). DCC proteins did not bind to the GST protein control (Fig. 7C). p120RasGAP N-SH2 interacted with DCC, DCC-Y1361F, and DCC 1–1421 lacking the P3 region (Fig. 7, A and D). However, its binding to DCC 1–1327 truncated before the P2 region was strongly reduced (Fig. 7, A and D). Surprisingly, DCC-Y1418F was still able to interact with p120RasGAP N-SH2 (Fig. 7D). Because FAK interacts with both the P3 region of DCC and p120RasGAP (17, 52, 53), we suspected that DCC-Y1418F might be pulled down indirectly by p120RasGAP N-SH2 via an interaction with FAK (Fig. 7G). The phosphorylation of Tyr-397 on FAK has been shown to mediate its interaction with the N-SH2 domain of p120RasGAP (53). Indeed, GST-N-SH2 pulled down FAK- Tyr(P)-397 along with DCC and DCC mutant proteins (Fig. 7D), suggesting that p120RasGAP N-SH2 is also able to interact with DCC and FAK independently of DCC-Tyr-1418. Furthermore, the expression of either DCC 1–1421 or DCC 1–1327 severely impaired the phosphorylation of FAK on Tyr-397 in total cell lysates, confirming that the P3 region of DCC is important for the phosphorylation of FAK on Tyr-397 in HEK293 cells (Fig. 7B), as reported previously (14, 17). Consequently, the expression of DCC 1–1421 or DCC 1–1327 impaired or completely abolished the interaction of FAK- Tyr(P)-397 with GST-N-SH2, respectively. It also suggested that phosphorylated Tyr-1418, which is able to directly interact in vitro with p120RasGAP N-SH2 (Fig. 1), might mediate the binding of DCC 1–1421 lacking the P3 region with GST-N-SH2 (Fig. 7D).
FIGURE 7.
The N terminus of p120RasGAP interacts with the C terminus of DCC. A, the intracellular domain of rat DCC (amino acids 1120–1445) contains three conserved regions (P1, P2, and P3). The conserved tyrosine residue in the phospho-deficient mutants DCC-Y1418F and DCC-Y1361F was substituted for a phenylalanine residue. The truncation mutants DCC 1–1421 and 1–1327 are truncated before P3 or P2, respectively. B, DCC, DCC-Y1418F, DCC-Y1361F, DCC 1–1421, and DCC 1–1327 were expressed in HEK293 cells. C—F, proteins from cell lysates were pulled down using purified GST control protein (C), GST-p120RasGAP N-SH2 (D), C-SH2 (E), or SH2-SH3-SH2 (NT) (F). Associated proteins and total cell lysates (TCL) were resolved by SDS-PAGE and immunoblotted (IB) with antibodies against DCC, FAK- Tyr(P)-397, and tubulin. GST fusion proteins were stained with Ponceau S. G, the N-SH2 and C-SH2 domains of p120RasGAP interact with phosphorylated Tyr-1418 and Tyr-1361, respectively. Alternatively, the N-SH2 domain can also interact with FAK- Tyr(P)-397 and DCC independently of DCC-Tyr-1418.
It has been shown previously that the 2 SH2 domains of p120RasGAP bind simultaneously to two adjacent tyrosine residues on binding partners such as p190RhoGAP and tyrosine kinase receptors (54–57). Therefore, we examined whether p120RasGAP C-SH2 could also interact with a phosphotyrosine residue in the intracellular domain of DCC. We selected Tyr-1361 as a candidate binding site for C-SH2 because it is the closest to DCC-Tyr-1418 (Fig. 7A). GST-C-SH2 interacted with DCC, DCC-Y1418F, and DCC 1–1421 proteins, whereas its interaction with DCC 1–1327 and DCC-Y1361F was impaired (Fig. 7E), demonstrating that p120RasGAP C-SH2 interacted preferentially with DCC via the phosphorylated Tyr-1361 residue. FAK- Tyr(P)-397 was also pulled down indirectly with p120RasGAP C-SH2 via a DCC or DCC-Y1418F interaction, but its interaction with DCC 1–1327, DCC-Y1361F, and DCC 1–1421 was reduced severely reduced (Fig. 7E). Finally, the SH2-SH3-SH2 domains of p120RasGAP interacted with FAK- Tyr(P)-397 and DCC, DCC-Y1418F, DCC-Y1361F, and DCC 1–1421 but much less with DCC 1–1327 (Fig. 7F). Together, these data demonstrate cooperative binding of the N- and C-SH2 domains of p120RasGAP with the C terminus region of DCC and FAK (Fig. 7G).
Discussion
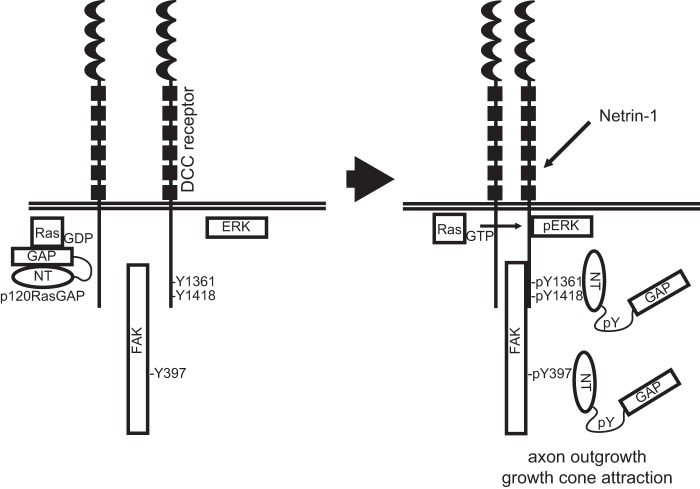
In this study, we report a novel interaction between p120RasGAP and the netrin-1 receptor DCC in embryonic cortical neurons. We demonstrate that p120RasGAP is essential for netrin-1 to induce axon outgrowth and attraction of embryonic cortical neurons and that the N terminus of p120RasGAP serves as a scaffolding protein to mediate netrin-1-induced cortical axon outgrowth (Fig. 8). To our knowledge, this study is the first one to support a positive role for p120RasGAP during axon outgrowth and guidance.
FIGURE 8.
Proposed model for the role of p120RasGAP in netrin-1/DCC-mediated axon outgrowth and guidance. In the absence of netrin-1, the GAP domain of p120RasGAP maintains Ras inactive (Ras-GDP). In response to netrin-1 stimulation, DCC- Tyr(P)-1418/Tyr-1361, FAK (Tyr(P)-397), pERK, and tyrosine-phosphorylated p120RasGAP associate in a multiprotein signaling complex. Through these interactions, mediated by the N-terminal SH2-SH3-SH2 domains (NT) of p120RasGAP, Ras-GTP is released to induce sustained ERK activation in response to netrin-1, leading to axon outgrowth and growth cone attraction.
As a GAP, p120RasGAP stimulates the GTPase activity of Ras to inactivate the GTPase (58–60). Indeed, our findings demonstrate that p120RasGAP-deficient cortical neurons exhibit an aberrant increase in Ras/ERK activities, further supporting the hypothesis that p120RasGAP acts as a negative regulator of Ras activity in cortical neurons in the absence of netrin-1 stimulation. Endo and Yamashita (34) have proposed a model suggesting that p120RasGAP is sequestered and inhibited by the neogenin receptor via an interaction with FAK- Tyr(P)-397. The repulsive guidance cue RGMa releases p120RasGAP from that interaction to mediate the inhibition of Ras activity (34). Other studies have shown that the GAP activity of p120RasGAP is inhibited when its N terminus interacts with p190RhoGAP, FAK, p200RhoGAP, or SOCS-3 (53, 61–63). Tyrosine phosphorylation of these proteins, except for p200RhoGAP, mediated the interaction (53, 61, 63). Here we find that netrin-1 induces the formation of a DCC-p120RasGAP protein complex in cortical neurons. The N-SH2 and C-SH2 domains bind to the phosphorylated tyrosine residues Tyr-1418 and Tyr-1361 of DCC, respectively, which are both conserved among vertebrate DCC orthologs. The phosphorylation of FAK-Tyr-397 also mediates interactions with both the N-SH2 domain of p120RasGAP and DCC (17, 53). Previous studies from Pawson and co-workers (42, 64) have suggested that p120RasGAP regulates the level and duration of PDGF-dependent Ras/ERK activation in fibroblasts. Conversely, the absence of p120RasGAP recruitment to the EphB2 receptor delayed and reduced ephrinB1-dependent ERK inhibition in neuroblastoma cells (33). Extracellular factors such as PDGF, EGF, and FGF have been reported to induce tyrosine phosphorylation of p120RasGAP and activation of Ras (61, 65–69). In our study, we demonstrate that tyrosine phosphorylation of p120RasGAP is concurrent with the interaction of DCC, FAK, and ERK in response to netrin-1. On the basis of these results, we propose that netrin-1 mediates the tyrosine phosphorylation of p120RasGAP, leading to the assembly of a DCC multiprotein complex essential to mediate netrin-1-mediated axon extension and guidance (Fig. 8).
This novel role as a positive modulator of the attractive function of netrin-1 is in marked contrast with previous reports that describe p120RasGAP as a negative regulator of axon outgrowth and guidance in primary neurons (33–35, 70, 71). We further substantiate the evidence for a positive role of p120RasGAP by demonstrating that the N terminus of p120RasGAP is sufficient to mediate axon outgrowth in response to netrin-1. In addition to the PH, C2, and C-terminal GAP domains, the N terminus of p120RasGAP, comprising one SH3 and two SH2 domains, interacts with various proteins to regulate cell survival, proliferation, and migration (36, 37). In cortical neurons, the depletion of p120RasGAP expression leads to a significant increase in axon length of unstimulated neurons and inhibition of netrin-1-induced axon outgrowth. In p120RasGAP-deficient neurons, re-expression of full-length or the N-terminal SH2-SH3-SH2 domains of p120RasGAP is sufficient to restore the axon length to one that is comparable with unstimulated control neurons. However, netrin-1-induced axon outgrowth is only restored by expression of the N-terminal SH2-SH3-SH2 domains of p120RasGAP in these neurons. These results suggest that overexpression of full-length p120RasGAP inhibits netrin-1-mediated outgrowth because of its GAP activity, which represses axon extension, as reported in previous studies (33–35, 70, 71). Therefore, the N terminus of p120RasGAP is sufficient to regulate netrin-1-mediated axon outgrowth independently of the GAP domain. Among its GAP-independent functions, it has been demonstrated that the N terminus of p120RasGAP can also serve as a Ras effector and RhoA inhibitor. First, expression of the N terminus increases Ras and ERK activation in fibroblasts (33) and promotes Ras-dependent differentiation of PC12 cells (72, 73). These studies and others suggest that the N terminus of p120RasGAP forms interactions that promote Ras signaling (58, 74, 75). Second, cell migration is impaired severely in p120RasGAP-deficient mouse fibroblasts, in part because of cell polarity defects and the lack of focal adhesion turnover at the leading edge of cells (42). Both defects are rescued by the expression of the p120RasGAP N terminus (42). Focal adhesion turnover at the leading edge of migrating fibroblasts is regulated by a protein complex comprising p120RasGAP, FAK, and p190RhoGAP that inhibits RhoA activation and promotes cell migration (42, 76, 77). In our study, the recruitment of p120RasGAP to DCC in response to netrin-1 indicates that p120RasGAP regulates specific functions in growth cones, the “leading edge of neurons.” We show that netrin-1 promotes the interaction of p120RasGAP with DCC, pFAK, and pERK in cortical neurons. Other proteins involved in netrin-1/DCC signaling, such as Nck, Src, and ezrin, also interact with p120RasGAP (14, 16, 78–81). With two SH2 and one SH3 domain, the N terminus of p120RasGAP has the ability to engage in a wide range of protein interactions and to form multiple and diverse multiprotein complexes. Therefore, we conclude that netrin-1 activates the scaffolding function of the p120RasGAP N terminus to regulate netrin-1/DCC signaling in neuronal growth cones and promote netrin-1-mediated axon outgrowth.
In conclusion, the depletion of p120RasGAP expression severely impairs netrin-1/DCC-mediated cellular functions in neurons. Netrin-1 cannot induce axon outgrowth or growth cone turning in p120RasGAP-deficient cortical neurons. In fact, netrin-1 decreases axon outgrowth in these neurons. It will be of great interest to address whether this reduction in axon outgrowth results from the loss of p190RhoGAP regulation in p120RasGAP-deficient cortical neurons. We propose that p120RasGAP acts as a molecular clutch and scaffold that primes and engages DCC in attractive netrin-1 signaling by recruiting key regulators (Fig. 8). Until now, the functions of the GAP domain have been studied separately from the functions of the N terminus. Studies that examine how the molecular interactions of p120RasGAP are integrated to initiate a unified cellular response will certainly be more successful at deciphering the intricacies of p120RasGAP functions in the future. The significance of these findings is not only limited to the mechanisms of axon growth and guidance because Ras/p120RasGAP activities and netrin-1/DCC signaling are implicated in the regulation of vascular development and the progression of cancer (36, 37, 82, 83). Notably, the autosomal dominant disorder capillary malformation-arteriovenous malformation is caused by heterozygous mutations in the p120RasGAP locus, RASA1 (84–87). The study of netrin-1 signal transduction outside of the nervous system will undoubtedly add to our understanding of the physiological functions of p120RasGAP.
Author Contributions
J. A. B. and N. L. V. designed the study and wrote the article. J. A. B., N. L. V., and A. E. F. analyzed the data. J. A. B. performed the experiments shown in all figures. P. M. D. performed the experiments shown in Figs. 3 and 4, C–E, J, and K with J. A. B. R. A. and J. A. B. performed the experiments and analyzed the data shown in Fig. 5. T. E. K. provided purified netrin-1 VI-V for the turning assays.
Supplementary Material
Acknowledgments
We thank Jonathan DeGeer for critical reading of the manuscript. We also thank M. Arpin (CNRS, France), D. Bowie, and L. Larose (McGill University, Canada) and T. Pawson (Samuel Lunenfeld Institute, Canada) for reagents. We thank Min Fu and the imaging core facility of the Research Institute of the McGill University Health Centre for assistance with confocal microscopy.
This research was supported by Canadian Institute of Health Research Grant MOP-14701 and the Canada Foundation for Innovation Leaders Opportunity Fund (to N. L. V.). The authors declare that they have no conflicts of interest with the contents of this article.

This article contains supplemental Fig. S1.
- DCC
- deleted in colorectal cancer
- FAK
- focal adhesion kinase
- GAP
- GTPase-activating protein
- SH
- Src homology
- DIV
- day(s) in vitro
- NT
- N terminus
- ANOVA
- analysis of variance.
References
- 1. Bashaw G. J., and Klein R. (2010) Signaling from axon guidance receptors. Cold Spring Harb. Perspect. Biol. 2, a001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lykissas M. G., Batistatou A. K., Charalabopoulos K. A., and Beris A. E. (2007) The role of neurotrophins in axonal growth, guidance, and regeneration. Curr. Neurovasc. Res. 4, 143–151 [DOI] [PubMed] [Google Scholar]
- 3. Sánchez-Camacho C., and Bovolenta P. (2009) Emerging mechanisms in morphogen-mediated axon guidance. BioEssays 31, 1013–1025 [DOI] [PubMed] [Google Scholar]
- 4. Lai Wing Sun K., Correia J. P., and Kennedy T. E. (2011) Netrins: versatile extracellular cues with diverse functions. Development 138, 2153–2169 [DOI] [PubMed] [Google Scholar]
- 5. Keino-Masu K., Masu M., Hinck L., Leonardo E. D., Chan S. S., Culotti J. G., and Tessier-Lavigne M. (1996) Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell 87, 175–185 [DOI] [PubMed] [Google Scholar]
- 6. Shu T., Valentino K. M., Seaman C., Cooper H. M., and Richards L. J. (2000) Expression of the netrin-1 receptor, deleted in colorectal cancer (DCC), is largely confined to projecting neurons in the developing forebrain. J. Comp. Neurol. 416, 201–212 [DOI] [PubMed] [Google Scholar]
- 7. Serafini T., Colamarino S. A., Leonardo E. D., Wang H., Beddington R., Skarnes W. C., and Tessier-Lavigne M. (1996) Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell 87, 1001–1014 [DOI] [PubMed] [Google Scholar]
- 8. Fazeli A., Dickinson S. L., Hermiston M. L., Tighe R. V., Steen R. G., Small C. G., Stoeckli E. T., Keino-Masu K., Masu M., Rayburn H., Simons J., Bronson R. T., Gordon J. I., Tessier-Lavigne M., and Weinberg R. A. (1997) Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature 386, 796–804 [DOI] [PubMed] [Google Scholar]
- 9. Srour M., Rivière J. B., Pham J. M., Dubé M. P., Girard S., Morin S., Dion P. A., Asselin G., Rochefort D., Hince P., Diab S., Sharafaddinzadeh N., Chouinard S., Théoret H., Charron F., and Rouleau G. A. (2010) Mutations in DCC cause congenital mirror movements. Science 328, 592. [DOI] [PubMed] [Google Scholar]
- 10. Depienne C., Cincotta M., Billot S., Bouteiller D., Groppa S., Brochard V., Flamand C., Hubsch C., Meunier S., Giovannelli F., Klebe S., Corvol J. C., Vidailhet M., Brice A., and Roze E. (2011) A novel DCC mutation and genetic heterogeneity in congenital mirror movements. Neurology 76, 260–264 [DOI] [PubMed] [Google Scholar]
- 11. Grant A., Fathalli F., Rouleau G., Joober R., and Flores C. (2012) Association between schizophrenia and genetic variation in DCC: a case-control study. Schizophr. Res. 137, 26–31 [DOI] [PubMed] [Google Scholar]
- 12. Kim J. M., Park S. K., Yang J. J., Shin E. S., Lee J. Y., Yun J. Y., Kim J. S., Park S. S., and Jeon B. S. (2011) SNPs in axon guidance pathway genes and susceptibility for Parkinson's disease in the Korean population. J. Hum. Genet. 56, 125–129 [DOI] [PubMed] [Google Scholar]
- 13. Meriane M., Tcherkezian J., Webber C. A., Danek E. I., Triki I., McFarlane S., Bloch-Gallego E., and Lamarche-Vane N. (2004) Phosphorylation of DCC by Fyn mediates Netrin-1 signaling in growth cone guidance. J. Cell Biol. 167, 687–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li W., Lee J., Vikis H. G., Lee S. H., Liu G., Aurandt J., Shen T. L., Fearon E. R., Guan J. L., Han M., Rao Y., Hong K., and Guan K. L. (2004) Activation of FAK and Src are receptor-proximal events required for netrin signaling. Nat. Neurosci. 7, 1213–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ren X. R., Hong Y., Feng Z., Yang H. M., Mei L., and Xiong W. C. (2008) Tyrosine phosphorylation of netrin receptors in netrin-1 signaling. Neuro-Signals 16, 235–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Antoine-Bertrand J., Ghogha A., Luangrath V., Bedford F. K., and Lamarche-Vane N. (2011) The activation of ezrin-radixin-moesin proteins is regulated by netrin-1 through Src kinase and RhoA/Rho kinase activities and mediates netrin-1-induced axon outgrowth. Mol. Biol. Cell 22, 3734–3746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ren X. R., Ming G. L., Xie Y., Hong Y., Sun D. M., Zhao Z. Q., Feng Z., Wang Q., Shim S., Chen Z. F., Song H. J., Mei L., and Xiong W. C. (2004) Focal adhesion kinase in netrin-1 signaling. Nat. Neurosci. 7, 1204–1212 [DOI] [PubMed] [Google Scholar]
- 18. Liu G., Beggs H., Jürgensen C., Park H. T., Tang H., Gorski J., Jones K. R., Reichardt L. F., Wu J., and Rao Y. (2004) Netrin requires focal adhesion kinase and Src family kinases for axon outgrowth and attraction. Nat. Neurosci. 7, 1222–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu X. J., Wang C. Z., Dai P. G., Xie Y., Song N. N., Liu Y., Du Q. S., Mei L., Ding Y. Q., and Xiong W. C. (2007) Myosin X regulates netrin receptors and functions in axonal path-finding. Nat. Cell Biol. 9, 184–192 [DOI] [PubMed] [Google Scholar]
- 20. Wei Z., Yan J., Lu Q., Pan L., and Zhang M. (2011) Cargo recognition mechanism of myosin X revealed by the structure of its tail MyTH4-FERM tandem in complex with the DCC P3 domain. Proc. Natl. Acad. Sci. U.S.A. 108, 3572–3577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hirano Y., Hatano T., Takahashi A., Toriyama M., Inagaki N., and Hakoshima T. (2011) Structural basis of cargo recognition by the myosin-X MyTH4-FERM domain. EMBO J. 30, 2734–2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dent E. W., Gupton S. L., and Gertler F. B. (2011) The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harb. Perspect. Biol. 3, a001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vitriol E. A., and Zheng J. Q. (2012) Growth cone travel in space and time: the cellular ensemble of cytoskeleton, adhesion, and membrane. Neuron 73, 1068–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hall A., and Lalli G. (2010) Rho and Ras GTPases in axon growth, guidance, and branching. Cold Spring Harb. Perspect. Biol. 2, a001818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li X., Saint-Cyr-Proulx E., Aktories K., and Lamarche-Vane N. (2002) Rac1 and Cdc42 but not RhoA or Rho kinase activities are required for neurite outgrowth induced by the Netrin-1 receptor DCC (deleted in colorectal cancer) in N1E-115 neuroblastoma cells. J. Biol. Chem. 277, 15207–15214 [DOI] [PubMed] [Google Scholar]
- 26. Shekarabi M., and Kennedy T. E. (2002) The netrin-1 receptor DCC promotes filopodia formation and cell spreading by activating Cdc42 and Rac1. Mol. Cell Neurosci. 19, 1–17 [DOI] [PubMed] [Google Scholar]
- 27. Moore S. W., Correia J. P., Lai Wing Sun K., Pool M., Fournier A. E., and Kennedy T. E. (2008) Rho inhibition recruits DCC to the neuronal plasma membrane and enhances axon chemoattraction to netrin 1. Development 135, 2855–2864 [DOI] [PubMed] [Google Scholar]
- 28. Briançon-Marjollet A., Ghogha A., Nawabi H., Triki I., Auziol C., Fromont S., Piché C., Enslen H., Chebli K., Cloutier J. F., Castellani V., Debant A., and Lamarche-Vane N. (2008) Trio mediates netrin-1-induced Rac1 activation in axon outgrowth and guidance. Mol. Cell. Biol. 28, 2314–2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Skaper S. D. (2012) The neurotrophin family of neurotrophic factors: an overview. Methods Mol. Biol. 846, 1–12 [DOI] [PubMed] [Google Scholar]
- 30. Forcet C., Stein E., Pays L., Corset V., Llambi F., Tessier-Lavigne M., and Mehlen P. (2002) Netrin-1-mediated axon outgrowth requires deleted in colorectal cancer-dependent MAPK activation. Nature 417, 443–447 [DOI] [PubMed] [Google Scholar]
- 31. Campbell D. S., and Holt C. E. (2003) Apoptotic pathway and MAPKs differentially regulate chemotropic responses of retinal growth cones. Neuron 37, 939–952 [DOI] [PubMed] [Google Scholar]
- 32. Ming G. L., Wong S. T., Henley J., Yuan X. B., Song H. J., Spitzer N. C., and Poo M. M. (2002) Adaptation in the chemotactic guidance of nerve growth cones. Nature 417, 411–418 [DOI] [PubMed] [Google Scholar]
- 33. Elowe S., Holland S. J., Kulkarni S., and Pawson T. (2001) Downregulation of the Ras-mitogen-activated protein kinase pathway by the EphB2 receptor tyrosine kinase is required for ephrin-induced neurite retraction. Mol. Cell. Biol. 21, 7429–7441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Endo M., and Yamashita T. (2009) Inactivation of Ras by p120GAP via focal adhesion kinase dephosphorylation mediates RGMa-induced growth cone collapse. J. Neurosci. 29, 6649–6662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hancock M. L., Preitner N., Quan J., and Flanagan J. G. (2014) MicroRNA-132 is enriched in developing axons, locally regulates Rasa1 mRNA, and promotes axon extension. J. Neurosci. 34, 66–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pamonsinlapatham P., Hadj-Slimane R., Lepelletier Y., Allain B., Toccafondi M., Garbay C., and Raynaud F. (2009) p120-Ras GTPase activating protein (RasGAP): a multi-interacting protein in downstream signaling. Biochimie 91, 320–328 [DOI] [PubMed] [Google Scholar]
- 37. King P. D., Lubeck B. A., and Lapinski P. E. (2013) Nonredundant functions for Ras GTPase-activating proteins in tissue homeostasis. Sci. Signal 6, re1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Algrain M., Turunen O., Vaheri A., Louvard D., and Arpin M. (1993) Ezrin contains cytoskeleton and membrane binding domains accounting for its proposed role as a membrane-cytoskeletal linker. J. Cell Biol. 120, 129–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Serafini T., Kennedy T. E., Galko M. J., Mirzayan C., Jessell T. M., and Tessier-Lavigne M. (1994) The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell 78, 409–424 [DOI] [PubMed] [Google Scholar]
- 40. Kennedy T. E., Wang H., Marshall W., and Tessier-Lavigne M. (2006) Axon guidance by diffusible chemoattractants: a gradient of netrin protein in the developing spinal cord. J. Neurosci. 26, 8866–8874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tcherkezian J., Brittis P. A., Thomas F., Roux P. P., and Flanagan J. G. (2010) Transmembrane receptor DCC associates with protein synthesis machinery and regulates translation. Cell 141, 632–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kulkarni S. V., Gish G., van der Geer P., Henkemeyer M., and Pawson T. (2000) Role of p120 Ras-GAP in directed cell movement. J. Cell Biol. 149, 457–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. DeGeer J., Boudeau J., Schmidt S., Bedford F., Lamarche-Vane N., and Debant A. (2013) Tyrosine phosphorylation of the Rho guanine nucleotide exchange factor Trio regulates netrin-1/DCC-mediated cortical axon outgrowth. Mol. Cell. Biol. 33, 739–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hayashi K., Yonemura S., Matsui T., and Tsukita S. (1999) Immunofluorescence detection of ezrin/radixin/moesin (ERM) proteins with their carboxyl-terminal threonine phosphorylated in cultured cells and tissues. J. Cell Sci. 112, 1149–1158 [DOI] [PubMed] [Google Scholar]
- 45. Yam P. T., Langlois S. D., Morin S., and Charron F. (2009) Sonic hedgehog guides axons through a noncanonical, Src-family-kinase-dependent signaling pathway. Neuron 62, 349–362 [DOI] [PubMed] [Google Scholar]
- 46. Métin C., Deléglise D., Serafini T., Kennedy T. E., and Tessier-Lavigne M. (1997) A role for netrin-1 in the guidance of cortical efferents. Development 124, 5063–5074 [DOI] [PubMed] [Google Scholar]
- 47. Richards L. J., Koester S. E., Tuttle R., and O'Leary D. D. (1997) Directed growth of early cortical axons is influenced by a chemoattractant released from an intermediate target. J. Neurosci. 17, 2445–2458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wu C., Lai C. F., and Mobley W. C. (2001) Nerve growth factor activates persistent Rap1 signaling in endosomes. J. Neurosci. 21, 5406–5416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Duan J.-H., Wang Y., Duarte D., Vasko M. R., Nicol G. D., and Hingtgen C. M. (2011) Ras signaling pathways mediate NGF-induced enhancement of excitability of small-diameter capsaicin-sensitive sensory neurons from wildtype but not Nf1+/− mice. Neurosci. Lett. 496, 70–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kent C. B., Shimada T., Ferraro G. B., Ritter B., Yam P. T., McPherson P. S., Charron F., Kennedy T. E., and Fournier A. E. (2010) 14-3-3 proteins regulate protein kinase a activity to modulate growth cone turning responses. J. Neurosci. 30, 14059–14067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yam P. T., Kent C. B., Morin S., Farmer W. T., Alchini R., Lepelletier L., Colman D. R., Tessier-Lavigne M., Fournier A. E., and Charron F. (2012) 14-3-3 proteins regulate a cell-intrinsic switch from sonic hedgehog-mediated commissural axon attraction to repulsion after midline crossing. Neuron 76, 735–749 [DOI] [PubMed] [Google Scholar]
- 52. Serpente N., Birling M. C., and Price J. (1996) The regulation of the expression, phosphorylation, and protein associations of pp125FAK during rat brain development. Mol. Cell Neurosci. 7, 391–403 [DOI] [PubMed] [Google Scholar]
- 53. Hecker T. P., Ding Q., Rege T. A., Hanks S. K., and Gladson C. L. (2004) Overexpression of FAK promotes Ras activity through the formation of a FAK/p120RasGAP complex in malignant astrocytoma cells. Oncogene 23, 3962–3971 [DOI] [PubMed] [Google Scholar]
- 54. Holland S. J., Gale N. W., Gish G. D., Roth R. A., Songyang Z., Cantley L. C., Henkemeyer M., Yancopoulos G. D., and Pawson T. (1997) Juxtamembrane tyrosine residues couple the Eph family receptor EphB2/Nuk to specific SH2 domain proteins in neuronal cells. EMBO J. 16, 3877–3888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hu K. Q., and Settleman J. (1997) Tandem SH2 binding sites mediate the RasGAP-RhoGAP interaction: a conformational mechanism for SH3 domain regulation. EMBO J. 16, 473–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kazlauskas A., Ellis C., Pawson T., and Cooper J. A. (1990) Binding of GAP to activated PDGF receptors. Science 247, 1578–1581 [DOI] [PubMed] [Google Scholar]
- 57. Margolis B., Li N., Koch A., Mohammadi M., Hurwitz D. R., Zilberstein A., Ullrich A., Pawson T., and Schlessinger J. (1990) The tyrosine phosphorylated carboxyterminus of the EGF receptor is a binding site for GAP and PLC-γ. EMBO J. 9, 4375–4380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gideon P., John J., Frech M., Lautwein A., Clark R., Scheffler J. E., and Wittinghofer A. (1992) Mutational and kinetic analyses of the GTPase-activating protein (GAP)-p21 interaction: the C-terminal domain of GAP is not sufficient for full activity. Mol. Cell. Biol. 12, 2050–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Marshall M. S., Hill W. S., Ng A. S., Vogel U. S., Schaber M. D., Scolnick E. M., Dixon R. A., Sigal I. S., and Gibbs J. B. (1989) A C-terminal domain of GAP is sufficient to stimulate ras p21 GTPase activity. EMBO J. 8, 1105–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vogel U. S., Dixon R. A., Schaber M. D., Diehl R. E., Marshall M. S., Scolnick E. M., Sigal I. S., and Gibbs J. B. (1988) Cloning of bovine GAP and its interaction with oncogenic ras p21. Nature 335, 90–93 [DOI] [PubMed] [Google Scholar]
- 61. Moran M. F., Polakis P., McCormick F., Pawson T., and Ellis C. (1991) Protein-tyrosine kinases regulate the phosphorylation, protein interactions, subcellular distribution, and activity of p21ras GTPase-activating protein. Mol. Cell. Biol. 11, 1804–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shang X., Moon S. Y., and Zheng Y. (2007) p200 RhoGAP promotes cell proliferation by mediating cross-talk between Ras and Rho signaling pathways. J. Biol. Chem. 282, 8801–8811 [DOI] [PubMed] [Google Scholar]
- 63. Cacalano N. A., Sanden D., and Johnston J. A. (2001) Tyrosine-phosphorylated SOCS-3 inhibits STAT activation but binds to p120 RasGAP and activates Ras. Nat. Cell Biol. 3, 460–465 [DOI] [PubMed] [Google Scholar]
- 64. van der Geer P., Henkemeyer M., Jacks T., and Pawson T. (1997) Aberrant Ras regulation and reduced p190 tyrosine phosphorylation in cells lacking p120-Gap. Mol. Cell. Biol. 17, 1840–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Molloy C. J., Bottaro D. P., Fleming T. P., Marshall M. S., Gibbs J. B., and Aaronson S. A. (1989) PDGF induction of tyrosine phosphorylation of GTPase activating protein. Nature 342, 711–714 [DOI] [PubMed] [Google Scholar]
- 66. Ellis C., Moran M., McCormick F., and Pawson T. (1990) Phosphorylation of GAP and GAP-associated proteins by transforming and mitogenic tyrosine kinases. Nature 343, 377–381 [DOI] [PubMed] [Google Scholar]
- 67. Kaplan D. R., Morrison D. K., Wong G., McCormick F., and Williams L. T. (1990) PDGF β-receptor stimulates tyrosine phosphorylation of GAP and association of GAP with a signaling complex. Cell 61, 125–133 [DOI] [PubMed] [Google Scholar]
- 68. Liu X. Q., and Pawson T. (1991) The epidermal growth factor receptor phosphorylates GTPase-activating protein (GAP) at Tyr-460, adjacent to the GAP SH2 domains. Mol. Cell. Biol. 11, 2511–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Woodcock S. A., and Hughes D. A. (2004) p120 Ras GTPase-activating protein associates with fibroblast growth factor receptors in Drosophila. Biochem. J. 380, 767–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dail M., Richter M., Godement P., and Pasquale E. B. (2006) Eph receptors inactivate R-Ras through different mechanisms to achieve cell repulsion. J. Cell Sci. 119, 1244–1254 [DOI] [PubMed] [Google Scholar]
- 71. Oinuma I., Ishikawa Y., Katoh H., and Negishi M. (2004) The Semaphorin 4D receptor Plexin-B1 is a GTPase activating protein for R-Ras. Science 305, 862–865 [DOI] [PubMed] [Google Scholar]
- 72. Leblanc V., Tocque B., and Delumeau I. (1998) Ras-GAP controls Rho-mediated cytoskeletal reorganization through its SH3 domain. Mol. Cell. Biol. 18, 5567–5578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Nakata H., and Watanabe Y. (1996) Proliferation and differentiation of PC12 cells were affected by p21ras GTPase activating proteins and its deletion mutant proteins. Biochem. Biophys. Res. Commun. 218, 538–543 [DOI] [PubMed] [Google Scholar]
- 74. Tocque B., Delumeau I., Parker F., Maurier F., Multon M. C., and Schweighoffer F. (1997) Ras-GTPase activating protein (GAP): a putative effector for Ras. Cell Signal. 9, 153–158 [DOI] [PubMed] [Google Scholar]
- 75. Yatani A., Okabe K., Polakis P., Halenbeck R., McCormick F., and Brown A. M. (1990) ras p21 and GAP inhibit coupling of muscarinic receptors to atrial K+ channels. Cell 61, 769–776 [DOI] [PubMed] [Google Scholar]
- 76. McGlade J., Brunkhorst B., Anderson D., Mbamalu G., Settleman J., Dedhar S., Rozakis-Adcock M., Chen L. B., and Pawson T. (1993) The N-terminal region of GAP regulates cytoskeletal structure and cell adhesion. EMBO J. 12, 3073–3081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tomar A., Lim S. T., Lim Y., and Schlaepfer D. D. (2009) A FAK-p120RasGAP-p190RhoGAP complex regulates polarity in migrating cells. J. Cell Sci. 122, 1852–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Viswanatha R., Wayt J., Ohouo P. Y., Smolka M. B., and Bretscher A. (2013) Interactome analysis reveals ezrin can adopt multiple conformational states. J. Biol. Chem. 288, 35437–35451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ger M., Zitkus Z., and Valius M. (2011) Adaptor protein Nck1 interacts with p120 Ras GTPase-activating protein and regulates its activity. Cell Signal. 23, 1651–1658 [DOI] [PubMed] [Google Scholar]
- 80. Li X., Meriane M., Triki I., Shekarabi M., Kennedy T. E., Larose L., and Lamarche-Vane N. (2002) The adaptor protein Nck-1 couples the netrin-1 receptor DCC (deleted in colorectal cancer) to the activation of the small GTPase Rac1 through an atypical mechanism. J. Biol. Chem. 277, 37788–37797 [DOI] [PubMed] [Google Scholar]
- 81. Park S., Liu X., Pawson T., and Jove R. (1992) Activated Src tyrosine kinase phosphorylates Tyr-457 of bovine GTPase-activating protein (GAP) in vitro and the corresponding residue of rat GAP in vivo. J. Biol. Chem. 267, 17194–17200 [PubMed] [Google Scholar]
- 82. Larrieu-Lahargue F., Thomas K. R., and Li D. Y. (2012) Netrin ligands and receptors: lessons from neurons to the endothelium. Trends Cardiovasc. Med. 22, 44–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mehlen P., and Guenebeaud C. (2010) Netrin-1 and its dependence receptors as original targets for cancer therapy. Curr. Opin. Oncol. 22, 46–54 [DOI] [PubMed] [Google Scholar]
- 84. Bayrak-Toydemir P., and Stevenson D. (2014) in GeneReviews (Internet) (Pagon R. A., Adam M. P., Ardinger H. H., Bird T. D., Dolan C. R., Fong C.-T., Smith R. J. H., and Stephens K. eds.) December 19, 2013 Ed, University of Washington, Seattle [Google Scholar]
- 85. Eerola I., Boon L. M., Mulliken J. B., Burrows P. E., Dompmartin A., Watanabe S., Vanwijck R., and Vikkula M. (2003) Capillary malformation-arteriovenous malformation, a new clinical and genetic disorder caused by RASA1 mutations. Am. J. Hum. Genet. 73, 1240–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Revencu N., Boon L. M., Mendola A., Cordisco M. R., Dubois J., Clapuyt P., Hammer F., Amor D. J., Irvine A. D., Baselga E., Dompmartin A., Syed S., Martin-Santiago A., Ades L., Collins F., Smith J., Sandaradura S., Barrio V. R., Burrows P. E., Blei F., Cozzolino M., Brunetti-Pierri N., Vicente A., Abramowicz M., Désir J., Vilain C., Chung W. K., Wilson A., Gardiner C. A., Dwight Y., Lord D. J., Fishman L., Cytrynbaum C., Chamlin S., Ghali F., Gilaberte Y., Joss S., Boente Mdel C., Léauté-Labrèze C., Delrue M. A., Bayliss S., Martorell L., González-Enseñat M. A., Mazereeuw-Hautier J., O'Donnell B., Bessis D., Pyeritz R. E., Salhi A., Tan O. T., Wargon O., Mulliken J. B., and Vikkula M. (2013) RASA1 mutations and associated phenotypes in 68 families with capillary malformation-arteriovenous malformation. Hum. Mutat. 34, 1632–1641 [DOI] [PubMed] [Google Scholar]
- 87. Revencu N., Boon L. M., Mulliken J. B., Enjolras O., Cordisco M. R., Burrows P. E., Clapuyt P., Hammer F., Dubois J., Baselga E., Brancati F., Carder R., Quintal J. M., Dallapiccola B., Fischer G., Frieden I. J., Garzon M., Harper J., Johnson-Patel J., Labrèze C., Martorell L., Paltiel H. J., Pohl A., Prendiville J., Quere I., Siegel D. H., Valente E. M., Van Hagen A., Van Hest L., Vaux K. K., Vicente A., Weibel L., Chitayat D., and Vikkula M. (2008) Parkes Weber syndrome, vein of Galen aneurysmal malformation, and other fast-flow vascular anomalies are caused by RASA1 mutations. Hum. Mutat. 29, 959–965 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.